Abstract
Lysyl oxidase-like 2 (LOXL2) and 3 (LOXL3) are members of the lysyl oxidase family of enzymes involved in the maturation of the extracellular matrix. Both enzymes share a highly conserved catalytic domain, but it is unclear whether they perform redundant functions in vivo. In this study, we show that mice lacking Loxl3 exhibit perinatal lethality and abnormal skeletal development. Additionally, analysis of the genotype of embryos carrying double knockout of Loxl2 and Loxl3 genes suggests that both enzymes have overlapping functions during mouse development. Furthermore, we also show that ubiquitous expression of Loxl2 suppresses the lethality associated with Loxl3 knockout mice.
Keywords: lysyl oxidases, Loxl2, Loxl3, epistasis analysis, embryonic lethality
1. Introduction
The lysyl oxidase (LOX) family is composed of five lysine-tyrosylquinone (LTQ)-dependent copper amine oxidases: LOX and four LOX-like paralogs LOXL1–4. All members are characterised by a highly conserved carboxyl (C)-terminal amine oxidase catalytic domain. This domain includes a histidine-rich copper-binding motif and a lysyl–tyrosyl–quinone (LTQ) cofactor, both essential for catalytic activity, and a cytokine receptor-like (CRL) domain whose function remains unknown [1,2,3,4]. By contrast, the amino (N)-terminal region diverges among all members. In the case of LOXL2–4, it presents four scavenger receptor cysteine-rich (SRCR) domains whose functional role has not been well characterised yet, although they could be involved in protein–protein interactions [5]. Based on N-domain diversification and sequences comparisons, these proteins have been classified into two subfamilies: one constituted by LOX and LOXL1, while LOXL2, LOXL3, and LOXL4 belong to the second one [2,4]. The physiological function of LOX enzymes is the maturation of the extracellular matrix (ECM). Lysyl oxidases catalyse the oxidative deamination of ε-amino groups of peptidyl–lysine and hydroxylysine residues to produce highly reactive aldehydes—allysine residues—that undergo a spontaneous condensation, thus establishing intra- or inter-cross-linkages in collagen and elastin [4,6]. Besides their critical role in ECM maturation, lysyl oxidase proteins are also associated with diverse pathologies, including fibrosis, cancer, and cardiovascular diseases (reviewed in [7,8,9,10,11,12,13]).
The generation and characterisation of genetically modified mouse models with gain or loss of function of Lox [14,15,16], Loxl1 [17,18], Loxl2 [19,20], Loxl3 [21,22] and Loxl4 [23] have highlighted the critical role of LOX enzymes in mammalian development. The genetic ablation of Loxl2 or Loxl3 leads to perinatal lethality with incomplete penetrance caused by different alterations. Loxl2 KO mice lethality is associated with congenital heart defects and/or distension of the hepatic blood vessels [19], while Loxl3 KO mice lethality has been linked to impaired embryonic development of the palate shelves, vertebral column and pulmonary system [21,24]. Moreover, Loxl3 was shown to be key for proper muscle development [22]. These different phenotypes indicate that there is no functional compensation between both enzymes during embryonic development despite presenting 71% of identity in the catalytic domain [3]. Nevertheless, this assumption has not been demonstrated to date. Therefore, we decided to study the functional relationship of Loxl2 and Loxl3 using in vivo models. To this end, we generated double transgenic mice carrying either double Loxl2 and Loxl3 knockout (KO) genes or a Loxl2 knockin (KI) gene in a Loxl3 KO background. We have observed that double Loxl2/Loxl3 KO results in embryonic lethality, whereas the ubiquitous expression of Loxl2 can suppress the perinatal lethality associated with Loxl3 KO mice.
2. Results
2.1. Deletion of Loxl3 Provokes Perinatal Lethality
We first confirmed the described perinatal lethality of Loxl3 KO mice [21,22]. To this end, we generated heterozygous Loxl3+/LacZ mice as recently described [25] and analysed the lethality associated with the Loxl3LacZ/LacZ genotype upon breeding heterozygous mice. The quantification of the offspring after weaning revealed that the percentage of Loxl3LacZ/LacZ mice was 5.64%, a five-fold lower frequency than expected from a Mendelian ratio (Table 1). Since at E18.5 the expected number of Loxl3LacZ/LacZ embryos was observed (Table 1), we concluded that Loxl3 KO mice died perinatally. Of note, the percentage of Loxl2−/− surviving animals after weaning is 10.7%, two-fold lower than expected [19].
Table 1.
Genotype frequency of the offspring from Loxl3+/LacZ heterozygous mice crossings. Value reflecting the perinatal embryonic lethality associated with Loxl3LacZ/LacZ genotype is indicated in bold.
| Genotype | After Weaning (%) | E18.5 (%) | Expected (%) |
|---|---|---|---|
| Loxl3 +/+ | 30.09 | 18.52 | 25 |
| Loxl3 +/LacZ | 64.26 | 55.56 | 50 |
| Loxl3LacZ /LacZ | 5.64 | 25.93 | 25 |
| Total number of animals analysed | 319 | 81 |
2.2. Loxl3 KO Mice Present Skeletal Abnormalities
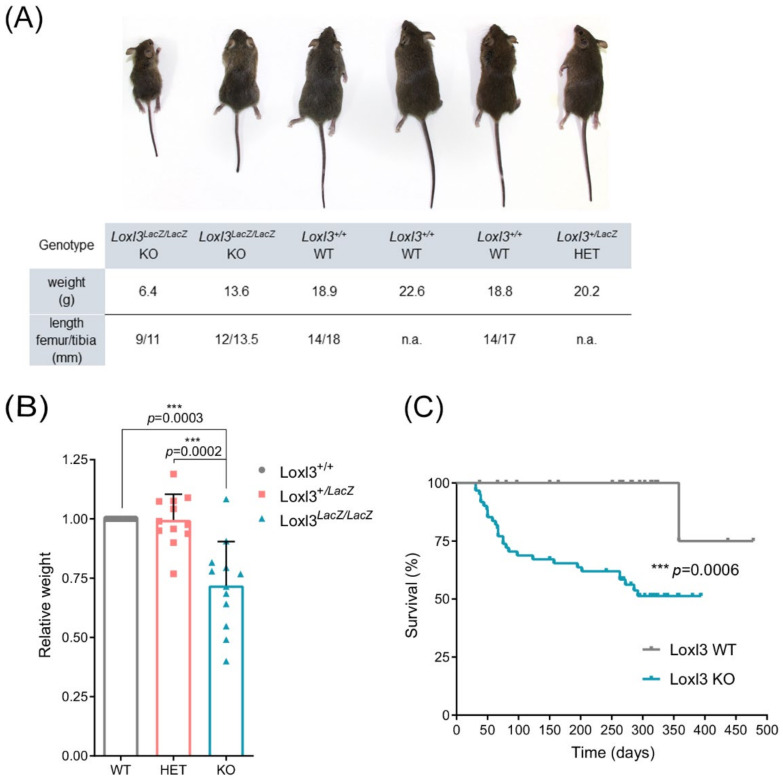
We then analysed the phenotype of Loxl3 KO surviving mice. Loxl3LacZ/LacZ mice showed significantly smaller size, consistent with lower weight and shorter length of tibia and femur (Figure 1A,B), and a poor health appearance that resulted in a higher mortality rate than their corresponding littermates, determined by closely observing Loxl3+/+ (n = 26) and Loxl3LacZ/LacZ (n = 61) mice for more than a year. In addition, most Loxl3LacZ/LacZ adult animals presented alterations in locomotion, imbalance and spinal deformities (Video S1), the latter observed by Zhang et al. in newborn Loxl3 KO mice [21]. However, in Loxl3LacZ/LacZ mice, we did not detect any of the craniofacial abnormalities described previously [21], suggesting that the perinatal lethality we observed was not due to cleft palate.
Figure 1.
Loxl3 KO adult mice display reduced size and poor overall survival. (A) Representative littermate (8 weeks old) obtained from crossing Loxl3 heterozygous (Loxl3LacZ/+) mice. The weight of mice and length of femur and tibia from each mouse is depicted below. (B) Relative weight of wild-type, WT (Loxl3+/+), heterozygous, HET (Loxl3+/LacZ) and KO (Loxl3LacZ/LacZ) mice from 12 representative littermates (n = 39) from the breeding of Loxl3 heterozygous (Loxl3+/LacZ) mice. Female and male mice were included, and their weight was normalised to the weight of wild-type female and male mice in each littermate. p values were calculated by Student’s two-tailed paired t test. (C) Kaplan–Meier survival curve of Loxl3+/+ (Loxl3 WT, n = 26) and Loxl3LacZ/LacZ (Loxl3 KO, n = 61) mice. Perinatal death was not taken into consideration. Most adult Loxl3 KO mice died spontaneously or had to be euthanised due to their poor health status. p value was calculated by Mantel–Cox test.
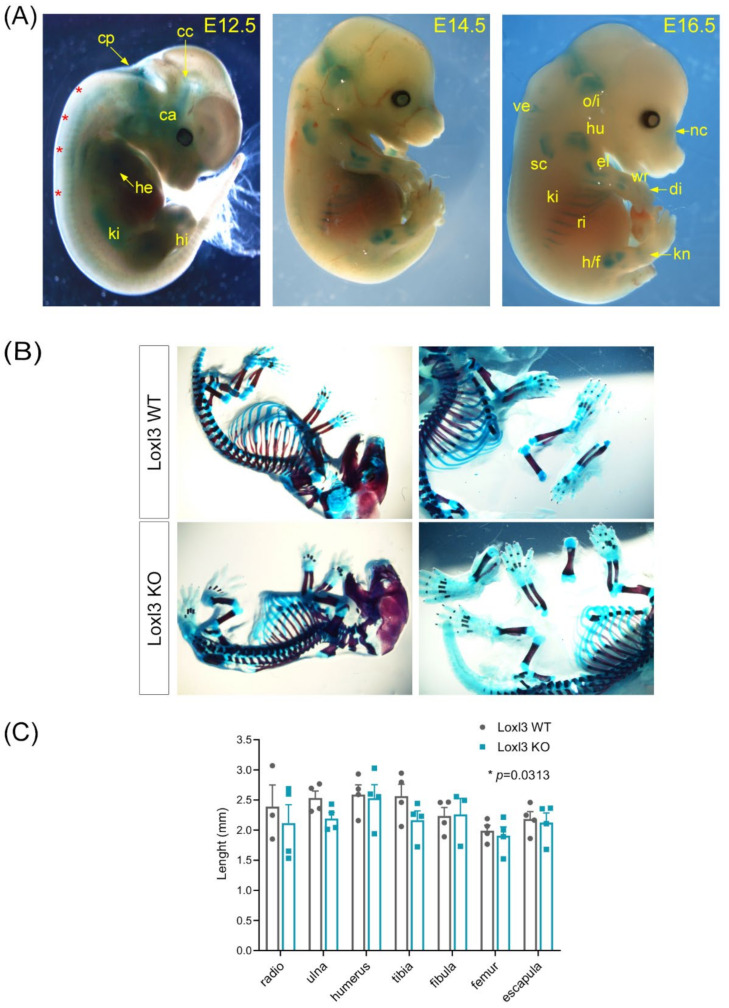
To investigate the causes of the Loxl3 KO phenotype, we first analysed the expression of Loxl3 in Loxl3+/LacZ embryos at E12.5, 14.5 and 16.5 developmental stages. To this end, we took advantage of the Loxl3LacZ allele that expresses the LacZ gene under the control of the Loxl3 promoter [25]. In this way, it was possible to analyse the expression of Loxl3 during embryonic development by X-Gal staining. As can be seen in Figure 2A, we detected the expression of Loxl3 at E12.5 in various regions of the head (chondrocranium and cochlear area), fourth ventricle of the choroid plexus, axial zone, kidney, heart, and faint expression in the hindlimbs. At E14.5 and E16.5 stages, the expression of Loxl3 is comparable and more spatially defined, showing an intense staining in pre-osseous cartilaginous structures such as cartilage precursors of the occipital and interparietal bones, nasal area, scapula and costal cartilages, as well as in the epiphysis of long bones, hip or humeral head, elbow, wrist and slight expression in vertebrae (Figure 2A). These data indicate that Loxl3 may have a role in mouse skeletal development.
Figure 2.
Loxl3 expression during embryogenesis is associated with mouse skeletal development. (A) Loxl3 expression pattern in embryos at indicated developmental stages as depicted by X-Gal staining. Red asterisks indicate axial staining; cp, choroid plexus; cc, chondrocranium; ca, cochlear area; he, heart; ki, kidney; hi, hindlimbs; o/i, occipital and interparietal cartilaginous precursors; ve, vertebrae; hu, humerus; nc, nasal cartilages; el, elbow; sc, scapula; wr, wrist; di, digits; ri, ribs; s/f, hip/femur; kn, knee. (B) Representative images of Loxl3 WT (Loxl3+/+) and KO (Loxl3LacZ/LacZ) E18.5 embryos stained with alcian blue (cartilage)/alizarin red (bone) used to measure mineralised tissues. (C) Length (mm) of indicated bones from Loxl3 WT (Loxl3+/+) and KO (Loxl3LacZ/LacZ) embryos (E18.5). Individual values and mean with SEM from WT (n = 4) and KO (n = 4) littermate embryos are shown. p value was calculated by Wilcoxon matched-pairs signed rank test.
To gain insight into this possible function of Loxl3, littermate E18.5 embryos (n = 4 WT and n = 4 KO) were stained with alcian blue/alizarin red, a technique that distinguishes cartilage from mineralised bone (Figure 2B). The measurement of the length of the different ossified skeletal structures from the embryos showed a significant difference in the global relative length of these ossified elements in Loxl3LacZ/LacZ embryos compared to wild-type controls (Figure 2C).
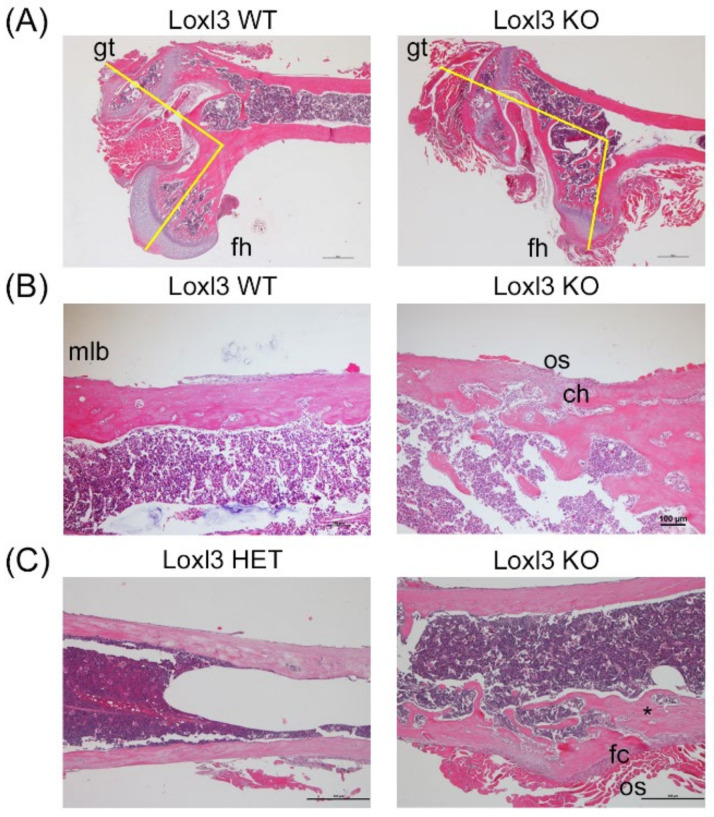
To analyse the observed defects in postnatal skeletal development, paraffin sections from the femur of one-month-old Loxl3+/+ and Loxl3LacZ/LacZ littermates were stained with haematoxylin/eosin. Histopathological analysis showed that some Loxl3LacZ/LacZ mice exhibit dysplasia in the upper femoral epiphysis, with atrophy of the head of the femur, hypertrophy of the greater trochanter and an abnormal angle arrangement of these two structures (Figure 3A). This could contribute to the locomotor problems that we observed in some of the Loxl3 KO mice (Video S1). Additionally, the mature lamellar bone of the diaphysis of Loxl3LacZ/LacZ mice is interrupted by zones of immature reticular embryonic bone, with osteoblasts and chondrocytes inside (Figure 3B). In certain areas, the diaphysis compact bone is replaced by cancellous bone (Figure 3C). This anomalous organisation would be responsible for the greater skeletal fragility detected de visu when processing the bones of animals that lacked Loxl3.
Figure 3.
Embryonic loss of Loxl3 promotes abnormal skeletal development. (A) Images showing femoral head dysplasia found in several Loxl3 KO (Loxl3LacZ/LacZ) mice with atrophy of the femoral head (fh), hypertrophy of the greater trochanter (gt) and an abnormal angle between the fh and gt, displayed by yellow lines. (B) The lamellar diaphysis bone in Loxl3 KO (Loxl3LacZ/LacZ) animals is interrupted by woven bone, including few chondrocytes (ch) and active osteoblasts (os) in the periphery compared to the mature lamellar bone (mlb) in WT mice. (C) The diaphysis from Loxl3 KO (Loxl3LacZ/LacZ) femur displays a fibro-cartilaginous (fc) area with local deformation and bone marrow disorganisation not present in a littermate heterozygous mouse (HET, Loxl3+/LacZ). The asterisk depicts a connective channel between the periphery and deep inter-trabecular spaces. Scale bars, 100 µm.
Altogether, our results suggest that Loxl3 contributes to skeletal development and that aberrant bone maturation might be the underlying cause or significantly contribute to the perinatal lethality observed in Loxl3 KO mice.
2.3. Double Knockout of Loxl2 and Loxl3 Genes Leads to Embryonic Lethality
We then proceeded to analyse the viability of double Loxl2/Loxl3 KO (Loxl2−/−; Loxl3LacZ/LacZ) mice by breeding Loxl2+/− [19] and Loxl3+/LacZ heterozygous mice. We could not find any double Loxl2/Loxl3 KO mouse among the offspring from the crossings (n = 293) (Table 2).
Table 2.
Genotype frequency of the offspring from Loxl2+/−; Loxl3+/LacZ crossings. Value reflecting the postnatal lethality associated with double Loxl2/Loxl3 KO is indicated in bold.
| Genotype | After Weaning (%) | Expected (%) |
|---|---|---|
| Loxl2+/+; Loxl3+/+ | 13.31 | 6.25 |
| Loxl2+/+; Loxl3+/LacZ | 23.89 | 12.25 |
| Loxl2+/+; Loxl3LacZ/LacZ | 3.75 | 6.25 |
| Loxl2+/−; Loxl3+/+ | 20.14 | 12.5 |
| Loxl2+/−; Loxl3+/LacZ | 34.81 | 25 |
| Loxl2+/−; Loxl3LacZ/LacZ | 0.68 | 12.5 |
| Loxl2−/−; Loxl3+/+ | 2.05 | 6.25 |
| Loxl2−/−; Loxl3+/LacZ | 1.37 | 12.5 |
| Loxl2−/−; Loxl3LacZ/LacZ | 0 | 6.25 |
| Total number of mice analysed | 293 |
To discern whether the observed lethality in the double KO mice occurred during embryogenesis or postnatally, we harvested embryos at E9.5, E11.5 and E13.5 developmental stages. Genotype analysis indicated that Loxl2−/−; Loxl3LacZ/LacZ embryos appeared with a normal Mendelian frequency until E9.5, but not a single double Loxl2/Loxl3 KO was observed at either E11.5 and E13.5 stages (Table 3). These data suggest that Loxl2 and Loxl3 are dispensable for early embryonic development up to E9.5 but are required for normal development thereafter.
Table 3.
Genotype frequency of embryos obtained from Loxl2+/−; Loxl3+/LacZ crossings. Values reflecting the embryonic lethality associated with double Loxl2/Loxl3 KO are indicated in bold.
| Genotype | E9.5 (%) | E11.5 (%) | E13.5 (%) | Expected (%) |
|---|---|---|---|---|
| Loxl2+/+; Loxl3+/+ | 4.82 | 9.38 | 11.11 | 6.25 |
| Loxl2+/+; Loxl3+/LacZ | 10.84 | 12.5 | 15.38 | 12.25 |
| Loxl2+/+; Loxl3LacZ/LacZ | 4.82 | 12.5 | 3.42 | 6.25 |
| Loxl2 +/− ; Loxl3 +/+ | 14.46 | 28.13 | 16.24 | 12.5 |
| Loxl2+/−; Loxl3+/LacZ | 39.76 | 28.13 | 38.46 | 25 |
| Loxl2+/−; Loxl3LacZ/LacZ | 9.64 | 6.25 | 8.55 | 12.5 |
| Loxl2−/−; Loxl3+/+ | 7.23 | 3.13 | 3.42 | 6.25 |
| Loxl2−/−; Loxl3+/LacZ | 3.61 | 0 | 3.42 | 12.5 |
| Loxl2−/−; Loxl3LacZ/LacZ | 4.82 | 0 | 0 | 6.25 |
| Total number of mice analysed | 83 | 32 | 117 |
2.4. Ubiquitous Expression of Loxl2 Ameliorates Perinatal Lethality in Loxl3 KO Mice
We previously developed a mouse model ubiquitously expressing Loxl2 from the ROSA26 locus (R26Loxl2/+) [19] that now allow us to analyse if Loxl2 overexpression could rescue the Loxl3 KO perinatal phenotype. To that end, we generated double heterozygous R26Loxl2/+; Loxl3+/LacZ mice, bred them and analysed the offspring at weaning, around 21 days postnatally (n = 150). The expected Mendelian ratios of Loxl3 KO mice expressing Loxl2 ubiquitously were 6.25% for R26Loxl2/Loxl2; Loxl3LacZ/LacZ and 12.5% for R26Loxl2/+; Loxl3LacZ/LacZ mice (Table 4). The observed percentage of mice with the latter genotype fitted with the expected ratio, whereas less (2%) but still some R26Loxl2/Loxl2; Loxl3LacZ/LacZ mice survived up to adulthood. No Loxl3 KO mouse that was not overexpressing Loxl2 (R26+/+; Loxl3LacZ/LacZ) was found (Table 4), although the embryonic lethality in our single Loxl3 KO model was not completely penetrant. These results suggest that ubiquitous Loxl2 expression rescues or strongly alleviates Loxl3 KO-associated lethality. Only three R26Loxl2/Loxl2; Loxl3LacZ/LacZ mice compared to 19 mice with the R26Loxl2/+; Loxl3LacZ/LacZ genotype were found, suggesting that increased Loxl2 protein levels in R26Loxl2/Loxl2 compared to R26Loxl2/+ background [19] have a deleterious effect in mice lacking one or both wild-type Loxl3 alleles (Table 4).
Table 4.
Genotype frequency of offspring from R26Loxl2/+; Loxl3+/LacZ crossings. Values reflecting the suppression of the embryonic lethality associated with Loxl3 KO are indicated in bold.
| Genotype | After Weaning (%) | Expected (%) |
|---|---|---|
| R26Loxl2/Loxl2 ; Loxl3LacZ/LacZ | 2 | 6.25 |
| R26Loxl2/+; Loxl3LacZ/LacZ | 12.67 | 12.25 |
| R26+/+; Loxl3LacZ/LacZ | 0 | 6.25 |
| R26Loxl2/Loxl2 ; Loxl3+/LacZ | 5.33 | 12.5 |
| R26Loxl2/+ ; Loxl3+/LacZ | 44 | 25 |
| R26+/+; Loxl3+/LacZ | 7.33 | 12.5 |
| R26Loxl2/Loxl2 ; Loxl3+/+ | 12.67 | 6.25 |
| R26Loxl2/+ ; Loxl3+/+ | 14 | 12.5 |
| R26+/+; Loxl3+/+ | 2 | 6.25 |
| Total number of animals analysed | 319 |
3. Discussion
The perinatal lethality shown by Loxl3LacZ/LacZ mice as well as by other Loxl3 KO models [21,22] points to a key role for Loxl3 in embryonic development. The embryonic expression pattern of Loxl3 and the phenotypes associated with its genetic inactivation indicate that the main role of Loxl3 is essentially linked to ECM maturation and tissue homeostasis. Although the study by Zhang and collaborators [21] attributed the complete lethality they observed by P1 to the critical role of Loxl3 in palatogenesis, our Loxl3 KO mouse model, in which we were not able to detect craniofacial defects, showed impaired skeletal development as indicated previously [21,22]. These differences, probably due to their different genetic backgrounds, allowed us to characterise Loxl3 KO adult mice, which presented smaller size as well as locomotive, balance and skeletal abnormalities, resulting in a markedly shorter lifespan than Loxl3 wild-type mice. Our data indicate that Loxl3 might have a role in endochondral ossification, plausibly linked to an altered ECM maturation provoked by the absence of Loxl3 during embryogenesis. We have shown that Loxl3 is expressed in pre-ossified cartilage structures at least from E12.5, being detected in the long bones, mostly in the epiphysis, at E14.5 and E16.5. Defects in cartilage maturation would explain the cleft palate and spinal deformity observed in Loxl3 KO mice [21] and in a zebrafish model lacking a LOXL3 orthologue [26]. Furthermore, a recent study using a targeted Loxl3 deletion has demonstrated the relevance of Loxl3 in inner ear function, related to its role in type II collagen crosslinking [27] and required for the functional structure provided by the ECM. Human LOXL3 has been proposed as a candidate gene responsible for recessive autosomal Stickler syndrome [28], a collagenopathy characterised by bone and cartilage abnormalities as well as by different degrees of hearing impairment [29] that might also cause imbalance, similar to the instability we observed in Loxl3 KO adult mice.
Deletion of Loxl2 causes incomplete perinatal lethality due to heart failure [19], whereas Lox-targeted mice died after birth due to cardiovascular instability [15]. The neonatal lethality we observed in our Loxl3 KO mice might be related to cardiac defects as well as impaired lung development [24], which may contribute to death due to the physical stresses associated with parturition. Independent gene disruption of Lox, Loxl2 and Loxl3 results in lethality, indicating that the presence of other lysyl oxidases cannot compensate for their individual loss. These facts, together with the different phenotypes caused by the constitutive abrogation of Loxl2 or Loxl3, led to presume that no functional overlap exists between both enzymes, even if their catalytic domains present 71% identity. Nevertheless, this assumption has never been proven in in vivo models. We approached this question experimentally in two ways. First, we performed an epistasis analysis of Loxl2 and Loxl3 deletions in the mouse and observed that double Loxl2 and Loxl3 KO embryos die between E9.5 and E11.5 developmental stages, in contrast to the perinatal lethality observed in the independent single Loxl2 [19] or Loxl3 KO mutants. This result supports the idea that Loxl2 and Loxl3 functions may overlap to some degree in normal development and that they must operate in a common biochemical pathway required for embryonic viability after E9.5. Second, a rescue experiment of Loxl3LacZ/LacZ perinatal lethality by ubiquitous expression of Loxl2 was performed. The results showed that Loxl2 ubiquitous expression ameliorates the perinatal lethality associated with Loxl3LacZ/LacZ genotype, suggesting that the lack of functional complementation during embryonic development is not due to alternative functions of Loxl2 and Loxl3 proteins but either to their limiting expression levels or differential temporal and/or tissue distribution during development.
LOXL3, to the best of our knowledge, is the only lysyl oxidase with a crucial role in skeletal development. Further analyses of adult Loxl3 KO mice expressing Loxl2 ubiquitously would be required to determine whether Loxl2 rescues the skeletal abnormalities associated with Loxl3 deficiency.
4. Materials and Methods
4.1. Mice
All mouse studies were performed in accordance with protocols approved by the Universidad Autónoma de Madrid Ethics Committee (ref # CEI-25-587) and the Comunidad de Madrid (PROEX 122/17). Mice were bred in a mixed genetic background (C57BL/6, CD1 and 129v strains). Loxl2 constitutive KO mice (Loxl2−/−) and mice expressing Loxl2 ubiquitously (R26Loxl2/Loxl2) were generated and genotyped as previously described [19]. To generate Loxl3 constitutive KO mice (Loxl3LacZ/LacZ), embryonic stem cell clones bearing a knockout first allele (Loxl3tm1a(EUCOMM)Wtsi) were obtained from EUCOMM and were genotyped as described in [25]. Mouse strains have been deposited in the European Mouse Mutant Archive (EMMA) with accession numbers EM12882, EM13122 and EM13124.
4.2. Histology
Bones were dissected, cleared from muscle tissue and fixed in 4% formaldehyde overnight at 4 °C. Samples were decalcified with 0.5 M EDTA pH 8.0 for 48 h before embedding in paraffin. Five-micrometre thick sections were stained with haematoxylin and eosin solutions for morphological analysis.
4.3. Whole-Mount X-Gal Staining of Embryos
Mouse embryos at the indicated stages were collected in ice-cold phosphate-buffered saline (PBS) in 2 mL tubes. PBS was replaced by freshly prepared fixative solution (1% formaldehyde, 0.2% glutaraldehyde, 0.02% NP40 and 0.01% sodium deoxycholate in PBS) for 30 min at 4 °C on a rocking platform. After 3 washes in PBS at room temperature, embryos were embedded in fresh staining solution (0.5 mg/mL X-Gal, 0.25 mM K3Fe(CN6), 0.25 mM K4Fe(CN6), 0.01% NP40, 40 mM MgCl2 in PBS; to which 0.01% sodium deoxycholate was added for embryos ≥ E13.5) overnight at 37 °C in a rotating wheel. The enzymatic reaction was stopped with several washes in PBS, and embryos were photographed using a Leica stereo-microscope with a DF550 camera.
4.4. Skeletal Stainings
Alcian blue/alizarin red stainings of cartilage and bones were performed based on the protocol detailed in [30]. Briefly, embryos were collected in ice-cold PBS and stored overnight in tap water. The following morning, embryos were boiled in hot tap water for 30 s, deskinned, eviscerated and fixed in 95% ethanol for 3–5 days. Cartilage staining was performed, incubating the samples overnight in alcian blue stain (150 mg/L alcian blue 8GX; 20% glacial acetic acid in ethanol; filtered) with gentle rotation. Next, embryos were rinsed twice with 95% ethanol and extensively washed for >16 h with several changes of 95% ethanol. Embryos were cleared in 1% KOH for 10–15 min, followed by bone staining in alizarin red solution (50 mg/L alizarin red in 1% KOH; filtered) for 1–3 h. Embryos were further cleared by incubation in 1% KOH for 30 min, followed by an 80:20, 60:40, 40:60 and 20:80 1% KOH/glycerol series until destaining was complete. Dissected limbs and embryos were photographed using a Leica stereo-microscope with a DF550 camera.
4.5. Statistical Analyses
Unless otherwise indicated, numerical data are expressed as mean ± SEM. No statistical methods were used to predetermine sample/group sizes. Sample sizes and normalisation methods are indicated in each figure legend. Statistical analyses were performed using GraphPad Prism 8.0 software, and the corresponding method is indicated in each figure legend. The statistical significance of difference between groups is indicated by the number or asterisks (*, 0.01 < p < 0.05; **, 0.001 < p < 0.01; ***, p < 0.001).
Acknowledgments
We would like to thank Alberto Martín (CIC Salamanca) and Fernando Salvador (SOLTI) for the development of the Loxl2 mouse models, Vanesa Santos (IIBM CSIC-UAM) for technical support, and Raquel Arocha (Animal Care Facility, IIBM, CSIC-UAM) for help with the maintenance of mice colonies. P.G.S. is thankful to Víctor Ruiz (IIBM CSIC-UAM) for his help with embryo photographing and Cristina Cebrián (CCHMC, Ohio, US) for sharing her knowledge on embryo stainings. We thank Javier López-Ríos, Gretel Nusspaumer (CABD Sevilla) and Ángela Nieto (IN Alicante) for their input on the phenotypic characterisation of Loxl3 KO mice. We particularly thank Gema Moreno-Bueno for her continuous support, and the authors are grateful to the members of Amparo Cano’s and Paco Portillo’s laboratories for helpful discussions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23105730/s1, Video S1: Altered locomotion in an adult Loxl3 KO mouse.
Author Contributions
P.G.S., A.C. and F.P. conceived and designed the study. J.B.-T., A.F., A.V.-N. and S.M. supervised and genotyped the mouse colonies. P.G.S., S.M., J.B.-T. and A.F. performed the experiments. P.D. performed pathological analyses of mice and embryos, made intellectual contributions, and discussed the results. P.G.S., A.C. and F.P. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the protocols approved by the Use Committee for Animal Care from the UAM (Ref. # CEI-25–587) and the Comunidad de Madrid (PROEX 122/17).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by grants from the Spanish Ministry of Science and Innovation MCIN SAF2016-76504-R (to A.C. and F.P.), PID2019-111052RB-100 (to F.P.) and from the Spanish Institute of Health Carlos III CIBERONC CCB16/12/00295 (to A.C.), all of them partly supported from EU-FEDER funds, and Worldwide Cancer Research (16–0295 to A.C., F.P. and P.G.S.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Finney J., Moon H.-J., Ronnebaum T., Lantz M., Mure M. Human copper-dependent amine oxidases. Arch. Biochem. Biophys. 2014;546:19–32. doi: 10.1016/j.abb.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grau-Bové X., Ruiz-Trillo I., Rodriguez-Pascual F. Origin and evolution of lysyl oxidases. Sci. Rep. 2015;5:10568. doi: 10.1038/srep10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hajdú I., Kardos J., Major B., Fabó G., Lőrincz Z., Cseh S., Dormán G. Inhibition of the LOX enzyme family members with old and new ligands. Selectivity analysis revisited. Bioorg. Med. Chem. Lett. 2018;28:3113–3118. doi: 10.1016/j.bmcl.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Lucero H.A., Kagan H.M. Lysyl oxidase: An oxidative enzyme and effector of cell function. Cell. Mol. Life Sci. 2006;63:2304–2316. doi: 10.1007/s00018-006-6149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez V.G., Moestrup S.K., Holmskov U., Mollenhauer J., Lozano F. The Conserved Scavenger Receptor Cysteine-Rich Superfamily in Therapy and Diagnosis. Pharmacol. Rev. 2011;63:967–1000. doi: 10.1124/pr.111.004523. [DOI] [PubMed] [Google Scholar]
- 6.Trackman P.C. Enzymatic and non-enzymatic functions of the lysyl oxidase family in bone. Matrix Biol. 2016;52:7–18. doi: 10.1016/j.matbio.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cano A., Santamaría P.G., Moreno-Bueno G. LOXL2 in epithelial cell plasticity and tumor progression. Future Oncol. 2012;8:1095–1108. doi: 10.2217/fon.12.105. [DOI] [PubMed] [Google Scholar]
- 8.Barker H.E., Cox T.R., Erler J.T. The rationale for targeting the LOX family in cancer. Nat. Rev. Cancer. 2012;12:540–552. doi: 10.1038/nrc3319. [DOI] [PubMed] [Google Scholar]
- 9.Trackman P.C. Lysyl Oxidase Isoforms and Potential Therapeutic Opportunities for Fibrosis and Cancer. Expert Opin. Ther. Targets. 2016;20:935–945. doi: 10.1517/14728222.2016.1151003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodríguez C., Martínez-González J. The Role of Lysyl Oxidase Enzymes in Cardiac Function and Remodeling. Cells. 2019;8:1483. doi: 10.3390/cells8121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Pascual F., Rosell-Garcia T. Lysyl Oxidases: Functions and Disorders. J. Glaucoma. 2018;27((Suppl. 1)):S15–S19. doi: 10.1097/IJG.0000000000000910. [DOI] [PubMed] [Google Scholar]
- 12.Yang N., Cao D.-F., Yin X.-X., Zhou H.-H., Mao X.-Y. Lysyl oxidases: Emerging biomarkers and therapeutic targets for various diseases. Biomed. Pharmacother. 2020;131:110791. doi: 10.1016/j.biopha.2020.110791. [DOI] [PubMed] [Google Scholar]
- 13.Ye M., Song Y., Pan S., Chu M., Wang Z.-W., Zhu X. Evolving roles of lysyl oxidase family in tumorigenesis and cancer therapy. Pharmacol. Ther. 2020;215:107633. doi: 10.1016/j.pharmthera.2020.107633. [DOI] [PubMed] [Google Scholar]
- 14.Mäki J.M., Räsänen J., Tikkanen H., Sormunen R., Mäkikallio K., Kivirikko K.I., Soininen R. Inactivation of the Lysyl Oxidase Gene Lox Leads to Aortic Aneurysms, Cardiovascular Dysfunction, and Perinatal Death in Mice. Circulation. 2002;106:2503–2509. doi: 10.1161/01.CIR.0000038109.84500.1E. [DOI] [PubMed] [Google Scholar]
- 15.Hornstra I.K., Birge S., Starcher B., Bailey A.J., Mecham R.P., Shapiro S.D. Lysyl Oxidase Is Required for Vascular and Diaphragmatic Development in Mice. J. Biol. Chem. 2003;278:14387–14393. doi: 10.1074/jbc.M210144200. [DOI] [PubMed] [Google Scholar]
- 16.Galán M., Martí-Pàmies I., Varona S., Rodríguez-Calvo R., Briones A.M., Navarro M.A., de Diego A., Osada J., Orriols M., Guadall A., et al. Lysyl oxidase (LOX) in vascular remodelling. Insight from a new animal model. Thromb. Haemost. 2014;112:812–824. doi: 10.1160/TH14-01-0024. [DOI] [PubMed] [Google Scholar]
- 17.Liu X., Zhao Y., Gao J., Pawlyk B., Starcher B., Spencer J.A., Yanagisawa H., Zuo J., Li T. Elastic fiber homeostasis requires lysyl oxidase–like 1 protein. Nat. Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 18.AlSofi L., Daley E., Hornstra I., Morgan E.F., Mason Z.D., Acevedo J.F., Word R.A., Gerstenfeld L.C., Trackman P.C. Sex-Linked Skeletal Phenotype of Lysyl Oxidase Like-1 Mutant Mice. Calcif. Tissue Res. 2015;98:172–185. doi: 10.1007/s00223-015-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martín A., Salvador F., Moreno-Bueno G., Floristán A., Ruiz-Herguido C., Cuevas E.P., Morales S., Santos V., Csiszar K., Dubus P., et al. Lysyl oxidase-like 2 represses Notch1 expression in the skin to promote squamous cell carcinoma progression. EMBO J. 2015;34:1090–1109. doi: 10.15252/embj.201489975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang J., Savvatis K., Kang J.S., Fan P., Zhong H., Schwartz K., Barry V., Mikels-Vigdal A., Karpinski S., Kornyeyev D., et al. Targeting LOXL2 for cardiac interstitial fibrosis and heart failure treatment. Nat. Commun. 2016;7:13710. doi: 10.1038/ncomms13710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J., Yang R., Liu Z., Hou C., Zong W., Zhang A., Sun X., Gao J. Loss of lysyl oxidase-like 3 causes cleft palate and spinal deformity in mice. Hum. Mol. Genet. 2015;24:6174–6185. doi: 10.1093/hmg/ddv333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraft-Sheleg O., Zaffryar-Eilot S., Genin O., Yaseen W., Soueid-Baumgarten S., Kessler O., Smolkin T., Akiri G., Neufeld G., Cinnamon Y., et al. Localized LoxL3-Dependent Fibronectin Oxidation Regulates Myofiber Stretch and Integrin-Mediated Adhesion. Dev. Cell. 2016;36:550–561. doi: 10.1016/j.devcel.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Li H., Guo J., Jia Y., Kong W., Li W. LOXL4 Abrogation Does Not Exaggerate Angiotensin II-Induced Thoracic or Abdominal Aortic Aneurysm in Mice. Genes. 2021;12:513. doi: 10.3390/genes12040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J., Liu Z., Zhang T., Lin Z., Li Z., Zhang A., Sun X., Gao J. Loss of Lysyl Oxidase-like 3 Attenuates Embryonic Lung Development in Mice. Sci. Rep. 2016;6:33856. doi: 10.1038/srep33856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vázquez-Naharro A., Bustos-Tauler J., Floristán A., Yuste L., Oltra S.S., Vinyals A., Moreno-Bueno G., Fabra À., Portillo F., Cano A., et al. Loxl3 Promotes Melanoma Progression and Dissemination Influencing Cell Plasticity and Survival. Cancers. 2022;14:1200. doi: 10.3390/cancers14051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Boxtel A.L., Gansner J.M., Hakvoort H.W., Snell H., Legler J., Gitlin J.D. Lysyl oxidase-like 3b is critical for cartilage maturation during zebrafish craniofacial development. Matrix Biol. 2011;30:178–187. doi: 10.1016/j.matbio.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z., Bai X., Wan P., Mo F., Chen G., Zhang J., Gao J. Targeted Deletion of Loxl3 by Col2a1-Cre Leads to Progressive Hearing Loss. Front. Cell Dev. Biol. 2021;9:683495. doi: 10.3389/fcell.2021.683495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alzahrani F., Al Hazzaa S.A., Tayeb H., Alkuraya F.S. LOXL3, encoding lysyl oxidase-like 3, is mutated in a family with autosomal recessive Stickler syndrome. Qual. Life Res. 2015;134:451–453. doi: 10.1007/s00439-015-1531-z. [DOI] [PubMed] [Google Scholar]
- 29.Robin N.H., Moran R.T., Ala-Kokko L. Stickler Syndrome. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Gripp K.W., Mirzaa G.M., Amemiya A., editors. GeneReviews® [Internet] University of Washington, Seattle; Seattle, WA, USA: 2021. [Google Scholar]
- 30.Tissières V., Geier F., Kessler B., Wolf E., Zeller R., Lopez-Rios J. Gene Regulatory and Expression Differences between Mouse and Pig Limb Buds Provide Insights into the Evolutionary Emergence of Artiodactyl Traits. Cell Rep. 2020;31:107490. doi: 10.1016/j.celrep.2020.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.