Abstract
Chloroquine (CQ) and hydroxychloroquine (HCQ) have recently become the focus of global attention as possible treatments for Coronavirus Disease 2019 (COVID-19). The current systematic review aims to assess their safety in short treatments (≤14 days), whether used alone or in combination with other drugs. Following the PRISMA and SWiM recommendations, a search was conducted using four health databases for all relevant English-, Chinese-, and Spanish-language studies from inception through 30 July 2021. Patients treated for any condition and with any comparator were included. The outcomes of interest were early drug adverse effects and their frequency. A total of 254 articles met the inclusion criteria, including case and case-control reports as well as cross-sectional, cohort, and randomised studies. The results were summarised either qualitatively in table or narrative form or, when possible (99 studies), quantitatively in terms of adverse event frequencies. Quality evaluation was conducted using the CARE, STROBE, and JADAD tools. This systematic review showed that safety depended on drug indication. In COVID-19 patients, cardiac adverse effects, such as corrected QT interval prolongation, were relatively frequent (0–27.3% and up to 33% if combined with azithromycin), though the risk of torsade de pointes was low. Compared to non-COVID-19 patients, COVID-19 patients experienced a higher frequency of cardiac adverse effects regardless of the regimen used. Dermatological adverse effects affected 0–10% of patients with autoimmune diseases and COVID-19. A broad spectrum of neuropsychiatric adverse effects affected patients treated with CQ for malaria with variable frequencies and some cases were reported in COVID-19 patients. Gastrointestinal adverse effects occurred regardless of drug indication affecting 0–50% of patients. In conclusion, CQ and HCQ are two safe drugs widely used in the treatment of malaria and autoimmune diseases. However, recent findings on their cardiac and neuropsychiatric adverse effects should be considered if these drugs were to be proposed as antivirals again.
Keywords: chloroquine, hydroxychloroquine, adverse reactions, drug safety, systematic review
1. Introduction
Chloroquine (CQ) and hydroxychloroquine (HCQ), two safe drugs widely used in the treatment of malaria and autoimmune diseases, have become a global focus of attention due to early findings on their antiviral effectiveness against the novel SARS-CoV-2 coronavirus, which leads to what is known as coronavirus disease 2019 (COVID-19) [1,2]. In the context of the absence of specifically approved drugs for the treatment of SARS-CoV-2 pneumonia, previous evidence of the effects of CQ against coronaviruses [3,4], findings on the effects of CQ and HCQ on SARS-CoV-2 in vitro [1,2], and positive preliminary observational findings in China [5,6], justified clinical research on these drugs [7,8]. At the outbreak of the COVID-19 pandemic, these drugs were authorised as part of national emergency use programmes or clinical trials by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for patients affected by SARS-CoV-2 pneumonia [9,10], and the massive use of these treatments spread to different countries. Clinical research on and the use of these drugs were focused on three aspects: (i) treatment of patients with SARS-CoV-2 pneumonia, (ii) post-exposure prophylaxis of contacts [11], and (iii) prevention of SARS-CoV-2 infection among healthcare personnel [12].
Short-course CQ and HCQ regimens have traditionally been considered safe. Mild to moderate toxicity may occur occasionally, with symptoms including headache, malaise, dizziness, visual alterations, mild gastrointestinal and neurologic events, and itching being more or less common depending on the population treated [13]. However, increasing concern arose when these drugs were used in patients with COVID-19, whether alone or in combination with other drugs, due to their cardiac and neuropsychiatric adverse effects [14,15]. Large randomised clinical trials have ruled out the benefits of CQ or HCQ for COVID-19 outcomes and there is ongoing concern about the consequences that these treatments may have in patients with or without COVID-19 [16,17,18].
Besides short-term CQ and HCQ regimens, these drugs have been widely used for the long-term treatment of autoimmune inflammatory diseases. HCQ is currently recommended to treat systemic lupus erythematosus (SLE), and CQ and HCQ have been used for decades to treat rheumatoid arthritis (RA) and are commonly considered safe [14,15].
We hypothesise that the toxicity of short-course CQ or HCQ is low, but that an additive effect could occur when they are used in combination with other drugs or in concrete clinical conditions. The present work, therefore, has two main objectives: first, it seeks to assess and summarise the available literature on the early toxicity of CQ and HCQ alone or in combination with other drugs that have been used to treat COVID-19 in different clinical situations (such as malaria and other parasitic infections, or autoimmune conditions); and second, more specifically, it will assess the impact of drug combinations and pathological situations on the frequency of adverse drug effects in short-course regimens with CQ or HCQ.
2. Methods
We performed a systematic review of the literature on the safety of short-course treatments with CQ and HCQ with the goal of assessing the adverse effects of these drugs either alone or in combination with other drugs used to treat COVID-19, whether antivirals such as remdesivir, lopinavir plus ritonavir (LPVr), boosted darunavir (DRV), tenofovir, favipiravir, arbidol and ribavirin, antibiotics such as levofloxacin or azithromycin (AZM), immunomodulatory agents such as baricitinib, tocilizumab (TCZ), sarilumab, anakinra and interferons, corticosteroids such as dexamethasone, prednisone, prednisolone and methylprednisolone, anticoagulant heparins or low-weight heparins, or neutralizing antibodies and serotherapies. In our selection criteria we included case reports as well as case-control, cross-sectional, cohort, and randomised studies. Adverse effects associated with short-course regimens (≤14 days) were assessed by including both studies reporting information on short-term CQ and HCQ treatment regimens (e.g., for malaria) and those reporting long-term treatment regimens (e.g., for autoimmune diseases) in which early adverse effects were assessed or described. The main outcome of interest was the safety of CQ and HCQ and the frequency of adverse drug reactions during short-course regimens.
This systematic review was carried out in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) methodology [19] and was registered in the International Prospective Register of Systematic Reviews of the Centre for Reviews and Dissemination (PROSPERO) (registration number: CRD42020180708) [20].
2.1. Literature Search
We searched MEDLINE using PubMed, Embase using Ovid, CENTRAL (the Cochrane Central register of Controlled Trials), and LILACS (Literatura Latinoamericana y del Caribe en Ciencias de la Salud) for articles published from inception through 30 July 2021. We applied two searches, one designed to identify studies describing the safety of CQ or HCQ by itself and another designed to identify studies in which CQ or HCQ were combined with the drugs mentioned above. The combined MeSH and search terms used in the two search strategies in MEDLINE through PubMed are described in Tables S1 and S2 of Supplementary Material. Equivalent search strategies were applied for the Embase, CENTRAL, and LILACS searches. Once these searches were completed and all relevant articles obtained, the list of references at the end of each article was checked to identify additional relevant studies. No date restrictions were imposed. Only articles published in Spanish, Chinese, or English were included. Abstracts, posters, or book chapters were not included. Although a specific search was not performed on preprint databases, those articles in preprint form found through database searches or reference checks were included in the selection process.
2.2. Selection Process
Articles identified through the preliminary search were then screened for relevance to our study in two steps. First, the title and abstract of each article were independently checked by two reviewers for at least minimally relevant information on CQ, HCQ, and their safety. The resulting two lists of articles were compared and any differences were resolved by a third reviewer. The second step involved evaluating the full text of each article to confirm its relevance for this review. Articles were only included in the final set if they reported cases of adverse drug reactions to CQ or HCQ, or were case-control, cross-sectional, cohort, or randomised studies that reported information on the safety of these drugs for adult patients 18 years or older (it only was admitted if part of the population included adolescents ≥12 years in large cross-sectional, cohort, or randomised studies, not in the cases). Articles were excluded if they reported adverse drug reactions that occurred beyond the first 14 days of treatment; if they were related to intoxications (intakes of more than five times the Defined Daily Dose of 0.5 g of CQ base or 0.516 g of HCQ base) [21]; if the route of administration was not the oral route; if they were related to work-related exposure; if they were surveys of health professionals; if they assessed the validity of a diagnostic or screening technique; if they contained preclinical data (including in vitro or animal experimentation); if they were protocols, surveys, reviews, systematic reviews, scoping reviews, or meta-analyses; if the adverse drug reaction was associated with a combination of drugs other than those mentioned; if they did not indicate the temporal relationship between drug intake and the appearance of adverse drug reactions; or if they contained duplicate information (the same case or sample of patients reported in separate articles). Two reviewers performed this process independently. Subsequently, the results were compared, and discrepancies were resolved by a third reviewer to produce the final set of articles for synthesis.
2.3. Data Collection and Data Items
Working separately, two reviewers extracted from each of the articles a set of specific data about study design, participants, quality, and the results in a specific datasheet. The two resulting data compilations were compared, and any discrepancies were discussed and resolved with the participation of a third reviewer. We gathered data related to the design, the participants, the quality, and the results of each study. The final data selected for synthesis in this systematic review can be seen in Supplementary Material.
2.4. Quality Assessment, Risk of Bias in Individual Studies and across Studies
The CARE (CAse REport) Checklist was used to evaluate the quality of reporting in case reports and case series reports [22]. Case-control, cross-sectional, and cohort studies were assessed using the combined STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) checklist [23]. The different items on the checklists mentioned were rated as “Yes” (=1 point), “Partly” (=0.5 point), “No” (=0 points), or “Not applicable”. We calculated an overall score for the quality of each study by dividing the total number of points scored per article by the number of items to produce a percentage. A low score indicated low quality, hence a higher risk of bias. We considered studies scoring between 75% and 100% to be of high quality, those between 50% and 74% of moderate quality, and those below 50% of low quality. If more than 50% of the assessed items were rated as “Not applicable”, the study was dismissed for quality assessment but not eliminated from our dataset. The quality of randomised studies was judged using the JADAD scale, which assigns a score ranging from 0 to 5 points such that the higher the score, the better the methodological quality [24]
2.5. Data Synthesis and Summary Measures
2.5.1. Case Series, Case Reports, and Case-Control Studies
Data from these studies were synthesised in either table or narrative form. Case and case series reports were grouped according to drug or drugs reported (CQ or HCQ alone or in combination), drug indication, and the organ system affected by an adverse effect. No study was eliminated based on the risk of bias. Case-control studies were presented in a table showing reported adverse drug reactions. Presentation was ordered according to quality evaluation scores, presence of probability scales such as the Naranjo Adverse Drug Reaction Probability Scale for case and case series reports [25], and relevance of the evidence.
2.5.2. Cross-Sectional, Cohort, and Randomised Studies
We presented the synthesised evidence following the SWiM (Synthesis Without Meta-analysis) guidelines [26] in table and narrative form. Studies were divided into studies on the safety of CQ or HCQ alone and studies in which CQ and/or HCQ were combined with one of the eligible drugs and also grouped according to drug indication. The rationale for this grouping of studies was our focus on drug safety and the influence of drug combinations and indications on this outcome. Initially, we did not use a standardised metric to present exposure and/or direction effects or p values, so we reported these effects in their original format, namely as mean differences, standardised mean differences, risk ratios, odds ratios, or risk differences. However, whenever possible, we calculated the frequency of each toxicity for each study, and a summary reporting the frequency of adverse events reported as a range of percentages was presented for each group mentioned above. Those studies reporting data on pregnant patients were synthesised using narratives and tables but not included in the quantitative data synthesis. In the case of combinations, only those cases in which more than one study reported enough data were included for quantitative data synthesis. Combinations in which only one study was found were presented separately. Whenever possible, data on adverse drug reactions frequency-adjusted for patient status or comorbidities or any other confounding factors were considered. Due to the heterogeneity of the populations included, drug exposure data, reported adverse drug reactions, and study methodologies, we did not consider a meta-analysis of the outcome effects. Studies were prioritised according to quality evaluation scores, sample size, and relevance of the evidence.
3. Results
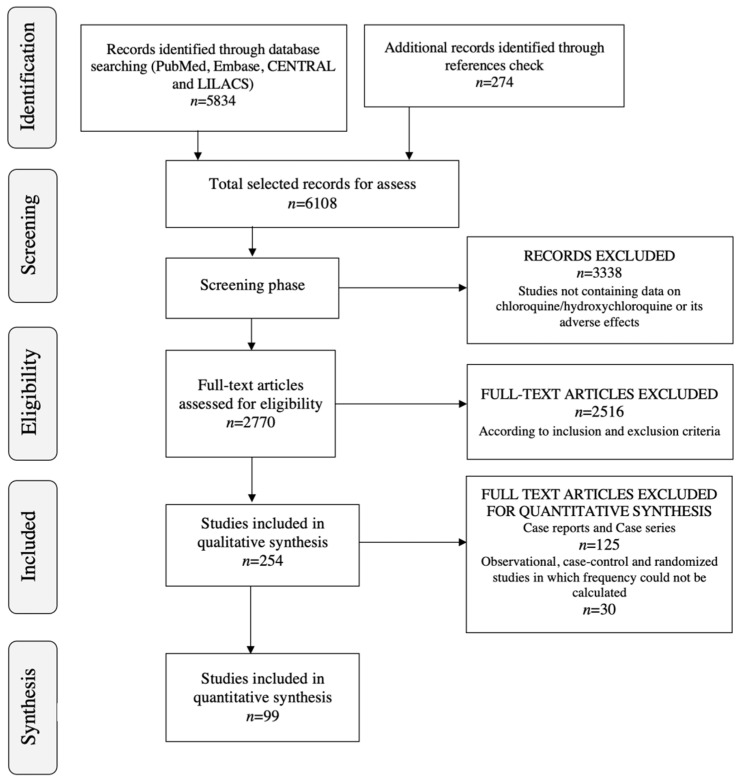
The preliminary online database search yielded a set of 6108 articles, of which 2942 articles were identified through MEDLINE using PubMed, 1977 were identified through Embase using Ovid, 683 were identified through CENTRAL, 232 were identified through LILACS, and 274 more were identified by checking article reference lists. Of this initial set of 6108, 3338 articles were excluded in the title and abstract screening process. Of the remaining 2770 articles, an additional 2516 were excluded for the content eligibility reasons described above (eligibility criteria are summarised in Table S3 of Supplementary Material). The full selection process yielded a final set of 254 studies for this systematic review (Figure 1) [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280].
Figure 1.
Safety of chloroquine and hydroxychloroquine. Selection process, flow diagram.
3.1. Study Characteristics and Results of Individual Studies
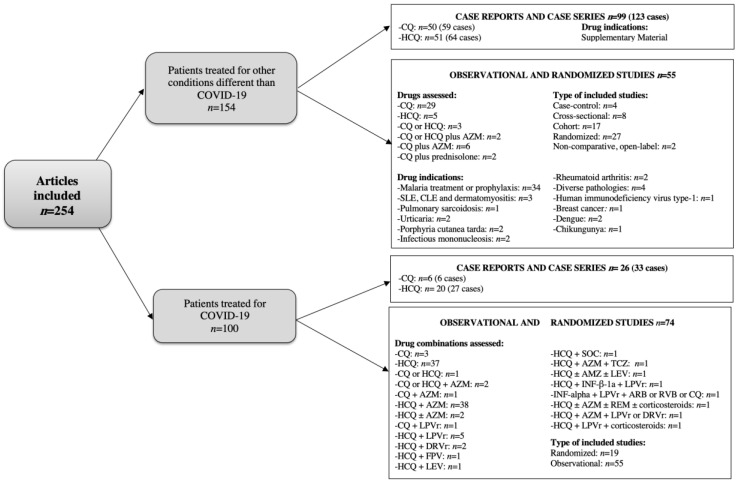
Figure 2 describes the main characteristics of the articles included.
Figure 2.
Study characteristics and results of individual studies, flow diagram.
3.1.1. Patients Treated for Conditions Other Than COVID-19
Case and Case Series Reports
A total of 99 articles reporting 123 cases were found [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125]. These cases are summarised in Table 1 and Table 2 and described more fully in Tables S4–S12 of Supplementary Material part 2.
Table 1.
Summary of studies included: case reports and case series reports related to HCQ adverse events.
| Organ Affected | N° of Patients (n° of Studies) |
Adverse Effect (N° of Patients) (First Author, Year) |
Indication of HCQ (n° of Patients) |
Long-Term Consequences (n° of Patients) |
|---|---|---|---|---|
| Dermatological | 41 (28) | DRESS (3) (Volpe A et al., 2008, Randhawa A et al., 2018, Girijala RL et al., 2019) [48,60,62] |
Seronegative polyarthritis (2) Suspected Sjögren’s like process (1) |
Resolution (3) |
| Severe pruritus (1) (Gül U et al., 2006) [45] |
Discoid lupus erythematosus (1) | Resolution (1) | ||
| Pemphigus vulgaris (1) (Ghaffarpour G et al., 2006) [44] |
Rheumatoid arthritis (1) | After 3 w the lesions cleared with only a mild post-inflammatory hyperpigmentation (1) | ||
| Sweet’s syndrome (1) (Manzo C et al., 2019) [63] |
Sjögren syndrome (1) | Resolution (1) | ||
| Inverse psoriasis (1) (Ullah A et al., 2019) [64] |
Rheumatoid arthritis (1) | Resolution (1) | ||
| Mild cutaneous eruptions (1) (Matsuda T et al., 2017) [57] |
Lupus erythematosus (1) | Resolution (1) | ||
| AGEP (11) (Assier-Bonnet et al., 1996, Evans CC et al., 2004, Atzori L et al., 2007, Bailey K et al., 2013, Soria A et al., 2015, Pearson KC et al., 2016, Mercogliano C et al., 2018, Matsuda-Hirose H et al., 2020) [38,43,47,51,54,55,59,65] |
SLE and related disorders (3) NA (1) Erythematous facial dermatitis (1) Photosensitivity (1) Arthritis and related rheumatic disorders (4) Mucinosis (1) |
Resolution (10) NA (1) |
||
| Acute pustular psoriasis (1) (Welsch MJ et al., 2003) [42] |
Sjögren syndrome (1) | Resolution (1) | ||
| Stevens-Johnson syndrome (1) (Leckie MJ et al., 2002) [41] |
Rheumatoid arthritis (1) | The rash improved but persisted (1) | ||
| Erythema multiforme (1) (Abou Assalie N et al., 2017) [56] |
SLE (1) | Resolution (1) | ||
| Fatal toxic epidermal necrolysis (2) (Murphy M et al., 2001, Cameron MC et al., 2014) [40,52] |
Seropositive nodular rheumatoid disease (1) SLE (1) |
Death (2) | ||
| Psoriasis (1) (Gray RG et al., 1985) [34] |
Seronegative rheumatoid arthritis (1) | Resolution (1) | ||
| Severe psoriasis exacerbation (1) (Luzar MJ et al., 1982) [31] |
Psoriatic arthropathy (1) | Resolution (1) | ||
| Hypersensitivity rash (5) (Mates M et al., 2006, Awad P et al., 2013) [46,50] |
Arthritis and related rheumatic disorders (4) Chronic cutaneous lupus (1) |
Resolution (1) NA (4) |
||
| Erythema annulare centrifugum (1) (Hudson LD et al., 1985) [35] |
Suspected SLE (1) | Resolution (1) | ||
| Pustular eruption (1) (Pastushenko I et al., 2015) [53] |
Rheumatoid arthritis (1) | Resolution (1) | ||
| Photosensitivity (2) (Soria A et al., 2015) [54] |
Rheumatism (1) Autoimmune bullous skin disease (1) |
NA (2) | ||
| AGEP/DRESS (1) (Soria A et al., 2015) [54] |
Granuloma annulare (1) | Resolution (1) | ||
| Urticaria (2) (Soria A et al., 2015) [54] |
Jessner-Kanof (1) Cutaneous lupus erythematous (1) |
Resolution (2) | ||
| MPE (2) (Soria A et al., 2015) [54] |
Gougerot-Sjögren syndrome (1) Cutaneous lupus erythematous (1) |
Resolution (2) | ||
| Generalised pustular rash (1) (Lotem M et al., 1990) [36] |
Pemphigus erythematosus (1) | Resolution (1) | ||
| Psychiatric | 2 (2) | Psychosis (1) (Ward WQ et al., 1985) [74] |
Lupus erythematosus (1) | Resolution (1) |
| Auditory and visual hallucination (1) (Ganjei Z et al., 2021) [125] |
Discoid lupus erythematosus (1) | Resolution (1) | ||
| Neurologic | 1 (1) | Significant psychomotor agitation (1) (Manzo C et al., 2017) [63] |
Rheumatoid arthritis (1) | Resolution (1) |
| Cardiac | 3 (3) | Complete heart block (1) (Comín-Colet J et al., 2001) [96] |
SLE (1) | Resolution (1) |
| Implanted pacemaker failure (1) (Huang PH et al., 2003) [97] |
Rheumatoid arthritis (1) | Resolution (1) | ||
| QT-interval prolongation (1) Morgan ND et al., 2013 [99] |
SLE (1) | QT relatively normal after a year (1) | ||
| Hematologic and metabolic | 4 (4) | Hypoglycaemic coma (1) (Shojania K et al., 1999) [101] |
Rheumatoid polyarthritis (1) | Resolution (1) |
| Hypoglycaemia (1) (Winter EM et al., 2011) [102] |
Osteoarthritis (1) | Resolution (1) | ||
| Thrombocytopaenia (1) (Demir D et al., 2014) [104] |
Used erroneously as a pain killer (1) | Resolution (1) | ||
| Thrombotic thrombocytopaenic purpura (1) (Fromm LM et al., 2017) [105] |
Rheumatoid arthritis (1) | Death related to cardiac failure (1) | ||
| Hepatic | 4 (4) | Severe acute hepatitis (1) (Giner Galvañ V et al., 2007) [111] |
Arthritis (1) | Resolution (1) |
| Liver injury (1) (Sunkara B et al., 2018) [112] |
Subacute cutaneous lupus erythematosus (1) | Resolution (1) | ||
| Fulminant hepatic failure (1) (Makin AJ et al., 1994) [114] |
SLE (1) | Death (1) | ||
| Bullous rash and acute hepatitis (1) (Kutz DC et al., 1995) [115] |
SLE (1) | Resolution (1) | ||
| Other | 6 (6) | Porphyria variegata precipitation (1) (Baler GR et al., 1976) [116] |
SLE (1) | Resolution (1) |
| Severe vacuolar myopathy (1) (Bolaños-Meade J et al., 2005) [119] |
cGVHD (1) | Resolution (1) | ||
| Anaphylaxis (1) (Donado CD et al., 2010) [121] |
SLE (1) | Resolution (1) | ||
| Two episodes of urinary incontinence (1) (Carnovale C et al., 2013) [122] |
Rheumatoid arthritis (1) | Resolution (1) | ||
| Diffuse interstitial lung disease (1) (Català R et al., 2015) [123] |
Polymorphic light eruption (1) | Resolution (1) | ||
| Acute eosinophilic pneumonia (1) (Ishiguro Y et al., 2019) [124] |
Chilblain lupus erythematosus (1) | Resolution (1) | ||
| Sense organs | 3 (3) | Severe positional vertigo (1) (Prince DS et al., 1975) [107] |
Rheumatoid arthritis (1) | Resolution (1) |
| Severe vestibular toxicity (1) (Malik MK et al., 1977) [108] |
Malaria (1) | Bilateral complete canal paresis (1) | ||
| Complete ageusia (1) (Fleury O et al., 2009) [110] |
SLE (1) | Resolution (1) |
AGEP: Acute generalised exanthematous pustulosis, cGVHD: chronic graft-versus-host disease, DRESS: Drug rash with eosinophilia and systemic symptoms, HCQ: Hydroxychloroquine, MPE = maculopapular exanthema, NA: not available/not applicable, SLE: systemic lupus erythematosus, w: weeks.
Table 2.
Summary of studies included: case reports and case series reports related to CQ adverse events.
| Organ Affected | N° of Patients (n° of Studies) |
Adverse Effect (n° of Patients) (First Author, Year) |
Indication of CQ (n° of Patients) |
Long-Term Consequences (n° of Patients) |
|---|---|---|---|---|
| Dermatological | 12 (12) | Exacerbation of psoriasis and arthritis (1) (Fisher S, 1961) [27] |
Psoriasis (1) | Death due to toxaemia from staphylococcic peritonitis (1) |
| Eczema (1) (Skog E, 1975) [28] |
Malaria prophylaxis (1) | NA (1) | ||
| Toxic epidermal necrolysis (1) (Kanwar AJ, 1976) [29] |
Suspected malaria (1) | Resolution (1) | ||
| Exacerbation of psoriasis (1) (Olsen TG, 1981) [30] |
Malaria (1) | Resolution (1) | ||
| Severe pruritus (1) (Spencer HC, 1982) [32] |
Malaria (1) | Resolution (1) | ||
| Pruritus (1) (Bhasin V, 1984) [33] |
Malaria (1) | Resolution (1) | ||
| Erythrodermic psoriasis (1) (Vestey JP, 1992) [37] |
Psoriasis (1) | Psoriasis remained well controlled with usual treatment (1) | ||
| Pustular eruption (1) (Wilairatana P, 1998) [39] |
Malaria (1) | After discontinuation, the eruption quickly resolved with mild desquamation (1) | ||
| Stevens–Johnson syndrome (1) (Das JK, 2011) [49] |
Malaria (1) | Resolution (1) | ||
| Photosensitivity (1) (Soria A, 2015) [54] |
SLE (1) | NA (1) | ||
| Palmo-plantar exfoliation (1) (Nair PA, 2017) [58] |
Malaria (1) | NA (1) | ||
| Urticaria (1) (Balamurugesan K, 2019) [61] |
Malaria (1) | Resolution (1) | ||
| Psychiatric | 20 (15) | Psychosis (9) (Burrell Z, 1958; Dornhorst AC, 1963; Rab SM, 1963; Oscar L, 1964; Kabir SM, 1969; Bomb BS, 1975; Ward WQ, 1985; Choughule A, 2019) [66,67,68,69,70,71,74,81] |
Acute myocardial infarction (1) Rheumatoid arthritis (1) Hepatic or intestinal amoebiasis (4) Malaria (3) |
Resolution (8) NA (1) |
| Moderate to severe depression (2) (Das EM, 1981) [72] |
Malaria (2) | Resolution (2) | ||
| Mania (5) (Akhtar S, 1993; Plesnicar BK, 2013) [75,78] |
Malaria (4) Rheumathoid arthritis (1) |
Resolution (3) Disorder remains beyond 5 months (1) Mild attention deficit and memory difficulties (1) |
||
| Psychotic disorder with symptoms of depersonalization and anxiety (1) (Telgt DS, 2005) [76] |
Malaria (1) | Resolution (1) | ||
| Organic delusional (schizophrenia-like) disorder (1) (Sahoo S, 2007) [77] |
Malaria (1) | Resolution (1) | ||
| Exacerbation of bipolar disorder (maniac episode with psychotic features) (1) (Bogaczewicz J, 2014) [79] |
SLE and arthritis (1) | Resolution (1) | ||
| Paranoid-like disorder (1) (Bogaczewicz A, 2016) [80] |
SLE (1) | Resolution (1) | ||
| Neurologic | 15 (11) | Seizures (4), grand mal seizure (1) (Torrey EF, 1968; Martin AN 2016) [82,92] |
Hepatic or intestinalamoebiasis (4) Prophylactic treatment of gastrointestinal parasitic infection (1) |
Resolution (4) NA (1) |
| Involuntary movements (1) (Umez-Eronini EM, 1977) [83] |
Fever (1) | Resolution (1) | ||
| Akathisia and persistent protrusion of the tongue (1) (Singh RP, 1981) [84] |
Malaria (1) | Resolution (1) | ||
| Auditory hallucinations, acute psychotic behaviour, difficulty in swallowing, protrusion of the tongue, and marked extrapyramidal rigidity (1) (Singh RP, 1981) [84] |
Malaria (1) | Resolution (1) | ||
| Serious tonic-clonic convulsion (1) (Fish DR, 1988) [85] |
Malaria prophylaxis (1) | Serious consequences (1) | ||
| Severe cerebral ataxia with extrapyramidal movements (1) (James RF, 1988) [86] |
Malaria (1) | Resolution (1) | ||
| Transient global amnesia (1) (Cras P, 1990) [87] |
Malaria prophylaxis (1) | Resolution (1) | ||
| Retinopathy and persisting mild ocular myasthenia (1) (De Bleecker J, 1991) [88] |
Malaria (1) | Symptoms persisted more than 10 years after drug discontinuation (1) | ||
| Tonic-clonic seizures (2) (Adamolekun B, 1992, Ebenso BE, 1998) [89,91] |
Suspected malaria (1) Erythema nodosum leprosum (1) |
Resolution (2) | ||
| Non-convulsive status epilepticus (1) (Mülhauser P, 1995) [90] |
Malaria prophylaxis (1) | Resolution (1) | ||
| Cardiac | 3 (3) | Cardiovascular collapse (1) (Sogani RK, 1986) [94] |
Dermatologic problem (1) | NA (1) |
| Cardiac arrhythmia (1) (Siqueira-Batista R, 1998) [95] |
Malaria (1) | Resolution (1) | ||
| Syncopal attacks and torsade de pointes (1) (Yelve K, 2012) [98] |
Hepatic and intestinal amoebiasis (1) | Resolution (1) | ||
| Hematologic and metabolic | 2 (2) | Hypoglycaemia (1) (Abu-Shakra M, 1994) [100] |
Psoriatic arthritis (1) | Resolution (1) |
| Methaemoglobinaemia (1) (Rizvi I, 2012) [103] |
Fever (1) | Resolution (1) | ||
| Sense organs | 3 (3) | Diplopia and persistent blurred near vision (1) (Rubin ML, 1970) [106] |
Hypercalcemia associated with sarcoidosis (1) | Resolution (1) |
| Vestibular toxicity (1) (Malik MK, 1977) [108] |
Malaria (1) | NA (1) | ||
| Loss of hearing (1) (Dwivedi GS,1978) [109] |
Malaria (1) | Tinnitus and hearing loss have so far persisted for 5.5 months without improvement (1) | ||
| Hepatic | 1 (1) | Hepatotoxic reaction (1) (Liu AC, 1995) [113] |
Malaria prophylaxis (1) | Resolution (1) |
| Other | 3 (3) | CQ overdose with severe headache, dizziness on standing, nausea and blurred vision (1) (Davis TM, 2003) [117] |
Malaria (1) | Resolution (1) |
| Severe myopathy (1) (Richter JG, 2003) [118] |
SLE with arthralgia and renal involvement (1) | NA (1) | ||
| Acute eosinophilic pneumonitis (1) (Knudsen L, 2009) [120] |
Mild rosacea (1) | NA (1) |
CQ: Chloroquine, NA: not available/not applicable, SLE: systemic lupus erythematosus.
Case-Control, Cross-Sectional, Cohort, and Randomised Studies
Forty-seven articles reported data on the safety of CQ or HCQ when used alone and nine others provided data for when CQ or HCQ were combined with one of the drugs of interest [126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180]. Table 3 and Table 4 show the frequency of adverse drug reactions as reported in these studies. Data from case-control and other observational studies in which the frequency could not be calculated are presented in Table 5. Full data from these studies can be found in Tables S13–S20 of Supplementary Material part 2.
Table 3.
Summary of included studies reporting data on the frequency of early adverse events of CQ or HCQ alone (except COVID-19).
| First Author, Year | Type of Study | Drug, Sample Size | Gastrointestinal Disorders | Hepatobiliary | Neurological | Sense Organs | Dermatological | Other | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diarrhea | Nausea/Vomiting | Abdominal Pain/Dyspepsia | Bilirubin/GGT Increase | Transaminase Increase | Headache/Dizziness | ||||||
| Malaria treatment and prophylaxis | |||||||||||
| Weinke T, 1992 [126] | OBS | CQ n = 301 |
NA | NA | 4.7% | NA | NA | Headache 0.3% Dizziness 0.3% |
Tinnitus 0.7% | Exanthema 0% Pruritus 3.3% |
Psychosis 0% ECG changes 0% |
| Bussaratid V, 2000 [127] | OBS | CQ n = 1189 |
NA | NA | NA | NA | NA | NA | NA | Pruritus 1.9% | NA |
| Olayemi O, 2003 [128] | OBS | CQ n = 200 |
NA | NA | NA | NA | NA | NA | NA | Pruritus 64.5% | NA |
| Gama H, 2009 [129] | OBS | CQ n = 542 |
NA | NA | NA | NA | NA | NA | NA | Pruritus 30.1% | NA |
| Jeevangi SR, 2010 [130] | OBS | CQ n = 128 |
NA | Nausea 9.4% Vomiting 9.4% |
NA | NA | NA | NA | Tinnitus 9.4% | NA | Anorexia 9.4% |
| Ballut PC, 2013 [131] | OBS | CQ n = 510 |
NA | NA | NA | NA | NA | NA | NA | Pruritus 20.4% | NA |
| Gozal D, 1991 [138] | RAN | CQ n = 78 |
3.8% | Nausea 11.5% Vomiting 8.9% |
24.4% | NA | NA | Headache 10.2% | Visual disturbances 1.3% | Pruritus 1.3% | Anorexia 16.7% Oral ulcers 19.2% |
| McClean K, 1992 [139] | RAN | CQ n = 18 |
NA | NA | NA | NA | NA | NA | NA | Pruritus 44.4% | NA |
| Yanze MF, 2001 [171] | RAN | CQ n = 60 |
8.3% | Nausea 10.0% Vomiting 3.3% |
11.7% | NA | NA | Headache 3.3% Dizziness 5.0% |
NA | Pruritus 5.0% | NA |
| Dunne MW, 2005 [140] | RAN | CQ n = 102 |
NA | Nausea 4.9% Vomiting 7.8% |
NA | NA | NA | Headache 1.0% | NA | Cutaneous drug eruption 2.9% Pruritus 7.8% |
Myalgia 0% |
| Tagbor H, 2006 [172] | RAN | CQ n = 225 |
NA | Nausea 22.2% Vomiting 31.1% |
NA | NA | NA | Dizziness 43.1% | NA | Pruritus 39.1% | Weakness 47.1% |
| Ratcliff A, 2007 [141] | RAN | CQ n = 40 |
NA | Vomiting 10.0% | NA | NA | NA | NA | NA | NA | NA |
| Massaga JJ, 2008 [142] | RAN | CQ n = 20 |
NA | NA | 30% | NA | 0% | Headache 25.0% | NA | NA | Weakness 20.0% Fever 15.0% |
| Dunne MW, 2005 (2) [146] | RAN | CQ n = 16 |
6% | Nausea 0.0% Vomiting 0.0% |
NA | NA | NA | Dizziness 19.0% | NA | Pruritus 19.0% | Pharyngitis 6.0% Fatigue 13.9% |
| Poravuth Y, 2011 [149] | RAN | CQ n = 228 |
NA | Vomiting 1.8% | NA | 0% | 0.43% | Headache 1.3% Dizziness 2.2% |
NA | NA | Fatigue 0.4% Anorexia 0.9% QT prolongation 2.7% |
| Watt G, 1988 [151] | RAN | CQ n = 10 |
20% | Vomiting 10.0% | NA | NA | NA | NA | NA | Pruritus 10.0% | NA |
| Systemic lupus erythematosus and cutaneous lupus erythematosus | |||||||||||
| Kishi CJ, 2018 [13] | OBS | HCQ n = 31 |
6.4% | NA | 3.2% | NA | NA | Dizziness 3.2% | Visual disturbances 3.2% |
Erythema 6.4% | NA |
| Gonzalez CD, 2019 [134] | OBS | HCQ/CQ n = 136 |
NA | NA | NA | NA | NA | NA | NA | Cutaneous drug eruption 4.0% | NA |
| Chasset F, 2018 [132] | OBS | HCQ/CQ n = 64 |
NA | NA | NA | NA | NA | NA | NA | Exanthema 1.6%c | NA |
| Pulmonary sarcoidosis | |||||||||||
| Baltzan M, 1999 [145] | RAN | CQ n = 23 |
NA | NA | 4.3% | NA | NA | NA | NA | Cutaneous drug eruption 4.3% | Anxiety 4.3% |
| Rheumatoid arthritis | |||||||||||
| Haar D, 1993 [143] | RAN | HCQ n = 28 |
NA | NA | 3.6% | NA | NA | NA | NA | NA | NA |
| Dermatomyositis | |||||||||||
| Gonzalez CD, 2019 [134] | OBS | HCQ/CQ n = 44 |
NA | NA | NA | NA | NA | NA | NA | Cutaneous drug eruption 5.0% | NA |
| Refractory chronic urticaria and chronic autoimmune urticaria | |||||||||||
| Seth S, 2017 [137] | OBS | HCQ n = 45 |
NA | NA | NA | NA | NA | NA | NA | Pruritus 2.2% | NA |
| Reeves GE, 2004 [148] | RAN | HCQ n = 9 |
NA | NA | NA | NA | NA | NA | NA | NA | Significant toxicity 0% |
| Porphyria cutanea tarda | |||||||||||
| Petersen CS, 1992 [135] | OBS | HCQ n = 72 |
NA | Nausea 20.8% Vomiting 12.5% |
20.8% | Icterus 1.4% | 95.8% | Headache 25.0% | NA | NA | Arthralgia 5.5% Hepatomegaly 2.8% Myalgia 26.4% |
| Rossmann-Ringdahl, 2007 [136] | OBS | CQ n = 57 |
NA | NA | NA | NA | 100% | NA | NA | NA | NA |
| Human immunodeficiency virus type 1 | |||||||||||
| Sperber K, 1995 [147] | RAN | HCQ n = 19 |
NA | NA | NA | NA | NA | NA | NA | NA | Adverse reactions 0% |
| Breast cancer | |||||||||||
| Arnaout A, 2019 [150] | RAN | CQ n = 46 |
17.4% | Nausea and/or abdominal cramps 23.9% | NA | NA | NA | Dizziness 8.7% |
Visual symptoms 8.7% Documented visual changes 0% Auditory symptoms 2.2% |
NA | Fatigue 2.2% Muscle weakness 8.7% Dry mouth 4.3% |
| Chikungunya acute infection | |||||||||||
| De Lamballerie X, 2008 [154] | RAN | CQ n = 27 |
NA | NA | NA | NA | NA | NA | NA | NA | Mild adverse reactions (mainly nausea and pruritus) 25.9% |
| Dengue | |||||||||||
| Tricou V, 2010 [155] | RAN | CQ n = 153 |
NA | Vomiting 4.1% | NA | NA | NA | NA | NA | NA | NA |
| Borges MC, 2013 [156] | RAN | CQ n = 19 |
NA | NA | NA | NA | NA | NA | Blurred vision 5.2% |
NA | Loss of consciousness 5.2% |
| Infectious mononucleosis | |||||||||||
| Cowley RG, 1962 [152] | OBS | CQ n = 20 |
Gastrointestinal complaints (anorexia, nausea, vomiting) 60% | NA | NA | NA | NA | NA | NA | ||
| Schumacher HR, 1963 [153] | OBS | CQ n = 5 |
NA | NA | NA | NA | NA | NA | NA | NA | Complications 0% |
CQ: chloroquine, ECG: electrocardiogram, GGT: gamma-glutamyl transferase, HCQ: hydroxychloroquine, NA: not available/not applicable, OBS: observational, RAN: randomised.
Table 4.
Summary of studies: early adverse events of CQ in combination with AZM in patients affected by malaria or who received prophylactic treatment.
| Type of Study, Arm and Sample Size | Randomised AZM Plus CQ n = 114 |
Randomised AZM Plus CQ n = 113 |
Randomised AZM Plus CQ n = 1446 |
Randomised AZM Plus CQ n = 64 |
Single-Arm AZM Plus CQ n = 168 |
Single-Arm AZM (2 g) Plus CQ n = 110 |
Randomised AZM(1 g) Plus CQ n = 197 |
Randomised AZM (0.5 g) Plus CQ n = 81 |
|---|---|---|---|---|---|---|---|---|
| Author, year | Sagara I, 2014 [173] | Kimani J, 2016 [179] | Dunne MW, 2005 [146] | Phiri K, 2016 [180] | Kshirsagar NA, 2017 [174] | |||
| Any AEs | 78.1% | 70.8% | 68.9% | 20% | NA | 44% | 26% | 10% |
| Abdominal pain/discomfort | 7.0% | 11.5% | 8.3–8.5% | NA | NA | 0% | 3% | 0% |
| Asthenia | 5.3% | 8.0% | 16.6% | NA | NA | NA | NA | NA |
| Blood/lymphatic disorders | NA | NA | 14.3% | NA | NA | NA | NA | NA |
| Dehydration | NA | NA | NA | NA | NA | 4% | 0% | 0% |
| Diarrhea | 5.3% | 9.7% | 14.2% | 3% | NA | 12% | 4% | 0% |
| Dizziness | 9.6% | 15.9% | 32.0% | 0% | 19.6% | NA | NA | NA |
| Fatigue | 0% | 3.5% | 5.6% | NA | 4.2% | NA | NA | NA |
| Gastritis | NA | NA | NA | NA | NA | 4% | 2% | 1% |
| Headache | 13.2% | 17.7% | 20.7% | NA | 6.0% | 0% | 2% | 0% |
| Infections | NA | NA | 30.1% | Pharyngitis 0% | Parasitic infection 7.1% Upper respiratory infection 4.2% |
NA | NA | NA |
| Nausea | 7.9% | 8.8% | 14.9% | 6% | 3.6% | 30% | 0% | 0% |
| Pain | 1.8% | 5.3% | NA | NA | NA | NA | NA | NA |
| Palpitations | 2.6% | 0% | NA | NA | NA | NA | NA | NA |
| Paraesthesia | NA | NA | NA | NA | NA | 0% | 3% | 0% |
| Pruritus | 50.9% | 28.3% | NA | 2% | Pruritus 7.7% Generalised pruritus 5.4% |
4% | 15% | 6% |
| Visual disorders | NA | NA | 10.1% | NA | NA | NA | NA | NA |
| Vomiting | 15.8% | 3.5% | 45.2% | 8% | 20.8% | 18% | 4% | 1% |
AEs: adverse events, AZM: azithromycin, CQ: chloroquine, g: grams, NA: not available/not applicable.
Table 5.
Adverse effects reported on case-control studies and observational studies not included in data synthesis.
| First Author, Year | Drug, Indication |
Adverse Effect |
|---|---|---|
| CQ or HCQ alone | ||
| Obasikene G, 2012 [162] | CQ malaria |
Ototoxicity |
| Ajayi AA, 1989 [157] | CQ malaria |
Pruritus |
| Castro-Cavadía CJ, 2020 [166] | CQ malaria |
AEs were confused in frequency and intensity with malaria symptoms and signs |
| Schneider C, 2013 [163] | CQ malaria |
Neuropsychiatric disorder |
| Sarathi P, 2014 [165] | CQ malaria |
Psychiatric manifestation |
| Dugué A, 2004 [167] | HCQ NA |
Muscular adverse events |
| Sidoroff A, 2007 [169] | CQ and HCQ NA |
AGEP |
| George AO, 2004 [161] | CQ malaria |
Pruritus |
| Patel KJ, 2007 [168] | CQ NA |
Gastritis |
| Emerole CG, 2014 [164] | CQ malaria |
Loss of visual acuity |
| Ajayi AA, 1998 [175] | CQ malaria |
Pruritus |
| Katugampola G,1990 [158] | CQ malaria |
Worsening of psoriasis |
| Frías Salcedo JA, 1992 [159] | CQ malaria |
Visual and gastrointestinal disturbances or pruritus and headache |
| Yanze MF, 2001 [171] | CQ malaria |
Headache, diarrhea, abdominal pain, nausea, pruritus, dizziness, and vomiting |
| Walsh DS, 1999 [170] | CQ malaria |
Abdominal discomfort and diarrhea |
| Garcia P, 2020 [280] | HCQ COVID-19 |
Psychiatric disorders |
| CQ or HCQ combined with other drug | ||
| Vouri SM, 2020 [177] | CQ and HCQ plus AZM autoimmune disease | Sudden cardiac arrest, ventricular arrhythmias, and cardiac symptoms |
| Sarayani A, 2021 [178] | CQ and HCQ plus AZM NA | CQ and HCQ appeared not to be associated with a safety risk related to torsade de pointes or QT prolongation when used alone, when used with AZM they were associated with a potential safety risk |
| Ajayi AA, 1991 [175] | CQ plus prednisolone malaria |
Pruritus |
| Adebayo RA, 1997 [176] | CQ plus prednisolone malaria |
Pruritus |
AE: adverse event, AGEP: acute generalised exanthematous pustulosis, AZM: azithromycin, COVID-19: Coronavirus Disease 2019, CQ: chloroquine, HCQ: hydroxychloroquine, NA: not available/not applicable, TCZ: tocilizumab.
3.1.2. Patients Treated for COVID-19
Case and Case Series Reports
A total of 26 articles reporting cases related to the safety of HCQ or CQ during treatment for COVID-19 were found [181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206]. Table 6 synthesises the data from the cases reporting HCQ and CQ adverse drug reactions in COVID-19-affected patients and Table S21 of Supplementary Material part 2 provides full details.
Table 6.
Summary of studies included: COVID-19 case reports related to chloroquine or hydroxychloroquine adverse effects in patients with suspected or confirmed COVID-19 or who were prophylactically treated.
| Organ Affected | Number of Patients | Adverse Effect, Drug Combination If Required (Number of Patients) (First Author, Year) | Long-Term Consequences |
|---|---|---|---|
| CQ | |||
| Cardiac | 2 | Major QT prolongation and recurrent torsade de pointes (1) (Szekely Y, 2020) [188] |
ECGs showed gradual normalization of QT interval |
| Wide complex tachycardia, along with AZM (1) (Gracia-Ramos AE, 2021) [189] |
Death after cardiac arrest | ||
| Hematologic and metabolic | 1 | G6PD deficiency-associated haemolysis and methaemoglobinaemia (1) (Kuipers MT, 2020) [184] |
The patient’s methaemoglobin normalized within 6 days |
| Psychiatric | 3 | Psychotic symptoms, along with AZM (1) (Benjelloun R, 2020) [195] |
Resolution after 48 h |
| Acute and intense anxiety, along with AZM (1) (Benjelloun R, 2020) [195] |
No | ||
| Psychosis episode (1) (Ambar Akkaoui M, 2021) [193] |
NA | ||
| HCQ | |||
| Cardiac | 6 | Right bundle brunch block and critically prolonged QTc (1) (Asli R, 2020) [181] |
Resolution |
| QT interval prolongation in a patient on AZM (1) (Mitra RL, 2020) [186] |
Death owing to progressive metabolic acidosis and multiorgan system failure | ||
| QTc prolongation and torsade de pointes, along with dexamethasone (1) (Aslam W, 2021) [190] |
NA | ||
| Suspected HCQ-induced sinus bradycardia and QTc interval prolongation (1) (Kang Y, 2020) [191] |
A temporary pacemaker was implanted | ||
| QTc prolongation, along with AZM (1) (Patel J, 2020) [192] |
No | ||
| Sinus bradycardia, along with AZM and corticosteroids (1) (Patel J, 2020) [192] |
No | ||
| Dermatological | 12 | Psoriasis exacerbation (1) (Kutlu Ö, 2020) [185] |
NA |
| AGEP with erythema multiforme-like lesions (1) (Robustelli Test E, 2020) [187] |
Slow but progressive resolution | ||
| Rash (1) (Kurd R, 2020) [202] |
NA | ||
| AGEP (1) (Enos T, 2020) [199] |
Resolved with prednisone after 38 days | ||
| AGEP (1) (Delaleu J, 2020) [198] |
NA | ||
| Erythema multiforme (1) (Monte-Serrano J, 2020) [197] |
NA | ||
| Urticaria with maculopapular rash, palmoplantar itching (1) (Sardana K, 2020) [196] |
NA | ||
| Urticaria (1) (Sardana K, 2020) [196] |
NA | ||
| Palmoplantar itching (1) (Sardana K, 2020) [196] |
NA | ||
| DRESS syndrome, along with AZM and LPVr (1) (Castro Jiménez A, 2021) [200] |
NA | ||
| Purpuric erythematous rash with non-follicular pustules, on the trunk and limps, with intense involvement of armpits and scalp (1) (Abadías-Granado I, 2021) [201] |
No | ||
| Purpuric erythematous rash with non-follicular pustules and targetoid lesions on the back (1) (Abadías-Granado I, 2021) [201] |
No | ||
| Hematologic, muscular and metabolic | 6 | Worsening of haemolysis (1) (Beauverd Y, 2020) [183] |
NA |
| Haemolysis in a G6DP-deficient patient (1) (Maillart E, 2020) [204] |
NA | ||
| Haemolytic anemia in a G6DP-deficient patient (1) (Aguilar J, 2020) [206] |
NA | ||
| Acute haemolytic anemia in a G6DP-deficiency patient (1) (Chaney SI, 2020) [205] |
NA | ||
| Thrombotic thrombocytopaenic purpura (1) (Arıkan F, 2020) [203] |
No | ||
| Hepatic | 1 | Hepatotoxicity (1) (Falcão MB, 2020) [182] |
NA |
| Ophthalmology | 1 | Myasthenic syndrome (1) (Koc G, 2020) [194] |
No |
| Gastrointestinal | 1 | Nausea, vomiting, diarrhea (1) (Patel J, 2020) [192] |
NA |
AGEP: acute generalised exanthematous pustulosis, AZM: azithromycin, CQ: chloroquine, DRESS: drug rash with eosinophilia and systemic symptoms, ECGs: electrocardiograph, G6PD: glucose-6-phosphate dehydrogenase, HCQ: hydroxychloroquine, LPVr: lopinavir plus ritonavir, NA: not available/not applicable.
Case-Control, Cross-Sectional, Cohort, and Randomised Studies
A total of 74 articles reporting data on the safety of HCQ or CQ in patients treated for COVID-19 were found [207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280]. Table 7 and Table 8 show the frequency of adverse drug reactions as reported in these studies. The full data is provided in Table S22 of the Supplementary Material part 2.
Table 7.
Frequency of adverse effects of CQ or HCQ alone when treating COVID-19 reported in observational and randomised studies.
| First Author, Year | Type of Study, Drug and Sample Size | Cardiac | Gastrointestinal Disorders | Hepatobiliary | Neurological | Sense Organs | Dermatological | Other | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QT Prolongation | Prolonged QTc ≥ 500 ms | Prolongued QTc ≥ 60 ms | Ventricular Arrythmia | Torsade Depointes | Arrhythmogenic Death | Diarrhea | Nausea/Vomiting | Abdominal Pain/Dyspepsia | Bilirubin/GGT Increase | Transaminase Increase | Headache/Dizziness | |||||
| Seyhan AU, 2020 [241] | OBS HCQ n = 51 |
NA | 1.96% | 1.96% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Abella BS, 2021 [270] |
RAN HCQ n = 132 |
NA | NA | NA | NA | NA | NA | 32% | 9% | 6% | NA | NA | Headache 0% Dizziness 2% |
NA | Rash 5% | Paraesthesia 2% |
| Bernardini A, 2021 [246] | OBS HCQ n = 40 |
40% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Furtado RHM, 2020 [247] | RAN HCQ n = 183 |
21% | NA | NA | CRVA 3% | NA | 0% | NA | NA | 24% | 3% | NA | NA | NA | NA | NA |
| Satlin MJ, 2020 [250] | OBS HCQ n = 153 |
NA | NA | NA | Monomorphic VT 0.6% | 0% | NA | NA | NA | NA | NA | Grade 3 11% Grade 4 9% |
NA | NA | NA | NA |
| Hsia BC, 2020 [253] | OBS HCQ n = 40 |
NA | NA | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| OBS CQ n = 5 |
NA | NA | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Skipper CP, 2020 [252] |
RAN HCQ n = 212 |
NA | NA | NA | NA | NA | NA | 23.6% | 31.1% | NA | NA | NA | Headache 0.9% Dizziness 9.4% |
Ringing in ears 3.8% Changes in vision 1.9 Taste, dry mouth 0% |
Rash 2.8 | NA |
| Boulware DR, 2020 [271] | RAN HCQ n = 414 |
NA | NA | NA | NA | NA | NA | NA | 22.9% | 23.2% | NA | NA | 3.7% | Tinnitus 2.3% Visual changes 0.9% Taste change or dry mouth 0.9% |
Skin reaction 1.1% | NA |
| Falcão F, 2020 [239] | OBS HCQ n = 20 |
10% | NA | NA | NA | NA | NA | 5% | Nausea 10% Vomiting 5% |
0% | Liver cholestasis 0% | 10% | NA | Ocular disorders 0% | Skin and subcutaneous disorders 10% | NA |
| Sogut O, 2021 [240] | OBS HCQ n = 152 |
64.5% | 0% | 0% | NA | NA | 0% | 22.3% | NA | NA | 16.4% | NA | Itching and redness 2.6% | NA | ||
| Mitjà O, 2021 [268] | RAN HCQ n = 1116 |
NA | NA | NA | NA | NA | NA | 42.6% | NA | NA | 21.7% | NA | NA | General disorder: myalgia, fatigue, malaise 8.6% | ||
| Barnabas RV, 2021 [269] | RAN HCQ n = 407 |
NA | NA | NA | NA | NA | NA | NA | 3.4% | 6.1% | NA | NA | Headache 1.2% Dizziness 1.5% |
Taste change or dry mouth 0.2% Visual changes 1% Tinnitus 0% |
Rash 2.7% | Fatigue 1% |
| Nagaraja BS, 2020 [272] | OBS HCQ n = 156 |
NA | NA | NA | NA | NA | NA | 7.22% | Nausea 10.24% Vomiting 1.20% |
7.22% | NA | NA | Headache 6% Dizziness 3.6% |
Tinnitus 0.6% Transient visual blurring 2.4% |
Hair fall 1.8% Oral ulcer 1.2% Itching 0.6% |
Psychiatric 4.8% Nightmare 0.6% Nervousness 1.20% Fatigue, lethargyWeakness 7.2% |
| Özdemir IH, 2020 [256] | OBS HCQ n = 45 |
NA | NA | NA | NSVT 0% SVT 0% VF 0% |
0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Cavalcanti AB, 2021 [257] | RAN HCQ n = 221 |
14.6% | NA | NA | VT 0% | NA | NA | NA | Nausea 4.5% Vomiting 0% |
NA | 2.5% | 8.5% | NA | Hypoacusia 0% | Itching 0.5% | Hypoglycaemia 0.5% |
| Çap M, 2020 [258] | OBS HCQ n = 66 |
6% | NA | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Lauriola M, 2020 [259] |
OBS HCQ n = 17 |
NA | NA | NA | NA | NA | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Ramireddy A, 2020 [264] | OBS HCQ n = 10 |
NA | NA | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Tanriverdİ E, 2021 [260] | OBS HCQ n = 30 |
NA | NA | NA | 0% | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Arshad S, 2020 [261] |
OBS HCQ n = 1202 |
NA | NA | NA | NA | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Pereira MR, 2020 [265] | OBS HCQ n = NA |
0% | NA | NA | 0% | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Jain S, 2020 [230] | OBS HCQ n = 415 |
23.6% | NA | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Hor CP, 2020 [228] | OBS HCQ n = 2 |
100% | NA | NA | 0% | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Paccoud O, 2020 [227] |
OBS HCQ n = 38 |
5.3% | NA | NA | NA | NA | NA | 2.6% | NA | NA | NA | NA | Headache 2.6% | NA | NA | NA |
| Lagier JC, 2020 [226] |
OBS HCQ n = 101 |
NA | NA | 2% | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Reis G, 2021 [233] | RAN HCQ n = 207 |
NA | NA | NA | NA | NA | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Faruqui AR, 2021 [267] | OBS HCQ n = 1303 (HCQ + AZ: 0.8%; CQ: 0.5%) |
NA | NA | NA | NA | NA | NA | NA | Nausea 8.7% Vomiting 1.4% |
7.0% | NA | NA | NA | Photosensitivity 0.5% | NA | NA |
| Eftekhar SP, 2021 [236] |
OBS HCQ n = 29 |
10.3% | NA | NA | 0% | 3.4% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Mazzanti A, 2020 [229] |
OBS HCQ n = 50 |
NA | NA | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Karolyi M, 2021 [231] |
OBS CQ n = 20 |
NA | NA | NA | NA | NA | NA | 0% | 5% | NA | NA | 10% | NA | NA | NA | NA |
| Mitjà O, 2020 [232] | RAN HCQ n = 136 |
NA | NA | NA | NA | NA | 0% | 88.1% | NA | NA | 37.5% | Ear and labyrinth disorders 3% Eye disorders 3% |
6.5% | Psychiatric disorders 1.2% | ||
| Lofgren SM, 2020 [266] | RAN HCQ once-daily n = 576 |
NA | NA | NA | NA | NA | 0% | Upset stomach or nausea 25.3% Diarrhea, vomiting, or abdominal pain 22.7% |
NA | NA | Headache 2.6% Irritability, dizziness, vertigo 6.8% |
Tinnitus 2.8% Visual changes 1.2% Taste change or dry mouth 0.5% |
Skin reaction 1.7% | Panic 0% | ||
| RAN HCQ once-weekly n = 473 |
NA | NA | NA | NA | NA | 0% | Upset stomach or nausea 17.5% Diarrhea, vomiting, or abdominal pain 12.9% |
NA | NA | Irritability, dizziness, vertigo 5.7% | Tinnitus 2.1% Visual changes 1.5% |
Skin reaction 2.7% | Sleep disturbance 2.1% | |||
| RAN HCQ twice-weekly n = 463 |
NA | NA | NA | NA | NA | 0% | Upset stomach or nausea 19.4% Diarrhea, vomiting, or abdominal pain 17.1% |
NA | NA | Irritability, dizziness, vertigo 5.2% | Tinnitus 1.5% Visual changes 0.9% |
Skin reaction 5.0% | Sleep disturbance 1.5% | |||
| Bessière F, 2020 [207] |
OBS HCQ n = 22 |
NA | 5% | NA | NA | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Mercuro NJ, 2020 [215] |
OBS HCQ n = 37 |
NA | 19% | 8% | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Rosenberg ES, 2020 [218] | OBS HCQ n = 271 |
14.4% | NA | NA | NA | NA | NA | 17.0% | NA | NA | NA | NA | NA | NA | NA | NA |
| Saleh M, 2020 [219] | OBS HCQ or CQ n = 82 |
NA | 8.5% | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Van den Broek MPH 2020 [220] | RAN CQ n = 95 |
NA | 23% | NA | NA | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Chen Z, 2020 [222] | RAN HCQ n = 31 |
NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | Headache 3.2% | NA | 3.2% | NA |
| Huang M, 2020 [223] |
RAN CQ n = 10 |
NA | NA | NA | NA | NA | NA | 50.0% | Nausea 40% Vomiting 50% |
10% | NA | NA | Headache 0% Dizziness 0% |
NA | 10% | Psychosis 0% |
| Fernández-Ruiz M, 2020 [212] |
OBS HCQ n = 4 |
NA | 25% | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Chen J, 2020 [224] | OBS HCQ n = 15 |
NA | NA | NA | NA | NA | NA | 13.3% | NA | NA | 6.66% | NA | NA | NA | NA | Weakness 6.6% |
AEs: adverse effects, AF: atrial fibrillation, BBB: bundle branch block, CRVA: clinically rellevant ventricular arryhtmia, COVID-19: coronavirus disease 2019, CQ: chloroquine, ECG: electrocardiograph, HCQ: hydroxychloroquine, ms: milliseconds, NA: not available/not applicable, NSVT: non-sustained ventricular tachycardia, OBS: observational, QTc: corrected QT interval, RAN: randomised, SVT: sustained ventricular tachycardia, SVT: supraventricular tachycardia, VF: ventricula fibrillation, VT: ventricular tachycardia.
Table 8.
Adverse effects of CQ or HCQ in combination with other drugs used to treat COVID-19 reported in observational and randomised studies.
| First Author, Year | Type of Study, Drug and Sample Size | Cardiac | Gastrointestinal | Hepatobiliary | Neurological | Sense Organs | Dermatological | Other | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QT Prolongation | Prolonged QTc ≥ 500 ms | Prolongued QTc ≥ 60 ms | Ventricular Arrythmia | Torsade de Pointes | Arrhythmogenic Death | Diarrhea | Nausea/Vomiting | Abdominal Pain/Dyspepsia/Other | Bilirubin Increase/GGT Increase | Transaminase Increase | Headache/Dizziness | |||||
| Gao X, 2020 [273] | OBS INF-alpha + LPVr + arbidol or rivabirin or CQ n = 26 |
NA | NA | NA | NA | NA | NA | 11.5% | NA | NA | Abnormal liver function 61.5% | NA | NA | NA | Rash 7.7% |
Dyslipidemia 42.3% |
| Seyhan AU, 2020 [241] | OBS HCQ + AZM n = 93 |
NA | 1.07% | 2.15% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Lamback EB, 2021 [237] | OBS HCQ + AZM n = 101 |
7.9% | NA | NA | NA | NA | NA | 7.9% | NA | NA | NA | NA | NA | NA | ||
| Saleh M, 2020 [245] | OBS HCQ ± AZM n = 6.476 (HCQ n = 2847 HCQ + AZM n = 3629) |
NA | NA | NA | VF 0.06% SMVT 0.08% NSMVT 0.27% SPVT 0.015% NSPVT 0% |
0.015% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Bernardini A, 2021 [246] | OBS HCQ + AZM n = 53 |
70% | 8% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Furtado RHM, 2020 [247] | RAN HCQ + AZM n = 214 |
20% | NA | NA | CRVA 3% | NA | NA | NA | NA | 25% | Bilirrubin increase > 50% 4% |
NA | NA | NA | NA | NA |
| Giaime P, 2020 [248] | OBS HCQ + AZM n = 21 |
NA | 4.8% | NA | NA | NA | NA | NA | 19% | NA | NA | NA | NA | Visual impairment 0% | Dermatitis 0% | Hypoglicaemia 23.8% |
| Kalligeros M, 2020 [249] | OBS HCQ + AZM n = 32 |
3.1% | 9.4% | NA | VC 0.9% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA | Seizure 3.1% |
| Kelly M, 2021 [276] | OBS HCQ + AZM n = 82 |
13.4% | NA | NA | NA | NA | NA | NA | NA | NA | NA | Elevated liver function tests 65% | NA | NA | NA | NA |
| Hsia BC, 2020 [253] | OBS HCQ + AZM n = 33 |
NA | NA | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| OBS CQ + AZM n = 4 |
NA | NA | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Moschini L, 2021 [251] |
OBS HCQ + AZM n = 52 |
NA | 13% (day 3), 20% (day 7) | NA | MVA 1.9% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| OBS HCQ + DRVr n = 61 |
NA | NA | NA | MVA 1.6% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| O’Connell TF, 2021 [238] | OBS HCQ + AZM n = 415 |
NA | 21% | NA | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Falcão F, 2020 [239] | OBS HCQ + AZM n = 52 |
5.7% | NA | NA | NA | NA | NA | 1.9% | Nausea 3.8% Vomiting 1.9% |
3.8% | Bilirrubin increase 0% GGT increase 1.9% Liver cholestasis 7.7% |
3.84% Hepatotoxicity 7.7% |
NA | Ocular disorders 1.9% | Skin and subcutaneous disorders1.9% | NA |
| OBS HCQ + LPVr n = 22 |
0% | NA | NA | NA | NA | NA | 40.9% | Nausea 4.5% Vomiting 4.5% |
4.5% | Bilirrubin increase 13.6% GGT increase 13.6% Liver cholestasis 4.5% |
54.54% Hepatotoxicity 0% |
NA | Ocular disorders 4.5% |
Skin and subcutaneous disorders 0% |
NA | |
| OBS HCQ + AZM + LPVr n = 7 |
14.2% | NA | NA | NA | NA | NA | 71.4% | Nausea 14.2% Vomiting 0% |
0% | Bilirrubin increase 14.3% GGT increase 14.2% Liver cholestasis 14.2% |
42.8% Hepatotoxicity 14.2% |
NA | Ocular disorders 0% | Skin and subcutaneous disorders0% | NA | |
| Chen CP, 2020 [274] | RAN HCQ ± AMZ ± OSM ± LEV n = 21 |
0% | NA | NA | NA | NA | NA | 5.3% | 5.3% | Gastritis 5.3% | NA | NA | Headache 21.1% Dizziness 5.3% |
Photophobia 5.3% | NA | NA |
| Meriglier E, 2021 [242] | OBS HCQ + LPVr n = 21 |
NA | NA | NA | NA | NA | NA | 23.8% | 9.52% | NA | NA | 0% | Headache 0% | NA | 0% | NA |
| OBS HCQ + DRVr n = 25 |
NA | NA | NA | NA | NA | NA | 32% | 0% | NA | NA | 4% | Headache 0% | NA | 0% | NA | |
| Self WH, 2020 [275] | RAN HCQ ± AZM ± REM ± corticosteroids n = 242 |
NA | 5.9% | NA | 2.1% | NA | NA | NA | NA | NA | NA | 20.7% | NA | NA | NA | Seizure 0.4% Symptomatic hypoglycaemia 4.1% |
| Fteiha B, 2021 [243] | OBS HCQ± AZM n = 90 |
NA | 7.8% | 12% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| RECOVERY Collaborative Group, 2020 [244] | RAN HCQ± AZM n = 1561 |
NA | NA | NA | VT or fibrillation 0.7% | 0.064% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Nagaraja BS, 2020 [272] | OBS HCQ + AZM n = 7 |
NA | NA | NA | NA | NA | NA | 28.6% | NA | NA | NA | NA | NA | NA | ||
| Echarte-Morales J, 2020 [254] |
OBS HCQ + AZM n = 54 |
9.3% | 3.7% | 11.1% | NA | NA | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| OBS HCQ + AZM + LPVr n = 114 |
11.4% | 6.1% | 18.4% | NA | NA | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Jiménez-Jáimez J, 2020 [255] | OBS HCQ + AZM + LPVr or DRVr n = 114 |
NA | 1.8% | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| OBS HCQ ± AZM n = 105 |
NA | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| Özdemir IH, 2020 [256] | OBS HCQ + AZM n = 56 |
NA | NA | NA | NSVT 0% SVT 0% |
0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Cavalcanti AB, 2021 [257] | RAN HCQ + AZM n = 217 |
14.7% | NA | NA | VT 0% |
NA | NA | NA | Nausea 2.5% Vomiting 0% |
NA | Bilirrubin increase 0.4% | 10.9% | NA | Hypoacusia 0% | Itching 0% | Hypoglycaemia 0% |
| Çap M, 2020 [258] | OBS HCQ + FVP n = 66 |
3% | NA | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Rodriguez-Garcia JL, 2020 [277] |
OBS HCQ + LPVr + corticosteroids n = 50 |
NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | Delirium 4% Hyperglycemic decompensation 10% |
| Ip A, 2020 [278] | OBS HCQ ± AZM (HCQ: n = 441 HCQ + AZM n = 1473) |
NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Lauriola M, 2020 [259] |
OBS HCQ + AZM n = 297 |
NA | NA | NA | NA | NA | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Tanriverdİ E, 2021 [260] | OBS HCQ + AZM n = 26 |
NA | NA | NA | 0% | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Arshad S, 2020 [261] |
OBS HCQ + AZM n = 783 |
NA | NA | NA | NA | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Bun SS, 2020 [262] | OBS HCQ + AZM n = 71 |
NA | 2.8% | NA | NA | NA | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Dastan F, 2020 [279] |
RAN HCQ + INF-β-1a + LPVr n = 20 |
NA | NA | NA | NA | NA | 0% | NA | NA | NA | 0% | 0% | NA | NA | NA | NA |
| Maraj I, 2020 [263] | OBS HCQ + AZM n = 91 |
23% | 14% | NA | 2.2% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Ramireddy A, 2020 [264] | OBS HCQ + AZM n = 61 |
11.4% | NA | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Pereira MR, 2020 [265] | OBS HCQ + AZM n = NA |
0% | NA | NA | 0% | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Uğurlu Ilgin B, 2021 [235] | OBS HCQ + OSM + AZM n = 43 |
42.85% | 8.8% | NA | 0% | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| OBS HCQ + OSM + LEV n = 48 |
NA | 0% | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA | |||
| Hor CP, 2020 [228] | OBS HCQ + AZM n = 11 |
NA | NA | 9.1% | 0% | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Lagier JC, 2020 [226] |
OBS HCQ + AZM ≥ 3 days n = 3119 HCQ + AZM < 3 days n = 218 |
NA | 0.03% | 0.6% | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Dabbous HM, 2021 [234] | OBS HCQ + OSM n = 50 |
NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 0% | NA | NA | NA | NA |
| Eftekhar SP, 2021 [236] |
OBS HCQ + AZM n = 143 |
24.5% | NA | NA | 1.4% | 0.7% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Mazzanti A, 2020 [229] |
OBS HCQ + AZM n = 39 |
NA | NA | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| OBS HCQ + LPVr n = 53 |
NA | NA | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| OBS HCQ + LPVr + AZM n = 9 |
NA | NA | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Borba MGS, 2020 [221] | RAN CQ low dose + AZM ± OSM n = 40 |
NA | 7.9% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| RAN CQ high dose + AZM ± OSM n = 41 |
NA | 21.2% | 5.8% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Tang W, 2020 [211] | RAN HCQ + SOC n = 70 |
NA | NA | NA | NA | NA | NA | 10% | NA | NA | NA | NA | NA | NA | NA | NA |
| Chong VH, 2020 [208] | OBS HCQ + LPVr n = 11 |
NA | 18.2% | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Fernández-Ruiz M, 2020 [212] | OBS HCQ + LPVr n = 6 |
NA | NA | NA | NA | NA | NA | NA | NA | 16.7% | NA | NA | NA | NA | NA | NA |
| Colaneri M, 2020 [225] | OBS HCQ + AZM + TCZ n = 21 |
NA | NA | NA | NA | NA | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Saleh M, 2020 [245] | OBS CQ or HCQ + AZM n = 119 |
NA | 9.2% | NA | NA | 0% | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Rosenberg ES, 2020 [218] | OBS HCQ + AZM n = 735 |
11.0% | NA | NA | NA | NA | NA | 11.6% | NA | NA | NA | NA | NA | NA | NA | NA |
| Molina JM, 2020 [217] |
OBS HCQ + AZM n = 11 |
9.09% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Million M, 2020 [216] |
OBS HCQ + AZM n = 1061 |
NA | 0% | 0.8% | NA | 0% | 0% | 1.1% | Nausea 0.2% Vomiting 0.1% |
Abdominal pain 0.3% | NA | NA | Headache 0.3% | Transient blurred vision 0.2% | Erythematous and bullous rash 0.1% | Insomnia 0.2% |
| Bessière F, 2020 [207] |
OBS HCQ + AZM n = 18 |
NA | 33% | NA | NA | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Chorin E, 2020 [209] |
OBS HCQ + AZM n = 251 |
NA | 13% | NA | NA | 0.4% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Cipriani A, 2020 [210] |
OBS HCQ + AZM n = 22 |
NA | 4.54% | 18% | 4.54% | NA | 0% | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Gautret P, 2020 [213] |
OBS HCQ + AZM n = 80 |
NA | NA | NA | NA | NA | NA | 5.0% | 2.5% | NA | NA | NA | NA | Blurred vision 1.2% | NA | NA |
| Mahévas M, 2020 [214] | OBS HCQ ± AZM n = 84 |
NA | 1.2% | 8.3% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Mercuro NJ, 2020 [215] |
OBS HCQ + AZM n = 53 |
NA | 21% | 13% | NA | 1.88% | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
AF: atrial fibrillation, AVB: atrioventricular block, AZM: azithromycin, BBB: bundle branch block, COVID-19: coronavirus disease 2019, CQ: chloroquine, CRVA: clinically rellevant ventricular arryhtmia, DRVr: darunavir/ritonavir, ECG: electrocardiograph, GGT: gamma-glutamyl transferase, HCQ: hydroxychloroquine, INF-β: interferon-beta, LEV: levofloxacin, LPVr: lopinavir/ritonavir, MVA: malignant ventricular arryhtmia, NA: not available/not applicable, NSMVT: non-sustained monomorphic ventricular tachycardia, NSPVT: non-sustained polymorphic ventricular tachycardia, NSVT: non-sustained ventricular tachycardia, ms: milliseconds, OBS: observational, OSM: oseltamivir, QTc: corrected QT interval, RAN: randomised, REM: remdesivir, SMVT: sustained monomorphic ventricular tachycardia, SOC: standard of care, SPVT: sustained polimorphic ventricular tachycardia, SVT: sustained ventricular tachycardia, SVT: supraventricular tachycardia, TCZ: tocilizumab, VC: ventricular contractions, VF: ventricular fibrillation, VT: ventricular tachycardia.
3.2. Quality Assessment
For case series and case reports, overall CARE Checklist scores ranged from 34% to 100%. Only two studies could not be assessed because more than 50% of the checklist items were judged “Not applicable”, one of them reporting a case of acute psychosis after CQ administration and the other reporting a case of acute generalised exanthematous pustulosis with HCQ [47,66]. A total of 73 studies were rated as high quality, 40 as moderate quality, and 11 as low quality. Although 11 articles were rated as having low reporting quality, the adverse effects were clearly described in all cases, so this did not affect their inclusion in the qualitative synthesis of the results. A total of 84 observational studies were assessed using the combined STROBE checklist. A total of 47 studies were rated as high quality, 29 as moderate quality, and 6 as low quality. The quality of 2 other studies could not be assessed because more than 50% of the checklist items were “Not applicable”. In the case of the randomised studies, 46 articles including 49 clinical trials were assessed using the JADAD scale. A total of 16 studies received scores less than 3, whereas 33 studies received scores greater than or equal to 3.
3.3. Data Synthesis of the Systematic Review Findings
The study data on the frequency of adverse events was quantitatively synthesised and is reported as a range of percentages ordered by indication and drug combination in Table 9. Table 5 shows the adverse effects reported in the case-control studies as well as those that could not be added to the data synthesis.
Table 9.
Systematic review findings synthesised by the frequency of adverse events reported as a range of percentages.
| Treatment Indication | Malaria Treatment and Prophylaxis | Autoimmune Diseases | Porphyria Cutanea Tarda | Malaria Treatment and Prophylaxis | COVID-19 Prophylaxis | COVID-19 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drug | CQ | CQ/HCQ | CQ/HCQ | CQ plus AZM | HCQ | CQ/HCQ | CQ/HCQ + AZM | HCQ + LPVr | HCQ + DRVr | HCQ + AZM + LPVr |
| Cardiac adverse events | ||||||||||
| Arrhythmia | NA | NA | NA | NA | 0–0.2% (1) | 0–16.2% (3) | 0–20.4% (3) | NA | NA | NA |
| Palpitations | NA | NA | NA | 0–2.6% (2) | 0.4–2.4% (3) | NA | NA | NA | NA | NA |
| Cardiac arrest | NA | NA | NA | NA | NA | 0–13.7% (3) | 7–15.5% (2) | NA | NA | NA |
| ECG changes | 0% (1) | NA | NA | NA | NA | 0–27.3% (2) | 0–27.1% (2) | 19.0% (1) | 0–16% (1) | NA |
| Prolonged QTc ≥500 ms | NA | NA | NA | NA | NA | 0–25% (8) | 0–33% (18) | 18.2% (1) | NA | 6.1% (1) |
| QTc change ≥60 ms | NA | NA | NA | NA | NA | 0–8% (4) | 0–18% (9) | NA | NA | 18.4% (1) |
| Torsade de pointes | NA | NA | NA | NA | NA | 0–3.4% (19) | 0–1.88% (19) | 0% (2) | 0% (1) | 0% (2) |
| Arrhythmogenic deaths | NA | NA | NA | NA | 0% (1) | 0% (19) | 0% (18) | 0% (2) | 0% (1) | 0% (3) |
| Dermatological adverse events | ||||||||||
| Cutaneous Drug Eruptions | 2.9% (1) | 4.0–6.4% (4) | NA | NA | 0.6–5% (5) | 0.6–10.0% (9) | 0–1.9% (4) | 0% (2) | 0% (1) | 0% (1) |
| Exanthema | 0% (1) | 1.6% (1) | NA | NA | NA | NA | NA | 0% (1) | 0% (1) | 0% (1) |
| Pruritus | 3.3–64.5% (12) | 2.2% (1) | NA | 2.0–50.9% (6) | NA | NA | NA | 0% (1) | 0% (1) | 0% (1) |
| Gastrointestinal adverse events | ||||||||||
| Diarrhea | 3.8–20.0% (4) | 6.4% (1) | NA | 0–12.0% (6) | 7.2–32% (2) | 0–50% (7) | 1.1–11.6% (4) | 23.8–40.9% (2) | 32% (1) | 71.4% (1) |
| Anorexia | 0.9–16.7% (3) | NA | NA | NA | 4.8% (1) | NA | NA | NA | NA | NA |
| Nausea | 0–22.0% (6) | NA | 20.8% (1) | 0–30.0% (6) | 3.4–25.3% (4) | 4.5–40% (3) | 0.2–3.8% (3) | 4.5–9.5% (2) | 0% (1) | 14.2% (1) |
| Vomiting | 0–31.1% (9) | NA | 12.5% (1) | 1.0–18.0% (6) | 1.2–1.4% (2) | 0–50% (3) | 0–1.9% (3) | 4.5% (1) | 0% (1) | 0% (1) |
| Abdominal pain or discomfort, dyspepsia or GI intolerance | 4.7–30.0% (4) | 3.2–4.3% (3) | 20.8% (1) | 0–11.5% (5) | 6–23.2% (5) | 0–24% (3) | 0.3–25.0% (3) | 4.5–16.7% (2) | NA | 0% (1) |
| Psychiatric and neurological adverse events | ||||||||||
| Anxiety/nervousness | NA | 4.3% (1) | NA | NA | 0.6–1.2% (1) | NA | NA | NA | NA | NA |
| Insomnia/Sleep disturbances | NA | NA | NA | NA | 1.5–2.1% (1) | NA | 0.2% (1) | NA | NA | NA |
| Psychosis | 0% (1) | NA | NA | NA | NA | 0% (1) | NA | NA | NA | NA |
| Dizziness | 0.3–43.1% (5) | 3.2% (1) | NA | 0–15.9% (3) | 1.5–3.6% (3) | 0–9.4% (2) | NA | NA | NA | NA |
| Headache | 0.3–25.0% (6) | NA | 25.0% (1) | 0–17.7% (2) | 0–6% (4) | 0–3.2% (4) | 0.3% (1) | 0% (1) | 0% (1) | NA |
| Paraesthesia | NA | NA | NA | 0–3.0% (3) | 2% (1) | NA | NA | NA | NA | NA |
| Hematologic and metabolic adverse events | ||||||||||
| Thrombocytopaenia | NA | NA | NA | NA | NA | 0–7% (3) | 0–7.1% (3) | 4.5–9.1% (2) | NA | 0% (1) |
| Hypoglycaemia | NA | NA | NA | NA | 1.1% (1) | 0.5% (1) | 0–23.8% (2) | 0 (1) | 0% (1) | NA |
| Sense organs adverse events | ||||||||||
| Blurred vision | NA | NA | NA | NA | NA | NA | 0.2–1.2% (2) | NA | NA | NA |
| Tinnitus | 0.7–9.4% (2) | NA | NA | NA | 0–2.8% (4) | 3.8% (1) | 0% (1) | NA | NA | NA |
| Visual disturbances/ocular disorders | 1.3% (1) | 3.2% (1) | NA | NA | 0.9–2.4% (4) | 0–3% (3) | 0–1.9% (2) | 4.5% (1) | NA | 0% (1) |
| Hepatic adverse events | ||||||||||
| Hepatomegaly | NA | NA | 2.8% (1) | NA | NA | NA | NA | NA | NA | NA |
| Icterus/Bilirubin or GGT increase | 0% (1) | NA | 1.4% (1) | NA | NA | 0–6.6% (4) | 0–4% (3) | NA | NA | 14.3% (1) |
| Transaminase increase | 0–0.43% (2) | NA | 95.8–100.0% (2) | NA | NA | 0–11% (5) | 3.8–10.9% (2) | 0–54.5% (3) | 4% (1) | 42.8% (1) |
| Other adverse events | ||||||||||
| Asthenia/Weakness | 20.0–47.1% (2) | NA | NA | 5.3–8.0% (2) | 7.2% (1) | 6.6% (1) | NA | NA | NA | NA |
| Fatigue | 0.4–1.9% (2) | NA | 13.9% (1) | 0–3.5% (3) | 1% (1) | NA | NA | NA | NA | NA |
| Fever | 15.0% (1) | NA | 37.5–43.8% (2) | NA | NA | NA | NA | NA | NA | NA |
| Myalgia | 0% (1) | NA | 26.4% (1) | NA | NA | NA | NA | NA | NA | NA |
AZM: azithromycin; CQ: chloroquine; DRVr: darunavir plus ritonavir; ECG: electrocardiogram; GGT: gamma-glutamyl transferase; GI: gastrointestinal; HCQ: hydroxychloroquine; LPVr: lopinavir plus ritonavir; NA: not available/not applicable; QTc: corrected QT interval.
3.3.1. Patients Treated for Conditions Other Than COVID-19
Of the 47 studies reporting data on CQ or HCQ alone, 31 provided data that could be added to the quantitative data synthesis [126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166], but in the case of 15 others, the frequency could not be calculated so they were not included [167,168,169,170,171,172,173,174,175,176,177,178,179,180,181]. The data from one additional study reported data from pregnant patients and was likewise excluded from the quantitative synthesis [172]. Of the nine studies reporting data on CQ or HCQ combined with other drugs, three reported data that could be added to the quantitative data synthesis [146,173,174], whereas four others did not report adverse event frequency [175,176,177,178], and two reported data from pregnant patients [179,180]. One article reported data that could be added to our synthesis both for CQ alone and for CQ plus AZM [146].
3.3.2. Patients Treated for COVID-19
Out of a total of 74 studies, 66 provided data that could be added to the quantitative synthesis [207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272]. Seven of these studies contained data on COVID-19 prophylactic treatments [266,267,268,269,270,271,272]. The remaining studies lacked information about these drug combinations (e.g., they were the only studies reporting data in this specific combination) or reported data with which the frequency could not be calculated and were therefore not included in the quantitative synthesis [273,274,275,276,277,278,279,280].
3.4. Summary of the Evidence across Studies
3.4.1. Cardiac Adverse Drug Reactions
Cases
Cases of a complete heart block, an implanted pacemaker failure, and a QT-interval prolongation were described in patients treated with HCQ for autoimmune conditions [97,98,100], and cases of cardiovascular collapse, non-specified cardiac arrhythmia, and syncopal attacks with torsade de pointes were described in patients treated with CQ for malaria, amoebiasis, and a dermatological problem [95,96,99]. In patients being treated for COVID-19, six cases of cardiac adverse effects with QT interval prolongation were described, consisting of a case of QT interval prolongation and recurrent torsade de pointes with CQ [188], a case of right bundle branch block and critical QT interval prolongation with HCQ [181], a case of torsade de pointes in a patient treated with HCQ plus dexamethasone [190], a case of suspected HCQ-induced sinus bradycardia and QT interval prolongation [191], a case of QT prolongation in a patient treated with HCQ plus AZM [192], and a case of death due to progressive metabolic acidosis and multiple organ system failure in a patient being treated with HCQ plus AZM [186]. Additionally, a case of death from cardiac arrest in a patient who developed wide complex tachycardia during CQ plus AZM treatment [189], and a case of sinus bradycardia with HCQ plus AZM were reported [192].
Observational and Randomised Studies
Studies including patients treated with CQ or HCQ for malaria, autoimmune conditions, or porphyria cutanea tarda (PCT) did not report any cases of cardiac adverse effects. One retrospective cohort study assessing cases of cardiac symptoms, cardiac arrest, and ventricular arrhythmias in patients treated with CQ plus AZM for autoimmune diseases did not find significant differences in these events in comparison with amoxicillin treatment [177]. One study assessing cases of torsade de pointes, QT prolongation, and death in patients treated with CQ or HCQ plus AZM for diverse pathologies did not find any potential safety concerns for HCQ or CQ alone. Conversely, this study found a significant safety risk for torsade de pointes and QT prolongation when AZM was used alone [178]. In two randomised studies on the combination of CQ plus AZM for malaria, only three cases of palpitations were identified after evaluating 227 patients [173]. However, this was not observed in four other studies, which did not describe electrocardiographic evaluations [146,174,179,180]. In subjects with COVID-19, cardiac adverse drug reactions were the most common adverse drug reactions reported. Prolongation of the corrected QT interval ≥500 ms was observed in 0–25% of subjects treated with HCQ or CQ alone [207,212,215,219,220,240,241,246], 0–33% of subjects treated with HCQ or CQ plus AZM [207,209,210,214,215,216,221,226,238,241,245,246,248,249,251,254,262,263], 18.2% of subjects treated with HCQ plus LPVr [208], and 6.1% of subjects treated with HCQ plus AZM plus LPVr [254]. Prolongation of the corrected QT interval ≥60 ms was observed in 0–8% of subjects treated with HCQ alone [215,226,240,241], in 0–18% of subjects treated with HCQ plus AZM [210,214,215,216,221,226,228,241,254], and in 18.4% of subjects treated with HCQ plus AZM plus LPVr [254]. Torsade de pointes was only observed in 5 out of 15,039 patients in CQ or HCQ plus AZM COVID-19 studies [207,209,212,215,216,219,220,226,228,230,235,236,245,249,250,253,255,256,258,260,261,264,265]. No subjects in studies on HCQ plus LPVr or DRVr, or HCQ plus AZM plus LPVr or DRVr developed torsade de pointes [207,208,215,219,220,229,255]. Arrhythmogenic deaths were not reported in any study. The discontinuation of CQ or HCQ due to cardiac adverse drug reactions was observed in 2.4% of patients treated with HCQ or CQ alone [219], in 0–9.5% of patients treated with CQ or HCQ plus AZM [212,214,216,217,219], and in 0–36.4% of patients treated with HCQ plus LPVr [208,212].
3.4.2. Dermatological Adverse Drug Reactions
Cases
A total of 41 cases of mild to severe dermatological adverse effects, including 2 cases of fatal toxic epidermal necrolysis, were described in patients treated with HCQ for autoimmune diseases [31,34,35,36,38,40,41,42,43,44,45,46,47,48,50,51,52,53,54,55,56,57,59,60,62,63,64,65]. A smaller number of cases were reported in patients treated with CQ for autoimmune conditions or malaria although one fatal case was reported [27,28,29,30,32,33,37,39,49,54,58,61]. In connection with treatment for COVID-19, 12 cases were reported in patients treated with HCQ including cases of psoriasis exacerbation, rash, acute generalised exanthematous pustulosis (AGEP), drug rash with eosinophilia and systemic symptoms (DRESS), urticaria, palmoplantar itching, and purpuric erythematous rashes [185,187,196,197,198,199,200,201,202].
Observational and Randomised Studies
Pruritus has been described as occurring with high frequency (2–64.5%) in black African patients treated with CQ (with or without AZM) for malaria [138,139,140,170,171]. Two studies revealed a favourable effect of prednisolone to prevent pruritus without reporting other adverse effects [160,176]. Severe reactions were less often described in patients treated with CQ for malaria or autoimmune conditions. Erythema, exanthema, and maculopapular and vesiculopapular rashes in patients treated with CQ for malaria [140]; cutaneous drug eruptions, erythema, urticaria, and macular and papular exanthemas in patients treated with CQ or HCQ for SLE; and cutaneous lupus erythematosus (CLE) and dermatomyositis [132,133,134], and a generalised maculopapular rash in one patient treated with CQ for pulmonary sarcoidosis were described [145], with frequencies ranging from 0% to 6.4%. In COVID-19-affected patients, moderate to severe skin reactions were described in 0% to 10.0% of subjects although the highest frequency corresponded to a study that only included 10 patients [216,222,223,232,239,240,248,252,257]. We did not find increases in skin adverse effects when HCQ or CQ were combined with AZM, DRVr, or LPVr [239,242]. In the majority of the studies included that referred to patients with malaria, PCT, or COVID-19, there was no reference at all to dermatological toxicities.
3.4.3. Neurologic and Psychiatric Adverse Drug Reactions
Cases
A broad spectrum of neurological and psychiatric events was described in patients treated with CQ for malaria, amoebiasis, arthritis, acute myocardial infarction, erythema nodosum leprosum, or COVID-19 [66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,125,193,195]. Nevertheless, in the case of treatment with HCQ, only one case of psychomotor agitation in a patient with RA and one case of psychosis in a patient with SLE were reported [74,93].
Observational and Randomised Studies
Anxiety was reported in one patient treated with CQ in a study on pulmonary sarcoidosis [145], and anxiety and nervousness in patients treated with HCQ for COVID-19 prophylaxis [272]. Insomnia was reported in 0.18% of patients with HCQ plus AZM for COVID-19 [216], and sleep disturbances were reported in patients treated with HCQ for COVID-19 prophylaxis [266,272]. Dizziness was reported in 3.2% of patients with SLE treated with HCQ [133], 0.3–19% of patients with malaria treated with CQ [126,146,171], 0–15.9% of patients treated with CQ plus AZM for malaria [140,173], 1.5–3.6% of patients treated for COVID-19 prophylaxis [266,269,270,272], 9.4% of patients treated with HCQ for COVID-19 [252], and 0–0.3% of patients treated with HCQ plus AZM for COVID-19. Headache was reported in 0.3–25% of patients with malaria treated with CQ [126,138,140,142,171], 0–17.7% of patients treated with CQ plus AZM for malaria [173,174], 25% of patients with PCT treated with HCQ [135], 0–3.2% of patients treated with CQ or HCQ [222,223,227,252], and 0.28% for patients treated with HCQ plus AZM for COVID-19 [216], but was not reported in patients with autoimmune conditions. Paraesthesia was reported in 0–3% of patients treated with CQ plus AZM for malaria [174] and 2% of patients treated with HCQ for COVID-19 prophylaxis [270], but not reported in other conditions. One study assessing cases of depression as well as accidents/injuries in patients treated with CQ or HCQ plus AZM for diverse pathologies did not find potentially meaningful pharmacovigilance signs for CQ or HCQ, either alone or in combination with AZM [178]. However, a pharmacovigilance analysis suggested that COVID-19 patients exposed to HCQ could suffer psychiatric disorders and that HCQ was associated with an increased risk of reporting psychiatric disorders compared with other treatments [280]. Psychosis was not observed in patients treated with CQ for malaria nor in patients with COVID-19 [126,223] and was not reported in the rest of the studies.
3.4.4. Gastrointestinal and Hepatic Adverse Drug Reactions
Cases
Prior to COVID-19, five cases of liver injury were reported, one involving treatment with CQ and four involving HCQ, including one fatal case [111,112,113,114,115]. One case of hepatotoxicity in a COVID-19-affected patient treated with HCQ was also reported [182].
Observational and Randomised Studies
Nausea and vomiting affected 0–11.5% of patients treated with CQ for malaria [130,138,140,141,146,171,172], 12.5–20.8% of patients treated with CQ or HCQ for PCT [135], 0–30% of patients treated with CQ plus AZM for malaria [146,173,174], 40–50% of patients treated with CQ for COVID-19 (data from a small study of 10 patients) [223], 0–31.3% of patients treated with HCQ for COVID-19 [239,252,257], 0–19% of patients treated with HCQ plus AZM for COVID-19 [213,216,231,239,248,257], 4.5–9.5% of patients treated with HCQ plus LPVr for COVID-19 [239,242], and 0–14.2% of patients treated with HCQ plus AZM plus LPVr for COVID-19 [239], Diarrhea was reported in 3.8–8.3% of patients treated for malaria with CQ [138,146,171], 6.4% of patients treated with HCQ for autoimmune conditions [133], and 0–12% of patients treated with CQ plus AZM for malaria [133,146,173,174,179,180]. In the case of COVID-19 patients, diarrhea was observed in 50% of patients treated with CQ [223], 0–23.6% of patients treated with HCQ [218,224,227,231,239,252], 1.1–11.6% of patients treated with HCQ plus AZM [213,216,218,239], 23.8–40.9% of patients treated with HCQ plus LPVr [239,242], 32% of patients treated with HCQ plus DRVr [242], 71.4% of patients treated with HCQ plus AZM plus LPVr [239], and 10% of patients treated with HCQ plus standard of care (SOC) including antiviral agents, antibiotics, or systemic glucocorticoids [211]. Abdominal discomfort or pain or other gastrointestinal discomforts were reported in 4.7–30.0% of patients treated with CQ for malaria [126,138,142,171], 3.2–4.3% of patients treated with CQ or HCQ for autoimmune conditions [133,145], 20.0% of patients treated with HCQ for PCT [135], 0–11.5% of patients treated with CQ plus AZM for malaria [173,174], 10% of patients with COVID-19 treated with CQ [223], 0–24% of patients treated with HCQ [239,247], 0.3–25% of patients treated with HCQ plus AZM [216,239,247], and 4.5–16.7% of patients treated with HCQ plus LPVr [212,239]. Dyspepsia and gastritis affected 3.6% of patients treated with HCQ for RA [143] and 1–4% of patients treated with CQ plus AZM for malaria [174]. Transaminase increase and other hepatic disorders were reported in the majority of patients treated with CQ or HCQ and affected by PCT but observed less often in patients treated for other conditions such as malaria, autoimmune conditions, or COVID-19 [135,136].
3.4.5. Other Findings
Cases of hypoglycaemia (including a case of hypoglycaemic coma) [99,100,101,102], thrombocytopaenia [104], thrombotic thrombocytopaenic purpura [105,203], methaemoglobinaemia, sense organ adverse effects (including cases of severe positional vertigo [107], severe vestibular toxicity [108], loss of hearing [109], complete ageusia [110], diplopia and blurred vision) [106], porphyria variegata [116], severe myopathies [118,119], myasthenic syndrome [194], anaphylaxis [121], urinary incontinence [122], diffuse interstitial lung disease [123], and acute eosinophilic pneumonias [120], were described in connection with short-term CQ and HCQ treatments.
Various cases of haemolysis and methaemoglobinaemia were described in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency treated with HCQ or CQ for COVID-19 [183,184,204,205,206].
4. Discussion
The evidence collected does not show that COVID-19 patients treated with CQ or HCQ alone or in combination with studied drugs suffered a greater proportion of dermatologic, gastrointestinal, hepatic, metabolic, or haematological adverse effects compared with subjects receiving these drugs for indications other than COVID-19. However, no clinical benefits were found when those drugs were used to treat or prevent COVID-19 [281,282,283].
Although the ocular toxicity of CQ and HCQ is extremely important in long-term regimens with these drugs [284], it was rarely mentioned in connection with short-term regimens or in the first weeks of long-term regimens. The cases of patients with G6PD deficiency are of special interest because the related toxicity has been shown in such patients with and without COVID-19, and CQ and HCQ treatment must therefore be avoided in these patients [183,184]. During the first days of treatment, gastrointestinal adverse effects should be considered as they were reported in most indications. In the case of cardiac events, it is noteworthy that in more than 70 years of use, only a few cases of early cardiac adverse effects were found [94,95,96,97,98,99]. In contrast, in the much shorter period that has elapsed since the start of the COVID-19 pandemic, a greater number of cardiac cases have been reported involving COVID-19-affected patients regardless of the drug regimen used. In addition, the concomitant use of other drugs such as AZM should be considered. This systematic review shows that cardiac adverse effects such as QTc prolongation were frequent in COVID-19-affected-patients treated with CQ or HCQ (0–27.3%, and up to 33% if combined with AZM), though the risk of torsade de pointes was low. These data were extracted from 55 observational and 18 randomised studies with an overall favourable quality assessment. In 52 of these studies, at least some electrocardiographic changes were reported in patients treated with HCQ or CQ and a consistent and large effect was found. The results of this systematic review coincide with those of the previous one (that only included COVID-19 studies) that showed a significantly higher rate of adverse events with CQ or HCQ treatment but no significant differences in the case of serious adverse events (including cardiac arrhythmias and life-threatening events) [285]. A recent systematic review that focused on the cardiac safety of CQ and HCQ in COVID-19-affected patients has also shown an important association between CQ and HCQ use and the risk of drug-induced QT prolongation with a relatively higher incidence of torsade de pointes, ventricular tachycardia, or cardiac arrest [286]. Beyond the medication effects, several cardiac manifestations have been described in patients with COVID-19 including acute myopericarditis, acute coronary syndrome, congested heart failure, cardiogenic shock, and cardiac arrhythmias as a result of the injuries caused by the virus and systemic inflammation [287]. These cardiac manifestations were not only shown with CQ or HCQ use, but also with other drugs used in the treatment of COVID-19 such as corticosteroids, rivabirin, LPVr, and AZM [288,289,290,291]. This suggests that COVID-19 could have a role in these cardiac safety reports. Nevertheless, publication or measurement bias cannot be ruled out in pre-COVID-19 published data since these cardiac effects were rarely assessed or mentioned.
The cardiac abnormalities found in this study could be explained by CQ and HCQ electrophysiological effects, AZM combination, and COVID-19 concurrence. CQ and HCQ can cause acute cardiac functional changes by inhibition of ion channels with membrane-stabilizing effects that can lead to conduction disturbances [14]. Laboratory electrophysiological studies revealed CQ blocked the inward sodium current, the l-type calcium current, and the potassium currents such as the rapid delayed rectifier outward currents explaining prolongations and reductions in maximum velocity of cardiac action potentials and QT interval prolongation [292,293]. Moreover, synergistic effects of HCQ and AZM on the electrophysiological and contractile functions of human-induced pluripotent stem cell-derived cardiomyocytes have been observed in the short-term [294]. Furthermore, AZM might act as a weak CYP3A4 inhibitor involved in the metabolism of these drugs [295]. In addition to CQ, HCQ, and AZM effects, some common clinical concerns in elderly patients with COVID-19, such as dyselectrolythemia or dehydration, could increase the risk of arrhythmias [296,297]. In the case of neuropsychiatric symptoms associated with HCQ and CQ, different pharmacological mechanisms have been proposed such as serotonin or cholinergic imbalances induction or lysosomal dysfunction, although this has not been elucidated to date [280,298].
This systematic review only partially fulfils the proposed objectives. In patients not affected by COVID-19, only the combinations with AZM and glucocorticoids could be assessed, whereas in the case of patients affected by COVID-19, quantitative data synthesis could only be performed in combinations with AZM, LPVr, and boosted DRV but there were no sufficient studies reporting on the other possible combinations. The studies examined here were performed in very different settings and the methodologies used for the assessment of drug safety were very different, thus limiting our ability to compare the results reported. Furthermore, some adverse effects were not monitored with the same intensity in different contexts, so the question remains as to whether they did not occur or were simply not measured. Data synthesis was performed according to drug indication and whether CQ or HCQ were used alone or in combination with a second drug, but not according to how the dose regimen used could have influenced the adverse effects (e.g., drug regimens used in COVID-19 were generally longer than those used in malaria and in higher daily doses than those used in autoimmune diseases). Moreover, this systematic review was not focused on finding differences between CQ and HCQ safety. Despite these limitations, this systematic review clearly suggests that the use of CQ or HCQ tended to increase the cardiac risks for patients being treated for COVID-19, although these rarely resulted in severe consequences and the risk of torsade de pointes was low. Taking into account these considerations, in the future great caution should be exercised when testing potentially arrhythmogenic drugs in patients affected by severe acute viral or inflammatory pathologies.
5. Conclusions
Early adverse effects of CQ and HCQ may manifest as cardiac, dermatologic, neuropsychiatric, gastrointestinal, hepatic, metabolic, or haematological events. In the evidence reviewed, the occurrence and frequency of these toxicities were variable depending on the drug indication and the characteristics of the population being treated. Unlike pre-COVID-19 patients who received CQ or HCQ treatment, cardiac adverse drug effects occurred often in COVID-19 patients. Although severe consequences were rarely reported, this data must be considered, especially if CQ or HCQ are combined with other drugs such as AZM. This systematic review provides a comprehensive synthesis of the reported evidence on the short-term safety of CQ and HCQ treatment and provides important data for further research on the use of these drugs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/1424-8247/15/5/634/s1, Table S1 search terms and MeSH terms used in the bibliographic search on the safety of chloroquine and hydroxychloroquine alone; Table S2: search terms and MeSH terms used in the bibliographic search on the safety of chloroquine and hydroxychloroquine in combination with other drugs used for the treatment of COVID-19 disease; Table S3: articles exclusion according to eligibility criteria; Table S4: total of adverse effects related to CQ/HCQ: case reports and case series; Table S5: characteristics of included studies: case reports and case series related to dermatological adverse events; Table S6: characteristics of included studies: case reports and case series related to psychiatric adverse events; Table S7: characteristics of included studies: case reports and case series related to neurologic adverse events; Table S8: characteristics of included studies: case reports and case series related to cardiac adverse events; Table S9: characteristics of included studies: case reports and case series related to hematologic and metabolic adverse events; Table S10: characteristics of included studies: case reports and case series related to sense organs adverse events; Table S11: characteristics of included studies: case reports and case series related to hepatic adverse events; Table S12: characteristics of included studies: case reports and case series related to other adverse events; Table S13: characteristics of included studies: observational studies, patients affected by malaria or who received prophylactic treatment; Table S14: characteristics of included studies: observational studies, patients affected by cutaneous and/or systemic lupus erythematosus and dermatomyositis; Table S15: characteristics of included studies: observational studies, patients affected by porphyria cutanea tarda; Table S16: characteristics of included studies: observational studies, patients affected by diverse pathologies; Table S17: characteristics of included studies: clinical trials, patients affected by malaria or who received prophylactic treatment; Table S18: characteristics of included studies: clinical trials, patients affected by rheumatoid arthritis; Table S19: characteristics of included studies: clinical trials, patients affected by other pathologies; Table S20: characteristics of included studies: clinical trials and observational studies, patients affected by malaria or who received prophylactic treatment with CQ or HCQ in combination with other drugs; Table S21: characteristics of included studies: case reports and case series in patients with COVID-19; Table S22: characteristics of included studies: observational studies and clinical trials (COVID-19 treatment and prophylaxis).
Author Contributions
Conceptualization, S.M., A.M.V., M.B.P., C.R.-B., A.P.-R., L.V.J., R.P., J.R. and C.Q.; methodology, S.M., A.M.V., M.B.P., C.R.-B., A.P.-R., L.V.J., R.P., J.R. and C.Q.; software, S.M., A.M.V., M.B.P., C.R.-B., A.P.-R., L.V.J. and C.Q.; validation, S.M., A.M.V., M.B.P., C.R.-B., A.P.-R., L.V.J., R.P., J.R. and C.Q.; formal analysis, S.M., A.M.V., M.B.P., C.R.-B.,A.P.-R., L.V.J., R.P., J.R. and C.Q.; investigation, S.M., A.M.V., M.B.P., C.R.-B., A.P.-R., L.V.J., R.P., J.R. and C.Q.; resources, S.M. and C.Q.; data curation, S.M., A.M.V., M.B.P., C.R.-B., A.P.-R., L.V.J., J.R. and C.Q.; writing—original draft preparation, S.M., A.M.V., M.B.P., C.R.-B. and A.P-R.; writing—review and editing, S.M., A.M.V., M.B.P., C.R.-B., A.P.-R., L.V.J. and C.Q.; visualization, S.M., A.M.V., M.B.P., C.R.-B., A.P.-R., L.V.J. and C.Q.; supervision, S.M., A.M.V., M.B.P., C.R.-B., A.P.-R., L.V.J. and C.Q.; project administration, S.M. and C.Q. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in article and Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;7:1732–1739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devaux C.A., Rolgin J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: What to expect for COVID-19? Int. J. Antimicrob. Agents. 2020;55:105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 asso-ciated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 6.Kang S., Peng W., Zhu Y., Lu S., Zhou M., Lin W., Wu W., Huang S., Jiang L., Luo X., et al. Recent progress in understanding 2019 novel coronavirus (SARS-CoV-2) associated with human respiratory disease: Detection, mechanisms and treatment. Int. J. Antimicrob. Agents. 2020;55:105950. doi: 10.1016/j.ijantimicag.2020.105950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovane R.A., Rezai S., Cleland E., Henderson C.E. Current pharmacological modalities for management of novel coronavirus disease 2019 (COVID-19) and the rationale for their utilization: A review. Rev. Med. Virol. 2020;30:e2136. doi: 10.1002/rmv.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keshtkar-Jahromi M., Bavari S. A Call for Randomized Controlled Trials to test the Efficacy of Chloroquine and Hy-droxychloroquine as Therapeutics against Novel Coronavirus Disease (COVID-19) Am. J. Trop. Med. Hyg. 2020;102:932–933. doi: 10.4269/ajtmh.20-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Food and Drug Administration Coronavirus (COVID-19) Update: Daily Roundup March 30, 2020. [(accessed on 25 April 2020)]; Available online: https://www.fda.gov/news-events/pressannouncements/coronavirus-covid-19-update-dailyroundup-march-30-2020.
- 10.European Medicines Agency COVID-19: Chloroquine and Hydroxychloroquine Only to Be Used in Clinical Trials or Emergency Use Programmes. [(accessed on 25 April 2020)]; EMA/170590/2020. Available online: https://www.ema.europa.eu/en/news/covid-19-chloroquine-hydroxychloroquine-only-be-used-clinical-trials-emergency-use-programmes.
- 11.Mitjà O., Clotet B. Use of antiviral drugs to reduce COVID-19 transmission. Lancet Glob. Health. 2020;8:e639–e640. doi: 10.1016/S2214-109X(20)30114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Revollo B., Tebe C., Peñafiel J., Blanco I., Perez-Alvarez N., Lopez R., Rodriguez L., Ferrer J., Ricart P., Moret E., et al. Hydroxychloroquine pre-exposure prophylaxis for COVID-19 in healthcare workers. J. Antimicrob. Chemother. 2020;76:827–829. doi: 10.1093/jac/dkaa477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor W.R., White N.J. Antimalarial drug toxicity: A review. Drug Saf. 2004;27:25–61. doi: 10.2165/00002018-200427010-00003. [DOI] [PubMed] [Google Scholar]
- 14.Chatre C., Roubille F., Vernhet H., Jorgensen C., Pers Y.M. Cardiac Complications Attributed to Chloroquine and Hy-droxychloroquine: A Systematic Review of the Literature. Drug Saf. 2018;41:919–931. doi: 10.1007/s40264-018-0689-4. [DOI] [PubMed] [Google Scholar]
- 15.Sato K., Mano T., Iwata A., Toda T. Neuropsychiatric adverse events of chloroquine: A real-world pharmacovigilance study using the FDA Adverse Event Reporting System (FAERS) database. Biosci. Trends. 2020;14:139–143. doi: 10.5582/bst.2020.03082. [DOI] [PubMed] [Google Scholar]
- 16.Kashour Z., Riaz M., Garbati M.A., AlDosary O., Tlayjeh H., Gerberi D., Murad M.H., Sohail M.R., Kashour T., Tleyjeh I.M. Efficacy of chloroquine and hydroxychloroquine in COVID-19 patients: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2021;71:30–42. doi: 10.1093/jac/dkaa403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui X., Sun J., Minkove S.J., Li Y., Cooper D., Couse Z., Eichacker P.Q., Torabi-Parizi P. Effects of chloroquine or hydroxychloroquine treatment on non-SARS-CoV2 viral infections: A systematic review of clinical studies. Rev. Med. Virol. 2021;31:e2228. doi: 10.1002/rmv.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiolet T., Guihur A., Rebeaud M.E., Mulot M., Peiffer-Smadja N., Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: A systematic review and me-ta-analysis. Clin. Microbiol. Infect. 2021;27:19–27. doi: 10.1016/j.cmi.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marin S., Martin A., Bosch M., Rodríguez C., Pérez-Ricart A., Quiñones C. A Systematic Review on the Early Toxicity and Its Long-Term Consequences of Oral Chloroquine and Hydroxychloroquine on Patients Suffering from Malaria, Amebiasis, Connective Tissue Diseases, Rheumatoid Conditions, Porphyria Cutanea Tarda and COVID-19. [(accessed on 30 July 2021)]. PROSPERO 2020 CRD42020180708. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020180708.
- 21.World Health Organization WHO Collaborating Centre for Drug Statistics Methodology [Online] [(accessed on 1 April 2020)]. Available online: http://www.whocc.no/atc_ddd_index.
- 22.Gagnier J.J., Kienle G., Altman D.G., Moher D., Sox H., Riley D., CARE Group The CARE Guidelines: Consensus-Based Clinical Case Reporting Guideline Development. Glob. Adv. Health Med. 2013;2:38–43. doi: 10.7453/gahmj.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P., STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Jadad A.R., Moore A., Carroll D., Jenkinson C., Reynolds D.J., Gavaghan D.J., McQuay H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Contr. Clin. Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 25.Naranjo C.A., Busto U., Sellers E.M., Sandor P., Ruiz I., Roberts E.A., Janecek E., Domecq C., Greenblatt D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 26.Campbell M., McKenzie J.E., Sowden A., Katikireddi S.V., Brennan S.E., Ellis S., Hartmann-Boyce J., Ryan R., Shepperd S., Thomas J., et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. BMJ. 2020;368:l6890. doi: 10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher S. Psoriatic erythroderma, rheumatoid arthritis, and death, as a sequence to a drug reaction. J. Maine Med. Assoc. 1961;52:57–59. [PubMed] [Google Scholar]
- 28.Skog E. Systemic eczematous contact-type dermatitis induced by iodochlorhydroxyquin and chloroquine phosphate. Contact Dermat. 1975;1:187. doi: 10.1111/j.1600-0536.1975.tb05372.x. [DOI] [PubMed] [Google Scholar]
- 29.Kanwar A.J., Singh O.P. Toxic epidermal necrolysis-drug induced. Indian J. Dermatol. 1976;21:73–77. [PubMed] [Google Scholar]
- 30.Olsen T.G. Chloroquine and Psoriasis. Ann. Intern. Med. 1981;94:546–547. doi: 10.7326/0003-4819-94-4-546_2. [DOI] [PubMed] [Google Scholar]
- 31.Luzar M.J. Hydroxychloroquine in psoriatic arthropathy: Exacerbations of psoriatic skin lesions. J. Rheumatol. 1982;9:462–464. [PubMed] [Google Scholar]
- 32.Spencer H.C., Poulter N.R., Lury J.D., Poulter C.J. Chloroquine-associated pruritus in a European. Br. Med. J. (Clin. Res. Ed.) 1982;285:1703–1704. doi: 10.1136/bmj.285.6356.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhasin V., Goel S., Srivastava V.K. Role of pantothenic acid in chloroquine induced skin toxicity. J. Indian Med. Assoc. 1984;82:447–448. [PubMed] [Google Scholar]
- 34.Gray R.G. Hydroxychloroquine provocation of psoriasis. J. Rheumatol. 1985;12:391. [PubMed] [Google Scholar]
- 35.Hudson L.D. Erythema annulare centrifugum: An unusual case due to hydroxychloroquine sulfate. Cutis. 1985;36:129–130. [PubMed] [Google Scholar]
- 36.Lotem M., Ingber A., Segal R., Sandbank M. Generalized pustular drug rash induced by hydroxychloroquine. Acta Derm. Venereol. 1990;70:250–251. [PubMed] [Google Scholar]
- 37.Vestey J., Savin J. Psoriasis worsened by antimalarial prophylaxis. J. Infect. 1992;24:211–212. doi: 10.1016/0163-4453(92)93058-X. [DOI] [PubMed] [Google Scholar]
- 38.Assier-Bonnet Saada V., Bernier M., Clerici T., Saïag P. Acute generalized exanthematous pustulosis induced by hydroxychloroquine. Dermatology. 1996;193:70–71. doi: 10.1159/000246211. [DOI] [PubMed] [Google Scholar]
- 39.Wilairatana P., Looareesuwan S., Riganti M., Teja-Isavadharm P., Keeratithakul D., Eickmeyer S., Walsh D.S. Pustular eruption in a malaria patient treated with chloroquine. Int. J. Dermatol. 1998;37:713–714. doi: 10.1046/j.1365-4362.1998.00430.x. [DOI] [PubMed] [Google Scholar]
- 40.Murphy M., Carmichael A.J. Fatal toxic epidermal necrolysis associated with hydroxychloroquine. Clin. Exp. Dermatol. 2001;26:457–458. doi: 10.1046/j.1365-2230.2001.00857-3.x. [DOI] [PubMed] [Google Scholar]
- 41.Leckie M.J., Rees R.G. Stevens-Johnson syndrome in association with hydroxychloroquine treatment for rheumatoid arthritis. Rheumatology. 2002;41:473–474. doi: 10.1093/rheumatology/41.4.473. [DOI] [PubMed] [Google Scholar]
- 42.Welsch M.J. Acute pustular psoriasis complicated by leukocytoclastic vasculitis. J. Drugs Dermatol. 2003;2:193–197. [PubMed] [Google Scholar]
- 43.Evans C.C., Bergstresser P.R. Acute generalized exanthematous pustulosis precipitated by hydroxychloroquine. J. Am. Acad. Dermatol. 2004;50:650–651. doi: 10.1016/S0190-9622(03)02733-6. [DOI] [PubMed] [Google Scholar]
- 44.Ghaffarpour G., Jalali M.H., Yaghmaii B., Mazloomi S., Soltani-Arabshahi R. Chloroquine/hydroxychloroquine-induced pemphigus. Int. J. Dermatol. 2006;45:1261–1263. doi: 10.1111/j.1365-4632.2006.03075.x. [DOI] [PubMed] [Google Scholar]
- 45.Gül U., Cakmak S.K., Kiliç A., Gönül M., Bilgili S. A case of hydroxychloroquine induced pruritus. Eur. J. Dermatol. 2006;16:586–587. [PubMed] [Google Scholar]
- 46.Mates M., Zevin S., Breuer G.S., Navon P., Nesher G. Desensitization to hydroxychloroquine--experience of 4 patients. J. Rheumatol. 2006;33:814–816. [PubMed] [Google Scholar]
- 47.Atzori L., Pinna A., Pilloni L., Ferreli C., Aste N., Zucca M., Pau M., Aste N. Acute generalized exanthematous pustulosis: The experience of an Italian drug-surveillance centre. G. Ital. Dermatol. Venereol. 2007;142:303–310. [Google Scholar]
- 48.Volpe A., Marchetta A., Caramaschi P., Biasi D., Bambara L.M., Arcaro G. Hydroxychloroquine-induced DRESS syndrome. Clin. Rheumatol. 2008;27:537–539. doi: 10.1007/s10067-007-0772-1. [DOI] [PubMed] [Google Scholar]
- 49.Das J.K., Medhi J., Chakravarty R., Soibam R. Mucous membrane grafting for the post-Steven-Johnson syndrome symblepharon: A case report. Indian J. Ophthalmol. 2011;59:231–233. doi: 10.4103/0301-4738.81039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Awad P., Wulf A., Kawas D. Reacción adversa a hidroxicloroquina. Rev. Chil. Dermatol. 2013;29:197–198. [Google Scholar]
- 51.Bailey K., McKee D., Wismer J., Shear N. Acute Generalized Exanthematous Pustulosis Induced by Hydroxychloroquine: First Case Report in Canada and Review of the Literature. J. Cutan. Med. Surg. 2013;17:414–418. doi: 10.2310/7750.2013.12105. [DOI] [PubMed] [Google Scholar]
- 52.Cameron M.C., Word A.P., Dominguez A. Hydroxychloroquine-induced fatal toxic epidermal necrolysis complicated by angioinvasive rhizopus. Dermatol. Online J. 2014;20:4. doi: 10.5070/D32011024620. [DOI] [PubMed] [Google Scholar]
- 53.Pastushenko I., Gracia-Cazaña T., Morales-Moya A.L., Grasa M.P. Acute cutaneous pustular eruption due to hydroxychloroquine. Med. Clin. 2014;143:e13. doi: 10.1016/j.medcli.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Soria A., Barbaud A., Assier H., Avenel-Audran M., Tétart F., Raison-Peyron N., Amarger S., Girardin P., Francès C., FISARD (French Investigators for Skin Adverse Reaction to Drugs) Cutaneous Adverse Drug Reactions with Antimalarials and Allergological Skin Tests. Dermatology. 2015;231:353–359. doi: 10.1159/000438787. [DOI] [PubMed] [Google Scholar]
- 55.Pearson K.C., Morrell D.S., Runge S.R., Jolly P. Prolonged pustular eruption from hydroxychloroquine: An unusual case of acute generalized exanthematous pustulosis. Cutis. 2016;97:212–216. [PubMed] [Google Scholar]
- 56.Abou Assalie N., Durcan R., Durcan L., Petri M.A. Hydroxychloroquine-induced erythema multiforme. J. Clin. Rheumatol. 2017;23:127–128. doi: 10.1097/RHU.0000000000000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuda T., Ly N.T.M., Kambe N., Nguyen C., Ueda-Hayakawa I., Son Y., Okamoto H. Early cutaneous eruptions after oral hydroxychloroquine in a lupus erythematosus patient: A case report and review of the published work. J. Dermatol. 2017;45:344–348. doi: 10.1111/1346-8138.14156. [DOI] [PubMed] [Google Scholar]
- 58.Nair P.A., Patel T. Palmoplantar exfoliation due to chloroquine. Indian J. Pharmacol. 2017;49:205–207. doi: 10.4103/ijp.IJP_659_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mercogliano C., Khan M., Lin C., Mohanty E., Zimmerman R. AGEP overlap induced by hydroxychloroquine: A case report and literature review. J. Community Hosp. Intern. Med. Perspect. 2018;8:360–362. doi: 10.1080/20009666.2018.1547089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Randhawa A., Wylie G. A case of an acute cutaneous drug reaction with hydroxychloroquine. Scott. Med. J. 2018;63:91–94. doi: 10.1177/0036933018763277. [DOI] [PubMed] [Google Scholar]
- 61.Balamurugesan K., Davis P., Ponprabha R., Sarasveni M. Chloroquine induced urticaria: A newer adverse effect. J. Fam. Med. Prim. Care. 2019;8:2545–2547. doi: 10.4103/jfmpc.jfmpc_413_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Girijala R.L., Siddiqi I., Kwak Y., Wright D., Patel D.B., Goldberg L.H. Pustular DRESS Syndrome Secondary to Hydroxychloroquine with EBV Reactivation. J. Drugs Dermatol. 2019;18:207–209. [PubMed] [Google Scholar]
- 63.Manzo C., Pollio N., Natale M. Sweet’s Syndrome Following Therapy with Hydroxychloroquine in a Patient Affected with Elderly-Onset Primary Sjogren’s Syndrome. Medicines. 2019;6:111. doi: 10.3390/medicines6040111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ullah A., Zeb H., Khakwani Z., Murphy F.T. Hydroxychloroquine-induced inverse psoriasis. BMJ Case Rep. 2019;12:e224619. doi: 10.1136/bcr-2018-224619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsuda-Hirose H., Sho Y., Yamate T., Nakamura Y., Saito K., Takeo N., Nishida H., Ishii K., Sugiura K., Hatano Y. Acute generalized exanthematous pustulosis induced by hydroxychloroquine successfully treated with etretinate. J. Dermatol. 2019;47:e53–e54. doi: 10.1111/1346-8138.15185. [DOI] [PubMed] [Google Scholar]
- 66.Burrell Z.L., Martinez A.C. Chloroquine and Hydroxychloroquine in the Treatment of Cardiac Arrhythmias. N. Engl. J. Med. 1958;258:798–800. doi: 10.1056/NEJM195804172581608. [DOI] [PubMed] [Google Scholar]
- 67.Dornhorst A.C., Robinson B.J. Chloroquine psychosis? Lancet. 1963;1:118. doi: 10.1016/S0140-6736(63)91108-5. [DOI] [Google Scholar]
- 68.Rab S.M. Two Cases of Chloroquine Psychosis. BMJ. 1963;1:1275. doi: 10.1136/bmj.1.5340.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oscar L. Toxic psychosis due to quinacrine and chloroquine. JAMA. 1964;187:373–375. doi: 10.1001/jama.1964.03060180059026. [DOI] [PubMed] [Google Scholar]
- 70.Kabir S.M. Chloroquine psychosis. Trans. R. Soc. Trop. Med. Hyg. 1969;63:549. doi: 10.1016/0035-9203(69)90056-X. [DOI] [PubMed] [Google Scholar]
- 71.Bomb B.S., Bedi H.K., Bhatnagar L.K. Chloroquine psychosis. Trans. R. Soc. Trop. Med. Hyg. 1975;69:123. doi: 10.1016/0035-9203(75)90118-2. [DOI] [PubMed] [Google Scholar]
- 72.Das E.M., Mohan D. Chloroquine-related depression. Indian J. Psychiatry. 1981;23:184–185. [PMC free article] [PubMed] [Google Scholar]
- 73.Mohan D., Mohandas E., Rajat R. Chloroquine psychosis: A chemical psychosis? J. Natl. Med. Assoc. 1981;73:1073–1076. [PMC free article] [PubMed] [Google Scholar]
- 74.Ward W.Q., Walter-Ryan W.G., Shehi G.M. Toxic psychosis: A complication of antimalarial therapy. J. Am. Acad. Dermatol. 1985;12:863–865. doi: 10.1016/S0190-9622(85)70109-0. [DOI] [PubMed] [Google Scholar]
- 75.Akhtar S., Mukherjee S. Chloroquine Induced Mania. Int. J. Psychiatry Med. 1993;23:349–356. doi: 10.2190/8DRE-DBNH-MXXG-7AJF. [DOI] [PubMed] [Google Scholar]
- 76.Telgt D.S., van der Ven A.J., Schimmer B., Droogleever-Fortuyn H.A. Serious psychiatric symptoms after chloroquine treatment following experi-mental malaria infection. Ann. Pharmacother. 2005;39:551–554. doi: 10.1345/aph.1E409. [DOI] [PubMed] [Google Scholar]
- 77.Sahoo S., Kumar M., Sinha V.K. Chloroquine-Induced Recurrent Psychosis. Am. J. Ther. 2007;14:406–407. doi: 10.1097/MJT.0b013e31802e4b0e. [DOI] [PubMed] [Google Scholar]
- 78.Plesnicar B., Velikonja I., Vitorovic S. Two Challenge and Rechallenge Episodes of Chloroquine-Induced Psychotic Mania in a Patient with Rheumatoid Arthritis. Aktuel. Rheumatol. 2013;38:177–179. doi: 10.1055/s-0032-1327626. [DOI] [Google Scholar]
- 79.Bogaczewicz J., Sobów T., Robak E., Bienkowski P., Sysa-Jędrzejowska A., Woźniacka A. Exacerbations of bipolar disorder triggered by chloroquine in systemic lupus erythematosus—A case report. Lupus. 2014;23:188–193. doi: 10.1177/0961203313513818. [DOI] [PubMed] [Google Scholar]
- 80.Bogaczewicz A., Sobów T., Bienkowski P., Kowalski J., Wozniacka A. Chloroquine-induced subacute paranoid-like disorder as a complication of dermatological treatment. Int. J. Dermatol. 2016;55:1378–1380. doi: 10.1111/ijd.13266. [DOI] [PubMed] [Google Scholar]
- 81.Choughule A., Salunkhe R. Chloroquine induced psychosis in an adult patient with amoebic liver abscess: A case report. Indian J. Ment. Health. 2019;6:115–118. [Google Scholar]
- 82.Torrey E.F. Chloroquine seizures. JAMA. 1968;204:867–870. doi: 10.1001/jama.1968.03140230025006. [DOI] [PubMed] [Google Scholar]
- 83.Umez-Eronini E.M., Eronini E.A. Chloroquine induced involuntary movements. BMJ. 1977;1:945–946. doi: 10.1136/bmj.1.6066.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh R.P., Sinha A.K. Neuropsychiatric toxicity of chloroquine. J. Indian Med. Assoc. 1981;77:133–134. [PubMed] [Google Scholar]
- 85.Fish D.R., Espir M.L. Convulsions associated with prophylactic antimalarial drugs: Implications for people with epilepsy. BMJ. 1988;297:526–527. doi: 10.1136/bmj.297.6647.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.James R.F. Cerebellar ataxia in patients with malaria treated with chloroquine. Postgrad. Med. J. 1988;64:167. doi: 10.1136/pgmj.64.748.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cras P., Martin J.J. Transient global amnesia following ingestion of chloroquine. J. Neurol. Neurosurg. Psychiatry. 1990;53:926. doi: 10.1136/jnnp.53.10.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Bleecker J., De Reuck J., Quatacker J., Meire F. Persisting chloroquine-induced myasthenia? Acta Clin. Belg. 1991;46:401–406. doi: 10.1080/17843286.1991.11718197. [DOI] [PubMed] [Google Scholar]
- 89.Adamolekun B. Seizures associated with chloroquine therapy. Cent. Afr. J. Med. 1992;38:350–352. [PubMed] [Google Scholar]
- 90.Mulhauser P., Allemann Y., Regamey C. Chloroquine and Nonconvulsive Status Epilepticus. Ann. Intern. Med. 1995;123:76. doi: 10.7326/0003-4819-123-1-199507010-00021. [DOI] [PubMed] [Google Scholar]
- 91.Ebenso B.E. Seizures following chloroquine treatment of type II lepra reaction: A case report. Lepr. Rev. 1998;69:178–181. doi: 10.5935/0305-7518.19980021. [DOI] [PubMed] [Google Scholar]
- 92.Martin A.N., Tsekes D., White W.J., Rossouw D. Chloroquine-induced bilateral anterior shoulder dislocation: A unique aetiology for a rare clinical problem. BMJ Case Rep. 2016;2016:bcr2015214292. doi: 10.1136/bcr-2015-214292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Manzo C., Gareri P., Castagna A. Psychomotor Agitation Following Treatment with Hydroxychloroquine. Drug Saf. Case Rep. 2017;4:6. doi: 10.1007/s40800-017-0048-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sogani R.K., Sharma D.K., Gupta V. Cardiovascular collapse following small dose of chloroquin in healthy young adult. J. Assoc. Physicians India. 1986;34:534. [PubMed] [Google Scholar]
- 95.Siqueira-Batista R., Ramos Júnior A.N., Pessanha B.S., Sforza-de-Almeida M.P., Potsch D.F. Chloroquine and cardiac arrhythmia: Case report. East Afr. Med. J. 1998;75:117–119. [PubMed] [Google Scholar]
- 96.Comín-Colet J., Sánchez-Corral M.A., Alegre-Sancho J.J., Valverde J., López-Gómez D., Sabaté X., Juan-Mas A., Esplugas E. Complete heart block in an adult with systemic lupus erythe-matosus and recent onset of hydroxychloroquine therapy. Lupus. 2001;10:59–62. doi: 10.1191/096120301673172543. [DOI] [PubMed] [Google Scholar]
- 97.Huang P.H., Tuan T.C., Lin Y.J., Ding Y.A., Kong C.W. Implanted pacemaker failure caused by the antirheumatic drug hydroxychloroquine. Lupus. 2003;12:725–727. doi: 10.1191/0961203303lu435xx. [DOI] [PubMed] [Google Scholar]
- 98.Yelve K., Phatak S., Patil M.A., Pazare A.R. Syncope in a patient being treated for hepatic and intestinal amoebiasis. BMJ Case Rep. 2012;2012:bcr2012006687. doi: 10.1136/bcr-2012-006687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morgan N.D., Patel S.V., Dvorkina O. Suspected Hydroxychloroquine-Associated QT-Interval Prolongation in a Patient with Systemic Lupus Erythematosus. J. Clin. Rheumatol. 2013;19:286–288. doi: 10.1097/RHU.0b013e31829d5e50. [DOI] [PubMed] [Google Scholar]
- 100.Abu-Shakra M., Lee P. Hypoglycemia: An unusual adverse reaction to chloroquine. Clin. Exp. Rheumatol. 1994;12:95. [PubMed] [Google Scholar]
- 101.Shojania K., Koehler B.E., Elliott T. Hypoglycemia induced by hydroxychloroquine in a type II diabetic treated for polyarthritis. J. Rheumatol. 1999;26:195–196. [PubMed] [Google Scholar]
- 102.Winter E.M., van der Meer A.S., Eustatia-Rutten C., Janssen M., Shawkat E., Hussain N., Myers J.E., Gillham J., Helbert M. Hydroxychloroquine as a glucose lowering drug. BMJ Case Rep. 2011;2011:bcr0620114393. doi: 10.1136/bcr.06.2011.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rizvi I., Zaman S., Zaidi N., Asif M.S., Abdali N. Acute life-threatening methaemoglobinaemia following ingestion of chloroquine. BMJ Case Rep. 2012;2012:bcr1220115383. doi: 10.1136/bcr.12.2011.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Demir D., Öcal F., Abanoz M., Dermenci H. A case of thrombocytopenia associated with the use of hydroxychloroquine following open heart surgery. Int. J. Surg. Case Rep. 2014;5:1282–1284. doi: 10.1016/j.ijscr.2014.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fromm L.M. Suspected hydroxychloroquine-induced thrombotic thrombocytopaenic purpura. J. Pharm. Pract. Res. 2018;48:72–75. doi: 10.1002/jppr.1248. [DOI] [Google Scholar]
- 106.Rubin M.L., Thomas W.C., Jr. Diplopia and loss of accommodation due to chloroquine. Arthritis Rheum. 1970;13:75–82. doi: 10.1002/art.1780130108. [DOI] [PubMed] [Google Scholar]
- 107.Prince D.S., Hardin J.G. Hydroxychloroquine-induced vertigo. JAMA. 1975;233:984. doi: 10.1001/jama.1975.03260090050024. [DOI] [PubMed] [Google Scholar]
- 108.Malik M.K., Bhatia B.P.R., Malik G.K. Chloroquine causing vestibular toxicity. Indian J. Otolaryngol. Head Neck Surg. 1977;29:191. doi: 10.1007/BF02992014. [DOI] [Google Scholar]
- 109.Dwivedi G.S., Mehra Y.N. Ototoxicity of Chloroquine Phosphate. A case report. J. Laryngol. Otol. 1978;92:701–703. doi: 10.1017/S0022215100085960. [DOI] [PubMed] [Google Scholar]
- 110.Fleury O., Droitcourt C., Polard E., Chevrant-Breton J. Reversible ageusia as an adverse effect of hydroxychloroquine treatment. J. Eur. Acad. Dermatol. Venereol. 2009;23:604–605. doi: 10.1111/j.1468-3083.2008.02984.x. [DOI] [PubMed] [Google Scholar]
- 111.Giner Galvañ V., Oltra M.R., Rueda D., Esteban M.J., Redón J. Severe acute hepatitis related to hydroxychloroquine in a woman with mixed connective tissue disease. Clin. Rheumatol. 2007;26:971–972. doi: 10.1007/s10067-006-0218-1. [DOI] [PubMed] [Google Scholar]
- 112.Sunkara B., Roofeh D., Silver S., Pearson T.L., Ettel M., McCune W.J. The devil’s in the dosing: Severe drug-induced liver injury in a hydroxychloroquine-naive patient with subacute cutaneous lupus erythematosus and porphyria cutanea tarda. Lupus. 2018;27:1383–1386. doi: 10.1177/0961203318768884. [DOI] [PubMed] [Google Scholar]
- 113.Liu A.C. Hepatotoxic reaction to chloroquine phosphate in a patient with previously unrecognized porphyria cutanea tarda. West. J. Med. 1995;162:548–551. [PMC free article] [PubMed] [Google Scholar]
- 114.Makin A.J., Wendon J., Fitt S., Portmann B.C., Williams R. Fulminant hepatic failure secondary to hydroxychloroquine. Gut. 1994;35:569–570. doi: 10.1136/gut.35.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kutz D.C., Bridges A.J. Bullous rash and brown urine in a systemic lupus erythematosus patient treated with hydroxychlo-roquine. Arthritis Rheum. 1995;38:440–443. doi: 10.1002/art.1780380325. [DOI] [PubMed] [Google Scholar]
- 116.Baler G.R. Porphyria precipitated by hydroxychloroquine treatment of systemic lupus erythematosus. Cutis. 1976;17:96–98. [PubMed] [Google Scholar]
- 117.Davis T.M.E., Syed D.A., Ilett K.F., Barrett P.H.R. Toxicity Related to Chloroquine Treatment of Resistant Vivax Malaria. Ann. Pharmacother. 2003;37:526–529. doi: 10.1345/aph.1C311. [DOI] [PubMed] [Google Scholar]
- 118.Richter J.G., Becker A., Ostendorf B., Specker C., Stoll G., Neuen-Jacob E., Schneider M. Differential diagnosis of high serum creatine kinase levels in systemic lupus ery-thematosus. Rheumatol. Int. 2003;23:319–323. doi: 10.1007/s00296-003-0309-0. [DOI] [PubMed] [Google Scholar]
- 119.Bolaños-Meade J., Zhou L., Hoke A., Corse A., Vogelsang G., Wagner K.R. Hydroxychloroquine causes severe vacuolar myopathy in a patient with chronic graft-versus-host disease. Am. J. Hematol. 2005;78:306–309. doi: 10.1002/ajh.20294. [DOI] [PubMed] [Google Scholar]
- 120.Knudsen L., Gugger M., Dumont P., Nicod L., von Garnier C. A Rare Cause of Acute Respiratory Failure and Elevated Eosinophils in Broncho-Alveolar Lavage Fluid. Respiration. 2009;77:224–228. doi: 10.1159/000197806. [DOI] [PubMed] [Google Scholar]
- 121.Donado C.D., Díez E.M. Successful Desensitization for Hydroxychloroquine Anaphylaxis. J. Rheumatol. 2010;37:1975–1976. doi: 10.3899/jrheum.091453. [DOI] [PubMed] [Google Scholar]
- 122.Carnovale C., Perrone V., Borsadoli C., Mambrini A., Speziali A., Froldi G., Antoniazzi S., Magistro L., Clementi E., Radice S. A case of urinary incontinence by hydroxychloroquine in a geriatric patient. J. Clin. Pharm. Ther. 2013;38:169–171. doi: 10.1111/jcpt.12024. [DOI] [PubMed] [Google Scholar]
- 123.Català R., Azón Masoliver A. Hernández Flix SInterstitial lung disease induced by hydroxychloroquine. Med. Clin. 2015;145:415–416. doi: 10.1016/j.medcle.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 124.Ishiguro Y., Muro Y., Murase C., Takeichi T., Kono M., Adachi R., Takahashi K., Akiyama M. Drug-induced acute eosinophilic pneumonia due to hydroxychloroquine in a chilblain lupus patient. J. Dermatol. 2019;46:e356–e357. doi: 10.1111/1346-8138.14905. [DOI] [PubMed] [Google Scholar]
- 125.Ganjei Z., Bahmani K. A case report of hydroxychloroquine-induced auditory and visual hallucination. Int. J. Clin. Pharmacol. Ther. 2021;59:254–256. doi: 10.5414/CP203789. [DOI] [PubMed] [Google Scholar]
- 126.Weinke T., Held T., Trautmann M., Rögler G., Mravak S., Alexander M., Pohle H.D. Malaria therapy in 452 patients, with special reference to the use of quinine. J. Infect. 1992;25:173–180. doi: 10.1016/0163-4453(92)94012-M. [DOI] [PubMed] [Google Scholar]
- 127.Bussaratid V., Walsh D.S., Wilairatana P., Krudsood S., Silachamroon U., Looareesuwan S. Frequency of pruritus in Plasmodium vivax malaria patients treated with chloroquine in Thailand. Trop. Dr. 2000;30:211–214. doi: 10.1177/004947550003000410. [DOI] [PubMed] [Google Scholar]
- 128.Olayemi O., Fehintola F., Osungbade A., Aimakhu C., Udoh E., Adeniji A. Pattern of chloroquine-induced pruritus in antenatal patients at the University College Hospital, Ibadan. J. Obstet. Gynaecol. 2003;23:490–495. doi: 10.1080/0144361031000153693. [DOI] [PubMed] [Google Scholar]
- 129.Gama H., Ismael A., Sitoi F., Matola A., Barros H., Lunet N. Factors associated with chloroquine-induced pruritus during malaria treatment in Mozam-bican university students. Gac. Sanit. 2009;23:306–310. doi: 10.1016/j.gaceta.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 130.Jeevangi S.R., Manjunath S., Awanti S.M. Prescription pattern of anti-malarial drugs in a tertiary care hospital. Asian Pac. J. Trop. Med. 2010;3:337–420. [Google Scholar]
- 131.Ballut P.C., Siqueira A.M., Orlando A.C., Alexandre M.A., Alecrim M.G.C., Lacerda M.V. Prevalence and risk factors associated to pruritus in Plasmodium vivax patients using chloroquine in the Brazilian Amazon. Acta Trop. 2013;128:504–508. doi: 10.1016/j.actatropica.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 132.Chasset F., Arnaud L., Jachiet M., Monfort J.-B., Bouaziz J.-D., Cordoliani F., Bagot M., Barbaud A., Francès C. Changing antimalarial agents after inefficacy or intolerance in patients with cutaneous lupus erythematosus: A multicenter observational study. J. Am. Acad. Dermatol. 2018;78:107–114.e1. doi: 10.1016/j.jaad.2017.08.045. [DOI] [PubMed] [Google Scholar]
- 133.Kishi C., Motegi S.I., Yasuda M., Ishikawa O. Therapeutic efficacy and adverse events of hydroxychloroquine administration in Jap-anese systemic/cutaneous lupus erythematosus patients. J. Dermatol. 2018;45:1020–1022. doi: 10.1111/1346-8138.14512. [DOI] [PubMed] [Google Scholar]
- 134.Gonzalez C.D., Hansen C., Clarke J.T. Adverse cutaneous drug reactions with antimalarials in cutaneous lupus and derma-tomyositis: A retrospective cohort study. J. Am. Acad. Dermatol. 2019;81:859–860. doi: 10.1016/j.jaad.2019.04.068. [DOI] [PubMed] [Google Scholar]
- 135.Petersen C.S., Thomsen K. High dose hydroxychloroquine treatment of porphyria cutanea tarda. J. Am. Acad. Dermatol. 1992;26:614–619. doi: 10.1016/0190-9622(92)70090-3. [DOI] [PubMed] [Google Scholar]
- 136.Rossmann-Ringdahl I., Olsson R. Porphyria cutanea tarda: Effects and risk factors for hepatotoxicity from high-dose chlo-roquine treatment. Acta Derm. Venereol. 2007;87:401–405. doi: 10.2340/00015555-0260. [DOI] [PubMed] [Google Scholar]
- 137.Seth S., Khan D.A. The Comparative Safety of Multiple Alternative Agents in Refractory Chronic Urticaria Patients. J. Allergy Clin. Immunol. Pract. 2016;5:165–170. doi: 10.1016/j.jaip.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 138.Gozal D., Hengy C., Fadat G. Prolonged malaria prophylaxis with chloroquine and proguanil (chloroguanide) in a nonim-mune resident population of an endemic area with a high prevalence of chloroquine resistance. Antimicrob. Agent Chemother. 1991;35:373–376. doi: 10.1128/AAC.35.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McClean K.L., Hitchman D., Shafran S.D. Norfloxacin Is Inferior to Chloroquine for Falciparum Malaria in Northwestern Zambia: A Comparative Clinical Trial. J. Infect. Dis. 1992;165:904–907. doi: 10.1093/infdis/165.5.904. [DOI] [PubMed] [Google Scholar]
- 140.Dunne M.W., Dev V., Valecha N., Singh N., Shukla M., Devi C.U., Patki K., Yadav R.S., Bhattacharyya P.C., Mohapatra M.K., et al. A Double-blind, randomized study of azithromycin compared to chloroquine for the treatment of plasmodium vivax malaria in india. Am. J. Trop. Med. Hyg. 2005;73:1108–1111. doi: 10.4269/ajtmh.2005.73.1108. [DOI] [PubMed] [Google Scholar]
- 141.Ratcliff A., Siswantoro H., Kenangalem E., Wuwung M., Brockman A., Edstein M., Laihad F., Ebsworth E., Anstey N., Tjitra E., et al. Therapeutic response of multidrug-resistant Plasmodium falciparum and P. vivax to chloroquine and sulfadoxine–pyrimethamine in southern Papua, Indonesia. Trans. R. Soc. Trop. Med. Hyg. 2007;101:351–359. doi: 10.1016/j.trstmh.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Massaga J.J., Lusingu J.P., Makunde R., Malebo H.M., Chile M.M., Akida J.A., Lemnge M.M., Rønn A.M., Theander T.G., Bygbjerg I.C., et al. Biological and haematological safety profile of oral amodiaquine and chloroquine in healthy volunteers with or without Plasmodium falciparum infection in northeast Tanzania. Tanzan. J. Health Res. 2008;10:144–150. doi: 10.4314/thrb.v10i3.14354. [DOI] [PubMed] [Google Scholar]
- 143.Haar D., Sølvkjær M., Unger B., Rasmussen K.-J.E., Christensen L., Hansen T.M. A Double-blind Comparative Study of Hydroxychloroquine and Dapsone, Alone and in Combination, in Rheumatoid Arthritis. Scand. J. Rheumatol. 1993;22:113–118. doi: 10.3109/03009749309099254. [DOI] [PubMed] [Google Scholar]
- 144.Van Schaardenburg D., Vlkema R., Dijkmans B.A., Papapoulos S., Zwinderman A.H., Han K.H., Pauwels E.K., Breedveld F.C. Prednisone treatment of elderly-onset rheumatoid arthritis. Disease activity and bone mass in comparison with chloroquine treatment. Arthritis Rheumatol. 1995;38:334–342. doi: 10.1002/art.1780380307. [DOI] [PubMed] [Google Scholar]
- 145.Baltzan M., Mehta S., Kirkham T.H., Cosio M.G. Randomized Trial of Prolonged Chloroquine Therapy in Advanced Pulmonary Sarcoidosis. Am. J. Respir. Crit. Care Med. 1999;160:192–197. doi: 10.1164/ajrccm.160.1.9809024. [DOI] [PubMed] [Google Scholar]
- 146.Dunne M.W., Singh N., Shukla M., Valecha N., Bhattacharyya P.C., Dev V., Patel K., Mohapatra M.K., Lakhani J., Benner R., et al. A multicenter study of azithromycin, alone and in combination with chloroquine, for the treatment of acute uncomplicated Plasmodium falciparum malaria in India. J. Infect. Dis. 2005;191:1582–1588. doi: 10.1086/429343. [DOI] [PubMed] [Google Scholar]
- 147.Sperber K., Louie M., Kraus T., Proner J., Sapira E., Lin S., Stecher V., Mayer L. Hydroxychloroquine treatment of patients with human immunodeficiency virus type 1. Clin. Ther. 1995;17:622–636. doi: 10.1016/0149-2918(95)80039-5. [DOI] [PubMed] [Google Scholar]
- 148.Reeves G.E.M., Boyle M.J., Bonfield J., Dobson P., Loewenthal M. Impact of hydroxychloroquine therapy on chronic urticaria: Chronic autoimmune urticaria study and evaluation. Intern. Med. J. 2004;34:182–186. doi: 10.1111/j.1444-0903.2004.00532.x. [DOI] [PubMed] [Google Scholar]
- 149.Poravuth Y., Socheat D., Rueangweerayut R., Uthaisin C., Pyae Phyo A., Valecha N., Rao B.H., Tjitra E., Purnama A., Borghini-Fuhrer I., et al. Pyronaridine-artesunate versus chloroquine in patients with acute Plas-modium vivax malaria: A randomized, double-blind, non-inferiority trial. PLoS ONE. 2011;6:e14501. doi: 10.1371/journal.pone.0014501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Arnaout A., Robertson S.J., Pond G.R., Lee H., Jeong A., Ianni L., Kroeger L., Hilton J., Coupland S., Gottlieb C., et al. A randomized, double-blind, window of opportunity trial evaluating the effects of chloroquine in breast cancer patients. Breast Cancer Res. Treat. 2019;178:327–335. doi: 10.1007/s10549-019-05381-y. [DOI] [PubMed] [Google Scholar]
- 151.Watt G., Long G.W., Padre L.P., Alban P., Sangalang R., Ranoa C.P. Chloroquine and quinine: A randomized, double-blind comparison of efficacy and side effects in the treatment of Plasmodium falciparum malaria in the Philippines. Trans. R. Soc. Trop. Med. Hyg. 1988;82:205–208. doi: 10.1016/0035-9203(88)90411-7. [DOI] [PubMed] [Google Scholar]
- 152.Cowley R.G., Myers J.E., Jr. Chloroquine in the treatment of infectious mononucleosis. Ann. Intern. Med. 1962;57:937–945. doi: 10.7326/0003-4819-57-6-937. [DOI] [PubMed] [Google Scholar]
- 153.Schumacher H.R., Jacobson W.A., BeMiller C.R. Treatment of Infectious Mononucleosis. Ann. Intern. Med. 1963;58:217–228. doi: 10.7326/0003-4819-58-2-217. [DOI] [PubMed] [Google Scholar]
- 154.De Lamballerie X., Boisson V., Reynier J.C., Enault S., Charrel R.N., Flahault A., Roques P., Le Grand R. On chikungunya acute infection and chloroquine treatment. Vector Borne Zoonotic Dis. 2008;8:837–839. doi: 10.1089/vbz.2008.0049. [DOI] [PubMed] [Google Scholar]
- 155.Tricou V., Minh N.N., VAN T.P., Lee S.J., Farrar J., Wills B., Tran H.T., Simmons C.P. A Randomized Controlled Trial of Chloroquine for the Treatment of Dengue in Vietnamese Adults. PLoS Negl. Trop. Dis. 2010;4:e785. doi: 10.1371/journal.pntd.0000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Borges M.C., Castro L.A., da Fonseca B.A.L. Chloroquine use improves dengue-related symptoms. Mem. Inst. Oswaldo Cruz. 2013;108:596–599. doi: 10.1590/S0074-02762013000500010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ajayi A.A., Oluokun A., Sofowora O., Akinleye A. Epidemiology of antimalarial-induced pruritus in Africans. Eur. J. Clin. Pharmacol. 1989;37:539–540. doi: 10.1007/BF00558141. [DOI] [PubMed] [Google Scholar]
- 158.Katugampola G., Katugampola S. Chloroquine and Psoriasis. Int. J. Dermatol. 1990;29:153–154. doi: 10.1111/j.1365-4362.1990.tb04095.x. [DOI] [PubMed] [Google Scholar]
- 159.Frías Salcedo J.A., Flores Vargas D. Acute reactions to chloroquine phosphate at the beginning of antimalaria chemopro-phylaxis. Rev. Sanid Mil. 1992;46:1–3. [Google Scholar]
- 160.Ajayi A.A., Olotu T.C., Sofowora G.G. Knowledge, attitude and practice of prednisolone prevention of chloroquine induced pruritus among Nigerian health workers. Trop. Dr. 1998;28:210–211. doi: 10.1177/004947559802800407. [DOI] [PubMed] [Google Scholar]
- 161.George A.O. Chloroquine induced pruritus--questionnaire based epidemiological study. Afr. J. Health Sci. 2005;11:87–92. doi: 10.4314/ajhs.v11i3.30785. [DOI] [PubMed] [Google Scholar]
- 162.Obasikene G., Adobamen P., Okundia P., Ogusi F.O. Prevalence of ototoxicity in University of Benin Teaching Hospital, Benin city: A 5-year review. Niger. J. Clin. Pract. 2012;15:453–457. doi: 10.4103/1119-3077.104527. [DOI] [PubMed] [Google Scholar]
- 163.Schneider C., Adamcova M., Jick S.S., Schlagenhauf P., Miller M.K., Rhein H.-G., Meier C.R. Antimalarial chemoprophylaxis and the risk of neuropsychiatric disorders. Travel Med. Infect. Dis. 2013;11:71–80. doi: 10.1016/j.tmaid.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 164.Emerole C.G., Nneli R.O., Osim E.E. Gender and environmental influences on visual acuity in Owerri, Nigeria. Niger. J. Physiol. Sci. 2014;29:17–22. [PubMed] [Google Scholar]
- 165.Sarathi P., Sen D., Majumdar R. Psychosis following chloroquine ingestion: A 10-year comparative study from a malar-ia-hyperendemic district of India. Gen. Hosp. Psychiatry. 2014;36:181–186. doi: 10.1016/j.genhosppsych.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 166.Castro-Cavadía C.J., Carmona-Fonseca J. Assessment of the efficacy and safety of chloroquine monotherapy for the treatment of acute uncomplicated gestational malaria caused by P. vivax, Cordoba, Colombia, 2015–2017. Rev. Colomb. Obstet. Ginecol. 2020;71:21–33. doi: 10.18597/rcog.3370. [DOI] [PubMed] [Google Scholar]
- 167.Dugué A., Bagheri H., Lapeyre-Mestre M., Tournamille J.F., Sailler L., Dedieu G., Salvayre R., Thouvenot J.P., Massip P., Montastruc J.L. Detection and incidence of muscular adverse drug reactions: A prospective analysis from laboratory signals. Eur. J. Clin. Pharmacol. 2004;60:285–292. doi: 10.1007/s00228-004-0760-1. [DOI] [PubMed] [Google Scholar]
- 168.Patel K.J., Kedia M.Ş., Bajpai D., Mehta S.S., Kshirsagar N.A., Gogtay N.J. Evaluation of the prevalence and economic burden of adverse drug reactions presenting to the medical emergency department of a tertiary referral centre: A prospective study. BMC Clin. Pharmacol. 2007;7:8. doi: 10.1186/1472-6904-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sidoroff A., Dunant A., Viboud C., Halevy S., Bavinck J.B., Naldi L., Mockenhaupt M., Fagot J.-P., Roujeau J.-C. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case–control study (EuroSCAR) Br. J. Dermatol. 2007;157:989–996. doi: 10.1111/j.1365-2133.2007.08156.x. [DOI] [PubMed] [Google Scholar]
- 170.Walsh D.S., Looareesuwan S., Wilairatana P., Heppner J.D.G., Tang D.B., Brewer T.G., Chokejindachai W., Viriyavejakul P., Kyle D.E., Milhous W.K., et al. Randomized Dose-Ranging Study of the Safety and Efficacy of WR 238605 (Tafenoquine) in the Prevention of Relapse of Plasmodium vivax Malaria in Thailand. J. Infect. Dis. 1999;180:1282–1287. doi: 10.1086/315034. [DOI] [PubMed] [Google Scholar]
- 171.Yanze M.F., Duru C., Jacob M., Bastide J.M., Lankeuh M. Rapid therapeutic response onset of a new pharmaceutical form of chloroquine phosphate 300 mg: Effervescent tablets. Trop. Med. Int. Health. 2001;6:196–201. doi: 10.1046/j.1365-3156.2001.00681.x. [DOI] [PubMed] [Google Scholar]
- 172.Tagbor H., Bruce J., Browne E., Randal A., Greenwood B., Chandramohan D. Efficacy, safety, and tolerability of amodiaquine plus sulphadoxine-pyrimethamine used alone or in combination for malaria treatment in pregnancy: A randomised trial. Lancet. 2006;368:1349–1356. doi: 10.1016/S0140-6736(06)69559-7. [DOI] [PubMed] [Google Scholar]
- 173.Sagara I., Oduro A.R., Mulenga M., Dieng Y., Ogutu B., Tiono A.B., Mugyenyi P., Sie A., Wasunna M., Kain K.C., et al. Efficacy and safety of a combination of azithromycin and chloroquine for the treatment of uncomplicated Plasmodium falciparum malaria in two multi-country randomized clinical trials in African adults. Malar. J. 2014;13:458. doi: 10.1186/1475-2875-13-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kshirsagar N.A., Gogtay N.J., Moran D., Utz G., Sethia A., Sarkar S., Vandenbroucke P. Treatment of adults with acute uncomplicated malaria with azithromycin and chloroquine in India, Colombia, and Suriname. Res. Rep. Trop. Med. 2017;8:85–104. doi: 10.2147/RRTM.S129741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ajayi A.A., Akinleye A.O., Udoh S.J., Ajayi O.O., Oyelese O., Ijaware C.O. The effects of prednisolone and niacin on chloroquine-induced pruritus in malaria. Eur. J. Clin. Pharmacol. 1991;41:383–385. doi: 10.1007/BF00314973. [DOI] [PubMed] [Google Scholar]
- 176.Adebayo R.A., Sofowora G.G., Onayemi O., Udoh S.J., Ajayi A.A. Chloroquine-induced pruritus in malaria fever: Contribution of malaria para-sitemia and the effects of prednisolone, niacin, and their combination, compared with antihistamine. Br. J. Clin. Pharmacol. 1997;44:157–161. doi: 10.1046/j.1365-2125.1997.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vouri S.M., Thai T.N., Winterstein A.G. An evaluation of co-use of chloroquine or hydroxychloroquine plus azithromycin on cardiac outcomes: A pharmacoepidemiological study to inform use during the COVID19 pandemic. Res. Soc. Adm. Pharm. 2020;17:2012–2017. doi: 10.1016/j.sapharm.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sarayani A., Cicali B., Henriksen C.H., Brown J.D. Safety signals for QT prolongation or Torsades de Pointes associated with azithromycin with or without chloroquine or hydroxychloroquine. Res. Soc. Adm. Pharm. 2021;17:483–486. doi: 10.1016/j.sapharm.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kimani J., Phiri K., Kamiza S., Duparc S., Ayoub A., Rojo R., Robbins J., Orrico R., Vandenbroucke P. Efficacy and Safety of Azithromycin-Chloroquine versus Sulfadoxine-Pyrimethamine for Intermittent Preventive Treatment of Plasmodium falciparum Malaria Infection in Pregnant Women in Africa: An Open-Label, Randomized Trial. PLoS ONE. 2016;11:e0157045. doi: 10.1371/journal.pone.0157045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Phiri K., Kimani J., Mtove G.A., Zhao Q., Rojo R., Robbins J., Duparc S., Ayoub A., Vandenbroucke P. Parasitological Clearance Rates and Drug Concentrations of a Fixed Dose Combination of Azithromycin-Chloroquine in Asymptomatic Pregnant Women with Plasmodium Falciparum Parasitemia: An Open-Label, Non-Comparative Study in Sub-Saharan Africa. PLoS ONE. 2016;11:e0165692. doi: 10.1371/journal.pone.0165692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Asli R., Abdullah M.S., Chong P.L., Metussin D., Momin R.N., Mani B.I., Chong V.H. Case report: Right bundle brunch block and QTc prolongation in a patient with novel coronavirus disease (COVID-19) treated with hydroxychloroquine. Am. J. Trop. Med. Hyg. 2020;103:79–82. doi: 10.4269/ajtmh.20-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Falcão M.B., Pamplona de Góes Cavalcanti L., Filgueiras Filho N.M., de Brito C.A.A. Case Report: Hepatotoxicity Associated with the Use of Hydroxychloroquine in a Patient with COVID-19. Am. J. Trop. Med. Hyg. 2020;102:1214–1216. doi: 10.4269/ajtmh.20-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Beauverd Y., Adam Y., Assouline B., Samii K. COVID-19 infection and treatment with hydroxychloroquine cause severe hae-molysis crisis in a patient with glucose-6-posphate dehydrogenase deficiency. Eur. J. Haematol. 2020;105:357–359. doi: 10.1111/ejh.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kuipers M.T., van Zwieten R., Heijmans J., Rutten C.E., de Heer K., Kater A.P., Nur E. G6PD deficiency-associated hemolysis and methemoglobinemia in a COVID-19 patient treated with chloroquine. Am. J. Hematol. 2020;95:E194–E196. doi: 10.1002/ajh.25862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kutlu Ö., Metin A. A case of exacerbation of psoriasis after oseltamivir and hydroxychloroquine in a patient with COVID-19: Will cases of psoriasis increase after COVID-19 pandemic? Dermatol. Ther. 2020;33:e13383. doi: 10.1111/dth.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mitra R.L., Greenstein S.A., Epstein L.M. An algorithm for managing QT prolongation in coronavirus disease 2019 (COVID 2019) patients treated with either chloroquine or hydroxychloroquine in conjunction with azithromycin: Possible benefits of in-travenous lidocaine. Heart Rhythm. Case Rep. 2020;6:244–248. doi: 10.1016/j.hrcr.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Robustelli Test E., Vezzoli P., Carugno A., Raponi F., Gianatti A., Rongioletti F., Sena P. Acute generalized exanthematous pustulosis with erythema multiforme-like lesions in a COVID-19 woman. Eur. Acad. Dermatol. Venereol. 2020;12:475–478. doi: 10.1111/jdv.16613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Szekely Y., Lichter Y., Abu Shrkihe B., Bruck H., Oster H.S., Viskin S. Chloroquine-induced torsades de pointes in a patient with coronavirus disease 2019. Heart Rhythm. 2020;17:1452–1455. doi: 10.1016/j.hrthm.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gracia-Ramos A.E., Cortes-Ortiz A. Wide complex tachycardia in a patient with COVID-19 treated with chloro-quine/azithromycin. Oxf. Med. Case Rep. 2021;1:omaa124. doi: 10.1093/omcr/omaa124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Aslam W., Lamb C.R., Ali N. Torsades de pointes in SARS-CoV-2 (COVID-19) pneumonia: Medicine reconciliation and careful monitoring of QTc interval may help prevent cardiac complications. BMJ Case Rep. 2021;14:e239963. doi: 10.1136/bcr-2020-239963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kang Y., Wang H., Chen H., Wang B., Yang Y., Zhao X., Ran Q., Wei J. Suspected Hydroxychloroquine-Induced Sinus Bradycardia and QTc Prolongation in a Patient with COVID-19. Int. Heart J. 2020;61:1056–1058. doi: 10.1536/ihj.20-271. [DOI] [PubMed] [Google Scholar]
- 192.Patel J., Patel R., Rodriguez L.-M., Blanco A., Hamza A. Cardiovascular Considerations of Experimental Hydroxychloroquine Therapy on Patients Diagnosed with COVID-19: A Case Series Review. Cureus. 2020;12:e9151. doi: 10.7759/cureus.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ambar Akkaoui M., Lejoyeux M., Geoffroy P.A. Chloroquine-Induced First-Episode Psychosis in a Patient Self-medicated for COVID-19. Biol. Psychiatry. 2021;89:e9. doi: 10.1016/j.biopsych.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Koc G., Odabasi Z., Tan E. Myasthenic Syndrome Caused by Hydroxychloroquine Used for COVID-19 Prophylaxis. J. Clin. Neuromuscul. Dis. 2020;22:60–62. doi: 10.1097/CND.0000000000000316. [DOI] [PubMed] [Google Scholar]
- 195.Benjelloun R., Otheman Y., El Kettani C. Psychiatric side effects of chloroquine in COVID-19 patients: Two case reports. Pan Afr. Med. J. 2020;35:83. doi: 10.11604/pamj.supp.2020.35.24064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sardana K., Mathachan S.R., Deepak D., Khurana A., Sinha S. Cutaneous side effects of hydroxychloroquine in health care workers in a COVID referral hospital—Implications for clinical practice. J. Dermatolog. Treat. 2020;22:1–3. doi: 10.1080/09546634.2020.1781041. [DOI] [PubMed] [Google Scholar]
- 197.Monte-Serrano J., Cruañes-Monferrer J., García-García M., García-Gil M.F. Hydroxychloroquine-induced erythema multiforme in a patient with COVID-19. Med. Clin. (Engl. Ed.) 2020;155:231. doi: 10.1016/j.medcle.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Delaleu J., Deniau B., Battistella M., de Masson A., Bensaid B., Jachiet M., Lazaridou I., Bagot M., Bouaziz J.D., Saint-Louis CORE (COVID Research) Group Acute generalized exanthematous pustulosis induced by hydroxychloroquine prescribed for COVID-19. J. Allergy Clin. Immunol. Pract. 2020;8:2777–2779. doi: 10.1016/j.jaip.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Enos T., Jeong H.S., Vandergriff T., Jacobe H.T., Chong B.F. Acute generalized exanthematous pustulosis induced by empiric hydroxychloroquine for presumed COVID-19. Dermatol. Ther. 2020;33:e13834. doi: 10.1111/dth.13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Castro Jiménez A., Navarrete Navarrete N., Gratacós Gómez A.R., Florido López F., García Rodríguez R., Gómez Torrijos E. First case of DRESS syndrome caused by hydroxychloroquine with a positive patch test. Contact Dermat. 2021;84:50–51. doi: 10.1111/cod.13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Abadías-Granado I., Palma-Ruiz A., Cerro P., Morales-Callaghan A., Gómez-Mateo M., Gilaberte Y., Schwartz R. Generalized pustular figurate erythema first report in two COVID-19 patients on hydroxychloroquine. J. Eur. Acad. Dermatol. Venereol. 2021;35:e5–e7. doi: 10.1111/jdv.16903. [DOI] [PubMed] [Google Scholar]
- 202.Kurd R., Zuckerman M., Ben-Chetrit E. Hydroxychloroquine-related Rash in COVID-19 Infected Patient. Isr. Med. Assoc. J. 2020;22:525. [PubMed] [Google Scholar]
- 203.Arıkan F., Yıldız Y., Ercan T., Oruç Ö., Akçay S., Yılmaz F., Toptaş T., Tuğlular T. Hydroxychloroquine-Associated Thrombotic Thrombocytopenic Purpura. Turk. J. Hematol. 2020;37:302–304. doi: 10.4274/tjh.galenos.2020.2020.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Maillart E., Leemans S., Van Noten H., Vandergraesen T., Mahadeb B., Salaouatchi M.T., De Bels D., Clevenbergh P. A case report of serious haemolysis in a glucose-6-phosphate dehydrogenase-deficient COVID-19 patient receiving hydroxychloroquine. Infect. Dis. (Lond.) 2020;52:659–661. doi: 10.1080/23744235.2020.1774644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chaney S., Basirat A., McDermott R., Keenan N., Moloney E. COVID-19 and hydroxychloroquine side-effects: Glucose 6-phosphate dehydrogenase deficiency (G6PD) and acute haemolytic anaemia. QJM. 2020;113:890–891. doi: 10.1093/qjmed/hcaa267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Aguilar J., Averbukh Y. Hemolytic Anemia in a Glucose-6-Phosphate Dehydrogenase-Deficient Patient Receiving Hy-droxychloroquine for COVID-19: A Case Report. Perm. J. 2020;24:158. doi: 10.7812/TPP/20.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bessière F., Roccia H., Delinière A., Charrière R., Chevalier P., Argaud L., Cour M. Assessment of QT Intervals in a Case Series of Patients with Coronavirus Disease 2019 (COVID-19) Infection Treated with Hydroxychloroquine Alone or in Combination with Azithromycin in an Intensive Care Unit. JAMA Cardiol. 2020;5:1067–1069. doi: 10.1001/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chong V.H., Chong P.L., Metussin D., Asli R., Momin R.N., Mani B.I., Abdullah M.S. Conduction abnormalities in hydroxychloroquine add on therapy to lop-inavir/ritonavir in COVID-19. J. Med. Virol. 2020;92:2322–2324. doi: 10.1002/jmv.26004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chorin E., Wadhwani L., Magnani S., Dai M., Shulman E., Nadeau-Routhier C., Knotts R., Bar-Cohen R., Kogan E., Barbhaiya C., et al. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020;17:1425–1433. doi: 10.1016/j.hrthm.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cipriani A., Zorzi A., Ceccato D., Capone F., Parolin M., Donato F., Fioretto P., Pesavento R., Previato L., Maffei P., et al. Arrhythmic profile and 24-hour QT interval variability in COVID-19 patients treated with hydroxychloroquine and azithromycin. Int. J. Cardiol. 2020;316:280–284. doi: 10.1016/j.ijcard.2020.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W., Wu Y., Xiao W., Liu S., Chen E., et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: Open label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fernández-Ruiz M., Andrés A., Loinaz C., Delgado J.F., López-Medrano F., Juan R.S., González E., Polanco N., Folgueira M.D., Lalueza A., et al. COVID-19 in solid organ transplant recipients: A single-center case series from Spain. Am. J. Transplant. 2020;20:1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Sevestre J., Mailhe M., Doudier B., Aubry C., Amrane S., et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med. Infect. Dis. 2020;34:101663. doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mahévas M., Tran V.-T., Roumier M., Chabrol A., Paule R., Guillaud C., Fois E., Lepeule R., Szwebel T.-A., Lescure X., et al. Clinical efficacy of hydroxychloroquine in patients with COVID-19 pneumonia who require oxygen: Observational comparative study using routine care data. BMJ. 2020;369:m1844. doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mercuro N.J., Yen C.F., Shim D.J., Maher T.R., McCoy C.M., Zimetbaum P.J., Gold H.S. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing postivie for coronavirus disease 2019 (COVID-2019) JAMA Cardiol. 2020;5:1036–1041. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Million M., Lagier J.-C., Gautret P., Colson P., Fournier P.-E., Amrane S., Hocquart M., Mailhe M., Esteves-Vieira V., Doudier B., et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Travel Med. Infect. Dis. 2020;35:101738. doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Molina J., Delaugerre C., Le Goff J., Mela-Lima B., Ponscarme D., Goldwirt L., de Castro N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Mal. Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rosenberg E.S., Dufort E.M., Udo T., Wilberschied L.A., Kumar J., Tesoriero J., Weinberg P., Kirkwood J., Muse A., DeHovitz J., et al. Association of Treatment with Hydroxychloroquine or Azithromycin with In-Hospital Mortality in Patients with COVID-19 in New York State. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Saleh M., Gabriels J., Chang D., Soo Kim B., Mansoor A., Mahmood E., Makker P., Ismail H., Goldner B., Willner J., et al. Effect of chloroquine, hydroxychloroquine and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ. Arrhythmia Electrophysiol. 2020;13:e008662. doi: 10.1161/CIRCEP.120.008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Van den Broek M.P.H., Möhlmann J.E., Abeln B.G.S., Liebregts M., van Dijk V.F., van de Garde E.M.W. Chloroquine-induced QTc prolongation in COVID-19 patients. Neth. Heart J. 2020;28:406–409. doi: 10.1007/s12471-020-01429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., Mourão M.P.G., Brito-Sousa J.D., Baía-da-Silva D., Guerra M.V.F., et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection: A randomized clinical Trial. JAMA Netw. Open. 2020;3:e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 222.Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D., Zhuang R., Hu B., Zhang Z. Efficacy of hydroxychloroquine in patients with COVID-19: Results of a randomized clinical trial. MedRxiv. 2020 doi: 10.1101/2020.03.22.20040758. [DOI] [Google Scholar]
- 223.Huang M., Tang T., Pang P., Li M., Ma R., Lu J., Shu J., You Y., Chen B., Liang J., et al. Treating COVID-19 with Chloroquine. J. Mol. Cell Biol. 2020;12:322–325. doi: 10.1093/jmcb/mjaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chen J., Liu D., Liu L., Liu P., Xu Q., Xia L., Ling Y., Huang D., Song S., Zhang D., et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J. Zhejiang Univ. 2020;49:215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Colaneri M., Bogliolo L., Valsecchi P., Sacchi P., Zuccaro V., Brandolino F., Montecucco C., Mojoli F., Giusti E.M., Bruno R., et al. Tocilizumab for Treatment of Severe COVID-19 Patients: Preliminary Results from SMAtteo COvid19 REgistry (SMACORE) Microorganisms. 2020;8:695. doi: 10.3390/microorganisms8050695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lagier J.-C., Million M., Gautret P., Colson P., Cortaredona S., Giraud-Gatineau A., Honoré S., Gaubert J.-Y., Fournier P.-E., Tissot-Dupont H., et al. Outcomes of 3,737 COVID-19 patients treated with hydroxychloroquine/azithromycin and other regimens in Marseille, France: A retrospective analysis. Travel Med. Infect. Dis. 2020;36:101791. doi: 10.1016/j.tmaid.2020.101791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Paccoud O., Tubach F., Baptiste A., Bleibtreu A., Hajage D., Monsel G., Tebano G., Boutolleau D., Klement E., Godefroy N., et al. Compassionate Use of Hydroxychloroquine in Clinical Practice for Patients with Mild to Severe COVID-19 in a French University Hospital. Clin. Infect. Dis. 2020;73:e4064–e4072. doi: 10.1093/cid/ciaa791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hor C.P., Hussin N., Nalliah S., Ooi W.T., Tang X.Y., Zachariah S., Singh G.P.S.J., Rani R.A., Perumal K., Cheah W.K. Experience of short-term hydroxychloroquine and azithromycin in COVID-19 patients and effect on QTc trend. J. Infect. 2020;81:e117–e119. doi: 10.1016/j.jinf.2020.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Mazzanti A., Briani M., Kukavica D., Bulian F., Marelli S., Trancuccio A., Monteforte N., Manciulli T., Morini M., Carlucci A., et al. Association of Hydroxychloroquine with QTc Interval in Patients with COVID-19. Circulation. 2020;142:513–515. doi: 10.1161/CIRCULATIONAHA.120.048476. [DOI] [PubMed] [Google Scholar]
- 230.Jain S., Workman V., Ganeshan R., Obasare E.R., Burr A., DeBiasi R.M., Freeman J.V., Akar J., Lampert R., Rosenfeld L.E. Enhanced electrocardiographic monitoring of patients with Coronavirus Disease 2019. Heart Rhythm. 2020;17:1417–1422. doi: 10.1016/j.hrthm.2020.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Karolyi M., Pawelka E., Mader T., Omid S., Kelani H., Ely S., Jilma B., Baumgartner S., Laferl H., Ott C., et al. Hydroxychloroquine versus lopinavir/ritonavir in severe COVID-19 patients: Results from a real-life patient cohort. Wien Klin. Wochenschr. 2021;133:284–291. doi: 10.1007/s00508-020-01720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mitjà O., Corbacho-Monné M., Ubals M., Tebé C., Peñafiel J., Tobias A., Ballana E., Alemany A., Riera-Martí N., Pérez C.A., et al. Hydroxychloroquine for Early Treatment of Adults with Mild Covid-19: A Randomized-Controlled Trial. Clin. Infect. Dis. 2021;73:e4073–e4081. doi: 10.1093/cid/ciaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Reis G., Moreira Silva E.A.D.S., Medeiros Silva D.C., Thabane L., Singh G., Park J.J.H., Forrest J.I., Harari O., Quirino Dos Santos C.V., Guimarães de Almeida A.P.F., et al. Effect of Early Treatment with Hydroxychloroquine or Lopinavir and Ritonavir on Risk of Hospitalization Among Patients with COVID-19: The TOGETHER Randomized Clinical Trial. JAMA Netw. Open. 2021;4:e216468. doi: 10.1001/jamanetworkopen.2021.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dabbous H.M., El-Sayed M.H., El Assal G., Elghazaly H., Ebeid F.F.S., Sherief A.F., Elgaafary M., Fawzy E., Hassany S.M., Riad A.R., et al. Safety and efficacy of favipiravir versus hydroxychloroquine in management of COVID-19: A randomised controlled trial. Sci. Rep. 2021;11:7282. doi: 10.1038/s41598-021-85227-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 235.Uğurlu Ilgın B., Akbulut Koyuncu İ.M., Kızıltunç E. Effect of triple antimicrobial therapy on electrocardiography parameters in patients with mild-to-moderate coronavirus disease 2019. Anatol. J. Cardiol. 2021;25:184–190. doi: 10.14744/AnatolJCardiol.2020.79138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Eftekhar S.P., Kazemi S., Barary M., Javanian M., Ebrahimpour S., Ziaei N. Effect of Hydroxychloroquine and Azithromycin on QT Interval Prolongation and Other Cardiac Arrhythmias in COVID-19 Confirmed Patients. Cardiovasc. Ther. 2021;2021:6683098. doi: 10.1155/2021/6683098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lamback E.B., de Oliveira M.A., Haddad A.F., Vieira A.F.M., Neto A.L.F., Maia T.d.S., Chrisman J.D.R., Spineti P.P.D.M., de Mattos M.A., Costa E. Hydroxychloroquine with azithromycin in patients hospitalized for mild and moderate COVID-19. Braz. J. Infect. Dis. 2021;25:101549. doi: 10.1016/j.bjid.2021.101549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.O’Connell T.F., Bradley C.J., Abbas A.E., Williamson B.D., Rusia A., Tawney A.M., Gaines R., Schott J., Dmitrienko A., Haines D.E. Hydroxychloroquine/Azithromycin Therapy and QT Prolongation in Hospitalized Patients with COVID-19. JACC Clin. Electrophysiol. 2021;7:16–25. doi: 10.1016/j.jacep.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Falcão F., Viegas E., Carmo I., Soares J., Falcao M., Solano M., Cavaco P., Mendes D., Rijo J., Povoa P., et al. A prospective, observational study to evaluate adverse drug reactions in patients with COVID-19 treated with remdesivir or hydroxychloroquine: A preliminary report. Eur. J. Hosp. Pharm. 2021;28:248–253. doi: 10.1136/ejhpharm-2020-002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sogut O., Can M.M., Guven R., Kaplan O., Ergenc H., Umit T.B., Demir O., Kaya M., Akdemir T., Cakmak S. Safety and efficacy of hydroxychloroquine in 152 outpatients with confirmed COVID-19: A pilot observational study. Am. J. Emerg. Med. 2020;40:41–46. doi: 10.1016/j.ajem.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Seyhan A.U., Doganay F., Yilmaz E., Topal N.P., Ak R. Investigation of QT Prolongation with Hydroxychloroquine and Azithromycin for the Treatment of COVID-19. J. Coll. Physicians Surg. Pak. 2020;30:153–157. doi: 10.29271/jcpsp.2020.supp2.s153. [DOI] [PubMed] [Google Scholar]
- 242.Meriglier E., Rivoisy C., Hessamfar M., Bernard N., Aureau I., Lapoirie J., Contis A., Sacher F., Sacristan B., Lahouati M., et al. Safety of hydroxychloroquine and darunavir or lopinavir in COVID-19 infection. J. Antimicrob. Chemother. 2020;76:482–486. doi: 10.1093/jac/dkaa441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fteiha B., Karameh H., Kurd R., Ziff-Werman B., Feldman I., Bnaya A., Einav S., Orlev A., Ben-Chetrit E. QTc prolongation among hydroxychloroquine sulphate-treated COVID-19 patients: An observational study. Int. J. Clin. Pract. 2021;75:e13767. doi: 10.1111/ijcp.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Recovery Collaborative Group Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Saleh M., Gabriels J., Chang D., Fishbein J., Qiu M., Mountantonakis S.E., Epstein L.M., Northwell COVID-19 Research Consortium Safely Administering Potential QTc Prolonging Therapy Across a Large Health Care System in the COVID-19 Era. Circ. Arrhythm. Electrophysiol. 2020;13:e008937. doi: 10.1161/CIRCEP.120.008937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bernardini A., Ciconte G., Negro G., Rondine R., Mecarocci V., Viva T., Santini F., de Innocentiis C., Gianelli L., Witkowska E., et al. Assessing QT interval in COVID-19 patients:safety of hydroxychloro-quine-azithromycin combination regimen. Int. J. Cardiol. 2021;324:242–248. doi: 10.1016/j.ijcard.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Furtado R.H.M., Berwanger O., Fonseca H.A., Corrêa T.D., Ferraz L.R., Lapa M.G., Zampieri F.G., Veiga V.C., Azevedo L.C.P., Rosa R.G., et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): A randomised clinical trial. Lancet. 2020;396:959–967. doi: 10.1016/S0140-6736(20)31862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Giaime P., Guenoun M., Pedinielli N., Narbonne H., Bergounioux J.-P., Solas C., Guilhaumou R., Sampol J., Ollier J., Sichez H., et al. Hydroxychloroquine and azithromycin tolerance in haemodialysis patients during COVID-19 infection. Nephrol. Dial. Transplant. 2020;35:1346–1353. doi: 10.1093/ndt/gfaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kalligeros M., Shehadeh F., Atalla E., Mylona E.K., Aung S., Pandita A., Larkin J., Sanchez M., Touzard-Romo F., Brotherton A., et al. Hydroxychloroquine use in hospitalised patients with COVID-19: An observational matched cohort study. J. Glob. Antimicrob. Resist. 2020;22:842–844. doi: 10.1016/j.jgar.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Satlin M.J., Goyal P., Magleby R., Maldarelli G.A., Pham K., Kondo M., Schenck E.J., Rennert H., Westblade L.F., Choi J.J., et al. Safety, tolerability, and clinical outcomes of hydroxychloroquine for hospitalized patients with coronavirus 2019 disease. PLoS ONE. 2020;15:e0236778. doi: 10.1371/journal.pone.0236778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Moschini L., Loffi M., Regazzoni V., Di Tano G., Gherbesi E., Danzi G.B. Effects on QT interval of hydroxychloroquine associated with ritonavir/darunavir or azithromycin in patients with SARS-CoV-2 infection. Heart Vessels. 2021;36:115–120. doi: 10.1007/s00380-020-01671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Skipper C.P., Pastick K.A., Engen N.W., Bangdiwala A.S., Abassi M., Lofgren S.M., Williams D.A., Okafor E.C., Pullen M.F., Nicol M.R., et al. Hydroxychloroquine in Nonhospitalized Adults with Early COVID-19: A Randomized Trial. Ann. Intern. Med. 2020;173:623–631. doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hsia B.C., Greige N., Quiroz J.A., Khokhar A.S., Daily J., Di Biase L., Ferrick K.J., Fisher J.D., Krumerman A. QT prolongation in a diverse, urban population of COVID-19 patients treated with hydroxychloroquine, chloroquine, or azithromycin. J. Interv. Card Electrophysiol. 2020;59:337–345. doi: 10.1007/s10840-020-00822-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Echarte-Morales J., Minguito-Carazo C., Del Castillo-García S., Borrego-Rodríguez J., Rodríguez-Santamarta M., Sánchez-Muñoz E., Bergel-García R., González-Maniega C., Prieto-González S., Menéndez-Suarez P., et al. Effect of hydroxychloroquine, azithromycin and lop-inavir/ritonavir on the QT corrected interval in patients with COVID-19. J. Electrocardiol. 2021;64:30–35. doi: 10.1016/j.jelectrocard.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Jiménez-Jáimez J., Macías-Ruiz R., Bermúdez-Jiménez F., Rubini-Costa R., Ramírez-Taboada J., Flores P.I.G., Gallo-Padilla L., García J.D.M., García C.M., Suárez S.M., et al. Absence of relevant QT interval prolongation in not critically ill COVID-19 patients. Sci. Rep. 2020;10:21417. doi: 10.1038/s41598-020-78360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zdemir İ.H., Özlek B., Özen M.B., Gündüz R., Çetin N., Bilge A.R. Hydroxychloroquine/azithromycin treatment, QT interval and ventricular arrhythmias in hospitalised patients with COVID-19. Int. J. Clin. Pract. 2021;75:e13896. doi: 10.1111/ijcp.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C., Veiga V.C., Avezum A., Damiani L.P., Marcadenti A., Kawano-Dourado L., Lisboa T., et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N. Engl. J. Med. 2020;383:2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Çap M., Bilge Ö., Işık F., Burak C., Karagöz A., İnci Ü., Akyüz A., Aslan B., Altıntaş B., Altındağ R., et al. The effect of favipiravir on QTc interval in patients hospitalized with coronavirus disease 2019. J. Electrocardiol. 2020;63:115–119. doi: 10.1016/j.jelectrocard.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lauriola M., Pani A., Ippoliti G., Mortara A., Milighetti S., Mazen M., Perseghin G., Pastori D., Grosso P., Scaglione F. Effect of Combination Therapy of Hydroxychloroquine and Azithromycin on Mortality in Patients with COVID-19. Clin. Transl. Sci. 2020;13:1071–1076. doi: 10.1111/cts.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tanriverdİ E., Çörtük M., Yildirim B.Z., Uğur Chousein E.G., Turan D., Çinarka H., Özgül M.A., Çetinkaya E. Hydroxychloroquine plus azithromycin and early hospital admission are benefi-cial in COVID-19 patients: Turkish experience with real-life data. Turk. J. Med. Sci. 2021;51:10–15. doi: 10.3906/sag-2005-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Arshad S., Kilgore P., Chaudhry Z.S., Jacobsen G., Wang D.D., Huitsing K., Brar I., Alangaden G.J., Ramesh M.S., McKinnon J.E., et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Bun S.S., Taghji P., Courjon J., Squara F., Scarlatti D., Theodore G., Baudouy D., Sartre B., Labbaoui M., Dellamonica J., et al. QT Interval Prolongation Under Hydroxychloroquine/Azithromycin Association for Inpa-tients with SARS-CoV-2 Lower Respiratory Tract Infection. Clin. Pharmacol. Ther. 2020;108:1090–1097. doi: 10.1002/cpt.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Maraj I., Hummel J.P., Taoutel R., Chamoun R., Workman V., Li C., Tran L., DelVecchio A., Howes C., Akar J.G. Incidence and determinants of QT interval prolongation in COVID-19 patients treated with hydroxychloroquine and azithromycin. J. Cardiovasc. Electrophysiol. 2020;31:1904–1907. doi: 10.1111/jce.14594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ramireddy A., Chugh H., Reinier K., Ebinger J., Park E., Thompson M., Cingolani E., Cheng S., Marban E., Albert C.M., et al. Experience with Hydroxychloroquine and Azithromycin in the Coronavirus Dis-ease 2019 Pandemic: Implications for QT Interval Monitoring. J. Am. Heart Assoc. 2020;9:e017144. doi: 10.1161/JAHA.120.017144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Pereira M.R., Mohan S., Cohen D.J., Husain S.A., Dube G.K., Ratner L.E., Arcasoy S., Aversa M.M., Benvenuto L.J., Dadhania D.M., et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am. J. Transplant. 2020;20:1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lofgren S.M., Nicol M.R., Bangdiwala A.S., Pastick K.A., Okafor E.C., Skipper C.P., Pullen M.F., Engen N.W., Abassi M., Williams D.A., et al. Safety of Hydroxychloroquine Among Outpatient Clinical Trial Participants for COVID-19. Open Forum Infect. Dis. 2020;7:ofaa500. doi: 10.1093/ofid/ofaa500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kshirsagar N., Faruqui A.R., Xavier D., Kamat S., Chandy S., Medhi B., Tripathi R., Shetty Y., Raj J., Kaushal S., et al. Safety of hydroxychloroquine in healthcare workers for COVID-19 prophylaxis. Indian J. Med. Res. 2021;153:219–226. doi: 10.4103/ijmr.IJMR_2294_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Mitjà O., Corbacho-Monné M., Ubals M., Alemany A., Suñer C., Tebé C., Tobias A., Peñafiel J., Ballana E., Pérez C.A., et al. A Cluster-Randomized Trial of Hydroxychloroquine for Prevention of Covid-19. N. Engl. J. Med. 2021;384:417–427. doi: 10.1056/NEJMoa2021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Barnabas R.V., Brown E.R., Bershteyn A., Stankiewicz Karita H.C., Johnston C., Thorpe L.E., Kottkamp A., Neuzil K.M., Laufer M.K., Deming M., et al. Hydroxychloroquine as Postexposure Prophylaxis to Prevent Severe Acute Respiratory Syndrome Coronavirus 2 Infection: A Randomized Trial. Ann. Intern. Med. 2021;174:344–352. doi: 10.7326/M20-6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Abella B.S., Jolkovsky E.L., Biney B.T., Uspal J.E., Hyman M.C., Frank I., Hensley S.E., Gill S., Vogl D.T., Maillard I., et al. Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-exposure SARS-CoV-2 Prophylaxis Among Health Care Workers: A Randomized Clinical Trial. JAMA Intern. Med. 2021;181:195–202. doi: 10.1001/jamainternmed.2020.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C., Skipper C.P., Nascene A.A., Nicol M.R., Abassi M., et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N. Engl. J. Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Nagaraja B.S., Ramesh K.N., Dhar D., Mondal M.S., Dey T., Saha S., Khan M.A., Rutul S.D., Pratik K., Manjula J., et al. HyPE study: Hydroxychloroquine prophylaxis-related adverse events’ analysis among healthcare workers during COVID-19 pandemic: A rising public health concern. J. Public Health. 2020;42:493–503. doi: 10.1093/pubmed/fdaa074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Gao X., Ma C., Ma Y., Wang X., Wei J., Feng T., Zhao G., Xu L., Zhou W., Zheng X., et al. Clinical efficacy and safety of different antiviral regimens in patients with coronavirus disease 2019. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32:1423–1427. doi: 10.3760/cma.j.cn121430-20201019-00679. [DOI] [PubMed] [Google Scholar]
- 274.Chen C.P., Lin Y.C., Chen T.C., Tseng T.Y., Wong H.L., Kuo C.Y., Lin W.P., Huang S.R., Wang W.Y., Liao J.H., et al. A multicenter, randomized, open-label, controlled trial to evaluate the efficacy and tol-erability of hydroxychloroquine and a retrospective study in adult patients with mild to moderate coronavirus disease 2019 (COVID-19) PLoS ONE. 2020;15:e0242763. doi: 10.1371/journal.pone.0242763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Self W.H., Semler M.W., Leither L.M., Casey J.D., Angus D.C., Brower R.G., Chang S.Y., Collins S.P., Eppensteiner J.C., Filbin M.R., et al. Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients with COVID-19: A Randomized Clinical Trial. JAMA. 2020;324:2165–2176. doi: 10.1001/jama.2020.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Kelly M., O’Connor R., Townsend L., Coghlan M., Relihan E., Moriarty M., Carr B., Melanophy G., Doyle C., Bannan C., et al. Clinical outcomes and adverse events in patients hospitalised with COVID-19, treated with off-label hydroxychloroquine and azithromycin. Br. J. Clin. Pharmacol. 2021;87:1150–1154. doi: 10.1111/bcp.14482. [DOI] [PubMed] [Google Scholar]
- 277.Rodriguez-Garcia J.L., Sanchez-Nievas G., Arevalo-Serrano J., Garcia-Gomez C., Jimenez-Vizuete J.M., Martinez-Alfaro E. Bari-citinib improves respiratory function in patients treated with corticosteroids for SARS-CoV-2 pneumonia: An observational cohort study. Rheumatology (Oxford) 2021;60:399–407. doi: 10.1093/rheumatology/keaa587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Ip A., Berry D.A., Hansen E., Goy A.H., Pecora A.L., Sinclaire B.A., Bednarz U., Marafelias M., Berry S.M., Berry N.S., et al. Hydroxychloroquine and tocilizumab therapy in COVID-19 patients—An observational study. PLoS ONE. 2020;15:e0237693. doi: 10.1371/journal.pone.0237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Dastan F., Nadji S.A., Saffaei A., Marjani M., Moniri A., Jamaati H., Hashemian S.M., Baghaei P., Abedini A., Varahram M., et al. Subcutaneous administration of interferon beta-1a for COVID-19: A non-controlled prospective trial. Int. Immunopharmacol. 2020;85:106688. doi: 10.1016/j.intimp.2020.106688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Garcia P., Revet A., Yrondi A., Rousseau V., Degboe Y., Montastruc F. Psychiatric Disorders and Hydroxychloroquine for Coronavirus Disease 2019 (COVID-19): A VigiBase Study. Drug Saf. 2020;43:1315–1322. doi: 10.1007/s40264-020-01013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Lewis K., Chaudhuri D., Alshamsi F., Carayannopoulos L., Dearness K., Chagla Z., Alhazzani W., GUIDE Group The efficacy and safety of hydroxychloroquine for COVID-19 prophylaxis: A systematic review and meta-analysis of randomized trials. PLoS ONE. 2021;16:e0244778. doi: 10.1371/journal.pone.0244778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.ElSawah H.K., Elsokary M.A., Elrazzaz M.G., Elshafie A.H. Hydroxychloroquine for treatment of nonsevere COVID-19 patients: Systematic review and meta-analysis of controlled clinical trials. J. Med. Virol. 2020;93:1265–1275. doi: 10.1002/jmv.26442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Chen C., Pan K., Wu B., Li X., Chen Z., Xu Q., Li X., Lv Q. Safety of hydroxychloroquine in COVID-19 and other diseases: A systematic review and meta-analysis of 53 randomized trials. Eur. J. Clin. Pharmacol. 2021;77:13–24. doi: 10.1007/s00228-020-02962-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Yam J.C., Kwok A.K.H. Ocular toxicity of hydroxychloroquine. Hong Kong Med. J. 2006;12:294–304. [PubMed] [Google Scholar]
- 285.Kumar J., Jain S., Meena J., Yadav A. Efficacy and safety of hydroxychloroquine/chloroquine against SARS-CoV-2 infection: A systematic review and meta-analysis. J. Infect. Chemother. 2021;27:882–889. doi: 10.1016/j.jiac.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Tleyjeh I.M., Kashour Z., AlDosary O., Riaz M., Tlayjeh H., Garbati M.A., Tleyjeh R., Al-Mallah M.H., Sohail M.R., Gerberi D., et al. Cardiac Toxicity of Chloroquine or Hydroxychloroquine in Patients with COVID-19: A Systematic Review and Meta-regression Analysis. Mayo Clin. Proc. Innov. Qual. Outcomes. 2021;5:137–150. doi: 10.1016/j.mayocpiqo.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Liu J., Virani S.S., Alam M., Denktas A.E., Hamzeh I., Khalid U. Coronavirus disease-19 and cardiovascular disease: A risk factor or a risk marker? Rev. Med. Virol. 2021;31:e2172. doi: 10.1002/rmv.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Gori T., Lelieveld J., Münzel T. Perspective: Cardiovascular disease and the Covid-19 pandemic. Basic Res. Cardiol. 2020;115:1–4. doi: 10.1007/s00395-020-0792-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ray W.A., Murray K.T., Hall K., Arbogast P.G., Stein C.M. Azithromycin and the Risk of Cardiovascular Death. New Engl. J. Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Gourieux B., Reisz F., Belmas A.S., Danion F., Fourtage M., Nai T., Reiter-Schatz A., Ruch Y., Walther J., Nivoix Y., et al. Prescribing practices of lopinavir/ritonavir, hydroxychloroquine and azithromycin during the COVID-19 epidemic crisis and pharmaceutical interventions in a French teaching hospital. Eur. J. Hosp. Pharm. 2021;28:242–247. doi: 10.1136/ejhpharm-2020-002449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kelleci Cakir B., Bayraktar-Ekincioglu A., Demirkan K. Benefit versus toxicity risk of digoxin in patients with COVID-19. Eur. J. Hosp. Pharm. 2021:1. doi: 10.1136/ejhpharm-2021-002880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.White N.J. Cardiotoxicity of antimalarial drugs. Lancet Infect. Dis. 2007;7:549–558. doi: 10.1016/S1473-3099(07)70187-1. [DOI] [PubMed] [Google Scholar]
- 293.Zequn Z., Yujia W., Dingding Q., Jiangfang L. Off-label use of chloroquine, hydroxychloroquine, azithromycin and lop-inavir/ritonavir in COVID-19 risks prolonging the QT interval by targeting the hERG channel. Eur. J. Pharmacol. 2021;893:173813. doi: 10.1016/j.ejphar.2020.173813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Li W., Luo X., Poetsch M.S., Oertel R., Nichani K., Schneider M., Strano A., Hasse M., Steiner R.P., Cyganek L., et al. Synergistic Adverse Effects of Azithromycin and Hydroxychloroquine on Human Cardio-myocytes at a Clinically Relevant Treatment Duratio n. Pharmaceuticals. 2022;15:220. doi: 10.3390/ph15020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zhao Y., Zhang J., Zheng K., Thai S., Simpson R.J., Kinlaw A.C., Xu Y., Wei J., Cui X., Buse J.B., et al. Serious Cardiovascular Adverse Events Associated with Hydroxychloroquine/Chloroquine Alone or with Azithromycin in Patients with COVID-19: A Pharmacovigilance Analysis of the FDA Adverse Event Reporting System (FAERS) Drugs Real World Outcomes. 2022;9:231–241. doi: 10.1007/s40801-022-00300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Marcianò G., Roberti R., Palleria C., Mirra D., Rania V., Casarella A., De Sarro G., Gallelli L. SARS-CoV-2 Treatment: Current Therapeutic Options and the Pursuit of Tailored Therapy. Appl. Sci. 2021;11:7457. doi: 10.3390/app11167457. [DOI] [Google Scholar]
- 297.Ross S.B., Wilson M.G., Papillon-Ferland L., Elsayed S., Wu P.E., Battu K., Porter S., Rashidi B., Tamblyn R., Pilote L., et al. COVID-SAFER: Deprescribing Guidance for Hydroxychloroquine Drug Interactions in Older Adults. J. Am. Geriatr. Soc. 2020;68:1636–1646. doi: 10.1111/jgs.16623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Das P., Rai A., Chopra A., Philbrick K. Psychosis Likely Induced by Hydroxychloroquine in a Patient with Chronic Q Fever: A Case Report and Clinically Relevant Review of Pharmacology. Psychosomatics. 2014;55:409–413. doi: 10.1016/j.psym.2013.06.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in article and Supplementary Material.