Abstract
Background.
A subset of triple-negative breast cancer (TNBC) is characterized by aggressive disease, rapid relapse, and mortality within 24 months of diagnosis, termed “rapid relapse” TNBC (rrTNBC). The objective of this study is to define the association between sociodemographic variables and surgical management among rrTNBC patients in the Surveillance, Epidemiology and End Results (SEER) Program.
Methods.
TNBC patients diagnosed from January 1, 2010 to December 31, 2014 with local or regional disease were identified in SEER. Patients were stratified as rrTNBC, defined as disease specific mortality ≤ 24 months after diagnosis, and non-rrTNBC. Chi-squared tests, t tests, and multivariable logistic regression were used to assess the association of rapid relapse with sociodemographic variables and surgical management.
Results.
The cohort included 8% (1378/17,369) rrTNBCs. A higher proportion of rrTNBC patients had no surgery (11.7%) compared with non-rrTNBC (2.6%). Omission of axillary staging among patients who had surgery was 6.2% rrTNBC versus 4.5% non-rrTNBC. Black race (odds ratio [OR] 1.22, 95% confidence interval [CI] 1.05–1.43; p = 0.01; white ref), Medicaid or no insurance (Medicaid OR 1.53, 95% CI 1.31–1.79; p < 0.001; no insurance OR 1.74, 95% CI 1.31–2.32; p < 0.001; private ref), single status (OR 1.19, 95% CI 1.01–1.39; p = 0.03; married ref), no breast (OR 2.35, 95% CI 1.77–3.11; p < 0.001; mastectomy ref), and no axillary surgery (OR 1.44, 95% CI 1.13–1.83; p = 0.003 axillary surgery ref) were associated with rapid relapse.
Conclusions.
Medicaid or no insurance, single status, black race, and no surgery are associated with higher odds of rrTNBC in SEER. These results indicate an interplay between socioeconomic factors, clinical and genomic variables may be disproportionately contributing to worse outcomes among a subset of TNBC patients.
Triple-negative breast cancer (TNBC) is defined by absence of the estrogen receptor (ER), progesterone receptor (PR), and HER2 protein. TNBC makes up approximately 10–15% of breast cancer cases yet accounts for more than 30–40% of breast cancer mortality.1 Relative to other breast cancer subsets, TNBCs tend to present larger tumor size and are more likely to metastasize to visceral sites such as lung and brain.2,3 Historically, median survival for patients with TNBC after metastatic diagnosis was approximately 18 months, improved to approximately 25 months among patients with PD-L1 positive TNBC receiving the recently FDA-approved regimen of chemotherapy plus immunotherapy.1,3–6 In several, large, TNBC cohort studies, the median distant metastasis-free survival ranged from 19.7 to 31.2 months.3,4,7,8 Patients who have short disease-free interval after primary therapy have a particularly poor prognosis. Clinically, these patients demonstrate resistance to standard therapies and rapid progression of disease.9–11 To further understand this aggressive subset of TNBCs, we define “rapid relapse” TNBC (rrTNBC) as relapse or death within 24 months of diagnosis.
The factors that mediate rapid relapse versus late or no relapse are poorly understood. We hypothesize that rrTNBC is impacted by biological features of the tumor, patient sociodemographic features, and access to clinical care. We previously investigated the association of genomic and basic clinical features with rrTNBC among 453 primary TNBCs and found that while there were certain genomic features associated with rrTNBC, stage at diagnosis remained a top contributing feature even in the context of thousands of genomic features.12 Stage at diagnosis is associated with both biological features and sociodemographic features, such as race, insurance, income, and education.13–18 In a multi-institution retrospective study, we observed that rrTNBC was significantly associated with higher stage, Medicaid/indigent insurance, lower income, and younger age at diagnosis.19 However, the prior study was more limited in scope (~ 3000 TNBCs).
In this study, we evaluated the association between sociodemographic variables and surgical management with rrTNBC among patients identified in the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute.
METHODS
Cohort
Data from the SEER Program were used to identify TNBC patients aged 18–64 years and diagnosed from January 1, 2010 to December 31, 2014 with local or regional disease.20 SEER is a collection of high-quality, population-based cancer registries with very high estimated completeness of reporting. These registries capture data covering approximately 30% of the U.S. population. Data reflecting sociodemographic and clinical factors of patients diagnosed with TNBC and included in 18 SEER registries were obtained from case listing sessions using SEER*Stat software (version 8.3.6).21 Additional data reflecting radiation and chemotherapy factors were obtained as a result of special permission from the SEER Program.
Due to our interest in neighborhood socioeconomic status, the NCI census tract-level index in SEER was included as a variable. The NCI census tract-level index is a time-dependent composite score that includes: median household income, education index, median house value, percent working class, median rent, percent unemployed, and percent below 150% of poverty line.22,23 The index was generated by linking census tract information on cancer cases in SEER to the 2000 U.S. Decennial Census long-form survey and the American Community Survey (ACS) 5-year estimates from 2006 to 2016.24
Patients
Patients were divided into two groups: rrTNBC and non-rrTNBC. rrTNBC was defined as disease specific mortality ≤ 24 months after diagnosis, based on existing data from large TNBC cohort studies and our prior work.3,4,7,8,19 We excluded patients with distant disease at diagnosis or unknown stage, patients with < 24 months of follow-up or with missing follow-up data or who died from a cause other than this cancer diagnosis, or patients missing insurance data.
Statistical Analyses
Sociodemographic and clinical variables included insurance type, age at diagnosis by decade, year of diagnosis, marital status, race/ethnicity, education (% less than high school), median household income, histology, SEER registry, stage, grade, rural urban continuum, and NCI census tract-level index. Insurance type was categorized as uninsured, Medicaid, or insured (non-Medicaid/unknown). Education and median household income are county-level variables presented as continuous variables (percentages or means). For the NCI census tract-level index we calculated and used a normalized value (normalized to a 0–100 scale) to improve interpretability. A higher score indicates a higher neighborhood socioeconomic status.
Surgical management and treatment variables included surgery (no surgery vs. breast-conserving surgery [BCS] vs. mastectomy), axillary surgery (no breast or axillary surgery vs. breast surgery but no axillary surgery vs. breast surgery and sentinel lymph node biopsy vs. breast surgery and axillary lymph node dissection), radiation (no/unknown vs. yes with BCS vs. yes with mastectomy), and chemotherapy (no/unknown vs. yes). Based on prior studies, sentinel lymph node biopsy was defined as removal of < 10 nodes and axillary lymph node dissection was defined as removal of ≥ 10 nodes.25
Chi-squared and t tests were performed for associations between each covariate of interest with rapid relapse versus no rapid relapse. Features with a p value < 0.05 were included in the multivariable logistic model. For the logistic model, the outcome was rapid relapse versus no rapid relapse and the predictors were stage, race/ethnicity, insurance, education, income, NCI census tract-level index, marital status, age at diagnosis, grade, histology, surgery, axillary surgery, and radiation. Finally, we performed four sensitivity analyses to assess the final model: (1) removed patients who did not have breast or axillary surgery, (2) removed inflammatory breast cancers, (3) removed patients who did not receive chemotherapy, and (4) removed black patients. All statistical analyses were performed with SAS version 9.4. The Ohio State University Office of Responsible Research Practices deemed this study institutional review board exempt.
RESULTS
There were 17,369 TNBC patients aged 18–64 years who were diagnosed between 2010 and 2014 with local or regional disease (Fig. 1). The cohort included 8% (1378/17,369) rrTNBCs. Overall, a majority of this cohort was insured, aged 51–60 years, married/partnered, non-Hispanic white race, had ductal histology, local stage, poorly differentiated grade, and lived-in large metropolitan areas (Table 1). In bivariable analyses, statistically significant differences (p < 0.05) were observed for rrTNBC versus non-rrTNBC patients based on insurance type (p < 0.01), age at diagnosis (p = 0.02), marital status (p < 0.001), race/ethnicity (p < 0.001), education (p < 0.001), median household income (p < 0.001), histology (p < 0.001), SEER registry (p = 0.06; Supplemental Table 1), stage (p < 0.001), grade (p < 0.001), and NCI census tract-level index (p < 0.001) (Table 1). Among treatment variables, statistically significant differences were observed for rrTNBC versus non-rrTNBC patients based on breast surgery (p < 0.001), axillary surgery (p < 0.001), and radiation (p < 0.001) (Table 2). Notably, there was no significant difference between the groups on receipt of chemotherapy (p = 0.26). On subset analysis stratified by stage, a higher percentage of rrTNBC patients were more likely to have surgical management omitted compared to patients in the non-rrTNBC group (Supplementary Table 2).
FIG. 1.
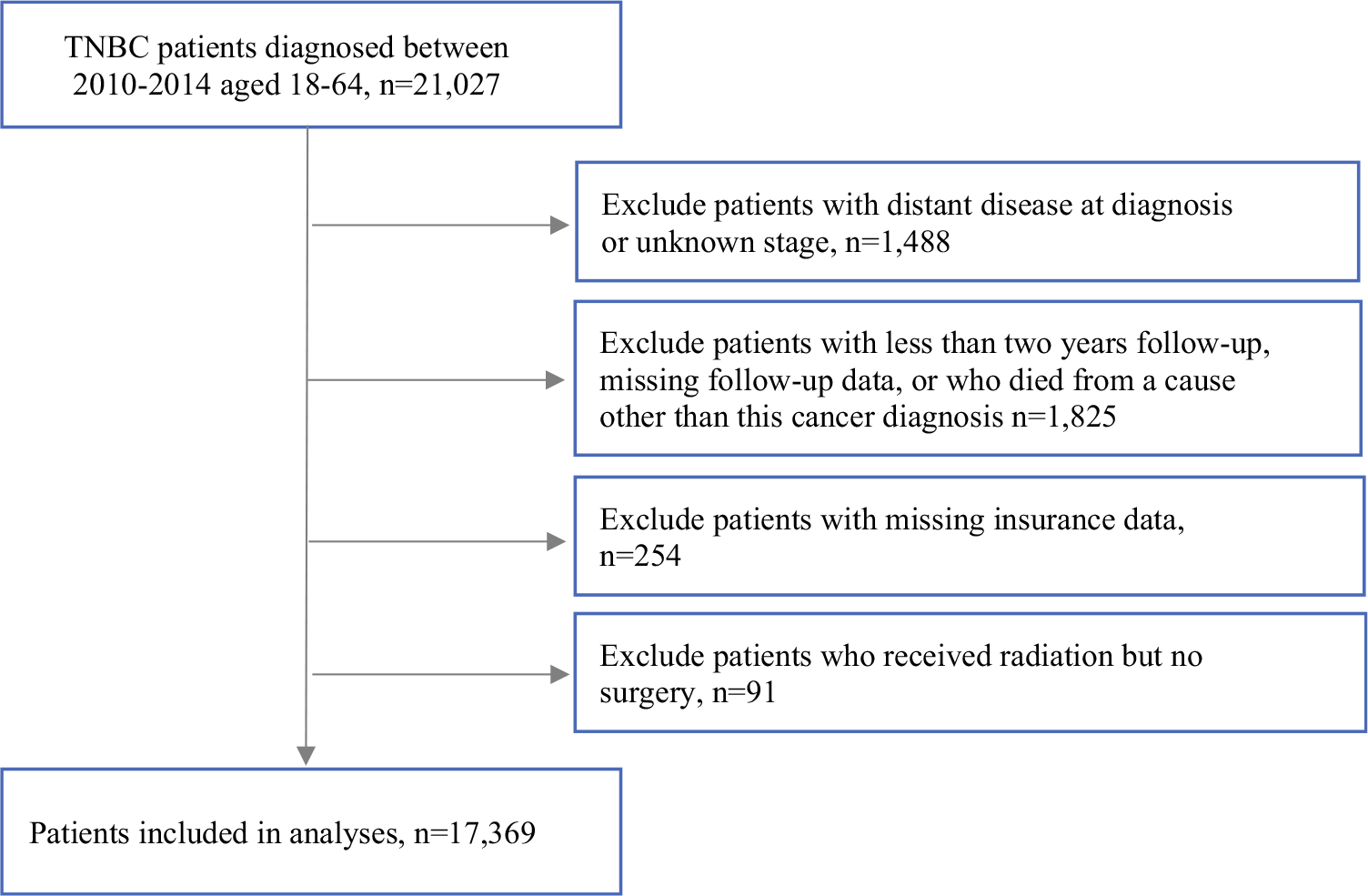
CONSORT diagram of study population from SEER
TABLE 1.
Description of study sociodemographic and clinical variables
| Variable | Total (N = 17,369) N (%) |
Rapid relapse* (N = 1378) N (%) |
No rapid relapse (N = 15,991) N (%) |
Chi-squared/t test p value |
|---|---|---|---|---|
|
| ||||
| Insurance type | < 0.01 | |||
| Uninsured | 526 (3.0) | 82 (6.0) | 444 (2.8) | |
| Medicaid | 2813 (16.2) | 372 (27.0) | 2441 (15.3) | |
| Insured (non-Medicaid/UK) | 14,030 (80.8) | 924 (67.1) | 13,106 (82.0) | |
| Age at diagnosis (years) | 0.02 | |||
| ≤ 40 | 2543 (14.6) | 234 (17.0) | 2309 (14.4) | |
| 41–50 | 5142 (29.6) | 421 (30.6) | 4721 (29.5) | |
| 51–60 | 6926 (39.9) | 528 (38.3) | 6398 (40.0) | |
| 61–64 | 2758 (15.9) | 195 (14.2) | 2563 (16.0) | |
| Year of diagnosis | 0.07 | |||
| 2010 | 3633 (20.9) | 280 (20.3) | 3353 (21.0) | |
| 2011 | 3811 (21.9) | 289 (21.0) | 3522 (22.0) | |
| 2012 | 3536 (20.4) | 284 (20.6) | 3252 (20.3) | |
| 2013 | 3399 (19.6) | 251 (18.2) | 3148 (19.7) | |
| 2014 | 2990 (17.2) | 274 (19.9) | 2716 (17.0) | |
| Marital status | < 0.001 | |||
| Single | 3446 (20.8) | 363 (27.7) | 3083 (20.2) | |
| Married/partnered | 10,152 (61.3) | 689 (52.5) | 9463 (62.1) | |
| Separated/divorced | 2323 (14.0) | 202 (15.4) | 2121 (13.9) | |
| Widowed | 629 (3.8) | 59 (4.5) | 570 (3.7) | |
| Race/ethnicity | < 0.001 | |||
| Non-Hispanic white | 9875 (56.9) | 687 (49.9) | 9188 (57.5) | |
| Non-Hispanic black | 3772 (21.7) | 410 (29.8) | 3362 (21.0) | |
| Hispanic | 2365 (13.6) | 202 (14.7) | 2163 (13.5) | |
| Non-Hispanic other | 1357 (7.8) | 79 (5.7) | 1278 (8.0) | |
| % Less than high school (mean (95%o CI)) | 14.0 (13.9,14.1) | 14.8 (14.5,15.1) | 13.9 (13.8,14.0) | < 0.001 |
| Median household income (mean (95% CI)) | 63,892 (63,638,64,147) | 61,511 (60,629,62,394) | 64,097 (63,832,64,362) | < 0.001 |
| Histology | < 0.001 | |||
| Ductal | 15,148 (87.2) | 1144 (83.0) | 14,004 (87.6) | |
| Lobular | 130 (0.8) | 20 (1.5) | 110 (0.7) | |
| Mixed | 659 (3.8) | 60 (4.4) | 599 (3.8) | |
| Other | 1432 (8.2) | 154 (11.2) | 1278 (8.0) | |
| Stage | < 0.001 | |||
| Local | 11,048 (63.6) | 368 (26.7) | 10,680 (66.8) | |
| Regional | 6321 (36.4) | 1010 (73.3) | 5311 (33.2) | |
| Grade | < 0.001 | |||
| Well differentiated | 296 (1.8) | 7 (0.6) | 289 (1.9) | |
| Moderately differentiated | 2416 (14.6) | 140 (11.0) | 2276 (14.9) | |
| Poorly differentiated | 13,829 (83.6) | 1129 (88.5) | 12,700 (83.2) | |
| Rural urban continuum | 0.07 | |||
| Large metro | 10,777 (62.1) | 843 (61.2) | 9934 (62.2) | |
| Metro | 4831 (27.8) | 373 (27.1) | 4458 (27.9) | |
| Urban | 1524 (8.8) | 134 (9.7) | 1390 (8.7) | |
| Rural | 224 (1.3) | 27 (2.0) | 197 (1.2) | |
| NCI census tract-level index (mean (95% CI)) | 72.3 (72.0,72.7) | 69.0 (67.7,70.3) | 72.6 (72.3,73.0) | < 0.001 |
Rapid relapse was defined as disease specific mortality within 24 months post-diagnosis
TABLE 2.
Comparison of treatment among rapid relapse versus no rapid relapse triple-negative breast cancers
| Variable | Total N (%) |
Rapid relapse N (%) |
No rapid relapse N (%) |
Chi-squared p value |
|---|---|---|---|---|
|
| ||||
| Surgery | < 0.001 | |||
| No surgery | 743 (4.3) | 198 (14.4) | 545 (3.4) | |
| BCS | 7924 (45.7) | 299 (21.8) | 7625 (47.8) | |
| Mastectomy | 8670 (50.0) | 874 (63.8) | 7796 (48.8) | |
| Axillary surgery | < 0.001 | |||
| No breast or axillary surgery | 573 (3.3) | 158 (11.7) | 415 (2.6) | |
| Breast surgery but no axillary surgery | 797 (4.6) | 83 (6.2) | 714 (4.5) | |
| Breast surgery and sentinel lymph node biopsy (1–9 nodes removed) | 11,512 (66.9) | 496 (36.8) | 11,016 (69.5) | |
| Breast surgery and axillary lymph node dissection (≥ 10 nodes removed) | 4316 (25.1) | 610 (45.3) | 3706 (23.4) | |
| Radiation | < 0.001 | |||
| No/unknown | 8750 (50.4) | 776 (56.3) | 7974 (49.9) | |
| Yes with BCS | 5839 (33.6) | 165 (12.0) | 5674 (35.5) | |
| Yes with mastectomy | 2780 (16.0) | 437 (31.7) | 2343 (14.7) | |
| Chemotherapy | 0.26 | |||
| No/unknown | 2795 (16.1) | 207 (15.0) | 2588 (16.2) | |
| Yes | 14,574 (83.9) | 1171 (85.0) | 13,403 (83.8) | |
From the multivariable logistic model, odds of rrTNBC were higher for regional versus local stage (odds ratio [OR] 4.36, 95% confidence interval [CI] 3.77–5.03; p < 0.001), black race versus white race (OR 1.22, 95% CI 1.05–1.43; p = 0.01), Medicaid insurance (OR 1.53, 95% CI 1.31–1.79; p < 0.001), and no insurance (OR 1.74, 95% CI 1.31–2.32; p < 0.001) versus private insurance, single versus married/partnered marital status (OR 1.19, 95% CI 1.01–1.39; p = 0.03), lobular (OR 2.58, 95% CI 1.48–4.51; p < 0.001), and other (OR 1.39, 95% CI 1.12–1.73; p = 0.003) versus ductal histology, no breast surgery (OR 2.35, 95% CI 1.77–3.11 p < 0.001) versus mastectomy, and no axillary surgery (OR 1.44, 95% CI 1.13–1.83; p = 0.003) versus axillary surgery (Table 3). Odds of rrTNBC were lower for non-Hispanic other versus white race (OR 0.69, 95% CI 0.52–0.92; p = 0.01), well-differentiated (OR 0.33, 95% CI 0.15–0.77; p = 0.01), and moderately differentiated (OR 0.71, 95% CI 0.58–0.87; p = 0.001) versus poorly differentiated grade, and radiation with BCS versus no radiation (OR 0.44, 95% CI 0.34–0.57; p < 0.001). We visualized the association between the normalized NCI census tract-level index (higher number corresponds to higher socioeconomic status) and mortality in Fig. 2; however, the association was not significant in the multivariable logistic model.
TABLE 3.
Multivariable logistic model for rapid relapse versus no rapid relapse triple-negative breast cancers
| Variable | OR | 95% CI | p value |
|---|---|---|---|
|
| |||
| Stage | |||
| Local | Ref | Ref | Ref |
| Regional | 4.36 | (3.77, 5.03) | < 0.001 |
| Race/ethnicity | |||
| Non-Hispanic white | Ref | Ref | Ref |
| Non-Hispanic black | 1.22 | (1.05, 1.43) | 0.01 |
| Hispanic | 0.86 | (0.70, 1.05) | 0.14 |
| Non-Hispanic other | 0.69 | (0.52, 0.92) | 0.01 |
| Insurance type | |||
| Uninsured | 1.74 | (1.31, 2.32) | < 0.001 |
| Medicaid | 1.53 | (1.31, 1.79) | < 0.001 |
| Insured (non-Medicaid/UK) | Ref | Ref | Ref |
| % Less than high school (Mean (95% CI)) | 1.01 | (0.99, 1.02) | 0.44 |
| Median household income (Mean (95%o CI)) | 1.00 | (1.00, 1.00) | 0.95 |
| NCI census tract-level index | 1.00 | (0.99, 1.00) | 0.35 |
| Marital status | |||
| Single | 1.19 | (1.01, 1.39) | 0.03 |
| Married/partnered | Ref | Ref | Ref |
| Separated/divorced | 1.15 | (0.95, 1.38) | 0.15 |
| Widowed | 1.30 | (0.95, 1.78) | 0.10 |
| Age at diagnosis | |||
| ≤40 | 0.94 | (0.78, 1.13) | 0.49 |
| 41–50 | 0.96 | (0.83, 1.12) | 0.63 |
| 51–60 | Ref | Ref | Ref |
| 61–64 | 1.02 | (0.84, 1.24) | 0.86 |
| Grade | |||
| Well differentiated | 0.33 | (0.15, 0.77) | 0.01 |
| Moderately differentiated | 0.71 | (0.58, 0.87) | 0.001 |
| Poorly differentiated | Ref | Ref | Ref |
| Histology | |||
| Ductal | Ref | Ref | Ref |
| Lobular | 2.58 | (1.48, 4.51) | < 0.001 |
| Mixed | 1.32 | (0.98, 1.80) | 0.07 |
| Other | 1.39 | (1.12, 1.73) | 0.003 |
| Surgery | |||
| No surgery | 2.35 | (1.77, 3.11) | < 0.001 |
| BCS | 0.89 | (0.72, 1.11) | 0.31 |
| Mastectomy | Ref | Ref | Ref |
| Axillary surgery | |||
| No axillary surgery | 1.44 | (1.13, 1.83) | 0.003 |
| Axillary surgery | Ref | Ref | Ref |
| Radiation | |||
| No | Ref | Ref | Ref |
| Yes with BCS | 0.44 | (0.34, 0.57) | < 0.001 |
| Yes with mastectomy | 1.15 | (0.98, 1.35) | 0.09 |
FIG. 2.
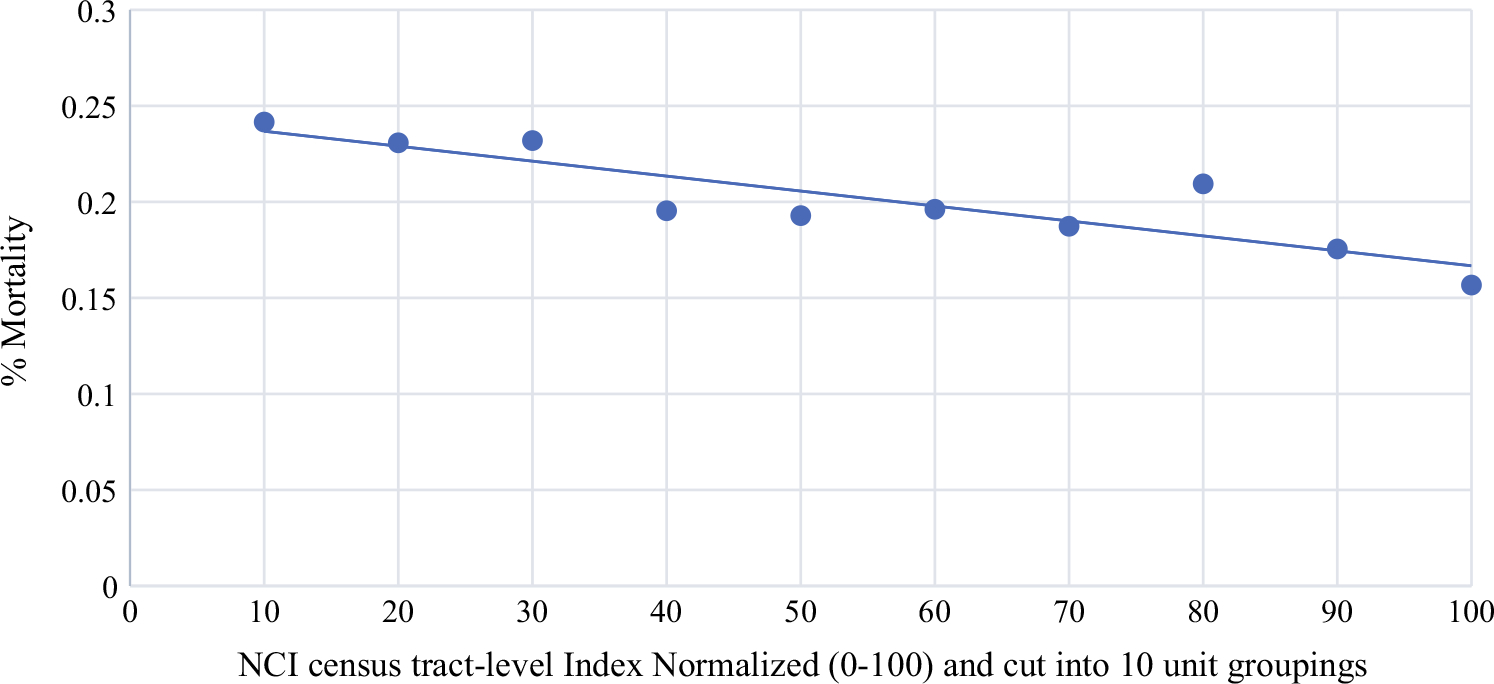
Relationship between NCI census tract-level index and mortality among triple-negative breast cancers. *Higher NCI census-tract level indices correspond with high socioeconomic status
To further understand potential interactions among the multiple significant features, we performed four sensitivity analyses of the final model by removing one group at a time and comparing to the original final model (Supplementary Table 3). First, we removed patients who did not receive breast or axillary surgery. The odds of rrTNBC for non-Hispanic other race and single marital status changed slightly leading to a change from significance to non-significance, but the model was overall stable. Next, we removed inflammatory breast cancer patients and found no significant changes in the final model. We then removed patients who did not receive chemotherapy. The odds of rrTNBC for non-Hispanic other race, single marital status, well-differentiated grade, and other histology changed marginally from significance to nonsignificance. Finally, we removed black patients. The odds of rrTNBC for other histology changed marginally from significance to non-significance, but the model was overall stable.
Because receipt of surgery was significantly associated with rrTNBC in all models, we investigated the documented reasons underlying lack of surgery, which included 204/1378 rrTNBC patients (14.8%; Supplementary Table 4). The most common reason cited was surgery was “not recommended” in 84.0% (467/556) of patients who did not receive surgery.
DISCUSSION
In our review of a contemporary breast cancer population in the SEER program, black race, Medicaid or uninsured status, omission of breast and axillary surgery, higher stage, not undergoing radiation therapy, and single marital status were associated with an increased odds of rapid relapse among patients presenting with TNBC. Notably, there was no significant difference between the two groups in the use of chemotherapy. These study results suggest that clinical outcomes, such as rapid relapse, in TNBC patients may be influenced by a combination of socioeconomic factors and disparities in surgical management.
To date, it has been well established in the literature that patients presenting with TNBC are more likely to present with advanced stages of disease, have shorter times to recurrence and have a worse mortality than those with hormone-positive breast cancers.7,26 Additionally, there are a subset of TNBC patients whose clinical courses are characterized by resistance to treatment, rapid progression, and subsequent early mortality.11 Genomic analysis by Stover et. al. found that TNBC with mortality or relapse ≤ 24 months (rapid relapse) after diagnosis or within 5 years of diagnosis exhibited lower expressions of immune signatures compared to patients who did not relapse.12 Furthermore, additional analysis suggests that genomic differences explained only a fraction of the disparities in clinical outcomes between those in the rapid relapse group versus those who did not relapse.12 Based on our study results, we anticipate that outcomes in the rrTNBC patients are most likely driven by interactions between tumor biology and social determinants of health such as access to care, quality of care, and socioeconomic status.
Socioeconomic factors, such as insurance, marital status, and neighborhood socioeconomic status, have been implicated in stage of presentation, quality of care, and mortality among breast cancer patients.27–30 Specifically, Medicaid and uninsured patients are more likely to present with later stages of disease and subsequently have a lower survival than their privately insured counterparts.31,32 In Martinez et al.’s evaluation of California Cancer Registry, unmarried breast cancer patients had a higher mortality rates than their married counterparts.33 Additionally, it has been well established that there are racial disparities in breast cancer presentation and mortality.34 For instance, black women have a higher incidence of TNBC, a worse mortality from breast cancer (all subtypes) than white women, and are more likely to receive nonguideline concordant locoregional managment.35–37 Within the context of these studies and the disparities literature in general, our study results of an association between rrTNBC and single marital status, black race, Medicaid insurance or no insurance, later stage of presentation, and disparities in surgical care are not unexpected. Possible explanations for our results are a complex interplay between intermediary social determinants of health (e.g., access to healthcare and quality of healthcare, patient material resources, etc.) and structural social determinants of health (e.g., economic and social policies).38 In particular, the population of patients identified as experiencing rapid relapse fall into the category of patients who typically face barriers in healthcare due to problems with access, financial hardship, issues with transportation, marginal knowledge about their disease, and subsequent low quality care.27,39–41 To this end, our findings highlight a subset of TNBC patients whose difficulty accessing and receiving care appears to be exacerbating the clinical course of this very aggressive cancer subtype.
Current National Comprehensive Cancer Network (NCCN) Guidelines recommend breast surgery (breast conservation surgery or mastectomy) and axillary staging for all TNBC patients presenting with localized and regional disease.42 However, in this study we found a higher percentage of patients in the rrTNBC did not undergo breast surgery or axillary staging. Moreover, rrTNBC patients were more likely to undergo breast surgery without axillary staging, which is not in tune with guideline concordant care. Supplementary analysis revealed the most frequently listed reason for omission of surgery was because it was not recommended. Unfortunately, because SEER does not provide specific reasons for the recommendation to omit surgery, it is difficult to discern why surgery was not recommended. It could be argued that the omission of surgery might have been secondary to underlying comorbidities and/or performance status. However, it should be noted that to mitigate the effects of comorbidities and/or performance status, our sample focused on younger patients (ages 18–64 years) and disease-specific mortality instead of overall mortality. Other disparities in locoregional control include rrTNBC patients not receiving radiation therapy after breast-conservation surgery at higher rates than the nonrelapse group—a practice that portends a lower survival.43
We anticipate that the omission of surgical management among the rrTNBC population is most likely attributable to an interplay between patient preference, clinical presentation (e.g., locally advanced disease), provider recommendations, and institutional resource availability. Moreover, we expect the cause of mortality for this subset of patients is progression of untreated disease rather than rapid relapse after no evidence of disease. Patient-related variables contributing to disparities in locoregional management could include social determinants of health, such as issues with transportation, increased distance from treatment facilities, low educational achievement, economic instability, stress, and poor social networks.39,44,45 Possible institution and provider-related variables may include poor patient-physician relationships, lack of oncology specialists, and low hospital breast cancer volumes, which have been associated with adherence to guideline concordant care and mortality.46–49
The strength of this study is the use of a large racially, ethnically, and socioeconomically diverse population-based registry to define socioeconomic and treatment factors contributing to rapid relapse among breast cancer patients. Moreover, the use of a census-tract based socioeconomic index in conjunction with insurance status enabled the study to operationalize both individual and neighborhood socioeconomic status. Census tract populations are more homogenous than county or zip code groupings.50 Insurance status can serve as a proxy for individual SES due to income specific requirements for insurance types, such as Medicaid.
Limitations of this study include the lack of comorbidity and performance status data in SEER, which are both important when making recommendations for surgery. Additionally, treatment information on radiation and chemotherapy in SEER do not explain why treatment was omitted, how many doses patients received, or the type of chemotherapeutic agents administered. Of note, SEER codes no and unknown into one category for radiation and chemotherapy making it difficult to discern whether treatment was truly omitted. Consequently, data involving radiation and chemotherapy need to be interpreted with caution.51 The authors acknowledge that the NCI-census tract index used in this study is based on neighborhood SES and therefore may not be reflective of individual SES.
CONCLUSIONS
Rapid relapse among TNBC patients is associated with a combination of aggressive tumor biology and socioeconomic factors. Moreover, patients with rrTNBC are less likely to be recommended guideline concordant surgical care or to undergo appropriate locoregional management. Future studies should interrogate more granular treatment facility, provider, and patient-related variables that might be contributing to disparities in surgical management among rrTNBC patients.
Supplementary Material
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1245/s10434021-09688-3.
DISCLOSURES The authors declare that they have no disclosures to report.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
REFERENCES
- 1.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. Cancer. 2007;109(9):1721–8. 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 2.Lin NU, Vanderplas A, Hughes ME, et al. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. Cancer. 2012;118(22):5463–72. 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113(10):2638–45. 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–34. 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 5.Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Multicenter Study Research Support, Non-U.S. Gov’t. Clin Breast Cancer. 2009;9(1):29–33. 10.3816/CBC.2009.n.005. [DOI] [PubMed] [Google Scholar]
- 6.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nabpaclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21. 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 7.van Roozendaal LM, Smit LHM, Duijsens G, et al. Risk of regional recurrence in triple-negative breast cancer patients: a Dutch cohort study. Breast Cancer Res Treat. 2016;156(3):465–72. 10.1007/s10549-016-3757-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh J, Agarwal S, Ganguly S, et al. Patterns of recurrence in triple negative breast cancer patients (automated IHC): an Indian Tertiary Care Center data. J Clin Oncol. 2018;36(15_suppl):e13128. 10.1200/JCO.2018.36.15_suppl.e13128. [DOI] [Google Scholar]
- 9.Cancer Genome Atlas N Comprehensive molecular portraits of human breast tumours. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. Nature. 2012;490(7418):61–70. 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis C, Shah SP, Chin SF, et al. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t. Nature. 2012;486(7403):346–52. 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stover DG, Coloff JL, Barry WT, Brugge JS, Winer EP, Selfors LM. The role of proliferation in determining response to neoadjuvant chemotherapy in breast cancer: a gene expression-based meta-analysis. Clin Cancer Res. 2016;22(24):6039–50. 10.1158/1078-0432.CCR-16-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Nock W, Wyse M, et al. Machine learning predicts rapid relapse of triple negative breast cancer. bioRxiv. 2019:613604. 10.1101/613604. [DOI] [Google Scholar]
- 13.Hunter CP. Epidemiology, stage at diagnosis, and tumor biology of breast carcinoma in multiracial and multiethnic populations. Cancer. 2000;88(S5):1193–202. . [DOI] [PubMed] [Google Scholar]
- 14.Lantz PM, Mujahid M, Schwartz K, et al. The influence of race, ethnicity, and individual socioeconomic factors on breast cancer stage at diagnosis. Am J Public Health. 2006;96(12):2173–8. 10.2105/AJPH.2005.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz KL, Crossley-May H, Vigneau FD, Brown K, Banerjee M. Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer Causes Control. 2003;14(8):761–6. [DOI] [PubMed] [Google Scholar]
- 16.Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987–2005). Cancer Epidemiol Biomark Prev. 2009;18(1):121–31. 10.1158/1055-9965.EPI-080679. [DOI] [PubMed] [Google Scholar]
- 17.Huo D, Hu H, Rhie SK, et al. Comparison of breast cancer molecular features and survival by African and European ancestry in the cancer genome atlas. JAMA Oncol. 2017;3(12):1654–62. 10.1001/jamaoncol.2017.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iqbal J, Ginsburg O, Rochon PA, Sun P, Narod SA. Differences in breast cancer stage at diagnosis and cancer-specific survival by race and ethnicity in the United States. JAMA. 2015;313(2):165–73. 10.1001/jama.2014.17322. [DOI] [PubMed] [Google Scholar]
- 19.Asad S, Barcenas C, Bleicher R, et al. Sociodemographic factors associated with rapid relapse in triple negative breast cancer: a multi-institution study. J Natl Compr Cancer Netw. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Institute NC. Surveillance, Epidemiology, and End Results (SEER) Program. 2020. www.seer.cancer.gov
- 21.Institute NC. SEER*Stat software version 8.3.6. www.seer.cancer.gov/seerstat
- 22.Yu M, Tatalovich Z, Gibson JT, Cronin KA. Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes Control. 2014;25(1):81–92. 10.1007/s10552-013-0310-1. [DOI] [PubMed] [Google Scholar]
- 23.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703–11. 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute S, Epidemiology, and End Results Program. Census tract–level SES and rurality database (2000–2015). National Cancer Institute. Accessed 26 May 2020. https://seer-cancer-gov.proxy.lib.ohio-state.edu/seerstat/databases/census-tract/index.html. [Google Scholar]
- 25.Axelsson CK, Mouridsen HT, Zedeler K. Axillary dissection of level I and II lymph nodes is important in breast cancer classification. The Danish Breast Cancer Cooperative Group (DBCG). Eur J Cancer. 1992;28a(8–9):1415–8. 10.1016/0959-8049(92)90534-9. [DOI] [PubMed] [Google Scholar]
- 26.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28(10):1684–91. 10.1200/jco.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 27.Dreyer MS, Nattinger AB, McGinley EL, Pezzin LE. Socioeconomic status and breast cancer treatment. Breast Cancer Res Treat. 2018;167(1):1–8. 10.1007/s10549-017-4490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halpern MT, Bian J, Ward EM, Schrag NM, Chen AY. Insurance status and stage of cancer at diagnosis among women with breast cancer. Cancer. 2007;110(2):403–11. 10.1002/cncr.22786. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y-l Wang D-w, Yang Z-c, et al. Marital status is an independent prognostic factor in inflammatory breast cancer patients: an analysis of the surveillance, epidemiology, and end results database. Breast Cancer Res Treat. 2019;178(2):379–88. 10.1007/s10549-019-05385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdel-Rahman O Impact of NCI Socioeconomic index on the outcomes of nonmetastatic breast cancer patients: analysis of SEER Census Tract-Level Socioeconomic Database. Clin Breast Cancer. 2019;19(6):e717–22. 10.1016/j.clbc.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Obeng-Gyasi S, Timsina L, Miller KD, Ludwig KK, Fisher CS, Haggstrom DA. The implications of insurance status on presentation, surgical management, and mortality among nonmetastatic breast cancer patients in Indiana. Surgery. 2018;164(6):1366–71. 10.1016/j.surg.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Shi R, Taylor H, McLarty J, Liu L, Mills G, Burton G. Effects of payer status on breast cancer survival: a retrospective study. BMC Cancer. 2015;15:211. 10.1186/s12885-015-12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martínez ME, Unkart JT, Tao L, et al. Prognostic significance of marital status in breast cancer survival: a population-based study. PLoS One. 2017;12(5):e0175515. 10.1371/journal.pone.0175515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daly B, Olopade OI. A perfect storm: how tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65(3):221–38. 10.3322/caac.21271. [DOI] [PubMed] [Google Scholar]
- 35.DeSantis CE, Miller KD, Goding Sauer A, Jemal A, Siegel RL. Cancer statistics for African Americans, 2019. CA Cancer J Clin. 2019;69(3):211–33. 10.3322/caac.21555. [DOI] [PubMed] [Google Scholar]
- 36.Newman LA, Kaljee LM. Health disparities and triple-negative breast cancer in African American women: a review. JAMA Surg. 2017;152(5):485–93. 10.1001/jamasurg.2017.0005. [DOI] [PubMed] [Google Scholar]
- 37.Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357–62. 10.1200/jco.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 38.Solar O A conceptual framework for action on the social determinants of health. Social Determinants of Health Discussion Paper 2 (Policy and Practice). 2010. Accessed 11 March 2020.
- 39.Jagsi R, Pottow JA, Griffith KA, et al. Long-term financial burden of breast cancer: experiences of a diverse cohort of survivors identified through population-based registries. J Clin Oncol. 2014;32(12):1269–76. 10.1200/jco.2013.53.0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin. 2018;68(2):153–65. 10.3322/caac.21443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freedman RA, Virgo KS, He Y, et al. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117(1):180–9. 10.1002/cncr.25542. [DOI] [PubMed] [Google Scholar]
- 42.Network NCC. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines), Breast Cancer. Accessed 21 June 2020. Available at https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 43.Malmström P, Holmberg L, Anderson H, et al. Breast conservation surgery, with and without radiotherapy, in women with lymph node-negative breast cancer: a randomised clinical trial in a population with access to public mammography screening. Eur J Cancer. 2003;39(12):1690–7. 10.1016/s0959-8049(03)00324-1. [DOI] [PubMed] [Google Scholar]
- 44.Jagsi R, Abrahamse PH, Lee KL, et al. Treatment decisions and employment of breast cancer patients: results of a population-based survey. Cancer. 2017;123(24):4791–9. 10.1002/cncr.30959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gu J, Groot G, Boden C, Busch A, Holtslander L, Lim H. Review of factors influencing women’s choice of mastectomy versus breast conserving therapy in early stage breast cancer: a systematic review. Clin Breast Cancer. 2018;18(4):e539–54. 10.1016/j.clbc.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Greenup RA, Obeng-Gyasi S, Thomas S, et al. The effect of hospital volume on breast cancer mortality. Ann Surg. 2018;267(2):375–81. 10.1097/sla.0000000000002095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kingsmore D, Hole D, Gillis C. Why does specialist treatment of breast cancer improve survival? The role of surgical management. Br J Cancer. 2004;90(10):1920–5. 10.1038/sj.bjc.6601846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willems S, De Maesschalck S, Deveugele M, Derese A, De Maeseneer J. Socio-economic status of the patient and doctor-patient communication: does it make a difference? Patient Educ Counsel. 2005;56(2):139–46. [DOI] [PubMed] [Google Scholar]
- 49.Sheppard VB, Isaacs C, Luta G, et al. Narrowing racial gaps in breast cancer chemotherapy initiation: the role of the patient–provider relationship. Breast Cancer Res Treat. 2013;139(1):207–16. 10.1007/s10549-013-2520-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter? The Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156(5):471–82. [DOI] [PubMed] [Google Scholar]
- 51.Noone AM, Lund JL, Mariotto A, et al. Comparison of SEER treatment data with medicare claims. Med Care. 2016;54(9):e55–64. 10.1097/mlr.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


