Abstract
Divercin V41 (DV41) is a class IIa bacteriocin produced by Carnobacterium divergens V41. This antilisterial peptide is homologous to pediocin PA-1 and contains two disulfide bonds. To establish the structure-activity relationships of this specific family of bacteriocin, chemical modifications and enzymatic hydrolysis were performed on DV41. Alteration of the net charge of this cationic bacteriocin by succinylation and acetylation revealed that, in a certain range, the electrostatic interactions were surprisingly not necessary for the activity of DV41. Cleavage of DV41 by endoproteinase Asp-N released two fragments N1[1–17] and N2[18–43] corresponding to the conserved hydrophilic N-terminal and the variable hydrophobic C-terminal sequences, respectively. Inhibitory assays showed that only the C-terminal fragment was active, and after trypsin cleavage at Lys42 or disulfide reduction it lost its inhibitory activity. These results suggested that both hydrophobicity and folding imposed by the Cys25-Cys43 disulfide bond were essential for antilisterial activity of the C-terminal hydrophobic peptide. Chemical oxidation of tryptophan residues by N-bromosuccinimide demonstrated that these residues were crucial for inhibitory activity since modification of any one of them rendered DV41 inactive. On the contrary, only the modification of all the three tyrosine residues caused a total loss of antilisterial activity. These latter results strengthened previous results suggesting that the N-terminal domain containing the YGNGV consensus sequence was not involved in the binding of DV41 to a potential specific receptor on listerial cells.
Lactic acid bacterium (LAB) bacteriocins are antimicrobial peptides which could be useful as natural and nontoxic food preservatives. These compounds could also be considered for other applications in human health and may provide new approaches for dealing with antibiotic-resistant bacteria (22). LAB bacteriocins can be generally divided in two main classes, the lantibiotics and nonlantibiotic peptides. Among the latter, the class IIa bacteriocins (17) are cationic heat-stable and membrane-active peptides, containing one or two disulfide bonds. Members of this group are amphiphilic with a hydrophilic conserved NH2-terminal domain and a hydrophobic variable COOH-terminal of equivalent size. The structure of leucocin A, a class IIa member with one disulfide bond, has been recently obtained by nuclear magnetic resonance (12). The hydrophilic domain forms a three-strand antiparallel β-sheet stabilized by an intramolecular disulfide bond, while the C-terminal domain forms an amphipathic helix in lipid micelles. This structure is compatible with the pore-forming properties of pediocin-like bacteriocins (5).
If it is now accepted that the membrane is the primary target of bacteriocins, it is still not known how the peptide inserts into membrane to form a structured pore. In the first step of adsorption onto membranes, the necessity of a specific receptor is still discussed (4, 5). As for other membrane active peptides and proteins (8, 9, 19, 29), this process could only require the highly hydrophobic and cationic character of the bacteriocin. A preliminary response can be done by changing chemically, enzymatically, or genetically the chemical structure of some amino acid side chains. For example, Fleury et al. (11) and Quadri et al. (24) have recently shown from synthetic mesentericin Y105 and recombinant carnobacteriocin B2 mutants, respectively, that changing just a residue in both the N- and the C-terminal domains induces important and often complete loss of the inhibitory activity of the corresponding bacteriocins. Chen et al. (3) in studies with synthetic peptides have suggested that the N-terminal is involved in the electrostatic interaction of class IIa bacteriocins with anionic membrane phospholipids of target cells.
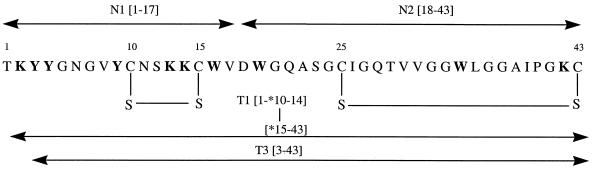
As a part of these structure-function studies we have performed, for the first time, different chemical and enzymatic modifications on divercin V41 (DV41), a class IIa bacteriocin containing two disulfide bonds. DV41 is a 43-amino-acid peptide of 4,509 Da with two disulfide bonds, produced by Carnobacterium divergens V41 (21, 23) (Fig. 1). The results highlighted the respective roles of the conserved N-terminal and variable C-terminal domains, as well as of some amino acid side chains.
FIG. 1.
Amino acid sequence of divercin V41 from C. divergens V41 (21). The modified residues are in boldface letters. N1 and N2 are the resulting fragments obtained after endoproteinase Asp-N digestion, and T1 and T3 are the resulting fragments after trypsin digestion. An asterisk indicates the cysteine residue involved in the disulfide bridge that maintains the two fragments.
MATERIALS AND METHODS
Chemicals and enzymes.
Tetranitromethane (TNM), dithiothreitol (DTT), hydroxylamine, and trifluoroacetic acid (TFA) were purchased from Sigma-Aldrich Fine chemicals (St. Quentin Fallavier, France). Iodoacetamide, succinic anhydride, and N-bromosuccinimide (NBS) were from Merck (Darmstadt, Germany). Acetic anhydride was from Prolabo (Gradignan, France). Sequencing-grade endoproteinase Asp-N and trypsin were from Boehringer Mannheim (Meylan, France). High-performance liquid chromatography (HPLC)-grade acetonitrile (ACN) was purchased from SDS (Marseille, France).
Bacterial strains, culture conditions, and production of DV41 and inhibitory activity.
C. divergens V41 isolated from fish viscera (ENITIAA, Nantes, France) and Listeria innocua F (IFREMER, Nantes, France) were maintained at −80°C in MRS broth (6) containing 15% (vol/vol) glycerol. Before use, the strain was cultivated twice for 24 h at 30°C in MRS broth (Biokar, Beauvais, France).
C. divergens V41 was grown on a Tween-deficient MRS medium. Temperature was maintained at 20°C, and pH was regulated at 6.5 by automatic addition (SET 2M; SGI, Toulouse, France) of 6 N NaOH (23). Inhibitory activity was tested against L. innocua F, grown in Elliker medium (Biokar, Beauvais, France) containing 10 g of agar per liter. The 10 μl of DV41 or modified DV41 was spotted onto agar plates containing the indicator strain. Bacterial activity was monitored by monitoring the appearance of an inhibition zone (1).
DV41 was purified from the supernatant of a 2-liter culture of C. divergens V41 by using Triton X-114 phase-partitioning technique (2). Briefly, Triton X-114 was added to the culture medium at up to 2% (wt/vol) at 4°C. After heating at 35°C and phase separation, the lower detergent-rich phase was removed, diluted 10 times with water and loaded onto a carboxymethyl cellulose C200 (Amicon, Beverly, Mass.) column (20 by 2.5 cm). The excess of the nonionic detergent was washed out by elution with deionized water. DV41 was eluted with a gradient of from 100% deionized water to 100% 0.7 M NaCl in deionized water. Fractions containing DV41 were detected by absorbance at 280 nm and by the inhibition spot assay. DV41 was desalted and purified by preparative reversed-phase HPLC (RP-HPLC) as described below.
The protein concentration was determined by the bicinchoninic acid procedure as described by the supplier with bovine serum albumin as a standard (Pierce Europe, Oud Beijerland, The Netherlands).
Chemical modifications.
DV41 was reduced and alkylated according to the method of Yan et al. (30). Then, 200 μg of peptide was dissolved in 0.1 M Tris-HCl (pH 8.5) buffer containing 6 M guanidine chloride. A 50 M excess of DTT was added. The reaction was allowed to proceed overnight at room temperature under a nitrogen atmosphere. Then, iodoacetamide was added (10 times the mass of DTT used), and the mixture was placed in the dark for 1 h under nitrogen.
DV41 was succinylated according to the method of Klotz (18). A total of 100 mol of succinic anhydride per mol of amino group (ɛ-NH2 and NH2-terminal) was added in small aliquots to a vigorously stirred solution of DV41. The pH was maintained at 8.0 to 8.5 by the addition of 1 M NaOH with a pH stat (645 Multi-Dosimat). The reaction was allowed to proceed during 1 h at room temperature.
Acetylation of DV41 was carried out according to the method of Riordan and Vallee (25). A 3 M excess of acetic anhydride was added per mol of NH2 group. A basic pH (ca. 10) was maintained by the addition of 1 M NaOH with a pH stat.
Tryptophan residues were oxidized by the addition of NBS at room temperature according to the procedure described by Spande et al. (28). Oxidation of tryptophan to oxindolealanine was followed by measuring the absorbance of these chromophores on a Cary E1 spectrophotometer (Varian) and by the decrease of the intensity of tryptophan fluorescence on a Fluoromax spectrofluorimeter (SPEX, Edison, N.J.). DV41 (11 μmol) was solubilized in a 50 mM sodium acetate solution (pH 4.5), and small aliquots (1 μl) of 10 mM NBS in the same buffer were added. For spectrophotometry, a scan was recorded at from 240 to 350 nm to monitor the increase of absorbance at 250 nm (oxindolealanine), while the absorbance at 280 nm corresponding to tryptophan residues decreased. For spectrofluorimetry, the emission spectra of tryptophan residues were recorded from 300 to 400 nm with an excitation wavelength set at 295 nm.
Nitration of tyrosine residues was performed as described by Sokolovsky et al. (27). Aliquots from a freshly prepared stock solution of 25 mM TNM in 96% ethanol were mixed thoroughly with pure DV41 (20 to 30 μM) solubilized in 0.1 M NH4HCO3 (pH 8.0). Nitration proceeded for 60 min at 25°C.
Enzymatic cleavage of DV41.
Endoproteinase Asp-N was used as recommended by the supplier. The lyophilized enzyme was suspended in 50 μl of distilled water, resulting in a buffer concentration of 10 mM Tris-HCl (pH 7.5). DV41 was solubilized in a 50 mM sodium phosphate buffer (pH 8.0). The enzyme was added in a ratio of 1:50 (wt/wt). Digestion was allowed to proceed at 37°C for 18 h.
For the cleavage with trypsin, the lyophilized enzyme was solubilized in deionized water containing 0.01% (vol/vol) TFA. The peptide was dissolved in a 20 mM ammonium acetate buffer (pH 8), and the enzyme was added in a ratio of 1:100 (wt/wt). Digestion was allowed to proceed at 37°C for 12 h.
Purification of modified DV41 and endoproteinase peptides.
After nitration, excess reagent was eliminated by using batch ion-exchange chromatography. Carboxymethyl cellulose C200 gel was added to the reacting medium, and adsorption was allowed to take place for 30 min at room temperature. The gel was then washed with deionized water, and finally the modified DV41 was eluted by using 0.1 M Tris-HCl (pH 8) buffer containing 0.5 M NaCl.
All of the modified and cleaved peptides except acetylated DV41 were finally purified by analytical RP-HPLC. The samples were loaded on a C18 Nucleosyl column (250 by 4.6 mm, 5-μm-diameter particles, 300 Å; CIL, Bordeaux, France). Elution was performed at 50°C with a linear gradient from 100% deionized water with 0.06% TFA (solvent A) to 100% ACN with 0.04% TFA (solvent B) in 50 min at 1 ml/min. Peptides detected by absorbance at 220 nm were collected manually. To prevent from cross-contamination, only a fraction of the corresponding absorbance peaks (generally near the top of the peak) was collected.
To purify acetylated DV41, semipreparative RP-HPLC was performed at 50°C. The sample was loaded on a C18 Nucleosyl column (250 by 10 mm, 300 Å, 5-μm-diameter particles; CIL). After the loading, the salt was washed away by elution with 100% solvent A for 10 min. Then, the peptides were subjected to a linear gradient from 80% solvent A to 100% solvent B in 30 min at 3 ml/min and were detected by their absorbance at 280 nm.
Mass spectrometry and amino acid sequencing.
Molecular mass was determined by using an API-III Plus mass spectrometer (Sciex, Thornhill, Canada) equipped with an atmospheric pressure ionization source (Electro-Spray mass spectrometer [ES-MS]). The sample analysis was carried out either by an on-line coupling between MS and RP-HPLC (LC-MS) or by using infusion pump syringe at a flow rate of 5 μl/min. RP-HPLC columns (Symmetry C18; Waters Corp., Milford, Mass.) were eluted at a flow rate of 250 μl/min with a split to the MS ionization source that was set at a flow rate of 30 μl/min. The instrument scale for the mass-to-charge (m/z) ratio was calibrated with the ions of the ammonium adduct of polypropylene glycol. Scan data were obtained with Tune 2.5, and mass calculation was done with Biomultiview 1.2 (Software package Sciex).
The N-terminal amino acid sequence of DV41 was obtained by Edman degradation performed on a model 447A gas-phase sequencer equipped with an on-line 120A phenythiohydantion amino acid analyzer (Applied Biosystems, Foster City, Calif.).
RESULTS
Endoproteinase cleavages of DV41.
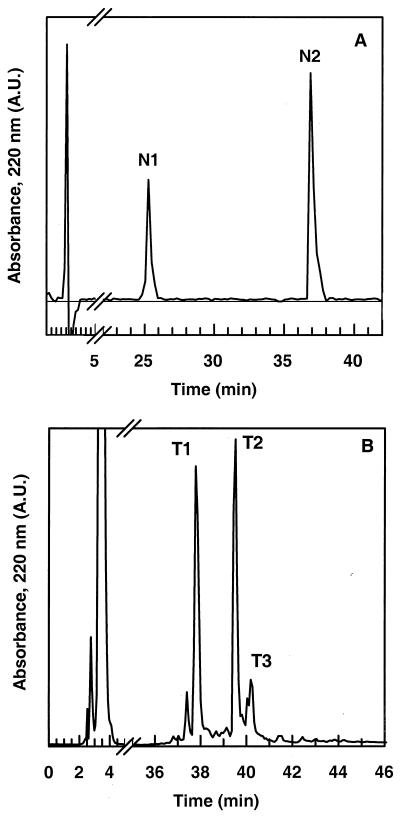
Endoproteinase cleavages were performed on nonreduced DV41. The endoproteinase Asp-N cleaves at the N-terminal end of the aspartic and cysteic acids. Since DV41 possesses only one aspartic residue at position 18, two peptides were obtained, N1 and N2, which were eluted in RP-HPLC at 25 and 38% ACN, respectively (Fig. 2A). ES-MS revealed that N1 stood for the NH2-terminal fragment, 1-17, while N2 corresponded to the C-terminal fragment, 18-43 (Fig. 1 and Table 1). N2 and the native DV41 were eluted at the same retention time. Since ES-MS revealed trace amounts of DV41 in the N2 peak, N1 and N2 peptides were first separated on the basis of their net charge by use of a batch cation exchanger. N1 and especially DV41, as positively charged peptides (net charge +3), were retained on the cation exchanger, while N2, with a null net charge, was not. The unbound material was desalted and purified by RP-HPLC, yielding pure N2 for biological inhibitory assays. Both N1 and the native DV41 were retained on the ion exchanger and further eluted with 0.1 M Tris (pH 8) containing 0.5 M NaCl. Finally, RP-HPLC afforded peptide N1. Antimicrobial assays revealed that N1 was totally inactive, whereas N2 still displayed activity against L. innocua (Table 1).
FIG. 2.
RP-HPLC of endoproteinase digests. (A) Asp-N digest of divercin V41. N1 and N2 correspond to fragments 1-17 and 18-43, respectively. (B) RP-HPLC of tryptic digest of divercin V41. T1 and T3 correspond to fragments 1-14–15-43 and 3-43, respectively, and T2 corresponds to DV41.
TABLE 1.
Experimental and calculated molecular masses of the fragments obtained after endoproteinase hydrolysis
| Endoproteinase | Molecular mass from ES-MS (Da) | Calculated molecular mass (Da) | Identified fragment(s) | Inhibitory activity against L. innocua F |
|---|---|---|---|---|
| Native divercin | 4,508.6 ± 0.3 | 4,509.2 | 1–43 | +c |
| Endoproteinase Asp-N | ||||
| N1: NH2 terminus | 2,012.1 ± 0.1 | 2,011.3 | 1–17a | − |
| N2: COOH terminus | 2,516.1 ± 0.1 | 2,515.9 | 18–43 | + |
| Trypsin | ||||
| T1: cleavage at Lys14 | 4,527.4 ± 0.4 | 4,527.2 | 1–*10-14 | + |
| | | ||||
| *15–43b | ||||
| T3: cleavage at Lys2 | 4,279.5 ± 0.4 | 4,279.9 | 3–43 | + |
| Asp-N followed by trypsin | ||||
| Cleavage at Lys2 and Lys13 | 1,672.19 ± 0.3 | 1,671.9 | 3–*10-13 | − |
| | | ||||
| *15–17 | ||||
| Cleavage at Lys42 | 2,534.3 ± 0.5 | 2,534.0 | 18–*25-42 | |
| | | − | |||
| *43 |
Boldface letters indicate the cleavage site.
Cysteine residues involved in the disulfide bridge that maintains the two fragments are indicated by asterisks.
+ and −, presence and absence of inhibitory activity, respectively.
Trypsin specifically cleaves peptide bonds at the carboxyl side chains of lysine and arginine. DV41 displayed only four potential cleavage sites at Lys2, Lys13, Lys14, and Lys42 (Fig. 1). Three main peaks were obtained by RP-HPLC after 12 h of incubation of DV41 with trypsin (Fig. 2B). One of them (T2) displayed the same retention time as DV41, and ES-MS confirmed that T2 corresponded to the nonhydrolyzed bacteriocin (Table 1). At an enzyme/DV41 mass ratio of 1:100, trypsin hydrolysis was not complete. At higher enzyme/DV41 ratios, i.e. 1:50 and 1:20, we only observed an increase of T1 and T3 in regard to T2, and no other cleavage sites were obtained (results not shown). According to ES-MS, T3 was shown to be the 3-43 fragment, indicating that DV41 was cleaved at Lys2 (Table 1). ES-MS showed that tryptic peptide T1 had a molecular mass of 4,527 Da, which corresponded to the mass of DV41 plus 18 Da (Table 1). This revealed that the bacteriocin was indeed cleaved into two fragments that were still attached by a disulfide bridge (Fig. 1). Amino acid sequencing of T1 yielded two subsequences, TKYYG and XWVD. X corresponded to a blank cycle in Edman degradation and was interpreted as a cysteine residue involved in a disulfide bond. These results suggested that the cleavage site was at Lys14. Therefore, T1 stood for fragments 1-14 and 15-43 linked by the disulfide bond Cys10-Cys15. T1 and T3 were still able to inhibit the growth of L. innocua. These results are in good agreement with those obtained with Asp-N cleavage, since both T1 and T2 contained the active N2[18-43] fragment.
When trypsin was added to an Asp-N hydrolysate, two new main peaks were collected by analytical HPLC (results not shown). The first peak, with a molecular mass of 1,672 Da (Table 1), corresponded to peptide 3-13–15-17, in other words, to peptide N1 cleaved at Lys2, Lys13, and Lys14. As expected, this peptide was not active since N1 was not active. The second peptide, with a molecular mass of 2,534 Da (Table 1), corresponded to the fragment N2 [18-43] cleaved at Lys42. This fragment was composed of peptide 18-42 linked to Cys43 by the Cys25-Cys43 disulfide bond. This peptide showed no inhibitory activity (Table 1). These results suggested that the folding imposed by the disulfide bond Cys25-Cys43 was essential for the activity of the N2 peptide.
Role of disulfide bonds on the antimicrobial activity of DV41.
To determine the contribution of the disulfide bridges Cys10-Cys15 and Cys25-Cys43 (Fig. 1) to the antimicrobial activity of DV41, they were reduced by DTT and alkylated with iodoacetamide. This alkylating agent was chosen because it does not change the net charge of the peptide as does iodoacetic acid or hydrophobicity as does 4-vinylpyridine. The alkylation was complete, as confirmed by ES-MS (Table 2), and the corresponding peptide was totally unable to inhibit growth of L. innocua. Therefore, it can be deduced that disulfide bonds were mandatory for antilisterial activity.
TABLE 2.
Experimental and calculated masses of chemically modified peptides
| Chemical modification(s) | Molecular mass from ES-MS (Da) | Calculated molecular mass (Da) | Modification | Inhibitory activity against L. innocua F |
|---|---|---|---|---|
| Reduction and alkylation | 4,741.9 ± 0.1 | 4,741.1 | Reduction of SS bonds and formation of S-amidomethyl-cysteines | − |
| Nitration | 4,554.2 ± 0.4 (Y1) | 4,553.6 | 1 Tyr-NO2 | + |
| 4,598.2 ± 0.6 (Y2) | 4,599.5 | 2 Tyr-NO2 | + | |
| 4,643.2 ± 0.2 (Y3) | 4,643.5 | 3 Tyr-NO2 | − | |
| Succinylation | 5,208.6 ± 0.1 | 5,207.6 | Succinylation of Ser-OH, ɛ-NH2 (Lys) and NH2 terminus | − |
| Restoration of Ser after succinylation (hydroxylamine, 0.5 M) | 5,033.6 ± 0.6 | 5,034.3 | Succinylation of ɛ-NH2 (Lys) and NH2 terminus | − |
| Formation of hydroxamic-Thr | ||||
| Acetylation | 4,720.8 ± 0.1 | 4,720.6 | Acetylation of ɛ-NH2 (Lys) and NH2 terminus | + |
| Oxidation | 842.7 ± 0.5 (W1) | Unknown compound | NDa | |
| 4,555.2 ± 1.5 (W2) | 4,557.4 | Three Trp modified | − | |
| 4,524.2 ± 1.5 (W3) | 4,525.4 | One Trp modified | − | |
| 4,525.4 ± 1.5 (W4) | 4,525.4 | One Trp modified | − | |
| 4,509.5 ± 0.5 (W5) | 4,509.4 | DV41 | + | |
| 65,986.0 ± 16.0 (W5) | Unknown compound | ND |
ND, not determined.
Modification of the net charge of DV41.
Acylation of DV41 was performed to show whether its cationic nature (net charge +3) was essential for inhibitory activity. First, succinylation yielded negatively charged succinyl lysine together with the N-terminal and two serine residues, as revealed by ES-MS (Table 2). Serine residues were restored by treating the succinylated DV41 with 0.5 M hydroxylamine for 1 h. As a consequence, the threonine residues were also converted to hydroxamic acid. In both cases, the succinylated DV41 was not active against L. innocua. Second, DV41 was acetylated by acetic anhydride neutralizing the lysine positive charge. In this case, only the four lysine residues and the NH2 terminus were modified (Table 2). The acetylated peptide conserved its inhibitory activity.
Modification of the aromatic side chains.
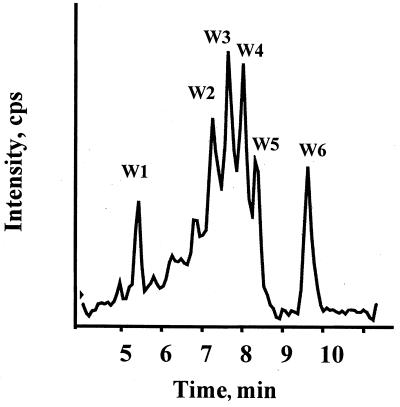
LC-MS revealed the presence of different compounds after the addition of NBS (Fig. 3). W2 corresponded to DV41 with three oxidized tryptophan residues, while W3 and W4 corresponded to DV41 with one oxidized tryptophan (Table 2). The proportions of W3 and W4 were similar, and the slight difference of their retention times indicated that each corresponded to DV41 modified at different tryptophan positions. W5 corresponded to unmodified DV41. Two other compounds, W1 and W6, were observed by LC-MS with molecular masses of about 843 and 66,000 Da, respectively (Fig. 3 and Table 2). W6 might correspond to an aggregate of covalently oligomerized DV41 (modified and native) by disulfide bond rearrangement. These unknown compounds were not further characterized. Even with just one oxidized tryptophan residue, no inhibitory activity was observed. Therefore, each tryptophan residue was essential to antimicrobial activity.
FIG. 3.
LC-MS of oxidized DV41 by NBS. See Table 2 for details.
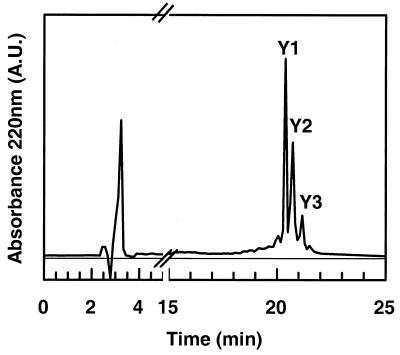
Finally the tryrosine residues were modified by nitration with TNM. Three main peaks, Y1, Y2, and Y3, were collected by RP-HPLC, and ES-MS allowed us to classify them as DV41 with one, two, and three modified residues (Fig. 4 and Table 2). Only the peptide with all the three nitrated tyrosine residues did not exhibit any inhibitory activity against L. innocua.
FIG. 4.
RP-HLPC of nitrated DV41 with 25 mM TNM in 96% ethanol at 25°C for 60 min. Y1, Y2, and Y3 represent modified DV41 with 1, 2, and 3 nitrotyrosine derivatives, respectively.
DISCUSSION
While some studies have been done by using amino acid substitution and or by truncating the peptide sequence (11, 24), no similar data are available for class IIa bacteriocins containing two disulfide bonds, such as pediocin PA-1. In this study, we used DV41, a bacteriocin which is highly homologous to pediocin PA-1 and which has been recently characterized (21).
The cationic nature of membrane-active peptides is generally essential for their insertion in membranes, probably because they interact strongly with anionic membrane phospholipids, as demonstrated with nisin (7) and pediocin PA-1 (2). Upon succinylation, DV41 is transformed into a highly anionic peptide (net charge of −7) and loses its activity, probably because repulsive forces impair interaction with the anionic phospholipids of the membrane. However, acetylated DV41 still displays inhibitory activity. This observation could be contradictory since acetylated DV41 is anionic (net charge, −2) and succeeds in interacting with the membrane. In the case of membrane-active cationic peptides or proteins, acetylation of lysine leads generally to a loss of membrane toxicity (13, 14). However, Gallagher et al. (12) reported similar results on leucocin A, a class IIa bacteriocin with just one disulfide bond. In DV41, as in leucocin A, most of the acetylated residues are found in the N-terminal hydrophilic part. The inhibitory activity of acetylated DV41 is in agreement with Asp-N cleavage, which shows that the C-terminal hydrophobic domain, i.e., fragment 18-43, with a null net charge is still active. Furthermore, it is worth noting that acetylation of lysine also increases peptide hydrophobicity. These results provide the first evidence that the hydrophobicity of DV41 and especially of the C-terminal peptide is more important than the cationic character of the bacteriocin for the inhibitory activity. This is in agreement with the oxidation of the hydrophobic tryptophan residues which induced a loss of inhibitory activity in DV41. Similar results have been obtained with Trp37-deleted mesentericin Y105 (11) and Phe33-Ser33 substitution in the C-terminal of carnobacteriocin B2 (24). These results could suggest that increased hydrophobicity of C-terminal domain would drive insertion in target membrane to such a degree that electrostatic interaction would no longer be rate limiting. However, for mesentericin Y105, the C-terminal end alone does not display any antibacterial activity (11). The hydrophobicity of the C terminus of DV41 is not sufficient to maintain antimicrobial activities since the cleavage at Lys42 by trypsin induced a loss of antilisterial activity of the Asp-N 18-43 fragment. This cleavage and disulfide reduction are detrimental to the folding of the C-terminal part. Therefore, it is obvious that in the case of DV41, both folding and hydrophobicity are needed for the expression of inhibitory activity. These results strengthen the previous hypothesis that the disulfide bond found in the C terminus is mandatory to the activity of class IIa bacteriocin containing two disulfide bonds (5).
The importance of the C-terminal hydrophobic part on the inhibitory activity has already been addressed by Fimland et al. (10). However, in the case of mesentericin Y105, Fleury et al. (11) have shown that the N terminus is also essential to the bacteriocin activity. It has been suggested that, in addition to its role in the recognition by a receptor, it could also play a role in the electrostatic interaction with anionic lipids of the target membrane (3). In the case of DV41, these electrostatic bonds are not necessary since acetylation still maintains an inhibitory activity. Furthermore, the trypsin cleavages which either remove the two residues at N terminus or break the loop stabilized by the disulfide bond Cys10-Cys15 generates peptide fragments which are still able to inhibit the growth of L. innocua. The latter cleavage by trypsin is in agreement with previous observations suggesting that the disulfide bond in the N-terminal conserved hydrophilic domain is not essential to the antilisterial activity (5). Only the modification of aromatic tyrosine and especially tryptophan residues are detrimental to the activity of DV41. However, it was necessary to modify all three tyrosine residues to obtain a complete loss of inhibitory activity. On the contrary, replacing Tyr3 by phenylalanine in carnobacteriocin B2 (24) and truncated mesentericin Y105 (fragment 4-37) (11) yielded peptides with a drastic decrease in activity. Therefore, our results are in agreement with previous results (3) providing evidence that the conserved YGNGV sequence is not directly involved in membrane recognition. The formation of the bulky tyrosine nitroderivatives probably impairs peptide-peptide interaction and therefore impairs proper oligomerization of the bacteriocin within the membrane to form a functional pore.
We have previously postulated that the tryptophan residues of the N-terminal domain could play a major role in the interaction and orientation of bacteriocin on the membrane surface (1). The present study further supports the importance of these residues for the inhibitory activity of class IIa bacteriocins. This is not surprising since tryptophan residues are known to play an essential role in the adsorption and orientation of amphiphilic peptides and proteins in membranes due to their capacity to form both hydrogen and hydrophobic bonds with the polar and nonpolar groups of polar lipids (9, 16, 20, 26). In particular, Trp16 and Trp19 at the joining part of the N- and C-terminal domains could play a major role in the proper orientation of these domains within the membrane (1). In the case of the anionic acetylated DV41, both tryptophan residues and peptide hydrophobicity could promote membrane insertion to such a degree that electrostatic interaction would no longer be rate limiting.
Finally, this structure-function study shows that, in the case of DV41, N-terminal conserved peptide is not necessary in order to have an antimicrobial activity. However, when this domain is present its structural integrity and some key residues such as tryptophan are essential for maintaining the inhibitory activity. This domain could facilitate and could be necessary for a proper orientation of the peptide at the surface of membranes and for a subsequent anchoring of the hydrophobic C-terminal within the hydrophobic core of membrane bilayers to form a functional pore. However, the hydrophobicity of the C-terminal domain and especially the presence of a disulfide bond which imposes folding constraints on this domain are essential for a proper orientation of DV41 within membranes. At the moment, it is not possible to generalize these conclusions to the other class IIa bacteriocins with two disulfide bonds, but these results open new perspectives for further studies with peptide variants provided by peptide synthesis or site-directed mutagenesis.
ACKNOWLEDGMENTS
This work was supported by the French Ministry of Agriculture (DGER) and by the Region Pays de la Loire.
REFERENCES
- 1.Bhugaloo-vial P, Dousset X, Metivier A, Sorokine O, Anglade P, Boyaval P, Marion D. Purification and amino acid sequences of piscicocins V1a and V1b, two class IIa bacteriocins secreted by Carnobacterium pisicola V1 that display significantly different levels of significantly different levels of specify. Appl Environ Microbiol. 1996;62:4410–4416. doi: 10.1128/aem.62.12.4410-4416.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blochet J E, Chevalier C, Forest E, Pebay-Peyroula E, Gautier M F, Joudrier P, Pézolet M, Marion D. Complete amino acid sequence of puroindoline, a new basic and cystine rich protein with a unique tryptophan-rich domain, isolated from wheat endosperm by Triton X-114 phase partitioning. FEBS Lett. 1993;329:336–340. doi: 10.1016/0014-5793(93)80249-t. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Ludescher R D, Montville T J. Electrostatic interactions, but not the YGNGV consensus motif, govern the binding of pediocin PA-1 and its fragments to phospholipid vesicles. Appl Environ Microbiol. 1997;63:4770–4777. doi: 10.1128/aem.63.12.4770-4777.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Shapira R, Einsenstein M, Montville T J. Functional characterization of pediocin PA-1 binding to liposomes in the absence of a protein receptor and its relationship to a predicted tertiary structure. Appl Environ Microbiol. 1997;63:524–531. doi: 10.1128/aem.63.2.524-531.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chikindas M L, Garcia-Garcera M J, Driessen A J M, Ledeboer A A T M, Nissen-Meyer J, Nes I F. Pediocin PA-1, a bacteriocin from Pediococcus acidilactici PA1.0, forms hydrophilic pores in the cytoplasmic membrane of target cells. Appl Environ Microbiol. 1993;59:577–3584. doi: 10.1128/aem.59.11.3577-3584.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Man J C, Rogosa M, Sharpe E. A medium for the cultivation of Lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- 7.Demel R A, Peelen T, Siezen R J, De Kruijf B, Kuipers O P. Nisin Z, mutant nisin Z and lactacin 481 interactions with anionic lipids correlate with antimicrobial activity. A monolayer study. Eur J Biochem. 1996;235:267–274. doi: 10.1111/j.1432-1033.1996.00267.x. [DOI] [PubMed] [Google Scholar]
- 8.Dempsey E C. The actions of mellitin on membranes. Biochim Biophys Acta. 1990;1031:143–161. doi: 10.1016/0304-4157(90)90006-x. [DOI] [PubMed] [Google Scholar]
- 9.Dubreil L, Compoint J P, Marion D. Interaction of puroindolines with wheat flour polar lipids determines their foaming properties. J Agric Food Chem. 1997;45:108–116. [Google Scholar]
- 10.Fimland G, Blingsmo O R, Sletten K, Jung G, Nes I F, Nissen-Meyer J. New biologically active hybrid bacteriocins constructed by combining regions from various pediocin-like bacteriocins: the C terminal region is important for determining specificity. Appl Environ Microbiol. 1996;62:3313–3318. doi: 10.1128/aem.62.9.3313-3318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleury F, Dayem M A, Montagne J J, Choiboiseau E, Le Caer J P, Nicolas P, Delfour A. Covalent structure, synthesis and structure-function studies of mesentericin Y10537, a defensive peptide from gram-positive bacteria Leuconostoc mesenteroides. J Biol Chem. 1996;24:14421–14429. doi: 10.1074/jbc.271.24.14421. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher F N L, Sailer M, Niemczura W P, Nakashima T T, Stiles M E, Vederas J C. Three-dimensional structure of leucocin A in trifluoroethanol and dodecylphosphocholine micelles: spatial location of residues critical for biological activity in type II bacteriocins from lactic acid bacteria. Biochemistry. 1997;36:15062–15072. doi: 10.1021/bi971263h. [DOI] [PubMed] [Google Scholar]
- 13.Gatineau E, Takechi M, Bouet F, Mansuelle P, Rochat H, Harvey A L, Montenay-Garestier T, Menez A. Delineation of the functional site of a snake venom cardiotoxin: preparation, structure and function of monoacylated derivatives. Biochemistry. 1990;29:6480–6489. doi: 10.1021/bi00479a021. [DOI] [PubMed] [Google Scholar]
- 14.Habersetzer-Rochat C, Sampieri F. Structure function relationships of scorpion neurotoxin. Biochemistry. 1976;15:2254–2261. doi: 10.1021/bi00656a002. [DOI] [PubMed] [Google Scholar]
- 15.Harvey A L, Rowan E G, Vatampour H, Engstrom A, Westerlund B, Karlsson E. Changes to biological activity following acetylation of dendrotoxin I from Dendroapsis polylepis (black mamba) Toxicon. 1997;35:1263–1273. doi: 10.1016/s0041-0101(97)00016-0. [DOI] [PubMed] [Google Scholar]
- 16.Hu W, Lazo N D, Cross T A. Tryptophan dynamics and structural refinement in a lipid bilayer environment: solid state NMR of the gramicidin channel. Biochemistry. 1995;34:14138–14146. doi: 10.1021/bi00043a019. [DOI] [PubMed] [Google Scholar]
- 17.Klaenhammer T R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol Rev. 1993;12:39–86. doi: 10.1111/j.1574-6976.1993.tb00012.x. [DOI] [PubMed] [Google Scholar]
- 18.Klotz I M. Succinylation. Methods Enzymol. 1967;11:576. [Google Scholar]
- 19.Lackey J H, Gonzalez-Manas J M, Van der Goot F G, Pattus F. The membrane insertion of colicins. FEBS Lett. 1992;307:26–29. doi: 10.1016/0014-5793(92)80895-n. [DOI] [PubMed] [Google Scholar]
- 20.Landolt-Marticorena C, Williams K A, Deber C M, Reithmeier R A F. Non-random distribution of amino acids in the trans-membrane segments of human type I single membrane proteins. J Mol Biol. 1993;229:602–608. doi: 10.1006/jmbi.1993.1066. [DOI] [PubMed] [Google Scholar]
- 21.Metivier A, Pilet M F, Dousset X, Sorokine O, Anglade P, Piard J C, Marion D, Cenatiempo Y, Fremaux C. DV41, a new cystibiotic secreted by Carnobacterium divergens V41: primary and genomic organization. Microbiology. 1998;144:2837–2844. doi: 10.1099/00221287-144-10-2837. [DOI] [PubMed] [Google Scholar]
- 22.Nissen-Meyer J, Nes I F. Ribosomally synthesized antimicrobial peptides: their structure, biogenesis, and mechanism of action. Arch Microbiol. 1997;167:66–77. [PubMed] [Google Scholar]
- 23.Pilet M F, Dousset X, Barré R, Novel G, Desmazeaud M, Piard J C. Evidence for two bacteriocins produced by Carnobacterium divergens V41 and Carnobacterium piscicola V1 isolated from fish and active against Listeria monocytogenes. J Food Prot. 1995;3:256–262. doi: 10.4315/0362-028X-58.3.256. [DOI] [PubMed] [Google Scholar]
- 24.Quadri L E N, Yan L Z, Stiles M E, Vederas J C. Effect of amino acid substitution on the activity of Carnobacterium B2. J Biol Chem. 1997;272:3384–3388. doi: 10.1074/jbc.272.6.3384. [DOI] [PubMed] [Google Scholar]
- 25.Riordan J F, Vallee B L. Acetylation. Methods Enzymol. 1967;11:565. [Google Scholar]
- 26.Schiffer M, Chang C H, Stevens F J. The functions of tryptophan residues in membrane proteins. Prot Eng. 1992;5:231–214. doi: 10.1093/protein/5.3.213. [DOI] [PubMed] [Google Scholar]
- 27.Sokolovsky A, Riordan J F, Vallee B L. Tetranitromethane: a reagent for the nitration of tyrosyl residues in proteins. Biochemistry. 1966;5:3582–3589. doi: 10.1021/bi00875a029. [DOI] [PubMed] [Google Scholar]
- 28.Spande T F, Green N M, Witkop B. The reactivity towards N-bromosuccinimide of tryptophan in enzymes, zymogens and inhibited enzymes. Biochemistry. 1966;5:1926–1933. doi: 10.1021/bi00870a020. [DOI] [PubMed] [Google Scholar]
- 29.White S H, Wimley W C, Selsted M E. Structure, function, and membrane integration of defensins. Struct Biol. 1995;5:521–527. doi: 10.1016/0959-440x(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 30.Yan L, Tseng J L, Frindland G H, Desiderio D M. Characterization of an opioid peptide-containing protein and of bovine α-lactalbumin by electrospray ionization and liquid secondary ion mass spectrometry. Am Soc Mass Spectr. 1994;5:377–386. doi: 10.1016/1044-0305(94)85053-4. [DOI] [PubMed] [Google Scholar]