Abstract
(1) Background: Nutrition therapy guided by indirect calorimetry (IC) is the gold standard and is associated with lower morbidity and mortality in critically ill patients. When performing IC during continuous venovenous hemofiltration (CVVH), the measured VCO2 should be corrected for the exchanged CO2 to calculate the ‘true’ Resting Energy Expenditure (REE). After the determination of the true REE, the caloric prescription should be adapted to the removal and addition of non-intentional calories due to citrate, glucose, and lactate in dialysis fluids to avoid over- and underfeeding. We aimed to evaluate this bioenergetic balance during CVVH and how nutrition therapy should be adapted. (2) Methods: This post hoc analysis evaluated citrate, glucose, and lactate exchange. Bioenergetic balances were calculated based on these values during three different CVVH settings: low dose with citrate, high dose with citrate, and low dose without citrate. The caloric load of these non-intentional calories during a CVVH-run was compared to the true REE. (3) Results: We included 19 CVVH-runs. The bioenergetic balance during the low dose with citrate was 498 ± 110 kcal/day (range 339 to 681 kcal/day) or 26 ± 9% (range 14 to 42%) of the true REE. During the high dose with citrate, it was 262 ± 222 kcal/day (range 56 to 262 kcal/day) or 17 ± 11% (range 7 to 32%) of the true REE. During the low dose without citrate, the bioenergetic balance was −189 ± 77 kcal/day (range −298 to −92 kcal/day) or −13 ± 8% (range −28 to −5%) of the true REE. (4) Conclusions: Different CVVH settings resulted in different bioenergetic balances ranging from −28% up to +42% of the true REE depending on the CVVH fluids chosen. When formulating a caloric prescription during CVVH, an individual approach considering the impact of these non-intentional calories is warranted.
Keywords: continuous renal replacement therapy, continuous venovenous hemofiltration, indirect calorimetry, resting energy expenditure, citrate, non-intentional calories
1. Introduction
The individualization of medical nutrition by the assessment of resting energy expenditure (REE) with indirect calorimetry (IC) is associated with lower mortality in adult patients admitted to the intensive care unit [1]. Based on the measured REE, progressive nutrition to achieve normocaloric feeding between day 3 and 5 as compared to hypocaloric feeding, is associated with lower morbidity and mortality [2,3]. Overfeeding, especially in the first days of admission, is also deleterious and is associated with higher mortality [4,5]. The beneficial effects on disease evolution and outcome of IC-guided nutrition therapy can probably be extrapolated in other cohorts, including patients with acute kidney injury receiving renal replacement therapy [6,7]. Acute kidney injury, a frequent (prevalence of 13–78%) condition in the intensive care unit, leads to toxin accumulation and can be treated with renal replacement therapy [8]. Continuous renal replacement therapy (CRRT) is usually preferred over intermittent dialysis in critically ill patients as it is hemodynamically better tolerated, offers prompt correction of life-threatening metabolic imbalances, and allows for the adequate control of fluid balance [9]. Depending on the technique used for solute removal, dialysis is called continuous venovenous hemofiltration (CVVH) or continuous venovenous hemodiafiltration (CVVHDF). Citrate is frequently used as an anticoagulant for the extracorporeal circuit of CRRT [10]. Although citrate reaches high concentrations in the extracorporeal circuit, the concentration in the systemic circulation is much lower. Citrate is partially removed in the effluent during CRRT [10,11]. When the extracorporeal blood enters the systemic blood circulation, citrate dissolves in the total blood volume and is cleared by the liver resulting in very low plasma levels [12].
Due to the exchange of solutes during CRRT, nutrition therapy is more challenging. First, CRRT induces CO2 exchange outside the lungs, leading to inaccurate REE measurements with IC [13]. The earlier manuscript of this MECCIAS trial clarified how the CO2 is exchanged during CVVH and how a correction factor can be integrated into the REE measurements by IC to calculate the ‘true REE’ [14,15]. Second, non-intentional calories are exchanged during CRRT [7,16]. During CVVHDF, up to 1434 kcal/day were administered due to citrate, glucose, and lactate [11,12]. Not all dialysis fluids contain the same amount of glucose and lactate, which results in different bioenergetic balances (Table 1). Therefore, depending on the dialysis fluid used, different amounts of non-intentional calories are given to the patient [11,12]. Furthermore, due to the hepatic clearance, citrate use is contraindicated in liver function impairment. CRRT regimens have been developed with citrate-free fluids, which can affect the bioenergetic balance [10].
Table 1.
Composition of different dialysis fluids.
| Prismocitrate ® 18/0 | Prismocal B22 ® | Biphozyl ® | NaCl 0.9% | |
|---|---|---|---|---|
| Na (mmol/L) | 140 | 140 | 140 | 154 |
| K (mmol/L) | 0 | 4 | 4 | 0 |
| Cl (mmol/L) | 86 | 120.5 | 122 | 154 |
| Mg (mmol/L | 0 | 0 | 0.75 | 0 |
| P (mmol/L) | 0 | 0 | 1–2 | 0 |
| HCO3 (mmol/L) | 0 | 22 | 22 | 0 |
| Citrate (mmol/L) | 18 | 0 | 0 | 0 |
| Glucose (mmol/L) | 0 | 6.1 | 0 | 0 |
| Lactate (mmol/L) | 0 | 3 | 0 | 0 |
®: registered trademark.
Finally, besides the different compositions of dialysis fluids, the flows used during CRRT can also impact the exchange of non-intentional calories. Dialysis fluid flows are tailored to the patient’s individual need, resulting in different exchange rates of non-intentional calories [10,11,12]. To our knowledge, no data exist on the relative proportion of non-intentional calories related to the true REE during CVVH.
In this study, we aimed to analyze the bioenergetic balance of different CVVH settings during the MECCIAS trial and compared it to the true REE to achieve general considerations and recommendations.
2. Materials and Methods
A retrospective analysis was performed on all patients included in the prospective MECCIAS trial (MEtabolic Consequences of Continuous venovenous hemofiltration on Indirect cAlorimetry) [15]. The prospective trial explored the influence of CO2 exchange on the REE of different settings of CVVH: during a standard of care low dose CVVH with citrate, during a high dose CVVH with citrate, and during a low dose CVVH without citrate. The low dose CVVH with citrate was performed according to a local low dose protocol with citrate predilution with a targeted effluent dose of 25 to 30 mL/kg/h. For the high dose CVVH setting, the postdilution fluid was intended to be increased so an effluent dose of 50 mL/kg/h was achieved. The CVVH was then adjusted again to the first low dose settings and the citrate predilution was replaced by normal saline (NaCl 0.9%). For this retrospective report, we included all of the CVVH runs with glucose and lactate measurements in effluent. Glucose and lactate were measured using a point of care blood gas analyzer (ABL 90 flex®, radiometer; Bronshoj, Denmark). The bioenergetic balance of the CVVH was defined as the net combined exchange of citrate, glucose and lactate converted into kcal per 24 h. The loss of glucose and lactate was calculated by multiplying the effluent dose with the measured glucose and lactate concentration of the fluid, respectively. Citrate in the extracorporeal circulation before the filter was calculated by multiplying the predilution flow with the citrate content of the fluid and dividing this by the flow of predilution plus the flow of blood [citrate]ECC = [citrate]pre × Qpre/(Qpre + Q blood). The sieving coefficient of the citrate is approximatively 1 [17,18]. Therefore, the citrate concentration in the effluent is the same as in the blood after the predilution fluid is added. The removal was calculated by multiplying the theoretical concentration in effluent with the effluent flow [17]. The following calculations were performed, to convert the amount of the different carbohydrates that are lost in the effluent to energy loss: 1 g of citrate was considered to contain 2.5 kcal; 1 g of glucose was considered to contain 3.75 kcal; and 1 g of lactate was considered to contain 3.6 kcal [11,12]. The bioenergetic balance of the CVVH was compared with the true REE, as described in the MECCIAS trial. The true REE was measured and calculated during each different run, as different components of the CVVH will influence this measurement and calculation [15].
Statistical advice was sought at the statistical department of the Vrije Universiteit Brussel. Due to the low number of samples, normality was assumed to be not assessable. Due to missing data, an unpaired analysis was performed. A one-way ANOVA was performed to compare the multiple groups and a student t-test was performed to compare the two groups. p < 0.05 was considered a statistically significant difference. A Prism version 7.0c (GraphPad, San Diego, CA, USA) was used for the statistical analysis.
3. Results
Nineteen CVVH runs from nine different patients were included for the post hoc analysis as they contained a blood gas analysis of the lactate and glucose concentrations of the effluent. The runs consisted of eight low dose CVVHs with citrate, four high dose CVVHs with citrate, and seven low dose CVVHs without citrate. The CVVH settings during the different runs of CVVH are presented in Table 2. The patient characteristics can be found in Appendix A.
Table 2.
Continuous venovenous hemofiltration (CVVH) settings.
| Low Dose CVVH with Citrate |
High Dose CVVH with Citrate |
Low Dose CVVH without Citrate |
|
|---|---|---|---|
| n = | 8 | 4 | 7 |
| Blood flow (mL/min) | 150 ± 0 | 150 ± 0 | 150 ± 0 |
| Predilution flow (mL/h) | 1756 ± 264 | 1700 ± 147 | 1721 ± 296 |
| Postdilution flow | 506 ± 431 | 2300 ± 1036 | 464 ± 490 |
| Postdilution fluid (n) | |||
|
1 | 0 | 1 |
|
3 | 2 | 3 |
|
4 | 2 | 3 |
| Effluent flow (mL/h) | 2363 ± 476 | 4075 ± 974 | 2279 ± 551 |
Values are expressed in mean ± standard deviation; ®: registered trademark. CVVH: continuous venovenous hemofiltration.
3.1. Bioenergetic Balance
The absolute and relative bioenergetic balances are depicted in Table 3. The mean absolute bioenergetic balance (over 24 h) during the low dose CVVH with citrate was 498 ± 110 kcal ranging from 339 kcal to 681 kcal. During the high dose CVVH with citrate, the mean absolute bioenergetic balance was 262 ± 222 kcal with a range of 56 kcal to 565 kcal, which was a statistically significant difference (p = 0.030) compared to the low dose CVVH with citrate. The mean absolute bioenergetic balance during the low dose CVVH without citrate was −189 ± 77 kcal with a range of −298 kcal to −92 kcal, which was a statistically significant difference compared to the low dose CVVH with citrate (p < 0.0001) and the high dose CVVH with citrate (p = 0.001).
Table 3.
Absolute and relative bioenergetic balances during the different Continuous venovenous hemofiltration (CVVH) settings.
| Low Dose CVVH with Citrate |
High Dose CVVH with Citrate |
Low Dose CVVH without Citrate |
||
|---|---|---|---|---|
| Absolute bioenergetic balance (kcal/day) | Mean | 498 ± 110 | 262 ± 222 | −189 ± 77 |
| Range | 339 to 681 | 56 to 565 | −298 to −92 | |
| Relative bioenergetic balance (%) | Mean | 26 ± 9 | 17 ± 11 | −13 ± 8 |
| Range | 14 to 42 | 7 to 32 | −28 to −5 |
Mean values are expressed with their standard deviation (±).
The mean relative bioenergetic balance during the low dose CVVH with citrate compared to the true REE was 26 ± 9%, with a range of 14% to 42%. This was not a statistically significant difference from the high dose CVVH with citrate (p = 0.128), as the mean relative bioenergetic balance was 17 ± 11% compared to the true REE, with a range of 7% to 32%. During the low dose CVVH without citrate, the mean relative bioenergetic balance compared to the true REE was −13 ± 8%, with a range of −28% to −5 %, which was a statistically significant difference (p < 0.0001 and p = 0.001, respectively) compared to the two previous settings.
The adaptation for nutrition therapy ranges between −298 kcal and up to 681 kcal per day.
3.2. Non-Intentional Calories
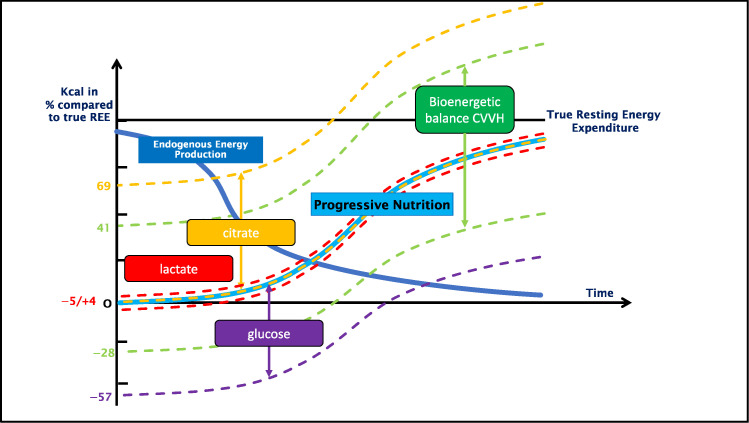
Table 4 shows the exchange, due to the CVVH, of the different non-intentional calorie-containing molecules: citrate, glucose, and lactate. The relative energetic balance of the different molecules compared to the true REE can be found in Figure 1.
Table 4.
Exchange of non-intentional calories during the different Continuous venovenous hemofiltration (CVVH) settings.
| Low Dose CVVH with Citrate |
High Dose CVVH with Citrate |
Low Dose CVVH without Citrate |
p-Value | ||
|---|---|---|---|---|---|
| Gain due to dialysis fluid of non-intentional caloric containing molecules | Citrate (mmol/24 h) | 759 ± 114 | 734 ± 64 | 0 | <0.001 |
| Glucose (g/24 h) | 6 ± 14 | 38 ± 45 | 7 ± 15 | 0.083 | |
| Lactate (mmol/24 h) | 16 ± 38 | 104 ± 124 | 19 ± 40 | 0.083 | |
| Loss in effluent of non-intentional caloric containing molecules | Citrate (mmol/24 h) | 168 ± 47 | 281 ± 73 | 0 | <0.001 |
| Glucose (g/24 h) | 64 ± 28 | 107 ± 40 | 57 ± 22 | 0.032 | |
| Lactate (mmol/24 h) | 64 ± 34 | 127 ± 74 | 60 ± 38 | 0.070 | |
| Total balance of non-intentional caloric containing molecules | Citrate (mmol/24 h) | 591 ± 81 | 453 ± 60 | 0 | <0.001 |
| Glucose (g/24 h) | −59 ± 24 | −69 ± 53 | −50 ± 20 | 0.607 | |
| Lactate (mmol/24 h) | −48 ± 16 | −22 ± 84 | −42 ± 14 | 0.567 | |
| Absolute caloric balance (kcal/day) |
Citrate | 736 ± 101 | 564 ± 75 | 0 | <0.001 |
| Glucose | −222 ± 90 | −262 ± 202 | −187 ± 74 | 0.584 | |
| Lactate | −16 ± 5 | −7 ± 27 | 3 ± 15 | 0.032 | |
| Relative caloric balance compared to the true Resting Energy Expenditure (REE) (%) |
Citrate | 40 ± 14% (26 to 69%) | 44 ± 16% (34 to 69%) | 0% | <0.001 |
| Glucose | −12 ± 7% (−25 to −5%) | −24 ± 24% (−57 to 0%) | −13 ± 8% (−28 to −5%) | 0.300 | |
| Lactate | −1 ± 1% (−2 to 0%) | −1 ± 3% (−5 to 1%) | 0 ± 1% (−1 to 3%) | 0.200 |
Values are expressed in mean ± standard deviation. In the relative caloric balance, the minimum and maximum were also added between ( ).
Figure 1.
Bioenergetic balance of the CVVH compared to the true REE in relation to the ideal caloric delivery over time during progressive nutrition. This figure depicts the exchange of citrate, lactate and glucose in percentages compared to the true REE. It is placed against the need for progressive nutrition therapy, as recommended by the ESPEN guidelines [16], in relation to the endogenous energy production due to catabolism. The graph follows the recommendation of progressive nutrition therapy, illustrating the impact on total caloric load if the exchange of the different nutrients was not taken into consideration.
4. Discussion
The current study shows a large variability of bioenergetic balance during CVVH. Although in CRRT modes other than CVVH a similar effect has been described, to our knowledge this is a new finding [11,12]. The lowest bioenergetic balance was noticed during the CVVH without citrate, which correlates with previous findings, although there, lactate was used in the dialysis fluids [11]. Citrate is the most dominant contributor to positive bioenergetic balances (Figure 1).
Furthermore, citrate increases the REE [15]. Currently used predicting equations of REE do not take this change into consideration. Therefore, the predictive efforts without IC have failed to reproduce the individual REE systematically and accurately in intensive care unit (ICU) patients [19,20]. The MECCIAS-trial showed that during the CVVH, IC could measure the REE and, subsequently, could objectify the effect of citrate during the CVVH on individual metabolism [15]. By comparing the true REE measured by IC with the bioenergetic balance in the CVVH, we introduce a new concept. The MECCIAS-trial showed that citrate use during CVVH induced a raise in the REE of 330 kcal or approximatively 20% compared to the CVVH without citrate [15]. We found that the low dose CVVH with citrate delivers 40% of the calories needed compared to the true REE. The increase in calory delivery is more than the increase in REE. There would still be an overload of calories, which would largely overshoot the altered REE due to the citrate itself. Therefore, measuring the bioenergetic balance is necessary to avoid overfeeding.
Considerable variability in bioenergetic balances during CVVH with citrate was observed. The first explanation for this variability is the differences in the CVVH settings. Although the low dose CVVH with citrate induced the highest bioenergetic balance compared to the high dose CVVH with citrate, the delivery of citrate was clinically insignificantly different during the low dose CVVH versus the high dose CVVH. The difference in high or low dose did not contribute to the differences in the citrate-induced higher bioenergetic balance. On the other hand, the effluent dose was higher during the high dose CVVH. Therefore, more citrate was filtrated during the high dose CVVH resulting in lower bioenergetic balances of citrate, and this can explain the higher bioenergetic balance in the low dose CVVH compared to the high dose CVVH.
A second explanation for the variability in bioenergetic balances during the CVVH with citrate is the adaptation of the CVVH settings and fluids to individual needs. Due to the patient-related and technique-related issues, the citrate delivery varies between individuals [10]. Personalized CVVH therapy with personalized citrate delivery and removal explains the considerable variability in bioenergetic balances. Subsequently, if a precise feeding strategy is aspired, the variability of the bioenergetic balance of CVVH compared and combined with the measured REE dictates an individual approach. By doing so, under-, and probably more frequently overfeeding will be avoided.
The other novel finding in this manuscript was the in vivo negative balances during the low dose CVVH without citrate. Negative bioenergetic balances have been described in a theoretical in vitro model evaluating CRRT without glucose or citrate [21]. This is in contrast with previous in vivo reports in which glucose and lactate were identified with a dominant role on the impact of positive bioenergetic balances during CVVH [11,12]. The earlier reported studies used glucose and lactate containing fluids during CVVH, whereas our dialysis protocol uses glucose and lactate-free solutions. More recent CVVH guidelines advise using lactate-free buffer solutions [10]. The dialysis protocol of the intensive care department at UZ Brussel also uses glucose-free dialysis fluids as stress-induced hyperglycemia is frequently encountered in the ICU [22]. During the low dose CVVH without citrate, glucose loss was the primary cause of negative bioenergetic balances. Lactate impacted the bioenergetic balances less. If caloric prescriptions were made without accounting for these losses of calories, it would leave the patient highly underfed and might impair prognosis [2,4,16].
Applying a fixed correction factor for the caloric prescription depending on the CVVH setting without calculating the individual bioenergetic balance seems a practical approach; however, this is difficult to implement in clinical practice because of the multitude of influences on adequacy [23,24]. If a patient individualized calculation would not be feasible, we could try to implement the knowledge of the bioenergetic balance presented in this paper: During the low dose CVVH with citrate, the mean bioenergetic balance was 26% with a minimum of 14 to 42%. Normocaloric feeding implies a delivery of 70–100% of the REE [16]. This means that if bioenergetic balances would not be calculated, to avoid over- and underfeeding in all of the subjects, exactly 56% (56% + 14% = 70%) to 58% (58% + 42% = 100%) of the true REE should be delivered as calories to the patients. During the high dose CVVH with citrate, 63% to 68% of the true REE should be delivered as calories. During the low dose CVVH without citrate, the caloric prescription should be 105% to 98% of the true REE. Translation of such a narrow caloric target in clinical practice is practically impossible because of feeding challenges. These small margins would leave no room for errors in clinical practice where a lot of interfering factors can, but not necessarily will, influence the adequacy of the delivered nutrients, and thus, the calories [23,24]. A larger portion of the patients would be under- or overfed if such correction factors were applied. It seems preferable to measure the caloric balances and adapt accordingly.
Another consideration in CVVH is the loss of proteins in the effluent [25]. In the original MECCIAS trial, no information on protein losses were recorded as it was out of the scope. Although the energetic load of delivered proteins is added to the caloric delivery during nutrition therapy, the extra amount that should be given is to compensate for the losses. Therefore, it would not impact the caloric prescription as it is merely a supplementation [25]. However, further research integrating all of these different nutrition factors to optimize nutrition in CVVH patients seems necessary.
5. Conclusions
The bioenergetic balance of CVVH has considerable variation and can range from −28% up to +42% of the true REE. During CVVH with citrate, and predilution and postdilution without glucose or lactate, citrate is the predominant source of non-intentional calories. The absence of glucose in dialysis fluid contributes to negativizing the bioenergetic balance. Lactate has a minor impact on the bioenergetic balance during CVVH. When formulating a caloric prescription in a CVVH patient, an individual approach considering the impact of these non-intentional calories upon the bioenergetic balance is warranted.
Acknowledgments
The authors would like to thank Marie-Claire Van Malderen for the administrative work related to this study.
Appendix A
Table A1.
Patient characteristics.
| Amount of Subjects (ntotal) | 9 |
|---|---|
| Mean age | 70 ± 12 years |
| Gender | |
|
6 |
|
3 |
| Mean weight | 89 ± 28 kg |
| Mean BMI | 30.3 ± 6.7 kg/m2 |
| Mean APACHE II score | 30 ± 12 |
| Ventilation (n) | |
|
4 |
|
5 |
| 30-day mortality (n/ntotal) | 7/9 |
When data are presented as the mean, the standard deviation is also provided (±).
Author Contributions
Conceptualization, J.J. and E.D.W.; methodology: J.J. and E.D.W.; formal analysis, J.J.; investigation, J.J. and E.D.W.; resources, J.J. and E.D.W.; data curation, J.J.; writing—original draft preparation, J.J.; writing—review and editing, J.J., A.V.H., T.O. and E.D.W.; visualization, J.J., T.O. and E.D.W.; supervision, J.J. and E.D.W.; project administration, J.J.; funding acquisition, J.J. and E.D.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The original prospective MECCIAS-trial upon which this post hoc analysis was performed is registered at https://clinicaltrials.gov/ct2/show/NCT03314363 (NCT03314363, accessed on 19 October 2017) and was approved by the ethical committee UZ Brussel (approval number B.U.N. 143201731636). The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Raw data can be obtained upon written request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
The department of critical care of Universitair Ziekenhuis Brussel has received a grant from Baxter Healthcare Corporation, with grant number “dewaele_elisabeth_102417” as a replacement fee and logistic support to perform the original prospective trial.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Duan J.Y., Zheng W.H., Zhou H., Xu Y., Huang H.B. Energy delivery guided by indirect calorimetry in critically ill patients: A systematic review and meta-analysis. Crit. Care. 2021;25:88. doi: 10.1186/s13054-021-03508-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidegger C.P., Berger M.M., Graf S., Zingg W., Darmon P., Costanza M.C., Thibault R., Pichard C. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: A randomised controlled clinical trial. Lancet. 2013;381:385–393. doi: 10.1016/S0140-6736(12)61351-8. [DOI] [PubMed] [Google Scholar]
- 3.Pertzov B., Bar-Yoseph H., Menndel Y., Bendavid I., Kagan I., Glass Y.D., Singer P. The effect of indirect calorimetry guided isocaloric nutrition on mortality in critically ill patients-a systematic review and meta-analysis. Eur. J. Clin. Nutr. 2022;76:5–15. doi: 10.1038/s41430-021-00919-0. [DOI] [PubMed] [Google Scholar]
- 4.Zusman O., Theilla M., Cohen J., Kagan I., Bendavid I., Singer P. Resting energy expenditure, calorie and protein consumption in critically ill patients: A retrospective cohort study. Crit. Care. 2016;20:367. doi: 10.1186/s13054-016-1538-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weijs P.J., Looijaard W.G., Beishuizen A., Girbes A.R., Oudemans-van Straaten H.M. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit. Care. 2014;18:701. doi: 10.1186/s13054-014-0701-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arabi Y.M., Aldawood A.S., Al-Dorzi H.M., Tamim H.M., Haddad S.H., Jones G., McIntyre L., Solaiman O., Sakkijha M.H., Sadat M., et al. Permissive Underfeeding or Standard Enteral Feeding in High- and Low-Nutritional-Risk Critically Ill Adults. Post Hoc Analysis of the PermiT Trial. Am. J. Respir. Crit. Care Med. 2017;195:652–662. doi: 10.1164/rccm.201605-1012OC. [DOI] [PubMed] [Google Scholar]
- 7.Fiaccadori E., Sabatino A., Barazzoni R., Carrero J.J., Cupisti A., De Waele E., Jonckheer J., Singer P., Cuerda C. ESPEN guideline on clinical nutrition in hospitalized patients with acute or chronic kidney disease. Clin. Nutr. 2021;40:1644–1668. doi: 10.1016/j.clnu.2021.01.028. [DOI] [PubMed] [Google Scholar]
- 8.Case J., Khan S., Khalid R., Khan A. Epidemiology of acute kidney injury in the intensive care unit. Crit. Care Res. Pract. 2013;2013:479730. doi: 10.1155/2013/479730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider A.G., Bellomo R., Bagshaw S.M., Glassford N.J., Lo S., Jun M., Cass A., Gallagher M. Choice of renal replacement therapy modality and dialysis dependence after acute kidney injury: A systematic review and meta-analysis. Intensive Care Med. 2013;39:987–997. doi: 10.1007/s00134-013-2864-5. [DOI] [PubMed] [Google Scholar]
- 10.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron. Clin. Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 11.Balik M., Zakharchenko M., Leden P., Otahal M., Hruby J., Polak F., Rusinova K., Stach Z., Tokarik M., Vavrova J., et al. Bioenergetic gain of citrate anticoagulated continuous hemodiafiltration—A comparison between 2 citrate modalities and unfractionated heparin. J. Crit. Care. 2013;28:87–95. doi: 10.1016/j.jcrc.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 12.New A.M., Nystrom E.M., Frazee E., Dillon J.J., Kashani K.B., Miles J.M. Continuous renal replacement therapy: A potential source of calories in the critically ill. Am. J. Clin. Nutr. 2017;105:1559–1563. doi: 10.3945/ajcn.116.139014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oshima T., Berger M.M., De Waele E., Guttormsen A.B., Heidegger C.P., Hiesmayr M., Singer P., Wernerman J., Pichard C. Indirect calorimetry in nutritional therapy. A position paper by the ICALIC study group. Clin. Nutr. 2017;36:651–662. doi: 10.1016/j.clnu.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Jonckheer J., Spapen H., Debain A., Demol J., Diltoer M., Costa O., Lanckmans K., Oshima T., Honoré P.M., Malbrain M., et al. CO2 and O2 removal during continuous veno-venous hemofiltration: A pilot study. BMC Nephrol. 2019;20:222. doi: 10.1186/s12882-019-1378-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonckheer J., Demol J., Lanckmans K., Malbrain M., Spapen H., De Waele E. MECCIAS trial: Metabolic consequences of continuous veno-venous hemofiltration on indirect calorimetry. Clin. Nutr. 2020;39:3797–3803. doi: 10.1016/j.clnu.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Singer P., Blaser A.R., Berger M.M., Alhazzani W., Calder P.C., Casaer M.P., Hiesmayr M., Mayer K., Montejo J.C., Pichard C., et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019;38:48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 17.Mariano F., Morselli M., Bergamo D., Hollo Z., Scella S., Maio M., Tetta C., Dellavalle A., Stella M., Triolo G. Blood and ultrafiltrate dosage of citrate as a useful and routine tool during continuous venovenous haemodiafiltration in septic shock patients. Nephrol. Dial Transpl. 2011;26:3882–3888. doi: 10.1093/ndt/gfr106. [DOI] [PubMed] [Google Scholar]
- 18.Chadha V., Garg U., Warady B.A., Alon U.S. Citrate clearance in children receiving continuous venovenous renal replacement therapy. Pediatr. Nephrol. 2002;17:819–824. doi: 10.1007/s00467-002-0963-6. [DOI] [PubMed] [Google Scholar]
- 19.De Waele E., Opsomer T., Honoré P.M., Diltoer M., Mattens S., Huyghens L., Spapen H. Measured versus calculated resting energy expenditure in critically ill adult patients. Do mathematics match the gold standard? Minerva Anestesiol. 2015;81:272–282. [PubMed] [Google Scholar]
- 20.Zusman O., Kagan I., Bendavid I., Theilla M., Cohen J., Singer P. Predictive equations versus measured energy expenditure by indirect calorimetry: A retrospective validation. Clin. Nutr. 2019;38:1206–1210. doi: 10.1016/j.clnu.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Stevenson J.M., Heung M., Vilay A.M., Eyler R.F., Patel C., Mueller B.A. In vitro glucose kinetics during continuous renal replacement therapy: Implications for caloric balance in critically ill patients. Int. J. Artif. Organs. 2013;36:861–868. doi: 10.5301/ijao.5000232. [DOI] [PubMed] [Google Scholar]
- 22.Mizock B.A. Alterations in carbohydrate metabolism during stress: A review of the literature. Am. J. Med. 1995;98:75–84. doi: 10.1016/S0002-9343(99)80083-7. [DOI] [PubMed] [Google Scholar]
- 23.De Waele E., Spapen H., Honore P.M., Mattens S., Rose T., Huyghens L. Bedside calculation of energy expenditure does not guarantee adequate caloric prescription in long-term mechanically ventilated critically ill patients: A quality control study. Sci. World J. 2012;2012:909564. doi: 10.1100/2012/909564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Jonghe B., Appere-De-Vechi C., Fournier M., Tran B., Merrer J., Melchior J.C., Outin H. A prospective survey of nutritional support practices in intensive care unit patients: What is prescribed? What is delivered? Crit. Care Med. 2001;29:8–12. doi: 10.1097/00003246-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Stapel S.N., de Boer R.J., Thoral P.J., Vervloet M.G., Girbes A.R.J., Oudemans-van Straaten H.M. Amino Acid Loss during Continuous Venovenous Hemofiltration in Critically Ill Patients. Blood Purif. 2019;48:321–329. doi: 10.1159/000500998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data can be obtained upon written request to the corresponding author.