Abstract
Lumpy skin disease virus (LSDV) causes lumpy skin disease in cattle and buffaloes, which is associated with significant animal production and economic losses. Since the 2000s, LSDV has spread from Africa to several countries in the Middle East; Europe; and Asia; including, more recently, several south-east Asian countries. In November 2020, Myanmar reported its first LSD outbreak. This study reports on the first incursion of LSD in Myanmar and the molecular analysis of the LSDV detected. Staff from the Livestock Breeding and Veterinary Department (LBVD) of the Ministry of Agriculture, Livestock, and Irrigation collected samples from cattle with suspected LSD infection. The Food and Agriculture Organization (FAO) of the United Nations’ Emergency Centre for Transboundary Animal Diseases (ECTAD) and the Joint International Atomic Energy Agency (IAEA)/FAO program’s Animal Health and Production laboratory provided LSDV diagnostic support to two regional veterinary diagnostic laboratories in Myanmar. Samples from 13 cattle tested positive by real-time PCR. Selected samples underwent sequence analysis in IAEA laboratories. The results show that the Myanmar LSDV sequences clustered with LSDV isolates from Bangladesh and India, LSDV Kenya, and LSDV NI-2490. Further characterization showed that the Myanmar LSDV is 100% identical to isolates from Bangladesh and India, implying a common source of introduction. These findings inform diagnosis and development of control strategies.
Keywords: lumpy skin disease, LSDV, Myanmar, molecular analysis, outbreak
1. Introduction
Lumpy skin disease virus (LSDV) belongs to the Capripoxvirus genus of the Poxviridae family and is closely related to both sheeppox virus (SPPV) and goatpox virus (GTPV) [1]. LSDV is the causative agent of lumpy skin disease (LSD) in cattle and buffaloes. Cattle movements can spread LSDV over long distances. Because arthropod vectors such as Stomoxys spp. transmit the virus locally, LSD outbreaks are difficult to contain [2].
The main clinical sign of LSD is nodules on the skin, but lesions also occur on mucous membranes and internal organs. Although it usually results in less than 10% mortality, LSD causes substantial production losses and hide damage, affecting cattle trade, including export. Because of its economic impact, the World Organisation for Animal Health (OIE) lists LSD as a notifiable disease [3,4].
In Myanmar, cattle and buffaloes contribute to food security and constitute important social and financial assets in the livelihoods of most of the rural population. Smallholder farmers use bovine animals for plowing and transport on relatively unmechanized farms. The cattle have additional economic importance due to the valuable export trade of live cattle from Myanmar to meet the demand for beef in China. LSD can seriously affect rural livelihoods and food security in Myanmar.
Cattle are unevenly distributed around the country, with more than half the national population concentrated in three central dry zone regions: the Sagaing region, Mandalay region, and Magwe region. The 2018 national livestock census estimated the total draught cattle population at 9.6 million, with 2.2 million farms raising an average of four draught cattle apiece. Dairy cattle are relatively less numerous, with a reported national herd of about 129,000 raised on 32,000 farms, mainly concentrated around Yangon and Mandalay’s two large urban centers. On the other hand, draught cattle are relatively numerous in upland areas, including Kayin state, Kayah state, Kachin state, Shan state (east, south, and north), and Rakhine state [5]. (In Myanmar, states and regions are similar administrative areas.)
The national draught buffalo population is 1.9 million, kept on about 420,000 farms with an average of four draught buffaloes per farm. Buffaloes are more commonly found in Sagaing region and upland states than in other states or regions. Both male and female buffaloes are used for plowing, often individually. Only around 7000 farms raise dairy buffaloes [5]. The national population of around 36,000 mithun or gayal (Bos frontalis) is kept in about 10,000 holdings, mainly in Chin state [5]. The susceptibility of this species to LSDV is unknown.
LSDV is endemic in Africa. Since 2015, its range increased, with outbreaks, for example, in several Balkan countries [6]. Its potential for wider distribution is long recognized, including its possibility to become firmly established in Asia [4]. In 2019, three countries bordering Myanmar detected and reported LSDV for the first time in Asia, namely, Bangladesh in July 2019 [7,8], India in August 2019 [9], and China in August 2019 [10]. Nepal reported its first outbreak in June 2020 [11], and Vietnam reported an outbreak in November 2020 [12].
Following the reports of LSD in neighboring countries, the Livestock Breeding and Veterinary Department (LBVD) of the Ministry of Agriculture, Livestock, and Irrigation (MOALI) was alert to the possibility of the virus’s incursion into Myanmar. Therefore, the LBVD prepared for this eventuality by conducting a qualitative risk analysis in July 2020, by raising awareness in its field staff, by participating in online training organized by the Food and Agriculture Organization (FAO) of the United Nations, and by procuring laboratory diagnostic reagents through support from the Joint International Atomic Energy Agency (IAEA)/FAO program’s Animal Health and Production laboratory.
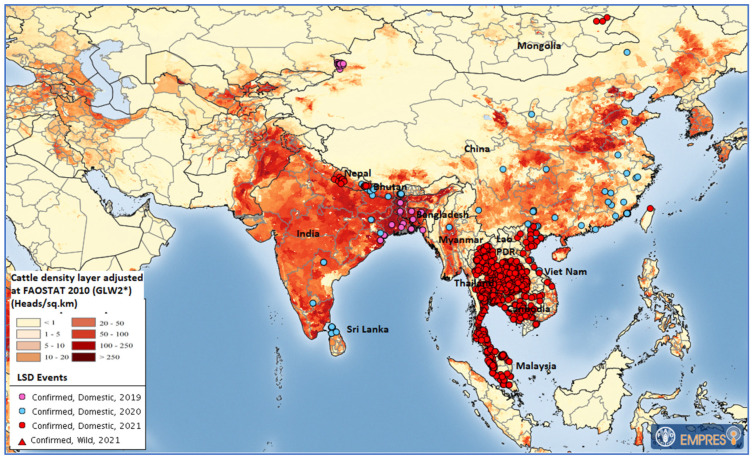
In November 2020, Myanmar reported its first LSD outbreak with six cases in cattle in Sagaing region [13]. No further outbreak was reported from the field, but as no LSD vaccine is authorized for use in Myanmar, it is feasible that unreported spread occurred in the country. Reported outbreaks in neighboring countries from 2019 to December 2021 are shown in Figure 1.
Figure 1.
Locations of confirmed LSD outbreaks in Asia from 2019 to December 2021 (map source: FAO Emergency Prevention System Global Animal Disease Information System (EMPRES-i); data: OIE and national authorities; cattle density layer source: GLW2*: The Gridded Livestock of the World, Version 2, 2014, https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0096084 (accessed on 21 April 2022). Maps disclaimer: The boundaries and names shown and the designations used on these map(s) do not imply the expression of any opinion whatsoever on the part of FAO concerning the legal status of any country, territory, city, or area, or of its authorities, or concerning the delimitation of its frontiers and boundaries. Dashed lines on maps represent approximate border lines for which there may not yet be full agreement. FAO Regional Office for Asia and the Pacific (RAP) has verified that all maps contained in the work are in conformity with UN maps, and FAO RAP accepts all responsibility in the event of reputational damage to FAO or FAO member countries as a result of inappropriate boundaries.
Molecular analysis of LSDV from countries that share a border with Myanmar showed that the virus types sampled in China and India/Bangladesh are different (see Discussion). Therefore, molecular analysis of the Myanmar virus was carried out to inform disease control through vaccination and provide clues to the virus’s origin.
This study confirms that LSDV is present in Myanmar. Furthermore, it describes the Myanmar virus isolates’ genetic and phylogenetic relationship with other known LSDVs.
2. Materials and Methods
2.1. Sample Collection, Transport, Storage, and DNA Extraction
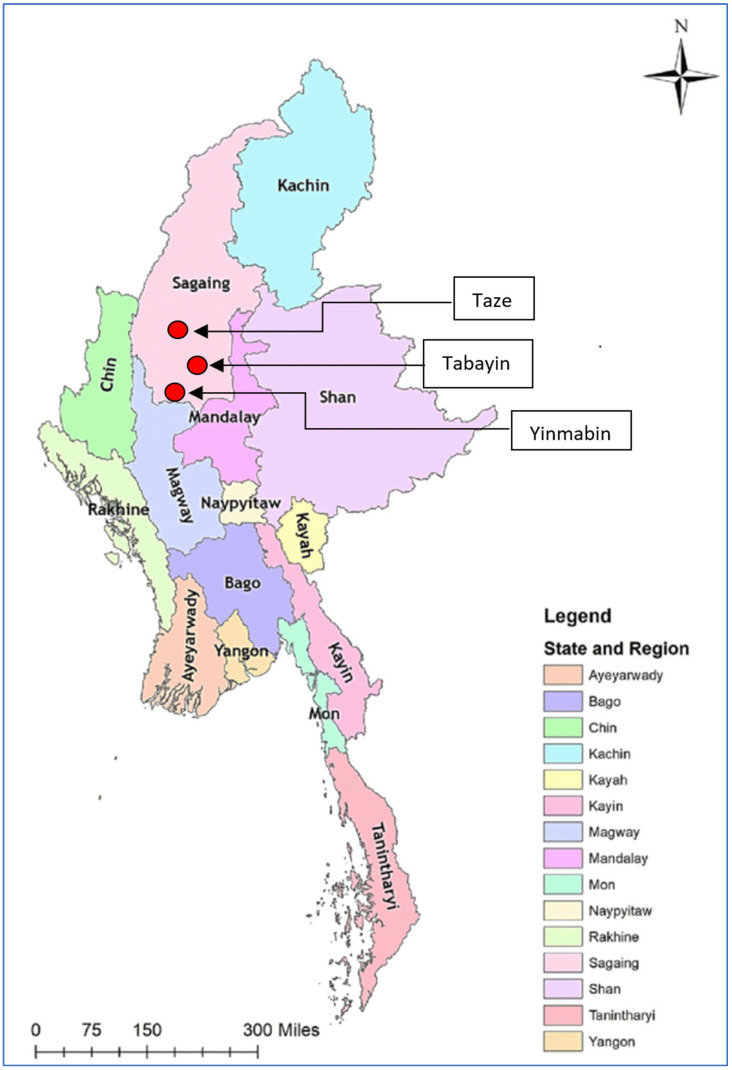
In 2020, nodular lesions on cattle suggestive of LSD were reported in the Sagaing region in northern Myanmar. Therefore, in November 2020, LBVD staff conducted a field investigation in three Sagaing townships, namely, Taze, Tabayin, and Yinmarbin, and collected samples for laboratory confirmation. The locations of the sample sites are shown in Figure 2.
Figure 2.
Map showing locations (red circles) of three townships in Sagaing region where LSD samples were collected.
Following the established LBVD-approved procedures, the LBVD staff collected samples from 13 adult cattle with clinical signs. From the total of 180 cattle that appeared to be infected due to their clinical signs consistent with LSD, the LBVD selected 13 with typical, advanced, nodular skin lesions for sample collection. Another field sampling criterion was practicality: some affected cattle were not used to handling and were maintained at premises that lacked the facilities to safely restrain adult bovines. Thus, the LBVD followed a purposive sample selection procedure based on clinical appearance and operator safety.
Samples comprising whole blood, scabs, and nodule biopsy materials were placed in labeled double plastic zip-lock bags. The samples were transported on ice in insulated cool boxes to the Yangon Veterinary Diagnostic Laboratory (VDL) and the Mandalay VDL. If diagnostic testing could not start immediately, the samples were stored in a −80 °C freezer.
The samples were prepared for PCR testing by grinding approximately 1 g of tissue from nodular lesions in 9 mL phosphate buffer saline (PBS) and centrifuging for 10 min at 2500 rpm. Total DNA was extracted from the sample supernatants according to the DNA extraction kit manufacturers’ instructions (in Yangon VDL, using Invitrogen Pure link viral DNA/RNA mini kit (Thermo-Fisher Scientific); in Mandalay VDL, using both the Invitrogen Pure link viral DNA/RNA mini kit and the Qiagen AllPrep DNA/RNA extraction kit/DNAeasy Mini Kit). The DNA was eluted using 50 μL of elution buffer and stored at −20 °C until use.
2.2. Detection of Capripoxvirus and Differential Diagnosis
All collected samples were tested for the presence of the capripoxvirus (CaPV) genome using real-time PCR as previously described [14] with some modifications. The PCR reactions were prepared in a 20 μL reaction volume containing 10 μL of 2X iQsupermix (Bio-Rad, Hercules, CA, USA), 400 nM each of the forward and reverse primers, 250 nM of the probe, and 2 μL of template DNA. The PCR program conditions were initial denaturation at 95 °C for 10 min, followed by 45 cycles at 95 °C for 15 s and at 60 °C for 60 s, with the fluorescence reading at the end. Known positive samples of SPPV, GTPV, and LSDV were included as controls.
To further characterize the CaPV positive samples, a previously developed high-resolution melting (HRM) assay [15] was employed to distinguish the LSDV-positive samples from other closely related CaPVs. This assay categorizes CaPVs based on the melting temperature (Tm) of the PCR products. Briefly, the PCR was set up in a 20 μL reaction volume containing 1X of SsoFast EvaGreen Supermix (Bio-Rad, Hercules, CA, USA), 200 nM each of the forward and reverse primers, and 2 μL of template DNA. The PCR was performed in a CFX96 PCR machine with an initial denaturation step at 95 °C for 4 min, followed by 40 cycles at 95 °C for 1 s, 59 °C for 5 s, and 70 °C for 5 s with fluorescence reading at the end, followed by 95 °C for 30 s, 65 °C for 1 min, and melting between 65 and 85 °C at 10 s/0.2 °C with the fluorescence reading at each °C, then 37 °C for 1 min.
2.3. Amplification and Sequencing of LSDV Target Genes
Following the screening for positive CaPV samples at the LBVD laboratories, 10 samples with low Cq values were selected for sequencing and further genetic characterization. Four LSDV genes, namely, the RNA polymerase 30 kDa subunit (RPO30), G protein-coupled receptor (GPCR), extracellular enveloped virus (EEV) glycoprotein, and CaPV homolog of the variola virus B22R, were amplified as previously described [16,17,18]. The PCR reactions were conducted in a total reaction volume of 20 μL consisting of 1X PCR buffer (Qiagen, Hilden, Germany), 500 nM of each forward and the reverse primers, 200 uM of dNTPs, 1.25 U of Taq DNA polymerase (Qiagen, Hilden, Germany), and 2 μL of template DNA. The PCR products were analyzed on a 2% agarose gel in 1X TAE buffer and visualized on a Gel Documentation System (Bio-Rad, Hercules, USA). The PCR products were subsequently purified and sent for sequencing from both directions using forward and reverse primers at LGC Genomics (Berlin, Germany).
2.4. Sequence Analysis
The obtained sequences were assembled using the Vector NTI software v11.5 (Invitrogen, Waltham, MA, USA). Reference sequences for LSDVs, SPPVs, and GTPVs were retrieved from GenBank and included in the datasets, followed by alignment with MEGA v7.0.26 using the muscle algorithm and the codon option [19]. A comparative alignment of the partial EEV glycoprotein and the B22R gene was performed and visualized on BioEdit v7.2.5. Finally, Bayesian phylogenetic inferences for the complete RPO30 and GPCR genes were performed using BEAST v1.8.4 [20]. The log files were inspected using the TRACER program to guarantee that the MCMC run reached convergence by determining the optimum burn-in value number based on the effective sample sizes (ESS). TreeAnnotator was used to generate the maximum clade credibility (MCC) files, and the phylogenetic trees with the associated metadata were visualized using ggtree package on R [21]. A section of the alignment of the GPCR between nucleotide positions 80 and 120 was visualized together with the GPCR tree.
3. Results
3.1. Clinical Findings
In the three affected townships (Figure 2), LBVD staff found 180 clinically affected cattle. The main presenting sign observed was skin nodules. Other reported signs included swollen, edematous legs, swelling of the brisket, and ocular and nasal discharge. Figure 3 shows a typical case. No buffalo case was reported.
Figure 3.
Bull in Myanmar with LSD lesions. Photo: Dr Ohnmar Aye (Assistant Director, LBVD, Tabayin township).
From the data on township cattle populations, the morbidity at the township level ranged from 1% to 1.6%, as shown in Table 1 below. No mortality was reported.
Table 1.
Mortality and morbidity of cattle in affected townships.
| Township | No. of Affected Animals | Species | Clinical Signs | No. of Samples | Samples Type | No. of Animals at Risk in Township | Morbidity % | No. of Dead Animals |
|---|---|---|---|---|---|---|---|---|
| Tabayin | 90 | cattle | Nodule, leg swollen, brisket swollen, nasal and ocular discharges | 7 | Exposed nodular lesion, blood | 5696 | 1.6% | - |
| Taze | 50 | cattle | Nodule, leg swollen | 3 | Exposed nodular lesion | 3571 | 1.4% | - |
| Yinmarbin | 40 | cattle | Nodule, leg swollen | 3 | Exposed nodular lesion | 4000 | 1% | - |
Reporting officers observed stable flies, mosquitoes, and ticks in the affected townships. Farmers attempted to control the biting insects by burning smoky fires near where they housed their cattle. The LBVD collected some vectors for LSDV testing with PCR. The collected vectors comprised ten mosquitoes and four ticks from animals in the Yinmarbin township. Stable flies were not available at the time of sample collection, perhaps due to smoky fires. The LBVD laboratories found no PCR-positive results in either of the collected vector species.
3.2. Molecular Diagnosis of CaPV
All 13 samples collected from LSD-suspected animals in the three townships were confirmed to be CaPV positive (+) by RT-PCR (Table 2) and further identified as LSDVs based on the Tm of the PCR products using HRM assay (highlighted in grey in Table 2).
Table 2.
List of samples analyzed in this study. Grey-highlighted samples were further analyzed with HRM assay and sent for sequencing.
| Sr. | Host | Origin in Myanmar | Sample Type | Date of Collection | Vaccination History | CaPV RT-PCR | HRM Tm | Cq Value |
|---|---|---|---|---|---|---|---|---|
| 1 | Cattle | Taze township, Sagaing region | Nodule | Oct 2020 | Non-vaccinated | (+) | ||
| 2 | Cattle | Yinmabin township, Sagaing region | Serum | Oct 2020 | Non-vaccinated | (+) | ||
| 3 | Cattle | Tabayin township, Sagaing region | Nodule | Nov 2020 | Non-vaccinated | (+) | 77.6 | 19.08 |
| 4 | Cattle | Tabayin township, Sagaing region | Nodule | Nov 2020 | Non-vaccinated | (+) | 77.6 | 16.46 |
| 5 | Cattle | Tabayin township, Sagaing region | Whole Blood | Nov 2020 | Non-vaccinated | (+) | 77.4 | 32.14 |
| 6 | Cattle | Tabayin township, Sagaing region | Nodule | Nov 2020 | Non-vaccinated | (+) | 77.6 | 17.18 |
| 7 | Cattle | Tabayin township, Sagaing region | Nodule | Nov 2020 | Non-vaccinated | (+) | 77.6 | 14.91 |
| 8 | Cattle | Tabayin township, Sagaing region | Nodule | Nov 2020 | Non-vaccinated | (+) | 77.6 | 25.44 |
| 9 | Cattle | Tabayin township, Sagaing region | Nodule | Nov 2020 | Non-vaccinated | (+) | 77.6 | 26.01 |
| 10 | Cattle | Tabayin township, Sagaing region | Nodule | Nov 2020 | Non-vaccinated | (+) | 77.6 | 23.89 |
| 11 | Cattle | Tabayin township, Sagaing region | Nodule | Nov 2020 | Non-vaccinated | (+) | 77.8 | 23.87 |
| 12 | Cattle | Taze township, Sagaing region | Serum | Nov 2020 | Non-vaccinated | (+) | ||
| 13 | Cattle | Yinmabin township, Sagaing region | Nodule | Nov 2020 | Non-vaccinated | (+) | 77.6 | 33.8 |
(+) CaPV positive samples.
3.3. LSDV Gene Amplification and Sequencing
The ten samples with low Cq values, which were confirmed LSDV positive, were subjected to conventional PCR for the amplification of the complete RPO30 and GPCR genes and the partial EEV glycoprotein and B22R genes. Out of the ten samples, six were successfully amplified and sequenced for all four targeted LSDV genes. The sequences were edited on BioEdit to 606, 1146, 327, and 832 bp for the RPO30, GPCR, EEV, and B22R genes, respectively, and deposited in GenBank with OM674460–OM674483 accession numbers.
3.4. Sequence Analysis
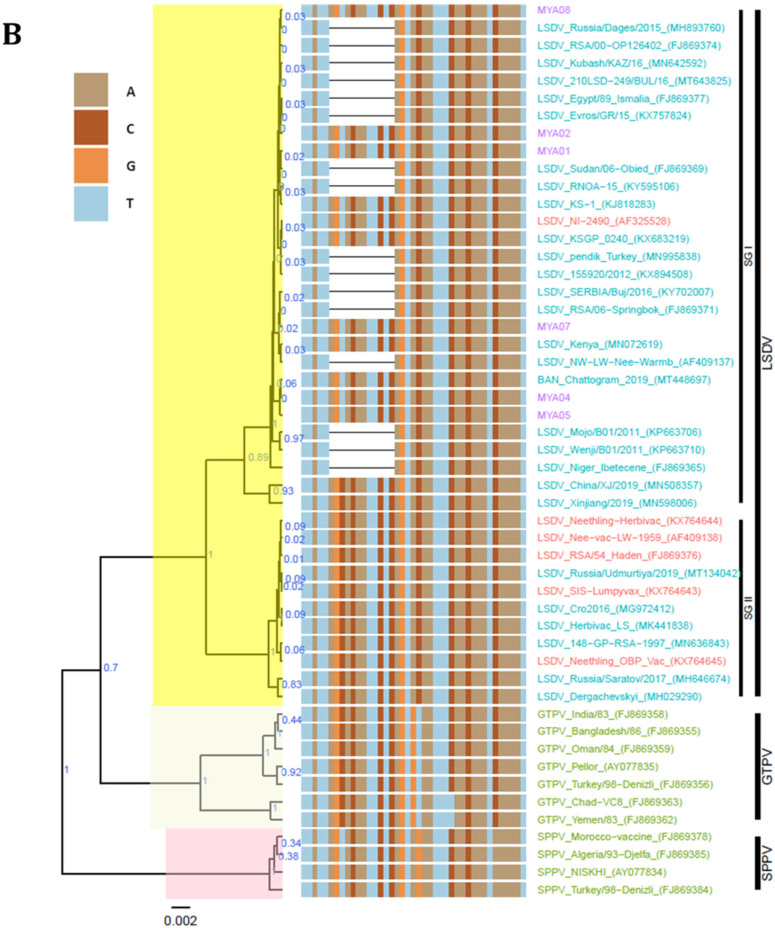
To compare and determine the phylogenetic relationship of the Myanmar LSDV isolates with other CaPVs, the obtained sequences were aligned with the corresponding reference gene sequences for LSDVs, SPPVs, and GTPVs retrieved from GenBank. The multiple sequence alignments of all four targeted genes showed that all Myanmar LSDVs in this study are identical. Moreover, the EEV glycoprotein sequence alignment showed that the Myanmar LSDVs share 100% sequence identity with other LSDVs, such as Bangladesh LSDV, LSDV KSGP-0240, LSDV NI-2490, and LSDV Kenya, that have a 27-nucleotide insertion (175–201). This 27-nucleotide insertion distinguishes Myanmar LSDVs from the LSDV Neethling-derived vaccines, recombinant LSDVs from Russia, and LSDVs from China (Figure 4A). The B22R fragment alignment further showed that the Myanmar LSDVs are identical to the Bangladesh LSDVs, LSDV NI-2490, and LSDV Kenya, but different from LSDV Neethling- and LSDV KSGP-0240-derived vaccines that have a nucleotide insertion at positions 102 and 745, respectively (Figure 4B).
Figure 4.
Multiple sequence alignments of the partial nucleotide sequences of (A) the EEV glycoprotein gene aligned with representative LSDV sequences, showing the 27-nucleotide deletion absent in Myanmar isolates (highlighted in the box), and (B) the B22R gene aligned with representative LSDV sequences retrieved from GenBank. The nucleotide insertion in LSDV Neethling and LSDV KSGP-0240 vaccines that is absent in Myanmar isolates is shown in the blocks. The Myanmar isolates are highlighted in violet, and the dots indicate the identical nucleotides in the alignments.
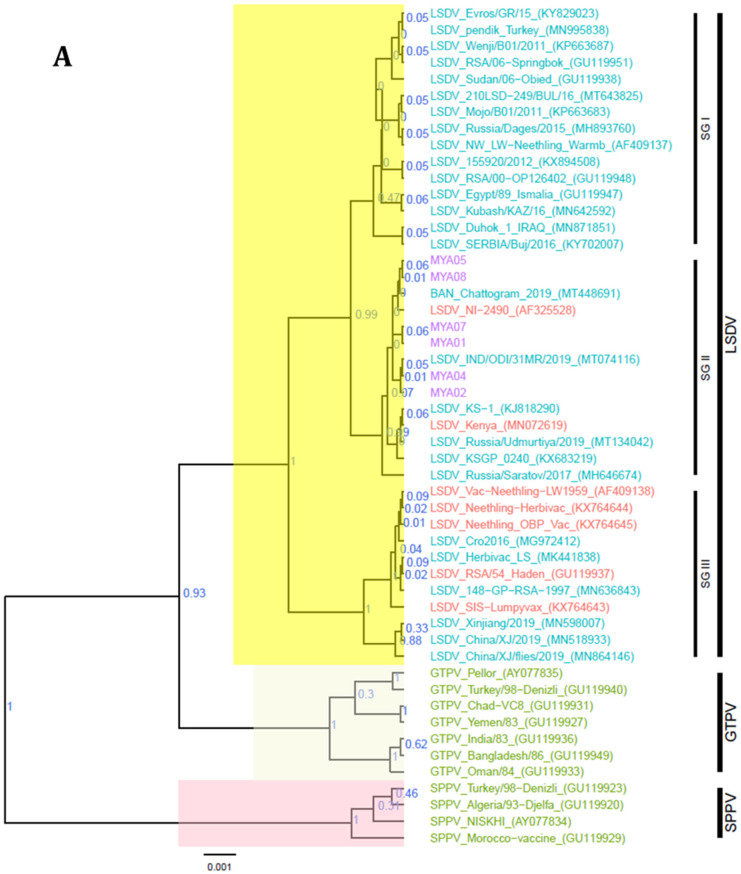
The RPO30 gene phylogenetic tree showed that the Myanmar LSDV sequences clustered in the LSDV subgroup SG II with field LSDV strains from Bangladesh and India, LSDV Kenya, and LSDV NI-2490. Notably, the vaccine strain LSDV KSGP-0240 and the Russian recombinant LSDVs (LSDV Udmurtiya/2019 and LSDV Saratov/2017) also clustered in SG II (Figure 5A). In addition, the GPCR phylogenetic tree further clustered the Myanmar LSDVs with the Bangladesh and Indian LSDVs, LSDV Kenya, LSDV NI-2490, and the vaccine strain LSDV KSGP-0240, but separate from the Russian recombinant LSDVs. The GPCR sequence alignment also showed a 12-nucleotide insertion in Myanmar LSDVs, a marker for LSDV vaccine strains (LSDV SGP_O-240 and LSDV Neethling vaccine), which has also been reported in Bangladesh and Indian LSDVs, LSDV NI-2490, LSDV Kenya, and recombinant LSDVs from Russia (Figure 5B).
Figure 5.
Maximum clade credibility (MCC) tree based on (A) the complete RPO30 gene sequences and (B) the complete GPCR gene sequences of CaPVs, plotted together with a portion of the GPCR multiple sequence alignment between 80 and 120 nucleotide positions. LSDV isolates from Myanmar are highlighted in violet.
4. Discussion
For decades, LSDV was endemic in Africa, where it caused substantial losses in cattle production, leading to food insecurity. Since the 2000s, the disease has spread to areas on other continents, such as the Middle East and the Balkans [22]. Recently, disease outbreaks have been reported in several south and south-east Asian countries, including Bangladesh, India, China, Vietnam, and Nepal [23]. Following the LSDV outbreaks in these neighboring countries, the Livestock Breeding and Veterinary Department (LBVD) of the Ministry of Agriculture, Livestock, and Irrigation (MOALI) of Myanmar conducted a risk assessment for LSDV outbreak in the country. In addition, the LBVD raised awareness among its staff through training on prevention and diagnosis of the disease.
Nevertheless, in November 2020, Myanmar reported its first LSDV case in cattle in the Sagaing region. The cattle showed typical clinical signs of LSDV, such as skin nodules and nasal and ocular discharges. Additionally, investigations into the affected holdings in Myanmar showed that they were infested by stable flies and mosquitoes, which are known to transmit LSDV [24,25].
We further described the genetic profiles of LSDV isolates circulating in Myanmar based on well-established molecular tools for CaPVs. The LSDV-suspected cases were first confirmed through qPCR and further characterized with HRM assay. This diagnostic tool has proven to differentiate CaPVs based on the PCR product’s melting temperature and the melting curve analysis, and it rules out other poxviruses, such as orthopoxviruses and parapoxviruses [15]. Further characterization of the samples based on four CaPVs markers (RPO30, GPCR, EEV glycoprotein, and B22R) showed that the LSD virus isolated in Myanmar is 100% identical to the LSDV isolates circulating in neighboring countries, such as Bangladesh and India, implying a common source of the LSDV introduction into these countries [26,27,28,29]. Although the origin of the index case is unknown, the genetic profile aligns it with a virus previously isolated from the Indian subcontinent. This is not surprising, as it was previously shown that there is frequent, informal livestock trade with South Asia [30].
Notably, the Myanmar LSDV was different from the Chinese LSDV, whose EEV sequences have a 27-nucleotide deletion and are also phylogenetically clustered separately based on the RPO30 gene sequences. Importantly, our results show that the Myanmar LSDV isolates are closely related to the historical Kenyan LSDVs, that is, LSDV Kenya and LSDV NI-2490, but different from the Russian recombinant LSDVs and the vaccine-derived LSDVs, that is, LSDV KSGP-0240 and LSDV Neethling.
To date, LSDV has been identified in cattle as the primary host as well as in buffaloes [18]. However, the possibility cannot be ruled out that other closely related domesticated species found in Myanmar, such as gayal (Bos frontalis), could be susceptible and at risk of LSDV infection.
Myanmar has an economically significant and growing export trade in beef cattle with China. Effective LSD control is a prerequisite for sustaining this trade. Considering that no vaccine program against LSDV is established in Myanmar yet, these findings provide helpful molecular information for the proper diagnosis and development of control strategies such as vaccination against LSDV in Myanmar and its neighboring countries.
Author Contributions
Conceptualization, M.T.M., I.K.M. and C.E.L.; methodology, T.B.K.S.; software, I.K.M.; formal analysis, I.K.M. and. T.B.K.S.; investigation, M.M.K. (Myint Myint Khin), W.W.M., W.Z.T., O.A. and I.K.M.; resources, Y.T.W. and G.C.; visualization, E.P.; writing—original draft preparation, M.T.M., M.M.K. (Maung Maung Kyin), D.H., I.K.M. and C.E.L.; writing—review and editing, M.T.M., D.H., I.K.M., G.C. and C.E.L.; supervision, M.T.M., D.H., W.W.M., Y.T.W., G.C. and C.E.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the VETLAB network initiative of the Joint FAO/IAEA Division and funded through the Peaceful Uses Initiatives (PUI) of Japan.
Institutional Review Board Statement
The authors confirm that the ethical polices of the journal, as stated on the journal’s ethical guidelines, have been adhered to. The samples used in this study were those collected and submitted to Mandalay and Yangon Veterinary Diagnostic Laboratories, Myanmar, for LSDV diagnosis. Ethical approval was not required.
Informed Consent Statement
Not applicable (the study did not involve humans).
Data Availability Statement
The DNA sequences generated and used in the analysis for this study are available in GenBank under the accession numbers OM674460–OM674483.
Conflicts of Interest
The authors declare no conflict of interest. The views expressed in this publication are those of the author(s) and do not necessarily reflect the views or policies of the Food and Agriculture Organization of the United Nations.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Buller R.M., Arif B.M., Black D.N., Dumbell K.R., Esposito J.J., Lefkowitz E.J., McFadden G., Moss B., Mercer A.A., Moyer R.W. Virus Taxonomy: Classification and Nomenclature of Viruses. Eighth Report of the International Committee on Taxonomy of Viruses. Elsevier; Amsterdam, The Netherlands: Academic Press; San Diego, CA, USA: 2005. Family Poxviridae; pp. 117–133. [Google Scholar]
- 2.Baldacchino F., Muenworn V., Desquesnes M., Desoli F., Charoenviriyaphap T., Duvallet G. Transmission of Pathogens by Stomoxys Flies (Diptera, Muscidae): A Review. Parasite. 2013;20:26. doi: 10.1051/parasite/2013026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.OIE . Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. OIE; Paris, France: 2021. Lumpy Skin Disease. [Google Scholar]
- 4.Babiuk S., Bowden T.R., Boyle D.B., Wallace D.B., Kitching R.P. Capripoxviruses: An Emerging Worldwide Threat to Sheep, Goats and Cattle. Transbound. Emerg. Dis. 2008;55:263–272. doi: 10.1111/j.1865-1682.2008.01043.x. [DOI] [PubMed] [Google Scholar]
- 5.Livestock Breeding and Veterinary Department . National Livestock Baseline Survey 2018 Report. Livestock Breeding and Veterinary Department; Yangon, Myanmar: 2019. [Google Scholar]
- 6.Lumpy Skin Disease (LSD)—OIE—Asia. [(accessed on 17 March 2022)]. Available online: https://rr-asia.oie.int/en/projects/lumpy-skin-disease-lsd/
- 7.Talukdar F., Fakir A., Hasan Z., Osmani T.M.G., Choudhury G.A., Budhay S., Sadekuzzaman M., Brum E., Bhowmic H.R., Noorjahan B. Identification of Lumpy Skin Disease (LSD): First-Time in Bangladesh during the Investigation of Unknown Skin Disease of Cattle. Int. J. Infect. Dis. 2020;101:360–361. doi: 10.1016/j.ijid.2020.09.946. [DOI] [Google Scholar]
- 8.Hasib F.M.Y., Islam M.S., Das T., Rana E.A., Uddin M.H., Bayzid M., Nath C., Hossain M.A., Masuduzzaman M., Das S., et al. Lumpy Skin Disease Outbreak in Cattle Population of Chattogram, Bangladesh. Vet. Med. Sci. 2021;7:1616–1624. doi: 10.1002/vms3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sudhakar S.B., Mishra N., Kalaiyarasu S., Jhade S.K., Hemadri D., Sood R., Bal G.C., Nayak M.K., Pradhan S.K., Singh V.P. Lumpy Skin Disease (LSD) Outbreaks in Cattle in Odisha State, India in August 2019: Epidemiological Features and Molecular Studies. Transbound. Emerg. Dis. 2020;67:2408–2422. doi: 10.1111/tbed.13579. [DOI] [PubMed] [Google Scholar]
- 10.Lu G., Xie J., Luo J., Shao R., Jia K., Li S. Lumpy Skin Disease Outbreaks in China, since 3 August 2019. Transbound. Emerg. Dis. 2021;68:216–219. doi: 10.1111/tbed.13898. [DOI] [PubMed] [Google Scholar]
- 11.Acharya K.P., Subedi D. First Outbreak of Lumpy Skin Disease in Nepal. Transbound. Emerg. Dis. 2020;67:2280–2281. doi: 10.1111/tbed.13815. [DOI] [PubMed] [Google Scholar]
- 12.Tran H.T.T., Truong A.D., Dang A.K., Ly D.V., Nguyen C.T., Chu N.T., Van Hoang T., Nguyen H.T., Nguyen V.T., Dang H.V. Lumpy Skin Disease Outbreaks in Vietnam, 2020. Transbound. Emerg. Dis. 2021;68:977–980. doi: 10.1111/tbed.14022. [DOI] [PubMed] [Google Scholar]
- 13.OIE-WAHIS. [(accessed on 17 March 2022)]. Available online: https://wahis.oie.int/#/report-info?reportId=28135.
- 14.Bowden T.R., Babiuk S.L., Parkyn G.R., Copps J.S., Boyle D.B. Capripoxvirus Tissue Tropism and Shedding: A Quantitative Study in Experimentally Infected Sheep and Goats. Virology. 2008;371:380–393. doi: 10.1016/j.virol.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gelaye E., Mach L., Kolodziejek J., Grabherr R., Loitsch A., Achenbach J.E., Nowotny N., Diallo A., Lamien C.E. A Novel HRM Assay for the Simultaneous Detection and Differentiation of Eight Poxviruses of Medical and Veterinary Importance. Sci. Rep. 2017;7:srep42892. doi: 10.1038/srep42892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badhy S.C., Chowdhury M.G.A., Settypalli T.B.K., Cattoli G., Lamien C.E., Fakir M.A.U., Akter S., Osmani M.G., Talukdar F., Begum N., et al. Molecular Characterization of Lumpy Skin Disease Virus (LSDV) Emerged in Bangladesh Reveals Unique Genetic Features Compared to Contemporary Field Strains. BMC Vet. Res. 2021;17:61. doi: 10.1186/s12917-021-02751-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelaye E., Belay A., Ayelet G., Jenberie S., Yami M., Loitsch A., Tuppurainen E., Grabherr R., Diallo A., Lamien C.E. Capripox Disease in Ethiopia: Genetic Differences between Field Isolates and Vaccine Strain, and Implications for Vaccination Failure. Antivir. Res. 2015;119:28–35. doi: 10.1016/j.antiviral.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Koirala P., Meki I.K., Maharjan M., Settypalli B.K., Manandhar S., Yadav S.K., Cattoli G., Lamien C.E. Molecular Characterization of the 2020 Outbreak of Lumpy Skin Disease in Nepal. Microorganisms. 2022;10:539. doi: 10.3390/microorganisms10030539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drummond A.J., Suchard M.A., Xie D., Rambaut A. Bayesian Phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu G., Smith D.K., Zhu H., Guan Y., Lam T.T.Y. Ggtree: An r Package for Visualization and Annotation of Phylogenetic Trees with Their Covariates and Other Associated Data. Methods Ecol. Evol. 2017;8:28–36. doi: 10.1111/2041-210X.12628. [DOI] [Google Scholar]
- 22.Tuppurainen E.S.M., Venter E.H., Shisler J.L., Gari G., Mekonnen G.A., Juleff N., Lyons N.A., De Clercq K., Upton C., Bowden T.R., et al. Review: Capripoxvirus Diseases: Current Status and Opportunities for Control. Transbound. Emerg. Dis. 2017;64:729–745. doi: 10.1111/tbed.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das M., Chowdhury M.S.R., Akter S., Mondal A.K., Uddin M.J., Rahman M.M., Rahman M.M. An Updated Review on Lumpy Skin Disease: Perspective of Southeast Asian Countries. J. Adv. Biotechnol. Exp. Ther. 2021;4:322–333. doi: 10.5455/jabet.2021.d133. [DOI] [Google Scholar]
- 24.Tuppurainen E.S.M., Stoltsz W.H., Troskie M., Wallace D.B., Oura C.A.L., Mellor P.S., Coetzer J.A.W., Venter E.H. A Potential Role for Ixodid (Hard) Tick Vectors in the Transmission of Lumpy Skin Disease Virus in Cattle. Transbound. Emerg. Dis. 2011;58:93–104. doi: 10.1111/j.1865-1682.2010.01184.x. [DOI] [PubMed] [Google Scholar]
- 25.Sprygin A., Pestova Y., Wallace D.B., Tuppurainen E., Kononov A.V. Transmission of Lumpy Skin Disease Virus: A Short Review. Virus Res. 2019;269:197637. doi: 10.1016/j.virusres.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Lamien C.E., Le Goff C., Silber R., Wallace D.B., Gulyaz V., Tuppurainen E., Madani H., Caufour P., Adam T., El Harrak M., et al. Use of the Capripoxvirus Homologue of Vaccinia Virus 30kDa RNA Polymerase Subunit (RPO30) Gene as a Novel Diagnostic and Genotyping Target: Development of a Classical PCR Method to Differentiate Goat Poxvirus from Sheep Poxvirus. Vet. Microbiol. 2011;149:30–39. doi: 10.1016/j.vetmic.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 27.Le Goff C., Lamien C.E., Fakhfakh E., Chadeyras A., Aba-Adulugba E., Libeau G., Tuppurainen E., Wallace D.B., Adam T., Silber R., et al. Capripoxvirus G-Protein-Coupled Chemokine Receptor: A Host-Range Gene Suitable for Virus Animal Origin Discrimination. J. Gen. Virol. 2009;90:1967–1977. doi: 10.1099/vir.0.010686-0. [DOI] [PubMed] [Google Scholar]
- 28.Menasherow S., Erster O., Rubinstein-Giuni M., Kovtunenko A., Eyngor E., Gelman B., Khinich E., Stram Y. A High-Resolution Melting (HRM) Assay for the Differentiation between Israeli Field and Neethling Vaccine Lumpy Skin Disease Viruses. J. Virol. Methods. 2016;232:12–15. doi: 10.1016/j.jviromet.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Chibssa T.R., Settypalli T.B.K., Berguido F.J., Grabherr R., Loitsch A., Tuppurainen E., Nwankpa N., Tounkara K., Madani H., Omani A., et al. An HRM Assay to Differentiate Sheeppox Virus Vaccine Strains from Sheeppox Virus Field Isolates and Other Capripoxvirus Species. Sci. Rep. 2019;9:6646. doi: 10.1038/s41598-019-43158-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bo L.L., Lwin K.S., Ungvanijban S., Knowles N.J., Wadsworth J., King D.P., Abila R., Qiu Y. Foot-and-Mouth Disease Outbreaks due to an Exotic Serotype Asia 1 Virus in Myanmar in 2017. Transbound. Emerg. Dis. 2019;66:1067–1072. doi: 10.1111/tbed.13112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The DNA sequences generated and used in the analysis for this study are available in GenBank under the accession numbers OM674460–OM674483.