Abstract
This is a retrospective and observational study on 1511 patients with SARS-CoV-2, who were diagnosed with COVID-19 by real-time PCR testing and hospitalized due to COVID-19 pneumonia. 1511 patients, 879 male (58.17%) and 632 female (41.83%) with a mean age of 60.1 ± 14.7 were included in the study. Survivors and non-survivors groups were statistically compared with respect to survival, discharge, ICU admission and in-hospital death. Although gender was not statistically significant different between two groups, 80 (60.15%) of the patients who died were male. Mean age was 72.8 ± 11.8 in non-survivors vs. 59.9 ± 14.7 in survivors (p < 0.001). Overall in-hospital mortality was found to be 8.8% (133/1511 cases), and overall ICU admission was 10.85% (164/1511 cases). The PSI/PORT score of the non-survivors group was higher than that of the survivors group (144.38 ± 28.64 versus 67.17 ± 25.63, p < 0.001). The PSI/PORT yielding the highest performance was the best predictor for in-hospital mortality, since it incorporates the factors as advanced age and comorbidity (AUROC 0.971; % 95 CI 0.961–0.981). The use of A-DROP may also be preferred as an easier alternative to PSI/PORT, which is a time-consuming evaluation although it is more comprehensive.
Keywords: COVID-19 pneumonia, prediction, prognosis, severity, mortality, risk scores
1. Introduction
Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2), known as the “COVID-19 pandemic”, is globally associated with high mortality. SARS-CoV-2 has infected over 364,191,457 million people in the world, causing over 5,631,457 million casualties [1]. COVID-19 usually exhibits mild or moderate (81%) clinical appearance, even though 14% of cases are severe and 5% are critical. According to the Chinese Center for Disease Control and Prevention report, including 72,314 COVID-19 cases, the overall mortality rate was reported as 2.3%. This rate was 14.8% for patients older than 80 years, and 49% in critical cases [2].
COVID-19 is a multisystemic viral disease which may show varying clinical presentations such as asymptomatic disorders, injuries associated with organs as liver, heart, kidney, neurological manifestatations, coagulopathy, sepsis, septic shock and multiple organ dysfunction [3,4,5]. Among the above mentioned problems, especially acute respiratory failure, adult respiratory distress syndrome (ARDS) and/or multiorgan failure are known as the major complications of COVID-19, leading to death. In critical cases, rare complications such as cytokine storm and macrophage activation syndrome may also be observed [6,7].
As our knowledge of COVID-19 has evolved, certain clinical predictors leading to poor prognosis have been identified. These well-established risk factors for severe COVID-19 are: comorbidites related to old age, hypertension, cardiovascular diseases, diabetes, obesity, chronic lung disease (especially chronic obstructive and interstitial lung diseases), immunocompromised state, end-stage renal disease, liver disease and malignancy [8,9,10]. It is also reported that lymphopenia, increased levels of ferritin, d-dimer, troponin I, lactate dehydrogenase (LDH) and intereukin-6 were associated with poor prognosis and higher mortality [7,8,11,12,13,14].
As there is still an uncertainty about the progress of COVID-19 disease, it becomes increasingly important to develop clinical risk classification tools to identify the patients under risk, to prognose their clinical progress and to predict their mortality rate [15]. Common prognostic scales, widely used in community-acquired pneumonia (CAP) and sepsis as proven disease severity scoring systems in order to predict the disease consequences, are also referred to in COVID-19 [16,17,18]. On the other hand, novel models specifically designed for COVID-19 are also being developed [19,20,21,22,23].
Previously published studies did not show differentiating factors among patients with MERS and those without MERS [24]. On the other hand, Rainer et al. demonstrated that scoring systems might help identify patients who should receive more specific tests for influenza or SARS [25]. Nowadays, CAP severity indices which are known to be significantly associated with mortality are examined almost in all pneumonia cases. As an example, a multi-center prospective study showed that during a H1N1 virus pandemic severity of pneumonia was identified by PSI/PORT score [26]. When the utility of severity indices in viral pneumonia were examined, PSI/PORT was an important indicator for assessing the prognosis of patients suffering from CAP in which the causative agent to be a respiratory virus or not [27]. Several studies showed that CURB-65 score was a useful tool in influenza, non-influenza, bacterial and mixed viral-bacterial agents in CAP [28,29].
Pneumonia Severity Index (PSI/PORT) [30], A-DROP (Age, dehydration, oxygen saturation, orientation, blood pressure) [31], National Early Warning Score 2 (NEWS2) [32], Modified Early Warning Score (MEWS) [33], CURB-65 (confusion, urea, respiratory rate, blood pressure, age) [34], expanded CURB-65 (hypoalbuminemia, LDH, thrombocytopenia, confusion, urea, respiratory rate, blood pressure, age) [35] and the quick Sequential (Sepsis-Related) Organ Failure Assessment (qSOFA) [36] are some of the other scoring systems used in COVID-19 cases as well. However, their performance on prognosis and mortality prediction is not presented in sufficent detail [17,37,38,39,40,41,42,43].
The quick COVID-19 Severity Index (qCSI: mental status, respiratory rate and systolic blood pressure) [19] and ISARIC 4C Mortality score (4C Mortality score) [20] are specifically developed for COVID-19 as novel risk assessment tools.
Scoring systems can be used for early identification of high-risk patients, accurate assessment of disease severity, prediction of disease progress and determination of patient specific treatment approach [16,18,44,45].
In this retrospective study, we aimed to identify simple, useful and accurate scoring systems by studying the conventional methods used for community acquired pneumonia (CAP) and sepsis, and those specifically used for COVID-19 such as qCSI and 4C mortality.
2. Materials and Methods
2.1. Study Design and Participants
This retrospective observational study was carried out on 1909 patients with SARS-CoV-2, who were diagnosed with COVID-19 pneumonia by real-time PCR testing and hospitalized at Prof. Dr. Murat Dilmener Emergency Hospital, pandemic 3rd level, in Istanbul, from 1 September 2020 to 31 December 2020. The diagnosis of COVID-19 was determined on the basis of World Health Organization (WHO) guidelines [46]. All cases enrolled in the study were over the age of 18, and none of them had been taken to the intensive care unit (ICU). They were managed in accordance with the COVID-19 treatment protocol of the Turkish Health Ministry [47]. The research was approved by the ethics committee of the University of Health Sciences, Bakırköy Dr. Sadi Konuk Training and Research Hospital (approval number 2021/91), and was conducted following the principles of the Declaration of Helsinki.
Retrospectively, medical records were obtained from the hospital electronic database. All the parameters relevant for scoring the disease severity were measured and recorded. A standardized form was used for data collection, which included demographics, past medical history, underlying chronic diseases, vital signs, the severity of admission, laboratory findings and chest computed tomography (CT) scans results. In addition, definitive outcomes (death, discharge or ICU admission) were obtained from the hospital information system.
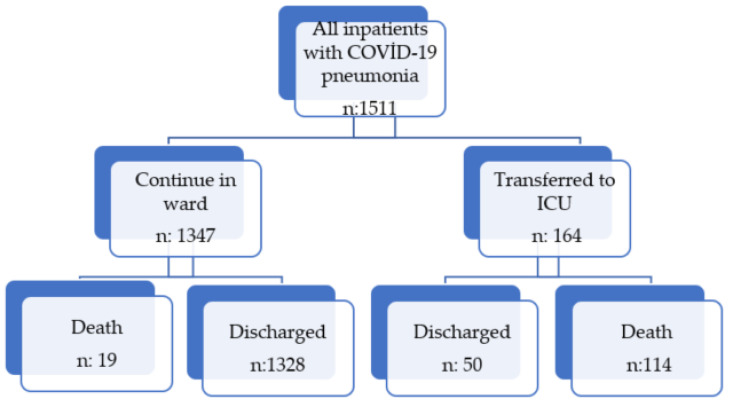
Patients with COVID-19 were categorized into two groups (non-severe and severe illness) according to National Institutes of Health (NIH) classification based on disease severity [48]. Severe cases were defined as having at least any one of the following criteria: respiratory distress (>30 breaths/min); oxygen saturation < 94% at rest; arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2 < 300 mmHg), or lung infiltrates > 50%. Radiologist-evaluated chest CT scans were classifed into three categories, mild, moderate and severe involvement [49]. Patients with no radiological involvement were excluded in our study. Only moderate and severe cases were included in our study. Patients who had incomplete data or those who had pneumonia arising from other pathogens were all excluded from the study. Another criterion for exclusion was pregnancy. After the exclusion, 1511 patients (older than 18 years) who were hospitalized with the diagnosis of COVID-19 pneumonia were included in the study. The patient flowchart can be seen in Figure 1.
Figure 1.
Flow chart of study population.
2.2. Scores Selections and Definitions
The scores on hospitalization of each patient were calculated for nine severity scoring rules, including PSI/PORT, A-DROP, NEWS-2, MEWS, CURB-65, Expanded CURB-65, qSOFA, qCSI, and 4C Mortality as defined previously in the literature [19,20,30,31,32,33,34,35,36]. All the components of each scoring system were accurately registered in the medical records.
2.3. Statistical Analysis
All statistical analyses were performed in commercially available SPSS software v.22 (Statistical Package for the Social Sciences Inc., Chicago, IL, USA). Patient characteristics were summarized using descriptive statistics (mean, standard deviation, median, frequency, percentages, minimum, maximum, Q1–Q3). The conformity of the quantitative variables to the normal distribution was tested with the Shapiro-Wilk test and graphical examinations. Student t-test was used for the comparison of normally distributed quantitative variables between two groups, and the Mann-Whitney U test was used for comparisons between two groups of non-normally distributed quantitative variables. Categorical variables were compared using the Chi-square test and Fisher’s exact test. Diagnostic screening tests (sensitivity, specificity, positive predictive value and negative predictive value) and ROC (area under the curve of the receiver operating characteristic) analysis were used to determine the cutoff value for all nine scoring systems. ROC Curve analysis and Binomial exact test were used to determine in-hospital mortality prediction. Multivariate logistic regression analysis was performed to identify other risk factors on mortality. A p-value < 0.05 was accepted as statistically significant.
3. Results
3.1. Comparison of Basic Clinical Characteristics between the Two Groups
1511 patients, 879 male (58.17%) and 632 female (41.83%) with a mean age of 60.1 ± 14.7, were included in the study. Although gender difference was not statistically significant, 80 (60.15%) of the patients who died were male. Mean age was 72.8 ± 11.8 in non-survivors vs. 59.9 ± 14.7 in survivors (p < 0.001). 386 patients (25.54%) had only a single comorbidity, while 693 (45.86%) had two or more. The most common comorbidity was hypertension (48.04%, 726/1511), followed by diabetes mellitus (33.28%, 503/1511) and coronary artery disease (14.36%, 217/1511). The number of comorbidities was higher in patients who died (78.19% vs. 42.74%, p < 0.001). Hypertension, coronary artery disease, atrial fibrillation (AF), congestive heart failure and cerebrovascular disease were significantly more common in non-survivors than in survivors (p < 0.001). We observed a difference between cohorts in terms of malignancy and chronic kidney diasease (p = 0.01), while no such difference existed for diabetes, dyslipidemia, COPD and asthma. Ultimately, the non-survivor group was older and had more comorbidities than the survivor group (p < 0.001 for both). Respiratory rate, oxygen saturation by pulse oximetry (SpO2) under oxygen support, supplemental oxygen requirement and heart rate were significantly higher in non-survivors.
General information and baseline characteristics of the patients are shown in Table 1. Laboratory findings, CT scores, disease severity status and outcomes of the patients are given in Table 2.
Table 1.
The baseline characteristics of the patients.
| Non-Survivor Group (n: 133) |
Survivors (n: 1378) |
p | |
|---|---|---|---|
| Age, years | 72.8 ± 11.8 | 59.9 ± 14.7 | <0.001 |
| Male sex, n (%) 879 (58.17) |
80 (60.15) | 799 (58) | NS |
| Comorbidity no, % | <0.001 | ||
| 0 | 12 (9) | 420 (30.47) | |
| 1 (386, 25.54) | 17 (12.78) | 369 (26.77) | |
| ≥2 (693, 45.86) | 104 (78.19) | 589 (42.74) | |
| Comorbidities, n (%) | |||
| Hypertension, 726 (48.04) | 92 (69.6) | 634 (46.2) | <0.001 |
| Diabetes, 503 (33.28) | 52 (39.3) | 451(32.9) | NS |
| Coronary artery disease, 217 (14.36) | 41 (31) | 176 (12.8) | <0.001 |
| Atrial fibrillation, 85 (5.62) | 25 (18.79) | 60 (4.35) | <0.001 |
| Congestive heart failure, 91 (6.02) | 22 (16.54) | 69 (5) | <0.001 |
| Dyslipidemia, 73 (4.83) | 8 (6.01) | 65 (4.71) | NS |
| Cerebrovascular disease, 53 (3.5) | 14 (10.6) | 39 (2.8) | <0.001 |
| Chronic obstructive pulmonary disease, 62 (4.1) |
9 (6.8) | 53 (3.8) | NS |
| Asthma, 135 (8.93) | 12 (9) | 123 (8.9) | NS |
| Malignancy, 75 (4.96) | 13 (9.77) | 62 (4.49) | 0.01 |
| Chronic kidney disease, 67 (4.43) | 12 (9) | 55 (4) | 0.01 |
| Physical findings | |||
| Body temperature, °C | 36.95 ± 0.64 | 36.91 ± 0.67 | NS |
| Respiratory rate, per minute | 30.26 ± 4.64 | 20.08 ± 4.34 | <0.001 |
| SpO2, under oxygen support, mean | 92.95 ± 2.19 | 94.46 ± 1.90 | <0.001 |
| O2 support, L/per min | 15.77 ± 9.94 | 3.86 ± 5.88 | <0.001 |
| Systolic blood pressure, mmHg | 129.50 ± 22.28 | 126.51 ± 18.23 | NS |
| Diastolic blood pressure, mmHg | 70.14 ± 12.29 | 70.69 ± 10.28 | NS |
| Heart rate, per minute | 86.18 ± 20.58 | 82.68 ± 14.23 | 0.01 |
Table 2.
Laboratory findings, CT scores, disease severity status and outcomes of the patients.
| Non-Survivor Group (n: 133) |
Survivors (n: 1378) |
p | |
|---|---|---|---|
| Laboratory findings | |||
| Neutrophil count, cells/mL | 6.87 ± 3.76 | 5.40 ± 2.89 | <0.001 |
| Lymphocytes count, cells/mL | 0.83 ± 0.56 | 1.21 ± 0.58 | <0.001 |
| N/L ratio (neutrophil/lymphocytes) |
11.52 ± 9.99 | 5.74 ± 5.05 | <0.001 |
| Platelet count, 103/mm3 | 215.30 ± 103.60 | 250.74 ± 105.14 | <0.001 |
| Hematocrit, % | 36.70 ± 5.58 | 37.52 ± 4.72 | NS |
| Glucose, mg/dL | 171.91 ± 73.92 | 151.46 ± 71.50 | 0.003 |
| Urea, mg/dL | 71.80 ± 48.31 | 39.94 ± 25.62 | <0.001 |
| Creatinine, mg/dL | 1.43 ± 1.54 | 0.94 ± 0.80 | <0.001 |
| Alanine transaminase, ALT, U/L | 35.17 ± 30.87 | 43.66 ± 39.94 | 0.01 |
| Aspartate aminotransferase, AST, U/L | 49.42 ± 35.30 | 42.82 ± 30.90 | 0.04 |
| Lactate dehydrogenase, LDH, U/L | 480.73 ± 226. 37 | 344.84 ± 155.73 | <0.001 |
| Potassium, mEq/L | 4.27 ± 0.60 | 4.22 ± 0.51 | NS |
| Sodium, mEq/L | 136.83 ± 5.70 | 137.19 ± 3.81 | NS |
| C-reactive protein, mg/L | 145.04 ± 78.54 | 101.63 ± 77.11 | <0.001 |
| Procalcitonin, ng/mL | 0.79 ± 2.28 | 0.69 ± 7.87 | NS |
| Ferritin, (µg/L) | 770.39 ± 693.89 | 502.60 ± 562.91 | <0.001 |
| D-dimer, (µg FEU/mL) | 1.32 ± 1.33 | 0.84 ± 1.21 | <0.001 |
| Fibrinogen, mg/dL | 545.66 ± 146.22 | 511.43 ± 134.19 | 0.01 |
| International normalized ratio, INR | 1.16 ± 0.29 | 1.06 ± 0.20 | <0.001 |
| Troponin I, ng/mL | 123.63 ± 449.44 | 18.49 ± 119.07 | <0.001 |
| Albumin, g/dL | 32.78 ± 5.10 | 35.93 ± 5.23 | <0.001 |
| Disease Severity Status n (%) | <0.001 | ||
| Moderate, 596 (39.44) | 3 (2.25) | 593(43.03) | |
| Severe, 915 (60.56) | 130 (97.75) | 785 (56.97) | |
| CT involvement n, (%) | <0.001 | ||
| Mild, 329 (21.77) | 13 (9.77) | 316 (22.93) | |
| Moderate, 727 (48.11) | 44 (33.8) | 683 (49.56) | |
| Severe, 455 (30.11) | 76 (56.43) | 379 (27.50) | |
| Outcomes, n (%) | |||
| Hospital length of stay, days | 14.52 ± 8.78 | 11.27 ± 6.50 | <0.001 |
| Admission to ICU, 164 (10.85) | 114 (85.71) | 50 (3.62) | <0.001 |
3.2. Comparison of Laboratory Tests and Chest CT Scans between the Two Groups
Patients had decreased lymphocyte, platelet, albumine and elevated neutrophil count, N/L ratio, C-reactive protein (CRP), urea, creatinine, LDH, ferritin, D-dimer, INR, troponin I levels in non-survivors (p < 0.001). In addition to that, glucose, ALT, AST and fibrinogen levels were higher in non-survivors (p values, respectively 0.003, 0.01, 0.04 and 0.01). There were no significant differences in procalcitonin and hematocrit levels between two groups. Concurrently, deceased patients had significantly higher disease severity status and chest CT scores than the survivors (p < 0.001). Additionally, 97.75% (130/133) of deceased patients had severe disease status.
3.3. Score Distribution
The PSI/PORT score of the non-survivor group was higher than that of the survivor group (144.38 ± 28.64 versus 67.17 ± 25.63, p < 0.001). Similarly, all the other eight scores were also found to be higher in non-survivors than survivors (p < 0.001). As seen in Table 3, prognostic scores were higher in deceased patients.
Table 3.
Prognostic scores of patients.
| Score, Mean ± SD | Non-Survivor Group | Survivors | p | |
|---|---|---|---|---|
| CURB-65 | Mean ± SD | 2.33 ± 1.05 | 0.75 ± 0.84 | <0.001 |
| Median (Q1–Q3) | 2 (2–3) | 1 (0–1) | ||
| Expanded CURB-65 | Mean ± SD | 4.18 ± 1.36 | 2.34 ± 1.85 | <0.001 |
| Median (Min–Max) | 4 (1–7) | 2 (0–6) | ||
| A-DROP | Mean ± SD | 2.56 ± 0.94 | 0.57 ± 0.78 | <0.001 |
| Median (Q1–Q3) | 3 (2–3) | 0 (0–1) | ||
| qSOFA | Mean ± SD | 1.41 ± 0.61 | 0.52 ± 0.61 | <0.001 |
| Median (Q1–Q3) | 1 (1–2) | 0 (0–1) | ||
| qCSI | Mean ± SD | 7.02 ± 2.03 | 2.75 ± 2.90 | <0.001 |
| Median (Q1–Q3) | 7 (6–9) | 2 (0–5) | ||
| PSI/PORT | Mean ± SD | 144.38 ± 28.64 | 67.17 ± 25.63 | <0.001 |
| Median (Q1–Q3) | 145 (124–168) | 62 (49–81) | ||
| NEWS2 | Mean ± SD | 8.29 ± 2.21 | 3.92 ± 2.71 | <0.001 |
| Median (Q1–Q3) | 8 (7–10) | 4 (2–6) | ||
| MEWS | Mean ± SD | 3.44 ± 1.20 | 1.69 ± 1.05 | <0.001 |
| Median (Q1–Q3) | 3 (3–4) | 2 (1–2) | ||
| 4C Mortality | Mean ± SD | 13.96 ± 3.45 | 7.77 ± 3.99 | <0.001 |
| Median (Min–Max) | 14 (3–20) | 8 (0–21) |
When the mortality rates were investigated in terms of pneumonia scores, the mortality rate in the high-risk group was found to be 6.88% (104 cases) for those having a NEWS2 score of 7 or higher. The mortality rate reduced to 6.22% (94 cases) for patients having Expanded CURB-65 scores of 4 or higher, and to 5.69% (86 cases) for those classified as PSI/PORT Class V. When the same analysis was performed in low-risk groups, there was no mortality among all 872 patients categorized as Class II with regard to PSI/PORT, and for patients having 4C Mortality score between 0 and 3, only a single mortality was observed.
ICU admissions were 8% (122 cases) in NEWS2 (≥7 points), 5.75% (87 cases) in PSI/PORT Class V and 0.13% (2 cases) in 4C Mortality (0–3 points). Prediction scores distributions for mortality and ICU admission are given in Table 4.
Table 4.
The rates of in-hospital fatality and ICU admission across risk groups based on scores.
| Risk Scores | No of Patients n (%) |
Death n (%) |
ICU Admission n (%) |
Death in ICU n (%) |
|---|---|---|---|---|
| CURB-65 | ||||
| 0–1 | 1114 (73.72) | 26 (1.72) | 53 (3.5) | 21 (1.38) |
| ≥2 | 397 (26.27) | 107 (7) | 111 (7.34) | 93 (6.16) |
| ≥3 | 100 (6.61) | 65 (4.3) | 66 (4.36) | 60 (3.97) |
| 4 | 17 (1.12) | 15 (0.99) | 15 (0.99) | 14 (0.92) |
| EXPANDED CURB-65 | ||||
| 0–1 | 402 (26.6) | 4 (0.26) | 8 (0.52) | 4 (0.26) |
| ≥2 | 1109 (73.09) | 129 (8.53) | 156 (10.32) | 110 (7.27) |
| ≥3 | 689 (45.59) | 118 (7.8) | 138 (9.13) | 102 (6.75) |
| ≥4 | 338 (22.36) | 94 (6.22) | 102 (6.75) | 81 (5.36) |
| A-DROP | ||||
| 0–1 | 1208 (79.94) | 21 (1.38) | 54 (3.57) | 20 (1.32) |
| ≥2 | 303 (20.05) | 112 (7.41) | 110 (7.27) | 94 (6.22) |
| ≥3 | 103 (6.81) | 75 (4.96) | 70 (4.63) | 65 (4.3) |
| PSI/PORT | ||||
| ≤70 (CLASS II) | 872 (57.71) | 0 | 8 (0.53) | 0 |
| 71–90 (CLASS III) | 282 (18.66) | 5 (0.33) | 12 (0.8) | 2 (0.13) |
| 91–130 (CLASS IV) | 242 (16) | 42 (2.77) | 57 (3.77) | 36 (2.38) |
| >130 (CLASS V) | 115 (7.61) | 86 (5.69) | 87 (5.75) | 76 (5) |
| ≥107 | 341 (22.56) | 122 (8.07) | 132 (8.73) | 108 (7.14) |
| MEWS | ||||
| 0–2 | 1175 (77.76) | 25 (1.65) | 42 (2.77) | 17 (1.12) |
| 3 | 336 (22.23) | 108 (7.14) | 122 (8.07) | 97 (6.41) |
| 3–4 | 289 (19.12) | 84 (5.55) | 95 (6.28) | 74 (4.89) |
| ≥5 | 47 (3.11) | 24 (1.58) | 27 (1.78) | 23 (1.52) |
| NEWS2 | ||||
| 0–4 | 801 (53) | 3 (0.19) | 12 (0.79) | 2 (0.13) |
| 5–6 | 339 (22.43) | 26 (1.72) | 30 (1.98) | 20 (1.32) |
| ≥6 | 547 (36.2) | 118 (7.8) | 136 (9) | 101 (6.68) |
| ≥7 | 371 (24.55) | 104 (6.88) | 122 (8) | 92 (6) |
| qCSI | ||||
| ≤3 | 741 (49) | 8 (0.52) | 13 (0.86) | 4 (0.26) |
| 4–6 | 544 (36) | 31 (2) | 37 (2.44) | 24 (1.58) |
| ≥6 | 439 (29) | 114 (7.54) | 135 (8.93) | 103 (6.81) |
| 7–9 | 222 (14.69) | 93 (6.15) | 110 (7.27) | 85 (5.6) |
| 10–12 | 4 (0.26) | 1 (0.06) | 4 (0.26) | 1 (0.06) |
| 4C MORTALITY | ||||
| 0–3 | 223 (14.75) | 1 (0.06) | 2 (0.13) | 0 |
| 4–8 | 589 (38.98) | 8 (0.53) | 20 (1.32) | 7 (0.46) |
| 9–14 | 573 (37.92) | 49 (3.2) | 79 (5.22) | 49 (3.2) |
| ≥15 | 126 (8.33) | 66 (4.36) | 63 (4.16) | 58 (3.83) |
| ≥12 | 363 (24) | 109 (7.21) | 112 (7.41) | 94 (6.22) |
| qSOFA | ||||
| 0 | 751 (49.7) | 4 (0.26) | 14 (0.92) | 2 (0.13) |
| 1 | 629 (41.6) | 77 (5) | 96 (6.35) | 67 (4.43) |
| 2 | 117 (7.74) | 46 (3) | 47 (3.11) | 40 (82.64) |
| 3 | 14 (0.92) | 6 (0.39) | 7 (0.46) | 5 (0.33) |
Among all nine scores that PSI/PORT presented the highest discrimination (AUROC 0.971; 95% CI 0.961–0.981), followed by A-DROP (AUROC 0.929; 95% CI 0.911–0.948), NEWS2 (AUROC 0.885; 95% CI 0.860–0.909), qCSI (AUROC 0.882; 95% CI 0.853–0.911), 4C-Mortality (AUROC 0.875; 95% CI 0.845–0.906), MEWS (AUROC 0.870; 95% CI 0.842–0.898), CURB-65 (AUROC 0.859; 95% CI 0.823–0.896), Expanded CURB-65 (AUROC 0.836; 95% CI 0.800–0.873), and qSOFA (AUROC 0.818; 95% CI 0.786–0.850) in predicting in-hospital death (Table 5, Figure 2). Overall, PSI/PORT score showed higher sensitivity and specificity for in-hospital mortality than the other scores. When the optimal cut-off value of PSI/PORT was taken as 107, the sensitivity and specificity were obtained as 91.7% and 91.9%, respectively. The cut-off value for the A-DROP score, which turned out to be the second best result, was assumed to be 2 yielded a sensitivity and specificity of 84.2% and 86.1%, respectively (Table 5).
Table 5.
Discriminative accuracy of scores in predicting hospital mortality.
| Scores | AUROC (95% CI) | Std. Error | Cutoff | Se (%) | Sp (%) | PPV | NPV | p |
|---|---|---|---|---|---|---|---|---|
| PSI/PORT | 0.971 (0.961–0.981) | 0.005 | ≥107 | 91.7 | 91.9 | 52,1 | 99.1 | <0.001 |
| A-DROP | 0.929 (0.911–0.948) | 0.009 | ≥2 | 84.2 | 86.1 | 37.0 | 98.3 | <0.001 |
| NEWS2 | 0.885 (0.860–0.909) | 0.012 | ≥7 | 78.2 | 80.6 | 28.0 | 97.5 | <0.001 |
| qCSI | 0.882 (0.853–0.911) | 0.015 | ≥6 | 85.7 | 76.4 | 26.0 | 98.2 | <0.001 |
| 4C-MORTALITY | 0.875 (0.845–0.906) | 0.016 | ≥12 | 81.9 | 81.6 | 30.0 | 97.9 | <0.001 |
| MEWS | 0.870 (0.842–0.898) | 0.014 | ≥3 | 81.2 | 83.5 | 32.1 | 97.9 | <0.001 |
| CURB-65 | 0.859 (0.823–0.896) | 0.019 | ≥2 | 80.5 | 78.9 | 27.0 | 97.7 | <0.001 |
| EXPANDED CURB-65 | 0.836 (0.800–0.873) | 0.018 | ≥4 | 70.7 | 82.3 | 27.8 | 96.7 | <0.001 |
| qSOFA | 0.818 (0.786–0.850) | 0.016 | ≥1 | 97.0 | 54.2 | 17.0 | 99.5 | <0.001 |
Abbreviations: AUROC: area under the receiver operating characteristic; CI: confidence interval; std. error: standard error; Se: Sensitivity; Sp: Specificity; NPV, negative predictive value; PPV, positive predictive value.
Figure 2.
Receiver operating characteristic curves for PSI/PORT, A-DROP, NEWS2, qCSI, 4C-Mortality, MEWS, CURB-65, expanded CURB-65, and qSOFA scores for in-hospital mortality in COVID-19 pneumonia patients.
Binomial exact test has figured out PSI/PORT in predicting mortality as a superior reference, compared with A-DROP, NEWS2, qCSI, 4C-Mortality, MEWS, CURB-65, expanded CURB-65 and qSOFA (p < 0.001). Similarly taking A-DROP as a reference, A-DROP was superior compared with NEWS2 (p = 0.002), qCSI (p = 0.005), 4C-Mortality (p < 0.001), MEWS (p < 0.001), CURB-65 (p < 0.001), expanded CURB-65 (p < 0.001) and qSOFA (p < 0.001). There was no statistically significant difference between CURB-65, expanded CURB-65 and qSOFA (p > 0.005).
3.4. Outcomes in Two Groups
In our study, in-hospital mortality was found to be 8.8% (133/1511 cases), and overall ICU admission was 10.85% (164/1511 cases). While the total number of patients who died in ward and ICU was found to be 133, the remainder (1378) were discharged. 114 patients died in ICU, and 19 died while they were being treated in the ward. It was found that the mean hospitalization stay of the deceased patients was longer (11.27 vs. 14.52 days, p < 0.001), and the frequency of admission to the ICU was higher (85.71% vs. 3.62%, p < 0.001).
Mortality was associated with advanced age, presence of certain comorbidities (hypertension, coronary artery disease, AF, congestive heart failure, cerebrovascular disease), hypoxia or tachypnea on admission, higher urea, creatinine, D-dimer, troponin I, ferritine, CRP, neutrophile count, neutrophil-to-lymphocyte ratio, lower lymphocyte count, platelet count and albumine on admission.
Backward stepwise logistic regression test was performed on 32 parameters predicting mortality including age, number of comorbidities, presence of certain comorbidities (hypertension, coronary artery disease, AF, congestive heart failure, cerebrovascular disease, chronic kidney disease, malignancy), SpO2, O2 support, heart rate, neutrophil-to-lymphocyte ratio, platelet count, troponin I, D-dimer, INR, fibrinogen, ferritin, CRP, glucose, urea, creatinine, albumine, AST, ALT, LDH, disease severity status, CT score, hospital length of stay, PSI/PORT, A-DROP, NEWS2, qCSI, 4C-Mortality, MEWS, CURB-65, expanded CURB-65 and qSOFA; and the independent risk factors for mortality came out to be PSI/PORT, A-DROP, MEWS, qSOFA scores, O2 support, PLT and CRP ve LDH (Table 6).
Table 6.
Multivariate logistic regression analysis for risk factors on mortality.
| p | Odds Ratio | %95 CI | ||
|---|---|---|---|---|
| Lower | Upper | |||
| PSI/PORT (≥107) | 0.001 ** | 25.172 | 11.232 | 56.413 |
| A-DROP (≥2) | 0.001 ** | 4.686 | 2.303 | 9.532 |
| MEWS (≥3) | 0.009 ** | 2.458 | 1.255 | 4.814 |
| qSOFA (≥1) | 0.003 ** | 5.714 | 1.774 | 18.399 |
| O2 support, L/per min | 0.001 ** | 1.065 | 1.027 | 1.105 |
| Platelet count, PLT, 103/mm3 | 0.024 * | 0.997 | 0.995 | 1.000 |
| C-reactive protein, CRP, mg/L | 0.046 * | 0.996 | 0.992 | 1.000 |
| Lactate dehydrogenase, LDH, U/L | 0.002 ** | 1.003 | 1.001 | 1.004 |
| Constant | 0.001 ** | 0.001 | ||
* p < 0.05; ** p < 0.01.
In this model, PSI/PORT score ≥ 107 predicts a 25.172 times mortality (%95 CI: 11.232–56.413). A-DROP score ≥ 2 predicts a 4.686 times higher mortality (%95 CI: 2.303–9.523). MEWS score ≥ 3 predicts a mortality risk 2.458 times higher (%95 CI: 1.255–4.814). qSOFA score ≥ 1 predicts a 5.714 times higher mortality (%95 CI: 1.774–18.399). One unit lower O2 support predicts 1.065 times higher mortality (%95 CI: 1.027–1.105). Thrombocytopenia increases mortality risk by 0.997 (%95 CI: 0.995–1.000). One mg/dl CRP rise increases mortality risk by 0.996 times (%95 CI: 0.992–1.000). One unit increase in LDH increases mortality risk by 1.003 times (%95 CI: 1.001–1.004) (Table 6). PSI/PORT, A-DROP, MEWS, qSOFA scores, O2 support, PLT, CRP and LDH came out as independent risk factors predicting in-hospital mortality.
4. Discussion
We aimed to analyze the utility of the well-known CAP severity indices; CURB-65, Expanded CURB-65, PSI-PORT, NEWS2, MEWS and A-DROP, as well as qCSI 4-C Mortality and qSOFA scores introduced for COVID-19 in predicting mortality and progression to severe disease.
In our study, in-hospital mortality was found to be as 8.8% (133 cases). This result seems to be lower than those in other studies though it stands as a higher value when compared with 0.77% (cases: 11,249,216, deaths: 86,661), which comes from estimations based on national data [1,8,50].
In our study, mortality was associated with factors such as; advanced age presence of certain comorbidities (hypertension, coronary artery disease, AF, congestive heart failure, cerebrovascular disease), hypoxia or tachypnea on admission, heart rate, higher urea, creatinine, D-dimer, troponin I, ferritine, CRP, neutrophile count, neutrophil-to-lymphocyte ratio, lower lymphocyte count, platelet count and albumine on admission similar to previous studies [4,8,51,52,53,54].
Multivariable logistic regression model revealed PSI/PORT, A-DROP, MEWS, qSOFA scores, O2 support, PLT, CRP and LDH as independent predictors for mortality. This may be the result of SARS-Cov-2 infection associated with hypoxia, thrombogenesis, inflammation and organ injury in concordance with previous studies [8,13,14,51,55,56].
Although no relationship was found between diabetes and mortality, hyperglycemia was found to be more frequent in those patients who died. Similarly, the disease severity and chest CT scores were found to be related with mortality [8,52,53,57,58,59].
The higher score at admission have higher risk of ICU care and death in patients with COVID-19 pneumonia. High prognostic scores indicate worse prognosis. All prognostic scores were higher in deceased patients. The mortality in low-risk groups that were designated to manage outside the hospital was 0 in PSI/PORT Class II, 1.73% (21/1208) in A-DROP low risk and 2.33% (26/1114) in CURB-65 low-risk.
When the high-risk groups were investigated the mortality rate was observed to be as 17.35% (42/242) in PSI/PORT Class IV and 74.78% (86/115) in PSI/PORT Class V. 30-day mortality in CAP patients in PSI Class and Mortality in the Pneumonia PORT Validation Cohort group were reported to be 0.6%, 9.3% and 27% for class II, IV and V patients, respectively [30].
All nine scoring systems evaluated in this study performed well in predicting in-hospital mortality. However, PSI/PORT (AUROC 0.971; 95% CI 0,961–0,981) had the highest predicting power, followed by A-DROP (AUROC 0.929; 95% CI 0.911–0.948). PSI/PORT score had both the highest sensitivity (94%) and the specificity (90%). Our results yielded an AUROC value of 0.971 for PSI-PORT, which was higher than those obtained in other COVID-19 studies with the results of AUROC values of 0.85 (95% CI 0.81–0.88) [16], 0.85 (95% CI 0.78–0.90) [37] and 0.83 (95% CI 0.82–0.84) [60]. While expanded CURB-65 had 0.836 AUROC (95% CI 0.800–0.873) and 82.3% specificity but with the lowest sensitivity of 70.7%, qSOFA had 0.818 AUROC (95% CI 0.786–0.850) and 97% sensitivity, but with the lowest specificity of 54.2% (Table 5).
Previous studies have already shown that PSI/PORT score was demonstrated to have a strong predictive performance in CAP cases. We have obtained even a higher performance on our COVID-19 pneumonia cohort than those found on the CAP, in which PSI/PORT presented an AUROC of 0.78 (95% CI 0.73–0.82) to predict composite primary endpoint (death within 28 days by any cause, or transfer to ICU) [61] and 0.812 (95% CI 0.673–0.951) to predict 30-day mortality [62].
PSI/PORT score consists of certain parameters such as age, male gender, comorbidities, metabolic abnormalities, tachypneia and hypoxemia which are shown to have a relation with mortality in COVID-19 patients [3,4,8,9,18,19,51,63,64]. The higher performance of PSI/PORT score may be attributed to the fact that the mean age of non-survivors was 72.8 ± 11.8, or that 78.19% (104/133) of them had two or more comorbidities and lower SpO2 levels compared with survivors. PSI/PORT score may be the best predictor for mortality, since it gives extra credit for such factors as advanced age, clustered comorbidities and hypoxemia. However, PSI/PORT may have some disadvantages as not to include chronic lung diseases as COPD and asthma; only evaluating heart failure in cardiologic parameters; limiting the respiratory rate above 30 per minute; underestimating severe pneumonia in young healthy patients due to absolute age parameters; automatic classification of individuals without any comorbidities over age 50 as Class II; and the fact that clinicians may find considering 19 parameters for score quantification more time-consuming.
Previous studies have shown that the A-DROP scoring system was accurate and clinically useful for assessing the severity of both bacterial and atypical pneumonia [65,66]. Recently, Miyashita et al. reported that a high A-DROP score, indicating severe or extremely severe pneumonia, was associated with a high mechanical ventilation rate or high death rate [67].
A-DROP score is a score which is calculated by adding such parameters as advanced age (male ≥ 70 years, female ≥ 75 years versus > 65 years in CURB-65), and respiratory failure (arterial oxygen saturation ≤ 90% or arterial oxygen pressure ≤ 60 mmHg) parameters to CURB-65. A-DROP score had the highest second rank in predicting in-hospital mortality with 0.929 AUROC (95% CI 0.911–0.948). In contradiction, there are also studies reporting that A-DROP score performs better than the PSI/PORT in predicting the in-hospital mortality of COVID-19 patients AUROCs for A-DROP 0.87 (95% CI 0.84–0.90) vs. PSI 0.85 (95% CI 0.81–0.88) [16] and AUROCs for A-DROP 0.875 (95% CI; 0.822–0.937) vs. PSI 0.873 (95% CI 0.820–0.925) [68]. These findings suggest that involving advanced age [4,8,18], which is related to higher COVID-19 mortality, places A-DROP score in a superior situation compared to CURB-65 in predicting mortality. In our study, the mean age of deceased patients was 72 years, and they had lower SpO2 levels. Zhou et al. also reported that the mean age of non-survivors with COVID-19 was 69 years [8,18] and Xie et al. reported hypoxemia was independently associated with in-hospital mortality.
In our study, the capability of PSI /PORT (AUROC 0.971) and A-DROP (AUROC 0.929) to predict hospital mortality was better than other studies in COVID-19 pneumonia, with the results of AUROC PSI/PORT values of 0.85 (95% CI 0.81–0.88) [16], 0.85 (95% CI 0.78–0.90) [37], 0.835 (95% CI 0.826–0.845) [60], 0.874 (95% CI 0.808–0.939) [18], 0.873(95% CI 0.820–0.925) [68], while AUROC A-DROP results were 0.87 (95% CI 0.84–0.90) [16] and 0.875 (95% CI 0.822–0.937) [68].
NEWS2 score, having the respiratory parameters (respiratory rate, oxygen saturation, supplemental oxygen), performed as the third-highest system in predicting mortality. Its lack of a scale to indicate increased oxygen requirement, its insensitivity to hypoxic respiratory failure (type 1) often encountered in COVID-19 cases and its seldom monitoring of hypercapnic respiratory failure (type 2), neglecting the age, comorbidities and organ dysfunctioning, come forward as the disadvantages of NEWS2 [7,11,32,50,69,70].
In our study NEWS2 ≥ 7 also predicted in-hospital mortality yielding the AUROC 0.885 (95% CI 0.860–0.909) which turned out to be better than those in the previous studies with AUROC 0.81 (95% CI 0.77–0.85) [16], 0.809 (95% CI 0.727–0.891) [17], 0.822 (0.690–0.953) [43].
qCSI score index, which includes only three respiratory parameters, was found to have a higher predictive performance with AUROC value 0.882 (95% CI 0.853–0.911) compared with Navaa et al., and Covino et al. AUROC 0.711 (95% CI 0.656–761) and AUROC 0.749 (95% CI 0.685–0.806), respectively [23,71]. Haimovich et al. had a AUROC 0.81 (95% CI 0.73–0.89) in predicting acute respiratory failure in accordance with our findings [19].
4C Mortality which was specifically developed for COVID-19 cases, came out to be the fifth rank in our score performance results, although it included parameters as age, inflammatory markers and respiratory measures yet underestimating comorbidities dealing them only by number. 4C Mortality score with AUROC 0.78 (95% CI 0.75–0.81) was reported in the second place after the PSI/PORT score having 0.79 (95% CI 0.77–0.82) AUROC in performance in a recent study [72]. 4C Mortality was found to have a good prognostic value for mortality on patients over the age of 60 (AUROC 0.799; 95% CI 0.738–0.851) and qCSI had a similar effectiveness as reported in (AUROC 0.749; 95% CI 0.685–0.806) [71].
qSOFA (AUROC 0.818; 95% CI 0.786–0.850), CURB-65 (AUROC 0.859; 95% CI 0.823–0.896), Expanded CURB-65 (AUROC 0.836; 95% CI 0.800–0.873) and MEWS (AUROC 0.870; 95% CI 0.842–0.898) scores were inferior in predicting in-hospital mortality of patients with COVID-19 pneumonia, as confirmed by previous studies with the results of AUROC for qSOFA 0.73 (95% CI 0.69–0.78), CURB-65 0.85 (95% CI 0.81–0.89) [16], qSOFA 0.63 (95% CI 0.6–0.66), CURB 65 0.74 (95% CI 0.72–0.77) [72], MEWS 0.670 (95% CI 0.573–0.767) [17], MEWS 0.586 (95% CI 0.531–0.640), qSOFA 0.673 (95% CI 0.620–0.723) [73], expanded CURB-65 0.885 (95% CI 0.827–0.942) [74].
Kodama et al. reported that expanded CURB-65 score was a good predictor, with 0.832 (95% CI 0.763–0.901) AUROC of an increase in oxygen requirement patients with SARS-CoV-2 pneumonia [75].
Fan et al. has also compared in-hospital mortality of different risk scores systems like our study [16]. Although both studies took A-DROP, CURB-65 and 4C Mortality scores by the same cut off values; we have used a different cut off value as 107 points for PSI/PORT score, which is a mid-value between Class III and IV patients. Fan et al. has found the greatest score as A-DROP; our results suggest that a cut-off value of 107 points with PSI/PORT score yield a more valid score for predicting in-hospital mortality.
In our study, in-hospital mortality was found to be as 8.8% (133 cases). As it was reported to WHO, that there have been 11,249,216 confirmed COVID-19 cases, of which 86.661 were fatal (0.77%) in Turkey during the time interval from January 2020 to January 2022 [1,8,50]. It is suggested that the relative highness of our mortality value, in comparison with the national statistics can be attributed to our cohorts, consisting of moderate and severe cases with an average age of 60.12 ± 14.73. An additional factor might be that the study was conducted in a hospital dedicated entirely to COVID-19 patients. Varying mortality rates were reported in other studies ranging from 2.3% to 36%. The characteristics of the patients included in these studies, different treatment regimens applied and different mortality criteria (e.g., in-hospital, 48 h, 72 h, 7-day or 30-day measures) used might have been the major reasons why they yielded such a wide range of different mortality rates [2,69,71,76].
Limitation
This study has some limitations. It is a single-center study involving only hospitalized patients with moderate and severe disease in a retrospective design. Omicron and delta variants were not included, since the study was conducted during the early stages of the COVID-19 pandemic.
5. Conclusions
In conclusion, the accuracies of a variety of severity scores to predict in-hospital mortality in COVID-19 pneumonia patients were examined in our study. We found that PSI/PORT score showed the highest accuracy of in-hospital death prediction compared to other widely used CAP-specific and COVID specific score systems, such as qCSI and 4C Mortality. The PSI/PORT with a cut-off value of 107 points yielding the greatest performance was the best predictor for mortality, since it incorporated the factors such as advanced age and comorbidities. On the other hand, determination of the PSI/PORT required a longer time, since it involved the measurement and evaluation of a wide range of clinical parameters. Although all these data have to be validated in newer patient groups involving Cmicron and delta variants; our findings suggest that the use of A-DROP may also be preferred as a practical alternative to PSI/PORT, which is more time-consuming.
Acknowledgments
The authors wish to thank Ahmet Ademoglu and Biomedical Engineering Institute of Bogazici University for his help with statistical analyses and interpretation of data, and Isil Uzunhasan for her help in revision of the manuscript.
Author Contributions
Conceptualization, I.K.A. and M.B.; methodology, I.K.A. and M.B.; software, M.B.; validation, I.K.A. and M.B.; formal analysis, I.K.A., M.B., A.U.G. and R.K.; investigation, I.K.A. and M.B.; resources, I.K.A., M.B., R.K. and A.U.G.; data curation, I.K.A., M.B., A.U.G. and E.C.U.; writing—original draft preparation, I.K.A. and M.B.; writing—review and editing, I.K.A. and M.B.; visualization, I.K.A., M.B., A.U.G., R.K. and E.C.U.; project administration, I.K.A. and M.B.; supervision, K.K.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of University of Health Sciences, Bakırköy Dr. Sadi Konuk Training and Research Hospital (approval protocol code 2021/91 on the 15 February 2021).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization WHO Coronavirus (COVID-19) Dashboard. [(accessed on 28 January 2022)]. Available online: https://covid19.who.int.
- 2.Wu Z., McGoogan J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., Villamizar-Peña R., Holguin-Rivera Y., Escalera-Antezana J.P., Alvarado-Arnez L.E., Bonilla-Aldana D.K., Franco-Paredes C., Henao-Martinez A.F., et al. Clinical, Laboratory and Imaging Features of COVID-19: A Systematic Review and Meta-Analysis. Travel Med. Infect. Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duan J., Wang X., Chi J., Chen H., Bai L., Hu Q., Han X., Hu W., Zhu L., Wang X., et al. Correlation between the Variables Collected at Admission and Progression to Severe Cases during Hospitalization among Patients with COVID-19 in Chongqing. J. Med. Virol. 2020;92:2616–2622. doi: 10.1002/jmv.26082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middeldorp S., Coppens M., Haaps T.F., Foppen M., Vlaar A.P., Müller M.C.A., Bouman C.C.S., Beenen L.F.M., Kootte R.S., Heijmans J., et al. Incidence of Venous Thromboembolism in Hospitalized Patients with COVID-19. J. Thromb. Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu B., Huang S., Yin L. The Cytokine Storm and COVID-19. J. Med. Virol. 2021;93:250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:934. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus—Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donoso-Navarro E., Arribas Gómez I., Bernabeu-Andreu F.A. IL-6 and Other Biomarkers Associated with Poor Prognosis in a Cohort of Hospitalized Patients with COVID-19 in Madrid. Biomark Insights. 2021;16:117727192110133. doi: 10.1177/11772719211013363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical Predictors of Mortality Due to COVID-19 Based on an Analysis of Data of 150 Patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C., Ye J., Chen Q., Hu W., Wang L., Fan Y., Lu Z., Chen J., Chen Z., Chen S., et al. Elevated Lactate Dehydrogenase (LDH) Level as an Independent Risk Factor for the Severity and Mortality of COVID-19. Aging. 2020;12:15670–15681. doi: 10.18632/aging.103770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umeh C., Tuscher L., Ranchithan S., Watanabe K., Gupta R. Predictors of COVID-19 Mortality in Critically Ill ICU Patients: A Multicenter Retrospective Observational Study. Cureus. 2022;14:e20952. doi: 10.7759/cureus.20952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N., Cuomo-Dannenburg G., Thompson H., Walker P.G.T., Fu H., et al. Estimates of the Severity of Coronavirus Disease 2019: A Model-Based Analysis. Lancet Infect. Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan G., Tu C., Zhou F., Liu Z., Wang Y., Song B., Gu X., Wang Y., Wei Y., Li H., et al. Comparison of Severity Scores for COVID-19 Patients with Pneumonia: A Retrospective Study. Eur. Respir. J. 2020;56:2002113. doi: 10.1183/13993003.02113-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu H., Yao N., Qiu Y. Predictive Value of 5 Early Warning Scores for Critical COVID-19 Patients. Disaster Med. Public Health Prep. 2020:1–8. doi: 10.1017/dmp.2020.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.García Clemente M.M., Herrero Huertas J., Fernández Fernández A., De La Escosura Muñoz C., Enríquez Rodríguez A.I., Pérez Martínez L., Gómez Mañas S., Iscar Urrutia M., López González F.J., Madrid Carbajal C.J., et al. Assessment of Risk Scores in COVID-19. Int. J. Clin. Pract. 2021;75:e13705. doi: 10.1111/ijcp.13705. [DOI] [PubMed] [Google Scholar]
- 19.Haimovich A.D., Ravindra N.G., Stoytchev S., Young H.P., Wilson F.P., van Dijk D., Schulz W.L., Taylor R.A. Development and Validation of the Quick COVID-19 Severity Index: A Prognostic Tool for Early Clinical Decompensation. Ann. Emerg. Med. 2020;76:442–453. doi: 10.1016/j.annemergmed.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight S.R., Ho A., Pius R., Buchan I., Carson G., Drake T.M., Dunning J., Fairfield C.J., Gamble C., Green C.A., et al. Risk Stratification of Patients Admitted to Hospital with COVID-19 Using the ISARIC WHO Clinical Characterisation Protocol: Development and Validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji D., Zhang D., Xu J., Chen Z., Yang T., Zhao P., Chen G., Cheng G., Wang Y., Bi J., et al. Prediction for Progression Risk in Patients With COVID-19 Pneumonia: The CALL Score. Clin. Infect. Dis. 2020;71:1393–1399. doi: 10.1093/cid/ciaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grifoni E., Valoriani A., Cei F., Vannucchi V., Moroni F., Pelagatti L., Tarquini R., Landini G., Masotti L. The CALL Score for Predicting Outcomes in Patients With COVID-19. Clin. Infect. Dis. 2020;72:ciaa686. doi: 10.1093/cid/ciaa686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez-Nava G., Yanez-Bello M.A., Trelles-Garcia D.P., Chung C.W., Friedman H.J., Hines D.W. Performance of the Quick COVID-19 Severity Index and the Brescia-COVID Respiratory Severity Scale in Hospitalized Patients with COVID-19 in a Community Hospital Setting. Int. J. Infect. Dis. 2021;102:571–576. doi: 10.1016/j.ijid.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Tawfiq J.A., Hinedi K., Ghandour J., Khairalla H., Musleh S., Ujayli A., Memish Z.A. Middle East Respiratory Syndrome Coronavirus: A Case-Control Study of Hospitalized Patients. Clin. Infect. Dis. 2014;59:160–165. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rainer T.H., Lee N., Ip M., Galvani A.P., Antonio G.E., Wong K.T., Chan D.P.N., Ng A.W.H., Shing K.K., Chau S.S.L., et al. Features Discriminating SARS from Other Severe Viral Respiratory Tract Infections. Eur. J. Clin. Microbiol. Infect. Dis. 2007;26:121–129. doi: 10.1007/s10096-006-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qu J.-X., Gu L., Pu Z.-H., Yu X.-M., Liu Y.-M., Li R., Wang Y.-M., Cao B., Wang C., For Beijing Network for Adult Community-Acquired Pneumonia (BNACAP) Viral Etiology of Community-Acquired Pneumonia among Adolescents and Adults with Mild or Moderate Severity and Its Relation to Age and Severity. BMC Infect. Dis. 2015;15:89. doi: 10.1186/s12879-015-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim M.-A., Park J.S., Lee C.W., Choi W.-I. Pneumonia Severity Index in Viral Community Acquired Pneumonia in Adults. PLoS ONE. 2019;14:e0210102. doi: 10.1371/journal.pone.0210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F., Wang Y., Liu Y., Liu X., Gu L., Zhang X., Pu Z., Yang G., Liu B., Nie Q., et al. Disease Severity and Clinical Outcomes of Community-Acquired Pneumonia Caused by Non-Influenza Respiratory Viruses in Adults: A Multicentre Prospective Registry Study from the CAP-China Network. Eur. Respir. J. 2019;54:1802406. doi: 10.1183/13993003.02406-2018. [DOI] [PubMed] [Google Scholar]
- 29.Zhan Y., Yang Z., Chen R., Wang Y., Guan W., Zhao S. Respiratory Virus Is a Real Pathogen in Immunocompetent Community-Acquired Pneumonia: Comparing to Influenza like Illness and Volunteer Controls. BMC Pulm. Med. 2014;14:144. doi: 10.1186/1471-2466-14-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fine M.J., Auble T.E., Yealy D.M., Hanusa B.H., Weissfeld L.A., Singer D.E., Coley C.M., Marrie T.J., Kapoor W.N. A Prediction Rule to Identify Low-Risk Patients with Community-Acquired Pneumonia. N. Engl. J. Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 31.Miyashita N., Matsushima T., Oka M. The JRS Guidelines for the Management of Community-Acquired Pneumonia in Adults:An Update and New Recommendations. Intern. Med. 2006;45:419–428. doi: 10.2169/internalmedicine.45.1691. [DOI] [PubMed] [Google Scholar]
- 32.Royal College of Physicians . National Early Warning Score (NEWS) 2: Standardising the Assesment of Acute-Ilness Severity in the NHS. Updated Report of Working Party. RCP; London, UK: 2017. [(accessed on 28 January 2022)]. Available online: https://www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2. [Google Scholar]
- 33.Subbe C.P. Validation of a Modified Early Warning Score in Medical Admissions. QJM. 2001;94:521–526. doi: 10.1093/qjmed/94.10.521. [DOI] [PubMed] [Google Scholar]
- 34.Lim W.S. Defining Community Acquired Pneumonia Severity on Presentation to Hospital: An International Derivation and Validation Study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J., Xu F., Zhou H., Wu X., Shi L., Lu R., Farcomeni A., Venditti M., Zhao Y., Luo S., et al. Expanded CURB-65: A New Score System Predicts Severity of Community-Acquired Pneumonia with Superior Efficiency. Sci. Rep. 2016;6:22911. doi: 10.1038/srep22911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seymour C.W., Liu V.X., Iwashyna T.J., Brunkhorst F.M., Rea T.D., Scherag A., Rubenfeld G., Kahn J.M., Shankar-Hari M., Singer M., et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:762. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satici C., Demirkol M.A., Sargin Altunok E., Gursoy B., Alkan M., Kamat S., Demirok B., Surmeli C.D., Calik M., Cavus Z., et al. Performance of Pneumonia Severity Index and CURB-65 in Predicting 30-Day Mortality in Patients with COVID-19. Int. J. Infect. Dis. 2020;98:84–89. doi: 10.1016/j.ijid.2020.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holten A.R., Nore K.G., Tveiten C.E.V.W.K., Olasveengen T.M., Tonby K. Predicting Severe COVID-19 in the Emergency Department. Resusc. Plus. 2020;4:100042. doi: 10.1016/j.resplu.2020.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wynants L., Van Calster B., Collins G.S., Riley R.D., Heinze G., Schuit E., Bonten M.M.J., Dahly D.L., Damen J.A., Debray T.P.A., et al. Prediction Models for Diagnosis and Prognosis of COVID-19: Systematic Review and Critical Appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferreira M., Blin T., Collercandy N., Szychowiak P., Dequin P.-F., Jouan Y., Guillon A. Critically Ill SARS-CoV-2-Infected Patients Are Not Stratified as Sepsis by the QSOFA. Ann. Intensive Care. 2020;10:43. doi: 10.1186/s13613-020-00664-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gidari A., De Socio G.V., Sabbatini S., Francisci D. Predictive Value of National Early Warning Score 2 (NEWS2) for Intensive Care Unit Admission in Patients with SARS-CoV-2 Infection. Infect. Dis. 2020;52:698–704. doi: 10.1080/23744235.2020.1784457. [DOI] [PubMed] [Google Scholar]
- 42.Jang J.G., Hur J., Hong K.S., Lee W., Ahn J.H. Prognostic Accuracy of the SIRS, QSOFA, and NEWS for Early Detection of Clinical Deterioration in SARS-CoV-2 Infected Patients. J. Korean Med. Sci. 2020;35:e234. doi: 10.3346/jkms.2020.35.e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Myrstad M., Ihle-Hansen H., Tveita A.A., Andersen E.L., Nygård S., Tveit A., Berge T. National Early Warning Score 2 (NEWS2) on Admission Predicts Severe Disease and in-Hospital Mortality from COVID-19—A Prospective Cohort Study. Scand. J. Trauma Resusc. Emerg. Med. 2020;28:66. doi: 10.1186/s13049-020-00764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sai F., Liu X., Li L., Ye Y., Zhu C., Hang Y., Huang C., Tian L., Huang H., Xu X. Clinical Characteristics and Risk Factors for Mortality in Patients with Coronavirus Disease 2019 in Intensive Care Unit: A Single- Center, Retrospective, Observational Study in China. Ann. Palliat. Med. 2021;10:2859–2868. doi: 10.21037/apm-20-1575. [DOI] [PubMed] [Google Scholar]
- 45.Martín-Rodríguez F., Martín-Conty J.L., Sanz-García A., Rodríguez V.C., Rabbione G.O., Cebrían Ruíz I.C., Oliva Ramos J.R., Castro Portillo E., Polonio-López B., Enríquez de Salamanca Gambarra R., et al. Early Warning Scores in Patients with Suspected COVID-19 Infection in Emergency Departments. J. Pers. Med. 2021;11:170. doi: 10.3390/jpm11030170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization WHO Coronavirus Disease (COVID-19)/Technical Guidance. [(accessed on 28 January 2022)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications.
- 47.Republic of Turkey Ministry of Health COVID-19 (SARS-CoV-2 Infection) Guide. [(accessed on 28 January 2022)]; Available online: https://covid19.saglik.gov.tr/TR-66926/eriskin-hasta-tedavisi.html.
- 48.National Institutes of Health (NIH) Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. [(accessed on 28 January 2022)]; Available online: http://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum. [PubMed]
- 49.Yang R., Li X., Liu H., Zhen Y., Zhang X., Xiong Q., Luo Y., Gao C., Zeng W. Chest CT Severity Score: An Imaging Tool for Assessing Severe COVID-19. Radiol. Cardiothorac. Imaging. 2020;2:e200047. doi: 10.1148/ryct.2020200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie J., Covassin N., Fan Z., Singh P., Gao W., Li G., Kara T., Somers V.K. Association Between Hypoxemia and Mortality in Patients With COVID-19. Mayo Clin. Proc. 2020;95:1138–1147. doi: 10.1016/j.mayocp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., Aaron J.G., Claassen J., Rabbani L.E., Hastie J., et al. Epidemiology, Clinical Course, and Outcomes of Critically Ill Adults with COVID-19 in New York City: A Prospective Cohort Study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y., Tobin K.A., Cerfolio R.J., Francois F., Horwitz L.I. Factors Associated with Hospital Admission and Critical Illness among 5279 People with Coronavirus Disease 2019 in New York City: Prospective Cohort Study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rath D., Petersen-Uribe Á., Avdiu A., Witzel K., Jaeger P., Zdanyte M., Heinzmann D., Tavlaki E., Müller K., Gawaz M.P. Impaired Cardiac Function Is Associated with Mortality in Patients with Acute COVID-19 Infection. Clin. Res. Cardiol. 2020;109:1491–1499. doi: 10.1007/s00392-020-01683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang W., Liang H., Ou L., Chen B., Chen A., Li C., Li Y., Guan W., Sang L., Lu J., et al. Development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients With COVID-19. JAMA Intern. Med. 2020;180:1081. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao J., Zhong L., Wu M., Ji J., Liu Z., Wang C., Xie Q., Liu Z. Risk Factors for Mortality in Critically Ill Patients with COVID-19: A Multicenter Retrospective Case-Control Study. BMC Infect. Dis. 2021;21:602. doi: 10.1186/s12879-021-06300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi Y., Pandita A., Hardesty A., McCarthy M., Aridi J., Weiss Z.F., Beckwith C.G., Farmakiotis D. Validation of Pneumonia Prognostic Scores in a Statewide Cohort of Hospitalised Patients with COVID-19. Int. J. Clin. Pract. 2021;75:e13926. doi: 10.1111/ijcp.13926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan M., Yin W., Tao Z., Tan W., Hu Y. Association of Radiologic Findings with Mortality of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. PLoS ONE. 2020;15:e0230548. doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gallo Marin B., Aghagoli G., Lavine K., Yang L., Siff E.J., Chiang S.S., Salazar-Mather T.P., Dumenco L., Savaria M.C., Aung S.N., et al. Predictors of COVID-19 Severity: A Literature Review. Rev. Med. Virol. 2021;31:1–10. doi: 10.1002/rmv.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Artero A., Madrazo M., Fernández-Garcés M., Muiño Miguez A., González García A., Crestelo Vieitez A., García Guijarro E., Fonseca Aizpuru E.M., García Gómez M., Areses Manrique M., et al. Severity Scores in COVID-19 Pneumonia: A Multicenter, Retrospective, Cohort Study. J. Gen. Intern. Med. 2021;36:1338–1345. doi: 10.1007/s11606-021-06626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The PROGRESS Study Group. Ahnert P., Creutz P., Horn K., Schwarzenberger F., Kiehntopf M., Hossain H., Bauer M., Brunkhorst F.M., Reinhart K., et al. Sequential Organ Failure Assessment Score Is an Excellent Operationalization of Disease Severity of Adult Patients with Hospitalized Community Acquired Pneumonia—Results from the Prospective Observational PROGRESS Study. Crit. Care. 2019;23:110. doi: 10.1186/s13054-019-2316-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asai N., Watanabe H., Shiota A., Kato H., Sakanashi D., Hagihara M., Koizumi Y., Yamagishi Y., Suematsu H., Mikamo H. Efficacy and Accuracy of QSOFA and SOFA Scores as Prognostic Tools for Community-Acquired and Healthcare-Associated Pneumonia. Int. J. Infect. Dis. 2019;84:89–96. doi: 10.1016/j.ijid.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 63.Liu D., Cui P., Zeng S., Wang S., Feng X., Xu S., Li R., Gao Y., Yu R., Wang Y., et al. Risk Factors for Developing into Critical COVID-19 Patients in Wuhan, China: A Multicenter, Retrospective, Cohort Study. EClinicalMedicine. 2020;25:100471. doi: 10.1016/j.eclinm.2020.100471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gupta S., Hayek S.S., Wang W., Chan L., Mathews K.S., Melamed M.L., Brenner S.K., Leonberg-Yoo A., Schenck E.J., Radbel J., et al. Factors Associated with Death in Critically Ill Patients with Coronavirus Disease 2019 in the US. JAMA Intern. Med. 2020;180:1436. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shindo Y., Sato S., Maruyama E., Ohashi T., Ogawa M., Imaizumi K., Hasegawa Y. Comparison of Severity Scoring Systems A-DROP and CURB-65 for Community-Acquired Pneumonia. Respirology. 2008;13:731–735. doi: 10.1111/j.1440-1843.2008.01329.x. [DOI] [PubMed] [Google Scholar]
- 66.Kohno S., Seki M., Watanabe A., the CAP Study Group Evaluation of an Assessment System for the JRS 2005: A-DROP for the Management of CAP in Adults. Intern. Med. 2011;50:1183–1191. doi: 10.2169/internalmedicine.50.4651. [DOI] [PubMed] [Google Scholar]
- 67.Miyashita N., Nakamori Y., Ogata M., Fukuda N., Yamura A., Ishiura Y., Nomura S. A Warning Related to Predicting the Severity of COVID-19 Pneumonia Using the A-DROP Scoring System. J. Infect. Chemother. 2022;28:359–360. doi: 10.1016/j.jiac.2021.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ucan E.S., Ozgen Alpaydin A., Ozuygur S.S., Ercan S., Unal B., Sayiner A.A., Ergan B., Gokmen N., Savran Y., Kilinc O., et al. Pneumonia Severity Indices Predict Prognosis in Coronavirus Disease-2019. Respir. Med. Res. 2021;79:100826. doi: 10.1016/j.resmer.2021.100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bradley P., Frost F., Tharmaratnam K., Wootton D.G. Utility of Established Prognostic Scores in COVID-19 Hospital Admissions: Multicentre Prospective Evaluation of CURB-65, NEWS2 and QSOFA. BMJ Open Respir. Res. 2020;7:e000729. doi: 10.1136/bmjresp-2020-000729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R., et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Covino M., De Matteis G., Burzo M.L., Russo A., Forte E., Carnicelli A., Piccioni A., Simeoni B., Gasbarrini A., Franceschi F., et al. Predicting In-Hospital Mortality in COVID-19 Older Patients with Specifically Developed Scores. J. Am. Geriatr. Soc. 2021;69:37–43. doi: 10.1111/jgs.16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lazar Neto F., Marino L.O., Torres A., Cilloniz C., Meirelles Marchini J.F., Garcia de Alencar J.C., Palomeque A., Albacar N., Brandão Neto R.A., Souza H.P., et al. Community-Acquired Pneumonia Severity Assessment Tools in Patients Hospitalized with COVID-19: A Validation and Clinical Applicability Study. Clin. Microbiol. Infect. 2021;27:e1–e1037. doi: 10.1016/j.cmi.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Covino M., Sandroni C., Santoro M., Sabia L., Simeoni B., Bocci M.G., Ojetti V., Candelli M., Antonelli M., Gasbarrini A., et al. Predicting Intensive Care Unit Admission and Death for COVID-19 Patients in the Emergency Department Using Early Warning Scores. Resuscitation. 2020;156:84–91. doi: 10.1016/j.resuscitation.2020.08.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.CURB-65 plus Hypoalbuminemia: A New Score System for Prediction of the in-Hospital Mortality Risk in Patients with SARS-CoV-2 Pneumonia. Infez Med. 2021;29:408–415. doi: 10.53854/liim-2903-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kodama T., Obinata H., Mori H., Murakami W., Suyama Y., Sasaki H., Kouzaki Y., Kawano S., Kawana A., Mimura S. Prediction of an Increase in Oxygen Requirement of SARS-CoV-2 Pneumonia Using Three Different Scoring Systems. J. Infect. Chemother. 2021;27:336–341. doi: 10.1016/j.jiac.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi S., Liu X., Xiao J., Wang H., Chen L., Li J., Han K. Prediction of Adverse Clinical Outcomes in Patients with Coronavirus Disease 2019. J. Clin. Lab. Anal. 2021;35:e23598. doi: 10.1002/jcla.23598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy reasons.