Abstract
Membrane fatty acid composition and thermal resistance (D value) of Pediococcus sp. were determined for mid-exponential-phase (ME) and stationary-phase (ST) cells grown in tryptic soy broth (TSB) and tryptone-glucose-yeast extract (TGY) at 28 and 37°C. As the cells entered the stationary phase of growth, the unsaturated fatty acid, C18:1 n11c, produced during the exponential phase of growth was converted to its cyclic form, C19:0 Δ9c. This shift in membrane fatty acid composition was accompanied by an increase in the D values of this bacterium. Data from this study suggest that the membrane fatty acid composition of Pediococcus sp. is dependent on the growth conditions and that membrane fatty acid composition plays a critical role in thermal resistance. Thermal inactivation curves of Pediococcus sp. cells grown in TGY at 28°C indicated the presence of a cell population that is heterogeneous in thermal resistance. The growth of this bacterium in TGY at 37°C and in TSB at 28 and 37°C resulted in cell populations that were uniform in thermal resistance with a lag time for thermal inactivation. Thermal inactivation curves of ME and ST cultures were similar. The data presented here suggest that the cell population’s uniformity of thermal inactivation is independent of the growth phase of the culture.
Bacterial cytoplasmic membrane, which consists mainly of lipids, has been shown to be a site for thermal injury (6, 8, 9, 32). This cytoplasmic membrane, the boundary between the cytoplasm and the external environment, regulates the flow of nutrients and metabolic products in and out of the cell, thereby permitting homeostasis of the cytoplasmic environment (3, 13). Growth conditions such as the composition of growth medium (1, 2, 12, 13, 17, 24), the growth phase (age) of the cells (13, 17, 18, 20, 32), the incubation temperature at which the bacteria were cultured (1, 6, 8, 13, 17, 18, 22–24, 26, 30), and the pH (3, 17, 23, 24) markedly affect the composition of the membrane lipid. These changes in the composition of the membrane lipid affect mainly the fluidity of the cellular membrane and are thought to occur in order to maintain both membrane integrity and functionality in the face of the external conditions (30). The major way in which bacteria maintain this ideal membrane fluidity is by changing their fatty acid composition (1, 22, 30). For example, as the growth temperature decreases, fatty acids with lower melting points are incorporated into the lipid bilayer, which lowers the order-disorder transition temperature of the membrane, thereby maintaining fluidity and compensating for the decreased temperature (1, 22, 30). This process has been described as a homeoviscous adaptation by Sinensky (26), a process in which the membrane fluidity is maintained (relatively) constant through lipid changes in response to changes in growth temperature.
Thermal inactivation of bacteria was shown to be dependent on environmental parameters such as the growth medium (2, 4, 9, 10, 27), the growth temperature (6, 8–10, 20, 21), the growth phase (10, 20), and the pH (2, 10). Also, changes in thermal resistance of bacteria due to changes in growth conditions were positively correlated with alterations in the membrane fluidity (6, 8, 9, 32, 33). These researchers reported that an increase in the fluidity of the bacterial membrane due to the changes in growth conditions corresponded to a decrease in thermal resistance. Furthermore, the use of procaine, a membrane fluidizing agent, in thermal inactivation studies resulted in an increase in the membrane fluidity, with a subsequent decrease in the thermal resistance of Escherichia coli (6, 33).
Pediococcus sp. (formerly, Micrococcus freudenreichii [2]) is a gram-positive, spherical, nonmotile, non-spore-forming, facultative anaerobe. This bacterium, originally isolated from milk and dairy utensils (28), is a heat-resistant, spoilage, nonpathogenic organism that has been used as a test organism in milk and milk byproduct pasteurization studies (14, 28, 29). These characteristics, i.e., nonpathogenicity and thermal resistance, have made this bacterium an attractive test organism for studying the destruction of bacteria by microwave energy in a food pilot plant (15, 16). Our data, obtained from a nonthermal, semicontinuous pilot plant process utilizing microwave energy, suggested that the lethal effect of electromagnetic energy on Pediococcus sp. was dependent on the growth medium and the processing fluid (15). Similarly, growth of this bacterium in different growth media resulted in different thermal resistance in all of the heating menstrua tested (2). This suggested that the thermal resistance of Pediococcus sp. was dependent on the growth medium. While this bacterium is considered to be a test organism in milk pasteurization studies, little is known about the influence of growth conditions on the membrane fatty acid composition and the thermal resistance of this organism.
This study is a part of ongoing research to develop a cold pasteurization process utilizing electromagnetic energy (15, 16). The data from our pilot plant microwave process indicated that cells of Pediococcus sp. grown on tryptone-glucose-yeast extract (TGY) were more sensitive to microwave energy than cells grown in tryptic soy broth (TSB) (15) and that bacterial inactivation by microwave energy was dependent on the growth temperature (unpublished data). Also, we previously reported that the thermal resistance of Pediococcus sp. was dependent on the growth medium (2). It was the objective of the present study to determine the influence of growth medium, growth phase (age), and growth temperature on membrane fatty acid composition and the thermal resistance of Pediococcus sp.
MATERIALS AND METHODS
Microorganism, culture maintenance, and growth media.
Pediococcus sp. strain NRRL B-2354 was supplied by L. K. Nakamura (U.S. Department of Agriculture, Peoria, Ill.). The culture was maintained on tryptose agar (TA; Difco Laboratories, Detroit, Mich.) at 4°C, with biweekly transfers to maintain strain viability. The three growth media used were TSB (Difco Laboratories) prepared in distilled water according to the manufacturer’s guidelines and supplemented with glucose to a final concentration of 0.5% (wt/vol), TGY broth formulated in our laboratory (tryptone, 5 g; yeast extract, 5 g; glucose, 1 g; dibasic potassium phosphate, 1 g; double-distilled and deionized water, 1 liter; pH 7.00), and TGYG broth (TGY broth supplemented with glucose to a final concentration of 0.5% [wt/vol]). All ingredients were mixed prior to autoclaving, and the medium pH did not change after autoclaving.
Inoculum development, growth conditions, sample preparation, and thermal inactivation.
A late-exponential-phase culture grown in the appropriate medium at either 28 or 37°C was used to inoculate 50 to 100 ml of the same medium at a 1% level (vol/vol). Growth was monitored by measuring the optical density at 600 nm with a Shimadzu UV-160 spectrophotometer (Shimadzu Scientific Instruments, Inc., Columbia, Md.). Cultures were grown to the mid-exponential (A600 = 0.5) or stationary (A600 = 1.0) phase of growth, harvested by centrifugation at 16,000 × g for 10 min at 4°C, and washed once with cold sterile distilled water. The cell pellet was suspended in tap water as the heating menstruum to a target level of 8 log CFU/ml. Culture samples (9.5 ml) were loaded onto a Techne submerged-coil heating apparatus model tempette TE-8D (Protocol Instruments Limited, West Byfleet, United Kingdom) and kept at 60°C. Heating time and sampling frequency was based on the culture growth conditions. After being heated, the samples were quickly stored on ice.
Assessment of bacterial viability.
The bacterial suspensions were serially diluted in 0.1% peptone (Difco) and surface plated on TA plates by using the spiral plating system, model D (Spiral Systems Instruments, Inc., Bethesda, Md.). The plates were then incubated at 37°C for 18 to 24 h, and the survivors were enumerated by using a laser bacterial colony counter, model 500A (Spiral Systems Instruments, Inc.). Cell densities were reported as CFU per milliliter of sample.
D values.
D values (the times needed in order to inactivate 90% of the population) were calculated as the negative reciprocal slope of the linear portion of survivor curves (which were obtained by plotting logarithms of survival counts versus their corresponding heating times). Linear regression lines were fitted to the linear portion of two sets of independent data.
Fatty acid analysis.
The total fatty acids were extracted and methyl esterified from 40 to 80 mg (wet weight) of cell pellets as previously described (1). A Hewlett-Packard 5890 gas-liquid chromatograph (Hewlett-Packard, Avondale, Pa.) equipped with a split-splitless injector, flame ionization detector, integrator, and a 30-m-by-0.25-mm SPB-1 (0.25-μm film thickness) fused silica capillary column (Supelco, Inc., Bellefonte, Pa.) was used for the separation and detection of the fatty acid methyl esters (FAME). The carrier gas flow (helium) was adjusted to 24 cm/s, and the injector and detector temperatures were maintained at 250 and 280°C, respectively. The sample (3 to 5 μl) was injected in the split mode (ratio of 100:1), and the column temperature was held at 150°C for 4 min before it was raised to 250°C at a rate of 4°C/min. FAME were identified by comparing the retention times of a qualitative standard bacterial FAME mixture (Matreya, Inc., Pleasant Gap, Pa.).
All chemicals were analytical-grade reagents. Glassware was cleaned with Nochromix acid solution (Godax Laboratories, Inc., Takoma Park, Md.) and rinsed repeatedly with distilled water before use.
Statistical analysis.
Standard errors were calculated from the regression analysis by using SAS software (SAS Institute, Inc., Cary, N.C.). Duncan’s multiple-range test was used to determine the significant differences (unless otherwise mentioned, P < 0.05) among membrane lipid fatty acids and the D values of Pediococcus cells grown under different conditions.
RESULTS
Effect of growth conditions on membrane fatty acid composition.
Pediococcus cells were grown to mid-exponential phase (ME) and stationary phase (ST) in TGY and TSB at 28 and 37°C, and their membrane fatty acid composition was determined. Total saturated fatty acids (SFA), total unsaturated fatty acids (USFA), and total cyclic fatty acids (CFA) were reported as the sum of the mean values of three independent replications ± the standard deviation (Tables 1 and 2) and were used in determining the significant differences among membrane fatty acids of Pediococcus cells grown under different conditions. The major fatty acids were, in order, USFA, SFA, and CFA, with USFA representing up to 72% of the total fatty acids (Tables 1 and 2).
TABLE 1.
Effect of growth mediuma on the membrane fatty acidb composition of Pediococcus cells grown at 28°C until ST or ME
| Acyl chainc | % Total fatty acid on:
|
||||
|---|---|---|---|---|---|
| TGY
|
TSB
|
TGYG
|
|||
| ST | ME | ST | ME | ST | |
| SFA | |||||
| C13:0 | 0.09 | 0.12 | ND | 0.14 | 0.08 |
| C14:0 | 5.75 | 5.41 | 8.59 | 4.41 | 2.54 |
| C15:0 | 0.62 | 0.99 | 0.12 | 0.36 | 1.03 |
| C16:0 | 12.93 | 16.30 | 16.15 | 17.56 | 11.81 |
| C17:0 | NDd | ND | ND | 0.11 | 0.30 |
| C18:0 | 1.06 | 1.62 | 1.11 | 2.34 | 2.33 |
| C20:0 | 0.31 | 0.29 | 0.45 | 0.51 | 1.04 |
| Totale | 20.76 ± 3.64 | 24.73 ± 2.28 | 26.41 ± 1.47 | 25.42 ± 4.44 | 19.13 ± 4.38 |
| USFA | |||||
| C16:1 n9c | 18.71 | 16.20 | 16.95 | 11.47 | 11.31 |
| C18:2 n9c, n12c | 0.21 | ND | 0.92 | 0.19 | ND |
| C18:1 n9c | ND | 0.25 | ND | 0.39 | ND |
| C18:1 n11c | 46.24 | 55.51 | 27.70 | 59.72 | 57.78 |
| Totale | 65.16 ± 0.24 | 71.97 ± 1.21 | 45.56 ± 3.00 | 71.76 ± 3.74 | 69.09 ± 14.10 |
| CFA | |||||
| C17:0 Δ9c | 0.40 | 0.07 | 2.25 | 0.09 | 0.11 |
| C19:0 Δ9c | 13.69 | 3.24 | 24.69 | 2.73 | 11.15 |
| Totale | 14.10 ± 3.42 | 3.31 ± 1.09 | 26.93 ± 2.73 | 2.83 ± 0.74 | 11.26 ± 8.93 |
| Others | ND | ND | 1.09 | ND | 0.57 |
Cells were grown in 100 ml of TGY, TSB, or TGYG medium at 28°C until ST (A600 = 1.0) or ME (A600 = 0.5), centrifuged, and washed once with sterile distilled water. Wet cells (40 to 80 mg) were used in the preparation and analysis of FAME as described in Materials and Methods.
Data are presented as percent total fatty acid and represent the mean of three replications.
n, location of double bond; Δ, location of cyclopropane ring; c, cis.
ND, not detected (species absent or below detection limit).
Data are the total of the mean values ± the standard deviation of replicate values.
TABLE 2.
| Acyl chainc | % Total fatty acid on:
|
Tcd (°C) | |
|---|---|---|---|
| TGY | TSB | ||
| SFA | |||
| C13:0 | NDe | 0.05 | 13.5 |
| C14:0 | 7.14 | 8.99 | 23.0 |
| C15:0 | 0.56 | 0.13 | 34.2 |
| C16:0 | 18.98 | 17.50 | 41.0 |
| C17:0 | ND | ND | 47.8 |
| C18:0 | 2.37 | 1.49 | 58.0 |
| C20:0 | 0.33 | 0.19 | 66.0 |
| Totalf | 29.38 ± 8.70 | 28.35 ± 7.79 | |
| USFA | |||
| C16:1 n9c | 15.56 | 18.02 | −36.0 |
| C18:2 n9c, n12c | 0.39 | 0.16 | −17.6 |
| C18:1 n9c | 4.35 | 2.90 | −22.0 |
| C18:1 n11c | 40.91 | 24.97 | −19.0 |
| Totalf | 61.22 ± 12.45 | 46.05 ± 7.37 | |
| CFA | |||
| C17:0 Δ9c | 0.26 | 1.88 | −19.9 |
| C19:0 Δ9c | 9.15 | 23.60 | −0.5 |
| Totalf | 9.41 ± 5.17 | 25.48 ± 4.43 | |
| Others | ND | 0.13 | |
Data represent the mean of three replications.
Cells were grown in 100 ml of TGY or TSB medium at 37°C until ST (A600 = 1.0), centrifuged, and washed once with sterile distilled water. Wet cells (40 to 80 mg) were used in the preparation and analysis of FAME as described in Materials and Methods.
n, location of double bond; Δ, location of cyclopropane ring; c, cis.
Tc, phospholipid gel to liquid-crystalline-phase transition temperature (25).
ND, not detected (species absent or below detection limit).
Data are the total of the mean values ± the standard deviation of replicate values.
The ST cells of Pediococcus sp. grown in TGY at 28°C showed a significant 4.3-fold increase in CFA compared to the ME cells grown in TGY at 28°C (Table 1). Growth of Pediococcus cells in TSB at 28°C to ST resulted in a 9.5-fold significant increase in CFA and a 36.5% significant decrease in USFA compared to ME cells grown in TSB at 28°C (Table 1). Pediococcus cells grown in TGYG at 28°C to ST had a membrane fatty acid profile similar to that of ST cells grown in TGY (Table 1).
Growth of Pediococcus cells at 37°C in TGY to ST resulted in a 1.4-fold significant (P = 0.07) increase in SFA (Table 2) compared to ST cells grown in TGY at 28°C (Table 1). When grown in TSB at 37°C, membrane fatty acid composition of ST cells (Table 2) was similar to that of ST cells grown at 28°C (Table 1). Also, the membrane fatty acid compositions of ME cells grown in TGY and TSB at 37°C (data not shown) was similar to those of ME cells grown in TGY and TSB at 28°C (Table 1).
Pediococcus cells grown in TSB at 28°C to ST exhibited a 1.9-fold significant increase in CFA and a 30.1% significant decrease in USFA compared to ST cells grown in TGY at 28°C (Table 1). When grown at 37°C, TSB-grown ST cells showed a 2.7-fold significant increase in CFA and a 24.8% significant decrease in USFA compared to TGY-grown ST cells (Table 2).
Effect of growth conditions on thermal resistance.
ME and ST cultures grown in TGY and TSB at 28 and 37°C were used in studying the effect of growth conditions on the thermal resistance of Pediococcus sp. The thermal resistance (D value) at 60°C was determined in tap water since cells were shown to be stable in this heating menstruum (2). Logarithms of surviving Pediococcus cells (in CFU per milliliter) were plotted against heating time, and the D values were obtained by linear regression from the linear portion of the survivor curves (Fig. 1 and 2). The coefficient of correlation (r2) range was 0.920 to 0.994. D values were reported as the mean of two independent replications ± the standard deviation (Table 3) and were used to determine the significance of growth conditions on the thermal resistance of Pediococcus sp.
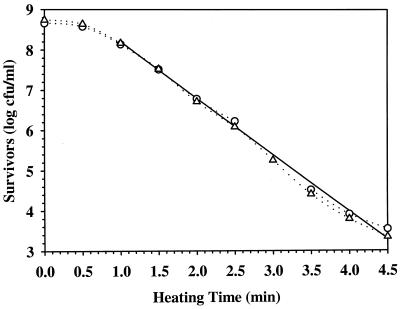
FIG. 1.
Thermal inactivation curve of TSB-grown Pediococcus cells in tap water at 60°C. Circles and triangles represent the data of two independent studies. The solid straight line is the average regression plot of the straight portion of the two survivor curves (dotted lines). Cells were grown to ME at 28°C, washed once with sterile distilled water, and suspended in tap water to ca. 8 log CFU/ml.
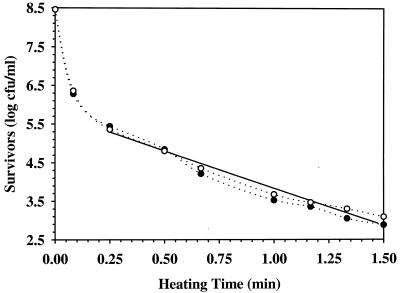
FIG. 2.
Thermal inactivation curve of TGY-grown Pediococcus cells in tap water at 60°C. Circles and triangles represent the data of two independent studies. The solid straight line is the average regression plot of the straight portion of the two survivor curves (dotted lines). Cells were grown to ME at 28°C, washed once with sterile distilled water, and suspended in tap water to ca. 8 log CFU/ml.
TABLE 3.
Effect of growth conditions on D values of Pediococcus sp. at 60°C
| Culture growth conditionsa | D value (min)b | Lag time (min)c | pH at end of growthd |
|---|---|---|---|
| TGY, ME, 28°C | 0.51 ± 0.04 | NDe | 6.34 |
| TGY, ST, 28°C | 1.09 ± 0.06 | ND | 5.15 |
| TGY, ST, 37°C | 2.81 ± 0.38 | 0.25 | 4.86 |
| TSB, ME, 28°C | 0.71 ± 0.02 | 0.25 | 6.17 |
| TSB, ST, 28°C | 4.46 ± 0.06 | 1.00 | 5.28 |
| TSB, ST, 37°C | 5.28 ± 1.75 | 2.00 | 4.68 |
| TGYG, ST, 28°C | 0.70 ± 0.02 | ND | 4.28 |
Cells were grown in the appropriate medium (TGY, TSB, or TGYG) until ME or ST at the appropriate temperature (28 or 37°C), centrifuged, washed once with sterile distilled water, and resuspended in tap water to a final cell concentration of ca. 8 log CFU/ml.
Data represent the mean values of two replications ± the standard deviation of replicate values.
Initial period for thermal resistance.
Medium pH was measured prior to harvesting of the cells.
ND, not detected.
A representative example of the effect of growth medium on survivor curves at 60°C is shown in Fig. 1 and 2, where the thermal inactivation curves demonstrated a biphasic inactivation characterized by a shoulder and/or tailing. While ME cells grown in TSB at 28°C exhibited a lag time for thermal inactivation (Fig. 1) of 0.25 min (Table 3), TGY-grown ME cells exhibited an initial period of higher thermal sensitivity (Fig. 2). Survivor curves of ST cells grown in TSB and TGY at 28°C (data not shown) were similar to those of ME cells grown in TSB (Fig. 1) and TGY (Fig. 2) at 28°C, with TSB-grown ST cells exhibiting a 1.00-min lag time for thermal inactivation (Table 3). Growth of Pediococcus cells in TGY and TSB at 37°C to ST resulted in survivor curves (data not shown) similar to that of ME cells grown in TSB at 28°C (Fig. 1), with lag times for thermal inactivation of 0.25 and 2.00 min, respectively (Table 3). When compared to ST cells grown in TGY at 28°C, the growth of Pediococcus cells in TGYG at 28°C to ST cells resulted in cell population with uniform thermal resistance (data not shown), a result similar to that seen with ME cells grown in TSB at 28°C (Fig. 1), but no lag time for inactivation was detected (Table 3).
Growth of Pediococcus cells at 28°C to ST in TGY and TSB resulted in 2.1- and 6.3-fold significant increases in D values, respectively, compared to ME cells grown in the same medium (Table 3). ST cells grown at 37°C in TGY showed a 2.6-fold significant increase in D value compared to ST cells grown in TGY at 28°C (Table 3).
The growth of Pediococcus cells in TSB at 28 and 37°C to ST resulted in 4.1- and 1.9-fold significant increases in D values, respectively, compared to ST cells grown in TGY at 28 and 37°C (Table 3).
DISCUSSION
Fatty acids play an important role in determining the physiochemical properties of cellular and membrane lipids. Microorganisms possess species-specific fatty acid profiles (30), and the membrane fatty acid composition of Pediococcus cultures (Tables 1 and 2) was consistent with that of the genus Pediococcus (19). Fatty acids derived from ST cultures grown in TGY and TSB at different temperatures revealed a marked shift in membrane fatty acid composition as cells entered the ST of growth, where a significant proportion of the C18:1 n11c in ME cells was converted to C19:0 Δ9c in ST cells (Table 1). The formation of CFA is considered to be a postsynthetic modification of the phospholipid bilayer that occurs predominantly as cultures enter the ST (3, 5, 24, 31). The CFA are formed by CFA synthase through the addition of a methylene group from S-adenosyl-l-methionine to the cis double bond of the USFA moiety of the phospholipid. The conversion of USFA to CFA as the cells enter the ST is believed to serve as a protective measure against lipid oxidation (18), low pH (3), and thermal inactivation (32). On the other hand, bacterial mutant strains that completely lacked the ability to synthesize CFA were able to grow and survive normally under virtually all conditions (31). The survival of these mutant strains was somewhat reduced after repeated cycles of freezing and thawing, indicating that this fatty acid modification is not essential but may be beneficial under certain conditions (31).
The most commonly found CFA in the bacterial membrane are C19:0 Δ11c, which is derived from C18:1 n11c; C17:0 Δ9c, which is derived from C16:1 n9c; and C19:0 Δ9c, which is derived from C18:1 n9c (24). While the conversion of C18:1 n11c to C19:0 Δ11c was reported in ST cells of E. coli (18, 24, 31), Brown et al. (3) and Yatvin et al. (32) did not indicate the location of the cyclopropane ring on the cyclic fatty acid C19:0 that was derived from C18:1 n11c. The data reported here indicate that the C19:0 Δ9c in the Pediococcus cytoplasmic membrane was derived from C18:1 n11c (Tables 1 and 2) rather than from C18:1 n9c. To check the validity of this data, we added the known FAME C18:1 n11c, C18:1 n9c, C19:0 Δ11c, or C19:0 Δ9c to the membrane FAME samples and analyzed them by gas-liquid chromatography. The results indicated that C19:0 Δ9c fatty acid was indeed derived from C18:1 n11c. This indicates that unlike other microorganisms, Pediococcus sp. might contain an enzyme system that is capable of carrying on the conversion of C18:1 n11c to C19:0 Δ9c instead of C19:0 Δ11c.
The experiments described here focus attention on C19:0 Δ9c as playing a critical role in thermal resistance of Pediococcus sp. This fatty acid becomes one of the major fatty acids as cultures enter ST irrespective of the growth medium and the growth temperature (Tables 1 and 2). The phase transition temperature (Tc) of phosphatidylcholine containing C19:0 Δ9c (−0.5°C) is significantly higher than that of phosphatidylcholine containing C18:1 n11c (−19°C) (Table 2). Also, C19:0 Δ9c, which has less rotational freedom than C18:1 n11c, imparts increased rigidity to the cytoplasmic membrane (7, 32). Thus, the increase in CFA and the decrease in USFA would cause a decrease in membrane fluidity, thereby increasing the thermal resistance (6, 8, 32).
Pediococcus cells grown in TGY and TSB at 28°C to ST showed a significant increase in CFA, a significant decrease in USFA (Table 2), and a significant increase in D values (Table 3) compared to the ME cells. These data suggest that ST cells are more thermotolerant than ME cells (11, 20, 32). The increase in CFA within membrane fatty acids increases the membrane rigidity and hence decreases the fluidity of the cell membrane (3, 7, 18, 20, 32). This decrease in membrane fluidity could explain the increase in the thermal resistance of these ST cultures (Table 3). A similar correlation between thermal resistance (6, 8, 32) or acid resistance (3) and the CFA content of cytoplasmic membrane fluidity was reported for E. coli.
Growth of Pediococcus cells in TSB to ST resulted in a significant increase in CFA and a significant decrease in USFA compared to ST cultures grown in TGY at 28°C (Table 1) and 37°C (Table 2). The ST cells of Pediococcus sp. grown in TSB at 28 and 37°C had significantly higher D values and exhibited up to a 1.75-min increase in lag time for thermal inactivation compared to TGY-grown ST cells (Table 3). The increase in membrane rigidity as a result of producing more CFA and less USFA could explain the increase in the D values of TSB-grown ST cells compared to the TGY-grown ST cells. While the total levels of SFA, USFA, and CFA of ME cells grown in TGY and TSB at 28°C were similar (Table 1), TSB-grown ME cells seemed to contain higher concentrations of the individual fatty acids with higher melting points (see Table 2 for Tc values) compared to TGY-grown ME cells. Also, TSB-grown ME cells possessed a 1.4-fold higher D value and a 0.25-min increase in lag time for thermal inactivation compared to ME cells grown in TGY at 28°C (Table 3). Thus, the change in membrane fluidity due to higher concentrations of fatty acids with higher melting points could explain the change in the thermal resistance of this bacterium. These data indicate that the TSB-grown cells were more resistant to thermal inactivation than cells grown in TGY. These differences in D values (Table 3) and initial thermal protection (Fig. 1) or sensitivity (Fig. 2) appear to result from effects of the growth medium regardless of the growth phase used. A similar report (2) suggested that the changes in D values and the initial thermal protection or sensitivity of Pediococcus ST cells seemed to be an effect of the growth medium regardless of the heating menstruum used. This effect could be due to the higher concentrations of glucose and/or the nitrogen source in TSB medium compared to that of TGY medium. Concentrations of glucose and/or the nitrogen source in the growth medium have been known to influence the bacterial cell membrane fluidity (1, 2, 12, 17), where an increase in membrane fluidity was reported to result in a decrease in the thermal resistance of the bacterial cell (3, 6, 8, 9, 32, 33).
The D values of TSB (containing 0.5% glucose)-grown cells of Pediococcus sp. were significantly higher than those of TGY (containing 0.1% glucose)-grown cells in all heating menstrua tested (2). These results, in conjunction with the data presented here (see above), suggest that the increase in thermal resistance of Pediococcus cells is a result of an increasing glucose concentration in the growth medium. To check the validity of this hypothesis, we studied the effect of glucose on thermal inactivation of Pediococcus sp. Cells were grown in TGYG (TGY broth containing 0.5% glucose) to ST at 28°C, and the D values were determined in tap water at 60°C. There was a 36% decrease in the D value of ST cells grown in TGYG compared to TGY-grown ST cells (Table 3). These data suggest that the increase in glucose concentration in the growth medium had a negative effect on the thermal resistance of this bacterium. Smith et al. (27) reported that the presence of glucose in the growth medium potentiated the heat injury of Staphylococcus aureus. When ST Pediococcus cells were grown in TGY or TGYG at 28°C, the pH values of the growth media at the end of growth were 5.15 and 4.28, respectively. Thus, the decrease in thermal resistance as a result of increasing the glucose concentration in the growth medium might be related to the pH of the medium at the end of growth. The data presented in Table 3 show that the increase in D values of cells grown in TGY and TSB at 37°C was associated with a decrease in medium pH at the end of growth compared to growth at 28°C. However, data presented in Table 3 indicated that ST cells of Pediococcus sp. grown in TGYG at 28°C had a low pH (4.28), yet the cells were more sensitive to thermal inactivation than the TGY-grown ST cells (Table 3). Thus, the decrease in pH was merely indicative of glucose metabolism (27).
ST cells of Pediococcus sp. grown in TGYG medium at 28°C showed a 31% decrease in CFA (Table 1) and a 36% decrease in the D value (Table 3) compared to ST cells grown in TGY. This decrease in the CFA could increase the membrane fluidity and thus explain the decrease in the thermal resistance of this bacterium (6, 8, 32). However, the presence of 0.5% glucose in the TSB medium resulted in a decreased membrane fluidity in ST cells grown at 28°C (Table 1), with a concomitant increase in the D value (Table 3) compared to TGYG-grown ST cells. This suggests that nutrient sources other than glucose present in TSB compared to TGY probably affected the membrane fluidity. Since the composition of growth medium was reported to affect the bacterial membrane fluidity through the alteration of fatty acid content (see above), determination of thermal resistance would ultimately depend on growth medium.
We sought to investigate the effect of growth temperature on membrane fatty acid composition and thermal resistance of this bacterium. ME cultures of Pediococcus sp. grown in TGY and TSB at 28°C (Table 1) had similar fatty acid profiles compared to ME cultures grown at 37°C (data not shown). Also, ST cells grown in TSB at 37°C (Table 2) showed a similar fatty acid profile (slight increase in SFA) compared to those of ST cells grown at 28°C (Table 1), a ca. 1.2-fold increase in D value, and a 1-min increase in lag time for thermal inactivation (Table 3). When grown in TGY at 37°C (Table 2), ST cells showed a significant increase in SFA compared to ST cells grown at 28°C (Table 1), a significant increase in D value (ca. 2.6-fold), and a 0.25-min increase in lag time for thermal inactivation (Table 3). This suggests that cyclization of fatty acid does not play a role in temperature adaptation (18, 22). On the other hand, the increase in SFA, which has a significantly higher Tc (Table 2) than USFA, can result in lower membrane fluidity and hence can explain the increase in the D values of Pediococcus sp. in response to the increase in growth temperature.
The increase in growth temperature of Pediococcus sp. revealed that the mode of adaptation of fatty acid composition was dependent on the growth medium (Tables 1 and 2). The activity of CFA synthase responsible for synthesis of C19:0 Δ9c fatty acid from the C18:1 n11c intermediate was possibly produced at lower levels when cells were grown in TGY compared to TSB-grown cells (Tables 1 and 2).
The biphasic nature of the survivor curves suggests that two discrete populations were present (2, 10, 11). The survivor curves of Pediococcus sp. grown in TSB at 28°C to ME exhibit a lag time for thermal inactivation and a linear decrease in cell concentration during thermal inactivation (Fig. 1). This suggests that the cell population is uniform in its thermal resistance (2, 10, 11). On the other hand, ME cells grown in TGY exhibited an initial period of higher thermal sensitivity (Fig. 2), suggesting the presence of a cell population heterogeneous in thermal resistance (2, 10, 11). Similar survivor curves were reported for Pediococcus cultures grown in TGY and TSB at 28°C to ST (2), suggesting that the cell population’s uniformity in thermal resistance is independent of the culture growth phase. Similar biphasic thermal inactivation kinetics in Salmonella enteritidis PT4 have been reported to be independent of culture age (11). When Pediococcus cultures were grown in TGY and TSB at 37°C to ST, cell populations were uniform in thermal resistance (data not shown), as previously seen with ME cells grown in TSB at 28°C (Fig. 1), with a lag time for thermal inactivation (Table 3). When compared to ST cells grown in TGY at 28°C, the ST cell population of Pediococcus sp. grown in TGYG at 28°C was uniform in thermal resistance (data not shown), with no lag time for thermal inactivation (Table 3). Thus, the data suggest that the thermal inactivation curves of Pediococcus cell populations are dependent on the growth medium and growth temperature and not on the culture growth phase.
In conclusion, thermal resistance of Pediococcus sp. was shown here to be dependent on many factors, including growth medium, growth temperature, and growth phase (see above), as well as the heating menstruum (2). These growth conditions, as well as the methodology used for bacterial recovery, could make it difficult to compare D values obtained in different laboratories. This study is important because it shows that until a universal growth medium suitable for D value determination is developed, the interpretation and prediction of the bacterial thermal resistance in foods from data obtained under one growth condition and/or one experimental procedure are not advisable.
ACKNOWLEDGMENT
We are grateful to John Phillips for his valuable help with the statistical analysis.
REFERENCES
- 1.Annous B A, Becker L A, Bayles D O, Labeda D P, Wilkinson B J. Critical role of anteiso-C15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl Environ Microbiol. 1997;63:3887–3894. doi: 10.1128/aem.63.10.3887-3894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annous B A, Kozempel M F. Influence of growth medium on thermal resistance of Pediococcus sp. NRRL B-2354 (formerly, Micrococcus freudenreichii) in liquid foods. J Food Prot. 1998;61:578–581. doi: 10.4315/0362-028x-61.5.578. [DOI] [PubMed] [Google Scholar]
- 3.Brown J L, Ross T, McMeekin T A, Nichols P D. Acid habituation of Escherichia coli and the potential role of cyclopropane fatty acids in low pH tolerance. Int J Food Microbiol. 1997;37:163–173. doi: 10.1016/s0168-1605(97)00068-8. [DOI] [PubMed] [Google Scholar]
- 4.Casadei M A, Esteves de Matos R, Harrison S T, Gaze J E. Heat resistance of Listeria monocytogenes in dairy products as affected by the growth medium. J Appl Microbiol. 1998;84:234–239. doi: 10.1046/j.1365-2672.1998.00334.x. [DOI] [PubMed] [Google Scholar]
- 5.Cronan J E, Jr, Rock C O. Biosynthesis of membrane lipids. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B Jr, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Vol. 1. Washington, D.C: ASM Press; 1996. pp. 612–636. [Google Scholar]
- 6.Dennis W H, Yatvin K B. Correlation of hyperthermic sensitivity and membrane microviscosity in E. coli K1060. Int J Radiat Biol. 1981;39:265–271. doi: 10.1080/09553008114550341. [DOI] [PubMed] [Google Scholar]
- 7.Dufourc E J, Smith I C P, Jarrell H C. Role of cyclopropane moieties in the lipid properties of biological membranes: a 2H NMR structural and dynamic approach. Biochemistry. 1984;23:2300–2309. [Google Scholar]
- 8.Hansen E W. Correlation of fatty acid composition with thermal resistance of E. coli. Dan Tidsskr Farm. 1971;45:339–344. [PubMed] [Google Scholar]
- 9.Hansen E W, Skadhauge K. The influence of growth temperature on the thermal resistance of E. coli. Dan Tidsskr Farm. 1971;45:24–28. [PubMed] [Google Scholar]
- 10.Hansen N-H, Riemann H. Factors effecting the heat resistance of nonsporing organisms. J Appl Bacteriol. 1963;26:314–333. [Google Scholar]
- 11.Humpheson L, Adams M R, Anderson W A, Cole M B. Biphasic thermal inactivation kinetics in Salmonella enteritidis PT4. Appl Environ Microbiol. 1998;64:459–464. doi: 10.1128/aem.64.2.459-464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Julák J, Mára M. Effect of glucose of glycerin in cultivation media on the fatty acid composition of Listeria monocytogenes. J Hyg Epidemiol Microbiol Immunol. 1973;17:329–338. [PubMed] [Google Scholar]
- 13.Kadner R J. Cytoplasmic membrane. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B Jr, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Vol. 1. Washington, D.C: ASM Press; 1996. pp. 58–87. [Google Scholar]
- 14.Kornacki J L, Marth E H. Thermal inactivation of Salmonella senftenberg and Micrococcus freudenreichii in retentates from ultrafiltered milks. Lebensm-Wiss U-Technol. 1993;26:21–27. [Google Scholar]
- 15.Kozempel M F, Annous B A, Cook R D, Scullen O J, Whiting R C. Inactivation of microorganisms with microwaves at reduced temperatures. J Food Prot. 1998;61:582–585. doi: 10.4315/0362-028x-61.5.582. [DOI] [PubMed] [Google Scholar]
- 16.Kozempel M F, Scullen O J, Cook R D, Whiting R C. Preliminary investigation using a batch flow process to determine bacteria destruction by microwave energy at low temperatures. Lebensm-Wiss U-Technol. 1997;30:691–696. [Google Scholar]
- 17.Lechevalier M P, Moss C W. Lipids in bacterial taxonomy—a taxonomist’s view. CRC Crit Rev Microbiol. 1977;6:109–210. doi: 10.3109/10408417709102311. [DOI] [PubMed] [Google Scholar]
- 18.McGarrity J T, Armstrong J B. The effect of temperature and other growth conditions on the fatty acid composition of Escherichia coli. Can J Microbiol. 1981;27:835–840. doi: 10.1139/m81-128. [DOI] [PubMed] [Google Scholar]
- 19.O’Leary W M, Wilkinson S G. Gram-positive bacteria. In: Ratledge C, Wilkinson S G, editors. Microbial lipids. Vol. 1. London, England: Academic Press; 1988. pp. 117–201. [Google Scholar]
- 20.Rees C E D, Dodd C E R, Gibson P T, Booth I R, Stewart G S S B. The significance of bacteria in stationary phase to food microbiology. Int J Food Microbiol. 1995;28:263–275. doi: 10.1016/0168-1605(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 21.Rowan N J, Anderson J G. Effects of above-optimum growth temperature and cell morphology on thermotolerance of Listeria monocytogenes cells suspended in bovine milk. Appl Environ Microbiol. 1998;64:2065–2071. doi: 10.1128/aem.64.6.2065-2071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell N J. Mechanisms of thermal adaptation in bacteria: blueprints for survival. Trends Biochem Sci. 1984;9:108–112. [Google Scholar]
- 23.Russell N J, Evans R I, ter Steeg P F, Hellemons J, Verheul A, Abee T. Membranes as a target stress adaptation. Int J Food Microbiol. 1995;28:255–261. doi: 10.1016/0168-1605(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 24.Schweizer S. Biosynthesis of fatty acids and related compounds. In: Ratledge C, Wilkinson S G, editors. Microbial lipids. Vol. 2. London, England: Academic Press; 1989. pp. 3–50. [Google Scholar]
- 25.Silvius J R. Thermotropic phase transitions of pure lipids in model membranes and their modification by membrane proteins. In: Jost P C, Griffith O H, editors. Lipid-protein interactions. Vol. 2. New York, N.Y: John Wiley & Sons; 1982. pp. 239–281. [Google Scholar]
- 26.Sinensky M. Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci USA. 1974;71:523–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith J L, Bencivengo M M, Kalinowski S M. Absence or presence of glucose in growth medium and its effect on heat injury in Staphylococcus aureus. J Ind Microbiol. 1986;1:75–78. [Google Scholar]
- 28.Speck M L. The resistance of Micrococcus freudenreichii in laboratory high-temperature-short-time pasteurization of milk and ice cream mix. J Dairy Sci. 1947;30:975–981. [Google Scholar]
- 29.Speck M L, Lucas H L. Some observations on the high-temperature-short-time pasteurization of chocolate milk. J Dairy Sci. 1951;34:333–341. [Google Scholar]
- 30.Suutari M, Laakso S. Microbial fatty acids and thermal adaptation. Crit Rev Microbiol. 1994;20:285–328. doi: 10.3109/10408419409113560. [DOI] [PubMed] [Google Scholar]
- 31.Taylor F, Cronan J E., Jr Selection and properties of Escherichia coli mutants defective in the synthesis of cyclopropane fatty acids. J Bacteriol. 1976;125:518–523. doi: 10.1128/jb.125.2.518-523.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yatvin M B, Gipp J J, Klessig D R, Dennis W H. Hyperthermic sensitivity and growth stage in Escherichia coli. Radiat Res. 1986;106:78–88. [PubMed] [Google Scholar]
- 33.Yatvin M B, Schmitz B J. Radiation killing of E. coli K1060: role of membrane fluidity, hypothermia and local anaesthetics. Int J Radiat Biol. 1980;37:513–519. doi: 10.1080/09553008014550641. [DOI] [PubMed] [Google Scholar]