Abstract
Obesity is a risk factor for coronary atherosclerosis. However, the influence of adipose tissue in carotid atherosclerosis is not completely understood. No systematic review/meta-analysis was previously performed to understand if obesity is a risk factor for carotid atherosclerosis. This paper aims to provide an opportunistic review of the association between obesity and carotid atherosclerosis and define the role of the different adipose tissue depots in the characteristics of carotid stenosis. The databases PubMed and Cochrane Library were searched on 15–27 April and 19 May 2021. A total of 1750 articles published between 1985 and 2019 were identified, 64 were preselected, and 38 papers (35,339 subjects) were included in the final review. The most frequent methods used to determine obesity were anthropometric measures. Carotid plaque was mostly characterized by ultrasound. Overall obesity and visceral fat were not associated with the presence of carotid plaque when evaluated separately. Waist-hip ratio, however, was a significant anthropometric measure associated with the prevalence of carotid plaques. As it reflected the ratio of visceral and subcutaneous adipose tissue, the balance between these depots could impact the prevalence of carotid plaques.
Keywords: obesity, visceral adipose tissue, subcutaneous adipose tissue, carotid atherosclerotic disease
1. Introduction
The worldwide prevalence of obesity has increased in the past decades. Obesity is an independent risk factor for atherosclerosis, stroke, and cardiovascular disease [1,2,3]. Cardiovascular outcomes and mortality are more dependent on fat distribution than on the total amount of adipose tissue [1]. Previous studies demonstrated that a higher ratio of visceral adipose tissue (VAT) to subcutaneous adipose tissue (SAT) was associated with an increased risk of poor cardiovascular outcomes [1]. VAT is metabolically active and secret adipokines that cause vascular inflammation and insulin resistance [2]. Conversely, SAT is associated with a neutral or even beneficial metabolic impact [2].
Increased abdominal VAT/SAT ratio was inversely correlated with the extent and severity of coronary artery plaques, higher total mortality, and incidence of major adverse cardiac events (MACE) [1,2].
The influence of obesity on carotid atherosclerosis disease remains unclear [4]. No previous systematic review/metanalyses evaluated the relationship between obesity on carotid atherosclerosis.
2. Methods
2.1. Data Sources and Search
The PubMed and Cochrane Library databases were searched on 15–27th of April and 19 May 2021. The query was as follows:
(‘Carotid artery stenosis OR ‘Carotid artery atherosclerosis’) AND (‘visceral adipose tissue’ OR ‘visceral fat’ OR ‘fat tissue’ OR ‘Obesity’ OR ‘subcutaneous adipose tissue’ OR ‘subcutaneous fat’).
This review was conducted according to established methods for reviews in cardiovascular medicine (PRISMA criteria).
2.2. Inclusion and Exclusion Criteria
Studies were included in the current opportunistic review if they met the following criteria: (1) correlating general obesity, VAT, or SAT with the prevalence of carotid artery plaque, (2) evaluating the influence of general obesity, VAT/SAT on carotid artery symptomatology, (3) retrospective or prospective observational clinical studies, (4) performed in humans, (5) full-text available, (6) studies published in English, French, Spanish, and Portuguese.
Other studies were excluded for the following reasons: (1) analyzing intima-media thickness, (2) performed in children, (3) studies reported only as abstracts or with incomplete data, letters, reviews, case reports, nonclinical studies, (4) reviews or meta-analysis.
If the studies had overlapping subjects, the one with the largest sample size was included in the final analysis.
No attempt was made to contact the authors of the included studies to enquire about missing or incomplete data. No studies were excluded because of concerns about missing data.
2.3. Data Extraction—Outcomes-Definitions
After removing the duplicated articles, two authors (J.F. and A.C.) independently selected the full-text articles after screening the title and abstract. Disagreements were resolved by consensus.
For each study, the subsequent data were collected: first author, year of publication, country of the research center, type of study design, total number of patients, age, men (percentages), aims, inclusion and exclusion criteria, and main conclusions (Table 1, Table 2 and Table 3).
Table 1.
Characteristics of the studies included in the opportunistic review.
| Study | Year | Country | Type of Study | Total Number of Patients | Quality of Studies MINOR CRITERIA |
|---|---|---|---|---|---|
| Bogousslavsky et al. [5] | 1985 | Switzerland | Cross-sectional | 477 | 3 |
| Lakka et al. [6] | 2001 | Finland | Longitudinal | 774 | 12 |
| Hunt et al. [7] | 2002 | USA | Cross-sectional | 750 | 14 |
| Hegazi et al. [8] | 2003 | USA | Cross-sectional | 52 | 12 |
| Czernichow et al. [9] | 2005 | France | Cross-sectional | 1014 | 13 |
| Hadjiev et al. [10] | 2003 | Bulgaria | Cross-sectional | 500 | 14 |
| De Souza et al. [11] | 2005 | Brazil | Cross-sectional | 144 | 14 |
| Montalcini et al. [12] | 2006 | Italy | Cross-sectional | 313 | 14 |
| Lear et al. [12] | 2007 | Canada | Cross-sectional | 794 | 14 |
| Park et al. [13] | 2007 | Korea | Cross-sectional | 378 | 14 |
| Irace et al. [14] | 2009 | Italy | Cross-sectional | 1842 | 12 |
| Yu et al. [15] | 2009 | Hong Kong | Cross-sectional | 518 | 13 |
| Terzis et al. [16] | 2011 | Greece | Longitudinal | 106 | 14 |
| Kadoglou et al. [17] | 2012 | Greece | Longitudinal | 112 | 12 |
| Solomon et al. [18] | 2012 | South Africa | Cross-sectional | 203 | 12 |
| Rodríguez-Flores et al. [19] | 2013 | Mexico | Cross-sectional | 185 | 14 |
| Maksimovic et al. [20] | 2013 | Serbia | Cross-sectional | 657 | 14 |
| Galarza-Delgado et al. [21] | 2013 | Mexico | Cross-sectional | 124 | 14 |
| Cuspidi et al. [22] | 2013 | Italy | Cross-sectional | 3752 | 12 |
| Chiquete et al. [23] | 2014 | Mexico | Cross-sectional | 533 | 12 |
| Irie et al. [24] | 2014 | Japan | Cross-sectional | 179 | 12 |
| Yan et al. [25] | 2014 | China | Cross-sectional | 911 | 12 |
| Yuan et al. [26] | 2016 | USA | Cross-sectional | 1315 | 12 |
| Pan et al. [27] | 2016 | China | Cross-sectional | 474 | 12 |
| Radmard et al. [28] | 2016 | Iran | Cross-sectional | 191 | 12 |
| Sandfort et al. [29] | 2016 | USA | Longitudinal | 106 | 12 |
| Mitevska et al. [30] | 2017 | Macedonia | Cross sectional | 60 | 14 |
| Higuchi et al. [31] | 2017 | Japan | Cross-sectional | 980 | 12 |
| Mancusi et al. [32] | 2017 | Italy | Cross-sectional | 8815 | 14 |
| Nishizawa et al. [33] | 2017 | Brazil | Cross-sectional | 240 | 12 |
| Omisore et al. [34] | 2018 | Nigeria | Cross-sectional | 162 | 14 |
| Imahori et al. [35] | 2018 | Norway | Cross-sectional | 4906 | 12 |
| Laugesen et al. [36] | 2018 | Denmark | Cross-sectional | 169 | 12 |
| Yoshida et al. [37] | 2018 | Japan | Cross-sectional | 352 | 14 |
| Scicali et al. [38] | 2018 | Italy | Cross-sectional | 276 | 12 |
| Rovella et al. [4] | 2018 | Italy | Cross-sectional | 390 | 12 |
| Geraci et al. [39] | 2019 | Italy | Cross-sectional | 468 | 14 |
| Haberka et al. [40] | 2019 | Poland | Cross-sectional | 391 | 14 |
Table 2.
Study aims, inclusion and exclusion criteria, methods to study adipose tissue.
| Study | Age (Years) |
Men (%) | Aims | Criteria | Method Used to Determine | |||
|---|---|---|---|---|---|---|---|---|
| Inclusion | Exclusion | Overall Obesity | VAT | SAT | ||||
| Bogousslavsky et al. [5] | 363 (76.1%) |
Determine the relative importance of each cardiovascular risk factor according to the progression of local atheromatous obstruction | Patients with atheromatous internal carotid artery occlusion or stenosis, compared with matched control subjects without internal carotid artery disease and with matched patients with coronary heart disease but without internal carotid artery disease | Age < 50 years old, without anatomic verification of stenosis, with dissection, dysplasia, posttraumatic occlusion, or intake of oral contraceptives | According to medical charts |
NA | NA | |
| Lakka et al. [6] | NA | 774 (100%) |
Whether WHR and WC are directly related to a 4-year increase in the indicators of common carotid atherosclerosis independent of BMI and other risk factors for atherosclerosis | Men, 42–60 years old, Complete information about anthropometric measures and carotid atherosclerosis |
Coronary heart disease, stroke, claudication | BMI | WC WHR |
HC WHR |
| Hunt et al. [7] | 42.2 ± 15.9 | 289 (38.5%) |
The extent to which the presence or absence of carotid artery plaque was under genetic control | Age: 40–60-year-old, that the proband has a living spouse who was willing to participate in the study, and that the proband has at least 6 first-degree relatives, excluding parents, who were at least 16 years of age and living in the San Antonio area | NA | BMI | WC | NA |
| Hegazi et al. [8] | 51 ± 9 | 18 (35%) |
Examine the relationship between obesity and regional patterns of adiposity, insulin resistance, and five independent measures of subclinical atherosclerosis | Volunteers with a prior diagnosis of Type 2 diabetes of known duration not longer than 5 years, age: 20–70 years, and stable weight, with overall good general health | Insulin treatment, current use of tobacco, prior history of myocardial infarction, stroke, or peripheral vascular disease | BMI DXA |
CT scan. | CT scan. |
| Czernichow et al. [9] | 59.4 ± 4.7 | 504 (49.7%) |
Association of body composition assessed by bioimpedance analysis and anthropometric indicators of fat repartition with carotid structure and function | Volunteers Women aged: 35–60 years Men aged: 35–60 years |
Disease likely to hinder participation or threaten 5 years survival. Extreme beliefs or behavior regarding diet |
Bioimpedance BMI |
WC WHR |
HC WHR |
| Hadjiev et al. [10] | 2003 | NA | This population-based biennial epidemiological survey has been designed to assess the prevalence of the multiple vascular risk factors, their distribution patterns, and outcomes among the Bulgarian urban population. | Without signs and symptoms of cerebrovascular disease, aged 50–79 years were enrolled in the study | NA | BMI | NA | NA |
| De Souza et al. [11] | 34.0 ± 11.7 | 0 (0%) |
Estimated the prevalence of atherosclerotic plaque in carotid arteries in systemic lupus erythematous patients and controls and verified possible associations between risk factors and carotid plaque | Women fulfilled the update American College of Rheumatology criteria for systemic lupus erythematosus |
Controls excluded if they had an autoimmune disease | BMI | NA | NA |
| Montalcini et al. [12] | 57.2 ± 7.37 | 0 (0%) |
Investigate whether the subclinical carotid atherosclerosis prevalence is different in obese postmenopausal women with and without metabolic syndrome | Postmenopausal, Caucasian, aged 45–75 years | Diabetes cardiovascular disease arrhythmia |
BMI | NA | NA |
| Lear et al. [3] | 46.9 ± 8.7 | 389 (48.6%) |
Hypothesized that the association between VAT and atherosclerosis is independent of total body fat, established risk factors, and measures of central adiposity | Healthy men and women (between 30 and 65 years of age) matched for ethnicity and BMI | Recent weight change, previous diagnosis of cardiovascular disease, significant comorbidity, had significant prosthetics or amputations, currently taking medications for cardiovascular risk factors | Dual-energy X-ray absorptiometry, CT scan BMI |
CT scan WC WHR |
CT scan WHR |
| Park et al. [13] | 65.3 ± 12.2 | 204 (54%) |
Elucidate the relationship between metabolic syndrome and cerebrovascular stenosis | Consecutive patients with acute ischemic stroke (large artery atherosclerosis, small artery occlusion, cardiac embolism, and ischemic stroke of undetermined etiology) | Strokes of other determined etiology (venous thrombosis, arterial dissection, or moyamoya disease and those with transient ischemic attack). Patients unable to stand with assistance, patients who had no relevant lesions on diffusion-weighted imaging, poor MR angiographic images, incomplete work up | NA | WC | NA |
| Irace et al. [14] | 30–80 | 1002 (54.4%) |
Evaluate the contribution of generalized adiposity, to carotid atherosclerosis, in participants with or without metabolic syndrome | Caucasians | BMI < 18.5 kg/m2, age < 30 years | BMI | WC | NA |
| Yu et al. [15] | 56.4 ± 3.3 | 0 (0%) |
Determine the prevalence of carotid plaque and identity its associated risk factors | Postmenopausal Chinese women aged 50–64 years | Surgical menopause, presence of cardiovascular disease, cancer and renal failure | BMI | WC WHR |
WHR |
| Terzis et al. [16] | 40.5 ± 1.1 | 60 (56.6%) |
Assess associations between actual long-term changes in BMI since adolescence and early and advanced stages of subclinical atherosclerosis among a population of healthy young adults | This longitudinal study was based on a cohort initially recruited consecutively from two Athens high schools in 1983, collecting data on cardiovascular risk factors from a population of consecutive adolescents aged 12–17 years | Loss/change of contact information or decline to participate, or not alive | BMI | WC | NA |
| Kadoglou et al. [17] | 65 ± 7.7 | 86 (76.8%) |
Assess if apelin and visfatin correlate with carotid plaque echogenicity | Aged 56–80 years and overweight (BMI > 25 kg⁄m2 fat-mass > 30%) and with unilateral or bilateral carotid atherosclerosis without indications for intervention, not receiving lipid-lowering treatment | Cerebral hemorrhage, sources of cardioembolism, concurrent conditions, diseases interfering with the expression of inflammatory mediators during the previous 3 months | BMI Bioimpedance |
WHR | WHR |
| Solomon et al. [18] | 56.4 ± 10.9 | 0 (0%) |
Ascertain the association between clinical obesity and atherosclerosis | African black women and Caucasian women who met the American College of Rheumatology criteria for rheumatoid arthritis | Infected with HIV | BMI | WC WHtR WHR |
WHR |
| Rodríguez-Flores et al. [19] | NA | 107 (57.8%) |
Analyze the relationship between cardiovascular risk factors, including obesity, with the severity of atherosclerosis in different arterial territories | Cadavers of men and women aged 0 to 90 years | Arteries were not taken for examination in the following circumstances: when tissues had suffered advanced damage that precluded their analysis when the cadaver arrived more than 36 h after death, or in cases with previously reported congenital heart disease | BMI | NA | NA |
| Maksimovic et al. [20] | 65.3 ± 8.4 | 412 (62.7%) |
Investigate the relationship between abdominal obesity, and other atherosclerotic risk factors in patients with symptomatic carotid atherosclerotic disease | Subjects who had symptoms of cerebral ischemia and carotid stenosis of ≥50% | Age < 18 years, malignant disease, rheumatoid arthritis, or previous endarterectomy | NA | WC | NA |
| Galarza-Delgado et al. [21] | 55.5 ± 13.1 | 13 (10.5%) |
Association between the presence of rheumatoid nodules and plaque of the carotid artery | Met at least 4 American Colege of Rheumatology criteria for rheumatoid arthritis greater than 16 years |
Pregnant patient, History of carotid surgery |
BMI | WC | NA |
| Cuspidi et al. [22] | 53.3 ± 12.6 | 1977 (52.7%) |
Risk of developing left ventricular hypertrophy and carotid atherosclerosis is different in men and women with metabolic syndrome | Uncomplicated essential hypertension | Previous clinically overt cardiovascular disease, secondary causes of hypertension, life-threatening conditions | NA | WC | NA |
| Chiquete et al. [23] | 69.2 | 211 (39.6%) |
Identify risk factors associated with moderate to severe carotid stenosis | History of ischemic stroke or transient ischemic attack or at least two cardiovascular risk factors (Age ≥ 55 years, hypertension, dyslipidemia, smoking habits, obesity or diabetes) | NA | BMI | NA | NA |
| Irie et al. [24] | 65 ± 7 | 147 (82%) |
Clarify the parameters related to the echogenicity of carotid plaque | Age ≥ 40 years, Type 2 diabetes, presence of carotid plaques | History of ischemic stroke, coronary heart disease, peripheral artery disease, elevated liver enzymes, renal insufficiency | BMI | NA | NA |
| Yan et al. [25] | 68.1 (4.9) | 370 (40.6%) |
Investigate the association of the metabolic syndrome components with subclinical atherosclerosis | Age ≥ 60 years | Patients with clinical stroke, coronary heart disease, or heart failure | BMI | WC | NA |
| Yuan et al. [26] | 58.9 (9.7) | 552 (42.0%) |
Assess relationships between anthropometric measures and adipose tissue volumes with subclinical cardiovascular disease in carotid arteries | Type 2 diabetes | Prior coronary artery procedures. Absence of coronary artery calcification | BMI | WC CT scan |
CT scan |
| Pan et al. [27] | NA | 231 (48.7%) |
Identify risk factors associated with carotid atherosclerosis | Relatively healthy populations residing in Northeast China | Excessive alcohol consumption, severe hepatitis B or C, liver disease, mental illness, severe cardiac or pulmonary insufficiency, and cancer | BMI | WC | NA |
| Radmard et al. [28] | 57 ± 5.7 | 92 (48.2%) |
Association between quantitative measures of central adiposity with indicators of carotid atherosclerosis | Aged over 50 | Contraindications for MRI | BMI | WHR WHtR MRI |
WHR WHtR MRI |
| Sandfort et al. [29] | 65 | 67 (63%) |
Evaluate the change of atherosclerosis in the carotid artery wall in hyperlipidemic participants during treatment with statins and determine cardiovascular risk factors associated with change in extent of atherosclerosis | Age ≥ 55 years and an indication for lipid-lowering therapy | Contraindication for statin therapy, use of nonstatin lipid-lowering therapy, and ineligibility for MRI scan | BMI | NA | NA |
| Mitevska et al. [30] | 67 ± 6 | 34 (56.7%) |
Evaluate the risk factor profile, presence of asymptomatic carotid artery disease, and predictors of coronary artery disease in asymptomatic Type 2 diabetic patients | Asymptomatic patients with Type 2 diabetes | Typical stable angina pectoris, previously known or established as coronary artery disease, left ventricular ejection fraction < 50% at rest, severe valvular disease, atrial fibrillation, left bundle branch block, presence of a pacemaker, severe chronic pulmonary disease | BMI | NA | NA |
| Higuchi et al. [31] | 59.0 ± 11.5 | 655 (67%) |
Evaluate the clinical impact of visceral fat accumulation on the cerebrovascular lesions | Japanese aged ≥ 40 years | NA | BMI | WHR CT scan |
WHR CT scan |
| Mancusi et al. [32] | 54.0 ± 11.5 | 5104 (57.9%) |
Impact of obesity on carotid target organ damage | Hypertensive patients without prevalent cardiovascular disease | Cardiovascular disease: myocardial infarction, angina, coronary revascularization, stroke, transitory ischemic attack, or congestive heart failure requiring hospitalization | BMI | NA | NA |
| Nishizawa et al. [33] | 64.8 ± 15.3 | 151 (62.9%) | Investigate the association between abdominal visceral fat with atherosclerosis in the aorta, coronary, carotid, and cerebral arteries in an autopsy study | Aged ≥ 30 years | Family provided inconsistent information during the clinical interview, the family had less than weekly contact with the deceased, the next of kin was unable to participate due to emotional suffering, subjects who had lost 10% or more of regular weight during the six months prior to death, arteries or visceral fat was retained at autopsy by the pathologist, subjects with post mortem interval ≥ 24 h, and subjects with signs of body autolysis according to the Crossley criteria |
NA | Omental, mesenteric, mesocolon, and perirenal fat were dissected after the autopsy and weighed. The VAT was the sum of the omental, mesenteric, mesocolon and perirenal fat | NA |
| Omisore et al [34]. | 52.0 ± 15.1 | 80 (49.4%) |
Evaluated the impact of traditional cardiovascular risk factors on carotid atherosclerosis in a sample of Nigerian adults | Adults aged 18 years and older | NA | BMI | NA | NA |
| Imahori et al. [35] | (60–72) | 2184 (44.5%) |
Evaluate the associations between adiposity measures and the presence of carotid plaque | Right carotid artery | NA | BMI | WC, WHR, WHtR |
WHR, WHtR |
| Laugesen et al. [36] | 59 ± 9.4 | 86 (50.9%) |
Assess plaque composition by carotid magnetic resonance imaging | Age > 18 years, diagnosis of Type 2 diabetes mellitus and known duration of diabetes mellitus < 5 years | Acute or chronic infectious disease, end-stage renal failure, pregnancy or lactation, prior or present cancer, and contraindications to MRI (including body weight > 120 kg). | Fat percentage was assessed by whole-body dual-energy X-ray absorptiometry. BMI |
WHR | WHR |
| Yoshida et al. [37] | 61.8 ± 11.9 | 60 (17%) |
Determine the association between obesity and/or VAT and the risk for atherosclerosis | Age > 18 years, Japanese rheumatoid arthritis patients | Presence of an internal or external electronic device, severely degraded health status, presence of fractures and pain preventing assessment of visceral and subcutaneous fat, WC < 57 cm, poor physical health on the day of examination, and concurrent cancer and hepatitis treatment, dialysis, and/or sex-hormone suppression or replacement therapy | BMI | Bioimpedance | Bioimpedance |
| Scicali et al. [38] | 56.8 ± 8.0 | 183 (66.3%) | Investigate the presence of carotid plaque in overweight patients | BMI: 25–29.9 kg/m2, age: 40–70 years, and at least one cardiovascular risk factor (hypertension, dyslipidemia, or current smoking) | Previous history of diabetes, coronary heart disease, cerebrovascular disease, peripheral artery disease, or clinical evidence of advanced renal disease | BMI | WHR WC |
NA |
| Rovella et al. [4] | 69.8 (7.2) | 273 (70%) |
Evaluate by histology the role of obesity in the destabilization of carotid plaques | Submitted to carotid endarterectomy AND Symptomatic patients with thrombo-embolism due to carotid atherosclerosis OR Asymptomatic patients with carotid stenosis ≥ 60% | Cardiac source of embolization, stenosis greater than 50% of Willis circle |
BMI | NA | NA |
| Geraci et al. [39] | 58 ± 14 | 279 (59.6%) |
Relationship between anthropometric indices of adiposity and carotid atherosclerosis | Caucasian patients, age: 30–80 years with essential hypertension | BMI > 40 kg/m2, Renovascular, endocrine, or malignant hypertension, Carotid thromboendarterectomy and/or percutaneous carotid angioplasty, Pre-existing cardiovascular comorbidities |
BMI BSA |
ABSI BRI WC |
WHtR |
| Haberka et al. [40] | 61.8 ± 8 | 255 (65.2%) | Association between obesity, fat depots, and carotid artery stenosis in patients with high cardiovascular risk | Scheduled for elective coronary angiography | Heart failure, severe primary heart valve disease or any other extracardiac chronic disease causing at least 10% unintentional weight loss (prior 3 months), secondary causes of obesity or medical intervention aimed at weight loss, neck or abdomen surgery, neck radiotherapy, a very poor carotid artery image quality, and confirmed diagnosis of a genetic predisposition for CV diseases | BMI | Ultrasound WC |
Ultrasound |
Table 3.
Data collected about carotid plaque and conclusion.
| Study | Carotid Plaque | Conclusion | |||
|---|---|---|---|---|---|
| Evaluation Method | Definition | Grade | Symptomatic | ||
| Bogousslavsky et al. [5] | Angiography US doppler |
Defined on radiological clinic grounds and confirmed pathologically in patients who underwent endarterectomy | Determined | Symptomatic (157 patients) Asymptomatic (320 patients) |
Obesity was significantly more frequent in patients with internal artery occlusion or stenosis than in controls |
| Lakka et al. [6] | Ultrasound | Plaque height was calculated as the average of the differences between the maximal and minimal IMT of the right and left common carotid artery and was used as an indicator of how steeply atherosclerotic lesions protruded into the lumen | NA | Asymptomatic | Abdominal obesity, as indicated by high WHR and by high WC, is associated with accelerated progression of carotid atherosclerosis independent of overall obesity and other risk factors in middle-aged men with no prior atherosclerotic diseases |
| Hunt et al. [7] | US doppler | Focal widening of the IMT relative to the adjacent wall segment, measuring at least 1.5 mm in thickness | NA | Symptomatic and asymptomatic | The prevalence of carotid artery plaque increased with WC and decreased with BMI |
| Hegazi et al. [8] | Ultrasound | Plaque was defined as a distinct area of hyperechogenicity and/or protrusion into the lumen of the vessel with at least 50% greater thickness than the surrounding area | Plaque was defined as a distinct area of hyperechogenicity and/or protrusion into the lumen of the vessel with at least 50% greater thickness than the surrounding. For each segment, the degree of plaque was graded as follows: 0 = no plaque, 1 = 1 small plaque. <30% of vessel diameter, 2 = 1 medium plaque between 30% of vessel diameter or multiple small plaques, and 3 = 1 large plaque >50% of the vessel diameter or multiple plaques with at least 1 medium plaque. The grades were summed across the right and left carotid arteries to create an overall measure of the extent of focal plaque | Asymptomatic | Measures of adiposity were not significantly different in patients with higher plaques score |
| Czernichow et al. [9] | Ultrasound | Localized eco structures encroaching upon the vessel lumen for which the distance between the media-adventitia internal side of lesion was >1 mm | NA | NA | No association was found between the presence of carotid plaques and body composition |
| Hadjiev et al. [10] | Duplex scanning was employed | NA | Classification of carotid stenosis according to NASCET | Asymptomatic | Obesity was not associated with asymptomatic carotid stenosis of 50% or greater |
| De Souza et al. [11] | Ultrasound | Distinct area of hyperechogenicity and/or a focal protrusion of the vessel wall into the lumen | NA | Symptomatic (11 patients) Asymptomatic (133 patients) |
The prevalence of carotid artery plaque was significantly associated with obesity |
| Montalcini et al. [12] | US doppler | Was defined as an echogenic focal structure encroaching the vessel lumen with a distinct area 50% greater than the intima-media thickness of neighboring sites. Stenosis was defined as a peak systolic velocity >120 cm/s, and occlusion was defined as the absence of a Doppler signal | NA | Asymptomatic | BMI was not associated with carotid atherosclerosis Women with metabolic syndrome who were overweight or obese had approximately three times higher adjusted odds of having carotid atherosclerosis |
| Lear et al. [3] | Ultrasound | Focal plaques were identified as wall thickness that was increased compared with the surrounding IMT | NA | Asymptomatic | There were no differences in BMI, total fat mass, percent body fat, total abdominal adipose tissue, and SAT in those with versus without carotid plaques. In those with plaques, VAT was a significant, independent predictor of plaque area after adjusting for age, sex, ethnicity, education, household income, family history of CVD, smoking, and percent body fat |
| Park et al. [13] | Magnetic resonance | Degree of luminal narrowing of ≥ 50% | NA | Symptomatic | None of the metabolic syndrome components were shown to be associated with extracranial internal carotid artery stenosis |
| Irace et al. [14] | US doppler | Localized lesion encroaching the lumen of thickness at least 1.3 mm, no spectral broadening or only in the deceleration phase of systole and systolic peak velocity less than 120 cm/s. Stenosis was defined as spectral broadening throughout systole and/or peak flow velocity of at least 120 cm/s | NA | NA | Overweight and obesity, however, do not independently associate with carotid atherosclerosis |
| Yu et al. [15] | Ultrasound | Plaque was defined as a focal wall thickening of at least 1.5 mm | The degree of plaque at the six segments was graded according to the following criteria: grade 0, no observable plaque, grade 1, one small plaque < 30% of vessel diameter, grade 2, one medium plaque between 30% and 50% of the vessel diameter or multiple small number plaques, and grade 3, one large plaque > 50% vessel diameter or multiple plaques with at least one medium plaque | Asymptomatic | A high WHR was independently associated with the presence of plaque |
| Terzis et al. [16] | Ultrasound | Plaque was defined as a focal structure encroaching into the arterial lumen of at least 0.5 mm or 50% of the surrounding IMT value, or a thickness of 1.5 mm as measured from the media-adventitia interface to the intima-lumen interface | NA | Asymptomatic | The presence of atheromatous plaques was independently associated with BMI |
| Kadoglou et al. [17] | US doppler | Localized thickening of the vessel wall of more than 2.5 mm | Classification of carotid stenosis according to the recommendations of the Society of Radiologists in Ultrasound | Symptomatic (35 patients) Asymptomatic (51 patients) |
Increased fat mass correlated with carotid plaque vulnerability, as expressed by the gray scale median score |
| Solomon et al. [18] | Ultrasound | Focal structure that encroaches into the arterial lumen of a least 0.5 mm or 50% of the surrounding intima-media thickness value or demonstrates a thickness of >1.5 mm as measured from the media-adventitia interface to the intima-lumen interface | NA | NA | WHR was significantly related to carotid artery plaque in African Caucasian women, whereas none of the obesity measures were associated with carotid artery plaque in black women |
| Rodríguez-Flores et al. [19] | Histopathological study | Classification of atherosclerosis lesions according to the American Heart Association | NA | NA | BMI did not independently predict the risk of development of advanced lesions |
| Maksimovic et al. [20] | Ultrasound | NA | Classification of carotid stenosis according to NASCET | Symptomatic | Patients with and without abdominal obesity did not significantly differ, either in the degree of carotid stenosis or in the degree of its clinical manifestation |
| Galarza-Delgado et al. [21] | Ultrasound | Focal structure that invades the lumen of the artery by at least 0.5 mm or 50% of the value of intima-media thickness, or when the thickness is equal to or greater than 1.5 mm when measured from the adventitia-media interphase to the intima-arterial lumen interphase | NA | NA | Presence of plaque was associated with abdominal circumference |
| Cuspidi et al. [22] | Ultrasound | NA | NA | Asymptomatic | No association was found between abdominal obesity and carotid plaque |
| Chiquete et al. [23] | US doppler | NA | Classification of carotid stenosis according to NASCET | Symptomatic (30 patients) Asymptomatic (503 patients) |
There was no association between obesity and carotid stenosis ≥ 50% or between obesity and symptomatic carotid stenosis |
| Irie et al. [24] | Ultrasound | Focal structure encroaching into the arterial lumen or demonstrating a thickness >1.0 mm as measured from the media–adventitia interface to the intra-lumen interface | NA | Asymptomatic | The presence of echolucent carotid plaques with low gray-scale median values was related to high BMI |
| Yan et al. [25] | US doppler | Focal encroachment of internal carotid artery walls on either side | NA | Asymptomatic | There was no significant association between abdominal obesity or overweight/obesity with carotid plaques |
| Yuan et al. [26] | CT scan | Calcium mass score | NA | NA | No association was found between BMI, WC, and VAT, SAT determined on CT scan and carotid calcification |
| Pan et al. [27] | Ultrasound | NA | NA | NA | In females, the prevalence of carotid atherosclerosis was significantly higher in obese than in the control group |
| Radmard et al. [28] | Ultrasound | Localized thickening of >1.2 mm, not involving the whole circumference of the artery | NA | NA | Subjects with the highest amount of VAT were more prone to have more than one carotid plaque in comparison with participants showing the highest values of SAT or other conventional anthropometric indices |
| Sandfort et al. [29] | Magnetic resonance | NA | Total wall volume measurements | NA | Obesity was associated with the progression of carotid atherosclerosis in a low- to moderate-risk population treated with optimal statin therapy |
| Mitevska et al. [30] | US doppler | Detection of an IMT > 1.3 mm or a focal structure emerging from the wall of at least 0.5 mm or 50% of the surrounding IMT value | Carotid stenosis greater than 60% was considered significant | Asymptomatic | Multivariate analysis showed that obesity was not an independent predictor for the presence of carotid plaques |
| Higuchi et al. [31] | US doppler | NA | Stenosis was regarded as significant if stenosis rate ≥70 | Asymptomatic | Visceral fat ≥ 100 cm2 was independently associated with cervical plaque. BMI and WHR were not |
| Mancusi et al. [32] | Ultrasound | IMT ≥ 1.5 mm | NA | Asymptomatic | Obesity was associated with a modestly increased prevalence of carotid plaques |
| Nishizawa et al. [33] | Autopsy | The largest atheroma plaque in the carotid artery was determined to calculate the stenosis index | The stenosis index was calculated by subtracting the lumen area from the outer area, dividing the difference by the outer area, and multiplying the result by 100 | NA | Visceral fat was not associated with carotid artery stenosis index |
| Omisore et al. [34] | Ultrasound | Plaque was defined as focal thickening of at least 50% greater than that of the surrounding vessel wall, with a minimum thickness of at least 1.5 mm | NA | NA | Carotid plaques were associated with obesity |
| Imahori et al. [35] | Ultrasound | Localized protrusion of the vessel wall into the lumen of at least 50% compared with the adjacent intima-media thickness | NA | NA | BMI, WC, and WHtR were not associated with the presence of carotid plaques. The main measure of central obesity (WHR) showed the strongest and most consistent association with plaque presence and with plaque area |
| Laugesen et al. [36] | Magnetic resonance | NA | Carotid artery plaque burden was measured as maximum wall thickness derived from the lumen area and total vessel area outlines, maximum wall area, and maximum normalized wall index | Asymptomatic | Obesity was associated with increased carotid plaque necrotic core volume and calcification |
| Yoshida et al. [37] | Ultrasound | Localized elevated lesions with a maximum thickness of more than 1 mm | NA | NA | Visceral adiposity is an independent predictor of atherosclerosis |
| Scicali et al. [38] | Ultrasound | IMT greater than 1.5 mm | NA | Asymptomatic | The presence of carotid plaque was associated with high WHR |
| Rovella et al. [4] | Histology | Collected at carotid endarterectomy | NA | Symptomatic (265 patients) Asymptomatic (125 patients) |
Obesity is an independent risk factor for carotid plaque destabilization |
| Geraci et al. [39] | US doppler | Focal structure encroaching into the arterial lumen of at least 0.5 mm or 50% of the surrounding carotid IMT value or carotid IMT > 1.5 mm | NA | Asymptomatic | ABSI was the only anthropometric adiposity index independently associated with the presence of carotid atherosclerotic plaque |
| Haberka et al. [40] | US doppler | Presence of plaques in the common carotid artery, bulb, and internal carotid artery | Classification of carotid stenosis according to NASCET | NA | None of the obesity measurements revealed an association with carotid atherosclerosis severity |
Concerning the adipose tissue, the following information was recorded: the method used to determine the overall obesity, the VAT, and SAT (Table 3).
The information collected about carotid stenosis were: imaging methods used to determine the presence of carotid stenosis; the definition of carotid plaque; the grade of carotid stenosis; and if the carotid plaque was symptomatic (Table 3).
The quality of each study was assessed by one author (J. F.) using the MINORS (methodological index for non-randomized studies) criteria (see supplement material) [41]. Each item was scored as 0 (not reported), 1 (reported but inadequate), or 2 (re-ported and adequate). The global ideal score is 16 for non-comparative studies and 24 for comparative studies [41]. The final classification is presented in Table 1.
3. Results
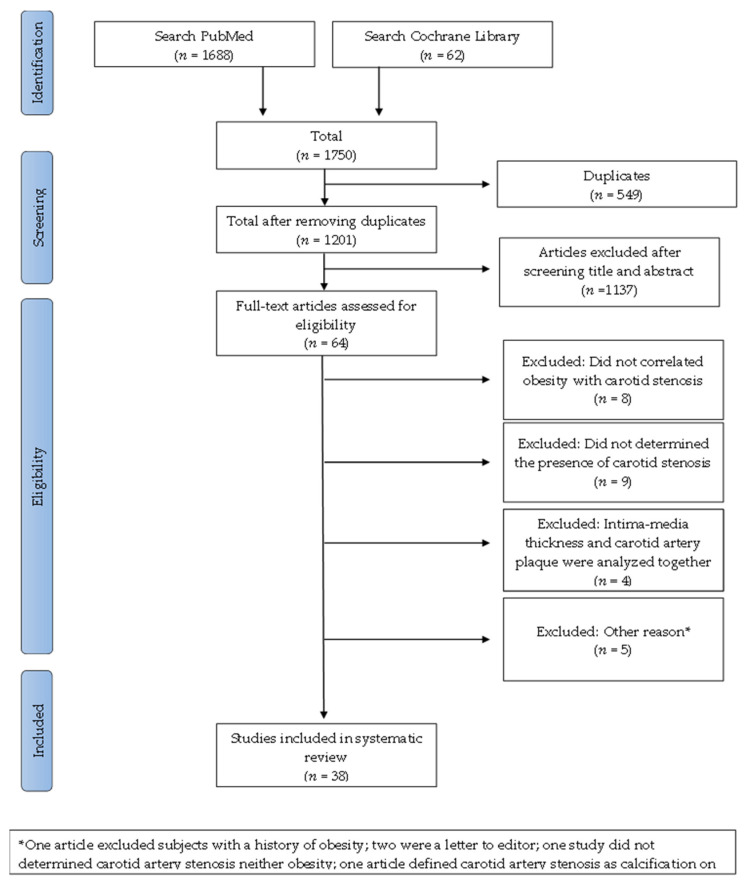
A total of 1750 articles were found, and after removing duplicates, 1201 articles were left, as shown in Figure 1 [42,43,44,45,46,47,48,49].
Figure 1.
Flow diagram for this opportunistic review that aims to analyze the correlation between obesity and carotid atherosclerosis.
Of the 64 papers, 26 were rejected after reading the full text due to the following reasons: eight articles did not correlate obesity with carotid artery stenosis [42,43,44,45,46,47,48,49]; Nine did not determine the presence of carotid artery stenosis [50,51,52,53,54,55,56,57,58]; In four articles, intima-media thickness and carotid artery plaque were both correlated with obesity [59,60,61,62]; One article excluded subjects with a history of obesity [63]; Two papers were a letter to the editor [52,64]; One study did not measure carotid artery stenosis or obesity [65]; One article defined carotid artery stenosis as calcification seen on radiographs [66].
The 38 studies included in this opportunistic review were published in 1985 (one article) and between 2001 and 2019. The studies were conducted in 32 different countries, the majority in Europe (18 papers), eight studies in North America, eight in Asia, two in South America, and two in Africa (Table 1).
Analyzing the studies included in this opportunist review by regions, there was a higher number of papers describing a positive association between obesity and carotid plaque characteristics in the research work performed in Asia and Africa. However, just two papers were identified from Africa. The majority of papers included were from Europe, where 10 papers found a relationship and an equal number did not.
-
-
The majority of the studies (34) were cross-sectional, and four were longitudinal.
-
-
A total of 35,339 subjects (49.20% men) were included: 26,492 from Europe, 4239 from Asia, 3859 from North America, 384 from South America, and 365 from Africa (Table 1). Some studies only included a specific group of patients: Type 2 diabetes (5 papers), women (4), patients with an autoimmune disease (4), or hypertension (3) (Table 2).
The authors used different methods to determine the quantity of adipose tissue and to characterize the atherosclerotic plaque. The most frequent method used to determine obesity was anthropometric measures: Thirty-three articles assessed overall obesity with BMI, 19 evaluated visceral obesity with WC, and 11 estimated the relationship between the visceral and subcutaneous adipose tissue with WHR (Table 2). Six authors used medical imaging to quantify the VAT and SAT: Four authors used CT scans [3,8,26,31], one author used ultrasound [40], and another MRI [28]. Other methods were used: bioimpedance (three papers) [9,17,37]; dual-energy X-ray absorptiometry (two) [3,36]; and DXA (one) [8] (Table 2). In one paper, omental, mesenteric, mesocolon, and perirenal fat were dissected after the autopsy and weighed [33]. The VAT was the sum of the omental, mesenteric, mesocolon, and perirenal fat [33].
The carotid plaque was evaluated with ultrasound in 19 articles, with doppler ultrasound in 12 papers, and with MRI in three articles (Table 3).
Two articles analyzed the histological composition of the carotid plaque (number of macrophages, foam cells, cap characteristics, and the quantity of lipids and calcium) [4,19]. One study determined the area of the cadavers of the largest atheroma plaque in the carotid artery of cadavers to calculate the stenosis index [33].
The carotid plaque was defined in most papers as a focal structure encroaching the vessel lumen or as a widening in the intima media-thickness (Table 3). However, there was no homogeneity in the definition, and different publications used different measures of intima-media thickness to define a plaque (Table 3). Sixteen articles only analyzed asymptomatic carotid plaques, six papers studied both symptomatic and asymptomatic, and two articles symptomatic. Fourteen articles did not specify if the carotid artery caused symptomatology. (Table 3). This opportunistic review included 1533 symptomatic and 19,799 asymptomatic patients.
3.1. Overall Obesity and the Prevalence of Carotid Plaques
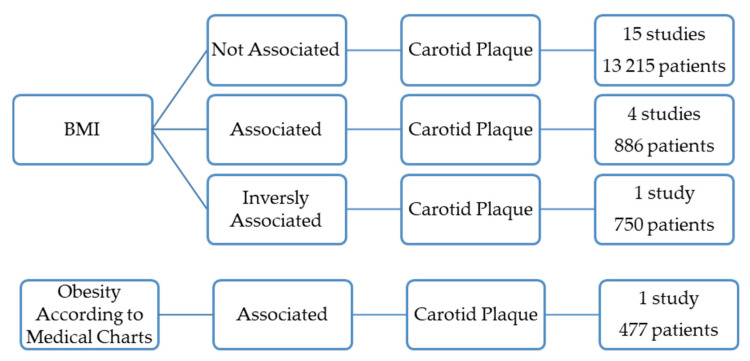
Overall obesity, determined by BMI, was not associated with the presence of carotid plaques in 15 papers, totaling 13,215 patients (Figure 2) [3,9,10,12,14,15,18,21,25,28,30,31,35,39,40].
Figure 2.
Results of the studies that correlate overall obesity with the presence of the carotid atherosclerotic plaques.
Five studies (1363 patients) found, however, a positive association between the prevalence of carotid plaques and overall obesity (Figure 2) [5,11,16,27,34].
One of these was a prevalence study conducted on 474 healthy residents in Northeast China [27]. This study sought to determine the risk factors associated with carotid atherosclerosis identified by ultrasound. However, there was no definition of atherosclerotic plaque [27]. The prevalence was significantly higher in obese females than in the control females. Obesity was defined as BMI ≥ 28 kg/m2 [27]. The study included 231 males, and no association was found for this gender [27].
Another study conducted on 144 women with systemic lupus erythematous found that the prevalence of carotid artery plaque was significantly associated with obesity, determined by BMI [11].
The oldest study included in this review included 477 patients who performed angiography or Doppler ultrasound and concluded that obesity was significantly more frequent in patients with internal artery occlusion or stenosis than in controls. Obesity was defined according to medical charts [5].
Two papers totaling 268 patients found that the prevalence of carotid plaques was associated with overall obesity determined by BMI [16,34].
However, one paper with 750 individuals concluded that the prevalence of carotid artery plaques was inversely related to BMI (Figure 2) [7].
3.2. Overall Obesity and the Characteristics of the Carotid Plaques
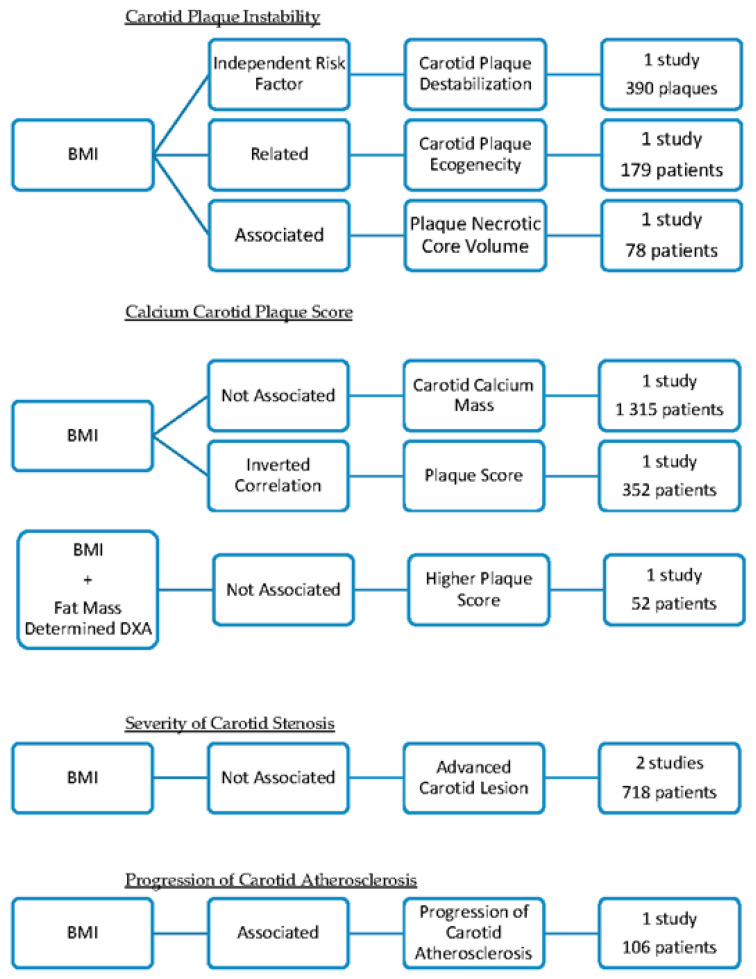
Three papers suggested that overall obesity could be associated with carotid plaque instability (Figure 3) [4,24,36].
Figure 3.
Summary of the studies that correlate overall obesity with the characteristics of the carotid atherosclerotic plaques.
-
(1).
Histological analysis of carotid plaques (390) concluded that obesity, defined as BMI ≥ 30 kg/m2 was an independent risk factor for carotid plaque destabilization, particularly in males [4]. Obesity was correlated with the presence of unstable carotid plaques, characterized by a high degree of inflammation, thinning, and rupture of the cap [4].
-
(2).
One study analyzed the plaque echogenicity by gray-scale median using ultrasound and concluded that low gray-scale median values were related to high BMI [24]. Plaques with a low gray-scale median had a higher probability of causing embolization and symptoms. This research included 179 diabetic patients [24].
-
(3).
One research paper found that obesity (BMI > 30.0 kg/m2) was associated with increased carotid plaque necrotic core volume and calcification independently of diabetes mellitus status [36]. The carotid plaque composition was assessed by magnetic resonance imaging. Obesity was determined by BMI [36]. The study included 78 patients with short-duration Type 2 diabetes mellitus and 91 sex- and aged-matched control subjects [36].
This opportunistic review identified three articles that found that obesity did not correlate with calcium score or carotid plaque score (Figure 3) [8,26,37].
-
(1).
No association was found between BMI and calcium mass score of the carotid arteries determined with a CT scan [26]. This study was performed on 1315 diabetic patients [26].
-
(2).
One study found that the measures of adiposity (BMI, the systemic fat mass, and the fat-free mass determined with DXA) were not significantly different in patients with higher plaques score [8]. For each segment, the degree of plaque was graded as follows: 0 = no plaque; 1 = 1 small plaque. <30% of vessel diameter; 2 = 1 medium plaque between 30% of vessel diameter or multiple small plaques; and 3 = 1 large plaque >50% of the vessel diameter or multiple plaques with at least 1 medium plaque [8]. The grades were summed across the right and left carotid arteries to create an overall measure of the extent of focal plaque [8]. This study included 52 patients [8].
-
(3).
One paper found an inverse correlation between BMI and plaque score, defined as the total plaque thickness for the visualization sites in the IMT measurement on the right and left size [37]. The study was performed on 352 rheumatoid arthritis patients [37].
Two different papers did not find any relation between BMI and the severity of atherosclerotic plaque (Figure 3) [19,23].
-
(1).
A histological study analyzed the relationship between obesity and the severity of atherosclerosis carotid plaque [19]. The atherosclerotic lesions were described according to the American Heart Association classification [19]. BMI did not independently predict the risk of developing advanced carotid atherosclerotic lesions (including 185 cadavers of men and women) [19].
-
(2).
No association was found between obesity (BMI > 27 kg/m2) and carotid stenosis ≥ 50% (determined with NASCET criteria) in 533 patients [23]. In this study, there was no association between obesity and symptomatic carotid stenosis [23].
Another paper concluded that obesity (determined by BMI) was associated with the progression of carotid atherosclerosis, studied with magnetic resonance [29]. This research project determined the change in the carotid artery wall in 106 hyperlipidemic participants during the course of treatment with statins (Figure 3) [29].
3.3. Visceral Adipose Tissue and the Prevalence of Carotid Plaque
Nineteen articles analyzed the relationship between VAT and carotid stenosis.
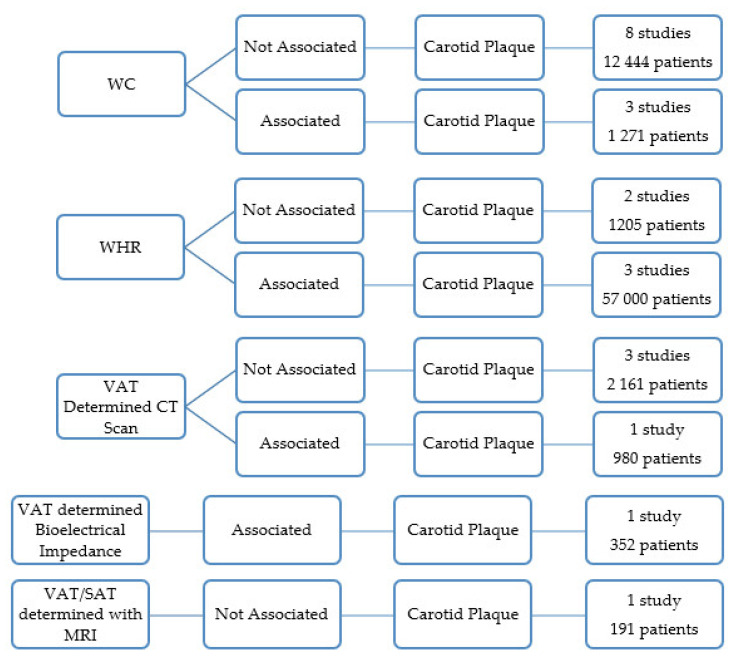
Eight papers performed in 12,444 patients showed no association between waist circumference (WC) and the presence of carotid plaques [9,13,15,16,22,25,35,39,40]. Three studies, however, found that the presence of plaque was associated with WC in 1271 subjects (Figure 4) [7,21].
Figure 4.
Results of the papers correlating visceral obesity with the presence of the carotid atherosclerotic plaques.
Three other studies that included 5 700 subjects found that WHR was associated with the prevalence of carotid plaques (Figure 4) [15,35,38].
WHR was significantly related to the presence of carotid artery plaque in African Caucasian women, whereas none of the obesity measures were associated with carotid artery plaque in black women [18]. The study was limited to 203 African black and African Caucasian women who met the American College of Rheumatology criteria for rheumatoid arthritis (Figure 4) [18].
In two research works, there was no association between WHR and the presence of carotid plaques (1205 subjects) (Figure 4) [9,28].
A research work analyzed the relationship between new anthropometric measures that reflected the quantity of abdominal adipose tissue and the carotid plaque [39]. The anthropometric measures were the A Body Shape Index (ABSI) and Body Roundness Index (BRI) [39]. ABSI was independently associated with the presence of carotid atherosclerotic plaque. The study included 468 subjects with arterial hypertension [39].
Four articles used CT scans to quantify the VAT and another used MRI [3,8,26,28,31]. The VAT area determined with a CT scan in 980 healthy Japanese was independently associated with cervical plaque [31]. In three studies with 2161 subjects, the VAT area determined with a CT scan was not associated with the presence of carotid plaques [3,8,26]. One paper published the relationship between VAT and SAT as determined by MRI and the presence of carotid plaque [28]. The study included 191 subjects, and no relation was found between the variables (Figure 4) [28].
One paper concluded that VAT measured with bioelectrical impedance was associated with a higher prevalence of atherosclerotic carotid plaques [37]. This study was limited to 352 patients with rheumatoid arthritis (Figure 4) [37].
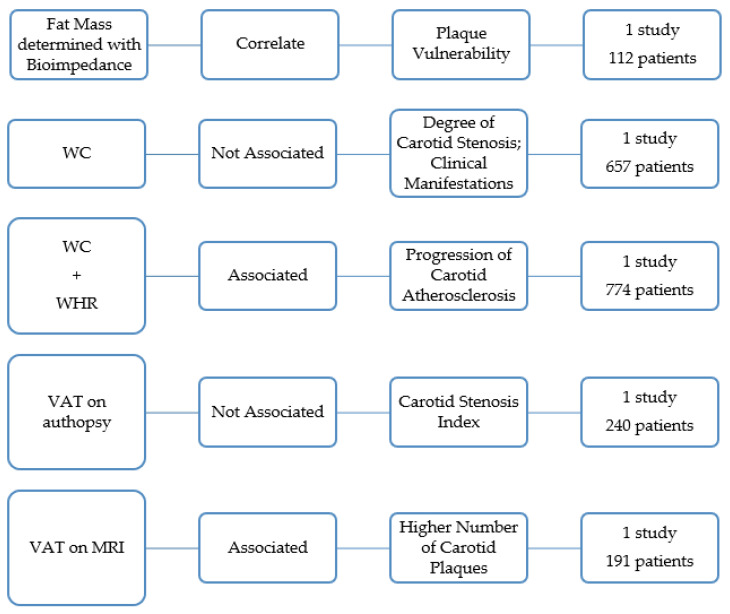
3.4. Visceral Adipose Tissue and the Characteristics of the Carotid Plaque
A research work of 112 subjects found that increased fat mass (determined with bioimpedance) correlates with carotid plaque vulnerability, as expressed by the gray scale median score (Figure 5) [17].
Figure 5.
Overview of the studies that analyze the association between visceral obesity and the characteristics of the carotid atherosclerotic plaques.
A research work determined WC in 657 patients with symptoms of cerebral ischemia and carotid stenosis of ≥50% [20]. The author concluded that patients with and without abdominal obesity did not significantly differ either in the degree of carotid stenosis or in the degree of its clinical manifestation [20]. Haberka also reached a similar conclusion determining the central obesity with WC and ultrasound [8]. In this study, the amount of visceral fat was not related to carotid stenosis severity (Figure 5) [40].
A study in 774 men without atherosclerosis showed that abdominal obesity, as indicated by high WHR and high WC, was associated with accelerated progression of carotid atherosclerosis independent of overall obesity and other risk factors in middle-aged men with no prior atherosclerotic diseases (Figure 5) [6].
An autopsy study with 240 deceased subjects concluded that VAT was not associated with carotid artery stenosis index [33]. The VAT was the sum of the weight of the omental, mesenteric, mesocolon and perirenal fat [33]. The largest atheroma plaque in the carotid artery was determined [33]. The stenosis index was calculated by subtracting the lumen area from the outer area, dividing the difference by the outer area, and multiplying the result by 100 (Figure 5) [33].
One paper showed that subjects with the highest amount of VAT were more prone to have more than one carotid plaque compared to participants showing the highest values of SAT or other conventional anthropometric indices [28]. The quantity of VAT and SAT was quantified by MRI in 191 subjects (Figure 5) [28].
3.5. Subcutaneous Adipose Tissue and Carotid Plaque
Six studies evaluated SAT, and none found any relationship with carotid plaques [3,8,26,28,31,40].
4. Discussion and Conclusions
To the best of our knowledge, this is the first opportunistic review analyzing the relationship between obesity and carotid artery disease.
The general assumption emerging from our analysis suggests no association between overall obesity (determined with BMI) or visceral obesity (determined in the majority of the studies with WC) and the presence of carotid plaque. However, three studies (5700 patients) found that WHR was associated with the prevalence of carotid plaques [15,35,38]. BMI is not always a measure of fatness. Individuals with more muscle mass may be incorrectly classified as obese [67]. BMI has poor specificity for excess adiposity, and it does not characterize the excess of centrally distributed obesity [68]. Low BMI values may also be associated with the loss of lean body mass (muscle) [68]. In contrast with BMI, WC and WHR specifically address abdominal obesity and correlate better with overall atherosclerotic disease prevalence [68]. WC and WHR may correlate better with body fatness [67] and may more accurately reflect the additional risk conferred by obesity [68]; actually, they are more associated with mortality and cardiovascular events than BMI [68].
Although WC is well-described as a measure of VAT and a marker of obesity’s associated metabolic risks, WHR has superior performance in estimating atherosclerotic risk [68]. One possible explanation is that WHR is an indexed value (to lower body girth) and provides a more precise assessment of relative central adiposity across the spectrum of body size, compared with WC [68]. WHR is considered the main anthropometric measure of central obesity [15,35,38]. Another explanation is that increased hip circumference may protect against atherosclerosis [68]. Fat in the lower body may function as a protective reservoir against ectopic (abdominal) adiposity [68]. WHR is independently associated with prevalent atherosclerosis and provides better dis- crimination than either BMI or WC [68].
There is a correlation between the WHR and the ratio of VAT-to-SAT cross-sectional area (quantified by CT images taken in the abdominal region) [69]. VAT is an endocrine organ that can secrete adipokines, including cytokines and chemokines [70,71]. VAT is associated with cardiovascular disease and can be used as a cardiometabolic risk marker, while SAT has a beneficial metabolic impact in the opposite direction [71]. Abdominal visceral fat correlates with the prevalence of coronary artery disease and mortality. Subcutaneous fat may play a protective role against the development of coronary artery disease by improving insulin sensitivity or the secretion of adipokines. The VAT/SAT ratio is a unique parameter relevant to vascular inflammation or poor cardiovascular outcomes [71,72]. VAT/SAT ratio is associated with a higher total mortality and incidence of MACE, independently of traditional vascular risk factors, and with the presence of obstructive coronary artery disease [2].
In this review, we found a relationship between obesity and other characteristics of carotid atherosclerotic plaque. Overall obesity (738 patients) [4,24,36] and an increase in fat mass (112 patients) [17] were associated with carotid plaque instability. Obesity and visceral fat (880 patients) are correlated with the progression of carotid atherosclerosis [6,29]. However, no study related obesity to carotid artery symptomatology.
Studies conducted with cardiac patients found that obesity was also positively associated with the progression of coronary plaque [72,73]. Excess VAT is correlated with higher serum levels of fasting glucose, triglycerides, lower HDL cholesterol, greater prevalence of hypertension, tobacco use, and artery inflammation [72,73]. The systemic inflammation causes endothelial dysfunction, formation, and progression of atherosclerotic plaques [73,74]. Importantly, inflammation is also responsible for atherosclerotic plaque instability [43]. These biological facts could also explain why in this opportunistic review, we found that obesity did not correlate with calcium score, carotid plaque score [8,26,37], or the severity of atherosclerotic plaque [19,20,23,40].
None of the studies included in this review found any relationship between SAT and carotid plaques [3,8,26,28,31,40]. Further studies to characterize the subcutaneous fat should be developed.
4.1. Strengths and Limitations
A key strength of this review is the standardized data extraction, the quality assessment procedures, and searches conducted by two authors. This manuscript covers a broader range of patients and countries from 1985 to 2019 (most articles were published in the last 10 years). It includes published research in English, French, Portuguese, and Spanish language journals. The main limitation of this revision is that it did not conduct a meta-analysis. The articles included are not homogenous. There are different definitions of carotid plaque and obesity. The authors included different subjects and used different methods to determine obesity and carotid plaque. The opportunistic review included observational studies, and its bias was not entirely avoided.
Another bias of this study is the inability to determine the impact of obesity on atherosclerotic plaque independently of the cardiovascular risk. Obesity can increase the prevalence of certain factors such as dyslipidemia which contributes to atherosclerosis. However, there was no description of cardiovascular risk factors in all included papers.
Another limitation is that two research questions were not answered: (i). We did not find the relation between VAT and symptomatic carotid artery disease (ii). neither was the role played by subcutaneous tissue in carotid stenosis.
4.2. Implications for Practice
The results of the current investigation provide key information for practice. First, the importance of determining the WHR to infer the relation between VAT and SAT. The BMI, more frequently used in clinical activity, can erroneously represent the cardiovascular risk. Secondly, it may be useful to address obese patients to increase lean body mass. This study also has an impact on future research. The authors should use rigorous methods to determine obesity to homogenize the results, and facilitate the comparisons accurately. The role of subcutaneous tissue in carotid atherosclerosis still needs more investigations to clearly determine its role in vascular diseases. The relationship between obesity, subcutaneous fat, and carotid symptomatology should be more deeply investigated. Behavioral and pharmacological interventions could be developed to decrease the VAT/SAT ratio. Studies focused on obesity and inflammation could be important in atherosclerosis control.
4.3. Concluding Remarks
Considering the data analyzed, obesity and visceral obesity were not associated with the presence of carotid plaque. The ratio between VAT and SAT could influence the prevalence of carotid plaques. Obesity could be related to carotid plaque instability and progression, but its association with carotid symptomatology has not been proved and should be investigated in future studies.
Acknowledgments
We thank Rita Alonso for structuration of this article.
Abbreviations
BMI—body mass index; BSA—body surface area BRI—body roundness index; ABSI—a body shape index; CT scan—computerized tomography scan; DXA—Dual-energy X-ray absorptiometry; HC—hip circumference; IMT—intima-media thickness; MACE—major adverse cardiac events; MINORS—methodological index for non-randomized studies; MRI—magnetic resonance imaging; NA—not applicable; NASCET—North American symptomatic carotid endarterectomy trial; VAT—visceral adipose tissue; SAT—subcutaneous adipose tissue; WC—waist circumference; WHR—waist-to-hip ratio; WHtR—waist-to-height ratio.
Author Contributions
Conceptualization, J.F.; Writing—original draft preparation, J.F. and A.C.; Formal Analysis, J.F. and A.C.; Investigation, J.F., P.C. and I.V.; Design, P.C.; Writing—review and editing, P.C., A.L.-F., A.M. (Amílcar Mesquita), J.C., A.M. (Armando Mansilha) and M.C.-N.; Validation, P.C., I.V., C.C., C.S., A.L.-F., A.M. (Amílcar Mesquita), J.C., A.M. (Armando Mansilha) and M.C.-N.; Supervision, A.M. (Armando Mansilha). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Portuguese Society of Vascular Surgery. This work was developed under the scope of project NORTE-01-0145-FEDER- 000013, supported by the Northern Portugal Regional Operational Programme (NORTE 2020) under the Portugal Partnership Agreement, through the European Regional Development Fund (FEDER), and by National funds, through the Foundation for Science and Technology (FCT)—project UIDB/50026/2020 and UIDP/50026/2020.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tanaka T., Kishi S., Ninomiya K., Tomii D., Koseki K., Sato Y., Okuno T., Sato K., Koike H., Yahagi K., et al. Impact of abdominal fat distribution, visceral fat, and subcutaneous fat on coronary plaque scores assessed by 320-row computed tomography coronary angiography. Atherosclerosis. 2019;287:155–161. doi: 10.1016/j.atherosclerosis.2019.06.910. [DOI] [PubMed] [Google Scholar]
- 2.Ladeiras-Lopes R., Sampaio F., Bettencourt N., Fontes-Carvalho R., Ferreira N., Leite-Moreira A., Gama V. The Ratio Between Visceral and Subcutaneous Abdominal Fat Assessed by Computed Tomography Is an Independent Predictor of Mortality and Cardiac Events. Rev. Española De Cardiol. 2017;70:331–337. doi: 10.1016/j.recesp.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Lear S., Humphries K., Kohli S., Frohlich J., Birmingham C., Mancini J. Visceral Adipose Tissue, a Potential Risk Factor for Carotid Atherosclerosis Results of the Multicultural Community Health Assessment Trial (M-CHAT) Stroke. 2007;38:2422–2429. doi: 10.1161/STROKEAHA.107.484113. [DOI] [PubMed] [Google Scholar]
- 4.Rovella V., Anemona L., Cardellini M., Scimeca M., Saggini A., Santeusanio G., Bonanno E., Montanaro M., Legramante I.M., Ippoliti A., et al. The role of obesity in carotid plaque instability: Interaction with age, gender, and cardiovascular risk factors. Cardiovasc. Diabetol. 2018;17:1–9. doi: 10.1186/s12933-018-0685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogousslavsky J., Regli F., Melle V. Risk factores and Concomitants of Internal Carotid Artery Occlusion or Stenosis. Arch. Neuril. 1985;42:864–867. doi: 10.1001/archneur.1985.04060080042014. [DOI] [PubMed] [Google Scholar]
- 6.Lakka T., Lakka M., Salomen R., Kaplan G., Salomen J. Abdominal obesity is associated with accelerated progression of carotid atherosclerosis in men. Atherosclerosis. 2001;154:497–505. doi: 10.1016/S0021-9150(00)00514-1. [DOI] [PubMed] [Google Scholar]
- 7.Hunt K.J., Duggirala R., Göring H.H., Williams J.T., Almasy L., Blangero J., O’Leary D.H., Stern M.P. Genetic Basis of Variation in Carotid Artery Plaque in the San Antonio Family Heart Study. Stroke. 2002;33:2775–2780. doi: 10.1161/01.STR.0000043827.03966.EF. [DOI] [PubMed] [Google Scholar]
- 8.Hegazi R.A., Sutton-Tyrrell K., Evans R.W., Kuller L.H., Belle S., Yamamoto M., Edmundowicz D., Kelley D.E. Relationship of Adiposity to Subclinical Atherosclerosis in Obese Patients with Type 2 Diabetes. Obes. Res. 2002;11:1597–1605. doi: 10.1038/oby.2003.212. [DOI] [PubMed] [Google Scholar]
- 9.Czernichow S., Bertrais S., Oppert J.-M., Galan P., Blacher J., Ducimetière P., Hercberg S., Zureik M. Body composition and fat repartition in relation to structure and function of large arteries in middle-aged adults (the SU.VI.MAX study) Int. J. Obes. 2005;29:826–832. doi: 10.1038/sj.ijo.0802986. [DOI] [PubMed] [Google Scholar]
- 10.Hadjiev D., Mineva P., Vukov M. Multiple modifiable risk factores for first ischemic stroke: A population-based epidemiological study. Eur. J. Neurol. 2003;10:577–582. doi: 10.1046/j.1468-1331.2003.00651.x. [DOI] [PubMed] [Google Scholar]
- 11.De Souza A. Atherosclerotic plaque in carotid arteries in systemic lupus erythematosus: Frequency and associated risk factors. São Paulo Med. J. 2005;123:137–142. doi: 10.1590/S1516-31802005000300010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montalcini T., Gorgone G., Gazzaruso C., Sesti G., Perticone F., Pujia A. Carotid atherosclerosis associated to metabolic syndrome but not BMI in healthy menopausal women. Diabetes Res. Clin. Pract. 2007;76:378–382. doi: 10.1016/j.diabres.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Park J.-H., Kwon H.-M., Roh J.-K. Metabolic syndrome is more associated with intracranial atherosclerosis than extracranial atherosclerosis. Eur. J. Neurol. 2007;14:379–386. doi: 10.1111/j.1468-1331.2007.01682.x. [DOI] [PubMed] [Google Scholar]
- 14.Irace C., Scavelli F., Carallo C., Serra R., Cortese C., Gnasso A. Body mass index, metabolic syndrome and carotid atherosclerosis. Coron. Artery Dis. 2009;20:94–99. doi: 10.1097/MCA.0b013e3283219e76. [DOI] [PubMed] [Google Scholar]
- 15.Yu R., Ho S., Chan S., Woo J., Ahuja A. Carotid atherosclerosis and the risk factors in early postmenopausal Chinese women. Maturitas. 2009;63:233–239. doi: 10.1016/j.maturitas.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Terzis I.D., Papamichail C., Psaltopoulou T., Georgiopoulos G.A., Lipsou N., Chatzidou S., Kontoyiannis D., Kollias G., Iacovidou N., Zakopoulos N., et al. Long-Term BMI Changes Since Adolescence and Markers of Early and Advanced Subclinical Atherosclerosis. Obesity. 2011;20:414–420. doi: 10.1038/oby.2011.137. [DOI] [PubMed] [Google Scholar]
- 17.Kadoglou N.P.E., Sailer N., Moumtzouoglou A., Kapelouzou A., Gerasimidis T., Kostakis A., Liapis C.D. Adipokines: A novel link between adiposity and carotid plaque vulnerability. Eur. J. Clin. Investig. 2012;42:1278–1286. doi: 10.1111/j.1365-2362.2012.02728.x. [DOI] [PubMed] [Google Scholar]
- 18.Solomon A., Norton G.R., Woodiwiss A.J., Dessein P.H. Obesity and carotid atherosclerosis in African black and Caucasian women with established rheumatoid arthritis: A cross-sectional study. Arthritis Res. Ther. 2012;14:R67. doi: 10.1186/ar3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodríguez-Flores M., Rodríguez-Saldaña J., Cantú-Brito C., Aguirre-García J., Alejandro G. Prevalence and severity of atherosclerosis in different arterial territories and its relation with obesity. Cardiovasc. Pathol. 2013;22:332–338. doi: 10.1016/j.carpath.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Maksimovic M., Vlajinac H., Radak D., Marinkovic J., Maksimovic J., Jorga J. Relationship between Abdominal Obesity and Other Cardiovascular Risk Factors: Cross Sectional Study of Patients with Symptomatic Carotid Disease. Srp. Arh. Celok. Lek. 2013;141:460–465. doi: 10.2298/SARH1308460M. [DOI] [PubMed] [Google Scholar]
- 21.Galarza-Delgado D.A., Esquivel-Valerio J.A., Garza-Elizondo M.A., Góngora-Rivera F., Muñoz-De Hoyos J.L., Serna-Pena G. Carotid atherosclerosis in patients with rheumatoid arthritis and rheumatoid nodules. Reum. Clin. 2013;9:136–141. doi: 10.1016/j.reuma.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Cuspidi C., Sala C., Lonati L., Negri F., Rescaldani M., Re A., Meani S., Mancia G. Metabolic syndrome, left ventricular hypertrophy and carotid atherosclerosis in hypertension: A gender-based study. Blood Press. 2013;22:138–143. doi: 10.3109/08037051.2012.744151. [DOI] [PubMed] [Google Scholar]
- 23.Chiquete E., Torres-Octavo B., Cano-Nigenda V., Valle-Rojas D., Domínguez-Moreno R., Tolosa-Tort P., Flórez-Cardona J.A., Flores-Silva F., Reyes-Melo I., Higuera-Calleja J., et al. Caracterización de factores asociados con estenosis carotídea en una población de alto riesgo. Rev. Neurol. 2014;58:541–547. doi: 10.33588/rn.5812.2013226. [DOI] [PubMed] [Google Scholar]
- 24.Irie Y., Katakami N., Kaneto H., Takahara M., Sakamoto K., Kosugi K., Shimomura I. The risk factors associated with ultrasonic tissue characterization of carotid plaque in type 2 diabetic patients. J. Diabetes Its Complicat. 2014;28:523–527. doi: 10.1016/j.jdiacomp.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Yan Z., Liang Y., Jiang H., Cai C., Sun B., Qiu C. Metebolic Syndrome and Subclinical Carotid Atherosclerosis Among Chinese Elderly People Living in a Rural Community. Metab. Syndr. Relat. Disord. 2014;12:269–276. doi: 10.1089/met.2013.0135. [DOI] [PubMed] [Google Scholar]
- 26.Yuan M., Hsu F.-C., Bowden N.W., Xu J., Smith S.C., Wagenknecht L.E., Comeau M.E., Divers J., Register T.C., Carr J.J., et al. Relationships Between Measures of Adiposity with Subclinical Atherosclerosis in Patients with Type 2 Diabetes. Obesity. 2016;24:1810–1818. doi: 10.1002/oby.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan X., Lai Y., Gu J., Wang H., Liu A., Shan Z. Factors Significantly Associated with the Increased Prevalence of Carotid Atherosclerosis in a Northeast Chinese Middle-aged and Elderly Population. Medicine. 2016;95:1–7. doi: 10.1097/MD.0000000000003253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radmard A.R., Poustchi H., Ansari L., Khorasanizadeh F., Yoonessi A., Taheri A.P.H., Rahmanian M.S., Jafari E., Malekzadeh R., Merat S. Abdominal fat distribution and carotid atherosclerosis in a general population: A semi-automated methods using magnetic resonance imaging. Jpn. J. Radiol. 2016;34:414–422. doi: 10.1007/s11604-016-0540-8. [DOI] [PubMed] [Google Scholar]
- 29.Sandfort V., Lai S., Ahlman M.A., Mallek M., Liu S., Sibley C.T., Turkbey E.B., Lima J.A.C., Bluemke D.A. Obesity Is Associated with Progression of Atherosclerosis During Statin Treatment. J. Am. Heart Assoc. 2016;5:e003621. doi: 10.1161/JAHA.116.003621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitevska I., Baneva N., Bosevski M., Kostovska E. Prevalence of risk factors and asymptomatic carotid atherosclerosis in diabetic patients screened for silent myocardial ischemia by SPECT myocardial imaging. Nucl. Med. Ver. 2017;20:3–9. doi: 10.5603/NMR.a2016.0039. [DOI] [PubMed] [Google Scholar]
- 31.Higuchi S., Kabeya Y., Kato K. Visceral-to-subcutaneous fat ratio is independently related to small and large cerebrovascular lesions even in healthy subjects. Atherosclerosis. 2017;259:41–45. doi: 10.1016/j.atherosclerosis.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Mancusi C., Gerdts E., Losi M.A., D’Amato A.D., Manzi M.V., Canciello G., Trimarco V., De Luca N., Simonse G., Izzo R. Differential effect of obesity on prevalence of cardiac and carotid target organ damage in hypertension (The Campania Salute Network) Int. J. Cardiol. 2017;244:260–264. doi: 10.1016/j.ijcard.2017.06.045. [DOI] [PubMed] [Google Scholar]
- 33.Nishizawa A., Suemoto C.K., Farias-Itao D.S., Campos F.M., Silva K.C.S., Bittencourt M.S., Grinberg L.T., Leite R.E.P., Ferretti-Rebustini R.E.L., Farfel J.M., et al. Morphometric measurements of systemic atherosclerosis and visceral fat: Evidence from na autopsy study. PLoS ONE. 2017;12:e0186630. doi: 10.1371/journal.pone.0186630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omisore A., Famurewa O., Komolafe M., Asaleya C., Fawale M., Afolabi B. Association of traditional cardiovascular risk factors with carotid atherosclerosis among adults at a teaching hospital in South-western Nigeria. Cardiovasc. J. Afr. 2018;28:183–188. doi: 10.5830/CVJA-2018-014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imahori Y., Mathiesen E.B., Leon D.A., Hopstock L.A., Hughes A.D., Johnsen S.H., Jørgensen L., Emaus N., Morgan K.E. The contribution of obesity to carotid atherosclerotic plaque burden in a general population sample in Norway: The Tromsø Study. Atherosclerosis. 2018;273:15–20. doi: 10.1016/j.atherosclerosis.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Laugesen E., Høyem P., Thrysoe S., Hansen E.S.S., Mikkelsen A.F.S., Kerwin W.S., Poulsen P.L., Hansen T.K., Kim W.Y. Negative Carotid Artery Remodeling in Early Type 2 Diabetes Mellitus and Increased Carotid Plaque Vulnerability in Obesity as Assessed by Magnetic Resonance Imaging. J. Am. Heart Assoc. 2018;7:e008677. doi: 10.1161/JAHA.118.008677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida T., Hashimoto M., Kawahara R., Yamamoto H., Tanaka M., Ito H., Masuda I., Hosoda K., Yamamoto W., Uozumi R., et al. Non-obese visceral adiposity is associated with the risk of atherosclerosis in Japanede patients with rheumatoid arthritis: A cross-sectional study. Rheumatol. Int. 2018;38:1679–1689. doi: 10.1007/s00296-018-4095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scicali R., Rosenbaum D., Pino A. Na increased waist-to-hip ratio is a key determinant of a atherosclerotic burden in overweight subjects. Acta Diabetol. 2018;55:741–749. doi: 10.1007/s00592-018-1144-9. [DOI] [PubMed] [Google Scholar]
- 39.Geraci G., Zammuto M., Gaetani R., Mattina A., D’Ignoto F., Geraci C., Noto D., Averna M., Cottone S., Mulè G. Relationship of a Body Shape Index and Body Roundness Index With carotid atherosclerosis in arterial hypertension. Nitrition Metab. Cardiovasc. Dis. 2019;29:822–829. doi: 10.1016/j.numecd.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 40.Haberka M., Skilton M., Biedroń M., Szostak-Janiak K., Partyka M., Matla M., Gąsior Z. Obesity, visceral adiposity and carotid atherosclerosis. J. Diabetes Its Complicat. 2019;33:302–306. doi: 10.1016/j.jdiacomp.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Buford T.W., Anton S.D., Judge A.R., Marzetti E., Wohlgemuth S.E., Carter C.S., Leeuwenburgh C., Pahor M., Manini T.M. Models of accelerated sarcopenia: Critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res. Rev. 2010;9:369–383. doi: 10.1016/j.arr.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puttonen S., Kivimäki M., Elovainio M., Pulkki-Råback L., Hintsanen M., Vahtera J., Telama R., Juonala M., Viikari J.S., Raitakari O.T., et al. Shift work in young adults and carotid artery intima–media thickness: The Cardiovascular Risk in Young Finns study. Atherosclerosis. 2009;205:608–613. doi: 10.1016/j.atherosclerosis.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Pearson T.L. Cardiovascular risk in minority and underserved women in Appalachian Tennessee: A descriptive study. J. Am. Acad. Nurse Pract. 2010;22:210–216. doi: 10.1111/j.1745-7599.2010.00495.x. [DOI] [PubMed] [Google Scholar]
- 44.Varleta P., Concepción R., Vargas P., Casanova H. Grosor íntima media carotídeo y asociación con factores de riesgo cardiovascular tradicionales y metabólicos. Ver. Med. Chile. 2013;141:695–703. doi: 10.4067/S0034-98872013000600002. [DOI] [PubMed] [Google Scholar]
- 45.Alkali N., Bwala S., Akano A., Osi-Ogbu O., Alabi P., Ayeni O. Stroke risk factors, subtypes, and 30 day case fatality in Abuja, Nigeria. Niger. Med. J. 2013;54:129–136. doi: 10.4103/0300-1652.110051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodríguez-Saldaña J., Rodriguez-Flores M., Cantú-Brito C., Aguirre-Garcia J. A Pathological Study of the Epidemiology of Atherosclerosis in Mexico City. Cardiol. Res. Pr. 2014;2014:2642058. doi: 10.1155/2014/264205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maddaloni E., Cavallari I., De Pascalis M., Keenan H., Park K., Manfrini S., Buzzetti R., Patti G., Di Sciascio G., Pozzilli P. Relation of Body Circumferences to Cardiometabolic Disease in Overweight-Obese Subjects. Am. J. Cardiol. 2016;118:822–827. doi: 10.1016/j.amjcard.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 48.Masson W., Siniawski D., Toledo G., Vita T., Fernández G., Castillo S.G., Valle J., Cagide A. Estimación de la «edad vascular» basada en el índice de masa corporal en una población en prevención primaria. Asociación con la aterosclerosis carotídea subclínica. Med. Clínica. 2013;140:255–259. doi: 10.1016/j.medcli.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 49.Serena J., Segura T., Roquer J., García-Gil M., Castillo J. The ARTICO study: Identification of patients at high risk of vascular recurrence after a first non-cardioembolic stroke. BMC Neurol. 2015;15:1–7. doi: 10.1186/s12883-015-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manchev I.C., Mineva P.P., Hadjiev D.I. Prevalence of Stroke Risk Factors and Their Outcomes: A Population-Based Longitudinal Epidemiological Study. Cerebrovasc. Dis. 2001;12:303–307. doi: 10.1159/000047725. [DOI] [PubMed] [Google Scholar]
- 51.Kahraman S., Yilmaz R., Akinci D., Arici M., Altun B., Erdem Y., Yasavul U., Turgan C. U-Shaped Association of Body Mass Index with Inflammation and Atherosclerosis in Hemodialysis Patients. J. Ren. Nutr. 2005;15:377–386. doi: 10.1053/j.jrn.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 52.Yasuda T., Matsuhisa M., Fujiki N., Sakamoto F., Tsuji M., Fujisawa N., Kimura M., Ishibashi R., Kaneto H., Yamasaki Y., et al. Is Central Obesity a Good Predictor of Carotid Atherosclerosis in Japanese Type 2 Diabetes with Metabolic Syndrome? Endocrime J. 2007;54:695–702. doi: 10.1507/endocrj.K06-210. [DOI] [PubMed] [Google Scholar]
- 53.Lajunen T., Vikatmaa P., Bloigu A., Ikonen T., Lepäntalo M., Pussinen P.J., Saikku P., Leinonen M. Chlamydial LPS and high-sensitivity CRP levels in serum are associated with an elevated body mass index in patients with cardiovascular disease. Innate Immun. 2008;14:375–382. doi: 10.1177/1753425908099172. [DOI] [PubMed] [Google Scholar]
- 54.De Michele M., Panico S., Iannuzzi A., Celentano E., Ciardullo A.V., Galasso R., Sacchetti L., Zarrilli F., Bond M.G., Rubba P. Association of Obesity and Central Fat Distribution with Carotid Artery Wall Thickening in Middle-Aged Women. Stroke. 2002;33:2923–2928. doi: 10.1161/01.STR.0000038989.90931.BE. [DOI] [PubMed] [Google Scholar]
- 55.Lind L., Siegbahn A., Ingelsson E., Sundstrom J., Arnlov J. A Detailed Cardiovascular Characterization of Obesity Without the Metabolic Syndrome. Arter. Thromb. Vasc. Biol. 2011;31:e27–e34. doi: 10.1161/ATVBAHA.110.221572. [DOI] [PubMed] [Google Scholar]
- 56.Jackson R., Sidawy A., Amdur R., Macsata R. Obesity is an Independent Risk Factor for Death and Cardiac Complications after Carotid Endarterectomy. J. Am. Coll Surg. 2012;214:148–155. doi: 10.1016/j.jamcollsurg.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 57.Abreu T., Ferreira E., Filho S., Sales K., Lopes F., Oliveira A. Prevalence of carotid artery calcifications detected on panoramic radiographs and confirmed by Doppler ultrasonography: Their relationship with systemic conditions. Indian J. Dent. Res. 2015;26:345–350. doi: 10.4103/0970-9290.167644. [DOI] [PubMed] [Google Scholar]
- 58.Yamauchi T., Kuno T., Takada H., Nagura Y., Kanmatsuse K., Takahashi S. The impact of visceral fat on multiple risk factors and carotid atherosclerosis in chronic haemodialysis patients. Nephrol. Dial. Transpl. 2003;18:1842–1847. doi: 10.1093/ndt/gfg261. [DOI] [PubMed] [Google Scholar]
- 59.Hwang I., Suh S., Seo A., Ahn H., Yim E. Association between Metabolic Components and Subclinical Atherosclerosis in Korean Adults. Korean J. Fam. Med. 2012;33:229–236. doi: 10.4082/kjfm.2012.33.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Novo S., Peritore A., Trovato R.L., Guarneri F.P., Di Lisi D., Muratori I., Novo G. Preclinical atherosclerosis and metabolic syndrome increase cardio- and cerebrovascular events rate: A 20-year follow up. Cardiovasc. Diabetol. 2013;12:155. doi: 10.1186/1475-2840-12-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X., Li W., Song F., Wang L., Fu Q., Cao S., Gan Y., Zhang W., Yue W., Yan F., et al. Carotid Atherosclerosis Detected by Ultrasonography: A National Cross-Sectional Study. J. Am. Heart Assoc. 2018;7:e00870. doi: 10.1161/JAHA.118.008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sforza E., Boissier C., Martin M.S., Feasson S., Barthélémy J.C., Roche F. Carotid artery atherosclerosis and sleep disordered breathing in healthy elderly subjects: The Synapse cohort. Sleep Med. 2013;14:66–70. doi: 10.1016/j.sleep.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto M., Egusa G., Hara H., Yamakido M. Association of intraabdominal fat and carotid atherosclerosis in non-obese middle-aged men with normal glucose tolerance. Int. J. Obes. 1997;21:948–951. doi: 10.1038/sj.ijo.0800501. [DOI] [PubMed] [Google Scholar]
- 64.Nejmanze P. Differential Effects of Body Adiposity and Serum Lipids on Right and Left Carotid Artery Lesions. Stroke. 2003;34:e185–e186. doi: 10.1161/01.STR.0000094662.73951.3B. [DOI] [PubMed] [Google Scholar]
- 65.Node K., Inoue T., Boyko V., Goldberg I., Fisman E.Z., Adler Y., Schwammenthal E., Matas Z., Behar S., Tenenbaum A. Long-term effects of peroxisome proliferator-activated receptor ligand bezafibrate on N-terminal pro-B type natriuretic peptide in patients with advanced functional capacity impairment. Cardiovasc. Diabetol. 2009;8:1–7. doi: 10.1186/1475-2840-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friedlander A., Altman L. Carotid artery atheromas in postmenopausal womem–Their prevalence on panoramic radiographs and their relationship to atherogenic risk factors. Am. Dent. Assoc. 2001;132:1130–1136. doi: 10.14219/jada.archive.2001.0340. [DOI] [PubMed] [Google Scholar]
- 67.Wang H., Chen Y., Eitzman D. Imaging Body Fat–Techniques and Cardiometabolic Implications. Arterioscler. Thromb. Vasc. Biol. 2014;34:2217–2223. doi: 10.1161/ATVBAHA.114.303036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.See R., Abdullah S.M., McGuire D.K., Khera A., Patel M.J., Lindsey J.B., Grundy S.M., de Lemos J.A. The Association of Differing Measures of Overweight and Obesity with Prevalent Atherosclerosis-The Dallas Heart Study. J. Am. Coll. Cardiol. 2007;50:752–759. doi: 10.1016/j.jacc.2007.04.066. [DOI] [PubMed] [Google Scholar]
- 69.Shuster A., Patlas M., Pinthus J., Mourtzakis M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 2012;85:1–10. doi: 10.1259/bjr/38447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Há E., Bauer R. Emerging Roles for Adipose Tissue in Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2018;38:e137–e144. doi: 10.1161/ATVBAHA.118.311421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao Y., Wang Y., Lu C., Zeng C., Chang D., Ju S. Correlations between the abdominal fat related parameters and severity of coronary artery disease assessed by computed tomography. Quant. Imaging Med. Surg. 2018;8:579–587. doi: 10.21037/qims.2018.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Figueroa A.L., Takx R.A., MacNabb M.H., Abdelbaky A., Lavender Z.R., Kaplan R.S., Truong Q.A., Lo J., Ghoshhajra B.B., Grinspoon S.K., et al. Relationship between measures of adiposity, arterial inflammation, and subsequent cardiovascular events. Circ. Cardiovasc. Imaging. 2016;9:e004043. doi: 10.1161/CIRCIMAGING.115.004043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Imai A., Komatsu S., Ohara T., Kamata T., Yoshida J., Miyaji K., Takewa M., Kodama K. Visceral abdominal fat accumulation predicts the progression of noncalcified coronary plaque. Atherosclerosis. 2012;222:524–529. doi: 10.1016/j.atherosclerosis.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 74.Hansson G., Libby P., Tabas I. Inflammation and plaque vulnerability. J. Intern. Med. 2015;278:483–493. doi: 10.1111/joim.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]