Abstract
We examined physiological adaptations which allow the psychrotroph Rhodococcus sp. strain Q15 to assimilate alkanes at a low temperature (alkanes are contaminants which are generally insoluble and/or solid at low temperatures). During growth at 5°C on hexadecane or diesel fuel, strain Q15 produced a cell surface-associated biosurfactant(s) and, compared to glucose-acetate-grown cells, exhibited increased cell surface hydrophobicity. A transmission electron microscopy examination of strain Q15 grown at 5°C revealed the presence of intracellular electron-transparent inclusions and flocs of cells connected by an extracellular polymeric substance (EPS) when cells were grown on a hydrocarbon and morphological differences between the EPS of glucose-acetate-grown and diesel fuel-grown cells. A lectin binding analysis performed by using confocal scanning laser microscopy (CSLM) showed that the EPS contained a complex mixture of glycoconjugates, depending on both the growth temperature and the carbon source. Two glycoconjugates [β-d-Gal-(1-3)-d-GlcNAc and α-l-fucose] were detected only on the surfaces of cells grown on diesel fuel at 5°C. Using scanning electron microscopy, we observed strain Q15 cells on the surfaces of octacosane crystals, and using CSLM, we observed strain Q15 cells covering the surfaces of diesel fuel microdroplets; these findings indicate that this organism assimilates both solid and liquid alkane substrates at a low temperature by adhering to the alkane phase. Membrane fatty acid analysis demonstrated that strain Q15 adapted to growth at a low temperature by decreasing the degree of saturation of membrane lipid fatty acids, but it did so to a lesser extent when it was grown on hydrocarbons at 5°C; these findings suggest that strain Q15 modulates membrane fluidity in response to the counteracting influences of low temperature and hydrocarbon toxicity.
A significant problem in biodegradation of hydrocarbon contaminants is the very low solubility of the compounds in the aqueous phase. This is especially true at lower temperatures, at which longer-chain alkanes (>C10) are generally more insoluble or exist as solids; this hinders the biodegradative activity of psychrotrophic and psychrophilic bacteria. The resulting decreased bioavailability is responsible for the recalcitrance of the compounds commonly observed in temperate and cold environments. Nevertheless, cold-adapted hydrocarbon-degrading microorganisms can degrade these substrates at temperatures as low as 0°C (45–47) and, therefore, are physiologically able to take up and assimilate the compounds at low temperatures.
Although the actual uptake of alkanes by bacteria is thought to be a passive transport process, microorganisms possess a number of adaptive mechanisms for accumulating and transporting hydrocarbons into the cell for initial enzymatic catabolism (17, 36, 44). Bacteria transport and assimilate soluble alkanes that are dissolved in the aqueous phase. Indeed, it was initially thought that bacteria could utilize only solubilized hydrocarbons (7). However, alkanes are degraded at rates which exceed the rates of dissolution of hydrocarbons in the aqueous phase, indicating that other uptake mechanisms are also utilized by hydrocarbon-degrading microorganisms (25, 41). Many bacteria are capable of emulsifying hydrocarbons in solution by producing surface-active agents, such as biosurfactants (10, 17, 29). These amphiphatic compounds reduce surface tension by accumulating at the interface of immiscible fluids or of a fluid and a solid and increasing the surface areas of insoluble compounds, which leads to increased bioavailability and subsequent biodegradation of the hydrocarbons (25). Microorganisms may also take up insoluble hydrocarbons by adhering to hydrocarbons at the water-hydrocarbon liquid or solid interface (7, 17, 33, 42, 44). To facilitate adhesion to hydrophobic substrates, hydrocarbon-degrading bacteria may increase cell surface hydrophobicity by modifying cell surface components (43). In addition, microbial cells may produce extracellular polymeric substances (EPS) in the form of capsules or mucoid secretions that may interact with hydrophobic substrates, such as hydrocarbons (48, 49).
Bacteria are also known to adapt to changes in environmental conditions, such as growth temperature or growth in the presence of hydrocarbon substrates, by altering the lipid composition of the cytoplasmic membrane in order to maintain or adjust membrane bilayer fluidity (26, 36). Alterations to the fatty acid moieties of membrane lipids are thought to be the most effective means of maintaining the liquid crystalline state in membranes, which is essential for optimal membrane function (16). During growth at low temperatures, bacteria can modulate the viscosity of membrane lipids to maintain or increase membrane fluidity by decreasing the degree of saturation, by shortening the acyl chain length, by increasing the cis/trans fatty acid ratio, and by increasing the relative amount of branched fatty acids (9, 16). The effects of adding hydrophobic growth substrates like hydrocarbons are completely opposite the effects observed during low-temperature growth and mimic changes observed during bacterial growth at high temperatures. In this case, an increased degree of saturation or conversion of cis fatty acids to trans fatty acids is commonly observed (14, 36). These changes act as protective mechanisms against hydrocarbon-associated toxicity by rendering the membrane less permeable to hydrocarbons (36).
In the present study, we examined physiological adaptations involved in alkane assimilation at a low temperature by Rhodococcus sp. strain Q15, a psychrotroph that is capable of mineralizing a variety of alkanes and can grow on and emulsify diesel fuel and Bunker C crude oil at both 5 and 24°C (47). The ability of strain Q15 to produce biosurfactant and its ability to alter its cell membrane by changing the fatty acid composition in response to both growth temperature and carbon source were also examined. Possible structural and morphological adaptations of strain Q15 cells grown at 5°C on various alkanes were also examined by transmission electron microscopy (TEM), scanning electron microscopy (SEM), and confocal scanning laser microscopy (CSLM).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Rhodococcus sp. strain Q15 was originally isolated from Bay of Quinte, Lake Ontario, Canada (18), and was characterized by Whyte et al. (47). Strain Q15 was grown on Trypticase soy agar at 24°C and was maintained at 4°C. In growth experiments performed with different carbon sources, strain Q15 was grown on a mineral salts medium (MSM) (12) supplemented with 0.005% yeast extract and 0.2% (wt/vol) hexadecane, 0.2% (wt/vol) diesel fuel, 0.2% (wt/vol) octacosane, or 0.2% (wt/vol) glucose-acetate (1:1, wt/wt) as the carbon and energy source.
Surface tension and hydrophobicity analyses of Rhodococcus sp. strain Q15.
In order to detect the formation of a biosurfactant(s) by Rhodococcus sp. strain Q15, cells were grown in a water bath shaker (150 rpm) at 5 or 24°C in 125-ml screw-cap flasks containing 50 ml of MSM supplemented with hexadecane, diesel fuel, or glucose-acetate. Cellular growth was monitored by determining the optical density at 600 nm (OD600). Each culture was transferred to a Pyrex beaker (50 by 70 mm), and the surface tension was measured with a model 21 Surface Tensiomat (Fisher Scientific, Toronto, Ontario, Canada). To determine if strain Q15 biosurfactant was released into the medium or remained associated with the cells, 25 ml of culture medium was centrifuged (12,000 × g, 10 min, 4°C), and the cell-free supernatant was separated from the cell pellet. The latter was resuspended in 25 ml of fresh MSM, and the surface tensions of both fractions were determined. All surface tension measurements were performed in duplicate. The cell surface hydrophobicities of strain Q15 grown on various carbon sources at 5°C and collected by centrifugation were determined by the microbial adhesion to hydrocarbon (MATH) test essentially as previously described (32, 34) by using dodecane as the test liquid hydrocarbon.
TEM and SEM analyses of Rhodococcus sp. strain Q15 cells.
To prepare thin sections for TEM, glucose-acetate-grown cells were collected by centrifugation and fixed with 2% (vol/vol) glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.3) containing 0.015% (wt/vol) ruthenium red. Cells were enrobed in agar and fixed with 1% (wt/vol) osmium tetroxide and 1% (wt/vol) uranyl acetate, both of which contained 0.015% (wt/vol) ruthenium red. Samples were dehydrated by using an ethanol series and were embedded in LR White resin. Cells grown on diesel fuel were first deposited on a thin layer of Noble agar in a small glass petri dish and covered with another thin layer of Noble agar. The glutaraldehyde-ruthenium red fixative was added to the agar containing the cells. After fixation for 2 h, the agar was cut into small blocks, and the specimens were processed as described above. Some thin sections were poststained with uranyl acetate and lead citrate. All specimens were examined with a Philips model EM300 TEM operating at 60 kV.
For SEM, crystals of octacosane on which strain Q15 cells were grown were transferred to 1 ml of 2% (vol/vol) glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.3). The cells were postfixed with 1% (wt/vol) osmium tetroxide in buffer, dehydrated by using ethanol and acetone, and then critical point dried. The crystals were taped onto silicon chips, gold coated, and examined with a Hitachi model S-4500 SEM.
CSLM analysis of Rhodococcus sp. strain Q15.
A Bio-Rad model MRC 1000 CSLM consisting of a krypton-argon laser system mounted on a Nikon model Microphot-SA microscope was used to nondestructively obtain images of strain Q15 cells grown at 5 or 24°C on MSM containing either glucose-acetate, diesel fuel, or octacosane. Cells grown at 5°C were kept at 5°C during staining and preparation for observation and analysis, and all microscopy was performed by using a microscope stage cooled at 5°C (Physitemp Instruments, Clifton, N.J.). Observations of some diesel fuel- and glucose-acetate-grown cells were carried out in 0.5-mm-deep well slides covered with no. 1 coverslips (Fisher Scientific, Montreal, Quebec, Canada), which allowed imaging of undisturbed cell mass and diesel fuel microdroplets. The microscope was equipped with a 60×, 1.4-numerical-aperture oil immersion lens (Nikon Corporation, Chiyoda-ku, Tokyo). The CSLM was operated as described previously (21). Optical thin sections in the xy plane, as well as xz sagittal images, were obtained. In addition, SYTO 9 (Molecular Probes, Eugene, Oreg.) was used as a nucleic acid stain to positively stain strain Q15 cells (24). Strain Q15 biofilm material was positively stained with Nile Red (Eastman Kodak Co., Rochester, N.Y.), a hydrophobic compound-specific benzophenoxazinone dye (20). A staining solution containing 5 μg of Nile Red ml−1 (final concentration) in 50% aqueous glycerol was prepared from a 1-mg ml−1 stock solution of Nile Red dissolved in acetone. A panel of fluor-conjugated lectins, derived from Arachis hypogaea, Canavalia ensiformis, Tetragonolobus purpureas, Triticum vulgaris, and Ulex europeaus (Sigma Chemical Co., St. Louis, Mo.), was used to detect and assess the presence of EPS and to provide information on the chemical nature of EPS. The methods used have been described in detail previously (22, 23, 49).
Analysis of cell membrane fatty acid composition in Rhodococcus sp. strain Q15.
Three 100-ml cultures of cells grown until the late exponential phase on MSM containing either glucose-acetate, hexadecane, or diesel fuel were centrifuged (10 min, 9,000 × g, 4°C) and washed twice with 0.1 M potassium phosphate buffer (pH 7.0). The free and loosely bound lipids of pelleted cells were extracted basically as previously described by Bligh and Dyer (6). Methyl esters of fatty acids were prepared as previously described (27). The fatty acid methyl esters were analyzed by gas chromatography by using a model Sigma 2000 capillary chromatograph (Perkin-Elmer, Norwalk, Conn.) equipped with a type DB-225 column (J&W Scientific, Folsom, Calif.) and a flame ionization detector (Perkin-Elmer). Helium was the carrier gas, and the flow rate was 2.5 ml/min. Samples (approximately 1 ml) were injected via a split injection port with a split ratio of 1:9. The initial temperature of the column was 180°C, and the temperature was increased at a rate of 2°C/min to 220°C; then the temperature was increased at a rate of 5°C/min to 235°C and then kept constant for 7 min. The temperatures of both the injection port and the detector were kept constant at 240°C. Following the gas chromatography analysis, the mass percentage of each fatty acid was calculated by comparing the area of each individual fatty acid peak to the total area.
RESULTS
Surface tension and hydrophobicity analyses of Rhodococcus sp. strain Q15.
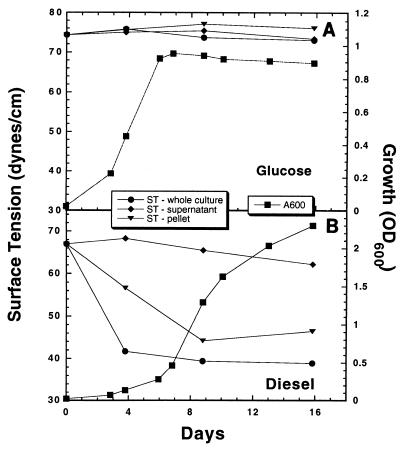
The surface tensions of culture media were measured during strain Q15 growth at 5 or 24°C on various carbon sources. During growth at 5°C on glucose-acetate, the surface tension of the culture medium remained ∼70 mN/m (Fig. 1). The surface tension of the culture medium decreased to ∼40 mN/m during growth on diesel fuel at 5°C (Fig. 1). Following separation of diesel fuel-grown cells from the culture supernatant, the biosurfactant activity was present in cell pellets resuspended in fresh MSM but not in the cell-free supernatant, indicating that the biosurfactant remained associated with the cell envelope and was not excreted into the medium. As in diesel fuel-grown cells, biosurfactant activity was observed in cells grown on hexadecane at 5°C; this activity also was present in the resuspended cell pellet (data not shown). Similar results were obtained at 24°C, at which the surface tension was ∼36 mN/m in hexadecane- and diesel fuel-grown cells but remained at 70 mN/m in glucose-acetate-grown cells. Determining cellular growth by measuring the OD600 proved to be difficult when strain Q15 was grown with either hexadecane or diesel fuel as the carbon and energy source due to the formation of large, adhesive cellular flocs with low buoyant densities. However, in the case of diesel fuel-grown cells, reproducible results could be obtained by covering the cuvette with Parafilm, inverting the cuvette five times, and then immediately determining the OD600.
FIG. 1.
Growth of Rhodococcus sp. strain Q15 at 5°C with glucose-acetate (A) or diesel fuel (B) as the carbon and energy source and changes in the surface tensions in the cultures, the supernatants, and the pellet fractions. The surface tension values are means based on duplicate determinations. ST, surface tension; A600, absorbance at 600 nm.
The relative cell surface hydrophobicity of strain Q15 grown at 5°C, as measured by the MATH test, was greater in pelleted cells following growth on diesel fuel (41.1% ± 5.5% [mean ± standard deviation]) and hexadecane (20.8% ± 6.5%) than in cells grown on glucose-acetate (9.3% ± 1.7%). Similar results were obtained for cells grown at 24°C.
TEM and SEM analyses of Rhodococcus sp. strain Q15.
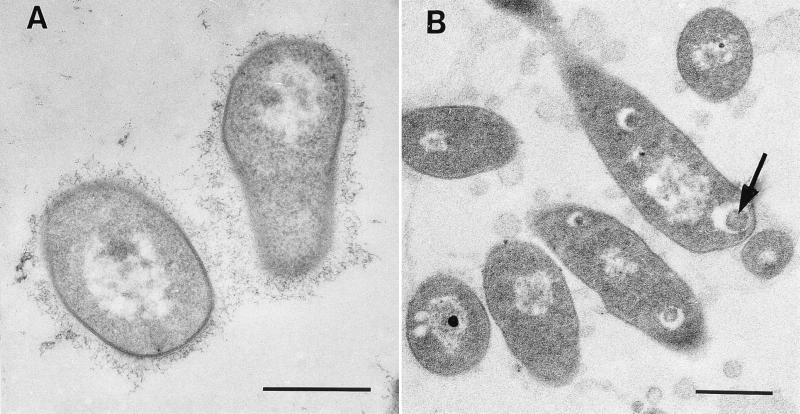
Strain Q15 grew as freely suspended cells in glucose-acetate medium. Cells fixed in the presence of ruthenium red were surrounded by a delicate web of fibers (Fig. 2A). This finely contrasted zone of capsular material was very extensive but did not cause the cells to adhere to each other. Floc phase cells of strain Q15 grown on diesel fuel were also fixed in the presence of ruthenium red. Because the floc material was difficult to collect by centrifugation, the flocs were gently deposited on a thin layer of Noble agar in a small glass petri dish, covered with another thin layer of agar, and then exposed to glutaraldehyde containing ruthenium red. In thin sections, finely contrasted clusters of EPS were found surrounding and connecting many cells which were clumped together within the floc (Fig. 2B). Large, spherical, electron-transparent inclusions were present in the diesel fuel-grown cells (Fig. 2B) but not in glucose-acetate-grown cells. Some inclusions contained electron-dense material, as shown in Fig. 2B. The inclusions were not membrane bound. The cell envelope profiles were the same in glucose-acetate- and diesel fuel-grown cells.
FIG. 2.
TEM micrographs of Rhodococcus sp. strain Q15 cells obtained during growth on glucose-acetate or diesel fuel at 5°C. (A) Strain Q15 grown on glucose-acetate and fixed in the presence of ruthenium red. The organism occurred as single cells which were surrounded by EPS consisting of a delicate web of fibers consistent with bacterial capsular material. (B) Floc phase cells of strain Q15 grown on diesel fuel and fixed in the presence of ruthenium red. In thin sections, finely contrasted clusters of EPS material surrounded cells within the flocs. The arrow indicates an intracellular inclusion. Bars = 500 nm.
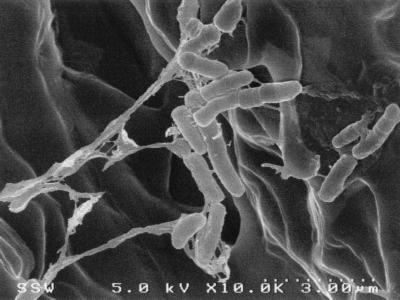
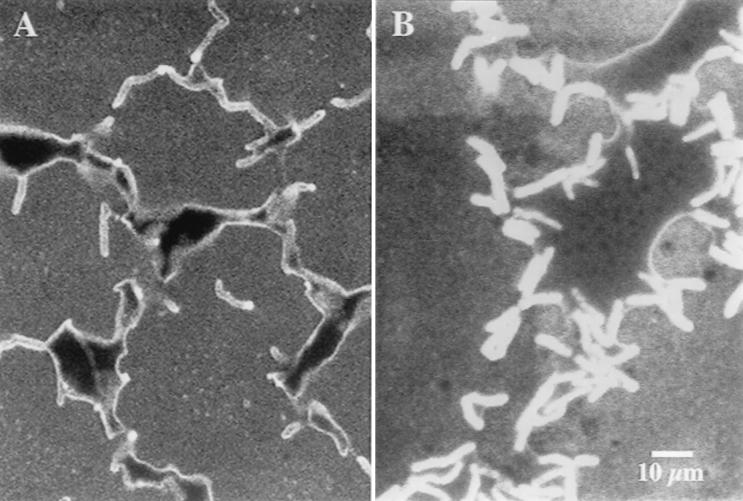
Strain Q15 cells grown on octacosane at 5°C were observed by SEM on the surfaces of and adhering to octacosane crystals (Fig. 3). Similar observations of Q15 cells on the surfaces of octacosane crystals were made by CSLM (data not shown). EPS was visible by SEM as strands and fibers between cells and clumps on the surfaces of the cells.
FIG. 3.
SEM micrograph of Rhodococcus sp. strain Q15, showing groups of cells colonizing the surface of an octacosane crystal during growth at 5°C. Strands and fibers (fingerlike projections) of EPS were observed on the cell surface between cells and between cells and the surface of the octacosane crystal.
CSLM analysis of Rhodococcus sp. strain Q15.
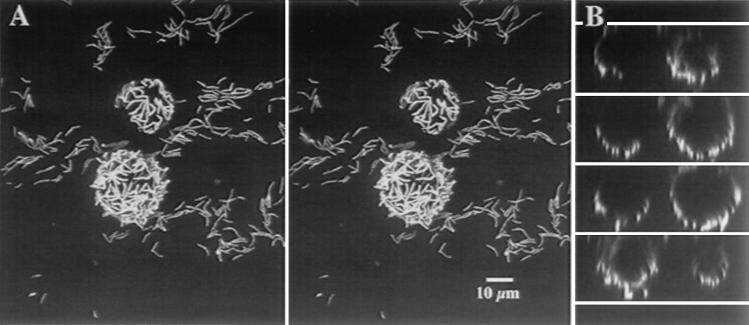
CSLM allows direct in situ observation of fully hydrated, intact samples, such as biofilms, which avoids the problems associated with dehydration artifacts during TEM and SEM preparation and analysis. We observed that 5°C strain Q15 cells completely surrounded and adhered to the surfaces of diesel fuel microdroplets which were ∼10 to 16 μm in diameter (Fig. 4A). xz optical sectioning through a diesel fuel microdroplet showed that strain Q15 cells were not inside the diesel fuel microdroplets (Fig. 4B). At 24°C, diesel fuel microdroplets were not observed.
FIG. 4.
CSLM of Rhodococcus sp. strain Q15 cells grown on diesel fuel at 5°C. (A) Three-dimensional projections presented as a stereo pair showing a z series through an SYTO 9-stained strain Q15 biofilm grown on diesel fuel as the sole C source. Note the basal layer of attached cells on the slide surface and the microdroplets of diesel fuel surrounded by cells of strain Q15. (B) Series of xz optical sections through the diesel fuel microdroplets shown in panel A. The upper surface was a glass coverslip overlying a well slide. The cells grown on diesel fuel were transferred to the well slide and covered with a glass coverslip, and microdroplets were observed after they rose to contact the upper glass surface. The cells were maintained at 5°C, and no fixation or immobilization of the droplets was performed.
A CSLM analysis performed with fluor-conjugated lectins was used to characterize the EPS present in biological matrices, such as biofilms and cell surfaces (48). Lectins are plant- and animal-derived proteins which bind to glycoconjugate residues (glycolipids, glycoproteins, and polysaccharides) with high levels of specificity, which allows characterization of their chemical nature and physical distribution in biological matrices. In the present study, CSLM image analysis revealed that the five lectins used bound, to different extents to the exterior surfaces of strain Q15 cells depending on both the growth temperature and the carbon source (Table 1). For example, the lectins from C. ensiformis and T. purpureas bound to cells during growth on diesel fuel at 5°C (Fig. 5). At 5°C, the EPS surrounding diesel fuel-grown cells contained a mixture of glycoconjugates that may have included fucose, glucose, mannose, (GluNAc)2, and β-d-Gal-(1-3)-d-GlcNAc, as well as perhaps GlcNAc and NeuNAc (Table 1). These observations indicated that the chemical composition of the EPS in the biofilm matrix was complex. Interestingly, β-d-Gal-(1-3)-d-GlcNAc, and (to a lesser extent) fucose, appeared only on the surfaces of cells grown on diesel fuel at 5°C, suggesting that formation of these compounds may have been an adaptive response to low-temperature growth on this substrate. In contrast to the results obtained after growth on diesel fuel at 5°C, none of the five lectins bound to cells grown on glucose-acetate (Table 1) at 5°C, although some of them did bind strongly to Q15 EPS at 24°C. It was also apparent that there was extensive production of EPS that was not bound to cells at 24°C, particularly EPS binding the C. ensiformis lectin.
TABLE 1.
Effects of carbon source (glucose-acetate or diesel fuel) and temperature (5 or 24°C) on lectin binding to cells and exopolymer of Rhodococcus sp. strain Q15
| Lectin-fluor conjugatea | Glycoconjugate(s) | Growth at 5°C on:
|
Growth at 24°C on:
|
||
|---|---|---|---|---|---|
| Glucose-acetate | Diesel fuel | Glucose-acetate | Diesel fuel | ||
| Arachis hypogaea-TRITC | β-Gal(1->3)-GalNAc | No binding | Binding to cell surface and surfaces of diesel fuel microdroplets | No binding | No binding |
| Canavalia ensiformis A-FITC | α-Glucose, α-mannose | Weak cell surface binding | Binding to cell surface and surfaces of diesel fuel microdroplets | Strong binding to exopolymer | Strong binding to exopolymer |
| Tetragonolobus purpureas-TRITC | α-l-Fucose | Weak cell surface binding | Binding to cell surface | Weak cell surface binding | No binding |
| Triticum vulgaris-TRITC | (GlcNAc)2, NeuNAc | Very weak cell surface binding | Weak binding to cells and exopolymer | Weak binding to cells and exopolymer | Weak cell surface binding |
| Ulex europeaus-TRITC | α-l-Fucose, (GlcNAc)2 | No binding | Binding to cell surface | Strong binding to exopolymer | No binding |
TRITC, tetramethyl rhodamine isothiocyanate; FITC, fluorescein isothiocyanate.
FIG. 5.
CSLM xy images showing binding of the C. ensiformis (A) and T. purpureas (B) lectins to cells of strain Q15 grown at 5°C with diesel fuel as the sole carbon source. C. ensiformis lectin appeared to bind to the exterior surfaces of the diesel fuel microdroplets in the biofilm, creating a bright boundary region. There is also some evidence that there were binding sites within the diesel fuel droplets. The T. purpureas lectin binding was extensive at the cell surface and occurred between cells and within the microdroplets. In addition, there is evidence that an emulsion was formed within the microdroplets and in the vicinity of Q15 cells.
Analysis of cell membrane fatty acid composition in Rhodococcus sp. strain Q15.
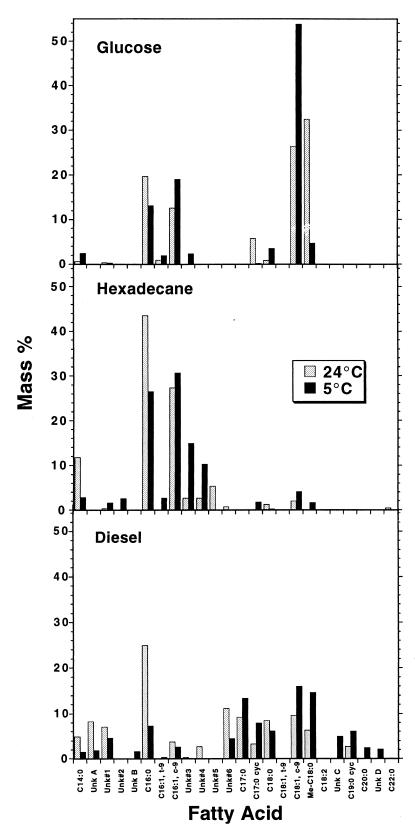
Rhodococcus sp. strain Q15 cells were grown on MSM supplemented with either glucose-acetate, diesel fuel, or hexadecane at 5 or 24°C to determine changes in the fatty acid composition of the readily extractable lipids in response to both changes in temperature and different carbon sources. Overall, the acyl chain lengths of the observed fatty acids ranged from C14 to C18; C16 and C18 fatty acids were generally the most prominent fatty acids regardless of the temperature or carbon source. However, the carbon source greatly influenced the overall composition of the fatty acid profile (Fig. 6). Compared with glucose-acetate-grown cells, relatively small amounts of C18 fatty acids were detected in hexadecane-grown cells and the relative amounts of C16 and C14:0 fatty acids were greater, especially at 24°C. The fatty acid profiles of strain Q15 cells grown on diesel fuel contained the greatest variety of fatty acid species, which perhaps reflected the relatively complex chemical composition of the diesel fuel substrate.
FIG. 6.
Comparison of fatty acid profiles of Rhodococcus sp. strain Q15 cells grown on glucose-acetate, hexadecane, or diesel fuel at 5 or 24°C. Unk, unknown, putative fatty acid; c, cis, t, trans; cyc, cylcopropane; Me, methyl. The values are means based on triplicate samples (standard deviation, <10% of mean). Unknown fatty acids 3 and 4, which were present in relatively large quantities in cells grown on hexadecane at 5°C, were tentatively identified by gas chromatography-mass spectrometry as C16:1 and methyl-C15:0, respectively.
We also observed differences in specific fatty acids in response to growth temperature. In glucose-grown cells, a decrease in the amount of saturated C16:0 and increases in the amounts of cis-9-C16:1 and trans-9-C16:1 occurred at 5°C compared with 24°C. A similar shift was observed for C18 fatty acids. In hexadecane-grown cells, a shift from saturated to unsaturated fatty acids was observed for the C16 and C14 fatty acids at 5°C. Relatively large quantities of putative fatty acids 3 and 4, which were tentatively identified by gas chromatography-mass spectrometry analysis as C16:1 and methyl-C15:0, respectively, were present in cells grown on hexadecane at 5°C, indicating that these compounds might be important in maintaining optimal membrane fluidity at this low temperature. For cells grown on diesel fuel, the amounts of the putative fatty acids were relatively high as well. There was a large decrease in the amount of C16:0 when cells grown on diesel fuel at 5°C were compared with cells grown on diesel fuel at 24°C, but no subsequent increase in the amount of C16:1 was apparent. For the C18 fatty acids, relative increases in the amounts of both cis-9-C18:1 and 10-methyl-C18:0 were apparent at 5°C. In both diesel fuel-and hexadecane-grown cells the relative amounts of both C17:0-cyclopropane and C19:0-cyclopropane, as well as the relative amount of C17:0, increased at 5°C. Interestingly, the opposite result was obtained with glucose-acetate-grown cells, in which C17:0-cyclopropane was found only during growth at 24°C. The exact physiological role(s) of cyclopropane fatty acids is presently unknown, but formation of these fatty acids in bacterial cellular membranes may be an adaptation to starvation or other forms of growth stasis (13).
To elucidate global adaptation mechanisms of membrane fatty acids in strain Q15 in response to growth at a low temperature on hydrocarbon substrates, the degree of saturation (i.e., saturated/unsaturated ratio) of fatty acids, the ratio of saturation at 24 and 5°C, the average acyl chain length, and the cis/trans ratio were determined and compared (Table 2). Patterns indicating specific adaptations of strain Q15 to growth on hydrocarbons at a low temperature were not clearly delineated by comparing average acyl chain lengths and cis/trans ratios. A comparison of the degrees of saturation strongly indicated that the fatty acids changed from relatively saturated fatty acids at 24°C to relatively unsaturated fatty acids at 5°C, regardless of the growth substrate. However, this shift was approximately two-fold less in hydrocarbon-grown cells than in glucose-acetate-grown cells, as measured by the 24°C/5°C ratio of saturation (Table 2).
TABLE 2.
Degree of saturation (saturated/unsaturated ratio), average acyl chain length (numbers of C atoms), and cis/trans ratio of fatty acids in Rhodococcus sp. strain Q15 cells grown on different carbon sources at 24 or 5°C
| Carbon source | Temp (°C) | Degree of satu-ration (saturated/ unsaturated ratio) | 24°C/5°C ratio of saturation | Average acyl chain length | cis/trans ratioa |
|---|---|---|---|---|---|
| Glucose-acetate | 24 | 1.35 | 4.2 | 17.2 | 42.6 |
| 5 | 0.32 | 17.2 | 32.2 | ||
| Hexadecane | 24 | 1.92 | 2.3 | 15.9 | 29.3:0 |
| 5 | 0.83 | 16.1 | 13.2 | ||
| Diesel fuel | 24 | 3.33 | 2.1 | 16.6 | 13.3:0 |
| 5 | 1.56 | 17.6 | 46.9 |
Determining the cis/trans ratio for the fatty acids obtained from hydrocarbon-grown cells was possible only for fatty acids derived from cells grown at 5°C, since no trans fatty acids were detected in the lipids which were obtained from cells grown at 24°C.
DISCUSSION
Physiological adaptations for alkane uptake at a low temperature by the alkane-degrading psychrotrophic organism Rhodococcus sp. strain Q15 were characterized in order to better understand how this bacterium utilizes insoluble hydrocarbon substrates at low temperatures. This psychrotroph produced biosurfactant which was associated with the cell envelope and was capable of lowering the surface tension during growth on diesel fuel or hexadecane at both 5 and 24°C. To the best of our knowledge, this is the first report of biosurfactant activity at low temperatures. Cold-active solubilizing agents like the strain Q15 biosurfactant(s) could prove to be very beneficial for successful bioremediation of hydrocarbon-contaminated sites at low ambient temperatures by increasing the bioavailability of recalcitrant hydrocarbons like long-chain alkanes.
As biosurfactant activity was observed only during growth on hydrocarbons and not during growth on glucose-acetate and as biosurfactant activity was observed during the late lag and early exponential phases of growth, the production of biosurfactant(s) by strain Q15 was induced and may be a prerequisite for strain Q15 growth on hydrocarbons. Although the biosurfactant(s) produced by strain Q15 was not characterized, Rhodococcus erythropolis, which is phylogenetically closely related to strain Q15 (47), produces cell-bound biosurfactants (30) during growth on alkanes, and these biosurfactants have been identified as trehalose mycolate lipids (19, 30, 31). These compounds, as well as lipoglycan, lipoprotein, and phospholipids, other known surface-active agents (10, 29), are found on the outer asymmetric lipid bilayer recently hypothesized for the rhodococcal cell envelope (38).
Microscopic examination revealed morphological changes, including electron-transparent cellular inclusions, in strain Q15 cells when they were grown in the presence of hydrocarbon substrates. Inclusions are a common feature in bacteria grown on hydrocarbons, and similar structures have been observed in a variety of gram-negative and gram-positive bacteria, including rhodococci and other actinomycetes (1, 35), and are believed to be hydrophobic storage compounds (35, 37).
The presence of EPS in diesel fuel- and hexadecane-grown cells was anticipated because the cells formed flocs during growth on these hydrocarbons. Patches or clusters of finely contrasted EPS were observed in cells of strain Q15 grown on diesel fuel. The cell envelope and cytoplasm of strain Q15 were stained well by the ruthenium red concentration used in our studies, but the method used resulted in low contrast for the EPS. The presence of EPS was not anticipated in strain Q15 cells grown on glucose-acetate because the cells did not aggregate during growth. However, glucose is known to stimulate the production of capsules in many bacteria, and encapsulated bacteria do not usually aggregate. The delicate web of fibers surrounding strain Q15 cells grown on glucose-acetate is consistent with images of capsules of other bacteria stabilized only by ruthenium red (4, 26). The morphological differences between the EPS of glucose-acetate- and diesel fuel-grown cells may reflect a difference in the chemistry of the exopolymers. This idea is supported by CSLM observations which revealed that significant changes to or adaptations of the EPS of strain Q15 had occurred during growth on diesel fuel at 5°C, including the appearance of two specific glycoconjugates found in this bacterium’s EPS under these growth conditions.
The ability of strain Q15 to adhere to both solid (octacosane) and liquid (diesel fuel) hydrocarbon substrates is especially intriguing and may be the key mechanism by which this organism takes in and assimilates alkane substrates at low temperatures. The adhesion observed may be related to the increased cell surface hydrophobicity of strain Q15 cells observed during growth on hydrocarbons, as determined by the MATH test performed with pelleted cells. The observation that strain Q15 cells completely surrounded hydrophobic diesel fuel microdroplets strongly indicates that the strain Q15 cell surface is hydrophobic rather than hydrophilic. In addition, CSLM images of strain Q15 cells grown at 5°C on diesel fuel and stained with the hydrophobic stain Nile Red appeared to bind to a greater extent to the surfaces of diesel fuel-grown cells than to the surfaces of glucose-acetate-grown cells, indicating that there was an increase in cell surface hydrophobicity and/or lipid content in the diesel fuel-grown cells (data not shown). The presence and chain lengths of mycolic acids in the cell envelopes of a variety of coryneform bacteria, including Rhodococcus strains, were related to the increased cell surface hydrophobicities of these organisms (5, 29).
The adhesion process may also be related to changes in or adaptations to the cell surface of strain Q15 when the organism is grown on hydrocarbons at 5°C. The ability of an alkane-degrading Acinetobacter sp. to adhere to fuel oil droplets was attributed to the production of EPS, which allowed the cells to anchor themselves to the surface (3). Indeed, EPS is thought to bridge the gap between the secondary minimum (reversible attachment) and the primary minimum (irreversible attachment), as described by the DLVO theory (11); in this case this allows direct contact between the surfaces (i.e., EPS) of strain Q15 cells and the hydrocarbon substrate. With close contact between the strain Q15 cell and hydrocarbon surfaces achieved, a cell surface-associated biosurfactant(s) could then solubilize the alkane substrates, facilitating cellular uptake. A cell surface-associated polysaccharide in another Rhodococcus sp. possesses biosurfactant activity and increases cell surface hydrophobicity (28). The EPS may also play a role in floc formation in strain Q15, enabling the cells to remain in close physical contact with hydrocarbons, in the same manner described previously for R. erythropolis during growth on pentadecane (40) and for alkane-degrading yeasts (37).
Membrane fatty acid analysis indicated that the counteracting effects of growth at a low temperature and growth on hydrocarbons resulted in a balanced response by Rhodococcus sp. strain Q15. This psychrotroph adapted to growth at a low temperature by decreasing the degree of saturation of the membrane lipid fatty acids in order to maintain optimal membrane fluidity, like Rhodococcus rhodochrous (39). However, the extent of this adaptation was not as great when strain Q15 was grown on aliphatic substrates, indicating that strain Q15 cells adapted or protected themselves during growth on the aliphatic substrates. The relatively greater degree of saturation (two- to fivefold) observed at 5°C when strain Q15 was grown on aliphatic substrates than when it was grown on glucose-acetate was indicative of the counteractive effects of hydrocarbon substrates on cytoplasmic membrane fatty acid composition. As the greatest relative degree of fatty acid saturation was found in strain Q15 cells at both 5 and 24°C when they were grown on diesel fuel, which is a complex mixture of long- and short-chain alkanes, cyclic hydrocarbons, and aromatic compounds, the relatively greater saturation may be a protective mechanism required by strain Q15 to limit the toxicity of the short-chain alkanes present in diesel fuel. This protective mechanism may be especially important during low-temperature growth because volatilization of short-chain alkanes (<C10) is impeded, which results in increased solubility of these compounds in the aqueous phase and, consequently, increased microbial toxicity (2, 25). Conversion of cis fatty acids to their trans forms, an additional protective mechanism to reduce hydrocarbon toxicity observed in a number of Pseudomonas spp. (8, 14, 15), does not seem to occur in Rhodococcus sp. strain Q15 as trans fatty acids were not detected or were present only at low levels in the fatty acid profiles of cells grown under the conditions tested. It should be noted that during extraction of the lipids from hexadecane- and diesel fuel-grown cells, the contents of the inclusions which were observed in these cells during TEM analysis might have been isolated as well. Consequently, the fatty acid profiles obtained may not precisely reflect the fatty acid profiles of the membrane lipids; rather, they may reflect the fatty acid profiles of the whole cells.
In conclusion, we observed numerous and complex physiological cellular responses and adaptations involved in alkane assimilation at a low temperature by Rhodococcus sp. strain Q15. These included (i) production of a cell-bound biosurfactant(s), (ii) augmentation of cell surface hydrophobicity, (iii) production of intracellular inclusions, (iv) modification of the EPS, and (v) alteration of membrane fatty acid composition. We are currently isolating and characterizing the EPS of strain Q15 in order to better understand its role in hydrocarbon utilization at low temperatures.
ACKNOWLEDGMENTS
The SEM and TEM expertise of Dale Weber of the University of Waterloo and Judy Sholdice of the University of Western Ontario was greatly appreciated.
Footnotes
Publication 41842 of the National Research Council Canada.
REFERENCES
- 1.Alvarez H M, Mayer F, Fabritius D, Steinbüchel A. Formation of intracytoplasmic lipid inclusions by Rhodococcus opacus strain PD630. Arch Microbiol. 1996;165:377–386. doi: 10.1007/s002030050341. [DOI] [PubMed] [Google Scholar]
- 2.Atlas R M. Microbial degradation of petroleum hydrocarbons: an environmental perspective. Microbiol Rev. 1981;45:180–209. doi: 10.1128/mr.45.1.180-209.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldi F, Ivošević N, Minacci A, Pepi M, Fani R, Svetličić V, Žutić V. Adhesion of Acinetobacter venetianus to diesel fuel droplets studied with in situ electrochemical and molecular probes. Appl Environ Microbiol. 1999;65:2041–2048. doi: 10.1128/aem.65.5.2041-2048.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bayer M E, Thurow H. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J Bacteriol. 1977;130:911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendinger B, Rijnaarts H H M, Altendorf K, Zehnder A B. Physiochemical cell surface and adhesive properties of coryneform bacteria related to the presence of chain length of mycolic acids. Appl Environ Microbiol. 1993;59:3973–3977. doi: 10.1128/aem.59.11.3973-3977.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 7.Britton L N. Microbial degradation of aliphatic hydrocarbons. In: Gibson D T, editor. Microbial degradation of organic compounds. New York, N.Y: Marcel Dekker, Inc.; 1984. pp. 89–129. [Google Scholar]
- 8.Chen Q, Janssen D B, Witholt B. Growth on octane alters the membrane lipid fatty acids of Pseudomonas oleovorans due to the induction of alkB and synthesis of octanol. J Bacteriol. 1995;177:6894–6901. doi: 10.1128/jb.177.23.6894-6901.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper D G, Goldenberg B G. Surface active agents from two Bacillus species. Appl Environ Microbiol. 1987;53:224–229. doi: 10.1128/aem.53.2.224-229.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai J D, Banat I M. Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev. 1997;61:47–64. doi: 10.1128/mmbr.61.1.47-64.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert P, Evans D J, Brown M R W. Formation and dispersal of bacterial biofilms in vivo and in situ. J Appl Bacteriol Symp Suppl. 1993;74:67S–78S. doi: 10.1111/j.1365-2672.1993.tb04343.x. [DOI] [PubMed] [Google Scholar]
- 12.Greer C W, Hawari J, Samson R. Influence of environmental factors on 2,4-dichlorophenoxyacetic acid degradation by Pseudomonas cepacia isolated from peat. Arch Microbiol. 1990;154:317–322. doi: 10.1007/BF00276525. [DOI] [PubMed] [Google Scholar]
- 13.Grogan D W, Cronan J E. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol Mol Biol Rev. 1997;61:429–441. doi: 10.1128/mmbr.61.4.429-441.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heipieper H J, Diefenbach R, Keweloh H. Conversion of cis unsaturated fatty acids to trans, a possible mechanism for the protection of phenol-degrading Pseudomonas putida P8 from substrate toxicity. Appl Environ Microbiol. 1992;58:1847–1852. doi: 10.1128/aem.58.6.1847-1852.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heipieper H J, de Bont J A M. Adaptation of Pseudomonas putida S12 to ethanol and toluene at the level of fatty acid composition of membranes. Appl Environ Microbiol. 1994;60:4440–4444. doi: 10.1128/aem.60.12.4440-4444.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbert R A. The ecology and physiology of psychrophilic microorganisms. In: Herbert R A, Codd G A, editors. Microbes in extreme environments. New York, N.Y: Academic Press; 1986. pp. 1–23. [Google Scholar]
- 17.Hommel R K. Formation and physiological role of biosurfactants produced by hydrocarbon-utilizing microorganisms. Biodegradation. 1990;1:107–119. doi: 10.1007/BF00058830. [DOI] [PubMed] [Google Scholar]
- 18.Inniss W E, Mayfield C I. Growth rates of psychrotrophic sediment microorganisms. Water Res. 1978;12:231–236. [Google Scholar]
- 19.Kretschmer A, Bock H, Wagner F. Chemical and physical characterization of interfacial-active lipids from Rhodococcus erythropolis grown on n-alkane. Appl Environ Microbiol. 1982;44:864–870. doi: 10.1128/aem.44.4.864-870.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamont H C, Silvester W B, Torrey J G. Nile red fluorescence demonstrates lipid in the envelope of vesicles from N2-fixing cultures of Frankia. Can J Microbiol. 1987;34:656–660. [Google Scholar]
- 21.Lawrence J R, Korber D R, Hoyle B D, Costerton J W, Caldwell D E. Optical sectioning of microbial biofilms. J Bacteriol. 1991;173:6558–6567. doi: 10.1128/jb.173.20.6558-6567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrence J R, Korber D R, Wolfaardt G M, Caldwell D E. Analytical imaging and microscopy techniques. In: Hurst C J, Knudsen G R, McInerney M, Stetzenbach L D, Walter M V, editors. Manual of environmental microbiology. Washington, D.C: American Society for Microbiology Press; 1996. pp. 29–51. [Google Scholar]
- 23.Lawrence J R, Wolfaardt G M, Neu T R. The study of biofilms using confocal laser scanning microscopy. In: Wilkinson M H F, Schut F, editors. Digital image analysis of microbes. Imaging, morphometry, fluorometry and motility techniques and applications. Sussex, United Kingdom: John Wiley and Sons Ltd.; 1997. pp. 431–446. [Google Scholar]
- 24.Lawrence J R, Neu T R, Swerhone G D W. Application of multiple parameter imaging for the quantification of algal, bacterial and exopolymer components of microbial biofilms. J Microbiol Methods. 1998;32:253–261. [Google Scholar]
- 25.Leahy J G, Colwell R R. Microbial degradation of hydrocarbons in the environment. Microbiol Rev. 1990;54:305–315. doi: 10.1128/mr.54.3.305-315.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mataftschier E A. Exostructure of Rhizobium meliloti. FEMS Microbiol Lett. 1982;13:171–175. [Google Scholar]
- 27.Morrison W R, Smith L M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J Lipid Res. 1964;5:600–608. [PubMed] [Google Scholar]
- 28.Neu T R, Poralla K. An amphiphilic polysaccharide from an adhesive Rhodococcus strain. FEMS Microbiol Lett. 1988;49:389–392. [Google Scholar]
- 29.Neu T R. Significance of bacterial surface-active compounds in interaction of bacteria with interfaces. Microbiol Rev. 1996;60:151–166. doi: 10.1128/mr.60.1.151-166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rapp P, Bock H, Wray V, Wagner F. Formation, isolation and characterization of trehalose dimycolates from Rhodococcus erythropolis grown on n-alkanes. J Gen Microbiol. 1979;115:491–503. [Google Scholar]
- 31.Ristau E, Wagner F. Formation of novel anionic trehalose-tetraesters from Rhodococcus erythropolis under growth limiting conditions. Biotechnol Lett. 1983;5:95–100. [Google Scholar]
- 32.Rosenberg M, Gutnick D, Rosenberg E. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett. 1980;9:29–33. [Google Scholar]
- 33.Rosenberg M, Rosenberg E. Role of adherence in growth of Acinetobacter calcoaceticus RAG-1 on hexadecane. J Bacteriol. 1981;148:51–57. doi: 10.1128/jb.148.1.51-57.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenberg M. Basic and applied aspects of microbial adhesion at the hydrocarbon:water interface. Crit Rev Microbiol. 1991;18:159–173. doi: 10.3109/10408419109113512. [DOI] [PubMed] [Google Scholar]
- 35.Scott C C L, Finnerty W R. A comparative analysis of the ultrastructure of hydrocarbon oxidizing micro-organisms. J Gen Microbiol. 1976;94:342–350. doi: 10.1099/00221287-94-2-342. [DOI] [PubMed] [Google Scholar]
- 36.Sikkema J, De Bont J A M, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singer M E, Finnerty W R. Microbial metabolism of straight-chain and branched alkanes. In: Atlas R M, editor. Petroleum microbiology. New York, N.Y: Macmillan Publishing Co.; 1984. pp. 1–59. [Google Scholar]
- 38.Sutcliffe I C. Macroamphiphilic cell envelope components of Rhodococcus equi and closely related bacteria. Vet Microbiol. 1997;56:297–299. doi: 10.1016/s0378-1135(97)00097-7. [DOI] [PubMed] [Google Scholar]
- 39.Takaichi S, Ishidsu J. Influence of growth temperature on compositions of carotenoids and fatty acids from carotenoid glucoside ester and from cellular lipids in Rhodococcus rhodochrous RNMS1. Biosci Biotechnol Biochem. 1993;57:1886–1889. [Google Scholar]
- 40.Takeda M, Kurane R, Nakamura I. Localization of a biopolymer produced by Rhodococcus erythropolis grown on n-pentadecane. Agric Biol Chem. 1991;55:2665–2666. [Google Scholar]
- 41.Thomas J M, Yordy J R, Amador J A, Alexander M. Rates of dissolution and biodegradation of water-insoluble organic compounds. Appl Environ Microbiol. 1986;52:290–296. doi: 10.1128/aem.52.2.290-296.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volkering F, Breure A M, Rulkens W H. Microbiological aspects of surfactant use for biological soil remediation. Biodegradation. 1998;8:410–417. doi: 10.1023/a:1008291130109. [DOI] [PubMed] [Google Scholar]
- 43.Watkinson R, Morgan P. Physiology of aliphatic hydrocarbon-degrading microorganisms. Biodegradation. 1990;1:79–92. doi: 10.1007/BF00058828. [DOI] [PubMed] [Google Scholar]
- 44.Watkinson R J. Interaction of microorganisms with hydrocarbons. In: Harrison D E F, Higgins I J, Watkinson R J, editors. Hydrocarbons in biotechnology. London, United Kingdom: Heyden; 1980. pp. 11–24. [Google Scholar]
- 45.Whyte L G, Greer C W, Inniss W E. Assessment of the biodegradation potential of psychrotrophic microorganisms. Can J Microbiol. 1996;42:99–106. doi: 10.1139/m96-016. [DOI] [PubMed] [Google Scholar]
- 46.Whyte L G, Bourbonnière L, Greer C W. Biodegradation of petroleum hydrocarbons by psychrotrophic Pseudomonas strains possessing both alkane (alk) and naphthalene (nah) catabolic pathways. Appl Environ Microbiol. 1997;63:3719–3723. doi: 10.1128/aem.63.9.3719-3723.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whyte L G, Hawari J, Zhou E, Bourbonniére L, Inniss W E, Greer C W. Biodegradation of variable-chain-length alkanes at low temperatures by a psychrotrophic Rhodococcus sp. Appl Environ Microbiol. 1998;64:2578–2584. doi: 10.1128/aem.64.7.2578-2584.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolfaardt G M, Lawrence J R, Headley J V, Robarts R D, Caldwell D E. Microbiol exopolymers provide a mechanism for bioaccumulation of contaminants. Microb Ecol. 1994;27:279–291. doi: 10.1007/BF00182411. [DOI] [PubMed] [Google Scholar]
- 49.Wolfaardt G M, Lawrence J R, Robarts R D, Caldwell D E. In situ characterization of biofilm exopolymers involved in the accumulation of chlorinated organics. Microb Ecol. 1998;35:213–223. doi: 10.1007/s002489900077. [DOI] [PubMed] [Google Scholar]