Abstract
We have previously identified methylene blue, a tricyclic phenothiazine dye approved for clinical use for the treatment of methemoglobinemia and for other medical applications as a small-molecule inhibitor of the protein–protein interaction (PPI) between the spike protein of the SARS-CoV-2 coronavirus and ACE2, the first critical step of the attachment and entry of this coronavirus responsible for the COVID-19 pandemic. Here, we show that methylene blue concentration dependently inhibits this PPI for the spike protein of the original strain as well as for those of variants of concern such as the D614G mutant and delta (B.1.617.2) with IC50 in the low micromolar range (1–5 μM). Methylene blue also showed promiscuous activity and inhibited several other PPIs of viral proteins (e.g., HCoV-NL63–ACE2, hepatitis C virus E–CD81) as well as others (e.g., IL-2–IL-2Rα) with similar potency. This nonspecificity notwithstanding, methylene blue inhibited the entry of pseudoviruses bearing the spike protein of SARS-CoV-2 in hACE2-expressing host cells, both for the original strain and the delta variant. It also blocked SARS-CoV-2 (B.1.5) virus replication in Vero E6 cells with an IC50 in the low micromolar range (1.7 μM) when assayed using quantitative PCR of the viral RNA. Thus, while it seems to be a promiscuous PPI inhibitor with low micromolar activity and has a relatively narrow therapeutic index, methylene blue inhibits entry and replication of SARS-CoV-2, including several of its mutant variants, and has potential as a possible inexpensive, broad-spectrum, orally bioactive small-molecule antiviral for the prevention and treatment of COVID-19.
Keywords: ACE2, coronavirus, COVID-19, delta (B.1.617.2) variant, drug repositioning, methylene blue, oral antiviral, protein–protein interaction, SARS-CoV-2, spike protein
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a betacoronavirus that emerged in late 2019 and is responsible for the ongoing COVID-19 pandemic [1,2,3], is the most infectious agent in a century [4]. Among the seven coronaviruses (CoVs) currently known to infect humans, which include four causing only common colds (HCoV 229E, OC43, NL63, and HKU1) and two that caused previous recent epidemics of high mortality (SARS-CoV-1 ~10% and MERS-CoV ~35%), SARS-CoV-2 caused by far the biggest health and economic damage, despite its lower overall mortality compared with the last two. The success of the recent vaccination program notwithstanding, there still is a considerable therapeutic need for antivirals, as a significant portion of the population is unable or unwilling to be vaccinated and mutations are diminishing vaccine efficacy so that SARS-CoV-2 infections are likely to continue in the foreseeable future [5]. Orally bioavailable antivirals are especially of interest since oral treatments can be taken easily following the first symptoms. While efforts toward the repurposing of approved drugs for SARS-CoV-2 had only very limited success so far [6,7], two new drugs with classic antiviral mechanisms (i.e., inhibition of protease activity or viral reproduction) have shown promise and were granted emergency use authorization by the United States Food and Drug Administration (FDA) for the treatment of COVID-19: molnupiravir (Emory University, Ridgeback Biotherapeutics, and Merck) [8] and nirmatrelvir (Pfizer; as part of the nirmatrelvir/ritonavir combination Paxlovid) [9].
Because of our interest in small-molecule inhibitors (SMIs) of protein–protein interactions (PPIs) [10,11], we focused on an alternative target and searched for possible inhibitors of the PPIs between the CoV spike (S) proteins and their cognate cell-surface receptors needed to initiate cell attachment and virus entry (angiotensin converting enzyme 2, ACE2, for both SARS-CoV-1 and SARS-CoV-2) [12]. While SMIs for PPIs are more challenging to identify than antibodies, progress has been made, and three SMIs of PPIs are now approved for clinical use (venetoclax [13], lifitegrast [14], and fostemsavir [15]) [11,16,17,18]. In particular, fostemsavir targets gp120 binding to CD4 to block HIV attachment and entry, further validating a PPI inhibitory strategy for antiviral drug discovery. Based on this premise, we initiated a screening to identify possible SMIs of this PPI [12], during which we discovered that methylene blue (MeBlu), a tricyclic small-molecule dye, which is approved for the treatment of acquired methemoglobinemia and some other clinical uses [19,20,21,22], inhibits the SARS-CoV-2–hACE2 PPI and the entry of SARS-CoV-2 spike-bearing pseudoviruses into ACE2-expressing cells [23]. Here, we show that MeBlu inhibits these even for mutants such as D614G and variants of concern (VoC) such as delta (B.1.617.2; first detected in India, October 2020) that have emerged since then. The D614G mutation increases the infectivity, transmission rate, and efficiency of cell entry for SARS-CoV-2 [24] and is present in all later variants of high infectivity. Delta is a VoC that shows increased transmissibility and reduction in neutralization by postvaccination sera [25]. We also show that MeBlu inhibits the replication of a native/infective SARS-CoV-2 strain (B.1.5) in Vero E6 cells. While we found MeBlu to have apparent promiscuous PPI inhibitory activity and a relatively narrow therapeutic index, this inexpensive and clinically long-used drug still could be of interest as an oral treatment for the prevention and therapy of COVID-19, especially in nonindustrialized nations with limited access to proprietary vaccines and antivirals.
2. Results
2.1. Inhibition of SARS-CoV-2 Spike–hACE2 PPIs
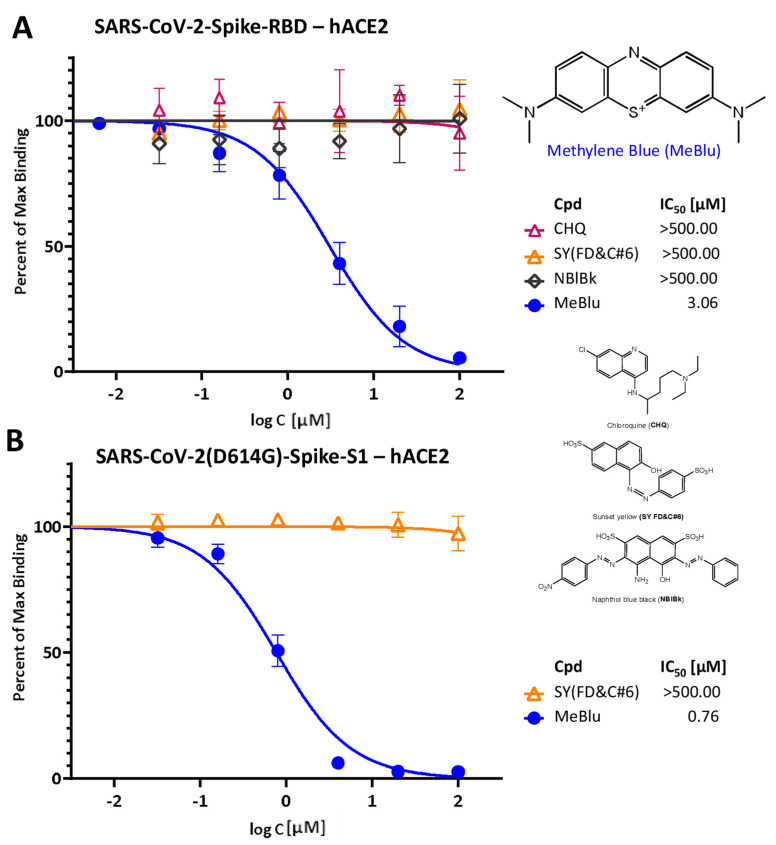
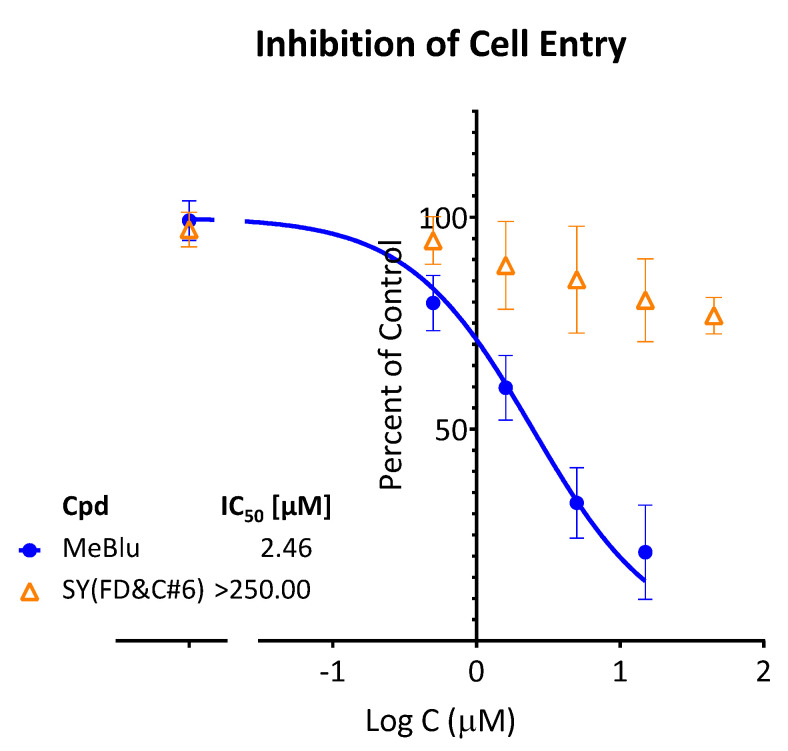
We have identified MeBlu as an inhibitor of the SARS-CoV-2 S protein–ACE2 PPI, a PPI essential for the viral attachment and entry of this novel, highly infectious coronavirus [23]. In our ELISA-type assay, MeBlu inhibited the interaction between the receptor binding domain (RBD) of the SARS-CoV-2 spike of the original strain and hACE2 in typical concentration responsive manner with an IC50 of 3.06 μM (95% CI: 2.56–3.67 μM), whereas two other dyes included as control, sunset yellow FCF (FD&C yellow #6; SY) and naphthol blue black (NBlBk), as well as chloroquine showed no such activity (IC50 > 500 μM; Figure 1A). In general agreement with this, surface plasmon resonance (SPR) measurements using single and multicycle kinetics indicated that MeBlu binds to the SARS-CoV-2 spike RBD in the low micromolar range (Supplementary Information, Figure S1). However, the binding behavior appears to be more complex than can be described by a standard 1:1 binding interaction, and since it was unclear from the SPR data alone what model would best describe this behavior, kinetics/affinity values were not determined. Single-cycle kinetics indicated that MeBlu also binds hACE2 in the low micromolar range, again, in a manner that could not be described well by a standard 1:1 binding interaction (data not shown).
Figure 1.
Concentration-dependent inhibition of SARS-CoV-2 spike protein binding to human ACE2 by methylene blue (MeBlu). Concentration–response curves obtained in ELISA-type assay showing the inhibition of the binding of SARS-CoV-2 spike RBD (His tagged) (A) and D614G mutant S1 (B) to hACE2 in the presence of increasing concentrations of test compounds. Contrary to MeBlu, neither chloroquine (CHQ) nor the two other dyes included as control (sunset yellow FCF, SY and naphthol blue black, NBlBk) showed inhibitory activity (chemical structures shown on the right). Data in (A) incorporate measurements from our previous work [23] combined with one confirmatory remeasurement here and are included for reference and comparison purposes. Data (mean ± SD) were normalized and fitted with standard inhibition curves; obtained IC50 values are shown.
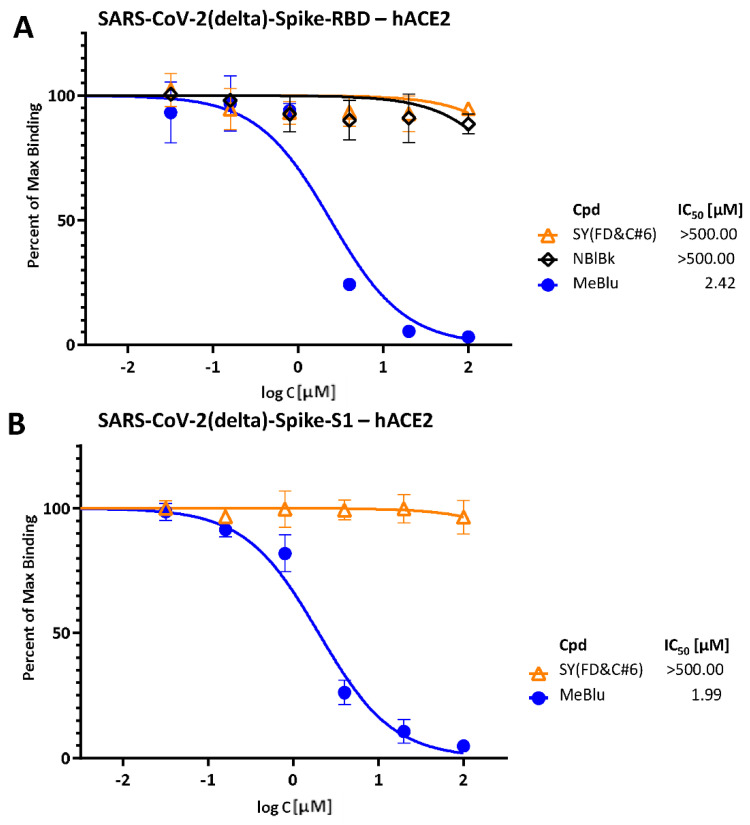
Notably, especially in light of the emergence of several SARS-CoV-2 mutants and variants of concerns (VoCs) [26], MeBlu also inhibited the binding of mutant spike proteins to hACE2 with very similar activities. For example, MeBlu inhibited binding of the D614G mutant spike protein (S1 portion) with an IC50 of 0.76 μM (95% CI: 0.65–0.90; Figure 1B). Inhibitory activity remained about the same for the delta (B.1.617.2) variant of concern, assayed here either as the S1 protein or as its RBD only: IC50s of 1.99 μM (95% CI: 1.67–2.36) and 2.42 μM (95% CI: 1.77–3.30), respectively (Figure 2), indicating potential for broad-spectrum activity.
Figure 2.
Concentration-dependent inhibition of SARS-CoV-2 delta (B.1.617.2) spike RBD (A) and S1 protein (B) binding to hACE2 by MeBlu. Concentration–response curves obtained in a similar setup as in Figure 1 but with the spike protein RBD (A) and S1 (B) of the delta (B.1.617.2) variant in the presence of increasing concentrations of test compounds. Data (mean ± SD) were normalized and fitted with standard inhibition curves; obtained IC50 values are shown on the right for each figure.
2.2. Inhibition of Other PPIs
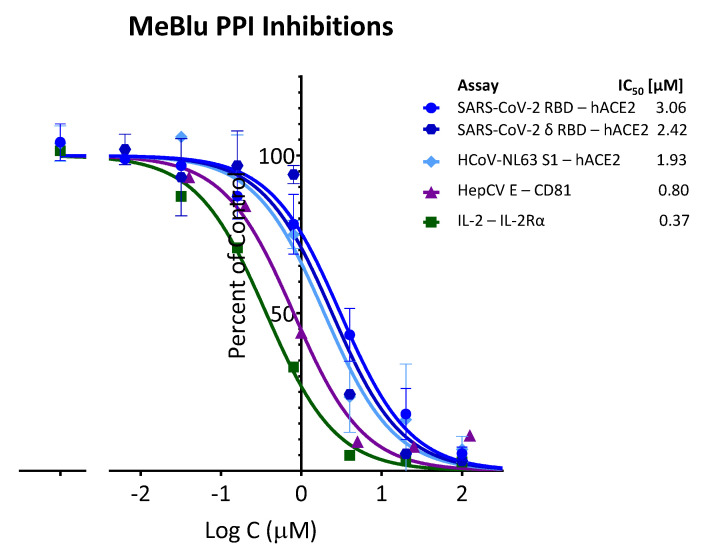
It has to be noted, however, that based on our assays, MeBlu seems to be a promiscuous PPI inhibitor with low micromolar activity. Promiscuous, i.e., nonselective inhibition of multiple targets, is a known problem in medicinal chemistry and screening assays [27,28]. MeBlu inhibited several PPIs we explored; in fact, it had slightly better activity (lower IC50) in a number of them than it had for the SARS-CoV-2 spike–hACE2 PPI. For example, compared with the 3.06 and 2.42 μM for inhibiting the RBD and delta RBD–hACE2 PPIs, respectively, we obtained IC50s of 1.93 μM (95% CI: 1.35–2.78) for HCoV-NL63 S1–hACE2, a PPI important for the attachment and entry of this other human coronavirus (HCoV-NL63) that uses ACE2 as its receptor, and 0.80 μM (95% CI: 0.61–1.07) for HCV E–CD81, a PPI used by the hepatitis C virus (Figure 3). In addition, MeBlu also inhibited TNF superfamily (TNFSF) PPIs such as TNF-R1−TNFα and CD40–CD154 as mentioned before [23], and the IL-2–IL-2Rα PPI assayed here (IC50 0.37 μM; 95% CI: 0.27–0.50), which we selected for having a complex interface [29] and is thus less likely to be susceptible to inhibition by a small molecule.
Figure 3.
Promiscuous PPI inhibitory activity of MeBlu. Methylene blue inhibited several protein–protein interactions as assayed here in concentration-dependent manner following typical law of mass action response (i.e., unity Hill slope, nH = 1). In addition to coronavirus spike protein interactions with human ACE2, including SARS-CoV-2, its delta variant of concern, and the cold-causing HCoV-NL63, it also inhibited the PPIs of hepatitis C virus envelope glycoprotein E with CD81 as well as others such as IL-2–IL-2Rα with similar potency. Data (mean ± SD) for each assay as indicated were normalized and fitted with standard inhibition curves; obtained IC50 values are shown at right.
2.3. SARS-CoV-2 Pseudovirus Entry Inhibition
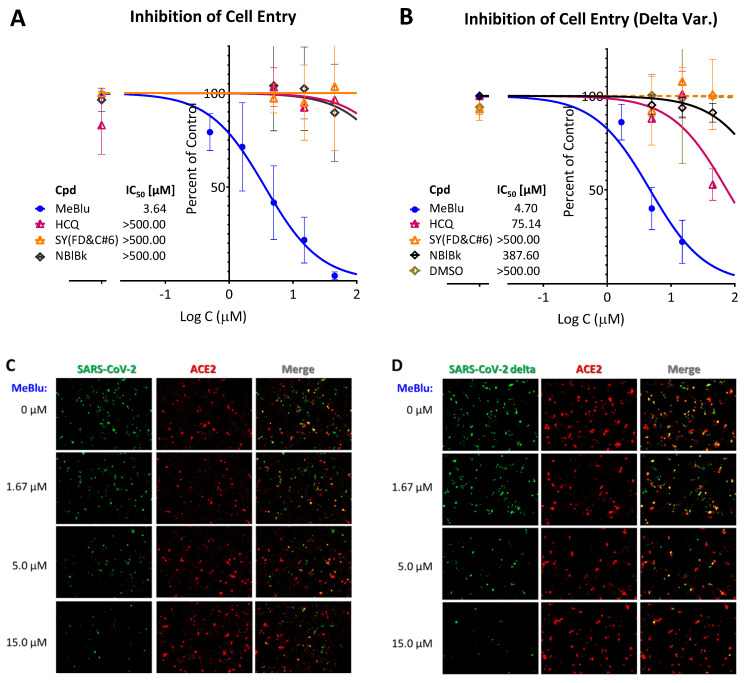
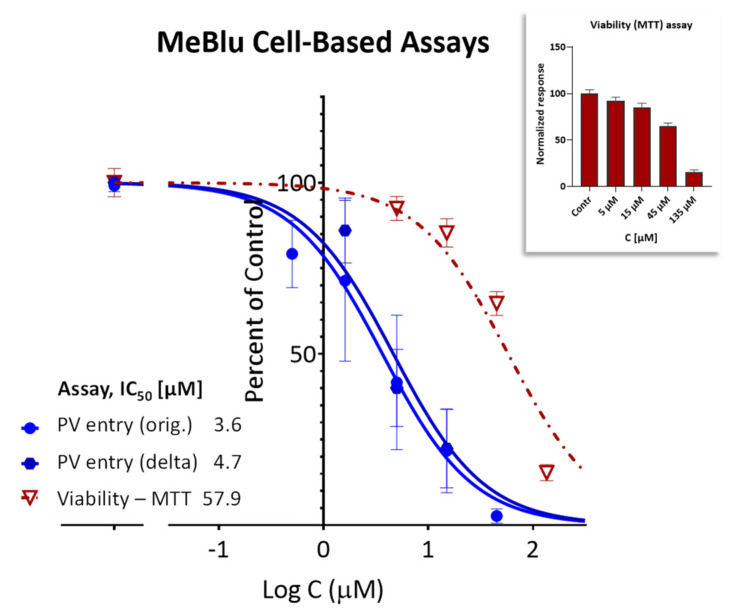
Importantly, this nonspecific PPI inhibitory activity notwithstanding, MeBlu also inhibited the entry of two different pseudoviruses bearing the SARS-CoV-2 S spike protein into ACE2-expressing cells. Such pseudovirus assay allows the quantification of viral entry using biosafety level 1 containment because they do not replicate in human cells. First, it has been performed with a baculovirus bearing SARS-CoV-2 spike proteins S and generated using BacMam-based tools. As this pseudovirus also expresses bright green fluorescent protein, entry is indicated by expression of green fluorescence in the nucleus of host cells (that express ACE2 and a red fluorescence reporter). If the entry is blocked, the cell nucleus remains dark. MeBlu showed clear concentration-dependent inhibitory activity with IC50s of 3.6 μM (95% CI: 2.4–5.4 μM) for the original strain and 4.7 μM (95% CI: 3.4–6.3) for the delta (B.1.617.2) variant (Figure 4A,B). Here, a set of representative images from serial dilution experiments are also included for illustration (Figure 4C,D). Cytotoxicity evaluation performed under similar conditions gave TC50s of 57.9 μM (95% CI: 44.9–72.2) indicating clear separation between inhibitory activity and toxicity (Figure 5); nevertheless, MeBlu had a relatively narrow therapeutic (selectivity) index (TI) of just slightly higher than 10 (i.e., a quantitative indicator of the relative safety highlighting the separation between toxic and effective concentrations or doses, e.g., TI = TC50/IC50, which here in this cell-based assay came out to 15.9 and 12.3 for the original and delta strains, respectively).
Figure 4.
Concentration-dependent inhibition of the entry of SARS-CoV-2 pseudoviruses (BacMam) into ACE2-expressing cells by MeBlu. Quantification of entry of pseudoviruses bearing the original (A) or the delta (B.1.617.2) variant (B) of the SARS-CoV-2 S protein (plus green fluorescent protein reporters; BacMam based) in ACE2 (plus red fluorescence)-expressing host cells (HEK293T). Values were obtained as before from the quantification of the amount of green present [12,23], as green fluorescence is expressed only in pseudovirus infected cells. Data are shown as percent of control on semilogarithmic scale and fitted with classic sigmoidal curve used to calculate IC50s for MeBlu as well as the control compounds included (hydroxychloroquine, HCQ, sunset yellow, SY, and naphthol blue black, NBlBk). A set of corresponding representative images from serial dilution experiments with MeBlu and the BacMam-based pseudovirus bearing the SARS-CoV-2 S proteins are shown in (C,D) for illustration.
Figure 5.
Therapeutic index of MeBlu in the present cell-based assay. Concentration-dependent activity (inhibition of the entry of SARS-CoV-2 BacMam-based pseudoviruses) and safety (effect on viability) of MeBlu in HEK293T cells. Inhibitory effects on the cell entry of pseudoviruses expressing the original and the delta mutant of the spike protein of SARS-CoV-2 (dark and light blue, respectively) shown in parallel with effects on cell viability (dark red; assessed via MTT assay) under the same conditions (48 h in the presence of MeBlu +48 h culture; also shown separately as a column graph in the inset).
Entry inhibition by MeBlu for the original strain was also confirmed with a different pseudovirus, a SARS-CoV-2 spike plus GFP reporter bearing VSV-ΔG pseudovirus, i.e., vesicular stomatitis virus that lacks the VSV envelope glycoprotein) [30]. As described before [12], GFP fluorescence quantified using a live imaging system (Incucyte) served as a measure of infection, and normalized values were fitted with concentration–response curves to obtain IC50 (Figure 6). The value obtained in this assay with a different cell line (ACE2/Furin-overexpressing Vero E6 cells) IC50 = 2.5 μM (95% CI: 1.9–3.2 μM) was very consistent with that from the previous one, confirming the antiviral potential of MeBlu.
Figure 6.
Concentration-dependent inhibition of the entry of SARS-CoV-2 pseudovirus (VSV-ΔG) into hACE2/Furin-expressing cells by MeBlu. Entry of VSV-ΔG pseudoviruses bearing the SARS-CoV-2 S protein (plus GFP reporters) in ACE2/Furin overexpressing host cells (Vero E6) in the presence of increasing concentrations of MeBlu was quantified via GFP fluorescence in a live imaging system. Normalized data are shown on a semilogarithmic scale and fitted with a classic sigmoidal curve used to calculate IC50.
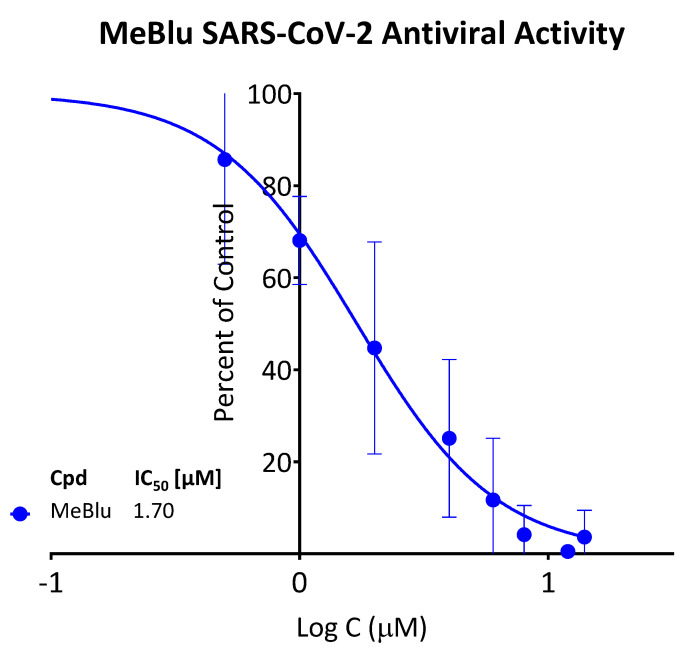
2.4. SARS-CoV-2 Antiviral Activity
Finally, the antiviral activity of MeBlu was also confirmed in a viral RNA reduction assay in Vero E6 cells infected with SARS-CoV-2 (B.1.5) at a multiplicity of infection (MOI) of 0.01 as described before [31,32]. In this assay with live virus, MeBlu was effective in blocking SARS-CoV-2 replication in Vero E6 cells with an IC50 of 1.70 μM (95% CI: 1.44–1.98) (Figure 7).
Figure 7.
Concentration-dependent inhibition of SARS-CoV-2 replication in Vero E6 cells by MeBlu. Vero E6 cells were infected with SARS-CoV-2 at an MOI of 0.01; treatments were performed for 48 h as described in the Methods. The viral yield in cell supernatant was quantified by droplet-digital PCR.
3. Discussion
Our results, summarized above, indicate that MeBlu inhibits the interaction of the SARS-CoV-2 spike protein with hACE2, including for the delta variant of concern (B.1.617.2), and reinforce the potential of MeBlu, a dye compound included in the WHO List of Essential Medicines [19,20,21,22] as an inexpensive preventive or therapeutic antiviral treatment for COVID-19. MeBlu, which was first synthesized at BASF (Badische Anilin und Soda Fabrik) by Heinrich Caro in 1876 [21], was, in fact, the first fully synthetic medicine, as it was used for the treatment of malaria since 1891 (until it was replaced by chloroquine during WW2) [20]. MeBlu is approved by the FDA for clinical use in the U.S. for the treatment of methemoglobinemia and is also used for some other therapeutic applications [20,22]. In the presence of light, MeBlu has broad-spectrum virucidal activity, and it has been used like this since 1991 to inactivate viruses in blood products prior to transfusions [33]. MeBlu-treated convalescent plasma has also been explored as treatment for COVID-19 patients, e.g., [34]. In addition to our results, others have also found evidence of possible antiviral and, in particular, anti-SARS-CoV-2 activity for MeBlu, even in the absence of UV-induced activation, as summarized in Table 1.
Table 1.
Summary of published peer-reviewed studies showing anti-SARS-CoV-2 activity for MeBlu.
| Assay | Cell Line | IC50 (μM) | Comment | Reference |
|---|---|---|---|---|
| PPI inhibition (SARS-CoV-2-S—hACE2) | N/A | 1–5 | Including VoCs (e.g., delta) | [23] and this work (VoC) |
| SARS-CoV-2 pseudovirus cell entry | HEK293T | 3–5 | Including VoCs (e.g., delta) | [23] and this work (VoC) |
| SARS-CoV-2 antiviral activity | Vero E6 | 1.7 | Inhibition of SARS-CoV-2 (B.1.5) replication | This work |
| Binding to SARS-CoV-2 S RBD | N/A | 0.36 | Bio-layer interferometry | [35] |
| SARS-CoV-2 antiviral activity | Vero E6 | ~0.3 | Also active against influenza virus H1N1 | [36] |
| SARS-CoV-2 antiviral activity | Vero E6 | 0.4–1.1 | clinically isolated SARS-CoV-2 strains (IHUMI-3 and IHUMI-6) | [37,38] |
| SARS-CoV-2 cytopathicity | Caco-2 | 2.03 | HTS hit, confirmed in concentration response (Supp Info in Res Square preprint) | [39] |
| VSV-SARS-CoV-2-Sdel18 pseudovirus entry | Vero E6 | 9 | HTS hit, confirmed in concentration response | [40] |
| Image-based multicycle replication assay (hCoV-229E) | Huh7 | 1.4 | HTS hit, confirmed in concentration response | [41] |
For example, Cagno, Tapparel, and coworkers at the University of Geneva (Geneva, Switzerland) found that MeBlu showed preventive or therapeutic virucidal activity against SARS-CoV-2 (as well as influenza virus H1N1) at low micromolar concentrations and in the absence of UV activation [36]. Their results also suggest that the antiviral activity of MeBlu might result from multiple mechanisms of action, as the extent of genomic RNA degradation was higher in the presence of light and after long exposure. Gendrot, Pradines, and coworkers at the Institut de Recherche Biomédicale des Armées (Marseille, France) found non-photoactivated MeBlu to inhibit SARS-CoV-2 replication in Vero E6 in vitro with an IC50 of 0.3 ± 0.03 μM [37] and then confirmed it later with two clinically isolated SARS-CoV-2 strains (IHUMI-3 and IHUMI-6; IC50s of 0.4 and 1.1 μM, respectively) [38]. In line with the observations of Cagno and coworkers, the study by Gendrot and coworkers in Vero E6 cells also suggested that MeBlu interacts at both entry and postentry stages of SARS-CoV-2 infection [38]. MeBlu was also identified in several drug repurposing (repositioning) high-throughput screening (HTS) assays looking for anti-SARS-CoV-2 activity and then confirmed as having low micromolar activity in concentration–response studies. For example, H. L. Xiong and coworkers at Xiamen University (Xiamen, China) found MeBlu to have an IC50 of 9 μM in a VSV-SARS-CoV-2-Sdel18 pseudovirus assay when tested as one of the 12 selected compounds of promising activity identified from the screening of 1403 FDA-approved drugs [40]. Finally, an image-based multicycle replication assay-based repurposing screening of a chemical library of 5440 compounds that are approved, in clinical trial or in preclinical development, by Murer, Greber, and coworkers at the University of Zurich (Zurich, Switzerland) identified MeBlu as one of two promising candidates (the other being mycophenolic acid) that have low micromolar activity against SARS-CoV-2 (1.4 μM) [41]. While Cagno et al. reported a good therapeutic index for MeBlu in Vero E6 cell-based assay (TI = 46.1/0.11 = 420) [36], this study by Murer et al. with Huh7 cells found it to be even lower (TI = 8.7/1.4 = 6.2) [41] than that obtained here with HEK293T cells (TI = 12–16).
Regarding the corresponding clinical applicability of MeBlu, it is important that concentration levels that seem to provide antiviral activity for MeBlu (e.g., IC50 = 0.5–5.0 μM) are well within the range of those obtained following normal clinical dosage (~3 mg/kg) even with oral administration. For example, 19 μM peak blood concentration were measured after 500 mg p.o. [42], and 6–7 μM trough levels were obtained after doses of 207 mg/day (69 mg, t.i.d., p.o.) [43]. The oral bioavailability (F ≈ 80%) and the terminal elimination half-life of MeBlu (t1/2 ≈ 14 h) [42] are in very reasonable ranges for once-daily oral administration. While MeBlu is generally safe, it is known to cause dose-dependent toxicity, in line with its relatively narrow therapeutic index seen here (TI ≈ 10–15; Figure 5). Undesired side effects (e.g., nausea, vomiting, and hemolysis) are increasingly common at doses higher than 7 mg/kg [19,21,22]. MeBlu is contraindicated in persons taking serotonin reuptake inhibitors or having hereditary glucose-6-phosphate dehydrogenase (G6PD) deficiency.
Along these lines, it has to be mentioned that while MeBlu clearly acts as an inhibitor of the SARS-CoV-2 S–hACE2 PPI (Figure 1 and Figure 2), it also seems to be a promiscuous PPI inhibitor, which clearly limits its specificity and, hence, usefulness. For example, it also inhibited TNFSF PPIs (e.g., CD40–CD40L, TNF-R1–TNFα) [23] as well as the IL-2R–IL-2 PPIs in our assays with similar low micromolar potency (Figure 3). The three-ring phenothiazine framework of MeBlu resembles somewhat that of xanthene dyes such as erythrosine B, rose Bengal, and phloxine, which we found before to also be promiscuous PPI inhibitors [44]. Furthermore, MeBlu is well-known to show polypharmacology and act on a multitude of targets [20]. While these further limit specificity and can cause unwanted effects, at least some of these effects could actually be beneficial in treating COVID-19 [45,46,47]. For example, its main mechanism of action behind its clinical use is its ability to reduce the oxidized form of hemoglobin when in a state of methemoglobinemia and increase its oxygen-binding capacity, which increases oxygen delivery to tissues [22]. This could be an important additional benefit in COVID-19 patients who often exhibit very low oxygen levels, i.e., silent (or happy) hypoxemia [48].
There is some, albeit limited, clinical evidence supporting the use of MeBlu to prevent or treat COVID-19, and most of it should be treated with caution as it was published not in internationally well-recognized peer-reviewed medical journals. The first support for a possible preventive role came from a retrospective study of 2500 patients treated with MeBlu (75 mg t.i.d.) as part of their cancer care in France. This found that none of them developed influenza-like illness during the early phases of the COVID-19 pandemic (March 2020) [49]. Currently, there are three trials documented as having been initiated with MeBlu for COVID-19 treatment (according to ClinicalTrial.gov accessed on 7 February 2022): one in Mexico (NCT04619290; 1 mL Prexablu and 50 min low-level light therapy s.i.d. for 7 days), one in Switzerland (NCT04635605; MeBlu 100 mg b.i.d. for 5 days), and one in Iran (NCT04370288; MeBlu 1 mg/kg as part of a three-drug last therapeutic option add-on cocktail with vitamin C 1500 mg/kg and N-acetyl cysteine 2000 mg/kg in critically ill COVID-19 patients). The last one completed Phase 2 and 3 studies with promising results claimed. In Phase 1, four of the five treated patients responded well to the treatment [50]. The Phase 2 extension was a randomized, controlled, open-label clinical trial involving 80 hospitalized patients with severe COVID-19. It indicated that the addition of MeBlu to the treatment protocol significantly improved oxygen saturation (SpO2) and respiratory distress (p < 0.001), resulting in decreased hospital stays (7.3 ± 4.7 vs. 11.7 ± 6.6 days, p = 0.004) and mortality (12.5% vs. 22.5%) [51]. A Phase 3 randomized, controlled, open-label clinical trial was completed in 223 hospitalized patients with confirmed severe cases of COVID-19. This also found that the addition of oral MeBlu to the standard care significantly shortened hospital stays (6.2 ± 3.1 vs. 10.6 ± 9.2, p < 0.001) and decreased mortality (12.2% vs. 21.4%, p = 0.07) [52].
Intravenous MeBlu (1 mg/kg) has been explored as a rescue therapy in 50 moderate-to-severe hypoxic COVID-19 patients with acute respiratory distress syndrome (ARDS) with promising results and no major side effects or adverse events [53]. Finally, inhaled applications have also been explored in some developing countries as respiratory treatments [54]. Two studies with nebulized MeBlu conducted in patients with COVID-19 infections in India claimed noticeable benefits: decrease in inflammatory markers and oxygen requirements and a trend toward reduced hospital stays (9.17 vs. 12 days) in an observational study with 63 patients divided into three groups [55] and beneficial effects on oxygen saturation and requirements in seven patients with severe COVID-19 and ARDS who did not respond to antiviral (remedesivir and favipiravir) and anti-inflammatory (tocilizumab, steroids, and heparin) treatments and oxygen inhalation but subsequently responded to MeBlu inhalation [56].
4. Materials and Methods
4.1. Binding Assays
Methylene blue and other test compounds used here were obtained from Sigma-Aldrich (St. Louis, MO, USA) and used as such. Purities (and catalog numbers) were as follows: methylene blue >95% (M4159), chloroquine >98.5% (C6628), hydroxychloroquine >98% (H0915), naphthol blue back >99% (70490), and sunset yellow FCF 90% (465224). Proteins used in the binding assays were obtained from Sino Biological (Wayne, PA, USA) as follows: human ACE2-Fc (10108-H05H), SARS-CoV-2 S1 (His tag, 40591-V08H), RBD (His tag, 40592-V08H), D614G S1 (His tag, 40591-V08H3), delta S1 (His tag, 40591-V08H23), delta RBD (His tag, 40592-V08H90), and HCoV-NL63 S1 (His tag, 40600-V08H). HepCV E (His tag, 9146-HC) and CD81-Fc (9144-CD) were from R&D Systems (Minneapolis, MN, USA); IL-2 (His tag, IL2-H52H8) and IL-2Rα-Fc (ILA-H5251) were from ACROBiosystems (Newark, DE, USA). Mutations in the delta S1 variant (40591-V08H23) are T19R, G142D, E156G, 157–158 deletion, L452R, T478K, D614G, and P681R. Mutations in the delta RBD variant (40592-V08H90) are L452R and T478K. Binding inhibition assays were performed in a 96-well cell-free format as described before [12,23]. Briefly, microtiter plates (Nunc F Maxisorp, 96-well; Thermo Fisher Scientific, Waltham, MA, USA) were coated overnight at 4 °C with 100 μL/well of Fc-conjugated receptor proteins diluted in PBS pH 7.2. This was followed by blocking with 200 μL/well of 2% BSA (A7030, Sigma-Aldrich, St. Louis, MO, USA) for 1 h at room temperature. Plates were then washed twice using washing solution (PBS pH 7.4, 0.05% Tween-20) and tapped dry before the addition of the tagged ligand protein and test compounds diluted in binding buffer (20 mM HEPES, pH 7.2) to give a total volume of 100 μL/well. After 1 h incubation, three washes were conducted, and a further 1 h incubation with anti-His HRP conjugate (652504; BioLegend, San Diego, CA, USA) diluted (1:20,000) in 2% BSA was used to detect the bound His-tagged ligand. Plates were washed four times before the addition of 100 μL/well of HRP substrate TMB (3,3′,5,5′-tetramethylbenzidine) and kept in the dark for up to 15 min. The reaction was stopped using 20 μL of 1 M H2SO4; absorbance values were read at 450 nm. The plated concentrations of Fc-conjugated receptor proteins were 1.0 μg/mL ACE2 for SARS-CoV-2 RBD and D614G S1 variant, 0.5 μg/mL ACE2 for delta RBD and delta S1, 2.0 μg/mL ACE2 for HCoV-NL63, 1.0 μg/mL IL-2Rα for IL-2, and 2.0 μg/mL CD81 for HepCV E. The concentrations of the ligands used in the inhibitory assays were 0.5 μg/mL for RBD, 1.0 μg/mL for D614G S1 variant and delta S1, 0.5 μg/mL for delta RBD, 20 μg/mL for HCoV-NL63, 0.03 μg/mL for IL-2, and 2.0 μg/mL for HepCV E. These values were selected following preliminary testing to optimize response (i.e., to produce a high-enough signal at conditions close to half-maximal response, EC50). For all compounds, 10 mM stock solutions in DMSO were used.
4.2. SARS-CoV-2 Pseudovirus Assays
For the BacMam-based assays, fluorescent biosensors from Montana Molecular (C1100R and C1110G, C1123G; Bozeman, MT, USA) were used per the instructions of the manufacturer with minor modifications as described before [12,23]. Mutations in the delta variant (C1123G) are T19R, V70F, T95I, G142D, E156-, F157-, R158G, A222V, W258L, K417N, L452R, T478K, D614G, P681R, and D950N. Briefly, HEK293T cells (CRL-3216; ATCC, Manassas, VA, USA) were seeded onto 96-well plates at a density of 5 × 104 cells per well in 100 μL complete medium (DMEM supplemented with 10% fetal bovine serum). A transduction mixture containing ACE2 BacMam Red-Reporter virus (1.8 × 108 VG/mL) and 2 mM sodium butyrate prepared in complete medium was added (50 μL per well) and incubated for 24 h at 37 °C and 5% CO2. Medium was removed, washed once with PBS, and replaced with 100 μL fresh medium containing test compound at selected concentrations, preincubating for 30 min at 37 °C and 5% CO2. A transduction mixture containing pseudo-SARS-CoV-2 Green-Reporter pseudovirus or pseudo-SARS-CoV-2 spike Delta variant Green-Receptor pseudovirus (3.3 × 108 VG/mL) and 2 mM sodium butyrate prepared in complete medium was added (50 μL per well) and incubated for 48 h at 37 °C and 5% CO2. Medium was removed, washed once with PBS, replaced with 150 μL fresh medium, and cells were incubated for an additional 48 h at 37 °C and 5% CO2. Cell fluorescence was detected using an EVOS FL microscope (Life Technologies, Carlsbad, CA, USA) and was quantified in ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA) [57] using the Analyze Particles tool after thresholding for the corresponding colors.
For the VSV-ΔG-based assay, the SARS-CoV-2 S-bearing pseudovirus generated in-house was used as described before [12,30]. Vero E6 cells (African Green Monkey renal epithelial cells; ATCC cat. no. CRL-1586) engineered to overexpress hACE2/Furin were seeded in 24-well plates to obtain a confluence of 80%. The medium was replaced with 250 μL cell-culture medium (DMEM) supplemented with 2% fetal bovine serum, 1% penicillin/streptomycin/glutamine, and the compounds of interest for 30 min at 37 °C. Cells were inoculated with the SARS-CoV-2 spike protein pseudotyped VSV-ΔG (multiplicity of infection = 0.05) by adding complete media to bring the final volume to 400 μL, and 20 h post infection; plates were scanned with a 10× objective using the Incucyte ZOOM imaging system (Sartorius, Ann Arbor, MI, USA). Normalized GFP expression (GCU) values per image were obtained by dividing the Total Green Object Integrated Intensity (Green Calibrated Units (GCU) × μm2/image) values of each image by its corresponding Total Phase Area (μm2/image) as described before [12,30].
4.3. Cytotoxicity Assay
For the MTT assay, HEK293T cells were seeded onto 96 wells with the density of 5.0 × 104 cells per well. Cells were treated with MeBlu for 48 h and then replaced with fresh medium for an additional 48 h. After that, cells were incubated with MTT reagent at 37 °C for 2 h. The medium was removed, and dimethyl sulfoxide was added. Cell viability was evaluated using a microplate reader at 570 nm absorbance (SpectraMax iD3, Molecular DEVICES, San Jose, CA, USA).
4.4. Surface Plasmon Resonance
SPR was performed at Reaction Biology (Malvern, PA, USA) using single and multicycle kinetics to assess the binding to the SARS-CoV-2 spike RBD (His-tagged, Arg319-Ph541; RayBiotech; Peachtree Corners, GA, USA) and determine the kinetics/affinity. Measurements were performed on a Biacore 8K (Cytiva) with a series S CM5 (Cytiva) sensor chip. Spike RBD was immobilized via amine coupling at pH 5.5 to immobilization levels of ~1500–2300 RU (single-cycle) and ~2500 RU (multi-cycle). A running buffer of PBS-p+ (20 mM phosphate buffer with 2.7 mM KCl, 137 mM NaCl, and 0.05% surfactant Tween20) with 1% DMSO was used. All data was solvent corrected, reference subtracted, and blank subtracted using the Biacore Insight Evaluation Software. To try and obtain kinetic/affinity parameters, the data was evaluated using a 1:1 kinetic binding model. However, the binding behavior appears to be more complex than can be approximated using a 1:1 binding model, which prevents a quantitative analysis of the SPR binding data.
4.5. In Vitro SARS-CoV-2 Antiviral Assay
Assay was performed under BSL-4 conditions [31,32]. Briefly, Vero E6 cells (European Collection of Authenticated Cell Cultures) were seeded onto 96-well plates at a density of 3 × 104 cells per well in 100 µL cell-culture media that consisted of DMEM (Lonza, Basel, Switzerland) 1% penicillin–streptomycin (Lonza, Basel, Switzerland) and 2% heat-inactivated fetal bovine serum (Gibco, Waltham, MA, USA) the day before the experiment. On the following day, cells were treated with the test compound (MeBlu) at predefined concentrations (14, 12, 8, 6, 4, 2, 1, and 0.5 μM) obtained by diluting DMSO stock solutions with the cell-culture media. Immediately after treatment, cells were infected with SARS-CoV-2 (hCoV-19/Hungary/SRC_isolate_2/2020, GISAID ID: EPI_ISL_483637) at MOI: 0.01. Cells were incubated for 30 min at 37 °C, then the supernatant was replaced with fresh maintenance media supplemented with the compounds at the appropriate concentration. Nucleic acid extraction was made from the supernatant using a magnetic-bead-based nucleic acid isolation system (Zybio, Chongqing, China; EXM 3000 Nucleic Acid Isolation System) 48 h postinfection. Viral copy numbers were determined using SARS-CoV-2 RdRp gene-specific primers and probe (see [31]) and droplet digital PCR (Bio-Rad Laboratories Inc., Hercules, CA, USA; QX200 Droplet Digital PCR System). Copy numbers were normalized to the mean for the untreated, infected wells; n = 3 biological replicates. IC50 values were determined using nonlinear regression analysis (four-parameter logistic model; GraphPad Prism 8).
4.6. Statistics and Data Fitting
Binding inhibition and cell assays were performed in at least duplicates per plates, virus assays in at least triplicates, and assays were performed as at least two or three independent experiments. As before [12,23], binding data were converted to percent inhibition and fitted with standard log inhibitor vs. normalized response models [58] using nonlinear regression in GraphPad Prism (GraphPad, La Jolla, CA, USA) to establish half-maximal inhibitory concentrations (IC50). All activity data are presented as concentration–response curves (mean ± SD) and quantitative descriptors (i.e., IC50, TC50) are included with their 95% confidence interval (CI) per recommended guidelines on reporting data and statistical analysis [59].
5. Conclusions
In conclusion, we found that MeBlu is a low-micromolar inhibitor of the PPI between SARS-CoV-2 spike and its cognate receptor ACE2, including for mutants such as the delta variant of concern. MeBlu also inhibited the cell entry of spike-bearing pseudoviruses both for the original strain and the delta variant, and it blocked SARS-CoV-2 (B.1.5.) virus replication in Vero E6 cells. Thus, MeBlu, a drug approved for clinical use, shows potential as a repurposed (repositioned) antiviral agent for the prevention and treatment of COVID-19. While MeBlu shows strong polypharmacology, seems to be a nonspecific PPI inhibitor, and has a relatively narrow therapeutic index, it still has potential as an inexpensive, widely available, and orally administrable treatment for diseases caused by SARS-CoV-2, its variants of concern, and possibly other spike-expressing CoVs.
Acknowledgments
We are grateful to György Miklós Keserű for his help facilitating the collaboration with the National Laboratory of Virology group at the University of Pécs, Hungary.
Abbreviations
The following abbreviations are used in this manuscript:
| ACE2 | angiotensin converting enzyme 2 |
| CoV | coronavirus |
| MeBlu | methylene blue |
| NBlBk | naphthol blue black |
| PPI | protein–protein interaction |
| SARS | severe acute respiratory syndrome |
| SMI | small-molecule inhibitor |
| SPR | surface plasmon resonance |
| SY | sunset yellow FCF |
| TNF | tumor necrosis factor |
| VoC | variant of concern |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph15050621/s1, Figure S1: Binding of MeBlu to SARS-CoV-2 spike RBD as assessed via surface plasmon resonance (SPR).
Author Contributions
Conceptualization, P.B.; methodology, S.-T.C., H.P., R.E., J.M.C.C., L.A.S. and P.B.; formal analysis, S.-T.C., H.P., R.E., J.M.C.C. and P.B.; investigation, S.-T.C., H.P., A.K., R.E. and J.M.C.C.; resources, L.A.S., F.J. and P.B.; data curation, S.-T.C., H.P., R.E., J.M.C.C. and P.B.; writing—original draft preparation, P.B.; writing—review and editing, S.-T.C., H.P., A.K., R.E., J.M.C.C., L.A.S., F.J. and P.B.; supervision, L.A.S., F.J. and P.B.; project administration, P.B.; funding acquisition, L.A.S., F.J. and P.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Diabetes Research Institute Foundation, Thematic Excellence Program 2021 Health and National Defense, National Security Subprograms of the Hungarian National Research, Development and Innovation Office of the University of Pécs (grant number TKP2021-EGA-10 and TKP2021-NVA-07), the European Commission, Comprehensive Development for Implementing Smart Specialization Strategies at the University of Pécs (grant number EFOP-3.6.1.-16-2016-00004), the National Institute of Health (grant number 1R01HL140468) and the Miami Heart Research Institute.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Matheson N.J., Lehner P.J. How does SARS-CoV-2 cause COVID-19? Science. 2020;369:510–511. doi: 10.1126/science.abc6156. [DOI] [PubMed] [Google Scholar]
- 2.Santacroce L., Charitos I.A., Carretta D.M., De Nitto E., Lovero R. The human coronaviruses (HCoVs) and the molecular mechanisms of SARS-CoV-2 infection. J. Mol. Med. 2021;99:93–106. doi: 10.1007/s00109-020-02012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.V’Kovski P., Kratzel A., Steiner S., Stalder H., Thiel V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021;19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiwari V., Beer J.C., Sankaranarayanan N.V., Swanson-Mungerson M., Desai U.R. Discovering small-molecule therapeutics against SARS-CoV-2. Drug Discov. Today. 2020;25:1535–1544. doi: 10.1016/j.drudis.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nabel K.G., Clark S.A., Shankar S., Pan J., Clark L.E., Yang P., Coscia A., McKay L.G.A., Varnum H.H., Brusic V., et al. Structural basis for continued antibody evasion by the SARS-CoV-2 receptor binding domain. Science. 2022;375:eabl6251. doi: 10.1126/science.abl6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Solidarity Trial Consortium Repurposed antiviral drugs for COVID-19—Interim WHO Solidarity trial results. N. Engl. J. Med. 2020;384:497–511. doi: 10.1056/nejmoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tummino T.A., Rezelj V.V., Fischer B., Fischer A., O’Meara M.J., Monel B., Vallet T., White K.M., Zhang Z., Alon A., et al. Drug-induced phospholipidosis confounds drug repurposing for SARS-CoV-2. Science. 2021;373:541–547. doi: 10.1126/science.abi4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., Kovalchuk E., Gonzalez A., Delos Reyes V., Martin-Quiros A., Caraco Y., Williams-Diaz A., Brown M.L., et al. Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N. Engl. J. Med. 2021;386:509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owen D.R., Allerton C.M.N., Anderson A.S., Aschenbrenner L., Avery M., Berritt S., Boras B., Cardin R.D., Carlo A., Coffman K.J., et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374:1586–1593. doi: 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- 10.Chen J., Song Y., Bojadzic D., Tamayo-Garcia A., Landin A.M., Blomberg B.B., Buchwald P. Small-molecule inhibitors of the CD40-CD40L costimulatory protein-protein interaction. J. Med. Chem. 2017;60:8906–8922. doi: 10.1021/acs.jmedchem.7b01154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bojadzic D., Buchwald P. Toward small-molecule inhibition of protein-protein interactions: General aspects and recent progress in targeting costimulatory and coinhibitory (immune checkpoint) interactions. Curr. Top. Med. Chem. 2018;18:674–699. doi: 10.2174/1568026618666180531092503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bojadzic D., Alcazar O., Chen J., Chuang S.T., Condor Capcha J.M., Shehadeh L.A., Buchwald P. Small-molecule inhibitors of the coronavirus spike—ACE2 protein-protein interaction as blockers of viral attachment and entry for SARS-CoV-2. ACS Infect. Dis. 2021;7:1519–1534. doi: 10.1021/acsinfecdis.1c00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souers A.J., Leverson J.D., Boghaert E.R., Ackler S.L., Catron N.D., Chen J., Dayton B.D., Ding H., Enschede S.H., Fairbrother W.J., et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 14.Gadek T.R., Burdick D.J., McDowell R.S., Stanley M.S., Marsters J.C., Jr., Paris K.J., Oare D.A., Reynolds M.E., Ladner C., Zioncheck K.A., et al. Generation of an LFA-1 antagonist by the transfer of the ICAM-1 immunoregulatory epitope to a small molecule. Science. 2002;295:1086–1089. doi: 10.1126/science.295.5557.1086. [DOI] [PubMed] [Google Scholar]
- 15.Meanwell N.A., Krystal M.R., Nowicka-Sans B., Langley D.R., Conlon D.A., Eastgate M.D., Grasela D.M., Timmins P., Wang T., Kadow J.F. Inhibitors of HIV-1 attachment: The discovery and development of temsavir and its prodrug fostemsavir. J. Med. Chem. 2018;61:62–80. doi: 10.1021/acs.jmedchem.7b01337. [DOI] [PubMed] [Google Scholar]
- 16.Arkin M.R., Wells J.A. Small-molecule inhibitors of protein-protein interactions: Progressing towards the dream. Nat. Rev. Drug Discov. 2004;3:301–317. doi: 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]
- 17.Milroy L.G., Grossmann T.N., Hennig S., Brunsveld L., Ottmann C. Modulators of protein-protein interactions. Chem. Rev. 2014;114:4695–4748. doi: 10.1021/cr400698c. [DOI] [PubMed] [Google Scholar]
- 18.Scott D.E., Bayly A.R., Abell C., Skidmore J. Small molecules, big targets: Drug discovery faces the protein-protein interaction challenge. Nat. Rev. Drug Discov. 2016;15:533–550. doi: 10.1038/nrd.2016.29. [DOI] [PubMed] [Google Scholar]
- 19.Clifton J., 2nd, Leikin J.B. Methylene blue. Am. J. Ther. 2003;10:289–291. doi: 10.1097/00045391-200307000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Schirmer R.H., Adler H., Pickhardt M., Mandelkow E. “Lest we forget you—methylene blue...”. Neurobiol. Aging. 2011;32:2325.e7–2325.e16. doi: 10.1016/j.neurobiolaging.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Oz M., Lorke D.E., Hasan M., Petroianu G.A. Cellular and molecular actions of Methylene Blue in the nervous system. Med. Res. Rev. 2011;31:93–117. doi: 10.1002/med.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bistas E., Sanghavi D. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2020. Methylene blue. [PubMed] [Google Scholar]
- 23.Bojadzic D., Alcazar O., Buchwald P. Methylene blue inhibits the SARS-CoV-2 spike—ACE2 protein-protein interaction—a mechanism that can contribute to its antiviral activity against COVID-19. Front. Pharmacol. 2021;11:600372. doi: 10.3389/fphar.2020.600372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L., Jackson C.B., Mou H., Ojha A., Peng H., Quinlan B.D., Rangarajan E.S., Pan A., Vanderheiden A., Suthar M.S., et al. SARS-CoV-2 spike-protein D614G mutation increases virion spike density and infectivity. Nat. Commun. 2020;11:6013. doi: 10.1038/s41467-020-19808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC, Center for Disease Control and Prevention SARS-CoV-2 Variant Classifications and Definitions. [(accessed on 14 February 2022)];2021 Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html.
- 26.Kupferschmidt K. Evolving threat. Science. 2021;373:844–849. doi: 10.1126/science.373.6557.844. [DOI] [PubMed] [Google Scholar]
- 27.McGovern S.L., Caselli E., Grigorieff N., Shoichet B.K. A common mechanism underlying promiscuous inhibitors from virtual and high-throughput screening. J. Med. Chem. 2002;45:1712–1722. doi: 10.1021/jm010533y. [DOI] [PubMed] [Google Scholar]
- 28.Aldrich C., Bertozzi C., Georg G.I., Kiessling L., Lindsley C., Liotta D., Merz K.M., Jr., Schepartz A., Wang S. The ecstasy and agony of assay interference compounds. J. Med. Chem. 2017;60:2165–2168. doi: 10.1021/acs.jmedchem.7b00229. [DOI] [PubMed] [Google Scholar]
- 29.Arkin M.R., Tang Y., Wells J.A. Small-molecule inhibitors of protein-protein interactions: Progressing toward the reality. Chem. Biol. 2014;21:1102–1114. doi: 10.1016/j.chembiol.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Condor Capcha J.M., Lambert G., Dykxhoorn D.M., Salerno A.G., Hare J.M., Whitt M.A., Pahwa S., Jayaweera D.T., Shehadeh L.A. Generation of SARS-CoV-2 spike pseudotyped virus for viral entry and neutralization assays: A 1-week protocol. Front. Cardiovasc. Med. 2020;7:618651. doi: 10.3389/fcvm.2020.618651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bajusz D., Wade W.S., Satala G., Bojarski A.J., Ilas J., Ebner J., Grebien F., Papp H., Jakab F., Douangamath A., et al. Exploring protein hotspots by optimized fragment pharmacophores. Nat. Commun. 2021;12:3201. doi: 10.1038/s41467-021-23443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bereczki I., Papp H., Kuczmog A., Madai M., Nagy V., Agócs A., Batta G., Milánkovits M., Ostorházi E., Mitrović A., et al. Natural apocarotenoids and their synthetic glycopeptide conjugates inhibit SARS-CoV-2 replication. Pharmaceuticals. 2021;14:1111. doi: 10.3390/ph14111111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lozano M., Cid J., Muller T.H. Plasma treated with methylene blue and light: Clinical efficacy and safety profile. Transfus. Med. Rev. 2013;27:235–240. doi: 10.1016/j.tmrv.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Alemany A., Millat-Martinez P., Corbacho-Monne M., Malchair P., Ouchi D., Ruiz-Comellas A., Ramirez-Morros A., Rodriguez Codina J., Amado Simon R., Videla S., et al. High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: A randomised, placebo-controlled trial. Lancet Respir. Med. 2022;10:278–288. doi: 10.1016/S2213-2600(21)00545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coghi P., Yang L.J., Ng J.P.L., Haynes R.K., Memo M., Gianoncelli A., Wong V.K.W., Ribaudo G. A drug repurposing approach for antimalarials interfering with SARS-CoV-2 spike protein receptor binding domain (RBD) and human angiotensin-converting enzyme 2 (ACE2) Pharmaceuticals. 2021;14:954. doi: 10.3390/ph14100954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cagno V., Medaglia C., Cerny A., Cerny T., Zwygart A.C., Cerny E., Tapparel C. Methylene blue has a potent antiviral activity against SARS-CoV-2 and H1N1 influenza virus in the absence of UV-activation in vitro. Sci. Rep. 2021;11:14295. doi: 10.1038/s41598-021-92481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gendrot M., Andreani J., Duflot I., Boxberger M., Bideau M.L., Mosnier J., Jardot P., Fonta I., Rolland C., Bogreau H., et al. Methylene blue inhibits the replication of SARS-CoV-2 in vitro. Int. J. Antimicrob. Agents. 2020;56:106202. doi: 10.1016/j.ijantimicag.2020.106202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gendrot M., Jardot P., Delandre O., Boxberger M., Andreani J., Duflot I., Le Bideau M., Mosnier J., Fonta I., Hutter S., et al. In vitro evaluation of the antiviral activity of methylene blue alone or in combination against SARS-CoV-2. J. Clin. Med. 2021;10:3007. doi: 10.3390/jcm10143007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellinger B., Bojkova D., Zaliani A., Cinatl J., Claussen C., Westhaus S., Keminer O., Reinshagen J., Kuzikov M., Wolf M., et al. A SARS-CoV-2 cytopathicity dataset generated by high-content screening of a large drug repurposing collection. Sci. Data. 2021;8:70. doi: 10.1038/s41597-021-00848-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiong H.L., Cao J.L., Shen C.G., Ma J., Qiao X.Y., Shi T.S., Ge S.X., Ye H.M., Zhang J., Yuan Q., et al. Several FDA-approved drugs effectively inhibit SARS-CoV-2 infection in vitro. Front. Pharmacol. 2021;11:609592. doi: 10.3389/fphar.2020.609592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murer L., Volle R., Andriasyan V., Petkidis A., Gomez-Gonzalez A., Yang L., Meili N., Suomalainen M., Bauer M., Sequeira D., et al. Identification of broad anti-coronavirus chemical agents for repurposing against SARS-CoV-2 and variants of concern. Curr. Res. Vir. Sci. 2022;3:100019. doi: 10.1016/j.crviro.2022.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walter-Sack I., Rengelshausen J., Oberwittler H., Burhenne J., Mueller O., Meissner P., Mikus G. High absolute bioavailability of methylene blue given as an aqueous oral formulation. Eur. J. Clin. Pharmacol. 2009;65:179–189. doi: 10.1007/s00228-008-0563-x. [DOI] [PubMed] [Google Scholar]
- 43.Baddeley T.C., McCaffrey J., Storey J.M., Cheung J.K., Melis V., Horsley D., Harrington C.R., Wischik C.M. Complex disposition of methylthioninium redox forms determines efficacy in tau aggregation inhibitor therapy for Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2015;352:110–118. doi: 10.1124/jpet.114.219352. [DOI] [PubMed] [Google Scholar]
- 44.Ganesan L., Margolles-Clark E., Song Y., Buchwald P. The food colorant erythrosine is a promiscuous protein-protein interaction inhibitor. Biochem. Pharmacol. 2011;81:810–818. doi: 10.1016/j.bcp.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 45.Scigliano G., Scigliano G.A. Acute respiratory distress syndrome from COVID-19: A perfect storm from free radicals? Proposal for a new treatment. Med. Hypotheses. 2020;144:110120. doi: 10.1016/j.mehy.2020.110120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scigliano G., Scigliano G.A. Methylene blue in COVID-19. Med. Hypotheses. 2021;146:110455. doi: 10.1016/j.mehy.2020.110455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dabholkar N., Gorantla S., Dubey S.K., Alexander A., Taliyan R., Singhvi G. Repurposing methylene blue in the management of COVID-19: Mechanistic aspects and clinical investigations. Biomed. Pharmacother. 2021;142:112023. doi: 10.1016/j.biopha.2021.112023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tobin M.J., Laghi F., Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am. J. Respir. Crit. Care Med. 2020;202:356–360. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henry M., Summa M., Patrick L., Schwartz L. A cohort of cancer patients with no reported cases of SARS-CoV-2 infection: The possible preventive role of methylene blue. Substantia. 2020;4:888. doi: 10.13128/Substantia-888. [DOI] [Google Scholar]
- 50.Alamdari D.H., Moghaddam A.B., Amini S., Keramati M.R., Zarmehri A.M., Alamdari A.H., Damsaz M., Banpour H., Yarahmadi A., Koliakos G. Application of methylene blue-vitamin C-N-acetyl cysteine for treatment of critically ill COVID-19 patients, report of a phase-I clinical trial. Eur. J. Pharmacol. 2020:173494. doi: 10.1016/j.ejphar.2020.173494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alamdari D.H., Lotfabadi S.H., Moghaddam A.B., Safari H., Mozdourian M., Javidarabshahi Z., Peivandi-Yazdi A., Ali-Zeraati A., Sedaghat A., Poursadegh F., et al. Methylene blue for treatment of hospitalized COVID-19 patients: A randomized, controlled, open-label clinical trial, phase 2. Rev. De Investig. Clínica. 2021;73:190–198. doi: 10.24875/ric.21000028. [DOI] [PubMed] [Google Scholar]
- 52.Alamdari D.H., Lotfabadi S.H., Darban B.M., Agheli-Rad M., Saadatian S., Hashemi S.H., Ahmadabadi F.B., Morovatdar N., Arastoo M., Bhushan B. Methylene blue for treatment of hospitalized COVID-19 patients, randomized, controlled, open-label clinical trial, Phase 3. [(accessed on 7 February 2022)];Aristotle Biomed. J. 2021 3:12–18. Available online: https://ejournals.lib.auth.gr/ABJ/article/view/8158. [Google Scholar]
- 53.Mahale N., Godavarthy P., Marreddy S., Gokhale S.D., Funde P., Rajhans P.A., Akole P.V., Pawar B., Bhurke B., Dalvi P., et al. Intravenous methylene blue as a rescue therapy in the management of refractory hypoxia in COVID-19 ARDS patients: A case series. Indian J. Crit. Care Med. 2021;25:934–938. doi: 10.5005/jp-journals-10071-23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Golwalkar D. Treatment for COVID-19 Using Methylene Blue. Medium. 2020. [(accessed on 21 August 2020)]. Available online: https://medium.com/@dr.deepak.golwalkar/treatment-for-covid-19-using-methylene-blue-d23fc5a31a4d.
- 55.Patidar V., Sharma A., Bhoraskar S., Tripathi A.P., Dhaneriya S. The role of nebulized methylene blue (NMB) in the management of COVID-19 cases: An observational study. Int. J. Med. Arts. 2022;4:2129–2132. doi: 10.21608/ijma.2022.112347.1416. [DOI] [Google Scholar]
- 56.Bawaskar H.S., Bawaskar P.H. Role of methylene blue in the management of mild, moderate and severe COVID-19 disease. J. Fam. Med. Prim. Care. 2022;11:812–814. doi: 10.4103/jfmpc.jfmpc_2071_21. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buchwald P. A single unified model for fitting simple to complex receptor response data. Sci. Rep. 2020;10:13386. doi: 10.1038/s41598-020-70220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Michel M.C., Murphy T.J., Motulsky H.J. New author guidelines for displaying data and reporting data analysis and statistical methods in experimental biology. J. Pharmacol. Exp. Ther. 2020;372:136–147. doi: 10.1124/jpet.119.264143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article and supplementary material.