Abstract
We investigated the capability of the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22 (T-22) to solubilize in vitro some insoluble or sparingly soluble minerals via three possible mechanisms: acidification of the medium, production of chelating metabolites, and redox activity. T-22 was able to solubilize MnO2, metallic zinc, and rock phosphate (mostly calcium phosphate) in a liquid sucrose-yeast extract medium, as determined by inductively coupled plasma emission spectroscopy. Acidification was not the major mechanism of solubilization since the pH of cultures never fell below 5.0 and in cultures containing MnO2 the pH rose from 6.8 to 7.4. Organic acids were not detected by high-performance thin-layer chromatography in the culture filtrates. Fe2O3, MnO2, Zn, and rock phosphate were also solubilized by cell-free culture filtrates. The chelating activity of T-22 culture filtrates was determined by a method based on measurement of the equilibrium concentration of the chrome azurol S complex in the presence of other chelating substances. A size exclusion chromatographic separation of the components of the culture filtrates indicated the presence of a complexed form of Fe but no chelation of Mn. In liquid culture, T. harzianum T-22 also produced diffusible metabolites capable of reducing Fe(III) and Cu(II), as determined by the formation of Fe(II)-Na2-bathophenanthrolinedisulfonic acid and Cu(I)-Na2-2,9-dimethyl-4,7-diphenyl-1,10-phenanthrolinedisulfonic acid complexes. This is the first report of the ability of a Trichoderma strain to solubilize insoluble or sparingly soluble minerals. This activity may explain, at least partially, the ability of T-22 to increase plant growth. Solubilization of metal oxides by Trichoderma involves both chelation and reduction. Both of these mechanisms also play a role in biocontrol of plant pathogens, and they may be part of a multiple-component action exerted by T-22 to achieve effective biocontrol under a variety of environmental conditions.
Numerous microorganisms, especially those associated with roots, have the ability to increase plant growth and productivity (5, 20). In a few cases, this effect has been suggested to involve solubilization of otherwise unavailable mineral nutrients (8, 11).
In soil, both macro- and micronutrients undergo a complex dynamic equilibrium of solubilization and insolubilization that is greatly influenced by the soil pH and microflora and that ultimately affects their accessibility to plant roots for absorption. Phosphorus is commonly deficient in most natural soils, since it is fixed as insoluble iron and aluminum phosphates in acidic soils (especially those with pH lower than 5.0) or calcium phosphates in alkaline soils (pH above 7.0). However, insoluble calcium phosphate can be dissolved and made available to plants by soil and rhizosphere microorganisms via a mechanism that is thought to involve the release of organic acids (8, 11).
Iron and manganese have been particular foci of studies on their solubilization by soil microflora, their availability to plants, and their effects on plant diseases (12). Some antagonistic root-colonizing pseudomonads react to limiting iron conditions by using a high-affinity iron uptake system based on the release of Fe3+-chelating molecules (siderophores). This chelated iron is not available to plant pathogens, whose activity is thereby reduced (3), while plant roots can take up chelated iron either directly or after reduction of Fe3+ by plasma membrane reductases (30). Manganese is a microelement required for diverse physiological functions in plants and plays a major role in both plant growth and disease resistance (12). Manganese can occur in several oxidation states, but it is available to plants only in the reduced form (Mn2+). Higher oxidation states are insoluble. The oxidation state of soil manganese depends on both the soil conditions (pH values below 6 favor reduction and values above 6.5 favor oxidation) and the activity of rhizosphere microorganisms that can either oxidize or reduce manganese and thus influence its availability (15).
Thus, microbial interactions with plant roots are known to profoundly affect plant nutrient status and, for manganese at least, to affect plant resistance to pathogens (15). Trichoderma species are among the most commonly studied biocontrol microbes (14, 25), and they also exhibit plant-growth-promoting activity (6, 10, 19, 31). The classical mechanisms suggested for biocontrol are mycoparasitism, antibiosis, competition (6), and induction of defense responses in host plants (32). Most probably, other mechanisms must also operate to provide the increase in plant growth. In spite of their theoretical and practical importance, the mechanisms responsible for the growth response due to Trichoderma harzianum have not been investigated extensively. Since growth enhancement has been observed in the absence of any detectable disease (5) and in sterile soil (31), it is not thought to be a side effect of suppression of disease or minor plant pathogens. Other mechanisms, including production of hormone-like metabolites and release of nutrients from soil or organic matter, have been proposed (19, 31). However, there is almost no experimental data on the ability of Trichoderma spp. to solubilize plant nutrients. Should these fungi be found to possess solubilizing abilities, this mechanism might account for at least some of their plant-growth-promoting and biocontrol abilities and would provide new opportunities to study their interactions with plants. If some strains of Trichoderma possess the ability to solubilize many different nutrients, it would not be surprising to find that multiple mechanisms are involved, even for a single element. For example, solubilization of iron may involve reduction from Fe3+ to Fe2+ as well as chelation of Fe3+ by siderophores or chelating agents (9). Therefore, studies of this general mechanism will probably require the purification of large numbers of biomolecules and the subsequent study of their interactions with both plants and microbes.
The purpose of the present work was to determine whether T. harzianum is able to solubilize various nutrients in vitro. In this study, we have demonstrated for the first time that this fungus is able to solubilize several sparingly soluble minerals and that with some elements, chelating agents are involved. This study provides a basis for future detailed studies on the numerous specific biomolecules involved in these interactions and, subsequently, how they affect both microbial and plant growth.
MATERIALS AND METHODS
T. harzianum strain.
The strain of Trichoderma used for this study was T. harzianum Rifai 1295-22 (T-22). T-22 is an effective biocontrol agent of several soilborne plant diseases, including Pythium, Rhizoctonia and Fusarium root rots (14). Strain T-22 has beneficial effects on plant growth and vigor and on the development and efficiency of the root systems of several crops including bean, potato, and corn (4, 14).
Solubilization of minerals in liquid culture.
For solubilization experiments, the following insoluble or sparingly soluble minerals were used: MnO2, Fe2O3, CuO, granular metallic zinc, and rock phosphate containing about 32% phosphorus (mostly as calcium phosphate). The various minerals (50 mg) were added to 100 ml of a sucrose-yeast extract (SY) medium (30 g of sucrose and 1 g of yeast extract per liter of distilled water) in 250-ml Erlenmeyer flasks. The pH of the medium after autoclave sterilization (121°C for 15 min) was 6.2 to 6.5. Flasks were inoculated with 1 ml of a conidial suspension of T-22 containing 106 conidia/ml. The cultures were incubated on a rotary shaker (160 rpm) at 25°C for 5 days. Three replicate flasks were sampled each day. Culture filtrates were filtered in turn through no. 5 paper (Whatman, Maidstone, England) and 0.45- and 0.22-μm-pore-size filters of mixed esters of cellulose (Millipore, Bedford, Mass.), and the pH of the filtrates was measured. Noninoculated flasks, processed in the same way, were used as checks. Clear culture filtrates were acidified with a few drops of concentrated HCl to prevent the loss of soluble ions and microbial growth until the analyses were completed. Acidified filtrates, or dilutions thereof, were analyzed by inductively coupled argon plasma emission spectroscopy (ICP) on a Jarrell-Ash model Trace Analyzer spectrometer, using an axial plasma and simultaneous acquisition of emission data for 24 elements including Ca, Fe, Mn, P, and Zn.
Solubilization of minerals by cell-free culture filtrates.
Sterile filtrates of three replicate 100-ml cultures of T-22 on SY medium and of three noninoculated check flasks incubated for 5 days at 25°C on a rotary shaker (160 rpm) were obtained by 0.22-μm-pore-size-filter sterilization. In some experiments, filtrates from each flask were divided into three 30-ml aliquots that were autoclaved at 121°C for 20 min, digested with 50 μg of protease K (Sigma, St. Louis, Mo.) per ml for 4 h at 37°C, or stored at −80°C. Then 5 ml of filtrate from each of the three above treatments was dispensed into oven-sterilized test tubes containing 5 mg of CuO, Fe2O3, MnO2, granular metallic zinc, or rock phosphate. Tubes without minerals were prepared in the same way to provide the initial content of elements in medium and culture filtrate (controls). The tubes were incubated at room temperature for 48 h on a reciprocal shaker. The broths were then filtered through 0.22-μm-pore-size filters, and the filtrates were analyzed by ICP.
Analysis of organic acids.
The culture filtrates were analyzed for the presence of oxalic acid, citric acid, dl-malic acid, succinic acid, dl-lactic acid, and fumaric acid by high-performance thin-layer chromatography. Both raw and 10-fold-concentrated culture filtrates were acidified to pH 2.0 with 0.1 N HCl and then applied, along with standard solutions of the above organic acids, to precoated cellulose plates (10 by 10 cm; thickness, 0.1 mm [Merck, Darmstadt, Germany]). The plates were developed with diethyl ether-formic acid-water (70:20:10), and acids were visualized with 0.045% bromophenol blue in 95% ethanol. The detection limit of the method was 10 μg/ml for all the acids analyzed.
Chemical assay for detection of chelators in culture filtrates.
The chelating activity of T-22 culture filtrates was assayed by the method described by Shenker et al. (27). This method is based on the measurement of the equilibrium concentration of the Cu-CAS (chrome azurol S) complex in the presence of other chelating substances. In the presence of complexing substances in the filtrates, the Cu is competitively lost from the Cu-CAS complex and a decrease in absorbance occurs. The method was adapted to use 96-well microtitration plates for spectrophotometric measurements. To avoid interference due to the presence of complexing substances (impurities, proteins, and small organic residues) in the SY medium, T-22 was grown in modified Richard’s solution (RMT) [containing, in grams per liter, KNO3, 10; KH2PO4, 5; MgSO4, 1.3; FeCl3, 0.02; sucrose, 20; ZnSO4 · 7 H2O, 0.0035; CuSO4 · 5 H2O, 0.0004; MnSO4 · H2O, 0.00031; and (NH4)6Mo7O24 · 4 H2O, 0.00013; adjusted to pH 7.0 with NaOH]. Culture filtrates were sampled daily over a 7-day period.
A stock solution of the Cu-CAS reagent was prepared with 200 μM CuCl2, 210 μM CAS (Sigma), and 40 mM (N-morpholino)ethanesulfonic acid (MES) (Sigma) with the pH adjusted to 5.7 with NaOH. The reagent solution and the culture filtrate were mixed in a 1:1 ratio in microtitration plate wells to a total volume of 100 μl, and the absorbance at 540 nm was measured. Noninoculated culture broth was used as a control.
Chromatographic separation of chelating metabolites.
Culture filtrates (25 ml) of T-22 grown in the presence of either Fe2O3 or MnO2 were lyophilized and redissolved in 5 ml of 20 mM NaCl. Concentrated culture filtrates, supplemented or not with an Fe2+ or Mn2+ salt (0.1 mM FeSO4 · 7 H2O or 0.3 mM MnSO4 · H2O), were then fractionated by gel permeation chromatography (GPC), and their elution profiles were compared with the elution profile of the noninoculated culture medium processed in the same way.
A Bio-Gel P-2 (Bio-Rad, Hercules, Calif.) polyacrylamide gel column (20 by 300 mm) was packed as specified by the manufacturer. The column was loaded with 1 ml of sample and eluted with 20 mM NaCl at a flow rate of 30 ml/h (about 10 cm/h). Fractions (3 ml) were collected every 6 min, acidified with concentrated HCl, and analyzed by ICP.
Fe3+ and Cu2+ reduction activity of culture filtrates.
Cultures of T-22 in RMT were grown, sampled in triplicate, and processed as described above. Reduction of Fe3+ and Cu2+ was assayed by the colorimetric reaction of the reduced forms (Fe2+ and Cu+) with disodium-bathophenanthrolinedisulfonic acid (BPDS; Sigma) and disodium 2,9-dimethyl-4,7-diphenyl-1,10-phenanthrolinedisulfonic acid (BCDS, Sigma), respectively (30). The reduction of Fe3+ and Cu2+ was quantified by spectrophotometric measurement of the Fe(II)-BPDS and Cu(I)-BCDS complexes. Assays were performed in 96-well plates. Each well contained (in a total volume of 100 μl) 30 μl of culture filtrate, 0.2 mM CaSO4, 5 mM MES buffer (pH 5.5), and either 0.1 mM Fe(III)-EDTA and 0.3 mM disodium BPDS for quantification of Fe3+ reduction, or 0.2 mM CuSO4, 0.6 mM trisodium citrate and 0.4 mM disodium BCDS for quantification of Cu2+ reduction. Noninoculated culture medium was used as a control. The increase in the absorbance [at 540 nm for Fe(II)-BPDS and 492 nm for Cu(I)-BCDS] of the assay solutions was measured after 4 h of incubation at 37°C in the dark. Preliminary experiments demonstrated that reduction of both Cu(II) and Fe(III) increased linearly with time during these assays.
RESULTS
Solubilization of minerals in liquid culture and by cell-free culture filtrates.
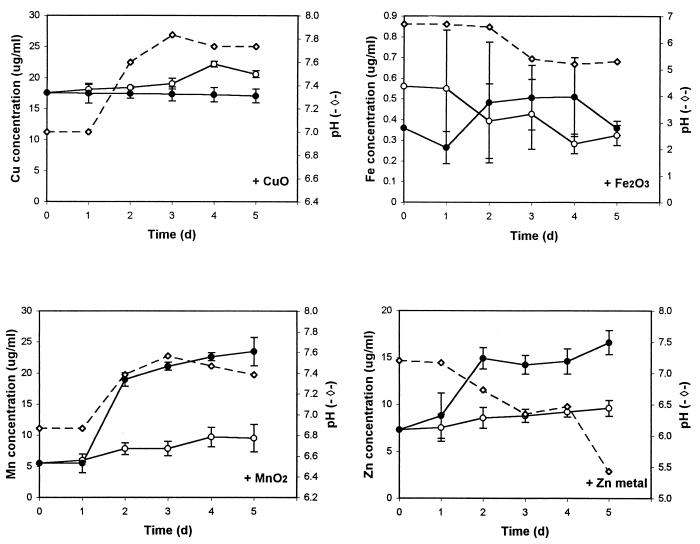
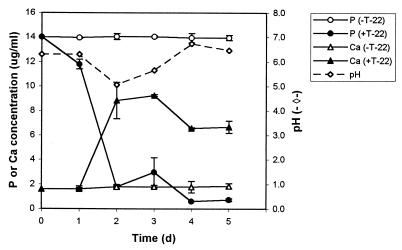
Cultures were sampled daily for 5 days of growth. During this period, T-22 reached the maximal level of biomass in the sucrose-yeast medium, as determined by the dry weight of cultures (data not shown). T-22 was able to solubilize MnO2, Zn (Fig. 1), and rock phosphate (Fig. 2) in the culture medium. In all the cultures, the pH values never fell below 5.0, and in cultures containing MnO2, the pH rose from 6.8 to 7.4. Data on solubilization of CuO and Fe2O3 in liquid culture were not conclusive because of highly variable data due to initial partial solubility of these minerals. Solubilization of rock phosphate was indicated by an increase in the concentration of soluble Ca (Fig. 2). However, the level of phosphorus in the culture filtrate decreased, probably due to uptake by T-22. In cultures supplemented with rock phosphate, the solubility of Fe, as well as that of Ca, increased (up to fivefold [0.1 μg/ml]) over the level in the control flasks, as result of Fe impurities in the rock phosphate (data not shown).
FIG. 1.
Concentrations of Fe, Cu, Mn, and Zn in T-22 cultures (●) and in control flasks (○) of an SY medium supplemented with CuO, Fe2O3, MnO2, and metallic zinc, respectively. The dashed line shows the pH of the cultures. Error bars indicate standard deviations of three replicate determinations.
FIG. 2.
Solubilization of rock phosphate. Concentrations of P and Ca in the SY medium in the presence (+) or absence (−) of T-22 are shown. Error bars indicate standard deviations of three replicate determinations.
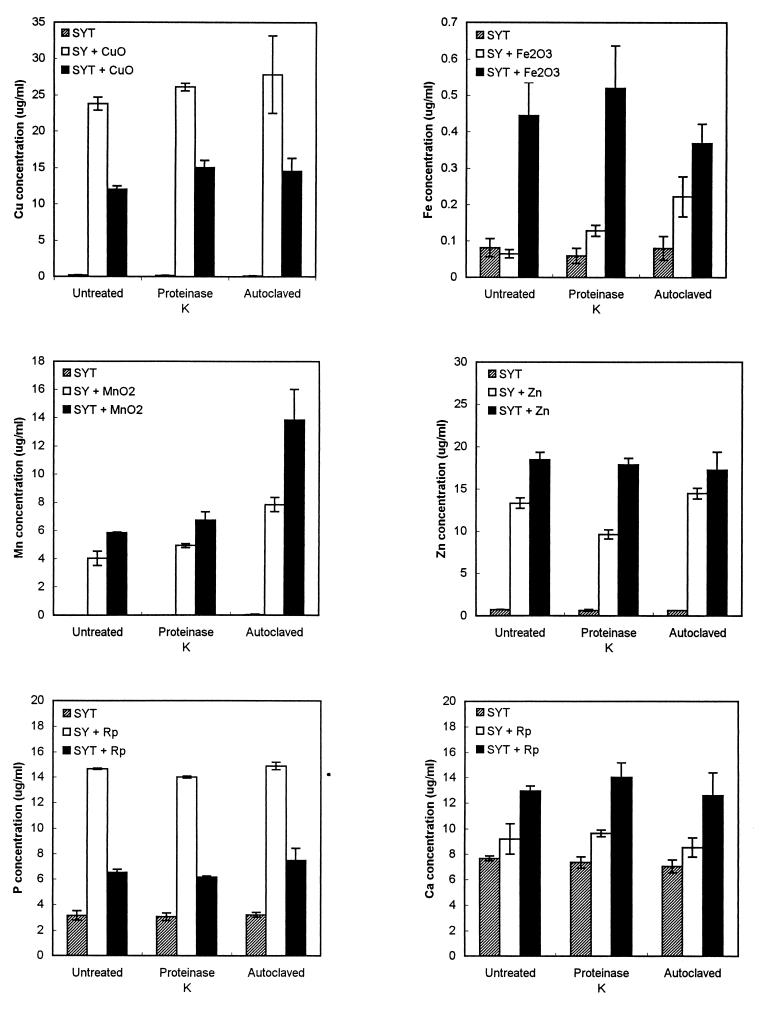
Experiments were also conducted to determine the ability of cell-free culture filtrates to solubilize various materials. Cell-free culture filtrates were able to solubilize every material tested (Fig. 3). However, the control filtrate consisting of noninoculated SY medium also was able to solubilize the same materials. SY medium alone released significantly more Cu from CuO and more P from rock phosphate than did the cell-free culture filtrates. In every other case, the filtrates released significantly more soluble materials than did SY medium (Fig. 3).
FIG. 3.
Solubilization of CuO, Fe2O3, MnO2, metallic zinc (Zn), and rock phosphate (Rp) by cell-free culture filtrates of T-22 grown in SY medium (SYT) in comparison with noninoculated medium filtrates (SY). Filtrates were either untreated, autoclaved (121°C for 20 min), or digested with protease K (50 μg/ml for 4 h at 37°C). Inoculated SY medium not supplemented with any of the above minerals provided the basic levels of the various elements in culture filtrates. Error bars indicate standard deviations of three replicate determinations.
To study the heat stability and the possible proteinaceous nature of the substances that solubilized the various materials, the solubilizing activity of autoclaved or proteinase K-treated culture filtrates was assayed in comparison with the crude culture filtrate (Fig. 3). With the exception of MnO2, no differences in solubilizing capability were found among untreated and treated filtrates, suggesting that solubilizing substances are heat stable and not proteinaceous. Fe2O3, MnO2, metallic zinc, and rock phosphate were all solubilized by cell-free culture filtrates. The high level of Cu detected in noninoculated filtrates in the presence of CuO is consistent with the previous observation that this mineral is rather soluble in the medium.
Production of organic acids and chelating metabolites.
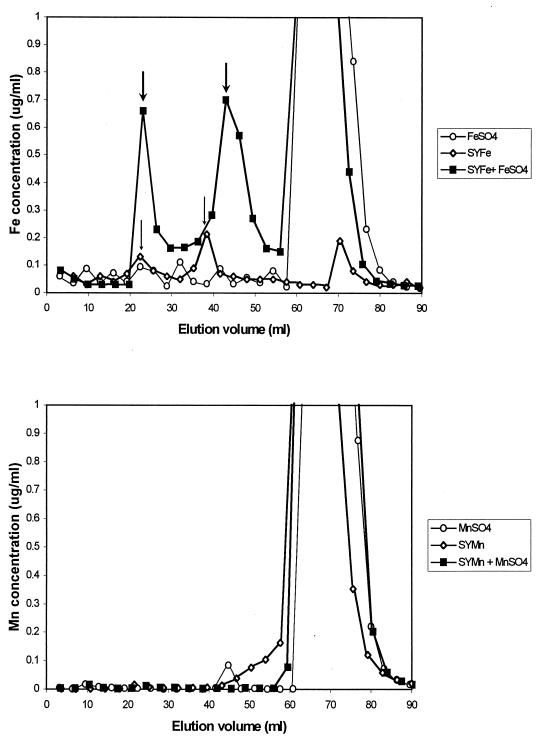
Oxalic acid, citric acid, dl-malic acid, succinic acid, dl-lactic acid, and fumaric acid were not found in the culture filtrates at the detection limit of 10 μg/ml. The chemical assay (Cu-CAS) for chelating activity showed a decrease in the absorbance at 540 nm of the T-22 culture filtrates, indicating the presence of complexing metabolites. The chelating activity was first detected in 3-day-old culture filtrates, and its level did not change (data not shown). However, the Cu-CAS assay only indicated that chelating metabolites were produced in culture; it did not provide any information about the actual state (free or chelated) of metal ions in the culture filtrates. For this reason, we performed a GPC separation of the components of the culture filtrates. In a preliminary experiment, this method proved to be suitable to separate free Fe2+ ions from EDTA-chelated Fe3+ (data not shown). The chromatogram of the filtrates from cultures supplemented with MnO2 showed one single exit peak of Mn at the elution time of free ions (Mn2+), while the chromatogram of the filtrates from cultures supplemented with Fe2O3 showed two additional peaks, suggesting that Fe ions were complexed by metabolites from T-22 (Fig. 4).
FIG. 4.
GPC of the culture filtrates of T-22 grown on SY medium (◊) in the presence of Fe2O3 (top) or MnO2 (bottom). The exit of free Fe2+ or Mn2+ ions occurred at an elution volume greater than 50 ml (○). When the filtrate SYFe was applied along with 0.1 mM FeSO4 · 7 H2O (■), an increase in the height of two peaks at 25 and 40 ml (arrows) occurred as a result of the complexed state of the Fe ions.
Fe3+ and Cu2+ reduction activity of culture filtrates.
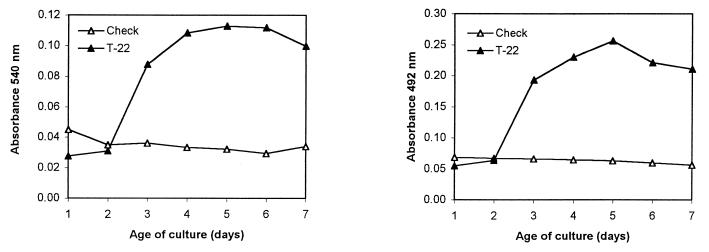
In liquid culture, strain T-22 produced diffusible metabolites capable of reducing Fe(III) and Cu(II), as determined by the formation of Fe(II)-BPDS and Cu(I)-BCDS complexes (Fig. 5). The level of reducing metabolites in culture filtrates paralleled the growth and weight increase of the cultures, reaching a maximum on days 5 to 6 and decreasing later. Extensive dialysis of the culture filtrates through a membrane with a 6,000 to 8,000-Da cutoff resulted in total loss of the Fe(III)-reducing activity. This suggests that the T-22 Fe(III)-reducing metabolite is either a small molecule or a larger molecule that requires a small cofactor for activity. On the other hand, Cu(II)-reducing activity was still present after dialysis, albeit at a lower level (about 65% of the initial activity). Therefore, at least a portion of copper-reducing activity is associated with a macromolecule.
FIG. 5.
Fe(III) and Cu(II) reduction by T-22 culture filtrates. The amount of Fe(II)-BPDS or Cu(I)-BCDS was determined spectrophotometrically by the increase of the absorbance (540 and 492 nm, respectively) after 4 h of incubation at 37°C (for more details, see the text).
DISCUSSION
Most of the recent literature concerning microbial solubilization of minerals in soil and their potential use for enhancement of soil fertility deals with P-solubilizing bacteria and vesicular-arbuscular mycorrhizal fungi. However, the ability of a few filamentous nonmycorrhizal fungi, especially Aspergillus spp. and Penicillium spp., to solubilize mineral nutrients has also been shown (1, 17, 23), and a commercial formulation of Penicillium bilaii Chalabuda has been registered in Canada as a biological enhancer of plant nutrition (8).
The release of organic acids that both sequester cations and acidify the microenvironment near roots is thought to be a major mechanism of solubilization of P, as well as Mn, Fe, and Zn, by plants and non-vesicular-arbuscular mycorrhizal fungi (8). However, it has been pointed out that in some instances solubilization occurs in the absence of detectable chelating agents or organic acids, mainly as a result of the acidification of the medium (2, 18). In our study, oxalic acid, citric acid, dl-malic acid, succinic acid, dl-lactic acid, and fumaric acid were not detected in the culture filtrates of T-22. Furthermore, under the experimental conditions used, solubilization of rock phosphate, MnO2, Fe2O3, and Zn occurred at very slightly acidic or alkaline pH values. These data indicate that neither reduction of pH nor production of simple organic acids accounted for the solubilization of the various materials in this study. Soluble P apparently was removed from the culture as rapidly as it was solubilized and was sequestered in the T-22 mycelium. Halvorson et al. (13) suggest that continuous removal of P from the solution and the consequent disturbance of the equilibrium between insoluble and soluble P can account for solubilization of P by fungi under alkaline conditions. A major feature of strain T-22 is its capability to grow along roots during their elongation, thus colonizing the whole root system and benefiting the crop for its entire life (rhizosphere competence) (28). Therefore, P could be solubilized and stored in the Trichoderma biomass to be released in a readily available form in close proximity to the roots after lysis of the mycelium with age. Interestingly, it has been reported that the phytopathogenic fungi Pythium and Rhizoctonia are unable to solubilize phosphates (1). Thus, the high competitive efficiency of T-22 in P uptake might play a role in the suppression of these plant pathogens.
The analysis of the fractions obtained by GPC has shown that compounds in T-22 culture filtrates are able to chelate iron. Therefore, chelation accounts at least partially for solubilization of this nutrient, whereas it does not seem to be involved in the solubilization of manganese. With regard to the chemical nature of the complexing substances, organic acids were not detected in T-22 culture filtrates. However, besides organic acids, several other metabolites or constituents of fungi, such as peptides, proteins, phenolics, and chitin, might exhibit complexing properties, and their production and involvement in Fe chelation should be investigated. As far as we are aware, hydroxamate siderophores, especially coprogens and dimerum acid, are produced in other fungi but not Trichoderma. These compounds are potent chelators of iron (9, 22). Fe-chelated coprogens and other hydroxamate siderophores are taken up by plants and provide Fe to them (7). If similar compounds are produced by T-22, they would be readily available to plants since the fungus is located on root surfaces. Purification and characterization of the siderophores produced by T-22 is under way. A major role in disease control and plant growth enhancement has been proposed for siderophores produced by some biocontrol bacteria (21). Unlike bacterial siderophores, the production of the Trichoderma metabolite(s) able to chelate iron does not require Fe deficiency.
In the assimilatory process of metal ions, siderophores and other chelating substances often act in association with a reductive enzymatic mechanism. For instance, iron is absorbed in the reduced form, Fe2+, by root cells of nongrass plant species. However, in well-aerated soils, the Fe(III) oxidation state of Fe predominates, and it must be reduced to Fe(II) in order to be taken up by plants in sufficient quantities for their metabolic needs. Fe uptake by the roots is thought to be regulated by the activity of a plasma membrane redox system, namely, Fe(III) chelate reductase, which reduces Fe(III) from various Fe(III) chelates to Fe(II). The Fe(III) chelate reductase also seems to be capable of reducing other metals, such as Mn(III, IV) and Cu(II), and its activity is stimulated by Fe and Cu deficiency conditions (24). Furthermore, it is thought to play a general role in the regulation of cation absorption by root cells (30). Our data show that strain T-22 produces diffusible metabolites capable of reducing Fe(III) and Cu(II). Similar to the production of chelating substances, synthesis of reducing metabolites appears to be a constitutive trait of T-22. Presumably, the Fe- and/or Cu-reducing metabolite(s) also reduces Mn4+ to the soluble form, Mn2+, since solubilization of MnO2 occurs in the absence of either acidification or chelation. Björkman et al. (4) reported that T-22 was able to enhance the vigor of cold- and toxicant-stressed maize seeds and to restore the vigor of hypochlorite-treated seeds. Our findings are consistent with the hypothesis that the beneficial effect of T-22 is related to its ability to restore or prevent the oxidative damage of hypochlorite by means of a reducing mechanism of some kind. Although our data suggest that solubilization of Mn, and possibly of Fe and Cu, occurs by means of a reducing mechanism, proteins apparently are not involved. The reducing activity was produced extracellularly, and solubilization occurred even after heat or proteinase K treatment of the culture filtrates. On the other hand, at least the Cu(II)-reducing activity was not lost after extensive dialysis of the culture filtrates (6,000 to 8,000-Da cutoff), and this suggests the involvement of macromolecules.
Although the reducing activity found in T-22 culture filtrates accounts, at least partially, for solubilization of metal oxides, solubilization of metallic zinc occurs by a different process. The stable oxidation state for Zn is Zn2+, which is the form required by plants (29) and fungi (26). Hence, solubilization of metallic zinc (oxidation state 0) depends on its oxidation to Zn2+. T-22 proved to have the ability in culture to accelerate the oxidative dissolution of metallic zinc, releasing Zn2+. In this case, the effect of the fungus may include the release of complexing ligands which sequester Zn2+, thereby increasing the dissolution of metallic zinc in the culture medium.
While the importance of mineral nutrition for plant growth and vigor is obvious, the role of nutrients, especially micronutrients, in plant disease resistance and root stress tolerance is less well known but is gaining increasing recognition (12, 16). Many of the micronutrients are required for polyphenol formation and other aspects of phenolic metabolism, and hence they are crucial for plant defense capabilities. Of the micronutrients, Mn may prove to be the most important in the development of resistance of plants to both root and foliar diseases of fungal origin. Manganese is required for several physiological functions of plants such as photosynthesis, N metabolism (especially reduction of nitrate), and synthesis of aromatic ring compounds as precursors for some amino acids, hormones (auxins), phenols, and lignin. As a consequence, it plays a major role in the growth and disease resistance of plants. The association of several plant diseases (e.g., take-all disease of wheat, wilt of tomato, blast of rice, and common scab of potato) with low Mn availability in soil has long been known (16). In the cases of scab of potato and take-all of wheat, caused by Streptomyces scabies and Gaeumannomyces graminis var. tritici, respectively, the Mn-oxidizing capability of the pathogen proved to be part of the mechanism of pathogenicity (15, 16). Although the correlation between the availability of other micronutrients and plant diseases has been investigated less extensively, it is generally accepted that the addition of micronutrients to plant crops results in a decrease in the incidence of many diseases (12).
The information gathered in this study indicates that the biocontrol and plant-growth-promoting fungus T. harzianum T-22 has the ability to solubilize many plant nutrients from their solid-phase compounds (e.g., rock phosphate, MnO2, Fe2O3, and metallic zinc), at least in vitro. To the best of our knowledge, this is the first report of the ability of a Trichoderma strain to solubilize insoluble or sparingly soluble minerals. A wide range of mechanisms and chemical entities may be involved in the solubilization of different materials. For example, the fungus produced substances that chelated Fe but not Mn. Furthermore, Fe3+ and Cu2+ were reduced to Fe2+ and Cu+ but these reductions were due to different substances. In addition, the substances that chelate Fe are unlikely to be the same materials that reduce Fe3+ to Fe2+; the fungal metabolites known to chelate iron are not also known to have reducing ability (9). In preliminary further studies, there appear to be several substances that chelate iron, based on thin-layer chromatography separations. Finally, the mechanism for the nonreductive solubilization of metallic zinc is not known but probably is dissimilar to those already cited, as is the mechanism for solubilization of rock phosphate. Chelation and/or redox activity are known to play a role in biocontrol of plant pathogens and might be part of a multiple-component action exerted by T-22 to achieve effective biocontrol under a variety of environmental conditions.
This summation indicates that T. harzianum possesses a range of different mechanisms to solubilize and, in some cases, chelate various plant nutrient compounds. Further progress in this new field requires that we understand these mechanisms and identify the chemical components involved in them. These mechanistic and biochemical studies are under way. Only when these studies are complete can we design definitive strains and studies to determine how these substances influence Trichoderma-plant interactions.
ACKNOWLEDGMENTS
C. Altomare’s research at NYSAES/Cornell University was supported in part by a CNR and a NATO-CNR senior fellowship. Research in the United States was supported in part by grant IS-2880-97 from the US-Israel Binational Agricultural Research and Development Fund.
C. Altomare heartily thanks D. Houghton, K. L. Ondik, and R. Petzoldt for their helpful assistance and their friendship. The valuable assistance of K. L. Ondik and A. Llobell in the revision of the manuscript is also gratefully acknowledged.
REFERENCES
- 1.Agnihotri V P. Solubilization of insoluble phosphates by some soil fungi isolated from nursery seedbeds. Can J Microbiol. 1970;16:877–880. doi: 10.1139/m70-148. [DOI] [PubMed] [Google Scholar]
- 2.Asea P E A, Kucey R M N, Stewart J W B. Inorganic phosphate solubilization by two Penicillium species in solution culture and soil. Soil Biol Biochem. 1988;20:459–464. [Google Scholar]
- 3.Baker R, Elad Y, Sneh B. Physical, biological and host factors in iron competition in soils. In: Swinburne T R, editor. Iron siderophores, and plant diseases. New York, N.Y: Plenum Publishing Corp.; 1986. pp. 77–84. [Google Scholar]
- 4.Björkman T, Blanchard L M, Harman G E. Growth enhancement of shrunken-2 (sh2) sweet corn by Trichoderma harzianum 1295-22: effect of environmental stress. J Am Soc Hortic Sci. 1998;123:35–40. [Google Scholar]
- 5.Chang Y-C, Chang Y-C, Baker R, Kleifeld O, Chet I. Increased growth of plants in the presence of the biological control agent Trichoderma harzianum. Plant Dis. 1986;70:145–148. [Google Scholar]
- 6.Chet I. Trichoderma-application, mode of action, and potential as a biocontrol agent of soilborne plant pathogenic fungi. In: Chet I, editor. Innovative approaches to plant disease control. New York, N.Y: John Wiley & Sons, Inc.; 1987. pp. 137–160. [Google Scholar]
- 7.Crowley D E, Reid C P R, Szaniszlo P J. Utilization of microbial siderophores in iron acquisition by oat. Plant Physiol. 1988;87:680–685. doi: 10.1104/pp.87.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham J E, Kuiack C. Production of citric and oxalic acids and solubilization of calcium phosphate by Penicillium bilaii. Appl Environ Microbiol. 1992;58:1451–1458. doi: 10.1128/aem.58.5.1451-1458.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dori S, Solel Z, Kashman Y, Barash I. Characterization of hydroxamate siderophores and siderophore-mediated iron uptake in Gaeumannomyces graminis var. tritici. Physiol Mol Plant Pathol. 1990;37:97–106. [Google Scholar]
- 10.Duffy B K, Simon A, Weller D M. Combination of Trichoderma koningii with fluorescent pseudomonads for control of take-all on wheat. Phytopathology. 1996;86:188–194. [Google Scholar]
- 11.Goldstein A H. Recent progress in understanding the molecular genetics and biochemistry of calcium phosphate sulubilization by gram negative bacteria. Biol Agric Hortic. 1995;12:185–193. [Google Scholar]
- 12.Graham R D, Webb M J. Micronutrients and disease resistance and tolerance in plants. In: Welch R M, editor. Micronutrients in agriculture. Madison, Wis: Soil Science Society of America; 1991. pp. 329–370. [Google Scholar]
- 13.Halvorson H O, Keynan A, Kornberg H L. Utilization of calcium phosphates for microbial growth at alkaline pH. Soil Biol Biochem. 1990;22:887–890. [Google Scholar]
- 14.Harman G E, Björkman T. Potential and existing uses of Trichoderma and Gliocladium for plant disease control and plant growth enhancement. In: Harman G E, Kubicek C P, editors. Trichoderma and Gliocladium. Vol. 2. London, United Kingdom: Taylor and Francis; 1998. pp. 229–265. [Google Scholar]
- 15.Huber D M, McCay-Buis T S. A multiple component analysis of the take-all disease of cereals. Plant Dis. 1993;77:437–447. [Google Scholar]
- 16.Huber D M, Wilhelm N S. The role of manganese in resistance to plant diseases. In: Graham R D, Hannam R J, Uren N C, editors. Manganese in soils and plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. pp. 155–173. [Google Scholar]
- 17.Illmer P, Schinner F. Solubilization of inorganic phosphates by microorganisms isolated from forest soils. Soil Biol Biochem. 1992;24:389–395. [Google Scholar]
- 18.Illmer P, Schinner F. Solubilization of inorganic calcium phosphates—solubilization mechanisms. Soil Biol Biochem. 1995;27:257–263. [Google Scholar]
- 19.Kleifeld O, Chet I. Trichoderma harzianum—interaction with plants and effect on growth response. Plant Soil. 1992;144:267–272. [Google Scholar]
- 20.Kloepper J W, Hume D J, Scher F M, Singleton C, Tipping B, Laliberte M, Frauley K, Kutchaw T, Simonson C, Lifshitz R, Zaleska I, Lee L. Growth-promoting rhizobacteria on canola (rapeseed) Plant Dis. 1988;72:42–46. [Google Scholar]
- 21.Loper J E, Buyer J S. Siderophores in microbial interactions on plant surfaces. Mol Plant-Microbe Interact. 1991;4:5–13. [Google Scholar]
- 22.Manulis S, Kashman Y, Barash I. Identification of siderophores and siderophore-mediated uptake of iron in Stemphylium botryosum. Phytochemistry. 1987;26:1317–1320. [Google Scholar]
- 23.Molla M A Z, Chowdhury A A, Islam A, Hoque S. Microbial mineralization of organic phosphate in soil. Plant Soil. 1984;78:393–399. [Google Scholar]
- 24.Norvell W A, Welch R M, Adams M L, Kochian L V. Reduction of Fe(III), Mn(III), and Cu(II) chelates by roots of pea (Pisum sativum L.) or soybean (Glycine max) Plant Soil. 1993;155/156:123–126. [Google Scholar]
- 25.Papavizas G C. Trichoderma and Gliocladium: biology, ecology, and potential for biocontrol. Annu Rev Phytopathol. 1985;23:23–54. [Google Scholar]
- 26.Ross I S. Uptake of zinc by fungi. In: Winkelmann G, Winge D R, editors. Metal ions in fungi. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 237–257. [Google Scholar]
- 27.Shenker M, Hadar Y, Chen Y. Rapid method for accurate determination of colorless siderophores and synthetic chelates. Soil Sci Soc Am J. 1995;59:1612–1618. [Google Scholar]
- 28.Sivan A, Harman G E. Improved rhizosphere competence in a protoplast fusion progeny of Trichoderma harzianum. J Gen Microbiol. 1991;137:23–29. [Google Scholar]
- 29.Welch R M. Micronutrient nutrition in plants. Crit Rev Plant Sci. 1995;14:49–82. [Google Scholar]
- 30.Welch R M, Norvell W A, Schaefer S C, Shaff J E, Kochian L V. Induction of iron(III) and copper(II) reduction in pea (Pisum sativum L.) roots by Fe and Cu status: does the root-cell plasmalemma Fe(III)-chelate reductase perform a general role in regulating cation uptake? Planta. 1993;190:555–561. [Google Scholar]
- 31.Windham M T, Elad Y, Baker R. A mechanism for increased plant growth induced by Trichoderma spp. Phytopathology. 1986;76:518–521. [Google Scholar]
- 32.Yedidia L, Benhamou N, Chet I. Induction of defense responses in cucumber plants (Cucumis sativus L.) in the biocontrol agent Trichoderma harzianum. Appl Environ Microbiol. 1999;65:929–935. doi: 10.1128/aem.65.3.1061-1070.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]