Abstract
Vestibular schwannoma (VS) is a benign tumor that originates from Schwann cells in the vestibular component. Surgical treatment for VS has gradually declined over the past few decades, especially for small tumors. Gamma knife radiosurgery has become an accepted treatment for VS, with a high rate of tumor control. For neurofibromatosis type 2 (NF2)-associated VS resistant to radiotherapy, vascular endothelial growth factor (VEGF)-A/VEGF receptor (VEGFR)-targeted therapy (e.g., bevacizumab) may become the first-line therapy. Recently, a clinical trial using a VEGFR1/2 peptide vaccine was also conducted in patients with progressive NF2-associated schwannomas, which was the first immunotherapeutic approach for NF2 patients. Targeted therapies for the gene product of SH3PXD2A-HTRA1 fusion may be effective for sporadic VS. Several protein kinase inhibitors could be supportive to prevent tumor progression because merlin inhibits signaling by tyrosine receptor kinases and the activation of downstream pathways, including the Ras/Raf/MEK/ERK and PI3K/Akt/mTORC1 pathways. Tumor-microenvironment-targeted therapy may be supportive for the mainstays of management. The tumor-associated macrophage is the major component of immunosuppressive cells in schwannomas. Here, we present a critical overview of targeted therapies for VS. Multimodal therapy is required to manage patients with refractory VS.
Keywords: schwannoma, NF2, bevacizumab, VEGF, SH3PXD2A-HTRA1 fusion, molecular targeted therapy
1. Introduction
Schwann cells originate from neural crest cells, which migrate with growing neurites during nerve development. Schwann cells, which form the myelin sheath of an axon, support neuronal function and regeneration [1].
Schwannoma (Sch) is one of the common benign intracranial tumors with an incidence of 1 per 100,000 [2]. Sch often presents between the ages of 40 and 60 years [2]. Among these cases, 80–90% originate from the vestibular nerve. About 5–10% of vestibular Schs (VSs) are observed as bilateral in neurofibromatosis 2 (NF2) patients. A total of 95% of NF2 patients show bilateral VSs [3]. About 60% of unilateral VSs and 90% of bilateral VSs show NF2 gene mutation and the dysfunction of its transcription product, moesin–ezrin–radixin-like (merlin) protein [4].
Currently, the mainstays of management are observation, surgery, and radiosurgery. Surgery with facial and auditory monitoring remains the only curative treatment for growing VSs of all sizes. Stereotactic radiosurgery is considered as a widely accepted treatment option for small-sized VSs. For larger tumors, combined treatment strategies are mostly recommended. In particular, gamma knife radiosurgery (GKRS) has become an accepted treatment for VS [5]. However, additional treatment is needed for some refractory cases. Tumor volume ≥15 cm3 is a significant factor predicting poor tumor control following GKRS [6]. There is no approved medical therapy for VS. For refractory VS with high risks of surgical treatment or GKRS, medical therapies that can slow tumor growth are urgently needed. Here, we review the molecular biology and its relevance to treatment for VS.
2. NF2 Gene
NF2 is an autosomal-dominant disease caused by a biallelic loss of the NF2 gene on chromosome 22. Although 50% of NF2 patients have an affected parent with the disease, the remaining 50% have de novo gene mutations [7].
Although 60% of patients with de novo NF2 show mosaic NF2, the actual diagnostic rate of this condition remains low at 20% because of the difficulties in detecting NF2 variants with a low variant allele frequency [8]. Teranishi et al. improved the diagnostic rate of mosaic NF2 using targeted deep sequencing of DNA. The mosaic NF2 phenotype was found to be different from that in the NF2 germline variant in terms of tumor growth and hearing outcome [8].
Differentiated Schwann cells become quiescent because merlin regulates this contact-dependent inhibition of proliferation. Merlin plays a significant role in regulating the actin cytoskeleton, adhesion junction formation, and cell proliferation [9]. Merlin can regulate multiple tumorigenic pathways, including retrovirus-associated DNA sequences (Ras)/rapidly accelerated fibrosarcoma (Raf)/mitogen extracellular signal-regulated kinase (MEK)/extracellular-signal-regulated kinases (ERK), and mammalian target of rapamycin complex 1 (mTORC1)/phosphoinositide 3-kinase (PI3K)/Akt [10,11].
3. SH3PXD2A-HTRA1 Fusion
In 2016, alternative tumorigenic mechanisms were proposed, including a recurrent in-frame fusion transcript of the HTRA1 and SH3PXD2A genes. The gene product of SH3PXD2A-HTRA1 fusion promotes proliferation and invasion. In a previous study, the frequency of this fusion gene was investigated [12]. The fusion gene SH3PXD2A-HTRA1, activating the MAPK pathway, has been associated with 10% of sporadic Schs. Agnihotri et al. suggested that SH3PXD2A-HTRA1 fusion promoted tumorigenesis and sensitivity to an MEK-ERK inhibitor [12]. Even though SH3PXD2A-HTRA1 fusion has been shown to be a driver of tumorigenesis, the fusion transcript was extremely rare in Norwegian sporadic VS patients [13]. Further investigation is warranted to elucidate the importance of this fusion gene.
4. Protein-Kinase-Related Pathway
4.1. VEGF-A/VEGFRs
The vascular endothelial growth factor receptor (VEGFR) family mainly includes VEGFR-1 (Flt-1), VEGFR-2 (Flk-1/KDR), and VEGFR-3 (Flt-4), which are important regulators of physiological and pathological angiogeneses [14]. Merlin deletion leads to the downregulation of the protein semaphorin 3F, which inhibits VEGF-mediated angiogenesis [15]. A previous study has shown that the concentrations of VEGF-A and VEGFR-1 are related to the growth rate of VS [16].
Tumor shrinkage and hearing improvement have been identified after the administration of bevacizumab (anti-VEGF-A antibody) in about 41% and 20% of progressive VSs in NF2 patients, respectively [17]. Bevacizumab may be considered as first-line medical therapy for rapidly growing VS. In a recent meta-analysis, the median treatment duration was 16 months [18,19]. Recently, the first phase III randomized clinical trial using bevacizumab was conducted in Japan [20,21]. Furthermore, progressive sporadic VS also exhibited significant tumor shrinkage after bevacizumab administration [22].
However, some aspects of bevacizumab treatment are problematic, such as the need for frequent parenteral administration, side effects, apparent drug resistance, and rebound tumor progression [23]. In the majority of published case series of bevacizumab usage for VS, their conclusions on efficacy were based on relatively short follow-ups. Long-term follow-up studies using a large number of patients are warranted. A clinical trial using a VEGFR-1/2 peptide vaccine was also conducted in patients with progressive NF2-derived Schs, showing hearing improvement and tumor volume reduction. Memory cytotoxic T lymphocytes have the possibility to persist in the long-term [24]. This was the first immunotherapeutic approach for NF2 patients.
4.2. ErbB
The ErbB family’s cell membrane receptors include the epidermal growth factor receptor (EGFR) (HER1/ErbB-1), HER2 (neu/ErbB-2), HER3 (ErbB-3), and HER4 (ErbB-4). MAPK/ERK and PI3K/Akt signaling pathways are considerably downstream of ErbB-2 activation [25]. ErbB receptors were activated in both sporadic and NF2-related VSs, and EGFR expression levels correlated with Sch size [26]. Furthermore, EGF was upregulated in NF2-related VS but not in sporadic VS, suggesting that an EGFR inhibitor might have efficacy in NF2 patients [27,28].
The predominant ErbB receptor dimerization patterns in VS are EGFR and ErbB2 heterodimers [29]. Trastuzumab, a humanized anti-ErbB2 monoclonal antibody, could significantly reduce tumor growth; however, this antibody did not induce significant cell death in VS xenografts [29].
Lapatinib is a potent and reversible tyrosine kinase inhibitor, showing a dual inhibitory effect on the EGF activation of EGFR/ErbB2 [30]. A phase II clinical trial showed that lapatinib has minor toxicity and the minor effects of reducing tumor volume and improving hearing in NF2-related progressive VS [30]. This treatment failure was due to the ErbB3 upregulation caused by the inhibition of ErbB2. Erlotinib is a reversible, small-molecule EGFR-specific tyrosine kinase inhibitor [30]. However, erlotinib was ineffective in NF2-related VSs for tumor shrinkage and improving hearing outcome. Bevacizumab has shown better benefits in the treatment of NF2 patients compared with lapatinib and erlotinib [31].
4.3. PDGFR
Platelet-derived growth factor (PDGF) regulated the migration of mesenchymal stem cells via PI3K signaling [32]. The PDGF receptor (PDGFR) family includes PDGFR-α, PDGFR-β, colony-stimulating factor1 receptor (CSF1-R), fetal liver kinase-2 (Flk-2), and c-kit [32]. Compared with normal nerves, the expressions of c-kit, PDGFR-α, and PDGFR-β are increased in sporadic and NF2-related VSs [33]. Imatinib mesylate (STI571) is an inhibitor of the BCR-ABL fusion kinase for chronic myelogenous leukemia [34]. Imatinib mesylate inhibits the activation of c-KIT, PDGFR-α, and PDGFR-β and their downstream signaling pathways, leading to increased apoptosis in the immortalized NF2-null VS cell line. Moreover, imatinib has an inhibitory effect for angiogenesis in both sporadic and NF2-related VSs [35].
Nilotinib (Bcr-Abl tyrosine kinase inhibitor) is 10–30-fold more potent than imatinib in inhibiting Bcr-Abl tyrosine kinase activity and proliferation [36]. Nilotinib also inhibited cell proliferation more effectively compared with imatinib in Sch cell lines. Anti-tumor effects were related to the inhibition of PDGFR-α and PDGFR-β, as well as their downstream signaling mediators, Akt and mTOR [36].
Ponatinib inhibits SRC, fibroblast growth factor receptor (FGFR), PDGFR, and VEGFR1–3, stimulating a robust G1 cell cycle arrest of merlin-deficient human Schwann cells [37]. However, in the clinical setting, targeting PDGF/PDGFR signaling did not show significant benefits in the treatment of NF2 patients.
4.4. HGFR
The hepatocyte growth factor receptor (HGFR), known as c-mesenchymal–epithelial transition (c-MET), is a glycosylated receptor tyrosine kinase and plays a role in driving tumorigenesis [38,39]. The activation of the HGF/c-MET pathway in sporadic VS can promote the inflammation network and cancer progression [40]. This pathway can also protect cells from apoptosis induced by chemotherapy or radiotherapy through PI3K/Akt signaling [41].
Therefore, crizotinib (a c-MET and anaplastic lymphoma kinase inhibitor) can enhance the radiation-induced DNA damage of NF2-related Sch cells, enhancing radio sensitivity. This effect leads to a reduction in radiation dose and protects hearing [42]. A phase II clinical trial using crizotinib for NF2 and progressive sporadic VSs in children and adults is ongoing (NCT04283669). The simultaneous use of the c-MET inhibitor “cabozantinib” and the Src inhibitor “saracatinib” can reduce the viability of human VS cells with he NF2 mutation, which is more effective than using either inhibitor alone [43].
There is a crosstalk between c-MET and VEGF-A in VSs. Sonam et al. found that c-MET and VEGF-A protein levels decreased using c-MET-targeted siRNA, while VEGF-A- targeted siRNA reduced c-MET expression. The combined inhibition of VEGF-A and c-MET may be an effective therapy [40].
4.5. PI3K/Akt/mTOR
PI3K/Akt/mTOR signaling contributes to a variety of processes that are critical in anabolic reactions and cell growth and survival. PI3K/Akt/mTOR signaling is elevated in VS [44]. Therefore, the PI3K/Akt pathway is also an attractive treatment target for VS [44].
OSU-03012 is an ATP-competitive inhibitor of PAK activity and suppresses the phosphorylation of Akt, which inhibits VS cell growth and promotes apoptosis [45]. Additionally, OSU-HDAC42 (AR-42), a novel phenylbutyrate-derived histone deacetylase inhibitors, can inhibit the downstream Akt expression of PI3K through protein phosphatase-1-mediated Akt dephosphorylation, showing the effect of G2 cell cycle arrest and cell apoptosis in a VS animal model [46].
mTOR is a downstream signal of the PI3K/Akt pathway [47]. A previous study has shown that an mTORC1 inhibitor (rapamycin) can inhibit the growth of merlin-deficient tumors in vivo. Rapamycin can lead to tumor shrinkage in NF2 patients with growing VSs [48].
Everolimus (RAD001), a derivative of rapamycin, can inhibit mTORC1 and reduce tumor angiogenesis. Although a phase II study has shown that everolimus is ineffective in progressive NF2-related VS patients [49], another study has shown that everolimus reduced the tumor volume in 55.6% of patients with NF2-related VS [50,51]. The effect of everolimus is still debatable.
5. Cytokines and Chemokines
C-X-C motif chemokine ligand 12 (CXCL12) binds to C-X-C chemokine receptor type 4 (CXCR4). The CXCL12/CXCR4 axis plays a pivotal role in tumor development, survival, angiogenesis, metastasis, and the tumor microenvironment. In addition, this chemokine axis promotes chemoresistance in cancer therapy. CXCR4 is also considered to be correlated with the tumorigenesis and functional disturbance of sporadic and NF2-related VSs [52]. CXCR4-directed positron emission tomography/computed tomography imaging with radiolabeled CXCR4-targeted ligand [68Ga]-Pentixafor was used to evaluate CXCR4 expression in VS patients [53]. These results provide a possibility for the use of Plerixafor (AMD3100) as a CXCR4-targeting drug [52,53].
Multiple cytokines and chemokines, including CXCL12, CXCL16, interleukin (IL)-1β, IL-6, IL-34, macrophage colony-stimulating factor (M-CSF), and tumor necrosis factor-α (TNF-α), are also associated with tumor progression and hearing disturbance [54].
In addition to the direct compression of auditory nerve fibers by tumors, in cases of NF2-associated deafness, detrimental paracrine substances, such as proinflammatory cytokines from tumors, have been proposed as a mechanism of cochlear hearing loss [55]. A novel therapeutic strategy targeting cytokines and chemokines may support other treatment strategies.
6. Tumor Microenvironment
Sch consists of different cell types, including tumorigenic Schwann cells, axons, macrophages, T cells, fibroblasts, blood vessels, and an extracellular matrix. The tumor microenvironment plays a relevant role in the development and progression of Sch. There are few studies regarding the tumor microenvironment in Sch [56,57].
Fast-growing VSs expressed high M-CSF and IL-34 levels that could regulate the chemotaxis of tumor-associated macrophages (TAMs). TAMs produce growth factors and anti-inflammatory cytokines to suppress the host immune response, resulting in tumor progression. VEGF in the hypoxic tumor microenvironment is a key factor for transitioning from the M1 to the M2 macrophage phenotype [58]. A greater TAM infiltration was found in growing sporadic VSs compared with non-growing sporadic VSs [54,59,60].
Programmed death-1 (PD-1) is expressed on CD8+T cells. Programmed death-ligand 1 (PD-L1) is expressed on tumor cells in numerous malignant tumors and binds to PD-1 to negatively regulate the immune response of CD8+T cells [58]. In 11 NF2-associated Schs, both high levels of programmed death-ligand 1 (PD-L1) expression and the presence of TAMs and T lymphocytes were identified in nearly all specimens [61]. In another study of 44 sporadic Schs, an increased presence of TAMs and an elevated PD-L1 expression were significantly associated with tumor progression [62].
Regulatory T cells (Tregs) (CD4 + CD25 + Foxp3+) play an active and significant role in the progression of tumors, and they play an important role in suppressing tumor-specific immunity [58]. In NF2 patients, the number of Foxp3-positive cells in Sch with a progressive course was significantly higher than in those without a progressive course, suggesting that growth may be associated with Foxp3-positive Tregs [24,63].
A previous study investigated the hypoxic tumor microenvironment of patients with NF2 Sch. Hypoxia was important for the shorter progression-free survival of NF2 Sch [59]. An immunotherapy that specifically targets the tumor microenvironment may emerge as a new class of Sch therapeutics.
7. Inflammation and Stress Reaction
7.1. COX2
The expression of cyclooxygenase 2 (COX-2) is associated with sporadic and NF2-related VS proliferation. Mutations in the NF2 gene can activate the Hippo pathway, in which YAP can promote the transcription of COX-2 for prostaglandin production. Prostaglandin E2 (PGE2) catalyzed by COX-2 has multiple roles in cell proliferation, apoptosis, angiogenesis, inflammation, and immune monitoring. COX-2 inhibitors may have the potential to inhibit the growth of VS [64,65].
A negative correlation between aspirin users and sporadic VS growth has been demonstrated [66,67]. In addition to inhibiting COX-2, aspirin can also suppress the activated NF-κB pathway in VS, which may be another potential mechanism. However, other studies demonstrated that there is no growth inhibitory effect for celecoxib on NF2-related VS or aspirin on sporadic VS [66,67]. Other studies have shown that NSAIDs, glucocorticoids, and other immunosuppressive drugs could not alter the expression of COX-2 in sporadic Sch [68].
7.2. Hsp90
Heat shock protein 90 (HSP90) is a ubiquitous molecule. The absence of Hsp90 results in proteasomal degradation [69]. The dysregulation of the Hippo pathway is necessary for schwannomagenesis, and MAPK signaling acts as a modifier for Sch formation. Furthermore, the pharmacological co-inhibition of YAP/TAZ transcriptional activity and MAPK signaling shows a synergistic size reduction in a mouse Sch model [70].
In a recent study, a novel small-molecule inhibitor compound of HSP90, NXD30001 (pochoxime A), was able to show reduced growth of NF2-deficient tumors in vivo. There are no current clinical trials using an HSP90 inhibitor [71].
The molecular patterns and mutations described for VS are summarized in Table 1.
Table 1.
Molecular patterns and mutations currently described for VS.
| Targeted Pathway | |
|---|---|
| NF2 (merlin)-related pathway | |
| 1 | Ras/Raf/MEK/ERK signaling |
| 2 | PI3K/Akt/mTORC1 signaling |
| SH3PXD2A-HTRA1-fusion-related pathway | |
| 1 | MAPK signaling |
| Protein-kinase-related pathway | |
| 1 | VEGF-A/VEGFR signaling |
| 2 | ErbB family signaling |
| 3 | PDGF/PDGFR signaling |
| 4 | HGF/HGFR (c-MET) signaling |
| Cytokines and chemokines | |
| 1 | CXCL12/CXCR4 signaling |
| 2 | IL-1β, IL-6, IL-34, M-CSF, TNF-α |
| Tumor microenvironment | |
| 1 | Tumor-associated macrophage |
| 2 | Regulatory T cell |
| 3 | PD-1/PD-L1 |
| 4 | Hypoxia |
| Inflammation and stress reaction | |
| 1 | COX2 |
| 2 | Hsp90 |
c-MET, c-mesenchymal–epithelial transition; COX2, cyclooxygenase 2; CXCL12, C-X-C motif chemokine ligand 12; CXCR4, C-X-C chemokine receptor type 4; ERK, extracellular-signal-regulated kinases; HGFR, hepatocyte growth factor receptor; Hsp90, heat shock protein 90; IL, interleukin; MAPK, mitogen-activated protein kinase; M-CSF, macrophage colony-stimulating factor; MEK, mitogen extracellular signal-regulated kinase; mTORC1, mammalian target of rapamycin complex 1; NF, neurofibromatosis; PDGFR, platelet-derived growth factor; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; PI3K, phosphoinositide 3-kinase; Raf, rapidly accelerated fibrosarcoma; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor.
8. Drug Repositioning
Mifepristone (RU486), a progesterone and glucocorticoid receptor antagonist that has already been approved for medical abortion, was chosen as the most promising candidate drug [72]. In a preclinical study, mifepristone reduced cellular proliferation in primary human VS cultures regardless of NF2 mutation. A phase II clinical trial on mifepristone in VS is currently being planned [72].
In VS, genes associated with NLRP3 were significantly upregulated in patients with poor hearing. NLRP3 mutation is associated with cochlear autoinflammation in conjunction with DFNA34-mediated hearing loss and age-rated hearing loss. The activation of NLRP3 triggers the production of IL-1β [73]. A recombinant human IL-1 receptor antagonist reversed the hearing loss observed in a family with sensorineural hearing loss and NLRP3 mutations [54].
9. Gene Therapy
Gene therapy offers the potential to treat a wide range of inherited and acquired human diseases. The direct modulation of affected genes in specific cell types represents the most powerful treatment strategy for NF2 patients. Delivery platforms typically include viral vectors, such as retroviruses, adenoviruses, and adeno-associated viruses (AAVs), as well as nonviral vectors, including nanoparticles and polymers [74].
A direct injection of an AAV serotype 1 vector encoding caspase-1 (ICE) under the Schwann-cell specific promoter led to the regression of Sch in a mouse model. Recently, a direct injection of AAV1 encoding the apoptosis-associated speck-like protein reduced tumor growth and resolved tumor-associated pain in a human xenograft Sch model [75].
Nonviral vectors, such as liposomal-, polymeric-, and peptide-based nanoparticles, offer an attractive alternative for gene delivery. Liposomes were used to deliver genome-editing agents to the cochlea of neonatal mice with dominant genetic deafness. By decorating the nanoparticle surface with a peptide targeting Schwann cells, peptide-based nanoparticles were used to deliver genetic materials, resulting in a decreased secretion of an ototoxic inflammatory cytokine from tumor cells [76].
10. Ongoing Clinical Trials
Table 2 shows ongoing clinical trials using multimodal treatment strategies for Sch. The superselective intraarterial infusion of bevacizumab is performed to control tumor progression (NCT01083966). Because of the promising results found with bevacizumab, it may be safely used by direct intracranial superselective intraarterial infusion up to a dose of 10mg/kg in order to enhance survival and hearing function. Another six trials are using medical treatment strategies. Crizotinib, AR-42 (OSU-HDAC42), everolimus, selumetinib (MEK 1/2 inhibitor), and tanezumab (a monoclonal antibody against nerve growth factor as a treatment for pain) are being evaluated in the trials. A previous meta-analysis suggests that there is insufficient evidence to recommend aspirin usage in patients with VS [77,78]. High-quality trials are warranted to determine the efficacy of aspirin in reducing VS growth (NCT03079999).
Table 2.
Active and recruiting clinical trials using medical therapeutic approaches for schwannoma.
| ClinicalTrials.Gov Identifier | ID | RP | EE | Age | TS |
|---|---|---|---|---|---|
| NCT01083966 | 8, 2011 | Lenox Hill Brain Tumor Center | 30 | ≥18 | Superselective intraarterial intracranial infusion of bevacizumab |
| NCT04283669 | 2, 2020 | University of Alabama at Birmingham | 19 | ≥6 | Crizotinib |
| NCT03079999 | 6, 2018 | Massachusetts Eye and Ear Infirmary | 300 | ≥12 | Aspirin |
| NCT02282917 | 9, 2015 | Massachusetts Eye and Ear | 5 | ≥18 | AR-42 (OSU-HDAC42) |
| NCT01345136 | 7, 2015 | University of California | 4 | 16–65 | Everolimus |
| NCT03095248 | 5, 2017 | Children’s Hospital Medical Center | 34 | 3–45 | Selumetinib |
| NCT04163419 | 4, 2020 | Massachusetts General Hospital | 46 | ≥18 | Tanezumab |
ER, estimated enrollment; ID, initiation date; RP, responsible party; TS, treatment strategy.
11. Future Direction
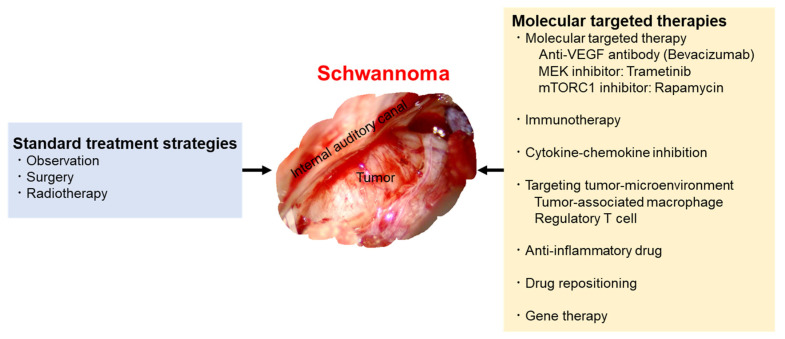
Bevacizumab has recently been considered as the first-line medical therapy for rapidly growing VS. Furthermore, new therapeutic strategies targeting the SH3PXD2A-HTRA1 fusion gene, several protein kinases, and the tumor microenvironment may be supportive for the mainstays of management. An immunotherapeutic approach may also be needed to control multiple tumor progression in the long term. In addition to the standard treatment strategy, including surgery and radiotherapy, these targeted medical therapies are needed for multiple and large tumors of VS (Figure 1). Multimodal therapy is required to manage patients with refractory VS.
Figure 1.
Multimodal treatment and management strategies.
The mainstays of management are observation, surgery, and radiation therapy. Bevacizumab has recently been considered as the first-line medical therapy for rapidly growing vestibular schwannomas. Furthermore, new therapeutic strategies targeting the SH3PXD2A-HTRA1 fusion gene, several protein kinases, and the tumor microenvironment may be supportive for the mainstays of management.
Author Contributions
Conception and Design: R.T.; Manuscript Writing: R.T.; Manuscript Reviewing: M.T.; Supervision: M.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no potential conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jessen K.R., Mirsky R., Lloyd A.C. Schwann Cells: Development and Role in Nerve Repair. Cold Spring Harb. Perspect. Biol. 2015;7:a020487. doi: 10.1101/cshperspect.a020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher J.L., Pettersson D., Palmisano S., Schwartzbaum J., Edwards C.G., Mathiesen T., Prochazka M., Bergenheim T., Florentzson R., Harder H., et al. Loud Noise Exposure and Acoustic Neuroma. Am. J. Epidemiol. 2014;180:58–67. doi: 10.1093/aje/kwu081. [DOI] [PubMed] [Google Scholar]
- 3.Slattery W.H. Neurofibromatosis type 2. Otolaryngol. Clin. N. Am. 2015;48:443–460. doi: 10.1016/j.otc.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Evans D.G.R., Ramsden R.T., Shenton A., Gokhale C., Bowers N.L., Huson S.M., Pichert G., Wallace A. Mosaicism in neurofibromatosis type 2: An update of risk based on uni/bilaterality of vestibular schwannoma at presentation and sensitive mutation analysis including multiple ligation-dependent probe amplification. J. Med. Genet. 2007;44:424–428. doi: 10.1136/jmg.2006.047753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao L., Alahmari M., Temel Y., Hovinga K. Therapy of Sporadic and NF2-Related Vestibular Schwannoma. Cancers. 2020;12:835. doi: 10.3390/cancers12040835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C.W., Tu H.T., Chuang C.Y., Chang C.S., Chou H.H., Lee M.T., Huang C.F. Gamma Knife radiosurgery for large vestibular schwannomas greater than 3 cm in diameter. J. Neurosurg. 2018;128:1380–1387. doi: 10.3171/2016.12.JNS161530. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z.-Y., Wu Y.-Y., Cai X.-Y., Fang W.-L., Xiao F.-L. Molecular Diagnosis of Neurofibromatosis by Multigene Panel Testing. Front. Genet. 2021;12:603195. doi: 10.3389/fgene.2021.603195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teranishi Y., Miyawaki S., Hongo H., Dofuku S., Okano A., Takayanagi S., Ota T., Yoshimura J., Qu W., Mitsui J., et al. Targeted deep sequencing of DNA from multiple tissue types improves the diagnostic rate and reveals a highly diverse phenotype of mosaic neurofibromatosis type 2. J. Med. Genet. 2021;58:701–711. doi: 10.1136/jmedgenet-2020-106973. [DOI] [PubMed] [Google Scholar]
- 9.Gladden A.B., Hebert A.M., Schneeberger E.E., McClatchey A.I. The NF2 Tumor Suppressor, Merlin, Regulates Epidermal Development through the Establishment of a Junctional Polarity Complex. Dev. Cell. 2010;19:727–739. doi: 10.1016/j.devcel.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pećina-Šlaus N. Merlin, the NF2 Gene Product. Pathol. Oncol. Res. 2013;19:365–373. doi: 10.1007/s12253-013-9644-y. [DOI] [PubMed] [Google Scholar]
- 11.Santarpia L., Lippman S.M., El-Naggar A.K. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin. Ther. Targets. 2012;16:103–119. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agnihotri S., Jalali S., Wilson M.R., Danesh A., Li M., Klironomos G., Krieger J.R., Mansouri A., Khan O., Mamatjan Y., et al. The genomic landscape of schwannoma. Nat. Genet. 2016;48:1339–1348. doi: 10.1038/ng.3688. [DOI] [PubMed] [Google Scholar]
- 13.Taule-Sivertsen P., Bruland O., Håvik A.L., Bratland E., Lund-Johansen M., Knappskog P.M. The SH3PXD2A-HTRA1 fusion transcript is extremely rare in Norwegian sporadic vestibular schwannoma patients. J. Neuro-Oncol. 2021;154:35–40. doi: 10.1007/s11060-021-03796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simons M., Gordon E., Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016;17:611–625. doi: 10.1038/nrm.2016.87. [DOI] [PubMed] [Google Scholar]
- 15.Wong H.K., Shimizu A., Kirkpatrick N.D., Garkavtsev I., Chan A.W., di Tomaso E., Klagsbrun M., Jain R.K. Merlin/NF2 regulates angiogenesis in schwannomas through a Rac1/semaphorin 3F-dependent mechanism. Neoplasia. 2012;14:84–94. doi: 10.1593/neo.111600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uesaka T., Shono T., Suzuki S.O., Nakamizo A., Niiro H., Mizoguchi M., Iwaki T., Sasaki T. Expression of VEGF and its receptor genes in intracranial schwannomas. J. Neuro-Oncol. 2007;83:259–266. doi: 10.1007/s11060-007-9336-0. [DOI] [PubMed] [Google Scholar]
- 17.Plotkin S.R., Duda D.G., Muzikansky A., Allen J., Blakeley J., Rosser T., Campian J.L., Clapp D.W., Fisher M.J., Tonsgard J., et al. Multicenter, Prospective, Phase II and Biomarker Study of High-Dose Bevacizumab as Induction Therapy in Patients with Neurofibromatosis Type 2 and Progressive Vestibular Schwannoma. J. Clin. Oncol. 2019;37:3446–3454. doi: 10.1200/JCO.19.01367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi J., Lu D., Gu R., Sun H., Yu L., Pan R., Zhang Y. Reliability and toxicity of bevacizumab for neurofibromatosis type 2-related vestibular schwannomas: A systematic review and meta-analysis. Am. J. Otolaryngol. 2021;42:103148. doi: 10.1016/j.amjoto.2021.103148. [DOI] [PubMed] [Google Scholar]
- 19.Morris K.A., Golding J.F., Axon P.R., Afridi S., Blesing C., Ferner R.E., Halliday D., Jena R., Pretorius P.M., Evans G., et al. Bevacizumab in neurofibromatosis type 2 (NF2) related vestibular schwannomas: A nationally coordinated approach to delivery and prospective evaluation. Neuro-Oncol. Pract. 2016;3:281–289. doi: 10.1093/nop/npv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujii M., Ichikawa M., Iwatate K., Bakhit M., Yamada M., Kuromi Y., Sato T., Sakuma J., Saito K. Bevacizumab Therapy of Neurofibromatosis Type 2 Associated Vestibular Schwannoma in Japanese Patients. Neurol. Med. Chir. 2020;60:75–82. doi: 10.2176/nmc.oa.2019-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii M., Kobayakawa M., Saito K., Inano A., Morita A., Hasegawa M., Mukasa A., Mitsuhara T., Goto T., Yamaguchi S., et al. Rationale and Design of BeatNF2 Trial: A Clinical Trial to Assess the Efficacy and Safety of Bevacizumab in Patients with Neurofibromatosis Type 2 Related Vestibular Schwannoma. Curr. Oncol. 2021;28:726–739. doi: 10.3390/curroncol28010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karajannis M.A., Hagiwara M., Schreyer M., Haque S. Sustained imaging response and hearing preservation with low-dose bevacizumab in sporadic vestibular schwannoma. Neuro-Oncology. 2019;21:822–824. doi: 10.1093/neuonc/noz051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura R., Tanaka T., Miyake K., Yoshida K., Sasaki H. Bevacizumab for malignant gliomas: Current indications, mechanisms of action and resistance, and markers of response. Brain Tumor Pathol. 2017;34:62–77. doi: 10.1007/s10014-017-0284-x. [DOI] [PubMed] [Google Scholar]
- 24.Tamura R., Fujioka M., Morimoto Y., Ohara K., Kosugi K., Oishi Y., Sato M., Ueda R., Fujiwara H., Hikichi T., et al. A VEGF receptor vaccine demonstrates preliminary efficacy in neurofibromatosis type 2. Nat. Commun. 2019;10:5758. doi: 10.1038/s41467-019-13640-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wee P., Wang Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers. 2017;9:52. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad Z.K., Brown C.M., Cueva R.A., Ryan A.F., Doherty J.K. ErbB Expression, Activation, and Inhibition with Lapatinib and Tyrphostin (AG825) in Human Vestibular Schwannomas. Otol. Neurotol. 2011;32:841–847. doi: 10.1097/MAO.0b013e31821f7d88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doherty J.K., Ongkeko W., Crawley B., Andalibi A., Ryan A.F. ErbB and Nrg: Potential Molecular Targets for Vestibular Schwannoma Pharmacotherapy. Otol. Neurotol. 2008;29:50–57. doi: 10.1097/mao.0b013e31815d4429. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y., Long J., Ren J., Huang X., Zhong P., Wang B. Potential Molecular Biomarkers of Vestibular Schwannoma Growth: Progress and Prospects. Front. Oncol. 2021;11:731441. doi: 10.3389/fonc.2021.731441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark J.J., Provenzano M., Diggelmann H.R., Xu N., Hansen S.S., Hansen M.R. The ErbB inhibitors trastuzumab and erlotinib inhibit growth of vestibular schwannoma xenografts in nude mice: A preliminary study. Otol. Neurotol. 2008;29:846–853. doi: 10.1097/MAO.0b013e31817f7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ammoun S., Cunliffe C.H., Allen J., Chiriboga L., Giancotti F.G., Zagzag D., Hanemann C.O., Karajannis M.A. ErbB/HER receptor activation and preclinical efficacy of lapatinib in vestibular schwannoma. Neuro-Oncology. 2010;12:834–843. doi: 10.1093/neuonc/noq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plotkin S.R., Halpin C., McKenna M.J., Loeffler J.S., Batchelor T.T., Barker F.G., 2nd Erlotinib for progressive vestibular schwannoma in neurofibromatosis 2 patients. Otol. Neurotol. 2010;31:1135–1143. doi: 10.1097/MAO.0b013e3181eb328a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salha S., Gehmert S., Brébant V., Anker A., Loibl M., Prantl L., Gehmert S. PDGF regulated migration of mesenchymal stem cells towards malignancy acts via the PI3K signaling pathway. Clin. Hemorheol. Microcirc. 2018;70:543–551. doi: 10.3233/CH-189319. [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee J., Kamnasaran D., Balasubramaniam A., Radovanovic I., Zadeh G., Kiehl T.-R., Guha A. Human Schwannomas Express Activated Platelet-Derived Growth Factor Receptors and c-kit and Are Growth Inhibited by Gleevec (Imatinib Mesylate) Cancer Res. 2009;69:5099–5107. doi: 10.1158/0008-5472.CAN-08-4475. [DOI] [PubMed] [Google Scholar]
- 34.O’Dwyer M.E., Druker B.J. STI571: An inhibitor of the BCR-ABL tyrosine kinase for the treatment of chronic myelogenous leukaemia. Lancet Oncol. 2000;1:207–211. doi: 10.1016/S1470-2045(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 35.Yener U., Avsar T., Akgün E., Şeker A., Bayri Y., Kılıç T. Assessment of antiangiogenic effect of imatinib mesylate on vestibular schwannoma tumors using in vivo corneal angiogenesis assay laboratory investigation. J. Neurosurg. 2012;117:697–704. doi: 10.3171/2012.6.JNS112263. [DOI] [PubMed] [Google Scholar]
- 36.Ammoun S., Schmid M.C., Triner J., Manley P., Hanemann C.O. Nilotinib alone or in combination with selumetinib is a drug candidate for neurofibromatosis type 2. Neuro-Oncology. 2011;13:759–766. doi: 10.1093/neuonc/nor056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrilli A.M., Garcia J., Bott M., Plati S.K., Dinh C.T., Bracho O.R., Yan D., Zou B., Mittal R., Telischi F.F., et al. Ponatinib promotes a G1 cell-cycle arrest of merlin/NF2-deficient human schwann cells. Oncotarget. 2017;8:31666–31681. doi: 10.18632/oncotarget.15912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu J., Su X., Li Z., Deng L., Liu X., Feng X., Peng J. HGF/c-MET pathway in cancer: From molecular characterization to clinical evidence. Oncogene. 2021;40:4625–4651. doi: 10.1038/s41388-021-01863-w. [DOI] [PubMed] [Google Scholar]
- 39.Konstorum A., Lowengrub J.S. Activation of the HGF/c-Met axis in the tumor microenvironment: A multispecies model. J. Theor. Biol. 2018;439:86–99. doi: 10.1016/j.jtbi.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dilwali S., Roberts D., Stankovic K.M. Interplay between VEGF-A and cMET signaling in human vestibular schwannomas and schwann cells. Cancer Biol. Ther. 2015;16:170–175. doi: 10.4161/15384047.2014.972765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu S.-Y., Duan H.-F., Li Q.-F., Yang Y.-F., Chen J.-L., Wang L.-S., Wang H. Hepatocyte growth factor protects endothelial cells against gamma ray irradiation-induced damage. Acta Pharmacol. Sin. 2009;30:1415–1420. doi: 10.1038/aps.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Y., Liu P., Zhang N., Chen J., Landegger L.D., Wu L., Zhao F., Zhao Y., Zhang Y., Zhang J., et al. Targeting the cMET pathway augments radiation response without adverse effect on hearing in NF2 schwannoma models. Proc. Natl. Acad. Sci. USA. 2018;115:E2077–E2084. doi: 10.1073/pnas.1719966115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuse M.A., Plati S.K., Burns S.S., Dinh C.T., Bracho O., Yan D., Mittal R., Shen R., Soulakova J.N., Copik A.J., et al. Combination Therapy with c-Met and Src Inhibitors Induces Caspase-Dependent Apoptosis of Merlin-Deficient Schwann Cells and Suppresses Growth of Schwannoma Cells. Mol. Cancer Ther. 2017;16:2387–2398. doi: 10.1158/1535-7163.MCT-17-0417. [DOI] [PubMed] [Google Scholar]
- 44.Welling D.B., Lasak J.M., Akhmametyeva E., Ghaheri B., Chang L.-S. cDNA Microarray Analysis of Vestibular Schwannomas. Otol. Neurotol. 2002;23:736–748. doi: 10.1097/00129492-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 45.Lee T.X., Packer M.D., Huang J., Akhmametyeva E.M., Kulp S.K., Chen C.-S., Giovannini M., Jacob A., Welling D.B., Chang L.-S. Growth inhibitory and anti-tumour activities of OSU-03012, a novel PDK-1 inhibitor, on vestibular schwannoma and malignant schwannoma cells. Eur. J. Cancer. 2009;45:1709–1720. doi: 10.1016/j.ejca.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bush M.L., Oblinger J., Brendel V., Santarelli G., Huang J., Akhmametyeva E.M., Burns S.S., Wheeler J., Davis J., Yates C.W., et al. AR42, a novel histone deacetylase inhibitor, as a potential therapy for vestibular schwannomas and meningiomas. Neuro-Oncology. 2011;13:983–999. doi: 10.1093/neuonc/nor072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porta C., Paglino C., Mosca A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front. Oncol. 2014;4:64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.James M.F., Stivison E., Beauchamp R.L., Han S., Li H., Wallace M.R., Gusella J.F., Stemmer-Rachamimov A.O., Ramesh V. Regulation of mTOR Complex 2 Signaling in Neurofibromatosis 2–Deficient Target Cell Types. Mol. Cancer Res. 2012;10:649–659. doi: 10.1158/1541-7786.MCR-11-0425-T. [DOI] [PubMed] [Google Scholar]
- 49.Lane H.A., Wood J.M., McSheehy P.M., Allegrini P.R., Boulay A., Brueggen J., Littlewood-Evans A., Maira S.-M., Martiny-Baron G., Schnell C.R., et al. mTOR Inhibitor RAD001 (Everolimus) Has Antiangiogenic/Vascular Properties Distinct from a VEGFR Tyrosine Kinase Inhibitor. Clin. Cancer Res. 2009;15:1612–1622. doi: 10.1158/1078-0432.CCR-08-2057. [DOI] [PubMed] [Google Scholar]
- 50.Goutagny S., Raymond E., Esposito-Farese M., Trunet S., Mawrin C., Bernardeschi D., Larroque B., Sterkers O., Giovannini M., Kalamarides M. Phase II study of mTORC1 inhibition by everolimus in neurofibromatosis type 2 patients with growing vestibular schwannomas. J. Neuro-Oncol. 2015;122:313–320. doi: 10.1007/s11060-014-1710-0. [DOI] [PubMed] [Google Scholar]
- 51.Karajannis M.A., Legault G., Hagiwara M., Giancotti F.G., Filatov A., Derman A., Hochman T., Goldberg J.D., Vega E., Wisoff J.H., et al. Phase II study of everolimus in children and adults with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro-Oncology. 2014;16:292–297. doi: 10.1093/neuonc/not150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breun M., Schwerdtfeger A., Martellotta D.D., Kessler A.F., Perez J.M., Monoranu C.M., Ernestus R.-I., Matthies C., Löhr M., Hagemann C. CXCR4: A new player in vestibular schwannoma pathogenesis. Oncotarget. 2018;9:9940–9950. doi: 10.18632/oncotarget.24119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breun M., Monoranu C.M., Kessler A.F., Matthies C., Löhr M., Hagemann C., Schirbel A., Rowe S.P., Pomper M.G., Buck A.K., et al. [68Ga]-Pentixafor PET/CT for CXCR4-Mediated Imaging of Vestibular Schwannomas. Front. Oncol. 2019;9:503. doi: 10.3389/fonc.2019.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hannan C.J., Lewis D., O’Leary C., Donofrio C.A., Evans D.G., Roncaroli F., Brough D., King A.T., Coope D., Pathmanaban O.N. The inflammatory microenvironment in vestibular schwannoma. Neuro-Oncol. Adv. 2020;2:vdaa023. doi: 10.1093/noajnl/vdaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fujioka M., Okano H., Ogawa K. Inflammatory and immune responses in the cochlea: Potential therapeutic targets for sensorineural hearing loss. Front. Pharmacol. 2014;5:287. doi: 10.3389/fphar.2014.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Helbing D.-L., Schulz A., Morrison H. Pathomechanisms in schwannoma development and progression. Oncogene. 2020;39:5421–5429. doi: 10.1038/s41388-020-1374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tamura R., Tanaka T., Yamamoto Y., Akasaki Y., Sasaki H. Dual role of macrophage in tumor immunity. Immunotherapy. 2018;10:899–909. doi: 10.2217/imt-2018-0006. [DOI] [PubMed] [Google Scholar]
- 58.Tamura R., Tanaka T., Akasaki Y., Murayama Y., Yoshida K., Sasaki H. The role of vascular endothelial growth factor in the hypoxic and immunosuppressive tumor microenvironment: Perspectives for therapeutic implications. Med. Oncol. 2019;37:2. doi: 10.1007/s12032-019-1329-2. [DOI] [PubMed] [Google Scholar]
- 59.Tamura R., Morimoto Y., Sato M., Kuranari Y., Oishi Y., Kosugi K., Yoshida K., Toda M. Difference in the hypoxic immunosuppressive microenvironment of patients with neurofibromatosis type 2 schwannomas and sporadic schwannomas. J. Neuro-Oncol. 2020;146:265–273. doi: 10.1007/s11060-019-03388-5. [DOI] [PubMed] [Google Scholar]
- 60.Nisenbaum E., Misztalm C., Szczupak M., Thielhelm T., Peña S., Mei C., Goncalves S., Bracho O., Ma R., Ivan M.E., et al. Tumor-Associated Macrophages in Vestibular Schwannoma and Relationship to Hearing. OTO Open. 2021;5:2473974X211059111. doi: 10.1177/2473974X211059111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Z., Liu X., Guo R., Wang P. TIM-3 plays a more important role than PD-1 in the functional impairments of cytotoxic T cells of malignant Schwannomas. Tumor Biol. 2017;39:1010428317698352. doi: 10.1177/1010428317698352. [DOI] [PubMed] [Google Scholar]
- 62.Wang S., Liechty B., Patel S., Weber J.S., Hollmann T.J., Snuderl M., Karajannis M.A. Programmed death ligand 1 expression and tumor infiltrating lymphocytes in neurofibromatosis type 1 and 2 associated tumors. J. Neuro-Oncol. 2018;138:183–190. doi: 10.1007/s11060-018-2788-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacobs J.F., Idema A.J., Bol K.F., Nierkens S., Grauer O.M., Wesseling P., Grotenhuis J.A., Hoogerbrugge P.M., de Vries I.J., Adema G.J. Regulatory T cells and the PD-L1/PD-1 pathway mediate immune suppression in malignant human brain tumors. Neuro Oncol. 2009;11:394–402. doi: 10.1215/15228517-2008-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakanishi M., Rosenberg D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2013;35:123–137. doi: 10.1007/s00281-012-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Finetti F., Travelli C., Ercoli J., Colombo G., Buoso E., Trabalzini L. Prostaglandin E2 and Cancer: Insight into Tumor Progression and Immunity. Biology. 2020;9:434. doi: 10.3390/biology9120434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hong B., Krusche C.A., Schwabe K., Friedrich S., Klein R., Krauss J.K., Nakamura M. Cyclooxygenase-2 Supports Tumor Proliferation in Vestibular Schwannomas. Neurosurgery. 2011;68:1112–1117. doi: 10.1227/NEU.0b013e318208f5c7. [DOI] [PubMed] [Google Scholar]
- 67.Kandathil C.K., Dilwali S., Wu C.-C., Ibrahimov M., McKenna M.J., Lee H., Stankovic K.M. Aspirin Intake Correlates with Halted Growth of Sporadic Vestibular Schwannoma In Vivo. Otol. Neurotol. 2014;35:353–357. doi: 10.1097/MAO.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 68.Mackeith S., Wasson J., Baker C., Guilfoyle M., John D., Donnelly N., Mannion R., Jefferies S., Axon P., Tysome J.R. Aspirin does not prevent growth of vestibular schwannomas: A case-control study. Laryngoscope. 2018;128:2139–2144. doi: 10.1002/lary.27114. [DOI] [PubMed] [Google Scholar]
- 69.Whitesell L., Lindquist S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 70.Chen Z., Li S., Mo J., Hawley E., Wang Y., He Y., Brosseau J.-P., Shipman T., Clapp D.W., Carroll T.J., et al. Schwannoma development is mediated by Hippo pathway dysregulation and modified by RAS/MAPK signaling. JCI Insight. 2020;5:e141514. doi: 10.1172/jci.insight.141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanaka K., Eskin A., Chareyre F., Jessen W., Manent J., Kawakita M., Chen R., White C., Vitte J., Jaffer Z.M., et al. Therapeutic Potential of HSP90 Inhibition for Neurofibromatosis Type 2. Clin. Cancer Res. 2013;19:3856–3870. doi: 10.1158/1078-0432.CCR-12-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sagers J.E., Brown A.S., Vasilijic S., Lewis R.M., Sahin M.I., Landegger L.D., Perlis R.H., Kohane I.S., Welling D.B., Patel C.J., et al. Computational repositioning and preclinical validation of mifepristone for human vestibular schwannoma. Sci. Rep. 2018;8:5437. doi: 10.1038/s41598-018-23609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakanishi H., Kawashima Y., Kurima K., Chae J.J., Ross A.M., Pinto-Patarroyo G., Patel S.K., Muskett J.A., Ratay J.S., Chattaraj P., et al. NLRP3 mutation and cochlear autoinflammation cause syndromic and nonsyndromic hearing loss DFNA34 responsive to anakinra therapy. Proc. Natl. Acad. Sci. USA. 2017;114:E7766–E7775. doi: 10.1073/pnas.1702946114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ren Y., Landegger L.D., Stankovic K.M. Gene Therapy for Human Sensorineural Hearing Loss. Front. Cell. Neurosci. 2019;13:323. doi: 10.3389/fncel.2019.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prabhakar S., Taherian M., Gianni D., Conlon T.J., Fulci G., Brockmann J., Stemmer-Rachamimov A., Sena-Esteves M., Breakefield X.O., Brenner G.J. Regression of Schwannomas Induced by Adeno-Associated Virus-Mediated Delivery of Caspase-1. Hum. Gene Ther. 2013;24:152–162. doi: 10.1089/hum.2012.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmed S.G., Abdelnabi A., Maguire C.A., Doha M., Sagers J.E., Lewis R.M., Muzikansky A., Giovannini M., Stemmer-Rachamimov A., Stankovic K.M., et al. Gene therapy with apoptosis-associated speck-like protein, a newly described schwannoma tumor suppressor, inhibits schwannoma growth in vivo. Neuro-Oncology. 2019;21:854–866. doi: 10.1093/neuonc/noz065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ignacio K.H.D., Espiritu A.I., Diestro J.D.B., Chan K.I., Dmytriw A.A., Omar A.T., 2nd Efficacy of aspirin for sporadic vestibular schwannoma: A meta-analysis. Neurol. Sci. 2021;42:5101–5106. doi: 10.1007/s10072-021-05193-3. [DOI] [PubMed] [Google Scholar]
- 78.Tamura R. Current Understanding of Neurofibromatosis Type 1, 2, and Schwannomatosis. Tamura R. Int. J. Mol. Sci. 2021;22:5850. doi: 10.3390/ijms22115850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.