Abstract
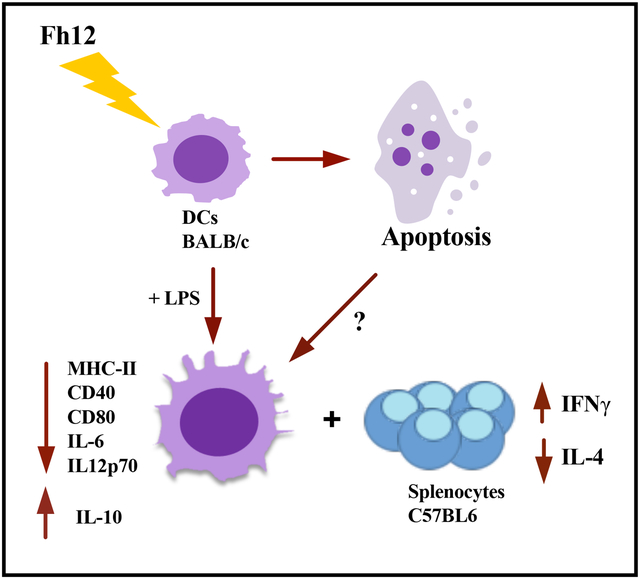
In a previous study we demonstrated that Fasciola hepatica fatty acid binding protein (Fh12) significantly suppress macrophage function by inhibiting IL-6, IL-1β, tumor necrosis factor (TNF)-α and IL-12 production in TLR4-stimulated murine macrophages, an effect mediated through the signaling of CD14 co-receptor without affecting the viability of these cells. Given that dendritic cells (DCs) are immune cells that play a central role in the initiation of primary immune responses and that are the only antigen-presenting cells capable of stimulating naïve T-cells, in the present study we investigated the effect of Fh12 on DCs. We found that Fh12 exerts a strong suppressive effect on activation and function of DCs. However, in contrast to the effect observed on macrophages, Fh12 induces early and late apoptosis of DCs being this phenomenon dose-dependent and CD14-coreceptor independent. At low concentration Fh12 modulates the LPS-induced DCs maturation status by suppressing the MHC-II, and co-stimulatory molecules CD40 and CD80 surface expression together with the pro-inflammatory cytokines IL-12p70 and IL-6 production whereas increase the IL-10 levels. Besides, Fh12 decreased the ability of LPS-activated DCs to induce IFN-γ production against allogeneic splenocytes, while increasing IL-4 production. We have described for the first time the ability of Fh12 to modify selectively the viability of DCs by apoptosis induction. The selective diminution in DCs survival could be a F. hepatica strategy in order to prevent a host immune response during the earliest phases of infection.
Graphical Abstract
INTRODUCTION
Fasciola hepatica is a helminth parasite that causes fascioliasis, a chronic disease that affects around 17 million people worldwide (Caravedo & Cabada, 2020). Fascioliasis also leads to economic losses in livestock, estimated in more than $3.2 billion annually (Alba et al, 2021; Mehmood et al, 2017). The survival of helminths in the host over long periods of time is the result of a dynamic co-evolution that results in a predominant Th2/Treg immune response that is only achieved by suppressing the Th1-inflammatory response (Donnelly et al, 2008; O’Neill et al, 2001). Excretory-secretory products (ESPs) and tegumental antigens (FhTeg), which are a complex mixture of antigens, have been largely implicated as responsible for the immune modulation (Anuracpreeda et al, 2006; Brady et al, 1999; Corral-Ruiz & Sanchez-Torres, 2020; Donnelly et al., 2008). These antigens exert a strong influence on the activation status and function of the antigen-presenting cells (APCs) at the early stages of infection. In this regard, ESPs and FhTeg have shown to induce alternative activation of macrophages (Adams et al, 2014; Donnelly et al, 2005; Flynn et al, 2007) and partial activation of dendritic cells (DCs) (Hamilton et al, 2009). In line with these studies, a recent work reveals a significant decrease in the expression of the antigen presentation markers CD83 and MHC-II by DCs and follicular DCs is observed in the lymph nodes of sheep experimentally infected with F. hepatica, suggesting the inhibition of markers related to antigenic presentation induced by the parasite (Ruiz-Campillo et al, 2020). Moreover, ESPs have also shown to induce apoptosis of eosinophils (Serradell et al, 2007) and macrophages (Guasconi et al, 2012) during the early stages of parasite infection likely as a mechanism to prevent pro-inflammatory functions of these cells (Adam-Klages et al, 2005). Interestingly, apoptosis induced by extracellular parasites such helminths or their products, would not compromise the survival of the parasite. In contrast, it has been reported that intracellular parasites such as Leishmania mexicana decreases DCs apoptosis which correlates with a diminution in the MAP kinases phosphorylation. The apoptosis prevention might be a mechanism to avoid the cells dead, allowing the persistent of these type of parasites (Motran et al, 2017; Rodriguez-Gonzalez et al, 2016).
We have demonstrated that F. hepatica fatty acid binding protein (FABP), an antioxidant molecule with essential functions for parasite metabolism and that has been many times identified in the FhTeg and ESPs (Hacariz et al, 2012; Morphew et al, 2012; Wilson et al, 2011), possesses powerful anti-inflammatory functions. Native (Fh12) and recombinant (Fh15) variants of FABP have shown to significantly suppress the production of IL-1β and TNFa from bone-marrow derived macrophages (BMDM) stimulated with LPS in vitro via toll-like receptor-4 (TLR4) (Martin et al, 2015; Ramos-Benitez et al, 2017). Fh12 exerts this effect by suppressing the expression of the CD14-coreceptor and the phosphorylation of various kinases downstream TLR4 signaling cascade (Martin et al., 2015). Moreover, Fh12 has also shown to significantly suppress many pro-inflammatory cytokines in a mouse model of septic shock. Also, to impair the murine BMDM function by inhibiting their phagocytic capacity without provoking any toxic or apoptotic effect on these cells (Martin et al., 2015).
In the present study we focus on studying the effect of Fh12 on activation and functionality of DCs, which play a relevant role during the initiation of immune response in the recognition of helminth or their products and the subsequent promotion of Th2/Treg development. In contrast to the effect observed on macrophages, we found that Fh12 modulate the activation and function of DCs by promoting the early and late apoptosis. The apoptotic effect of Fh12 on DCs was found to be dose-dependent and not mediated by the CD14-coreceptor. Concurrently, we demonstrated that F12 can induce regulatory features on LPS-activated DCs, thereby impairing their capacity to prime naïve T-cells thus, inducing a phenotype able to promote the development of anti-inflammatory responses.
MATERIALS AND METHODS
Animals
Six- to 8-week-old inbred female C57BL/6 and BALB/c mice were indistinctly purchased from Charles River Laboratory (Wilmington, MA) or from the Faculty of Veterinary Sciences, National University of Litoral (UNL, Argentina). B6.129S4 CD14 knockout (CD14KO) female mice (C57BL/6 background), 6–8-week-old, were purchased from Jackson Laboratory (Bar Harbor, ME). The animal studies were performed at the Animal Resources Center of the Medical Sciences Campus, University of Puerto Rico in accordance with guidelines and protocols approved by the Ethics Institutional Animal Care and Use Committee (MSC-IACUC, Protocol No. 7870215) and Faculty of Chemical Sciences, National University of Córdoba (Approval Number HCD 1637) in strict accordance with the recommendation of the Guide to the Care and Use of Experimental Animals published by the Canadian Council on Animal Care (OLAWAssurance number A5802–01).
Fh12 Purification
Fh12 was purified from a whole-fluke extract of adult F. hepatica using as previously described (Espino et al, 2001). Briefly, whole fluke extract was first subjected to ultracentrifugation at 30,000 g followed by gel filtration chromatography with Sephadex G-50 (XK 26/1000 column). Fractions containing proteins in the range of 1.5–30kDa were collected, pooled and subjected to two consecutive preparative isoelectric focusing (IEF) at pH 3–10 (first separation) and pH 5–7 (second separation) using a Rotofor Cell (Bio-Rad). The individual IEF fractions were harvested, and their pH value and OD 280 nm determined. Each aliquot was subjected to SDS-PAGE and the proteins were visualized by coomasie blue. Fractions from the second IEF run that exhibited a single polypeptide band of around 12–15kDa were manually excised from the gel, washed twice with double-distilled water, digested with sequencing-grade trypsin (Promega, Madison, WI) and analyzed by MALDI and MS/MS as previously described (Morales & Espino, 2012). After confirming the presence of Fh12-FABP as a unique component, these fractions were pooled (Fig-1S).
Figure-1S. Purification of native F. hepatica fatty acid binding protein (Fh12).
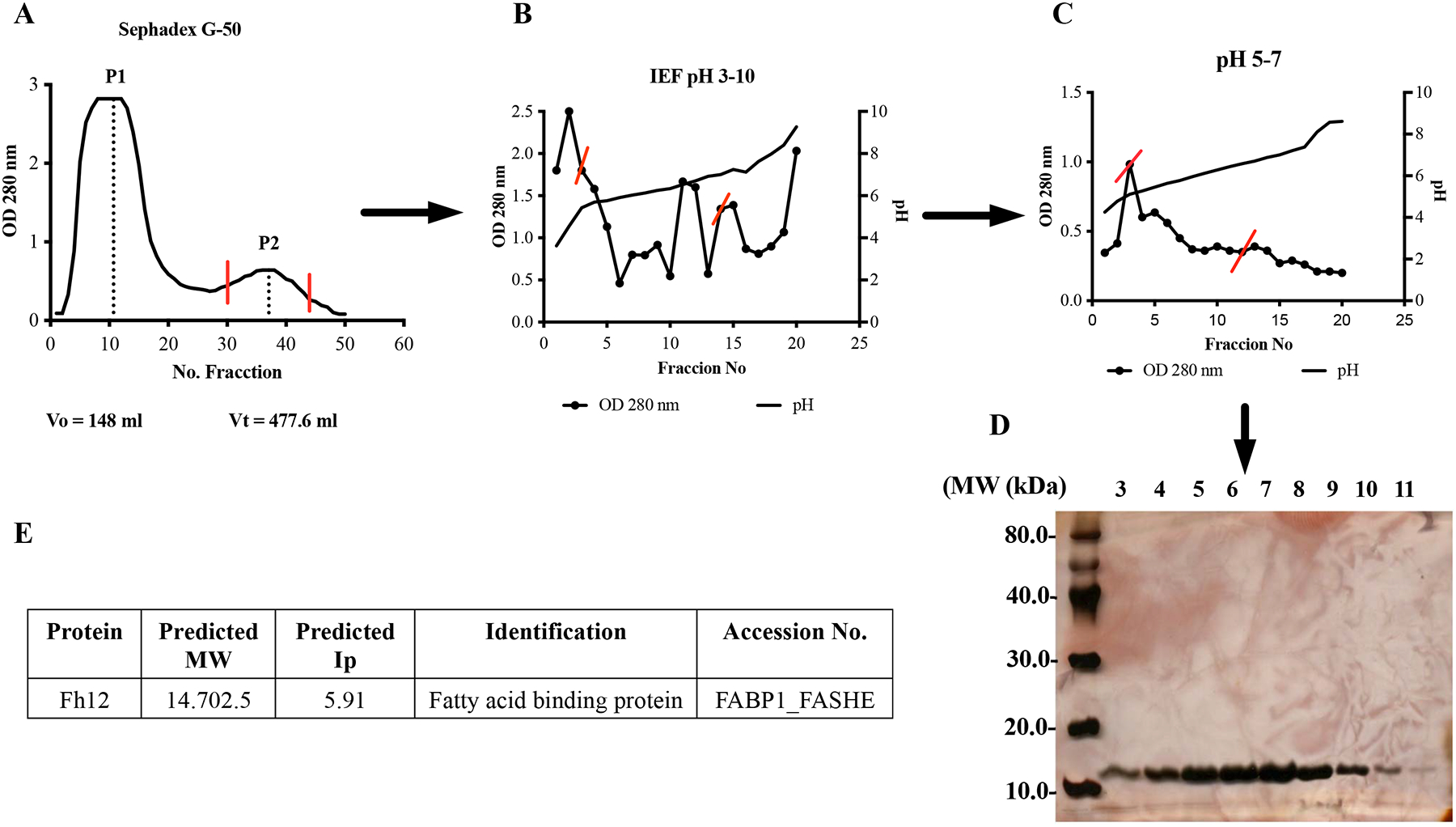
(A) F. hepatica whole worm extract was loaded onto a gel filtration chromatography with Sephadex G-50 (XK 26/100 column). Fractions eluted in peak-2 (P2) containing proteins among 1.5 to 30kDa were pooled. (B-C) P2 was dialyzed against 1% glycine containing 2% ampholytes pH 3–10 (first run) or pH 5–7 (second run) and then loaded onto a liquid isoelectric focusing system (Rotofor, Bio-Rad). Individual fractions were harvested and their pH and absorbance at 280nm were measured. Red lines on figures indicate fractions that were selected for pooling and subsequent purification step. (D) Fractions 3 to 11 from the IEF run with pH 5–7, which contain proteins with Ip between 4.61 to 5.9 were analyzed by 15% SDS-PAGE stained with silver stain to corroborate the presence of polypeptide of 12kDa. (E) Polypeptide band was excised from gel and analyzed by matrix-assisted laser desorption ionization 9MALDI) and tandem mass spectrometry (MS/MS). Analysis revealed the presence of fatty acid binding protein.
Endotoxin removal
Endotoxins were removed from the Fh12 by using the polymyxin-B (PMB) column according to the manufacturer’s instructions, and the levels of endotoxins were measured using the Chromogenic Limulus Amebocyte Lysate QCL-1000 Assay (Lonza, Walkersville, MD). Fh12 was considered endotoxin free when endotoxin levels gave like background levels (<0.1 EU/ml). Protein concentration was adjusted to 1 mg/ml as determined by the bicinchoninic acid (BCA) method using a Pierce protein assay kit (Pierce, Cambridge, NJ).
Myeloid DC generation and stimulation
DCs were generated as previously described (Falcon et al, 2010), with slight modifications. Briefly, bone marrow was collected from femurs and tibia of BALB/c mice and then seeded into bacteriological Petri dishes at 4 × 106 in 10 ml of complete RPMI 1640 medium supplemented with 2mM L-glutamine (Life Technologies, Gaithersburg, MD), 100U/ml penicillin and 100μg/ml streptomycin, 10% heat-inactivated endotoxin-free fetal calf serum (Sigma-Aldrich) and 20ng/ml GM-CSF (R&D System) at 37°C, 5% CO2. On day 3, an additional 10 ml of medium containing 20ng/ml GM-CSF was added. At day 6, 10 ml of culture supernatant was removed and replaced with fresh culture medium containing GM-CSF. On day 8, the supernatant was removed and replaced with medium without GM-CSF, and cells were harvested 18h later (day 9). After this time, >85% of harvested cells were DCs (MHC class II+, CD11c+).
For stimulation experiments, DCs were seeded into 96-wells plates at 4×105 cells per well in complete RPMI. To study the individual effect on the DCs, cells were treated for 18h with Fh12 (2.5μg/ml) alone and in other group of cells with 1μg/ml LPS (E. coli 0111: B4; Sigma-Aldrich) alone. For the inhibition experiments, DCs were treated first for 1h with Fh12 (2.5μg/ml) and further stimulated with LPS (1μg/ml). Cells treated with PBS were used as controls in both experiments. Supernatants from cultured DCs were tested to produce IL-10, IL-12p70 (eBioscience, USA) and IL-6 (BD Pharmingen, USA) by sandwich ELISA
Cell viability and Annexin-V and 7AAD assay
DCs treated with Fh12 (2.5, 5, 10 and 15 μg/ml) in the presence or absence of LPS (1μg/ml) was incubated with 50μl MTT (sodium 3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis (4- methoxy-6-nitro) benzene sulfonic acid hydrate) labeling reagent (Roche Life Science, USA) to each well. The absorbance was read at 480 nm. The percentage of viable, necrotic, and apoptotic DCs after treatments were determined by flow cytometry using surface Annexin V detection and 7-Amino-actinomycin D (7-AAD) incorporation (BD Biosciences, USA). DCs were washed with PBS, and then washed twice with annexin-V binding buffer (10mM HEPES, 140 mM NaCl, 2.5 mM CaCl, pH 7.4) and resuspended in 100μl of annexin-V binding buffer before being incubated with FITC-conjugated annexin-V (0.5μg / 2 × 105 cells) and 0.5μl (7-AAD) solution. After 15 min of incubation at room temperature (RT) in the dark, an extra amount of 400μl of annexin-V binding buffer was added to cells, and cells were analyzed by flow cytometry. DCs were gated based on their forward and side light scatter. If there is an alteration in the membrane integrity (due to externalization of phosphatidylserine), annexin-V detects both early- and late- apoptotic cells. Thus, the simultaneous addition of 7-AAD, which does not enter healthy cells with an intact plasma membrane, discriminates between early apoptotic (annexin V-positive and 7AAD-negative), late-apoptotic (both annexin V- and 7AAD-positive), necrotic (annexin V-negative and 7AAD-positive) and live (both annexin V- and 7AAD-negative) cells (Ardestani et al, 2012).
DCs Flow Cytometry
Expression of cell surface markers on DCs treated as described above was quantified by two-color flow cytometry. DCs were washed twice with PBS containing 2% fetal bovine serum (FBS), adjusted to 4 × 105 cells/ml, and fixed with 1% paraformaldehyde. Cells were stained with CD11c (BD BioSciences) antibody to ensure DCs percentage, proceeding with DCs batches with more than 85% of marker expression. Co-stimularory molecules were stained using allophycocyanin-, and phycoerythrin-conjugated antibodies specific for CD80, CD40, and MHC-II (BD BioSciences) for 30 min at 4°C. Appropriate labeled isotype-matched antibodies were used as controls. Cell acquisition was performed using FACS Calibur equipment and MACSQuant Analyzer - Miltenyi equipment, whereas analysis of results was performed using FlowJo software (FlowJo, LLC).
Allogeneic mixed lymphocyte reaction (MLR)
DCs from BALB/c mice were previously treated with Fh12 (2.5μg/ml), LPS (1μg/ml), or for 1h with Fh12 (2.5μg/ml) and further stimulated with LPS (1μg/ml) for 18h. Then the cells were cultured in U-bottom 96-well plates with C57BL/6 splenocytes (2×105 cells/well) for 5 days at a ratio of 5:1. For cytokine determination, supernatants were collected after 48h for IFN-γ, and 72h for IL-4 and IL-13 detection. Supernatants from cultured DCs were tested for the production of IFN-γ, IL-4 and IL-13 (eBioscience, USA) by sandwich ELISA.
Statistical analysis
All experiments were repeated twice with in different days with three replicates for each determination, and equivalent results were obtained. Data are expressed as mean ± standard error of the mean (S.E.M.) and analyzed statistically using the Student t-test. Comparisons of the values for multiple groups were made using one-way ANOVA. Statistical analyses were made using GraphPad Prism software (Prism-6). Statistical significance was assumed at the p-value of <0.05.
RESULTS
Fh12 induce apoptosis of myeloid DCs
Given in our previous studies we had demonstrated that the treatment of murine macrophages with different concentrations of Fh12 alone or combined with LPS suppressed the capacity of these cells to express pro-inflammatory mediators without affecting the cell viability (Martin et al., 2015); in the present study, we determined whether Fh12 could exert a similar effect on DCs. For this, bone marrow derived DCs were treated for 18h with different concentrations of Fh12 or with Fh12 for 1h and further stimulated with LPS. Results of the MTT-viability test revealed that the treatment of DCs with low Fh12 concentrations (among 2.5 to 5μg/ml) alone or combined with LPS seems to maintain the viability of cells as compared with those cells treated only with PBS or LPS alone.
However, when the DCs were treated with higher Fh12 concentrations of 10 and 15μg/ml, the absorbance values in the MTT assay significantly dropped, which indicates that at these Fh12 concentrations the viability of cells was seriously compromised (p<0.0001) (Fig. 1). To determine whether Fh12 could be inducing apoptosis, Fh12-treated DCs were stained with annexin-V and 7AAD and analyzed by flow cytometry. Results demonstrated that ~91% of cells remain viable when are treated with medium alone for 18h. However, when cells are treated with Fh12 the viability of cells drop significantly. At Fh12 concentrations of 2.5 or 5μg/ml ~ 52% and 41.9% of DCs remained viable, whereas that 46.5% (23.6% early apoptosis and 22.9% late apoptosis) and 52.9% (18.8% early apoptosis and 34.1% late apoptosis) was in apoptosis, respectively. The number of apoptotic cells increased significantly by 80.7% (22.1% early apoptosis and 58.6% late apoptosis) and 88.7% (18.3% early apoptosis and 70.4% late apoptosis) when the Fh12 concentration increased to 10μg/ml and 15μg/ml, respectively (Fig. 2). Besides, our results show that the lowest concentration of Fh12 induce approximately 47% of the cells in apoptosis, while low concentration of LPS (100ng/ml) induced only 17.5% of the apoptotic cells (Fig. 3) and both stimuli induce different activation status in DCs, suggesting that the apoptosis and the modulation of DCs activation might be independent events (Rescigno et al, 1998). Both phenomena could occur simultaneously, and it would be difficult to determine whether they are dependent or independent events. These results clearly demonstrate that Fh12 promotes the late apoptosis of DCs in a dose-dependent manner.
Figure-1. MTT-viability assay of Fh12 treated DCs.
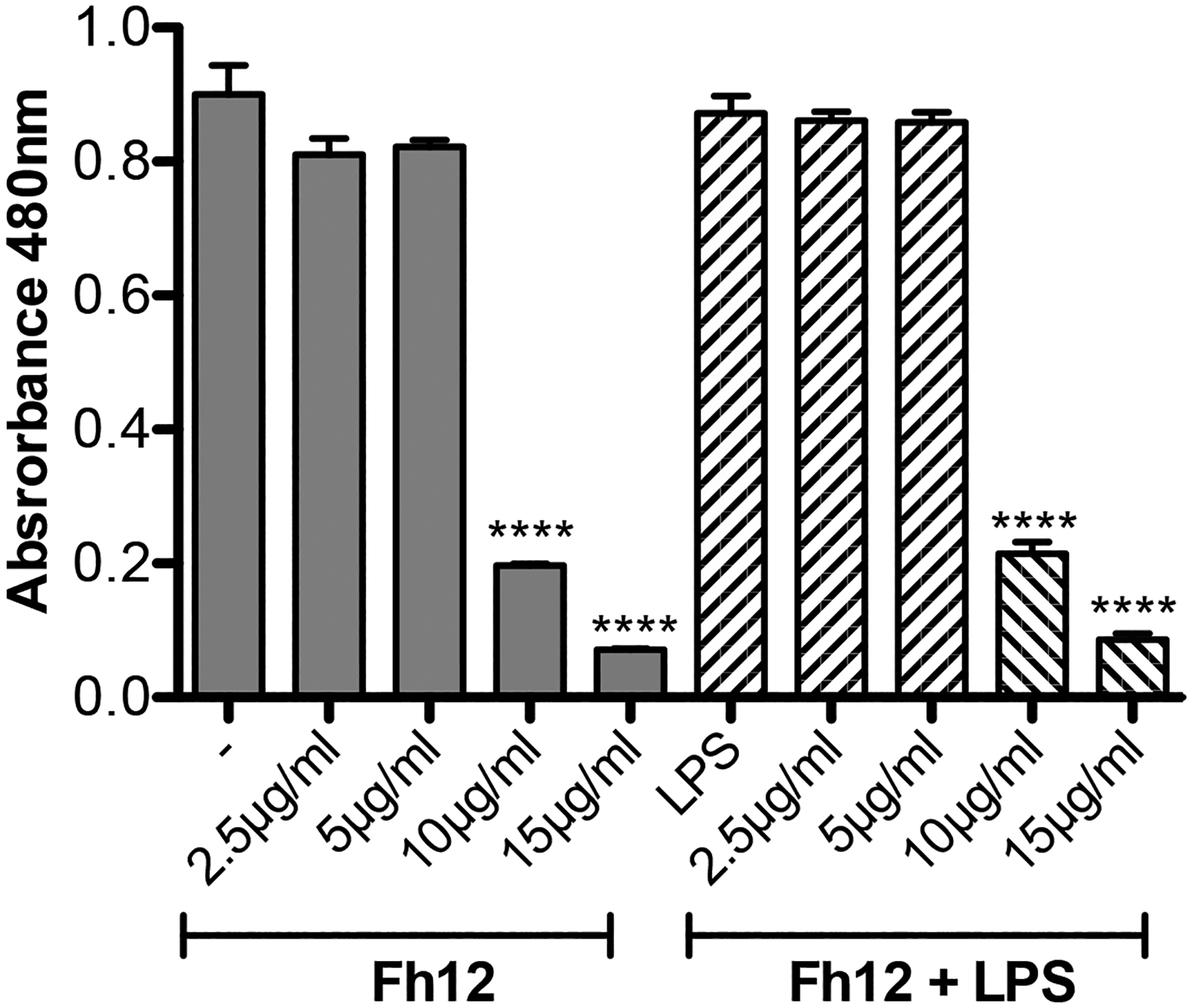
Murine DCs were seeded into 96-wells plates at 4x105 cells in complete RPMI plus 20ng/ml GM-CSF and then treated with Fh12 (2.5, 5, 10, 15μg/ml) in the presence or absence of 1μg/ml LPS (E. coli 0111:B4). Control cells were treated with Fh12 or LPS alone. After 18h of incubation at 37C, 5% CO2 50μl MTT (sodium 3′-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis (4- methoxy-6-nitro) benzene sulfonic acid hydrate) labeling reagent was added to each well and the absorbance was read at 480 nm. Viability of cells significantly dropped (****p<0.0001) at Fh12 concentrations ≥10μg/ml.
Figure-2. Fh12 induces apoptosis of DCs, as measured by Annexin-V binding to externalized phosphatidylserine.
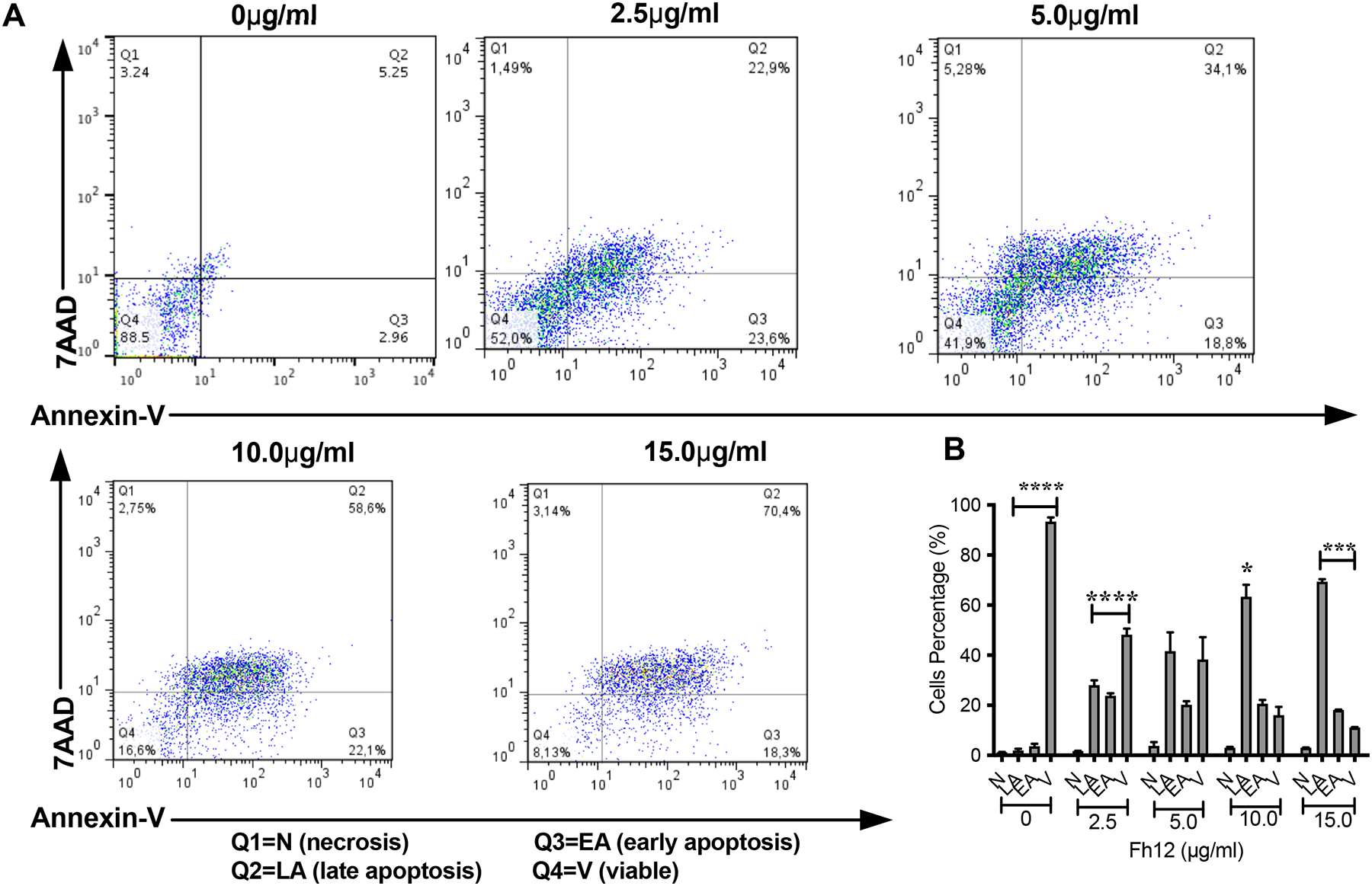
Myeloid DCs from C57BL/6 mice were treated with Fh12 (2.5, 5, 10, and 15 μg/ml) or remain untreated for 18 hours. After incubation, the cells were harvested and analyzed by two-color flow cytometry for Annexin-V and 7AAD. Cells were gated based on the CD11c expression. (A) Histogram representative of an experiment showing that the apoptosis of DCs induced by Fh12 is dose-dependent. (B) Average percentages and their SEM of at least three independent experiments showing that the percentage of DCs viable (V) at Fh12 concentration of 2.5μg/ml is significantly high (****p<0.0001) compared to the percentage of cells in early (EA) or late apoptosis (LA). In contrast, the percentage of DCs in apoptosis significantly increased at Fh12 concentration of 10μg/ml. (*p=0.0146) and 15μg/ml (***p<0.0008).
Figure-3. LPS does not promote significant apoptosis to DCs.
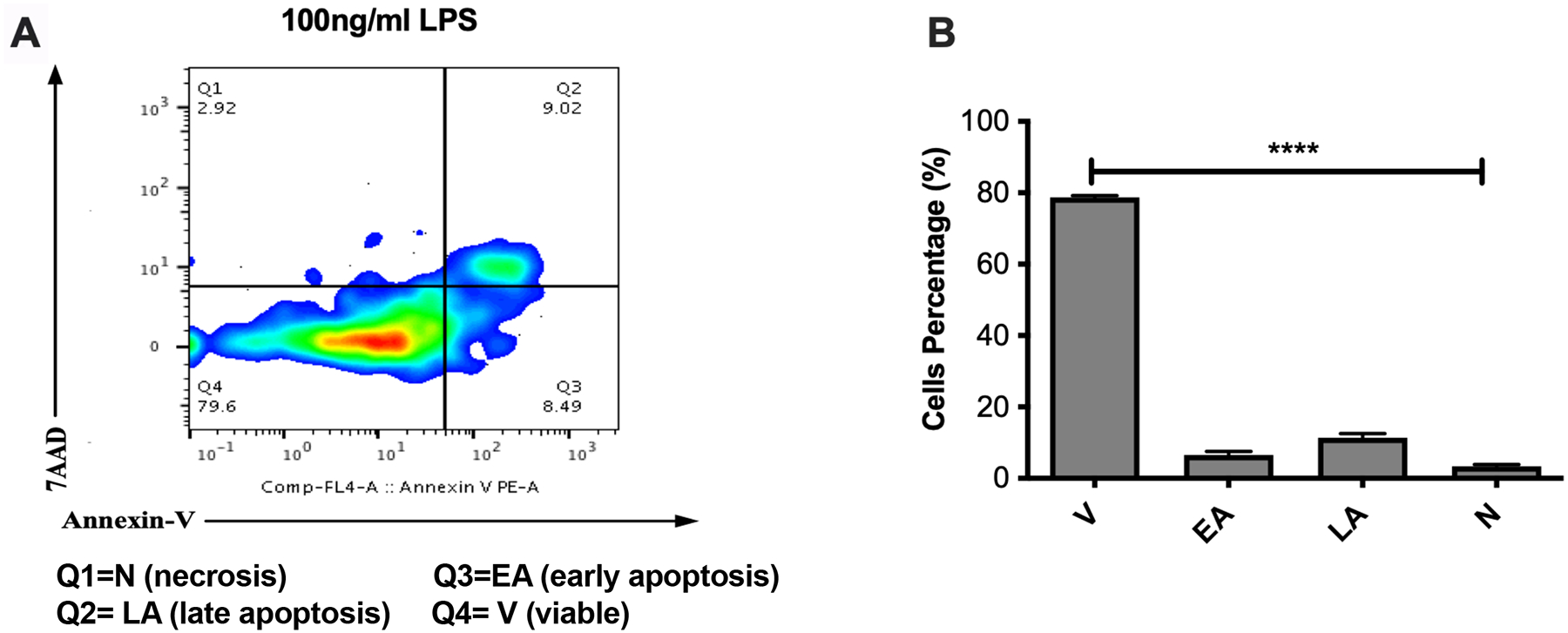
Myeloid DCs from C57BL/6 mice were treated with LPS (100ng/ml) or remain untreated for 18 hours. After incubation, the cells were harvested and analyzed by two-color flow cytometry for Annexin-V and 7AAD. Cells were gated based on the CD11c expression. (A) Histogram representative of an experiment showing that at this experimental conditions most of cells (79.6%) remain viable and that only the 17% of cells are apoptotic. (B) Average percentages and their SEM of at least three independent experiments showing that the percentage of DCs viable (V) at LPS concentration of 100ng/ml is significantly high (****p<0.0001) compared to the percentage of cells in early (EA) or late apoptosis (LA).
Fh12 induces apoptosis of DCs in absence of CD14 co-receptor
Since in our previous studies with murine macrophages we had demonstrated that Fh12 targeted the CD14 coreceptor as a mechanism to suppress the expression of pro-inflammatory cytokines and prevent the phagocytic capacity of macrophages (Martin et al., 2015), and given that CD14 has been involved in the regulation of programmed cell death (apoptosis) in immune and no-immune cells (Frey & Finlay, 1998; Heidenreich et al, 1997), we investigate whether Fh12 could require CD14 to induce apoptosis of DCs. Myeloid DCs were collected from CD14 KO mice and fully differentiated in vitro. Further, cells were cultured and treated with 15μg/ml Fh12. Results demonstrated that in the absence of CD14 Fh12 induces similar apoptosis levels that those observed in DCs of wild type animals (more than 70% Fig. 4). This result indicates that CD14 signaling is not involved in the apoptosis mechanism induced by Fh12.
Figure-4. Fh12 induces apoptosis of DCs in absence of CD14 coreceptor.
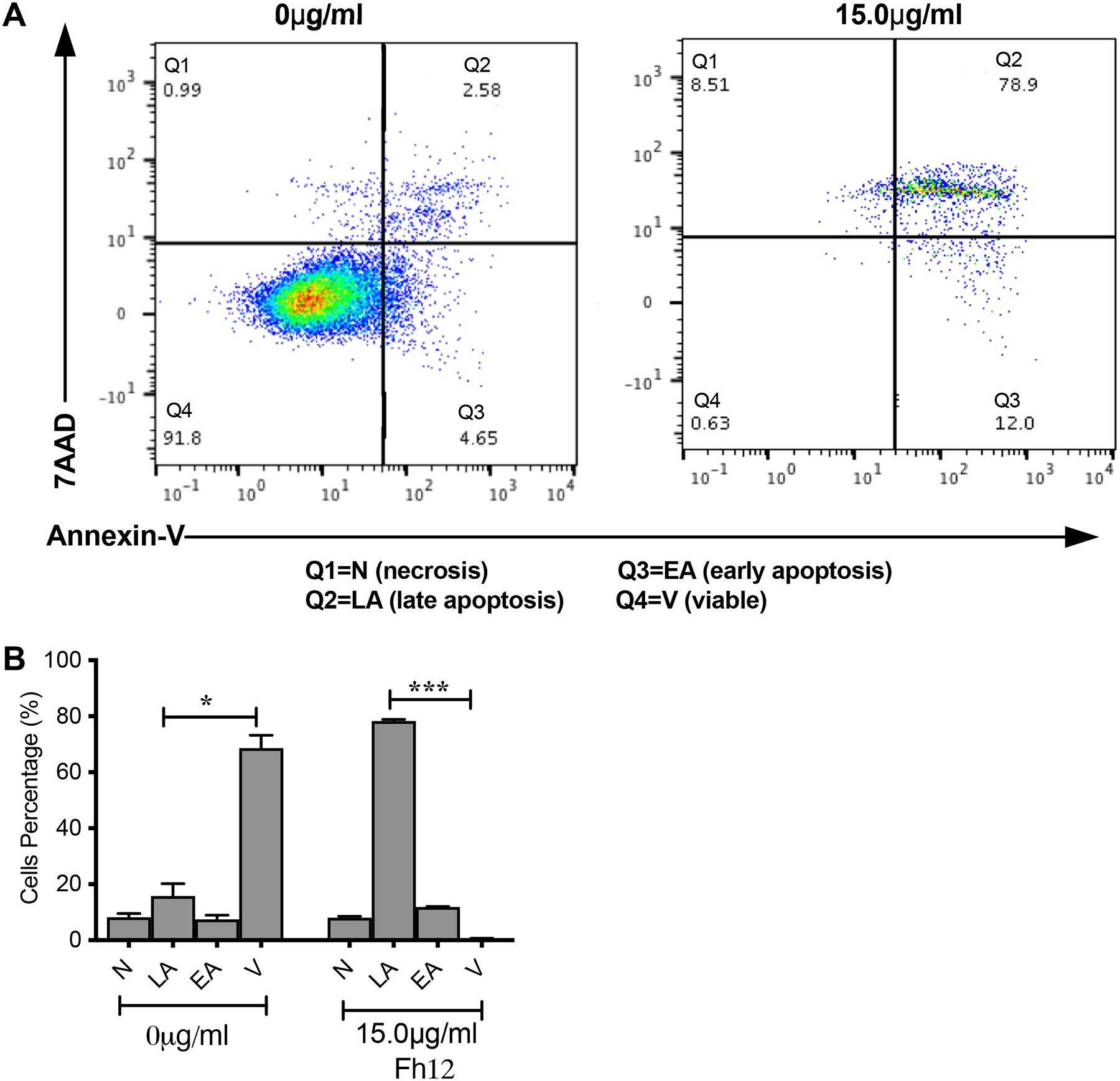
Myeloid DCs from CD14 KO C57BL/6 mice were treated with Fh12 (15μg/ml) for 18 hours or remain untreated. After incubation, the cells were harvested and analyzed by two-color flow cytometry for Annexin-V and 7AAD. Cells were gated based on the CD11c expression. (A) Histogram representative of an experiment showing the apoptosis of DCs induced by Fh12. (B) Average percentages and their SEM of at least three independent experiments showing that the percentage of DCs viable (V) compared to apoptotic (EA or LA) or necrotic cells (N) in untreated CD14 KO cells is significantly high (*p=0.144) whereas the number of DCs in late apoptosis (LA) compared to viable (V) cells is significantly high (****p<0.0001).
Fh12 did not induce DCs classical maturation but affected LPS-induced DCs activation
Having observed that after treatment with low Fh12 concentrations (2.5μg/ml) near 52% of DCs still remain viable, we investigated whether at this experimental conditions Fh12 could modify the activation status of LPS-activated DCs. The cytokines and the expression of surface proteins involved in the T-cell activation and polarization were analyzed in DCs cultured with medium alone, Fh12 (2.5μg/ml), LPS (1μg/ml) or for 1h with Fh12 and further stimulated with LPS. As expected, DCs stimulated with LPS produced significantly more IL-12p70 (p=0.0006) and IL-6 (p=0.0003) than untreated DCs and did not produce IL-10. However, although Fh12 alone did not induce the production of IL-12p70, IL-6, or IL-10, it significantly suppressed the LPS-induced IL-12p70 (p<0.031) and IL-6 (p<0.0458) production. In addition, in the presence of Fh12, LPS-stimulated cells produced significantly more IL-10 (p=0.0039) than untreated cells (Fig. 5). Moreover, the expression of the MHC-II and co-stimulatory molecules CD40 and CD80 induced by LPS-stimulation was found significantly downregulated by the Fh12 treatment (p=0.0072, p=0.0023, and p=0.0398, respectively) (Fig. 6). Together, our data showed that Fh12 was not able to induce classical DC maturation but exerted control over the DC activation induced by LPS. These not only by virtue of its ability to inhibit expression of co-stimulatory molecules essential for T-cell activation, but also by directly suppressing the secretion of cytokines critical for the Th1 cell polarization.
Figure-5. Levels of IL-6, IL-12p70, and IL-10 cytokines measured by ELISA in the supernatant of DCs treated with Fh12.
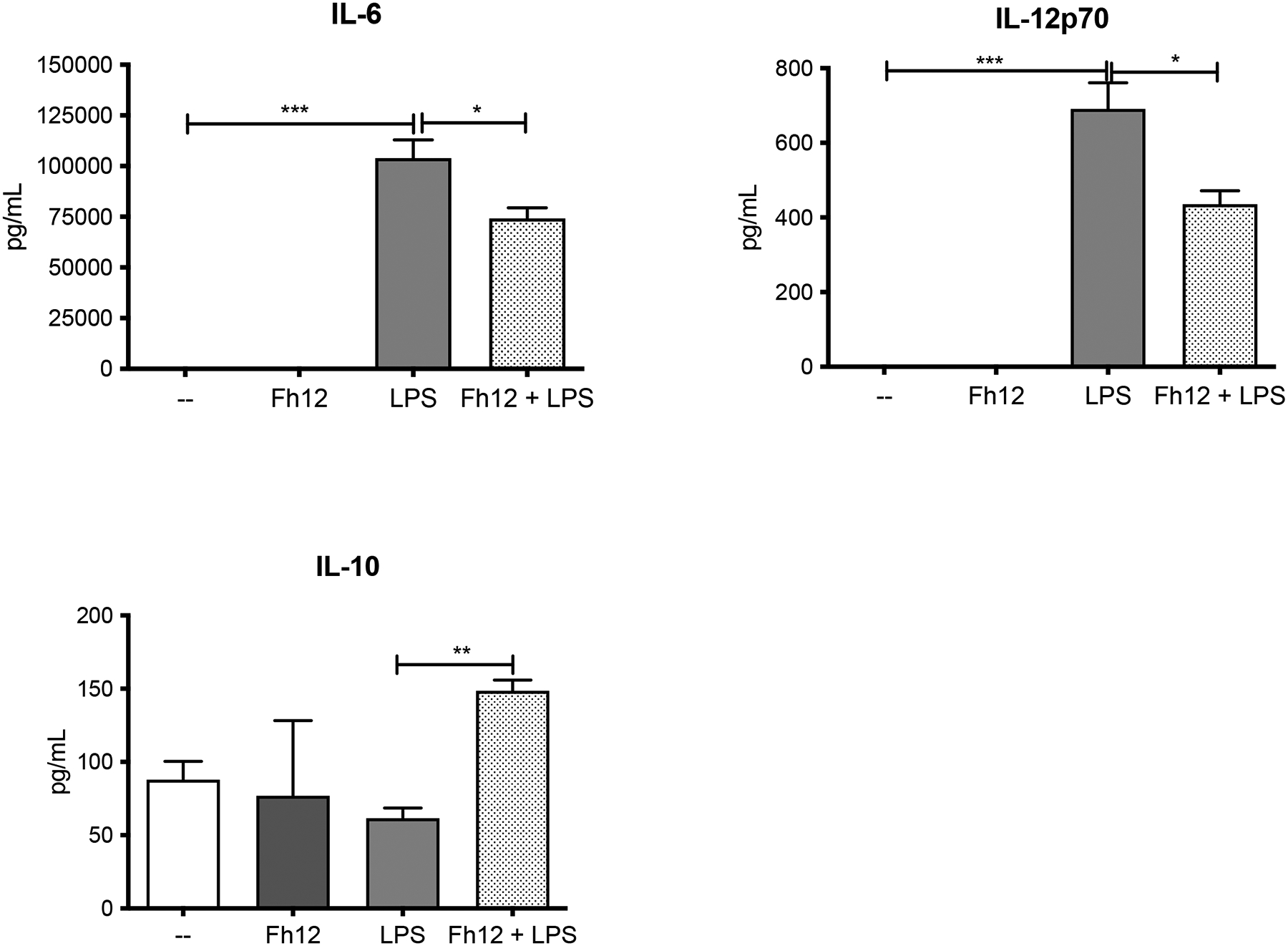
DCs collected from naïve BALB/c mice were cultured and treated with medium or Fh12 (2.5μg/ml) 1h before stimulation with LPS (1μg) for 18h LPS significantly produced high levels of IL-6 (***p=0.0003), which were significantly suppressed by Fh12 (***p<0.0458). LPS also induced the production of IL-12p70 (***p=0.0006), which also were significantly suppressed by Fh12 (*p<0.0315). Although Fh12 or LPS alone failed in inducing IL-10, in the presence LPS, Fh12 significantly increases the production of IL-10 (**p=0.0039). Data represent averages + S.E.M. of at least 3 replicated from three different experiments.
Figure-6. Fh12 inhibits MHC II and co-stimulatory molecules expression on LPS-activated DCs.
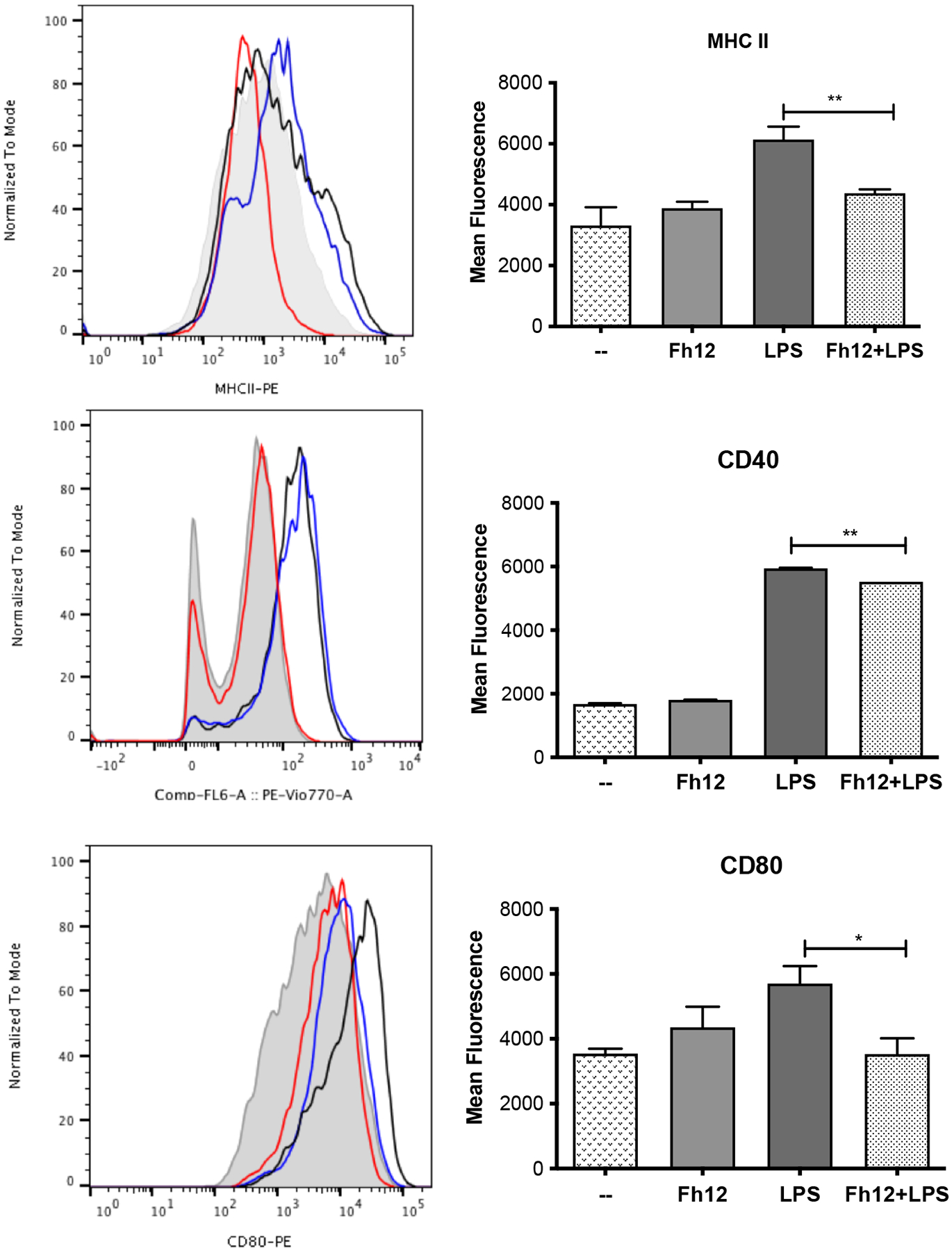
DCs from BALB/c mice were treated for 1h with Fh12 (2.5 μg/ml) and further stimulated with LPS (1μg) for 18h. After incubation, the cells were harvested and analyzed by two-color flow cytometry for MHC-II, CD40 and CD80. Cells were gated based on the expression of CD11c. Histograms (left panel) show LPS-stimulated cells (black line), Fh12-treated cells (red line), Fh12+LPS-stimulated cells (blue line) and untreated cells (--) (gray shaded histogram). Right panel represents mean fluorescence intensity of untreated DCs or treated with Fh12, LPS or Fh12/LPS, for MHC-II, CD40 and CD80, respectively (**p=0.0072, **p=0.0023, *p=0.0398).
Fh12-treated DCs showed reduced ability to induce allogeneic responses
Given our data showing that Fh12 inhibits the maturation of LPS-treated DCs, we reasoned that the ability of these cells to prime allospecific T-cell responses could be impaired. Then, we wanted to determine which T helper profile was promoted by Fh12-treated DCs in a mixed lymphocyte reaction. To this end, immature or LPS-treated DCs or treated for 1h with Fh12 and further stimulated with LPS from BALB/c mice were then cultured with allogeneic splenocytes from C57BL6. Cultures with untreated DCs were used as control. As expected, DCs stimulated with LPS and co-cultured with allogeneic splenocytes induced the secretion of significantly higher amounts of IFN-γ (p=0.0003) compared to untreated cells and failed to induce allogeneic IL-4 or IL-13 production. In contrast, Fh12-treated DCs induced significant amounts of IL-4 (p=0.0022) and IL-13 (p<0.0001) compared to untreated cells. Importantly, LPS-stimulated DCs that were pre-treated with Fh12 and then co-cultured with allogeneic splenocytes lack the ability to induce INF-γ production (p=0.0003) whereas significantly augmented their capacity to produce IL-4 (p=0.0073). Fh12 also showed a tendency to increase the production of IL-13 in DCs stimulated with LPS and then co-cultured with splenocytes, but these increases were not found significant (Fig. 7). These results suggest that Fh12 could be able to promote the activation of T-helper type-2 (Th2) responses of immature or LPS-mature DCs, whereas concurrently suppress the ability of these cells to promote the Th1-response.
Figure-7. Fh12 modulates DCs capacity to prime allogeneic responses.
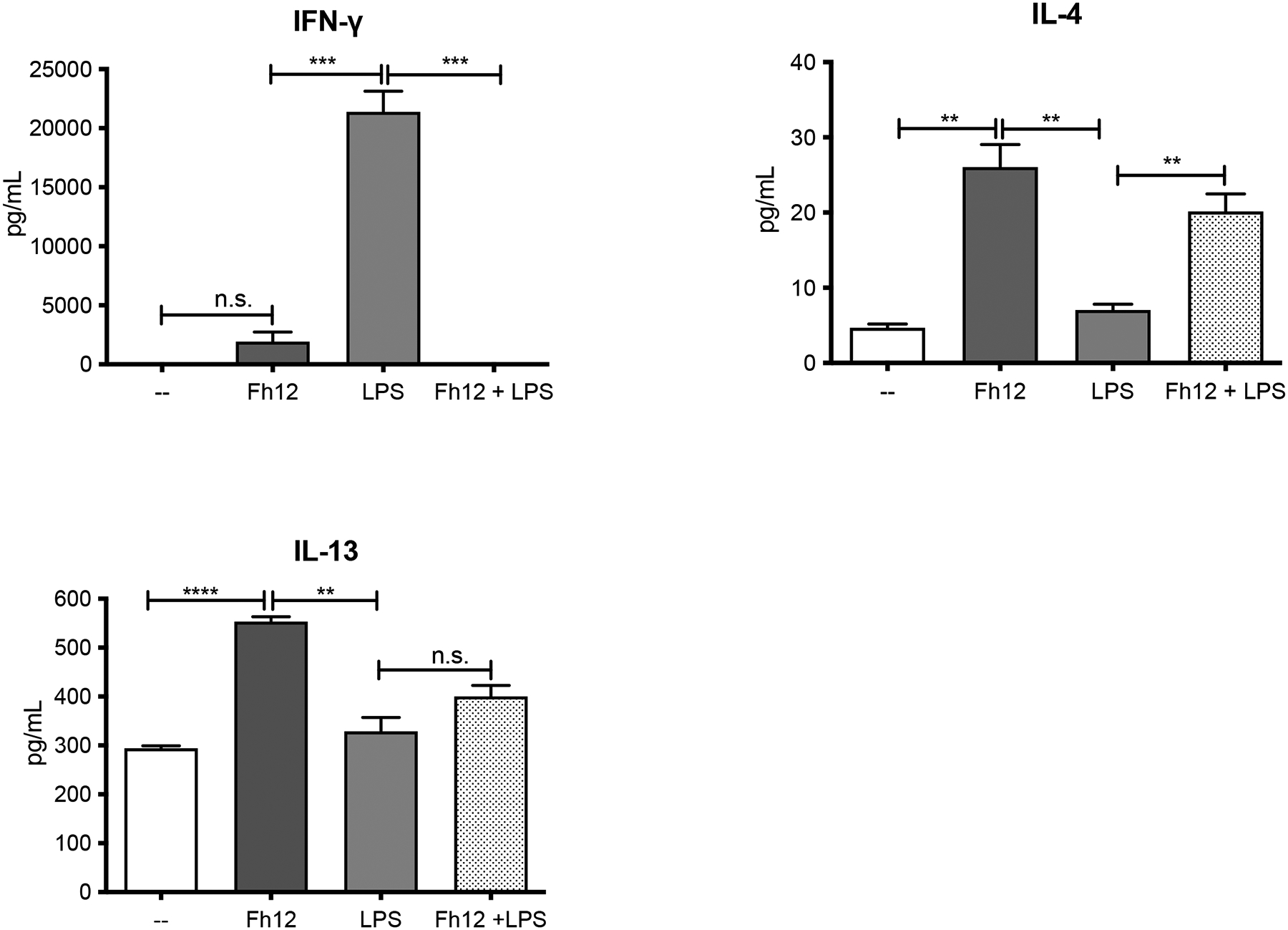
DCs collected from BALB/c mice were treated for 1h with Fh12 (2.5 μg/ml) and further stimulated with LPS (1μg/ml) for 18h. Cells were then washed and co-cultured with CD4+ Tcells from naïve C57BL/6 mice for 5 days. Fh12 significantly suppressed the LPS-induced IFN-γ levels (***p=0.0003). Fh12 significantly increased the levels of IL-4 either when it was added to the culture alone (p=0.0022) or before the LPS-stimulation (**p=0.0073). Fh12 alone significantly increased the production of IL-13 compared to untreated cells (p<0.0001) or stimulated with LPS (p=0.0017). However, in the presence of Fh12 + LPS, although it was observed a trend to increase the levels of IL-13, this increase was not significant. The data represent averages with their SEM of at least 3 replicates from three independent experiments.
DISCUSSION
F. hepatica can be considered a successful parasite because of its ability to migrate through the host tissues without suffering damage to finally allocate in the bile ducts, where it can survive for years. To achieve this survival, F. hepatica has co-evolved with the host and can induce a type of response that is not harmful to it. During its migration, the parasite excretes and/or secretes many products capable of modulating the immune response (Molina-Hernandez et al, 2015). Soon after the infection, the juvenile stage of F. hepatica crosses the intestinal wall and reaches the peritoneum inducing the recruitment of alternatively activated macrophages (Adams et al., 2014; Donnelly et al., 2005; Donnelly et al., 2008). Different reports have shown the ability of various products such as ESPs (Guasconi et al, 2011), and FhTeg (Hacariz et al, 2011) and different enzymes such as thioredoxin peroxidase (Donnelly et al., 2005), 2-Cys peroxiredoxin (Donnelly et al., 2008), fatty acid binding protein (Fh12) (Figueroa-Santiago, 2014) and more recently heme-oxygenase-1 (Carasi et al, 2017) to modulate macrophage activation toward an alternative profile. This phenotype promotes the secretion of anti-inflammatory factors, enhancing the differentiation of Th2 and Treg cells (Kreider et al, 2007). In addition to the modulation of macrophages activation, F. hepatica derived products such as ESPs (Falcon et al., 2010), FhTeg (Hamilton et al., 2009) and secreted proteins such as Kunitz type molecule (KTM) (Falcon et al, 2014) has been related to a down-modulation in DCs activation. It is reasonable to assume that distinct molecules, secreted or expressed by the parasite in its tegument, may participate during its migration in the prevention of an appropriate activation of DCs, contributing to an anti-inflammatory control.
Given that Fh12 is an immunogenic protein that plays an important role in nutrient acquisition and survival of the parasite and is present in ESPs and FhTeg, its interaction with DCs might be relevant. In this work, we investigate the effect of Fh12 on maturation and function of DCs. Intriguingly, our results showed that Fh12 induces the early and late apoptosis of DCs in a dose dependent manner. Additionally, Fh12 inhibited the ability of DCs to mature in the presence of LPS. Considering that Fh12 inhibits inflammatory cytokines in LPS matured macrophages through a mechanism that involves CD14, we hypothesize that this molecule might be involved in the apoptosis. However, our results are against this hypothesis since DCs from CD14 deficient mice in the presence of Fh12 showed similar apoptosis levels of those observed in DCs from normal animals, suggesting the independence of this phenomenon from CD14.
Being a CD14 a coreceptor that participates along with the toll-like receptor-4 (TLR4) and MD-2 in the recognition of bacterial LPS (Dowling, 2018; Kitchens, 2000) its importance in the recognition of Fh12 by DCs seems to be relative. In contrast, it has been shown that CD14 is involved in the apoptotic death of LPS-stimulated DCs via NFAT activation has been demonstrated (Zanoni et al, 2011). However, unlike what happens with DCs, macrophages do not die after LPS activation. The authors argue that after activation by LPS, significant differences are generated in the signal transduction pathways in DCs and macrophages. These last cells were unable to mobilize Ca, a crucial event to induce apoptosis. Interestingly tissue-resident macrophage survival after activation is a crucial event for inflammation resolution (Zanoni et al, 2009).
In a recent comprehensive review, Sakeri, 2017 (Zakeri, 2017) have described the main mechanisms used by helminths to induce apoptosis of host immune cells. Among them are the extrinsic pathway that involves different death receptors and are mediated by the formation of death-inducing signal complex (DISC), which in turn is responsible for caspases 8 and 10 activation. While in the intrinsic pathway is mediated by mitochondria upon membrane depolarization after DNA damage and cellular stress. The consequences of apoptosis in the host’s immune cells are a way in which these parasites modulate to have a more permissive environment that allows them to survive in chronic infections.
Helminth-induced apoptosis: a silent strategy for immunosuppression.
Interestingly, in previous reports, the induction of apoptosis in eosinophils by ES products of F. hepatica through the induction of reactive oxygen species (ROS) has been shown (Serradell et al., 2007). Recently, in a study based on immunohistochemistry and transmission electron microscopy findings authors described the presence of abundant caspase 3+ and nuclear-fragmented eosinophils in the liver of F. hepatica infected sheep (Escamilla et al, 2016). Since Fh12 is part of the F. hepatica ESPs, similar mechanisms of apoptosis induced by Fh12 in DCs might be occurring. Experiments using ROS, caspases, or other apoptosis mediator’s inhibitors, should be used to dissect the mechanism by which Fh12 exerts this effect on DCs. During the allogenic responses, a mismatch between the MHC-II molecules presents in the DCs and the TCR on T lymphocytes promotes the proliferation and production of cytokines, mainly IFN-γ. This phenomenon is exacerbated when the microenvironment generated during DCs stimulation is inflammatory (Herrera et al, 2004). Our results indicate that Fh12 exerts a negative regulation on DCs maturation induced by LPS inhibiting MHCII and co-stimulatory expression generating defective activated DCs with low production of proinflammatory molecules or cytokines. The poor maturation status of DCs might explain the impaired ability of these cells to induce allogeneic responses. This effect exerted by Fh12 does not appear to be redundant since other molecules derived from F. hepatica such as cathepsin L1 or glutathione S-transferase inhibit IL-23 secretion from LPS-matured DCs, with the consequence of a reduction in the inflammatory Th17 response.
Taken together, these results suggest that different antigens derived from F. hepatica might attenuate the DCs activation, which in turn is responsible for the decrease of both type Th1 and Th17 inflammatory responses (Dalton et al, 2013) what otherwise it would be harmful to the parasite. The control of DCs activation, together with the apoptosis of these cells, might be useful to reduce the impact of bacterial ligands translocation during the larvae passage through the intestinal wall (Ryan et al, 2020).Thus, our data suggest an immunomodulatory role for Fh12 on DCs function and its possible involvement in immunoevasion mechanisms.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Institute of Allergy and Infectious Diseases (NIAID) 1SC1AI155439-01 and grants from the National Institutes on Minority Health Disparities G12MD007600, R25GM061838 and 5R25GM061151. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- Adam-Klages S, Adam D, Janssen O, Kabelitz D (2005) Death receptors and caspases: role in lymphocyte proliferation, cell death, and autoimmunity. Immunologic research 33: 149–166 [DOI] [PubMed] [Google Scholar]
- Adams PN, Aldridge A, Vukman KV, Donnelly S, O’Neill SM (2014) Fasciola hepatica tegumental antigens indirectly induce an M2 macrophage-like phenotype in vivo. Parasite immunology 36: 531–539 [DOI] [PubMed] [Google Scholar]
- Alba A, Vazquez AA, Hurtrez-Bousses S (2021) Towards the comprehension of fasciolosis (re-)emergence: an integrative overview. Parasitology 148: 385–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anuracpreeda P, Wanichanon C, Chaithirayanon K, Preyavichyapugdee N, Sobhon P (2006) Distribution of 28.5 kDa antigen in the tegument of adult Fasciola gigantica. Acta Trop 100: 31–40 [DOI] [PubMed] [Google Scholar]
- Ardestani SK, Poorrajab F, Razmi S, Foroumadi A, Ajdary S, Gharegozlou B, Behrouzi-Fardmoghadam M, Shafiee A (2012) Cell death features induced in Leishmania major by 1,3,4-thiadiazole derivatives. Experimental parasitology 132: 116–122 [DOI] [PubMed] [Google Scholar]
- Brady MT, O’Neill SM, Dalton JP, Mills KH (1999) Fasciola hepatica suppresses a protective Th1 response against Bordetella pertussis. Infection and immunity 67: 5372–5378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carasi P, Rodriguez E, da Costa V, Frigerio S, Brossard N, Noya V, Robello C, Anegon I, Freire T (2017) Heme-Oxygenase-1 Expression Contributes to the Immunoregulation Induced by Fasciola hepatica and Promotes Infection. Frontiers in immunology 8: 883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravedo MA, Cabada MM (2020) Human Fascioliasis: Current Epidemiological Status and Strategies for Diagnosis, Treatment, and Control. Res Rep Trop Med 11: 149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Ruiz GM, Sanchez-Torres LE (2020) Fasciola hepatica-derived molecules as potential immunomodulators. Acta Trop 210: 105548. [DOI] [PubMed] [Google Scholar]
- Dalton JP, Robinson MW, Mulcahy G, O’Neill SM, Donnelly S (2013) Immunomodulatory molecules of Fasciola hepatica: candidates for both vaccine and immunotherapeutic development. Veterinary parasitology 195: 272–285 [DOI] [PubMed] [Google Scholar]
- Donnelly S, O’Neill SM, Sekiya M, Mulcahy G, Dalton JP (2005) Thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages. Infection and immunity 73: 166–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly S, Stack CM, O’Neill SM, Sayed AA, Williams DL, Dalton JP (2008) Helminth 2-Cys peroxiredoxin drives Th2 responses through a mechanism involving alternatively activated macrophages. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 22: 4022–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling DJ (2018) Recent Advances in the Discovery and Delivery of TLR7/8 Agonists as Vaccine Adjuvants. ImmunoHorizons 2: 185–197 [DOI] [PubMed] [Google Scholar]
- Escamilla A, Bautista MJ, Zafra R, Pacheco IL, Ruiz MT, Martinez-Cruz S, Mendez A, Martinez-Moreno A, Molina-Hernandez V, Perez J (2016) Fasciola hepatica induces eosinophil apoptosis in the migratory and biliary stages of infection in sheep. Veterinary parasitology 216: 84–88 [DOI] [PubMed] [Google Scholar]
- Espino AM, Rodriguez Medina JR, Hillyer GV (2001) Isolation and immunological characterization of fatty acid binding protein isoforms from Fasciola hepatica. The Journal of parasitology 87: 1028–1033 [DOI] [PubMed] [Google Scholar]
- Falcon C, Carranza F, Martinez FF, Knubel CP, Masih DT, Motran CC, Cervi L (2010) Excretory-secretory products (ESP) from Fasciola hepatica induce tolerogenic properties in myeloid dendritic cells. Veterinary immunology and immunopathology 137: 36–46 [DOI] [PubMed] [Google Scholar]
- Falcon CR, Masih D, Gatti G, Sanchez MC, Motran CC, Cervi L (2014) Fasciola hepatica Kunitz type molecule decreases dendritic cell activation and their ability to induce inflammatory responses. PLoS One 9: e114505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa-Santiago O, Espino AM 2014. (2014) Fasciola hepatica Fatty Acid Binding Protein Induces the Alternative Activation of Human Macrophages. Infection and immunity 82: 5005–5012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn RJ, Irwin JA, Olivier M, Sekiya M, Dalton JP, Mulcahy G (2007) Alternative activation of ruminant macrophages by Fasciola hepatica. Veterinary immunology and immunopathology 120: 31–40 [DOI] [PubMed] [Google Scholar]
- Frey EA, Finlay BB (1998) Lipopolysaccharide induces apoptosis in a bovine endothelial cell line via a soluble CD14 dependent pathway. Microbial pathogenesis 24: 101–109 [DOI] [PubMed] [Google Scholar]
- Guasconi L, Serradell MC, Garro AP, Iacobelli L, Masih DT (2011) C-type lectins on macrophages participate in the immunomodulatory response to Fasciola hepatica products. Immunology 133: 386–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasconi L, Serradell MC, Masih DT (2012) Fasciola hepatica products induce apoptosis of peritoneal macrophages. Veterinary immunology and immunopathology 148: 359–363 [DOI] [PubMed] [Google Scholar]
- Hacariz O, Sayers G, Baykal AT (2012) A proteomic approach to investigate the distribution and abundance of surface and internal Fasciola hepatica proteins during the chronic stage of natural liver fluke infection in cattle. J Proteome Res 11: 3592–3604 [DOI] [PubMed] [Google Scholar]
- Hacariz O, Sayers G, Mulcahy G (2011) A preliminary study to understand the effect of Fasciola hepatica tegument on naive macrophages and humoral responses in an ovine model. Veterinary immunology and immunopathology 139: 245–249 [DOI] [PubMed] [Google Scholar]
- Hamilton CM, Dowling DJ, Loscher CE, Morphew RM, Brophy PM, O’Neill SM (2009) The Fasciola hepatica tegumental antigen suppresses dendritic cell maturation and function. Infection and immunity 77: 2488–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich S, Schmidt M, August C, Cullen P, Rademaekers A, Pauels HG (1997) Regulation of human monocyte apoptosis by the CD14 molecule. J Immunol 159: 3178–3188 [PubMed] [Google Scholar]
- Herrera OB, Golshayan D, Tibbott R, Salcido Ochoa F, James MJ, Marelli-Berg FM, Lechler RI (2004) A novel pathway of alloantigen presentation by dendritic cells. J Immunol 173: 4828–4837 [DOI] [PubMed] [Google Scholar]
- Kitchens RL (2000) Role of CD14 in cellular recognition of bacterial lipopolysaccharides. Chemical immunology 74: 61–82 [DOI] [PubMed] [Google Scholar]
- Kreider T, Anthony RM, Urban JF Jr., Gause WC (2007) Alternatively activated macrophages in helminth infections. Curr Opin Immunol 19: 448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, Caban-Hernandez K, Figueroa-Santiago O, Espino AM (2015) Fasciola hepatica fatty acid binding protein inhibits TLR4 activation and suppresses the inflammatory cytokines induced by lipopolysaccharide in vitro and in vivo. J Immunol 194: 3924–3936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmood K, Zhang H, Sabir AJ, Abbas RZ, Ijaz M, Durrani AZ, Saleem MH, Ur Rehman M, Iqbal MK, Wang Y et al. (2017) A review on epidemiology, global prevalence and economical losses of fasciolosis in ruminants. Microbial pathogenesis 109: 253–262 [DOI] [PubMed] [Google Scholar]
- Molina-Hernandez V, Mulcahy G, Perez J, Martinez-Moreno A, Donnelly S, O’Neill SM, Dalton JP, Cwiklinski K (2015) Fasciola hepatica vaccine: we may not be there yet but we’re on the right road. Veterinary parasitology 208: 101–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales A, Espino AM (2012) Evaluation and characterization of Fasciola hepatica tegument protein extract for serodiagnosis of human fascioliasis. Clin Vaccine Immunol 19: 1870–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morphew RM, Eccleston N, Wilkinson TJ, McGarry J, Perally S, Prescott M, Ward D, Williams D, Paterson S, Raman M et al. (2012) Proteomics and in silico approaches to extend understanding of the glutathione transferase superfamily of the tropical liver fluke Fasciola gigantica. J Proteome Res 11: 5876–5889 [DOI] [PubMed] [Google Scholar]
- Motran CC, Ambrosio LF, Volpini X, Celias DP, Cervi L (2017) Dendritic cells and parasites: from recognition and activation to immune response instruction. Semin Immunopathol 39: 199–213 [DOI] [PubMed] [Google Scholar]
- O’Neill SM, Mills KH, Dalton JP (2001) Fasciola hepatica cathepsin L cysteine proteinase suppresses Bordetella pertussis-specific interferon-gamma production in vivo. Parasite immunology 23: 541–547 [DOI] [PubMed] [Google Scholar]
- Ramos-Benitez MJ, Ruiz-Jimenez C, Aguayo V, Espino AM (2017) Recombinant Fasciola hepatica fatty acid binding protein suppresses toll-like receptor stimulation in response to multiple bacterial ligands. Scientific reports 7: 5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P (1998) Dendritic cell survival and maturation are regulated by different signaling pathways. The Journal of experimental medicine 188: 2175–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Gonzalez J, Wilkins-Rodriguez A, Argueta-Donohue J, Aguirre-Garcia M, Gutierrez-Kobeh L (2016) Leishmania mexicana promastigotes down regulate JNK and p-38 MAPK activation: Role in the inhibition of camptothecin-induced apoptosis of monocyte-derived dendritic cells. Experimental parasitology 163: 57–67 [DOI] [PubMed] [Google Scholar]
- Ruiz-Campillo MT, Molina-Hernandez V, Bautista MJ, Pacheco IL, Zafra R, Buffoni L, Martinez-Moreno FJ, Martinez-Moreno A, Perez J (2020) Characterization of dendritic cells and follicular dendritic cells in the hepatic lymph nodes and liver of sheep experimentally infected with Fasciola hepatica. Veterinary research 51: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S, Shiels J, Taggart CC, Dalton JP, Weldon S (2020) Fasciola hepatica-Derived Molecules as Regulators of the Host Immune Response. Frontiers in immunology 11: 2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serradell MC, Guasconi L, Cervi L, Chiapello LS, Masih DT (2007) Excretory-secretory products from Fasciola hepatica induce eosinophil apoptosis by a caspase-dependent mechanism. Veterinary immunology and immunopathology 117: 197–208 [DOI] [PubMed] [Google Scholar]
- Wilson RA, Wright JM, de Castro-Borges W, Parker-Manuel SJ, Dowle AA, Ashton PD, Young ND, Gasser RB, Spithill TW (2011) Exploring the Fasciola hepatica tegument proteome. International journal for parasitology 41: 1347–1359 [DOI] [PubMed] [Google Scholar]
- Zakeri A (2017) Helminth-induced apoptosis: a silent strategy for immunosuppression. Parasitology 144: 1663–1676 [DOI] [PubMed] [Google Scholar]
- Zanoni I, Ostuni R, Capuano G, Collini M, Caccia M, Ronchi AE, Rocchetti M, Mingozzi F, Foti M, Chirico G et al. (2009) CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature 460: 264–268 [DOI] [PubMed] [Google Scholar]
- Zanoni I, Ostuni R, Marek LR, Barresi S, Barbalat R, Barton GM, Granucci F, Kagan JC (2011) CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 147: 868–880 [DOI] [PMC free article] [PubMed] [Google Scholar]


