Abstract
The causative agent of fish pasteurellosis, the organism formerly known as Pasteurella piscicida, has been reclassified as Photobacterium damselae subsp. piscicida on the basis of 16S rRNA gene sequence comparisons and chromosomal DNA-DNA hybridization data; thus, this organism belongs to the same species as Photobacterium damselae subsp. damselae (formerly Vibrio damselae). Since reassignment of P. damselae subsp. piscicida was based on only two strains, one objective of the present work was to confirm the taxonomic position of this fish pathogen by sequencing the 16S rRNA genes of 26 strains having different geographic and host origins. In addition, a nested PCR protocol for detection of P. damselae based on 16S rRNA was developed. This PCR protocol was validated by testing 35 target and 24 nontarget pure cultures, and the detection limits obtained ranged from 1 pg to 10 fg of DNA (200 to 20 cells). A similar level of sensitivity was observed when the PCR protocol was applied to fish tissues spiked with bacteria. The PCR approach described in this paper allows detection of the pathogen in mixed plate cultures obtained from asymptomatic fish suspected to be carriers of P. damselae subsp. piscicida, in which growth of this bacterium cannot be visualized. Our results indicate that the selective primers which we designed represent a powerful tool for sensitive and specific detection of fish pasteurellosis.
Pasteurellosis or pseudotuberculosis is one of the most important fish diseases in marine aquaculture, causing substantial economic losses especially in yellowtail (Seriola quinqueradiata), gilthead seabream (Sparus aurata), and seabass (Dicentrarchus labrax) cultures worldwide (for reviews see references 16 and 19). The causative agent of fish pasteurellosis was initially isolated from natural populations of white perch (Morone americanus) and striped bass (Morone saxatilis) in 1963 during a massive epizootic in the Chesapeake Bay (20). Janssen and Surgalla (12) proposed the name Pasteurella piscicida for this bacterium. Since then, this pathogen has been extensively characterized, and strains of the bacterium have been shown to be biochemically and serologically homogeneous (14, 16, 19), although minor variability has been detected by genetic methods, such as ribotyping analysis (17). Recently, P. piscicida has been reclassified as Photobacterium damselae subsp. piscicida (6, 21); thus, this organism belongs to the same species as Photobacterium damselae subsp. damselae (formerly Vibrio damsela). The two subspecies differ in important biochemical and physiological traits, such as motility, gas production from glucose, nitrate reduction, urease, lipase, amylase, and hemolysin production, and ranges of temperature and salinity for growth, as well as host specificity (3, 4, 16). Moreover, in contrast to P. damselae subsp. piscicida, which is serologically homogeneous, at least four serotypes are recognized within P. damselae subsp. damselae (3). Despite having marked phenotypic differences, the two subspecies of P. damselae exhibit a high degree of overall DNA base sequence similarity, as revealed by chromosomal DNA-DNA pairing (6). Similarly, the two subspecies reportedly have almost identical 16S rRNA gene sequences, although sequence comparisons have been performed with only three strains.
In recent years there has been much interest in the development of specific PCR protocols for detecting 16S rRNA genes of several bacterial fish pathogens, such as Vibrio anguillarum, Aeromonas salmonicida, Renibacterium salmoninarum, Flexibacter maritimus, and Flavobacterium species (for a review see reference 8). Previously, several studies have described the design of molecular aids for detection of P. damselae subsp. piscicida as the causative agent of pseutotuberculosis in cultured fish (1, 22, 23). However, these methods were based on nonconserved DNA regions (plasmid sequences and a gene library fragment of unknown function). Additionally, despite the wide distribution of this bacterium in the Northern Hemisphere, only Japanese isolates have been used to test the DNA-based diagnostic methods designed up to now.
There were two objectives of the present study. First, we wanted to perform a comparative 16S rRNA gene sequencing analysis with P. damselae strains from geographically diverse sources and a variety of homiotherm and poiquilotherm hosts in order to study a good cross-section of the natural diversity of this species. Second, we wanted to utilize the sequence information generated to develop a nested PCR protocol for rapid and specific diagnosis of pseudotuberculosis in fish. Development of such a PCR-based test for P. damselae is particularly important, since diagnosis of pasteurellosis may be hampered by the slow growth of the organism in laboratory media (16), which is easily obscured by the growth of other fast-growing bacteria, as well as by the fact that this fish pathogen has the capacity to rapidly form viable but nonculturable cells in the natural environment (15). In addition, a great number of fish harbor the bacterium in an asymptomatic carrier state, and fish that survive disease outbreaks remain covertly infected (13).
MATERIALS AND METHODS
Bacterial cultures and DNA extraction.
A total of 35 P. damselae strains, including representatives of both subspecies, were used in this study. The sources of isolation and geographic origins of these strains are listed in Table 1. Other Photobacterium and Vibrio species used to design and validate the PCR primers are shown in Table 2. All of the strains were grown aerobically on brain heart infusion agar (Pronadisa, Madrid, Spain) supplemented with 1% NaCl at 25°C. DNA was isolated by using the Instagene matrix (Bio-Rad) as recommended by the manufacturer.
TABLE 1.
P. damselae strains used in this study
| Straina | Origin | Country |
|---|---|---|
| P. damselae subsp. piscicida strains | ||
| DI 21 | Sparus aurata | Spain |
| DI 91 | Sparus aurata | Spain |
| B 21 | Dicentrarchus labrax | Spain |
| B 51 | Dicentrarchus labrax | Spain |
| C.1 | Sparus aurata | Spain |
| C.2 | Sparus aurata | Spain |
| R 46 | Sparus aurata | Spain |
| DS 11 | Sparus aurata | Spain |
| 619.1 | Sparus aurata | Portugal |
| 666.1 | Dicentrarchus labrax | Portugal |
| 693.2 | Sparus aurata | Portugal |
| 10831 | Dicentrarchus labrax | France |
| IT-1 | Sparus aurata | Italy |
| IT-2 | Sparus aurata | Italy |
| O69 A | Sparus aurata | Greece |
| O69 E | Sparus aurata | Greece |
| ATLIT 2 | Morone sp. | Israel |
| 2101 | Morone sp. | Israel |
| MP-7801 | Seriola quinqueradiata | Japan |
| EPOY-8803-II | Epinephelus akaara | Japan |
| P3333 | Seriola quinqueradiata | Japan |
| MZS 8001 | Seriola quinqueradiata | Japan |
| P3335 | Seriola quinqueradiata | Japan |
| ATCC 29690 | Seriola quinqueradiata | Japan |
| ATCC 17911 | Roccus americanus | United States |
| P. damselae subsp. damselae strains | ||
| RG 91 | Scophthalmus maximus | Spain |
| RG 153 | Scophthalmus maximus | Spain |
| RM 71 | Scophthalmus maximus | Spain |
| CDC 2227-81 | Human wound infection | United States |
| ATCC 33539 | Chromis punctipinnis | United States |
| ATCC 35083 | Carcharhinus plumbeus | United States |
| 192 | Tursiops truncatus | United States |
| PG-801 | Penaeus monodon | Taiwan |
| 158 | Anguilla anguilla | Belgium |
| 162 | Anguilla anguilla | Belgium |
CDC, Centers for Diseases Control; ATCC, American Type Culture Collection.
TABLE 2.
Organisms used in the 16S rRNA gene sequence comparisons and included in the PCR analysis as negative controls
| Organism | Straina | Accession no. |
|---|---|---|
| Photobacterium angustum | ATCC 25915T | X74685 |
| Photobacterium damselae | ATCC 33539T | X74700 |
| Photobacterium histaminum | JCM 8968T | D25308 |
| Photobacterium leiognathi | ATCC 25521T | X74686 |
| Photobacterium phosphoreum | ATCC 11040T | X74687 |
| Vibrio alginolyticus | ATCC 17749T | X74690 |
| Vibrio anguillarum | ATCC 12964T | X16895 |
| Vibrio campbelli | ATCC 25920T | X74692 |
| Vibrio fischeri | ATCC 7744T | X74702 |
| Vibrio fluvialis | NCTC 11327T | X76335 |
| Vibrio furnissii | ATCC 35016T | X74704 |
| Vibrio harveyi | ATCC 14126T | X74706 |
| Vibrio hollisae | ATCC 33564T | X74707 |
| Vibrio logei | ATCC 15832T | X74708 |
| Vibrio metschnikovii | NCTC 11170T | X74712 |
| Vibrio mimicus | ATCC 33653T | X74713 |
| Vibrio natriegens | ATCC 14048T | X74714 |
| Vibrio nereis | ATCC 25917T | X74716 |
| Vibrio nigripulchritudo | ATCC 27043T | X74717 |
| Vibrio orientalis | ATCC 33934T | X74719 |
| Vibrio parahaemolyticus | ATCC 17802T | X74721 |
| Vibrio pelagius | ATCC 25916T | X74722 |
| Vibrio proteolyticus | ATCC 15338T | X74723 |
| Vibrio splendidus | ATCC 33125T | X74724 |
| Vibrio tubiashi | ATCC 19109T | X74725 |
| Vibrio vulnificus | ATCC 29307T | X76334 |
ATCC, American Type Culture Collection; JCM, Japan Collection of Microorganisms; NCTC, National Collection of Type Cultures.
In addition, broodstock gilthead seabream (S. aurata) that were suspected carriers of pasteurellosis, as indicated by sensitive serological methods (13), were sacrificed, and then samples from kidneys and spleens were immediately directly streaked onto plates containing brain heart infusion agar supplemented with 1% NaCl, which were incubated at 25°C for 48 h. After this, at least one representative of each of the different morphological colony types was subcultured, subjected to standard biochemical and physiological tests (7), and identified by using the schemes of Holt et al. (10). Simultaneously, the total bacterial growth was scraped off the plates and resuspended in a saline solution, and a sample was used to extract the DNA of the mixed culture by the procedure described above.
Since healthy gilthead seabream may not necessarily be free of P. damselae subsp. piscicida, kidney tissues from fish species that are not susceptible to pasterellosis, such as trout and salmon, were employed as negative controls; these tissues were processed as described above for carrier gilthhead seabream.
Unless otherwise specified, between 1 and 10 μl of a DNA suspension was routinely used for each PCR.
Sequencing of 16S rRNA genes.
16S rRNA genes of 18 strains of P. damselae subsp. piscicida and eight strains of P. damselae subsp. damselae were amplified by PCR with universal primers pA and pH (11). The PCR were performed with a DNA thermal cycler (Biometra). A typical reaction mixture (100 μl) consisted of 0.5 μg of each specific primer, 2 U of Taq polymerase (Perkin-Elmer), 10 μl of 10× Taq polymerase buffer (Perkin-Elmer), 4 μl of a 50 mM MgCl2 solution, and each deoxynucleoside triphosphate at a concentration of 200 μM. The reaction mixtures were each overlaid with 30 μl of mineral oil (Sigma) and subjected to amplification at 95°C for 4 min, followed by 30 cycles consisting of 95°C for 1 min, 55°C for 1 min, and 72°C for 90 s. A final extension step consisting of 5 min at 72°C was carried out. The PCR products were cleaned by using a QIAquick PCR purification kit (Quiagen).
Primers (5) corresponding to internal conserved regions of the gene were used for sequencing reactions performed with a GeneAmp PCR System 9600 instrument (Perkin-Elmer); a Taq DyeDeoxy terminator cycle sequencing kit (Applied Biosystems, Foster City, Calif.) was used, and the program (35 cycles) consisted of denaturation at 96°C for 15 s, annealing at 48°C for 1 s, and elongation at 60°C for 4 min. The sequencing products were analyzed with a model 373A automatic DNA sequencer (Applied Biosystems).
Primers and DNA probe design.
16S rRNA gene sequences of 25 species belonging to the genera Photobacterium and Vibrio (Table 2) representing the closest relatives of P. damselae were retrieved from the GenBank database and compared with the sequences obtained in the present work. These sequences were aligned by using the program PILEUP (2). On the basis of the alignment, two regions of the 16S rRNA gene were chosen as highly variable regions within the family Vibrionaceae, and this allowed us to design three primers for an heminested PCR assay, as well as a short confirmatory DNA probe.
For the first round of PCR, forward primer Car1 (18-mer; 5′-GCTTGAAGAGATTCGAGT-3′; positions 1016 to 1033 in the Escherichia coli 16S rRNA gene) and reverse primer Car2 (18-mer; 5′-CACCTCGCGGTCTTGCTG-3′; positions 1266 to 1283) were developed; these primers flanked a 267-bp fragment of the 16S rRNA gene. For the second round of the heminested PCR, primer Car1 was used as the forward primer in combination with reverse primer Nestcar1 (17-mer; 5′-GGTCTTGCTGCCCTCTG-3′; positions 1259 to 1275) flanking a 259-bp fragment. An internal fragment designated CARSOND (18-mer; 5′-TACAATGGCATATACAGA-3′; positions 1245 to 1262) was labelled with digoxigenin (Pharmacia Biotech) and used as a DNA probe to assess the specificity of the fragment amplified in the nested PCR.
Sensitivity of the primers.
In order to test the sensitivity of primers Car1, Car2, and Nestcar1, spectrophotometrically quantified purified DNA of P. damselae subsp. piscicida ATCC 17911 was used. DNA dilutions containing from 400 ng to 10 fg of pure DNA were prepared as templates for PCR amplification.
In addition, 50-mg pieces of kidneys from rainbow trout were spiked with different dilutions in phosphate-buffered saline (108 to 10 cells/ml) of a culture suspension of P. damselae subsp. piscicida DI 21 and homogenized for 30 s. Tissue lysis and total DNA purification were performed with an EZNA Mollusc DNA kit (Omega Biotek) as recommended by the manufacturer. In this case, 10 μl of the extracted DNA solution was used in each PCR.
Nested PCR detection and Southern blotting.
Primers Car1, Car2, and Nestcar1 were used under the PCR conditions described above for 16S rRNA gene amplification, except that the annealing temperatures ranged from 55 to 65°C and the elongation cycle was 20 s long. A 0.5-μl portion of the first-round PCR product (obtained with primers Car1 and Car2) was used as the DNA template for the second-round or heminested PCR, in which primers Car1 and Nestcar1 were used. Amplification products were analyzed on 1% (wt/vol) agarose gels with TAE (0.04 M Tris-acetate, 1 mM EDTA) electrophoresis buffer and were visualized with a UV transilluminator after staining with ethidium bromide. A 1-kb DNA ladder (Bio-Rad) was included as a molecular weight marker.
The presence of the internal DNA CARSOND sequence was determined as follows. PCR products obtained from an amplification reaction in which primers Car1 and Car2 were used were separated in a 0.8% agarose gel. Transfer to nylon membranes, hybridization, and development of the labelling were carried out as described previously (17). In this analysis, 5 μl of probe solution (0.1 μg/μl) was used, and the preparation was hybridized for 8 h at 50°C.
Nucleotide sequence accession number.
The nucleotide sequence of the 16S rRNA gene of P. damselae subsp. piscicida ATCC 29690 has been deposited in the GenBank database under accession no. Y18496.
RESULTS
16S rRNA gene sequences.
Almost complete (>1,400-nucleotide) 16S rRNA gene sequences were obtained for 18 strains of P. damselae subsp. piscicida and for eight strains of P. damselae subsp. damselae. All of the strains examined exhibited 100% sequence identity, which confirmed the close phylogenetic relationship of the two subspecies and showed that the 16S rRNA gene was homogeneous regardless of the geographic origins, pathogenic properties, and host sources of the strains.
Experimental validation of the primers with pure cultures.
The specificity of primers Car1 and Car2 in a single-step PCR was tested experimentally by using 26 pure cultures of P. damselae subsp. piscicida and 10 pure cultures of P. damselae subsp. damselae, as well as 24 pure cultures of Vibrio and Photobacterium reference species listed in Table 2. PCR were performed at annealing temperatures of 55, 60, and 65°C.
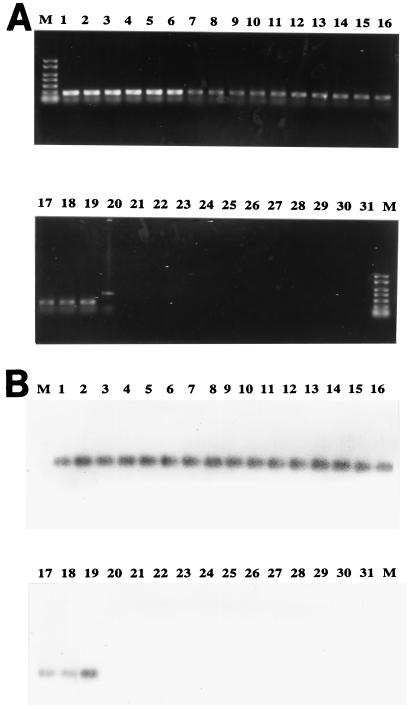
At the three different temperatures evaluated, all 36 P. damselae strains produced a unique and clear PCR band of the expected size (267 bp). However, at both 55 and 60°C most of the non-P. damselae strains produced nonspecific amplification bands (data not shown). Most of the PCR bands produced by the non-P. damselae strains were eliminated at an annealing temperature of 65°C; the only exception was a nonspecific higher-molecular-weight band produced by Vibrio splendidus. Consequently, 65°C was used for additional PCR experiments. Figure 1A shows the results of PCR analyses performed with primers Car1 and Car2 and with 19 pure cultures of P. damselae (including members of both subspecies) and 12 pure cultures of other Photobacterium and Vibrio species at an annealing temperature of 65°C. After Southern blotting and hybridization of the PCR products with the internal DNA probe CARSOND, all of the P. damselae amplification products exhibited a positive reaction, which confirmed the identities of the PCR products (Fig. 1B).
FIG. 1.
Specific PCR products (A) and hybridization of the products with digoxigenin-labelled probe CARSOND (B) obtained with pure cultures of 19 P. damselae strains and 13 isolates of related species. Lanes M, 1-kb molecular weight marker; lanes 1 to 13, P. damselae subsp. piscicida DI 21, C.1, R46, DS 11, ATCC 17911, ATCC 29690, 619.1, 10831, IT1, 069E, ATLIT-2, EPOY 8803, and MZS 8001, respectively; lanes 14 to 19, P. damselae subsp. damselae RG 91, RG 153, RM 71, CDC 2227-81, ATCC 33539, and ATCC 35083, respectively; lane 20, V. splendidus; lane 21, V. fischeri; lane 22, V. anguillarum; lane 23, V. pelagius; lane 24, V. harveyi; lane 25, V. proteolyticus; lane 26, V. alginolyticus; lane 27, P. leiognathi; lane 28, P. phosphoreum; lane 29, V. metschnikovii; lane 30, V. nereis; lane 31, V. campbelli.
Sensitivity of primers.
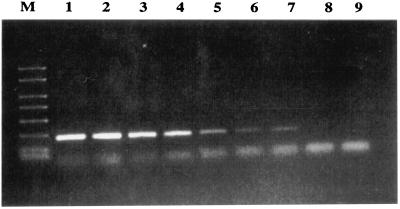
When primers Car1 and Car2 were tested for amplification with purified DNA of P. damselae subsp. piscicida, 267-bp PCR products were obtained with samples containing from 400 ng to 1 pg of DNA (Fig. 2). A second round of PCR (nested PCR) did not result in any further improvement in sensitivity, although the intensity of the bands increased substantially (data not shown). Application of this PCR protocol to DNA obtained from experimentally infected kidney tissue allowed us to detect as few as 100 bacterial cells.
FIG. 2.
Detection limit of the nested PCR designed in this work, as determined with different dilutions of purified DNA from strain DI 21 of P. damselae subsp. piscicida. Lane M, 1-kb molecular weight marker; lanes 1 to 8, 400 ng, 100 ng, 10 ng, 1 ng, 10 pg, 1 pg, 10 fg, and 1 fg of DNA, respectively; lane 9, no DNA.
Detection of P. damselae subsp. piscicida in mixed cultures.
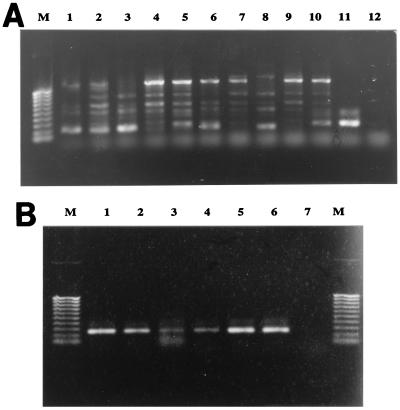
A bacteriological analysis of the different predominant colonies present in the plate cultures obtained from tissues of gilthead seabream individuals suspected to be carriers of P. damselae subsp. piscicida revealed a mixed bacterial population composed of V. splendidus and Vibrio pelagius, as well as other unidentified Vibrio and Pseudomonas species. DNA extracted from these mixed plate cultures were used as templates for a heminested PCR assay (as described above). A first PCR round performed with primers Car1 and Car2 generated multiple amplification products that ranged in size from 0.2 to 1 kb (Fig. 3A). These PCR products were used as templates for a second round of PCR in which primers Car1 and Nestcar1 were used. This heminested PCR approach yielded the specific 259-bp amplification product (Fig. 3B) which, as expected, hybridized with the internal DNA probe CARSOND (data not shown). DNA extracted from bacterial colonies obtained from fish used as negative controls did not yield any amplification product (Fig. 3A, lane 12). These results validated the assay used for specific and sensitive detection of the pathogen in plate cultures in which typical colonies were not visually evident due to overgrowth of other saprophytic microbiota.
FIG. 3.
Detection of P. damselae in mixed cultures obtained from asymptomatic carrier gilthead seabream by the PCR (A) and nested PCR (B) procedures developed in this study. (A) Lane M, 1-kb molecular weight marker; lanes 1 to 5, seabream 1 to seabream 5 (100 ng of DNA extracted from mixed bacterial culture); lanes 6 to 10, seabream 1 to seabream 5 (200 ng of DNA extracted from mixed bacterial culture); lane 11, positive control (pure DNA from strain ATCC 29690); lane 12, DNA from negative control. (B) Lanes M, 1-kb molecular weight marker; lanes 1 to 5, 0.1-μl portions of samples 1 to 5 from panel A; lane 6, positive control; lane 7, no DNA.
DISCUSSION
Our 16S rRNA gene sequence analyses confirmed unambiguously that the causative agent of fish pasteurellosis, which was formerly designated P. piscicida, clearly belongs to the genus Photobacterium, as reported previously (6). P. damselae subsp. piscicida and P. damselae subsp. damselae strains, irrespective of the host or geographic source, exhibited 100% 16S rRNA gene sequence identity. In a sequencing study that included one strain of P. damselae subsp. piscicida and two strains of P. damselae subsp. damselae, Gauthier et al. (6) found a single nucleotide difference between the two subspecies. In the present study, all 26 strains of P. damselae examined had the same 16S rRNA gene sequence.
Due to the difficulty of rapidly diagnosing pasteurellosis because of the slow growth of the causative agent and the persistence of the bacterium in asymptomatic fish, there is an urgent need to develop a sensitive and reliable method for fast detection of this pathogen, not only in clinically infected animals but also in egg stocks, in carrier fish, and in the environment, where it rapidly enters a viable yet nonculturable, state (15, 16). PCR and DNA probe-based detection procedures have previously been designed for P. damselae subsp. piscicida based on amplification of DNA fragments obtained from a genomic library of this bacterium (22) and on amplification of plasmid sequences (1, 23). However, these methods were based on nonconserved regions of the genome, and they have been tested exclusively with Japanese and American isolates of this fish pathogen, whose plasmid contents differ from the plasmid contents of European isolates (14).
Recently, a serological method for rapidly detecting fish pasteurellosis based on a magnetic bead enzyme immunoassay (Bionor Aquaeia-Pp kit) was evaluated in our laboratory, and a limit of detection of 104 cells was obtained (13). In this study, we developed a rapid procedure for detecting P. damselae by nested PCR. Our experiments to determine minimum DNA detection levels showed that between 10 fg and 1 pg of P. damselae DNA, corresponding to approximately 20 to 200 cells (9), can be accurately amplified during the first round of PCR. Interestingly, when the PCR protocol was used with DNA extracted from experimentally inoculated fish tissues (to simulate the expected field conditions), similar sensitivities were obtained. Therefore, we concluded that the PCR-based protocol which we describe here is at least 100 times more sensitive than the serological approach, which indicates that the method is suitable for fast, precise, and sensitive detection of this fish pathogen not only in diseased fish but also in asymptomatic carriers, in which bacteria can persist in low numbers and below the detection limits of serological techniques. Since we were able to detect P. damselae in experimentally infected tissues by this procedure (18), the accuracy of the nested PCR assay is currently being investigated with samples from different fish farms in order to evaluate its efficacy under field conditions.
Finally, it is pertinent that P. damselae subsp. piscicida and P. damselae subsp. damselae have identical 16S rRNA genes, and, therefore, the assay which we developed should detect both subspecies of P. damselae. However, since the two subspecies have different ecological habitats and distinct host specificities, our protocol can be used not only for rapid diagnosis of fish pasteurellosis but also for detection of P. damselae subsp. damselae in clinical samples. Additional studies will focus on the search for subspecies-specific genes which can be used as targets for discrimination of P. damselae strains at the subspecies level.
ACKNOWLEDGMENTS
This work was supported by grant XUGA 20003A96 from Xunta de Galicia, Spain and by grants MAR 96-1875 and MAR-99-0478 from the Comisión Interministerial de Ciencia y Tecnología (CICYT), Madrid, Spain. C. R. Osorio is grateful to the Ministerio de Educación y Ciencia of Spain for a predoctoral research fellowship.
We are grateful to colleagues for kindly supplying many of the P. damselae strains used in this study.
REFERENCES
- 1.Aoki T, Ikeda D, Katagiri T, Hirono I. Rapid detection of the fish pathogenic bacterium Pasteurella piscicida by polymerase chain reaction targetting nucleotide sequences of the species-specific plasmid pZP1. Fish Pathol. 1997;32:143–151. [Google Scholar]
- 2.Deveraux J, Haeberli P, Smithies D. A comprehensive set of sequence analysis programmes for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fouz B, Larsen J L, Nielsen B, Barja J L, Toranzo A E. Characterization of Vibrio damsela strains isolated from turbot Scophthalmus maximus in Spain. Dis Aquat Org. 1992;12:155–166. [Google Scholar]
- 4.Fouz B, Toranzo A E, Marco-Noales E, Amaro C. Survival of fish virulent strains of Photobacterium damselae subsp. damselae in seawater under starvation conditions. FEMS Microbiol Lett. 1998;168:181–186. doi: 10.1111/j.1574-6968.1998.tb13271.x. [DOI] [PubMed] [Google Scholar]
- 5.Funke G, Pascual Ramos C, Collins M D. Identification of some clinical strains of CDC coryneform group A-3 and A-4 bacteria as Cellulomonas hominis sp. nov. for some group A-3 strains. J Clin Microbiol. 1995;33:2091–2097. doi: 10.1128/jcm.33.8.2091-2097.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gauthier G, Lafay B, Ruimy R, Breittmayer V, Nicolas J L, Gauthier M, Christen R. Small-subunit rRNA sequences and whole DNA relatedness concur for the reassignment of Pasteurella piscicida (Snieszko et al.) Janssen and Surgalla to the genus Photobacterium as Photobacterium damsela subsp. piscicida comb. nov. Int J Syst Bacteriol. 1995;45:139–144. doi: 10.1099/00207713-45-1-139. [DOI] [PubMed] [Google Scholar]
- 7.Gerhardt P, Murray R G E, Wood W A, Krieg N R. Methods for general and molecular bacteriology. Washington, D.C: American Society for Microbiology; 1994. [Google Scholar]
- 8.Hiney M, Smith P. DNA-based diagnostics in aquaculture: can we overcome the problems of interpretation in the field. In: Barnes A C, Davidson G A D, Hiney M P, McIntosh D, editors. Methodology in fish research. Aberdeen, Scotland, United Kingdom: Fisheries Research Services; 1998. pp. 143–159. [Google Scholar]
- 9.Hiney M, Dawson M T, Heery D M, Smith P R, Gannon F, Powell R. DNA probe for Aeromonas salmonicida. Appl Environ Microbiol. 1992;58:1039–1042. doi: 10.1128/aem.58.3.1039-1042.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holt J G, Krieg N R, Sneath P H A, Staley J T, Williams S T. Bergey’s manual of determinative bacteriology. 9th ed. Baltimore, Md: Williams & Wilkins; 1994. [Google Scholar]
- 11.Hutson R A, Thompson D E, Collins M D. Genetic interrelationships of saccharolytic Clostridium botulinum types B, E and F and related clostridia as revealed by small-subunit rRNA gene sequences. FEMS Microbiol Lett. 1993;108:103–110. doi: 10.1111/j.1574-6968.1993.tb06081.x. [DOI] [PubMed] [Google Scholar]
- 12.Janssen W A, Surgalla M J. Morphology, physiology, and serology of a Pasteurella species pathogenic for white perch (Roccus americanus) J Bacteriol. 1968;96:1606–1610. doi: 10.1128/jb.96.5.1606-1610.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lores F, Magariños B, Osorio C R, Toranzo A E, Romalde J L. Abstracts of the 3rd International Symposium on Aquatic Animal Health. Baltimore, Md: American Fisheries Society; 1998. Accuracy of the AQUAEIA kit (BIONOR AS) for the detection of the pasteurellosis agent in diseased and carrier fish, abstr. no. S 1-5. [Google Scholar]
- 14.Magariños B, Romalde J L, Bandín I, Fouz B, Toranzo A E. Phenotypic, antigenic and molecular characterization of Pasteurella piscicida strains isolated from fish. Appl Environ Microbiol. 1992;58:3316–3322. doi: 10.1128/aem.58.10.3316-3322.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Magariños B, Romalde J L, Barja J L, Toranzo A E. Evidence of a dormant but infective state of the fish pathogen Pasteurella piscicida in seawater and sediment. Appl Environ Microbiol. 1994;60:180–186. doi: 10.1128/aem.60.1.180-186.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magariños B, Toranzo A E, Romalde J L. Phenotypic and pathobiological characteristics of Pasteurella piscicida. Annu Rev Fish Dis. 1996;6:41–64. [Google Scholar]
- 17.Magariños B, Osorio C R, Toranzo A E, Romalde J L. Applicability of Ribotyping for intraspecific classification and epidemiological studies of Pasteurella piscicida. Syst Appl Microbiol. 1997;20:634–639. [Google Scholar]
- 18.Osorio C R, Romalde J L, Barja J L, Collins M D, Toranzo A E. Abstracts of the 3rd International Symposium on Aquatic Animal Health. Baltimore, Md: American Fisheries Society; 1998. PCR-based detection of fish pasteurellosis, abstr. no. S 1-6. [Google Scholar]
- 19.Romalde J L, Magariños B. Immunization with bacterial antigens: pasteurellosis. In: Gudding R, Lillehaug A, Midtlyng P T, Brown F, editors. Fish vaccinology. Basel, Switzerland: Karger; 1997. pp. 167–177. [PubMed] [Google Scholar]
- 20.Snieszko S F, Bullock G L, Hollis E, Boone J G. Pasteurella sp. from an epizootic of white perch (Roccus americanus) in Chesapeake Bay tidewater areas. J Bacteriol. 1964;88:1814–1815. doi: 10.1128/jb.88.6.1814-1815.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Truper H G, DeĆlari L. Taxonomic note: necessary correction of epithets formed as substantives (nouns) “in apposition.”. Int J Syst Bacteriol. 1997;47:908–909. [Google Scholar]
- 22.Zhao J, Aoki T. A specific DNA hybridization probe for detection of Pasteurella piscicida. Dis Aquat Org. 1989;7:203–210. [Google Scholar]
- 23.Zhao J, Aoki T. Plasmid profile analysis of Pasteurella piscicida and use of a plasmid DNA probe to identify the species. J Aquat Anim Health. 1992;4:198–202. [Google Scholar]