Abstract
Cytochalasans from the endophytic fungi featured structure diversity. Our previous study has disclosed that cytochalasans from the endophytic fungus Phomopsis sp. shj2 exhibited an antimigratory effect. Further chemical investigation on Phomopsis sp. shj2 has led to the discovery of seven new cytochalasans (1–7), together with four known ones. Their structures were elucidated through extensive spectroscopic data interpretation and single-crystal X-ray diffraction analysis. Compounds 1–3 and 8–11 exhibited antimigratory effects against MDA-MB-231 in vitro with IC50 values in the range of 1.01−10.42 μM.
Keywords: endophytic fungus, Phomopsis, cytochalasan, antimigratory activity
1. Introduction
Endophytic fungi are emerging as rich resources for structurally unique and bioactive secondary metabolites, which arouse increasing research interest in the past decades [1,2,3]. Cytochalasans represent a large class of fungal polyketide synthase-nonribosomal peptide synthetase (PKS-NRPS) hybrid secondary metabolites. Recently, plenty of polycyclic cytochalasans have been identified [4,5,6,7,8] and synthesised [9,10]; moreover, they exhibited a broad spectrum of interesting biological activities, such as cytotoxic [4,5,8], immunoregulatory [11], and antimicrobial [6] activities. To date, more than 400 cytochalasans have been isolated from various fungal sources, such as Phomopsis [4], Xylaria [12], Chaetomium [13] and Phoma [14] genera.
Tumour spread is a major concern in cancer therapeutics as cancer metastasis is responsible for 90% of deaths from solid tumours [15]. Natural products with antimigratory activity represent a highly interesting field to explore for cancer chemoprevention and therapy. Fungi are emerging as a natural source, such as Diaporthe [16], Isaria [17], and Phenicillium [18,19] genera. Chemical investigations on endophytes of Isodon species have disclosed structurally diverse and bioactive natural products [19,20,21,22]. Phomopchalasins B and C were isolated from the endophytic fungus Phomopsis sp. shj2 from the stems of Isodon eriocalyx var. laxiflora and exhibited in vitro antimigratory effects against MDA-MB231 [19]. In our continuous efforts for more bioactive structures, the strain was further investigated by one strain-many compounds strategy (OSMAC), which led to the isolation of seven new cytochalasans (1–7), along with four known ones (Figure 1). Herein, we report the isolation, structure elucidation, and antimigratory activities of these cytochalasans.
Figure 1.
Structures of compounds 1–11.
2. Materials and Methods
2.1. General Experimental Procedures
Column chromatography (CC) was performed with silica gel (100–200 mesh, Qingdao Marine Chemical, Inc., Qingdao, China), Lichroprep RP-18 gel (40–63 μm, Merck, Darmstadt, Germany). Preparative HPLC and semi-preparative HPLC were performed on an Agilent 1200 liquid chromatograph with a Zorbax SB-C18 (9.4 mm × 25 cm) column. Fractions were monitored by TLC, and spots were visualized by heating silica gel plates sprayed with 10% H2SO4 in EtOH. Petroleum ether (PE, 60–90 °C), EtOAc, CHCl3, acetone, MeOH, and EtOH were of analytical grade and purchased from Sinopharm Chemical Reagent Co. Ltd., China. All solvents were distilled before use. NMR spectra were recorded on Bruker DRX-500, AV-600, and 800 spectrometers. ESIMS and HRESIMS experiments were performed on a Bruker HCT/Esquire spectrometer and a Waters AutoSpec Premier P776 spectrometer. CD spectra were measured on an Applied Photophysics Chirascan spectrophotometer. Optical rotations were measured with a JASCO P-1020 polarimeter. UV spectra were obtained using a Shimadzu UV-2401A spectrophotometer.
2.2. Fungal Material
The culture of Phomopsis sp. shj2 was isolated from the stems of Isodon eriocalyx var. laxiflora collected from Kunming Botanical Garden, Kunming, People’s Republic of China, in December 2012. The isolate was identified based on sequence (GenBank Accession No. KU533636) analysis of the ITS region of the rDNA. The fungal strain was cultured on slants of potato dextrose agar at 25 °C for 7 days. Agar plugs were cut into small pieces (about 0.5 × 0.5 × 0.5 cm3) under aseptic conditions, and 15 pieces were used to inoculate three Erlenmeyer flasks (250 mL), each containing 50 mL of media (0.4% glucose, 1% malt extract, and 0.4% yeast extract); the final pH of the media was adjusted to 6.5, and the flasks were sterilized by autoclave. Three flasks of the inoculated media were incubated at 28 °C on a rotary shaker at 180 rpm for 5 days to prepare the seed culture. Fermentation was carried out in 125 Fernbach flasks (500 mL), each containing 80 g of rice. Spore inoculum was prepared in sterile, distilled H2O to give a final spore/cell suspension of 1 × 106/mL. Distilled H2O (120 mL) was added to each flask, and the contents were soaked overnight before autoclaving at 15 psi for 30 min. After cooling to room temperature, each flask was inoculated with 5.0 mL of the spore inoculum and incubated at 28 °C for 42 days.
2.3. Extraction and Isolation
The fermented material was extracted with EtOAc (4 × 10.0 L) and the organic solvent was evaporated to dryness under vacuum to afford a crude extract (170 g). The crude extract was purified by CC (column chromatography on SiO2 with CHCl3/acetone gradient system 1:0, 9:1, 8:2, 7:3, 6:4 and 1:1) to yield six main fractions, Fr.s A–F. Fr. B was subjected to chromatography over silica gel CC (petroleum ether-EtOAc) to give subfractions B1–B9. Fr. B2 was further purified by silica gel CC (petroleum ether-acetone) to give 1 (10.7 mg). Fr. B8 was purified by semi-preparative HPLC (3 mL/min, detector UV λmax 210 nm, MeCN-H2O) to afford 11 (3.2 mg), 8 (25.1 mg), and 10 (3.7 mg). Fr. C was purified by chromatography over silica gel CC (petroleum ether-acetone) to give subfractions Fr.s C1–C10. The subfraction C8 was recrystallized to give 7 (20.5 mg). Fr. C5 was separated by semi-preparative HPLC (3 mL/min, detector UV λmax 210 nm, MeCN-H2O) to afford 3 (4.7 mg) and 9 (10.2 mg). Fr. D was subjected to Sephadex LH-20 (CH3Cl-MeOH) to yield subfractions D1–D6. The subfraction D5 was purified by recrystallization to afford 4 (1.2 mg). And Fr. D5 was further purified to afford 2 (20.3 mg). Fr. E was purified by semi-preparative HPLC (3 mL/min, detector UV λmax 210 nm, MeCN-H2O) to afford 5 (1.5 mg) and 6 (1.6 mg).
18-Acetoxycytochalasin H (1): white powder (MeOH); = +44.2 (c 0.23, MeOH), UV (MeOH) λmax (log ε): 203.2 (0.5151); 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS [M + Na]+ m/z 558.2826 (calcd for C32H41NO6Na, 558.2826).
Table 1.
1H NMR data (CDCl3, δ in ppm) of compounds 1–7.
| No. | 1 a,b | 2 a,c | 3 c,d | 4 a,c | 5 a,c | 6 a,e | 7 a,c |
|---|---|---|---|---|---|---|---|
| 3 | 3.25 (m) | 3.26 (m) | 3.33 (m) | 3.27 (m) | 3.23 (dt, 9.4, 4.3) | 3.54 (dt, J = 9.4, 4.3) | 3.28 (overlap) |
| 4 | 2.15 (m) | 2.14 (m) | 2.65 (m) | 2.58 (m) | 2.35 (t, 4.3) | 2.25 (t, 4.2) | 2.19 (t, 4.3) |
| 5 | 2.76 (m) | 2.78 (m) | 2.72 (m) | 2.90 (m) | 3.08 (m) | 2.11 (m) | 2.53 (m) |
| 6 | 2.01 (m) | ||||||
| 7 | 3.84 (d, 10.5) | 3.83 (d, 10.5) | 3.79 (d, 10.5) | 3.82 (d, 10.5) | 5.72 (s) | ||
| 8 | 2.93 (d, 10.5) | 2.96 (d, 10.5) | 2.94 (d, 10.5) | 2.90 (d, 10.5) | 3.94 (d, 9.3) | 3.79 (d, 9.4) | 3.26 (overlap) |
| 10 | 2.85 (dd, 13.5, 4.5) 2.65 (dd, 13.5, 9.6) |
2.86 (dd, 13.3, 3.8) 2.67 (m) |
2.81 (m) 2.79 (m) |
2.58 (m) 1.71 (m) |
2.90 (dd, 13.5, 4.3) 2.65 (dd, 13.5, 9.4) |
2.92 (dd, 13.5, 4.3) 2.64 (dd, 13.5, 9.4) |
2.91 (dd, 13.5, 4.3) 2.60 (dd, 13.5, 10.2) |
| 11 | 0.99 (d, 6.7) | 1.01 (d, 6.7) | 0.84 (d, 6.8) | 1.10 (d, 6.7) | 1.12 (d, 6.7) | 0.98 (d, 6.7) | 1.17 (d, 7.3) |
| 12 | 5.33 (s) 5.10 (s) |
5.35 (s) 5.11 (s) |
5.18 (s) 4.95 (s) |
5.32 (s) 5.11 (s) |
6.25 (s) 5.29 (s) |
1.12 (d, 7.0) | 4.53 (d, 12.8) 4.48 (d, 12.8) |
| 13 | 5.74 (dd, 15.5, 9.7) | 5.73 (dd, 15.1, 10.0) | 5.70 (dd, 15.0, 9.2) | 5.71 (dd, 15.5, 9.8) | 5.81 (dd, 15.6, 9.3) | 5.69 (dd, 15.5, 9.4) | 5.84 (dd, 15.3, 10.3) |
| 14 | 5.38 (m) | 5.43 (m) | 5.22 (m) | 5.35 (m) | 5.19 (m) | 5.16 (m) | 5.24 (m) |
| 15 | 2.01 (overlap) 1.79 (d, 12.4) |
2.00 (overlap) 1.79 (d, 11.3) |
1.90 (dd, 13.9, 3.1) 1.80 (m) |
1.98 (dd, 10.4, 4.7) 1.78 (m) |
2.04 (dd, 12.9, 4.4) 1.89 (m) |
2.01 (m) 1.81 (m) |
1.99 (m) 1.77 (overlap) |
| 16 | 1.65 (m) | 1.78 (m) | 1.66 (m) | 1.75 (m) | 1.76 (m) | 1.75 (m) | 1.75 (m) |
| 17 | 2.05 (dd, 14.3, 3.7), 1.75 (dd, 14.3, 3.0 |
1.69 (m) | 1.90 (m) 1.75 (m) |
1.78 (overlap) | 1.85 (overlap) 1.54 (dd, 14.3, 3.2) | 1.85 (m) 1.53 (dd, 14.3, 3.1) |
1.88 (dd, 14.3, 2.7) 1.54 (d, 14.3) |
| 19 | 5.56 (d, 16.6) | 5.52 (d, 16.7) | 5.84 (d, 16.7) | 5.73 (d, 16.7) | 5.52 (d, 16.6) | 5.49 (d, 16.6) | 5.52 (d, 16.6) |
| 20 | 5.85 (dd, 16.6, 2.3) | 5.79 (dd, 16.7, 2.4) | 5.97 (dd, 16.7, 2.2) | 5.99 (dd, 16.7, 2.6) | 5.90 (dd, 16.6, 2.6) | 5.85 (dd, 16.6, 2.5) | 5.91 (dd, 16.6, 2.6) |
| 21 | 5.63 (t, 2.3) | 5.54 (t, 2.4) | 4.02 (t, 2.2) | 4.12 (t, 2.6) | 5.65 (t, 2.6) | 5.60 (t, 2.5) | 5.68 (t, 2.6) |
| 22 | 1.02 (d, 6.9) | 1.01 (d, 6.5) | 0.99 (d, 6.3) | 1.01 (d, 6.3) | 1.04 (d, 7.0) | 1.03 (d, 6.9) | 1.04 (d, 6.3) |
| 23 | 1.58 (s) | 1.26 (s) | 1.53 (s) | 1.28 (s) | 1.34 (s) | 1.32 (s) | 1.34 (s) |
| 2′, 6′ | 7.14 (d, 7.4) | 7.15 (d, 7.4) | 7.21 (d, 7.3) | 7.15 (d, 7.4) | 7.12 (d, 7.4) | 7.15 (d, 7.3) | 7.14 (d, 7.2) |
| 3′, 5′ | 7.31 (t, 7.4) | 7.32 (t, 7.4) | 7.29 (t, 7.3) | 7.31 (t, 7.4) | 7.32 (t, 7.4) | 7.33 (t, 7.3) | 7.31 (t, 7.5) |
| 4′ | 7.25 (t, 7.4) | 7.25 (t, 7.4) | 7.26 (d, 7.3) | 7.24 (t, 7.4) | 7.25 (t, 7.4) | 7.25 (t, 7.3) | 7.24 (t, 7.2) |
| 12-OAc | 2.04, s | ||||||
| 18-OR | R = Ac 2.00 (s) |
R = Et 3.38 (m), 2.65 (m) 1.14 (t, 6.9) |
R = Ac 1.96 (s) |
R = Et 3.41 (m), 3.37 (m) 1.17 (t, 7.0) |
R = H | R = H | R = H |
| 21-OAc | 2.24 (s) | 2.25 (s) | 2.30 (s) | 2.28 (s) | 2.25 (s) |
a Recorded in CDCl3. b Recorded at 800 MHz. c Recorded at 600 MHz. d Recorded in acetone-d6. e Recorded at 500 MHz.
Table 2.
13C NMR data (CDCl3, δ in ppm) of compounds 1–7.
| No. | 1 a,b | 2 a,c | 3 d,e | 4 a,e | 5 a,e | 6 a,f | 7 a,e |
|---|---|---|---|---|---|---|---|
| 1 | 174.3 s | 174.3 s | 176.7 s | 175.8 s | 172.8 s | 173.5 s | 174.9 s |
| 3 | 53.9 d | 53.9 d | 54.2 d | 50.6 d | 54.0 d | 53.3 d | 56.1 d |
| 4 | 50.5 d | 50.9 d | 50.2 d | 53.9 d | 50.6 d | 51.1 d | 53.8 d |
| 5 | 33.0 d | 33.0 d | 33.8 d | 33.1 d | 34.2 d | 35.7 d | 34.7 d |
| 6 | 148.0 s | 148.0 s | 152.1 s | 148.6 s | 143.9 s | 45.8 d | 135.8 s |
| 7 | 70.0 d | 69.9 d | 71.6 d | 70.1 d | 198.7 s | 214.0 s | 134.6 d |
| 8 | 47.3 d | 47.4 d | 46.8 d | 46.0 d | 53.1 d | 52.0 d | 43.4 d |
| 9 | 52.1 s | 51.9 s | 54.5 s | 53.0 s | 52.9 s | 53.6 s | 56.2 s |
| 10 | 45.7 t | 45.8 t | 45.5 t | 45.8 t | 46.0 t | 46.3 t | 46.1 t |
| 11 | 14.1 q | 14.2 q | 14.2 q | 14.1 q | 14.4 q | 15.9 q | 13.2 q |
| 12 | 114.3 t | 114.3 t | 112.0 t | 113.9 t | 121.0 t | 16.0 q | 64.9 t |
| 13 | 127.6 d | 127.2 d | 130.2 d | 128.0 d | 123.0 d | 123.2 d | 128.3 d |
| 14 | 138.2 d | 138.6 d | 135.7 d | 137.8 d | 138.4 d | 137.9 d | 136.3 d |
| 15 | 42.7 t | 43.1 t | 43.7 t | 42.8 t | 43.1 t | 42.9 t | 42.8 t |
| 16 | 28.6 d | 28.0 d | 29.2 d | 27.8 d | 28.6 d | 28.5 d | 28.7 d |
| 17 | 51.5 t | 51.8 t | 52.9 t | 51.1 t | 53.7 t | 53.5 t | 53.5 t |
| 18 | 84.4 s | 78.5 s | 85.0 s | 78.5 s | 74.6 s | 74.5 s | 74.6 s |
| 19 | 136.6 d | 138.9 d | 135.1 d | 137.3 d | 137.7 d | 137.7 d | 137.3 d |
| 20 | 124.9 d | 125.8 d | 131.8 d | 130.8 d | 125.9 d | 125.9 d | 126.5 d |
| 21 | 77.4 d | 78.1 d | 76.7 d | 77.0 d | 77.9 d | 77.8 d | 77.0 d |
| 22 | 25.5 q | 26.2 q | 25.9 q | 26.0 q | 26.6 q | 26.6 q | 26.6 q |
| 23 | 26.3 q | 25.2 q | 27.0 q | 25.2 q | 31.5 q | 31.4 q | 31.6 q |
| 1′ | 137.5 s | 137.6 s | 138.9 s | 137.7 s | 137.0 s | 137.1 s | 137.7 s |
| 2′, 6′ | 129.1 d | 129.2 d | 130.8 d | 129.1 d | 129.2 d | 129.2 d | 129.1 d |
| 3′, 5′ | 129.1 d | 129.1 d | 129.2 d | 128.9 d | 129.1 d | 129.1 d | 129.0 d |
| 4′ | 127.2 d | 127.3 d | 127.4 d | 127.1 d | 127.4 d | 127.4 d | 127.3 d |
| 12-OR | 170.6 s, 21.1 q |
||||||
| 18-OR | R = Ac 170.1 s, 21.0 q |
R = Et 57.9 t, 16.3 q |
R = Ac 170.4 s, 22.2 q |
R = Et 57.5 t, 16.0 q |
R = H | R = H | |
| 21-OAc | 170.3 s, 22.4 q |
170.3 s, 21.1 q |
170.1 s, 21.1 q |
170.1 s, 21.1 q |
170.2 s, 21.2 q |
a Recorded in CDCl3. b Recorded at 100 MHz. c Recorded at 150 MHz. d Recorded in acetone-d6. e Recorded at 150 MHz. f Recorded at 125 MHz.
18-Ethoxycytochalasin H (2): white solid; = +39.0 (c 0.15, MeOH), UV (MeOH) λmax (log ε): 204.0 (0.4717); 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS [M + Na]+ m/z 544.3030 (calcd for C32H43NO5Na, 544.3039).
18-Acetoxycytochalasin J (3): = +22.0 (c 0.24, MeOH), UV (MeOH) λmax (log ε): 204.0 (0.5467); 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS [M + Na]+ m/z 516.2726 (calcd for C30H39NO5Na, 516.2720).
18-Ethoxycytochalasin J (4): = +50.0 (c 0.19, MeOH), UV (MeOH) λmax (log ε): 203.8 (0.5179); 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS [M + H]+ m/z 480.3111 (calcd for C30H42NO4, 480.3108).
7-Oxocytochalasin H (5): = −12.3 (c 0.19, MeOH), UV (MeOH) λmax (log ε): 205.0 (0.5284); 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS [M + Na]+ m/z 514.2559 (calcd for C30H37NO5Na, 514.2564).
Cytochalasin H3 (6): = −63.2 (c 0.19, MeOH), UV (MeOH) λmax (log ε): 205.5 (0.5241); 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS [M + Na]+ m/z 516.2719 (calcd for C30H39NO5Na, 516.2720).
Cytochalasin H4 (7): white solid; = −28.4 (c 0.18, MeOH), UV (MeOH) λmax (log ε): 203.2 (0.5250); IR (KBr) λmax 3471, 2958, 2925, 1740, 1688, 1640, 1454, 1441, 1384, 1232 cm−1; 1H and 13C NMR data, see Table 1 and Table 2; HRESIMS [M + H]+ m/z 536.3014 (calcd for C32H42NO6, 536.3007).
2.4. X-ray Crystal Structure Analysis
The intensity data for 1 and 3 were collected on a Bruker APEX DUO diffractometer using graphite-monochromated Cu Kα radiation. The structures of these compounds were solved by direct methods (SHELXS97), expanded using difference Fourier techniques, and refined by the program and full-matrix least-squares calculations. The non-hydrogen atoms were refined anisotropically, and hydrogen atoms were fixed at calculated positions. Crystallographic data for the structures of 1 (deposition number CCDC 2169670) and 3 (deposition number CCDC 2169671) have been deposited in the Cambridge Crystallographic Data Centre database. Copies of the data can be obtained free of charge from the CCDC at www.ccdc.cam.ac.uk (accessed on 1 May 2022).
Crystal data for 1: C32H41NO6, M = 535.66, orthorhombic, a = 9.5019 (6) Å, b = 15.8046 (9) Å, c = 19.6446 (11) Å, α = 90.00°, β = 90.00°, γ = 90.00°, V = 2950.1 (3) Å3, T = 100 (2) K, space group P212121, Z = 4, μ (CuKα) = 0.664 mm−1, 11817 reflections measured, 4764 independent reflections (Rint = 0.0569). The final R1 values were 0.0917 (I > 2σ (I)). The final wR (F2) values were 0.2549 (I > 2σ (I)). The final R1 values were 0.0937 (all data). The final wR (F2) values were 0.2565 (all data). The goodness of fit on F2 was 1.163. Flack parameter = 0.3 (5). The Hooft parameter is 0.15 (11) for 1883 Bijvoet pairs.
Crystal data for 3: C30H39NO5·H2O, M = 511.64, monoclinic, a = 9.7873 (3) Å, b = 9.4430 (3) Å, c = 15.5029 (4) Å, α = 90.00°, β = 103.6560 (10)°, γ = 90.00°, V = 1392.30 (7) Å3, T = 100 (2) K, space group P21, Z = 2, μ (CuKα) = 0.678 mm−1, 9344 reflections measured, 3802 independent reflections (Rint = 0.0470). The final R1 values were 0.0600 (I > 2σ (I)). The final wR (F2) values were 0.1736 (I > 2σ (I)). The final R1 values were 0.0681 (all data). The final wR (F2) values were 0.2047 (all data). The goodness of fit on F2 was 1.093. Flack parameter = 0.1 (3). The Hooft parameter is 0.31 (9) for 1240 Bijvoet pairs.
2.5. Antimigration Assay
Cell migration was determined using the Oris™ Pro Cell Migration Assay (Platypus Technologies, Madison, WI, USA), according to the manufacturer’s protocol. Briefly, MDA-MB-231 cells were seeded and incubated (37 °C, 5% CO2) for 1 h, and then indicated concentrations of compounds were added and incubated with cells for an additional 18 h. At the end of incubation, the cell viability was evaluated with MTS assays and the migration area of each group was calculated and analysed, and the results of each subgroup were presented as a percentage of DMSO-treated cells.
3. Results and Discussion
3.1. Structure Elucidation
The molecular formula of 18-acetoxycytochalasin H (1) was determined to be C32H41NO6 on the basis of HRESIMS ion at m/z 558.2826 [M + Na]+ (calcd. 558.2826), indicating 13 degrees of unsaturation. Its 1H NMR data (Table 1) showed typical signals of three tertiary methyl groups (δH 2.24, s; δH 2.00, s; δH 1.58, s), two secondary methyl groups (δH 0.99, d, J = 6.7 Hz; δH 1.02, d, J = 6.9 Hz), six olefinic protons (δH 5.85, dd, J = 16.6, 2.3 Hz; δH 5.74, dd, J = 15.5, 9.7 Hz; δH 5.56, d, J = 16.6 Hz; δH 5.38, m; δH 5.33, s; δH 5.10, s), two oxygenated methine groups (δH 5.63, d, J = 2.3 Hz; δH 3.84, d, J = 10.5 Hz), and one single-substituted phenyl (δH 7.31, t, J = 7.4 Hz, 2H; δH 7.25, t, J = 7.4 Hz, 1H; δH 7.14, d, J = 7.4 Hz, 2H). The 13C NMR data (Table 2) displayed resonances for 32 carbons, ascribed to 5 methyls, 4 methylenes (including 1 olefinic), 11 methines (4 olefinic and 2 oxygenated), 61 quaternary carbons (1 olefinic, 1 amide and 2 ester carbonyls), and 6 other signals assignable to the single-substituted phenyl group. Thus, the above-mentioned results indicated that 1 should be a new tetracyclic cytochalasin including a benzene ring, with structural similarity with cytochalasin H [23]. The manifest difference of the structure of 1 from that of cytochalasin H was an additional acetoxy group linked at C-18 (δC 84.4) in 1, which was further supported by the HMBC correlation from OAc (δH 2.24, s) to C-18. And the planar structure of 1 was established by extensive analysis of its 2D NMR spectra (Figure 2); its relative configuration was determined by the ROESY correlations (Figure 3) and comparative analysis of those of cytochalasin H. Fortunately, suitable crystals of 1 were obtained and subjected to X-ray diffraction analysis using Cu Kα radiation (Figure 4), which confirmed the above deductions and unambiguously determined the absolute configuration of 1 as 3S,4R,5S,7S,8R,9R,16S,18R,21R with the Hooft parameter 0.15 (11) for 1883 Bijvoet pairs (CCDC 2169670).
Figure 2.
Key HMBC (red arrows) and 1H-1H COSY (blue bold) correlations of compounds 1–7.
Figure 3.
Key ROESY correlations of compounds 1–7.
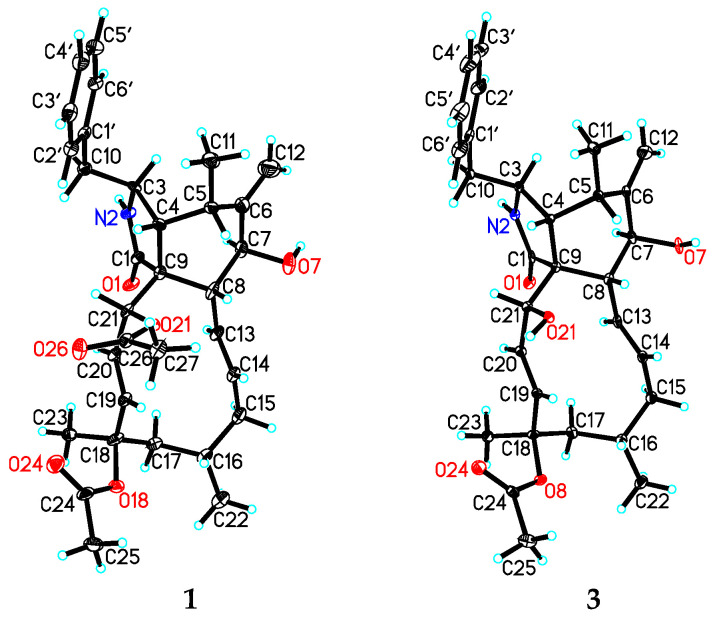
Figure 4.
X-ray crystallographic structures of compounds 1 and 3.
18-Ethoxycytochalasin H (2) was obtained as a white powder; its molecular formula was established as C32H43NO5 on the basis of the HRESIMS ion peak at m/z 544.3030 [M + Na]+ (calcd for C32H43NO5Na, 544.3039), indicating 12 degrees of unsaturation. Analyses of the NMR data of 2 with those of 1 indicated their structural similarities, except for an ethoxy group located at C-18 in 2 rather than the 18-OAc group in 1, which was confirmed by the 1H-1H COSY correlation of CH2 (δH 3.38, m; 2.65, m)/CH3 (1.14, t, J = 6.9 Hz) in the ethoxy group and the HMBC correlations from CH2-18-OEt (δH 3.38, m; 2.65, m) to C-18 (δC 78.5) (Figure 2). The relative configurations of C-3, C-4, C-5, C-7, and C-8 in 2 were determined to be the same as those of 1 by analysis of the ROESY spectrum (Figure 3). Considering the almost complete consistent CD spectra of 1 and 2 (see Supplementary Materials), the absolute configuration of 2 was determined as shown.
18-Acetoxycytochalasin J (3) had the molecular formula of C30H39NO5 based on the positive HRESIMS at m/z 516.2726 [M + Na]+ (calcd 516.2720), corresponding to 12 degrees of unsaturation. The 1D NMR data (Table 1 and Table 2) of 3 were similar to those of cytochalasin J [24], except for an additional acetoxy group located at C-18 in 3. The above deduction was further confirmed by the changed chemical shift of C-18, compared with the 13C NMR data of cytochalasin J [24], and the HMBC correlation from CH3-18-OAc (δH 1.96, s) to 18-OAc carbonyl (δC 170.4) (Figure 2); its structure including the relative configuration was finally established as shown by X-ray diffraction analysis (Figure 4). Considering the similar CD spectra of 1 and 3 (SI), the absolute configuration of 3 was determined to be 3S,4R,5S,7S,8R,9R,16S,18R,21R.
18-Ethoxycytochalasin J (4) had the molecular formula of C30H41NO4 on the basis of the positive HRESIMS (m/z 480.3111 [M + H]+, calcd 480.3108), corresponding to 11 degrees of unsaturation. Careful comparison of the 1H and 13C NMR spectra of 4 and 3 (Table 1 and Table 2) suggested their similar structures, except for an ethoxy group located at C-18 in 4 rather than an acetoxy group in 3. The above deduction was supported by the 1H-1H COSY correlations of CH2/CH3 (18-OEt) and the HMBC correlation from CH2-18-OEt (δH 3.41, m; 3.37, m) to C-18 (δC 78.5) (Figure 2). The absolute configuration of 4 was determined to be the same as that of 1 by analysis of their CD spectra; thus, the structure of 4 was established as shown (Figure 1).
7-Oxocytochalasin H (5) possessed the molecular formula of C30H37NO5 with 13 degrees of unsaturation, which was determined by the positive HRESIMS (m/z 514.2559 [M + Na]+, calcd 514.2564). Analysis of the 1H and 13 C NMR data (Table 1 and Table 2) of 5 and cytochalasin H [23] indicated their structural similarity. The manifest differences were that the C-7 oxymethine group in cytochalasin H was replaced by the C-7 carbonyl group (δC 198.7) in 5. The HMBC correlations from H-8 (δH 3.94, d, J = 9.3 Hz) and H2-12 (δH 6.25, s) to C-7 and other correlations in the 2D spectra of 5 confirmed the above deduction (Figure 2). The correlations of H-4/H-8, H2-10/H-4, and H3-11/H-3 in the ROESY spectrum indicated that H-3 was a-oriented and H-4, H-5, H-8 were β-oriented (Figure 3). Considering the same biogenetic pathway of 1 and 5, the structure of 5 was determined as shown (Figure 1).
Cytochalasin H3 (6) had the molecular formula of C30H39NO5 with 12 degrees of unsaturation, which was determined by the positive HRESIMS (m/z 516.2719 [M + Na]+, calcd 516.2720). Detailed analysis of 1H and 13C NMR data of 6 (Table 1 and Table 2) indicated that 6 possessed a similar structure to that of 5, except for the presence of a saturated C–C bond between C-6 (δC 45.8) and C-12 (δC 16.0) in 6 rather than a terminal double bond in 5, which was further confirmed by the 1H–1H COSY correlation of H-6/H3-12 and the HMBC correlations from H3-12 (δH 1.12, d, J = 7.0 Hz) to C-5 (δC 35.7) and C-7 (δC 214.0) (Figure 2). The ROESY correlations of H-3/H3-11, H3-11/H3-12, H2-10/H-4, and H-4/H-8 implied the α-orientation of H-3 and β-orientations of H-4, H-5, H-6, and H-8 (Figure 3). By analysis of the similar CD spectra of 6 and 1 and biogenetic consideration, the structure of 6 was determined as shown.
The molecular formula of cytochalasin H4 (7) was deduced to be C32H41NO6 with 13 degrees of unsaturation based on the positive HRESIMS (m/z 536.3014 [M + H]+, calcd 536.3007). The 13C NMR data (Table 1 and Table 2 of 7 displayed resonances for 32 carbons, ascribed to five methyls, four methylenes (including one oxygenated), 11 methines (5 olefinic and one oxygenated), six quaternary carbons (one olefinic, one amide and two ester carbonyls), and 6 other signals assignable to the single-substituted phenyl group. The above-mentioned results indicated the presence of an additional acetoxy group and an oxymethine group compared to those of the known RKS-1778 (10) [25]. The ROESY correlations of H-3/H3-11, H2-10/H-4, and H-4/H-8 implied the α-orientation of H-3 and β-orientations of H-4, H-5, and H-8 (Figure 3). The absolute configuration of 7 was determined as shown by analysis of their similar CD spectra of 7 and 10 and biogenetic consideration.
Compounds 8–11 were identified as cytochalasin H (8) [23], cytochalasin J1 (9) [24], RKS-1778 (10) [25], 21-acetoxycytochalasin J2 (11) [26] on the basis of their spectroscopic features and by comparison with the published data in the literature. Biogenetically, compounds 1–11 might be derived from a polyketide chain (octaketide) and an amino acid building block (phenylalanine) through a number of steps involving cycloaddition, oxidation, reduction, dehydration, acetylation, ethylation and methylation [27,28].
3.2. Antimigratory Activity
Our previous studies have revealed that phomopchalasins B and C displayed antimigratory effects [19]. In order to explore the potential of the cytochalasans on antimigration against tumours, eight compounds in sufficient natural amounts (Table 3) were evaluated for antimigratory activities against MDA-MB-231 in vitro. As a result, 1–3 and 8–11 exhibited in vitro antimigratory effects with IC50 values in the range of 1.01–10.42 μM (cytochalasin D as the positive control); it suggested the activity decreased when the C-18 hydroxy group was substituted with the acetoxy, ethoxy or methoxy group (8 vs. 1, 2, and 9). When a double bond was introduced between C-17 and C-18 rather than an ethoxy or methoxy group at C-18, the activity slightly improved (11 vs. 2 and 9). Compound 3 displayed antimigratory activity with an IC50 value of 6.38 μM. The introduction of an acetoxy group at C-21 may enhance the activity (1 vs. 3). When the unit of a terminal double bond (C6-C12) and a hydroxy group at C-7 was replaced by a trisubstituted alkene (C12-C6-C7), the activity slightly improved (8 vs. 10), but the further introduction of an acetoxy group at C-12 decreased the activity (10 vs. 7).
Table 3.
Antimigratory activities of the compounds against MDA-MB-231 in vitro.
| Compounds | IC50 (μM) | Compounds | IC50 (μM) |
|---|---|---|---|
| Cytochalasin D a | 0.78 | 8 | 1.25 |
| 1 | 3.14 | 9 | 7.31 |
| 2 | 10.42 | 10 | 1.01 |
| 3 | 6.38 | 11 | 6.41 |
| 7 | >25 |
a Positive control.
4. Conclusions
Seven new cytochalasans (1–7), together with four known ones, cytochalasin H (8), cytochalasin J1 (9), RKS-1778 (10), and 21-acetoxycytochalasin J2 (11), were isolated from Phomopsis sp. shj2. Their structures were elucidated through extensive spectroscopic data interpretation and single-crystal X-ray diffraction analysis. In the present study, eight cytochalasans were evaluated for their antimigratory activity. Compounds 1–3 and 8–11 exhibited antimigratory activity against MDA-MB-231 in vitro with IC50 values in the range of 1.01−10.42 μM. The results will lay a foundation for further study of the structure–activity relationship for the discovery of antitumour lead compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof8050543/s1, Section S1: NMR, HRESIMS, UV, ORD, and CD spectra of compound 1; Section S2: NMR, HRESIMS, UV, ORD, and CD spectra of compound 2; Section S3: NMR, HRESIMS, UV, ORD, and CD spectra of compound 3; Section S4: NMR, HRESIMS, UV, ORD, and CD spectra of compound 4; Section S5: NMR, HRESIMS, UV, ORD, and CD spectra of compound 5; Section S6: NMR, HRESIMS, UV, ORD, and CD spectra of compound 6; Section S7: NMR, HRESIMS, UV, ORD, and CD spectra of compound 7.
Author Contributions
Conceptualization, B.-C.Y. and P.-T.P.; methodology, W.-G.W., B.-C.Y., L.-M.K., J.-W.T., X.D., Y.L. and P.-T.P.; resources, W.-G.W., B.-C.Y., Y.L. and P.-T.P.; data curation, B.-C.Y. and L.-M.K.; writing—original draft preparation, B.-C.Y.; writing—review and editing, B.-CY. and P.-T.P.; project administration, B.-C.Y. and P.-T.P.; funding acquisition, B.-C.Y. and P.-T.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
X-ray crystallographic data of 1 and 3 (CIF) are available free of charge from the CCDC at https://www.ccdc.cam.ac.uk (accessed on 1 May 2022).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China (No. 81874298), the NSFC-Joint Foundation of Yunnan Province (U2002221), and the Yunnan Science Fund for Distinguished Young Scholars (2019FJ002), and the Postdoctoral Directional Training Foundation of Yunnan Province (B.-C.Y.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rai N., Keshri P.K., Verma A., Kamble S.C., Mishra P., Barik S., Singh S.K., Gautam V. Plant associated fungal endophytes as a source of natural bioactive compounds. Mycology. 2021;12:139–159. doi: 10.1080/21501203.2020.1870579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deshmukh S.K., Dufossé L., Chhipa H., Saxena S., Mahajan G.B., Gupta M.K. Fungal endophytes: A potential source of antibacterial compounds. J. Fungi. 2022;8:164. doi: 10.3390/jof8020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao Y., Liang W., Zhang Z., Wang Y., Zhang S., Liu J., Chang J., Ji C., Zhu D. Polyketide derivatives from the endophytic fungus Phaeosphaeria sp. LF5 isolated from Huperzia serrata and their acetylcholinesterase inhibitory activities. J. Fungi. 2022;8:232. doi: 10.3390/jof8030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y., Yang W., Zou G., Wang G., Kang W., Yuan J., She Z. Cytotoxic bromine- and iodine-containing cytochalasins produced by the mangrove endophytic fungus Phomopsis sp. QYM-13 using the OSMAC approach. J. Nat. Prod. 2022 doi: 10.1021/acs.jnatprod.1c01115. [DOI] [PubMed] [Google Scholar]
- 5.Miao S., Liu M., Qi S., Wu Y., Sun K., Zhang Z., Zhu K., Cai G., Gong K. Cytochalasins from coastal saline soil-derived fungus Aspergillus flavipes RD-13 and their cytotoxicities. J. Antibiot. 2022 doi: 10.1038/s41429-022-00527-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J.-Y., He J., Li Z.-H., Feng T., Liu J.-K. Zopfiellasins A–D, two pairs of epimeric cytochalasins from kiwi-associated fungus Zopfiella sp. and their antibacterial assessment. Molecules. 2021;26:5611. doi: 10.3390/molecules26185611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X., Wu Z., Bao A., Zhao Z., Chen Y., Zhao H., Wang J., Chen C., Tong Q., Zhu H., et al. Asperflavipines C–E and aspermichalasine A: Three cytochalasan heterotetramers and an unusual cytochalasan monomer from Aspergillus micronesiensis. Org. Chem. Front. 2022;9:2585–2592. doi: 10.1039/D2QO00309K. [DOI] [Google Scholar]
- 8.Yang X., Wu P., Xue J., Li H., Wei X. Cytochalasans from endophytic fungus Diaporthe sp. SC-J0138. Fitoterapia. 2020;145:104611. doi: 10.1016/j.fitote.2020.104611. [DOI] [PubMed] [Google Scholar]
- 9.Long X., Wu H., Ding Y., Qu C., Deng J. Biosynthetically inspired divergent syntheses of merocytochalasans. Chem. 2021;7:212–223. doi: 10.1016/j.chempr.2020.11.010. [DOI] [Google Scholar]
- 10.Bao R., Tian C., Zhang H., Wang Z., Dong Z., Li Y., Gao M., Zhang H., Liu G., Tang Y. Total syntheses of asperchalasines A–E. Angew. Chem. Int. Ed. 2018;57:14216–14220. doi: 10.1002/anie.201808249. [DOI] [PubMed] [Google Scholar]
- 11.Hua C., Yang Y., Sun L., Dou H., Tan R., Hou Y. Chaetoglobosin F, a small molecule compound, possesses immunomodulatory properties on bone marrow-derived dendritic cells via TLR9 signaling pathway. Immunobiology. 2013;218:292–302. doi: 10.1016/j.imbio.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Ye K., Ai H.-L., Liu J.-K. Identification and bioactivities of secondary metabolites derived from endophytic fungi isolated from ethnomedicinal plants of Tujia in Hubei Province: A review. Nat. Prod. Bioprospect. 2021;11:185–205. doi: 10.1007/s13659-020-00295-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Q.-C., Kong M.-Z., Zhao Q., Chen G.-D., Tian H.-Y., Li X.-X., Guo L.-D., Li J., Zheng Y.-Z., Gao H. Chaetoglobosin Y, a new cytochalasan from Chaetomium globosum. Fitoterapia. 2014;93:126–131. doi: 10.1016/j.fitote.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Evidente A., Andolfi A., Vurro M., Zonno M.C., Motta A. Cytochalasins Z4, Z5, and Z6, three new 24-oxa[14]cytochalasans produced by Phoma exigua var. heteromorpha. J. Nat. Prod. 2003;66:1540–1544. doi: 10.1021/np030252o. [DOI] [PubMed] [Google Scholar]
- 15.Gupta G.P., Massagué J. Cancer metastasis: Building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Nakashima K., Tomida J., Kamiya T., Hirai T., Morita Y., Hara H., Kawamura Y., Adachi T., Inoue M. Diaporthols A and B: Bioactive diphenyl ether derivatives from an endophytic fungus Diaporthe sp. Tetrahedron Lett. 2018;59:1212–1215. doi: 10.1016/j.tetlet.2018.02.032. [DOI] [Google Scholar]
- 17.Yahagi H., Yahagi T., Furukawa M., Matsuzaki K. Antiproliferative and antimigration activities of beauvericin isolated from Isaria sp. on pancreatic cancer cells. Molecules. 2020;25:4586. doi: 10.3390/molecules25194586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monteillier A., Allard P.-M., Gindro K., Wolfender J.-L., Cuendet M. Lung cancer chemopreventive activity of patulin isolated from Penicillium vulpinum. Molecules. 2018;23:636. doi: 10.3390/molecules23030636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan B.-C., Wang W.-G., Hu D.-B., Sun X., Kong L.-M., Li X.-N., Du X., Luo S.-H., Liu Y., Li Y., et al. Phomopchalasins A and B, two cytochalasans with polycyclic-fused skeletons from the endophytic fungus Phomopsis sp. shj2. Org. Lett. 2016;18:1108–1111. doi: 10.1021/acs.orglett.6b00214. [DOI] [PubMed] [Google Scholar]
- 20.Tang J.-W., Kong L.-M., Zu W.-Y., Hu K., Li X.-N., Yan B.-C., Wang W.-G., Sun H.-D., Puno P.-T. Isopenicins A–C: Two types of antitumor meroterpenoids from the plant endophytic fungus Penicillium sp. sh18. Org. Lett. 2019;21:771–775. doi: 10.1021/acs.orglett.8b04020. [DOI] [PubMed] [Google Scholar]
- 21.Xia J.-N., Hu K., Su X.-Z., Tang J.-W., Li X.-N., Sun H.-D., Puno P.-T. Discovery of ent-kaurane diterpenoids, characteristic metabolites of Isodon species, from an endophytic fungal strain Geopyxis sp. XY93 inhabiting Isodon parvifolia. Fitoterapia. 2022;158:105160. doi: 10.1016/j.fitote.2022.105160. [DOI] [PubMed] [Google Scholar]
- 22.Su X.-Z., Zhu Y.-Y., Tang J.-W., Hu K., Li X.-N., Sun H.-D., Li Y., Puno P.-T. Pestaloamides A and B, two spiro-heterocyclic alkaloid epimers from the plant endophytic fungus Pestalotiopsis sp. HS30. Sci. China Chem. 2020;63:1208–1213. doi: 10.1007/s11426-020-9762-0. [DOI] [Google Scholar]
- 23.Izawa Y., Hirose T., Shimizu T., Koyama K., Natori S. Six new 10-pheynl-[11]cytochalasans, cytochalasins N–S from Phomopsis sp. Tetrahedron. 1989;45:2323–2335. doi: 10.1016/S0040-4020(01)83434-7. [DOI] [Google Scholar]
- 24.Shang Z., Raju R., Salim A.A., Khalil Z.G., Capon R.J. Cytochalasins from an Australian marine sediment-derived Phomopsis sp. (CMB-M0042F): Acid-mediated intramolecular cycloadditions enhance chemical diversity. J. Org. Chem. 2017;82:9704–9709. doi: 10.1021/acs.joc.7b01793. [DOI] [PubMed] [Google Scholar]
- 25.Kakeya H., Morishita M., Onozawa C., Usami R., Horikoshi K., Kimura K., Yoshihama M., Osada H. RKS-1778, a new mammalian cell-cycle inhibitor and a key intermediate of the [11] cytochalasin group. J. Nat. Prod. 1997;60:669–672. doi: 10.1021/np970151o. [DOI] [PubMed] [Google Scholar]
- 26.Huang X., Zhou D., Liang Y., Liu X., Cao F., Qin Y., Mo T., Xu Z., Li J., Yang R. Cytochalasins from endophytic Diaporthe sp. GDG-118. Nat. Prod. Res. 2021;35:3396–3403. doi: 10.1080/14786419.2019.1700504. [DOI] [PubMed] [Google Scholar]
- 27.Scherlach K., Boettger D., Remme N., Hertweck C. The chemistry and biology of cytochalasans. Nat. Prod. Rep. 2010;27:869–886. doi: 10.1039/b903913a. [DOI] [PubMed] [Google Scholar]
- 28.Qiao K., Chooi Y.-H., Tang Y. Identification and engineering of the cytochalasin gene cluster from Aspergillus clavatus NRRL 1. Metab. Eng. 2011;13:723–732. doi: 10.1016/j.ymben.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
X-ray crystallographic data of 1 and 3 (CIF) are available free of charge from the CCDC at https://www.ccdc.cam.ac.uk (accessed on 1 May 2022).