Abstract
Ginkgo (Ginkgo biloba L.) is one of the most distinctive plants, characterized by excellent resistance to various environmental conditions. It is used as an ornamental plant and is recognized as a medicinal plant in both traditional and Western medicine. Its bioactive potential is associated with the presence of flavonoids and terpene trilactones, but many other compounds may also have synergistic effects. Flavonoid dimers—biflavonoids—are important constituents of ginkgophytopharmaceuticals. Currently, the presence of 13 biflavonoids has been reported in ginkgo, of which amentoflavone, bilobetin, sciadopitysin, ginkgetin and isoginkgetin are the most common. Their role in plants remains unknown, but their bioactivity and potential role in the management of human health are better investigated. In this review, we have provided an overview of the chemistry, diversity and biological factors that influence the presence of biflavonoids in ginkgo, as well as their bioactive and health-related properties. We have focused on their antioxidant, anticancer, antiviral, antibacterial, antifungal and anti-inflammatory activities as well as their potential role in the treatment of cardiovascular, metabolic and neurodegenerative diseases. We also highlighted their potential toxicity and pointed out further research directions.
Keywords: Ginkgo biloba L., biflavonoids, ginkgetin, isoginkgetin, bilobetin, sciadopitysin, amentoflavone, bioactive compounds
1. Introduction
Ginkgo biloba L., known as Maidenhair tree or ginkgo, is the only living dioecious species in the family Ginkgoaceae [1,2,3]. The species grows in small natural populations in southeastern China but is cultivated throughout China and the world [4,5]. The morphology of ginkgo is very conservative, and this species exhibits remarkable genetic stability [6,7]. The ginkgo species is characterized by a large genome and high tolerance to stress factors, including abiotic and biotic stress and dioecious reproduction [8]. High tolerance to stress is demonstrated not only by the fact that this tree is a living fossil but also through its survival of the Hiroshima atomic bomb, with miraculous recovery within 1–2 km of the blast. In horticulture, male trees are predominantly used due to the unpleasant odor from fruit that female trees produce [9]. The unpleasant odor of ginkgo seeds develops only when they are fully mature and is the result of two volatile compounds, butanoic and hexanoic acids, localized in the sarcotesta [10]. The species was introduced to other Asian and European countries, mainly for ornamental and greening purposes [11,12]. Today, ginkgo trees, often more than 200 years old, can be found as ornamental plants in many European cities (Figure 1).
Figure 1.
Approximately 200-year-old ginkgo tree in the old part of the city of Osijek, Croatia (photo taken in October 2021).
In traditional Chinese medicine, leaves and fruits have been used for several centuries with documented use dating back from 1280 to 1368 AD and the use of ginkgo nuts mentioned as early as the Yuan Dynasty [13]. For more than 500 years, various medicinal properties have been attributed to the nuts, including melioration of asthma, cough, and bladder infections, while leaves have been used mainly to treat skin infections and pulmonary dysfunction (reviewed by [14,15]). Although it is well used in Chinese medicine, the true value of this 200-million-year-old plant species was recognized only 40 years ago. The best-known properties of ginkgo are its beneficial effects on cognitive complaints, which became known through the commercial sale of a standardized extract formulation developed in Germany in 1965. However, commercial use under the name EGb761 was only in 1974 in France and is now used worldwide [15]. In addition to its medicinal properties, the fruits of ginkgo are used for the preparation of jams, especially for weddings and other celebrations. Roasted seeds are a delicacy in many East Asian countries such as Japan, China, Malaysia, and Korea [16].
Due to its long use in traditional medicine, ginkgo is considered a natural reservoir of molecules with health-promoting potential. The health-promoting properties of ginkgo are associated with the presence of specialized metabolites such as flavonoids, terpenoids and trilactones [17]. According to recent data, more than a hundred different flavonoid structures have been detected in ginkgo [18]. They exist as aglycones, glycosides, or dimeric forms called biflavonoids. Biflavonoids are dimers of flavone–flavone, flavone–flavonone, flavonone–flavonone subunits, and in rare cases, dimers of chalcones and isoflavones. As summarized by He et al. [19], a total of 592 biflavonoids are widely distributed in angiosperms, ferns, gymnosperms, and bryophytes, with most of them found in angiosperms. The vast majority of biflavonoids are from Clusiaceae, Thymelaeaceae, Ochnaceae, and Selaginellaceae, which account for about 50% of biflavonoids in all families [19]. Their presence is frequently reported in plants used in traditional and modern medicine, indicating their potential health benefits. Therefore, it is not surprising that biflavonoids have become the focus of scientific attention in recent years due to their pharmacological activities, including anti-inflammatory, antioxidant, antibacterial, antiviral, antidiabetic, antitumor, and cytotoxic properties, and may be used to treat Alzheimer’s and Parkinson’s disease [19]. To date, 13 different biflavonoids have been reported, of which ginkgetin, isoginkgetin, amentoflavone, bilobetin and sciadopitysin are the predominant biflavonoids [18].
Although biflavonoids are recognized as compounds with potent health-promoting properties, they have somehow been neglected in studies dealing with flavonoids in ginkgo. However, considering the use of ginkgo in traditional and modern medicine and the biological properties of biflavonoids, they may play an important role in the biological activity of ginkgo. Thus, in this review, we summarize data on ginkgo biflavonoids, their occurrence in various plant tissues, their biological activity, pharmacological potential, and safety to highlight this important class of natural products.
2. Botanical Characteristic of Ginkgo
Mature ginkgo trees typically reach a height of 20 to 40 m and a trunk diameter of one to four meters [20]. They form two types of shoots: long shoots with widely spaced leaves and axillary buds, and short shoots with clustered leaves with no internodes nor axillary buds. The leaves have long stalks and a characteristic fan-shape with dichotomously branched veins. They are light green in color and turn golden yellow in the fall (Figure 2). The tree bark is grey in color, smoots in older trees with longitudinal cracks turning the bark color to brown. The flowers are dioecious and grow only on short shoots.
Figure 2.
The color of young (left) and mature (right) G. biloba leaves.
The male catkins appear before the leaves and pollination occurs from early April to late May by the wind. The seeds produced are large (20–30 mm × 16–24 mm), with a thick seed coat containing embryos embedded in the tissue of the female gametophyte [21]. The structure of the seed coat is complex and consist of several layers: a soft, fleshy outer layer (the sarcotesta); a hard, stony middle layer; and a thin, membranous inner layer. Sex maturity in ginkgo is reached only after 20 years of age [9]. Vegetative reproduction is possible through embedded buds—lignotubers (basal chichi)—which are formed at the base of the main stem [22].
3. Phytochemicals in Ginkgo
As mentioned above, the phytochemicals of ginkgo have attracted the attention of scientists for several decades due to their long-standing use in traditional medicine. About 15 years ago, Singh et al. [23] categorized the phytochemicals found in ginkgo into the groups of terpenoids, polyphenols, allylphenols, organic acids, carbohydrates, fatty acids and lipids, inorganic salts and amino acids. In recent years, some new types of compounds such as lignans and new terpenoids have been identified [18]. Recently, Liu et al. [18] summarized all the data on phytochemicals in ginkgo published from 2015 to 2020 and found that 184 papers focused on phytochemicals found in ginkgo leaves during this period alone. In their article, Liu et al. [18] divided the phytochemicals associated with the health benefits on ginkgo into the group of flavonoids, terpenoids, alkylphenols and alkylphenolic acids, carboxylic acids, lignans, proanthocyanidins, polyprenols and polysaccharides. Characteristic of ginkgo are terpene trilactones such as ginkgolides and bilobalide, which are unique to ginkgo and are the only natural products that have a t-butyl group in their structure [23]. N-glycosides such as N-β-d-glucopyranosyl-1H-indole-3-acetic acid conjugates, N-[2-(1-β-d-glucopyranosyl)-1H-indol-3-yl) acetyl]-l-glutamic acid and N-[2-(1-β-d-glucopyranosyl)-1H-indol-3-yl)acetyl]-l-aspartic acid, have also been isolated from ginkgo parts, which are rarely found in nature as natural products [24]. Ginkgo seeds contain toxic compounds such as ginkgotoxin, ginkgotoxin-5-glucoside, ginkgolic acid, amygdalin-derived hydrocyanic acid and allergic proteins, which restricts daily consumption [25].
The standardized extract preparation of ginkgo leaves usually contains 24% flavonoids, 6% terpene trilactones (TTLs), and less than 5 ppm ginkgolic acid [17]. Flavonoids are a group of specialized plant metabolites that are important for plant–environment interactions [26,27] but have also been associated with numerous human health benefits [28,29,30,31,32]. Flavonoids are composed of a 15-carbon skeleton and consist of two benzene rings (ring A and ring B) connected by a 3-carbon linking linkage. Therefore, they are represented as C6-C3-C6 compounds. In many flavonoids, the linking chain also forms a heterocyclic pyran or pyrone ring (ring C). In the absence of this third ring system (ring C), flavonoids are referred to as chalcones and usually serve as precursors to the various flavonoid classes. In plants, flavonoids occur in two different forms, including free aglycones and glycoside-bound forms. Based on their structural differences, flavonoids are dividated into several groups- flavanones, flavanols, flavones, and flavonols. In addition, flavonoids include isoflavones, prenylflavonoids, glycosidoesters, flavonolignans, aurones, chalcones and biflavonoids.
According to the review article by Liu et al. [18], 110 flavonoids with unique structures have been detected in ginkgo, including flavonol and its glycosides, flavone and its glycosides, flavanone and its glycosides, isoflavone and its glycosides, flavan-3-ols, biflavonoids, and biginkgosides. Most of them, 52 reported structures, belong to the flavonol glycosides. The predominant flavonol aglycones in the glycosides are quercetin, kaempferol, and isorhamnetin, but also syringetin, myricetin, laricitrin, myricetin 3’,4’-dimethyl-ether, and patuletin were also found [18]. According to Wang et al. [33], the predominant compounds in ginkgo leaves during the different harvesting periods were flavonol glycosides, especially kaempferol-3-O-rutinoside and isorhamnetin-3-O-rutinoside. Other glycosides present in ginkgo are derivatives of flavones (mainly apigenin and luteolin), flavanones, isoflavones and flavan-3-ols [18]. In recent years, the presence of biginkgosides, flavonol glycoside cyclodimers, has been demonstrated in ginkgo [34]. To date, nine different structures of biginkosides have been described [34]. Several dimeric flavonoid structures have also been found in ginkgo which may be important contributors to the health benefits of ginkgo.
4. Biflavonoids in Ginkgo
In nature, in certain plant species, flavonoid dimerization may occur. Flavonoid dimers, known as biflavonoids, consist of two identical or non-identical flavonoid units joined in a symmetrical or asymmetrical manner, by an alkyl or an alkoxy-based linker of varying length. Most representative compounds of this class are formed by dimers of flavone–flavone, flavone–flavonone, flavonone–flavonone subunits, as well as dimers of chalcones and isoflavones, in rare cases. Their role in plants remains unknown. Based on in vitro biological activity and localization in the leaves, they might be involved in plant protection from pests and predators, act as growth regulators or protect leaves from UV radiation [35,36], but further experimental data should be collected to support these statements.
4.1. Chemistry and Diversity of Ginkgo Biflavonoids
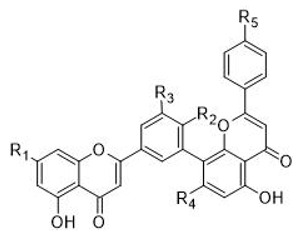
The first biflavonoid, ginkgentin, was isolated from ginkgo in 1929. as a yellow pigment [37] and since then, numerous biflavonoid compounds have been isolated from various plants. He et al. [19] reported that more than 592 biflavonoids have been structurally elucidated, but the actual number is probably even higher. In ginkgo, 13 different biflavonoids have been described so far [18]. Their structures are presented in a Table 1 and Table 2.
Table 1.
Chemical structures of the flavone-flavone biflavonoids in ginkgo.
| |||||
|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | |
| 1 amentoflavone | OH | OH | H | OH | OH |
| 2 sequoiaflavone | OCH3 | OH | H | OH | OH |
| 3 bilobetin | OH | OCH3 | H | OH | OH |
| 4 podocarpusflavone A | OH | OH | H | OH | OCH3 |
| 5 ginkgetin | OCH3 | OCH3 | H | OH | OH |
| 6 isoginkgetin | OH | OCH3 | H | OH | OCH3 |
| 7 5′-methoxybilobetin | OH | OCH3 | OCH3 | OH | OH |
| 8 sciadopitysin | OCH3 | OCH3 | H | OH | OCH3 |
| 9 7″-O-β-d-glucosyl-ginkgetin | OCH3 | OCH3 | H | O-Glc | OH |
| 10 7″-O-β-d-Glucosyl-isoginkgetin | OH | OCH3 | H | O-Glc | OCH3 |
| 11 amentoflavone 7″-O-d-glucopyranoside | OH | OH | H | O-Glc | OH |
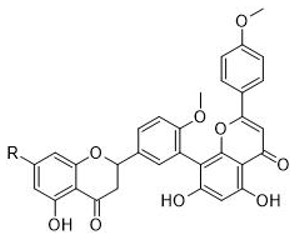
Table 2.
The structure of flavanone-flavone type of biflavonoide in ginkgo.
|
|
|---|---|
| R | |
| 12 2,3-Dihydroisoginkgetin | OH |
| 13 2,3-Dihydrosciadopitysin | OCH3 |
The most commonly reported biflavonoids in ginkgo are ginkgetin, isoginkgetin, amentoflavone, bilobetin and sciadopitysin, while the presence of sesquojaflavone, podocarpusflavone A, and 5’-metoxybilobetin has also been reported [18,33]. Amentoflavone is a dimer of two apigenins with six hydroxyl groups that can be easily replaced by a methoxyl group. Therefore, other biflavonoids can also be considered as derivatives of amentoflavone. In ginkgo sequoiaflavone (7-O-methylamentoflavone), bilobetin (4′-O-methylamentoflavone) and podocarpusflavone A (4′′′-O-methylamentoflavone) were found to be the natural derivatives with a single methoxyl group. Ginkgetin (7,4′-di-O-methylamentoflavone), isoginkgetin (4′,4′′′-di-O-methylamentoflavone) and 5’-methoxybilobetin have two methoxyl groups, while three methoxyl groups are present in sciadopitysin (7,4′,4′′′-tri-O-methylamentoflavone). Similar to monomeric flavonoids, biflavonoids tent to form glycosides. To date, the presence of 7′′-O-β-d-glucosyl-ginkgetin, 7′′-O-β-d-glucosyl-isoginkgetin and 7′′-O-β-d-glucosyl-amentoflavone has been reported in ginkgo [18]. In the structure of amentoflavone, the carbon–carbon double bonds of C2-C3 are easily hydrogenated. So far, the presence of 2,3-dihydroisoginkgetin and 2,3-dihydrosciadopitysin in ginkgo has been demonstrated [18]. Most of the ginkgo biflavonoids reported so far can be classified as a BB or flavone–flavone type [19].
As can be seen from the structures in Table 1 and Table 2, the separation and identification of biflavonoids can be challenging, because of the rather similar structure among some of them. It requires the use of modern techniques for separation and identification such as various hyphened methods, which are still expensive and not widely available. In addition, commercial availability of standards is limited to ginkgetin, isoginkgetin, amentoflavone, bilobetin and sciadopitysin, which may be the reason why these biflavonoids are the most commonly reported in ginkgo. To obtain a complete picture of the presence and metabolism of biflavonoids in ginkgo, further untargeted metabolomics experiments should be conducted to search for additional biflavonoids in ginkgo. These data would shed light on the still unknown role of biflavonoids in plant growth, development and communication with the environment.
4.2. Biological Factors Which Influence the Presence of Biflavonoids in Ginkgo
The amount and presence of individual biflavonoids depend on plant part, stage of development and environmental conditions during growth. Table 3 provides examples of selected reports on the presence of biflavonoids in various ginkgo plant parts and the used extraction and identification methods. Various organic solvents such as acetone, methanol, ethanol and petroleum ether have been used for the isolation of biflavonoids [38,39,40,41,42,43,44,45,46,47,48,49]. Recent data also show that sustainable extraction technologies [43] and modern extraction techniques [42] can be useful for the extraction of biflavonoids from ginkgo. The method of choice for the separation of compounds is liquid chromatography. Because of the characteristic UV absorption, DAD detectors can be used to detect biflavonoids. However, for accurate confirmation of structures DAD detectors are often combined with mass spectrometry and/or NMR, especially when standards are not commercially available.
Table 3.
Example of extraction and identification methods used for analysis of ginkgo biflavonoids from different plant parts.
| Plant Part | Biflavonoids | Extraction | Identification | |
|---|---|---|---|---|
| leaves | bilobetin, ginkgetin, isoginkgetin, sciadopitysin and dihyrosciadopitysin [38] |
acetone | NMR | |
| amentoflavone, bilobetin, ginkgetin, isoginkgetin, scyadopytisin [39] | 60% methanol | HPLC-DAD-MS | ||
| amentoflavone, bilobetin, ginkgetin/isoginkgetin, sciadopitysin [40] | methanol | HPLC- FTICR- MS | ||
| bilobetin, gingketin sciadopitysin [41] |
Soxhlet apparatus with 70% methanol | RP-HPTLC | ||
| bilobetin, gingketin, isoginkgetin, sciadopitysin [33] |
ultrasonic extraction using 50% aqueous ethanol | HPLC-MS/MS | ||
| bilobetin, ginkgetin, isoginkgetin, sciadopitysin [42] | MeOH-based deep eutectic solvent micellar system | UHPLC-QQQ-MS/MS | ||
| bilobetin, gingketin, isoginkgetin, sciadopitysin [43] | ultrasonic-assisted ionic liquid extraction | HPLC-DAD NMR |
||
| amentoflavone, sciadoptysin [44] | ethanol | UHPLC–ESI-MS/MS | ||
| stem | amentoflavone, sciadoptysin [44] | ethanol | UHPLC–ESI-MS/MS | |
| flowers | male | amentoflavone, bilobetin, isoginketin sciadopitysin [45] |
70% EtOH followed by silica gel column chromatography | NMR |
| fruit | amentoflavone, sciadoptysin [44] | ethanol | UHPLC–ESI-MS/MS | |
| exocarp | Bilobetin, ginkgetin Isoginkgetin [46] |
macroporous adsorption resin and then two-dimensional preparative HPLC-DAD system | HPLC-DAD | |
| sarcotesta | ginkgetin, isoginkgetin, sciadopitysin [47] | petroleum ether extract | HPLC-MS/MS NMR |
|
| seed coats | Isoginkgetin [48] | 70% ethanol | UPLC-ESI-MS | |
Lei et al. [43] extracted four biflavonoids—bilobetin, gingketin, isoginkgetin and sciadopitysin—from the leaves of ginkgo, of which sciadopitysin was the most abundant. The same biflavonoids in leaves were reported by Beck and Stengel [40]. In addition, they performed MALDI mass spectrometric imaging on leaf section and found that all detected biflavones were located at the leaf surface and the concentration was increased on the lower leaf surface compared to the upper surface. This could be related to their biological function. Pandey et al. [44] analysed the content of amentoflavone and sciadopitysin in leaves, stems, and fruits of ginkgo trees. They detected those biflavonoids in all analysed parts, although the sciadopitysin content was about 200 times higher in all samples. The content of amentoflavone in female trees was similar in all samples, while it was higher in male leaves. Sciadopitysin content in younger female leaves was lower than in the stem, while in older plants, it was more than twice that in the stem or fruit. Li et al. [45] reported the presence of amentoflavone, bilobetin, and isoginkgetin sciadopitysin but also amentoflavone 7″-O-d-glucopyranoside in male flowers. Chen et al. [48] found only isoginkgetin in leaves and seed coats among the 25 compounds identified in leaves, seed coats and embryoids of ginkgo, while it was absent in embryoids. Isoginkgetin was present in leaves at much higher concentrations than in seed coat samples. Zhou et al. [47] isolated sciadopitysin, ginkgetin and isoginkgetin from sarcotesta. Since the exocarp also contains biflavonoids, Shen et al. [46] developed an efficient and industrially suitable protocol for the targeted isolation of high-purity bilobetin, ginkgetin and isoginkgetin from the exocarp of ginkgo, which is considered industrial waste. They used a macroporous adsorption resin to enrich the biflavonoid content and then used a two-dimensional preparative normal-phase/reversed-phase HPLC-DAC system for isolatopn.
The presence of biflavonoids may also depend on the stage of leaf development. Wang et al. [33] reported the content of ginkgetin, isoginkgetin, bilobetin and sciadopitysin in leaves at six developmental stages. The content of biflavonoids ranged from 0 to 800 ng/g and depends significantly on the developmental stage. Sciadopitysin was present in all samples analyzed, but the amount was highest in the more advanced stages. The highest amounts in the last developmental stage were also reported for bilobetin and gingketin, while the content of isoginkgetin was the highest in the youngest leaves [33]. The age of the plant may also affect the accumulation of biflavonoids. According to Pandey et al. [44] 175-year-old trees accumulate more sciadopitysin in leaves and stem than 8-year-old trees.
Kaur et al. [41] studied the content of bilobetin, ginkgetin and sciadopitysin by reverse-phase high-performance thin-layer chromatography (RP-HPTLC) in ginkgo leaves collected from six locations in India. They reported ginkgetin and sciadopitysin in all analyzed samples, with ginkgetin being the most abundant in five of six localities. Bilobetin was present in samples from three locations. The authors indicated that the reason for this could be the difference in growth height and that plants cultivated in fields have higher levels of bioactive constituents than naturally growing old trees.
The mechanisms of the various biflavonoid accumulations and the influence of external factors are not explained. This may be because in physiological studies involving ginkgo flavonoids, biflavonoids have often been neglected, and only data on the changes in monomeric flavonoids have been reported [49,50,51]. In articles on the influence of environmental factors such as temperature [52], light [53], UV-B radiation [54] and salt stress [55] on ginkgo flavonoids, only data for monomeric flavonoids are reported. Further research including data on biflavonoids will help to understand the physiological role of biflavonoids in ginkgo.
5. Biological Activity of GinkgoBiflavonoids
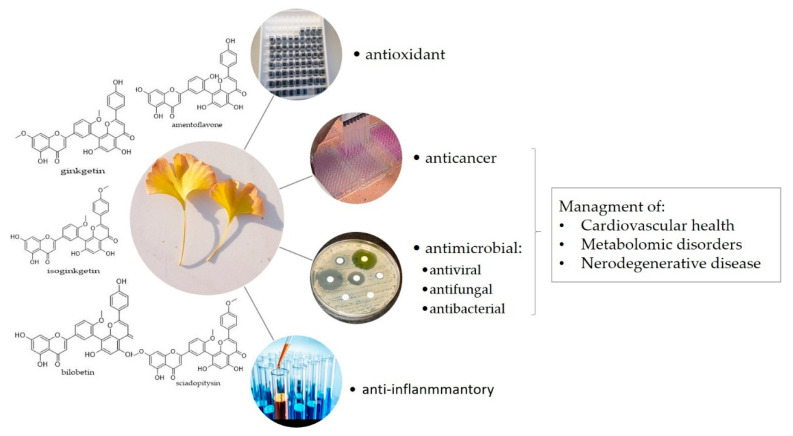
The biological properties and, consequently, the potential pharmacological application essentially depend on the chemical structure of flavonoids [26]. Modifications of flavonoids such as O-methylation, hydroxylation, glycosylation, and dimerization have a significant impact on the bioavailability and bioactivity of flavonoids and consequently on their potential role in plants and their potential health-related activities. Therefore, the biological activity of biflavonoids differs significantly from the biological activity of their monomeric structure. In the article, we have summarized the data on the biological activity of biflavonoids isolated from ginkgo in terms of their antioxidant, antimicrobial, antiviral and anti-inflammatory activities, and their potential role in management of health conditions such as neurodegenerative, cardiovascular, and metabolic diseases (Figure 3).
Figure 3.
Bioactivity of G. biloba biflavonoids.
5.1. Antioxidant Activity
Ginkgo extracts are known for their antioxidant activity and their benefit in preventing diseases related to oxidative stress (reviewed by [23,56,57]). However, according to Bedir et al. [58], biflavonoids are weaker antioxidants than other compounds identified from ginkgo. The biflavonoids amentoflavone, bilobetin, ginkgetin and sciadopitysin were compared with 25 other compounds isolated from ginkgo for antioxidant activity against HL-60 (promyeoloblasts) cells [58]. Compared to other flavonoids, the biflavonoids were not very potent inhibitors of respiratory burst. Among the four biflavonoids tested, amentoflavone had the best inhibitory effect, followed by ginkgetin. Interestingly, bilobetin and sciadopitysin were the compounds with the least antioxidant activity among the 29 specialized metabolites isolated from ginkgo.
On the other hand, amentoflavone, which was also isolated from other plant species, was reported to be a potent antioxidant [59,60], and it was suggested that dimerization, especially 3′,8″-dimerization, can increase the antioxidant capacity of flavonoids [61]. Amentoflavone showed significant antioxidant potential in vitro and in food-model systems; it exhibited strong antioxidant ability in scavenging DPPH, ABTS, superoxide, and hydroxyl radicals [59]. As summarized by Yu et al. [62] amentoflavone could be a potent molecule involved in the protection of various processes from different reactive oxygen species. Gan et al. [63] performed in vivo pharmacokinetic study on rat model to determine the plasma concentrations of amentoflavone and its metabolites. They reported that the low bioavailability of amentoflavone was due to extensive glucuronidation, but glucuronidation did not alter the antioxidant activity.
In view of all this, the contribution of biflavonoids to the antioxidant capacity of ginkgo needs to be further investigated, especially in in vivo models because the available data are contradictory.
5.2. Anticancer Activity
Ginkgo leaves have shown antiproliferative and anticancer activity in several in vitro studies against different cell lines [64,65], and these effects may be related to the presence of various flavonoids which have been targets for anticancer research for a decade [66]. In recent years, biflavonoids are also more and more studied as potential antitumor agents. As summarized by Menezes and Diederich [67], the protective effects of biflavonoids on cancer might be related to the inhibition of metabolism-related processes/pathways, enzymes, or proteins, such as STAT3/SHP-1/PTEN, kinesins, tissue kallikreins, aromatase, estrogen, protein modifiers, antioxidants, autophagy, and apoptosis-induction mechanisms.
In vitro bioassays and various cell lines are commonly used to test various natural products for their potential anticancer effects, and have also been applied to ginkgo biflavonoids. The results of in vitro studies on cell lines using ginkgo biflavonoids are summarized in Table 4. These assays are usually based on cell culture systems in which neoplastic cell lines are taken from human or other animal tumors and grown in vitro. The ability of the tested compounds to inhibit the growth (proliferation) of these cancer cells in culture is considered indicative of their potential value as cancer therapeutics in vivo.
Table 4.
Results of in vitro studies on cell lines using ginkgo biflavonoids.
| Testet Ginkgo Biflavonoids | Used Cell Line (s) | Main Findings |
|---|---|---|
| 7′′-O-β-d-glucopyranoside bilobetin isoginkgetin sciadopitysin [45] |
hepatocellular carcinoma, HepG2, cervical cancer cells, HeLa non-small-cell lung cancer, NCI-H460 |
bilobetin and isoginkgetin exhibited better anti-proliferative activities on different cancer lines. |
| ginkgetin [68] | breast cancer cells, MCF-7, T-47D, and MDA-MB | ginkgetin induces breast cancer cells with estrogen receptors via the inhibition of their expression |
| ginkgetin [69] | human prostate cancer, PC-3 cells. | ginkgetin induces apoptosis in PC-3 cells via activation of caspase 3 and inhibition of survival genes |
| ginkgetin [70] | human hepatocellular carcinoma, HepG2 | ginkgetin significantly reduced HepG2 cell viability in a dose-dependent manner and could be a cell apoptosis stimulator by affecting the balance between cell proliferation and apoptosis |
| ginkgetin [71] | human immortalised myelogenous leukemia, K562 | ginkgetin effectively inhibits K562 cell proliferation, and TNF-α plays a key role in ginkgetin-induced cell apoptosis |
| amentoflavone [72] | human bladder cancer cell line, TSGH8301 |
amentoflavone induces apoptosis and reduces expression of anti-apoptotic and metastasis-associated proteins |
| amentoflavone [73] | human ovarian cancer, SKOV3 and OVCAR-3 | amentoflavone significantly suppress cell proliferation, induce apoptosis and block cell cycle progression |
| amentoflavone [74], | human cervical cancer, SiHa and CaSki | amentoflavone activates PPARγ/PTEN expressions and induces apoptosis via suppressing E7 expression, cell cycle arrest at sub-G1 phase, and mitochondria-emanated intrinsic pathways |
The best studied biflavonoids from ginkgo in terms of their anticancer potential are ginkgetin and amentoflavone. As summarized by Adnan et al. [75], ginkgetin combats cancer progression by arresting the cell cycle, inducing apoptosis, stimulating autophagy, and targeting many deregulated signaling pathways such as JAK/STAT and MAPKs. It may act as a potent Hsp90 inhibitor [76]. Heat-shock protein 90 (Hsp90) is an important molecular chaperone that is overexpressed in cancer cells and may be a therapeutic drug target for cancer chemotherapy. Ginkgetin has been shown in several studies to be a promising compound agent that enhances the anti-cancer effects of other drugs/compounds. Lou et al. [77] reported that ginkgetin isolated from ginko leaves promoted an anticancer effect induced by cisplatin and that this mechanism was via ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR wild-type non-small-cell lung cancer. In synergy with resveratrol ginkgetin, it can suppress vascular endothelial growth factor (VEGF) and may represent a therapeutic strategy in cancer [78].
According to recent data, amentoflavone is involved in anti-cancer activity by mediating various signaling pathways such as extracellular signal-regulated kinase (ERK), nuclear factor kappa-B (NF-κB) and phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) (reviewed by [79]). In a comparative study of ginkgetin and sciadopitysin, Choi et al. [80] evaluated their effect on the inhibition of phosphatase in regenerating liver-3 (PRL-3), a key factor in the acquisition of metastatic properties of tumor cells. The authors showed that both compounds moderately inhibited PRL-3, but this effect was higher than in the monomeric flavonoids acacetin and apigenin.
Bilobetin, isoginkgetin and sciadopitysin have been less studied but may also be involved in the anticancer effect of ginkgo extracts. Li et al. [45] tested five biflavonoids isolated from ginkgo for their anti-proliferative activities on HepG2, HeLa, and NCI-H460 cell lines and reported that bilobetin and isoginkgetin exhibited the better anti-proliferative activities in different cancer lines. The significant morphological changes of the cells confirmed their anticancer effects in a dose-dependent manner. They were able to arrest the G2/M phase of the cell cycle, induce the apoptosis of HeLa cells in a dose-dependent manner, and activate the proapoptotic protein Bax and the executor caspase-3. Bilobetin could also inhibit the antiapoptotic protein Bcl-2 [45]. These could be the mechanisms underlying its anti-proliferation.
Although in vitro data suggest an anticancer effects of ginkgo biflavonoids, in vivo studies had to confirm these effects. For example, pharmacokinetic studies in rats showed that the oral bioavailability of amentoflavone is quite low and in plasma glucuronidated amentoflavone is predominantly found [63]. In the same study, they reported that glucuronidated amentoflavone metabolites have lower anticancer activity than amentoflavone. Therefore, further in vivo and clinical studies need to confirm ginkgo biflavonoids as potential anticancer agents.
5.3. Antiviral Activity
For more than 20 years, biflavonoids have been investigated as potential antiviral agents and shown inhibitory activities against several viruses including respiratory viruses (influenza A, influenza B, respiratory syncytial, parainfluenza type 3, adenovirus type 5, measles, severe acute respiratory syndrome including SARS-CoV2), herpesviruses (HSV-1, HSV-2, HCMV, VZV, and Epstein–Barr virus (EBV)), human immunodeficiency virus (HIV), protozoal (Leishmaniasis, Malaria) and viruses which cause dengue, coxsackieviral, hepatitis, etc. [37,67,81]. In recent years, during the coronavirus pandemic, amentoflavone has become a molecule of interest due to its antiviral effects against SARS-CoV-2 based on in vitro and in silico studies [82,83]. Amentoflavone in vitro has the potential to inhibit herpes simplex virus 1 (HSV-1) including Acyclovir (ACV) (antiviral drug) -resistant strains [84].
The anti-influenza virus properties of ginkgetin, isolated from leaves of ginkgo, were revealed via inhibiting the activity of sialidase, a key enzyme that plays an essential role in the life cycle of the influenza virus and is a crucial drug target for the development of effective anti-influenza medications. The antiviral activity of ginkgetin was determined against two types of influenza viruses sialidases (A/PR/8/34 (H1N1) and A/Guizhou/54/89 (H3N2)) with 50% inhibitory concentration values of 55.00 and 9.78 µg/mL, respectively [85]. In another investigation, Haruyama and Nagata [86] assessed the anti-infectivity properties of ginkgo leaf extract against influenza viruses A (H1N1 and H3N2) and B (B/Lee/40), and the results revealed notable antiviral actions against the investigated viruses. The authors have suggested that ginkgetin and other biflavonoid molecules present in extracts might be accountable for the antiviral effects against influenza viruses with a proposed mechanism via repressing the function of hemagglutinin in adsorption to host cells.
In a combined in vitro and in silico study, biflavonoids such as sciadopitysin, ginkgetin, isoginkgetin, amentoflavone, and bilobetin were examined for their anti-severe acute respiratory syndrome coronavirus 2 (anti-SARS-CoV-2) activities via targeting 3-chymotrypsin-like protease (3CLpro), an important enzyme that is required for the replication of coronaviruses, including SARS-CoV-2. The results of inhibition assay demonstrated that the tested compounds show notable anti-SARS-CoV-2 3CLpro actions. The results also showed that sciadopitysin is the most potent inhibitor of SARS-CoV-2 3CLpro among the test biflavonoids with reversible and mixed inhibition mechanisms. The mechanisms have been confirmed by inhibition kinetic studies and molecular docking simulations [87].
5.4. Anibacterial and Antifungal Activity
Extracts of ginko possess remarkably high inhibitory activity against a range of Gram-positive and Gram-negative bacteria [88], and this activity may be related to the presence of biflavonoids with antibacterial activity [19,67]. The best studied biflavonoid from ginkgo, in terms of its antimicrobial activity, is amentoflavone. Hwang et al. [89] tested the antimicrobial activity of amentoflavone against Enterococcus faecium, Staphylococcus aureus, Streptococcus mutans, Escherichia coli O-157, Escherichia coli, and Pseudomonas aeruginosa and showed that amentoflavone had a considerable antibacterial effect and synergistic interaction with antibiotics against various bacterial strains except S. mutans. Amentoflavone has confirmed antimicrobial activity against foodborne pathogens, including S. aureus and E. coli, which was also confirmed in food models using minced chicken meat and apple juice food models [59]. Due to its antimicrobial activity against the cyanobacterium Microcystis aeruginosa [90], it also has potential in the treatment of harmful cyanobacterial blooms. Cyanobacterial blooms are becoming a global environmental problem due to the destruction they cause in freshwater ecosystems. Amentoflavone [91] and ginkgetin [92] showed antimicrobial effects in vitro and in vivo in mouse models against Streptococcus suis, a zoonotic pathogen, which can cause considerable economic losses in the swine industry and severe public health issues. Bagla et al. [93] reported the antimicrobial activity of isoginkgetin against E. faecalis, S. aureus, P. aeruginosa and E. coli. The antimicrobial activity of bilobetin and sciadopitysin is poorly studied.
The biflavonoids of ginkgo are also potent antifungal agents. Krauze-Baranowskaa and Wiwart [94] tested amentoflavone, 7-O-methylamentoflavone (sequoiaflavone), bilobetin, ginkgetin, sciadopitysin and 2,3-dihydrosciadopitysin for their antifungal activity against Alternaria alternata, Cladosporium oxysporum and Fusarium culmorum. They reported that bilobetin exhibited significant antifungal activity and completely inhibited the growth of C. oxysporum and F. culmorum, but the activity of ginkgetin and 7-O-methylamentoflavone against A. alternata was stronger than that of bilobetin. In addition, biflavones without a methoxyl group such as amentoflavone and biflavonoid consisting of flavanone–flavone units such as 2,3-dihydrosciadopitysin were inactive or weak against A. alternata and C. oxysporum. The significant antifungal activity of biflavonoes with methoxyl group has been found mainly against C. oxysporum and increase in the number of methoxyl groups decreased antifungal action of biflavones against A. alternata [94]. Amentoflavone has been reported to be an effective molecule against the human pathogenic yeast Candida albicans and the antifungal mechanisms may be due to physiological changes inducing S-phase arrest in the intracellular environment [95]. Hwang et al. [96] reported that amentoflavone induces apoptosis in C. albicans and causes mitochondrial dysfunction.
5.5. Anti-Inflammatory Effect
Biflavonoids shown anti-inflammatory activity by inhibiting phospholipase A2 or regulating pro-inflammatory gene expression in vitro and in vivo [97]. Zhou et al. [98] compared the anti-inflammatory effects of ginkgetin and isoginkgetin using placental alkaline phosphatase reporter assay, developed to measure nuclear factor kappa B. Both biflavonoids showed inhibitory effects, with the effect of ginkgetin being greater than that of isoginkgetin. Kim et al. [99] studied in vitro the effects of five flavonoids, including amentoflavone and isoginkgetin on epidermal cyclooxygenase/lipoxygenase, as their anti-inflammation potential. In their study, the amentoflavone showed potent and selective inhibitory activity on cyclooxygenase, which was comparable to those of anti-inflammatory drug indomethacin. In the same study, ginkgetin showed a weak inhibitory effect on cyclooxygenase.
Li et al. [100] isolated 18 different chemical components from the flowers of ginkgo, including the biflavonoids amentoflavone, sciadopitysin, bilobetin and isoginkgetin. They evaluated their anti-inflammatory ability in the lipopolysaccharide induced RAW264.7 macrophages. Among all tested compounds, the most effective compounds were bilobetin and isoginkgetin. They exhibited significant dose-dependent inhibitory effects on tumor necrosis factor-α IL-6, PGE2, inducible NO synthase mRNA, and cyclooxygenase-2 mRNA levels. According to the authors, they can be promising candidates for the development of new anti-inflammatory agents.
5.6. Potential Role in the Management of Cardiovascular and Metabolomics Diseases
Extracts from ginkgo leaves are widely used in both Eastern and Western countries for the treatment of metabolic diseases (such as hyperlipidemia), but the bioactive compounds underlying these mechanisms have not been fully characterized [101]. Liu et al. [101] investigated the inhibitory potential and mechanism of isoginkgetin, ginkgetin, bilobetin and sciadopitysin isolated from ginkgo on pancreatic lipase, a key target regulating lipid absorption. Their results clearly demonstrated that all tested biflavones displayed strong-to-moderate inhibitory effects on pancreatic lipase. Wang et al. [102] also hypothesized that biflavonoids may play a potential role in the treatment of cerebrovascular disease. They isolated bilobetin, ginkgetin, isoginkgetin and sciadopitysin from ginkgo and screened them using oleic acid-induced lipid production in HepG2 cells. They also used reverse targeting and molecular dynamics simulation for screening the non-covalent effects of biflavonoids on the potential targets of atherosclerosis. The interactions between biflavonoids and potential targets were evaluated by an exogenous cell model. Among tested biflavonoids, ginkgetin significantly inhibited oleic acid-induced lipid production and reduced total cholesterol and triglyceride levels. The authors concluded that it can be promising natural medicine for the treatment of atherosclerosis. Dell’Agli et al. [103] studied the ability of five ginkgobiflavonoids, ginkgetin, sequoiaflavone, amentoflavone, bilobetin and sciadopitysin, to inhibit cAMP phosphodiesterase activity and promote vasorelaxation. They reported that all biflavones inhibited PDE5A1 in a concentration-dependent manner, with ginkgetin being the most potent, followed by bilobetin, sciadopitysin, amentoflavone and sequoiaflavone.
The best-studied biflavonoid from ginkgo regarding cardiovascular health protection is amentoflavone and several studies in recent years have highlighted its therapeutic potential. Kubota et al. [104] reported that amentoflavone significantly increased the heartbeat rate, and it is considered one of the main components of ginkgo that produces the beneficial chronotropic and inotropic effects. Amentoflavone can ameliorate the myocardial ischemia-reperfusion injury, as shown in in vivo and in vitro studies [105]. In an in vivo study in mice suffering from metabolic syndrome amentoflavone was shown to inhibit the changes in general metabolic parameters, including body weight, fat mass, insulin levels, and glucose tolerance activity, and it may markedly protect against cardiovascular dysfunction [106]. Amentoflavone prevents oxidized low-density lipoprotein-induced lipid accumulation and as such may be involved in protection from atherosclerotic cardiovascular disease [107].
5.7. Potential Role in the Management Neurodegenerative Disorders Treatment
The use of ginkgo for the treatment of neurodegenerative diseases is widely recognized [15]. As described by Uddin et al. [108], biflavonoids are molecules that have the potential to noticeably alter the aggregation of tau and Aβ and efficiently reduce the toxic effect of Aβ-oligomers compared to monoflavonoids. Thus, these are potent molecules for the treatment and prevention of Alzheimer’s disease.
Kang et al. [109] investigated the neuroprotective effects of nine naturally occurring bi-flavonoids on oxidative stress and amyloid-β-peptide-induced cell death in neuronal cells. In this study, bilobetin, ginkgetin, isoginkgetin, and sciadopitysin purified from ginkgo and amentoflavone from other source were investigated. Of the biflavonoids tested, amentoflavone, ginkgetin, and isoginkgetin showed the strongest neuroprotective effects against cytotoxic insults induced by oxidative stress and amyloid β. These results suggest their therapeutic potential against neurodegenerative diseases such as ischemic stroke and Alzheimer’s disease. Ginkgetin isolated from ginkgo showed neuroprotective effects against neurological damage in Parkinson’s disease in vivo and in vitro. The mechanism is via regulation of iron homeostasis by reducing oxidative damage, activating microglia, and enhancing neurotrophic potential [110,111]. As summarized by Adnan et al. [75], ginkgetin shows potent neuroprotective effects against oxidative stress-induced cell death, inhibits cerebral microbleeds, reduces neurological deficits, and stops apoptosis of neurons. Isoginkgetin also shows promising results in in vivo studies in rats as a novel therapeutic agent in the treatment of ischemic stroke injury [112]. As summarized by Varshney et al. [113], amentoflavone also has therapeutic and medicinal potential in the treatment of neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease, as well as in the treatment of depression and in the prevention of aging. However, amentoflavone shows poor brain permeability in mice in vivo [114]. After treatment with ginkgo extracts, the concentration of the active compounds in the brain was below the limit of quantification. There is also some evidence that amentoflavone may be useful in the treatment of psychiatric disorders [115].
Wang et al. [116] used network pharmacology to explore the potential mechanism of ginkgo in the treatment of neurodegenerative disorders. They reported that twenty-seven ginkgo constituents can affect biological processes such as oxidation reactions and activate the activities of transcription factors. However, they did not identify any of the biflavonoids as effective molecules for the treatment of neurodegenerative disorders.
6. Toxicity and Safety of Ginkgo Biflavonoids
Although herbal medicines are considered relatively safe, many herbal supplements have been reported to pose potential health risks, but because the complex chemical nature of supplements makes it difficult to evaluate their efficacy and safety. Mei et al. [117] summarized the data on the potential toxicology of ginkgo from experimental studies to human case reports and showed that the currently published data support both beneficial and adverse effects of ginkgo extracts and that it depends critically on which specific extract is used. Accordingly, the constituents of ginkgo extract that may be responsible for the toxicity, genotoxicity, and carcinogenicity of ginkgo have not yet been definitively identified.
The potential toxicity of biflavonoids from ginkgo has not been well studied. This may be because biflavonoids have only recently begun to attract greater scientific attention as potent bioactive compounds. Cardoso et al. [118] reported the mutagenic activity of amentoflavone in the Salmonella typhimurium assay. Amentoflavone and sciadopitysin could inhibit the activity of human UDP-glucuronosyltransferase (UGT), which is the most important class of detoxification enzymes and is highly expressed in metabolic organs such as liver, intestine, and kidney [119,120], but further studies are needed to explain this mechanism. Li et al. [121] investigated the toxicological effects of biflavonoids from ginkgo in an in vitro and in vivo study. Their results suggest that the investigated biflavonoids from ginkgo may have potential liver and kidney toxicity, so further studies on the safety and efficacy of biflavonoids from ginkgo are needed.
7. Conclusions and Further Directions
Ginkgo is a widely recognized medicinal plant and a natural reservoir of bioactive compounds. According to the available data, dimeric flavonoids—biflavonoids—are an important component of the bioactivity of ginkgo. So far, 13 biflavonoids have been identified in ginkgo, of which ginkgetin, isoginkgetin, amentoflavone, bilobetin, and sciadopitysin have been described most frequently. Most of the available work used only targeted analyses to determine biflavonoids in ginkgo, so further untargeted analyzes are needed to possibly identify additional biflavonoids structures. Their presence and content may be influenced by the growth stage, growth conditions, and age of the trees, and the accumulation of compounds in different parts of the plant varies. So far, leaves have mainly been studied, but biflavonoids are also detected in other plant parts such as stem, flowers and various seed parts. According to the available data, among the identified biflavonoids, sciadopitysin is often reported as one with the highest content. As far as we know, the role of biflavonoids in ginkgo plants is not known and further research in this direction would be useful.
The potential health benefits of biflavonoids from ginkgo are better understood. As we have summarized, the available data suggest their antioxidant, anticancer, antiviral, antibacterial, antifungal, and anti-inflammatory effects, as well as a possible role in the treatment of cardiovascular, metabolic, and neurodegenerative diseases. The best studied biflavonoid from ginkgo is amentoflavone, but according to the literature, other biflavonoids from ginkgo may also be potent pharmacological compounds. It is important to note that toxicological studies have also shown potential toxicity of ginkgo biflavonoids. Therefore, further research should consider these effects when evaluating their bioactive properties. The active concentration and safe range for ingestion should be determined.
In summary, biflavonoids play an important role in the biological activity of ginkgo, but there is still a lot to be clarified. They are also of interest from a plant biology/physiology point of view as they could play an important role in plant–environment interactions, and research in this area is needed due to the current problems with climate change. On the other hand, they are molecules that have the potential to be used in the pharmaceutical industry. However, most studies are conducted in vitro, and further in vivo and clinical studies should determine their efficacy and safety.
Acknowledgments
The authors thanks to Antonia Ćurić for the ginkgo photograph in Figure 1.
Author Contributions
Conceptualization, D.Š.; investigation, D.Š., E.K., S.D., S.T.S.H.; writing—original draft preparation, D.Š., E.K., S.D., S.T.S.H.; writing—review and editing, D.Š.; visualization, D.Š.; supervision, D.Š.; project administration, D.Š.; funding acquisition, D.Š. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work has been funded by Croatian Science Foundation project “Biflavonoids role in plants: Ginkgo biloba L. as a model system″ under the grant no. UIP-2019-04-1018.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Leistner E., Drewke C. Ginkgo biloba and ginkgotoxin. J. Nat. Prod. 2010;73:86–92. doi: 10.1021/np9005019. [DOI] [PubMed] [Google Scholar]
- 2.He B., Gu Y., Xu M., Wang J., Cao F., Xu L.A. Transcriptome analysis of Ginkgo biloba kernels. Front. Plant Sci. 2015;6:819. doi: 10.3389/fpls.2015.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Šmarda P., Veselý P., Šmerda J., Bureš P., Knápek O., Chytrá M. Polyploidy in a ‘living fossil’ Ginkgo biloba. New Phytol. 2016;212:11–14. doi: 10.1111/nph.14062. [DOI] [PubMed] [Google Scholar]
- 4.Gong W., Chen C., Dobeš C., Fu C.X., Koch M.A. Phylogeography of a living fossil: Pleistocene glaciations forced Ginkgo biloba L.(Ginkgoaceae) into two refuge areas in China with limited subsequent postglacial expansion. Mol. Phylogenetics Evol. 2008;48:1094–1105. doi: 10.1016/j.ympev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Tang C.Q., Yang Y., Ohsawa M., Yi S.-R., Momohara A., Su W.-H., Wang H.-C., Zhang Z.-Y., Peng M.-C., Wu Z.-L. Evidence for the persistence of wild Ginkgo biloba (Ginkgoaceae) populations in the Dalou Mountains, southwestern China. Am. J. Bot. 2012;99:1408–1414. doi: 10.3732/ajb.1200168. [DOI] [PubMed] [Google Scholar]
- 6.Bainian S., Liang X., Sanping X., Shenghui D., Yongdong W., Hui J., Turner S. Quantitative analysis of paleoatmospheric CO2 level based on stomatal characters of fossil Ginkgo from Jurassic to Cretaceous in China. Acta Geol. Sin. 2007;81:931–939. doi: 10.1111/j.1755-6724.2007.tb01016.x. [DOI] [Google Scholar]
- 7.Royer D.L., Hickey L.J., Wing S.L. Ecological conservatism in the “living fossil″ Ginkgo. Paleobiology. 2003;29:84–104. doi: 10.1666/0094-8373(2003)029<0084:ECITLF>2.0.CO;2. [DOI] [Google Scholar]
- 8.Guan R., Zhao Y., Zhang H., Fan G., Liu X., Zhou W., Shi C., Wang J., Liu W., Liang X., et al. Draft genome of the living fossil Ginkgo biloba. Gigascience. 2016;5:49. doi: 10.1186/s13742-016-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagata T., Hasebe M., Toriba T., Taneda H., Crane P.R. Sex conversion in Ginkgo biloba (Ginkgoaceae) J. Jpn. Bot. 2016;91:120–127. [Google Scholar]
- 10.Parliment T.H. Characterization of the putrid aroma compounds of Ginkgo biloba fruits. In Fruit Flavors: Biogenesis, Characterization, and Authentication; Rouseff, R., Leahy, M., Eds. Am. Chem. Soc. Symp. Ser. 1995;596:276–279. [Google Scholar]
- 11.Murakami M., Abe M., Kakumoto Y., Kawano H., Fukasawa H., Saha M., Takada H. Evaluation of ginkgo as a biomonitor of airborne polycyclic aromatic hydrocarbons. Atmos. Environ. 2012;54:9–17. doi: 10.1016/j.atmosenv.2012.02.014. [DOI] [Google Scholar]
- 12.Aziz T.A., Hussain S.A., Mahwi T.O., Ahmed Z.A., Rahman H.S., Rasedee A. The efficacy and safety of Ginkgo biloba extract as an adjuvant in type 2 diabetes mellitus patients ineffectively managed with metformin: A double-blind, randomized, placebo-controlled trial. Drug Des. Devel. Ther. 2018;12:735–742. doi: 10.2147/DDDT.S157113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goh L.M., Barlow P.J. Antioxidant capacity in Ginkgo biloba. Food Res. Int. 2002;35:815–820. doi: 10.1016/S0963-9969(02)00084-4. [DOI] [Google Scholar]
- 14.Noor-E-Tabassum Das R., Lami M.S., Chakraborty A.J., Mitra S., Tallei T.E., Idroes R., Mohamed A.A.-R., Hossain J., Dhama K., Mostafa-Hedeab G., et al. Ginkgo biloba: A Treasure of Functional Phytochemicals with Multimedicinal Applications. Evid.-Based Complement. Altern. Med. 2022;2022:8288818. doi: 10.1155/2022/8288818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbalho S.M., Direito R., Laurindo L.F., Marton L.T., Guiguer E.L., Goulart R.d.A., Tofano R.J., Carvalho A.C.A., Flato U.A.P., Capelluppi Tofano V.A., et al. Ginkgo biloba in the Aging Process: A Narrative Review. Antioxidants. 2022;11:525. doi: 10.3390/antiox11030525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKenna D.J., Jones K., Hughes K. Efficacy, safety, and use of Ginkgo biloba in clinical and preclinical applications. Altern. Ther. Health Med. 2001;7:88–90. [PubMed] [Google Scholar]
- 17.Jacobs B.P., Browner W.S. Ginkgo biloba: A living fossil. Am. J. Med. 2000;108:341–342. doi: 10.1016/S0002-9343(00)00290-4. [DOI] [PubMed] [Google Scholar]
- 18.Liu L., Wang Y., Zhang J., Wang S. Advances in the chemical constituents and chemical analysis of Ginkgo biloba leaf, extract, and phytopharmaceuticals. J. Pharm. Biomed. Anal. 2021;193:113704. doi: 10.1016/j.jpba.2020.113704. [DOI] [PubMed] [Google Scholar]
- 19.He X., Yang F., Huang X. Proceedings of Chemistry, Pharmacology, Pharmacokinetics and Synthesis of Biflavonoids. Molecules. 2021;26:6088. doi: 10.3390/molecules26196088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ražná K., Hrubík P. Cultural Extension of Ginkgo biloba L. in Slovakia. Agrobiodiversity Improv. Nutr. Health Life Qual. 2021;5:258–279. doi: 10.15414/ainhlq.2021.0025. [DOI] [Google Scholar]
- 21.Del Tredici P. Ginkgos and people: A thousand years of interaction. Arnoldia. 1991;51:2–15. [Google Scholar]
- 22.Barlow P.W., Kurczyńska E.U. The anatomy of the chi-chi of Ginkgo biloba suggests a mode of elongation growth that is an alternative to growth driven by an apical meristem. J. Plant Res. 2007;120:269–280. doi: 10.1007/s10265-006-0050-3. [DOI] [PubMed] [Google Scholar]
- 23.Singh B., Kaur P., Gopichand, Singh R.D., Ahuja P.S. Biology and chemistry of Ginkgo biloba. Fitoterapia. 2008;79:401–418. doi: 10.1016/j.fitote.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Cheng J.-T., Guo C., Cui W.-J., Zhang Q., Wang S.-H., Zhao Q.-H., Liu D.-W., Zhang J., Chen S., Chen C., et al. Isolation of two rare N-glycosides from Ginkgo biloba and their anti-inflammatory activities. Sci. Rep. 2020;10:5994. doi: 10.1038/s41598-020-62884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boateng I.D. A critical review of current technologies used to reduce ginkgotoxin, ginkgotoxin-5′-glucoside, ginkgolic acid, allergic glycoprotein, and cyanide in Ginkgo biloba L. seed. Food Chem. 2022;382:132408. doi: 10.1016/j.foodchem.2022.132408. [DOI] [PubMed] [Google Scholar]
- 26.Šamec D., Karalija E., Šola I., Vujčić Bok V., Salopek-Sondi B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants. 2021;10:118. doi: 10.3390/plants10010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nabavi S.M., Šamec D., Tomczyk M., Milella L., Russo D., Habtemariam S., Suntar I., Rastrelli L., Daglia M., Xiao J., et al. Flavonoid Biosynthetic Pathways in Plants: Versatile Targets for Metabolic Engineering. Biotechnol. Adv. 2020;38:107316. doi: 10.1016/j.biotechadv.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed T., Javed S., Javed S., Tariq A., Šamec D., Tejada S., Nabavi S.F., Braidy N., Nabavi S.M. Resveratrol and Alzheimer’s Disease: Mechanistic Insights. Mol. Neurobiol. 2017;54:2622–2635. doi: 10.1007/s12035-016-9839-9. [DOI] [PubMed] [Google Scholar]
- 29.Nabavi S.F., Khan H., D’onofrio G., Šamec D., Shirooie S., Dehpour A.R., Castilla S.A., Habtemariam S., Sobarzo-Sanchez E. Apigenin as Neuroprotective Agent: Of mice and men. Pharmacol. Res. 2018;128:359–365. doi: 10.1016/j.phrs.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Budzynska B., Faggio C., Kruk- Slomka M., Šamec D., Nabavi S.F., Sureda A., Devi K.P., Nabavi S.M. Rutin as neuroprotective agent: From bench to bedside. Curr. Med. Chem. 2019;26:5152–5164. doi: 10.2174/0929867324666171003114154. [DOI] [PubMed] [Google Scholar]
- 31.Arafah A., Rehman M.U., Mir T.M., Wali A.F., Ali R., Qamar W., Khan R., Ahmad A., Aga S.S., Alqahtani S., et al. Multi-Therapeutic Potential of Naringenin (4′,5,7-Trihydroxyflavonone): Experimental Evidence and Mechanisms. Plants. 2020;9:1784. doi: 10.3390/plants9121784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Šudomová M., Berchová-Bímová K., Mazurakova A., Šamec D., Kubatka P., Hassan S.T.S. Flavonoids Target Human Herpesviruses That Infect the Nervous System: Mechanisms of Action and Therapeutic Insights. Viruses. 2022;14:592. doi: 10.3390/v14030592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L.-T., Fan X.-H., Jian Y., Dong M.-Z., Yang Q., Meng D., Fu Y.-J. A sensitive and selective multiple reaction monitoring mass spectrometry method for simultaneous quantification of flavonol glycoside, terpene lactones, and biflavonoids in Ginkgo biloba leaves. J. Pharm. Biomed. Anal. 2019;170:335–340. doi: 10.1016/j.jpba.2019.03.058. [DOI] [PubMed] [Google Scholar]
- 34.Ma G.-L., Xiong J., Yang G.-X., Pan L.-L., Hu C.-L., Wang W., Fan H., Zhao Q.-H., Zhang H.-Y., Hu J.-F. Biginkgosides A–I, Unexpected Minor Dimeric Flavonol Diglycosidic Truxinate and Truxillate Esters from Ginkgo biloba Leaves and Their Antineuroinflammatory and Neuroprotective Activities. J. Nat. Prod. 2016;79:1354–1364. doi: 10.1021/acs.jnatprod.6b00061. [DOI] [PubMed] [Google Scholar]
- 35.DeForest J.C., Du L., Joyner P.M. 4′,4′′′,7,7′′-Tetra-O-methylcupressuflavone Inhibits Seed Germination of Lactuca sativa. J. Nat. Prod. 2014;77:1093–1096. doi: 10.1021/np4010739. [DOI] [PubMed] [Google Scholar]
- 36.Šamec D., Pierz V., Srividya N., Wüst M., Lange B.M. Assessing chemical diversity in Psilotum nudum (L.) Beauv., a Pantropical whisk fern that has lost many of its fern-like characters. Front. Plant Sci. 2019;10:868. doi: 10.3389/fpls.2019.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gontijo V.S., dos Santos M.H., Viegas C. Biological and Chemical Aspects of Natural Biflavonoids from Plants: A Brief Review. Mini Rev. Med. Chem. 2017;17:834–862. doi: 10.2174/1389557517666161104130026. [DOI] [PubMed] [Google Scholar]
- 38.Wollenweber E., Kraut L., Mues R. External Accumulation of Biflavonoids on Gymnosperm Leaves. Z. Nat. C. 1998;53:946–950. doi: 10.1515/znc-1998-11-1202. [DOI] [Google Scholar]
- 39.Lin L.-Z., Chen P., Ozcan M., Harnly J.M. Chromatographic Profiles and Identification of New Phenolic Components of Ginkgo biloba Leaves and Selected Products. J. Agric. Food Chem. 2008;56:6671–6679. doi: 10.1021/jf800488x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beck S., Stengel J. Mass spectrometric imaging of flavonoid glycosides and biflavonoids in Ginkgo biloba L. Phytochemistry. 2016;130:201–206. doi: 10.1016/j.phytochem.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Kaur P., Chaudhary A., Singh B., Gopichand Simultaneous quantification of flavonoids and biflavonoids in Ginkgo biloba using RP-HPTLC densitometry method. JPC-J. Planar Chromat. –Mod. TLC. 2011;24:507–512. doi: 10.1556/JPC.24.2011.6.10. [DOI] [Google Scholar]
- 42.Wang L.-T., Yang Q., Cui Q., Fan X.-H., Dong M.-Z., Gao M.-Z., Lv M.-J., An J.-Y., Meng D., Zhao X.-H., et al. Recyclable menthol-based deep eutectic solvent micellar system for extracting phytochemicals from Ginkgo biloba leaves. J. Clean. Prod. 2019;244:118648. doi: 10.1016/j.jclepro.2019.118648. [DOI] [Google Scholar]
- 43.Lei J., Jiang Y., Luo X., Zheng Y., Zhu L., Sun C., Linghu L., Qin C., Gang W. Ultrasonic-Assisted Ionic Liquid Extraction of Four Biflavonoids from Ginkgo biloba L. ChemistrySelect. 2021;6:3297–3307. doi: 10.1002/slct.202004605. [DOI] [Google Scholar]
- 44.Pandey R., Chandra P., Arya K.R., Kuma B. Development and validation of an ultra high performance liquid chromatography electrospray ionization tandem mass spectrometry method for the simultaneous determination of selected flavonoids in Ginkgo biloba. J. Sep. Sci. 2014;37:3610–3618. doi: 10.1002/jssc.201400853. [DOI] [PubMed] [Google Scholar]
- 45.Li M., Li B., Xia Z.-M., Tian Y., Zhang D., Rui W.-J., Dong J.-X., Xiao F.-J. Anticancer Effects of Five Biflavonoids from Ginkgo Biloba L. Male Flowers In Vitro. Molecules. 2019;24:1496. doi: 10.3390/molecules24081496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen N., Liu Y., Cui Y., Xin H. Large-scale targetedly isolation of biflavonoids with high purity from industrial waste Ginkgo biloba exocarp using two-dimensional chromatography coupled with macroporous adsorption resin enrichment. Ind. Crops Prod. 2022;175:114264. doi: 10.1016/j.indcrop.2021.114264. [DOI] [Google Scholar]
- 47.Zhou G., Yao X., Tang Y., Yang N., Pang H., Mo X., Zhu S., Su S., Qian D., Jin C., et al. Two new nonacosanetriols from Ginkgo biloba sarcotesta. Chem. Phys. Lipids. 2012;165:731–736. doi: 10.1016/j.chemphyslip.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Chen X., Zhong W., Shu C., Yang H., Li E. Comparative analysis of chemical constituents and bioactivities of the extracts from leaves, seed coats and embryoids of Ginkgo biloba L. Nat. Prod. Res. 2021;35:5498–5501. doi: 10.1080/14786419.2020.1788020. [DOI] [PubMed] [Google Scholar]
- 49.Lu Z., Zhu L., Lu J., Shen N., Wang L., Liu S., Wang Q., Yu W., Kato-Noguchi H., Li W., et al. Rejuvenation increases leaf biomass and flavonoid accumulation in Ginkgo biloba. Hortic. Res. 2022;9 doi: 10.1093/hr/uhab018. in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo Y., Gao C., Wang M., Fu F., El-Kassaby Y.A., Wang T., Wang G. Metabolome and transcriptome analyses reveal flavonoids biosynthesis differences in Ginkgo biloba associated with environmental conditions. Ind. Crops Prod. 2020;158:112963. doi: 10.1016/j.indcrop.2020.112963. [DOI] [Google Scholar]
- 51.Wu Y., Guo J., Zhou Q., Xin Y., Wang G., Xu L.-a. De novo transcriptome analysis revealed genes involved in flavonoid biosynthesis, transport and regulation in Ginkgo biloba. Ind. Crops Prod. 2018;124:226–235. doi: 10.1016/j.indcrop.2018.07.060. [DOI] [Google Scholar]
- 52.Wang G., Cao F., Chang L., Guo X., Wang J. Temperature has more effects than soil moisture on biosynthesis of flavonoids in Ginkgo (Ginkgo biloba L.) leaves. New For. 2014;45:797–812. doi: 10.1007/s11056-014-9437-5. [DOI] [Google Scholar]
- 53.Xu Y., Wang G., Cao F., Zhu C., Wang G., El-Kassaby Y.A. Light intensity affects the growth and flavonol biosynthesis of Ginkgo (Ginkgo biloba L.) New For. 2014;45:765–776. doi: 10.1007/s11056-014-9435-7. [DOI] [Google Scholar]
- 54.Zhao B., Wang L., Pang S., Jia Z., Wang L., Li W., Jin B. UV-B promotes flavonoid synthesis in Ginkgo biloba leaves. Ind. Crops Prod. 2020;151:112483. doi: 10.1016/j.indcrop.2020.112483. [DOI] [Google Scholar]
- 55.Xu N., Liu S., Lu Z., Pang S., Wang L., Wang L., Li W. Gene Expression Profiles and Flavonoid Accumulation during Salt Stress in Ginkgo biloba Seedlings. Plants. 2020;9:1162. doi: 10.3390/plants9091162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martínez-Solís I., Acero N., Bosch-Morell F., Castillo E., González-Rosende M.E., Muñoz-Mingarro D., Ortega T., Sanahuja M.A., Villagrasa V. Neuroprotective Potential of Ginkgo biloba in Retinal Diseases. Planta Med. 2019;85:1292–1303. doi: 10.1055/a-0947-5712. [DOI] [PubMed] [Google Scholar]
- 57.De Souza G.A., de Marqui S.V., Matias J.N., Elen Landgraf Guiguer E.L., Barbalho S.M. Effects of Ginkgo biloba on Diseases Related to Oxidative Stress. Planta Med. 2020;86:376–386. doi: 10.1055/a-1109-3405. [DOI] [PubMed] [Google Scholar]
- 58.Bedir E., Tatli I.I., Khan R.A., Zhao J., Takamatsu S., Walker L.A., Goldman P., Khan I.A. Biologically Active Secondary Metabolites from Ginkgo biloba. J. Agric. Food Chem. 2002;50:3150–3155. doi: 10.1021/jf011682s. [DOI] [PubMed] [Google Scholar]
- 59.Bajpai V.K., Park I., Lee J., Shukla S., Nile S.H., Chun H.S., Khan I., Oh S.-Y., Huh Y.S., Na M.K., et al. Antioxidant and antimicrobial efficacy of a biflavonoid, amentoflavone from Nandina domestica in vitro and in minced chicken meat and apple juice food models. Food Chem. 2019;271:239–247. doi: 10.1016/j.foodchem.2018.07.159. [DOI] [PubMed] [Google Scholar]
- 60.Li Y.-L., Chen X., Niu S.-Q., Zhou H.-Y., Li Q.-S. Protective Antioxidant Effects of Amentoflavone and Total Flavonoids from Hedyotis diffusa on H2O2-Induced HL-O2 Cells through ASK1/p38 MAPK Pathway. Chem. Biodivers. 2020;17:e2000251. doi: 10.1002/cbdv.202000251. [DOI] [PubMed] [Google Scholar]
- 61.Li X., Ouyang X., Cai R., Chen D. 3′,8″-Dimerization Enhances the Antioxidant Capacity of Flavonoids: Evidence from Acacetin and Isoginkgetin. Molecules. 2019;24:2039. doi: 10.3390/molecules24112039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu S., Yan H., Zhang L., Shan M., Chen P., Ding A., Li S.F.Y. A Review on the Phytochemistry, Pharmacology, and Pharmacokinetics of Amentoflavone, a Naturally-Occurring Biflavonoid. Molecules. 2017;22:299. doi: 10.3390/molecules22020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gan L., Ma J., You G., Mai J., Wang Z., Yang R., Xie C., Fei J., Tang L., Zhao J., et al. Glucuronidation and its effect on the bioactivity of amentoflavone, a biflavonoid from Ginkgo biloba leaves. J. Pharm. Pharmacol. 2020;72:1840–1853. doi: 10.1111/jphp.13247. [DOI] [PubMed] [Google Scholar]
- 64.Ahmed H.H., El-Abhar H.S., Hassanin E.A.K., Abdelkader N.F., Shalaby M. B Ginkgo biloba L. leaf extract offers multiple mechanisms in bridling N -methylnitrosourea–mediated experimental colorectal cancer. Biomed. Pharmacother. 2017;95:387–393. doi: 10.1016/j.biopha.2017.08.103. [DOI] [PubMed] [Google Scholar]
- 65.Silva A.M., Silva S.C., Soares J.P., Martins-Gomes C., Teixeira J.P., Leal F., Gaivão I. Ginkgo biloba L. Leaf Extract Protects HepG2 Cells Against Paraquat-Induced Oxidative DNA Damage. Plants. 2019;8:556. doi: 10.3390/plants8120556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Slika H., Mansour H., Wehbe N., Nasser S.A., Iratni R., Nasrallah G., Shaito A., Ghaddar T., Kobeissy F., Eid A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022;146:112442. doi: 10.1016/j.biopha.2021.112442. [DOI] [PubMed] [Google Scholar]
- 67.Menezes J.C.J.M.D.S., Diederich M.F. Bioactivity of natural biflavonoids in metabolism-related disease and cancer therapies. Pharmacol. Res. 2021;167:105525. doi: 10.1016/j.phrs.2021.105525. [DOI] [PubMed] [Google Scholar]
- 68.Park Y., Woo S.H., Seo S.-K., Kim H., Noh W.C., Lee J.K., Kwon B.-M., Min K.N., Choe T.B., Park I.-C. Ginkgetin induces cell death in breast cancer cells via downregulation of the estrogen receptor. Oncol. Lett. 2017;14:5027–5033. doi: 10.3892/ol.2017.6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.You O.H., Kim S.-H., Kim B., Sohn E.J., Lee H.-J., Shim B.-S., Yun M., Kwon B.-M., Kim S.-H. Ginkgetin induces apoptosis via activation of caspase and inhibition of survival genes in PC-3 prostate cancer cells. Bioorganic Med. Chem. Lett. 2013;23:2692–2695. doi: 10.1016/j.bmcl.2013.02.080. [DOI] [PubMed] [Google Scholar]
- 70.Liu Q., Chen L., Yin W., Nie Y., Zeng P., Yang X. Anti-tumor effect of ginkgetin on human hepatocellular carcinoma cell lines by inducing cell cycle arrest and promoting cell apoptosis. Cell Cycle. 2022;21:74–85. doi: 10.1080/15384101.2021.1995684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pan L.-L., Wu W.-J., Zheng G.-F., Han X.-Y., He J.-S., Cai Z. Ginkgetin inhibits proliferation of human leukemia cells via the TNF-α signaling pathway. Z. Nat. C. 2017;72:441–447. doi: 10.1515/znc-2016-0210. [DOI] [PubMed] [Google Scholar]
- 72.Chiang C.-H., Yeh C.-Y., Chung J.G., Chiang I.-T., Hsu F.-T. Amentoflavone Induces Apoptosis and Reduces Expression of Anti-apoptotic and Metastasis-associated Proteins in Bladder Cancer. Anticancer Res. 2019;39:3641–3649. doi: 10.21873/anticanres.13512. [DOI] [PubMed] [Google Scholar]
- 73.Liu H., Yue Q., He S. Amentoflavone suppresses tumor growth in ovarian cancer by modulating Skp2. Life Sci. 2017;189:96–105. doi: 10.1016/j.lfs.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 74.Lee S., Kim H., Kang J.W., Kim J.H., Lee D.H., Kim M.-S., Yang Y., Woo E.-R., Kim Y.M., Hong J., et al. The Biflavonoid Amentoflavone Induces Apoptosis via Suppressing E7 Expression, Cell Cycle Arrest at Sub-G1 Phase, and Mitochondria-Emanated Intrinsic Pathways in Human Cervical Cancer Cells. J. Med. Food. 2011;14:7–8. doi: 10.1089/jmf.2010.1428. [DOI] [PubMed] [Google Scholar]
- 75.Adnan M., Rasul A., Hussain G., Shah M.A., Zahoor M.K., Anwar H., Sarfraz I., Riaz A., Manzoor M., Adem S., et al. Ginkgetin: A natural biflavone with versatile pharmacological activities. Food Chem. Toxicol. 2020;145:111642. doi: 10.1016/j.fct.2020.111642. [DOI] [PubMed] [Google Scholar]
- 76.Bhadresha K., Upadhyay V., Kumar S.P., Pandya P., Jain N., Rawal R.M. Computational investigation of ginkgetin and theaflavin as potential inhibitors of heat shock protein 90 (Hsp90) J. Biomol. Struct. Dyn. 2021;25:1–7. doi: 10.1080/07391102.2021.1993344. [DOI] [PubMed] [Google Scholar]
- 77.Lou J.-S., Zhao L.-P., Huang Z.-H., Chen X.-Y., Xu J.-T., TAI W.C.-S., Tsim K.W.K., Chen Y.-T., Xie T. Ginkgetin derived from Ginkgo biloba leaves enhances the therapeutic effect of cisplatin via ferroptosis-mediated disruption of the Nrf2/HO-1 axis in EGFR wild-type non-small-cell lung cancer. Phytomedicine. 2021;80:153370. doi: 10.1016/j.phymed.2020.153370. [DOI] [PubMed] [Google Scholar]
- 78.Hu W.-H., Chan G.K.-L., Duan R., Wang H.-Y., Kong X.-P., Dong T.T.-X., Tsim K.W.-K. Synergy of Ginkgetin and Resveratrol in Suppressing VEGF-Induced Angiogenesis: A Therapy in Treating Colorectal Cancer. Cancers. 2019;11:1828. doi: 10.3390/cancers11121828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiong X., Tang N., Lai X., Zhang J., Wen W., Li X., Li A., Wu Y., Liu Z. Insights Into Amentoflavone: A Natural Multifunctional Biflavonoid. Front. Pharmacol. 2021;12:768708. doi: 10.3389/fphar.2021.768708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi S.-K., Oh H.-M., Lee S.-K., Jeong D.G., Ryu S.E., Son K.-H., Han D.C., Sung N.-D., Nam-In Baek N.-I., Kwon B.-M. Biflavonoids inhibited phosphatase of regenerating liver-3 (PRL-3) Nat. Prod. Res. 2006;20:341–346. doi: 10.1080/14786410500463312. [DOI] [PubMed] [Google Scholar]
- 81.Lin Y.-M., Flavin M.T., Schure R., Chen F.-C., Sidwell R., Barnard D.I., Huffmann J.H., Kern E.R. Antiviral Activities of Biflavonoids. Planta Med. 1999;65:120–125. doi: 10.1055/s-1999-13971. [DOI] [PubMed] [Google Scholar]
- 82.Singh A.V. Potential of amentoflavone with antiviral properties in COVID-19 treatment. Asian Biomed. 2021;15:153–159. doi: 10.2478/abm-2021-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hossain R., Islam M.T., Ray P., Jain D., Saikat A.S.M., Nahar L., Calina D. Amentoflavone, New Hope against SARS-CoV-2: An Outlook through its Scientific Records and an in silico Study. Pharm. Res. 2021;13:149–157. doi: 10.5530/pres.13.3.7. [DOI] [Google Scholar]
- 84.Li F., Song X., Su G., Wang Y., Wang Z., Jia J., Qing S., Huang L., Wang Y., Zheng K., et al. Amentoflavone Inhibits HSV-1 and ACV-Resistant Strain Infection by Suppressing Viral Early Infection. Viruses. 2019;11:466. doi: 10.3390/v11050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miki K., Nagai T., Suzuki K., Tsujimura R., Koyama K., Kinoshita K., Furuhata K., Yamada H., Takahashi K. Anti-Influenza Virus Activity of Biflavonoids. Bioorg. Med. Chem. Lett. 2007;17:772–775. doi: 10.1016/j.bmcl.2006.10.075. [DOI] [PubMed] [Google Scholar]
- 86.Haruyama T., Nagata K. Anti-Influenza Virus Activity of Ginkgo Biloba Leaf Extracts. J. Nat. Med. 2013;67:636–642. doi: 10.1007/s11418-012-0725-0. [DOI] [PubMed] [Google Scholar]
- 87.Xiong Y., Zhu G.-H., Wang H.-N., Hu Q., Chen L.-L., Guan X.-Q., Li H.-L., Chen H.-Z., Tang H., Ge G.-B. Discovery of Naturally Occurring Inhibitors against SARS-CoV-2 3CLpro from Ginkgo Biloba Leaves via Large-Scale Screening. Fitoterapia. 2021;152:104909. doi: 10.1016/j.fitote.2021.104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ražná K., Sawinska Z., Ivanišová E., Vukovic N., Terentjeva M., Stričík M., Kowalczewski P.Ł., Hlavačková L., Rovná K., Žiarovská J., et al. Properties of Ginkgo biloba L.: Antioxidant Characterization, Antimicrobial Activities, and Genomic MicroRNA Based Marker Fingerprints. Int. J. Mol. Sci. 2020;21:3087. doi: 10.3390/ijms21093087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hwang J.H., Choi H., Woo E.-R., Lee D.G. Antibacterial Effect of Amentoflavone and Its Synergistic Effect with Antibiotics. J. Microbiol. Biotechnol. 2013;23:953–958. doi: 10.4014/jmb.1302.02045. [DOI] [PubMed] [Google Scholar]
- 90.Lee J., Kim M., Jeong S.E., Yoon Park H., Jeon C.O., Park W. Amentoflavone, a novel cyanobacterial killing agent from Selaginella tamariscina. J. Hazard. Mater. 2019;384:121312. doi: 10.1016/j.jhazmat.2019.121312. [DOI] [PubMed] [Google Scholar]
- 91.Shen X., Niu X., Li G., Deng X., Wang J. Amentoflavone Ameliorates Streptococcus suis-Induced Infection In Vitro and In Vivo. Appl. Environ. Microbiol. 2018;84:e01804-18. doi: 10.1128/AEM.01804-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li G., Wang G., Wang S., Deng Y. Ginkgetin in vitro and in vivo reduces Streptococcus suis virulence by inhibiting suilysin activity. J. Appl. Microbiol. 2019;127:1556–1563. doi: 10.1111/jam.14365. [DOI] [PubMed] [Google Scholar]
- 93.Bagla V.P., McGaw L.J., Elgorashi E.E., Eloff J.N. Antimicrobial activity, toxicity and selectivity index of two biflavonoids and a flavone isolated from Podocarpus henkelii (Podocarpaceae) leaves. BMC Complement. Alter. Med. 2014;14:383. doi: 10.1186/1472-6882-14-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krauze-Baranowska M., Wiwart M. Antifungal Activity of Biflavones from Taxus baccata and Ginkgo biloba. Z. Nat. C. 2003;58:65–69. doi: 10.1515/znc-2003-1-212. [DOI] [PubMed] [Google Scholar]
- 95.Jung H.J., Park K., Lee I.-S., Kim H.S., Yeo S.H., Woo E.R., Lee D.G. S-Phase Accumulation of Candida albicans by Anticandidal Effect of Amentoflavone Isolated from Selaginella tamariscina. Biol. Pharm. Bull. 2007;30:1969–1971. doi: 10.1248/bpb.30.1969. [DOI] [PubMed] [Google Scholar]
- 96.Hwang I.S., Lee J., Jin H.G., Woo E.-R., Lee D.G. Amentoflavone Stimulates Mitochondrial Dysfunction and Induces Apoptotic Cell Death in Candida albicans. Mycopathologia. 2012;173:207–218. doi: 10.1007/s11046-011-9503-x. [DOI] [PubMed] [Google Scholar]
- 97.Kim H.P., Park H., Son K.H., Chang H.W., Kang S.S. Biochemical pharmacology of biflavonoids: Implications for anti-inflammatory action. Arch. Pharm. Res. 2008;31:265. doi: 10.1007/s12272-001-1151-3. [DOI] [PubMed] [Google Scholar]
- 98.Zhou H.-F., Xie C., Jian R., Kang J., Li Y., Zhuang C.-L., Yang F., Zhang L.-L., Lai L., Wu T., et al. Biflavonoids from Caper (Capparis spinosa L.) Fruits and Their Effects in Inhibiting NF-kappa B Activation. J. Agric. Food Chem. 2011;59:3060–3065. doi: 10.1021/jf105017j. [DOI] [PubMed] [Google Scholar]
- 99.Kim H.P., Mani I., Iversen L., Ziboh V.A. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and Iipoxygenase from guinea-pigs. Prostaglandins Lukot. Essent. Fatty Acids. 1998;58:17–24. doi: 10.1016/S0952-3278(98)90125-9. [DOI] [PubMed] [Google Scholar]
- 100.Li M., Li B., Hou Y., Tian Y., Chen L., Liu S., Zhang N., Dong J. Anti-inflammatory effects of chemical components from Ginkgo biloba L. male flowers on lipopolysaccharide-stimulated RAW264.7 macrophages. Phytoter. Res. 2019;33:989–997. doi: 10.1002/ptr.6292. [DOI] [PubMed] [Google Scholar]
- 101.Liu P.-K., Weng Z.-M., Ge G.-B., Li H.-L., Ding L.-L., Dai Z.-R., Hou X.-D., Leng Y.-H., Yu Y., Hou J. Biflavones from Ginkgo biloba as novel pancreatic lipase inhibitors: Inhibition potentials and mechanism. Int. J. Biol. Macromol. 2018;118:2216–2223. doi: 10.1016/j.ijbiomac.2018.07.085. [DOI] [PubMed] [Google Scholar]
- 102.Wang L.-T., Huang H., Chang Y.-H., Wang Y.-Q., Jian-DongWang J.-D., Cai Z.-H., Efferth T., Fu Y.-J. Biflavonoids from Ginkgo biloba leaves as a novel anti-atherosclerotic candidate: Inhibition potency and mechanistic analysis. Phytomedicine. 2022:154053. doi: 10.1016/j.phymed.2022.154053. in press . [DOI] [PubMed] [Google Scholar]
- 103.Dell’Agli M., Galli G.V., Bosisio E. Inhibition of cGMP-Phosphodiesterase-5 By Biflavones of Ginkgo biloba. Planta Med. 2006;72:468–470. doi: 10.1055/s-2005-916236. [DOI] [PubMed] [Google Scholar]
- 104.Kubota Y., Umegaki K., Tanaka N., Mizuno H., Nakamura K., Kunitomo M., Shinozuka K. Safety of Dietary Supplements: Chronotropic and Inotropic Effects on Isolated Rat Atria. Biol. Pharm. Bull. 2002;25:197–200. doi: 10.1248/bpb.25.197. [DOI] [PubMed] [Google Scholar]
- 105.Li W., Li D., Qin Y., Sun C., Wang Y., Gao L., Ling-Hu L., Zhang F., Cai W., Zhu L., et al. Cardioprotective effects of Amentoflavone by suppression of apoptosis and inflammation on an in vitro and vivo model of myocardial ischemia-reperfusion injury. Int. Immunopharmacol. 2021;101:108296. doi: 10.1016/j.intimp.2021.108296. [DOI] [PubMed] [Google Scholar]
- 106.Qin L., Zhao Y., Zhang B., Li Y. Amentoflavone improves cardiovascular dysfunction and metabolic abnormalities in high fructose and fat diet-fed rats. Food Funct. 2018;9:243–252. doi: 10.1039/C7FO01095H. [DOI] [PubMed] [Google Scholar]
- 107.Zhuang J.-L., Liu Y.-Y., Li Z.-Z., Zhuang Q.-Z., Tang W.-Z., Xiong Y., Huang X.-Z. Amentoflavone prevents ox-LDL-induced lipid accumulation by suppressing the PPARγ/CD36 signal pathway. Toxicol. Appl. Pharmacol. 2021;431:115733. doi: 10.1016/j.taap.2021.115733. [DOI] [PubMed] [Google Scholar]
- 108.Uddin S., Kabir T., Tewari D., Mathew B., Aleya L. Emerging Signal Regulating Potential of Small Molecule Biflavonoids to Combat Neuropathological Insults of Alzheimer Disease. Sci. Total Environ. 2020;700:134836. doi: 10.1016/j.scitotenv.2019.134836. [DOI] [PubMed] [Google Scholar]
- 109.Kang S.S., Lee J.Y., Choi Y.K., Song S.S., Kim J.S., Jeon S.J., Han Y.N., Son K.H., Han B.H. Neuroprotective effects of naturally occurring biflavonoids. Bioorganic Med. Chem. Lett. 2005;15:3588–3591. doi: 10.1016/j.bmcl.2005.05.078. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y.-Q., Wang M.-Y., Fu X.-R., Peng-Yu, Gao G.-F., Fan Y.-M., Duan X.-L., Zhao B.-L., Chang Y.Z., Shi Z.-H. Neuroprotective effects of ginkgetin against neuroinjury in Parkinson’s disease model induced by MPTP via chelating iron. Free Radic Res. 2015;49:1069–1080. doi: 10.3109/10715762.2015.1032958. [DOI] [PubMed] [Google Scholar]
- 111.Wang Y., Cheng R., Wu X., Miao M. Neuroprotective and neurotrophic effects of ginkgetin and bilobalide on MPTP-induced mice with Parkinson’ disease. Pharmazie. 2021;76:27–33. [Google Scholar]
- 112.Li Y., Meng L., Li B., Huang D., Huang X., Lin C., Li D., Qiu S., Wu Y., Wei Z., et al. Isoginkgetin attenuates endoplasmic reticulum stress-induced autophagy of brain after ischemic reperfusion injury. Bioengineered. :2021. doi: 10.1080/21655979.2021.1997564. in press . [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 113.Varshney M., Kumar B., Rana V.S., Sethiya N.K. An overview on therapeutic and medicinal potential of poly-hydroxy flavone viz. Heptamethoxyflavone, Kaempferitrin, Vitexin and Amentoflavone for management of Alzheimer’s and Parkinson’s diseases: A critical analysis on mechanistic insight. Crit. Rev. Food Sci. Nutr. 2021 doi: 10.1080/10408398.2021.1980761. in press . [DOI] [PubMed] [Google Scholar]
- 114.Colovic M., Fracasso C., Caccia S. Brain-to-plasma distribution ratio of the biflavone amentoflavone in the mouse. Drug Metab. Lett. 2008;2:90–94. doi: 10.2174/187231208784040988. [DOI] [PubMed] [Google Scholar]
- 115.Ishola I.O., Chatterjee M., Tota S., Tadigopulla N., Adeyemi O.O., Palit G., Shukla R. Antidepressant and anxiolytic effects of amentoflavone isolated from Cnestis ferruginea in mice. Pharmacol. Biochem. Behav. 2012;103:322–331. doi: 10.1016/j.pbb.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 116.Wang J., Chen X., Bai W., Wang Z., Xiao W., Zhu J. Study on Mechanism of Ginkgo biloba L. Leaves for the Treatment of Neurodegenerative Diseases Based on Network Pharmacology. Neurochem. Res. 2021;46:1881–1894. doi: 10.1007/s11064-021-03315-z. [DOI] [PubMed] [Google Scholar]
- 117.Mei N., Guo X., Ren Z., Kobayashi D., Wada K., Guo L. Review of Ginkgo biloba-induced toxicity, from experimental studies to human case reports. J. Environ. Sci. Health. C Environ. Carcinog. Ecotoxicol. Rev. 2017;35:1–28. doi: 10.1080/10590501.2016.1278298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cardoso CR P., de Syllos Cólus I.M., Bernardi C.C., Sannomiya M., Vilegas W., Varanda E.A. Mutagenic activity promoted by amentoflavone and methanolic extract of Byrsonima crassa Niedenzu. Toxicology. 2006;225:55–63. doi: 10.1016/j.tox.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 119.Lv X., Zhang J.B., Wang X.X., Hu W.-Z., Shi Y.-S., Liu S.-W., Hao D.-C., Zhang W.-D., Ge G.-B., Hou J., et al. Amentoflavone is a potent broad-spectrum inhibitor of human UDP-glucuronosyltransferases. Chem. Biol. Interact. 2018;284:48–55. doi: 10.1016/j.cbi.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 120.Wang X.X., Hou J., Ning J., Pan Y.-q., Hong M., Guo B. Inhibition of sciadopitysin against UDP-glucuronosyltransferases. Acta Pharm. Sin. 2016;51:749–755. [PubMed] [Google Scholar]
- 121.Li Y.-Y., Lu X.-Y., Sun J.-L., Wang Q.-Q., Zhang Y.-D., Zhang J.-B., Fan X.-H. Potential hepatic and renal toxicity induced by the biflavonoids from Ginkgo biloba. Chin. J. Nat. Med. 2019;17:672–681. doi: 10.1016/S1875-5364(19)30081-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.