Abstract
The roles of the kynurenine pathway (KP) of tryptophan (Trp) degradation in serotonin deficiency in major depressive disorder (MDD) and the associated inflammatory state are considered in the present study. Using molecular docking in silico, we demonstrate binding of antidepressants to the crystal structure of tryptophan 2,3-dioxygenase (TDO) but not to indoleamine 2,3-dioxygenase (IDO). TDO is inhibited by a wide range of antidepressant drugs. The rapidly acting antidepressant ketamine does not dock to either enzyme but may act by inhibiting kynurenine monooxygenase thereby antagonising glutamatergic activation to normalise serotonin function. Antidepressants with anti-inflammatory properties are unlikely to act by direct inhibition of IDO but may inhibit IDO induction by lowering levels of proinflammatory cytokines in immune-activated patients. Of six anti-inflammatory drugs tested, only salicylate docks strongly to TDO and apart from celecoxib, the other five dock to IDO. TDO inhibition remains the major common property of antidepressants and TDO induction the most likely mechanism of defective serotonin synthesis in MDD. TDO inhibition and increased free Trp availability by salicylate may underpin the antidepressant effect of aspirin and distinguish it from other nonsteroidal anti-inflammatory drugs. The controversial findings with IDO in MDD patients with an inflammatory state can be explained by IDO induction being overridden by changes in subsequent KP enzymes influencing glutamatergic function. The pathophysiology of MDD may be underpinned by the interaction of serotonergic and glutamatergic activities.
Keywords: 3-dioxygenase, Glutamatergic activity, Kynurenine monooxygenase, Kynurenine pathway, Serotonin, Tryptophan 2
Introduction
In accordance with the monoamine deficiency hypothesis of affective disorders, antidepressant drug development is based on inhibition of oxidation or reuptake of monoamine, particularly serotonin (5-hydroxytryptamine or 5-HT) and noradrenaline. More recently, inflammation has been implicated in major depressive disorder (MDD) and postulated to induce a serotonin deficiency by accelerating tryptophan (Trp) degradation along the kynurenine (Kyn) pathway (KP) by induction of the extrahepatic Trp-degrading enzyme indoleamine 2,3-dioxygenase (IDO1). The central serotonin pathway contributes very little (∼1%) to Trp degradation, whereas the KP contributes 95% [1,2]. Normally, the hepatic KP (rate-limited by Trp 2,3-dioxygenase or TDO, formerly Trp pyrrolase; EC 1.13.11.11) contributes 90%, whereas the extrahepatic pathway (rate-limited by IDO1; EC 1.13.11.17) contributes the remaining 5%, but, after immune activation, the extrahepatic pathway assumes a greater quantitative significance. The importance of liver TDO in control of Trp availability for cerebral serotonin synthesis is well established and evidenced more recently from studies demonstrating that deletion of the mouse TDO2 gene increases plasma [Trp] by 9.3- to 12.7-fold [3,4] and brain [Trp] by 10.6-fold in association with increased serotonin synthesis [5]. Deletion of the IDO1 or IDO2 genes makes little difference to Trp availability to the brain or to central serotonin synthesis [5]. Brain [Trp] is the major determinant of serotonin synthesis, because Trp hydroxylase (TPH2), the rate-limiting enzyme, is partially (≤50%) saturated with its Trp substrate [6], hence the suggestion of Trp as the key to boosting brain serotonin synthesis in MDD [7].
The KP produces a range of biologically active metabolites. Of particular importance to MDD are the antagonist kynurenic acid (KA) and agonist quinolinic acid (QA) of the N-methyl-D-aspartate (NMDA) type of receptors of the excitatory amino acid glutamate, and immunomodulation by these two metabolites and by 3-hydroxykynurenine (3-HK), 3-hydroxyanthranilic acid (3-HAA) picolinic acid (PA) and xanthurenic acid (XA). Overproduction of these metabolites can be deleterious to neuronal function and immune activity. Thus, modulation of NMDA receptor function can influence the state of neuronal excitability, which appears to be determined by the balance between KA and QA [8], and it is of interest that altered NMDA receptor function through modulation of KP activity has been implicated in the rapid antidepressant action of ketamine [9]. On the other hand, elevation of 3-HK, 3-HAA and QA induces immunosuppression by undermining T-cell function [1]. Increased production of Kyn metabolites, however, is not determined solely by TDO or IDO activity but also occurs through increased flux of plasma free (non-albumin-bound) Trp down the KP [10]. Also important is that KP metabolite levels can still be altered by changes in pathway enzyme activities in the absence of TDO/IDO induction or increased flux of free Trp.
The central serotonin deficiency in MDD is due to excessive peripheral and/or central Trp degradation via the KP. Accelerated Trp degradation in MDD is suggested by the widely reported decrease in circulating [Trp] [7] and from measurement of breath 13CO2 following administration of 1-13C-Trp [11]. The serotonin deficiency may involve liver TDO induction by cortisol, whose levels are elevated in at least 50% of depressed patients, or its activation by Trp-mediated catecholamine-related mechanisms as part of the stress response [7]. That immune activation acting through IDO induction in the periphery or the brain can also induce a central serotonin deficiency is exemplified by the incidence of depression in hepatitis C virus (HCV) patients treated with the IDO inducer interferon-α (IFN-α) (see [12] and references cited therein). The concept of IDO induction leading to serotonin deficiency in MDD may have arisen in part by extrapolation from the above and other studies with IFN-α. In HCV patients and related experimental models, the KP is already compromised by the virus and adding IFN-α simply exaggerates the KP changes and effects. Thus, HCV before initiation of antiviral therapy decreases plasma [Trp] by ∼9–24% and increases plasma [Kyn] by ∼18–24% [13,14]. In the mouse hepatitis virus model, the simultaneous elevation of Trp and Kyn levels in liver [15] is consistent with increased flux of plasma free (non-albumin-bound) Trp [16].
Although the immune status in MDD has received much attention [17], it is not clearly understood or well-defined. Not all MDD patients have an activated immune system and, when present, inflammation is of a mild and/or transient nature and its reversal is not associated with clinical outcome [9,17]. These features, compounded by heterogeneity of the disorder, associated comorbidities and biological factors, such as hypothalamic–pituitary–adrenal (HPA) axis activity and the immune status, illustrate the need for further studies. It is therefore reasonable to assume that immune activation in MDD, if strong enough, can impact Trp metabolism negatively through IDO induction, but that other mechanisms not involving IDO or overriding its effects must also operate in this disorder.
Whereas a range of mechanisms of actions of antidepressants exist, none of which is common to the majority of the drugs, except their ability to inhibit liver TDO activity both in vitro and after administration [7]. Antidepressants inhibit TDO activity significantly in doses as little as 0.5 mg/kg body wt [7]. Whereas much information is available on liver TDO inhibition by antidepressants, little is known about their potential effects on IDO activity, possibly because IDO activity is hardly detectable in the absence of immune activation and, even when IDO is highly expressed, investigators use the plasma [Kyn]/[Trp] ratio as an indirect measure of its activity, though this ratio is not specific to IDO, as it can also reflect changes in TDO activity, flux of plasma free Trp and activities of KP enzymes such as Kyn monooxygenase (KMO), and kynureninase [10]. The technique of molecular docking in silico is a useful tool for screening potential inhibitors of target proteins [18,19], but can also be applied to confirm binding of known inhibitors, e.g., with the TDO inhibitors 680C91 and LM10 as potential cancer therapies [20]. As far as we could ascertain, ours is the first group to use this technique to demonstrate binding (docking) of established antidepressant drugs to the TDO crystal structure. We showed that all eight antidepressants tested fitted well in the TDO active site with docking scores (kcal/mol) ranging from −109.796 (sertraline) to −139.706 (paroxetine) [21]. In the present paper, we report similar results with the TDO inhibitors tianeptine and venlafaxine and the failure of the non-antidepressant pargyline and the anti-inflammatory mefenamic acid, both of which do not inhibit TDO, to dock to it. Venlafaxine was chosen as the first joint serotonin-noradrenaline reuptake inhibitor (SNRI) to be shown to inhibit TDO. Tianeptine was chosen because its mode of action is still unclarified and, although it stimulates, rather than inhibits, serotonin reuptake, it is a clinically effective antidepressant, even in patients with poor response to serotonin-specific reuptake inhibitor (SSRI) monotherapy [22]. We also examined docking to IDO of the above 2 and 4 more antidepressants previously shown [21] to dock to TDO and compared their docking behaviours toward TDO and IDO with those of 6 nonsteroidal anti-inflammatory drugs (NSAIDs) not examined before. We further explored in some detail docking and other effects of aspirin, the only NSAID to dock to and inhibit TDO activity via its active metabolite salicylic acid, and ketamine, as its mode of action appears to be a novel one, modulating glutamatergic activity to restore serotonin homeostasis.
Materials and methods
Drugs and chemicals
Haematin hydrochloride and L-tryptophan were purchased from the Sigma chemical Co (St, Louis, Mo, U.S.A.) The antidepressants tianeptine sodium and venlafaxine were gift from Servier, France and Genetics Pharmaceutical, U.K., respectively. Pargyline and mefenamic acid were also gifts from Abbott Laboratories and Park Davis & Co, respectively. All other chemicals and reagents were from Sigma and/or BDH Chemicals (both of Poole, Dorset, U.K.) and were of the purest commercially available grades.
Computational studies
Docking to TDO of antidepressant and anti-inflammatory drugs and the non-antidepressant pargyline was performed using the Molegro virtual Docker (MVD) software as described previously [21]. Re-ranking was performed to improve accuracy. When re-ranked, 10 independent docking runs resulted in 10 solutions. The structures of the tested drugs (Supplementary Figure S1) were imported into the MVD work space in SDF format. All H atoms were added and important valence checked by using the utilities in MVD. The crystal structure of TDO from Xanthomonas campestris in complex with ferrous heme and Trp (Northeast Structural Genomics Target XcR13: PDB ID: 2NW8), was taken from the protein data bank (http://www.rcsb.org/pdb) [23] into MVD work region at reasonable resolution levels (≤2.8 Å). In each docking run, high-quality orientation was analysed and hydrogen bonds identified and labeled. A linear combination of E-inter and E-intra bonding ligand energy was inspected and analysed using an MVD score. The TDO PDB protein structure was not energy minimized with the heavy atoms constrained before docking, because minimizing energy before docking is not always necessary or convenient and may change the protein structure and therefore affect the outcome of desired simulation.
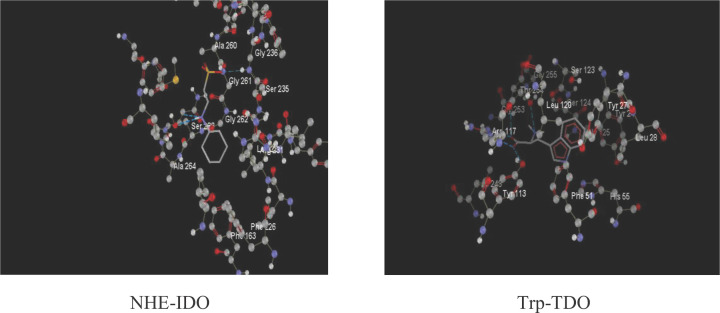
Docking to IDO was also performed with the same drugs and metabolites. The IDO crystal structure selected for this study (PDB ID: 2D0T), that of the 4-phenylimidazole bound form of human indoleamine 2,3-dioxygenase (PDB doi:10.2210/pdb2D0T/pdb) [24], was downloaded into the MVD workspace from the protein data bank (http://www.rcsb.org/pdb) at reasonable resolution criteria (≤2.8 Å). Binding of Trp to TDO and of NHE (2-[N-cyclohexylamino] ethanesulfonic acid) to IDO is illustrated in Figure 1.
Figure 1. Ligand binding to the crystal structures of tryptophan and indoleamine 2,3-dioxygenases.
NHE is an indole compound, 2-[N-cyclohexylamino] ethanesulfonic acid, that mimics IDO substrates sufficiently to demonstrate substrate binding to the enzyme. Its MolDock and rerank scores are −89.5365 and -68.5943, with an RMSD of 1.85566 and H bond energy of −5.4088. The corresponding values for the Trp-TDO binding are −139.546, −99.9363, 3.46611 and −3.16738. For binding of NHE to IDO, the amino acids at the active site were Ser 263, Gly 262, Ala 264,260, Gly 236, Ser 235 and Gly 261 and the ligand binding amino acids were Ser 263, Gly 262 and Ala 264. For binding of Trp to TDO, the corresponding amino acids were Arg 117, Gly 253, Thr 254, Tyr 113, Leu 120, Ser 124 and Ser 123, and Arg 117, Thr 254 and Tyr 113.
The MolDock scoring function used by MVD is derived from the PLP scoring functions originally proposed by Gehlhaar et al. (1995, 1998) and extended later by Yang and Chen (2004). The 10 solutions obtained from the 10 independent docking runs were re-ranked, in order to further increase the docking accuracy, by using a more complex scoring function. In MVD, along with the docking scoring function terms, a Lennard Jones 12-6 potential (Morris et al., 1998) (for these references, see [18,21]) and sp2 -sp2 torsion terms were also used. On the basis of pilot docking studies, the MolDock rerank scores were selected for ranking the inhibitor poses. The structure of all ligand were downloaded from pubchem and imported to the MVD workspace in ‘sdf’ format. In order to make accurate predictions, it is important that the imported structures have been properly prepared, that is, the atom connectivity and bond orders are correct and partial atomic charges are assigned. PDB files often have poor or missing assignment of explicit hydrogens, and the PDB file format cannot accommodate bond order information. All necessary valency checks and H atom addition were thus performed using the utilities provided in MVD. The binding site specifies the region of interest where the docking procedure will look for promising poses (ligand conformations). MolDock automatically identifies potential binding sites (also referred as cavities or active sites) by using its cavity detection algorithm. The cavities within a 30 × 30 × 30 Å3 cube centered at the experimentally known ligand position were used.
Preparation of liver homogenates and determination of tryptophan 2,3-dioxygenase activity
Locally bred male Albino Wistar rats weighing 180–290 g were housed five per cage at 22 ± 1°C and maintained on lab chow and drinking water. Rats were killed between 13:00 and 14:00 h and livers were removed within 10 s and perfused in situ with ice-cold saline (to remove the blood source of heme) before freezing in liquid nitrogen until analysis. Two-gram portions of the frozen livers were homogenized in 13 ml of ice-cold 0.14 M KCl-0.0025 M NaOH for 1min in ice-packed tubes using an Ultraturrax homogenizer and were used within 4–9 min of preparation. TDO activity was determined in liver homogenates by quantifying the formation of kynurenine from L-tryptophan in the absence (holoenzyme activity) or presence (total enzyme activity) of added hematin as described previously [25], with the apoenzyme activity calculated by difference.
A 15 ml portion of the homogenates was added to a mixture of 5 ml of 0.03 M L-Trp, 15 ml of 0.2 M sodium phosphate buffer, pH 7.0, and 25 ml of water at 0°C. For the total TDO activity, haematin hydrochloride was dissolved in 0.1 M NaOH and 0.l ml was included in the overall mixture to give a final concentration (2 µM) that was previously shown to be optimal for enzyme activation [26]. Samples (3 ml) of the assay mixture were incubated at 37°C for 0, 15, 30, 45, 60 and 75 min with shaking (120 oscillations/min) in stoppered 25 ml conical flasks in an atmosphere of O2. The reaction was stopped at each time intervals by the addition of 2 mL of 0.9 M trichloroacetic acid; the flasks and contents were shaken for a further 2 min and then filtered on Whatman no. 1 filter paper. To a measured portion of the filtrate (2.5 ml) was added 1.5 ml of 0.6 M NaOH and the kynurenine present was determined by measuring the E365 with a Unicam SP.500 spectrophotometer and by using ε = 4540 L.mol−1.cm−1. A lag phase persisted for the first 30–45min of incubation and enzyme activity was calculated from the increase in the E365 during the linear phase. For an extinction range of 0.1–1.0, the line representing the linear phase exactly covered points at three to four consecutive time intervals. The validity of this visual method was mathematically validated [25]. Drugs were dissolved in deionized water, neutralized to pH 7.3 with 1 M HCl and added at different concentrations (10 µM to 1 mM).
Statistical analysis
Data was analysed using unpaired t test. A probability P level of <0.05 was considered significant.
Results
Docking of drugs to TDO
Results of the docking of drugs to TDO and IDO are summarized in Table 1, detailed in Tables 2 and 3 and illustrated in Supplementary Figures S2–S6. Perfect fits of the various ligands to TDO and IDO is illustrated by all ligands having root mean square deviation (RMSD) values of zero, except indomethacin which had a value of 1. As shown in Table 2 and Supplementary Figure S2, both venlafaxine and tianeptine docked strongly to TDO, whereas the non-antidepressant pargyline did not. We showed previously [21] that the other four antidepressants listed in Tables 1 and 2 also docked strongly to TDO. The rapidly acting antidepressant ketamine, however, did not dock to TDO (Table 2 and Supplementary Figure S6). By contrast, only sodium salicylate among six NSAIDs docked to TDO (Table 2 and Supplementary Figure S3). Three diclofenac metabolites that mediate some of the pharmacological effects of their parent compound did not dock to TDO (Table 2 and Supplementary Figure S6).
Table 1. Summary of docking of antidepressant and anti-inflammatory drugs to TDO and IDO.
| Antidepressant drugs docking to | Anti-inflammatory drugs docking to | ||||
|---|---|---|---|---|---|
| Drug | TDO | IDO | Drug | TDO | IDO |
| Venlafaxine | + | - | Aspirin | - | + |
| Tianeptine | + | - | Salicylate | + | + |
| Amoxepine | + | - | Mefenamic acid | - | + |
| Paroxetine | + | - | Indomethacin | - | + |
| Moclobemide | + | - | Celecoxib | - | - |
| Fluvoxamine | + | - | Diclofenac | - | + |
| Ketamine | - | - | 3-Hydroxy | - | - |
| Pargyline* | - | 4-Hydroxy | - | - | |
| 4,5-Dihydroxy | - | - | |||
Does not inhibit TDO nor act as antidepressant.
Table 2. Molecular docking to TDO, re-ranking scores and hydrogen bond energy of antidepressants, pargyline and anti-inflammatory drugs.
| Drugs | Docking score (Kcal/mol) | Re-ranking score | Log P | M Wt | Torsion number | HBA | HBD | HBE |
|---|---|---|---|---|---|---|---|---|
| Antidepressants | ||||||||
| Venlafaxine | −93.3835 | −62.4479 | 2.74 | 277.4 | 5 | 3 | 1 | −2.34707 |
| Tianeptine | −68.980 | −51.092 | 2.87 | 437 | 8 | 5 | 3 | −3.26887 |
| Amoxepine* | −120.086 | −87.6386 | 3.08 | 288 | 1 | 3 | 1 | −0.748501 |
| Paroxetine* | −139.706 | −110.963 | 3.15 | 313 | 4 | 4 | 1 | −3.31147 |
| Moclobemide* | −121.275 | −86.5591 | 1.45 | 244 | 4 | 3 | 1 | −4.06079 |
| Fluvoxamine* | −133.451 | −90.2595 | 2.80 | 315 | 9 | 4 | 2 | −4.60011 |
| R Ketamine | 8045.94 | 79.6472 | 3.1 | 237.7 | 2 | 2 | 1 | −0.211 |
| Ketamine HCl | 6076 | 164.647 | 3.1 | 237.7 | 2 | 0 | 0 | 0 |
| Non-antidepressant | ||||||||
| Pargyline | 1952.76 | 15.3495 | −1.43 | 159.22 | 4 | 0 | 0 | 0 |
| Anti-inflammatory | ||||||||
| Aspirin | 1989.23 | 23.8794 | 1.25 | 179.14 | 3 | 3 | 1 | −2.9052 |
| Salicylate | −82.0858 | −66.7095 | −1.43 | 138 | 0 | 3 | 1 | −12.385 |
| Mefenamic acid | 5977.33 | 27.5291 | 3.84 | 240 | 3 | 2 | 1 | −2.56848 |
| Indomethacin | 4043.1 | 187.623 | 3.53 | 356.7 | 4 | 4 | 1 | −1.925 |
| Celecoxib | 9974.6 | 54.0047 | 3.5 | 381.4 | 4 | 7 | 1 | −1.487 |
| Diclofenac | 4011.2 | 52.289 | 4.14 | 296.14 | 4 | 2 | 0 | −2.182 |
| 3-Hydroxy | 3010.57 | 63.0403 | 4 | 312.14 | 4 | 4 | 4 | −5.4734 |
| 4-hydroxy | 1992.86 | 44.9216 | 3.7 | 312.14 | 4 | 4 | 3 | −6.0116 |
| 3,4-Dihydroxy | 4011.2 | 52.289 | 4.14 | 328.15 | 4 | 2 | 0 | −5.47 |
Abbreviations: HBA, hydrogen bond acceptor; HBD, hydrogen bond donor; HBE, hydrogen bond energy (kcal/mol). The * denotes data from our previous publication [21] [Dawood, S., Zarina, S. and Bano, S. (2014) Docking studies of antidepressants against single crystal structure of tryptophan 2,3-dioxygenase using Molegro Virtual Docker software. Pakistan J. Pharmaceut. Sci. 27, 1529–1539].
Table 3. Molecular docking to IDO, re-ranking scores and hydrogen bond energy of antidepressant and anti-inflammatory drugs.
| Drugs | Docking score (Kcal/mol) | Re-ranking score | Log P | M Wt | Torsion number | HBA | HBD | HBE |
|---|---|---|---|---|---|---|---|---|
| Antidepressants | ||||||||
| Venlafaxine | 8082.72 | 147.007 | 2.74 | 277.4 | 5 | 3 | 1 | 0 |
| Tianeptine | 6083 | 199 | 2.87 | 437 | 8 | 5 | 3 | −2.79 |
| Amoxepine | 7009.3 | 92.185 | 3.08 | 313.7 | 1 | 3 | 1 | −2.5 |
| Paroxetine | 5965.4 | 43.924 | 3.15 | 329.3 | 4 | 4 | 1 | −0.503 |
| Moclobemide | 4940 | −30.27 | 1.45 | 268.07 | 4 | 3 | 1 | −1.3 |
| Fluvoxamine | 4996.81 | 14.308 | 2.8 | 318.33 | 9 | 4 | 2 | −1.98 |
| R Ketamine | 9074.58 | 74.9601 | 3.1 | 237.72 | 2 | 0 | 0 | 0 |
| Ketamine HCl | 8081.89 | 90.2596 | 3.1 | 237.72 | 2 | 0 | 0 | 0 |
| Anti-inflammatory | ||||||||
| Aspirin | −110.253 | −95.3902 | 1.24 | 179.14 | 3 | 3 | 1 | −1.5 |
| Salicylate | −66.6195 | −52.8284 | -1.43 | 138 | 0 | 3 | 1 | −2.5 |
| Mefenamic acid | −115.731 | −95.9268 | 3.84 | 240 | 2 | 2 | 1 | −1.5 |
| Indomethacin | −161.647 | −118.1 | 3.53 | 356.7 | 4 | 4 | 1 | −4.16 |
| Celecoxib | 8011.03 | 77.4999 | 3.5 | 381.4 | 4 | 7 | 1 | −0.419 |
| Diclofenac | −147.324 | −115.0 | 4.14 | 296.14 | 5 | 2 | 0 | 0 |
| 3-Hydroxy | 3074.14 | 107.33 | 4 | 312.14 | 4 | 4 | 3 | −1.606 |
| 4-Hydroxy | 2025.25 | 50.9426 | 3.7 | 312.14 | 4 | 4 | 3 | −6.8767 |
| 4,5-Dihydroxy | 3047.71 | 82.6072 | 4.14 | 328.15 | 4 | 2 | 0 | −6.47 |
Abbreviations: HBA, hydrogen bond acceptor; HBD, hydrogen bond donor; HBE, hydrogen bond energy (kcal/mol)
Docking of drugs to IDO
None of the six antidepressants or ketamine docked to IDO (Table 3 and Supplementary Figures S4 and S6). Of the 6 NSAIDs tested, only celecoxib did not dock to IDO (Table 3 and Supplementary Figure S5). The three hydroxylated diclofenac metabolites also did not dock to IDO (Table 3 and Supplementary Figure S6).
TDO inhibition by venlafaxine and tianeptine
That TDO activity is inhibited directly in vitro by venlafaxine and tianeptine is shown in Table 4. In line with other antidepressants, both drugs inhibit the conjugation of the apoenzyme with its heme cofactor. Thus, inhibition is specific to the apoenzyme, with tianeptine causing 58, 75, 75 and 75% inhibition and venlafaxine causing 82, 73, 91 and 64% inhibition at concentrations of 10 µM and 0.1, 0.5 and 1 mM, respectively. The non-antidepressant pargyline and the NSAID mefenamic acid have previously been shown [27] not to inhibit TDO activity or to elevate brain [Trp] following their acute administration to rats.
Table 4. Inhibition of rat liver tryptophan 2,3 dioxygenase activity in vitro by tianeptine and venlafaxine.
| Drug | Concentration | Kynurenine formed (µmol/g wet wt of liver/h) | ||
|---|---|---|---|---|
| Holoenzyme | Total enzyme | Apoenzyme | ||
| Tianeptine | 0 | 1.2 ± 0.08 | 2.4 ± 0.18 | 1.2 ± 0.11 |
| 10 µM | 1.2 ± 0.10 | 1.7 ± 0.14* | 0.5 ± 0.12** | |
| 0.1 mM | 1.2 ± 0.11 | 1.5 ± 0.07** | 0.3 ± 0.04*** | |
| 0.5 mM | 1.1 ± 0.06 | 1.4 ± 0.17** | 0.3 ± 0.15** | |
| 1 mM | 1.0 ± 0.06 | 1.3 ± 0.13** | 0.3 ± 0.12* | |
| Venlafaxine | 0 | 2.0 ± 0.32 | 3.1 ± 0.24 | 1.1 ± 0.17 |
| 10 µM | 2.2 ± 0.27 | 2.4 ± 0.26 | 0.2 ± 0.18* | |
| 0.1 mM | 2.2 ± 0.27 | 2.5 ± 0.14 | 0.3 ± 0.13* | |
| 0.5 mM | 2.3 ± 0.30 | 2.4 ± 0.32 | 0.1 ± 0.06** | |
| 1 mM | 1.9 ± 0.30 | 2.3 ± 0.19* | 0.4 ± 0.12** | |
TDO activity was determined as described in the Materials and Methods either in the absence (holoenzyme activity) or presence (total enzyme activity) of added (2 µM) hematin. The apoenzyme activity was obtained by difference. Values are means ± SEM for at least three separate experiments per drug. The significance of the differences is indicated as follows: *P<0.05; **P<0.01; ***P<0.001.
Effects of sodium salicylate on tryptophan metabolism
As the active aspirin metabolite and only NSAID to dock to TDO, sodium salicylate exerts other important effects on Trp metabolism [28,29]. It inhibits TDO (Trp pyrrolase) activity at doses of ≥0.5 mg/kg and elevates plasma free [Trp] following displacement of the albumin-bound amino acid at doses of ≥2.5 mg/kg. However, a large dose of salicylate (400 mg/kg) exerts a biphasic effect on TDO activity, a major Trp-mediated activation following the initial inhibition [28].
Discussion
The present molecular docking results have demonstrated the binding of tianeptine and venlafaxine to the active site of the Trp 2,3-dioxygenase (TDO) crystal structure (Table 2 and Supplementary Figure S2). Their direct inhibition of TDO activity in vitro has also been demonstrated (Table 4). We previously found that other TDO-inhibitory antidepressants (a monoamine oxidase inhibitor, a tricyclic and 6 SSRIs) also dock strongly to TDO [21]. We also found in the present study that two drugs devoid of TDO inhibition (pargyline and mefenamic acid) do not dock to TDO. Taken together, our present and previous study demonstrate the validity and usefulness molecular docking for confirming the binding of TDO inhibitory antidepressants to the enzyme and absence of binding by non-inhibitors, and for identifying potential TDO inhibitors for use as antidepressants in future drug developmental studies. The present study is also the first to demonstrate that sodium salicylate, the active aspirin metabolite, is the only one of six established NSAIDs to dock to TDO and that of these six drugs, only celecoxib does not dock to IDO. Ketamine is the only antidepressant tested that failed to dock to TDO or IDO (Tables 2 and 3; Supplementary Figure S6). The mechanism of the antidepressant action of this rapidly acting drug is currently under investigation [9,30]. Its failure to bind to TDO or IDO, and thus unlikely to inhibit either enzyme, suggests that it may not enhance serotonin synthesis. Ketamine is also unlikely to exert its anti-inflammatory effects in humans via IDO inhibition. Potential mechanisms of the ketamine effects on the KP in MDD are discussed below.
Inflammation and the kynurenine pathway in MDD
Because inflammation has been proposed to induce the serotonin deficiency in MDD by IDO induction, a brief discussion of changes in IDO-inducing proinflammatory cytokines in MDD is useful. Elevation of cytokine levels is not universal among MDD patients [9,17] and the notable elevation of interleukins IL-1β, IL-6, IL-12, IL-18 and tumor-necrosis factor TNF-α in some patients does not appear to be associated with an inflamed subgroup but rather to a right shift of the immune marker distribution [31]. Changes in the [Kyn]/[Trp] ratio used to express IDO activity are contradictory, partly because of the non-specificity of this test, as an increase in this ratio is not limited to IDO induction and could be masked by changes in subsequent KP enzymes (Figure 2). For example, the low [Kyn] and [KA] levels in unmedicated patients [32] could be the result of elevated Kyn monooxygenase (KMO) activity depleting [Kyn], thereby depriving KAT of its substrate. IDO induction, however, does not play a role in MDD symptoms or response to antidepressant therapy [33,34]. Thus, it appears that IDO induction, if it occurs in patients with an inflamed state, is overridden by potential overexpression of KMO by IL-1β and IFN-γ [35–38]. The increases in [3-HAA] and [AA] by IFN-γ [38] suggest also activation of kynureninase, which has already been reported [35,36,39]. Many antidepressant drugs are postulated to possess anti-inflammatory properties. Their efficacy is, however, unlikely to be due to direct IDO inhibition, but, rather, to inhibition of KMO activation by proinflammatory cytokines, as has been demonstrated with ketamine [9] and escitalopram [40]. As KMO activation can result in increased production of the excitotoxic QA at the expense of the cytoprotective KA, future studies should focus on KMO as a potential determinant of neurological dysfunction in MDD.
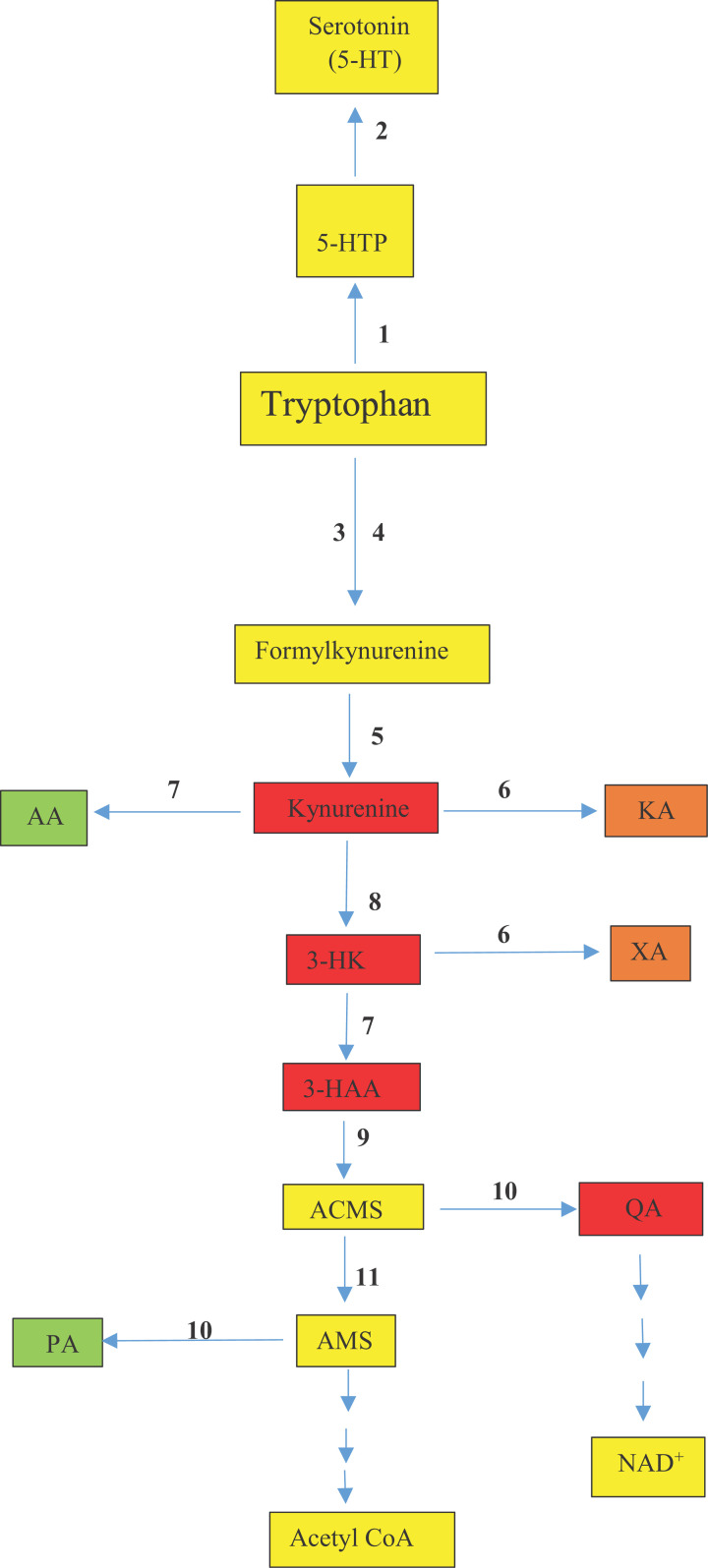
Figure 2. The kynurenine and serotonin pathways of tryptophan degradation.
Enzymes are numbered as follows: 1, tryptophan hydroxylase; 2, aromatic L-amino acid decarboxylase; 3, tryptophan 2,3-dioxygenase; 4, indoleamine 2,3-dioxygenase; 5, aryl hydrocarbon formamidase; 6, kynurenine aminotransferase; 7, kynureninase; 8, kynurenine monooxygenase; 9, 3-hydroxyanthranilic acid 3,4-dioxygenase; 10, non-enzymic cyclization; 11, 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase or picolinate carboxylase. Abbreviations: AA, anthranilic acid; ACMS, 2-amino-3-carboxymuconate-6-semialdehyde; KMO (kynurenine monooxygenase); 3-HAA, 3-hydroxyanthranilic acid; 3-HK, 3-hydroxykynurenine; 5-HT, 5-hydroxytryptamine; 5-HTP, 5-hydroxytryptophan; KA, kynurenic acid; NAD+, oxidised nicotinamide-adenine dinucleotide; PA, picolinic acid; QA, quinolinic acid; XA, xanthurenic acid. Colour code: red, proinflammatory; green, anti-inflammatory; amber, mixed-type; yellow, normal metabolite.
Neuronal activity, however, can influence and be influenced by serotonin function in MDD. The mutual interactions between serotonergic and glutamatergic activities are well established [41–45] and can be viewed simplistically as low serotonin losing control over glutamate neurotransmission and inhibition of the latter facilitating serotonin function.
The status of anti-inflammatory drugs in MDD therapy
With established NSAIDs, only aspirin possesses antidepressant properties in humans and experimental models of depression [46–54]. These favorable outcomes occur in studies performed over a relatively short- to medium-term period with daily doses of aspirin equivalent to 2.3 or 14.3 mg/kg body weight for a 70 kg adult. By contrast, long-term aspirin use, even in small doses, may be associated with increased depression [55,56]. These apparently contradictory clinical effects of aspirin can be explained by several mechanisms based on modulation of Trp metabolism and immune function by aspirin. The antidepressant effects of aspirin at therapeutic dose levels may involve TDO inhibition, but not direct IDO inhibition, and also Trp displacement from albumin-binding sites by its major hydrolytic metabolite salicylic acid. Thus, salicylate at doses as little as 0.5 and 1.0 mg/kg body weight inhibits rat liver TDO (Trp pyrrolase) activity without altering the binding of Trp to serum albumin, whereas larger doses continue to inhibit TDO activity, but, additionally, release Trp from albumin binding sites [29]. Both aspirin and salicylate do not inhibit IDO activity directly, even-though both dock to IDO (Table 3). They are, however, only partially effective in blocking IDO induction by IDO inducing treatments at a concentration (5 mM) [57] unlikely to be reached in therapeutic settings. Aspirin may, however, act in MDD patients with an inflamed state by decreasing IDO induction by interferon-γ [57], possibly by influencing cyclooxygenase 2 activity [58,59]. The incidence of depression with long-term use of even moderate doses of aspirin may involve activation and stabilization of TDO by the continued increase in Trp availability to the liver [25] leading to decreased serotonin synthesis. Increased TDO activity under these conditions leads to enhanced KP activity, as occurs in rheumatoid arthritis patients receiving long-term aspirin therapy exhibiting increased urinary excretion of Kyn, XA and 3-HAA [60]. We compared urinary Kyn levels within the control group in the study by Spiera [60] between those receiving aspirin and those not on aspirin and found that the former subgroup excreted more Kyn (3.00 ± 0.41 vs 1.94 ± 0.29 (mean ± SEM in mg/24 h; n = 8 vs 14; P = 0.044). Increased excretion of Kyn metabolites after Trp loading of rheumatoid patients was also attributed to drug (aspirin) therapy [61].
Conclusions and comments
The present results have demonstrated the preferential docking (binding) of antidepressant drugs to tryptophan 2,3-dioxygenase, in accord with their ability to inhibit this major enzyme of Trp degradation and primary determinant of cerebral serotonin synthesis. While rapidly acting ketamine does not dock to TDO, we propose that its unique mode of action in MDD therapy is a novel one: that of targeting KMO to undermine neuronal dysfunction, thereby restoring serotonin homeostasis. Findings with the SSRI escitalopram [40] are also consistent with this proposed mechanism. Current evidence suggests that KMO overexpression occurs in MDD and its metabolic consequences override IDO induction by proinflammatory cytokines in patients with an inflamed profile. MDD pathology can therefore be attributed to serotonin deficiency with or without glutamatergic dysfunction, with Kyn pathway modulation being a shared feature. Future studies in MDD are likely to yield fruitful results by focusing on the Kyn pathway and, in particular, the roles of TDO and KMO and related novel therapeutic mechanisms.
Supplementary Material
Acknowledgements
AA-BB held an honorary professorship in clinical biochemistry at Cardiff Metropolitan University during the period September 2006 to September 2016.
Abbreviations
- 3-HAA
3-hydroxyanthranilic acid
- 3-HK
3-hydroxykynurenine
- 5-HT
5-hydroxytryptamine
- 5-HTP
5-hydroxytryptophan
- AA
anthranilic acid
- ACMS
2-amino-3-carboxymuconate-6-semialdehyde
- AMS
2-amino-3-muconate-6-semialdehyde
- HBA
hydrogen bond acceptor
- HBD
hydrogen bond donor
- HBE
hydrogen bond energy
- IDO
indoleamine 2,3-dioxygenase
- KA
kynurenic acid
- KMO
kynurenine monooxygenase
- Kyn
kynurenine
- NAD+
oxidised nicotinamide-adenine dinucleotide
- PA
picolinic acid
- QA
quinolinic acid
- TDO
tryptophan 2,3-dioxygenase
- Trp
tryptophan
- XA
xanthurenic acid
Data Availability
The crystal structure of TDO from Xanthomonas campestris in complex with ferrous heme and Trp (Northeast Structural Genomics Target XcR13: PDB, ID, 2NW8), was taken from the protein data bank (http://www.rcsb.org/pdb). The IDO crystal structure selected for this study (PDB ID: 2D0T), that of the 4-phenylimidazole bound form of human indoleamine 2,3-dioxygenase, was taken from the protein data bank (PDB) (doi:10.2210/pdb2D0T/pdb). All other data are available directly from the authors.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
The authors declare that there are no sources of funding to be acknowledged.
CRediT Author Contribution
Shazia Dawood: Conceptualization, Data curation. Samina Bano: Supervision, Methodology, Writing—review & editing. Abdulla A.-B. Badawy: Writing—original draft, Writing—review & editing.
References
- 1.Badawy A.A.-B. (2017) Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int. J. Tryptophan Res. 10, 1–20 10.1177/1178646917691938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badawy A.A.-B. (2019) Tryptophan metabolism: a versatile area providing multiple targets for pharmacological intervention. Egypt. J. Basic. Clin. Pharmacol. 9, 1–30Article ID 101415 10.32527/2019/101415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanai M., Funakoshi H., Takahashi H., Hayakawa T., Mizuno S., Matsumoto K.et al. (2009) Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behaviour in mice. Mol. Brain 2, 8 10.1186/1756-6606-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terakata M., Fukuwatari T., Kadota E., Sano M., Kanai M., Nakamura T.et al. (2013) The niacin required for optimum growth can be synthesized from L-tryptophan in growing mice lacking tryptophan 2,3-dioxygenase. J. Nutr. 143, 1046–1051 10.3945/jn.113.176875 [DOI] [PubMed] [Google Scholar]
- 5.Too L.K., Li K.M., Suarna C., Maghzal G.J., Stocker R., McGregor I.S.et al. (2016) Deletion of TDO2, IDO-1 and IDO-2 differentially affects mouse behavior and cognitive function. Behav. Brain Res. 312, 102–117 10.1016/j.bbr.2016.06.018 [DOI] [PubMed] [Google Scholar]
- 6.Carlsson A. and Lindqvist M. (1978) Dependence of 5-HT and catecholamine synthesis on concentrations of precursor amino acids in rat brain. Naunyn-Schmiedeberg's Arch. Pharmacol. 303, 157–164 10.1007/BF00508062 [DOI] [PubMed] [Google Scholar]
- 7.Badawy A.A.-B. (2013) Tryptophan: the key to boosting brain serotonin synthesis in depressive illness. J. Psychopharmacol. 27, 878–893 10.1177/0269881113499209 [DOI] [PubMed] [Google Scholar]
- 8.Stone T.W. (1993) Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 45, 309–385 [PubMed] [Google Scholar]
- 9.Kopra E., Mondelli V., Pariante C. and Nikkheslat N. (2021) Ketamine's effect on inflammation and kynurenine pathway in depression: a systematic review. J. Psychopharmacol. 35, 934–945 10.1177/02698811211026426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badawy A.A.-B. and Guillemin G. (2019) The plasma [kynurenine]/[tryptophan] ratio and indoleamine 2,3-dioxygenase: time for appraisal. Int. J. Tryptophan Res. 12, 1–10 10.1177/1178646919868978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teraishi T., Hori H., Sasayama D., Matsuo J., Ogawa S., Ota M.et al. (2015) 13C- tryptophan breath test detects increased catabolic turnover of tryptophan along the kynurenine pathway in patients with major depressive disorder. Sci. Rep. 5, 15994 10.1038/srep15994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonaccorso S., Marino V., Biondi M., Grimaldi F., Ippoliti F. and Maes M. (2002) Depression induced by treatment with interferon alpha in patients affected by hepatitis C virus. J. Affect. Disord. 72, 237–241 10.1016/S0165-0327(02)00264-1 [DOI] [PubMed] [Google Scholar]
- 13.Raison C.L., Dantzer R., Kelley K.W., Schroecksnadel S., Ferenci P. and Fuchs D. (2010) CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol. Psychiatry 15, 393–403 10.1038/mp.2009.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zoller H., Jenal A., Staettermayer A.F., Schroecksnadel S., Ferenci P. and Fuchs D. (2015) Tryptophan breakdown in patients with HCV infection is influenced by IL28B polymorphism. Pharmaceuticals 8, 337–350 10.3390/ph8020337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duhalde Vega M., Aparicio J.L., Mandour M.F. and Retegui L.A. (2020) The autoimmune response elicited by mouse hepatitis virus (MHV-A59) infection is modulated by liver tryptophan-2,3-dioxygenase (TDO). Immunol. Lett. 217, 25–30 10.1016/j.imlet.2019.11.004 [DOI] [PubMed] [Google Scholar]
- 16.Badawy A.A.-B. (2020) Liver tryptophan 2,3-dioxygenase in the mouse hepatitis virus (MHV-A59) model. Immunol. Lett. 225, 23–24 10.1016/j.imlet.2020.05.007 [DOI] [PubMed] [Google Scholar]
- 17.Beurel E., Toups M. and Nemeroff C.B. (2020) The bidirectional relationship of depression and inflammation: double trouble. Neuron 107, 234–256 10.1016/j.neuron.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng X.-Y., Zhang H.-X., Mezei M. and Cui M. (2011) Molecular docking: a powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug Design 7, 146–157 10.2174/157340911795677602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreira L.G., dos Santos R.N., Oliva G. and Andricopulo A.D. (2015) Molecular docking and structure-based drug design strategies. Molecules 20, 13384–13421 10.3390/molecules200713384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolušić E., Larrieu P., Moineaux L., Stroobant V., Pilotte L., Colau D.et al. (2011) Tryptophan 2,3-dioxygenase (TDO) inhibitors 3-(2-(pyridyl)ethenyl) indoles as potential anticancer immunomodulators. J. Med. Chem. 54, 5320–5334 10.1021/jm2006782 [DOI] [PubMed] [Google Scholar]
- 21.Dawood S., Zarina S. and Bano S. (2014) Docking studies of antidepressants against single crystal structure of tryptophan 2, 3-dioxygenase using Molegro Virtual Docker software. Pakistan J. Pharmaceut. Sci. 27, 1529–1539 [PubMed] [Google Scholar]
- 22.Woo Y.S., Bahk W.-M., Jeong J.-H., Lee S.-H., Sung H.-M., Pae C.-U.et al. (2013) Tianeptine combination for partial or non-response to selective serotonin re-uptake inhibitor monotherapy. Psychiat. Clin. Neurosci. 67, 219–227 10.1111/pcn.12042 [DOI] [PubMed] [Google Scholar]
- 23.Forouhar F., Anderson J.L., Mowat C.G., Bruckmann C., Thackray S.J., Seetharaman J.et al. (2007) Molecular insights into substrate recognition and catalysis by tryptophan 2,3-dioxygenase. Proc. Natl. Acad. Sci. U. S. A. 104, 473–478 10.1073/pnas.0610007104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugimoto H., Oda S., Otsuki T., Hino T., Yoshida T. and Shiro Y. (2006) Crystal structure of human indoleamine 2,3-dioxygenase: catalytic mechanism of O2 incorporation by a heme-containing dioxygenase. Proc. Natl. Acad. Sci. U. S. A. 103, 2611–2616 10.1073/pnas.0508996103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badawy A.A.-B. and Evans M. (1975) The regulation of rat liver tryptophan pyrrolase by its cofactor haem-experiments with haematin and 5-aminolaevulinate and comparison with the substrate and hormonal mechanisms. Biochem. J. 150, 511–520 10.1042/bj1500511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badawy A.A.-B. and Evans M. (1974) Guinea-pig liver tryptophan pyrrolase – absence of detectable apoenzyme activity and of hormonal induction by cortisol and possible regulation y tryptophan. Biochem. J. 138, 445–451 10.1042/bj1380445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badawy A.A.-B. and Evans M. (1981) Inhibition of rat liver tryptophan pyrrolase activity and elevation of brain tryptophan concentration by administration of antidepressants. Biochem. Pharmacol. 30, 1211–1216 10.1016/0006-2952(81)90299-9 [DOI] [PubMed] [Google Scholar]
- 28.Badawy A.A.-B. and Smith M.J.H. (1971) The effects of salicylate on the activity of rat liver tryptophan pyrrolase in vitro and in vivo. Biochem. J. 123, 171–174 10.1042/bj1230171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badawy A.A.-B. (1982) Mechanisms of elevation of rat brain tryptophan concentration by various doses of salicylate. Br. J. Pharmacol. 76, 211–213 10.1111/j.1476-5381.1982.tb09208.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., Ye F., Zhang T., Lv S., Zhou L., Du D.et al. (2021) Structural basis of ketamine action on human NMDA receptors. Nature 596, 301–305 10.1038/s41586-021-03769-9 [DOI] [PubMed] [Google Scholar]
- 31.Osimo E.F., Pillingera T., Rodriguez I.M., Khandaker G.M., Pariante C.M. and Howes O.D. (2020) Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav. Immun. 87, 901–909 10.1016/j.bbi.2020.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogyu K., Kubo K., Noda Y., Iwata Y., Tsugawa S., Omura Y.et al. (2018) Kynurenine pathway in depression: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 90, 16–25 10.1016/j.neubiorev.2018.03.023 [DOI] [PubMed] [Google Scholar]
- 33.Quak J., Doornbos B., Roest A.M., Duivis H.E., Vogelzangs N., Nolen W.A.et al. (2014) Does tryptophan degradation along the kynurenine pathway mediate the association between pro-inflammatory immune activity and depressive symptoms? Psychoneuroendocrinology 45, 202–210 10.1016/j.psyneuen.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 34.Arteaga-Henriquez G., Burger B., Weidinger E., Grosse L., Moll N., Schuetz G.et al. (2021) Activation and deactivation steps in the tryptophan breakdown pathway in major depressive disorder: a link to the monocyte inflammatory state of patients. Progr. Neuropsychopharmacol. Biol. Psychiat. 107, 110226 10.1016/j.pnpbp.2020.110226 [DOI] [PubMed] [Google Scholar]
- 35.Ferrario R.G., Baratté S., Speciale C. and Salvati P. (1996) Kynurenine enzymatic pathway in human monocytes-macrophages. Adv. Exp. Med. Biol. 398, 167–170 10.1007/978-1-4613-0381-7_27 [DOI] [PubMed] [Google Scholar]
- 36.Saito K., Seishima M., Noma A., Markey S.P. and Heyes M.P. (1996) Cytokine and drug modulation of kynurenine pathway metabolism by blood mononuclear cells. Adv. Exp. Med. Biol. 398, 161–165 10.1007/978-1-4613-0381-7_26 [DOI] [PubMed] [Google Scholar]
- 37.Zunszain P.A., Anacker C., Cattaneo A., Choudhury S., Musaelyan K., Myint A.M.et al. (2012) Interleukin-1β: A new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology 37, 939–949 10.1038/npp.2011.277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones S.P., Franco N.F., Varney B., Sundaram G., Brown D.A., de Bie J.et al. (2015) Expression of the kynurenine pathway in human peripheral blood mononuclear cells: implications for inflammatory and neurodegenerative disease. PloS ONE 10, e0131389 10.1371/journal.pone.0131389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alberati-Giani D., Ricciardi-Castagnoli P., Köhler C. and Cesura A.M. (1996) Regulation of the kynurenine pathway by IFN-γ in murine cloned macrophages and microglial cells. Adv. Exp. Med. Biol. 398, 171–175 10.1007/978-1-4613-0381-7_28 [DOI] [PubMed] [Google Scholar]
- 40.Halaris A., Myint A.-M., Savant V., Meresh E., Lim E., Guillemin G.et al. (2015) Does escitalopram reduce neurotoxicity in major depression? J. Psychiatric Res. 66-67, 118–126 10.1016/j.jpsychires.2015.04.026 [DOI] [PubMed] [Google Scholar]
- 41.Ciranna L. (2006) Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: implications in physiological functions and in pathology. Curr. Neuropharmacol. 4, 101–114 10.2174/157015906776359540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aristieta A., Morera-Herreras T., Ruiz-Ortega J.A., Miguelez C., Vidaurrazaga I., Arrue A.et al. (2014) Modulation of the subthalamic nucleus activity by serotonergic agents and fluoxetine administration. Psychopharmacol 231, 1913–1924 10.1007/s00213-013-3333-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ugedo L. and De Deurwaerdère P. (2021) Serotonergic control of the glutamatergic neurons of the subthalamic nucleus. Prog. Brain Res. 261, 423–462 10.1016/bs.pbr.2020.11.003 [DOI] [PubMed] [Google Scholar]
- 44.Miyamoto Y., Yamada K., Noda Y., Mori H., Mishina M. and Nabeshima T. (2001) Hyperfunction of dopaminergic and serotonergic neuronal systems in mice lacking the NMDA receptor e1 subunit. J. Neurosci. 21, 750–757 10.1523/JNEUROSCI.21-02-00750.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fullana N., Gasull-Camós J., Tarrés-Gatius M., Castañé A., Bortolozzi A. and Artigas F. (2020) Astrocyte control of glutamatergic activity: downstream effects on serotonergic function and emotional behavior. Neuropharmacol 166, 107914 10.1016/j.neuropharm.2019.107914 [DOI] [PubMed] [Google Scholar]
- 46.Brunello N., Alboni S., Capone G., Benatti C., Blom J.M.C., Tascedda F.et al. (2006) Acetylsalicylic acid accelerates the antidepressant effect of fluoxetine in the chronic escape deficit model of depression. Int. Clin. Psychopharmacol. 21, 219–225 10.1097/00004850-200607000-00004 [DOI] [PubMed] [Google Scholar]
- 47.Mendelwicz J., Kriwin P., Oswald P., Souery D., Alboni S. and Brunello N. (2006) Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: A pilot open-label study. Int. Clin. Psychopharmacol. 21, 227–231 10.1097/00004850-200607000-00005 [DOI] [PubMed] [Google Scholar]
- 48.Laan W., Grobbee D.E., Selten J.-P., Heijnen V.C.J., Kahn R.S. and Burger H. (2010) Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J. Clin. Psychiat. 71, 520–527 10.4088/JCP.09m05117yel [DOI] [PubMed] [Google Scholar]
- 49.Stolk P., Souverein P.C., Wilting I., Leufkens H.G., Klein D.F., Rapoport S.I.et al. (2010) Is aspirin useful in patients on lithium? a pharmacoepidemiological study related to bipolar disorder Prostaglandins Leukotr. Essential Fatty Acids 82, 9–14 10.1016/j.plefa.2009.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Yang F., Liu Y.-F., Gao F.E. and Jiang W. (2011) Acetylsalicylic acid as an augmentation agent in fluoxetine treatment resistant depressive rats. Neurosci. Lett. 499, 74–79 10.1016/j.neulet.2011.05.035 [DOI] [PubMed] [Google Scholar]
- 51.Berk M., Dean O., Drexhage H., McNeil J.J., Moylan S., O'Neil A.et al. (2013) Aspirin: a review of its neurobiological properties and therapeutic potential for mental illness. BMC Med. 11, 74 10.1186/1741-7015-11-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sepehrmanesh Z., Fahimi H., Akasheh G., Davoudi M., Gilasi H. and Ghaderi A. (2017) The effects of combined sertraline and aspirin therapy on depression severity among patients with major depressive disorder: a randomized clinical trial. Electronic Physician 9, 5770–5777 10.19082/57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alboni S., Benatti C., Capone G., Tascedda F. and Brunello N. (2018) Neither all anti-inflammatory drugs nor all doses are effective in accelerating the antidepressant-like effect of fluoxetine in an animal model of depression. J. Affect. Disorders 235, 124–128 10.1016/j.jad.2018.04.063 [DOI] [PubMed] [Google Scholar]
- 54.Savitz J.B., Teague K., Misaki M., Macaluso M., Wurfel B.E., Meyer M.et al. (2018) Treatment of bipolar depression with minocycline and/or aspirin: an adaptive, 2×2 double-blind, randomized, placebocontrolled, phase IIA clinical trial. Transl. Psychiat. 8, 27 10.1038/s41398-017-0073-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berk M., Woods R.L., Nelson M.R., Shah R.C., Reid C.M., Storey E.et al. (2020) Effect of aspirin vs placebo on the prevention of depression in older people: a randomized clinical trial. JAMA Psychiatr. 77, 1012–1020 10.1001/jamapsychiatry.2020.1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berk M., Agustini B., Woods R.L., Nelson M.R., Shah R.C., Reid C.M.et al. (2021) Effects of aspirin on the long-term management of depression in older people: a double-blind randomised placebo-controlled trial. Molec. Psychiat. 26, 5161–5170 10.1038/s41380-021-01020-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schroecksnadel K., Winkler C., Wirleitner B., Schennach H. and Fuchs D. (2005) Aspirin down-regulates tryptophan degradation in stimulated human peripheral blood mononuclear cells in vitro. Clin. Exp. Immunol. 140, 41–45 10.1111/j.1365-2249.2005.02746.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lachininoto M.G., Nuzzolo E.R., Di Maggio A., Bonanno G., Mariotti A., Procoli A.et al. (2008) COX-2 Inhibition suppresses the interferon-γ-induced expression of indoleamine 2,3-dioxygenase (IDO) in human leukemia cell lines. Blood 112, 1623 10.1182/blood.V112.11.1623.1623 [DOI] [Google Scholar]
- 59.Boutaud O., Sosa I.R., Amin T., Oram D., Adler D., Hwang H.S.et al. (2016) Inhibition of the biosynthesis of prostaglandin E2 by low dose aspirin: implications for adenocarcinoma metastasis. Cancer. Prev. Res. (Phila) 9, 855–865 10.1158/1940-6207.CAPR-16-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spiera H. (1966) Excretion of tryptophan metabolites in rheumatoid arthritis. Arthritis Rheum. 9, 318–324 10.1002/art.1780090207 [DOI] [PubMed] [Google Scholar]
- 61.Bruckner F.E., Smith H.G., Lakatos C. and Chamberlain M.A. (1972) Tryptophan metabolism in rheumatoid neuropathies. Ann. Rheum. Dis. 31, 311–315 10.1136/ard.31.4.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The crystal structure of TDO from Xanthomonas campestris in complex with ferrous heme and Trp (Northeast Structural Genomics Target XcR13: PDB, ID, 2NW8), was taken from the protein data bank (http://www.rcsb.org/pdb). The IDO crystal structure selected for this study (PDB ID: 2D0T), that of the 4-phenylimidazole bound form of human indoleamine 2,3-dioxygenase, was taken from the protein data bank (PDB) (doi:10.2210/pdb2D0T/pdb). All other data are available directly from the authors.