Abstract
There are few data on the dynamics of SARS-CoV-2 viral manifestations in obese and overweight persons during each of the five waves that occurred in Romania during the last two years. As such, the purpose of this research was to characterize the variance in case severity, symptomatology, ICU hospitalizations, and mortality among overweight and obese individuals infected with the SARS-CoV-2 virus. We included 250 overweight and obese patients admitted to hospital with COVID-19, where 50 patients were selected from each of the five pandemic waves that existed in Romania until March 2022. A total of 113 patients with normal body mass index were included in the study. They were matched with overweight and obese patients by age, gender, and cardiovascular comorbidities to avoid the effect of confounding factors. Between the five waves of the COVID-19 pandemic in Romania, the present investigation found substantial changes in overweight and obese patient features. Obesity increases the risk of hospitalization, severe complications, and mortality from COVID-19. However, this unique demographic is disproportionately affected by obesity-related comorbidities, which contribute to these adverse outcomes. We advocate for the development of new guiding principles for the formulation of healthcare strategies aimed at high-prevalence special populations such as overweight and obese individuals, while also promoting pandemic containment and avoiding the recurrence of pandemic waves with the same guidelines that proved detrimental in terms of economic and human life loss.
Keywords: COVID-19, SARS-CoV-2 infection, obesity, overweight, disease severity
1. Introduction
The SARS-CoV-2 virus had a prompt worldwide spread since it was first identified in December 2019 in Wuhan City, Hubei Province, China [1,2,3]. The new coronavirus has a genetic sequence similar to that of the first coronavirus SARSCoV, the virus that caused the 2003 severe acute respiratory syndrome (SARS) pandemic [4,5]. They both include the coronavirus spike (S) protein. The cellular serine protease TMPRSS2 primes the S protein, allowing it to connect to the angiotensin converting enzyme 2 (ACE2) receptor and gain cellular entry [6]. In comparison to SARSCoV, the new SARS-CoV-2 has a greater affinity for ACE2, making it more easily transmissible [7,8]. Despite the association between SARS-CoV-2 and ACE2, there is presently no evidence that ACE inhibitors or angiotensin receptor blockers are associated with infection [9].
The most often seen symptoms of the 2019 coronavirus disease (COVID-19) are mostly unspecific, such as fever, fatigue and dry cough with high prevalence [10,11,12]. Pneumonia, pulmonary embolism, and acute respiratory distress syndrome (ARDS) are all respiratory symptoms and possible severe manifestations of severe COVID-19 [13,14,15]. All age groups are susceptible to infection, and the median age of hospitalized cohorts ranges between 50 and 60 years [16,17]. Initially, it was thought that men are more likely to be infected with the SARS-CoV-2 virus than women of similar age, and they account for a greater incidence among the hospitalized population requiring critical care, which may represent a difference in illness severity between the sexes [18,19]. However, the latest studies showed debatable results [20]. Additionally, these symptoms and manifestations have persisted more or less continuously during the course of the COVID-19 pandemic’s first 24 months; multiple studies indicate that distinct SARS-CoV-2 variants exhibit considerable differences in symptomatology and infection severity [21].
Several comorbidities have been studied in relationship with severe COVID-19, and it was observed than 25% of hospitalized patients and approximately three quarters of ICU patients with SARS-CoV-2 had at least one comorbid condition [22,23]. Comorbidities such as hypertension, cardiovascular disease, and diabetes mellitus were the most common and often mentioned in reports around the world [24,25], and all are known to be connected with obesity, and indeed, obesity is increasingly recognized as both a comorbidity and a risk factor [26,27]. Obesity prevalence rises with age in both men and women, having significant consequences for global health, since excess weight, as measured by an elevated body mass index (BMI), affects a large proportion of the world’s population, with almost 40% being overweight, and more than 10% obese [28]. Western nations have far higher rates of obesity. For example, in the United States, more than 40% of the total population is obese and another 32% are overweight [29,30], whereas in the United Kingdom, almost 30% of adults are obese and more than 30% are overweight [31,32].
Along with viral replication and antiviral treatment, obesity plays a critical role in COVID-19 development. Obesity was shown to be an independent risk factor for death in a major study and meta-analysis, with overweight patients also being at higher risk of having severe COVID-19 [33]. One possibility is that human ACE2 expression is greater in adipose tissue than in lung tissue [34]. Obese patients may have impaired lung function, a poor response to artificial ventilation, and a variety of other problems [35]. The growing body of research has concentrated on obesity and the adverse consequences associated with severe COVID-19.
Comprehensive investigations indicated that up to fifty percent of COVID-19 victims had metabolic and vascular abnormalities, establishing a clear relationship between COVID-19 and the metabolic and endocrine systems. Thus, not only are individuals with metabolic dysfunction at a higher risk of having severe COVID-19, but infection with SARS-CoV-2 may also result in the establishment of diabetes or a worsening of preexisting metabolic diseases [36]. At the molecular level, these effects can be explained by the effects of metabolic syndrome on mitochondria and inflammation. There are several pathways involving mitochondria and their functions in inflammation that may shed light on why SARS-CoV-2 affects overweight and obese patients. Several pathways connect aged mitochondria with decreased immunity, including over-stimulated or persistent inflammatory responses with interferon and cytokine production, mitochondrial biogenesis, and interference with apoptosis and mitophagy [37]. To our knowledge, however, data are few on the dynamics of SARS-CoV-2 viral manifestations in overweight and obese individuals during each of the five waves that were present in Romania in the past two years. Therefore, this study aimed to describe the variation in case severity, symptomatology, ICU admissions and mortality in the population of overweight and obese patients infected with the SARS-CoV-2 virus.
2. Materials and Methods
2.1. Study Design and Ethics
A multidisciplinary research protocol was designed as a retrospective cohort study of overweight and obese patients with COVID-19 that were admitted to hospital during a period that included five pandemic waves. The study took place in a tertiary hospital, and the setting comprised the departments of infectious disease, pulmonology, and radiology of the Infectious Diseases and Pulmonology Hospital, “Victor Babes” in the period starting March 2020 until March 2022.
The research protocol was approved by the Ethics Committee of the “Victor Babes” University of Medicine and Pharmacy in Timisoara, Romania. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of “Victor Babes” Clinical Hospital for Infectious Diseases and Pulmonology in Timisoara, which operates in accordance with Article 167 of Law No. 95/2006, Art. 28, Chapter VIII of Order 904/2006; with European Union’s GCP Directives 2005/28/EC, the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use; and with the Declaration of Helsinki—Recommendations Guiding Medical Practice. It was approved on 28 February 2022, with approval number 05.
2.2. Inclusion Criteria and Variables
A database and patient paper record search was conducted to determine the adult overweight patients and those suffering from obesity admitted to hospital with SARS-CoV-2 infection. The overweight status was considered as a body mass index (BMI) between 25 and 29.9 kg/m2, while the obese status was regarded as a BMI higher than 29.9 kg/m2. The COVID-19 status was defined by a positive polymerase chain reaction test (PCR) from oropharyngeal and nasal swabs. A predefined case report form was used to gather demographic, clinical, and outcome data from electronic medical records. The collected patient data were further stratified by the pandemic wave when the hospital admission was registered. The first wave was considered as the period between March and October 2020 when the main circulating variant was Wuhan-Hu-1 (NCBI Reference Sequence: NC_045512.2) [38]. The second COVID-19 wave was between October 2020 and February 2021, with the Clade variants (S:D614G) as the main circulating variants [39]. The third pandemic wave in Romania was between February and July 2021, having the Alpha (B.1.1.7) variant as the principal circulating viral strain [40]. The fourth wave of the COVID-19 pandemic was between July 2021 and December 2021, when the Delta (B1617.2) variant was causing most of the confirmed infections [41]. Lastly, the fifth pandemic wave in Romania was caused by the Omicron variant between December 2021 and March 2022 [42]. A total of 50 patients were included in the analysis for each wave, totaling 250 overweight and obese patients that were case-matched by age group, gender, and cardiovascular disease after using a stratified random sampling method.
The variables taken into consideration included background data (age, gender, area of residence, occupation, body mass index, smoking status, alcohol use), comorbidities (malignancies, chronic lung disease, cardiovascular disease, cerebrovascular disease, diabetes, autoimmune disease, chronic kidney disease, digestive and liver disease), and COVID-19 treatment. Based on the existing national guidelines [43], clinical picture and documented comorbidities, the COVID-19 patients received antiviral agents, broad-spectrum antibiotics, anticoagulant treatment, steroids and immune modulators for the duration of hospital admission. COVID-19 data (the pandemic wave, clinical severity, imaging severity, oxygen saturation on admission, respiratory rate on admission, heart rate on admission, temperature on admission, duration of hospital stay, duration from symptom onset until hospital admission, viral clearance, intensive care unit admission, duration of ICU stay, oxygen supplementation, mortality), signs and symptoms (cough, fever, dyspnea, headache, digestive symptoms, anosmia/ageusia, fatigue, myalgia/arthralgia, dysphagia), and laboratory data (red blood cells, white blood cells, hemoglobin, hematocrit, platelets, ferritin, erythrocyte sedimentation rate, c-reactive protein, fibrinogen, procalcitonin, D-dimers, interleukin-6, and the international normalized ratio) were assessed.
2.3. Statistical Analysis
IBM SPSS v.26 (Armonk, NY, USA: IBM Corp) and MedCalc v.20 (MedCalc Software bv, Ostend, Belgium) were used for statistical analysis. We calculated the absolute (n) and relative (%) frequencies of categorical variables and compared their proportions using Chi-square and Fisher’s exact test. The Mann–Whitney test was used to compare non-Gaussian variables that were defined be median and interquartile range (IQR). The mean and standard deviation of continuous variables with a normal distribution were compared using the Student’s t-test (unpaired, independent samples). Finally, a multivariate logistic regression analysis adjusted for confounding variables including age, COVID-19 vaccination, and comorbidities was used to identify independent risk factors for ICU admission and mortality in overweight and obese COVID-19 patients. A significance level of 0.05 was chosen as the alpha value.
3. Results
3.1. Normal Weight vs. Overweight Patients
A total of 250 overweight patients were included in the study, where 50 of them were infected with SARS-CoV-2 in each of the five waves of the COVID-19 pandemic in Romania. A control group of 113 patients with normal body mass index was included in the analysis, matched by age, gender, and cardiovascular comorbidities. The baseline characteristics comparison of normal weight and overweight patients with COVID-19 admitted to hospital presented in Table 1 did not identify significant differences between proportions of age, gender, area of residence, occupation, and alcohol use disorder in the group of patients with BMI 18.5–24.9 and those with BMI > 24.9. The smoking behavior was more common in overweight patients (51.6% vs. 38.1%, p-value = 0.016). Among the studied comorbidities, diabetes mellitus and digestive diseases were found in higher proportions in the overweight patient group (22.4% vs. 12.4%, p-value = 0.025), respectively (20.4% vs. 9.7%, p-value = 0.012). Regarding the COVID-19 treatment received during hospital admission, there were no important findings when comparing the proportions between overweight and obese patients except for antibiotics that were given with a higher frequency to obese patients (85.6% vs. 77.0%, p-value = 0.043).
Table 1.
Comparison of baseline characteristics between normal weight and overweight patients with COVID-19.
| Variables * | BMI 18.5–24.9 (n = 113) | BMI > 24.9 (n = 250) | p-Value |
|---|---|---|---|
| Background data | |||
| Age | 0.407 | ||
| 18–40 years | 21 (18.6%) | 33 (13.2%) | |
| 40–65 years | 54 (47.8%) | 129 (51.6%) | |
| >65 years | 38 (33.6%) | 88 (35.2%) | |
| Gender (men) | 62 (54.9%) | 137 (54.8%) | 0.990 |
| Area of residence (urban) | 68 (60.2%) | 143 (57.2%) | 0.594 |
| Occupation (employed) | 66 (58.4%) | 124 (49.6%) | 0.119 |
| Smoking | 43 (38.1%) | 129 (51.6%) | 0.016 |
| Alcohol use disorder | 9 (8.0%) | 17 (6.8%) | 0.690 |
| Comorbidities | |||
| Malignancy | 7 (6.2%) | 26 (10.4%) | 0.196 |
| Chronic lung disease | 11 (9.7%) | 39 (15.6%) | 0.133 |
| Cardiovascular disease | 51 (45.1%) | 114 (45.6%) | 0.934 |
| Cerebrovascular disease | 8 (7.1%) | 33 (13.2%) | 0.088 |
| Diabetes mellitus | 14 (12.4%) | 56 (22.4%) | 0.025 |
| Autoimmune disease | 3 (2.7%) | 12 (4.8%) | 0.341 |
| Chronic kidney disease | 4 (3.5%) | 14 (5.6%) | 0.402 |
| Digestive and liver disease | 11 (9.7%) | 51 (20.4%) | 0.012 |
| COVID-19 treatment | |||
| Antivirals | 103 (91.2%) | 239 (95.6%) | 0.092 |
| Corticosteroids | 99 (87.6%) | 211 (84.4%) | 0.422 |
| Antibiotics | 87 (77.0%) | 214 (85.6%) | 0.043 |
| Anticoagulants | 75 (66.4%) | 183 (73.2%) | 0.184 |
| Immune modulators | 28 (24.8%) | 83 (33.2%) | 0.106 |
* Data reported as n (%) and calculated using Chi-square test and Fisher’s exact unless specified differently; BMI—Body Mass Index.
The comparison of biological parameters and laboratory profiles of the patients included in the current study is presented in Table 2. It was observed that overweight and obese patients were suffering from anemia in higher proportion. The red blood cells and hematocrit were significantly found outside the normal range (65.6% vs. 54.0%, p-value = 0.034), respectively (49.2% vs. 27.4%, p-value < 0.001). The white blood cell count was also significantly altered compared to that of the group of patients with normal weight (71.6% vs. 60.2%, p-value = 0.030).
Table 2.
Comparison of biological profiles at admission between normal weight and overweight patients with COVID-19.
| Variables * | Normal Range | BMI 18.5–24.9 (n = 113) | BMI > 24.9 (n = 250) | p-Value |
|---|---|---|---|---|
| RBC (millions/mm3) | 4.35–5.65 | 61 (54.0%) | 164 (65.6%) | 0.034 |
| WBC (thousands/mm3) | 4.5–11.0 | 68 (60.2%) | 179 (71.6%) | 0.030 |
| Hemoglobin (g/dL) | 13.0–17.0 | 59 (52.2%) | 155 (62.0%) | 0.079 |
| Hematocrit (%) | 36–48 | 31 (27.4%) | 123 (49.2%) | <0.001 |
| Platelets (thousands/mm3) | 150–450 | 38 (33.6%) | 104 (41.6%) | 0.149 |
| Ferritin (ng/mL) | 20–250 | 33 (29.2%) | 96 (38.4%) | 0.090 |
| ESR (mm/h) | 0–22 mm/hr | 75 (66.4%) | 177 (70.8%) | 0.369 |
| CRP (mg/L) | 0–10 mg/L | 72 (63.7%) | 170 (68.0%) | 0.422 |
| Fibrinogen (g/L) | 2–4 g/L | 69 (61.1%) | 171 (68.4%) | 0.171 |
| Procalcitonin (ug/L) | 0–0.25 ug/L | 20 (17.7%) | 69 (27.6%) | 0.042 |
| D-dimers (ng/mL) | <250 | 13 (11.5%) | 44 (17.6%) | 0.139 |
| IL-6 (pg/mL) | 0–16 pg/mL | 32 (28.3%) | 98 (39.2%) | 0.045 |
* Data reported as % outside the normal range, and calculated using Chi-square test and Fisher’s exact unless specified differently; BMI—Body Mass Index.
Table 3 presents the signs and symptoms, as well as COVID-19 outcomes of patients included in the research. It was observed that overweight and obese patients were affected in significantly higher proportion by symptoms related to SARS-CoV-2 infection compared to patients with normal weight, excepting fever, digestive symptoms, anosmia/ageusia, and myalgia/arthralgia. Fatigue was the most common complaint of overweight patients with COVID-19 (86.8% vs. 72.6%, p-value < 0.001).
Table 3.
Comparison of SARS-CoV-2 infection signs, symptoms, and outcomes between normal weight and overweight patients with COVID-19.
| Variables * | BMI 18.5–24.9 (n = 113) | BMI > 24.9 (n = 250) | p-Value |
|---|---|---|---|
| Signs and Symptoms | |||
| Cough | 72 (63.7%) | 188 (75.2%) | 0.024 |
| Fever | 66 (58.4%) | 172 (68.8%) | 0.053 |
| Dyspnea | 13 (11.5%) | 56 (22.4%) | 0.014 |
| Headache | 9 (8.0%) | 42 (16.8%) | 0.024 |
| Digestive symptoms | 18 (15.9%) | 57 (22.8%) | 0.134 |
| Anosmia/ageusia | 34 (30.1%) | 81 (32.4%) | 0.661 |
| Fatigue | 82 (72.6%) | 217 (86.8%) | <0.001 |
| Myalgia/arthralgia | 36 (31.9%) | 95 (38.0%) | 0.259 |
| Dysphagia | 7 (6.2%) | 49 (19.6%) | 0.001 |
| COVID-19 Outcomes | |||
| Severe COVID-19 | 13 (11.5%) | 61 (24.4%) | 0.004 |
| Severe imaging features | 19 (16.8%) | 66 (26.4%) | 0.045 |
| Oxygen saturation on admission (<92%) | 14 (12.4%) | 63 (25.2%) | 0.005 |
| Respiratory rate on admission (>20/min) | 27 (23.9%) | 83 (33.2%) | 0.074 |
| Heart rate on admission (>100 bpm) | 35 (31.0%) | 107 (42.8%) | 0.032 |
| Duration of hospital stay | 14 (9–16) | 18 (8–25) | <0.001 |
| Duration from symptom onset until hospital admission | 5 (2–8) | 5 (1–7) | 0.319 |
| Viral clearance | 12 (7–19) | 15 (6–21) | <0.001 |
| ICU admission | 11 (9.7%) | 47 (18.8%) | 0.029 |
| Duration of ICU stay | 7 (3–14) | 11 (7–19) | <0.001 |
| Severe in-hospital complications | 11 (9.7%) | 46 (18.4%) | 0.035 |
| Oxygen supplementation | 42 (37.2%) | 148 (59.2%) | <0.001 |
| Mortality | 4 (3.5%) | 26 (10.4%) | 0.027 |
* Data reported as n (%), and calculated using Chi-square test and Fisher’s exact unless specified differently; BMI—Body Mass Index; ICU—Intensive Care Unit.
3.2. Dynamic Comparison of COVID-19 Pandemic Waves
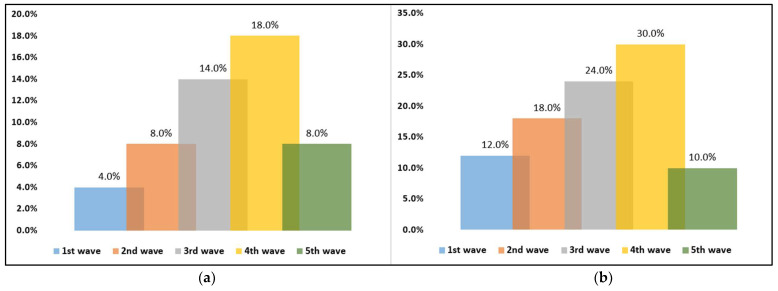
The dynamic comparison of overweight and obese patients with SARS-CoV-2 infection is presented in Table 4. It was observed that during the 4th wave when the Delta variant was the main circulating SARS-CoV-2 strain in Romania, patients were showing statistically significantly higher proportions in ICU admissions and mortality (Figure 1a,b), as well as significantly more altered clinical parameters at hospital admission. The duration of viral clearance was also statistically significantly higher in the 4th wave, with a median of 17 days, compared with 8 days during the first COVID-19 wave (p-value < 0.001). It was observed that medication received by the studied patients during the five pandemic waves was not significantly different, excepting the use of antibiotics (p-value = 0.007). Additionally, patients started to receive immune modulators from the third wave.
Table 4.
Baseline characteristics of study participants stratified by COVID-19 pandemic wave.
| 1st Wave (n = 50) | 2nd Wave (n = 50) | 3rd Wave (n = 50) | 4th Wave (n = 50) | 5th Wave (n = 50) | p-Value * | |
|---|---|---|---|---|---|---|
| Severe COVID-19 | 7 (14.0%) | 15 (30.0%) | 12 (24.0%) | 20 (40.0%) | 7 (14.0%) | 0.004 |
| Severe imaging features | 8 (16.0%) | 15 (30.0%) | 13 (26.0%) | 21 (42.0%) | 9 (18.0%) | 0.012 |
| Oxygen saturation on admission (<92%) | 6 (12.0%) | 11 (22.0%) | 18 (36.0%) | 21 (42.0%) | 7 (14.0%) | 0.001 |
| Respiratory rate on admission (>20/min) | 9 (18.0%) | 14 (28.0%) | 22 (44.0%) | 26 (52.0%) | 12 (52.0%) | 0.001 |
| Heart rate on admission (>100 bpm) | 12 (24.0%) | 20 (40.0%) | 27 (54.0%) | 33 (66.0%) | 15 (30.0%) | 0.025 |
| Duration of hospital stay | 14 (11–18) | 9 (7–12) | 18 (11–23) | 21 (13–25) | 9 (7–13) | <0.001 |
| Duration from symptom onset until hospital admission | 2 (1–4) | 5 (2.2–8.3) | 6 (2.3–9.2) | 6 (2.1–9.0) | 5 (1.9–7.4) | <0.001 |
| Viral clearance | 8 (3–13) | 12 (4–16) | 15 (7–21) | 17 (11–23) | 9 (4–15) | <0.001 |
| ICU admission | 6 (12.0%) | 9 (18.0%) | 12 (24.0%) | 15 (30.0%) | 5 (10.0%) | 0.002 |
| Duration of ICU stay | 7 (3–11) | 12 (4–15) | 12 (5–16) | 14 (9–19) | 9 (4–12) | 0.009 |
| Severe in-hospital complications | 6 (12.0%) | 9 (18.0%) | 12 (24.0%) | 14 (30.0%) | 5 (10.0%) | 0.021 |
| Oxygen supplementation | 22 (44.0%) | 27 (54.0%) | 37 (74.0%) | 45 (90.0%) | 17 (34.0%) | <0.001 |
| Mortality | 2 (4.0%) | 4 (8.0%) | 7 (14.0%) | 9 (18.0%) | 4 (8.0%) | 0.001 |
| COVID-19 treatment | ||||||
| Antivirals | 50 (100%) | 50 (100%) | 46 (92.0%) | 48 (96.0%) | 45 (90.0%) | 0.693 |
| Corticosteroids | 46 (92.0%) | 43 (86.0%) | 44 (88.0%) | 40 (80.0%) | 38 (76.0%) | 0.184 |
| Antibiotics | 47 (94.0%) | 48 (96.0%) | 42 (84.0%) | 40 (80.0%) | 37 (74.0%) | 0.007 |
| Anticoagulant | 39 (78.0%) | 38 (76.0%) | 36 (72.0%) | 38 (76.0%) | 32 (64.0%) | 0.528 |
| Immune modulators | 0 (0.0%) | 0 (0.0%) | 28 (56.0%) | 29 (58.0%) | 26 (52.0%) | 0.827 |
* Data reported as n (%) and calculated using Chi-square test and Fisher’s exact unless specified differently.
Figure 1.
(a,b) Dynamic comparison of ICU admissions and mortality in overweight and obese patients with COVID-19.
3.3. Risk Analysis
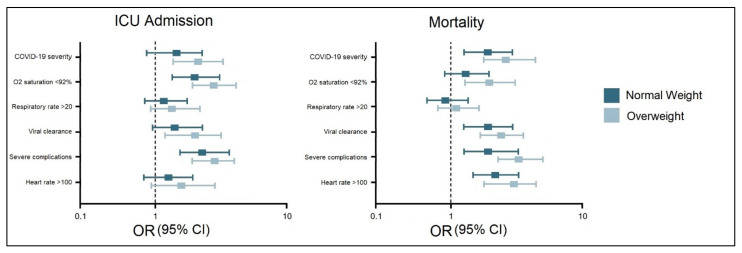
The multivariate risk factor analysis for ICU admission and mortality in overweight and obese patients determined that the odds were significantly higher compared to normal weight patients (Figure 2). COVID-19 severity, oxygen saturation below 92% at admission, long viral clearance, and severe complications were significant independent risk factors for ICU admission in overweight patients. In the same manner, COVID-19 severity, long viral clearance, severe complications, and a heart rate higher than 100 beats per minute were identified as independent risk factors for mortality in overweight and obese patients infected with SARS-CoV-2.
Figure 2.
Multivariate risk factor analysis for ICU admission and mortality in normal weight and overweight/obese patients with COVID-19.
4. Discussion
The current study determined significant changes in the characteristics of overweight and obese patients that were admitted to hospital between the five waves of the COVID-19 pandemic in Romania. Other studies have attempted to describe the variability of pandemics through viral mutations that occur when millions of individuals are infected. For example, the multi-wave pandemic dynamics were discussed in parallel between the Spanish influenza pandemic of the 20th century and the current and ongoing COVID-19 pandemic [44]. The researchers showed through an epidemic Renormalization Group (eRG) framework that the pace of disease propagation may be viewed as a time-dilation symmetry, and that the last stage of a wave corresponds to achieving a time-invariant state. The short period between two waves is shown to be a symptom of system instability, connected with a near-break of time scale invariance. Their findings indicate that the critical time for containing the next wave of a pandemic is the walking period between waves, during which the number of infections climbs linearly. Thus, preventing or delaying the entrance of the next wave is most effectively accomplished by restricting viral dissemination during this time period [45]. Although accurate methods were employed to forecast the growth of the COVID-19 disease’s case count at the start of the pandemic, it proved challenging to forecast the arrival of a second wave in the autumn of 2020 [46,47].
Other studies analyzed the early public health interventions to control the COVID-19 pandemic waves, although there were no population-specific measurements put in place and described. A South Korean study focused on the second and third pandemic waves and described that the third wave lasted longer than the second and was associated with a greater incidence of case fatalities, which is consistent with our findings [48]. The study concluded that to successfully suppress and manage the COVID-19 pandemic, early and timely interventions with reinforced social distancing regulations should be undertaken. If the first reaction is delayed, the COVID-19 pandemic may grow explosively inside local groups, making future social distancing methods difficult to implement. However, a further development of different SARS-CoV-2 viral strains during the fourth and fifth waves showed that the natural evolution of a pandemic is very hard to control, even though a full-scale vaccination program was successfully implemented in several countries [49,50].
A recent meta-analysis studied the impact of COVID-19 on overweight and obese patients, as well as the risk factors associated with hospitalization, ICU admission, and mortality. The researchers discovered that being overweight increases the risk of COVID-19-related hospitalization but not mortality, but that obesity and severe obesity increase the risk of both hospitalization and death associated with COVID-19 [51]. Additionally, a linear dose–response relationship was seen between obesity categories and COVID-19 results. Moreover, the association’s intensity reduced with time, consistent with the trend of COVID-19’s initial wave. However, there was no given analysis on each separate wave and the dynamics created by the alpha, beta, delta, and more recently, the omicron variant.
The formation of the SARS-CoV-2 alpha, beta, and delta variants of concern was accompanied by succeeding waves of infection, sometimes spanning throughout the whole globe [52]. For instance, the delta COVID-19 strain that was observed in our study to determine more severe infections had greater transmissibility and was accompanied by a larger viral load, a longer duration of infectiousness, and a high incidence of reinfection, owing to its capacity to evade natural immunity [53,54]. As a consequence, the delta strain swiftly became the worldwide prevalent variety. It continued to cause successive waves of infection and remained the major variant of concern in several nations throughout the fourth wave. These concerns regarding decreased vaccine effectiveness due to new variants have altered researchers’ views of the COVID-19 endgame, dispelling the myth that worldwide immunization alone will be sufficient to suppress SARS-CoV-2 infection [55]. The latest SARS-CoV-2 variants have emphasized the critical role of vaccination in conjunction with current public health preventative measures, such as masks, as a route to viral endemicity. Therefore, special populations such as overweight and obese patients who are at higher risk of developing severe complications during COVID-19 should be targeted by nationwide yearly vaccination campaigns such as the influenza vaccination campaign in Romania [56].
An important limitation of the current study is the risk of bias that might be determined by government laws and healthcare guidelines that were constantly changed during the COVID-19 pandemic. Therefore, several differences that were observed between the five waves of the pandemic might be caused by confounding factors such as regulations in COVID-19 patient admission, management, and treatment. Although the impact of obesity on the observed outcomes was justified, these confounding factors can not be ruled out. Moreover, hospitalized COVID-19 patients from the studied population received corticosteroids as the basis of treatment for SARS-CoV-2 infection according to the existing national guidelines at the time. Therefore, this treatment can increase the glycemic index of patients, and more so in overweight and obese that are likely to have metabolic syndrome. It is also worth mentioning the risk of selection bias that might occur in this study, since all patients were admitted at a tertiary hospital. Therefore, it is likely that the presented cases of COVID-19 could be of greater severity than the average. Another drawback of retrospective cohort studies is that since several healthcare providers were engaged in patient care, the assessment of risk variables and outcomes throughout the database is likely to be less precise and consistent than in a prospective cohort study.
5. Conclusions
Being overweight or obese determines a higher likelihood of hospitalization, severe complications, or death from COVID-19. However, this special population mostly suffers from obesity-associated comorbidities that contribute towards these negative outcomes. We advocate the creation of new guiding principles for the formulation of healthcare strategies aimed at special populations with high prevalence such as overweight and obese individuals, while also promoting the containment of the pandemic and avoiding the recurrence of pandemic waves with the same guidelines that proved detrimental in terms of economic and human life loss.
Author Contributions
Conceptualization: R.M.F.; methodology: R.M.F. software: F.B.; validation: C.C.; formal analysis: C.C.; investigation: D.M.; resources: D.M.; data curation: F.B. writing—original draft preparation: R.M.F.; writing—review and editing: O.R. and E.T.; visualization: O.R. and E.T.; supervision: C.O.; project administration: C.O. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of “Victor Babes” Clinical Hospital for Infectious Diseases and Pulmonology in Timisoara, approved on 28 February 2022, with approval number 05.
Informed Consent Statement
Written informed consent was obtained from the patients to publish this paper.
Data Availability Statement
Data available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang L., Wang K., Zhong H., Zhao N., Xu W., Yang Y., He Y., Liu S. The Effect of Coronavirus 2019 Disease Control Measures on the Incidence of Respiratory Infectious Disease and Air Pollutant Concentrations in the Yangtze River Delta Region, China. Int. J. Environ. Res. Public Health. 2022;19:1286. doi: 10.3390/ijerph19031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerbu B., Pantea S., Bratosin F., Vidican I., Turaiche M., Frent S., Borsi E., Marincu I. Liver Impairment and Hematological Changes in Patients with Chronic Hepatitis C and COVID-19: A Retrospective Study after One Year of Pandemic. Medicina. 2021;57:597. doi: 10.3390/medicina57060597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marincu I., Bratosin F., Vidican I., Bostanaru A.-C., Frent S., Cerbu B., Turaiche M., Tirnea L., Timircan M. Predictive Value of Comorbid Conditions for COVID-19 Mortality. J. Clin. Med. 2021;10:2652. doi: 10.3390/jcm10122652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrag M.A., Amer H.M., Bhat R., Hamed M.E., Aziz I.M., Mubarak A., Dawoud T.M., Almalki S.G., Alghofaili F., Alnemare A.K., et al. SARS-CoV-2: An Overview of Virus Genetics, Transmission, and Immunopathogenesis. Int. J. Environ. Res. Public Health. 2021;18:6312. doi: 10.3390/ijerph18126312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kayode A.J., Banji-Onisile F.O., Olaniran A.O., Okoh A.I. An Overview of the Pathogenesis, Transmission, Diagnosis, and Management of Endemic Human Coronaviruses: A Reflection on the Past and Present Episodes and Possible Future Outbreaks. Pathogens. 2021;10:1108. doi: 10.3390/pathogens10091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salleh M.Z., Deris Z.Z. In Silico Molecular Characterization of Human TMPRSS2 Protease Polymorphic Variants and Associated SARS-CoV-2 Susceptibility. Life. 2022;12:231. doi: 10.3390/life12020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pilut C.N., Citu C., Gorun F., Bratosin F., Gorun O.M., Burlea B., Citu I.M., Grigoras M.L., Manolescu D., Gluhovschi A. The Utility of Laboratory Parameters for Cardiac Inflammation in Heart Failure Patients Hospitalized with SARS-CoV-2 Infection. Diagnostics. 2022;12:824. doi: 10.3390/diagnostics12040824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Citu I.M., Citu C., Gorun F., Neamtu R., Motoc A., Burlea B., Rosca O., Bratosin F., Hosin S., Manolescu D., et al. Using the NYHA Classification as Forecasting Tool for Hospital Readmission and Mortality in Heart Failure Patients with COVID-19. J. Clin. Med. 2022;11:1382. doi: 10.3390/jcm11051382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bean D.M., Kraljevic Z., Searle T., Bendayan R., Kevin O., Pickles A., Folarin A., Roguski L., Noor K., Shek A., et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID-19 infection in a multi-site UK acute hospital trust. Eur. J. Heart Fail. 2020;22:967–974. doi: 10.1002/ejhf.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timircan M., Bratosin F., Vidican I., Suciu O., Tirnea L., Avram V., Marincu I. Exploring Pregnancy Outcomes Associated with SARS-CoV-2 Infection. Medicina. 2021;57:796. doi: 10.3390/medicina57080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Citu I.M., Citu C., Gorun F., Sas I., Tomescu L., Neamtu R., Motoc A., Gorun O.M., Burlea B., Bratosin F., et al. Immunogenicity Following Administration of BNT162b2 and Ad26.COV2.S COVID-19 Vaccines in the Pregnant Population during the Third Trimester. Viruses. 2022;14:307. doi: 10.3390/v14020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snider B., Patel B., McBean E. Asymptomatic Cases, the Hidden Challenge in Predicting COVID-19 Caseload Increases. Infect. Dis. Rep. 2021;13:340–347. doi: 10.3390/idr13020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandi N., Ciccarese F., Rimondi M.R., Balacchi C., Modolon C., Sportoletti C., Renzulli M., Coppola F., Golfieri R. An Imaging Overview of COVID-19 ARDS in ICU Patients and Its Complications: A Pictorial Review. Diagnostics. 2022;12:846. doi: 10.3390/diagnostics12040846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vîjîiac A., Stănciulescu D.I., Băetu A.E., Grigore I.-A., Vintilă D., Cojocaru C., Bădilă E., Moldovan H., Scafa-Udriște A. The Impact of COVID-19 Era on Pulmonary Embolism Patients: Increased Incidence of Hospitalizations and Higher Mortality—What Can Be Done? COVID. 2021;1:357–365. doi: 10.3390/covid1010030. [DOI] [Google Scholar]
- 15.Bogdan I., Citu C., Bratosin F., Malita D., Romosan I., Gurban C.V., Bota A.V., Turaiche M., Bratu M.L., Pilut C.N., et al. The Impact of Multiplex PCR in Diagnosing and Managing Bacterial Infections in COVID-19 Patients Self-Medicated with Antibiotics. Antibiotics. 2022;11:437. doi: 10.3390/antibiotics11040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L., Wang Y., Ye D., Liu Q. A review of the 2019 novel coronavirus (COVID-19) based on current evidence. Int. J. Antimicrob. Agents. 2020;55:105948. doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorbello M., El-Boghdadly K., Di Giacinto I., Cataldo R., Esposito C., Falcetta S., Merli G., Cortese G., Corso R.M., Bressan F., et al. The Italian coronavirus disease 2019 outbreak: Recommendations from clinical practice. Anaesthesia. 2020;75:724–732. doi: 10.1111/anae.15049. [DOI] [PubMed] [Google Scholar]
- 18.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adhikari S.P., Meng S., Wu Y., Mao Y.P., Ye R.X., Wang Q.Z., Sun C., Sylvia S., Rozelle S., Raat H., et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: A scoping review. Infect. Dis. Poverty. 2020;9:29. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giudice V., Iannaccone T., Faiella F., Ferrara F., Aversano G., Coppola S., De Chiara E., Romano M.G., Conti V., Filippelli A. Gender Differences in the Impact of COVID-19 Pandemic on Mental Health of Italian Academic Workers. J. Pers. Med. 2022;12:613. doi: 10.3390/jpm12040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soriano V., Ganado-Pinilla P., Sanchez-Santos M., Gómez-Gallego F., Barreiro P., de Mendoza C., Corral O. Main differences between the first and second waves of COVID-19 in Madrid, Spain. Int. J. Infect. Dis. 2021;105:374–376. doi: 10.1016/j.ijid.2021.02.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitra A.R., Fergusson N.A., Lloyd-Smith E., Wormsbecker A., Foster D., Karpov A., Crowe S., Haljan G., Chittock D.R., Kanji H.D., et al. Baseline characteristics and outcomes of patients with COVID-19 admitted to intensive care units in Vancouver, Canada: A case series. Can. Med. Assoc. J. 2020;192:E694–E701. doi: 10.1503/cmaj.200794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., Hosein Z., Padda I., Mangat J., Altaf M. Comorbidity and its Impact on Patients with COVID-19. SN Compr. Clin. Med. 2020;2:1069–1076. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilk-Sledziewska K., Sielatycki P.J., Uscinska N., Bujno E., Rosolowski M., Kakareko K., Sledziewski R., Rydzewska-Rosolowska A., Hryszko T., Zbroch E. The Impact of Cardiovascular Risk Factors on the Course of COVID-19. J. Clin. Med. 2022;11:2250. doi: 10.3390/jcm11082250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutierrez-Camacho J.R., Avila-Carrasco L., Murillo-Ruíz-Esparza A., Garza-Veloz I., Araujo-Espino R., Martinez-Vazquez M.C., Trejo-Ortiz P.M., Rodriguez-Sanchez I.P., Delgado-Enciso I., Castañeda-López M.E., et al. Evaluation of the Potential Risk of Mortality from SARS-CoV-2 Infection in Hospitalized Patients According to the Charlson Comorbidity Index. Healthcare. 2022;10:362. doi: 10.3390/healthcare10020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silveira E.A., de Souza Rosa L.P., de Carvalho Santos A.S.e.A., de Souza Cardoso C.K., Noll M. Type 2 Diabetes Mellitus in Class II and III Obesity: Prevalence, Associated Factors, and Correlation between Glycemic Parameters and Body Mass Index. Int. J. Environ. Res. Public Health. 2020;17:3930. doi: 10.3390/ijerph17113930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arias A., Rodríguez-Álvarez C., González-Dávila E., Acosta-Torrecilla A., Novo-Muñoz M.M., Rodríguez-Novo N. Arterial Hypertension in Morbid Obesity after Bariatric Surgery: Five Years of Follow-Up, a Before-And-After Study. Int. J. Environ. Res. Public Health. 2022;19:1575. doi: 10.3390/ijerph19031575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obesity and Overweight. World Health Organization; Geneva, Switzerland: 2020. [(accessed on 3 April 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. [Google Scholar]
- 29.Sanyaolu A., Okorie C., Qi X., Locke J., Rehman S. Childhood and Adolescent Obesity in the United States: A Public Health Concern. Glob. Pediatr. Health. 2019;6:2333794X19891305. doi: 10.1177/2333794X19891305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y., Beydoun M.A., Min J., Xue H., Kaminsky L.A., Cheskin L.J. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int. J. Epidemiol. 2020;49:810–823. doi: 10.1093/ije/dyz273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haase C.L., Eriksen K.T., Lopes S., Satylganova A., Schnecke V., McEwan P. Body mass index and risk of obesity-related conditions in a cohort of 2.9 million people: Evidence from a UK primary care database. Obes. Sci. Pract. 2020;7:137–147. doi: 10.1002/osp4.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agha M., Agha R. The rising prevalence of obesity: Part A: Impact on public health. Int. J. Surg. Oncol. 2017;2:e17. doi: 10.1097/IJ9.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai Z., Yang Y., Zhang J. Obesity is associated with severe disease and mortality in patients with coronavirus disease 2019 (COVID-19): A meta-analysis. BMC Public Health. 2021;21:1505. doi: 10.1186/s12889-021-11546-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Benna S. Association of high-level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes. Med. 2020;19:100283. doi: 10.1016/j.obmed.2020.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dixon A.E., Peters U. The effect of obesity on lung function. Expert Rev. Respir. Med. 2018;12:755–767. doi: 10.1080/17476348.2018.1506331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steenblock C., Schwarz P.E.H., Ludwig B., Linkermann A., Zimmet P., Kulebyakin K., Tkachuk A.V., Markov A.G., Lehnert H., de Angelis M.H., et al. COVID-19 and metabolic disease: Mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;9:786–798. doi: 10.1016/S2213-8587(21)00244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganji R., Reddy P.H. Impact of COVID-19 on Mitochondrial-Based Immunity in Aging and Age-Related Diseases. Front. Aging Neurosci. 2021;12:614650. doi: 10.3389/fnagi.2020.614650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lazar M., Sequencing of the SARS-CoV-2 Virus Genome in Romania National Institute of Medical-Military Research and Development “Cantacuzino”. [(accessed on 3 April 2022)]. Available online: https://cantacuzino.mapn.ro/pages/view/249.
- 39.Hodcroft E.B., Zuber M., Nadeau S., Vaughan T.G., Crawford K.H.D., Althaus C.L., Reichmuth M.L., Bowen J.E., Walls A.C., Corti D., et al. Spread of a SARS-CoV-2 variant through Europe in the summer of 2020. Nature. 2021;595:707–712. doi: 10.1038/s41586-021-03677-y. [DOI] [PubMed] [Google Scholar]
- 40.National Institute of Public Health Epidemiological Analysis of the First 136 COVID-19 Cases in Romania Confirmed with New Variants of SARS-CoV-2. [(accessed on 3 April 2022)]. Available online: https://www.cnscbt.ro/index.php/analiza-cazuri-confirmate-covid19/2291-cazuri-covid-19-cu-noi-variante-analiza-epidemiologica-a-primelor-136-cazuri/file.
- 41.National Committee for Coordination of Activities on Vaccination against COVID-19 What We Need to Know about the Delta Variant of the SARS-CoV-2 Virus. [(accessed on 3 April 2022)]; Available online: https://vaccinare-covid.gov.ro/wp-content/uploads/2021/07/Varianta-Delta-informare-de-presa-07.07.2021-1.pdf.
- 42.Ministry of Health in Romania Update of Infection Cases with the OMICRON Strain of SARS-CoV-2 Virus. [(accessed on 3 April 2022)]. Available online: https://www.ms.ro/2022/01/05/update-cazuri-de-infectare-cu-tulpina-omicron-a-virusului-sars-cov-2-2/
- 43.Legislative Portal Order no. 533 of 22 April 22 2021 on the Amendment of the Annex to the Order of the Minister of Health No. 487/2020 for the Approval of the Protocol for the Treatment of SARS-CoV-2 Virus Infection. Romanian Ministry of Health. [(accessed on 3 April 2022)]. Available online: https://www.dsptimis.ro/data_files/content/legislatie-covid/files/ordin-nr-533-27-04-2021.pdf.
- 44.Liang S.T., Liang L.T., Rosen J.M. COVID-19: A comparison to the 1918 influenza and how we can defeat it. Postgrad. Med. J. 2021;97:273–274. doi: 10.1136/postgradmedj-2020-139070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cacciapaglia G., Cot C., Sannino F. Multiwave pandemic dynamics explained: How to tame the next wave of infectious diseases. Sci. Rep. 2021;11:6638. doi: 10.1038/s41598-021-85875-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hâncean M.G., Perc M., Lerner J. Early spread of COVID-19 in Romania: Imported cases from Italy and human-to-human transmission networks. R. Soc. Open Sci. 2020;7:200780. doi: 10.1098/rsos.200780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou T., Liu Q., Yang Z., Liao J., Yang K., Bai W., Lu X., Zhang W. Preliminary prediction of the basic reproduction number of the Wuhan novel coronavirus 2019-nCoV. J. Evid. Based Med. 2020;13:3–7. doi: 10.1111/jebm.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seong H., Hyun H.J., Yun J.G., Noh J.Y., Cheong H.J., Kim W.J., Song J.Y. Comparison of the second and third waves of the COVID-19 pandemic in South Korea: Importance of early public health intervention. Int. J. Infect. Dis. 2021;104:742–745. doi: 10.1016/j.ijid.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Benhassine F., Rajah M.M., Planchais C., Porrot F., Robillard N., Puech J., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 50.Menni C., May A., Polidori L., Louca P., Wolf J., Capdevila J., Hu C., Ourselin S., Steves C.J., Valdes A.M., et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: A prospective community study from the ZOE COVID Study. Lancet Infect. Dis. 2022 doi: 10.1016/S1473-3099(22)00146-3. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sawadogo W., Tsegaye M., Gizaw A., Adera T. Overweight and obesity as risk factors for COVID-19-associated hospitalisations and death: Systematic review and meta-analysis. BMJ Nutr. Prev. Health. 2022:e000375. doi: 10.1136/bmjnph-2021-000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fontanet A., Autran B., Lina B., Kieny M.P., Abdool Karim S.S., Sridhar D. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet. 2021;397:952–954. doi: 10.1016/S0140-6736(21)00370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Townsend J.P., Hassler H.B., Wang Z., Miura S., Singh J., Kumar S., Ruddle N.H., Galvani A.P., Dornburg A. The durability of immunity against reinfection by SARS-CoV-2: A comparative evolutionary study. Lancet Microbe. 2021;2:e666–e675. doi: 10.1016/S2666-5247(21)00219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lazarevic I., Pravica V., Miljanovic D., Cupic M. Immune Evasion of SARS-CoV-2 Emerging Variants: What Have We Learnt So Far? Viruses. 2021;13:1192. doi: 10.3390/v13071192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diarra M., Kebir A., Talla C., Barry A., Faye J., Louati D., Opatowski L., Diop M., White L.J., Loucoubar C. Non-pharmaceutical interventions and COVID-19 vaccination strategies in Senegal: A modelling study. BMJ Glob. Health. 2022;7:e007236. doi: 10.1136/bmjgh-2021-007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kovács G., Kaló Z., Jahnz-Rozyk K., Kyncl J., Csohan A., Pistol A., Leleka M., Kipshakbaev R., Durand L., Macabeo B. Medical and economic burden of influenza in the elderly population in central and eastern European countries. Hum. Vaccin. Immunother. 2014;10:428–440. doi: 10.4161/hv.26886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request.