Abstract
Streptococcus thermophilus LY03 is a yogurt strain producing the same exopolysaccharide material in both milk and MRS broth. Actually, two types of exopolysaccharides are produced simultaneously. The two exopolysaccharides are identical in monomer composition (galactose and glucose in a 4:1 ratio) but differ in molecular size. Gel permeation chromatography revealed a high-molecular-mass exopolysaccharide (1.8 × 106) and a low-molecular-mass exopolysaccharide (4.1 × 105). Both exopolysaccharides can be isolated from the fermentation broth separately. The proportion in which they are produced is strongly dependent on the carbon/nitrogen ratio of the fermentation broth. A shift from a high-molecular-mass exopolysaccharide to a low-molecular-mass exopolysaccharide was observed with increasing initial complex nitrogen concentrations. All necessary biokinetic parameters to study the kinetics of S. thermophilus LY03 fermentations were obtained from a mathematical model which describes both S. thermophilus LY03 growth and exopolysaccharide production and degradation. The model is valid with various initial complex nitrogen concentrations and can be applied to simulate exopolysaccharide production in a milk medium.
Several lactic acid bacteria (LAB) are capable of forming sugar polymers or exopolysaccharides (EPS) (3). EPS produced by LAB consist of one sugar type (either glucose or fructose) such as dextran, mutan, levan, and alternan or are composed of different sugar monomers and classified as heteropolysaccharides. The latter group of EPS, containing two or more different types of monosaccharides (glucose, galactose, rhamnose, etc.) or their derivatives (for instance, N-acetyl amino sugars) can be produced by either mesophilic or thermophilic LAB (3, 4, 8). Although there have been recent investigations to unravel the structure (2, 12, 18, 19, 22, 27, 32, 33, 34, 35, 37, 40, 41), biosynthesis (11, 16), and molecular organization (13, 37, 39) of EPS, their production kinetics have not been studied as completely (9, 15, 24, 38). EPS from LAB are, however, of interest for their potential application in the food industry as texturizers, viscosifiers, and syneresis-lowering agents because of their pseudoplastic rheological behavior and water-binding capacity (3, 4, 38). Furthermore, they have two major advantages for their industrial use: first, they have a GRAS (generally regarded as safe) status and, second, they can be produced either in vitro, i.e., in a fermentor followed by the appropriate downstream processing (use as food additive), or in situ, i.e., in the food matrix during transformation of milk to yogurt (use of EPS-producing LAB starter cultures). The latter application mode is of utmost importance, since the thermophilic LAB strains Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus are used in the fermentation of milk to produce yogurt. The addition of stabilizers of plant or animal origin to natural yogurts is prohibited in most European Union countries.
S. thermophilus LY03, a strain isolated from an industrial yogurt starter culture, produces in milk enriched with yeast extract and peptone an EPS composed of the neutral sugars glucose and galactose in a 1:4 ratio (9). It was shown that EPS production strongly depends on the physical culture conditions used, i.e., temperature, pH, and oxygen tension (9), and on the carbon/nitrogen ratio applied (9). It was further shown that EPS production by S. thermophilus LY03 displays primary metabolite kinetics (9). This implies that it will be possible to improve EPS yields through enhancement of microbial growth and cell yield.
We describe here the influence of carbon/nitrogen ratios on EPS production by S. thermophilus LY03 in a customized MRS medium at a controlled optimal temperature and pH. It is shown that in MRS broth the same EPS is produced as in enriched milk medium. It is further shown that S. thermophilus LY03 produces a high-molecular-mass and a low-molecular-mass EPS, the proportion of which is dependent on the carbon/nitrogen ratio of the fermentation medium. A model is presented describing both S. thermophilus LY03 growth and EPS production. The model is valid with various initial complex nitrogen concentrations and can be applied to simulate EPS production in a milk medium.
MATERIALS AND METHODS
Microorganisms and media.
S. thermophilus LY03, a strain isolated from an English industrial yogurt starter culture (9) was used for all experiments. This strain was stored at −80°C in MRS broth (Oxoid, Basingstoke, England) containing 25% (vol/vol) glycerol. Before experimental use, the bacteria were propagated twice in MRS broth at 42°C for 12 h. A customized MRS broth was used as basic production medium; it contained (in grams · liter−1): lactose, 75; Lab-Lemco (Oxoid), 8; K2HPO4, 2; sodium acetate, 5; triammonium citrate, 2; MgSO4 · 7H2O, 0.2; MnSO4 · H2O, 0.038; and Tween 80 (1 ml · liter−1). Peptone and yeast extract (further referred to as initial complex nitrogen source) were used in a 2.5:1.0 ratio at concentrations varying from 14 to 70 g · liter−1, depending on the experiment.
Inoculum preparation.
The fermentor inoculum was always prepared in two steps. First, a test tube containing 10 ml of customized MRS broth, which was similar to the production medium used in the fermentation experiment afterwards, was inoculated with 100 μl of a freshly prepared S. thermophilus LY03 culture. After 12 h of incubation at 42°C, it was used to inoculate an Erlenmeyer flask containing 100 ml of production medium. After 12 h of growth at 42°C in an unshaken Erlenmeyer flask, this second preculture was used to inoculate the fermentor.
Fermentation runs.
All fermentations were carried out in 15 liters (working volume, 10 liters), with computer-controlled (MicroMFCS for Windows NT software) and in situ sterilizable laboratory fermentors (Biostat C; B. Braun Biotech International, Melsungen, Germany). Sterilization was performed at 121°C for 20 min; lactose was autoclaved separately (20 min at 121°C) and aseptically pumped into the fermentor. The fermentors were inoculated with 1% (vol/vol) of a preculture, which was prepared as described above. The pH was controlled at a fixed value of 6.2 by automatic addition of 10 N NaOH. Temperature was kept constant at 42°C. Agitation was performed at 100 rpm to keep the fermentation broth homogeneous. Production media with different yeast extract, peptone, and lactose concentrations were used to study the influence of the carbon/nitrogen ratio on S. thermophilus LY03 growth and the production of EPS.
Sampling and off-line analysis.
Samples were aseptically withdrawn from the fermentor to determine cell number (in CFU), cell dry mass (CDM), EPS content (polymer dry mass [PDM]), lactic acid concentration (LA), galactose concentration (Gal), and residual lactose concentration (S) as described elsewhere (9); pH and base supply were monitored on-line.
EPS were isolated as described previously (9), except that distinction was made between high-molecular-mass and low-molecular-mass EPS. Therefore, the first EPS precipitation with an isovolume of acetone and recovery of the floating fraction by spinning (further referred to as high-molecular-mass EPS fraction as experimentally determined by gel permeation chromatography [see below]) was followed by centrifugation (25,000 × g, 30 min, 4°C) of the remaining liquid to collect the suspended polysaccharide material (the so-called low-molecular-mass EPS fraction as experimentally determined by gel permeation chromatography [see below]). Both fractions were dried overnight at 42°C and weighed separately. Monomer analysis was performed as described previously (9).
Molecular mass estimation.
The molecular masses of the floating (the so-called high-molecular-mass EPS fraction) and the pelleted (the so-called low-molecular-mass EPS fraction) EPS fractions were estimated by gel permeation chromatography (Sephacryl S-500 column; Pharmacia, Uppsala, Sweden). A dextran standard series (molecular masses of 2.7 × 105, 4.1 × 105, 6.7 × 105, 7.5 × 105, 1.0 × 106, and 1.8 × 106) was used. Ammonium hydrogen carbonate (0.05 M) at a flow rate of 2 ml · min−1 was used as an elution buffer. Samples were collected in 8.5-ml fractions. All collected fractions were tested for their EPS content by using the anthrone spectrophotometric method (36).
Computer modelling and parameter calculation.
All model simulations were performed on an IBM-compatible Pentium PC. The model equations were integrated by Euler integration by using Microsoft Excel version 7.0. All modelled parameters were calculated by using the least-squares method, for which the difference between modelled and experimental values is reduced to a minimum.
RESULTS
Production and characterization of EPS produced by S. thermophilus LY03 in MRS broth.
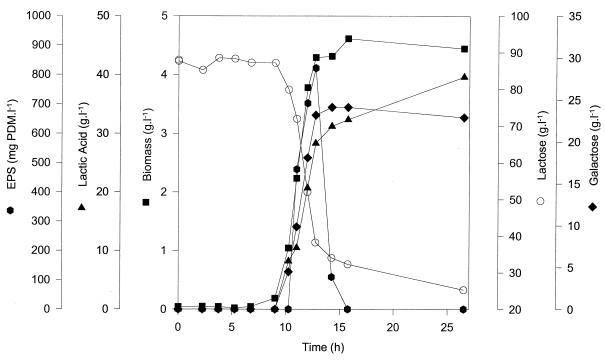
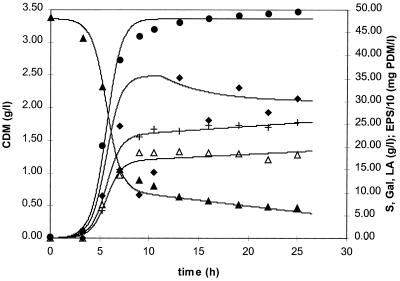
Screening for EPS-producing strains with different media and carbon sources showed that (enriched) milk was the only medium applicable for EPS production by S. thermophilus under nonoptimized conditions (9). S. thermophilus LY03 produces a heteropolysaccharide composed of galactose and glucose (4:1 ratio). Under optimal physical (pH, temperature, and oxygen tension) and chemical (carbon/nitrogen ratio) environmental conditions, maximal yields of approximately 550 mg of PDM · liter−1 were obtained in enriched milk (9). Applying those optimal physical and chemical conditions, EPS production could also be observed in MRS broth (containing either 75 or 100 g of initial lactose per liter and 28 g of initial complex nitrogen source per liter consisting of 20 g of peptone and 8 g of yeast extract per liter), even in higher concentrations (922 and 822 mg of PDM per liter, respectively, for biomass concentrations of 3.49 and 4.29 g of CDM per liter, respectively) than the ones observed in the “classical” milk production medium (9). The fermentation carried out in customized MRS broth with an initial lactose concentration of 100 g · liter−1 and an initial complex nitrogen concentration of 28 g · liter−1 is represented in Fig. 1. Exponential growth (μmax = 1.50 h−1; r2 = 0.999) took place during about 4 h; a maximal biomass concentration of 4.29 g of CDM per liter was obtained. During this active growth phase, EPS biosynthesis occurred. The product yield coefficients YEPS/S and YEPS/X, calculated from the PDM and the lactose consumption or CDM, respectively, were 0.017 g of PDM · g of lactose−1 and 0.192 g of PDM · g of CDM−1, respectively. The monosaccharide composition (galactose/glucose in a ratio of 4:1) of the EPS produced remained constant during the entire biosynthesis phase. Initiation of the stationary phase coincided with an immediate decrease of the EPS level, indicating a drastic degradation during this period. However, this EPS profile represents only the course of the high-molecular-mass EPS fraction; the total EPS yield is higher (see below). Lactic acid accumulation increased during the stationary phase as a result of maintenance metabolism. However, a discrepancy can be observed between the amount of lactic acid produced (too high) and the amount of galactose secreted (too low) from the mid-exponential phase upon further fermentation. This phenomenon was also observed during milk fermentations (9) and indicates a possible conversion of galactose into lactic acid, since galactose (derived from lactose) is supposed to be secreted stoichiometrically with the uptake of lactose (20) and is not used for EPS biosynthesis (7). Indeed, after ca. 16 h of fermentation, the amount of galactose secreted in the fermentation liquor decreases slightly, while lactic acid production increases more rapidly than can be derived from the consumption of lactose. Because of the high amount of residual lactose (2.5%) at the end of the fermentation with 100 g of lactose liter−1, 75 g of lactose liter−1 was used for further fermentations (residual lactose concentration of approximately 1.0%). In the latter case the biokinetic parameters μmax, YEPS/S, and YEPS/X averaged 1.38 h−1 (r2 = 0.998), 0.019 g of PDM · g of lactose−1, and 0.264 g of PDM · g of CDM−1, respectively.
FIG. 1.
Fermentation profile of S. thermophilus LY03 for a fermentation carried out in MRS medium at a constant temperature of 42°C, a constant pH of 6.2, an initial lactose concentration of 10.0%, and an initial complex nitrogen concentration of 2.8%.
Since the initial complex nitrogen concentration remained constant, whereas the initial lactose concentration varied in the fermentations mentioned above, it is clear that the carbon/nitrogen ratio influences the EPS yield, as was also the case in a milk medium (9). To study the influence of the carbon/nitrogen ratio on S. thermophilus LY03 growth and EPS production in detail, fermentations were carried out with various initial nitrogen concentrations (peptone and yeast extract in a 2.5/1.0 ratio) and an optimal initial lactose concentration of 7.5% (Table 1). A maximal EPS yield of 1,142 mg of PDM per liter could be obtained if an initial lactose concentration of 7.5% and an initial complex nitrogen concentration of 4.2% (3.0% peptone plus 1.2% yeast extract) was applied. This value is ca. 50% higher than those reported in the literature for S. thermophilus (4). It was further remarkable that no EPS material could be harvested at an initial complex nitrogen concentration of 7.5%. However, it could be shown that in the latter case only low-molecular-mass EPS material was produced (see below).
TABLE 1.
Influence of the carbon/nitrogen ratio on growth and EPS production by S. thermophilus LY03 in MRS medium with a constant initial lactose concentration of 7.5%a
| Initial N concn (%) | X (g of CDM liter−1) | μmaxb (h−1) (r2) | EPSmaxc (mg of PDM liter−1) (sampling time [h])
|
||
|---|---|---|---|---|---|
| HMM | LMM | HMM + LMM | |||
| 1.40 | 4.09 | 1.12 (0.988) | 410 (9.75) | 207 (24.00) | 592 (12.50) |
| 2.10 | 3.93 | 1.11 (0.999) | 445 (12.50) | 454 (24.00) | 789 (15.50) |
| 2.80 | 3.49 | 1.38 (0.998) | 922 (8.00) | 340 (7.25) | 922 (8.00) |
| 4.20 | 4.32 | 1.14 (1.000) | 821 (8.50) | 766 (10.00) | 1,142 (7.00) |
| 5.60 | 4.76 | 1.14 (0.976) | 700 (12.25) | 699 (24.00) | 833 (12.25) |
| 7.00 | 4.01 | 0.81 (0.997) | 0 (NR) | 901 (18.50) | 901 (18.50) |
The initial complex nitrogen consisted of a constant peptone/yeast extract ratio of 2.5:1.0. All fermentations were carried out in a Biostat C fermentor for 24 h at a constant temperature of 42°C and a controlled pH of 6.2. X, biomass concentration; HMM, high molecular mass; LMM, low molecular mass; NR, not relevant.
μmax is the maximal specific growth rate calculated from CDM measurements (r2 is the correlation coefficient and is shown in parentheses).
EPSmax is the maximal value for PDM achieved at the sampling time.
Differentiation of high- and low-molecular-mass EPS.
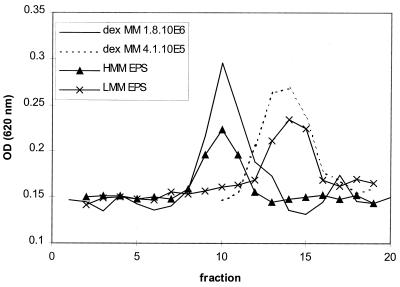
It could be shown that S. thermophilus LY03 produces two EPS fractions which could be isolated separately, i.e., as a floating fraction and as a pelleted fraction (see Materials and Methods). The monomeric compositions of the two fractions were compared and shown to be identical; i.e., galactose and glucose were in the same 4:1 ratio as was described previously for the EPS produced by this strain in a milk medium (9). Both fractions were applied to a gel permeation column and showed differences in molecular size (Fig. 2). The determination of the molecular masses of the EPS preparations has been carried out for different medium compositions (MRS medium with 7.5% lactose, 3.0% peptone, and 1.2% yeast extract; MRS medium with 7.5% lactose, 4.0% peptone, and 1.6% yeast extract; and a medium with 10% skimmed milk powder, 1.0% peptone, and 0.5% yeast extract) and at different time points of the fermentation course (i.e., the middle and end of the exponential-growth phase). The gel permeation chromatograms were comparable for all cases. The fraction that was easily isolated by spinning (the floating EPS material) possessed a molecular mass of 1.8 × 106, whereas the pelleted fraction isolated only after centrifugation possessed a molecular mass of 4.1 × 105. In addition, the proportion of these EPS fractions was dependent on the carbon/nitrogen ratio (Table 1). Indeed, a quantitative and detailed investigation of different S. thermophilus LY03 fermentations indicated that a shift from high- to low-molecular-mass EPS occurred in S. thermophilus LY03 fermentations with increasing initial complex nitrogen concentrations. However, both EPS fractions were produced simultaneously. At an initial complex nitrogen concentration of 7.5%, only low-molecular-mass EPS material was produced (Table 1). When the production course of these EPS fractions was compared during the active growth phase, it was remarkable that in all other cases the level of the low-molecular-mass fraction increased when that of the high-molecular-mass fraction decreased, indicating a possible breakdown of the high-molecular-mass EPS fraction. However, degradation of the low-molecular-mass EPS fraction also occurred. Isolation of both fractions from two different, independent fermentation experiments carried out in the same medium and under the same environmental conditions resulted in almost the same profile for high- and low-molecular-mass EPS titers. As a result, the total amount of EPS decreased slightly during the stationary phase, but a major EPS fraction always remained unchanged upon further fermentation; this fraction is referred to as EPSr.
FIG. 2.
Gel permeation chromatography of the floating (HMM EPS, high-molecular-mass EPS fraction) and the pelleted (LMM EPS, low-molecular-mass EPS fraction) EPS material isolated from a S. thermophilus LY03 fermentation. The fermentation mentioned here was carried out in MRS medium containing 7.5% lactose and 5.6% complex nitrogen at a constant temperature of 42°C and a constant pH of 6.2. The curves show the optical density (at 620 nm) of 8.5-ml fractions after determination of the reducing sugar content by the anthrone spectrophotometric method. The dextran standards with molecular masses of 4.1 × 105 and 1.8 × 106 are displayed.
Model development.
The dynamic growth equation used to describe growth of S. thermophilus LY03 is given by equation 1:
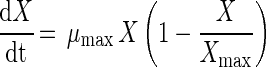 |
1 |
where X represents the CDM concentration (in grams per liter), μmax represents the maximum specific growth rate (per hour), and Xmax is the highest obtainable CDM concentration (in grams per liter). This is a commonly used equation to describe the growth of LAB (1, 21, 26, 29, 30). This logistic equation can be considered a mathematical description of the growth-associated production of an inhibitory product such as lactic acid (26). Another point of view could be that the fermentation broth contains components used as carbon and nitrogen sources by the bacteria which are more favored for growth than others, resulting in a decreased specific growth rate during the fermentation course and a limited maximum obtainable cell mass concentration (Xmax) (21).
Lactose consumption is described by the following maintenance energy model (31):
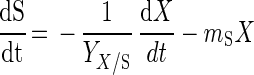 |
2 |
where S is the lactose concentration (in grams per liter), YX/S is the cell yield coefficient (in grams of CDM per gram of lactose), and mS is the maintenance coefficient (in grams of lactose per gram of CDM per hour).
S. thermophilus LY03 imports lactose via a lactose-galactose antiporter transport system and does not ferment galactose (20). This results in a galactose efflux during growth and maintenance of the bacteria, described by the following equation:
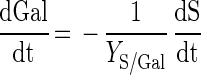 |
3 |
where Gal is the galactose concentration (in grams per liter) and YS/Gal is the yield coefficient for galactose (in grams of lactose consumed per gram of galactose excreted). Since S. thermophilus LY03 does not use galactose as a carbon source, 1 mol of lactose consumed should result in 1 mol of galactose excreted, which means that YS/Gal should be 2 g · g−1.
Since S. thermophilus LY03 is a homofermentative LAB strain, the lactic acid production can be given by equation 4
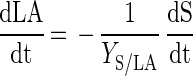 |
4 |
where LA is the lactic acid concentration (in grams per liter) and YS/LA is the yield coefficient for lactic acid (in grams of lactose consumed per gram of lactic acid produced). The latter should be 2 g · g−1. Indeed, lactose is hydrolyzed into glucose and galactose in equimolar ratios. Each mole of glucose which is then metabolized through glycolysis results in 2 mol of lactic acid. This explains why on-line monitoring of base consumption can simulate lactic acid production and hence lactose consumption.
The EPS production and degradation can be simulated by
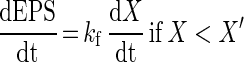 |
5 |
and
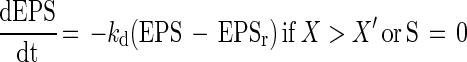 |
6 |
respectively, where EPS is the total concentration of PDM (in milligrams per liter), kf is the specific EPS formation (in milligrams of PDM per gram of CDM), kd is the EPS degradation rate (per hour), EPSr is the concentration of the total EPS fraction (in milligrams of PDM per liter) which remains unaffected in the fermentation broth upon prolonged fermentation, and X′ is the critical CDM concentration at which the EPS production ceases (in grams per liter). Equation 5 describes the production of EPS as a growth-associated product if the biomass concentration has not reached X′ yet. After this critical biomass concentration is reached, EPS degrades and the PDM level drops to a certain constant level (EPSr), since it was observed that a major EPS fraction always remained unattached in the fermentation broth. This degradation is described by equation 6 as a first-order reaction.
Simulation.
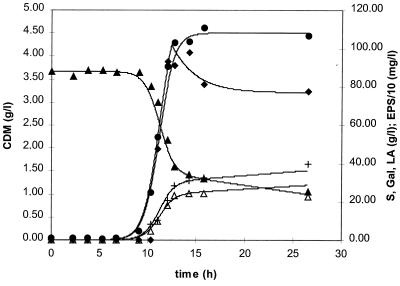
The use of the above-described mathematical model enabled the simulation of the data of the S. thermophilus LY03 fermentations as well as the estimation of relevant biokinetic parameters. Modelled data fit experimental values quite well (Fig. 3). Indeed, for all relevant biokinetic parameters (μmax, YX/S, mS, kf, and kd) and fermentation characteristics (Xmax, X′, EPSmax, and EPSr), only small differences were observed. For the maximal specific growth rate (μmax), a value of 1.28 h−1 was obtained from the model, while the value calculated from the experimental data was 1.50 h−1. YX/S, mS, kf, and kd were modelled as 0.085 g of CDM · g of lactose−1, 0.16 g of lactose · g of CDM−1 · h−1, 270 mg of PDM · g of CDM−1, and 0.40 h−1, respectively. Comparable small variances were also observed for Xmax; 4.29 g of CDM liter−1 was obtained from the experimental data and 4.50 g of CDM liter−1 was calculated from the model. For EPSmax values of 912 mg of PDM liter−1 (experimental data) and 1,018 mg of PDM liter−1 (modelled value) and for EPSr values of 776 mg of PDM liter−1 (experimental data) and 770 mg of PDM liter−1 (modelled value) were obtained. X′ was modelled as 3.80 g of CDM liter−1. All these data indicate that the proposed model is very appropriate to simulate S. thermophilus LY03 EPS fermentations in MRS broth.
FIG. 3.
Simulation of the experimental data of an S. thermophilus LY03 fermentation in MRS medium containing 10.0% lactose and 2.8% complex initial nitrogen at a constant temperature of 42°C and a constant pH of 6.2. Experimental values are indicated as follows: ●, CDM (in grams liter−1); ⧫, total PDM (in milligrams liter−1); ▴, residual lactose concentration (in grams liter−1); +, lactic acid produced (in grams liter−1); and ▵, galactose excreted (in grams liter−1). Concomitant simulated data are indicated by solid lines.
Influence of the C/N ratio on bacterial growth.
For increasing initial complex nitrogen concentrations at a constant initial lactose concentration of 7.5%, the maximal specific growth rate (ca. 1.4 h−1) did not vary substantially (Table 2). For the cell maintenance requirements, a constant value of 0.16 g of lactose · g of CDM−1 · h−1 was observed for all fermentations. In contrast, the cell yield coefficient YX/S increased with higher initial complex nitrogen concentrations (Table 2). For all fermentations carried out, a constant residual lactose concentration of approximately 15 g · liter−1 was observed. An initial complex nitrogen concentration of approximately 5.0% can be seen as an optimal value for bacterial growth. The highest obtainable CDM concentration (Xmax) parallels the YX/S changes (Table 2). The Xmax, μmax, and YX/S values seem to have an upper limit. For the fermentation carried out with 7.0% of the initial complex nitrogen source, YX/S dropped to a value of 0.7 g of CDM · g of lactose−1; also, Xmax and μmax were lower in this case.
TABLE 2.
Modelled values of the biokinetic parameters for growth and EPS production of S. thermophilus LY03a
| Initial N concn (%) | μmax (h−1) | Xmax (g of CDM liter−1) | YX/S (g of CDM g of S−1) | mS (g of S g of CDM−1 h−1) | kf (mg of PDM g of CDM−1) | EPSmax (mg of PDM liter−1) | kd (h−1) | EPSr (mg of PDM liter−1) | X′ (g of CDM liter−1) |
|---|---|---|---|---|---|---|---|---|---|
| 1.40 | 1.4 | 3.9 | 0.075 | 0.16 | 143 | 543 | 0.05b | 400 | 3.8 |
| 2.10 | 1.2 | 4.1 | 0.075 | 0.16 | 190 | 769 | 0.10b | 500 | 4.0 |
| 2.80 | 1.4 | 4.0 | 0.080 | 0.16 | 260 | 972 | 0.60 | 890 | 3.7 |
| 4.20 | 1.4 | 4.5 | 0.095 | 0.16 | 320 | 1,209 | 0.40 | 900 | 3.8 |
| 5.60 | 1.6 | 4.9 | 0.095 | 0.16 | 195 | 906 | 0.60 | 700 | 4.6 |
| 7.00 | 1.5 | 4.0 | 0.072 | 0.16 | 225 | 909 | 0.50 | 824 | 4.0 |
The μmax (maximal specific growth rate), Xmax (maximal attainable biomass concentration), YX/S (cell yield coefficient based on substrate consumption), mS (maintenance coefficient based on substrate consumption), X′ (critical biomass concentration), kf (specific EPS formation), EPSmax (maximal EPS yield), kd (EPS degradation rate), and EPSr (residual EPS concentration) are given. These values were obtained from simulations of S. thermophilus LY03 fermentations carried out at a constant temperature of 42°C and a constant pH of 6.2 in MRS medium with various initial complex nitrogen source concentrations (from 1.4 to 7.0% with a constant peptone/yeast extract ratio of 2.5:1.0) and a constant initial lactose concentration (7.5%).
Based on a limited number of data points.
For the yield coefficients YS/Gal and YS/LA, constant values of 2.2 g of lactose · g of galactose−1 and 1.8 g of lactose · g of lactic acid−1, respectively, were applied in the model. These values, different from the theoretical ones (cf. model development), seemed to be appropriate for modelling galactose excretion and lactic acid formation in all experiments. They confirm the hypothesis of conversion of galactose into lactic acid by S. thermophilus LY03.
Influence of the C/N ratio on EPS production.
The influence of the C/N ratio on EPS production was studied by estimation of the relevant parameters such as kf, EPSmax, EPSr, kd, and X′. The specific EPS formation constant (kf) is significantly influenced by increasing amounts of initial complex nitrogen. This value increased to a maximal value of 320 mg of PDM · g of CDM−1 at an initial complex nitrogen concentration of 4.2% and then declined for fermentations with higher initial complex nitrogen concentrations (Table 2). The same tendency was observed for the maximally observed total EPS yield (EPSmax) and the levels of EPSr (Table 2). This result underlines the importance of the carbon/nitrogen ratio for EPS production. For X′, the cell concentration at which the EPS production ceases, no significant variations were observed for different initial complex nitrogen concentrations. This is not surprising, since bacterial growth did not display significant changes at the end of the exponential-growth phase in all cases, and hence the EPS production always reached a maximum at almost the same moment in time because of the constant initial lactose concentration (7.5%) determining growth. This, together with the low variation between the Xmax values, resulted in constant X′ values. For kd, again no major changes were observed (Table 2). A value of 0.5 h−1 resulted in the best fits.
Simulation of S. thermophilus LY03 milk fermentations.
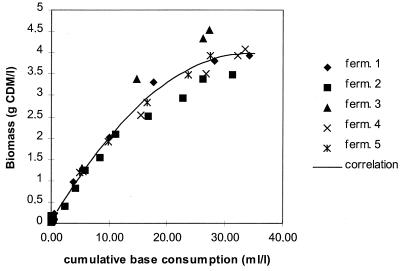
To use the above-developed model to simulate EPS production (which is growth associated) during milk fermentations, a correlation was first established between cell synthesis (biomass formation in grams of CDM per liter) and cumulative base consumption (correlated with lactic acid production and hence a measure of cell growth). Indeed, it is difficult to carry out CDM determinations in complex media such as milk; usually, the cell number or the cumulative base consumption is measured to monitor growth (9). To establish the correlation between biomass formation and cumulative base consumption, the experimental data of all the above-mentioned MRS fermentations were used. The results are represented in Fig. 4. A second-order correlation could be established (to determine the best-fit line). By using this second-order correlation, cell growth in milk can be simulated and hence also the course of EPS production, since the latter is linked to the former (see equation 5). An example of an S. thermophilus LY03 fermentation in enriched milk containing 10.0% skim milk powder enriched with 1.0% peptone and 0.5% yeast extract at a controlled temperature of 42°C and a pH of 5.5 is given in Fig. 5. Because the simulation of EPS biosynthesis represents the total EPS material (both the high- and the low-molecular-mass EPS fractions), a deviation can occur between modelled and experimental data, since only the high-molecular-mass fractions could be isolated from the complex milk medium experimentally. However, the double-peak fermentation profile reported earlier (9) is not apparent, since a low yield of high-molecular-mass EPS seems to be compensated for by a high yield of low-molecular-mass EPS. The maximal amount of EPS was modelled as 356 mg of PDM liter−1 (the sum of low- and high-molecular-mass EPS fractions), which perfectly coincides with the experimental value of 352 mg of PDM liter−1 (high-molecular-mass EPS) (9). This indicates that at this maximum for EPS production in milk, the fraction of low-molecular-mass EPS is almost zero. The average maximal specific growth rate (modelled value = 1.30 h−1; experimental value = 1.60 h−1) is comparable to the results in MRS broth (1.40 h−1). The cell yield coefficient YX/S (modelled value = 0.090 g of CDM · g of lactose−1) and the maintenance coefficient mS (modelled value = 0.08 g of lactose · g of CDM−1 · h−1) deviate from the values of the MRS fermentations (0.075 g of CDM · g of lactose−1 and 0.016 g of lactose · g of CDM−1 · h−1, respectively). These values indicate a lower energy demand for maintenance metabolism in milk medium and hence a more appropriate nitrogen source for cell synthesis. A lower EPS yield may indicate the presence of too little nitrogen in milk to stimulate EPS production. The modelled values of EPS degradation rate and residual EPS fractions averaged 0.21 h−1 and 300 mg of PDM liter−1, respectively. These values are lower than those obtained in MRS broth under comparable conditions (cf. Table 2).
FIG. 4.
Correlation between the cumulative base consumption and the biomass formation for fermentations carried out with S. thermophilus LY03 in different MRS media at a constant temperature of 42°C and a constant pH of 6.2.
FIG. 5.
Simulation of a S. thermophilus LY03 fermentation at a constant temperature of 42°C and a constant pH of 5.5 in enriched milk medium (10.0% skim milk powder, 1.0% peptone, 0.5% yeast extract). The simulations for cell growth, total EPS production, residual lactose concentration, lactic acid production, and galactose secretion are represented by solid lines. The concomitant experimental data are indicated as follows: ●, CDM (in grams liter−1) simulated according to the cumulative base consumption (cf. correlation in Fig. 4); ⧫, PDM (in milligrams liter−1); ▴, residual lactose content (in grams liter−1); +, lactic acid (in grams liter−1); ▵, galactose (in grams liter−1).
DISCUSSION
S. thermophilus LY03 is a yogurt strain producing an EPS of the heteropolysaccharide type consisting of galactose and glucose in a 4:1 ratio (9). From gel permeation chromatography data it can be concluded that S. thermophilus LY03 produces two types of EPS simultaneously: a high-molecular-mass EPS (molecular mass of 1.8 × 106) and a low-molecular-mass EPS (molecular mass of 4.1 × 105). Both EPS can be isolated from the fermentation broth separately. The proportion in which they are produced is strongly dependent on the carbon/nitrogen ratio of the fermentation broth. This was observed after detailed analysis of the fermentation profile and quantifying the separate EPS fractions through PDM determinations. Analysis of the EPS content from fermentation samples with the commonly used spectrophotometric methods does not differentiate between EPS fractions with respect to monomer composition or molecular size. Also Marshall et al. (25) found that Lactococcus lactis subsp. cremoris LC 330 produces two EPS simultaneously: an EPS with high molecular mass (>1.0 × 106) and one with low molecular mass (1.0 × 104), both with a different monomer composition. In contrast to the high-molecular-mass EPS, the production of the low-molecular-mass EPS was not influenced by the fermentation conditions (nitrogen limitation). Analysis of the EPS produced by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772, cultivated in a chemostat, also results in high-molecular-mass (1.7 × 106) and low-molecular-mass (4.0 × 104) EPS fractions, the proportion of which is dependent on the nature of the carbon source without any alteration of the monomer composition (17). Finally, Cerning (3) concluded that S. thermophilus strains produce two different EPS simultaneously, with molecular masses of 2.0 × 106 and 3.5 × 104. Because of the identical monomer compositions, she postulated that the latter one is a degradation product of the former.
The same production kinetics as those already observed in a milk medium (9) were again established in the customized MRS medium. Again, EPS was produced associated with growth, reaching a maximum at the beginning of the stationary phase. However, it could now be shown that the earlier described local high-molecular-mass EPS minimum, most often observed before the maximum occurs, was compensated for with an increase of low-molecular-mass EPS. Biosynthesis of the latter EPS fraction is most probably induced by a varying carbon/nitrogen ratio. Considering the totals of both fractions, the EPS production curve increased almost parallel with growth. It dropped to a constant level after it reached a maximum at the end of the exponential-growth phase.
This is the first study presenting a mathematical model to describe the growth and EPS production kinetics of LAB, particularly S. thermophilus. The model was developed by using some well-known metabolic LAB characteristics, namely, (i) increase of the biomass according to a logistic equation, (ii) non-growth-associated substrate consumption (maintenance metabolism), and (iii) homofermentative production of lactic acid (1, 21, 26, 29, 30). EPS production was described by using primary metabolite kinetics (9). After a critical biomass concentration is reached, EPS degradation takes place. This could be described by a first-order reaction. This model allowed the description of S. thermophilus LY03 growth, lactose consumption, galactose excretion, lactic acid formation, and EPS production in all of the cases tested. All relevant biokinetic parameters could be estimated, and it was shown that the carbon/nitrogen ratio has a major effect on EPS production.
Considering the constant maximum specific growth rates, the constant cell maintenance value, and the constant residual lactose concentrations for fermentations carried out with increasing initial complex nitrogen concentrations and a constant initial lactose concentration, it can be concluded that increasing the initial complex nitrogen concentrations had little effect on the bacterial growth. Nevertheless, these maximum specific growth rates are quite high, indicating that milk is a good growth medium and can be linked to the rather low mS values. It has been shown before with LAB that growth rate and maintenance metabolism are indeed inversely correlated (28). Limited Xmax, μmax, and YX/S values can be explained by the fact that some growth-limiting factor must begin to play an important role when the initial complex nitrogen concentration is increased. This factor can be seen as the formation of some growth-inhibiting component (other than lactic acid) or the lack of a necessary medium component other than nitrogen and lactose. Even sterilization of such high nitrogen concentrations can be responsible for the formation of some “toxic” components and hence growth-limiting conditions. The one exception with the highest nitrogen concentration can be explained by the same “growth-inhibiting” conditions. Furthermore, one can conclude that cell maintenance requirements (based on lactose) are not limited by the high lactic acid concentrations. The data also indicate that less energy (substrate consumption) is needed to obtain the same biomass concentration with an increasing initial complex nitrogen concentration. In addition, although S. thermophilus is a homofermentative bacterium, both yield coefficients YS/Gal and YS/LA differ from their theoretical values (1.8 and 2.2 g · g−1, respectively, instead of 2.0 g · g−1 for both). The only explanation for this can be found in the fact that small amounts of galactose are used as an energy source and are thus converted into lactic acid. We do not know at present the factor responsible for this phenomenon, although induction of galactose metabolism as a result of a variation of the carbon/nitrogen ratio during the growth cycle might be one explanation.
On the other hand, the initial complex nitrogen concentration drastically influences EPS production kinetics. The C/N ratio clearly influences specific EPS production; the kf doubled when higher initial complex nitrogen concentrations were applied. However, the increase of kf and the maximal EPS yield (EPSmax) were limited. This limitation of maximal EPS yield was also observed in milk medium (9). It has indeed been shown that some vitamins may affect the production of EPS relative to cell growth (14). The increase of the specific EPS formation (kf) can partly be explained by the increasing biomass yield coefficient YX/S, since less energy is required to achieve the same biomass concentration so that more energy becomes available for EPS biosynthesis. This is in contrast to some literature data stating that EPS production displays secondary metabolite kinetics (3, 4). Finally, a shift from high-molecular-mass EPS towards low-molecular-mass EPS was observed for increasing initial complex nitrogen concentrations, a phenomenon which cannot be explained by the current knowledge of EPS production mechanisms.
EPS degradation often takes place upon prolonged incubation; this may be due to glycohydrolase activity (5, 6, 12, 23). However, a large EPS fraction remained unaffected in the fermentation broth; this fraction, denoted by EPSr in the model, paralleled perfectly the maximum EPS yield and the specific EPS production. It seems that degradation takes place at a constant rate, confirming the hypothesis that EPS degradation would only depend on physical factors such as temperature and pH (9), thus indicating the presence of an EPS-degrading enzyme.
If all of the knowledge of EPS production kinetics are considered, the above-described mathematical model could be applied to simulate S. thermophilus LY03 growth and EPS production behavior in milk fermentations. This is important when applications of EPS-producing LAB starter strains in fermented milk production are being considered. Extending the model, taking into account all possible influences on growth and EPS production, will further contribute to the improvement of EPS production by S. thermophilus strains.
The influence of C/N ratios on EPS production by S. thermophilus LY03 in a customized MRS medium at a controlled optimal temperature and pH were described here. It is shown that in MRS broth the same EPS is produced as in enriched milk medium. It is further shown that S. thermophilus LY03 produces a high-molecular-mass and a low-molecular-mass EPS fraction, of which the proportion is dependent on the carbon/nitrogen ratio of the fermentation medium. A model is presented describing both S. thermophilus LY03 growth and EPS production. The model is valid with various initial complex nitrogen concentrations and can be applied to simulate EPS production in a milk medium.
ACKNOWLEDGMENTS
The research presented here was financially supported by the Institute Danone by means of a Navorsingskrediet voor Fundamenteel Voedingsonderzoek. We also acknowledge financial support from the Research Council of the Vrije Universiteit Brussel, the Flemish Institute for Encouragement of Scientific and Technological Research in Industry, the European Commission, and the Fund for Scientific Research–Flanders.
REFERENCES
- 1.Berkman T, Bozoglu T F, Özilgen M. Mixed culture growth kinetics of Streptococcus thermophilus and Lactobacillus bulgaricus. Enzyme Microb Technol. 1990;12:138–140. [Google Scholar]
- 2.Bubb W A, Urashima T, Fujiwara R, Shinnai T, Ariga H. Structural characterisation of the exocellular polysaccharide produced by Streptococcus thermophilus OR 901. Carbohydr Res. 1997;301:41–50. doi: 10.1016/s0008-6215(97)00083-9. [DOI] [PubMed] [Google Scholar]
- 3.Cerning J. Exocellular polysaccharides produced by lactic acid bacteria. FEMS Microbiol Rev. 1990;87:113–130. doi: 10.1111/j.1574-6968.1990.tb04883.x. [DOI] [PubMed] [Google Scholar]
- 4.Cerning J. Production of exopolysaccharides by lactic acid bacteria and dairy propionibacteria. Lait. 1995;75:463–472. [Google Scholar]
- 5.Cerning J, Bouillanne C, Desmazeaud M J. Exocellular polysaccharide production by Streptococcus thermophilus. Biotechnol Lett. 1988;4:255–260. [Google Scholar]
- 6.Cerning J, Bouillanne C, Landon M, Desmazeaud M J. Comparison of exocellular polysaccharide production by thermophilic lactic acid bacteria. Sci Aliments. 1990;10:443–451. [Google Scholar]
- 7.De Vos W M, Vaughan E E. Genetics of lactose utilization in lactic acid bacteria. FEMS Microbiol Rev. 1994;15:216–237. doi: 10.1111/j.1574-6976.1994.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 8.De Vuyst L, Degeest B. Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol Rev. 1999;23:153–177. doi: 10.1111/j.1574-6976.1999.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 9.De Vuyst L, Vanderveken F, Van de Ven S, Degeest B. Production by and isolation of exopolysaccharides from Streptococcus thermophilus grown in a milk medium and evidence for their growth-associated biosynthesis. J Appl Microbiol. 1998;84:1059–1068. doi: 10.1046/j.1365-2672.1998.00445.x. [DOI] [PubMed] [Google Scholar]
- 10.Doco T, Wieruszeski J-M, Fournet B, Carcano D, Ramos P, Loones A. Structure of an exocellular polysaccharide produced by Streptococcus thermophilus. Carbohydr Res. 1990;198:313–321. doi: 10.1016/0008-6215(90)84301-a. [DOI] [PubMed] [Google Scholar]
- 11.Escalante A, Wacher-Rodarte C, García-Garibay M, Farrés A. Enzymes involved in carbohydrate metabolism and their role on exopolysaccharide production in Streptococcus thermophilus. J Appl Microbiol. 1998;84:108–114. doi: 10.1046/j.1365-2672.1997.00330.x. [DOI] [PubMed] [Google Scholar]
- 12.Gancel F, Novel G. Exopolysaccharide production by Streptococcus salivarius ssp. thermophilus cultures. 1. Conditions of production. J Dairy Sci. 1994;77:685–688. [Google Scholar]
- 13.Griffin A M, Morris V J, Gasson M J. The cpsABCDE genes involved in polysaccharide production in Streptococcus salivarius ssp. thermophilus strain NCBF 2393. Gene. 1996;183:23–27. doi: 10.1016/s0378-1119(96)00405-2. [DOI] [PubMed] [Google Scholar]
- 14.Grobben G J, Chin-Joe I, Kitzen V A, Boels I C, Boer F, Sikkema J, Smith M R, de Bont J A M. Enhancement of exopolysaccharide production by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772 with a simplified defined medium. Appl Environ Microbiol. 1998;64:1333–1337. doi: 10.1128/aem.64.4.1333-1337.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grobben G J, Sikkema J, Smith M R, de Bont J A M. Production of extracellular polysaccharides by Lactobacillus delbrueckii ssp. bulgaricus NCFB 2772 grown in a chemically defined medium. J Appl Bacteriol. 1995;79:103–107. [Google Scholar]
- 16.Grobben G J, Smith M R, Sikkema J, De Bont J A M. Influence of fructose and glucose on the production of exopolysaccharides and the activities of enzymes involved in the sugar metabolism and the synthesis of sugar nucleotides in Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772. Appl Microbiol Biotechnol. 1996;46:279–284. [Google Scholar]
- 17.Grobben G J, van Casteren W H M, Schols H A, Oosterveld A, Sala G, Smith M R, Sikkema J, de Bont J A M. Analysis of the exopolysaccharides produced by Lactobacillus delbrueckii subsp. bulgaricus NCFB 2772 grown in continuous culture on glucose and fructose. Appl Microbiol Biotechnol. 1997;48:516–521. [Google Scholar]
- 18.Gruter M, Leeflang B R, Kuiper J, Kamerling J P, Vliegenthart J F G. Structure of the exopolysaccharide produced by Lactococcus lactis subspecies cremoris H414 grown in a defined medium or skimmed milk. Carbohydr Res. 1992;231:273–291. doi: 10.1016/0008-6215(92)84025-n. [DOI] [PubMed] [Google Scholar]
- 19.Gruter M, Leeflang B R, Kuiper J, Kamerling J P, Vliegenthart J F G. Structural characterisation of the exopolysaccharide produced by Lactobacillus delbrückii subspecies bulgaricus rr grown in skimmed milk. Carbohydr Res. 1993;239:209–226. doi: 10.1016/0008-6215(93)84216-s. [DOI] [PubMed] [Google Scholar]
- 20.Hutkins R W, Ponne C. Lactose uptake driven by galactose efflux in Streptococcus thermophilus: evidence for a galactose-lactose antiporter. Appl Environ Microbiol. 1991;57:941–944. doi: 10.1128/aem.57.4.941-944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lejeune R, Callewaert R, Crabbé K, De Vuyst L. Modelling the growth and bacteriocin production by Lactobacillus amylovorus DCE 471 in batch cultivation. J Appl Microbiol. 1998;84:159–168. [Google Scholar]
- 22.Lemoine J, Chirat F, Wieruszeski J-M, Strecker G, Favre N, Neeser J-R. Structural characterization of the exocellular polysaccharides produced by Streptococcus thermophilus SFi39 and SFi12. Appl Environ Microbiol. 1997;63:3512–3518. doi: 10.1128/aem.63.9.3512-3518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macura D, Townsley P M. Scandinavian ropy milk—identification and characterization of endogenous ropy lactic streptococci and their extracellular excretion. J Dairy Sci. 1984;67:735–744. [Google Scholar]
- 24.Manca De Nadra M C, Strasser De Saad A M, Pesce De Ruiz Holgado A A, Oliver G. Extracellular polysaccharide production by Lactobacillus bulgaricus CRL 420. Milchwissenschaft. 1985;40:409–411. [Google Scholar]
- 25.Marshall V M, Cowie E N, Moreton R S. Analysis and production of two exopolysaccharides from Lactococcus lactis subsp. cremoris LC330. J Dairy Res. 1995;62:621–628. [Google Scholar]
- 26.Mercier P, Yerushalmi L, Rouleau D, Dochain D. Kinetics of lactic acid fermentation on glucose and corn by Lactobacillus amylophilus. J Chem Technol Biotechnol. 1992;55:111–121. [Google Scholar]
- 27.Nakajima H, Hirota T, Toba T, Itoh T, Adachi S. Structure of the extracellular polysaccharide from slime-forming Lactococcus lactis subsp. cremoris SBT 0495. Carbohydr Res. 1992;224:245–253. doi: 10.1016/0008-6215(92)84110-e. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen J, Nikolajsen K, Villadsen J. Structured modelling of a microbial system. II. Experimental verification of a structured lactic acid fermentation model. Biotechnol Bioeng. 1991;38:11–23. doi: 10.1002/bit.260380103. [DOI] [PubMed] [Google Scholar]
- 29.Parente E, Ricciardi A. Influence of pH on the production of enterocin 1146 during batch fermentation. Lett Appl Microbiol. 1994;19:12–15. doi: 10.1111/j.1472-765x.1994.tb00891.x. [DOI] [PubMed] [Google Scholar]
- 30.Parente E, Ricciardi A, Addario G. Influence of pH on growth and bacteriocin production by Lactococcus lactis subsp. lactis 140NWC during batch fermentation. Appl Microbiol Biotechnol. 1994;41:388–394. [Google Scholar]
- 31.Pirt S J. The maintenance energy of bacteria in growing cultures. Proc R Soc Lond Ser B. 1965;163:224–231. doi: 10.1098/rspb.1965.0069. [DOI] [PubMed] [Google Scholar]
- 32.Robijn G W, van den Berg D J C, Haas H, Kamerling J P, Vliegenthart J F G. Determination of the structure of the exopolysaccharide produced by Lactobacillus sake 0-1. Carbohydr Res. 1995;276:117–136. doi: 10.1016/0008-6215(95)00172-p. [DOI] [PubMed] [Google Scholar]
- 33.Robijn G W, Thomas J R, Haas H, van den Berg D J C, Kamerling J P, Vliegenthart J F G. The structure of the exopolysaccharide produced by Lactobacillus helveticus 766. Carbohydr Res. 1995;276:137–154. doi: 10.1016/0008-6215(95)00171-o. [DOI] [PubMed] [Google Scholar]
- 34.Robijn G W, Wienk H L J, van den Berg D J C, Haas H, Kamerling J P, Vliegenthart J F G. Structural studies of the exopolysaccharide produced by Lactobacillus paracasei 34-1. Carbohydr Res. 1996;285:129–139. doi: 10.1016/s0008-6215(96)90178-0. [DOI] [PubMed] [Google Scholar]
- 35.Robijn G W, Thomas J R, Haas H, van den Berg D J C, Kamerling J P, Vliegenthart J F G. The structure of the exopolysaccharide produced by Lactobacillus helveticus 766. Carbohydr Res. 1995;276:137–154. doi: 10.1016/0008-6215(95)00171-o. [DOI] [PubMed] [Google Scholar]
- 36.Scott T A, Melvin E H. Determination of dextran with anthrone. Anal Chem. 1953;25:1656–1661. [Google Scholar]
- 37.Stingele F, Neeser J-R, Mollet B. Identification and characterization of the eps (exopolysaccharide) gene cluster from Streptococcus thermophilus Sfi6. J Bacteriol. 1996;178:1680–1690. doi: 10.1128/jb.178.6.1680-1690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Berg D J C, Robijn G W, Janssen A C, Giuseppin M L F, Vreeker R, Kamerling J P, Vliegenthart J F G, Ledeboer A M, Verrips T. Production of a novel extracellular polysaccharide by Lactobacillus sake 0-1 and characterization of the polysaccharide. Appl Environ Microbiol. 1995;61:2840–2844. doi: 10.1128/aem.61.8.2840-2844.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Kranenburg R, Marugg J D, Van Swam I I, Willem N J, De Vos W M. Molecular characterization of the plasmid-encoded eps gene cluster essential for exopolysaccharide biosynthesis in Lactococcus lactis. Mol Microbiol. 1997;24:387–397. doi: 10.1046/j.1365-2958.1997.3521720.x. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto Y, Murosaki S, Yamauchi R, Kato K, Sone Y. Structural study on an exocellular polysaccharide produced by Lactobacillus helveticus TY1-2. Carbohydr Res. 1994;261:67–78. doi: 10.1016/0008-6215(94)80006-5. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto Y, Nunome T, Yamauchi R, Kato K, Sone Y. Structure of an exocellular polysaccharide of Lactobacillus helveticus TN-4, a spontaneous mutant strain of Lactobacillus helveticus TY1-2. Carbohydr Res. 1995;275:319–332. doi: 10.1016/0008-6215(95)00077-7. [DOI] [PubMed] [Google Scholar]