Abstract
A disappointing number of new therapies for pulmonary hypertension (PH) have been successfully translated to the clinic. Adeno-associated viral (AAV) gene therapy has the potential to treat the underlying pathology of PH, but the challenge remains in efficient and safe delivery. The aims of this study were (1) to test the efficacy of endobronchial aerosolization delivery for AAV1-mediated sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a) gene therapy in a PH pig model and (2) to identify the most efficient airway administration modality for in-lung gene therapy in PH. We hypothesized that delivery to the distal bronchi increases lung viral uptake and avoids virus loss in off-target compartments. In part 1 of the study, PH was induced in pigs by surgically banding the pulmonary veins. Two months postsurgery, 1 × 1013 viral genomes (vg) of AAV1.SERCA2a or saline was endobronchially aerosolized using a bronchoscope. Two months after aerosolization, high vg copies (vgc) were detected in the lungs, accompanied by functional and morphometrical amelioration of PH. In part 2 of the study, we directly compared the endobronchial aerosolization gene delivery to the intratracheal aerosolization in PH pigs. Endobronchial delivery demonstrated higher viral expression (6,719 ± 927 vs. 1,444 ± 402 vgc/100 ng DNA, p = 0.0017), suggesting this delivery modality is a promising method for clinical AAV gene therapy for PH.
Keywords: airway delivery, bronchoscope, AAV, pulmonary hypertension, large animal
INTRODUCTION
Current pharmacological treatments for pulmonary hypertension (PH) aim to restore and maintain a healthy circulation in the pulmonary capillary bed. While these improve patient quality of life, none of the existing therapies can cure the disease: the mortality of PH remains high.1–3 Therapies able not only to ameliorate the symptoms but also to reverse vascular remodeling are desperately needed.
Gene therapy has the potential to target disease etiology and holds evident promise.4 Experimental gene therapy studies in rodent models demonstrated improvement of PH.5–7 Despite the encouraging results, a disappointingly low number of new targets translate to clinical studies: <3% of the active trials targeting PH are examining such therapies8 and even fewer demonstrated improved clinical outcomes.9 In an attempt to address this issue, two major bottlenecks of the PH gene therapy are encountered: lack of efficient gene delivery mode to the target tissues and lack of human-like preclinical models.9 Although the lung is amenable to localized delivery of therapeutics, innate obstacles such as airway branching, mucus, lung clearance, and local immunogenic factors make this organ a challenge for gene therapy.10
Furthermore, differences in the anatomy and (patho)physiology between the rodent and human respiratory systems render the direct translation of rodent lung gene therapy studies to humans a challenge. Therefore, there is an imperative need for efficient lung gene delivery methods and gene therapy studies in clinically relevant animal models that recapitulate human PH.
Our group previously demonstrated that restoration of calcium homeostasis through adeno-associated viral (AAV) vector-mediated overexpression of sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a) is beneficial for PH in several animal models.5,11–13 We tested intratracheal and nebulizer gene delivery in previous studies, but the delivery efficacy, specificity, and safety of these approaches were not ideal for clinical application. We hypothesized that targeted, localized gene delivery to the distal bronchi using bronchoscope (endobronchial delivery) maximizes viral uptake and improves specificity to the lungs.
MATERIALS AND METHODS
Animals
The study was performed in accordance with the Guidelines for the Care and Use of Laboratory Animals and was approved by the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee.
Yorkshire pigs were used with starting body weights of 10–15 kg. To test the feasibility of the endobronchial aerosolization method, preliminary experiments were performed in healthy male and female pigs. The main study was performed on female pigs that underwent surgery to induce PH.
Aim of the study
The aim of the study was (1) to test the applicability and efficacy of an endobronchially aerosolized AAV1.SERCA2a gene therapy and (2) to identify the most efficient in-lung delivery mode for gene therapy in PH. For the second aim, we compared the following two delivery methods:
-
1.
Endobronchial delivery: aerosolization of an AAV vector using a sprayer (Olympus PW-6C-1), which was advanced through the working channel of a flexible bronchoscope. The vector was directly administered to the peripheral bronchi.
-
2.
Intratracheal delivery: aerosolization using a rigid Microsprayer (Penn Century Inc.), which was advanced through the endotracheal tube. The vector was administered mid-tracheal, proximal to the carina.
Study design
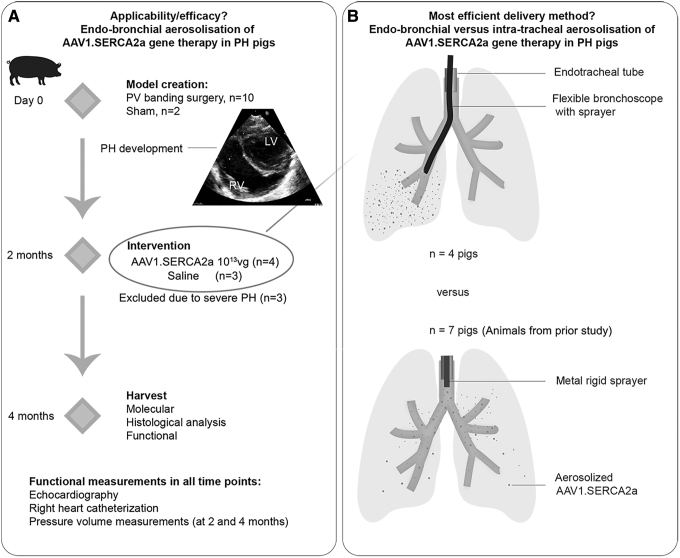
Figure 1 illustrates the study design. In part 1 of the study, a total of 12 animals were included. Ten animals underwent pulmonary vein banding to induce PH. Two healthy pigs were used as sham, which underwent all procedures but pulmonary vein banding. At the beginning of the study, the pigs underwent a clinical screening and baseline cardiac measurements using echocardiography and Swan-Ganz catheter. Blood was withdrawn to screen for neutralizing antibodies against AAV1. After baseline measurements, pigs underwent surgery for PH induction. Two months after the surgery, animals developed PH as evidenced by increased mean pulmonary arterial (PA) pressure consistent with our previous studies.11
Figure 1.
Study design. (A) The part 1 of the study aimed to assess the applicability, efficacy, and safety of endobronchial aerosolization of AAV1.SERCA2a. PH was surgically induced by PV banding in 10–15 kg pigs. Two months later, the animals were randomized based on the AAV1 neutralizing antibody titer: animals with low antibody titer were enrolled in the SERCA2a therapy arm (AAV1.SERCA2a, n = 4), while pigs with high antibody titer received saline (n = 3). The delivery was done using a flexible bronchoscope and a sprayer for aerosolization. Two months after injection, the animals underwent functional evaluation and tissues were harvested. One pig died 1 month after AAV1.SERCA2a therapy likely due to nontherapy-related issue and was excluded from the functional analysis. Echocardiography and right heart catheterization were performed at all 3 time points. Pressure-volume loops were acquired at the 4-month time point. (B) The part 2 of the study was aimed at assessing the most efficient delivery mode for in-lung gene therapy. The lung tissues from animals in the prior study,11 which received intratracheal AAV1.SERCA2a, were used (n = 7). Other than the delivery method, the same experimental protocol was used. AAV1.SERCA2a was aerosolized intratracheally using a metal sprayer, advanced through the endotracheal tube. The stiff metal composition of the sprayer precluded further advancements to bronchi. AAV, adeno-associated viral; PH, pulmonary hypertension; PV, pulmonary vein.
In our experience, animals that develop severe PH in the first 6–8 weeks have a significantly high mortality in the next few weeks (before expected gene expression using single-stranded AAV), associated with progressive and rapid development of right heart failure. Consequently, the exclusion criterion for the study was a mean PA pressure >40 mmHg before therapy randomization. Three animals met this criterion and were excluded from the main study. The randomization of the remaining AAV1.SERCA2a or saline group was done based on the antibody titers. Four pigs received 1 × 1013 viral genomes (vg) of AAV1.SERCA2a and three pigs received saline, which were administered using endobronchial delivery.
For part 2 of the study, a total of seven PH-diseased pigs that underwent intratracheal AAV1.SERCA2a aerosolization (1 × 1013 vg) using a metal sprayer were included. These animals underwent the same experimental protocol, with similar inclusion and randomization criteria, including the AAV1 neutralizing antibody screening. The functional efficacy of this gene therapeutic approach has been previously reported.11
Other specific methods are provided in Supplemental Data.
Statistics
Statistical analysis was performed with GraphPad Prism, version 9.0.2. Data are expressed as means unless otherwise stated. Data distribution was assessed using the D'Agostino Pearson and Shapiro–Wilk tests for normality. The t-test (for Gaussian distribution) or Mann–Whitney test (for non-Gaussian distribution) was used to assess differences between two groups. Differences among multiple means were assessed using analysis of variance (ANOVA) with the Tukey post-test for Gaussian distribution. For vascular remodeling analysis, Kolmogorov–Smirnov test was used to compare difference in distribution between the groups. For measurements over time, the repeated-measures ANOVA with Tukey post hoc test was used. The p-values <0.05 were considered significant.
RESULTS
Endobronchial aerosolization of AAV1.SERCA2a leads to robust lung viral uptake
We first performed preliminary experiments in healthy animals to test the hypothesis that endobronchial aerosolization using a flexible bronchoscope is an effective mode of lung targeting delivery. Evans Blue (1%) was injected as a reporter dye into the middle and lower lung lobes. In the postmortem lung examination, injected lobes were colored an intense blue. The upper noninjected lung lobes had little color deposition, indicating that uptake was mainly in the targeted areas (Supplementary Fig. S1).
Next, to test the AAV uptake of the endobronchial delivery, we injected a healthy animal with an AAV1 vector at the same dose as we intended for the main study (1 × 1013 vg). Four weeks after injection, high viral genome copies (vgc) were detected in the injected areas (Supplementary Table S1). The pig was healthy, and the functional cardiac measurements were normal.
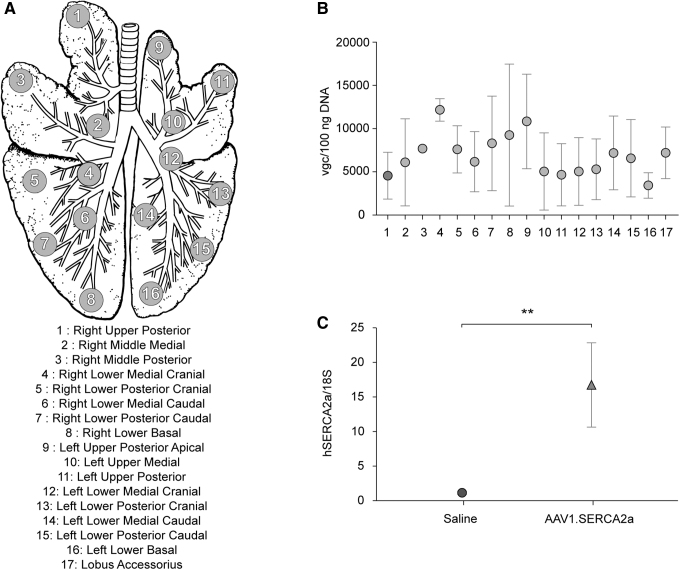
After these preliminary experiments, we proceeded to the main therapeutic study using diseased animals. The primary endpoint of the study was the detection of quantifiable vgc in the PH-diseased lungs. To assess the primary endpoint, we divided the pig lungs into 17 regions based on the bronchi architecture (Fig. 2A). We determined a high viral uptake in the injected regions with moderate heterogeneity (Fig. 2B). Lack of clear correlation between the proximal and distal lung viral uptake suggests more focused uptake of vectors in the target region. As expected, the lungs of saline-injected animals had vgc below the detection range of quantitative polymerase chain reaction (qPCR).
Figure 2.
Regional deposition of AAV1.SERCA2a in the lungs. (A) The lung of each endobronchially treated pig was removed and divided into 17 regions. The numbers indicate the locations from where the samples were taken for molecular and histological analyses. Region 17 (not included in the top image) is the accessory lobe that is common in pigs. The figure was adapted from Ref.31 and modified. (B) Quantitative analysis of vgc in DNA extracted from the lung regions from 4 pigs. (C) Quantitative analysis of hSERCA2a mRNA in real-time qPCR (n = 6 for saline-treated and n = 11 for AAV1.SERCA2a-treated lung tissue areas). Data are mean ± SEM, and individual values were analyzed using the Mann–Whitey test. **p < 0.01. mRNA, messenger RNA; qPCR, quantitative polymerase chain reaction; SEM, standard error of the mean; vgc, viral genome copies.
One animal died unexpectedly 1 month after the virus delivery. Necropsy by an animal pathologist identified an acute necrotizing hemorrhagic pneumonitis, most likely as a result of gastric acid regurgitation and aspiration that occurred near the time of death.
Although no functional measurements could be performed for this animal, we collected cardiac and lung tissues and quantified the vgc. Since the AAV is known to persist over time in the tissues, including the lungs,14 we do not assume a significant difference in the amount of virus in the lung between 1 and 2 months after injection. Indeed, the results were similar to the other injected animals, exhibiting high vgc in the injected lung areas.
Previous studies showed discrepancies between viral uptake and transgene expression in a large animal model.15 To determine if viral uptake indeed resulted in efficacious gene transduction, we isolated RNA and quantified hSERCA2a messenger RNA (mRNA) in the same lung areas that we examined for the vgc. SERCA2a-treated animals exhibited a significantly higher hSERCA2a mRNA compared to the saline-injected pigs (Fig. 2C).
Endobronchial AAV1.SERCA2a gene therapy ameliorates PH in a pig model
The aforementioned results confirmed the hypothesis that endobronchial delivery of AAV1.SERCA2a leads to efficient viral uptake in the diseased lung. To examine if this expression led to improved function as we demonstrated in previous studies,11,13 we analyzed echocardiographic and hemodynamic data at baseline, before AAV/saline delivery, and 2 months after treatment. Right ventricular (RV) pressure-volume measurements were acquired in the terminal study. As expected, pulmonary vein banding led to a significantly elevated mean PA pressure (9.2 ± 0.9 vs. 24 ± 1.0 mmHg, p < 0.0001 baseline vs. 2 months).
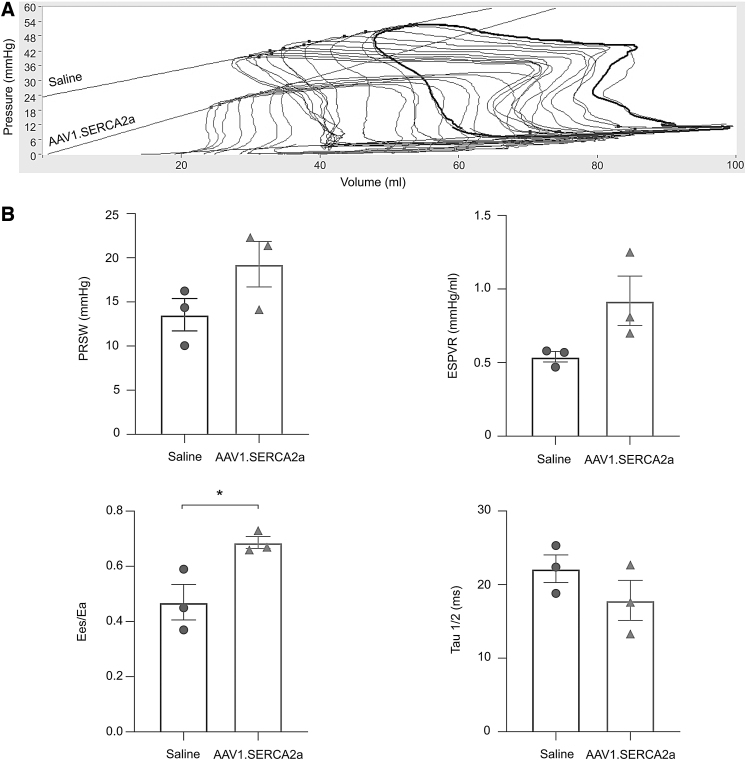
Two months after intervention (AAV1.SERCA2a or saline aerosolization), pigs in the AAV1.SERCA2a-treated group exhibited an amelioration of the mean PA pressure (Fig. 3A) together with lower pulmonary vascular resistance index (Fig. 3B). Invasively measured pressure-volume loops revealed lower RV systolic pressure, higher preload recruitable stroke work, and steeper end-systolic pressure-volume relationship after AAV1.SERCA2a treatment compared to the saline treatment, indicating prevention of RV dysfunction. This was accompanied by the maintained ventriculo-arterial coupling (Fig. 4B), an important prognostic parameter in PH.
Figure 3.

Evolution of pulmonary pressure and pulmonary vascular resistance index in saline and AAV1.SERCA2a-treated PH pigs. (A) Mean PA pressure measured by Swan-Ganz catheter. (B) Pulmonary vascular resistance index calculated as (mPA pressure – pulmonary capillary wedge pressure)/(cardiac output) × (body surface area). Data are mean ± SEM. Change over time was evaluated by repeated-measures ANOVA with Tukey post hoc test. *p < 0.05, **p < 0.01. ANOVA, analysis of variance; PA, pulmonary arterial; PVRI, pulmonary vascular resistance index; WU, wood units.
Figure 4.
RV function indices in saline and AAV1.SERCA2a-treated pigs. RV function was invasively assessed using pressure-volume relationship. (A) Representative pressure-volume loops during transient inferior vena cava occlusion. (B) Load insensitive indices of RV systolic function and Tau½ were all indicative of better RV function in AAV1.SERCA2a-treated pigs. Data are mean ± SEM. *p < 0.05. Ea, effective arterial elastance; Ees, end-systolic elastance; ESPVR, end-systolic pressure-volume relationship; PRSW, preload recruitable stroke work; RV, right ventricular.
In addition, Tau½ as a relaxation parameter was shorter in the AAV1.SERCA2a-treated animals compared to the saline-treated animals (Fig. 4B). Although the trend and degree of benefit seemed similar to our prior study,11 this study was underpowered to draw statistical significance in majority of these functional parameters. We therefore combined these data with our previous study and the results exhibited consistent benefit in the functional parameters after AAV1.SERCA2a gene therapy (Supplementary Fig. S2). Similarly, average RV weight was numerically smaller (Supplementary Fig. S3).
No obvious side effect was noticed in any of the treated animals, except for the one pig that died due to gastric regurgitation. One animal in the AAV1.SERCA2a group exhibited only a moderate effect on the PA pressure and RV function. This animal received the AAV only in the lower lobes due to the technical difficulty during delivery and the vgc result was consistent with the injection profile. We assume that the lower and nonhomogenous expression of AAV1.SERCA2a affected the functional efficacy of the aerosolized gene therapy. Functional benefit of AAV1.SERCA2a gene therapy on PH and RV has been previously shown in both rodent and large animal studies.5,11,13 Since our primary endpoint was met, and for economic and ethical reasons, we rationalized that no more animal inclusion was needed in this direction.
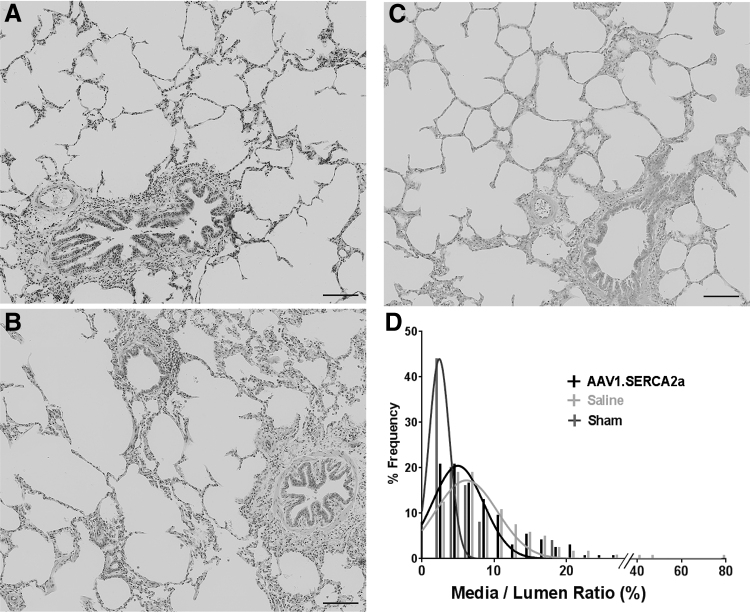
To assess whether the functional effect of the endobronchial therapy was accompanied by changes in vascular remodeling, we performed histological analysis. Media-to-lumen ratio was increased in PH pigs compared to the sham animals, but the distribution showed increased percentage of vasculatures with higher media-to-lumen ratio in pigs treated with saline (median 6.9) compared to the AAV1.SERCA2a (median 5.6, p = 0.04) (Fig. 5).
Figure 5.
Morphometrical analysis of the pulmonary vasculature in sham animals and PH-diseased pigs endobronchially treated with aerosolized saline or AAV1.SERCA2a. (A) Representative lung images stained with hematoxylin and eosin from (A) sham, (B) PH-diseased pig treated with aerosolized saline, and (C) PH-diseased pig treated with aerosolized AAV1.SERCA2a. Scale bar = 100 μm. (D) Distribution of media/lumen ratio with nonlinear fit curves (Gaussian fit) suggested less vascular wall thickening in the AAV1.SERCA2a-treated group compared to saline control. An average of 40 vasculatures was assessed per animal.
Endobronchial aerosolization leads to more efficient viral uptake compared to intratracheal aerosolization in PH-diseased lungs
To determine the optimal delivery mode to the lungs for future translational and clinical PH gene therapy studies, we compared the pulmonary vector uptake of two in-lung aerosolization delivery modes: the endobronchial aerosolization method using a sprayer in a flexible bronchoscope versus the previously tested intratracheal aerosolization using a rigid metal sprayer11 (Fig. 1B). We compared the amount of vgc in four different lung parts using these two methods. We found significantly higher vgc in the lungs of endobronchially aerosolized animals compared to intratracheal aerosolization (Fig. 6; 6,719 ± 927 vs. 1,444 ± 402 vgc/100 ng DNA, p = 0.0017).
Figure 6.
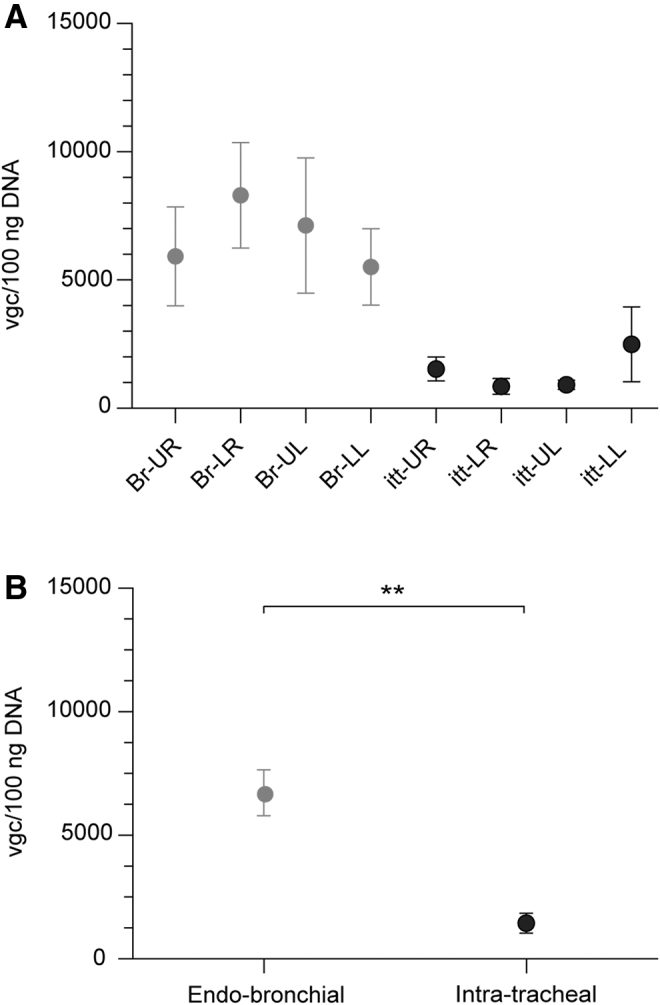
Vgc in the lungs of PH pigs, treated by endobronchial or intratracheal with AAV1.SERCA2a. The vgc were quantified with qPCR in DNA extracted from several regions in the pig lungs. (A) Vgc in four different lung segments (UR: upper right, LR: lower right, UL: upper left, LL: lower left). Four pigs in endobronchial delivery and seven pigs in intratrachial delivery were analyzed. (B) Vgc in the lungs of endobronchial and intratracheal treated animals. Data are mean ± SEM and individual values were analyzed using the Mann–Whitey test. **p < 0.01. Br, endobronchial; itt, intratracheal.
Part of our working hypothesis was that targeted delivery has the advantage of avoiding virus loss in third compartments, such as the trachea. To examine this, we quantified the vgc in the trachea of all AAV1.SERCA2a-treated animals. Three out of seven animals in the intratracheal delivery group showed detectable vgc in the proximal trachea (Supplementary Table S2). All the endobronchial treated pigs exhibited vgc below the detection level.
DISCUSSION
This study demonstrates the clinical applicability, safety, and efficacy of endobronchial AAV1.SERCA2a gene therapy in a pig model of PH. In recent years, a number of gene therapy targets have demonstrated beneficial effects in experimental PH models.6,7,16 The majority of these studies have been performed in vitro and in rodent PH models.17 While these proof-of-concept studies are essential, preclinical applications in large animal models, which more closely resemble the human pulmonary and cardiovascular system, bring clinical relevance.18,19
Large animal studies in the PH field denote a high degree of complexity, due to the severity of the models, the long observation period, and the high mortality, similar to humans. Nevertheless, we anticipate that large animal studies like the one presented here, aimed to test the efficacy along with the clinical applicability and pinpoint the optimal delivery method, are essential before clinical translation.
Accordingly, we systematically compared the efficiency of the currently available in-lung aerosolization delivery modes: the intratracheal delivery using a rigid sprayer versus the endobronchial delivery using a sprayer inserted in a flexible bronchoscope. Both delivery modes have been successfully used in previous studies in healthy humans and patients with cystic fibrosis.20,21 The metal sprayer used in the intratracheal delivery has also been used in healthy primates,15,22–24 mainly with the purpose of studying its therapeutic potential for cystic fibrosis.
To our knowledge, such a comparison has not been performed either in healthy or diseased large animals for in-lung AAV gene delivery. The use of PH-diseased large animals is of clinical relevance, since the efficacy of the delivery method is expected to differ in healthy and PH-diseased individuals, due to various factors such as cellular milieu or inflammatory reaction.
It is of note that disease pathology affects these lung conditions and our results might not apply to other classes of PH such as idiopathic pulmonary arterial hypertension, which accompanies more immune responses. Lack of a large animal model that mimics other PH phenotypes is a limitation of the field. Nevertheless, we believe our results offer clinically useful information, since PH due to left heart disease categorizes the most common forms of clinical PH and some of them are known to have advanced pulmonary vascular remodeling.25
In an effort to bridge basic and translational research, our laboratory has tested AAV1.SERCA2a gene therapy in Yorkshire pig11 and mini pig13 PH models. The rationale behind these large animal studies was based on long-standing evidence that overexpression of SERCA2a inhibits proliferation of vascular smooth muscle cells leading to amelioration of PH.5,12 In our previous studies, AAV1.SERCA2a was delivered either intratracheally11 or using a nebulizer in circuit with the endotracheal tube.13 Both methods successfully ameliorated PH. However, the translation of these delivery modes to human PH remained problematic.
In this study, we demonstrated that endobronchial delivery results in superior vector uptake (Fig. 6) and improved lung specificity compared to the intratracheal delivery. Endobronchial delivery also improved safety, because the delivery tools are more flexible compared to the metal sprayer and it allows direct visualization of delivery during AAV administration.
While the nebulizer has the advantage of being a noninvasive method, vector uptake in the oral cavity and the upper airways diminishes the amount of vector that reaches the target, diseased pulmonary vasculature.15 To account for this loss, a higher dose of AAV1.SERCA2a (5 × 1013 vg, as opposed to 1 × 1013 vg in our study) was required in the nebulizer study.13 Furthermore, the amount of virus that will be exhaled during or after the nebulization procedure is expected to be higher than in-lung delivery methods. Although often neglected in preclinical studies, associated consequences both for the patients' family environment and health care personnel are important safety concerns.19
The important concept underlying our study is the delivery efficiency, which can be defined as the ability to obtain the best possible results with the minimum waste of resources.26 In the case of pulmonary gene therapy, the disadvantages of an inefficient gene delivery are (1) systemic uptake and the associated off-target effects, (2) side effects associated with high-dose AAV therapy, and (3) increased vector cost.
Importantly, adverse clinical events due to high-dose AAV have been recently reported in multiple clinical studies, while the low-dose cohorts showed clinical improvement and no severe undesired effect.27,28 These clinical incidences highlight the importance of improving vector uptake and minimizing off-target distribution, namely, to improve the delivery efficiency, to ensure safety of AAV gene therapy. Our data suggest that endobronchial gene delivery offers high delivery efficiency relative to other airway delivery methods.
For part 1 of the study, we used AAV1.SERCA2a in diseased PH pigs. The primary endpoint of the study was met: the injected areas exhibited a high viral uptake, as measured by the vgc in DNA from lung tissues (Fig. 2). In addition to vgc detection, we demonstrated increased hSERCA2a mRNA expression. Demonstrating transgene mRNA expression is important because it directly leads to therapeutic efficacy. Reporter genes also provide information on distribution, but actual safety needs to be demonstrated using the therapeutic gene for clinical translation.
Functional measurements revealed an amelioration of the mean PA pressure and the pulmonary vascular resistance (Fig. 3), key parameters, which are used for the hemodynamic definition of PH. The increase in the RV afterload was associated with RV dysfunction in the pig PH model, which was ameliorated by AAV1.SERCA2a treatment (Fig. 4). The ventriculo-arterial coupling, as assessed invasively, was maintained in the AAV1.SERCA2a-treated pigs, whereas it fell below 0.5, a sign of decompensation in our previous studies.29 This index has been proven as an important prognostic factor for PH mortality.27
In addition, although the main study was underpowered, combining these data with the previous study indicated a clear signal in functional efficacy. Morphometrical analysis of the pulmonary vasculature revealed increased media thickness and obliteration of small vessels in PH-diseased pigs, consistent with previous studies.11,30 Meanwhile, AAV1.SERCA2a gene therapy ameliorated the vascular remodeling (Fig. 5). In total, these findings strengthen our prior evidence for the beneficial effect of intra-airway AAV1.SERCA2a gene therapy in PH5,11,13 and demonstrate efficacy and safety of the endobronchial delivery method.
Study limitations
Although the endobronchial aerosolized delivery led to an efficient virus uptake, it is important to keep in mind that the study was performed in ventilated animals. Given the technical difficulties of bronchoscopy in nonsedated animals, this is a parameter that needs to be addressed in future clinical studies.
The endobronchial aerosolization study was underpowered for assessing the functional efficacy of the AAV1.SERCA2a endobronchial gene therapy. Based on the mean and standard deviation of mean PA pressure, sample size calculation returned 12 animals per group to achieve statistical significance at 80% power and an alpha of 0.05. Given that SERCA2a overexpression has already been proven beneficial in previous animal studies and the primary endpoint of this study was met, we decided not to include additional animals.
CONCLUSIONS
This study is the first to demonstrate that endobronchial AAV1.SERCA2a aerosolization in diseased PH pigs leads to efficient virus uptake and ameliorates PH progression and RV failure. A direct comparison between in-lung delivery methods showed that endobronchial aerosolization is more efficient for AAV uptake compared to intratracheal aerosolization. There was minimal virus uptake in third compartments with the endobronchial delivery and we anticipate these results indicate that endobronchial delivery method might address the delivery bottleneck for PH gene therapy.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Lifan Liang for the Antibody Assays and the staff of the Center for Comparative Medicine and Surgery (CCMS) at the Icahn School of Medicine at Mount Sinai for their precious help with the animal care and experiments.
AUTHORs' CONTRIBUTIONS
O.B. was the primary researcher for all the experiments and the author of the article. S.T., K.Y., T.K., A.G., T.A., E.K., and K.I. performed experiments. O.B., K.Y., R.M., K.M.F., R.J.H., and K.I. analyzed and interpreted the data and critically reviewed the article.
AUTHOR DISCLOSURE
R.J.H. is a consultant to the Phospholamban Foundation, a nonprofit organization.
FUNDING INFORMATION
This work was supported by National Institutes of Health R01 HL139963 (to K.I.). O.B. was supported by the Deutsche Herzstiftung.
SUPPLEMENTARY MATERIAL
REFERENCES
- 1. Besinque GM, Lickert CA, Pruett JA. The myth of the stable pulmonary arterial hypertension patient. Am J Manag Care 2019;25:S47–S52. [PubMed] [Google Scholar]
- 2. Hoeper MM, Kramer T, Pan Z, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017;50:1700740. [DOI] [PubMed] [Google Scholar]
- 3. Mehari A, Valle O, Gillum RF. Trends in pulmonary hypertension mortality and morbidity. Pulm Med 2014;2014:105864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pilewski JM. Gene therapy for airway diseases: continued progress toward identifying and overcoming barriers to efficiency. Am J Respir Cell Mol Biol 2002;27:117–121. [DOI] [PubMed] [Google Scholar]
- 5. Hadri L, Kratlian RG, Benard L, et al. Therapeutic efficacy of AAV1.SERCA2a in monocrotaline-induced pulmonary arterial hypertension. Circulation 2013;128:512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McMurtry MS, Archer SL, Altieri DC, et al. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest 2005;115:1479–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reynolds AM, Xia W, Holmes MD, et al. Bone morphogenetic protein type 2 receptor gene therapy attenuates hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2007;292:L1182–L1192. [DOI] [PubMed] [Google Scholar]
- 8. Sutendra G, Michelakis ED. Pulmonary arterial hypertension: challenges in translational research and a vision for change. Sci Transl Med 2013;5:208sr5. [DOI] [PubMed] [Google Scholar]
- 9. Lecour S, Botker HE, Condorelli G, et al. ESC working group cellular biology of the heart: position paper: improving the preclinical assessment of novel cardioprotective therapies. Cardiovasc Res 2014;104:399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dolovich MB, Dhand R. Aerosol drug delivery: developments in device design and clinical use. Lancet 2011;377:1032–1045. [DOI] [PubMed] [Google Scholar]
- 11. Aguero J, Ishikawa K, Hadri L, et al. Intratracheal gene delivery of SERCA2a ameliorates chronic post-capillary pulmonary hypertension: a large animal model. J Am Coll Cardiol 2016;67:2032–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lipskaia L, Hadri L, Lopez JJ, et al. Benefit of SERCA2a gene transfer to vascular endothelial and smooth muscle cells: a new aspect in therapy of cardiovascular diseases. Curr Vasc Pharmacol 2013;11:465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Watanabe S, Ishikawa K, Plataki M, et al. Safety and long-term efficacy of AAV1.SERCA2a using nebulizer delivery in a pig model of pulmonary hypertension. Pulm Circ 2018;8:2045894018799738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zincarelli C, Soltys S, Rengo G, et al. Comparative cardiac gene delivery of adeno-associated virus serotypes 1–9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clin Transl Sci 2010;3:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beck SE, Laube BL, Barberena CI, et al. Deposition and expression of aerosolized rAAV vectors in the lungs of Rhesus macaques. Mol Ther 2002;6:546–554. [DOI] [PubMed] [Google Scholar]
- 16. Pozeg ZI, Michelakis ED, McMurtry MS, et al. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation 2003;107:2037–2044. [DOI] [PubMed] [Google Scholar]
- 17. Paulin R, Barrier M, Bonnet S. New therapeutics for pulmonary arterial hypertension: do gene therapies have translational values? Clin Invest 2011;1:363–366. [Google Scholar]
- 18. Bolli R, Ghafghazi S. Cell therapy needs rigorous translational studies in large animal models. J Am Coll Cardiol 2015;66:2000–2004. [DOI] [PubMed] [Google Scholar]
- 19. Secher T, Bodier-Montagutelli E, Guillon A, et al. Correlation and clinical relevance of animal models for inhaled pharmaceuticals and biopharmaceuticals. Adv Drug Deliv Rev 2020;167:148–169. [DOI] [PubMed] [Google Scholar]
- 20. Harvey BG, Hackett NR, Ely S, et al. Host responses and persistence of vector genome following intrabronchial administration of an E1(−)E3(−) adenovirus gene transfer vector to normal individuals. Mol Ther 2001;3:206–215. [DOI] [PubMed] [Google Scholar]
- 21. Harvey BG, Leopold PL, Hackett NR, et al. Airway epithelial CFTR mRNA expression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus. J Clin Invest 1999;104:1245–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beck SE, Jones LA, Chesnut K, et al. Repeated delivery of adeno-associated virus vectors to the rabbit airway. J Virol 1999;73:9446–9455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fischer AC, Smith CI, Cebotaru L, et al. Expression of a truncated cystic fibrosis transmembrane conductance regulator with an AAV5-pseudotyped vector in primates. Mol Ther 2007;15:756–763. [DOI] [PubMed] [Google Scholar]
- 24. Guggino WB, Benson J, Seagrave J, et al. A preclinical study in rhesus macaques for cystic fibrosis to assess gene transfer and transduction by AAV1 and AAV5 with a dual-luciferase reporter system. Hum Gene Ther Clin Dev 2017;28:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosenkranz S, Gibbs JS, Wachter R, et al. Left ventricular heart failure and pulmonary hypertension. Eur Heart J 2016;37:942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burches E, Burches M. Efficacy, effectiveness and efficiency in the health care: the need for an agreement to clarify its meaning. Int Arch Public Health Community Med 2020;4:035. [Google Scholar]
- 27. Mendell JR, Al-Zaidy S, Shell R, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med 2017;377:1713–1722. [DOI] [PubMed] [Google Scholar]
- 28. Wilson JM, Flotte TR. Moving forward after two deaths in a gene therapy trial of myotubular myopathy. Hum Gene Ther 2020;31:695–696. [DOI] [PubMed] [Google Scholar]
- 29. Aguero J, Ishikawa K, Hadri L, et al. Characterization of right ventricular remodeling and failure in a chronic pulmonary hypertension model. Am J Physiol Heart Circ Physiol 2014;307:H1204–H1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pereda D, Garcia-Alvarez A, Sanchez-Quintana D, et al. Swine model of chronic postcapillary pulmonary hypertension with right ventricular remodeling: long-term characterization by cardiac catheterization, magnetic resonance, and pathology. J Cardiovasc Transl Res 2014;7:494–506. [DOI] [PubMed] [Google Scholar]
- 31. Li Bassi G, Fernandez-Barat L, Saucedo L, et al. Endotracheal tube biofilm translocation in the lateral Trendelenburg position. Crit Care 2015;19:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.