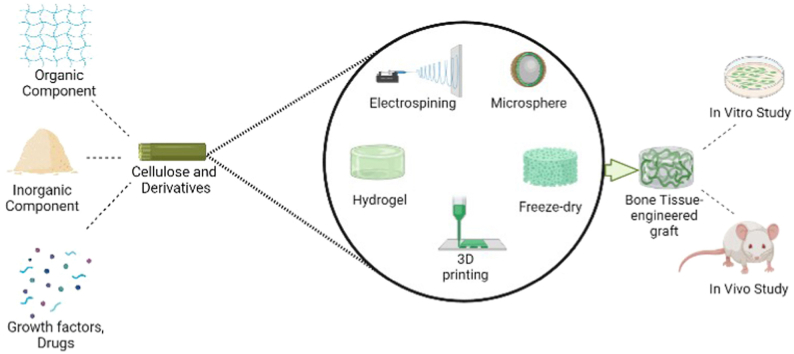
Abstract
Natural bone constitutes a complex and organized structure of organic and inorganic components with limited ability to regenerate and restore injured tissues, especially in large bone defects. To improve the reconstruction of the damaged bones, tissue engineering has been introduced as a promising alternative approach to the conventional therapeutic methods including surgical interventions using allograft and autograft implants. Bioengineered composite scaffolds consisting of multifunctional biomaterials in combination with the cells and bioactive therapeutic agents have great promise for bone repair and regeneration. Cellulose and its derivatives are renewable and biodegradable natural polymers that have shown promising potential in bone tissue engineering applications. Cellulose-based scaffolds possess numerous advantages attributed to their excellent properties of non-toxicity, biocompatibility, biodegradability, availability through renewable resources, and the low cost of preparation and processing. Furthermore, cellulose and its derivatives have been extensively used for delivering growth factors and antibiotics directly to the site of the impaired bone tissue to promote tissue repair. This review focuses on the various classifications of cellulose-based composite scaffolds utilized in localized bone drug delivery systems and bone regeneration, including cellulose-organic composites, cellulose-inorganic composites, cellulose-organic/inorganic composites. We will also highlight the physicochemical, mechanical, and biological properties of the different cellulose-based scaffolds for bone tissue engineering applications.
Keywords: Cellulose, Cellulose derivatives, Bone tissue engineering, Drug delivery system
Graphical abstract
Highlights
-
•
Cellulose and its derivatives are renewable and biodegradable natural polymers that with great potential for bone tissue engineering.
-
•
Cellulose-based materials can be used various therapeutics directly to the bone to achieve bone regeneration.
-
•
Bioinks made of cellulose-based materials hold great promise to develop patient specific solutions for bone repair using 3D printing.
-
•
Challenges associated with inaccuracies in existing preclinical models, sterilization regulatory barriers still need to be addressed before clinical translation.
Abbreviations
- BC
Bacterial cellulose
- CA
Cellulose acetate
- CMC
Carboxymethyl cellulose
- HPMC
Hydroxyl propyl methyl cellulose
- HEC
Hydroxyethyl cellulose
- EC
Ethyl cellulose
- RC
Regenerated cellulose
- TOCN
Tempo-oxidized cellulose nanofibril
- TOBC
Tempo-oxidized bacterial cellulose
- CNF
Cellulose nanofiber
- CNC
Cellulose nanocrystal
- CNW
Cellulose nanowisker
- SO-CNC
Surface-Oxidized CNCs
- BCN
Bacterial cellulose nanocrystal
- BF
Bamboo fiber
- SO
Spinacae olareacea
- CQ
Cissus quadrangularis
- ApA
3-Aminopropylphosphoric acid
- HMDA
Hexamethylenediamine
- DHT
Dehydrothermal treatment
- PLLA
Poly-l-lactide acid
- PLA
Poly lactic acid
- PHB
poly(3-hydroxybutyrate)
- HAp
Hydroxyapatite
- BG
Bioactive glass
- CS
Chitosan
- PAA
Polyacrylamide
- PVA
Polyvinyl alcohol
- PCL
Polycaprolactone
- PULL
Pullulan
- SA
Sodium alginate
- SF
Silk fibroin
- PU
Polyurethane
- XG
Xanthan gum
- MWCNT
Multiwall carbon nanotube
- BHA
Boron-doped hydroxyapatite
- SPI
Soy protein isolate
- GO
Graphene oxide
- GEL
Gelatin
- COL
Collagen
- HA
Hyaluronic acid
- PVP
Polyvinyl pyrrolidone
- PMMA
Polymethyl methacrylate
- BGP
β-glycerophosphate
- PHEMA
Poly (2hydroxyethyl methacrylate)
- Semi-IPNs
Semi-interpenetrating polymer networks
- hBN
Hexagonal boron nitride
- tBuOH
Tertbutanol
- CAM
Cellulose acetate membrane
- T-CNF
TEMPO-oxidized cellulose nanofibril
- EDC
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide
- NHS
N-hydroxysuccinimide
- STMP
Sodium trimetaphosphate
- NP
Nanoparticle
- GF
Growth factor
- TGF
Transforming growth factor
- FGF
Fibroblast growth factor
- BMP
Bone morphogenetic protein
- rhBMP-2
Recombinant human BMP-2
- p-rhOPN
Plant-derived recombinant human osteopontin
- ALP
Alkaline phosphatase
- RhVEGF
Recombinant human VEGF
- OGP
Osteogenic growth peptide
- PBS
Phosphate buffered saline
- CP
Calcium phosphate
1. Introduction
Bone is a rigid organ that constitutes several vital roles in the body including locomotion, mineral storage, soft tissue protection, and supplying the microenvironment for bone marrow. Even though bone indicates an inherent capacity to regenerate itself from small defects, advanced interventions are required to manage massive bone losses resulting from trauma, accident, surgery, congenital malformation, and tumor resection. These large bone defects lack self-regeneration capability and consequently affect the quality of life of patients [1,2]. Surgical reconstruction that utilizes autogenic and allogenic bone grafts in addition to metal implants has been used as a standard treatment for repairing bone defects. Autogenic bone grafts have been considered the gold standard in surgical operations. Unfortunately, they have several drawbacks, including insufficient donor sites, the need for additional surgery, and increased risk of infection at the implantation site. Allogenic grafts are another class of bone grafts, with insufficient donor sites along with the risk of pathogen transmission and immunological rejection. Inert non-bioactive metal implants have also been used to treat large bone defects. However, challenges associated with the integration of these implants with the surrounding tissue and the mechanical mismatch between the implant and the native bone have hindered their clinical use [3,4]. To mitigate these limitations, therapeutic approaches based on the engineering of tissues that use biomaterials, cells, and bioactive molecules have been emerged to accelerate the over the past few years [5,6].
An ideal bone scaffold must provide a desirable environment for cell attachment, growth, differentiation without inducing any toxic and immunological side effects [[7], [8], [9]]. Additionally, a bone mimicking scaffold with the interconnected porous network, suitable mechanical properties, and appropriate biodegradability is required to construct a functional bone similar to the native structure [10]. A pore size of 300 μm and larger has been recommended to allow acceptable mass transfer and vascularization for guiding cellular behavior in the direction of new bone formation [11]. Additionally, scaffolds should tolerate compressive loads between 5 and 224 MPa [12,13]. Although increasing pore size is desired for cell infiltration and tissue integration, the porosity will negatively affect the mechanical properties. Therefore, scaffolds should be carefully designed to optimize promote tissue remodeling post-implantation without compromising the mechanical properties of the implant. Over the years, various biomaterials from natural and synthetic polymers and ceramics have been examined for bone tissue engineering applications. The development of composite biomaterials that satisfy the needs of bone tissue engineering has been a significant focus of recent research efforts. Composite biomaterials have attracted much attention due to their improved properties compared with pure ceramic and polymer materials. Composite biomaterials are primarily intended to improve the degradation rate, mechanical properties, and bioactivity of scaffolds [14,15]. In addition, numerous studies have been focused on designing hydrogel-based composites that promote the infiltration of cells into the scaffold, increase nutrient transport, deliver bioactive molecules to the implantation site, and improve mechanical integrity at the defect site. Therefore, to achieve the requirements for bone regeneration, biomimetic matrixes were developed to create a suitable microenvironment to encourage osteoblast proliferation and osteogenesis. Also, composites are ideal materials for the controlled and sustained release of drugs and growth factors into the site of defects to enhance therapeutic outcomes. Furthermore, it can reduce the side effects of burst release of drugs [[16], [17], [18]].
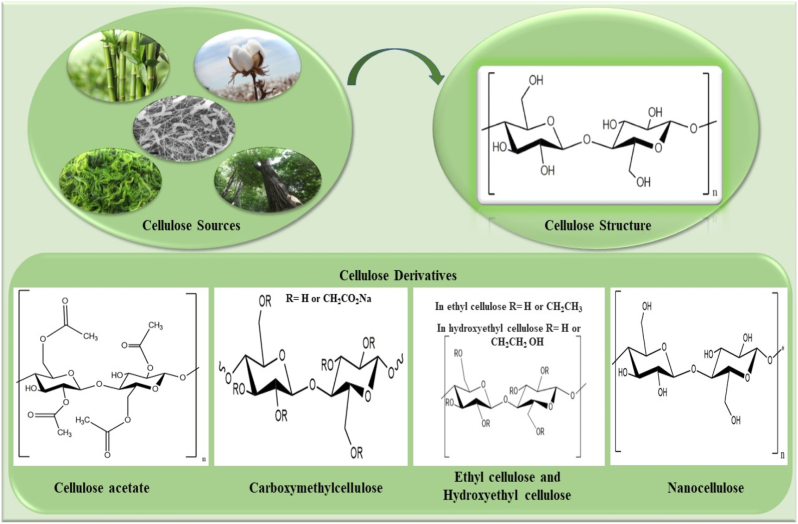
In bone tissue engineering, several types of polymeric materials are used to provide structural support and tissue regeneration. Proteins such as collagen, and gelatin offer a number of advantages, such as high biocompatibility, low toxicity, and enhanced cell responses; however, they fail to provide sufficient mechanical strength and stability in physiological conditions. Polysaccharides such as chitosan, alginate, and starch have a number of advantages, including biodegradability and biocompatibility, but are fragile and typically do not contain cell-binding moieties that promote cell attachment and infiltration. In contrast, synthetic polymers (e.g., PLA, PCL, PHB, and PVA) exhibit strong mechanical properties and tunable mechanical properties and stability in vivo. However, their bioactivity and cell attachment are insufficient for bone tissue engineering [19,20]. Among these polymers, cellulose is a linear polysaccharide abundantly found in natural sources from several plants (cotton, bast plants, wood, and bamboo) to some organisms (bacteria, fungi, algae). Notably, cellulose in the pristine or chemically modified form due to remarkable advantages such as high specific mechanical properties, non-immunogenicity, nontoxicity, source abundance, and low production cost, is one of the most common polysaccharides for fabricating bone substitutes [21,22]. It is worth noting that the origin of cellulose extraction intensely influences these characteristics. This syndiotactic homopolymer comprises d-glucopyranose ring units linked by β-1, 4-glycosidic linkage. High amounts of hydroxyl groups occur on the cellulose chains as a result of plentiful intra- and intermolecular hydrogen bonding in the biopolymer network. Coupled with this, hydrogen bonding gives some unique properties of stability, hydrophilicity, and high available sites for chemical modifications with different functional groups [[23], [24], [25]]. Cellulose esterification and etherification are the most critical modification processes from the application perspective. Cellulose acetate as a cellulose ester and the methylcellulose, ethylcellulose, hydroxyethylcellulose, and carboxymethyl cellulose as cellulose ethers are the well-known appealing cellulose derivatives in biomedicine and pharmaceutics (Fig. 1). Regenerated cellulose is another processed cellulose produced by chemical processing via dissolving cellulose in alkali and carbon disulfide to make a viscose solution [26,27].
Fig. 1.
Chemical formulation of cellulose and its derivatives [27].
Furthermore, crystalline cellulose derivatives, microcrystalline and nanocrystalline cellulose, are explored in different fields of tissue engineering since they exhibit some beneficial features of high surface area, biodegradability, and non-toxicity. Both crystalline derivatives are easily produced through the partial hydrolysis of amorphous regions of pristine cellulose. These crystalline structures vary with diameter from 5 to 20 nm and length of 100 nm to several micrometers for NCC, and diameter of 50 μm with the length of 100–1000 μm for MCC [28,29].
Cellulose and its derivatives have many favorable properties. To improve the material properties, cellulose-based composites were developed by combining two or more compounds, resulting in a suitable matrix with specific properties that cannot be achieved by any of the components individually. Extensive research efforts have been employed to adjust the mechanical properties, biodegradation, bioactivity, and superior biological properties of bone scaffolds by combining cellulose with different organic and inorganic compounds. In addition, cellulose-based composites are capable of loading therapeutic agents to enhance osteoinduction, osteoconduction, and anti-inflammation properties in bone repair [30,31]. Accordingly, cellulose-based composites have a great chance of being an ideal candidate for regenerative medicine and bone tissue engineering applications.
In this review, we will report and discuss the recent developments and applications of cellulose-based composite scaffolds for regenerating damaged bone tissue and delivering biomolecules to the injured bone site.
2. Cellulose-organic composite scaffolds
Polymers of synthetic and natural origin are broadly chosen as extracellular matrix mimicking biomaterial for advancing functional bone scaffolds. Scaffolds composed of natural polymers including proteins and polysaccharides meet inherent bioactivity. Yet, these materials are not recommended for load-bearing bone tissue engineering purposes due to poor mechanical strength. On the other hand, synthetic polymer-based scaffolds, i.e., PLLA, PCL, etc., degrade slowly and tolerate high mechanical forces compared to the natural polymers; however, they do not promote cell adhesion and growth. Accordingly, in recent years, there has been a significant effort made to improve cellulose scaffolds. Pure cellulose is not biodegradable in the human body and has a poor osseointegration that limits its clinical applications in bone tissue engineering. Broadly, it is pointed out that the incorporation of organic phases into a cellulose matrix enhanced the mechanical strength, biomineralization and stimulated osteogenic differentiation. Furthermore, biodegradability of cellulose-based composite are significantly better than those of pure cellulose materials [[32], [33], [34]]. In this section, bone scaffolds composed of organic biomaterials and pristine cellulose, cellulose ester, some cellulose ethers, or crystalline derivatives will be reviewed to understand the role of cellulose in the composite network.
2.1. Bacterial cellulose-organic composites
BC can be produced by microorganisms such as Gluconacetobacter xylinus, Gluconacetobacter, Agrobacterium tumefaciens, Sarcina ventriculi. Compared to plant cellulose, BC has higher crystallinity (above 60%) and chemical purity, containing no impurities of lignin or hemicellulose [35]. This highly crystalline linear biopolymer with thin organized nanofibers (∼20–100 nm) displays a large surface area, high water-holding capacity, and mechanical strength [36]. BC scaffolds were implanted in different bone defects created in the tibia, calvarial bones, and skull in rats and promoted new bone formation without any inflammatory reactions [37]. In polymeric-based bone composites, BC with nanofibrous crystalline structure enhances mechanical strength, while other polymeric parts are commonly added to the network for sharing biological properties. For example, BC-COL nanocomposite was fabricated to support bone repair under enhanced mechanical and biological conditions. Native human bone is the richest source of the connective tissue components comprising type I, type III, and type IV COL. Type I COL fibers as the main organic component (90% of the organic matrix) within the mineralized bone matrix are responsible for supplying tensile strength and expediting bone cell interactions [13]. Similarly, bone composite scaffolds of COL could stimulate bone cell adhesion, growth, and differentiation in an ideal biological environment. For instance, Saska et al. designed a flexible BC nanocomposite scaffold by incorporating type I COL into the polymeric structure [38].. Adding COL reduced the crystallinity, elastic modulus, and tensile strength of the final nanocomposite scaffold relative to the BC scaffold. It is expected that this flexibility facilitates the manipulation of the nanocomposite for surgical procedures. The in vitro examinations verified the osteogenic differentiation and ALP activity of the primary osteogenic cells cultured on this nanocomposite. A nanocomposite scaffold of BC-COL with an interconnected porous structure was also evaluated both in vitro and in vivo [39]. Using the freeze-drying method, ice crystals in BC-COL were transformed into water vapor, resulting in random pore size and interconnected pores, while the residual solvents were removed. This nanocomposite scaffold stimulated osteogenic differentiation of umbilical cord blood-derived MSCs through suitable cell ingrowth and nutrient transport within interconnected pores, and neovascularization was observed after subcutaneous transplantation of the nanocomposite in animal models. In addition, intrinsic water absorption and resistance to contraction of BC increased the water uptake and physical stability of the final scaffold, respectively. Using BC in another polymeric network, i.e., PHB, also enhanced the mechanical strength and biological properties. Good cytocompatibility, cell growth and proliferation were obtained in vitro experiments, and bone matrix production, as well as OSX expression and ALP activity referring biomineralization, was achieved from in vivo examination in critical sized bone defects induced in mice [40].
2.2. Cellulose acetate-organic composites
CA, the acetate ester form of cellulose, is the most abundant natural polysaccharide produced by organic reagents and solvents, and it should be mentioned that its structure is unique in component (occupying about two of third hydroxyl groups of main backbone chains with acetate groups). It is noteworthy that CA is an environmentally friendly material with adjustable biodegradability, wettability, and it is possible to use renewability of this material with the association of admirable processability [41]. Although CA has a lower degree of crystallinity, this material is more favorable due to desirable mechanical strength and electrospinability comparable with other forms of cellulose. Thus, the mentioned properties can prove its versatile application in the bone tissue engineering research [42]. Furthermore, CA can be explored for in vivo studies as a result of having an appropriate molecular weight (30 kDa) because it has been proved that the molecular weight of fewer than 50 kDa can be passed through the kidney [43]. Additionally, CA-based composite scaffolds reveal encouraging up osteoblast attachment, proliferation, migration, and differentiation in vitro due to hydrophilicity and flexibility, and it's bone reconstruction/regeneration in vivo have proved that CA can be explored and applied as a multifunctional platform for simulating bone tissue [44,45].
Considering the interesting findings from CA bone scaffolds, natural polymers involving COL and PULL were combined with CA to obtain progressed functional composite scaffolds for bone repair. Aravamudhan and colleagues achieved an optimized porous structure of CA microsphere matrices via sintering procedure and coated the prepared microspheres with COL type I [46]. For sintering, the CA microspheres were filled into a metal mold containing an adjusted solvent/non-solvent mixture of acetone: cyclohexane and remained until the mixture evaporated. This procedure resulted in the assembling of the microspheres into a unified structure to form a porous scaffold with optimized interconnected pores. After that, COL nanofibers were uniformly deposited on the obtained scaffold via a biomimetic approach to resemble biochemical and biophysical properties of bone matrix. The high coating efficiency of 42% of the COL nanofibers was determined that may be assigned to the hydrophilic nature of CA. This bioactive platform of COL-coated CA microspheres increased the viability and adhesion of human osteoblast cells in comparison with uncoated BC microspheres. This scaffold showed suitable compressive mechanical properties close to the trabecular bone that notably diminished under the wet condition. Regarding outstanding results from COL-coated CA microspheres, the same authors seeded the scaffold with bone marrow stromal cells for more evaluation [47]. Implantation of the cell-seeded composite scaffold into the mouse calvarial critical-sized defect model accelerated the bone healing process and encouraged new bone formation in the region of the bone defect. BMSCs along with the scaffold led to mineral deposition and bone formation by signaling the host cells to enter the defect. Atila et al. developed a cross-linked composite scaffold based on CA-PULL with improved pullulan maintenance using a trisodium trimetaphosphate as a crosslinking agent [42]. PULL is extracted from the fungus Aurobasidium pullulans, and it is a non-toxic, biocompatible polysaccharide with poor mechanical properties [48,49]. Composition with CA and crosslinking the final polymeric network with trisodium trimetaphosphate gave an opportunity to PULL to overcome its mechanical weakness. Finally, this crosslinked CA-PULL scaffold with an equal ratio of the polymers was suitable for bone tissue engineering owing to enhanced mechanical properties, structural integrity during degradation studies, appropriate porosity, uniform fiber morphologies, deposition of apatite-like structures, and cytocompatibility.
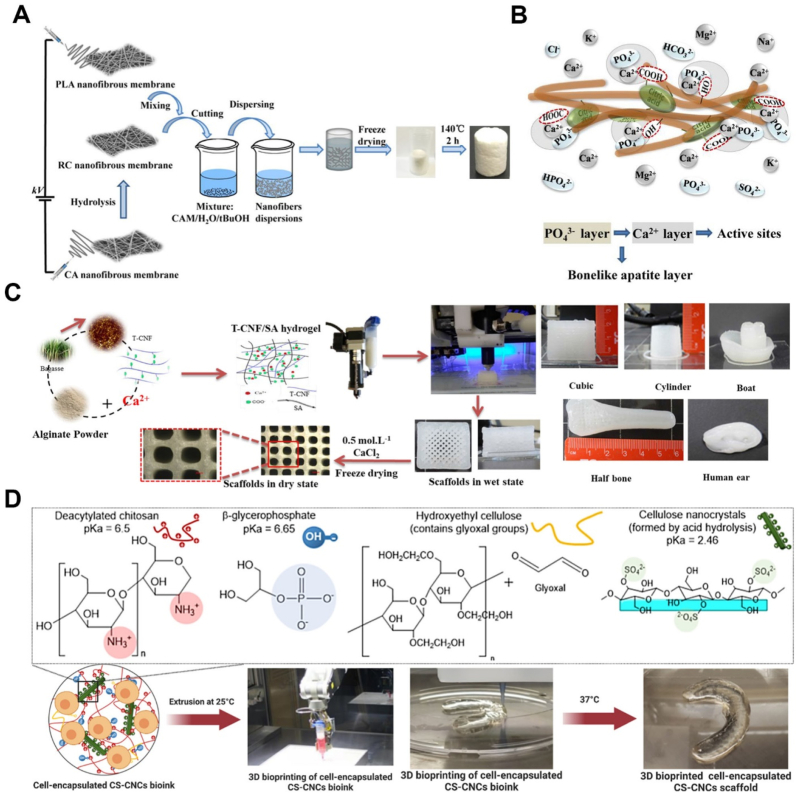
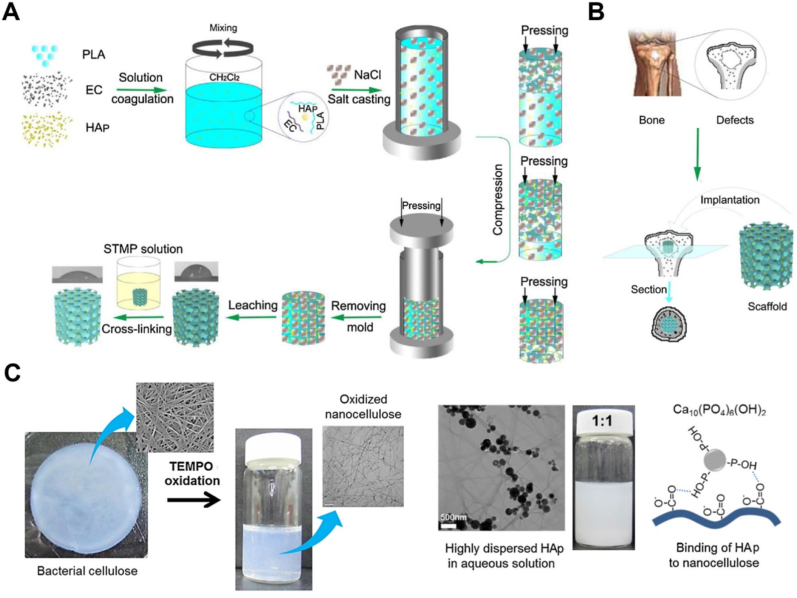
Special attention has been paid to combining CA with synthetic polymers to improve homogenous nanofibers production used for bone tissue engineering. In this regard, electrospun core-shell nanofibers of CA and PVP were developed by homogenous electrospinning without a coaxial needle for biomimetic growth of HAp [50]. PVP and CA owing to low surface energy and high mobility of CA chains were in the shell and core structure, respectively. Prior to mineralization, CA-PVP membranes were treated with deionized water to create the texture of grooves and cavities on the fibers regarding water solubility of PVP which increases the deposition of the mineral crystals. After dipping the treated fibers in SBF solution, mineral crystals with Ca/P ratio of 1.47 nucleated and grew on the surface of the CA-PVP structure. Chen et al. proposed an RC-PLA composite scaffold using a combination of electrospinning and freeze-drying methods for biomineralization and tissue regeneration after bone injuries (Fig. 2 A, B) [51]. Due to the low thickness and restricted shapes of electrospun nanofibers for bone graft, freeze-drying can be used to produce a stable 3D porous scaffold. PLA is a biocompatible polymer that is highlighted by its excellent mechanical properties and biodegradability; however, it has some restrictions of hydrophobicity and a lack of an active site for cell interaction [52]. To address these limitations, RC was generated from electrospun CA nanofibers and then a freeze-dried PLA-RC composite scaffold was prepared. By electrospinning, nanocomposite fibers could be produced that mimic the structure of extracellular matrix, while combining it with freeze drying resulted in a porous structure with appropriate thickness. CA is modified via an alkaline deacetylation process to eliminate the acetate group and produce numerous hydroxyl functional groups for creating esterification crosslinking sites [30]. They reported that the electrospun PLA and RC scaffold were cut, dispersed into the solvent, freeze-dried, and cross-link by citric acid. Water uptake evaluations revealed an increase of around 1300%–3500% that could positively affect cell adhesion and proliferation. Additionally, functional groups of RC-PLA (–COOH, –OH, =C O groups) induced the bio-mineralization process. As a result of the mineral phase deposition, an enhancement in Young's modulus and compressive stress was observed, which could be related to the porosity and confined deformation [51].
Fig. 2.
A. Schematic illustration for PLA/RC scaffold fabrication [51]. B. Calcium and phosphate nucleation by biomimetic method in PLA/RC scaffold [51]. C. 3D printed T-CNF/SA hydrogels in different forms [65]. D. 3D printed cell-encapsulated bio-ink was spontaneously gelled at 37 °C [71].
2.3. Carboxymethyl cellulose-organic composites
CMC is a hydrophilic polysaccharide formed by the chemical modification of cellulose with chloroacetate in an alkali environment. CMC comprises carboxymethyl groups in the C2, C3, or C6 positions of glucose units [53]. Negatively charged water-soluble CMC possesses chelating ability with multivalent cations such as Ca2+. Accordingly, Singh et al. synthesized CMC-SF composite nanofibers for promoting biomineralization in SF bone scaffolds. CMC-SF composite materials were electrospun into nanofibers to create close spatial proximity between bone and scaffold for better osteointegration due to mimic natural extracellular matrix. CMC plays a key role in the uniform nucleation and formation of spherical calcium phosphate crystals on the scaffold through the presence of binding sites on its chains, i.e., COOH and OH functional groups, for calcium cations attachment. As anticipated, CMC increased biomineralization and water uptake in the CMC-SF composite scaffold but demonstrated no significant effect on the mechanical properties. Furthermore, when MSCs has grown on the biomimetic CMC-SF composite substrate, the human MSCs differentiated into the osteogenic lineage [54].
To improve the bioactivity of composite scaffolds based on CMC with high mechanical properties, medicinal plant extracts such as CQ and SO were used in these bone substitutes. For instance, the incorporation of CQ with osteogenic activity into the CMC-CS polymeric network resulted in osteoblast proliferation and mineralization [55]. Moreover, the mechanical strength of the bioactive composite scaffold increased in dry and wet conditions owing to microstructural alterations and reduction of pore size. Also, the incorporation of CQ along with SO as an anti-inflammatory and antioxidant agent with respect, to the CMC-SA scaffold was cytocompatible to the MG-63 Human Osteosarcoma cell line [56].
2.4. Hydroxyethyl cellulose-organic composites
HEC is a water-soluble, non-ionic biopolymer with β-glucose linkage and the capability to bond to various chemical functional groups [57,58]. HEC-based composites have been manufactured by various technologies such as electrospinning and freeze-drying to serve as a bone tissue engineering scaffold. This cellulose ether must be blended with electrospinable biocompatible polymers for the electrospinning procedure due to its non-ionic nature. In this context, Chahal et al. prepared an electrospun nanofibrous composite of HEC-PVA for non-load bearing applications in bone tissue regeneration [59]. The elastic modulus and tensile strength of the nanofibrous composite scaffold increased because of the high tensile strength of HEC. Moreover, the cellular assays proved the cytocompatibility of the HEC composite to human osteosarcoma cells. In another study, Nizan et al. produced a freeze-dried 3D porous nanocomposite scaffold based on HEC-PVA reinforced with CNC [60]. The freeze-drying of HEC-PVA along with CNC nanoparticles provided high porosity and pore interconnectivity with improved nanoparticles stability and prevented nanoparticle aggregation. To reduce the solubility, degradability and increase the physical stability, the prepared nanocomposite was crosslinked by heat treatment at 80 °C. CNC nano-fillers were uniformly distributed within the nanocomposite matrix and created an interconnected pore structure through increasing reaction sites for hydrogen bonding with the polymer chains. The bone cells adhered and proliferated on the HEC-PVP-CNC nanocomposite more efficiently than the HEC-PVP due to its smaller pore size and higher surface roughness.
2.5. Nano- or micro-crystalline cellulose-organic composites
In recent years, CNC and CNF have drawn much attention in bone tissue engineering applications, especially mechanical reinforcement and biomineralization agents. Rod-like particles of CNC are prepared by maintaining crystal regions of cellulose and removing amorphous regions under harsh hydrolysis conditions in acidic media such as sulfuric acid and phosphoric acid. Mechanical techniques are performed for preparing flexible fiber-like CNF composed of both amorphous and crystalline regions with a diameter of less than 100 nm and a length of ≥500 nm [61,62].
CNF has been widely used in bone tissue applications and regenerative medicine, because of its printability, ions deposition, and ability to generate the bone-scaffold interface. In this context, GEL–CNF–based nanocomposite scaffolds were fabricated using CNF, CNF-COOH, or CNF-COOH-ApA [63]. All these nanocomposites demonstrated significantly greater growth and proliferation of MSCs relative to pure GEL scaffold. Furthermore, the addition of ApA moiety to the carboxylated-CNFs in GEL–CNF–COOH-ApA nanocomposite enhanced the HA-like crystals deposition by providing flexible phosphonate groups with high mobility and the capacity of tuning HA patterning through strong ionic bonds with Ca2+ ions. Similarly, a nanocomposite of GEL-TEMPO-oxidized CNF with appropriate mechanical stability and degradation rate obtained a conducive substrate for attachment, spreading, and osteogenic differentiation of hBMSC cells [64]. SA-TEMPO-oxidized CNF hydrogel with improved shape fidelity, mechanical, and biological properties was 3D printed for the bone repair application [65]. CNF enhanced the thixotropic behavior and printability of SA; hence, hydrogel viscosity rapidly recovered before and after applying the shear force. The pure SA hydrogel was soft and simply collapsed, whereas the SA-CNF hydrogel was stable due to balanced viscosity. The printed SA-CNFs hydrogel was cross-linked using calcium chloride solution to achieve a rigid stable scaffold. It was reported that carboxyl functional groups on both SA chains and TEMPO-oxidized CNFs tended to react with calcium cations which resulted in strong binding of the CNFs on the polymer chains and therefore improved mechanical strength. Likewise, it was found that the incorporation of TEMPO-oxidized CNFs improved the stiffness and compressive properties of the hydrogels. Furthermore, biomineralization in the presence of TEMPO-oxidized CNFs and a crosslinking agent resulted in the development of hydroxyapatite with an average crystal size of 25.4 nm, which is similar to natural apatite in native bone tissue (Fig. 2. C). An attempt to design suitable scaffolds for proliferation and osteogenic differentiation involves the use of a TEMPO-oxidized CNF coating via alkaline hydrolysis on PCL scaffold [66]. CNF gave hydrophilic properties and roughness on 3D printed PCL surface to facilitate protein adsorption, actin cytoskeleton formation, alkaline phosphatase activity, mineral formation, and osteogenic differentiation of human bone marrow-derived mesenchymal stem cells.
In bone tissue engineering, it has been shown that the addition of CNC to scaffolds improved matrix mechanical properties, mineralization density, and hydrophilicity [67]. For example, mechanical properties, hydrophilicity, degradability, and cytocompatibility of 3D printed PLLA scaffold was controlled by the addition of CNC fillers into the printer ink [67]. With the 3D printing technology, desired exterior outline could be made from PLLA- CNC to fit the bone defect to mimic the complex patterns of bone defects. A high crystallized strong nanocomposite scaffold was obtained by hydrogen bonding interaction between the hydroxyl group of CNCs and the carboxyl groups of the PLLA matrix. 3% wt.% CNC into the PLLA scaffold increased compressive strength and compressive modulus to 24.0 ± 0.25 MPa and 381 ± 9.54 MPa compared to PLLA scaffold (8.24 ± 0.22 MPa and 84.6 ± 8.95 MPa). By incorporation of CNC fillers into the PLLA scaffold, the water contact angle decreased (from 91.59° to 62.87°) and the weight loss increased (from 9.58 ± 0.25 to 11.65 ± 0.25). In another study, an electrospun PLA-CNC nanocomposite scaffold was fabricated for potential application in the bone tissue engineering [68]. In vivo experiments verified the defect size decreased with improvement in bone regeneration in the vicinity of the nanocomposite scaffold. Electrospun bioactive mats of aligned or random cellulose-CNCs nanofibers were fabricated and functionalized with rhBMP-2 [69]. The evaluation of osteo-differentiation and mineralization capacity for bone repair showed that aligned and functionalized cellulose-CNCs nanofibrous scaffolds increased the proportion of BMSCs and mineralized nodules formation that demonstrated in vitro osteogenic differentiation and ALP activity. Moreover, implanting nanocomposite scaffolds made of cellulose-CNCs-rhBMP-2 with aligned fibers in a rabbit calvarial bone defect promoted oriented collagen deposition on aligned fibers, cortical bone formation, and mineralization in the defective site. Recently, Hong et al. reported a nanocomposite composed of SO-CNCs and PCL with the ability to mineral formation [70]. In this regard, SO-CNCs obtained from sulfuric acid-hydrolyzed CNCs showed improved calcium phosphate mineral formation, and SO-CNC were non-toxic to MC3T3 preosteoblasts at concentrations of up to 3 mg/mL during a 24 h period. Additionally, the incorporation of SO-CNC into nanocomposites as nanofiller and the nucleating agent was shown to enhance the ultimate tensile strength (18.2 ± 0.3 MPa), Young's modulus (492.5 ± 44.1 MPa), hydrophilicity, and calcium-phosphate deposition in presence of carboxyl groups. Maturavongsadit et al. synthesized hydrogels based on CS-CNC along with BGP gelling agent and HEC for bone bioprinting [71]. The volume fraction and hydrogen bonding between CS and CNCs increased the viscosity of the hydrogels when the scaffold contained CNCs and MC3T3-E1 cells (5 million cells/mL). At 37 °C, HEC with glyoxal groups in the polymeric chains can create hydrogel via Schiff-base reaction between CS amine groups and HEC aldehyde groups. ALP activity, collagen synthesis, and high-capacity cell encapsulation of the generated hydrogels make them suitable for bone defect healing. (Fig. 2 D).
Owing to BCN's remarkable merits, this polymer can enhance the porous microstructure, mechanical reinforcement, and biological activity [72]. In contrast, the use of SA for bone tissue engineering is often limited by the lack of cell recognition sites and poor mechanical properties. To address this issue, SA combined with BCN, GEL, and CS have emerged as a way to produce the nanocomposite scaffold with well-defined properties [73]. In this regard, BCN was obtained from sulfuric acid hydrolysis of the pristine bacterial cellulose, and SA-BCN nanocomposite hydrogel alternatively immersed in CS and GEL solutions to obtain layer by layer assembly nanocomposite. Layer-by-layer electrostatic assembly for the surface modification of SA-BCN is effective way to enhance its uniform morphologies and pores due to the high specific surface area and formation of polyelectrolyte complex. CS-GEL layer by layer assembly on the SA-BCN surface improves stability and cytocompatibility owing to the polyelectrolyte complex formation. The produced SA matrix provided uniform morphologies and mechanical reinforcement by entrapping BCN, and the scaffold increased cell attachment due to the reinforcing effect and porous structure, in addition, gelatin as the outer layer improved cell adhesion.
PUs possesses versatile biocompatibility, biodegradability, and physicomechanical properties. which can be regulated by the addition of some polymers such as PAA and HEMA into the PUs matrix. Further, dispersion of CNWs reinforcing materials within the PU matrix significantly promoted mechanical resistance. For example, Shahrousvand et al. investigated the structures and mechanical properties of bimodal foam nanocomposites made of PU and CNW [74]. The combination of PU and CNW seems to have the potential to become an ideal nanocomposite scaffold due to the hydrogen bonding between urethane groups and active hydroxyl groups, which is an appealing choice for hMSC proliferation and osteogenic differentiation. In a similar study, Padash et al. developed PU-PAN-CNW foam matrices that could support proliferation and osteogenic differentiation of human mesenchymal stem cells for potential bone tissue engineering applications [75]. PAN is attractive for biomedical applications due to its high polar nitrile groups for interaction with the biomolecules. They reported an increase in the amount of cell viability (above 90%), osteogenic differentiation, ALP activity, and osteogenic gene expression in presence of nanostructured components that provided a suitable environment of biological properties. Shahrousvand et al. synthesized a Semi-IPNs of PU-PHEMA-CNWs which was seeded with hMSCs [76]. PHEMA is a hydrophilic polymer with suitable mechanical properties. But CNWs play a key role in improving the physical and mechanical properties of the matrix. PU-PHEMA-CNWs fabricated by solvent casting/particulate leaching possessed large and small pores related to salt particles and sugar, respectively. The small pores led to highly interconnected channels that were suitable for cell communication, while the large pores could support cell implantation. The nanocomposite exhibited a higher strength, stiffness, ALP activity, calcium deposition, and osteogenic differentiation was also noticed in presence of PHEMA and CNWs in the PU matrix. In addition to NC, the MCC can be incorporated in polymer matrices to create a suitable matrix for mechanical behavior and biological response improvement. For example, 3D printing has been used to produce composite scaffolds made of PCL and MCC [77]. Moreover, the use of a composite scaffold of PCL-MCC demonstrated that sheep bone marrow cells located significantly on proposed scaffolds, and the presence of MCC caused an increase in cell proliferation and reinforcement effect, likely due to the presence of microtopography and crystallinity normally absent on PCL alone.
It can be assumed from the literature on the cellulose-organic composite scaffolds that cellulosic biomaterials have remarkable effects on physical, mechanical, and biological characteristics. The fabrication method, physical and mechanical properties, and key biological performance of cellulose-organic scaffolds are summarized in Table 1. Hydroxyl functional groups on the cellulose chains act as positions for biomineralization, crosslinking, and intramolecular hydrogen bonding with other organic biomaterials. Therefore, we can have a stable polymeric composite with a tunable porous network and high tolerability for even load-bearing applications. Water holding capacity of the cellulose and some derivatives besides their biomineralization ability can be considered the key cues for directing bone cell behaviors. Taken together, these structural and biological properties can be mainly altered based on types of cellulosic biomaterials and manufacturing methods. Based on the findings, cellulose-based composites are becoming more popular due to their enhanced properties and applicability compared to single-phase materials. Accordingly, there is no one substance that can fulfill all the requirements for tissue regeneration. In order to overcome the disadvantages associated with each kind of material, they have been combined with other materials to use the synergistic advantageous properties. The combination of cellulose with an organic component (COL, SA, PULL, PLA, PHB and etc.) can be used to design successful biomaterials that enhance physical and biological properties. Further, the development of laboratory methods for producing scaffold materials has provided us with a broad range of options for manipulating the physiochemical properties of these materials. These methods such as electrospinning, freeze-drying, 3D printing, solvent casting/particulate leaching and etc. permit the proper processing and use of materials in the laboratory and clinic. In summary, freeze-dried scaffolds led to interconnected porous structures that facilitated the nutrients, oxygen and metabolic wastes exchange to promote the cell and blood vessel ingrowth. On the other hand, fibrous scaffolds made by electrospinning can significantly mimic the structure of the natural extracellular matrix, while, 3D printing provided complex designs for mimicking the shape, size, and dimension of bone defects. Depending on the tissue and its certain characteristics and properties, an appropriate process can be selected to manufacture.
Table 1.
Cellulose- organic composite scaffolds in bone tissue engineering.
| Composite | Fabrication method | Pore size (μm) | Porosity (%) | Mechanical properties | Type of study | Key biological results | Ref |
|---|---|---|---|---|---|---|---|
| BC-PHB | Salt leaching technique | 5–50 | ___ | Tensile Strength (MPa): 15 ± 1.0 Young Modulus (MPa): 1400 ± 101 |
In vitro, In vivo (critical size calvaria defect in mice) | Increased proliferation of 3T3-L1 preadipocytes, in vivo osteoblast differentiation, new bone formation, enhanced ALP activity and OSX expression | [40] |
| CA-PULL | Electrospinning | 20–100 | (41.98 ± 10.56)- (67.64 ± 4.89) | Young's modulus (MPa): (0.43 ± 0.01) to (1.68 ± 0.09) Elastic modulus (MPa): (2.97 ± 0.09) to (5.50 ± 0.79) |
In vitro | Enhanced adhesion, proliferation and differentiation of human osteogenic sarcoma cell line, promoted ALP activity | [42] |
| CA-COL | Oil-in water solvent-evaporation technique | 185.4 ± 8.6 | 33.9 ± 5.2 | Compressive modulus (MPa): 266-(75 ± 33)-22 (Dry scaffold), 130-(53 ± 13)-97 (Wet scaffold) Compressive strength (MPa): 12-(15 ± 2)-23 (Dry scaffold), 7-(15 ± 1)-24 (Wet scaffold) |
In vitro | Increased adhesion and proliferation of human osteoblast cells | [47] |
| PLA-RC | Electrospinning and Freeze-drying | Minor pores: smaller than 20. Major pores: 50 to 150 |
Around 96 | Young's modulus (kPa): 16.5 to 54.9 | In vitro | Increased biomineralization and bone-like apatite formation | [51] |
| Na-CMC-CS- CQ | Freeze-drying | 148–239 | ___ | Compression Moduli (kPa): 654.4 (dry condition), 87.65 (wet condition) |
In vitro | Enhanced adhesion, proliferation, and mineralization of osteoblasts, increased osteogenic activity and ALP activity | [55] |
| HEC-PVA | Electrospinning | (9.55 ± 0.17)- (5.98 ± 0.5) | ___ | Tensile Strength (MPa): 2.63 to 10.54 Elastic modulus (MPa): 188 to 349.25 |
In vitro | Increased attachment and proliferation of human osteosarcoma cells | [59] |
| HEC-PVA-CNC | Freeze-drying | 33.4-∼54.1 | 77 | ___ | In vitro | Increased adhesion and proliferation of human fetal osteoblast cells | [60] |
| TEMPO-oxidized CNF- GEL |
Freeze-drying | 8–150 | 71.4 ± 1.4 | ___ | In vitro | Increased attachment, spreading and osteogenic differentiation of hBMSCs, enhanced RUNX2 and SPP1 expression | [64] |
| CNC-PLLA | Selective laser sintering | 450–600 | ___ | Tensile strength (MPa): 7.93 ± 0.31 Modulus (GPa): 2.33 ± 0.07 |
In vitro | Enhanced adhesion, proliferation and differentiation of MG-63 cells, increased ALP activity | [67] |
| Cellulose-CNCs-BMP-2 | Electrospinning | 272.4 ± 31.64 nm | 77 | ___ | In vitro, In vivo (cranial bone in rabbit) | Increased osteogenic differentiation of BMSCs, enhanced ALP activity and calcium content, induced in vivo collagen assembly direction, cortical bone regeneration | [69] |
| BNC -SA-CS-GEL | Freeze-drying and Layer-by-layer assembly | 30–300 | 77.4 | compressive strengths (MPa): 0.27 | In vitro | Increased attachment, proliferation and differentiation of MC3T3 -E1 cells, enhanced ALP activity | [73] |
| CNW-PU | Solvent casting/particulate leaching | 20–150 | 82 | Tensile strength (kPa): 112 | In vitro | Promoted proliferation, adhesion, and osteogenic differentiation of hMSCs, increased ALP activity and calcium content | [74] |
| CNWs-PU-PHEMA | Solvent casting/particulate leaching | 20–150 | 85 | Tensile elasticity moduli (kPa): 80.5 Tensile strength (kPa): 89.8 |
In vitro | Enhanced osteogenic differentiation of hMSCs and bone mineralization | [76] |
| MCC-PCL | 3D Printing | 450–500 | 57 ± 2 | Compressive modulus (MPa): 7 | In vitro | Increased proliferation of sheep bone marrow cells | [77] |
3. Cellulose-inorganic composite scaffolds
Calcium phosphate-based bioceramics such as HAp are well-known osteoconductive inorganic materials, while bioactive glasses are generally recognized as osteoinductive biomaterials. Tissue-engineered scaffolds of both inorganic biomaterials are being developed for bone regeneration, even though their brittleness and low fracture toughness have remained a big challenge. One strategy being investigated to promote the structural integrity and mechanical properties of the inorganic scaffolds is to compose them with biopolymers. Here, we will review the literature on cellulose-inorganic composites to clarify the influence of the organic part, i.e., cellulose or its derivatives, on the properties of the final structure as a bone scaffold.
3.1. Bacterial cellulose-inorganic composites
Bone tissue engineering consists to reconstruct/regenerate damaged bone tissue, using organic and inorganic substances or a combination of those to simulate its functionality because bone natural tissue contains two major components; an organic matrix (COL) and an inorganic phase (HAp and CP). Among diverse types of synthetic and natural polymers [78], BC can be used to fabricate tissue-engineered scaffolds due to its prominent properties as we discussed in Section 2.1. From material point of view, as a result of non-animal origin, BC is compatible and does not cause any allergic response in comparison with COL. Interestingly, various studies reported that BC and COL fibers are similar in structure [79,80]. In addition, BC has high mechanical strength; 2 GPa tensile strength, and 138 GPa Young's modulus, and these results proved BC can provide mechanical performance close to the natural bone tissue which has been reported that the tensile strength and Young's modulus are approximately 15–20 GPa and 100–160 GPa, respectively. In addition, the textural properties of BC are distinguishable from plant cellulose; it has smaller nano-fibers and higher tensile strength due to the nano-fibril network structure [81]. Thus, the mentioned properties make this a potential bio-membrane for bone tissue regeneration. However, the main drawback of BC in bone tissue engineering is the inability to bond directly to the bone tissue [82]. To overcome these limitations, some studies have been reported based on incorporating HAp into pure BC. For instance, Fang et al. and Hong et al. [83,84], fabricated BC-HAp nanocomposite scaffold via soaking BC membrane into SBF solution in two separated studies. Their results showed that BMSCs adhered and proliferated on BC-HAp composite scaffold better than pure BC, and ALP activity and the expression of osteopontin, osteocalcin, bone sialoprotein was far better than those seeded on pure BC. It had been shown the amount of apatite growth and its crystallinity was associated with soaking time. Moreover, ionic interactions were occurred between negatively charged OH groups of BC and calcium ions of SBF, where calcium ions can bind phosphate ions to create the initial nuclei, then apatite formation was enhanced by uptake of phosphate and calcium ions from the surrounding SBF fluid. Similar results were observed by Sundberg et al. which fabricate interconnected macroporous BC composite scaffold through using paraffin porogen particles, then mineralized with HAp [85]. The results indicated that although HAp incorporation resulted in smaller pore size, it did not have a negative effect on cell/scaffold interaction and ALP activity. Additionally, Tazi et al. showed osteoblast attachment and growth can be promoted on BC-HAp composite scaffold compared with pure BC due to the presence of phosphate and calcium on the surface of the BC-HAp composite scaffold [86]. Another study has been reported based on in vivo evaluations, Saska et al. generated BC nanocomposite membrane via alternative soaking technique. Compared with plant cellulose, it had no allergic reactions, experimentally, and BC membrane enhanced apatite's particles growth substantially which may be related to better textural properties (nanofiber diameters ranged 10–50 nm) and larger surface area [87].
In addition, few studies have shown that pure BC cannot support apatite formation. Thus, different modification methods were employed to enhance physicochemical properties of BC scaffolds such as, the phosphorylation [88], electron beam radiation [89], surface modification [90,91], and alkaline treatment technique [92]. BC membrane suffers from a lack of apatite formation. It should be noted that hydroxyl groups of BCs cannot provide a potential site for nucleation and growth of apatite crystals. For example, Wan et al. fabricated a BC-HAp nanocomposite scaffold with soaking BC fibers into static culture containing CaCl2, then its surface was modified through phosphorylation procedures by phosphoric acid (H3PO4) in dimethyl formamide (DMF) [88]. The results showed that apatite formation improved with phosphorylated BC fibers which revealed durable ionic interaction between the phosphate groups and calcium ions. Another study was reported by Ahn et al. BC-HAp composite scaffold was developed by electron beam irradiation to alter physicochemical properties and enhance HAp adsorption [89]. The results showed that HAp adsorption was associated with incubation time. Moreover, mechanical properties were enhanced due to more HAp content. In addition, the biological properties were improved in comparison with pure BC. In the other study, Zimmermann et al. produced BC bone-like nanocomposite structure via a bioreactor/pump system, then the surface was modified with CMC, which resulted in a higher apatite formation [90]. Similar results were also observed by Wan et al. where the BC membrane was modified by CNT coating [93]. It resulted in a smaller nanofiber diameter because of the evaporation of excess gases at 600–1200 °C during the carbonization procedure, which enhanced carbon content, improved HAp formation as well. In addition, Gao et al. produced BC/polylysine nanocomposite scaffold in order to enhance the apatite formation [91]. Based on the reported results, polylysine (cationic charge, NH+3) and BC (anionic charge, OH−) resulted in the formation of BC-polylysine nanocomposite scaffold, which soaking BC-polylysine into CaCl2 enhanced Ca+2 adsorption onto the membrane due to electrostatic interaction. Thus, the applied method improved apatite formation. Niamsap et al. combined CNCs into BC-HAp nanocomposite scaffold as a dispersant agent for preventing HAp agglomeration [94]. The biological properties revealed no cytotoxicity potential while CNCs combinations resulted in superior physicochemical properties. Another study was designed to modify the biological properties of BC nanocomposite membrane with alkaline treatment technique, which resulted in improving apatite nucleation ability of BC, where Ca+2 activation enhanced ionic interaction between negatively charged hydroxyl groups and calcium ions [92]. The magnetized BC-HAp containing Fe3O4 nanocomposite scaffold was also synthesized by Torgbo et al. and it was revealed that adding magnetite NPs resulted in improving mechanical properties (Compressive strength: 9.87 MPa; Stiffness: 1.85 GPa) with appropriate porosity (81.1%), which is similar to human cancellous/trabecular alternative bone tissue [95]. Moreover, by adding Fe3O4 to the BC-HAp structure, the surface roughness increased and consequently resulted in enhanced osteoblast cells viability and proliferation.
In addition, appropriate pore size, biodegradability, and bioactivity were considered prominent to produce the bone-like structure. In this regard, some studies have been designed to address this issue. It should be noted that one reason for blending BC into HAp is the lack of proper pore size, which is noticeable in the scaffold characterization [83]. Regarding the previous studies, Zimmermann et al. and Grande et al. reported that the cell/scaffold interaction of BC nanocomposite membrane is limited due to the micro-textural properties of [90,96]. However, the structural properties of BC-HAp composite scaffold showed better interaction comparable with pure BC. To develop a well-designed substrate for bone tissue regeneration/reconstruction, oxidized BC-HAp nanocomposite scaffold was prepared by laser patterning technique because the nano-fibrous structure (about 50–200 nm) of pure BC was not suitable for vascularization and cell migration, and it did not show in vitro degradation experimentally. The results revealed osteoblastic cells were attached and proliferated on oxidized BC-HAp nanocomposite scaffold more than pure BC which was related to larger pore size (about 300 μm), and the in vitro degradation was increased up to 25% which made it a better candidate for bone tissue engineering [97]. Different approaches were designed to modify the membrane's porosity, such as in situ with porogens [[98], [99], [100]] or ex vivo with laser treatment [97,101,102]. However, the lack of interconnectivity also needs to be addressed. Another study was reported by Bayir et al. developed a novel BC-HAp composite scaffold by shredded agar technique, which was based on the dispersion of different percentages of agar NPs into Hestrin & Schramm solution homogenously to enhance pore size [81]. The results showed a larger pore size (275 μm) proper for SaOs-2 cell attachment and proliferation, and mineralization was enhanced compared with pure BC. In general, fully BC biodegradation in the body could prove BC application as an ideal biomaterial because its exclusive monomers are glucose which has an influential effect on cell growth. Hu et al. fabricated BC membrane, further incorporating cellulase enzyme to promote the rate of in vitro degradation, which showed 100% biodegradation after 4 weeks into SBF [103]. Moreover, scaffolds for guided bone tissue regeneration (GBTR) need to be bioactive and biocompatible [104], which can be suitable substrates for bone tissue formation and pharmaceutical applications [105]. In this regard, Luz et al. produced an oxidized BC membrane with the incorporation of Sr, which has a similar performance to Ca2+ to immobilized bioactive enzymes for enhancing osteogenesis and improving in vitro degradation [106]. The results showed oxidized BC adsorbed more Sr than pure BC because of enhanced targeted sites, which then enhanced water uptake capacity. The in vitro degradation and bone tissue regeneration also were enhanced substantially both in vitro and in vivo. In addition, Ramani et al. showed that incorporation of GO into BC-HAp structure produced with wet chemical precipitation technique tailored in vitro degradation in comparison with pure BC [79]. The mechanical properties were not reported, while in vitro degradation results mentioned that incorporation of GO into BC-HAp composite scaffold could make a compact network comparable with pure BC. Moreover, the osteogenic activity and cell compatibility could demonstrate a potential membrane for bone tissue regeneration.
Although, the incorporation of inorganic materials like HAp, CP, and BG can induce osteoconduction and osteoinduction, being brittle and mechanically weak have been recognized as the main drawbacks of the inorganic materials [107]. A combination of synthetic biopolymers and crosslinking agents like glutaraldehyde were studied previously to enhance the mechanical strength of these composites [37]. It had been proved that mechanical properties of HAp scaffold increased after the combination of BC to its microstructure [108]. Besides, blended BC-HAp showed far better osteoconductivity and biocompatibility than COL-HA [109]. Furthermore, different techniques were employed to improve the mechanical properties of the BC-HAp composite scaffold [90]. For instance, Gutiérrez-Hernández et al. used MWCNTs-loaded BC-HAp nanocomposite scaffold to reinforce mechanical/biological properties [110]. The results showed MWCNTs functionalized with carboxyl groups resulted in decreasing risk of toxicity due to negatively charged surface, which is more favorable for osteoblastic attachment and proliferation.
The bone-like apatite can be evaluated and created in vitro via SBF, so this technique has been recognized as a prominent approach to estimate the bioactivity of the composite [111]. To improve apatite layer formation, BC gel was placed in SBF solution containing Ca2+ and ions. In another study, BC membrane was soaked into static culture and mineral phase which improved apatite layer formation [112]. Another method, which was based on fabricating BC/nano-HAp composite scaffold is the electrospinning [113]. This technique is a versatile approach because it can make a proper membrane with adjustable fiber diameter, pore size, porosity, tensile strength, using a wide range of natural and synthetic polymers to develop a bone-like structure.
3.2. Plant cellulose-inorganic composites
Cellulose is a main structural component of plant cell walls and can be easily extracted from plant sources especially wood and cotton [[114], [115], [116], [117], [118]]. Additionally, plant-derived cellulose is inexpensive and plentifully available. Plant fibers offer a wide range of properties, especially excellent mechanical strength and flexibility, and provide adjustable biodegradation [115] which can promote HAp properties—such as improving mechanical properties and increasing the rate of in vitro degradation in order to produce a well-defined bone tissue-like construction [[119], [120], [121]]. Although collagen is a natural part of the bone structure that can be a suitable site for HAp nucleation and growth, it suffers from inadequate mechanical strength [115]. The physicochemical and biological effects of plant cellulose have been studied in many research areas [115,122]. Ma et al. used a bamboo source to prepare plant cellulose [115]. First, bamboo fibers (BF) were characterized, where BF properties were observed under different conditions and treatment including alkali-treated (NaOH solution), acid-treated (H2SO4 solution), and dissolved BF into NaOH. Then BF-HAp nanocomposite scaffolds were produced through the precipitation method. The results clearly show that alkali treatment and dissolved BF resulted in reduced apatite formation due to decreased hydrogen bonds compared with pure BF. On the other hand, the acid-treated technique enhanced HAp nucleation and growth. However, all prepared nanocomposite scaffolds revealed suitable mechanical strength, water absorption, bioactivity, and cell compatibility. In another study He et al. prepared cellulose from cotton which was produced into NaOH/urea aqueous solution at −12 °C, then Na2SO4 aqueous solution was used to produced cellulose-HAp nanocomposite scaffold [122]. The prepared porous cellulose-HAp nanocomposite scaffold showed good mechanical properties because of particle reinforcement agent and strong bonding between cellulose and HAp, which resulted in good cell compatibility, where 293T cells spread and proliferated well on the surface due to appropriate pore size.
Despite researchers have tried to address different requirements of bone tissue-like structure with the incorporation of HAp into cellulose scaffold including good textural properties (pore size and porosity), mechanical properties (elastic modulus and compressive strength), sufficient bioactivity (apatite nucleation and growth), and cell compatibility, it is not yet accessible to fabricate the scaffold with similar properties as same as bone tissue with just incorporation of HAp into cellulose. To achieve this goal, Saber-Samandari et al. investigated titanium dioxide (TiO2)-incorporated polyacrylamide-grafted cellulose (from cotton by dissolving into dimethylformamide and lithium chloride)-HAp nanocomposite scaffold via freeze-drying method [114]. The presence of TiO2 resulted in producing an interconnected nanocomposite scaffold with appropriate internal roughness, similar mechanical strength with trabecular bone tissue. In addition, the incorporation of TiO2 decreased water uptake capacity. The cell/scaffold interaction with L929 cells also showed good biological properties.
3.3. Cellulose acetate-inorganic composites
To induce bone formation and HAp mineralization on cellulose derivative, different techniques such as HAp-coating, grafting of adhesive peptides, oxidation, and phosphorylation have been explored [[123], [124], [125]]. Despite HAp can promote bone cell osteogenic differentiation and biomineralization, it suffers excessively from poor mechanical properties and poor dispersion and aggregation in the polymer solution [126,127]. To overcome poor mechanical strength and dispersion, some techniques were purposed, including surface functionalization and co-electrospun nanoparticles [128,129]. For example, Liu et al. produced GO-incorporated CA nanocomposite electrospun scaffold which resulted in decreasing nanofiber diameter (from 595 to 285 nm) due to higher electron charge density, improving substantially mechanical strength (Young's modulus and the tensile stress were 7.2 and 2.7 times more than pure CA), and biomineralization of GO-incorporated CA scaffold was much higher than pure CA because the anionic hydroxyl and carboxyl groups of GO enhanced Ca2+ deposition which resulted from in mineralization, apatite nucleation and growth as well as enhancing the osteogenesis of hMSCs [128].
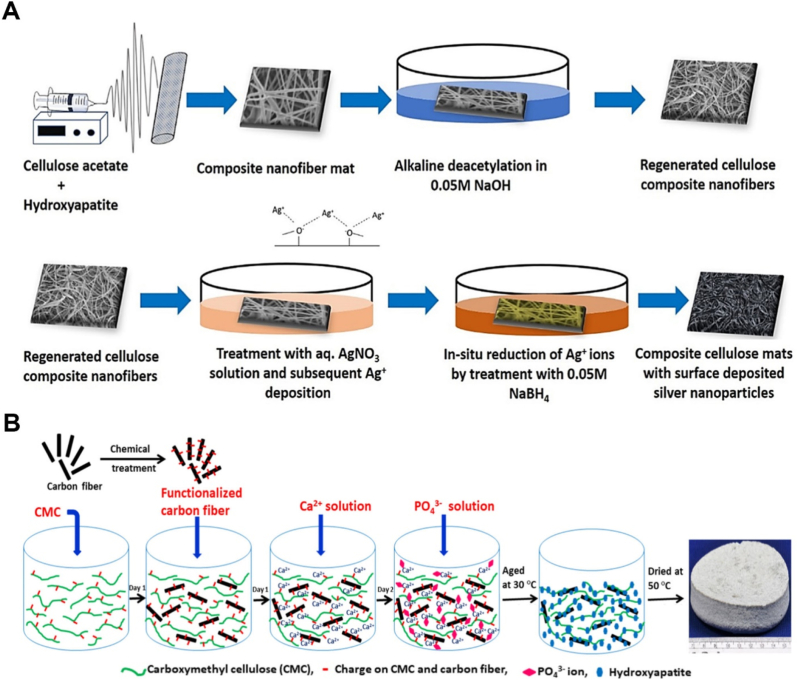
In addition, some studies were accomplished to produce RC using CA [130,131]. The main purpose of these investigations is to use unique properties of pure cellulose such as desirable mechanical strength, higher crystallinity, lower solubility, better hydrophilicity, and slow in vitro degradation which is more favorable in the bone tissue engineering [132]. For example, Sofi et al. developed RC nanocomposite electrospun scaffold via facilely alkaline de-acetylation technique [133]. Next, HAp and Ag NPs were incorporated into the prepared nanofibers via surface coating through electrostatic and van der Waals interactions (Fig. 3 A). The acetone-water solvent was used as a dispersion agent, then NaOH was used to de-acetylate RC nanofibers. The incorporation of HAp and Ag resulted in promoting apatite layer formation and biological properties. In addition, antibacterial activity was enhanced against S. aureus and E. coli.
Fig. 3.
A. Illustration of the fabricating regenerated cellulose fibers containing HAp and Ag NPs [133]. B. Schematic of creation of 3D carbon fiber reinforced CMC-HAp ternary composite [142].
3.4. Carboxymethyl cellulose-inorganic composites
During recent years, CMC has been drawn much attention in bone tissue engineering applications. CMC as a cellulose derivative is spectacular in structure because it is developed as follows: replacing some carboxymethyl groups with hydroxyl groups which results in higher biocompatibility and water solubility, designing for a wide range of biomedical demands [134], such as the cell adhesion properties to fabricate tissue-engineered scaffolds [135]. In addition, some studies have been reported that CMC can induce expression of BMSCs [136] and osteoblast cells [137]. Thus, CMC can be applied for bone tissue regeneration. For example, Qi et al. fabricated CMC-CP composite scaffold via alternative soaking technique into Na2HPO4 and CaCl2 solution, and for evaluation of osteoblast cells behavior and tissue formation, it was assessed both in vitro and in vivo [134]. The prepared scaffold enhanced osteoblast differentiation of hMSCs with higher Sp7 and Osterix expression (osteoblast markers), calcification, and ALP activity as well. Moreover, in vivo evaluations on the mouse calvarial model revealed an improvement in bone tissue regeneration compared to the control group.
Although prepared CMC as an anionic membrane offers biocompatibility and biodegradability, it suffers from a lack of appropriate mechanical strength [137]. CMC can improve apatite formation due to offering carboxyl groups (-COOH) which have been explored by different methods including in situ synthesis [138], co-precipitation [139], one-pot synthesis method [140,141], and wet precipitation [142]. Garai et al. fabricated a CMC-HAp nanocomposite scaffold with different percentages of CMC by using the biomineralization process (calcium nitrate aqueous solution) [143]. The results demonstrated that there was a linear relationship between porosity percentages and mechanical properties, where increasing porosity percentage resulted in higher mechanical characteristics. It should be noted that the mechanical properties (compressive modulus: 157–330 MPa; compressive strength: 1.74–12 MPa) of the CMC-HA nanocomposite scaffold were equal with the cancellous bone. Therefore, it can be concluded that, in addition to chemical compositions, the textural properties can affect directly on the mechanical properties and the cellular behaviors [144]. Another study also proved the importance of using HAp, where they fabricated CMC-HAp nanocomposite scaffold via one-pot technique [140]. The one-pot technique is a novel approach employing gel foam as pore-forming agent, which also the presence of gel enhanced its osteogenic activity. Moreover, another study was carried out by Sarkar et al. designed to enhance mechanical properties of CMC-HAp composite scaffold with the addition of carbon fiber (CF), where its compressive strength, flexural strength, and flexural modulus were increased significantly after a combination of CF into the membrane as same as human bone tissue (Fig. 3B) [142]. Therefore, excellent biocompatibility, biodegradability, and bioactivity make CMC a multifunctional platform for producing a bone tissue-like structure. However, their properties can be developed, and the mechanical properties need to be modified.
3.5. Nanocellulose-inorganic composites
As mentioned in section 2.5, CNCs and CNWs are well-known as promising and strong materials for producing bio-composite scaffolds because of reliable and adjustable properties, such as excellent biocompatibility, renewability, non-immunogenicity, and stiffness [145,146]. In addition, having Young's modulus ∼130 GPa, plus providing highly hydrophilic substrate as a result of a multitude of hydroxyl groups make CNCs and CNWs as potential candidates for bone tissue engineering comparable to COL or SF. Some studies are designed to show the potential of CNCs and CNWs through combining organic and inorganic materials because the inorganic phase improves the physicochemical properties of the organic material [147,148]. For instance, Fragal et al. fabricated a functionalized CNWs nanocomposite scaffold with carboxylic (COO−) or amine (NH2) groups by biomimetic mineralization process (CaCl2 solution) in order to induce targeted sites to increase HAp nucleation and formation, where Ca+2 and PO4−3 ions were bonded to the hydroxyl, carboxyl and amine groups which resulted in promoting hydrogen and anionic interactions [145]. All obtained data proved that this manipulated structure enhanced osteogenesis as well as cell/scaffold interaction, suggesting a prominent scaffold for bone demands. In another study, 45S5 BG was prepared with foam replication method, then coated with CNWs through dip-coating technique to improve its properties [148]. The prepared nanocomposite scaffold resulted in a highly porous and interconnected membrane, and mechanical properties were modified while it did not negatively affect bioactivity and cell compatibility (MG-63 cells) due to favorable pore size and morphological properties, and the wettability was also enhanced. Furthermore, a CNWs-based composite scaffold with specific surface compositions was designed to observe the chemical effect of different inorganic acids (HCl, H3PO4, and H3SO4) in the biomimetic method as well wet chemical precipitation. The results showed preparation of CNWs-based scaffold by biomimetic method resulted in higher HAp nucleation and growth than wet chemical precipitation. Interestingly, CNW(H3SO4)-HAp illustrated better physicochemical and biological properties among other composite scaffolds, i.e., CNW(HCl)-HAp and CNW(H3PO4)-HAp, which exerted unique cell viability and apatite growth. However, CNCs and CNWs are not degradable in the human body, and by just combining CNCs or CNWs with HAp, the composite scaffold would show poor mechanical properties [87,149]. Thus, simulating bone structure through using CNCs or CNWs has not yet completely addressed the requirements.
To fabricate a similar structure to bone tissue, some researches have been focused on combining CNCs or CNWs with other substitutes or using different approaches [[150], [151], [152], [153]]. For instance, Huang et al. designed a process to enhance mechanical properties of CNC [150]. To improve HAp nucleation, the sulfonic groups as an activate agent were replaced with ionic groups (like calcium ions). They fabricated CNC-HAp composite scaffold via freeze-casting method by applying different temperature. The HAp content was controlled with pH (reaching ∼ 47% at pH = 9.0), and the porosity was determined with HAp content and freezing temperature (from 70 to 91% with different percentages and temperature). The high mechanical properties (compression modulus and compression stress were 227.6 kPa and 61.7 kPa, respectively) was achieved due to its oriented structure and textural properties. To promote biodegradability and mechanical properties, CNC-HAp composite scaffold crosslinked with PEG and poly (methyl vinyl ether-alt-maleic acid) via esterification reaction, then freeze-casted for producing porous membrane [151]. The results illustrated that mechanical strength (Compression strength: 41.8 MPa) improved significantly (over 20 times higher than CNC-HAp). In addition, in vitro test of composite scaffolds showed the degradation rate was increased substantially, and the highly porous membrane (∼91%) was compatible as it improved bovine serum albumin protein stabilization and reduced the rate of inactivation.
In conclusion, the composition of cellulose-inorganic demonstrates favorable physical, chemical, mechanical, and biological properties especially for bone tissue regeneration. The fabrication method, physical and mechanical properties of cellulose-inorganic scaffolds and key biological results are shown in Table 2. Herein, the various cellulose-based materials (bacterial cellulose, plant cellulose, cellulose acetate, carboxymethyl cellulose, and nanocellulose) and adding inorganic materials to cellulose in bone tissue engineering are reviewed. In addition, as it is discussed above the differences of properties between cellulose-based composite materials and pure cellulose materials is mentioned to shed light the effect of adding inorganic substances into cellulose materials which demonstrate that the physico-chemical and biological properties showed better properties compared to pure cellulose. These composites exhibited desirable features due to apatite formation, osteogenic activity, osteoconduction and osteoinduction, which are unachievable by pure cellulose materials alone. The capability of cellulose and its derivatives to be modified and easy to be processed has made it a multifunctional platform for further studies towards bone tissue engineering.
Table 2.
Cellulose-inorganic composite scaffolds in bone tissue engineering.
| Composite | Fabrication method | Pore size (μm) | Porosity (%) | Mechanical properties | Type of study | Key biological results | Ref |
|---|---|---|---|---|---|---|---|
| BC-HAp Fe3O4 nanoparticles |
Ultrasonic radiation | _ | 81.1 | Compressive strength (MPa): 9.87 Stiffness (GPa): 1.85 |
In vitro | Enhanced viability and proliferation of osteoblast cells | [95] |
| BC-HAp | Laser patterning technique, then modified using periodate oxidation | 300 | _ | Tensile strength (MPa): 0.16 Elastic modulus (MPa): 1.05 |
In vitro | Induced attachment and proliferation of osteoblast cells | [97] |
| TiO2-cellulose-HAp | Freeze-drying method | 70–130 | 87 | Compressive strength (MPa): 4.1 | In vitro | Increased attachment, viability and proliferation of fibroblast cells | [114] |
| CMC-HAp-Gel | One-pot method | 2.5–900 | 80 | Compressive strength (MPa): 11.8 ± 1.5 Compressive modulus (GPa): 0.243 ± 0.031 |
In vitro | Enhanced osteogenic activity | [140] |
| CMC-HAp-PVA | Biomineralization process | 1–10 | 11–80 | Compressive strength (MPa): 1.74 to 12 Compressive modulus (MPa): 157 to 330 |
In vitro | Improved migration and proliferation of bone marrow-mesenchymal stem cells | [143] |
| CNW-45S5 BG | Foam replication | 200–550 | 93 | Compressive strength (MPa): 0.06 ± 0.01 | In vitro | No negative effect on bioactivity and cytocompatibility of MG-63 cells | [148] |
| CNC-HAp-PEG- PMVEMA | Freeze-casting | _ | 91 | Compression strength (MPa): 41.8 | In vitro | Improved bovine serum albumin protein stabilization and reduced the rate of inactivation | [151] |
4. Cellulose-organic/inorganic composite scaffolds
To prepare a functional scaffold based on cellulose mimicking bone tissue, we need a proper choice of composition of organic and inorganic biomaterials that should be organized in a homogenous integrated network via appropriate fabrication methods. Consequently, in this section, we will report the recent efforts for scientific research on the use of cellulose-organic/inorganic composites in bone regeneration applications.
4.1. Bacterial cellulose-organic/inorganic composites
Numerous investigations have been conducted on multicomponent organic/inorganic BC-GEL/HAp composite scaffolds for bone tissue engineering. In one study, a nanocomposite of enwrapped BC nanofibers into crosslinked GEL network was successfully synthesized and then a homogenous layer of HAp was bio-mineralized on the surface of the BC-GEL composite [154]. MSCs well interacted with this bioactive nanocomposite and recognized it as a suitable substrate for proliferation and growth. In another study, a BC membrane was modified via laser patterning technique in order to introduce parallel-microchannel arrays (approximately 200–300 μm in diameter) into the surface [155]. After that, the prepared BC membrane was coated with GEL and immersed into SBF for HAp deposition. This micro-pattern topography supplied a substrate with large and interconnected pores for attachment and proliferation of chondrogenic rat cells. Recently, a nanocomposite of BC-GEL/BHAp was fabricated and characterized for bone tissue engineering [156]. The mechanical and biological properties of the nanocomposite were significantly improved relative to the neat GEL scaffold. The biological results indicated an increase in attachment, proliferation, differentiation, and migration of Saos-2 cells within nano BHAp-containing composites. Here, boron had a determinative role in the direction of cellular activities. This essential trace element presents at a concentration around 56 ppm in healthy human bone, which stimulates new bone formation helping the variety of the metabolic actions and preventing tissue loss [157].
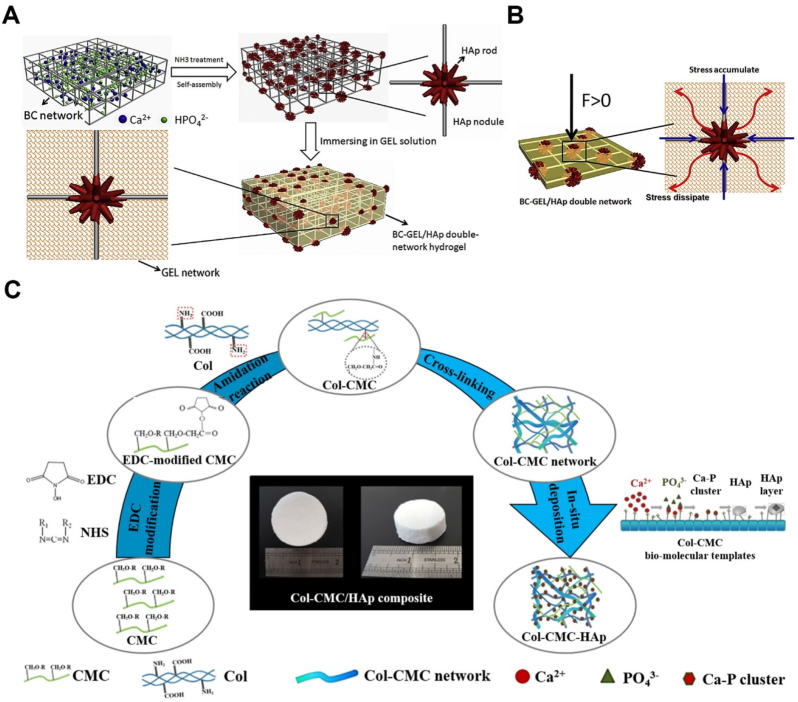
In the above-mentioned reports, the mechanical strength of the composites was not highlighted, instead, those studies mainly focused on the biological properties of the composite construct. Taking augmentation of the mechanical aspects into consideration, a double network scaffold of the BC-GEL containing HAp NPs was constructed. For this purpose, the BC-HAp network was prepared and at the next step, GEL was incorporated into the nanocomposite to modulate the brittleness and stiffness. The final nanocomposite scaffold revealed high compressive strength and tensile strength in comparison with BC-HAp and BC-GEL. The structure of the nanocomposite was mechanically stabilized through the self-assembly of HAp NPs at the nodules of the BC network and the dissipation of the accumulated stress at these sites by GEL molecules. According to the results of biological experiments, rat bone marrow-derived MSCs cultured on the BC-GEL/HAp showed better adhesion and higher proliferation than those cultured on the BC-GEL network (Fig. 4 A, B) [158].
Fig. 4.
A. Schematic of preparation process of the BC-GEL/HAp hydrogel [158]. B. Schematic of stress dissipating of the BC-GEL/HAp composite under external load [158]. C. Schematic of the formation mechanism of Col-CMC/HAp composites [176].
SF and PAA are other organic phases that were composed with nano HAp and BC to form three-phase nanocomposite scaffolds for bone repair. SF which owns a special crystalline structure has been extensively recognized as an organic reinforcing biomaterial, SFs have different amino acid sequences with the ability of tuning cell functions. Given that, Jiang et al. reinforced the BC-HAp framework by the addition of domestic SF (i.e., Bombyx mori silk (BMS)) and wild SF (i.e., Antheraea yamamai silk (AYS)) in order to fabricate high strength scaffold with anticipated bioactivity [159]. It should be noted that the composition, structure, and physicochemical properties of these native proteins vary remarkably depending on the source of extraction. AYSF has higher crystallinity than BMSF, and it further possesses tripeptide sequence Arg-Gly-Asp, which simulates the natural extracellular matrix molecules for cell adhesion. Based on the findings of this study, BC-AYSF/HAp had a higher elastic modulus (0.18 MPa) and higher fracture strength (0.29 MPa) than BC-BMSF/HAp and BC-HAp nanocomposites. Indeed, MC3T3-E1 cells cultured on the BC-AYSF/HAp nanocomposite demonstrated higher proliferation than the cells cultured on the BC-BMSF/HAp nanocomposite. In addition, a nanocomposite scaffold of nano HAp/cellulose-graft-PAA with high mechanical properties was explored for the bone regeneration [160]. The prepared nanocomposite exerted mechanical strength close to the trabecular bone along with good cytocompatibility. In another study, the incorporation of BC to a host composite of PVA-hBN increased some physical properties including pore size, viscosity, surface tension and elongation break [161]. PVA and hBN were employed at concentrations of 12 wt% and 0.25 wt%, respectively, but different amounts of BC were examined (concentration of 0.1, 0.25 and 0.5 wt%) that 0.5 wt% BC resulted the best mechanical and biological outcomes. By focusing on the biological properties, the hydrogel scaffolds of BC-PVP-β-TCP/HAp-CaCO3 and BC-CMC-β-TCP/HAp-CaCO3 were constructed with similar compressive strength to the trabecular bone (0.24–0.60 MPa) and good cell biocompatibility for bone tissue substitute [108,162].
4.2. Plant cellulose-organic/inorganic composites
In a series of studies, BFs were composed with PLGA and nano-HAp under a defined surface treatment, and then the effect of length and concentration of BFs was evaluated to achieve a desirable scaffold for bone regeneration [116,117,[119], [120], [121]]. BFs were treated using two different methods of alkali treatment and silane modification after alkali treatment to remove impurities and augment the interfacial interaction between BFs and polymer matrix. The treatments improved the mechanical performance, especially for silane modification. The length of the fibers (≤1 mm, 4–5 mm, and 9–10 mm) also affected some physical and mechanical properties, so treated composite consisting of the medium length BFs achieved the highest tensile properties, and the small length BFs exhibited the lowest water absorption and the slowest degradation rate [119]. The freeze-dried nanocomposite embedded with 5 wt% untreated BF (4–5 mm) could achieve the desirable porous structure and superior compressive properties relative to PLGA/HAp nanocomposites [116]. Further, the addition of BF accelerated the degradation rate of the organic/inorganic nanocomposite and exhibited a good cellular response to Human osteoblast cells (MG-63). In another study, the treated PLGA/HAp nanocomposite with 5 wt% BFs cut into 4–5 mm was prepared by solution mixing method [120]. The findings indicated no cytotoxicity and provided a suitable substrate for the attachment and proliferation of MG-63 cells.
Using plant extracted cellulose, a nanofibrous HAp-covered scaffold composed of PCL and lignin was prepared to stimulate cancellous bone (elastic modulus 5.57 GPa). PCL as a blender facilitated the electrospinning process, lignin as a biomineralization template induced deposition of clustered needle-like HAp nanocrystals on the surface of the lignin-PCL membrane. MC3T3-E1cells found the lignin-PCL/HAp membrane, an excellent substrate for adhesion with elongated morphology that confirmed their tendency for migration [163].
4.3. Cellulose acetate-organic/inorganic composite
Luo et al. fabricated CA composite electrospun scaffold for depositing CS-MWCNT via electrostatic self-assembly which was compared with a CS-SA scaffold in order to evaluation of the effect of MWCNTs on its properties [129]. The results indicated that the mechanical properties of the CA-CS-MWCNT nanocomposite scaffold were significantly improved when compared to the CA-CS-SA multilayered membrane. Moreover, the biological performance was significantly improved due to increased apatite formation due to MWCNTs' superior protein adsorption. In a recent study, Rad et al. fabricated a CA-GEL coated-disc shape scaffold of pure and 7% boron-doped nano-BG for the bone tissue engineering applications [164]. Polymeric coating significantly promoted compressive strength and Young's moduli, whereas no alteration was observed in the porosity. Additionally, growth and osteogenic differentiation of human dental pulp stem cells were higher in CA-GEL/boron-doped BG scaffold than CA-GEL/BG scaffold. In another study, the 2D nanofibrous membrane of CA and PCL was fabricated and subjected to deacetylation to change CA to RC [165]. After that, the treated fibers were immersed into the salt solutions for calcium hydroxide particle deposition. finally, several Ca treated layers of these mats were assembled to create a 3D multilayer composite. RC-PCL mats were soaked in a salt solution at varying time duration (12, 24 and 48 h) to cover with inorganic particles. The best results were obtained for the sample that was immersed for 24 h. This stiff nanofiber mat showed acceptable stress-bearing, increased porosity, high water uptake besides promoted cellular infiltration, mineralization, and osteogenesis.
4.4. Carboxymethyl cellulose-organic/inorganic composites
CMC is an anionic polymer with a high capacity for electrostatic interaction with cationic biomaterials such as CS, forming a polyelectrolyte complex [166,167]. While compared with pure CS, this polymeric blend generates a mechanically stable scaffold with high potential applications for hard tissue regeneration. Some efforts have been taken to intensify the biological and mechanical properties of this polymeric matrix by incorporating bioinorganic materials. A spiral-cylindrical scaffold mimicking structural bone properties was generated by rolling a nanocomposite membrane of CMC-CS/HAp in a concentric manner [149]. The obtained membranes with 60 wt% nano HAp (thickness ∼ 300 μm) were mechanically perforated to create pores with the size of 300 μm and pitch of 1.0 mm before forming in the final shape. This nanocomposite membrane obtained an excellent microenvironment for adhering, proliferating and differentiating primary osteoblasts derived from the calvaria of 3-day rats. In vivo evaluations in a concave radius defect model of rabbits demonstrated new bone formation along the spiral wall that tightly integrated with the scaffold. The valuable quantitative analysis within 12 weeks indicated a significant increase in bone volume density, bone mineral density, trabecular thickness, and number with time which confirmed the new bone regeneration. Sainitya et al. achieved significantly promoted bioactivity by the addition of mesoporous wollastonite particles into the freeze-dried CMC-CS scaffold [168]. The results showed that the high specific surface area and pore volume of the added mesoporous particles at 0.5% concentration increased biomineralization, protein adsorption, and osteoblastic differentiation of MG-63 cells. An antibacterial scaffold with the final structure close to cancellous bone was developed by blending different percent (0, 1, 2.5, 5 and 10%) of silver NPs decorated on carboxylate CNWs (CCNW-Ag) into CMC-CS composite [169]. Ag NPs were immobilized on CCNWs through high affinity toward carboxyl and hydroxyl groups of the cellulose whiskers [170]. By increasing the percentage of CCNW-Ag NPs, degradation rate and swelling ratio of the nanocomposite scaffolds gradually decreased, but mechanical strength increased. Sufficient protein adsorption, good cell adhesion and proliferation was observed for MG63 cells [169]. Tamburaci et al. compared the effect of the addition of Si-substituted HAp (SiHA) NPs into the CMC-CS polyelectrolyte scaffold with two natural silica NPs including diatomite and polyhedral oligomeric silsesquioxanes (POSS) [171]. Silicon, the second most abundant biogenic mineral encourages apatite layer formation on the structure and activates bone-related gene expression, hence stimulating new bone formation [172]. POSS has a nanocage structure with a very small size and large surface area [173], while diatomite is a rich source of (88–90%) hydrated amorphous silica [174]. The authors concluded that the novel silica NPs can be applied as an alternative to SiHAp that simulated the trabecular bone structure and enhanced the bioactivity. The following trends was observed for the compressive strength of the nanocomposites: CMC-CS/SiHAp (254.3 kPa) > CMC-CS/diatomite (230.3 kPa) > CMC-CS/POSS (228 kPa) > CMC-CS (179.3 kPa). All nanocomposites were appeared to be cytocompatible to MG-63 and SW1353 cells, albeit diatomite NPs showed the weakest cellular responses. In a recent study, a porous scaffold of CMC-CS was reinforced with whisker-like biphasic and triphasic calcium phosphate fibers [175]. Biphasic fibers consisted of two phases of HAp and monetite, and triphasic fibers contained HAp, β-tricalcium phosphate, and calcium. The triphasic fibers-reinforced scaffolds showed the highest cell viability and mechanical properties with compressive strengths up to three times greater than pure CS scaffolds. These bioinorganic particles interacted with the polymeric chains through the formation of hydrogen bonds and/or electrostatic interactions. Their reinforcing ability either in biological or mechanical aspects, homogenous distribution, and the integrity of the final composite would be associated with the size, geometry, and chemical formulation of these bioinorganic particles.
CMC can promote apatite nucleation, hydrophilicity, flexibility, and stop bleeding, where it was utilized as a matrix in triphasic composites. He et al., combined COL with CMC to provide a bimolecular template with high reaction sites for HAp deposition with the aim of constructing a high-strength triphasic composite [176]. In situ deposition of the inorganic HAp occurred during the dripping of the precursors, Ca (NO3)2 and Na2HPO4, solutions into the prepared CMC-COL solution. This biomolecular template with abundant carboxyl groups could act as active sites for coordination bonding with Ca2+ cations and then PO43−. This 3D micro-porous composite of CMC-COL containing deposited HAp nanocrystals and a rough texture on its surface showed an excellent in vitro biocompatibility (Fig. 4C). In another report, considering the good spin-ability of SF, a native bone mimics extracellular matrix scaffold was made by electrospinning of the CMC-SF blend and nano-BG [177]. The final structure supported the growth and osteogenic differentiation of MSCs by increasing ALP activity, biomineralization, and glycosaminoglycan secretion. Regarding the role of zinc and manganese elements, the nanofibrous composite scaffold of CMC-PVP incorporated with different percent of Zn-Mn HAp NPs was introduced for the bone tissue engineering [178]. Zinc is an essential element in the natural bone that helps biomineralization and bone growth, and manganese is another helpful trace element that affects cell adhesion of the extracellular matrix [179]. CMC-PVP containing 60 wt% of 0.1 M Zn and Mn substituted HAp exhibited enhanced physical and mechanical properties. This nanocomposite showed the highest antibacterial activity and lowest hemolysis value of 2.23% along with the highest cytocompatibility to human osteoblast cells.
Hemostasis in orthopedic osteotomy or bone cutting is a great challenge that can be controlled through different methods and materials. The bone waxes can mostly stop the bleeding of bone marrow, but they cannot be absorbed which causes infection and foreign body reactions. Therefore, a biodegradable composite scaffold of CMC-CS/BG with the ability to stop bleeding was cast to replace the traditional bone wax [180]. This composite with the same hemostatic performance as traditional bone wax showed the potential in the functional reconstruction of the segmental bone defect model in rabbits. According to X-ray images taken after 9 weeks, the amount of newly formed bone in animals treated with the composite was obviously more than that in the groups treated with the traditional bone wax. Recently, a modified tricalcium silicate cement was composed with CS-CMC/BG to develop an advanced bone wax with high strength, antibacterial activity, and high cell responses [181]. The organic phase was totally used for increasing fluidity, injectability, and tuning setting time, besides improving mechanical properties by forming a double network structure with hydrated cement components. Also, in vitro experiments verified the excellent adhesion and superior proliferation of MC3T3-E1 cells.
4.5. (Hydroxypropyl) methyl cellulose-organic/inorganic composites
HPMC belongs to the family of cellulose ethers that can be dissolved in water and some polar organic solvents. This hydrophilic biopolymer has found applications in different industries such as food, cosmetics, pharmaceutical, biomedical, and adhesives [182]. This anionic biopolymer can be an appropriate crosslinking agent for its own oppositely charged polymeric counterparts. Polyelectrolyte complex scaffold based on this anionic biopolymer and the cationic one, CS, was designed for hard tissue regeneration. Besides, HAp and BG particles as inorganic phases as well as antibacterial agents were added into this polyelectrolyte structure to improve mechanical and biological properties [[183], [184], [185]]. Conclusively, crosslinking by HMPC expedited the repair process following the promotion of cellular properties by giving the required structural support and morphological features.
4.6. Ethylcellulose and hydroxyethyl cellulose-organic/inorganic composites
EC, a pH-insensitive cellulose ether, is soluble in many polar solvents but insoluble in water, so it can be a non-swellable component in the matrix. Inversely, non-ionic HEC can be solved in water to give viscous solutions under different temperatures, but it is insoluble in the most polar solvents [186]. In bone tissue engineering, a composite of PLA with hydrophilic EC containing HAp was developed as a weight-bearing bone substitute [187]. Moreover, solvent casting/particulate leaching/compression molding technologies lead to bone substitutes with compact porous architecture. Owing to superior pore structure, the final triphasic composite showed enhanced mechanical properties along with dimensional stability during hydrolysis (Fig. 5A and B). In another study, electrospun EC grafted-PCL nanofibers were prepared and embedded layer-by-layer in SA solutions containing HAp NPs [188]. Finally, these constructions were freeze-dried to obtain the required macroporous scaffolds for bone regeneration. An increase in mechanical strength and biomineralization of EC grafted PCL/SA was observed by increasing HAp content (10, 20, and 30 wt%), whereas swelling, porosity, and degradability decreased with increasing the inorganic part. Wu et al. fabricated an epichlorohydrin-crosslinked HEC/SPI porous scaffold using freeze-drying method and functionalized it with HAp by in situ biomineralization for repairing non-load bearing large bone defects (compression strength: 0.22–110 MPa) [189]. In vitro experiments showed that the HEC/HAp composite with 70% SPI content promoted osteogenic differentiation of MC3T3-E1 cells by accelerating the expression of some osteogenic-related genes of Col-1, Runx2, OPN, and OCN. In vivo experiments on critical-sized cranial defects in a rat model confirmed desirable integration between the host bone and the composite after 12 weeks. Indeed, HEC was incorporated into HAp/SA scaffolds in order to control the degradation rate and water absorption [190]. HEC with a function of a protective colloid indicated a higher lysosomal degradation rate than SA. Meanwhile, this non-ionic cellulose derivative increased the water uptake of the final composite by increasing OH functional groups.
Fig. 5.
A. Preparation of PLA-EC/HAp porous scaffolds for bone grafting [187]. B. Bone grafting of PLA-EC/HAp porous scaffold [187]. C. Schematic of the TEMPO-oxidation of BC and the colloidal dispersion of HAp NPs. (HAp NPs/TOBC solutions with weight ratios of 1:1) [203].
4.7. Nano- or micro-crystalline cellulose-organic/inorganic composites
Nano or microcrystalline cellulose has been drawn much attention as a mechanical reinforcing agent for organic-inorganic composite scaffolds. A novel biomimetic Cellulose-PLLA/HAp nanocomposite was introduced for trabecular bone tissue engineering. Cotton-sourced MCC and HAp NPs were homogeneously dispersed into the polymeric network using a coupling agent, i.e., sodium dodecyl sulfate. Chemical bonding and effective interface between the PLLA matrix and the reinforcements increased the crystallinity of the nanocomposite up to 80%. Also, an improvement of compressive yield stress from 0.127 to 2.2 MPa was observed by increasing the weight ratio of MCC/PLLA and HAp/PLLA from 0.1 to 0.5 [191].
Reinforcing the GEL/BG scaffold with CNCs led to improved compressive strength, wettability, and favorable adhesion and proliferation of cells [192]. A nanocomposite scaffold of distributed nano-HAp particles and CNC into SF matrix with appropriate pore size, thermal stability and mechanical strength was designed for treatment of critical size calvarial defect in rat [193]. This composite indicated desirable cellular response to MC3T3-E1 cells which was significantly more than SF-HAp, SF-CNC, and CNC-HAp nanocomposites. In vivo examination after 12 weeks showed the highest bone mineral density values in CNC-SF/HAp nanocomposite among all experimental animal groups. This scaffold was biologically and mechanically developed by altering the composition to (carboxymethyl chitosan) CMCS-SF as organic and CNC and Sr-HAp nanocrystals as inorganic parts [194]. By comparing CNC-SF-CMCS/Sr-HAp to the SF-CMCS, the mechanical performance increased up to 3.3-fold and cell viability, as well as the expression of some osteogenic gene markers such as ALP, osteocalcin, osteopontin, and COL-1, enhanced remarkably. The swelling ratio of SF-CMCS significantly decreased by the addition of CNC and Sr-HAp due to reducing hydrophilicity and increasing the stability of the network. Shaheen et al. prepared a high mechanical strength scaffold of polyelectrolyte complex of CS and SA containing CNC and HAp [195]. CNC bridged the neighboring polymer chains via multifunctional cross-links, thus maximizing interfacial stress transfer. This bridging role of CNC stabilized the interconnected pores and enhanced the swelling ratio, porosity, and compressive strength. XG is an anionic branched polysaccharide with good water solubility, biocompatibility, beneficial shear-thinning property but a weak gelling behavior [196]. Kumar et al. successfully increased mechanical performance of XG-bioactive silica glass hybrid material [197] and SA-XG/halloysite nanotubes nanocomposite [198] by incorporating CNC in these structures. Another natural polymer, pectin, was selected as a hydrogel matrix and was mechanically and biologically augmented with CNCs and HAp [199]. Pectin polysaccharide is extracted from plant cell walls, and it has a branch structure with a high molar mass [200]. Nucleation and growth of HAp nanocrystals on pectin-CNC hydrogel was occurred by biomimetic method and wet chemical precipitation. In both methods, similar cell compatibility was observed, while the compression elastic moduli of the hydrogels by wet chemical precipitation (23–57 × 10−3 MPa) was much higher than the hydrogels obtained by the biomimetic method (180–330 × 10−3 MPa) [199]. In summary, the higher contents of cellulose crystals in composite scaffolds, the superior the mechanical properties. Additionally, a homogenous dispersion of these cellulose crystals along with their good interaction with other materials in composite structure may be determinative in the progress of the mechanical strength.
It is believed that CNC and oxidized cellulose nanofibers can help the dispersion of nanomaterials. In this regard, the electrostatic repulsion between anionic graphene oxide and weakly sulfated cellulose nanocrystals inhibited the surface aggregation of the nanomaterials in the composite film [201]. Similarly, TOCNs played a dispersant role in the preparation of printable composite with carbon nanotubes. The prepared nanofibers with higher aspect ratios than TOCNs and hence extremely high densities of carboxylate groups on their surfaces revealed excellent nanodispersibility [202]. According to the obtained results, a nanocomposite scaffold of TOBC-HAp and GEL was designed for bone tissue engineering [203]. HAp NPs adsorbed on the surface of TOBC fibers through hydrogen bonds and regularly spread into the polymeric network through the electrostatic repulsion between the fibers (Fig. 5C).
Additionally, it should be noted that CNWs were regenerated by different sources like softwood pulp that revealed physicochemical and structural properties like bone tissue. In this regard, Qi et al. developed TEMPO-oxidized CNWs hydrogel with completely oriented and fully compact nano-fibers, then this was mineralized through biomimetic technique (CaCl2 and K2HPO4 aqueous solution) in order to produce CNW-HAp nanocomposite scaffold, and then HAp aggregation was controlled through combining poly (acrylic acid) (PAA) into the solution [118]. In addition to appropriate mineralization, the mechanical properties were like natural bone tissue. Recently, CS and HA were established on the CNC-HAp template in a layer-by-layer architecture for making a stable composite scaffold with a high bioactivity [204]. For this purpose, in situ coating of HAp on CNC was firstly carried out in SBF, secondly, the HAp-deposited CNCs were cast and at the third step this template was alternatively dipped into the polyelectrolyte solutions to assemble CS-HA layers. This polyelectrolyte organic coating with thicknesses of 6.23 and 6.50 μm was achieved for 5 and 10 cycles, respectively. The hydrophilicity and surface affinity for cell viability also increased. Although a reduction of 1 GPa in Young modulus and 0.1 GPa in hardness was estimated after coating.
In conclusion, the pristine or chemically modified cellulose indicated various responsibilities in the organic/inorganic biocomposites from being a component of matrix materials or cross-linker to reinforcement or dispersant agent. It can be deduced cellulose, or its derivatives play a predominant role in strengthening the physical and mechanical features of the bone composites, however, their biological activities cannot be neglected considering the hydrophilic nature and ionic sites for nucleation of apatite crystals. The fabrication method and some important physical, mechanical, and biological properties of the bone composites based on cellulose-organic/inorganic biomaterials are summarized in Table 3.
Table 3.
Cellulose-organic/inorganic composite scaffolds in bone tissue engineering.
| Composite | Fabrication method | Pore size (μm) | Porosity (%) | Mechanical properties | Type of study | Key biological results | Ref |
|---|---|---|---|---|---|---|---|
| BC-GEL/BHAp | Freeze-drying | 45–210 | 68.49–80.94 | Young modulus (MPa): 9.61 to 11.33 Compressive strength (MPa): 35.02 to 94.9 |
In vitro | Increased attachment, proliferation and ALP activity of Saos-2 cell line | [156] |
| BC-GEL/HAp | Freeze-drying | 0.2–0.5 | ___ | Young modulus (MPa): 177 Fracture stress (MPa): 12.95 |
In vitro | Increased adhesion, proliferation and differentiation of MSCs | [158] |
| PAA-g-Cellulose/HAp | Freeze-drying | 72–125 | 85.7 | Elastic modulus (GPa): (0.11 ± 0.01) to (0.89 ± 0.01) Compressive strength (MPa): (1.21 ± 0.1) to (5.97 ± 0.2) |
In vitro | Desirable viability for human fibroblast gum cells | [160] |
| BC-PVA/hBN | 3D print | 265.6–290.1 | ___ | Tensile Strength (MPa): (0.05 ± 0.05) to (0.127 ± 0.05) Elongation at break (%): (48 ± 25) to (93 ± 23) |
In vitro | Increased viability and adhesion of human osteoblast cells | [161] |
| CA-GEL/B2O3-BG | Combined method: cold press molding-porogen leaching | ___ | 58.9 | Compressive strength (MPa): 0.82 | In vitro | Increased ALP activity and intracellular calcium in human dental pulp stem cells | [164] |
| CA-PCL/Ca | Electrospinning/gas foaming | ___ | (52.4 ± 3.1)- (75.4 ± 3.8) | Tensile strength (MPa): 4.0 to 5.0 Young's module (MPa): 7.3 to 9.1 |
In vitro | Increased cellular infiltration, mineralization and osteogenesis of MC3T3-E1 cells | [165] |
| CMC-CS/Ag-CNW | Freeze-drying | 150–500 | 80–90 | Compressive strength (MPa): 0.35 to 3.95 | In vitro | Increased adhesion and proliferation of MG63 cells, sufficient mineralization, excellent antimicrobial activity | [169] |
| CMC-CS/diatomite or POSS or SiHAp | Freeze-drying | 190–307 | 61–70 | Compressive strength (kPa): 179.3 to 254.3 | In vitro | Desirable viability for MG-63 & SW1353 cells, promoted osteogenic differentiation of MG-63 cells, increased ALP activity | [171] |
| CMC-CS/CaP | Freeze-drying | 35–250 | 61–75 | Compressive strength (kPa): 150 Compressive modulus (MPa): 3.08 |
In vitro | Increased attachment, proliferation and mineralization of MG63 cells | [175] |
| CMC-COL/HAp | Biomimetic template | 100–300 | (71 ± 4)-(75 ± 4) | Compressive strength (MPa): 3.17 to 7.06 | In vitro | Desirable viability of wild-type mouse embryonic fibroblasts cells | [176] |
| CMC-CS/(Zn-Mn HAp) | Electrospinning | ___ | 98 | Tensile Strength (MPa): 65.86 ± 1.81 Young's Modulus (MPa): 1149.03 ± 4.15 Elongation at Break (%): 139.94 ± 1.65 |
In vitro | Increased antimicrobial activity, hemocompatibility and human osteoblast cell viability | [178] |
| HPMC-CS/BG-ZnO | Freeze-drying | 90.5–132 | ___ | Compressive strength (MPa): 0.2869 to 0.4518 Elastic modulus (MPa): 1.5024 to 3.5082 |
In vitro | Increased proliferation and differentiation of MC3T3-E1 cells, good antibacterial activity | [184] |
| HPMC-CS/HAp | Freeze-drying | 41–273 | 0.53–0.66 | Compressive strength (MPa): (0.223 ± 0.018) to (0.324 ± 0.046) | In vitro | Increased proliferation and differentiation of MC3T3-E1 cells | [185] |
| EC-PLA/HAp | Combined method: solvent casting, particulate leaching, compression molding | 150–250 | 74.09–89.54 | Compressive modulus (MPa): (10.38 ± 2.38) to (35.21 ± 3.17) Compressive strength (MPa): (1.00 ± 0.21) to (1.57 ± 0.09) |
___ | ___ | [187] |
| EC-g-PCL-SA/HAp | Combined method: electrospinning and freeze drying | (160.4 ± 57.14)-(207.05 ± 83.26) | 68.05–84.15 | Compressive strength (MPa): (0.24 ± 0.25) to (0.43 ± 0.12) | In vitro | Increased proliferation and differentiation of human dental pulp stem cells, upregulation of osteogenic genes (BGLAP, Runx2, BMP2) | [188] |
| HEC-SA/HAp | Freeze-drying | ___ | (66.7 ± 3.2)-(87 ± 5.1) | Compressive Strength (MPa): (18.56 ± 0.76) to (23.96 ± 0.82) | In vitro | Increased protein adsorption, viability, and proliferation of MSCs | [190] |
| GEL-CNC/BG | Combined method: in situ composite and freeze drying | 120–320 | 67–78 | Compressive strength (MPa): (1.57 ± 0.25) to (3.61 ± 0.65) | In vitro | Increased adhesion, growth and proliferation of L929 fibroblasts cells | [192] |
| CNC-SF/HAp | Freeze-drying | 110 ± 7.3 | 90 ± 6.2 | Compressive stress (kPa): 200.7 ± 15.3 Compressive modulus (kPa): 617.5 ± 25.2 |
In vitro, In vivo (calvarial defect in rat) | Increased viability and ALP activity of MC3T3-E1 cells, increased bone mineral density in injured animals | [193] |
| CMC-SF/CNC/Sr-HAp | Freeze-drying | 64.22 ± 4.93 | 82.03 ± 1.45 | Compressive strength (KPa): 77.20 ± 4.52 | In vitro | Enhanced protein adsorption, ALP activity and osteogenic gene expression | [194] |
| CS-SA/CNC | Freeze-drying | 103.16–230 | 93.6 | ___ | In vitro | Desirable viability of MG-63 cells | [195] |
| Alg-XG/CNC-HNT | Freeze-drying | ___ | (88.5 ± 0.64)- (91.7 ± 0.81) | Compressive strength (kPa): (91.1 ± 1.2) to (114.4 ± 0.6) Stiffness (N/m): (3168.2 ± 145.2) to (9367.3 ± 113.0) |
In vitro | Desirable viability of MC3T3-E1 cells | [198] |
| CMC-CS/HA | Freeze-drying | 92.10–136.00 | 49.77 | Compressive strength (MPa): 3.89 | ___ | ___ | [205] |
| BC-GEL-PCL/HA | 3D print | 314.14 ± 23.2 | ___ | Tensile Strength (MPa): (1.08 ± 0.34) to (1.58 ± 0.19) Strain at Break (%): (3.29 ± 1.41) to (3.97 ± 2.27) |
In vitro | Increased viability of human osteoblast cells | [206] |
| CNC-PVA/HA | Freeze-drying | (180 ± 129)- (280 ± 124) | (87 ± 0.8)- (90 ± 0.2) | Compressive strength (MPa): 1.39 to 2.09 Compressive modulus (MPa): 10.67 to 16.01 |
___ | ___ | [207] |
| CNF-PCL/HA | Extrusion | ___ | 50.5 | Compressive modulus (MPa): 70.88 ± 8.60 Compressive strength (MPa): 12.12 ± 0.82 |
In vitro | Desirable cell viability | [208] |
| MC-SF/HA | Electrospinning | ___ | (66.24 ± 0.17)– (90.11 ± 0.12) | Tensile strength (MPa): 25.39 to 102.93 Elongation at break (%): 39.48 to 288 Young's modulus (MPa): 1335.33 to 9840.75 |
In vitro | High hemocompatibility, antimicrobial effect, increased proliferation, and ALP activity of human osteoblast cell line | [209] |
5. Cellulose-based composites as local delivery system
A wide range of therapeutic agents including growth factors, bioactive proteins, antibiotics, and anti-inflammatory drugs have been used in therapeutic interventions to incite and promote the natural healing process of damaged bone. Drug carriers become necessary to incorporate an effective dose of the therapeutic agents either physically or chemically and deliver them to the specific target site in a controlled manner over a demanded time. Thanks to the properties of cellulose and its derivatives, there has been a big chance for them to be candidates for drug delivery systems. Fig. 6 shows the scheme of different drug delivery systems based on cellulose-based biomaterials that can be applied for release of some growth factors or small molecule drugs.
Fig. 6.
A. Schematic of different drug delivery systems based on cellulose for bone tissue engineering. B. The ALP activity of BC-BMP-2 scaffolds after implantation (BMP-2 at 225 mg/ml for L-BMP-2/BC, and BMP-2 at 450 mg/ml for H-BMP-2/BC) [214]. C. Cumulative release of ceftazidime from EC microspheres and HAp/PU scaffolds (microspheres/scaffold-L and microspheres/scaffold-H refers to EC microspheres incorporated HAp/PU scaffolds containing 100 μg and 200 μg ceftazidime, respectively) [223].
5.1. Growth factor delivery
The natural bone healing process can be divided into three main phases: osteogenesis, osteoinduction, and osteoconduction. Growth factors, e.g., TGF, FGF, BMP, etc., play a crucial role in persuade bone healing during these phases [[210], [211], [212]]. Among them, BMP has been approved by the U.S. Food and Drug Administration (FDA) for commercial use. The natural or recombinant human BMPs, e.g., rhBMP-2 and rhBMP-7, are frequently used for clinical and experimental osseous defects as a robust osteoinductive factor [213]. From the therapeutic point of view, effective delivery of BMP into the target site is a vital issue. In this context, some cellulose-based scaffolds of BC, CMC, and CNC were developed to administer a therapeutic dose of this vital protein in the local the injured bone tissue.
Some current studies reported BMP entrapment into BC scaffolds using a simple soaking method based on physical interactions. In all reports, the obtained BC scaffolds were immersed into the GF solution to physically adsorb the cargo molecules onto the carriers. For instance, a nanofibrous osteogenic scaffold of BMP-2-coated BC was fabricated for sustained release of the growth factor in the site of the injured bone [214]. In vitro results demonstrated that the BMP-2-loaded scaffold induced the differentiation of the mouse fibroblast-like C2C12 cells into osteoblasts by increasing the ALP activity in a dose-dependent manner (Fig. 6B). The subcutaneously implanted scaffolds promoted cell infiltration with high efficiency of ectopic bone formation. The authors suggested that the BC scaffold was able to reduce the diffusivity of BMP-2 and effectively retain it at the site of injury. In another study, a high porous 3D microsphere of BC-COL was fabricated by the template method combined with the reverse-phase suspension method [215]. Before preparation of the microspheres, di-aldehyde BC was synthesized and grafted to COL chains via Schiff-base reaction for having a final strong structure. This combination method resulted in microspheres with high surface roughness and many interconnected pores in the internal network that were suitable voids for the physical trapping of growth factors. The BC-COL/BMP-2 porous microspheres successfully encouraged the growth and osteogenic differentiation of mice MC3T3-EL cells. On the contrary, in a recent study, an antibody-mediated osseous regeneration approach was recommended as an alternative to exogenous rhBMP-2 administration [216]. Using this approach, a bioactive osteoinductive membrane of BC-HA was functionalized with anti-BMP-2 for capturing endogenous BMP-2. In vitro assays verified that the antibody adsorption on the membrane lasted until day 7 and decreased by day 14 which was enough for the biological activity of the membrane. Totally an ordered nanofibrous structure of BC could provide a porous channel-like structure that was good for the physical entrapment of the GF molecules. On the other hand, these fibers seemed to be well-suited substrates for cell adhesion, growth, and proliferation just like collagen fibers, especially when they were loaded with appropriate GFs.
CMC and CNC are two cellulose derivatives used for sustained release of growth factors in bone tissue engineering. In one study, CMC-based hydrogel comprising calcium phosphate components with or without BMP-2 at two different concentrations of 0.1 and 0.5 mg were implanted in a rat tibial defect model [217]. The GF molecules were physically trapped into the hydrogel network by soaking method. A long-term release of the protein from the hydrogel was provided for more than one month. The hybrid materials containing 0.5 mg of BMP-2 permitted a greater bone formation than the other two hybrid material formulations because of the release of the effective dose of BMP-2. Furthermore, CNC was dispersed within a porous biphasic calcium phosphate scaffold as a drug carrier for rhBMP-2 and rhVEGF [218]. A solution of the growth factors and CNC were slowly dropped on the BCP scaffold by pipetting and then turning the delivery system several times to ensure the physical adsorption of the cargo molecules into the internal pores of the final structure. The growth factors released from the scaffold in a sustained manner for more than one month, an efficient therapeutic period for large bone defects. The prolonged release of both growth factors from the scaffold accelerates the new tissue formation in the early and late stages of bone regeneration. The prolonged sustained release from this delivery system was attributed to the slow degradation of CNC and thus gradual drug diffusion into the surrounding media. Also, CNC addition reduced the porosity and size of microspores of the scaffold.
OGP is an interesting new peptide for bone regeneration that stimulates the proliferation, differentiation, ALP activity, and biomineralization in osteoblastic lineage cells. Alongside, OGP regulates the expression of many growth factors such as TGF, FGF, and insulin-like growth factors [219]. OGP-containing cellulose-based biomaterials involving BC membrane [220], BC-HAp [221], and BC-COL/HAp composites [222] exhibited an increase in osteoinductive properties and bone healing. This growth peptide was physically stabilized into these BC-based scaffolds through the soaking method and forming hydrogen bonding interactions. The prepared osteoinductive membrane released the OGP in a day and exhibited the promoted osteogenic cell proliferation and mineralization process [220]. Pigossi et al. developed an OGP-loaded BC-HAp nanocomposite membrane for bone repair in the critical size calvarial defects in mice [221]. They observed enhanced new bone formation following the local administration of a therapeutic concentration of this peptide through the designed delivery system. BC-COL/HAp associated with OGP or its C-terminal pentapeptide OGP (10–14) developed osteoblastic phenotype in a similar manner without any cytotoxic, genotoxic, and mutagenic effects [222].
5.2. Antibiotic delivery
Infection and inflammation are the problematic challenges in bone repair that happen following traumatic injury or during tissue reconstruction. To address these issues, noteworthy investigations have been performed in the field of local delivery of antibiotics and anti-inflammatory drugs such as gentamycin, vancomycin, and sodium diclofenac. These small molecule drugs were impregnated into different cellulosic carriers such as BC membranes, EC microspheres, and CMC hydrogels or coatings for pharmaceutical applications in bone repair.
Liu et al. prepared a HAp/PU scaffold incorporated with ceftazidime-loaded EC microspheres for bone regeneration [223]. Ceftazidime-encapsulated EC microspheres were prepared using the emulsion-solvent evaporation method. The drug loading of 19.6 ± 1.8% was achieved. EC as a water-insoluble biomaterial was found suitable for delaying the release of the water-soluble drug, ceftazidime, although the loading efficiency of the delivery system was not very high because of the low affinity of EC to this small molecule drug. The drug release from the EC microspheres lasted 40 days which was 20 days lower than the release rate from the EC incorporated HAp/PU scaffold due to the presence of two polymeric barriers to mass transfer (Fig. 6C). Acrylic-based bone cement faced a major challenge of the initial burst release of antibiotics within 24–48 h after utilization and following that poor sustained delivery of the remained drug content in the cement. This result may be attributed to the low permeability of the hydrophobic PMMA to the hydrophilic drugs. To overcome this limitation, a high amount of antibiotics has been suggested to load into the network of the PMMA cement. The normal dose of antibiotics in PMMA cement is about 2 gr per 40 gr cement. Increasing the antibiotic dose from 3.5 to 5 gr per 40 gr PMMA impairs the mechanical properties of the cement, however, this high dose improves the release rate of the drug. The addition of BC to PMMA cement is a way to manage drug release and mechanical properties. Therefore, incorporating BC and a high dose of vancomycin hydrochloride and gentamycin sulfate in the PMMA cement resulted in a 1.3-fold drug release relative to the PMMA cement. This result was associated with increasing the hydrophilic portion of the PMMA cement by the addition of BC [224]. BC dressing saturated with gentamycin was created as an antibacterial carrier against biofilm-based infection in bone defects after surgery. This dressing significantly reduced the level of biofilm-forming pathogens in vitro and ex vivo due to keeping the effective contents of the antibiotic for several days in the damaged tissue. Indeed, BC dressing was introduced as an alternative to COL sponges that is broadly applied as a carrier in the medical product but meets the challenge of fast release of the loaded cargo [225]. Bipin and colleagues fabricated a delivery system based on the anionic CMC that was safely crosslinked by complexation with zirconia and further stabilized by cationic CS [167]. An antibacterial drug model, cefazolin, was encapsulated into the microcarriers by addition to CMC solution during the fabrication process before crosslinking and stabilization. As a control, the drug-coated microparticles were also fabricated by immersing them in the drug solution. The low incorporation into microparticles (encapsulation efficiency: 18%–20%) was achieved due to the repulsion between cefazolin and CMC with similar electric charge (negative) and loss of drug during preparation. The drug-encapsulated microcarriers exhibited the sustained release profile over 4 weeks whereas the drug-coated microcarriers released their cargo during <2 weeks. Zirconia not only exerted no adverse effects on the growth and proliferation of osteoblasts but also enhanced cell adhesion.
Bone implants should display high biocompatibility, beneficial interactions with the immune system, and therefore reduce the risk of rejection. Surface modification of the implants with biopolymers has been generally suggested as an easy and cost-effective procedure. Recently, an antibacterial saturated BC coating on the Ti6Al7Nb scaffold was developed in order to overcome the orthopedic implant-associated infection [226]. The manufactured Ti6Al7Nb scaffolds were immersed in a suspension of Komagataeibacter xylinus bacteria to produce a 3D polymeric coating layer of BC. The coating process was completed on average 7 days and subsequently, the BC matrix was impregnated with gentamycin by immersing into the drug solution. This delivery system was able to physically preserve the drug molecules into its microfibrillar structure and consequently obtain a desirable drug release for antibacterial effect against S. aureus. Another study reported a novel multi-layered sandwich-like coating of CMC and sodium diclofenac on the AISI 316LVM stainless steel for orthopedic applications [227]. The spin coating method was performed to coat the substrate using CMC and drug solutions in an alternating manner that gave a layer-by-layer structure of CMC and diclofenac. The release rate was tuned through the incorporation of different doses of the drug and the presence of CMC barrier layers against drug diffusion. Also, BC membrane was impregnated with essential oils (EO) obtained from cloves, eucalyptus, and thyme for antibacterial properties against microbe biofilm formation on the HA substrates [228]. There was no significant difference between the release rate of the oils from the membrane (about 50% of oil content was released after 72 h). Among this EO, thyme EO displayed the highest ability to eradicate S. aureus biofilm, while similar results were achieved for P. aeruginosa biofilm.
Considering the great potential of cellulose in pharmaceutical applications, this carbohydrate biopolymer has been also used for the delivery of other therapeutic agents in bone tissue engineering. For example, Klinthoopthamrong et al. developed a PAA-grafted BC membrane for conjugating with plant-derived recombinant human osteopontin (p-rhOPN) [229]. Carboxyl groups of PAA reacted with amino groups of p-rhOPN and formed amide bonds. This BC membrane was recommended as a good candidate for guided tissue regeneration by supporting adhesion and osteogenic differentiation of human periodontal ligament stem cells. Nanofibrous BC hydrogel loaded with fisetin, a phytoestrogen osteogenic inducer, supported bone marrow MSCs attachment, proliferation, and bone-specific matrix biosynthesis [230]. Additionally, vitamin D3 release from the delivery system of cellulose enriched HAp mesoporous silica NPs composite enhanced proliferation, adhesion, ALP activity and calcium deposition on osteoblasts like cells (MG63) [231]. For the drug loading, vitaminD3 was mixed with the material powders before freeze-drying. A freeze-dried 3D scaffold-based on CNF-cyclodextrin loaded with raloxifene hydrochloride succeeded in promoting cell adhesion, ALP expression, and calcium ion deposition by providing a long-term release within 20 days [232]. Raloxifene is a widely used estrogen receptor modulator in treating osteoporosis [233]. The drug release was controlled by lipophilic interaction between the molecules of raloxifene and cyclodextrin, and the hydrogen bonding between the molecules of the matrix materials.
In summary, cellulose and its derivatives indicated desirable experimental outcomes when they had been utilized as delivery systems for various therapeutic agents from proteins to small molecule drugs. The above-mentioned therapeutic agents could be easily loaded into the cellulose-based delivery systems and released over days to months. Taken together, in most delivery systems based on cellulosic materials, the drug molecules were physically entrapped into the final structure through the soaking method. Therefore, the porosity level and pore size of the final delivery system efficiently influenced the drug loading and release. The fibrillar structure of BC and CNC as a physical barrier to drug diffusion was also a determinative factor in the drug release. Accordingly, the fabrication method and the amount of crosslinking and blending with other polymers were found important parameters in tuning the delivery properties of the final scaffolds. Additionally, the abundance of OH functional groups and slow degradation of cellulose or its derivatives were effective in retaining and delivering the drug contents in a controlled manner. On the other hand, some characteristics of the drug molecules including size, chemical structure, and the degree of hydrophilicity impacted the interaction of the drug-cellulosic materials and thus tuning the loading efficiency and release behavior. These cellulosic materials can be promising candidates for the effective delivery of other therapeutic agents and as smart drug carries in the field of bone regeneration that can be achieved by the precise studies in the future.
6. Conclusion and future perspectives
In this review, we discussed the role and function of cellulose and its derivatives as a component of composite scaffolds for bone repair. The demand for using this low-cost, available, biocompatible, and biodegradable polysaccharide has been growing for tissue engineering and drug delivery applications. Cellulosic biomaterials are mechanically strong because of their crystallinity. They also have a high capability for chemical modification and interaction with other organic and inorganic biomaterials via many hydroxyl functional groups. Many cellulose-based scaffolds can be developed employing common approaches including electrospinning and freeze-drying or additive manufacturing methods such as 3D printing. Cellulose and its derivatives can be combined with different organic biomaterials to mechanically reinforce the network or biologically support them by providing apatite nucleation sites. Additionally, cellulose-inorganic composites, the cellulosic increases the flexibility of the scaffold. Further, immersion or biomineralization methods used for preparing cellulose-inorganic composites improves the uniformity of other bioactive ingredients within the composite network by taking advantage of the hydroxyl and carboxyl functional groups of cellulose/its derivatives. All in all, cellulose in the pristine or modified form is a promising biomaterial owing to its unique properties of hydrophilicity, dispersibility, and the capacity of biomineralization, mechanical reinforcement, and cross-linking. These unique properties have motivated researchers to develop even more complex composites with complementary properties made of cellulose and organic/inorganic biomaterials for bone repair. Engineered systems based on BC, cellulose ethers, i.e., CMC and EC, and CNC useful tools for delivering various therapeutic cargoes to the bone defects. Additive manufacturing has introduced as a technology with a promise of patient specific solution for generating synthetic bone analogue with biomimetic structures. Cellulose composites and their derivatives could be used as suitable ink for 3D bioprinting. One of the most important challenges in scaffold-based bone tissue engineering is vascularization. Further studies are required to rational design cellulose-based biocomposites that promote angiogenesis and vascularization. In conclusion, cellulose-based composite scaffolds are hopeful candidates for repairing and regenerating damaged bone tissue, however, further evaluations seem to need on the fabrication techniques, loading of bioactive agents, and combination with versatile biomaterials. Obstacles in the translation from bench to clinic often remain a major challenge. Challenges associated with inaccuracies in existing preclinical models, sterilization, intellectual property considerations, and regulatory barriers still need to be addressed before the clinical translation of these materials. Although cellulose-based materials have shown encouraging results in vitro and, in some pilot, in vivo studies, the path towards translating these materials into the clinic is expensive and time-consuming. Before testing these materials in humans, it is essential to validate their toxicity and performance in relevant animal models. Given that scaffolds made for bone tissue engineering will remain at the implantation site for a long period of time, safety validation should encompass cytotoxicity, sensitization, irritation, acute and chronic systemic toxicity, and hemocompatibility endpoints. Additionally, performance studies should be able to evaluate the ability of scaffolds to promote tissue regeneration in defected areas. Lastly, the manufacturing process should ensure scalability and reproducibility. Therefore, it is crucial to minimize the complexity of the design and development of cellulose-based composites.
Ethics
This review article does not include any studies that require institutional ethics approvals or consent from participants.
Declaration of competing interest
Authors do not have any conflict of interest in this manuscript.
Acknowledgments
M.A. and A.S. acknowledge the support received from the Natural Sciences and Engineering Research Council of Canada (NSERC).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Mohammad Sadegh Nourbakhsh, Email: s_nourbakhsh@semnan.ac.ir.
Mohsen Akbari, Email: makbari@uvic.ca.
References
- 1.Babilotte J., Guduric V., Le Nihouannen D., Naveau A., Fricain J.C., Catros S. 3D printed polymer–mineral composite biomaterials for bone tissue engineering: fabrication and characterization. J. Biomed. Mater. Res. B Appl. Biomater. 2019;107:2579–2595. doi: 10.1002/jbm.b.34348. [DOI] [PubMed] [Google Scholar]
- 2.Saravanan S., Leena R.S., Selvamurugan N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016;93:1354–1365. doi: 10.1016/j.ijbiomac.2016.01.112. [DOI] [PubMed] [Google Scholar]
- 3.Hannink G., Arts J.J.C. Bioresorbability, porosity and mechanical strength of bone substitutes: what is optimal for bone regeneration? Injury. 2011;42:S22–S25. doi: 10.1016/j.injury.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Tao F., Cheng Y., Shi X., Zheng H., Du Y., Xiang W., Deng H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2020;230 doi: 10.1016/j.carbpol.2019.115658. [DOI] [PubMed] [Google Scholar]
- 5.Kashte S., Jaiswal A.K., Kadam S. Artificial bone via bone tissue engineering: current scenario and challenges. Tissue Eng. Regen. Med. 2017;14:1–14. doi: 10.1007/S13770-016-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kesireddy V., Kasper F.K. Approaches for building bioactive elements into synthetic scaffolds for bone tissue engineering. J. Mater. Chem. B. 2016;4:6773–6786. doi: 10.1039/C6TB00783J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma H., Feng C., Chang J., Wu C. 3D-printed bioceramic scaffolds: from bone tissue engineering to tumor therapy. Acta Biomater. 2018;79:37–59. doi: 10.1016/j.actbio.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Shi C., Yuan Z., Han F., Zhu C., Li B. Polymeric biomaterials for bone regeneration. Ann. Jt. 2016;1 doi: 10.21037/aoj.2016.11.02. 27–27. [DOI] [Google Scholar]
- 9.Ogueri K.S., Jafari T., Escobar Ivirico J.L., Laurencin C.T. Polymeric biomaterials for scaffold-based bone regenerative engineering. Regen. Eng. Transl. Med. 2019;5:128–154. doi: 10.1007/S40883-018-0072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henkel J., Woodruff M.A., Epari D.R., Steck R., Glatt V., DIckinson I.C., Choong P.F.M., Schuetz M.A., Hutmacher Di.W. Bone regeneration based on tissue engineering conceptions — a 21st Century perspective. Bone Res. 2013;1:216–248. doi: 10.4248/br201303002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zadpoor A.A. Bone tissue regeneration: the role of scaffold geometry. Biomater. Sci. 2015;3:231–245. doi: 10.1039/C4BM00291A. [DOI] [PubMed] [Google Scholar]
- 12.Turnbull G., Clarke J., Picard F., Riches P., Jia L., Han F., Li B., Shu W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018;3:278–314. doi: 10.1016/j.bioactmat.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porter J., Ruckh T., Popat K.C. Bone tissue engineering: a review in bone biomimetics and drug delivery strategies. Biotechnol. Prog. 2009;25:1539–1560. doi: 10.1002/btpr.246. 2009. [DOI] [PubMed] [Google Scholar]
- 14.Swetha M., Sahithi K., Moorthi A., Srinivasan N., Ramasamy K., Selvamurugan N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2010;47:1–4. doi: 10.1016/j.ijbiomac.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Bahraminasab M., Janmohammadi M., Arab S., Talebi A., Nooshabadi V.T., Koohsarian P., Nourbakhsh M.S. Bone scaffolds: an incorporation of biomaterials, cells, and biofactors. ACS Biomater. Sci. Eng. 2021;7:5397–5431. doi: 10.1021/acsbiomaterials.1C00920. [DOI] [PubMed] [Google Scholar]
- 16.Tozzi G., De Mori A., Oliveira A., Roldo M. Composite hydrogels for bone regeneration. Materials. 2016;9:267. doi: 10.3390/MA9040267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blackwood K.A., Bock N., Dargaville T.R., Ann Woodruff M. Scaffolds for growth factor delivery as applied to bone tissue engineering. Int. J. Polym. Sci. 2012;2012 doi: 10.1155/2012/174942. [DOI] [Google Scholar]
- 18.Varghese S., Chaudhary J.P., Ghoroi C. One-step dry synthesis of an iron based nano-biocomposite for controlled release of drugs. RSC Adv. 2020;10:13394–13404. doi: 10.1039/D0RA01133A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tafazoli Moghadam E., Yazdanian M., Alam M., Tebyanian H., Tafazoli A., Tahmasebi E., Ranjbar R., Yazdanian A., Seifalian A. Current natural bioactive materials in bone and tooth regeneration in dentistry: a comprehensive overview. J. Mater. Res. Technol. 2021;13:2078–2114. doi: 10.1016/j.jmrt.2021.05.089. [DOI] [Google Scholar]
- 20.Reddy M.S.B., Ponnamma D., Choudhary R., Sadasivuni K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers. 2021;13 doi: 10.3390/polym13071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaman A., Huang F., Jiang M., Wei W., Zhou Z. Preparation, properties, and applications of natural cellulosic aerogels: a review. Energy Built Environ. 2020;1:60–76. doi: 10.1016/j.enbenv.2019.09.002. [DOI] [Google Scholar]
- 22.Seddiqi H., Oliaei E., Honarkar H., Jin J., Geonzon L.C., Bacabac R.G., Klein-Nulend J. Cellulose and its derivatives: towards biomedical applications. Cellulose. 2021;28:1893–1931. doi: 10.1007/S10570-020-03674-W. [DOI] [Google Scholar]
- 23.Heinze T. Cellulose: structure and properties. Adv. Polym. Sci. 2015;271:1–52. doi: 10.1007/12_2015_319. [DOI] [Google Scholar]
- 24.Klemm D., Heublein B., Fink H.P., Bohn A. Cellulose: fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005;44:3358–3393. doi: 10.1002/anie.200460587. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., Qian J., Zhao N., Liu T., Xu W., Suo A. Novel hydroxyethyl chitosan/cellulose scaffolds with bubble-like porous structure for bone tissue engineering. Carbohydr. Polym. 2017;167:44–51. doi: 10.1016/j.carbpol.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 26.Shokri J., Adibkia K. Application of cellulose and cellulose derivatives in pharmaceutical industries, pharmaceutical and electronic applications. 2013. [DOI]
- 27.Marques-Marinho F.D., Vianna-Soares C.D. Cellulose and its derivatives use in the pharmaceutical compounding practice. Cell. Med. Pharm. Electron. Appl. 2013 doi: 10.5772/56637. [DOI] [Google Scholar]
- 28.Salimi S., Sotudeh-Gharebagh R., Zarghami R., Chan S.Y., Yuen K.H. Production of nanocellulose and its applications in drug delivery: a critical review. ACS Sustain. Chem. Eng. 2019;7:15800–15827. doi: 10.1021/acssuschemeng.9B02744. [DOI] [Google Scholar]
- 29.Thoorens G., Krier F., Leclercq B., Carlin B., Evrard B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—a review. Int. J. Pharm. 2014;473:64–72. doi: 10.1016/j.ijpharm.2014.06.055. [DOI] [PubMed] [Google Scholar]
- 30.Oprea M., Voicu S.I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020;247 doi: 10.1016/j.carbpol.2020.116683. [DOI] [PubMed] [Google Scholar]
- 31.Dutta S.D., Patel D.K., Lim K.T. Functional cellulose-based hydrogels as extracellular matrices for tissue engineering. J. Biol. Eng. 2019;13 doi: 10.1186/S13036-019-0177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chocholata P., Kulda V., Babuska V. Fabrication of scaffolds for bone-tissue regeneration. Materials. 2019;12:568. doi: 10.3390/MA12040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Brien F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today. 2011;14:88–95. doi: 10.1016/S1369-7021(11)70058-X. [DOI] [Google Scholar]
- 34.Hickey R.J., Pelling A.E. Cellulose biomaterials for tissue engineering. Front. Bioeng. Biotechnol. 2019;7 doi: 10.3389/fbioe.2019.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jozala A.F., Celia De Lencastre-Novaes L., Lopes A.M., De V., Santos-Ebinuma C., Gava Mazzola P., Pessoa-Jr A., Grotto D., Gerenutti M., Chaud M.V. MINI-REVIEW Bacterial nanocellulose production and application: a 10-year overview. Appl. Microbiol. Biotechnol. 2016;100:2063–2072. doi: 10.1007/s00253-015-7243-4. [DOI] [PubMed] [Google Scholar]
- 36.Park J.K., Jung J.Y., Khan T. second ed. Handb. Hydrocoll.; 2009. Bacterial Cellulose; pp. 724–739. [DOI] [Google Scholar]
- 37.Torgbo S., Sukyai P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today. 2018;11:34–49. doi: 10.1016/j.apmt.2018.01.004. [DOI] [Google Scholar]
- 38.Saska S., Teixeira L.N., Tambasco De Oliveira P., Minarelli Gaspar A.M., Lima Ribeiro S.J., Messaddeq Y., Marchetto R. Bacterial cellulose-collagen nanocomposite for bone tissue engineering. J. Mater. Chem. 2012;22:22102–22112. doi: 10.1039/C2JM33762B. [DOI] [Google Scholar]
- 39.Noh Y.K., Dos Santos Da Costa A., Park Y.S., Du P., Kim I.H., Park K. Fabrication of bacterial cellulose-collagen composite scaffolds and their osteogenic effect on human mesenchymal stem cells. Carbohydr. Polym. 2019;219:210–218. doi: 10.1016/j.carbpol.2019.05.039. [DOI] [PubMed] [Google Scholar]
- 40.Codreanu A., Balta C., Herman H., Cotoraci C., Mihali C.V., Zurbau N., Zaharia C., Rapa M., Stanescu P., Radu I.C., Vasile E., Lupu G., Galateanu B., Hermenean A. Bacterial cellulose-modified polyhydroxyalkanoates scaffolds promotes bone formation in critical size calvarial defects in mice. Materials. 2020;13:1433. doi: 10.3390/MA13061433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L., He D., Wang G.S., Yu S.H. Bioinspired crystallization of CaCO3 coatings on electrospun cellulose acetate fiber scaffolds and corresponding CaCO3 microtube networks. Langmuir. 2011;27:7199–7206. doi: 10.1021/LA200738N. [DOI] [PubMed] [Google Scholar]
- 42.Atila D., Keskin D., Tezcaner A. Crosslinked pullulan/cellulose acetate fibrous scaffolds for bone tissue engineering. Mater. Sci. Eng. C. 2016;69:1103–1115. doi: 10.1016/j.msec.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 43.Bonino C.A., Krebs M.D., Saquing C.D., Jeong S.I., Shearer K.L., Alsberg E., Khan S.A. Electrospinning alginate-based nanofibers: from blends to crosslinked low molecular weight alginate-only systems. Carbohydr. Polym. 2011;85:111–119. doi: 10.1016/j.carbpol.2011.02.002. [DOI] [Google Scholar]
- 44.Gouma P., Xue R., Goldbeck C.P., Perrotta P., Balázsi C. Nano-hydroxyapatite—cellulose acetate composites for growing of bone cells. Mater. Sci. Eng. C. 2012;32:607–612. doi: 10.1016/j.msec.2011.12.019. [DOI] [Google Scholar]
- 45.Salgado A.J., Coutinho O.P., Reis R.L., Davies J.E. In vivo response to starch-based scaffolds designed for bone tissue engineering applications. J. Biomed. Mater. Res. 2007;80:983–989. doi: 10.1002/jbm.a.30946. [DOI] [PubMed] [Google Scholar]
- 46.Aravamudhan A., Ramos D.M., Nip J., Harmon M.D., James R., Deng M., Laurencin C.T., Yu X., Kumbar S.G. Cellulose and collagen derived micro-nano structured scaffolds for bone tissue engineering. J. Biomed. Nanotechnol. 2013;9:719–731. doi: 10.1166/jbn.2013.1574. [DOI] [PubMed] [Google Scholar]
- 47.Aravamudhan A., Ramos D.M., Nip J., Kalajzic I., Kumbar S.G. Micro-nanostructures of cellulose-collagen for critical sized bone defect healing. Macromol. Biosci. 2018;18 doi: 10.1002/mabi.201700263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amrita A. Arora, Sharma P., Katti D.S. Pullulan-based composite scaffolds for bone tissue engineering: improved osteoconductivity by pore wall mineralization. Carbohydr. Polym. 2015;123:180–189. doi: 10.1016/j.carbpol.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 49.Aydogdu H., Keskin D., Baran E.T., Tezcaner A. Pullulan microcarriers for bone tissue regeneration. Mater. Sci. Eng. C. 2016;63:439–449. doi: 10.1016/j.msec.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 50.Hou J., Wang Y., Xue H., Dou Y. Biomimetic growth of hydroxyapatite on electrospun CA/PVP core–shell nanofiber membranes. Polymer. 2018;10:1032. doi: 10.3390/polym10091032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen J., Zhang T., Hua W., Li P., Wang X. 3D Porous poly(lactic acid)/regenerated cellulose composite scaffolds based on electrospun nanofibers for biomineralization. Colloids Surf. A Physicochem. Eng. Asp. 2020;585 doi: 10.1016/j.colsurfa.2019.124048. [DOI] [Google Scholar]
- 52.Qu H., Fu H., Han Z., Sun Y. Biomaterials for bone tissue engineering scaffolds: a review. RSC Adv. 2019;9:26252–26262. doi: 10.1039/C9RA05214C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDonald J.E., Rooks D.J., McCarthy A.J. Methods for the isolation of cellulose-degrading microorganisms. Methods Enzymol. 2012;510:349–374. doi: 10.1016/B978-0-12-415931-0.00019-7. [DOI] [PubMed] [Google Scholar]
- 54.Singh B.N., Panda N.N., Mund R., Pramanik K. Carboxymethyl cellulose enables silk fibroin nanofibrous scaffold with enhanced biomimetic potential for bone tissue engineering application. Carbohydr. Polym. 2016;151:335–347. doi: 10.1016/j.carbpol.2016.05.088. [DOI] [PubMed] [Google Scholar]
- 55.Tamburaci S., Kimna C., Tihminlioglu F. Novel phytochemical Cissus quadrangularis extract–loaded chitosan/Na-carboxymethyl cellulose–based scaffolds for bone regeneration. J. Bioact. Compat Polym. 2018;33:629–646. doi: 10.1177/0883911518793913. [DOI] [Google Scholar]
- 56.Sharmila G., Muthukumaran C., Kirthika S., Keerthana S., Kumar N.M., Jeyanthi J. Fabrication and characterization of Spinacia oleracea extract incorporated alginate/carboxymethyl cellulose microporous scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020;156:430–437. doi: 10.1016/j.ijbiomac.2020.04.059. [DOI] [PubMed] [Google Scholar]
- 57.Zulkifli F.H., Hussain F.S.J., Harun W.S.W., Yusoff M.M. Highly porous of hydroxyethyl cellulose biocomposite scaffolds for tissue engineering. Int. J. Biol. Macromol. 2019;122:562–571. doi: 10.1016/j.ijbiomac.2018.10.156. [DOI] [PubMed] [Google Scholar]
- 58.Zulkifli F.H., Hussain F.S.J., Rasad M.S.B.A., Mohd Yusoff M. Nanostructured materials from hydroxyethyl cellulose for skin tissue engineering. Carbohydr. Polym. 2014;114:238–245. doi: 10.1016/j.carbpol.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 59.Chahal S., Hussain F.S.J., Kumar A., Rasad M.S.B.A., Yusoff M.M. Fabrication, characterization and in vitro biocompatibility of electrospun hydroxyethyl cellulose/poly (vinyl) alcohol nanofibrous composite biomaterial for bone tissue engineering. Chem. Eng. Sci. 2016;144:17–29. doi: 10.1016/j.ces.2015.12.030. [DOI] [Google Scholar]
- 60.Nizan N.S.N.H., Zulkifli F.H. Reinforcement of hydroxyethyl cellulose/poly (vinyl alcohol) with cellulose nanocrystal as a bone tissue engineering scaffold. J. Polym. Res. 2020;27:1–9. doi: 10.1007/S10965-020-02112-6. [DOI] [Google Scholar]
- 61.Du H., Liu W., Zhang M., Si C., Zhang X., Li B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019;209:130–144. doi: 10.1016/j.carbpol.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 62.George J., Sabapathi S.N. Cellulose nanocrystals: synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015;8:45. doi: 10.2147/nsa.S64386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gorgieva S., Girandon L., Kokol V. Mineralization potential of cellulose-nanofibrils reinforced gelatine scaffolds for promoted calcium deposition by mesenchymal stem cells. Mater. Sci. Eng. C. 2017;73:478–489. doi: 10.1016/j.msec.2016.12.092. [DOI] [PubMed] [Google Scholar]
- 64.Carlström I.E., Rashad A., Campodoni E., Sandri M., Syverud K., Bolstad A.I., Mustafa K. Cross-linked gelatin-nanocellulose scaffolds for bone tissue engineering. Mater. Lett. 2020;264 doi: 10.1016/j.matlet.2020.127326. [DOI] [Google Scholar]
- 65.Abouzeid R.E., Khiari R., Beneventi D., Dufresne A. Biomimetic mineralization of three-dimensional printed alginate/TEMPO-oxidized cellulose nanofibril scaffolds for bone tissue engineering. Biomacromolecules. 2018;19:4442–4452. doi: 10.1021/acs.biomac.8B01325. [DOI] [PubMed] [Google Scholar]
- 66.Rashad A., Mohamed-Ahmed S., Ojansivu M., Berstad K., Yassin M.A., Kivijärvi T., Heggset E.B., Syverud K., Mustafa K. Coating 3D printed polycaprolactone scaffolds with nanocellulose promotes growth and differentiation of mesenchymal stem cells. Biomacromolecules. 2018;19:4307–4319. doi: 10.1021/asc.biomac.8B01194. [DOI] [PubMed] [Google Scholar]
- 67.Shuai C., Yuan X., Yang W., Peng S., He C., Feng P., Qi F., Wang G. Cellulose nanocrystals as biobased nucleation agents in poly-l-lactide scaffold: crystallization behavior and mechanical properties. Polym. Test. 2020;85 doi: 10.1016/j.polymertesting.2020.106458. [DOI] [Google Scholar]
- 68.Patel D.K., Dutta S.D., Hexiu J., Ganguly K., Lim K.T. Bioactive electrospun nanocomposite scaffolds of poly(lactic acid)/cellulose nanocrystals for bone tissue engineering. Int. J. Biol. Macromol. 2020;162:1429–1441. doi: 10.1016/j.ijbiomac.2020.07.246. [DOI] [PubMed] [Google Scholar]
- 69.Zhang X., Wang C., Liao M., Dai L., Tang Y., Zhang H., Coates P., Sefat F., Zheng L., Song J., Zheng Z., Zhao D., Yang M., Zhang W., Ji P. Aligned electrospun cellulose scaffolds coated with rhBMP-2 for both in vitro and in vivo bone tissue engineering. Carbohydr. Polym. 2019;213:27–38. doi: 10.1016/j.carbpol.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 70.Hong J.K., Cooke S.L., Whittington A.R., Roman M. Bioactive cellulose nanocrystal-poly(ε-caprolactone) nanocomposites for bone tissue engineering applications. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.605924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maturavongsadit P., Narayanan L.K., Chansoria P., Shirwaiker R., Benhabbour S.R. Cell-laden nanocellulose/chitosan-based Bioinks for 3D bioprinting and enhanced osteogenic cell differentiation. ACS Appl. Bio Mater. 2021;4:2342–2353. doi: 10.1021/acsabm.0C01108. [DOI] [PubMed] [Google Scholar]
- 72.Liu J., Cheng F., Grénman H., Spoljaric S., Seppälä J., Eriksson J.E., Willför S., Xu C. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydr. Polym. 2016;148:259–271. doi: 10.1016/j.carbpol.2016.04.064. [DOI] [PubMed] [Google Scholar]
- 73.Yan H., Chen X., Feng M., Shi Z., Zhang D., Lin Q. Layer-by-layer assembly of 3D alginate-chitosan-gelatin composite scaffold incorporating bacterial cellulose nanocrystals for bone tissue engineering. Mater. Lett. 2017;209:492–496. doi: 10.1016/j.matlet.2017.08.093. [DOI] [Google Scholar]
- 74.Shahrousvand E., Shahrousvand M., Ghollasi M., Seyedjafari E., Jouibari I.S., babaei A., Salimi A. Preparation and evaluation of polyurethane/cellulose nanowhisker bimodal foam nanocomposites for osteogenic differentiation of hMSCs. Carbohydr. Polym. 2017;171:281–291. doi: 10.1016/j.carbpol.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 75.Padash A., Halabian R., Salimi A., Kazemi N.M., Shahrousvand M. Osteogenic differentiation of mesenchymal stem cells on the bimodal polymer polyurethane/polyacrylonitrile containing cellulose phosphate nanowhisker. Hum. Cell. 2020;34:310–324. doi: 10.1007/S13577-020-00449-0. [DOI] [PubMed] [Google Scholar]
- 76.Shahrousvand M., Ghollasi M., Zarchi A.A.K., Salimi A. Osteogenic differentiation of hMSCs on semi-interpenetrating polymer networks of polyurethane/poly(2-hydroxyethyl methacrylate)/cellulose nanowhisker scaffolds. Int. J. Biol. Macromol. 2019;138:262–271. doi: 10.1016/j.ijbiomac.2019.07.080. [DOI] [PubMed] [Google Scholar]
- 77.Alemán-Domínguez M.E., Giusto E., Ortega Z., Tamaddon M., Benítez A.N., Liu C. Three-dimensional printed polycaprolactone-microcrystalline cellulose scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2019;107:521–528. doi: 10.1002/jbm.b.34142. [DOI] [PubMed] [Google Scholar]
- 78.Burg K.J.L., Porter S., Kellam J.F. Biomaterial developments for bone tissue engineering. Biomaterials. 2000;21:2347–2359. doi: 10.1016/S0142-9612(00)00102-2. [DOI] [PubMed] [Google Scholar]
- 79.Ramani D., Sastry T.P. Bacterial cellulose-reinforced hydroxyapatite functionalized graphene oxide: a potential osteoinductive composite. Cellulose. 2014;21:3585–3595. doi: 10.1007/S10570-014-0313-4. [DOI] [Google Scholar]
- 80.Picheth G.F., Pirich C.L., Sierakowski M.R., Woehl M.A., Sakakibara C.N., de Souza C.F., Martin A.A., da Silva R., de Freitas R.A. Bacterial cellulose in biomedical applications: a review. Int. J. Biol. Macromol. 2017;104:97–106. doi: 10.1016/j.ijbiomac.2017.05.171. [DOI] [PubMed] [Google Scholar]
- 81.Bayir E., Bilgi E., Hames E.E., Sendemir A. Production of hydroxyapatite–bacterial cellulose composite scaffolds with enhanced pore diameters for bone tissue engineering applications. Cellulose. 2019;26:9803–9817. doi: 10.1007/S10570-019-02763-9. [DOI] [Google Scholar]
- 82.Märtson M., Viljanto J., Hurme T., Saukko P. Biocompatibility of cellulose sponge with bone. Eur. Surg. Res. 1998;30:426–432. doi: 10.1159/000008609. [DOI] [PubMed] [Google Scholar]
- 83.Fang B., Wan Y.Z., Tang T.T., Gao C., Dai K.R. Proliferation and osteoblastic differentiation of human bone marrow stromal cells on hydroxyapatite/bacterial cellulose nanocomposite scaffolds. Tissue Eng. 2009;15:1091–1098. doi: 10.1089/ten.tea.2008.0110. [DOI] [PubMed] [Google Scholar]
- 84.Hong L., Wang Y.L., Jia S.R., Huang Y., Gao C., Wan Y.Z. Hydroxyapatite/bacterial cellulose composites synthesized via a biomimetic route. Mater. Lett. 2006;60:1710–1713. doi: 10.1016/j.matlet.2005.12.004. [DOI] [Google Scholar]
- 85.Sundberg J., Götherström C., Gatenholm P. Biosynthesis and in vitro evaluation of macroporous mineralized bacterial nanocellulose scaffolds for bone tissue engineering. Bio Med. Mater. Eng. 2015;25:39–52. doi: 10.3233/bme-141245. [DOI] [PubMed] [Google Scholar]
- 86.Tazi N., Zhang Z., Messaddeq Y., Almeida-Lopes L., Zanardi L.M., Levinson D., Rouabhia M. Hydroxyapatite bioactivated bacterial cellulose promotes osteoblast growth and the formation of bone nodules. Amb. Express. 2012;2:1–10. doi: 10.1186/2191-0855-2-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saska S., Barud H.S., Gaspar A.M.M., Marchetto R., Ribeiro S.J.L., Messaddeq Y. Bacterial cellulose-hydroxyapatite nanocomposites for bone regeneration. Int. J. Biomater. 2011 doi: 10.1155/2011/175362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wan Y.Z., Huang Y., Yuan C.D., Raman S., Zhu Y., Jiang H.J., He F., Gao C. Biomimetic synthesis of hydroxyapatite/bacterial cellulose nanocomposites for biomedical applications. Mater. Sci. Eng. C. 2007;27:855–864. doi: 10.1016/j.msec.2006.10.002. [DOI] [Google Scholar]
- 89.Ahn S.J., Shin Y.M., Kim S.E., Jeong S.I., Jeong J.O., Park J.S., Gwon H.J., Seo D.E., Nho Y.C., Kang S.S., Kim C.Y., Huh J.B., Lim Y.M. Characterization of hydroxyapatite-coated bacterial cellulose scaffold for bone tissue engineering. Biotechnol. Bioproc. Eng. 2015;20:948–955. doi: 10.1007/S12257-015-0176-Z. [DOI] [Google Scholar]
- 90.Zimmermann K.A., Leblanc J.M., Sheets K.T., Fox R.W., Gatenholm P. Biomimetic design of a bacterial cellulose/hydroxyapatite nanocomposite for bone healing applications. Mater. Sci. Eng. C. 2011;31:43–49. doi: 10.1016/j.msec.2009.10.007. [DOI] [Google Scholar]
- 91.Gao C., Wan Y., Lei X., Qu J., Yan T., Dai K. Polylysine coated bacterial cellulose nanofibers as novel templates for bone-like apatite deposition. Cellulose. 2011;18:1555–1561. doi: 10.1007/S10570-011-9571-6. [DOI] [Google Scholar]
- 92.Shi S., Chen S., Zhang X., Shen W., Li X., Hu W., Wang H. Biomimetic mineralization synthesis of calcium-deficient carbonate-containing hydroxyapatite in a three-dimensional network of bacterial cellulose. J. Chem. Technol. Biotechnol. 2009;84:285–290. doi: 10.1002/jctb.2037. [DOI] [Google Scholar]
- 93.Wan Y., Zuo G., Yu F., Huang Y., Ren K., Luo H. Preparation and mineralization of three-dimensional carbon nanofibers from bacterial cellulose as potential scaffolds for bone tissue engineering. Surf. Coating. Technol. 2011;205:2938–2946. doi: 10.1016/j.surfcoat.2010.11.006. [DOI] [Google Scholar]
- 94.Niamsap T., Lam N.T., Sukyai P. Production of hydroxyapatite-bacterial nanocellulose scaffold with assist of cellulose nanocrystals. Carbohydr. Polym. 2019;205:159–166. doi: 10.1016/j.carbpol.2018.10.034. [DOI] [PubMed] [Google Scholar]
- 95.Torgbo S., Sukyai P. Fabrication of microporous bacterial cellulose embedded with magnetite and hydroxyapatite nanocomposite scaffold for bone tissue engineering. Mater. Chem. Phys. 2019;237 doi: 10.1016/j.matchemphys.2019.121868. [DOI] [Google Scholar]
- 96.Grande C.J., Torres F.G., Gomez C.M., Carmen Bañó M. Nanocomposites of bacterial cellulose/hydroxyapatite for biomedical applications. Acta Biomater. 2009;5:1605–1615. doi: 10.1016/j.actbio.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 97.Favi P.M., Webster Á.T.J., Ospina S.P., Gao M., Atehortua L. Preparation and characterization of biodegradable nano hydroxyapatite-bacterial cellulose composites with well-defined honeycomb pore arrays for bone tissue engineering applications. Cellulose. 2016;23:1263–1282. doi: 10.1007/s10570-016-0867-4. [DOI] [Google Scholar]
- 98.Bäckdahl H., Esguerra M., Delbro D., Risberg B., Gatenholm P. Engineering microporosity in bacterial cellulose scaffolds. J. Tissue Eng. Regen. Med. 2008;2:320–330. doi: 10.1002/term.97. [DOI] [PubMed] [Google Scholar]
- 99.Ikada Y. first ed. Academic Press; 2006. Tissue Engineering : Fundamentals and Applications; p. 27. [Google Scholar]
- 100.Petersen N., Gatenholm P. Bacterial cellulose-based materials and medical devices: current state and perspectives. Appl. Microbiol. Biotechnol. 2011;91:1277–1286. doi: 10.1007/S00253-011-3432-Y. [DOI] [PubMed] [Google Scholar]
- 101.Xiong G., Luo H., Gu F., Zhang J., Hu D., Wan Y. A novel in vitro three-dimensional macroporous scaffolds from bacterial cellulose for culture of breast cancer cells. J. Biomaterials Nanobiotechnol. 2013:316–326. doi: 10.4236/jbnb.2013.44040. 04. [DOI] [Google Scholar]
- 102.Xiong G., Luo H., Zhang C., Zhu Y., Wan Y. Enhanced biological behavior of bacterial cellulose scaffold by creation of macropores and surface immobilization of collagen. Macromol. Res. 2015;23:734–740. doi: 10.1007/S13233-015-3099-9. [DOI] [Google Scholar]
- 103.Hu Y., Zhu Y., Zhou X., Ruan C., Pan H., Catchmark J.M. Bioabsorbable cellulose composites prepared by an improved mineral-binding process for bone defect repair. J. Mater. Chem. B. 2016;4:1235–1246. doi: 10.1039/C5TB02091C. [DOI] [PubMed] [Google Scholar]
- 104.Bigham A., Salehi A.O.M., Rafienia M., Salamat M.R., Rahmati S., Raucci M.G., Ambrosio L. Zn-substituted Mg2SiO4 nanoparticles-incorporated PCL-silk fibroin composite scaffold: a multifunctional platform towards bone tissue regeneration. Mater. Sci. Eng. C. 2021;127 doi: 10.1016/j.msec.2021.112242. [DOI] [PubMed] [Google Scholar]
- 105.Hu Y., Catchmark J.M., Vogler E.A. Factors impacting the formation of sphere-like bacterial cellulose particles and their biocompatibility for human osteoblast growth. Biomacromolecules. 2013;14:3444–3452. doi: 10.1021/BM400744A. [DOI] [PubMed] [Google Scholar]
- 106.Luz E.P.C.G., das Chagas B.S., de Almeida N.T., de Fátima Borges M., Andrade F.K., Muniz C.R., Castro-Silva I.I., Teixeira E.H., Popat K., de Freitas Rosa M., Vieira R.S. Resorbable bacterial cellulose membranes with strontium release for guided bone regeneration. Mater. Sci. Eng. C. 2020;116 doi: 10.1016/j.msec.2020.111175. [DOI] [PubMed] [Google Scholar]
- 107.Bigham A., Foroughi F., Rezvani Ghomi E., Rafienia M., Neisiany R.E., Ramakrishna S. The journey of multifunctional bone scaffolds fabricated from traditional toward modern techniques. Bio-Design Manuf. 2020;3:281–306. doi: 10.1007/S42242-020-00094-4. [DOI] [Google Scholar]
- 108.Basu P., Saha N., Saha P. Inorganic calcium filled bacterial cellulose based hydrogel scaffold: novel biomaterial for bone tissue regeneration. Int. J. Polym. Mater. 2018;68:134–144. doi: 10.1080/00914037.2018.1525733. [DOI] [Google Scholar]
- 109.Kim T.G., Park S.H., Chung H.J., Yang D.Y., Park T.G. Microstructured scaffold coated with hydroxyapatite/collagen nanocomposite multilayer for enhanced osteogenic induction of human mesenchymal stem cells. J. Mater. Chem. 2010;20:8927–8933. doi: 10.1039/C0JM01062F. [DOI] [Google Scholar]
- 110.Gutiérrez-Hernández J.M., Escobar-García D.M., Escalante A., Flores H., González F.J., Gatenholm P., Toriz G. In vitro evaluation of osteoblastic cells on bacterial cellulose modified with multi-walled carbon nanotubes as scaffold for bone regeneration. Mater. Sci. Eng. C. 2017;75:445–453. doi: 10.1016/j.msec.2017.02.074. [DOI] [PubMed] [Google Scholar]
- 111.Zadpoor A.A. Relationship between in vitro apatite-forming ability measured using simulated body fluid and in vivo bioactivity of biomaterials. Mater. Sci. Eng. C. 2014;35:134–143. doi: 10.1016/j.msec.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 112.Hammonds R.L., Harrison M.S., Cravanas T.C., Gazzola W.H., Stephens C.P., Benson R.S. Biomimetic hydroxyapatite powder from a bacterial cellulose scaffold. Cellulose. 2012;19:1923–1932. doi: 10.1007/S10570-012-9767-4. [DOI] [Google Scholar]
- 113.Ao C., Niu Y., Zhang X., He X., Zhang W., Lu C. Fabrication and characterization of electrospun cellulose/nano-hydroxyapatite nanofibers for bone tissue engineering. Int. J. Biol. Macromol. 2017;97:568–573. doi: 10.1016/j.ijbiomac.2016.12.091. [DOI] [PubMed] [Google Scholar]
- 114.Saber-Samandari S., Yekta H., Ahmadi S., Alamara K. The role of titanium dioxide on the morphology, microstructure, and bioactivity of grafted cellulose/hydroxyapatite nanocomposites for a potential application in bone repair. Int. J. Biol. Macromol. 2018;106:481–488. doi: 10.1016/j.ijbiomac.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 115.Ma B., Jiang L., Tang C., Tang S., Su S., Shu Y. Preparation and properties of biomimetic hydroxyapatite-based nanocomposite utilizing bamboo fiber. Cellulose. 2019;27:2069–2083. doi: 10.1007/S10570-019-02920-0. [DOI] [Google Scholar]
- 116.Jiang L., Li Y., Xiong C., Su S., Ding H. Preparation and properties of bamboo fiber/nano-hydroxyapatite/poly(lactic-co-glycolic) composite scaffold for bone tissue engineering. ACS Appl. Mater. Interfaces. 2017;9:4890–4897. doi: 10.1021/acsami.6B15032. [DOI] [PubMed] [Google Scholar]
- 117.Jiang L., Li Y., Xiong C., Su S. Preparation and characterization of a novel degradable nano-hydroxyapatite/poly(lactic-co-glycolic) composite reinforced with bamboo fiber. Mater. Sci. Eng. C. 2017;75:1014–1018. doi: 10.1016/j.msec.2017.03.046. [DOI] [PubMed] [Google Scholar]
- 118.Qi Y., Cheng Z., Ye Z., Zhu H., Aparicio C. Bioinspired mineralization with hydroxyapatite and hierarchical naturally aligned nanofibrillar cellulose. ACS Appl. Mater. Interfaces. 2019;11:27598–27604. doi: 10.1021/acsami.9B09443. [DOI] [PubMed] [Google Scholar]
- 119.Jiang L., Li Y., Ma B., Ding H., Su S., Xiong C. Effect of bamboo fiber length on mechanical properties, crystallization behavior, and in vitro degradation of bamboo fiber/nanohydroxyapatite/poly(lactic-co-glycolic) composite. Ind. Eng. Chem. Res. 2018;57:4585–4591. doi: 10.1021/acs.iecr.7B05354. [DOI] [Google Scholar]
- 120.Jiang L., Ma B., Li Y., Ding H., Su S., Xiong C. Effect of bamboo fiber on the degradation behavior and in vitro cytocompatibility of the nano-hydroxyapatite/poly(lactide-co-glycolide) (n-HA/PLGA) composite. Cellulose. 2018;26:1099–1110. doi: 10.1007/S10570-018-2139-Y. [DOI] [Google Scholar]
- 121.Li Y., Jiang L., Xiong C., Peng W. Effect of different surface treatment for bamboo fiber on the crystallization behavior and mechanical property of bamboo fiber/nanohydroxyapatite/poly(lactic-co-glycolic) composite. Ind. Eng. Chem. Res. 2015;54:12017–12024. doi: 10.1021/acs.iecr.5B02724. [DOI] [Google Scholar]
- 122.He M., Chang C., Peng N., Zhang L. Structure and properties of hydroxyapatite/cellulose nanocomposite films. Carbohydr. Polym. 2012;87:2512–2518. doi: 10.1016/j.carbpol.2011.11.029. [DOI] [Google Scholar]
- 123.Salama A., El-Sakhawy M. Regenerated cellulose/wool blend enhanced biomimetic hydroxyapatite mineralization. Int. J. Biol. Macromol. 2016;92:920–925. doi: 10.1016/j.ijbiomac.2016.07.077. [DOI] [PubMed] [Google Scholar]
- 124.Sanaei-rad P., Jafarzadeh Kashi T.S., Seyedjafari E., Soleimani M. Enhancement of stem cell differentiation to osteogenic lineage on hydroxyapatite-coated hybrid PLGA/gelatin nanofiber scaffolds. Biologicals. 2016;44:511–516. doi: 10.1016/j.biologicals.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 125.Dascălu C.A., Maidaniuc A., Pandele A.M., Voicu S.I., Machedon-Pisu T., Stan G.E., Cîmpean A., Mitran V., Antoniac I.V., Miculescu F. Synthesis and characterization of biocompatible polymer-ceramic film structures as favorable interface in guided bone regeneration. Appl. Surf. Sci. 2019;494:335–352. doi: 10.1016/j.apsusc.2019.07.098. [DOI] [Google Scholar]
- 126.Xu X., Chen X., Liu A., Hong Z., Jing X. Electrospun poly(l-lactide)-grafted hydroxyapatite/poly(l-lactide) nanocomposite fibers. Eur. Polym. J. 2007;43:3187–3196. doi: 10.1016/j.eurpolymj.2007.05.024. [DOI] [Google Scholar]
- 127.Zhang X., Cai Q., Liu H., Zhang S., Wei Y., Yang X., Lin Y., Yang Z., Deng X. Calcium ion release and osteoblastic behavior of gelatin/beta-tricalcium phosphate composite nanofibers fabricated by electrospinning. Mater. Lett. 2012;73:172–175. doi: 10.1016/j.matlet.2012.01.049. [DOI] [Google Scholar]
- 128.Liu X., Shen H., Song S., Chen W., Zhang Z. Accelerated biomineralization of graphene oxide – incorporated cellulose acetate nanofibrous scaffolds for mesenchymal stem cell osteogenesis. Colloids Surf. B Biointerfaces. 2017;159:251–258. doi: 10.1016/j.colsurfb.2017.07.078. [DOI] [PubMed] [Google Scholar]
- 129.Luo Y., Wang S., Shen M., Qi R., Fang Y., Guo R., Cai H., Cao X., Tomás H., Zhu M., Shi X. Carbon nanotube-incorporated multilayered cellulose acetate nanofibers for tissue engineering applications. Carbohydr. Polym. 2013;91:419–427. doi: 10.1016/j.carbpol.2012.08.069. [DOI] [PubMed] [Google Scholar]
- 130.Maharjan B., Park J., Kaliannagounder V.K., Awasthi G.P., Joshi M.K., Park C.H., Kim C.S. Regenerated cellulose nanofiber reinforced chitosan hydrogel scaffolds for bone tissue engineering. Carbohydr. Polym. 2021;251 doi: 10.1016/j.carbpol.2020.117023. [DOI] [PubMed] [Google Scholar]
- 131.Chakraborty P.K., Adhikari J., Saha P. Facile fabrication of electrospun regenerated cellulose nanofiber scaffold for potential bone-tissue engineering application. Int. J. Biol. Macromol. 2019;122:644–652. doi: 10.1016/j.ijbiomac.2018.10.216. [DOI] [PubMed] [Google Scholar]
- 132.Miyamoto T., Takahashi S. ‐i, Ito H., Inagaki H., Noishiki Y. Tissue biocompatibility of cellulose and its derivatives. J. Biomed. Mater. Res. 1989;23:125–133. doi: 10.1002/jbm.820230110. [DOI] [PubMed] [Google Scholar]
- 133.Sofi H.S., Akram T., Shabir N., Vasita R., Jadhav A.H., Sheikh F.A. Regenerated cellulose nanofibers from cellulose acetate: incorporating hydroxyapatite (HAp) and silver (Ag) nanoparticles (NPs), as a scaffold for tissue engineering applications. Mater. Sci. Eng. C. 2021;118 doi: 10.1016/j.msec.2020.111547. [DOI] [PubMed] [Google Scholar]
- 134.Qi P., Ohba S., Hara Y., Fuke M., Ogawa T., Ohta S., Ito T. Fabrication of calcium phosphate-loaded carboxymethyl cellulose non-woven sheets for bone regeneration. Carbohydr. Polym. 2018;189:322–330. doi: 10.1016/j.carbpol.2018.02.050. [DOI] [PubMed] [Google Scholar]
- 135.Ninan N., Muthiah M., Park I.K., Elain A., Thomas S., Grohens Y. Pectin/carboxymethyl cellulose/microfibrillated cellulose composite scaffolds for tissue engineering. Carbohydr. Polym. 2013;98:877–885. doi: 10.1016/j.carbpol.2013.06.067. [DOI] [PubMed] [Google Scholar]
- 136.Clarke S.A., Hoskins N.L., Jordan G.R., Henderson S.A., Marsh D.R. In vitro testing of Advanced JAXTM Bone Void Filler System: species differences in the response of bone marrow stromal cells to β tri-calcium phosphate and carboxymethylcellulose gel. J. Mater. Sci. Mater. Med. 2007;18:2283–2290. doi: 10.1007/S10856-007-3099-1. [DOI] [PubMed] [Google Scholar]
- 137.Pasqui D., Torricelli P., De Cagna M., Fini M., Barbucci R. Carboxymethyl cellulose—hydroxyapatite hybrid hydrogel as a composite material for bone tissue engineering applications. J. Biomed. Mater. Res. 2014;102:1568–1579. doi: 10.1002/jbm.a.34810. [DOI] [PubMed] [Google Scholar]
- 138.Manjubala I., Basu P., Narendrakumar U. In situ synthesis of hydroxyapatite/carboxymethyl cellulose composites for bone regeneration applications. Colloid Polym. Sci. 2018;296:1729–1737. doi: 10.1007/S00396-018-4393-9. [DOI] [Google Scholar]
- 139.Kumar A.P., Khaja Mohaideen K., Alariqi S.A.S., Singh R.P. Preparation and characterization of bioceramic nanocomposites based on hydroxyapatite (HA) and carboxymethyl cellulose (CMC) Macromol. Res. 2010;18:1160–1167. doi: 10.1007/s13233-010-1208-3. [DOI] [Google Scholar]
- 140.Sarkar C., Anuvrat K., Garai S., Sahu S.K., Chakraborty J. One pot method to synthesize three-dimensional porous hydroxyapatite nanocomposite for bone tissue engineering. J. Porous Mater. 2019;27:225–235. doi: 10.1007/S10934-019-00805-Y. [DOI] [Google Scholar]
- 141.Sarkar C., Chowdhuri A.R., Kumar A., Laha D., Garai S., Chakraborty J., Sahu S.K. One pot synthesis of carbon dots decorated carboxymethyl cellulose- hydroxyapatite nanocomposite for drug delivery, tissue engineering and Fe3+ ion sensing. Carbohydr. Polym. 2018;181:710–718. doi: 10.1016/j.carbpol.2017.11.091. [DOI] [PubMed] [Google Scholar]
- 142.Sarkar C., Sahu S.K., Sinha A., Chakraborty J., Garai S. Facile synthesis of carbon fiber reinforced polymer-hydroxyapatite ternary composite: a mechanically strong bioactive bone graft. Mater. Sci. Eng. C. 2019;97:388–396. doi: 10.1016/j.msec.2018.12.064. [DOI] [PubMed] [Google Scholar]
- 143.Garai S., Sinha A. Biomimetic nanocomposites of carboxymethyl cellulose–hydroxyapatite: novel three dimensional load bearing bone grafts. Colloids Surf. B Biointerfaces. 2014;115:182–190. doi: 10.1016/j.colsurfb.2013.11.042. [DOI] [PubMed] [Google Scholar]
- 144.Orash Mahmoud Salehi A., Nourbakhsh M.S., Rafienia M., Baradaran-Rafii A., Heidari Keshel S. Corneal stromal regeneration by hybrid oriented poly (ε-caprolactone)/lyophilized silk fibroin electrospun scaffold. Int. J. Biol. Macromol. 2020;161:377–388. doi: 10.1016/j.ijbiomac.2020.06.045. [DOI] [PubMed] [Google Scholar]
- 145.Fragal E.H., Cellet T.S.P., Fragal V.H., Witt M.A., Companhoni M.V.P., Ueda-Nakamura T., Silva R., Rubira A.F. Biomimetic nanocomposite based on hydroxyapatite mineralization over chemically modified cellulose nanowhiskers: an active platform for osteoblast proliferation. Int. J. Biol. Macromol. 2019;125:133–142. doi: 10.1016/j.ijbiomac.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 146.Safwat E., Hassan M.L., Saniour S., Zaki D.Y., Eldeftar M., Saba D., Zazou M. Injectable TEMPO-oxidized nanofibrillated cellulose/biphasic calcium phosphate hydrogel for bone regeneration. J. Biomater. Appl. 2018;32:1371–1381. doi: 10.1177/0885328218763866. [DOI] [PubMed] [Google Scholar]
- 147.Fragal E.H., Cellet T.S.P., Fragal V.H., Companhoni M.V.P., Ueda-Nakamura T., Muniz E.C., Silva R., Rubira A.F. Hybrid materials for bone tissue engineering from biomimetic growth of hydroxiapatite on cellulose nanowhiskers. Carbohydr. Polym. 2016;152:734–746. doi: 10.1016/j.carbpol.2016.07.063. [DOI] [PubMed] [Google Scholar]
- 148.Li W., Garmendia N., De Larraya U.P., Ding Y., Detsch R., Grünewald A., Roether J.A., Schubert D.W., Boccaccini A.R. 45S5 bioactive glass-based scaffolds coated with cellulose nanowhiskers for bone tissue engineering. RSC Adv. 2014;4:56156–56164. doi: 10.1039/C4RA07740G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jiang H., Zuo Y., Zou Q., Wang H., Du J., Li Y., Yang X. Biomimetic spiral-cylindrical scaffold based on hybrid chitosan/cellulose/nano-hydroxyapatite membrane for bone regeneration. ACS Appl. Mater. Interfaces. 2013;5:12036–12044. doi: 10.1021/AM4038432. [DOI] [PubMed] [Google Scholar]
- 150.Huang C., Bhagia S., Hao N., Meng X., Liang L., Yong Q., Ragauskas A.J. Biomimetic composite scaffold from an in situ hydroxyapatite coating on cellulose nanocrystals. RSC Adv. 2019;9:5786–5793. doi: 10.1039/C8RA09523J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huang C., Hao N., Bhagia S., Li M., Meng X., Pu Y., Yong Q., Ragauskas A.J. Porous artificial bone scaffold synthesized from a facile in situ hydroxyapatite coating and crosslinking reaction of crystalline nanocellulose. Materialia. 2018;4:237–246. doi: 10.1016/j.mtla.2018.09.008. [DOI] [Google Scholar]
- 152.Jafari H., Shahrousvand M., Kaffashi B. Reinforced poly(ε-caprolactone) bimodal foams via phospho-calcified cellulose nanowhisker for osteogenic differentiation of human mesenchymal stem cells. ACS Biomater. Sci. Eng. 2018;4:2484–2493. doi: 10.1021/acsbiomaterials.7B01020. [DOI] [PubMed] [Google Scholar]
- 153.Ghaffari-Bohlouli P., Jafari H., Khatibi A., Bakhtiari M., Tavana B., Zahedi P., Shavandi A. Osteogenesis enhancement using poly (l-lactide-co-d, l-lactide)/poly (vinyl alcohol) nanofibrous scaffolds reinforced by phospho-calcified cellulose nanowhiskers. Int. J. Biol. Macromol. 2021;182:168–178. doi: 10.1016/j.ijbiomac.2021.04.029. [DOI] [PubMed] [Google Scholar]
- 154.Wang J., Wan Y., Han J., Lei X., Yan T., Gao C. Nanocomposite prepared by immobilising gelatin and hydroxyapatite on bacterial cellulose nanofibres. Micro & Nano Lett. 2011;6:133–136. doi: 10.1049/mnl.2010.0209. [DOI] [Google Scholar]
- 155.Jing W., Chunxi Y., Yizao W., Honglin L., Fang H., Kerong D., Yuan H. Laser patterning of bacterial cellulose hydrogel and its modification with gelatin and hydroxyapatite for bone tissue engineering. Soft Mater. 2013;11:173–180. doi: 10.1080/1539445X.2011.611204. [DOI] [Google Scholar]
- 156.Atila D., Karataş A., Evcin A., Keskin D., Tezcaner A. Bacterial cellulose-reinforced boron-doped hydroxyapatite/gelatin scaffolds for bone tissue engineering. Cellulose. 2019;26:9765–9785. doi: 10.1007/S10570-019-02741-1. [DOI] [Google Scholar]
- 157.Balasubramanian P., Büttner T., Miguez Pacheco V., Boccaccini A.R. Boron-containing bioactive glasses in bone and soft tissue engineering. J. Eur. Ceram. Soc. 2018;38:855–869. doi: 10.1016/j.jeurceramsoc.2017.11.001. [DOI] [Google Scholar]
- 158.Ran J., Jiang P., Liu S., Sun G., Yan P., Shen X., Tong H. Constructing multi-component organic/inorganic composite bacterial cellulose-gelatin/hydroxyapatite double-network scaffold platform for stem cell-mediated bone tissue engineering. Mater. Sci. Eng. C. 2017;78:130–140. doi: 10.1016/j.msec.2017.04.062. [DOI] [PubMed] [Google Scholar]
- 159.Jiang P., Ran J., Yan P., Zheng L., Shen X., Tong H. Rational design of a high-strength bone scaffold platform based on in situ hybridization of bacterial cellulose/nano-hydroxyapatite framework and silk fibroin reinforcing phase. J. Biomater. Sci. Polym. Ed. 2017;29:107–124. doi: 10.1080/09205063.2017.1403149. [DOI] [PubMed] [Google Scholar]
- 160.Beladi F., Saber-Samandari S., Saber-Samandari S. Cellular compatibility of nanocomposite scaffolds based on hydroxyapatite entrapped in cellulose network for bone repair. Mater. Sci. Eng. C. 2017;75:385–392. doi: 10.1016/j.msec.2017.02.040. [DOI] [PubMed] [Google Scholar]
- 161.Aki D., Ulag S., Unal S., Sengor M., Ekren N., Lin C.C., Yılmazer H., Ustundag C.B., Kalaskar D.M., Gunduz O. 3D printing of PVA/hexagonal boron nitride/bacterial cellulose composite scaffolds for bone tissue engineering. Mater. Des. 2020;196 doi: 10.1016/j.matdes.2020.109094. [DOI] [Google Scholar]
- 162.Basu P., Saha N., Alexandrova R., Andonova-Lilova B., Georgieva M., Miloshev G., Saha P. Biocompatibility and biological efficiency of inorganic calcium filled bacterial cellulose based hydrogel scaffolds for bone bioengineering. Int. J. Mol. Sci. 2018;19:3980. doi: 10.3390/ijms19123980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang D., Jang J., Kim K., Kim J., Park C.B. Tree to bone”: lignin/polycaprolactone nanofibers for hydroxyapatite biomineralization. Biomacromolecules. 2019;20:2684–2693. doi: 10.1021/asc.biomac.9B00451. [DOI] [PubMed] [Google Scholar]
- 164.Rad R.M., Alshemary A.Z., Evis Z., Keskin D., Tezcaner A. Cellulose acetate-gelatin-coated boron-bioactive glass biocomposite scaffolds for bone tissue engineering. Biomed. Mater. 2020;15 doi: 10.1088/1748-605X/AB8D47. [DOI] [PubMed] [Google Scholar]
- 165.Kim S.E., Tiwari A.P. Three dimensional polycaprolactone/cellulose scaffold containing calcium-based particles: a new platform for bone regeneration. Carbohydr. Polym. 2020;250 doi: 10.1016/j.carbpol.2020.116880. [DOI] [PubMed] [Google Scholar]
- 166.Rosca C., Popa M.I., Lisa G., Chitanu G.C. Interaction of chitosan with natural or synthetic anionic polyelectrolytes. 1. The chitosan–carboxymethylcellulose complex. Carbohydr. Polym. 2005;62:35–41. doi: 10.1016/j.carbpol.2005.07.004. [DOI] [Google Scholar]
- 167.Gaihre B., Jayasuriya A.C. Fabrication and characterization of carboxymethyl cellulose novel microparticles for bone tissue engineering. Mater. Sci. Eng. C. 2016;69:733–743. doi: 10.1016/j.msec.2016.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sainitya R., Sriram M., Kalyanaraman V., Dhivya S., Saravanan S., Vairamani M., Sastry T.P., Selvamurugan N. Scaffolds containing chitosan/carboxymethyl cellulose/mesoporous wollastonite for bone tissue engineering. Int. J. Biol. Macromol. 2015;80:481–488. doi: 10.1016/j.ijbiomac.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 169.Hasan A., Waibhaw G., Saxena V., Pandey L.M. Nano-biocomposite scaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modified cellulose nanowhiskers for bone tissue engineering applications. Int. J. Biol. Macromol. 2018;111:923–934. doi: 10.1016/j.ijbiomac.2018.01.089. [DOI] [PubMed] [Google Scholar]
- 170.Liu H., Song J., Shang S., Song Z., Wang D. Cellulose nanocrystal/silver nanoparticle composites as bifunctional nanofillers within waterborne polyurethane. ACS Appl. Mater. Interfaces. 2012;4:2413–2419. doi: 10.1021/AM3000209. [DOI] [PubMed] [Google Scholar]
- 171.Tamburaci S., Kimna C., Tihminlioglu F. Bioactive diatomite and POSS silica cage reinforced chitosan/Na-carboxymethyl cellulose polyelectrolyte scaffolds for hard tissue regeneration. Mater. Sci. Eng. C. 2019;100:196–208. doi: 10.1016/j.msec.2019.02.104. [DOI] [PubMed] [Google Scholar]
- 172.Arora M., Arora E. The Promise of Silicon: bone regeneration and increased bone density. J. Arthrosc. Jt. Surg. 2017;4:103–105. doi: 10.1016/j.jajs.2017.10.003. [DOI] [Google Scholar]
- 173.Xu D., Loo L.S., Wang K. Characterization and diffusion behavior of chitosan–poss composite membranes. J. Appl. Polym. Sci. 2011;122:427–435. doi: 10.1002/app.34146. [DOI] [Google Scholar]
- 174.Wang Y., Cai J., Jiang Y., Jiang X., Zhang D. Preparation of biosilica structures from frustules of diatoms and their applications: current state and perspectives. Appl. Microbiol. Biotechnol. 2013;97:453–460. doi: 10.1007/S00253-012-4568-0. [DOI] [PubMed] [Google Scholar]
- 175.Matinfar M., Mesgar A.S., Mohammadi Z. Evaluation of physicochemical, mechanical and biological properties of chitosan/carboxymethyl cellulose reinforced with multiphasic calcium phosphate whisker-like fibers for bone tissue engineering. Mater. Sci. Eng. C. 2019;100:341–353. doi: 10.1016/j.msec.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 176.He X., Tang K., Li X., Wang F., Liu J., Zou F., Yang M., Li M. A porous collagen-carboxymethyl cellulose/hydroxyapatite composite for bone tissue engineering by bi-molecular template method. Int. J. Biol. Macromol. 2019;137:45–53. doi: 10.1016/j.ijbiomac.2019.06.098. [DOI] [PubMed] [Google Scholar]
- 177.Singh B.N., Pramanik K. Generation of bioactive nano-composite scaffold of nanobioglass/silk fibroin/carboxymethyl cellulose for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2018;29:2011–2034. doi: 10.1080/09205063.2018.1523525. [DOI] [PubMed] [Google Scholar]
- 178.Kandasamy S., Narayanan V., Sumathi S. Zinc and manganese substituted hydroxyapatite/CMC/PVP electrospun composite for bone repair applications. Int. J. Biol. Macromol. 2020;145:1018–1030. doi: 10.1016/j.ijbiomac.2019.09.193. [DOI] [PubMed] [Google Scholar]
- 179.Pina S., Oliveira J.M., Reis R.L. Natural-based nanocomposites for bone tissue engineering and regenerative medicine: a review. Adv. Mater. 2015;27:1143–1169. doi: 10.1002/adma.201403354. [DOI] [PubMed] [Google Scholar]
- 180.Chen C., Li H., Pan J., Yan Z., Yao Z., Fan W., Guo C. Biodegradable composite scaffolds of bioactive glass/chitosan/carboxymethyl cellulose for hemostatic and bone regeneration. Biotechnol. Lett. 2014;37:457–465. doi: 10.1007/S10529-014-1697-9. [DOI] [PubMed] [Google Scholar]
- 181.Cao W., Zong S., Zhang Y., Qiu F., Tang J., Wu Z., Luan J. Preparation of a novel bone wax with modified tricalcium silicate cement and BGs. Mater. Sci. Eng. C. 2019;99:979–985. doi: 10.1016/j.msec.2019.02.049. [DOI] [PubMed] [Google Scholar]
- 182.Deshmukh K., Basheer Ahamed M., Deshmukh R.R., Khadheer Pasha S.K., Bhagat P.R., Chidambaram K. Biopolymer composites with high dielectric performance: interface engineering. Biopolym. Compos. Electron. 2017:27–128. doi: 10.1016/B978-0-12-809261-3.00003-6. [DOI] [Google Scholar]
- 183.Khan A.F., Afzal A., Chaudhary A.A., Saleem M., Shahzadi L., Jamal A., Yar M., Habib A., Rehman I.U. (Hydroxypropyl)methylcellulose mediated synthesis of highly porous composite scaffolds for trabecular bone repair applications. Sci. Adv. Mater. 2015;7:1177–1186. doi: 10.1166/sam.2015.2246. [DOI] [Google Scholar]
- 184.Zeeshan R., Mutahir Z., Iqbal H., Ali M., Iqbal F., Ijaz K., Sharif F., Shah A.T., Chaudhry A.A., Yar M., Luan S., Khan A.F. Ihtesham-ur-Rehman, Hydroxypropylmethyl cellulose (HPMC) crosslinked chitosan (CH) based scaffolds containing bioactive glass (BG) and zinc oxide (ZnO) for alveolar bone repair. Carbohydr. Polym. 2018;193:9–18. doi: 10.1016/j.carbpol.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 185.Iqbal H., Ali M., Zeeshan R., Mutahir Z., Iqbal F., Nawaz M.A.H., Shahzadi L., Chaudhry A.A., Yar M., Luan S., Khan A.F., ur Rehman I. Chitosan/hydroxyapatite (HA)/hydroxypropylmethyl cellulose (HPMC) spongy scaffolds-synthesis and evaluation as potential alveolar bone substitutes. Colloids Surf. B Biointerfaces. 2017;160:553–563. doi: 10.1016/j.colsurfb.2017.09.059. [DOI] [PubMed] [Google Scholar]
- 186.Kamel S., Ali N., Jahangir K., Shah S.M., El-Gendy A.A. Pharmaceutical significance of cellulose: a review. Express Polym. Lett. 2008;2:758–778. doi: 10.3144/expresspolymlett.2008.90. [DOI] [Google Scholar]
- 187.Mao D., Li Q., Bai N., Dong H., Li D. Porous stable poly(lactic acid)/ethyl cellulose/hydroxyapatite composite scaffolds prepared by a combined method for bone regeneration. Carbohydr. Polym. 2018;180:104–111. doi: 10.1016/j.carbpol.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 188.Hokmabad V.R., Davaran S., Aghazadeh M., Rahbarghazi R., Salehi R., Ramazani A. Fabrication and characterization of novel ethyl cellulose-grafted-poly (ϵ-caprolactone)/alginate nanofibrous/macroporous scaffolds incorporated with nano-hydroxyapatite for bone tissue engineering. J. Biomater. Appl. 2019;33:1128–1144. doi: 10.1177/0885328218822641. [DOI] [PubMed] [Google Scholar]
- 189.Wu M., Wu P., Xiao L., Zhao Y., Yan F., Liu X., Xie Y., Zhang C., Chen Y., Cai L. Biomimetic mineralization of novel hydroxyethyl cellulose/soy protein isolate scaffolds promote bone regeneration in vitro and in vivo. Int. J. Biol. Macromol. 2020;162:1627–1641. doi: 10.1016/j.ijbiomac.2020.08.029. [DOI] [PubMed] [Google Scholar]
- 190.Tohamy K.M., Mabrouk M., Soliman I.E., Beherei H.H., Aboelnasr M.A. Novel alginate/hydroxyethyl cellulose/hydroxyapatite composite scaffold for bone regeneration: in vitro cell viability and proliferation of human mesenchymal stem cells. Int. J. Biol. Macromol. 2018;112:448–460. doi: 10.1016/j.ijbiomac.2018.01.181. [DOI] [PubMed] [Google Scholar]
- 191.Eftekhari S., El Sawi I., Bagheri Z.S., Turcotte G., Bougherara H. Fabrication and characterization of novel biomimetic PLLA/cellulose/hydroxyapatite nanocomposite for bone repair applications. Mater. Sci. Eng. C. 2014;39:120–125. doi: 10.1016/j.msec.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 192.Gao W., Sun L., Zhang Z., Li Z. Cellulose nanocrystals reinforced gelatin/bioactive glass nanocomposite scaffolds for potential application in bone regeneration. J. Biomater. Sci. Polym. Ed. 2020;31:984–998. doi: 10.1080/09205063.2020.1735607. [DOI] [PubMed] [Google Scholar]
- 193.Chen X., Zhou R., Chen B., Chen J. Nanohydroxyapatite/cellulose nanocrystals/silk fibroin ternary scaffolds for rat calvarial defect regeneration. RSC Adv. 2016;6:35684–35691. doi: 10.1039/C6RA02038K. [DOI] [Google Scholar]
- 194.yun Zhang X., ping Chen Y., Han J., Mo J., feng Dong P., hong Zhuo Y., Feng Y. Biocompatiable silk fibroin/carboxymethyl chitosan/strontium substituted hydroxyapatite/cellulose nanocrystal composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019;136:1247–1257. doi: 10.1016/j.ijbiomac.2019.06.172. [DOI] [PubMed] [Google Scholar]
- 195.Shaheen T.I., Montaser A.S., Li S. Effect of cellulose nanocrystals on scaffolds comprising chitosan, alginate and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2019;121:814–821. doi: 10.1016/j.ijbiomac.2018.10.081. [DOI] [PubMed] [Google Scholar]
- 196.Zhang F., Luan T., Kang D., Jin Q., Zhang H., Yadav M.P. Viscosifying properties of corn fiber gum with various polysaccharides. Food Hydrocolloids. 2015;43:218–227. doi: 10.1016/j.foodhyd.2014.05.018. [DOI] [Google Scholar]
- 197.Kumar A., Rao K.M., Kwon S.E., Lee Y.N., Han S.S. Xanthan gum/bioactive silica glass hybrid scaffolds reinforced with cellulose nanocrystals: morphological, mechanical and in vitro cytocompatibility study. Mater. Lett. 2017;193:274–278. doi: 10.1016/j.matlet.2017.01.143. [DOI] [Google Scholar]
- 198.Kumar A., Rao K.M., Han S.S. Development of sodium alginate-xanthan gum based nanocomposite scaffolds reinforced with cellulose nanocrystals and halloysite nanotubes. Polym. Test. 2017;63:214–225. doi: 10.1016/j.polymertesting.2017.08.030. [DOI] [Google Scholar]
- 199.Catori D.M., Fragal E.H., Messias I., Garcia F.P., Nakamura C.V., Rubira A.F. Development of composite hydrogel based on hydroxyapatite mineralization over pectin reinforced with cellulose nanocrystal. Int. J. Biol. Macromol. 2021;167:726–735. doi: 10.1016/j.ijbiomac.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 200.Noreen A., Nazli Z.i.H., Akram J., Rasul I., Mansha A., Yaqoob N., Iqbal R., Tabasum S., Zuber M., Zia K.M. Pectins functionalized biomaterials; a new viable approach for biomedical applications: a review. Int. J. Biol. Macromol. 2017;101:254–272. doi: 10.1016/j.ijbiomac.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 201.Valentini L., Cardinali M., Fortunati E., Torre L., Kenny J.M. A novel method to prepare conductive nanocrystalline cellulose/graphene oxide composite films. Mater. Lett. 2013;105:4–7. doi: 10.1016/j.matlet.2013.04.034. [DOI] [Google Scholar]
- 202.Koga H., Saito T., Kitaoka T., Nogi M., Suganuma K., Isogai A. Transparent, conductive, and printable composites consisting of TEMPO-oxidized nanocellulose and carbon nanotube. Biomacromolecules. 2013;14:1160–1165. doi: 10.1021/BM400075F. [DOI] [PubMed] [Google Scholar]
- 203.Park M., Lee D., Shin S., Hyun J. Effect of negatively charged cellulose nanofibers on the dispersion of hydroxyapatite nanoparticles for scaffolds in bone tissue engineering. Colloids Surf. B Biointerfaces. 2015;130:222–228. doi: 10.1016/j.colsurfb.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 204.Huang C., Fang G., Zhao Y., Bhagia S., Meng X., Yong Q., Ragauskas A.J. Bio-inspired nanocomposite by layer-by-layer coating of chitosan/hyaluronic acid multilayers on a hard nanocellulose-hydroxyapatite matrix. Carbohydr. Polym. 2019;222 doi: 10.1016/j.carbpol.2019.115036. [DOI] [PubMed] [Google Scholar]
- 205.Aminatun D., Hikmawati P., Widiyanti T., Amrillah W., Nia Astri, Firdania I.T., Abdullah C.A.C. Study of mechanical and thermal properties in nano-hydroxyapatite/chitosan/carboxymethyl cellulose nanocomposite-based scaffold for bone tissue engineering: the roles of carboxymethyl cellulose. Appl. Sci. 2020;10:6970. doi: 10.3390/app10196970. [DOI] [Google Scholar]
- 206.Cakmak A.M., Unal S., Sahin A., Oktar F.N., Sengor M., Ekren N., Gunduz O., Kalaskar D.M. 3D printed polycaprolactone/gelatin/bacterial cellulose/hydroxyapatite composite scaffold for bone tissue engineering. Polymer. 2020;12 doi: 10.3390/polym12091962. 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kumar A., Negi Y.S., Choudhary V., Bhardwaj N.K. Microstructural and mechanical properties of porous biocomposite scaffolds based on polyvinyl alcohol, nano-hydroxyapatite and cellulose nanocrystals. Cellulose. 2014;21:3409–3426. doi: 10.1007/S10570-014-0339-7. [DOI] [Google Scholar]
- 208.Morouço P., Biscaia S., Viana T., Franco M., Malça C., Mateus A., Moura C., Ferreira F.C., Mitchell G., Alves N.M. Fabrication of poly(ε-caprolactone) scaffolds reinforced with cellulose nanofibers, with and without the addition of hydroxyapatite nanoparticles. BioMed Res. Int. 2016 doi: 10.1155/2016/1596157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Valarmathi N., Sumathi S. Biomimetic hydroxyapatite/silkfibre/methylcellulose composites for bone tissue engineering applications. New J. Chem. 2020;44:4647–4663. doi: 10.1039/C9NJ05592D. [DOI] [Google Scholar]
- 210.Tabata Y., Yamada K., Miyamoto S., Nagata I., Kikuchi H., Aoyama I., Tamura M., Ikada Y. Bone regeneration by basic fibroblast growth factor complexed with biodegradable hydrogels. Biomaterials. 1998;19:807–815. doi: 10.1016/S0142-9612(98)00233-6. [DOI] [PubMed] [Google Scholar]
- 211.Yamamoto M., Tabata Y., Hong L., Miyamoto S., Hashimoto N., Ikada Y. Bone regeneration by transforming growth factor beta1 released from a biodegradable hydrogel. J. Contr. Release. 2000;64:133–142. doi: 10.1016/S0168-3659(99)00129-7. [DOI] [PubMed] [Google Scholar]
- 212.Patel Z.S., Young S., Tabata Y., Jansen J.A., Wong M.E.K., Mikos A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bessa P.C., Casal M., Reis R.L. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) J. Tissue Eng. Regen. Med. 2008;2:81–96. doi: 10.1002/TERM.74. [DOI] [PubMed] [Google Scholar]
- 214.Shi Q., Li Y., Sun J., Zhang H., Chen L., Chen B., Yang H., Wang Z. The osteogenesis of bacterial cellulose scaffold loaded with bone morphogenetic protein-2. Biomaterials. 2012;33:6644–6649. doi: 10.1016/j.biomaterials.2012.05.071. [DOI] [PubMed] [Google Scholar]
- 215.Zhang W., chuan Wang X., yue Li X., le Zhang L., Jiang F. A 3D porous microsphere with multistage structure and component based on bacterial cellulose and collagen for bone tissue engineering. Carbohydr. Polym. 2020;236 doi: 10.1016/j.carbpol.2020.116043. [DOI] [PubMed] [Google Scholar]
- 216.Coelho F., Cavicchioli M., Specian S.S., Scarel-Caminaga R.M., de Aquino Penteado L., de Medeiros A.I., de Lima Ribeiro S.J., de Oliveira Capote T.S. Bacterial cellulose membrane functionalized with hydroxiapatite and anti-bone morphogenetic protein 2: a promising material for bone regeneration. PLoS One. 2019;14 doi: 10.1371/journal.pone.0221286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Song S.H., Yun Y.P., Kim H.J., Park K., Kim S.E., Song H.R. Bone formation in a rat tibial defect model using carboxymethyl cellulose/BioC/bone morphogenic protein-2 hybrid materials. BioMed Res. Int. 2014 doi: 10.1155/2014/230152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sukul M., Nguyen T.B.L., Min Y.K., Lee S.Y., Lee B.T. Effect of local sustainable release of BMP2-VEGF from nano-cellulose loaded in sponge biphasic calcium phosphate on bone regeneration. Tissue Eng. 2015;21:1822–1836. doi: 10.1089/ten.tea.2014.0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pigossi S.C., Medeiros M.C., Saska S., Cirelli J.A., Scarel-Caminaga R.M. Role of osteogenic growth peptide (OGP) and OGP(10–14) in bone regeneration: a review. Int. J. Mol. Sci. 2016;17:1885. doi: 10.3390/IJMS17111885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Saska S., Scarel-Caminaga R.M., Teixeira L.N., Franchi L.P., Dos Santos R.A., Gaspar A.M.M., De Oliveira P.T., Rosa A.L., Takahashi C.S., Messaddeq Y., Ribeiro S.J.L., Marchetto R. Characterization and in vitro evaluation of bacterial cellulose membranes functionalized with osteogenic growth peptide for bone tissue engineering. J. Mater. Sci. Mater. Med. 2012;239:2253–2266. doi: 10.1007/S10856-012-4676-5. 23 (2012) [DOI] [PubMed] [Google Scholar]
- 221.Pigossi S.C., De Oliveira G.J.P.L., Finoti L.S., Nepomuceno R., Spolidorio L.C., Rossa C., Ribeiro S.J.L., Saska S., Scarel-Caminaga R.M. Bacterial cellulose-hydroxyapatite composites with osteogenic growth peptide (OGP) or pentapeptide OGP on bone regeneration in critical-size calvarial defect model. J. Biomed. Mater. Res. 2015;103:3397–3406. doi: 10.1002/jbm.a.35472. [DOI] [PubMed] [Google Scholar]
- 222.Saska S., Teixeira L.N., de Castro Raucci L.M.S., Scarel-Caminaga R.M., Franchi L.P., dos Santos R.A., Santagneli S.H., Capela M.V., de Oliveira P.T., Takahashi C.S., Gaspar A.M.M., Messaddeq Y., Ribeiro S.J.L., Marchetto R. Nanocellulose-collagen-apatite composite associated with osteogenic growth peptide for bone regeneration. Int. J. Biol. Macromol. 2017;103:467–476. doi: 10.1016/j.ijbiomac.2017.05.086. [DOI] [PubMed] [Google Scholar]
- 223.Liu H., Zhang L., Shi P., Zou Q., Zuo Y., Li Y. Hydroxyapatite/polyurethane scaffold incorporated with drug-loaded ethyl cellulose microspheres for bone regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2010;95B:36–46. doi: 10.1002/jbm.b.31680. [DOI] [PubMed] [Google Scholar]
- 224.Mori R., Nakai T., Enomoto K., Uchio Y., Yoshino K. Increased antibiotic release from a bone cement containing bacterial cellulose. Clin. Orthop. Relat. Res. 2010;469:600–606. doi: 10.1007/S11999-010-1626-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Junka A., Bartoszewicz M., Dziadas M., Szymczyk P., Dydak K., Żywicka A., Owczarek A., Bil-Lula I., Czajkowska J., Fijałkowski K. Application of bacterial cellulose experimental dressings saturated with gentamycin for management of bone biofilm in vitro and ex vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2020;108:30–37. doi: 10.1002/jbm.b.34362. [DOI] [PubMed] [Google Scholar]
- 226.Dydak K., Junka A., Szymczyk P., Chodaczek G., Toporkiewicz M., Fijałkowski K., Dudek B., Bartoszewicz M. Development and biological evaluation of Ti6Al7Nb scaffold implants coated with gentamycin-saturated bacterial cellulose biomaterial. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Maver U., Xhanari K., Žižek M., Gradišnik L., Repnik K., Potočnik U., Finšgar M. Carboxymethyl cellulose/diclofenac bioactive coatings on AISI 316LVM for controlled drug delivery, and improved osteogenic potential. Carbohydr. Polym. 2020;230 doi: 10.1016/j.carbpol.2019.115612. [DOI] [PubMed] [Google Scholar]
- 228.Junka A., Żywicka A., Chodaczek G., Dziadas M., Czajkowska J., Duda-Madej A., Bartoszewicz M., Mikołajewicz K., Krasowski G., Szymczyk P., Fijałkowski K. Potential of biocellulose carrier impregnated with essential oils to fight against biofilms formed on hydroxyapatite. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-018-37628-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Klinthoopthamrong N., Chaikiawkeaw D., Phoolcharoen W., Rattanapisit K., Kaewpungsup P., Pavasant P., Hoven V.P. Bacterial cellulose membrane conjugated with plant-derived osteopontin: preparation and its potential for bone tissue regeneration. Int. J. Biol. Macromol. 2020;149:51–59. doi: 10.1016/j.ijbiomac.2020.01.158. [DOI] [PubMed] [Google Scholar]
- 230.Vadaye Kheiry E., Parivar K., Baharara J., Fazly Bazzaz B.S., Iranbakhsh A. The osteogenesis of bacterial cellulose scaffold loaded with fisetin, Iran. J. Basic Med. Sci. 2018;21:965. doi: 10.22038/ijbms.2018.25465.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sumathra M., Munusamy M.A., Alarfaj A.A., Rajan M. Osteoblast response to Vitamin D3 loaded cellulose enriched hydroxyapatite Mesoporous silica nanoparticles composite. Biomed. Pharmacother. 2018;103:858–868. doi: 10.1016/j.biopha.2018.04.078. [DOI] [PubMed] [Google Scholar]
- 232.Kamel R., El-Wakil N.A., Abdelkhalek A.F.A., Elkasabgy N.A. Nanofibrillated cellulose/cyclodextrin based 3D scaffolds loaded with raloxifene hydrochloride for bone regeneration. Int. J. Biol. Macromol. 2020;156:704–716. doi: 10.1016/j.ijbiomac.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 233.Terauchi M. Pharmacokinetics of selective estrogen receptor modulators(SERMs) Clin. Calcium. 2016;26:1571–1581. doi: 10.2165/00003088-200342040-00004. [DOI] [PubMed] [Google Scholar]