Abstract
Cell surface analysis often requires manipulation of cells prior to examination. The most commonly employed procedures are centrifugation at different speeds, changes of media during washing or final resuspension, desiccation (either air drying for contact angle measurements or freeze-drying for sensitive spectroscopic analysis, such as X-ray photoelectron spectroscopy), and contact with hydrocarbon (hydrophobicity assays). The effects of these procedures on electrophoretic mobility, adhesion to solid substrata, affinity to a number of Sepharose columns, structural integrity, and cell viability were systematically investigated for a range of model organisms, including carbon- and nitrogen-limited Psychrobacter sp. strain SW8 (glycocalyx-bearing cells), Escherichia coli (gram-negative cells without a glycocalyx), and Staphylococcus epidermidis (gram-positive cells without a glycocalyx). All of the cell manipulation procedures severely modified the physicochemical properties of cells, but with each procedure some organisms were more susceptible than others. Considerable disruption of cell surfaces occurred when organisms were placed in contact with a hydrocarbon (hexadecane). The majority of cells became nonculturable after air drying and freeze-drying. Centrifugation at a high speed (15,000 × g) modified many cell surface parameters significantly, although cell viability was considerably affected only in E. coli. The type of washing or resuspension medium had a strong influence on the values of cell surface parameters, particularly when high-salt solutions were compared with low-salt buffers. The values for parameters obtained with different methods that allegedly measure similar cell surface properties did not correlate for most cells. These results demonstrate that the methods used to prepare cells for cell surface analysis need to be critically investigated for each microorganism so that the final results obtained reflect the nature of the in situ microbial cell surface as closely as possible. There is an urgent need for new, reliable, nondestructive, minimally manipulative cell surface analysis techniques that can be used in situ.
The surfaces of microbial cells are vital to the organisms’ survival, since it is via them that the bacteria interact with the environment. Characterization of these surfaces is a rapidly expanding field of microbiology and encompasses both the macromolecular constitution and, on a more generic level, physicochemical properties, such as hydrophobicity and surface charge. Quantitative and qualitative measurement of these properties under laboratory conditions requires methods which provide data that is representative of the microorganism’s natural environment. The techniques routinely employed for characterizing the physicochemical nature of microbial cell surfaces include techniques involving sessile liquid drop contact angle measurement, microbial adhesion to hydrocarbon (MATH), infrared spectroscopy, X-ray photoelectron spectroscopy (XPS), electrophoretic mobility, electron microscopy, retention on chromatographic resins, and adhesion to inanimate materials. Most of these methods require cell preparation prior to analysis.
For contact angle measurement, a layer of bacterial cells is deposited on a membrane filter, and the contact angle of a drop of a diagnostic liquid on the bacterial filter cake is measured with a goniometer (5, 50, 56). In this system, the values of water contact angles depend on the degree of dehydration of the cells in the filter cake (1). Contact angles change continuously as the filter cake dries until the level of dehydration allows the angle at the liquid-surface interface to remain stable for 3 to 12 s. Excessive drying should be avoided as this may lead to collapse of hydrophobic structures on the cell surface and hence erratic contact angle values (52).
Cell surface hydrophobicity is often assessed by using the MATH assay (42). A cell suspension is mixed with hydrocarbon for a predetermined period to allow optimal interaction of the bacteria with the hydrocarbon phase (52). As a result, cells may remain in the liquid phase or partition either into the liquid-hydrocarbon interface or into the hydrocarbon phase, depending on their hydrophobicity. Many investigators have modified the original MATH test and have found that seemingly small variations in experimental conditions, such as the diameter of the test tubes, the pH of the suspension medium, and the volume of hydrocarbon used, can significantly alter the results (4, 17, 30). Aliphatic hydrocarbons are used in most cases, since aromatic hydrocarbons cause lysis of some bacterial species (55).
High-vacuum chemical analytical techniques, such as XPS, are increasingly used for chemical analysis of bacterial cells (43). The XPS technique requires that microorganisms be washed in desalted water and freeze-dried under a vacuum. Correlations between the data obtained with this cell surface technique and the data obtained with some of the other physiochemical methods have been reported for various yeasts and bacteria (8, 9, 24, 34), and many workers believe that these results accurately represent the properties of the hydrated cell envelope. However, other authors have suggested that the high vacuum necessary for the XPS technique and the resulting dehydration disrupt the cell surface to the extent that they seriously compromise the validity of the cell surface analysis in relation to hydrated samples (32).
Marshall et al. (32) pointed out the inherent dangers in extrapolating data from laboratory studies to microbial ecology. Any variation in or loss of surface polymers in vitro may completely alter the attachment mechanisms of an organism compared to its behavior in the natural environment. Considering the degree of variability introduced into any analytical protocol through operator error, as well as the delicate nature of microbial outer surface structures, it is questionable whether the parameters measured by the techniques used represent true microbial cell surface properties and whether the protocols used for cell preparation introduce artifacts and, therefore, additional sources of error. To our knowledge, there has not been a study which has comprehensively addressed how standard cell preparation procedures, which are integral to many cell surface analysis protocols, affect cell surface characteristics, structural integrity, and the survival of prokaryotic organisms.
In this study, we examined changes in the physicochemical surface properties of organisms subjected to high-speed centrifugation, air drying, and freeze-drying, which are techniques commonly used in many cell surface analysis protocols. These tests were performed with the following three microorganisms, each of which is representative of a different type of cell surface structure: Escherichia coli (a gram-negative rod), Staphylococcus epidermidis (a gram-positive coccus), and Psychrobacter sp. strain SW8 (a gram-negative coccobacillus with an extensive constitutive glycocalyx). The phenotypic variability of the results was assessed with carbon- and nitrogen-limited Psychrobacter sp. strain SW8. The structural integrity and viability of manipulated cell samples were scrutinized by using electron microscopy and viable cell counting. Changes in cell surface characteristics after treatment were assessed by assays for MATH, electrophoretic mobility, adhesion to solid substrata, and affinity to Sepharose gel columns. The latter two assays were employed because the interactions of cells with substrata depend essentially on the constitution of the outermost molecular layer of the cell surface. These methods are, therefore, sensitive indicators of the integrity of this part of the microbial interface.
MATERIALS AND METHODS
Reagents.
All of the chemicals used were analytical grade reagents obtained from BDH, Sigma, or Merck unless otherwise stated. A sodium chloride standard solution containing 24 g of NaCl per liter and buffered with 1 mM Na2HPO4 (pH 7.5) was prepared with water obtained from a Milli-Q ZFMQ 23004 reverse-osmosis unit (Millipore, Bedford, Mass.). The water used for resuspending cell preparations was also buffered with 1 mM Na2HPO4 to pH 7.5.
Microorganisms and cultivation.
The gram-negative marine organism Psychrobacter sp. strain SW8 was grown in continuous culture with an artificial seawater (ASW) minimal medium, as described by Schneider and Marshall (46). The chemostat temperature was 20°C, and feedback-controlled addition of 1 M HCl maintained the pH at 7.5. Sterile air was supplied to fermentors (culture volume, approximately 1 liter in a 1.5-liter vessel) at a rate of 3 liters of air per min, and the impeller speed was 500 rpm. A continuous supply of ASW medium was provided at a dilution rate of 0.08 h−1 by using peristaltic pumps (Watson-Marlow, Falmouth, Cornwall, England). The growth-limiting substrates were acetate for carbon-limited organisms and ammonia for nitrogen-limited cells. E. coli ATCC 8739 and S. epidermidis NCTC 11047 were grown in batch cultures with nutrient broth (Oxoid catalog no. CM3). Single loopfuls of either E. coli or S. epidermidis grown on nutrient agar slants were used to inoculate 250-ml Erlenmeyer flasks containing 20 ml of nutrient broth, which were then incubated for 16 h at 30°C on an orbital shaker. A 10% (vol/vol) inoculum from each culture was transferred into a flask containing fresh nutrient broth and grown to the mid-logarithmic phase (6 h for E. coli and 8 h for S. epidermidis). The optical densities at 546 nm (OD546) of the cultures were determined with a spectrophotometer (Ultraspec II; LKB, Bromma, Sweden).
Preparation of bacterial suspensions for analyses.
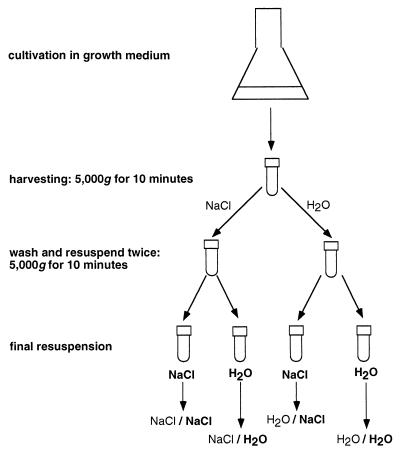
A flow diagram illustrating the procedure used for cell sample preparation is shown in Fig. 1. Unless otherwise stated, all bacterial cultures were harvested by centrifugation at 5,000 × g for 10 min. Each cell pellet was resuspended in either the NaCl solution or water and then centrifuged and washed twice before final resuspension either in fresh NaCl solution or water. Abbreviations are used below to indicate the washing and resuspension media used for preparation of cell samples; for example, NaCl/H2O indicates that the cell sample was washed twice in the NaCl solution before final resuspension in water. The abbreviations used are shown in Fig. 1. The buffered NaCl solution contained the same concentration of NaCl as ASW medium. The OD546 of the cell suspensions were adjusted to 1.0 for bacterial filter cake preparation (5) and to 0.3 for MATH assays (34), and a slightly turbid cell suspension (OD546, 0.1) was used for electrophoretic mobility measurements.
FIG. 1.
Schematic representation of the harvesting protocol and washing sequences used to prepare microorganisms for cell surface analysis. In each case the washing medium is listed before the resuspension medium (i.e., wash medium/resuspension medium).
Air-dried bacterial samples were prepared by the method described by van Oss et al. (56). Briefly, negative-pressure filtration was used to deposit cells onto cellulose acetate filters (pore size, 0.2 μm; diameter, 45 mm; Sartorius, Göttingen, Germany), and the filter cakes were subsequently dried on agar plates to avoid excessive desiccation. Sessile liquid droplets placed on bacterial cell lawns were used to measure contact angles with a manually adjusted goniometer. The optimum time to obtain measurements was determined by monitoring the stability of contact angles as a function of drying time (5). Filter cakes of each of the bacterial species tested provided stable contact angle values after approximately 90 min of air drying.
The protocol described by Amory et al. (2) was used as a model of the procedure used to prepare cells for analysis by high-vacuum techniques, such as XPS. Harvested bacterial suspensions were washed and concentrated (100:1) in water, and 0.05-ml aliquots were subsequently transferred to acid-washed glass vials (diameter, 8 mm; length, 100 mm). The cells were rapidly frozen by immersing the vials in liquid nitrogen for 15 min prior to vacuum desiccation (268 K) with a Speedvac centrifugal freeze-drier (model 5PS; Edward’s High Vacuum Ltd., Crawley, England). Freeze-dried samples were stored under a vacuum before they were resuspended in either the NaCl solution or water.
MATH test.
Cell surface hydrophobicity was measured by the MATH test, as modified by Neu and Marshall (36). Briefly, 4-ml samples of a cell suspension were transferred to individual test tubes (diameter, 1.7 cm; length, 15 cm), each of which contained 1 ml of dodecane. The test tubes were vortexed at full speed for 2 min (Vortex Genie; Scientific Industries Inc., Springfield, Mass.) and then left to stand for 15 min to allow phase separation. The OD546 of the aqueous phase was determined, and partitioning of the bacterial suspension was expressed as the percentage of cells adsorbed by the hydrocarbon phase: percent partitioning = [(0.3 − OD546 of aqueous phase)/0.3] × 100. The mean percentage of partitioning of an organism into the dodecane phase was calculated by using triplicate samples.
Electrophoretic mobility.
Cell surface charge was measured by using a Delsa Zetameter and Delsa 440 software (Coulter Instruments, Hialeah, Fla.). The electrophoretic mobilities of triplicate samples of cell preparations resulting from each cell surface analysis technique were determined by the four-angle Doppler shift method, which is based on application of the Smoluchowski equation (23). The results were expressed in micrometer-centimeters per volt-second.
Bacterial cell counting.
Bacterial suspensions resulting from each cell preparation technique were serially diluted in order to determine the total number of cells per milliliter with a hemocytometer (Improved Neubauer; Gordon-Keeble GP, Barton Mills, England). To determine viable cell counts, six 0.05-ml drops from 10−5, 10−6, and 10−7 dilutions of each sample were placed on nutrient agar plates and incubated at 30°C for 60 h. For each dilution series, the numbers of CFU per milliliter were counted, and the average based on triplicate plate counts was determined. The percentage of surviving organisms was determined by dividing CFU by the total count.
Bacterial adhesion to inanimate substrata.
Commercially available aluminum, stainless steel, perspex, and polypropylene were cut into coupons having uniform dimensions (38 by 19 by 3 mm) and were polished to a substratum surface roughness of less than 0.1 μm, as described by Schneider and Marshall (46). Each coupon was immersed in 95% ethanol for 1 h (or 10 s for the perspex coupons), thoroughly rinsed with water, and then transferred to a heated 10% (wt/vol) sodium dodecyl sulfate solution. After 1 h, the substrata were washed twice in boiling water and air dried under a protective hood. The residual organic contaminants remaining on the aluminum and stainless steel coupons after this procedure were removed by glow discharge for 90 s under a vacuum at 8 kV and 100 Torr with a Speedvac coating unit (Edward’s High Vacuum Ltd.).
Clean coupons were mounted in perspex chambers that had been cleaned with 95% ethanol and rinsed with boiling water; these chambers were constructed to have laminar fluid flow characteristics (channel depth, 1 mm) (46). The chambers were mounted vertically to eliminate the influence of gravity on cell attachment. A multichannel peristaltic pump (Ismatec, Glattbrugg, Switzerland) equipped with a pulsation dampening device was used to pump solutions to three parallel flow cells at a constant flow rate of 0.36 cm/s in each chamber. The chambers were equilibrated by flushing them with the appropriate solution (either the NaCl solution or water; both solutions were buffered with 1 mM phosphate buffer at pH 7.4) for at least 5 min before the substrata were exposed to bacterial suspensions for 10 min. Unattached cells were removed by washing the chambers with fresh suspension medium for an additional 15 min. The chambers were drained by reverse flushing at the same flow rate, and the substrata were allowed to dry.
Attached organisms were stained with a fluorochrome solution containing 1.0 μg of 4′,6′-diamidino-2-phenylindole (DAPI) per ml. Fluorescing organisms were examined at a magnification of ×500 under oil immersion (area of field of view, 2.01 × 10−2 mm2) by using an epifluorescence microscope (model BH2; Olympus, Tokyo, Japan) equipped with a type UG-1 exciter filter and a type L-420 barrier filter. Ten randomly selected fields were counted for each coupon. Statistically invalid field counts were identified and eliminated by using the method described by Schneider and Marshall (46). These counts occurred primarily when a randomly selected microscopic field coincided with a spot containing a large number of cells deposited from a liquid droplet that remained on the substratum after washing. First, the mean number of bacterial cells found attached to the 10 randomly selected fields counted for each test substratum was determined. Counts for individual fields that differed significantly from the mean were eliminated by using Grubb’s outlier test at P = 90% (15), and a new average for cells attached to the substratum was calculated; the procedure was repeated until no further outliers were found. In these experiments, 6.3% of individual field counts were eliminated. Grubb’s outlier test uses the following equation: Y = (|X* − X̄|)/ς, where X̄ is the mean of a series with n components, X* is the value most different from the mean value for the series, and ς is the standard deviation. rN is the highest value for Y at which there is 90% probability that X* is a valid value in a series with n components. If Y < rN, X* is within the main series; if Y > rN, X* is an outlier and is removed from the main series. In these cases, calculations were repeated for a new series with (n − 1) components. The cell counts obtained for each of the three substratum samples used in each experiment were compared by using Student’s t test at a P of <0.01 to identify possible inconsistencies in the results obtained with the different replicates of the same material, but no significant differences in attachment of cells were found. The mean and standard deviation for the number of microbes retained on a substratum in a particular experiment were then calculated by combining the field counts obtained from the three replicate coupons. Results obtained in adhesion assays were statistically reproducible, as evidenced by replicate experiments conducted throughout the study period with cells of the three organisms that had been treated at 5,000 × g.
Sepharose gel interaction chromatography.
Chromatography with Sepharose gels reveals interactions of microbial cells in columns packed with agarose beads. The surface chemistry of the beads can be modified in a controlled manner by covalent binding of aliphatic or charged residues. A range of commercially available beads with different well-characterized surface properties allows probing of a variety of cell surface parameters, such as hydrophobicity (33) and surface charge (24). Since bacteria may become bound to or entrapped by the Sepharose gel networks themselves (1), unsubstituted gels are used as controls. Sepharose gel interaction chromatography experiments were performed by using a modified method based on the technique developed by Smyth et al. (49). Pasteur pipettes plugged with glass wool were filled with 1.0 ml (bed volume) of either Sepharose CL-4B, phenyl-Sepharose CL-4B, octyl-Sepharose CL-4B, DEAE-Sepharose CL-6B, or carboxymethyl-Sepharose CL-6B gel (Pharmacia, Uppsala, Sweden) and equilibrated with either the NaCl solution or water. The void volume for all gels was determined to be 0.5 ml by using dextran blue. A 1-ml aliquot of a bacterial suspension (approximately 8 × 108 cells/ml) was applied to each column and was subsequently eluted with 1 ml of the appropriate resuspension medium. The OD546 of the eluates collected from each column were determined. Each test was performed in triplicate, and the means were calculated. The amounts of bacterial cells not retained in the gel were determined from the OD546 of the eluates. The affinity of each bacterial species for each gel was expressed as the percentage of cells initially retained based on the total number of cells applied to the column: % retention = [(OD546 of original SW8 suspension − OD546 of initial eluate)/(OD546 of original SW8 suspension)] × 100. The data resulting from different cell preparation techniques were compared by using Student’s t test (P < 0.01).
Electron microscopy.
Following each treatment cell morphology was examined by transmission electron microscopy of negatively stained specimens. Carbon-coated copper grids (400 mesh) were immersed for 20 s in bacterial suspensions (OD546, 0.3) resulting from each cell preparation technique and then stained with a 2% aqueous sodium phosphotungstate solution for 10 s. The excess liquid was removed by carefully touching the edges of the grids to filter paper and air drying the grids for 5 min prior to visualization with a model H-7000 transmission electron microscope (Hitachi, Tokyo, Japan).
Statistical comparison of data from different protocols.
A comparative analysis of data was performed by using CricketGraph, version 1.3.2. (Computer Associates, Islandia, N.Y.) for Macintosh. Graphs were constructed by using data obtained from the various cell surface analysis techniques, and correlation coefficients were calculated by the linear regression method.
RESULTS
Reference states.
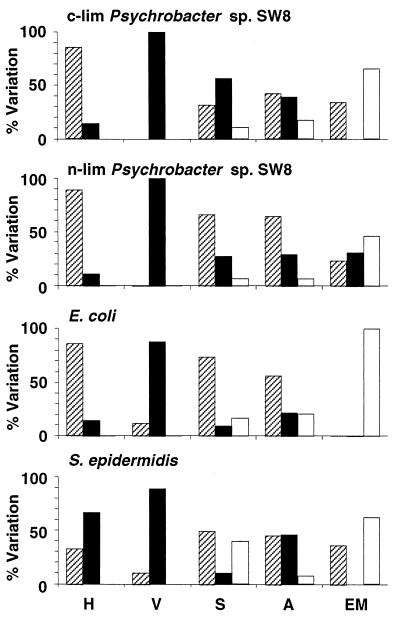
Comparative analysis of the effects of different cell preparation protocols on cell surface properties requires the arbitrary choice of a reference state. Cells harvested by centrifugation at 5,000 × g were used as reference cells (Table 1) because they most closely resembled unmanipulated organisms in the parameters that could be measured without extensive cell manipulation (e.g., viability and electrophoretic mobility). Organisms harvested by centrifugation at 5,000 × g were viable and negatively charged (Table 1). Carbon- and nitrogen-limited reference Psychrobacter sp. strain SW8 and S. epidermidis were very hydrophobic, as determined by the MATH assay, but E. coli was only moderately hydrophobic (Table 1). The retention of the two Psychrobacter sp. strain SW8 phenotypes on hydrophilic surfaces (stainless steel and aluminum) was between two and three times greater than the retention of this organism on hydrophobic surfaces (perspex and polypropylene) (Table 1). Similar numbers of E. coli cells attached to perspex, stainless steel, and aluminum, but significantly fewer E. coli cells attached to polypropylene. S. epidermidis was retained poorly on polypropylene, but the numbers of S. epidermidis cells that attached to aluminum were between two and three times greater than the numbers of S. epidermidis cells that attached to stainless steel and perspex. The affinity for DEAE-Sepharose of all 5,000-×-g-treated organisms was high. The affinities of the two phenotypes of Psychrobacter sp. strain SW8 were high for octyl-Sepharose, moderate for phenyl-Sepharose, low for carboxymethyl-Sepharose, and either moderate (carbon-limited cells) or low (nitrogen-limited cells) for Sepharose (Table 1). The affinities of E. coli for the four non-DEAE-Sepharose gels were generally moderate, but the affinities of S. epidermidis were low.
TABLE 1.
Reference values for parameters used for comparative analysis of the effects of cell preparation protocols on cell surface propertiesa
| Organism | Electrophoretic mobility (μm-cm/Vs) | Viabilityb (%) | Hydrophobicityc (%) | Attachment (103 cells/cm2) to:
|
% Retention on Sepharose columns
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stainless steel | Aluminum | Perspex | Polypropylene | Sepharose | Phenyl-Sepharose | Octyl-Sepharose | DEAE-Sepharose | Carboxymethyl-Sepharose | ||||
| Carbon-limited strain SW8 | −0.38 ± 0.01 | 85.4 ± 3.8 | 92.8 ± 1.9 | 5.2 ± 1.2 | 6.9 ± 2.4 | 2.0 ± 0.7 | 3.1 ± 0.9 | 57.4 ± 5.8 | 60.7 ± 7.7 | 80.6 ± 1.9 | 100 | 19.1 ± 3.3 |
| Nitrogen-limited strain SW8 | −0.51 ± 0.01 | 94.4 ± 3.8 | 92.2 ± 4.6 | 3.1 ± 0.9 | 3.7 ± 1.0 | 1.7 ± 0.6 | 1.2 ± 0.5 | 24.7 ± 1.6 | 41.9 ± 0.7 | 94.7 ± 0.6 | 100 | 22.6 ± 3.1 |
| E. coli | −3.33 ± 0.01 | 89.1 ± 3.7 | 77.6 ± 7.5 | 5.9 ± 2.2 | 7.5 ± 2.0 | 7.9 ± 1.6 | 0.9 ± 0.6 | 42.8 ± 2.3 | 45.3 ± 3.7 | 39.4 ± 1.0 | 91.3 ± 1.8 | 53.7 ± 5.1 |
| S. epidermidis | −1.04 ± 0.01 | 92.4 ± 3.8 | 91.3 ± 1.6 | 4.2 ± 1.2 | 8.2 ± 1.3 | 3.3 ± 1.1 | 0.4 ± 0.2 | 20.0 ± 2.7 | 26.3 ± 1.7 | 36.3 ± 2.1 | 97.7 ± 0.2 | 14.8 ± 2.3 |
Cells were centrifuged at 5,000 × g and then washed and resuspended in buffered solutions containing NaCl.
Percent viability [(number of CFU on nutrient agar)/(total number of cells)] × 100.
Percent hydrophobicity (MATH assay) = [(final optical density of the aqueous phase)/(optical density of the initial suspension prior to mixing with dodecane)] × 100.
The protocols used for manipulation of cells significantly modified cell surface properties in a manner which depended on the type of treatment, the nature of the microorganisms, and the type of washing or resuspension medium employed (Tables 2 through 7 and Fig. 2 through 4).
TABLE 2.
Impact of cell preparation protocols on viability, electrophoretic mobility, and hydrophobicity of organisms when different combinations of washing and resuspension media were used
| Organism | Treatment | % of reference valuea
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viability
|
Electrophoretic mobility
|
Hydrophobicity
|
|||||||||||
| NaCl/NaClb | NaCl/H2O | H2O/NaCl | H2O/H2O | NaCl/NaCl | NaCl/H2O | H2O/NaCl | H2O/H2O | NaCl/NaCl | NaCl/H2O | H2O/NaCl | H2O/H2O | ||
| Carbon-limited strain SW8 | 15,000 × gc | 100 | 100 | 100 | 100 | 100 | 100 | 119 | 100 | 100 | 27 | 100 | 100 |
| Freeze-drying | —d | —d | 3 | 3 | —d | —d | 83 | 0 | —d | —d | 47 | 11 | |
| Air drying | 65 | 60 | 56 | 46 | 82 | 85 | 71 | 80 | 100 | 100 | 40 | 45 | |
| MATH | 33 | 37 | 23 | 17 | 29 | 5 | 19 | 3 | |||||
| Nitrogen-limited strain SW8 | 15,000 × gc | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 20 | 100 | 72 |
| Freeze-drying | —d | —d | 5 | 5 | —d | —d | 220 | −104e | —d | —d | 100 | 17 | |
| Air drying | 64 | 60 | 55 | 60 | 124 | 70 | 135 | 65 | 100 | 100 | 53 | 52 | |
| MATH | 36 | 42 | 28 | 31 | 69 | 1 | 55 | 4 | |||||
| E. coli | 15,000 × gc | 79 | 77 | 66 | 66 | 15 | 72 | 112 | 74 | 100 | 125 | 100 | 55 |
| Freeze-drying | —d | —d | 2 | 17 | —d | —d | 100 | 80 | —d | —d | 100 | 170 | |
| Air drying | 25 | 32 | 24 | 23 | 29 | 82 | 91 | 84 | 70 | 23 | 50 | 0 | |
| MATH | 25 | 15 | 21 | 15 | 23 | 82 | 294 | 79 | |||||
| S. epidermidis | 15,000 × gc | 100 | 100 | 100 | 86 | 20 | 63 | 286 | 59 | 100 | 100 | 118 | 116 |
| Freeze-drying | —d | —d | 47 | 38 | —d | —d | —f | —f | —d | —d | —f | —f | |
| Air drying | 76 | 75 | 88 | 75 | 79 | 1 | 133 | 36 | 100 | 100 | 100 | 100 | |
| MATH | 100 | 78 | 100 | 72 | 100 | 2 | 88 | 47 | |||||
The values are percentages of reference values determined after centrifugation at 5,000 × g.
Washing medium/resuspension medium.
Centrifugation at 15,000 × g.
The bacterial cells used for XPS analysis were not washed in the NaCl solution.
The sign of the cell surface charge was reversed.
Freeze-dried samples of S. epidermidis could not be resuspended.
TABLE 7.
Impact of modification of the washing medium on viability, electrophoretic mobility, and hydrophobicity of test organisms subjected to different cell preparation protocols
| Organism | Treatment | % of reference value
|
|||||
|---|---|---|---|---|---|---|---|
| Viability
|
Electrophoretic mobility
|
Hydrophobicity
|
|||||
| H2O/NaCl (NaCl/NaCl)a |
H2O/H2O (NaCl/H2O) |
H2O/NaCl (NaCl/NaCl) |
H2O/H2O (NaCl/H2O) |
H2O/NaCl (NaCl/NaCl) |
H2O/H2O (NaCl/H2O) |
||
| Carbon-limited strain SW8 | 5,000 × gb | 100 | 100 | 100 | 100 | 81 | 71 |
| 15,000 × gc | 100 | 100 | 122 | 100 | 75 | 219 | |
| Freeze-drying | —d | —d | —d | —d | —d | —d | |
| Air drying | 100 | 100 | 100 | 100 | 30 | 31 | |
| MATH | 100 | 49 | 100 | 100 | |||
| Nitrogen-limited strain SW8 | 5,000 × gb | 86 | 100 | 100 | 100 | 72 | 86 |
| 15,000 × gc | 100 | 100 | 100 | 100 | 59 | 304 | |
| Freeze-drying | —d | —d | —d | —d | —d | —d | |
| Air drying | 100 | 100 | 100 | 100 | 39 | 39 | |
| MATH | 100 | 100 | 100 | 100 | |||
| E. coli | 5,000 × gb | 100 | 100 | 26 | 100 | 115 | 152 |
| 15,000 × gc | 100 | 100 | 194 | 100 | 100 | 67 | |
| Freeze-drying | —d | —d | —d | —d | —d | —d | |
| Air drying | 100 | 100 | 81 | 100 | 82 | 0 | |
| MATH | 100 | 100 | 321 | 100 | |||
| S. epidermidis | 5,000 × gb | 100 | 100 | 163 | 123 | 86 | 100 |
| 15,000 × gc | 100 | 100 | 2,305 | 114 | 100 | 100 | |
| Freeze-drying | —d | —d | —d | —d | —d | —d | |
| Air drying | 100 | 100 | 273 | 4,925 | 100 | 100 | |
| MATH | 100 | 100 | 142 | 2,911 | |||
Washing medium/resuspension medium (washing medium/resuspension medium with which the reference value was obtained).
Centrifugation at 5,000 × g.
Centrifugation at 15,000 × g.
The bacterial cells used for XPS analysis were not washed in the NaCl solution.
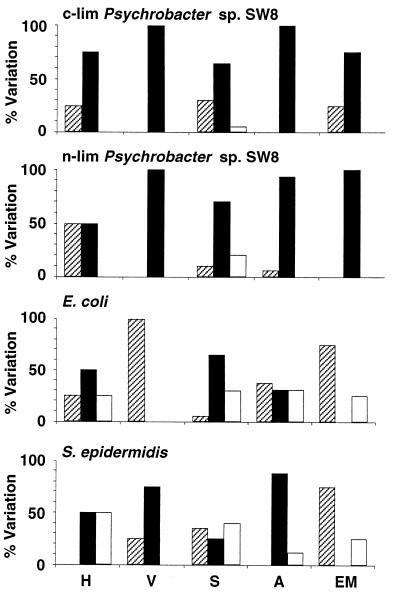
FIG. 2.
Effects of centrifugation at 15,000 × g on microbial physicochemical cell surface properties. H, hydrophobicity; V, viability; S, Sepharose column assays; A, attachment to solid substrata; EM, electrophoretic mobility. The percentages of experiments where the values of the parameters after treatment were reduced (cross-hatched bars), unchanged (solid bars), and increased (open bars) are shown. For information on reference data and modifications of the parameters see Tables 1 through 8. c-lim, carbon limited; n-lim, nitrogen limited.
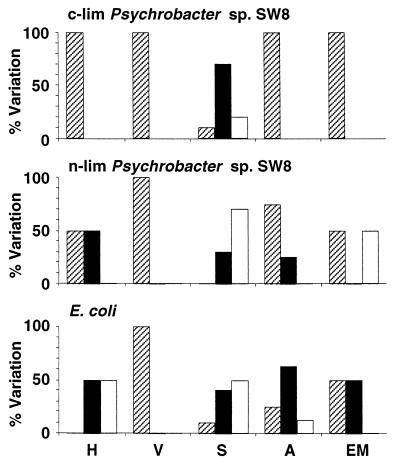
FIG. 4.
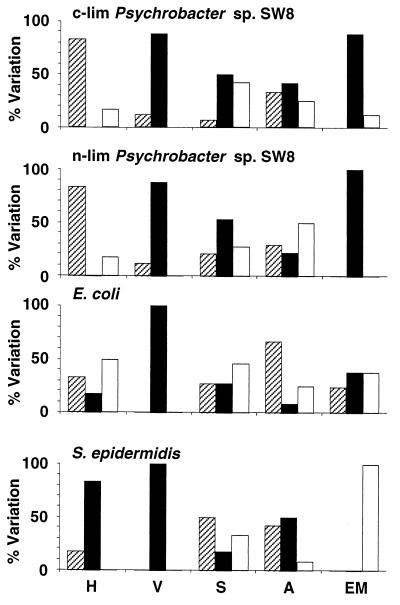
Effects of air drying on microbial physicochemical cell surface properties. H, hydrophobicity; V, viability; S, Sepharose column assays; A, attachment to solid substrata; EM, electrophoretic mobility. The percentages of experiments where the values of the parameters after treatment were reduced (cross-hatched bars), unchanged (solid bars), and increased (open bars) are shown. For information on reference data and modifications of the parameters see Tables 1 through 8. c-lim, carbon limited; n-lim, nitrogen limited.
High-speed centrifugation.
Centrifugation at 15,000 × g in many instances did not alter the viability of Psychrobacter sp. strain SW8 or S. epidermidis, but the culturability of E. coli was significantly diminished (Fig. 2 and Table 2). High-speed centrifugation affected hydrophobicity almost exclusively when water was used as the resuspension medium (e.g., in the combinations NaCl/H2O and H2O/H2O) (Table 2). In general, this cell manipulation procedure did not influence the electrophoretic mobility of Psychrobacter sp. strain SW8, but it reduced the surface charges of E. coli and S. epidermidis (Fig. 2 and Table 2). This technique had little effect on the adhesion of Psychrobacter sp. strain SW8 or S. epidermidis to solid surfaces, but for E. coli about two-thirds of the attachment results were different after high-speed centrifugation, with increased attachment and decreased attachment occurring at similar frequencies (Table 3 and Fig. 2). High-speed centrifugation modified about 30% of the Sepharose results obtained for Psychrobacter sp. strain SW8 and E. coli and approximately 70% of the Sepharose results obtained for S. epidermidis (Fig. 2). The majority of the effects occurred with the combinations NaCl/NaCl (E. coli and S. epidermidis), NaCl/H2O (Psychrobacter sp. strain SW8), and H2O/NaCl, as well as H2O/H2O (S. epidermidis) (Table 4).
TABLE 3.
Impact of cell preparation protocols on the attachment to different substrata of test organisms exposed to different combinations of washing and resuspension media
| Organism | Treatment | % of reference valuea
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NaCl/NaClb
|
NaCl/H2O
|
H2O/NaCl
|
H2O/H2O
|
||||||||||||||
| Stainless steel |
Aluminum | Perspex | Polypropylene | Stainless steel |
Aluminum | Perspex | Polypropylene | Stainless steel |
Aluminum | Perspex | Polypropylene | Stainless steel |
Aluminum | Perspex | Polypropylene | ||
| Carbon-limited strain SW8 | 15,000 × gc | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Freeze-drying | —d | —d | —d | —d | —d | —d | —d | —d | 40 | 13 | 33 | 17 | 5 | 4 | 25 | 4 | |
| Air drying | 54 | 17 | 35 | 13 | 100 | 100 | 100 | 100 | 28 | 22 | 100 | 100 | 20 | 9 | 25 | 0 | |
| Nitrogen-limited strain SW8 | 15,000 × gc | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0 |
| Freeze-drying | —d | —d | —d | —d | —d | —d | —d | —d | 48 | 100 | 18 | 100 | 97 | 15 | 9 | 40 | |
| Air drying | 100 | 100 | 29 | 0 | 100 | 100 | 100 | 100 | 352 | 656 | 100 | 100 | 26 | 10 | 0 | 0 | |
| E. coli | 15,000 × gc | 29 | 100 | 100 | 522 | 13 | 5 | 100 | 100 | 1,300 | 2,028 | 35 | 100 | 3,827 | 196 | 0 | 0 |
| Freeze-drying | —d | —d | —d | —d | —d | —d | —d | —d | 100 | 100 | 13 | 100 | 18 | age | 100 | 100 | |
| Air drying | 100 | 100 | 20 | 256 | 42 | 12 | 36 | 100 | 1,466 | 557 | 60 | 100 | 291 | 9 | 0 | 100 | |
| S. epidermidis | 15,000 × gc | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 413 | 100 | 100 | 209 | 100 | 100 |
| Freeze-drying | —d | —d | —d | —d | —d | —d | —d | —d | —f | —f | —f | —f | —f | —f | —f | —f | |
| Air drying | 390 | 100 | 21 | 400 | 100 | 190 | 19 | —g | 196 | 143 | 488 | 100 | 51 | 39 | 0 | —g | |
The values are percentages of reference values determined after centrifugation at 5,000 × g.
Washing medium/resuspension medium.
Centrifugation at 15,000 × g.
The bacterial cells used for XPS analysis were not washed in the NaCl solution.
ag, cells aggregated on the surface.
Freeze-dried S. epidermidis cells could not be resuspended.
No cells adhered to the reference substratum.
TABLE 4.
Impact of cell preparation protocols on the retention on Sepharose columns of test organisms exposed to different combinations of washing and resuspension media
| Organism | Treatment | % of reference valuea
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NaCl/NaClb
|
NaCl/H2O
|
H2O/NaCl
|
H2O/H2O
|
||||||||||||||||||
| Sepharose | PhenylSepharose | OctylSepharose | DEAESepharose | CarboxymethylSepharose | Sepharose | PhenylSepharose | OctylSepharose | DEAESepharose | CarboxymethylSepharose | Sepharose | PhenylSepharose | OctylSepharose | DEAESepharose | CarboxymethylSepharose | Sepharose | PhenylSepharose | OctylSepharose | DEAESepharose | CarboxymethylSepharose | ||
| Carbon-limited strain SW8 | 15,000 × gc | 100 | 100 | 100 | 100 | 100 | 51 | 60 | 48 | 90 | 215 | 100 | 100 | 88 | 100 | 100 | 100 | 100 | 74 | 100 | 100 |
| Freeze-drying | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | 136 | 146 | 100 | 100 | 100 | 100 | 100 | 67 | 100 | 100 | |
| Air drying | 55 | 35 | 65 | 100 | 100 | 25 | 26 | 50 | 100 | 178 | 52 | 63 | 90 | 100 | 100 | 38 | 21 | 17 | 100 | 73 | |
| Nitrogen-limited strain SW8 | 15,000 × gc | 100 | 1,714 | 100 | 100 | 100 | 100 | 151 | 275 | 100 | 100 | 100 | 138 | 100 | 100 | 100 | 55 | 100 | 52 | 100 | 100 |
| Freeze-drying | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | 243 | 161 | 100 | 100 | 178 | 304 | 408 | 352 | 100 | 234 | |
| Air drying | 167 | 213 | 100 | 100 | 299 | 210 | 215 | 379 | 100 | 165 | 100 | 142 | 100 | 100 | 148 | 203 | 227 | 224 | 100 | 171 | |
| E. coli | 15,000 × gc | 169 | 159 | 208 | 76 | 143 | 100 | 192 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 211 | 100 | 100 | 100 | 100 | 100 |
| Freeze-drying | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | 66 | 100 | 100 | 100 | 143 | 364 | 166 | 178 | 100 | 333 | |
| Air drying | 57 | 100 | 100 | 100 | 100 | 100 | 100 | 20 | 100 | 100 | 100 | 100 | 100 | 100 | 178 | 276 | 134 | 182 | 100 | 189 | |
| S. epidermidis | 15,000 × gc | 209 | 360 | 273 | 100 | 262 | 133 | 100 | 87 | 100 | 100 | 40 | 92 | 83 | 44 | 55 | 401 | 169 | 156 | 100 | 38 |
| Freeze-drying | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —e | —e | —e | —e | —e | —e | —e | —e | —e | —e | |
| Air drying | 100 | 147 | 100 | 100 | 100 | 21 | 18 | 12 | 100 | 44 | 58 | 100 | 100 | 72 | 47 | 224 | 100 | 100 | 100 | 49 | |
The values are percentages of reference values determined after centrifugation at 5,000 × g.
Washing medium/resuspension medium.
Centrifugation at 15,000 × g.
The bacterial cells used for XPS analysis were not washed in the NaCl solution.
Freeze-dried S. epidermidis cells could not be resuspended.
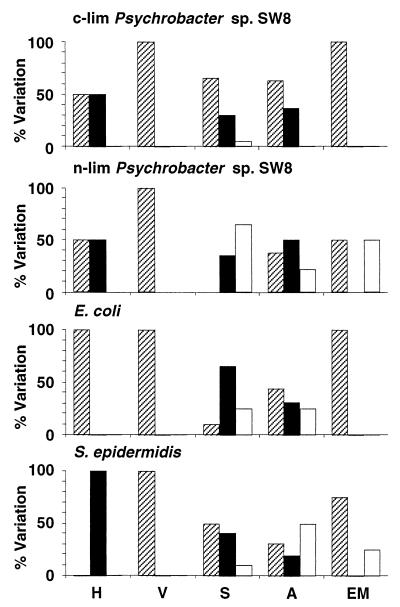
Freeze-drying.
Freeze-drying reduced the viabilities of the gram-negative organisms Psychrobacter sp. strain SW8 and E. coli by between 83 and 97% and the viability of the more resistant gram-positive organism S. epidermidis by approximately 60% (Table 2 and Fig. 3). No additional measurements could be obtained with freeze-dried S. epidermidis as attempts to resuspend desiccated samples in either the NaCl solution or water caused the majority of cells to aggregate and sediment. Freeze-drying significantly reduced the electrophoretic mobility of cells resuspended in water; the surface charge of nitrogen-limited Psychrobacter sp. strain SW8 was even reversed to slightly positive values (Table 2). When cells were resuspended in the NaCl solution, however, the cell surface charge of E. coli remained the same, while the charge of nitrogen- and carbon-limited Psychrobacter sp. strain SW8 was either strongly increased or reduced. Resuspension of freeze-dried cells in water reduced the hydrophobicity of Psychrobacter sp. strain SW8 but increased the hydrophobicity of E. coli (Table 2). Resuspension of the same cells in the NaCl solution had no effect on the hydrophobicity of E. coli, reduced the hydrophobicity of carbon-limited Psychrobacter sp. strain SW8, and increased the hydrophobicity of the nitrogen-limited phenotype. Significantly lower numbers of freeze-dried cells of Psychrobacter sp. strain SW8 attached to substrata in most experiments; in contrast, the E. coli attachment behavior was affected by freeze-drying in only a few experiments (Fig. 3). Freeze-drying also affected the interaction of microorganisms with Sepharose resins and generally increased their retention in the columns (Fig. 3). For the most part these effects did not occur in clear patterns; the exception was the consistently complete retention of all cells applied to DEAE-Sepharose columns (Table 4).
FIG. 3.
Effects of freeze-drying on microbial physicochemical cell surface properties. H, hydrophobicity; V, viability; S, Sepharose column assays; A, attachment to solid substrata; EM, electrophoretic mobility. The percentages of experiments where the values of the parameters after treatment were reduced (cross-hatched bars), unchanged (solid bars), and increased (open bars) are shown. For information on reference data and modifications of the parameters see Tables 1 through 8. c-lim, carbon limited; n-lim, nitrogen limited.
Air drying.
Air drying affected microbes in a manner similar to freeze-drying, but the effects were less intense (Fig. 4 and Tables 2 through 4). The viabilities of all of the microbes were reduced, but the effects were less dramatic than the effects of freeze-drying. The gram-positive organism S. epidermidis exhibited the best resistance to the process; the reductions in viability for S. epidermidis were relatively small (12 to 25%) compared to the reductions in viability observed for Psychrobacter sp. strain SW8 (35 to 54%) and E. coli (80%). Air drying modified virtually every electrophoretic mobility value measured and generally reduced the value (Fig. 4 and Table 2). The effects of air drying on hydrophobicity varied depending on the microbe and on the resuspension medium (Fig. 4 and Table 2). Using water as the resuspension medium for air-dried Psychrobacter sp. strain SW8 resulted in reduced hydrophobicity, but no such effect occurred with the NaCl solution. Air drying reduced the hydrophobicity of E. coli in all assays but had no effect on the hydrophobicity of S. epidermidis. Air drying altered the attachment of E. coli and S. epidermidis to surfaces in the majority of assays, but no clear relationship between increases or decreases in retention and the nature of the surface or of the suspension medium was identified (Fig. 4). For the most part the effects of air drying on the interactions of the two phenotypes of Psychrobacter sp. strain SW8 with solid substrata were similar. The exceptions were the interactions with stainless steel and aluminum; in these cases attachment of the two phenotypes subjected to NaCl/NaCl and H2O/NaCl treatments differed significantly (Table 3). Air drying also modified the interactions of cells with most of the Sepharose columns; the exceptions were the DEAE-Sepharose column experiments, in which complete retention of cells was always observed (Fig. 4 and Table 4). With most other column materials, air drying increased the retention of nitrogen-limited Psychrobacter sp. strain SW8 but reduced the retention of the carbon-limited phenotype (Table 4). The interactions of air-dried E. coli with Sepharose gels were generally not affected; the exceptions were cells washed in water, which exhibited increased affinity for all of the Sepharose gels other than the DEAE-Sepharose gels. Air drying consistently reduced the affinity of NaCl/H2O-treated S. epidermidis for Sepharose columns, but with the other medium combinations tested significant effects occurred only occasionally.
MATH.
Contact between cells and hydrocarbon reduced the viability of Psychrobacter sp. strain SW8 and E. coli by between 63 and 85% (Table 2) but either had no effect or caused only a small reduction of the viability of S. epidermidis, depending on the resuspension medium. The electrophoretic mobilities of both phenotypes of Psychrobacter sp. strain SW8 were reduced by approximately 95% when the cells were resuspended in water, a reduction similar to the reduction observed for NaCl/H2O-treated S. epidermidis (Table 2). Most of the reductions in electrophoretic mobility were smaller when the cells were resuspended in NaCl; the only exception was the H2O/NaCl-treated E. coli experiment, in which a large increase in electrophoretic mobility was observed after the cells were exposed to dodecane.
Change of resuspension medium.
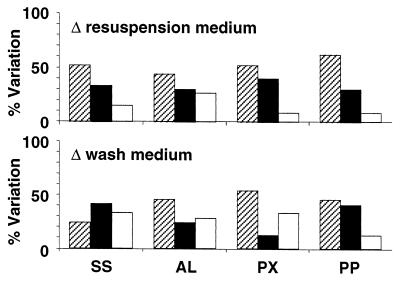
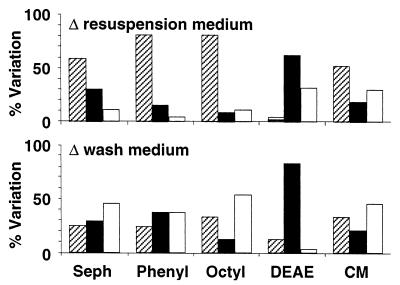
The cell surface properties of organisms resuspended in the NaCl solution were used as references for comparisons with similarly prepared cells resuspended in water (Fig. 5 and Tables 5 and 6). Resuspension of microorganisms in water did not change the viability of the microbes significantly (Fig. 5). Final resuspension of NaCl solution- or H2O-washed organisms in water increased their electrophoretic mobility considerably (by up to 700%) compared to cells resuspended in the NaCl solution (Table 5). Reductions in electrophoretic mobility were observed with freeze-dried and hydrocarbon-treated Psychrobacter sp. strain SW8, as well as with air-dried and hydrocarbon-treated S. epidermidis (Table 5). In most cases the hydrophobicity of cells was considerably reduced by resuspension in water; the exceptions were most S. epidermidis preparations, whose hydrophobicities were similar in the two resuspension media (Table 5). Switching the suspension medium from the NaCl solution to H2O altered the attachment of organisms to substrata in most instances and generally reduced the retention of organisms on surfaces (Fig. 6). Increased retention also occurred, but it occurred less frequently and primarily to aluminum (Fig. 6). With Psychrobacter sp. strain SW8 (but not E. coli or S. epidermidis), the washing medium appeared to determine whether attachment increased or decreased. With the NaCl solution as the washing medium, reductions in attachment predominated (83% of the results), but with H2O as the washing medium, reduced attachment (33%) was observed as frequently as increased attachment (29%). Retention of cells on Sepharose columns was affected by changes of the resuspension medium more than attachment to solid surfaces (Fig. 7). The change from the NaCl solution to H2O reduced the affinity of cells particularly to the hydrophobic columns (phenyl-Sepharose and octyl-Sepharose), but lower affinities were also observed for Sepharose and carboxymethyl-Sepharose. No effect or increased affinity occurred with the DEAE-Sepharose column, a column that often retained all of the organisms applied to it.
FIG. 5.
Effects of changes in resuspension medium on microbial physicochemical cell surface properties. H, hydrophobicity; V, viability; S, Sepharose column assays; A, attachment to solid substrata; EM, electrophoretic mobility. The percentages of experiments where the values of the parameters after treatment were reduced (cross-hatched bars), unchanged (solid bars), and increased (open bars) are shown. For information on reference data and modifications of the parameters see Tables 1 through 8. c-lim, carbon limited; n-lim, nitrogen limited.
TABLE 5.
Impact of modification of the resuspension medium on viability, electrophoretic mobility, and hydrophobicity of test organisms subjected to different cell preparation protocols
| Organism | Treatment | % of reference value
|
|||||
|---|---|---|---|---|---|---|---|
| Viability
|
Electrophoretic mobility
|
Hydrophobicity
|
|||||
| NaCl/H2O (NaCl/NaCl)a |
H2O/H2O (H2O/NaCl) |
NaCl/H2O (NaCl/NaCl) |
H2O/H2O (H2O/NaCl) |
NaCl/H2O (NaCl/NaCl) |
H2O/H2O (H2O/NaCl) |
||
| Carbon-limited strain SW8 | 5,000 × gb | 100 | 100 | 271 | 240 | 86 | 74 |
| 15,000 × gc | 100 | 100 | 246 | 210 | 24 | 69 | |
| Freeze-drying | —d | 100 | —d | 0 | —d | 17 | |
| Air drying | 100 | 100 | 281 | 270 | 82 | 100 | |
| MATH | 100 | 100 | 45 | 38 | |||
| Nitrogen-limited strain SW8 | 5,000 × gb | 100 | 100 | 490 | 490 | 73 | 88 |
| 15,000 × gc | 100 | 100 | 460 | 458 | 14 | 75 | |
| Freeze-drying | —d | 100 | —d | −108e | —d | 17 | |
| Air drying | 100 | 100 | 276 | 236 | 88 | 100 | |
| MATH | 100 | 100 | 9 | 32 | |||
| E. coli | 5,000 × gb | 100 | 87 | 145 | 564 | 50 | 66 |
| 15,000 × gc | 100 | 100 | 708 | 375 | 58 | 38 | |
| Freeze-drying | —d | 100 | —d | 478 | —d | 100 | |
| Air drying | 100 | 100 | 418 | 521 | 16 | 0 | |
| MATH | 55 | 100 | 505 | 151 | |||
| S. epidermidis | 5,000 × gb | 100 | 100 | 435 | 328 | 93 | 100 |
| 15,000 × gc | 100 | 100 | 1,362 | 672 | 100 | 100 | |
| Freeze-drying | —d | 100 | —d | —d | —d | —d | |
| Air drying | 100 | 100 | 5 | 88 | 91 | 100 | |
| MATH | 83 | 100 | 9 | 177 | |||
Washing medium/resuspension medium (washing medium/resuspension medium with which the reference value was obtained).
Centrifugation at 5,000 × g.
Centrifugation at 15,000 × g.
The bacterial cells used for XPS analysis were not washed in the NaCl solution.
Reversal of the electrophoretic mobility sign resulted in a reduction in the value.
TABLE 6.
Impact of modification of the resuspension medium on interaction with Sepharose columns and attachment to solid substrata of test organisms subjected to different cell preparation protocols
| Organism | Treatment | % of reference value
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NaCl/H2O (NaCl/NaCl)a
|
H2O/H2O (H2O/NaCl)
|
NaCl/H2O (NaCl/NaCl)
|
H2O/H2O (H2O/NaCl)
|
||||||||||||||||
| Sepharose | PhenylSepharose | OctylSepharose | DEAESepharose | CarboxymethylSepharose | Sepharose | PhenylSepharose | OctylSepharose | DEAESepharose | CarboxymethylSepharose | Stainless steel |
Aluminum | Perspex | Polypropylene | Stainless Steel |
Aluminum | Perspex | Polypropylene | ||
| Carbon-limited Psychrobacter sp. strain SW8 | 5,000 × gb | 100 | 100 | 109 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 13 | 13 | 10 | 13 | 100 | 175 | 400 | 383 |
| 15,000 × gc | 57 | 62 | 57 | 90 | 218 | 100 | 100 | 90 | 100 | 100 | 27 | 13 | 8 | 0 | 42 | 100 | 500 | 200 | |
| Freeze-drying | —d | —d | —d | —d | —d | 75 | 62 | 67 | 100 | 195 | —d | —d | —d | —d | 100 | 100 | 100 | 100 | |
| Air drying | 53 | 100 | 100 | 100 | 211 | 100 | 37 | 20 | 100 | 100 | 29 | 100 | 14 | 100 | 100 | 100 | 100 | 0 | |
| Nitrogen-limited Psychrobacter sp. strain SW8 | 5,000 × gb | 72 | 63 | 26 | 100 | 137 | 57 | 33 | 24 | 100 | 65 | 32 | 46 | 24 | 17 | 100 | 300 | 100 | 100 |
| 15,000 × gc | 100 | 56 | 69 | 100 | 184 | 35 | 20 | 13 | 100 | 52 | 40 | 38 | 12 | 0 | 52 | 225 | 100 | 0 | |
| Freeze-drying | —d | —d | —d | —d | —d | 71 | 84 | 85 | 100 | 86 | —d | —d | —d | —d | 30 | 100 | 100 | 22 | |
| Air drying | 100 | 64 | 95 | 100 | 75 | 100 | 53 | 55 | 100 | 75 | 34 | 56 | 100 | 100 | 11 | 5 | 0 | 0 | |
| E. coli | 5,000 × gb | 56 | 49 | 178 | 110 | 50 | 11 | 26 | 22 | 104 | 31 | 266 | 532 | 35 | 0 | 100 | 2,642 | 10 | 100 |
| 15,000 × gc | 35 | 59 | 73 | 144 | 45 | 15 | 19 | 23 | 109 | 15 | 100 | 33 | 42 | 0 | 540 | 256 | 0 | 0 | |
| Freeze-drying | —d | —d | —d | —d | —d | 58 | 36 | 34 | 100 | 73 | —d | —d | —d | —d | 25 | —e | 40 | 100 | |
| Air drying | 63 | 68 | 32 | 100 | 100 | 32 | 34 | 38 | 118 | 33 | 100 | 46 | 100 | 43 | 36 | 44 | 0 | 25 | |
| S. epidermidis | 5,000 × gb | 403 | 317 | 257 | 100 | 666 | 14 | 15 | 18 | 108 | 56 | 100 | 63 | 100 | 0 | 136 | 100 | 100 | 0 |
| 15,000 × gc | 237 | 82 | 82 | 103 | 256 | 143 | 27 | 34 | 246 | 38 | 212 | 66 | 100 | 0 | 100 | 100 | 39 | 0 | |
| Freeze-drying | —d | —d | —d | —d | —d | —f | —f | —f | —f | —f | —f | —d | —d | —d | —f | —f | —f | —f | |
| Air drying | 100 | 39 | 25 | 100 | 269 | 55 | 13 | 12 | 150 | 58 | 43 | 100 | 100 | 100 | 35 | 22 | 0 | 100 | |
Washing medium/resuspension medium (washing medium/resuspension medium with which the reference value was obtained).
Centrifugation at 5,000 × g.
Centrifugation at 15,000 × g.
The bacterial cells used for XPS analysis were not washed in the NaCl solution.
No cells adhered to the reference substratum.
Freeze-dried samples of S. epidermidis could not be resuspended.
FIG. 6.
Effects of changes of the resuspension and washing media on attachment of cells to substrata. SS, stainless steel; AL, aluminum; PX, perspex, PP, polypropylene. The percentages of experiments where the values of the parameters after treatment were reduced (cross-hatched bars), unchanged (solid bars), and increased (open bars) are shown. For information on reference data and modifications of the parameters see Tables 1 through 8.
FIG. 7.
Effects of changes of the resuspension and washing media on the retention of cells in Sepharose columns. Seph, unsubstituted Sepharose; Phenyl, phenyl-Sepharose; Octyl, octyl-Sepharose; DEAE, DEAE-Sepharose; CM, carboxymethyl-Sepharose. The percentages of experiments where the values of the parameters after treatment were reduced (cross-hatched bars), unchanged (solid bars), and increased (open bars) are shown. For information on reference data and modifications of the parameters see Tables 1 through 8.
Change of washing medium.
In an analysis of the effects of washing media on microbial cell surfaces, the properties of organisms initially washed in the NaCl solution were compared to the properties of similarly treated cells initially washed in water. Using different washing media generally did not alter cell viability (Tables 7 and 8 and Fig. 8). The electrophoretic mobilities determined for Psychrobacter sp. strain SW8 were similar for the two types of washing media (Fig. 8). The cell surface charge of E. coli was not modified when the washing medium was changed from the NaCl solution to water and the cells were resuspended in water but was significantly altered when the cells were resuspended in the NaCl solution (Table 7). A change of the washing medium from the NaCl solution to water always increased the electrophoretic mobility of S. epidermidis, irrespective of the final resuspension medium (Table 7). The same change of washing medium generally reduced the hydrophobicity of Psychrobacter sp. strain SW8, did not affect the hydrophobicity of S. epidermidis, and either increased or reduced the hydrophobicity of E. coli (Table 5 and Fig. 6). Replacing the NaCl solution with H2O as the washing medium generally reduced the attachment of cells to the hydrophobic substrata polypropylene and perspex and increased the attachment of cells to the hydrophilic substrata stainless steel and aluminum (Table 8). Changing the washing medium had less impact on the affinity of cells for Sepharose than changing the resuspension medium (Fig. 7). The interactions of E. coli and S. epidermidis with Sepharose resins were affected more by changing the washing medium than the interactions of Psychrobacter sp. strain SW8 (Fig. 8). Changing the washing medium from the NaCl solution to water when the NaCl resuspension medium was used generally increased the affinities of cells for the Sepharose columns, while the opposite occurred when H2O was used as the resuspension medium (Table 8).
TABLE 8.
Impact of modification of the washing medium on interaction with Sepharose columns and attachment to solid substrata of test organisms subjected to different cell preparation protocols
| Organism | Treatment | % of reference value
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2O/NaCl (NaCl/NaCl)a
|
H2O/H2O (NaCl/H2O)
|
H2O/NaCl (NaCl/NaCl)
|
H2O/H2O (NaCl/H2O)
|
||||||||||||||||
| Sepharose | PhenylSepharose | OctylSepharose | DEAESepharose | CarboxymethylSepharose | Sepharose | PhenylSepharose | OctylSepharose | DEAESepharose | CarboxymethylSepharose | Stainless steel |
Aluminum | Perspex | Polypropylene | Stainless steel |
Aluminum | Perspex | Polypropylene | ||
| Carbon-limited Psychrobacter sp. strain SW8 | 5,000 × gb | 100 | 100 | 115 | 100 | 241 | 100 | 100 | 112 | 100 | 203 | 100 | 27 | 15 | 19 | 629 | 778 | 600 | 575 |
| 15,000 × gc | 100 | 100 | 110 | 100 | 197 | 167 | 140 | 174 | 100 | 100 | 100 | 100 | 8 | 21 | 100 | 600 | 500 | 100 | |
| Freeze-drying | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | |
| Air drying | 100 | 203 | 260 | 100 | 155 | 146 | 100 | 37 | 100 | 83 | 39 | 100 | 43 | 100 | 100 | 100 | 100 | 0 | |
| Nitrogen-limited Psychrobacter sp. strain SW8 | 5,000 × gb | 136 | 146 | 106 | 100 | 188 | 100 | 100 | 100 | 100 | 100 | 100 | 37 | 100 | 33 | 310 | 282 | 275 | 250 |
| 15,000 × gc | 154 | 134 | 101 | 100 | 240 | 100 | 47 | 19 | 100 | 68 | 165 | 43 | 59 | 100 | 213 | 257 | 550 | 100 | |
| Freeze-drying | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | |
| Air drying | 79 | 100 | 100 | 100 | 100 | 100 | 80 | 58 | 100 | 100 | 255 | 256 | 400 | —e | 100 | 22 | 0 | 0 | |
| E. coli | 5,000 × gb | 219 | 188 | 221 | 100 | 100 | 42 | 100 | 27 | 100 | 53 | 10 | 9 | 51 | 33 | 7 | 46 | 14 | 100 |
| 15,000 × gc | 130 | 126 | 110 | 132 | 125 | 58 | 40 | 34 | 100 | 43 | 459 | 233 | 26 | 13 | 2,005 | 1,911 | 0 | 100 | |
| Freeze-drying | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | |
| Air drying | 362 | 213 | 207 | 100 | 194 | 182 | 100 | 245 | 100 | 78 | 167 | 37 | 150 | 17 | 48 | 35 | 0 | 10 | |
| S. epidermidis | 5,000 × gb | 404 | 326 | 272 | 94 | 588 | 14 | 17 | 9 | 100 | 49 | 100 | 65 | 24 | 100 | 100 | 100 | 46 | 100 |
| 15,000 × gc | 78 | 94 | 83 | 42 | 123 | 47 | 31 | 34 | 100 | 19 | 100 | 70 | 220 | 100 | 100 | 100 | 100 | 100 | |
| Freeze-drying | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | —d | |
| Air drying | 263 | 249 | 208 | 67 | 256 | 151 | 100 | 100 | 100 | 55 | 66 | 100 | 557 | 19 | 54 | 17 | 0 | 37 | |
Washing medium/resuspension medium (washing medium/resuspension medium with which the reference value was obtained.
Centrifugation at 5,000 × g.
Centrifugation at 15,000 × g.
The bacterial cells used for XPS analysis were not washed in the NaCl solution.
No cells adhered to the reference substratum.
FIG. 8.
Effects of changes of washing medium on microbial physicochemical cell surface properties. H, hydrophobicity; V, viability; S, Sepharose column assays; A, attachment to solid substrata; EM, electrophoretic mobility. The percentages of experiments where the values of the parameters after treatment were reduced (cross-hatched bars), unchanged (solid bars), and increased (open bars) are shown. For information on reference data and modifications of the parameters see Tables 1 through 8. c-lim, carbon limited; n-lim, nitrogen limited.
Electron microscopy.
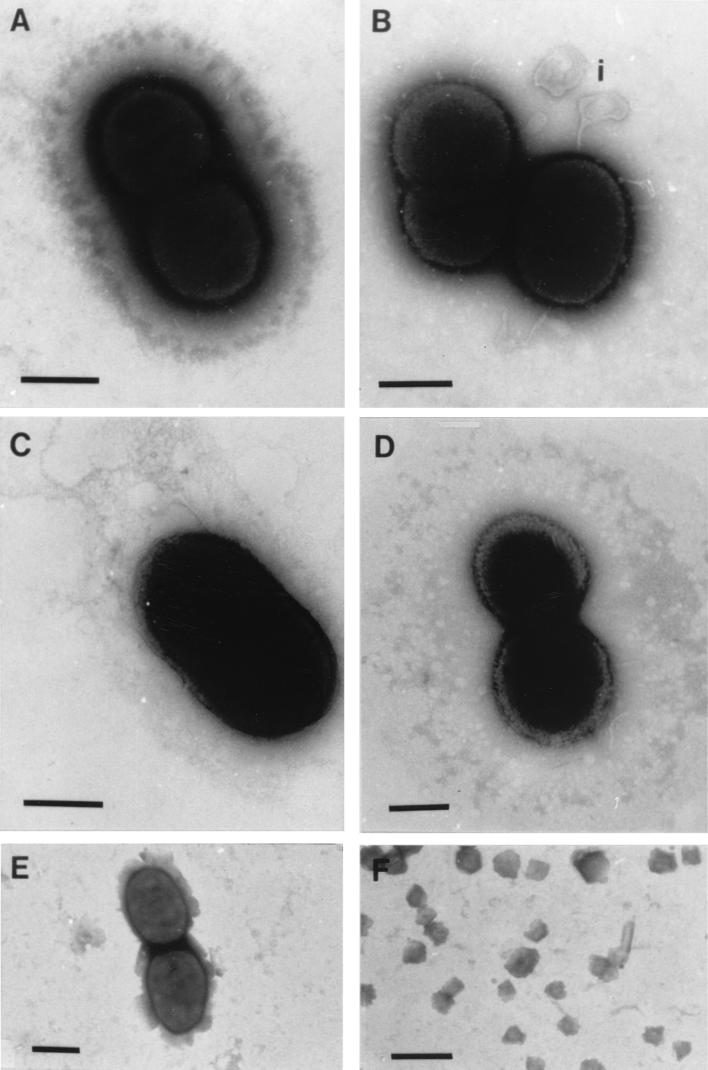
The gram-negative coccobacillus Psychrobacter sp. strain SW8, which occurs mostly as a doublet and has no flagella or fimbriae, is approximately 2 to 3 μm long (Fig. 9). Both carbon- and nitrogen-limited cells possess a thick constitutive glycocalyx which appears as a distinct structure external to the cytoplasm and cell wall in negatively stained preparations (Fig. 9A). The outermost region of the glycocalyx appears to be electron dense. No difference in the thickness of the glycocalyx was observed when the two SW8 phenotypes were compared. Centrifugation at 15,000 × g resulted in loss of the electron-dense outer layer of the glycocalyx of carbon-limited cells but not of the electron-dense outer layer of the glycocalyx of nitrogen-limited cells (Fig. 9B). Furthermore, blebbing of the outer cell membrane was observed in carbon-limited cell preparations resuspended in water (Fig. 9B). This effect was not observed in nitrogen-limited cells. Air-dried preparations of the organism appeared to have lost most of their glycocalyx structure, which left the outer cell membrane exposed (Fig. 9C). This was most apparent when we examined the surface morphology of carbon-limited cells, which was significantly modified following air drying. Similarly, cells of Psychrobacter sp. strain SW8 which remained intact following the freeze-drying procedure were for the most part devoid of a glycocalyx. However, the intact cells represented only a very small proportion of the sample viewed, as the morphology of most organisms was either grossly modified or totally disrupted by this protocol, particularly in the case of nitrogen-limited Psychrobacter sp. strain SW8 (Fig. 9E and F). Exposure of Psychrobacter sp. strain SW8 to dodecane in MATH assays resulted in complete removal of the glycocalyx, but the cells remained intact (Fig. 9D).
FIG. 9.
Negatively stained electron micrographs of Psychrobacter sp. strain SW8. (A) Cells harvested by centrifugation at 5,000 × g. Bar = 1 μm. (B) Carbon-limited phenotype harvested by centrifugation at 15,000 × g. Bar = 1 μm. i, blebbing of cell envelope. (C) Cells air dried on cellulose acetate filters. Bar = 2 μm. (D) Cells after mixing with dodecane in the MATH assay. Bar = 1 μm. (E and F) Freeze-dried cells. Bars = 2 and 5 μm, respectively.
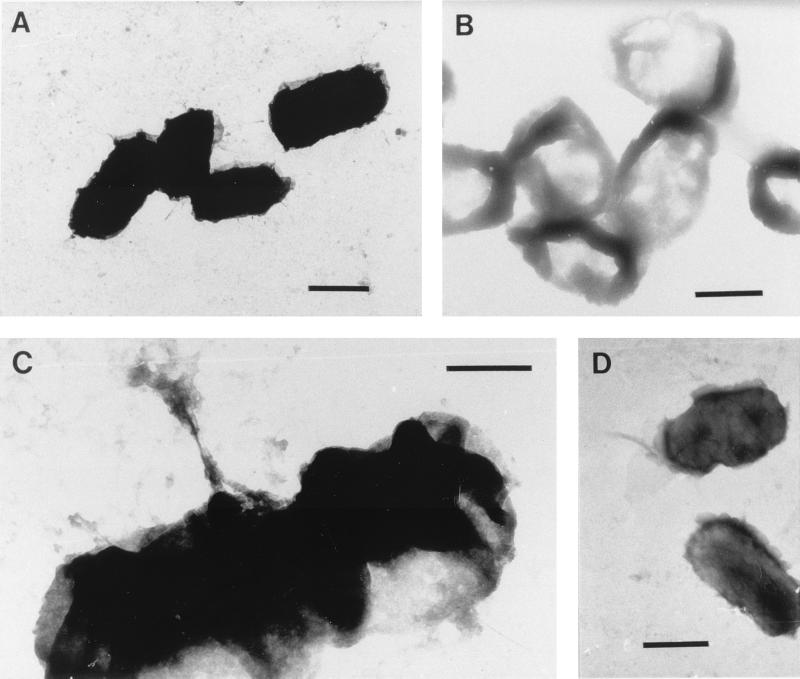
E. coli ATCC 8739 is a gram-negative rod which does not possess a glycocalyx or any distinguishable extracellular appendages. This organism is approximately 5 μm long and has an irregular outer cell surface (Fig. 10A). The surface of E. coli was compromised by high-speed centrifugation at 15,000 × g as distortion was clearly evident, particularly in preparations resuspended in water; however, the cells appeared not to disintegrate during the procedure. The significantly less dense intracellular material evident in electron micrographs of freeze-dried E. coli cells suggests that the cells lysed during the freeze-drying protocol and lost most of their plasma (Fig. 10B). The cells also had a tendency to aggregate when they were resuspended in liquid media. Air-dried E. coli cells had a strong tendency to aggregate, but otherwise these cells appeared to be similar to the controls. Micrographs of cells exposed to dodecane clearly showed damage to the E. coli cell surface; it appeared that the outermost surface of the organism was pulled away from the cell membrane during this procedure (Fig. 10C and D).
FIG. 10.
Negatively stained electron micrographs of E. coli ATCC 8739. (A) Cells harvested by centrifugation at 5,000 × g. Bar = 2 μm. (B) Freeze-dried cells. Bar = 2 μm. (C and D) Cells after mixing with dodecane in the MATH assay. Bars = 1 and 2 μm, respectively.
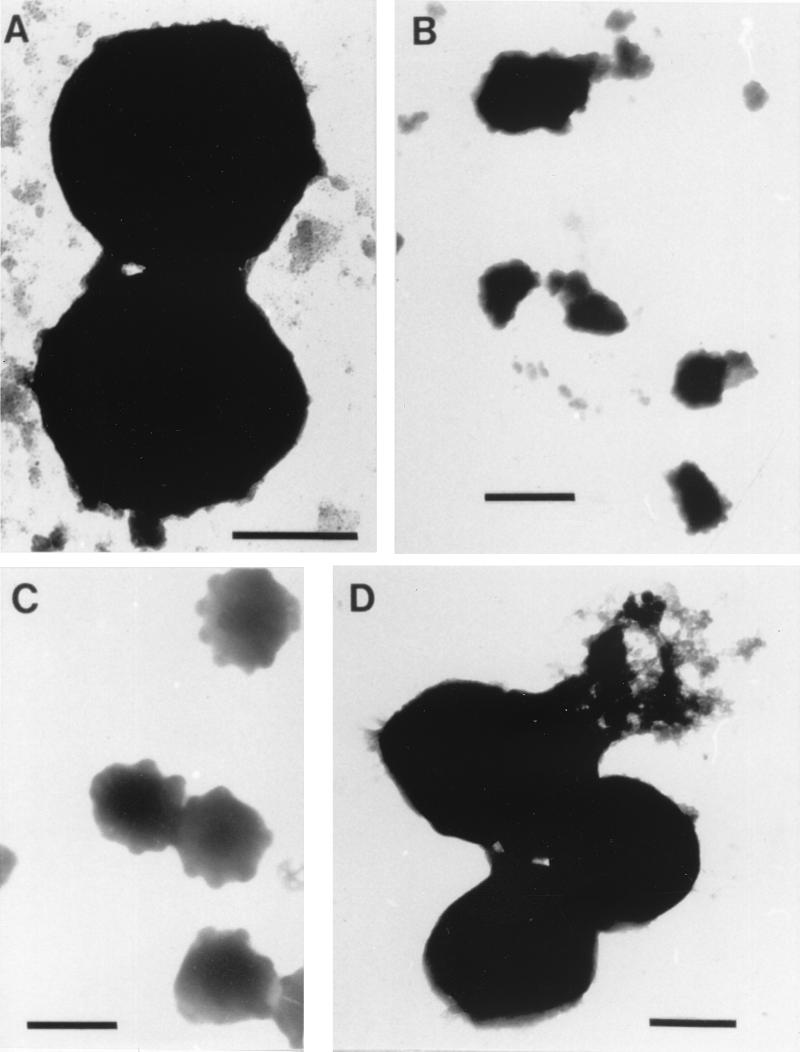
S. epidermidis NCTC 11047 is a gram-positive coccus with a diameter of 1 to 2 μm. This organism has a smooth surface and a solid spherical appearance (Fig. 11A). Cell morphology was unchanged after centrifugation at 15,000 × g. In contrast, the freeze-drying process caused significant structural damage to many S. epidermidis cells (Fig. 11B). Intact freeze-dried cells comprised less than 20% of the fields of view examined; the bulk of the sample consisted of completely disrupted cells and cell debris. While desiccation of S. epidermidis air drying resulted in severe modification of the cell surface, it appeared to be less harsh than freeze-drying and resulted in cell aggregation rather than lysis. Exposure to dodecane in MATH assays clearly affected the cell surface of this gram-positive bacterium, particularly organisms resuspended in water (Fig. 11C). Electron micrographs suggested that material was removed from the surface of the organism during this protocol.
FIG. 11.
Negatively stained electron micrographs of S. epidermidis NCTC 11047. (A) Cells harvested by centrifugation at 5,000 × g. Bar = 1 μm. (B) Freeze-dried cells. Bar = 2 μm. (C) Cells air dried on cellulose acetate filters. Bar = 2 μm. (D) Cells after mixing with dodecane in the MATH assay. Bar = 1 μm.
Correlation between different methods of cell surface analysis.
Since the results of both MATH assays and assays to measure retention on hydrophobic Sepharose columns (octyl-Sepharose and phenyl-Sepharose) depend on microbial cell surface hydrophobicity, we expected these parameters to be correlated with each other. However, good linear correlations (R ≥ 0.9) were found only occasionally (2 of 32 correlations) and occurred as often as good correlations with data which we did not expect to correlate, such as data for retention on plain Sepharose (39). Similarly, the surface charges of microbes should determine the interactions of the microbes with charged resins. Again, the number of linear correlations between affinity for DEAE-Sepharose or carboxymethyl-Sepharose and electrophoretic mobility was lower (6 of 32) than the number of correlations between data which we did not expect to be determined by surface charge (13 of 44 [39]). Affinity for Sepharose columns generally did not correlate with attachment to substrata having similar properties (e.g., hydrophobic columns and hydrophobic substrata or vice versa [39]).
DISCUSSION
The results of this systematic study clearly illustrate the shortcomings of techniques which may form part of a protocol for the preparation of microbial cells used for surface analysis. Both physicochemical and morphological properties of cells were altered during cell preparation. The MATH test, which is commonly used to assess cell hydrophobicity, was shown to physically change the constitution of microbial surfaces. Desiccation of microorganisms caused cellular disruption and reduced the numbers of viable organisms significantly. Harvesting by high-speed centrifugation generally reduced the net surface charge of E. coli or S. epidermidis but not the net surface charge of an organism with a dense glycocalyx, Psychrobacter sp. strain SW8. Washing and resuspending microorganisms in clean water also altered the physicochemical constitution of microbial cell surfaces. The considerable influence that the cell preparation protocol had on the outcome of cell surface analysis was most evident in the results obtained in adhesion assays and in Sepharose column experiments. These two techniques by themselves do not require additional cell manipulation and are sensitive primarily to the properties of the cell-medium interface, the primary objective of cell surface analysis. In summary, the results obtained with a set of microorganisms that represent the different types of cell surfaces in the microbial world suggest that techniques that involve the cell manipulations which we examined are not suitable for obtaining information about physicochemical properties of unmanipulated cell surfaces.
The physicochemical parameters of the organisms used in these experiments have been measured previously by other workers. The hydrophobicity, as determined by the MATH test, and electrophoretic mobility of Psychrobacter sp. strain SW8 controls were consistent with previous values for these parameters obtained for this organism (25, 46). The electrophoretic mobilities determined for E. coli and S. epidermidis at the time of harvesting were comparable to the electrophoretic mobilities previously reported for the same strains by Gilbert et al. (18). The results of the MATH assays performed with E. coli and S. epidermidis fell within hydrophobicity ranges for different strains of these organisms determined by Harkes et al. (22) and Cuperus et al. (9). The attachment characteristics of Psychrobacter sp. strain SW8 to stainless steel, aluminum, perspex, and polypropylene PP were consistent with the results of previous assays conducted with this organism (46). The strains of E. coli and S. epidermidis used in this study were also used in gel interaction chromatography experiments by Gilbert et al. (18), who reported retention levels of 60 and 92%, respectively, for these organisms on octyl-Sepharose. Our results fell within the ranges of hydrophobicity values previously reported for these organisms; discrepancies in the raw data were primarily due to the significantly higher concentration of NaCl in the elution buffers used by Gilbert et al. (18).
MATH.
The significantly reduced survival rates of organisms exposed to dodecane in MATH tests, as well as electron micrographs of morphologically modified cell surfaces, clearly illustrate the destructive effect of mixing bacteria with this hydrocarbon. Vanhaecke and Pijck (55) also obtained evidence that cell lysis occurs in a study in which 10 different strains of gram-negative bacteria were exposed to hexadecane, octane, and xylene. These authors measured the level of ATP in the medium as an indirect lysis indicator and found that hexadecane was the hydrocarbon which caused the least lysis of the microorganisms tested. Some cells were disrupted by simple vortexing. Proteins, polysaccharides, fatty acids, and other cell components released by lysis or removed from the cell surface during vortexing are likely to adsorb to the surfaces of both cells and hydrocarbons, thus modifying their physicochemical properties in a manner analogous to the physicochemical changes observed after adsorption of organic films on solid substrata (45). The great reductions in the electrophoretic mobilities of all of the organisms after mixing with dodecane indicate the importance of this process, which in this case led to a significant loss of charged groups at the cell surface and/or to the masking of these charged components by lysed cell debris. In addition, hydrocarbon droplets are likely to remain negatively charged after conditioning with cell material. Geertsema-Doornbusch et al. (17) reported that hexadecane droplets are negatively charged at pH 7, while van der Mei et al. (53) demonstrated that the electrophoretic mobility of hexadecane droplets is reduced after adsorption of proteins. The MATH assay is, therefore, unsuitable for measuring cell surface hydrophobicity in view of both the destructive nature of the protocol and the fundamental physicochemical inconsistencies of the test (use of negatively charged droplets to measure hydrophobicity).
Centrifugation at 15,000 × g.
The relatively small effects of centrifugation at 15,000 × g on the physicochemical parameters of Psychrobacter sp. strain SW8 are surprising in view of the considerable alterations to the cell surface structure. These effects contrasted with the loss of viability of E. coli and the significant alterations of many cell surface parameters of S. epidermidis. The disappearance of the electron-dense outer layer of the glycocalyx of carbon-limited Psychrobacter sp. strain SW8 observed by electron microscopy had practically no effect on the cell surface parameters of this organism. We have to consider, however, that the technique employed for morphological analysis of cells, electron microscopy, is itself prone to introduction of artifacts since cells have to be desiccated prior to analysis (32). The artifacts introduced by this analysis, however, are consistent for each type of cell. Differences in morphology between cells of the same organism subjected to different treatments are, therefore, indicative of real effects of the treatment rather than artifacts introduced by electron microscopy. The disappearance of the electron-dense layer may represent both a loss of material and a conformational alteration introduced during centrifugation. Hydrostatic pressures inside a 15-ml laboratory centrifuge tube centrifuged at 10,000 × g may be as high as 10 atm (20). The ensuing high shear rates could strip material from the cell surface and thus generate a new and very different microbe-environment interface. The physicochemical properties of this new interface would be similar to those of the original cell surface only if the stripped material was replaced by material with similar physicochemical properties from inside the glycocalyx. The blebbing observed in some cells indicated that weakening of the cell wall occurred during centrifugation, which eventually caused the cell wall to rupture and release the cell contents during desiccation for electron microscopy. The considerable decrease in viability and the widespread modifications of physicochemical cell surface properties suggest that E. coli cell surfaces did not resist centrifugation at 15,000 × g. Gilbert et al. (20) reported decreases of 25 and 40% in the viability of exponential-phase Pseudomonas aeruginosa following centrifugation at 5,000 × g and 10,000 × g respectively, and that centrifugation of E. coli at more than 10,000 × g increased the susceptibility of this organism to biocides (19). Most damage to cells occurred in the first few minutes of centrifugation (20). Adsorption of material released from dead cells may have contributed to modification of the cell surfaces since the alterations were more pronounced when a considerable proportion of the population had been inactivated by high-speed centrifugation.
Effects of desiccation.
The major cell disruptions caused by freeze-drying or air-drying procedures evident in electron micrographs and the significant decreases in viability should be interpreted with caution. Analyses of viability, of physicochemical parameters, and of electron microscopy results all require rehydration of dehydrated cells. This process may cause considerable damage to microbes, although it is well-known from electron microscopy and from recent investigations performed with environmental scanning microscopes that severe dehydration causes the collapse of biological macromolecular structures (3). If the conformation of cell structures were preserved in the dry state, then rehydration would not affect cell integrity. Damage to cell structures during the rehydration process, therefore, indicates that the constitution of these structures is altered when they are dry. A greater proportion of S. epidermidis than of the gram-negative organisms survived the desiccation processes primarily because highly cross-linked peptidoglycan constitutes approximately 50% of the gram-positive cell wall and provides these organisms with greater structural rigidity (58). The cell envelope of gram-negative organisms contains significantly less peptidoglycan, and consequently Psychrobacter sp. strain SW8 and E. coli had a much greater tendency to rupture during either desiccation process or when dried samples were resuspended in liquid media (51, 57). Preparation of lyophilized microbial stock cultures usually includes the use of a cryoprotectant, such as sucrose; however, this procedure is not suitable for preparing samples for cell surface analysis by high-vacuum techniques as the cryoprotectant coats the cells, thus masking surface components (2). In the case of freeze-dried S. epidermidis, which could not be resuspended, exposure of macromolecules in the peptidoglycan layer of this organism due to dehydration resulted in cross-linking of wall components between adjacent cells. Leaked intracellular material was also evident in electron micrographs of S. epidermidis, and the molecules may have contributed to the aggregation of resuspended organisms observed. Severe alterations of the physiological characteristics and viability of microorganisms subjected to freeze-drying have been demonstrated previously (10, 28, 51). The cytoplasmic membrane is considered the main site of dehydration damage due to changes in the physical state of membrane lipids and/or modification of the structure of key proteins (29, 31). Rehydration of freeze-dried cultures in liquid media is usually completed within a few seconds, which subjects the cells to very fast changes in the hydration state (11). Following rehydration, cells of Lactobacillus bulgaricus did not control the entry of sodium chloride or prevent the loss of internal constituents, such as β-galactosidase (6). Investigating the effects of freeze-drying on yeast cells, Dengis and Rouxhet (10) observed a variety of surface morphologies within the same species. These authors observed that some cell surface modification, such as removal of polymers or of hairlike structures, occurred during the first centrifugation of the cells. Cooling of cell samples had to be strictly monitored during the freeze-drying process to improve the reproducibility of analyses. Scanning electron microscope analysis of cells that were either melted or mechanically disrupted prior to freeze-drying indicated that variation in the results occurred primarily because of the release of intracellular material from lysed cells.
Organisms survived air drying considerably better than they survived freeze-drying, probably because of the lower level of dehydration. The better survival of Psychrobacter sp. strain SW8 than of E. coli following air drying was probably due to the protection against desiccation afforded to the former organism by its glycocalyx. Similarly, Ophir and Gutnick (38) found that mucoid strains of E. coli, Acinetobacter calcoaceticus, and Erwinia stewartii were significantly more resistant to desiccation than corresponding isogenic nonmucoid mutants of these organisms. When results obtained with air-dried cells are analyzed, it is important to consider that these organisms were deposited as a thick layer, the filter cake, on a membrane. Desiccation would have affected primarily the cells located in the top layers of the filter cake. The cells at the center and at the bottom of the filter cake remained hydrated to some extent, and these cells were probably the cells that survived the procedure. Therefore, the cell populations used to analyze cell surface parameters of air-dried organisms were heterogeneous and included cells exposed to different degrees of dehydration. The majority of the cells in a filter cake are embedded in the structure and are not exposed at the surface. These cells therefore determine the physiological properties of the population. With the exception of hydrophobicity of S. epidermidis, all of the parameters measured were significantly modified by air drying. Clearly, even cells embedded in filter cakes were affected considerably by the procedure.
Influence of resuspension medium.
Organisms resuspended in the NaCl solution were compared to organisms resuspended in water to determine the effects of modification of the resuspension medium on cell surface properties. The organisms used in this study had a net negative surface charge at pH 7.4. The negative charge of bacterial cells is derived from ionogenic groups which include amino and carboxylic acids and phosphate groups (22). Not all of these groups need to be exposed at the cell surface, however. In fact, ionogenic groups embedded in the cell wall may contribute significantly to the surface charge (54). The negatively charged cell surface attracts positively charged ions from the medium and repels the negatively charged counterparts. This process generates the Stern layer at the medium-cell interface that contains sorbed counterions (48). The thickness of this layer corresponds to the average diameter of the hydrated sorbed counterions. Further into the medium, the Stern layer is replaced by the diffuse double layer, a region of charge imbalance, where cations are accumulated and anions are depleted. The thickness of this region increases when the ion concentration in the medium decreases, since ions required for compensation of the charge on the cell surface have to be recruited from a larger volume of solution around the cell.
Most physicochemical parameters measured in this work required contact between the analyte (hydrocarbon droplet, solid substratum, Sepharose gel) and the cell surface. All analyte surfaces except the positively charged carboxymethyl-Sepharose surface were negatively charged in the buffers used. Attachment of cells to these surfaces, therefore, involved the contact of two negatively charged interfaces. This physicochemical phenomenon is best described by the DLVO theory (48), which is based on the energy changes that occur when two charged particles attach to each other in an electrolyte. Attachment requires that the forces of attraction (van der Waals forces, hydrogen bonds, ionic bonds) overcome the forces of repulsion (primarily electrostatic forces) in the immediate vicinity of the cell surface. The repulsive forces decrease exponentially with distance from the cell surface, while the attractive forces are maximal upon contact. Replacement of a phosphate-buffered solution containing NaCl at the concentration found in seawater by a medium containing the same amount of phosphate buffer but no salt should modify the electrostatic component of the interfaces involved in the process significantly. This obviously should influence the value of contact-dependent parameters of cells, such as adhesion to solid substrata, retention in Sepharose columns, and contact with hydrocarbon.
The DLVO theory and the Helmholtz-Smoluchowski equation predict that the change from a high-salt medium to a low-salt medium, such as phosphate-buffered H2O, should increase the electrophoretic mobility of the organisms in absolute terms (e.g., change the values to more negative numbers) and reduce hydrophobicity, affinity to Sepharose, and adhesion to substrata. These effects were indeed observed in the majority of our assays. However, situations in which the values of the parameters remained unchanged or even increased suggest that the interactions of cells with analytes in the assays were not solely determined by electrostatic forces. Reductions in electrophoretic mobility were observed with most cells exposed to hydrocarbons in the MATH assay and with some freeze-dried and air-dried cells (Table 5). Preferential stripping of charged cell surface components attached to the hydrocarbon droplets may have been responsible for the reduction in cell surface charge of the MATH-treated organisms. Reduced cell surface charges of air-dried or freeze-dried cells were most probably caused by a different process. The polymers of microbial cell surfaces are sufficiently flexible to allow their reorientation at the cell surface, depending on environmental conditions. Air and vacuum are hydrophobic phases (7). In such environments, a dynamic polymer surface such as the cell surface would reorientate to expose hydrophobic groups at the interface (7) in a manner analogous to the modification of the surface chemical composition of hydrogels, which depends on the hydration state of the polymers (40). When the surfaces of some freeze-dried cells were rehydrated, the macromolecules at the interface, which were in equilibrium with a hydrophobic medium, may not have been capable of reverting back rapidly to the hydrophilic interface characteristic of hydrated media. These cells would thus remain less charged and more hydrophobic than the original cells. DEAE-Sepharose is a positively charged surface. The fact that the affinity for this type of column was scarcely affected or even improved in some instances when the resuspension medium was changed from the NaCl solution to water reflects the strong attractive force between the surface of the DEAE-Sepharose column and the negatively charged cells. An additional factor that needs to be considered when the results of adhesion assays or Sepharose column experiments are analyzed is the fact that cell manipulation protocols, such as freeze-drying, centrifugation, and hydrocarbon contact, may disrupt the cell surface structure so that cell surface roughness is increased and macromolecular appendages (polysaccharides or proteins) protrude into the medium. Under certain conditions and on certain substrata, some of these appendages may bridge the repulsive electric double layer and establish sufficiently strong links to initiate adhesive contacts.
Influence of washing buffer.
Organisms resuspended in the NaCl solution were compared to organisms resuspended in water to determine the effects of modification of the washing medium on cell surface properties. The washing medium can have an effect on the cell surface properties of a microorganism only via modification of the cell surface itself, since it is not the final interaction environment. Osmotic effects are one possible mechanism by which a change from a high-salt washing medium to a low-salt medium may change the cell surface of a microbe. Microorganisms in aquatic environments have high intracellular concentrations of potassium ions and metabolites which produce cytoplasmic turgor pressure (27). This internal pressure is borne by the peptidoglycan layer and is maintained by the passive and active influx and efflux of ions from the surrounding liquid medium (57). Consequently, sudden exposure of microorganisms to lower-ionic-strength conditions disturbs the balance of ion transfer and results in conformational changes in the cell envelope. If these changes include modifications to the cell surface and if the modifications are stable in the resuspension buffer, then physicochemical cell surface properties are altered. The modifications can also result in the leakage of internal cell components, such as periplasmic and cytoplasmic enzymes and ions, as has been extensively reported previously for E. coli (33, 47, 59). These effects were not sufficiently strong to significantly affect the viability of the cells in this study. Coating of cell surfaces with leaked components, however, could have significantly modified their properties. Adsorption of ions from high-salt solutions or the lack of adsorption of ions is a third mechanism by which the washing medium may affect the cell surface properties of microbes. Adsorption of counterions may destabilize cell surfaces exposed to high shear stress during centrifugation, to dehydration during freeze-drying or air drying, and to vortexing and hydrocarbon contact during MATH. Carryover of adsorbed cations from a high-salt washing buffer into a low-salt resuspension medium should affect the cell surface properties measured in this medium compared with the properties of cells washed in low-salt medium. The type and density of adsorbed ions can vary greatly depending on the chemical composition of the cell wall, the presence of extracellular surface structures, the growth conditions, and the cell preparation protocols (13, 44). Cell surfaces damaged during cell preparation may therefore adsorb different ions, a factor which may exacerbate the effects of the cell manipulation procedure on cell surface properties.
Correlations between cell surface analysis techniques.
Poor correlation between different parameters which should measure similar properties of cell surfaces is a common problem (12, 14, 35, 41). It may appear surprising that tests in which cells are supposedly exposed to the same chemical groups, such as straight-chain hydrocarbons in MATH and octyl-Sepharose tests, produce results which do not correlate with each other, as has often been observed by other workers (12, 16, 26, 35, 50). This lack of correlation is in part a consequence of often missing or inaccurate information about the mechanism underlying the method. For example, the negative charge of hexadecane droplets at pH 7 implies that electrostatic interactions play a major role in the MATH assay. A cell surface charge measured by electrophoretic mobility includes charges generated by materials buried in the cell membrane or cell wall and may not accurately reflect the charge at the microbe-medium interface. The mechanisms of a cell surface analysis method need to be carefully considered to avoid overinterpretation of the data obtained with it. For example, cells used in adhesion assays may be centrifuged at 5,000 × g, but the same cells are subjected to vortexing in the presence of hydrocarbons before MATH tests. As our results clearly show, these two types of cells may have very little in common on their surfaces, and the MATH results may, therefore, not reflect the properties of the cell surface of the organisms used in the adhesion assay (or Sepharose column chromatography). Microbial cell surface analysis requires new methods that do not depend on extensive cell manipulation prior to measurement and that allow determinations of cell surface parameters of organisms obtained directly from the environment. Flow cytometry (37) and atomic force microscopy in the tapping mode in situ (21) in combination with appropriate cell surface markers or appropriately modified cantilever tips may permit nondestructive and noninvasive analysis of cell surface parameters.
Conclusion.
All of the microbes investigated in this work were affected to some degree by cell preparation techniques, but in each technique some organisms or different phenotypes of the same organism were more susceptible than others. Cells may even lose their viability after manipulation by some protocols. Therefore, it is important that the effects of cell surface analysis protocols on microbes be ascertained in every investigation pertaining to bacterial cell surface characteristics to ensure that cell samples are subjected to the least disruptive preparation methods and that the final results reflect the nature of the true microbial cell surface as realistically as possible. A second, very important issue that needs to be considered when cell surface analysis data are interpreted is the actual mechanism by which cell surface parameter data are obtained. The standard protocol used for harvesting bacteria, centrifugation at 5,000 × g, was found to be the protocol that was least disruptive to the structural integrity of cells. The composition of the buffer used in the cell surface analysis test should resemble the composition of the environment of the microbe as closely as possible. Nondestructive Sepharose column assays are preferable for hydrophobicity determinations. Last but not least, reliable, nondestructive cell surface analysis techniques need to be developed, perhaps by using some of the powerful new techniques, such as flow cytometry and atomic force microscopy.
ACKNOWLEDGMENTS
This study was supported by a grant from the Australian Research Council.
We thank Patrick Marks, Anita Vooros, and Martin Hoogland for technical assistance during the study. Comments from Andrew Leis and Henk Busscher were greatly appreciated.
REFERENCES
- 1.Absolom D R, Lamberti F V, Policova Z, Zingg W, van Oss C J, Neumann A W. Surface thermodynamics of bacterial adhesion. Appl Environ Microbiol. 1983;46:90–97. doi: 10.1128/aem.46.1.90-97.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amory D E, Mozes N, Hermesse M P, Leonard A J, Rouxhet P G. Chemical analysis of the surface of microorganisms by X-ray photoelectron spectroscopy. FEMS Microbiol Lett. 1988;49:107–110. [Google Scholar]
- 3.Beech I B, Cheung C W S, Johnson D B, Smith J R. Comparative studies of bacterial biofilms on steel surfaces using atomic force microscopy and environmental scanning electron microscopy. Biofouling. 1996;10:65–77. doi: 10.1080/08927019609386271. [DOI] [PubMed] [Google Scholar]
- 4.Bunt C R, Jones D S, Tucker I G. The effects of pH, ionic strength and organic phase on the bacterial adhesion to hydrocarbons (BATH) test. Int J Pharm (Amsterdam) 1993;9:93–98. [Google Scholar]
- 5.Busscher H J, Weerkamp A H, van der Mei H C, van Pelt A W J, DeJong H P, Arends J. Measurement of the surface free energy of bacterial cell surfaces and its relevance for adhesion. Appl Environ Microbiol. 1984;48:980–983. doi: 10.1128/aem.48.5.980-983.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro H B, Teixeira P M, Kirby R. Evidence of membrane damage in Lactobacillus bulgaricus following freeze-drying. J Appl Microbiol. 1997;82:87–94. [Google Scholar]
- 7.Chatelier R C, Xie X, Gengenbach T R, Griesser H J. Quantitative analysis of polymer surface restructuring. Langmuir. 1995;11:2576–2584. [Google Scholar]
- 8.Cowan M M, van der Mei H C, Rouxhet P G, Busscher H J. Physicochemical and structural investigation of the surfaces of some anaerobic subgingival bacteria. Appl Environ Microbiol. 1992;58:1326–1334. doi: 10.1128/aem.58.4.1326-1334.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuperus P L, van der Mei H C, Reid G, Bruce A W, Khoury A E, van der Kuijl-Booij M, Noordmans J, Busscher H J. Effects of ciprofloxacin and vancomycin on physico-chemical surface properties of Staphylococcus epidermidis, Escherichia coli, Lactobacillus casei and Lactobacillus acidophilus. Microbios. 1995;82:49–67. [PubMed] [Google Scholar]
- 10.Dengis P B, Rouxhet P G. Preparation of yeast cells for surface analysis by XPS. J Microbiol Methods. 1996;26:171–183. [Google Scholar]
- 11.de Valdez G F, de Giori G S, de Ruiz Holgado A P, Oliver G. Effect of rehydration medium on the recovery of freeze-dried lactic acid bacteria. Appl Environ Microbiol. 1985;50:1339–1341. doi: 10.1128/aem.50.5.1339-1341.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dillon J K, Fuerst J A, Hayward A C, Davis G H G. A comparison of five methods for assaying bacterial hydrophobicity. J Microbiol Methods. 1986;6:13–19. [Google Scholar]
- 13.Dolowy K. Bioelectrochemistry of cell surfaces. Prog Surf Sci. 1984;15:245–368. [Google Scholar]
- 14.Donlon B, Colleran E. A comparison of different methods to determine the hydrophobicity of acetogenic bacteria. J Microbiol Methods. 1993;17:27–37. [Google Scholar]
- 15.Funk W, Dammann V, Vonderheid C, Oehlmann G. Statistische Methoden in der Wasseranalytik. Weinheim, Germany: VCH Verlagsgesellschaft mbh; 1985. p. 25. [Google Scholar]
- 16.Gannon J T, Manilal V B, Alexander M. Relationship between cell surface properties and transport of bacteria through soil. Appl Environ Microbiol. 1991;57:190–193. doi: 10.1128/aem.57.1.190-193.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geertsema-Doornbusch G I, van der Mei H C, Busscher H J. Microbial cell surface hydrophobicity: the involvement of electrostatic interactions in microbial adhesion to hydrocarbons (MATH) J Microbiol Methods. 1993;18:61–68. [Google Scholar]
- 18.Gilbert P, Evans D J, Duguid I G, Evans E, Brown M R W. Cell surface properties of E. coli, and Staphylococcus epidermidis. In: Mozes N, Handley P S, Busscher H J, Rouxhet P G, editors. Microbial cell surface analysis: structural and physicochemical methods. New York, N.Y: VCH Publishers; 1991. pp. 339–356. [Google Scholar]
- 19.Gilbert P, Pemberton D, Wilkinson D E. Synergism within poly-hexamethylene biguanide biocide formulations. J Appl Bacteriol. 1990;69:593–598. doi: 10.1111/j.1365-2672.1990.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert P, Caplan F, Brown M R W. Centrifugation injury of Gram-negative bacteria. J Antimicrob Chemother. 1991;27:550–551. doi: 10.1093/jac/27.4.550. [DOI] [PubMed] [Google Scholar]
- 21.Hansma H G, Hoh J H. Biomolecular imaging with the atomic force microscope. Annu Rev Biophys Biomol Struct. 1994;23:115–139. doi: 10.1146/annurev.bb.23.060194.000555. [DOI] [PubMed] [Google Scholar]
- 22.Harkes G, van der Mei H C, Rouxhet P G, Dankert J, Busscher H J, Feijen J. Physicochemical characterisation of Escherichia coli: a comparison with Gram-positive bacteria. Cell Biophys. 1992;20:17–32. doi: 10.1007/BF02782652. [DOI] [PubMed] [Google Scholar]
- 23.Heimenz P. Electrophoresis and other electrokinetic phenomena. In: Lagowski J J, editor. Principles of colloid and surface chemistry. New York, N.Y: Marcel Dekker Inc.; 1977. pp. 452–487. [Google Scholar]
- 24.Herben P F G, Mozes N, Rouxhet P G. Variation of the surface properties of Bacillus licheniformis according to age, temperature and aeration. Biochim Biophys Acta. 1990;1033:184–188. doi: 10.1016/0304-4165(90)90010-t. [DOI] [PubMed] [Google Scholar]
- 25.Hodgson P H, Leslie G L, Schneider R P, Fane A G, Fell C J D, Marshall K C. Cake resistance and solute rejection in bacterial microfiltration: the role of the extracellular matrix. J Membr Sci. 1993;79:35–53. [Google Scholar]
- 26.Kjelleberg S, Hermansson M. Starvation-induced effects on bacterial surface characteristics. Appl Environ Microbiol. 1984;48:497–503. doi: 10.1128/aem.48.3.497-503.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labischinski H, Maidhof H. Bacterial peptidoglycan: overview and evolving concepts. In: Ghuysen J M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier Science B.V.; 1994. pp. 23–38. [Google Scholar]
- 28.Lang E, Malik K A. Maintenance of biodegradation capacities of aerobic bacteria during long-term preservation. Biodegradation. 1996;7:65–71. [Google Scholar]
- 29.Leslie S B, Israeli E, Lighthart B, Crowe J H, Crowe L M. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl Environ Microbiol. 1995;61:3592–3597. doi: 10.1128/aem.61.10.3592-3597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lichtenberg D, Rosenberg M, Sharfman N, Ofek I. A kinetic approach to bacterial adherence to hydrocarbon. J Microbiol Methods. 1985;4:141–146. [Google Scholar]
- 31.Lievense L C, Verbeek M A M, Noomen A, van’t Reit K. Mechanism of dehydration inactivation of Lactobacillus plantarum. Appl Microbiol Biotechnol. 1994;41:90–94. [Google Scholar]
- 32.Marshall K C M, Pembrey R, Schneider R P. The relevance of X-ray photoelectron spectroscopy for analysis of microbial cell surfaces: a critical review. Colloids Surf B Biointerfaces. 1994;2:371–376. [Google Scholar]
- 33.Morozov I I, Dergacheva I P, Ansimova N S. Effect of osmotic shock on the viability, optical density, and permeability of heated Escherichia coli cells. Mikrobiologiya. 1986;55:278–281. [PubMed] [Google Scholar]
- 34.Mozes N, Leonard A J, Rouxhet P G. On the relations between the elemental surface composition of yeasts and bacteria and their charge and hydrophobicity. Biochim Biophys Acta. 1988;945:324–334. doi: 10.1016/0005-2736(88)90495-6. [DOI] [PubMed] [Google Scholar]
- 35.Mozes N, Rouxhet P G. Methods for measuring hydrophobicity of microorganisms. J Microbiol Methods. 1987;6:99–112. [Google Scholar]
- 36.Neu T R, Marshall K C. Microbial “footprints”—a new approach to adhesive polymers. Biofouling. 1991;3:101–112. [Google Scholar]
- 37.Novo D, Perlmutter N G, Hunt R H, Shapiro H M. Accurate flow cytometric membrane potential measurement in bacteria using diethyloxacarbocyanine and a ratiometric technique. Cytometry. 1999;35:55–63. doi: 10.1002/(sici)1097-0320(19990101)35:1<55::aid-cyto8>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 38.Ophir T, Gutnick D L. A role for exopolysaccharides in the protection of microorganisms from desiccation. Appl Environ Microbiol. 1994;60:740–745. doi: 10.1128/aem.60.2.740-745.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pembrey R S. PhD. thesis. Sydney, New South Wales, Australia: University of New South Wales; 1999. [Google Scholar]
- 40.Ratner B D, Castner D G. Advances in X-ray photoelectron spectroscopy instrumentation, and methodology: instrument evaluation and new techniques with special reference to biomedical studies. Colloids Surf B Biointerfaces. 1994;2:333–346. [Google Scholar]
- 41.Rosenberg M. Bacterial adherence to hydrocarbons: a useful technique for studying cell surface hydrophobicity. FEMS Microbiol Lett. 1984;22:289–295. [Google Scholar]
- 42.Rosenberg M, Gutnik D, Rosenberg E. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett. 1980;9:29–33. [Google Scholar]
- 43.Rouxhet P G, Genet M J. Cell surface composition by XPS. In: Mozes N, Handley P S, Busscher H J, Rouxhet P G, editors. Microbial cell surface analysis: structural and physicochemical methods. New York, N.Y: VCH Publishers; 1991. pp. 173–220. [Google Scholar]
- 44.Rutter P R, Vincent B. Physicochemical interactions of the substratum, microorganisms and the fluid phase. In: Marshall K C, editor. Microbial adhesion and aggregation. Berlin, Germany: Springer-Verlag; 1980. pp. 21–38. [Google Scholar]
- 45.Schneider R P. Conditioning film-induced modification of substratum physicochemistry—analysis by contact angles. J Colloid Interface Sci. 1996;182:204–213. [Google Scholar]
- 46.Schneider R P, Marshall K C. Retention of the Gram-negative marine bacterium SW8 on surfaces: effects of microbial physiology, substratum nature and conditioning films. Colloids Surf B Biointerfaces. 1994;2:387–396. [Google Scholar]
- 47.Schwarz H, Koch A L. Phase and electron microscopic observations of osmotically induced wrinkling and the role of endocytotic vesicles in the plasmolysis of the Gram-negative cell wall. Microbiology. 1995;141:3161–3170. doi: 10.1099/13500872-141-12-3161. [DOI] [PubMed] [Google Scholar]
- 48.Shaw D J. Introduction to colloid and surface chemistry. 3rd ed. London, United Kingdom: Butterworths; 1980. [Google Scholar]
- 49.Smyth C J, Siegel J, Salton M R, Owen P. Immunochemical analysis of inner and outer membranes of Escherichia coli by crossed immunoelectrophoresis. J Bacteriol. 1978;133:306–319. doi: 10.1128/jb.133.1.306-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van der Mei H C, Weerkamp A H, Busscher H J. Physicochemical surface characteristics and adhesive properties of Streptococcus salivarius strains with defined cell surface structures. FEMS Microbiol Lett. 1987;40:15–19. [Google Scholar]
- 51.Van der Mei H C, Busscher H J. On the difference between contact angles measured on partly dehydrated and freeze-dried oral streptococci. J Colloid Interface Sci. 1989;136:297–300. [Google Scholar]
- 52.Van der Mei H C, Rosenberg M, Busscher H J. Assessment of microbial cell surface hydrophobicity. In: Mozes N, Handley P S, Busscher H J, Rouxhet P G, editors. Microbial cell surface analysis: structural and physicochemical methods. New York, N.Y: VCH Publishers; 1991. pp. 263–287. [Google Scholar]
- 53.Van der Mei H C, Meijer S, Busscher H J. Electrophoretic mobilities of protein-coated hexadecane droplets at different pH. J Colloid Interface Sci. 1998;205:185–190. doi: 10.1006/jcis.1998.5669. [DOI] [PubMed] [Google Scholar]
- 54.Vanderwal A, Minor M, Norde W, Zehnder A J B, Lyklema J. Conductivity and dielectric dispersion of Gram-positive bacterial cells. J Colloid Interface Sci. 1997;186:71–79. doi: 10.1006/jcis.1996.4615. [DOI] [PubMed] [Google Scholar]
- 55.Vanhaecke E, Pijck J. Bioluminescence assay for measuring the number of bacteria adhering to the hydrocarbon phase in the BATH test. Appl Environ Microbiol. 1988;54:1436–1439. doi: 10.1128/aem.54.6.1436-1439.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Oss C J, Gillmann C F, Neumann A W. Phagocytic engulfment and cell adhesiveness as cellular surface phenomena. New York, N.Y: Dekker; 1975. [Google Scholar]
- 57.Volkov V Y. Physiological and physicochemical mechanisms of bacterial resistance to freezing and drying. Microbiology (Washington, DC) 1994;63:5–16. [Google Scholar]
- 58.Wicken A J. Bacterial cell walls and surfaces. In: Savage D C, Fletcher M, editors. Bacterial adhesion. New York, N.Y: Plenum Publishing Co.; 1985. pp. 45–70. [Google Scholar]
- 59.Zaske S K, Dockins W S, McFeters G A. Cell envelope damage in Escherichia coli caused by short-term stress in water. Appl Environ Microbiol. 1980;40:386–390. doi: 10.1128/aem.40.2.386-390.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]