Abstract
Despite their reported therapeutic properties, not much is known about the immunomodulatory activity of essential oils present in Artemisia species. We isolated essential oils from the flowers and leaves of five Artemisia species: A. tridentata, A. ludoviciana, A. dracunculus, A. frigida, and A. cana. The chemical composition of the Artemisia essential oil samples had similarities and differences as compared to those previously reported in the literature. The main components of essential oils obtained from A. tridentata, A. ludoviciana, A. frigida, and A. cana were camphor (23.0–51.3%), 1,8-cineole (5.7–30.0%), camphene (1.6–7.7%), borneol (2.3–14.6%), artemisiole (1.2–7.5%), terpinen-4-ol (2.0–6.9%), α-pinene (0.8–3.9%), and santolinatriene (0.7–3.5%). Essential oils from A. dracunculus were enriched in methyl chavicol (38.8–42.9%), methyl eugenol (26.1–26.4%), terpinolene (5.5–8.8%), (E/Z)-β-ocimene (7.3–16.0%), β-phellandrene (1.3–2.2%), p-cymen-8-ol (0.9–2.3%), and xanthoxylin (1.2–2.2%). A comparison across species also demonstrated that some compounds were present in only one Artemisia species. Although Artemisia essential oils were weak activators of human neutrophils, they were relatively more potent in inhibiting subsequent neutrophil Ca2+ mobilization with N-formyl peptide receptor 1 (FPR1) agonist fMLF- and FPR2 agonist WKYMVM, with the most potent being essential oils from A. dracunculus. Further analysis of unique compounds found in A. dracunculus showed that farnesene, a compound with a similar hydrocarbon structure as lipoxin A4, inhibited Ca2+ influx induced in human neutrophils by fMLF (IC50 = 1.2 μM), WKYMVM (IC50 = 1.4 μM), or interleukin 8 (IC50 = 2.6 μM). Pretreatment with A. dracunculus essential oils and farnesene also inhibited human neutrophil chemotaxis induced by fMLF, suggesting these treatments down-regulated human neutrophil responses to inflammatory chemoattractants. Thus, our studies have identified farnesene as a potential anti-inflammatory modulator of human neutrophils.
Keywords: anti-inflammatory, Artemisia, calcium flux, chemotaxis, essential oils, farnesene, monoterpene, neutrophil
1. Introduction
The genus Artemisia is one of the most broadly distributed groups in the Asteraceae family [1]. Within this family, Artemisia is in the tribe Anthemideae and comprises about 530 species of plants that are found on all continents except for Antarctica. These plants are found mainly in the Northern Hemisphere, with only 25 species in the Southern Hemisphere [2]. According to the latest taxonomic revisions, there are 50 Artemisia species in North America, which belong to Artemisia (Miller) Less, Absinthium (Miller) Less, Dracunculus Besser, and Tridentatae (Rydb.) subgenera [3]. Artemisia is most prevalent in the western half of the United States, with 11 species in or near the Rocky Mountain region [4]. According to the United States Department of Agriculture, 15 Artemisia species have been described: A. arbuscula Nutt.,A. bigelovii Gray, A. californica Less., A. cana Pursh., A. filifolia Torr., A. frigida Willd., A. longiloba (Osterhout) Beetle, A. lugoviciana Nutt., A. nova A.Nels., A. pygmaea Gray, A. rigida (Nutt.) Gray, A. rothrockii Gray, A. spinescens Eaton, A. tridentata Nutt., and A. tripartita Rydb. [5].
The importance of Artemisia species in traditional medicine is evident in a number of ethnopharmacological reports [6,7,8]. Asteraceae-specific ethnobotanical reports recorded in the Native American Ethnobotany database revealed Artemisia species as the second most selected medicinal taxa after Achillea. A. tridentata was most commonly cited as a pulmonary aid, whereas A. dracunculus L., A. tridentata, and A. douglasiana Besser were cited for orthopedic uses [9]. Many Artemisia species are characterized by a strong odor due to essential oils that are mainly concentrated in their leaves and flowers. The pharmacological properties of Artemisia essential oils have been reported, including antibacterial, antiparasitic, antinociceptive, hypoglycemic, and antioxidant activities [10,11,12,13,14,15,16,17].
Essential oils from various plant species have been reported to exhibit immunomodulatory activity. For example, Ocimum kilimandscharicum Gürke leaf essential oils inhibited carrageenan-induced leukocyte rolling, adhesion, and fMLF-induced leukocyte chemotaxis [18]. Likewise, Juniperus essential oils inhibited the production of the proinflammatory cytokines tumor necrosis factor (TNF), interleukin (IL)-1β, and γ-interferon by lipopolysaccharide-stimulated leukocytes [19]. Recently, we found that essential oils from Artemisia kotuchovii, Ferula akitschkensis, F. iliensis, Hypericum perforatum, Rhododendron albiflorum, and Juniperus spp. can activate or inhibit human neutrophil function [20,21,22,23,24,25].
Here, we characterized the chemical composition and immunomodulatory activity of the flower and leaf essential oils from five Artemisia species collected in Montana and analyzed their chemical compositions and innate immunomodulatory activities. We show that several of the Artemisia essential oils potently inhibited intracellular Ca2+ mobilization [Ca2+]i in human neutrophils, with the most active being essential oils from A. dracunculus. Furthermore, we demonstrated farnesene, a unique component of A. dracunculus, inhibited human neutrophil activation and chemotaxis and is likely one of the main active components. Based on the critical role of neutrophils in inflammation, our data support the possibility that farnesene could have the potential for the development of new anti-inflammatory agents.
2. Results and Discussion
2.1. Essential Oil Composition
Essential oils were obtained from the flowers (designated by Fl) and leaves (designated by Lv) of five Artemisia species for subsequent phytochemical and biological characterization. Plant material was collected from wild plants in 2019 around Bozeman and Three Forks (MT, USA) (Table 1). After botanical identification of the plant material, the flowers and leaves were dried for 7–10 days at room temperature but protected from direct sunlight.
Table 1.
Location and date of collection of the plant material.
| Artemisia spp. | Location | Latitude (N) |
Longitude (E) |
Altitude (m) | Plant Material | Date of Collection; Specimen No. |
Yield (%) |
|---|---|---|---|---|---|---|---|
| A. ludoviciana | Three Forks, MT | 45.92721 | 111.50106 | 1235 | leaves/flowers | August 2019; 2019-IAS-A1 |
0.3/1.9 |
| A. dracunculus | Bozeman, MT | 45.71475° | 110.97890° | 1646 | leaves/flowers | August 2019; 2019-IAS-A2 |
0.9/0.9 |
| A. frigida | Three Forks, MT | 45.92553° | 111.49730° | 1240 | leaves/flowers | August 2019; 2019-IAS-A3 |
0.3/1.9 |
| A. cana | Three Forks, MT | 45.92274° | 111.49453° | 1238 | leaves/flowers | August 2019; 2019-IAS-A4 |
1.4/1.5 |
| A. tridentata | Bozeman, MT | 45.74118° | 110.98698° | 1415 | leaves/flowers | August 2019; 2019-IAS-A5 |
3.3/2.7 |
The yields (v/w) of Artemisia spp. essential oils ranged from 0.3 to 3.3% (Table 1). The composition of the extracted essential oils was determined using simultaneous GC-FID and GC/MS. Twenty-seven major compounds found in the essential oils are shown in Table 2 and Supplementary Table S1 shows the total of all compounds identified in these samples. The main classes of compounds in all Artemisia species were oxygenated monoterpenes, ranging from 1.9 (ADFl) to 84.8% (ACLv), and monoterpene hydrocarbons, which ranged from 6.5 (ACLv) to 25.8% (ADFl). Essential oils from A. tridentata, A. ludoviciana, A. frigida, and A. cana contained high amounts of oxygenated monoterpenes (77.4–84.8%), with 1,8-cineole and camphor being the most representative, which is consistent with previous reports for A. ludoviciana, A. frigida, and A. cana essential oils [26]. In addition, essential oils from the A. dracunculus were enriched in phenylpropanoids (65.3–69.0%), including methyl chavicol (estragole) and methyl eugenol, also supporting previous reports analyzing essential oils from aerial parts of this plant [26,27]. Note that this is the first report comparing individual leaf and flower essential oils from these Artemisia species.
Table 2.
Composition of essential oils (%) isolated from leaves and flowers of five Artemisia species.
| No | RRIa | RRIb | Compound | ATLv | ATFl | ALLv | ALFl | ADLv | ADFl | AFLv | AFFl | ACLv | ACFl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1032 | 1008–1039 # | α-Pinene | 2.1 | 1.8 | 2.5 | 3.9 | 0.1 | 0.1 | 2.2 | 3.3 | 0.8 | 2.0 |
| 2 | 1043 | 1011–1063 # | Santolinatriene | 0.7 | 1.7 | 3.5 | 2.2 | 2.4 | |||||
| 3 | 1076 | 1043–1086 # | Camphene | 7.7 | 7.7 | 7.1 | 5.5 | t | t | 4.0 | 6.0 | 1.6 | 4.1 |
| 4 | 1189 | 1179 @ | Artemiseole | 2.1 | 3.1 | 2.5 | 7.5 | 1.2 | |||||
| 5 | 1213 | 1186–1231 # | 1,8-Cineole | 21.8 | 23.8 | 16.3 | 23.1 | 12.5 | 5.7 | 30.0 | 21.9 | ||
| 6 | 1218 | 1188–1233 # | β-Phellandrene | 1.3 | 2.2 | ||||||||
| 7 | 1246 | 1211–1251 # | (Z)-β-Ocimene | 4.6 | 9.4 | ||||||||
| 8 | 1266 | 1232–1267 # | (E)-β-Ocimene | 2.7 | 6.6 | t | |||||||
| 9 | 1280 | 1246–1291 # | p-Cymene | 1.2 | 1.2 | 0.7 | 0.7 | 0.5 | 0.2 | 3.2 | 2.1 | 0.9 | 0.7 |
| 10 | 1290 | 1260–1300 # | Terpinolene | t | t | 0.2 | 0.3 | 8.8 | 5.5 | 0.1 | 0.3 | 0.1 | 0.1 |
| 11 | 1329 | 1312 ^ | Santolina epoxide * | 3.1 | |||||||||
| 12 | 1451 | 1400–1452 # | β-Thujone | 3.3 | 0.1 | ||||||||
| 13 | 1474 | 1453 ** | trans-Chrysanthenol | 2.0 | |||||||||
| 14 | 1532 | 1481–1537 # | Camphor | 51.3 | 41.7 | 41.1 | 26.6 | 23.0 | 37.7 | 32.5 | 35.9 | ||
| 15 | 1538 | 1533–1590 # | trans-Chrysanthenyl acetate | 8.1 | |||||||||
| 16 | 1553 | 1507–1564 # | Linalool | 3.8 | 2.5 | 0.1 | 0.2 | ||||||
| 17 | 1590 | 1549–1597 # | Bornyl acetate | 0.9 | 0.6 | 0.3 | 0.6 | 1.5 | 3.8 | 0.3 | 0.8 | ||
| 18 | 1611 | 1564–1630 # | Terpinen-4-ol | 2.7 | 2.9 | 2.4 | 3.8 | t | 3.3 | 6.9 | 2.0 | 2.3 | |
| 19 | 1687 | 1652–1690 # | Methyl chavicol | 42.9 | 38.8 | ||||||||
| 20 | 1719 | 1653–1728 # | Borneol | 2.4 | 3.3 | 9.6 | 8.1 | t | 6.6 | 14.6 | 2.9 | 2.3 | |
| 21 | 1737 | 1713–1748 # | (Z,E)-α-Farnesene | t | |||||||||
| 22 | 1748 | 1689–1748 # | Piperitone | 0.2 | 1.5 | 1.1 | |||||||
| 23 | 1758 | 1714–1763 # | (E,E)-α-Farnesene | 0.2 | 0.5 | ||||||||
| 24 | 1764 | 1751–1765 # | cis-Chrysanthenol | 0.5 | 0.8 | 1 | 0.5 | 3.9 | 0.5 | 0.2 | |||
| 25 | 1821 | 1807 *** | Fragranol | 3.0 | |||||||||
| 26 | 1827 | 1827 *** | Grandisol | 3.9 | |||||||||
| 27 | 1864 | 1813–1865 # | p-Cymen-8-ol | 0.2 | 0.1 | 0.1 | 2.3 | 0.9 | 0.4 | 0.3 | 0.1 | 0.1 | |
| 28 | 2030 | 1961–2033 # | Methyl eugenol | 26.1 | 26.4 | t | 0.1 | ||||||
| 29 | 2637 | 2608 ## | Xanthoxylin | 2.2 | 1.2 | ||||||||
| Summary of the composition of Artemisia spp. essential oils | |||||||||||||
| Compounds | ATLv | ATFl | ALLv | ALFl | ADLv | ADFl | AFLv | AFFl | ACLv | ACFl | |||
| Monoterpene Hydrocarbons | 13.1 | 14.4 | 13.5 | 18.7 | 20.3 | 25.8 | 11.6 | 17.4 | 6.5 | 11.3 | |||
| Oxygenated Monoterpenes | 84.5 | 79.8 | 82.5 | 77.4 | 4.0 | 1.9 | 79.6 | 79.8 | 84.8 | 81.0 | |||
| Sesquiterpene Hydrocarbons | 2.0 | 0.2 | 0.7 | 1.7 | 2.4 | 0.6 | 0.2 | 0.1 | 0.3 | ||||
| Oxygenated Sesquiterpenes | 0.8 | 1.6 | 1.1 | 1.2 | 1.7 | 1.1 | 2.5 | 0.6 | 0.1 | 0.4 | |||
| Phenylpropanoids | 1.3 | 0.2 | 69.0 | 65.3 | 0.1 | 0.1 | |||||||
| Others | t | t | 0.4 | 0.2 | 2.6 | 2.1 | 0.2 | 0.5 | t | 0.6 | |||
| Total | 98.0 | 97.1 | 98.9 | 97.8 | 98.2 | 97.7 | 94.5 | 98.4 | 91.5 | 93.6 | |||
Legend: The data are presented as relative % for each component identified. RRIa, relative retention index calculated based on retention of n-alkanes. RRIb, relative retention indexes reported in the literature: # [28], @ [26]; ^ [29]; ** [30], *** [31,32], ## [33]. % was calculated from flame ionization detector data. Trace amounts (t) were present at <0.1%. * Santolina epoxide was tentatively identified using Wiley and MassFinder mass spectra libraries and published RRI. All other compounds were identified by comparison with co-injected standards. Abbreviations for the oil samples: ATLv, A. tridentata leaves; ATFl, A. tridentata flowers; ALLv, A. ludoviciana leaves; ALFl, A. ludoviciana flowers; ADLv, A. dracunculus leaves; ADFl, A. dracunculus flowers; AFLv, A. frigida leaves; AFFl, A. frigida flowers; ACLv, A. cana leaves; ACFl, A. cana flowers.
Artemisia exhibits significant intraspecies variations in terpenes present in their essential oils [16,26,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]. For example, the variation in volatile components of these plants may occur during plant maturation at different altitudes. Our studies confirmed these findings and extended this analysis by providing the first report comparing essential oils isolated from preparations of A. tridentata flowers and leaves prepared from the same plant samples. Interestingly, we found that some compounds exhibited remarkable differences in terms of their presence in different parts of the same Artemisia species. For example, santolinatriene and artemiseole were found only in the flowers but not in the leaves of A. ludoviciana. Likewise, santolina epoxide and the monoterpene alcohol grandisol, a pheromone, were detected only in A. cana flowers. Similarly, trans-chrysanthenol and its ester trans-chrysanthenyl acetate were detected only in A. frigida leaves, and fragranol was found only in A. cana leaf essential oils and not those isolated from flowers. Therefore, it is important to evaluate different parts of plants in terms of target compounds. A comparison across species also demonstrated some differences in the presence of compounds, with some compounds (e.g., (Z)-β-ocimene, methyl chavicol, (E,E)-α-farnesene, xanthoxylin), being present in only one Artemisia species. It is also interesting that A. dracunculus essential oils had more unique compounds that were not present in essential oils from the other Artemisia species.
2.2. Effect of Artemisia Essential Oils and Pure Compounds on Neutrophil Ca2+ Influx
Neutrophils are the most common leukocyte in human blood and are required for innate immune responses [59]. These cells respond rapidly to infection and injury in various tissues. They represent the first line of defense and utilize multiple mechanisms of oxygen-dependent and oxygen-independent processes to protect against infection, including phagocytosis, cytokine secretion, and reactive oxygen species production [60,61]. Thus, neutrophils represent a potential target for the development of novel anti-inflammatory therapeutics. Indeed, natural products, such as essential oils, have exhibited neutrophil immunomodulatory activity [20,21,22,23,24].
Artemisia essential oils were evaluated for their immunomodulatory effects on human neutrophils. Initially, we measured the effects of the essential oils on [Ca2+]i, which is a key component of neutrophil activation and function [62]. We found that treatment of neutrophils with Artemisia essential oils only modestly enhanced [Ca2+]i at relatively high concentrations, with EC50 values ranging from in the range of 15.8 µg/mL (ALLv) to 48.7 µg/mL (ACLv). We also evaluated some of the individual compounds present in the various Artemisia essential oils to determine if they were responsible for neutrophil activation, including 1,8-cineole, (+)-camphor, (−)-camphor, (±)-bornyl acetate, farnesene, piperitone, and xanthoxylin. As shown in Table 3, 1,8-cineole, (+)-camphor, (−)-camphor, and piperitone did not affect human neutrophil Ca2+ flux, whereas farnesene, xanthoxylin, and (±)-bornyl acetate were weak activators, but only at relatively high concentrations. Note that previous analysis of some of the other major compounds that we also found here to be present in Artemisia essential oils (i.e., α-pinene, camphene, (E/Z)-β-ocimene, p-cymene, terpinolene, linalool, terpinen-4-ol, methyl chavicol, p-cymen-8-ol, and methyl eugenol) showed that they had no effect on human neutrophil [Ca2+]i [24,25].
Table 3.
Effect of essential oils from Artemisia spp. and pure component compounds on [Ca2+]i and cytotoxicity in human neutrophils.
| Essential Oil or Pure Compound |
Activation of [Ca2+]i | Inhibition of [Ca2+]i | Cytotoxicity | |
|---|---|---|---|---|
| fMLF-Induced | WKYMVM-Induced | |||
| EC50 (μg/mL) | IC50 (μg/mL) | IC50 (μg/mL) | ||
| ATLv | 23.6 ± 3.6 | 28.9 ± 4.1 | 3.4 ± 0.8 | Nontoxic |
| ATFl | 32.1 ± 4.5 | 19.5 ± 3.3 | 3.7 ± 0.1 | Nontoxic |
| ALLv | 15.8 ± 2.1 | 31.2 ± 3.9 | 8.1 ± 3.4 | Nontoxic |
| ALFl | 16.3 ± 4.2 | 21.4 ± 3.2 | 19.6 ± 6.3 | Nontoxic |
| ADLv | 25.3 ± 7.4 | 4.6 ± 1.3 | 5.3 ± 1.8 | 32.6 ± 4.1 |
| ADFl | 18.8 ± 3.2 | 2.2 ± 0.8 | 2.9 ± 0.8 | 24.7 ± 2.8 |
| AFLv | 44.7 ± 6.2 | 19.3 ± 4.2 | 32.4 ± 6.4 | 42.4 ± 3.2 |
| AFFl | 41.5 ± 3.5 | 13.0 ± 2.6 | 26.6 ± 6.5 | 31.4 ± 4.8 |
| ACLv | 48.7 ± 9.5 | 18.4 ± 7.6 | 36.5 ± 4.8 | Nontoxic |
| ACFl | 41.6 ± 11.7 | 22.6 ± 6.8 | 30.4 ± 7.1 | Nontoxic |
| EC50 (μM) | IC50 (μM) | IC50 (μM) | ||
| 1,8-Cineole | N.A. | N.A. | N.A. | Nontoxic |
| (−)-Camphor | N.A. | N.A. | N.A. | Nontoxic |
| (+)-Camphor | N.A. | N.A. | N.A. | Nontoxic |
| (±)-Bornyl acetate | 50.1 ± 11.5 | 42.6 ± 9.7 | 19.1 ± 0.1 | Nontoxic |
| Farnesene | 28.5 ± 2.6 | 1.1 ± 0.2 | 1.4 ± 0.5 | Nontoxic |
| Piperitone | N.A. | N.A. | N.A. | Nontoxic |
| Xanthoxylin | 53.3 ± 5.0 | 27.2 ± 6.6 | 52.7 ± 11.2 | Nontoxic |
Legend: EC50 and IC50 values were determined by nonlinear regression analysis of the dose-response curves. For the determination of cytotoxicity, neutrophils were incubated with indicated concentrations of the compounds for 90 min and cell viability was analyzed. N.A. indicates the samples had essentially no activity or no cytotoxicity, respectively (EC50 or IC50 > 55 µM for pure compounds or > 55 µg/mL for the oils). Presented as the mean ± SD of three independent experiments. Abbreviations for the oils: ATLv, A. tridentata leaves; ATFl, A. tridentata flowers; ALLv, A. ludoviciana leaves; ALFl, A. ludoviciana flowers; ADLv, A. dracunculus leaves; ADFl, A. dracunculus flowers; AFLv, A. frigida leaves; AFFl, A. frigida flowers; ACLv, A. cana leaves; ACFl, A. cana flowers.
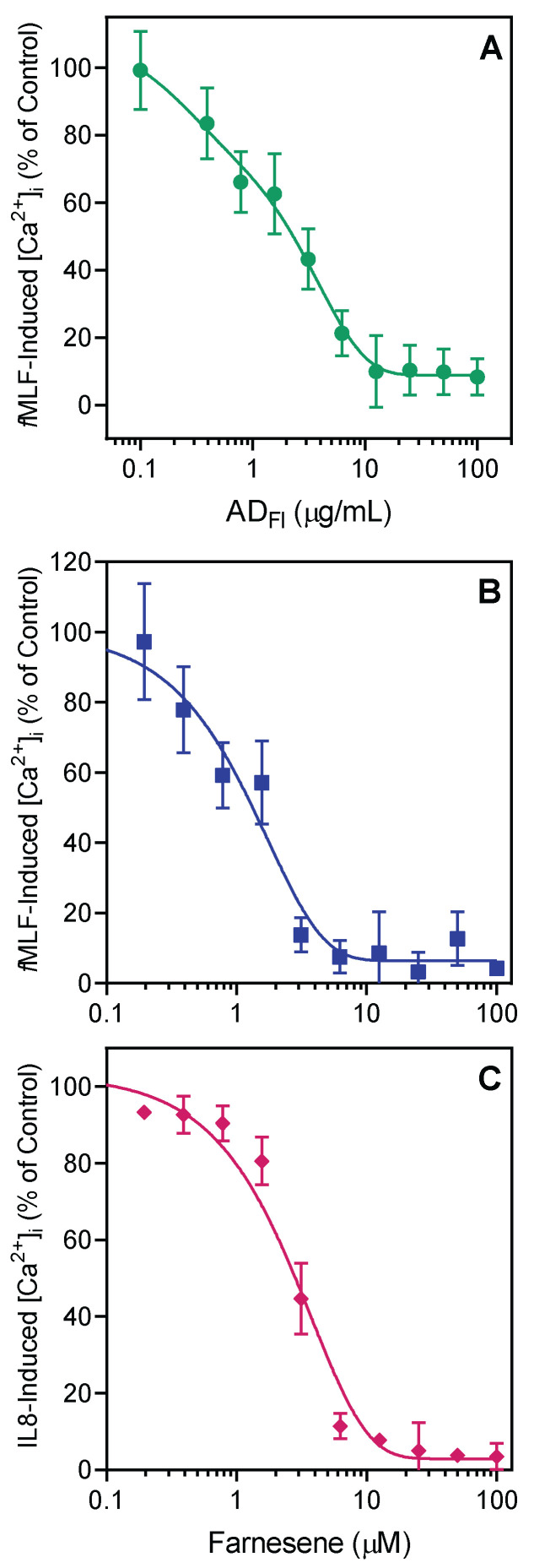
Since Artemisia essential oils and individual compound components exhibited only weak neutrophil activation, we considered whether they might have some effect on neutrophils activated by strong agonists, such as N-formyl peptide receptor 1 (FPR1) agonist fMLF and FPR2 agonist WKYMVM, as ligands can downregulate or alter neutrophil responses to subsequent treatment with heterologous or homologous agonists [63]. As shown in Table 3, Artemisia essential oils were relatively more potent inhibitors of [Ca2+]i in fMLF- and WKYMVM-stimulated neutrophils, with some IC50 values in the low micromolar range. Essential oils from A. dracunculus flowers (ADFl) were the most potent, with IC50 values of 2.2 and 2.9 μM for inhibition of fMLF- and WKYMVM-induced neutrophil [Ca2+]i, respectively. Representative data showing dose-dependent inhibition of fMLF-induced neutrophil [Ca2+]i, by ADFl are shown in Figure 1A.
Figure 1.
Effect of A. dracunculus flower essential oils (ADFl) and farnesene on fMLF- and IL-8-induced neutrophil [Ca2+]i. Human neutrophils were treated for 10 min with ADFl, farnesene, or 1% DMSO (negative control). The cells were then activated with 5 nM fMLF (A,B) or 25 nM of human IL-8 (C), and [Ca2+]i was monitored as described. The data are shown as the mean ± SD from one experiment. Representative of three (for fMLF) or two (for IL-8) independent experiments with similar results.
We also evaluated the effect of seven compounds present in Artemisia essential oils on fMLF- and WKYMVM-induced neutrophil [Ca2+]i and found that farnesene, xanthoxylin, and (±)-bornyl acetate inhibited fMLF- and WKYMVM-stimulated neutrophils, while the other compounds were inactive (Table 3). Xanthoxylin and (±)-bornyl acetate were also quite weak inhibitors, whereas the most potent inhibitor of peptide-induced neutrophil [Ca2+]i was farnesene (IC50 = 1.1 ± 0.2 and 1.4 ± 0.5 μM for fMLF- and WKYMVM-activated neutrophils, respectively). Representative data showing dose-dependent inhibition of fMLF-induced neutrophil [Ca2+]i, by farnesene are shown in Figure 1B. Likewise, farnesene also dose-dependently inhibited IL-8 induced neutrophil [Ca2+]i, with an IC50 = 2.6 ± 0.2 μM (Figure 1C). These data suggest that farnesene can modulate intracellular signaling pathways that are common to different G protein-coupled receptors (GPCRs), including FPR1/FPR2 and CXCR1/2 chemokine receptors resulting in down-regulation of the response to these chemoattractants.
2.3. Effect of A. dracunculus Essential Oils and Pure Compounds on Neutrophil Chemotaxis
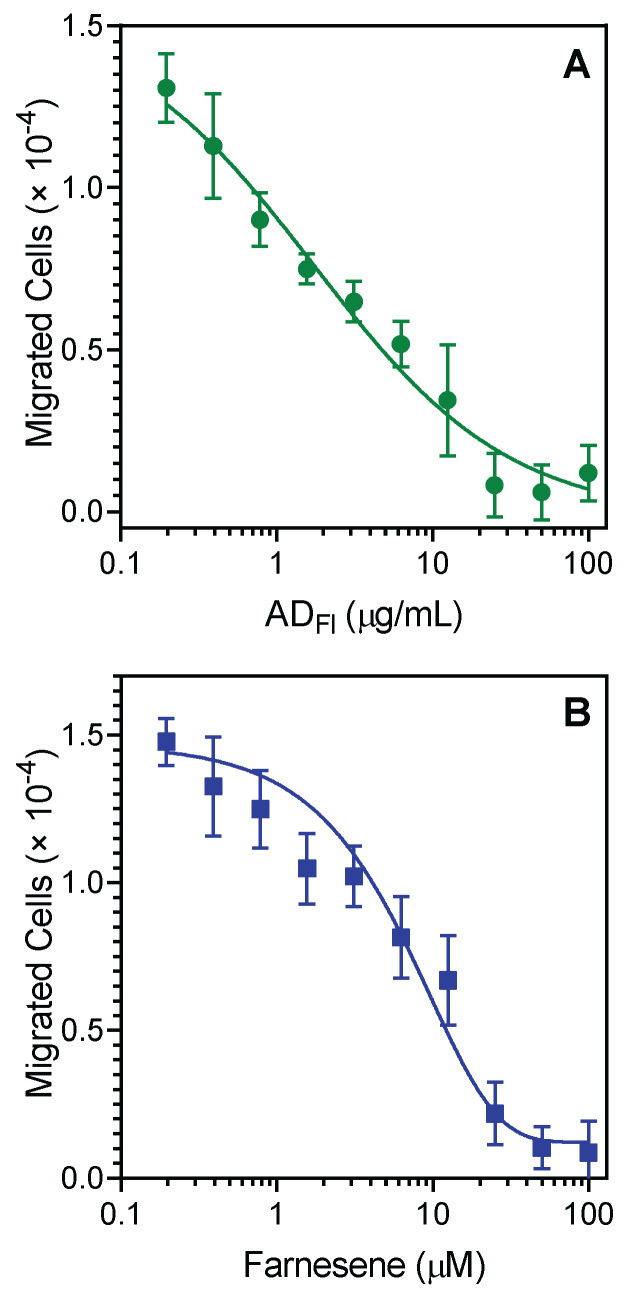
Since A. dracunculus essential oils and their unique component, farnesene, were effective inhibitors of agonist-induced neutrophil [Ca2+]i, we evaluated their effects on neutrophil chemotaxis. Pretreatment of neutrophils with ADFl for 30 min dose-dependently inhibited fMLF-induced human neutrophil chemotaxis toward fMLF, with an IC50 of 2.4 ± 1.1 µg/mL (Figure 2A). Likewise, pretreatment of neutrophils with farnesene also effectively inhibited neutrophil migration toward fMLF, with an IC50 of 3.3 ± 1.4 μM. A representative dose-dependent response for the inhibition of fMLF-induced chemotaxis in human neutrophils by farnesene is shown in Figure 2B. Analysis of (±)-bornyl acetate showed that this compound also inhibited fMLF-induced neutrophil chemotaxis, although with much lower potency (IC50 = 11.9 ± 1.9 μM). In contrast, xanthoxylin did not affect this response.
Figure 2.
Inhibition of neutrophil chemotaxis by essential oils from A. dracunculus flowers (ADFl) (A) and farnesene (B). Neutrophil chemotaxis toward 1 nM fMLF was measured. The data are from one experiment that is representative of two independent experiments.
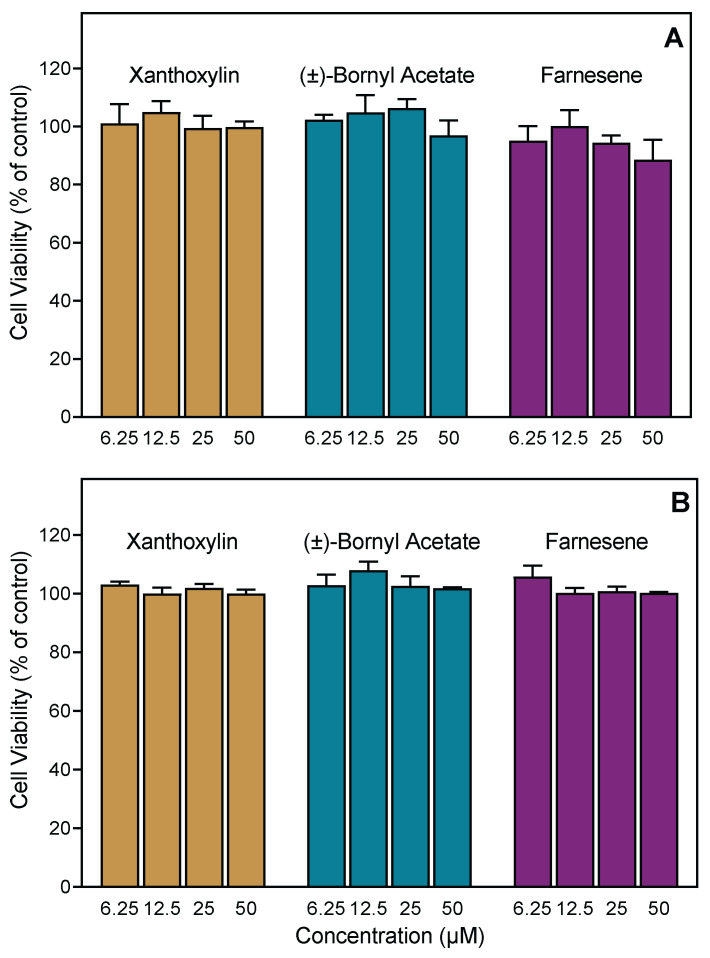
To ensure that Artemisia essential oils or active pure compounds were not cytotoxic, we evaluated the cytotoxicity of the essential oil samples (up to 55 µg/mL) and test compounds at various concentrations (up to 50 µM) in human neutrophils during a 1.5 h incubation period, which covers the times used to measure Ca2+ influx (up to 30 min) and cell migration (up to 1.5 h). As shown in Table 3, essential oils from A. tridentata, A. ludoviciana, and A. cana had no cytotoxicity in neutrophils, although essential oils from A. dracunculus and A. frigida had a little cytotoxicity at the highest concentrations (25–50 μM), which were >10-fold higher than those required to induce biological effects. The pure compounds (±)-bornyl acetate, farnesene, and xanthoxylin were nontoxic in human neutrophils during the 90 min incubation. In addition, these compounds did not affect the viability of human THP-1 monocytic cells after a 24 h incubation period (Figure 3). Thus, the biological effects of these compounds on neutrophil function are not due to compound cytotoxicity and support the conclusion that farnesene is a novel innate immunomodulator.
Figure 3.
Cytotoxicity of selected compounds. Human neutrophils (A) or human THP-1 cells (B) were incubated with indicated compounds for 90 min (A) or 24 h (B), and cell viability was analyzed. The data presented are the mean ± SD of triplicate samples from one experiment. Representative of two independent experiments with similar results.
2.4. Identification of Potential Protein Targets for Selected Compounds
Both farnesene and bornyl acetate inhibited neutrophil [Ca2+]i and chemotaxis, although farnesene was the most effective by far. Farnesene encompasses a set of six closely related sesquiterpenes, as the α-form can exist as four stereoisomers, and the β-isomer exists as two stereoisomers. We identified both (Z,E)-α-farnesene and (E,E)-α-farnesene in A. dracunculus, although (E,E)-α-farnesene was present at a higher level (Table 2). Both α- and β- forms of farnesene have been reported previously in essential oils from Artemisia spp. For example, (Z,E)-α-farnesene was found in A. maderaspatana L. [64] and (E,E)-α-farnesene was present in A. ordosica Krasch. and A. dracunculus [26,65,66]. (E)-β-farnesene was reported to be present in A. annua L. [67], A. magellanica Sch. Bip. [68], A. absinthium L. [69], A. lavandulifolia DC. [70], and A. biennis Willd. essential oils [26]. Finally, (Z)-β-farnesene was found in essential oils from A. absinthium [26], A. annua [71], and A. tournefortiana Reichb. [72]. Although the α- and β-forms of farnesene are well-known pheromones [73,74], we did not find any reports on the biological activity of these compounds in human cells.
Bornyl acetate has been reported previously to be a major component of essential oils isolated from Artemisia spp, including A. ludoviciana and A. frigida [17,53]. Bornyl acetate is also a volatile constituent present in numerous conifer essential oils and essential oils from Valeriana officinalis [75]. Previous studies have reported that bornyl acetate has anti-inflammatory properties in different experimental models. For example, bornyl acetate downregulated the levels of proinflammatory cytokines in vitro and in vivo and reduced the number of total cells, neutrophils, and macrophages in murine bronchoalveolar lavage fluid after intranasally injected lipopolysaccharide. Moreover, bornyl acetate inhibited the expression and production of IL-1β and TNF in human umbilical vein endothelial cells (HUVECs) and RAW 264.7 macrophages [76,77]. Bornyl acetate has also been reported to have analgesic effects [78].
To better understand the properties of these compounds, we calculated their most important physicochemical parameters using SwissADME [79]. The LogP values estimated using ALOGPS 2.1 program [80] and tPSA values allowed us to predict that (−) bornyl acetate, (+) bornyl acetate, (Z,E)-α-farnesene, and (E,E)-α-farnesene can all permeate the blood–brain barrier (BBB), according to the binary classification tree model [81] (Table 4).
Table 4.
Predicted physicochemical properties of farnesene isomers and bornyl acetate.
| Property | (E,E)-α-Farnesene | (Z,E)-α-Farnesene | Bornyl Acetate |
|---|---|---|---|
| Formula | C15H24 | C15H24 | C12H20O2 |
| M.W. | 204.35 | 204.35 | 196.29 |
| Heavy Atoms | 15 | 15 | 14 |
| Fraction Csp3 | 0.47 | 0.47 | 0.92 |
| Rotatable Bonds | 6 | 6 | 2 |
| H-Bond Acceptors | 0 | 0 | 2 |
| H-Bond Donors | 0 | 0 | 0 |
| MR | 72.32 | 72.32 | 56.33 |
| tPSA | 0.00 | 0.00 | 26.30 |
| LogP | 5.70 | 5.70 | 3.50 |
| BBB Permeation | Yes | Yes | Yes |
Legend: M.W., molecular weight (g/mol); MR, molar refractivity; tPSA, topological polar surface area (Å2); LogP, lipophilicity; BBB, blood–brain barrier.
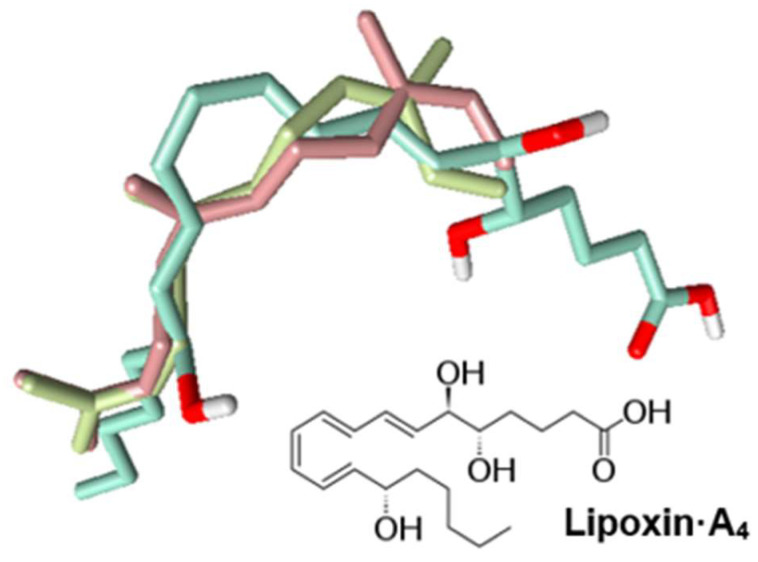
Farnesene is very lipophilic (Table 4). Thus, we propose that the neutrophil signaling inhibitory mechanisms may be based on the allosteric interaction of the farnesene chain with the membrane portion of the receptor, and we are currently investigating this mechanism. Indeed, other lipophilic compounds, such as the bile acids deoxycholic acid and chenodeoxycholic acid, have been reported to antagonize FPR1 at high concentrations (>100 µM) [82,83,84]. Moreover, lipoxin A4 was also reported as an allosteric modulator of a CB1 cannabinoid receptor and FPR2 [85,86]. To further investigate this issue, we aligned (E,E)-α-farnesene, (Z,E)-α-farnesene, and lipoxin A4 using FieldTemplater software (Figure 4) and found that the alignment was determined mainly by the hydrophobic hydrocarbon skeletons of the compounds. The combined similarity measure of the superimposition was relatively high (S = 0.690), suggesting that the farnesene molecules could mimic lipoxin A4 and maybe other related specialized pro-resolving mediators (known as SPMs), including resolvins, maresins, and protectins [87]. Indeed, many of these molecules have been demonstrated to act allosterically on a number of GPCRs (reviewed in [88]). Interestingly, we found previously that 6-methyl-3,5-heptadien-2-one (MHDO), a compound structurally similar to farnesene, also inhibited neutrophil activity [25], although its molecular targets were not identified.
Figure 4.
Chemical structure of lipoxin A4 and alignment of lipoxin-A4 (green), (E,E)-α-farnesene (pink), and (Z,E)-α-farnesene (khaki).
Not much is known about the specific cellular targets of farnesene or bornyl acetate. Thus, we performed reverse-pharmacophore mapping on the molecular structures of (Z,E)-α-farnesene, (E,E)-α-farnesene, and the (−) and (+) forms of bornyl acetate to identify potential biological targets. PharmMapper compared a database of pharmacophore patterns with these compounds and generated target information, such as pharmacophoric characteristics and normalized fitness scores. Note, however, that PharmMapper depends on the availability of structures for pharmacophore mapping, and most GPCRs are not represented. The proper optical isomers of the compounds were submitted to the PharmMapper server as mapping explicitly accounts for the three-dimensional structure of a molecule. The ten top-ranked potential targets found by PharmMapper are shown in Table 5.
Table 5.
Potential protein targets for bornyl acetate and farnesene were identified by PharmMapper.
| Rank | PDB ID | Target Name | Fit Score | Rank | PDB ID | Target Name | Fit Score |
|---|---|---|---|---|---|---|---|
| (−)-Bornyl Acetate | (+)-Bornyl Acetate | ||||||
| 1 | 1REU | BMP2 | 1 | 1 | 1J96 | AKR1C2 | 1 |
| 2 | 1J96 | AKR1C2 | 1 | 2 | 1REU | BMP2 | 1 |
| 3 | 1MX1 | LCE1 | 0.996 | 3 | 1OKL | CA2 | 0.9992 |
| 4 | 2AO6 | NR3C4 | 0.9925 | 4 | 2PIQ | NR3C4 | 0.9962 |
| 5 | 2PE0 | PDPK1 | 0.9923 | 5 | 2G01 | JNK1 | 0.9857 |
| 6 | 2G01 | JNK1 | 0.9848 | 6 | 1W8L | PPIase A | 0.9826 |
| 7 | 1IF4 | CA2 | 0.9815 | 7 | 2UZD | Cyclin-A2 | 0.9821 |
| 8 | 1W8L | PPIase A | 0.9811 | 8 | 1MX1 | LCE1 | 0.9784 |
| 9 | 1VJY | TGFBR1 | 0.979 | 9 | 1A28 | PgR | 0.9771 |
| 10 | 1A28 | PgR | 0.9636 | 10 | 2PE0 | PDPK1 | 0.9711 |
| (E,E)-α-Farnesene | (Z,E)-α-Farnesene | ||||||
| 1 | 1J96 | AKR1C2 | 1 | 1 | 1PME | JNK1 | 1 |
| 2 | 3HVC | p38α | 1 | 2 | 3HVC | p38α | 1 |
| 3 | 1E7A | Serum albumin | 1 | 3 | 3BGP | Pim-1 | 0.9998 |
| 4 | 1OJ9 | MAO-B | 1 | 4 | 2PG2 | KIF11 | 0.9993 |
| 5 | 1SHJ | Caspase-7 | 1 | 5 | 1E7A | Serum albumin | 0.999 |
| 6 | 1PME | ERK2 | 1 | 6 | 1OJ9 | MAO-B | 0.9989 |
| 7 | 1P49 | Steryl-sulfatase | 0.9989 | 7 | 1J96 | AKR1C2 | 0.9988 |
| 8 | 2PIN | NR1A2 | 0.9985 | 8 | 1L6L | Apo A-II | 0.9984 |
| 9 | 3BGP | Pim-1 | 0.9982 | 9 | 2PIN | NR1A2 | 0.9978 |
| 10 | 1L6L | Apo A-II | 0.9977 | 10 | 1P49 | Steryl-sulfatase | 0.9975 |
Legend: AKR1C2, aldo-keto reductase family 1 member C2; Apo A-II, apolipoprotein A-II; BMP2, bone morphogenetic protein 2; CA2, carbonic anhydrase 2; ERK2, extracellular signal-regulated kinase 2; JNK1, c-Jun N-terminal kinase 1; KIF11, kinesin family member 11; LCE1, liver carboxylesterase 1; MAO-B, monoamine oxidase B; NR1A2, thyroid hormone receptor β; NR3C4, androgen receptor; p38α, p38α mitogen-activated protein kinase; PDPK1, 3-phosphoinositide-dependent protein kinase 1; PgR, progesterone receptor; Pim-1, proto-oncogene serine/threonine-protein kinase; PPIase A, peptidyl-prolyl cis-trans isomerase A; TGFBR1, TGF-β receptor type-1.
PharmMapper analysis indicated that kinases could be among the potential targets for bornyl acetate and farnesene. Among the top ten ranked targets for the (±)-enantiomers of bornyl acetate were c-Jun N-terminal kinase 1 (JNK1), 3-phosphoinositide-dependent protein kinase 1 (PDPK1), and transforming growth factor-β receptor type-1 (TGFBR1), a serine/threonine-protein kinase. Likewise, JNK1, p38α mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase 2 (ERK2), and proto-oncogene serine/threonine-protein kinase (Pim-1) were kinases identified among the ten top-ranked targets for (E,E)- and (Z,E)-forms of α-farnesene (Table 5). MAPK signaling plays an important role in neutrophil signal transduction cascades [89], and studies have shown that members of the MAPK, JNK, and the p38 MAPK families of proteins are activated in response to neutrophil priming/activation (reviewed in [90]). It is also clear from previous studies that one or more of these MAPK pathways is induced by FPR agonists [91,92] and IL-8 [93]. Thus, these compounds and especially farnesene may be general inhibitors of neutrophil activation through GPCRs, and further studies are in progress to evaluate this idea and identify their specific molecular targets.
3. Materials and Methods
3.1. Materials
N-formyl-Met-Leu-Phe (fMLF), Trp-Lys-Tyr-Val-Met (WKYMVM), and farnesene (mixture of isomers) were from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). (±)-Bornyl acetate was from Cayman Chemical Company (Ann Arbor, MI, USA). Xanthoxylin was from TargetMol (Boston, MA, USA). Piperitone (mixture of isomers), (+)-camphor, and 1,8-cineole were from TCI America (Portland, OR, USA), and (−)-camphor was from Alfa Aesar (Ward Hill, MA, USA). Fluo-4AM was from Invitrogen (Carlsbad, CA, USA). Fetal bovine serum was from ATCC (Manassas, VA, USA). Hanks’ balanced salt solution without Ca2+ and Mg2+ (HBSS–) was from Life Technologies (Grand Island, NY, USA). We prepared HBSS+ by adding 1.3 mM CaCl2 and 1.0 mM MgSO4. Human interleukin-8 (IL-8) was purchased from Peprotech (Rocky Hill, NJ, USA).
3.2. Essential Oil Extraction
Essential oils were obtained by hydrodistillation of air-dried plant material. Hydrodistillation was performed with a Clevenger-type apparatus, as previously described [25]. To avoid artifacts, we used conditions accepted by the European Pharmacopoeia to avoid artifacts. Essential oil yields (w/v) were calculated based on the amount of air-dried plant material used. We prepared stock solutions of the essential oils in 10 mg/mL DMSO for biological evaluation. For gas-chromatographic (GC) analysis, samples were prepared in 10% w/v n-hexane.
3.3. GC-Flame Ionization Detector (GC-FID) and GC-Mass Spectrometry (GC-MS) Analysis
We performed GC-MS analysis using an Agilent 5975 GC-MSD system, as previously described [94]. An Agilent Innowax FSC column (60 m × 0.25 mm, 0.25 μm film thickness) was used with He as the carrier gas (0.8 mL/min). The GC oven temperature was kept at 60 °C for 10 min, increased to 220 °C at a rate of 4 °C/min, kept constant at 220 °C for 10 min, and then increased to 240 °C at a rate of 1 °C/min. The split ratio was adjusted to 40:1, and the injector temperature was 250 °C. MS spectra were monitored at 70 eV with a mass range of 35 to 450 m/z. GC analysis was carried out using an Agilent 6890 N GC system. To obtain the same elution order as with GC-MS, the line was split for FID and MS detectors, and a single injection was performed using the same column and appropriate operational conditions. The flame ionization detector (FID) temperature was 300 °C. The essential oil components were identified by co-injection with standards (whenever possible), which were purchased from commercial sources or isolated from natural sources. The identities of compounds were also using the MassFinder software 4.0 (Dr. Hochmuth Scientific Consulting, Hamburg, Germany), Adams Library, Wiley GC/MS Library (Wiley, Hoboken, NJ, USA), and NIST Library. Confirmation was also achieved using the in-house “Başer Library of Essential Oil Constituents” database. The database was created using chromatographic analysis of pure compounds run under identical conditions. Samples were spiked with a C8–C40 n-alkane standard solution (Fluka, Buchs, Switzerland) to determine relative retention indices (RRI). The FID chromatograms were used to calculate the relative amounts (%) of the separated compounds.
3.4. Isolation of Human Neutrophils
We obtained human neutrophils using blood collected from healthy donors. Blood collection was approved by the Institutional Review Board at Montana State University (Protocol #MQ041017). Neutrophils were isolated as described previously [95]. The isolated neutrophils were resuspended in HBSS–. Neutrophil preparations were routinely >95% pure and >98% viable, as determined by light microscopy and trypan blue exclusion, respectively.
3.5. Cell Culture
Human THP-1 monocytic cells obtained from ATCC (Manassas, VA, USA) were cultured in RPMI 1640 medium (Mediatech Inc., Herndon, VA, USA) supplemented with 10% (v/v) FBS, 100 μg/mL streptomycin, and 100 U/mL penicillin.
3.6. Ca2+ Mobilization Assay
Changes in intracellular Ca2+ concentrations ([Ca2+]i) were monitored with a FlexStation 3 (Molecular Devices, Sunnyvale, CA, USA). For these assays, neutrophils were loaded with 1.25 μg/mL Fluo-4AM and incubated in the dark at 37 °C for 30 min. The cells were then washed with HBSS-. The dye-loaded cells were resuspended in HBSS+ and pipetted into the wells of black microtiter plates at 2 × 105 cells/well. To measure the direct effects of samples on [Ca2+]i, the test samples were added to the wells (final concentration of DMSO was 1%), and fluorescence was monitored (λex = 485 nm, λem = 538 nm). Changes in fluorescence were monitored every 5 s at room temperature for 240 s. To evaluate the inhibitory effects of the test samples, the samples were added to the wells and incubated for 10 min, with the subsequent addition of 5 nM fMLF, 5 nM WKYMVM, or 25 nM IL-8. Responses were normalized to the response induced by control 5 nM fMLF, 5 nM WKYMVM, or 25 nM IL-8 alone without pretreatment. These responses were assigned as 100%. To calculate median effective concentrations (EC50 or IC50), we used curve fitting (at least five or six points) and nonlinear regression analysis of the dose–response curves. Curve fitting was performed with Prism 9 (GraphPad Software, Inc., San Diego, CA, USA).
3.7. Chemotaxis Assay
To evaluate effects of samples on neutrophil migration, we resuspended neutrophils in HBSS+ containing 2% (v/v) heat-inactivated FBS (2 × 106 cells/mL). We analyzed chemotaxis using 96-well ChemoTx chambers (Neuroprobe, Gaithersburg, MD). The neutrophils were first preincubated with the indicated concentrations of test samples at room temperature for 30 min. The pretreated cells were then pipetted into the chamber upper wells (4 × 104 cells/well). The lower wells contained 30 µL of HBSS+ with 2% (v/v) heat-inactivated FBS, the indicated test samples or control 1% DMSO, and 1 nM fMLF as the chemoattractant. Three lower wells were reserved for background controls (DMSO treated cells in the upper wells and DMSO without fMLF in the lower wells). We allowed the cells to migrate through the polycarbonate membrane filter for 60 min at 37 °C/5% CO2. We determined the number of migrated cells by measuring ATP in lysates of transmigrated cells and comparing this to a standard curve obtained with known neutrophil numbers, as described previously [23]. Calculation of median effective concentrations (IC50) was performed by nonlinear regression analysis of the dose-response curves.
3.8. Cytotoxicity Assay
We analyze cytotoxicity in human neutrophils or THP-1 monocytic cells. Cytotoxicity was analyzed using a CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, Madison, WI, USA). Briefly, the cells were incubated (104 cells/well) with the indicated concentrations of essential oil or compound for 90 min (for neutrophils) or 24 h (for THP-1 cells) at 37 °C/5% CO2. After incubation, we added substrate. The samples were analyzed using a Fluoroscan Ascent FL microplate reader.
3.9. Molecular Modeling
The PharmMapper Server [96] was used to identify potential protein targets for (E,E)-α-farnesene (CID: 5281516) and (Z,E)-α-farnesene (CID: 5362889) (CIDs are compound identifiers in PubChem). PharmMapper recognizes potential targets based on “invert” pharmacophore mapping. The protein biotargets are represented by sets of pharmacophore points in reference databases incorporated in the software. We used PubChem (https://pubchem.ncbi.nlm.nih.gov; accessed on 20 February 2022) as a source of initial 3D structures for our compounds. These structures were downloaded from PubChem in SDF format. We then uploaded the structures into PharmMapper. The system automatically generated up to 300 conformers of each compound based on the software option. We performed pharmacophore mapping using the “Human Protein Targets Only” database, which contained 2241 targets. We retrieved the top 250 potential targets for each compound evaluated. The potential targets were sorted by normalized fit score. We calculated compound physicochemical properties using SwissADME (http://www.swissadme.ch; accessed on 20 March 2022). Properties were calculated for the structures of (−)-bornyl acetate (CID: 93009), (+)-bornyl acetate (CID: 6950274), (E,E)-α-farnesene (CID: 5281516), and (Z,E)-α-farnesene (CID: 5362889). The alignment of lipoxin-A4, (E,E)-α-farnesene, and (Z,E)-α-farnesene was made with the use of FieldTemplater software (Cresset Group, England, UK).
3.10. Statistical Analysis
For statistical analysis, we performed a one-way analysis of variance (ANOVA), followed by Tukey's pair-wise comparisons. We considered differences at p < 0.05 to be statistically significant.
4. Conclusions
Our results demonstrate that: (1) Artemisia spp. essential oils contain compounds that exhibit neutrophil immunomodulatory activity, which might contribute to the reported pharmacological properties of extracts from these plants; (2) the biological effects of these compounds on neutrophil function are not due to compound cytotoxicity; (3) essential oils from A. dracunculus flowers (ADFl) contain farnesene isomers that may have anti-inflammatory activity due to their ability to inhibit neutrophil responses to inflammatory chemoattractants, establishing farnesene as a novel innate immunomodulator, and (4) synergetic effects may be possible with other essential oil constituents, such as bornyl acetate or xanthoxylin. Further work is in progress to define the mechanisms of farnesene action and evaluate its therapeutic potential.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph15050642/s1, Table S1: includes all compounds found in the essential oils from each Artemisia species.
Author Contributions
I.A.S. and M.T.Q. conceived and designed the project; I.A.S., L.N.K. and R.A.K. collected the plant material; I.A.S., G.Ö., T.Ö. and L.N.K. performed the experiments; A.I.K. conducted molecular modeling study; I.A.S., G.Ö., T.Ö., L.N.K. and A.I.K. analyzed and interpreted the data; I.A.S., G.Ö. and M.T.Q. drafted and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved by the Montana State University Institutional Review Board (protocol 2022-168, approved 23 March 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article and supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported in part by National Institutes of Health IDeA Program Grants GM115371 and GM103474; USDA National Institute of Food and Agriculture Hatch project 1009546; the Montana State University Agricultural Experiment Station; and the Tomsk Polytechnic University Development Program (Project Priority-2030-NIP/IZ-009-0000-2022).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wright C.W. Artemisia. CRC Press; London, UK: 2001. [Google Scholar]
- 2.Abad M.J., Bedoya L.M., Apaza L., Bermejo P. The Artemisia L. genus: A review of bioactive essential oils. Molecules. 2012;17:2542–2566. doi: 10.3390/molecules17032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia S., McArthur E.D., Pellicer J., Sanderson S.C., Valles J., Garnatje T. A molecular phylogenetic approach to western North America endemic Artemisia and allies (Asteraceae): Untangling the sagebrushes. Am. J. Bot. 2011;98:638–653. doi: 10.3732/ajb.1000386. [DOI] [PubMed] [Google Scholar]
- 4.Katial R.K., Lin F.L., Stafford W.W., Ledoux R.A., Westly C.R., Weber R.W. Mugwort and sage (Artemisia) pollen cross-reactivity: ELISA inhibition and immunoblot evaluation. Ann. Allerg. Asthma Immunol. 1997;79:340–346. doi: 10.1016/S1081-1206(10)63025-6. [DOI] [PubMed] [Google Scholar]
- 5.Francis J.K. Wildland Shrubs of the United States and its Territories: Thamnic Descriptions. Volume 1. International Institute of Tropical Forestry; Fort Collins, CO, USA; San Juan, Puerto Rico: 2004. pp. 47–90. Gen.Techn. Rep. IITF-GTR-26. [Google Scholar]
- 6.Liu Y., He Y., Wang F., Xu R., Yang M., Ci Z., Wu Z., Zhang D., Lin J. From longevity grass to contemporary soft gold: Explore the chemical constituents, pharmacology, and toxicology of Artemisia argyi H. Lév. & vaniot essential oil. J. Ethnopharmacol. 2021;279:114404. doi: 10.1016/j.jep.2021.114404. [DOI] [PubMed] [Google Scholar]
- 7.Ding J., Wang L., He C., Zhao J., Si L., Huang H. Artemisia scoparia: Traditional uses, active constituents and pharmacological effects. J. Ethnopharmacol. 2021;273:113960. doi: 10.1016/j.jep.2021.113960. [DOI] [PubMed] [Google Scholar]
- 8.Du Toit A., Van der Kooy F. Artemisia afra, a controversial herbal remedy or a treasure trove of new drugs? J. Ethnopharmacol. 2019;244:112127. doi: 10.1016/j.jep.2019.112127. [DOI] [PubMed] [Google Scholar]
- 9.Kachura A., Harris C.S. An ethnobotanical meta-analysis of North American medicinal Asteraceae. Botany. 2021;100:207–217. doi: 10.1139/cjb-2021-0079. [DOI] [Google Scholar]
- 10.Nagy J.G., Tengerdy R.P. Antibacterial action of essential oils of Artemisia as an ecological factor: I. Antibacterial action of the volatile oils of Artemisia tridentata and Artemisia nova on aerobic bacteria. Appl. Microbiol. 1967;15:819–821. doi: 10.1128/am.15.4.819-821.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagy J.G., Tengerdy R.P. Antibacterial action of essential oils of Artemisia as an ecological factor: II. Antibacterial action of the volatile oils of Artemisia tridentata (big sagebrush) on bacteria from the rumen of mule deer. Appl. Microbiol. 1968;16:441–444. doi: 10.1128/am.16.3.441-444.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Natvig D.O. Master’s Thesis. University of Montana; Missoula, MT, USA: 1976. The effects of volatile and other secondary metabolites from Artemisia tridentata on soil microfungi: An ecological approach. [Google Scholar]
- 13.Eichelbaum S.R. Screening of Plants for Antibacterial Properties: Growth Inhibition of Staphylococcus aureus by Artemisia tridentata. Florida International University; Miami, FL, USA: 2016. [Google Scholar]
- 14.Baldemir A., Karaman Ü., İllgün S., Kaçmaz G., Demirci B. Antiparasitic efficacy of Artemisia ludoviciana Nutt. (Asteraceae) essential oil for Acanthamoeba castellanii, Leishmania infantum and Trichomonas vaginalis. Indian J. Pharm. Educ. Res. 2018;52:416–425. doi: 10.5530/ijper.52.3.48. [DOI] [Google Scholar]
- 15.Anaya-Eugenio G.D., Rivero-Cruz I., Rivera-Chávez J., Mata R. Hypoglycemic properties of some preparations and compounds from Artemisia ludoviciana Nutt. J. Ethnopharmacol. 2014;155:416–425. doi: 10.1016/j.jep.2014.05.051. [DOI] [PubMed] [Google Scholar]
- 16.Pelarti M.S., Zarehshuran L.K., Babaeekhou L., Ghane M. Antibacterial, anti-biofilm and anti-quorum sensing activities of Artemisia dracunculus essential oil (EO): A study against Salmonella enterica serovar Typhimurium and Staphylococcus aureus. Arch. Microbiol. 2021;203:1529–1537. doi: 10.1007/s00203-020-02138-w. [DOI] [PubMed] [Google Scholar]
- 17.Anaya-Eugenio G.D., Rivero-Cruz I., Bye R., Linares E., Mata R. Antinociceptive activity of the essential oil from Artemisia ludoviciana. J. Ethnopharmacol. 2016;179:403–411. doi: 10.1016/j.jep.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Dos Santos E., Leitao M.M., Aguero Ito C.N., Silva-Filho S.E., Arena A.C., Silva-Comar F.M.S., Nakamura Cuman R.K., Oliveira R.J., Nazari Formagio A.S., Leite Kassuya C.A. Analgesic and anti-inflammatory articular effects of essential oil and camphor isolated from Ocimum kilimandscharicum Gurke leaves. J. Ethnopharmacol. 2021;269:113697. doi: 10.1016/j.jep.2020.113697. [DOI] [PubMed] [Google Scholar]
- 19.Darwish R.S., Hammoda H.M., Ghareeb D.A., Abdelhamid A.S.A., Bellah El Naggar E.M., Harraz F.M., Shawky E. Efficacy-directed discrimination of the essential oils of three Juniperus species based on their in-vitro antimicrobial and anti-inflammatory activities. J. Ethnopharmacol. 2020;259:112971. doi: 10.1016/j.jep.2020.112971. [DOI] [PubMed] [Google Scholar]
- 20.Ozek G., Schepetkin I.A., Yermagambetova M., Ozek T., Kirpotina L.N., Almerekova S.S., Abugalieva S.I., Khlebnikov A.I., Quinn M.T. Innate immunomodulatory activity of cedrol, a component of essential oils isolated from Juniperus species. Molecules. 2021;26:7644. doi: 10.3390/molecules26247644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schepetkin I.A., Ozek G., Ozek T., Kirpotina L.N., Khlebnikov A.I., Quinn M.T. Chemical composition and immunomodulatory activity of essential oils from Rhododendron albiflorum. Molecules. 2021;26:3652. doi: 10.3390/molecules26123652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schepetkin I.A., Ozek G., Ozek T., Kirpotina L.N., Khlebnikov A.I., Quinn M.T. Chemical composition and immunomodulatory activity of Hypericum perforatum essential oils. Biomolecules. 2020;10:916. doi: 10.3390/biom10060916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozek G., Schepetkin I.A., Utegenova G.A., Kirpotina L.N., Andrei S.R., Ozek T., Baser K.H.C., Abidkulova K.T., Kushnarenko S.V., Khlebnikov A.I., et al. Chemical composition and phagocyte immunomodulatory activity of Ferula iliensis essential oils. J. Leukoc. Biol. 2017;101:1361–1371. doi: 10.1189/jlb.3A1216-518RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schepetkin I.A., Kushnarenko S.V., Ozek G., Kirpotina L.N., Sinharoy P., Utegenova G.A., Abidkulova K.T., Ozek T., Baser K.H., Kovrizhina A.R., et al. Modulation of human neutrophil responses by the essential oils from Ferula akitschkensis and their constituents. J. Agric. Food Chem. 2016;64:7156–7170. doi: 10.1021/acs.jafc.6b03205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schepetkin I.A., Kushnarenko S.V., Ozek G., Kirpotina L.N., Utegenova G.A., Kotukhov Y.A., Danilova A.N., Ozek T., Baser K.H., Quinn M.T. Inhibition of human neutrophil responses by the essential oil of Artemisia kotuchovii and its constituents. J. Agric. Food Chem. 2015;63:4999–5007. doi: 10.1021/acs.jafc.5b01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopes-Lutz D., Alviano D.S., Alviano C.S., Kolodziejczyk P.P. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry. 2008;69:1732–1738. doi: 10.1016/j.phytochem.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Hosseini S.F., Amraie M., Salehi M., Mohseni M., Aloui H. Effect of chitosan-based coatings enriched with savory and/or tarragon essential oils on postharvest maintenance of kumquat (Fortunella sp.) fruit. Food Sci. Nutr. 2019;7:155–162. doi: 10.1002/fsn3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babushok V.I., Linstrom P.J., Zenkevich I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data. 2011;40:043101. doi: 10.1063/1.3653552. [DOI] [Google Scholar]
- 29.Hulley I.M., Sadgrove N.J., Tilney P.M., Ozek G., Yur S., Ozek T., Baser K.H.C., van Wyk B.E. Essential oil composition of Pentzia incana (Asteraceae), an important natural pasture plant in the Karoo region of South Africa. Afr. J. Range Sci. 2018;35:137–145. doi: 10.2989/10220119.2018.1495265. [DOI] [Google Scholar]
- 30.Darriet F. Caractérisation de Nouvelles Molécules et Variabilité Chimique de Trois Plantes du Contin-uum Corse-Sardaigne: Chamaemelum Mixtum, Anthemis Maritima et Eryngium Maritimum. University of Corsica Pasquale; Paoli, France: 2011. [Google Scholar]
- 31.Turkmenoglu F.P., Agar O.T., Akaydin G., Hayran M., Demirci B. Characterization of volatile compounds of eleven Achillea species from Turkey and biological activities of essential oil and methanol extract of A. hamzaoglui Arabaci & Budak. Molecules. 2015;20:11432–11458. doi: 10.3390/molecules200611432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demirci B., Baser K.H.C. The essential oil composition of Tanacetum macrophyllum (Waldst. et Kit.) Schultz. Bip. J. Essent. Oil Res. 2007;19:255–257. doi: 10.1080/10412905.2007.9699272. [DOI] [Google Scholar]
- 33.Brophy J.J., Goldsack R.J., Forster P.I., Southwell I.A. Leaf oils of the genus Acradenia (Rutaceae) J. Essent. Oil Res. 2001;13:136–139. doi: 10.1080/10412905.2001.9699639. [DOI] [Google Scholar]
- 34.Buttkus H.A., Bose R.J., Shearer D.A. Terpenes in the essential oil of sagebrush (Artemisia tridentata) J. Agric. Food Chem. 1977;25:288–291. doi: 10.1021/jf60210a021. [DOI] [Google Scholar]
- 35.Borek T.T., Hochrein J.M., Irwin A.N. Composition of the essential oils from Rocky Mountain juniper (Juniperus scopulorum), Big sagebrush (Artemisia tridentata), and White Sage (Salvia apiana) Sandia National Laboratories (SNL); Albuquerque, NM, USA; Livermore, CA, USA: 2003. [Google Scholar]
- 36.Powell J. Site factor relationships with volatile oils in big sagebrush. Rangel. Ecol. Manag. J. Range Manag. Arch. 1970;23:42–46. doi: 10.2307/3896006. [DOI] [Google Scholar]
- 37.Welch B.L., McArthur E.D. Variation of monoterpenoid content among subspecies and accessions of Artemisia tridentata grown in a uniform garden. Rangeland Ecol. Manag. 1981;34:380–384. doi: 10.2307/3897909. [DOI] [Google Scholar]
- 38.Epstein W.W., Gaudioso L.A. Santolinolide B [(2R, 3S, 4S)-4-hydroxy-2, 5-dimethyl-3-vinyl-5-hexenoic acid lactone]. A new irregular monoterpene from Artemisia tridentata tridentata. J. Org. Chem. 1979;44:3113–3117. doi: 10.1021/jo01332a006. [DOI] [Google Scholar]
- 39.Epstein W.W., Gaudioso L.A., Brewster G.B. Essential oil constituents of Artemisia tridentata rothrockii. The isolation and characterization of two new irregular monoterpenes. J. Org. Chem. 1984;49:2748–2754. doi: 10.1021/jo00189a021. [DOI] [Google Scholar]
- 40.Gunawardena K., Rivera S., Epstein W. The monoterpenes of Artemisia tridentata ssp. vaseyana, Artemisia cana ssp. viscidula and Artemisia tridentata ssp. spiciformis. Phytochemistry. 2002;59:197–203. doi: 10.1016/S0031-9422(01)00438-1. [DOI] [PubMed] [Google Scholar]
- 41.Collin G., St-Gelais A., Turcotte M., Gagnon H. Composition of the essential oil and of some extracts of the aerial parts of Artemisia ludoviciana var. latiloba Nutt. Am. J. Essential Oils Nat. Prod. 2016;4:28–38. [Google Scholar]
- 42.Ruiz-Cancino A., Cano A.E., Delgado G. Sesquiterpene lactones and flavonoids from Artemisia ludoviciana ssp. mexicana. Phytochemistry. 1993;33:1113–1115. doi: 10.1016/0031-9422(93)85032-M. [DOI] [Google Scholar]
- 43.Ohno N., Gershenzon J., Roane C., Mabry T.J. 11, 13-dehydrodesacetylmatricarin and other sesquiterpene lactones from Artemisia ludoviciana var. Ludoviciana and the identity of artecanin and chyrsartemin B. Phytochemistry. 1980;19:103–106. doi: 10.1016/0031-9422(80)85022-9. [DOI] [Google Scholar]
- 44.Geissman T., Saitoh T. Ludalbin, a new lactone from Artemisia ludoviciana. Phytochemistry. 1972;11:1157–1160. doi: 10.1016/S0031-9422(00)88471-X. [DOI] [Google Scholar]
- 45.Mumivand H., Ebrahimi A., Morshedloo M.R., Shayganfar A. Water deficit stress changes in drug yield, antioxidant enzymes activity and essential oil quality and quantity of Tarragon (Artemisia dracunculus L.) Ind. Crop. Prod. 2021;164:113381. doi: 10.1016/j.indcrop.2021.113381. [DOI] [Google Scholar]
- 46.Fraternale D., Flamini G., Ricci D. Essential oil composition and antigermination activity of Artemisia dracunculus (Tarragon) Nat. Prod. Commun. 2015;10:1469–1472. doi: 10.1177/1934578X1501000839. [DOI] [PubMed] [Google Scholar]
- 47.Sahakyan N., Andreoletti P., Cherkaoui-Malki M., Petrosyan M., Trchounian A. Artemisia dracunculus L. essential oil phytochemical components trigger the activity of cellular antioxidant enzymes. J. Food Biochem. 2021;45:e13691. doi: 10.1111/jfbc.13691. [DOI] [PubMed] [Google Scholar]
- 48.Gilemeister E., Hoffmann F. Die Aetherischen Ole. Volume 7. Academie Verlag.; Berlin, Germany: 1961. pp. 714–715. [Google Scholar]
- 49.Pushkareva E.S., Efremov A.A. Component composition of the essential oil of Artemisia frigida from Krasnoyarsk region and of its individual fractions. Sorption Chromatog. Proc. 2012;12:619–623. [Google Scholar]
- 50.Atazhanova G.A., Dembitskii A.D., Yakovleva T.D., Ishmuratova M.Y., Mikhailov V.G., Adekenov S.M. Composition of the essential oils of Artemisia radicans and A. frigida. Chem. Nat. Compd. 1999;35:427–429. doi: 10.1007/BF02282509. [DOI] [Google Scholar]
- 51.Korolyuk E., Tkachev A. Chemical composition of the essential oil from two wormwood species Artemisia frigida and Artemisia argyrophylla. Russ. J. Bioorg. Chem. 2010;36:884–893. doi: 10.1134/S1068162010070162. [DOI] [Google Scholar]
- 52.Zhigzhitzhapova S.V., Randalova T.E., Radnaeva L.D., Dylenova E.P., Chen S., Zhang F. Chemical composition of essentials oils of Artemisia frigida Willd. (Asteraceae) grown in the North and Central Asia. J. Essent. Oil Bear. Plants. 2017;20:915–926. doi: 10.1080/0972060X.2017.1377113. [DOI] [Google Scholar]
- 53.Liu X.C., Li Y., Wang T., Wang Q., Liu Z.L. Chemical composition and insecticidal activity of essential oil of Artemisia frigida Willd (Compositae) against two grain storage insects. Trop. J. Pharm. Res. 2014;13:587–592. doi: 10.4314/tjpr.v13i4.15. [DOI] [Google Scholar]
- 54.Borchuluun S., Wang Q., Hao J. Extraction of essential oil from the aerial parts of Artemisia frigida Willd by way of hydrodistillation. Proc. Mongolian Acad. Sci. 2020;60:25–30. doi: 10.5564/pmas.v60i2.1355. [DOI] [Google Scholar]
- 55.Borchuluun S., Wang Q., Xu Y., He X., Bao W., Pa B. Structure elucidation and NMR assignments of a new sesquiterpene of volatile oil from Artemisia frigida Willd. Nat. Prod. Res. 2021;35:2376–2380. doi: 10.1080/14786419.2019.1677653. [DOI] [PubMed] [Google Scholar]
- 56.Kelsey R.G., Shafizadeh F., Campbell J.A., Craig A.C., Campana C.F., Craig R.E. Canin from Artemisia cana Pursh ssp. cana. Crystal structure and identification of chrysartemin A. J. Org. Chem. 1983;48:125–127. doi: 10.1021/jo00149a026. [DOI] [Google Scholar]
- 57.Lee K., Simpson R., Geissman T. Sesquiterpenoid lactones of Artemisia. Constituents of Artemisia cana ssp. cana. The structure of canin. Phytochemistry. 1969;8:1515–1521. doi: 10.1016/S0031-9422(00)85924-5. [DOI] [Google Scholar]
- 58.Shafizadeh F., Bhadane N. Chemical constituents of sagebrush. V. Sesquiterpene lactones of sagebrush. New guaianolides from Artemisia cana subspecies viscidula. J. Org. Chem. 1972;37:3168–3173. doi: 10.1021/jo00985a028. [DOI] [Google Scholar]
- 59.Malech H.L., DeLeo F.R., Quinn M.T. The role of neutrophils in the immune system: An overview. Methods Mol. Biol. 2020;2087:3–10. doi: 10.1007/978-1-0716-0154-9_1. [DOI] [PubMed] [Google Scholar]
- 60.Keir H.R., Chalmers J.D. Neutrophil extracellular traps in chronic lung disease: Implications for pathogenesis and therapy. Eur. Respir. Rev. 2022;31:210241. doi: 10.1183/16000617.0241-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stojkov D., Gigon L., Peng S., Lukowski R., Ruth P., Karaulov A., Rizvanov A., Barlev N.A., Yousefi S., Simon H.U. Physiological and pathophysiological roles of metabolic pathways for NET formation and other neutrophil functions. Front. Immunol. 2022;13:826515. doi: 10.3389/fimmu.2022.826515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dixit N., Kim M.H., Rossaint J., Yamayoshi I., Zarbock A., Simon S.I. Leukocyte function antigen-1, kindlin-3, and calcium flux orchestrate neutrophil recruitment during inflammation. J. Immunol. 2012;189:5954–5964. doi: 10.4049/jimmunol.1201638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ali H., Richardson R.M., Haribabu B., Snyderman R. Chemoattractant receptor cross-desensitization. J. Biol. Chem. 1999;274:6027–6030. doi: 10.1074/jbc.274.10.6027. [DOI] [PubMed] [Google Scholar]
- 64.Jyotshna, Srivastava N., Singh B., Chanda D., Shanker K. Chemical composition and acetylcholinesterase inhibitory activity of Artemisia maderaspatana essential oil. Pharm. Biol. 2015;53:1677–1683. doi: 10.3109/13880209.2014.1001405. [DOI] [PubMed] [Google Scholar]
- 65.Zhang H., Zhou D., Luo Y., Wang J., Zong S. Identification of volatile compounds emitted by Artemisia ordosica (Artemisia, Asteraceae) and changes due to mechanical damage and weevil infestation. Z. Naturforsch. C. 2013;68:313–317. doi: 10.1515/znc-2013-7-809. [DOI] [PubMed] [Google Scholar]
- 66.Zhang H., Zong S., Luo Y., Wang T., Wang J., Cao C. Comparative study of the volatiles’ composition of healthy and larvae-infested Artemisia ordosica. Z. Naturforsch. C. 2013;68:8–12. [PubMed] [Google Scholar]
- 67.Padalia R.C., Verma R.S., Chauhan A., Chanotiya C.S., Yadav A. Variation in the volatile constituents of Artemisia annua var. CIM-Arogya during plant ontogeny. Nat. Prod. Commun. 2011;6:239–242. doi: 10.1177/1934578X1100600221. [DOI] [PubMed] [Google Scholar]
- 68.Gonzalez S.B., Gastaldi B., Catalan C., Di Leo Lira P., Retta D., van Baren C.M., Bandoni A.L. Artemisia magellanica. Chemical composition of the essential oil from an unexplored endemic species of Patagonia. Chem. Biodivers. 2019;16:e1900125. doi: 10.1002/cbdv.201900125. [DOI] [PubMed] [Google Scholar]
- 69.Govindarajan M., Benelli G. Artemisia absinthium-borne compounds as novel larvicides: Effectiveness against six mosquito vectors and acute toxicity on non-target aquatic organisms. Parasitol. Res. 2016;115:4649–4661. doi: 10.1007/s00436-016-5257-1. [DOI] [PubMed] [Google Scholar]
- 70.Cha J.D., Jeong M.R., Choi H.J., Jeong S.I., Moon S.E., Yun S.I., Kim Y.H., Kil B.S., Song Y.H. Chemical composition and antimicrobial activity of the essential oil of Artemisia lavandulaefolia. Planta Med. 2005;71:575–577. doi: 10.1055/s-2005-864164. [DOI] [PubMed] [Google Scholar]
- 71.Yu Z., Wang B., Yang F., Sun Q., Yang Z., Zhu L. Chemical compositionand anti-acetyl cholinesterase activity of flower essential oils of Artemisia annua at different flowering stages. Iran J. Pharm. Res. 2011;10:265–271. [PMC free article] [PubMed] [Google Scholar]
- 72.Qadir M., Maurya A.K., Agnihotri V.K., Shah W.A. Volatile composition, antibacterial and antioxidant activities of Artemisia tournefortiana Reichb. from Kashmir, India. Nat. Prod. Res. 2021;35:152–156. doi: 10.1080/14786419.2019.1613990. [DOI] [PubMed] [Google Scholar]
- 73.Xu T., Xu M., Lu Y.Y., Zhang W.Q., Sun J.H., Zeng R.S., Turlings T.C.J., Chen L. A trail pheromone mediates the mutualism between ants and aphids. Curr. Biol. 2021;31:4738–4747. doi: 10.1016/j.cub.2021.08.032. [DOI] [PubMed] [Google Scholar]
- 74.Wang B., Dong W., Li H., D’Onofrio C., Bai P., Chen R., Yang L., Wu J., Wang X., Wang B., et al. Molecular basis of (E)-β-farnesene-mediated aphid location in the predator Eupeodes corollae. Curr. Biol. 2022;32:951–962.e957. doi: 10.1016/j.cub.2021.12.054. [DOI] [PubMed] [Google Scholar]
- 75.Orhan I.E. A Review focused on molecular mechanisms of anxiolytic effect of Valerina officinalis L. in connection with its phytochemistry throughin vitro/in vivo studies. Curr. Pharm. Design. 2021;27:3084–3090. doi: 10.2174/1381612827666210119105254. [DOI] [PubMed] [Google Scholar]
- 76.Yang L., Liu J., Li Y., Qi G. Bornyl acetate suppresses ox-LDL-induced attachment of THP-1 monocytes to endothelial cells. Biomed. Pharmacother. 2018;103:234–239. doi: 10.1016/j.biopha.2018.03.152. [DOI] [PubMed] [Google Scholar]
- 77.Chen N., Sun G., Yuan X., Hou J., Wu Q., Soromou L.W., Feng H. Inhibition of lung inflammatory responses by bornyl acetate is correlated with regulation of myeloperoxidase activity. J. Surg. Res. 2014;186:436–445. doi: 10.1016/j.jss.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 78.Wu X., Xiao F., Zhang Z., Li X., Xu Z. Research on the analgesic effect and mechanism of bornyl acetate in volatile oil from amomum villosum. Zhong Yao Cai. 2005;28:505–507. [PubMed] [Google Scholar]
- 79.Daina A., Michielin O., Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. UK. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tetko I.V., Tanchuk V.Y. Application of associative neural networks for prediction of lipophilicity in ALOGPS 2.1 program. J. Chem. Inf. Comp. Sci. 2002;42:1136–1145. doi: 10.1021/ci025515j. [DOI] [PubMed] [Google Scholar]
- 81.Suenderhauf C., Hammann F., Huwyler J. Computational prediction of blood-brain barrier permeability using decision tree induction. Molecules. 2012;17:10429–10445. doi: 10.3390/molecules170910429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferrari C., Macchiarulo A., Costantino G., Pellicciari R. Pharmacophore model for bile acids recognition by the FPR receptor. J. Comput. Aid. Mol. Des. 2006;20:295–303. doi: 10.1007/s10822-006-9055-1. [DOI] [PubMed] [Google Scholar]
- 83.Chen X., Yang D., Shen W., Dong H.F., Wang J.M., Oppenheim J.J., Howard M.Z. Characterization of chenodeoxycholic acid as an endogenous antagonist of the G-coupled formyl peptide receptors. Inflamm. Res. 2000;49:744–755. doi: 10.1007/s000110050656. [DOI] [PubMed] [Google Scholar]
- 84.Chen X., Mellon R.D., Yang L., Dong H., Oppenheim J.J., Howard O.M. Regulatory effects of deoxycholic acid, a component of the anti-inflammatory traditional Chinese medicine Niuhuang, on human leukocyte response to chemoattractants. Biochem. Pharmacol. 2002;63:533–541. doi: 10.1016/S0006-2952(01)00917-0. [DOI] [PubMed] [Google Scholar]
- 85.Pamplona F.A., Ferreira J., de Lima O.M., Jr., Duarte F.S., Bento A.F., Forner S., Villarinho J.G., Bellocchio L., Wotjak C.T., Lerner R., et al. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc. Natl. Acad. Sci. USA. 2012;109:21134–21139. doi: 10.1073/pnas.1202906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ge Y., Zhang S., Wang J., Xia F., Wan J.B., Lu J., Ye R.D. Dual modulation of formyl peptide receptor 2 by aspirin-triggered lipoxin contributes to its anti-inflammatory activity. FASEB J. 2020;34:6920–6933. doi: 10.1096/fj.201903206R. [DOI] [PubMed] [Google Scholar]
- 87.Das U.N. Essential fatty acids and their metabolites in the pathobiology of inflammation and its resolution. Biomolecules. 2021;11:1873. doi: 10.3390/biom11121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park J., Langmead C.J., Riddy D.M. New advances in targeting the resolution of inflammation: Implications for specialized pro-resolving mediator GPCR drug discovery. ACS Pharmacol. Transl. 2020;3:88–106. doi: 10.1021/acsptsci.9b00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu D.J., Furuya W., Grinstein S. Involvement of multiple kinases in neutrophil activation. Blood Cells. 1993;19:343–351. [PubMed] [Google Scholar]
- 90.Bokoch G.M. Chemoattractant signaling and leukocyte activation. Blood. 1995;86:1649–1660. doi: 10.1182/blood.V86.5.1649.bloodjournal8651649. [DOI] [PubMed] [Google Scholar]
- 91.Schepetkin I.A., Khlebnikov A.I., Giovannoni M.P., Kirpotina L.N., Cilibrizzi A., Quinn M.T. Development of small molecule non-peptide formyl peptide receptor (FPR) ligands and molecular modeling of their recognition. Curr. Med. Chem. 2014;21:1478–1504. doi: 10.2174/0929867321666131218095521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.He H.Q., Ye R.D. The formyl peptide receptors: Diversity of ligands and mechanism for recognition. Molecules. 2017;22:455. doi: 10.3390/molecules22030455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Knall C., Young S., Nick J.A., Buhl A.M., Worthen G.S., Johnson G.L. Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J. Biol. Chem. 1996;271:2832–2838. doi: 10.1074/jbc.271.5.2832. [DOI] [PubMed] [Google Scholar]
- 94.Ozek G., Ishmuratova M., Tabanca N., Radwan M.M., Goger F., Ozek T., Wedge D.E., Becnel J.J., Cutler S.J., Can Baser K.H. One-step multiple component isolation from the oil of Crinitaria tatarica (Less.) Sojak by preparative capillary gas chromatography with characterization by spectroscopic and spectrometric techniques and evaluation of biological activity. J. Sep. Sci. 2012;35:650–660. doi: 10.1002/jssc.201100950. [DOI] [PubMed] [Google Scholar]
- 95.Schepetkin I.A., Kirpotina L.N., Khlebnikov A.I., Quinn M.T. High-throughput screening for small-molecule activators of neutrophils: Identification of novel N-formyl peptide receptor agonists. Mol. Pharmacol. 2007;71:1061–1074. doi: 10.1124/mol.106.033100. [DOI] [PubMed] [Google Scholar]
- 96.Liu X., Ouyang S., Yu B., Liu Y., Huang K., Gong J., Zheng S., Li Z., Li H., Jiang H. PharmMapper server: A web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acid. Res. 2010;38:W609–W614. doi: 10.1093/nar/gkq300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article and supplementary material.