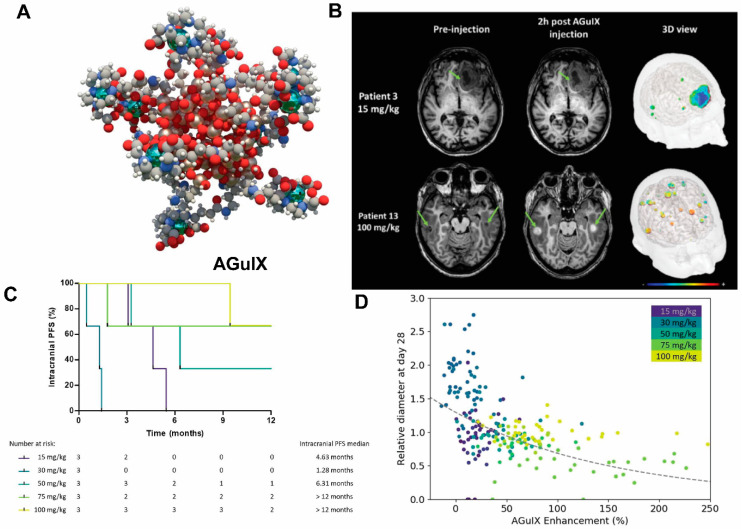
Figure 3.
AGuIX as radiosynthesizer and MRI contrast-enhancing NPs in the phase I clinical trial (NANO-RAD trial). (A) Schematic representation of AGuIX. Gadolinium ions are chelated by 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid derivatives. Polysiloxane core (Si, metallic grey; O, red; H, white; C, grey; N, blue) is surrounded by covalently grafted chelates of gadolinium (Gd, metallic green). (B) AGuIX contrast-enhanced MRI at 2 h in brain metastases of 2 patients with lung cancer following intravenous AGuIX administration at 15 and 100 mg/kg, respectively. T1-weighted MRI images were obtained without injection of contrast agent before and at 2 h after a single AGuIX intravenous administration at the indicated concentration. Green arrows are pointing highlighted metastases. The 3-D vizualization of entire brain with specific contrast enhancement into metastases was obtained from T1-weighted MRI mapping. (C) Intracranial progression-free survival (PFS) of multiple patients with brain metastases treated with a combination of whole-brain radiotherapy (WBRT) and different dose levels of intravenous AGuIX. The color of survival curves corresponds to different AGuIX doses. (D) Correlation between change in size of brain metastases and AGuIX signal variation. Correlation of measured metastasis sizes for patients with brain metastases and treated with whole brain radiotherapy and different AGuIX doses. Points colored according to patient number and administrated dose with darker colors corresponding to lower AGuIX doses. Metastasis diameter at 28 days normalized to diameter at Day 0 (V28/V0) as a function of AGuIX enhancement (points) compared with predicted trend (dashed line), showing good agreement and dependence of metastasis evolution on AGuIX uptake. AGuIX, Activation and Guidance of Irradiation by X-Ray. Figures were adapted based on Refs. [111,113] with permissions.