Abstract
Background:
While males are more likely diagnosed with cannabis use disorder (CUD), females are more susceptible to developing and maintaining CUD. Yet, for both sexes, CUD is associated with high rates of comorbid mental illness (MI).
Objectives:
To identify and compare sex differences in the prevalence of comorbid CUD amongst individuals with/without MIs.
Methods:
This systematic review generated pooled odds ratios (OR) and 95% confidence intervals (CI) from 37 studies (including clinical trials, cohort, and case-control studies) among individuals with and without MIs, quantifying sex differences in rates of comorbid CUD. A meta-analysis was also completed.
Results:
In the CUD-only group, males were twice as likely to have CUD than females (OR = 2.0, CI = 1.9–2.1). Among MIs, males were more likely than females to have CUD comorbid with schizophrenia (OR ~2.6, CI = 2.5–27) and other psychotic, mood, and substance use disorders (1 > OR <2.2, CI = 0.7–2.6). The reverse association (females > males) was observed for anxiety disorders and antisocial personality disorder (OR = 0.8, CI = 0.7–1.0). Among females. MIs increased the likelihood of having CUD, except for psychotic disorders and depression. A meta-analysis was inconclusive due to high heterogeneity across studies. Thus, comparisons across MI groups were not possible.
Conclusion:
While males are more likely to be diagnosed with CUD, there are important sex differences in the prevalence of CUD across MI diagnoses that should be taken into account when approaching CUD prevention and determining treatment efficacy.
Keywords: Cannabis, cannabis use disorder, sex differences, mental illness, comorbidity, schizophrenia, depression, bipolar disorder, meta-analysis
Introduction
The prevalence of cannabis use and cannabis use disorders (CUD) has been rising worldwide, which may reflect the changing legal landscape surrounding cannabis use and reduced perceptions of risk (1). Globally, consumption of cannabis (annual prevalence) is estimated at 2.5% (2), while 0.2% of the global population is diagnosed with CUD (3). As recreational and medical use have become more widespread, the United States reported a 50–120% increase in 2016 in the annual prevalence of cannabis use, daily cannabis use, and mild CUD (4). In Canada, the prevalence of weekly cannabis use doubled and occasional use increased by 50% after legalization (5,6). Moreover, the number of individuals seeking treatment for CUD globally has been on an upward trajectory, with Europe, Australia, and the United States (US) recently reporting increases of 30% (7). Detrimental consequences associated with CUD include increased risk of psychosis and psychosocial impairment (8), and high healthcare costs, with CUD-related hospital costs estimated ~$4.5 billion annually (9). Given that there are no approved pharmacological treatments, and behavioral treatments are only modestly effective for CUD, problematic cannabis use continues to be a significant public health concern (7).
It is well documented that CUD manifests differently between males and females in terms of development, severity, trajectory, and responsiveness to treatment (10,11). For instance, compared to females, males have an earlier age of onset of cannabis use (12) and a higher probability of initiating cannabis use (13). Males are also twice as likely to continue using cannabis than females (14) (past year prevalence 4.2% versus 1.7%, respectively), contributing to the higher rates of CUD observed among males (3.5%) compared to females (1.7%) (15). Social factors including cultural, familial, and socioeconomic may explain why males have a greater likelihood of CUD than females (10,16–18). For instance, studies show that males with CUD were more likely to be older than 45, have a high school education, or less and income over 20,000 USD and less likely to be unemployed or widowed/divorced compared to females (10,19). Yet, more recent data demonstrate that cannabis use is increasing at a faster rate among females relative to males. The National Survey of Drug Use and Health (NSDUH) found that between 2015 and 2018 the prevalence of cannabis use among females (aged 18–25) increased by 3.8% compared to males who showed increases of only 0.8% (20), suggesting that the sex difference in cannabis use prevalence is narrowing. The increasing rates of cannabis use among females raise concern given that females may have enhanced susceptibility to develop and maintain problematic cannabis use. For example, compared to males, females show greater sensitivity to the reinforcing properties of cannabis, have an accelerated progression to CUD (e.g., telescoping effects), experience more severe withdrawal symptoms, and exhibit poorer treatment outcomes (see Cooper and Craft, 2018 (21)). Thus, increasing rates of cannabis use among females are of high clinical relevance.
A significant factor that complicates the course and treatment of CUD is that nearly 100% of individuals with CUD are diagnosed with a mental illness (MI) (e.g., schizophrenia, depression, anxiety, or substance use disorders (SUDs)) (22). Yet, current treatments for CUD that are sex-specific (23) do not typically consider comorbid MIs.
To date, the influence of sex on the rates of CUD comorbid with MIs remains largely unexplored. Therefore, this systematic review and meta-analysis aimed to identify and compare sex differences among individuals with and without MI on rates of CUD, measured by pooled odds ratios (ORs). We then discuss cannabis use and putative neurobiological mechanisms that may contribute to sex differences in CUD comorbid with MIs. As policies related to cannabis use are rapidly evolving across the globe leading to increased accessibility, availability, and reduced perception of risk (7), more individuals (with and without MIs) are at risk for developing CUD (24); this may be particularly true for females who were previously deterred from obtaining cannabis illegally (25). Therefore, a better understanding of the difference in the rates of CUD comorbid with MIs between males and females is necessary to guide the development of sex-specific prevention strategies and treatment approaches for these patients.
Methods
Study selection
This systematic review and meta-analysis examined rates of CUD in cannabis users with and without comorbid MIs, as a function of sex, and followed the guidelines for Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISM-A) (26). This search strategy used Google Scholar, PubMed, Medline, and Psychlnfo databases to search for papers that included the keywords and were published in English from database inception to February 28th, 2020. The search included the keywords: ‘cannabis use disorder,’ ‘gender,’ and ‘sex.’ Keywords were also searched in combination (i.e., ‘cannabis use disorder’ AND ‘prevalence’ AND [‘mental disorder’ OR specific psychiatric comorbidity (e.g., ‘schizophrenia,’ ‘psychotic disorder,’ ‘depression,’ ‘bipolar disorder,’ ‘anxiety disorder,’ ‘mood disorder,’ ‘posttraumatic stress disorder (PTSD),’ ‘personality disorder,’ ‘substance use disorder (SUD)’)] AND (‘gender’ OR ‘sex’)). We included studies which referred to CUD as cannabis abuse and/or dependence. Three authors (KK, DJEL, RAR) initially assessed titles and abstracts identified by the search and reviewed the full text of the remaining articles for inclusion. Any conflicts that arose were resolved by the senior author (TPG).
Studies were included if they met the following criteria: (1) included population-based data on individuals with and without CUD and with or without comorbid MI; (2) for the CUD-only group, had a diagnosis of past year or lifetime CUD; (3) for the comorbid MI groups, had a diagnosis of past year or lifetime CUD and the presence of one diagnosis for MI; and (4) the sex distribution within the study sample was provided. Exclusion criteria included: (1) studies examining recreational or medical cannabis use not meeting criteria for CUD; (2) for the CUD-only group had a diagnosis for MI other than CUD; (3) treatment trials, reviews, case series/reports, commentaries, opinion, unpublished studies, conference posters/abstracts; and (4) when multiple studies were found reporting on the same population cohort, only the most recent study was included.
Statistical approach
To provide an estimate of the extent to which sex is associated with having CUD comorbid with MIs, ORs for individual studies, and pooled ORs for each MI, along with 95% Confidence Intervals (CI) were calculated using SPSS version 26 for Windows. For each study, diagnostic outcome (with or without CUD) and sex (male or female) were represented by arranging observed counts into 2 × 2 tables to calculate individual ORs. The counts used in the table were either available in the original text or calculated from the data provided in the article. For each MI, study level counts were summated into a 2 × 2 table from which an overall comorbid MI pooled OR, and respective 95% CI was calculated. Comparisons of pooled ORs were performed by displaying pooled ORs and their respective CIs in a forest plot (see Figure 2). OR >1 indicates a greater likelihood of CUD in males in comparison to females, while OR <1 indicates a lower likelihood of CUD in males in comparison to females (OR = 1 indicates equal likelihood of CUD in both sexes). We avoided statistical pairwise testing between pooled ORs, since these analyses were exploratory, and such an approach would result in low power due to correcting for multiple comparisons.
Figure 2. Forest plot of pooled ORs and 95% CIs for CUD comorbid with SMIs in males compared to females.
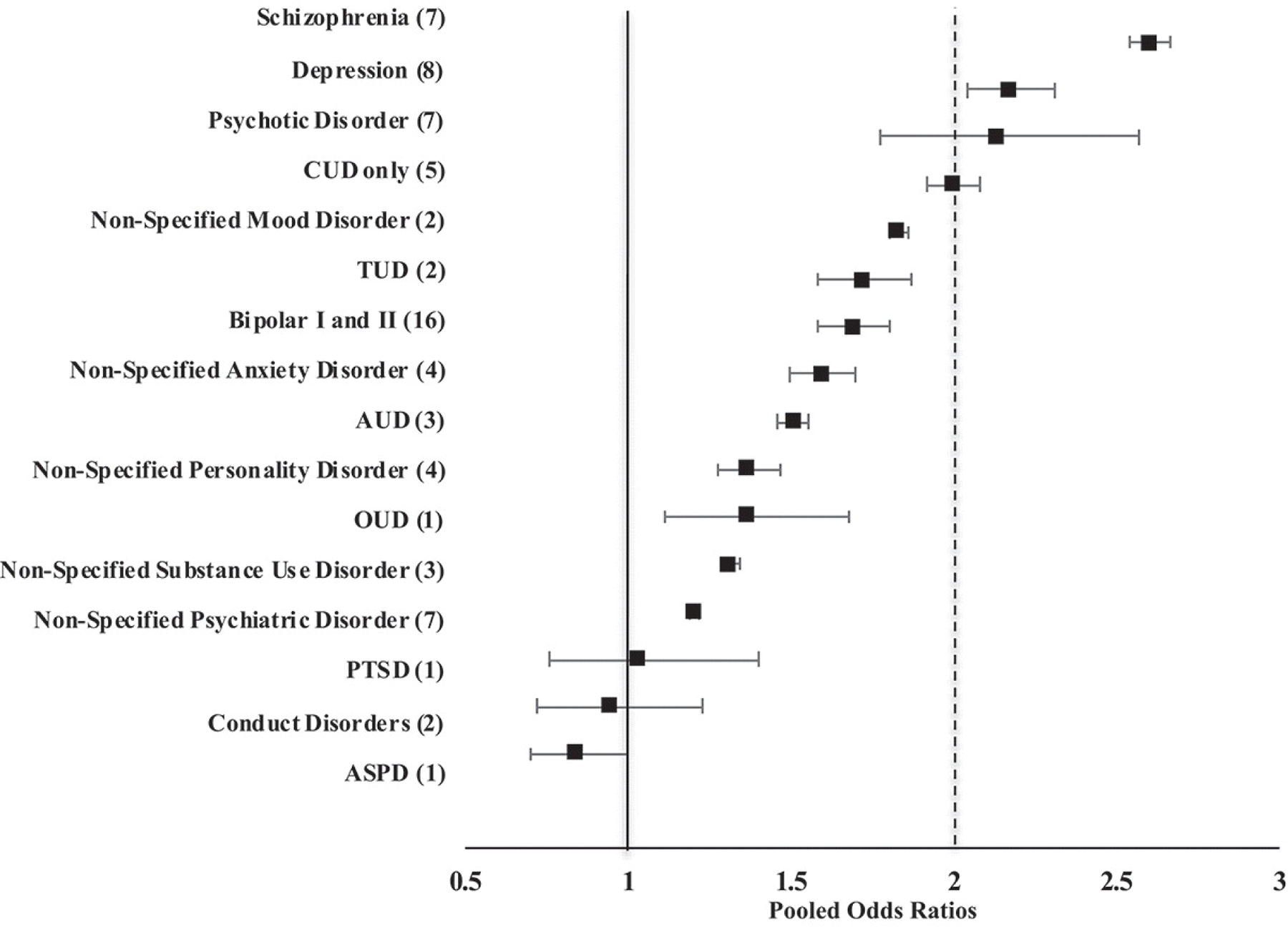
Numbers in brackets indicate the number of studies included in the pooled odds ratios (ORs). Studies were sorted by increasing ORs, with squares indicating ORs and horizontal lines indicating 95% confidence intervals (CIs). ASPD, antisocial disorder; AUD, alcohol use disorder; CUD, cannabis use disorder; OUD, opiate use disorder; PTSD, post-traumatic stress disorder; TUD, tobacco use disorder. Solid vertical line at 1 is the reference point where no sex difference exists. Dashed vertical line indicates the OR of the CUD-only group.
In order to calculate ORs several assumptions were made when the sample sizes were not provided in the study article. (1) If the sex distribution for the MI sample was not provided, rates from the literature were used (see Supplementary Table 1); (2) Unless specifically stated, we made the assumption that participants without CUD had the MI that was being examined; (3) When studies used data from a larger dataset (e.g., NESARC-I), we used the total sample from the original (larger) dataset; (4) If there was a discrepancy regarding the sample size listed in a study’s Methods section versus when calculated from sample data in their Results section, we used data calculated from data in the Results section. Of the 12 (of 37) studies where no sex distribution data for MI was provided, we attempted to contacted authors but were unable to obtain such data (see Supplementary Tables 1 and 4).
Meta-analysis
We used the METAFOR package in R statistical software (27) to estimate meta-analytic odds ratios for the association between sex and CUD, within groups of studies categorized by psychiatric diagnosis. We estimated random effects, multi-level meta-analytic models for each diagnosis group. Random effects meta-analysis is suitable when there is a high degree of heterogeneity between estimates (28). Meta-analytic estimates from random-effects models are interpreted as an average across multiple sampled populations, rather than a representative estimate from a single sampled population. Multi-level modeling allowed us to account for non-independence when multiple estimates from the same study were included in a model (29). We examined heterogeneity using the Q-statistic and I2 (30). The Q-statistic is interpreted as a hypothesis test with a null-hypothesis of no heterogeneity between study estimates. This statistic tends to be overpowered for large sample sizes; therefore, we also calculated I2.I2 is interpreted as the percent of heterogeneity between studies that is nonrandom. In other words, I2 represents the proportion of heterogeneity between studies that results from explainable population/sample differences, rather than random heterogeneity expected when multiple samples are pulled from the same underlying population. Higher I2 values indicate that random-effects modeling is the appropriate approach.
Results
Study selection results
See Figure 1 for the CONSORT diagram. A total of 5,493 studies were found in the overall search strategy, and 5,443 studies were excluded (3,866 were duplicates and content of 1,577 studies was not relevant). Fifty full-text articles were assessed for eligibility and 37 studies were included in this review (Table 1); see Supplementary Material, Table 2 for reasons of study exclusion. Diagnoses for MI included non-specified psychiatric disorder, schizophrenia, psychotic disorder, non-specified mood disorder (major depression, bipolar I and II), major depression, bipolar I and II, non-specified anxiety disorder, PTSD, non-specified personality disorder, antisocial disorder (ASPD), non-specified SUD, opioid use disorder (OUD), alcohol use disorder (AUD) and tobacco use disorder (TUD). Fourteen of these studies required at least one assumption to be made when calculating ORs for the individual studies and pooled ORs (see Supplementary Material, Table 4). The prevalence of CUD in males and females within each SMI for each study is reported in the Supplementary Material, Table 5.
Figure 1. Study selection PRISMA flow diagram.
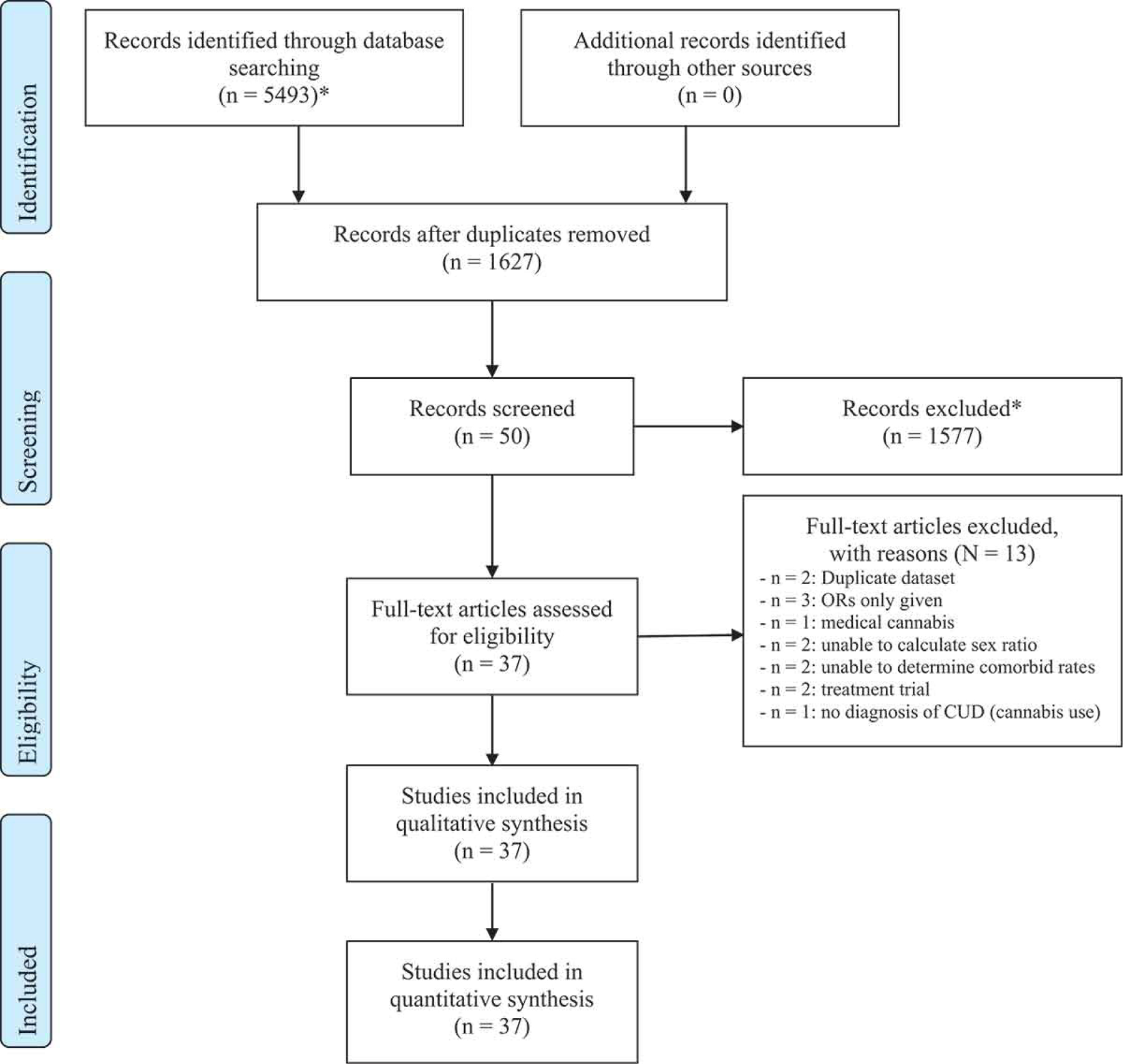
*Included animal studies, non-biological science studies, reviews, editorials, and commentaries
Table 1.
Odds and Pooled Odds Ratios for CUD in Samples with and without a Comorbid SMI as a Function of Sex
| Psychiatric Comorbidity | Study | Odds Ratio | 95% CI | p = |
|---|---|---|---|---|
| CUD-only | Stinson et al. 2006 | 2.756 | 2.312 to 3.284 | <0.0001 |
| Wu et al. 2014 | 2.044 | 1.940 to 2.153 | <0.0001 | |
| Kerridge et al. 2018 | 2.322 | 2.034 to 2.651 | <0.0001 | |
| Farmer et al. 2016 | 1.761 | 1.256 to 2.470 | 0.0010 | |
| Haberstick et al. 2014 | 1.581 | 1.408 to 1.776 | <0.0001 | |
|
| ||||
| Pooled studies | 1.996 | 1.913 to 2.083 | <0.0001 | |
|
| ||||
| Non-Specified Psychiatric Disorder | Lai and Sitharthan 2012 | 1.012 | 0.931 to 1.100 | 0.7841 |
| Khan et al. 2013 | 1.735 | 1.609 to 1.870 | <0.0001 | |
| Teesson et al. 2012 | 1.000 | 0.317 to 3.151 | 1.0000 | |
| Swift et al. 2001 | 4.072 | 2.967 to 5.590 | <0.0001 | |
| Mueser et al. 2000 | 2.254 | 1.387 to 3.663 | 0.0010 | |
| Zhu and Wu 2017 | 1.987 | 1.964 to 2.011 | <0.0001 | |
| Karam et al. 2002 | 2.727 | 0.993 to 7.491 | 0.0516 | |
|
| ||||
| Pooled studies | 1.201 | 1.188 to 1.215 | <0.0001 | |
|
| ||||
| Psychotic Disorder | Machielsen et al. 2010 | 3.731 | 1.513 to 9.201 | 0.0042 |
| Schimmelmann et al. 2012 | 1.717 | 0.773 to 3.814 | 0.1843 | |
| Lange et al. 2014 | 3.030 | 1.219 to 7.533 | 0.0170 | |
| Brunette et al. 2018 | 2.376 | 1.428 to 3.952 | 0.0009 | |
| Rabinowitz et al. 1998 | 0.301 | 0.078 to 1.165 | 0.0821 | |
| Kavanagh et al. 2004 | 2.392 | 1.656 to 3.455 | <0.0001 | |
| Khan et al. 2013 | 1.086 | 0.712 to 1.657 | 0.7012 | |
|
| ||||
| Pooled studies | 2.135 | 1.773 to 2.570 | <0.0001 | |
|
| ||||
| Schizophrenia | Zhu and Wu 2017 | 2.676 | 2.611 to 2.742 | <0.0001 |
| DeRosse et al. 2010 | 4.301 | 2.589 to 7.147 | <0.0001 | |
| Nesvag et al. 2015 | 4.046 | 3.209 to 5.101 | <0.0001 | |
| Lai and Sitharthan 2012 | 0.9995 | 0.885 to 1.129 | 0.9939 | |
| Libuy et al. 2018 | 0.983 | 0.603 to 1.603 | 0.9441 | |
| Rabinowitz et al. 1998 | 3.317 | 1.708 to 6.442 | 0.0004 | |
| Dubertret et al. 2006 | 3.778 | 1.779 to 8.026 | 0.0005 | |
|
| ||||
| Pooled studies | 2.600 | 2.539 to 2.662 | <0.0001 | |
|
| ||||
| Non-Specified Mood Disorder | Zhu and Wu 2017 | 1.825 | 1.799 to 1.852 | <0.0001 |
| Kerridge et al. 2018 | 12.216 | 7.867 to 18.970 | <0.0001 | |
|
| ||||
| Pooled studies | 1.830 | 1.804 to 1.857 | <0.0001 | |
|
| ||||
| Depression | Khan et al. 2013 | 2.111 | 1.819 to 2.452 | < 0.0001 |
| Rabinowitz et al. 1998 | 1.825 | 0.680 to 4.901 | 0.2327 | |
| Harder et al. 2008 | 3.363 | 2.467 to 4.586 | <0.0001 | |
| Feingold et al. 2017 | 2.150 | 1.487 to 3.110 | <0.0001 | |
| Nesvag et al. 2015 | 3.941 | 3.555 to 4.369 | <0.0001 | |
| Pacek et al. 2013 | 1.779 | 1.457 to 2.171 | <0.0001 | |
| Lai and Sitharthan 2012 | 1.000 | 0.876 to 1.140 | 0.9961 | |
| Kerridge et al. 2018 | 1.291 | 1.008 to 1.655 | 0.0431 | |
|
| ||||
| Pooled studies | 2.174 | 2.046 to 2.310 | < 0.0001 | |
|
| ||||
| Bipolar I and II | Khan et al. 2013 | 1.084 | 0.908 to 1.295 | 0.3713 |
| Hunt et al. 2016 | 2.206 | 1.956 to 2.489 | <0.0001 | |
| Cassidy et al. 2001 | 2.500 | 1.635 to 3.824 | <0.0001 | |
| Kawa et al. 2005 | 2.431 | 1.112 to 5.314 | 0.0260 | |
| Morgan et al. 2005 | 3.886 | 1.320 to 11.430 | 0.0137 | |
| Strakowski et al. 2007 | 1.631 | 0.756 to 3.518 | 0.2126 | |
| VanRossum et al. 2009 | 3.017 | 2.436 to 3.736 | <0.0001 | |
| Altshuler et al. 2010 | 1.238 | 0.743 to 2.064 | 0.4132 | |
| Braga et al. 2012 | 1.741 | 0.741 to 4.087 | 0.2031 | |
| Lev-Ran et al. 2013 | 1.644 | 1.122 to 2.409 | 0.0107 | |
| Weinstock et al. 2016 | 1.487 | 0.729 to 3.036 | 0.2757 | |
| Rabinowitz et al. 1998 | 2.589 | 1.386 to 4.838 | 0.0029 | |
| Kerridge et al. 2018 | 1.937 | 1.298 to 2.892 | 0.0012 | |
| Nesvag et al. 2015 | 2.686 | 2.238 to 3.225 | <0.0001 | |
| Lai and Sitharthan 2012 | 0.998 | 0.827 to 1.204 | 0.9816 | |
| Cotton et al. 2013 | 2.151 | 1.009 to 4.584 | 0.0473 | |
|
| ||||
| Pooled studies | 1.688 | 1.585 to 1.799 | <0.0001 | |
|
| ||||
| Non-Specified Anxiety Disorder | Khan et al. 2013 | 1.856 | 1.639 to 2.102 | <0.0001 |
| Zhu and Wu 2017 | 1.686 | 1.549 to 1.835 | <0.0001 | |
| Lai and Sitharthan 2012 | 1.007 | 0.791 to 1.283 | 0.9532 | |
| Kerridge et al. 2018 | 1.476 | 1.162 to 1.875 | 0.0014 | |
|
| ||||
| Pooled studies | 1.596 | 1.497 to 1.700 | <0.0001 | |
|
| ||||
| PTSD | Kerridge et al. 2018 | 1.031 | 0.762 to 1.396 | 0.8412 |
|
| ||||
| Non-Specified Personality Disorder | Khan et al. 2013 | 1.749 | 1.551 to 1.972 | <0.0001 |
| Zhu and Wu 2017 | 1.812 | 1.523 to 2.157 | <0.0001 | |
| Lai and Sitharthan 2012 | 1.000 | 0.857 to 1.167 | 0.9970 | |
| Kerridge et al. 2018 | 1.888 | 1.579 to 2.257 | <0.0001 | |
|
| ||||
| Pooled studies | 1.367 | 1.272 to 1.469 | <0.0001 | |
|
| ||||
| ASPD | Khan et al. 2013 | 0.836 | 0.701 to 0.998 | 0.047 |
|
| ||||
| Conduct Disorders | Khan et al. 2013 | 0.444 | 0.277 to 0.714 | 0.0008 |
| Zhu and Wu 2017 | 2.357 | 1.667 to 3.332 | <0.0001 | |
|
| ||||
| Pooled studies | 0.941 | 0.723 to 1.224 | 0.6493 | |
|
| ||||
| Non-Specified SUD | Khan et al. 2013 | 1.188 | 1.100 to 1.284 | <0.0001 |
| Zhu and Wu 2017 | 1.212 | 1.186 to 1.239 | <0.0001 | |
| Kerridge et al. 2018 | 2.330 | 2.016 to 2.694 | <0.0001 | |
|
| ||||
| Pooled studies | 1.311 | 1.284 to 1.338 | <0.0001 | |
|
| ||||
| OUD | Shand et al. 2011 | 1.363 | 1.108 to 1.677 | 0.0034 |
|
| ||||
| AUD | Khan et al. 2013 | 1.299 | 1.196 to 1.411 | <0.0001 |
| Zhu and Wu 2017 | 1.320 | 1.278 to 1.365 | <0.0001 | |
| Kerridge et al. 2018 | 4.097 | 3.351 to 5.009 | <0.0001 | |
|
| ||||
| Pooled studies | 1.505 | 1.460 to 1.551 | <0.0001 | |
|
| ||||
| TUD | Khan et al. 2013 | 1.374 | 1.244 to 1.519 | <0.0001 |
| Kerridge et al. 2018 | 2.253 | 1.911 to 2.656 | <0.0001 | |
|
| ||||
| Pooled studies | 1.720 | 1.579 to 1.873 | <0.0001 | |
ASPD, antisocial disorder; AUD, alcohol use disorder; OUD, opiate use disorder; PTSD, post-traumatic stress disorder; SMI, serious mental illness; TUD, tobacco use disorder.
Sex differences in the prevalence of CUD
Five studies were included in the CUD-only group (31–35) (Supplementary Material, Table 3). The pooled OR for the CUD-only males compared to CUD-only females was 2.0 (CI = 1.9–2.1, p < .01) as presented in Table 1. In the forest plot, the pooled OR for the CUD-only group is represented by a dashed vertical line (Figure 2).
Sex differences in the prevalence of CUD with comorbid MIs
Individual study ORs and pooled ORs for each MI are found in Table 1. Pooled ORs for CUD comorbid with MIs are compared in Figure 2. Except for schizophrenia/psychotic disorder and depression, we found that among females with MIs, the likelihood of CUD was lower (OR <2) than the CUD-only group.
Comorbid non-specified psychiatric disorders
Results from pooling seven studies (10,36–41) examining rates of CUD in non-specified comorbid psychiatric disorder produced an OR of 1.20 times greater for males than females (Cl = 1.19–1.22, p < .01).
Comorbid psychotic disorders
The pooled OR from seven studies examining psychotic disorders revealed that having a CUD was 2.1 times more likely for males than females (CI = 1.8–2.6, p < .01) (10,42–47). Similarly, the pooled OR from seven studies in schizophrenia was 2.6 times greater for males than females (36,40,47–51) (OR = 2.6, CI = 2.5–2.7, p< .01).
Comorbid mood disorders
The pooled OR from two studies in non-specified mood disorder revealed that the odds of having CUD were 1.83 times greater for males than females (CI = 1.80–1.86, p < .01) (33,40). From eight studies, the pooled OR of CUD and comorbid depression was 2.2 times greater for males compared to females (CI = 2.05 to 2.31, p < .01) (10,33,36,47,49,52–54). From 16 studies, the pooled OR of CUD and comorbid bipolar I and II was 1.7 times greater for males than females (CI = 1.6–1.8, p < .01) (10,33,36,47,49,55–65).
Comorbid anxiety disorders
The pooled OR from four studies of non-specified anxiety disorder revealed that the odds of having CUD were 1.6 times greater for males compared to females (CI = 1.5–1.7, p < .01) (10,33,36,40). Based on one study, the OR of CUD was 1.03 greater for males than females (CI = 0.8–1.4, p < .01) with comorbid PTSD (33).
Comorbid personality disorders
The pooled OR of four studies in non-specified personality disorder revealed that the odd of having CUD was 1.4 times greater for males than females (CI = 1.3–1.5, p < .01) (10,33,36,40). The pooled OR from the two studies examining CUD comorbid with conduct disorder was 0.94; however, the sex difference was not statistically significant (CI = 0.7–1.2, p = .65) (10,40). Based on one study, the OR was 0.84 greater for females than males with ASPD (CI = 0.7–1.0, p < .05) (10).
Comorbid SUDs
The result of pooling three studies (10,33,40) examining CUD comorbid with non-specified SUD was 1.31 greater for males than females (CI = 1.28–1.34, p < .01). The OR from the only study of CUD and comorbid OUD was 1.36 greater for males compared to females (CI = 1.11–1.68, p < .01) (66). The pooled OR from three studies (10,33,40) for CUD and comorbid AUD was 1.51 greater for males than females (CI = 1.46–1.55, p < .01). Lastly, the pooled OR from two studies (10,33) on TUD was 1.72 greater for males than females (CI= 1.58–1.87, p< .01).
Summary of meta-analysis
When we conducted our meta-analysis, we observed that our I2 values were consistently very high. With the exception of one I2 estimate of 68.8% (psychotic disorders; presented in Supplementary Material, Table 6), all others were >85%. This high degree of heterogeneity indicates that a random-effects model and interpretation are appropriate; in other words, the meta-analytic ORs (Table 6) are best interpreted as an average across studies from multiple populations, rather than a representative estimate of the association for a single diagnostic population. These large values are likely the result of included studies having extremely large sample sizes and therefore very small standard errors; generating large heterogeneity statistics, which compared between study variation and within study variation. With such a high degree of heterogeneity evident, comparisons between diagnoses are not meaningful.
Discussion
Our systematic review demonstrated that the odds of having CUD comorbid with MIs do indeed differ by sex, and the magnitude of this effect varied by MI diagnosis. Among the CUD-only group, males were twice as likely as females to be diagnosed with CUD. Across disorders, the strongest sex effect was observed for schizophrenia, with males having nearly three times greater odds for CUD compared to females. Males were also more likely to have CUD comorbid with mood, psychotic, SUDs, anxiety, and non-specified personality disorders than females, although to a lesser extent than schizophrenia based on lower pooled ORs and non-overlapping CIs. We observed overlapping CIs between depression, psychotic disorders, and the CUD-only group, indicative of equivalent ORs. On the other hand, females demonstrated modestly greater odds of having CUD comorbid with ASPD compared to males. Conduct disorder showed no sex difference in the rate of CUD. Importantly, for MIs other than schizophrenia, psychotic disorders, and depression, females were at greater odds of having CUD compared to females without a comorbid SMI, suggesting that in females having a MI increased the likelihood of CUD, or vice versa, that having a CUD increased the likelihood of a MI.
In terms of our meta-analysis, the quantitative meta-analytic averages did not provide additional evidence of variability in the association between sex and CUD, by diagnosis, nor did the results indicate a lack of variability. Therefore, the meta-analysis results were inconclusive. Future research focused on the association between sex and CUD within specific diagnoses will benefit from understanding why there is such a high degree of variability within findings. Our investigation focused on differences between diagnoses, and therefore a deeper understanding within specific diagnoses is beyond the scope of this report.
Indeed, understanding the factors that contribute to the sex differences found in our systematic review will be essential to aid in the development of novel therapies for individuals with CUD and comorbid MIs. Below we discuss potential mechanisms involving the endocannabinoid (eCB) system and age of onset of MIs which may contribute to sex differences in the association between CUD and MI.
The endocannabinoid system
The eCB system is implicated in various regulatory functions critical for homeostasis. Homeostasis is maintained primarily through type 1 cannabinoid receptors (CB1R) that are expressed in high concentrations in several brain regions, such as the basal ganglia, hippocampus, cerebral cortex, and cerebellum (67). Delta-9-tetrahydrocannabinol (THC), the primary psychoactive constituent of cannabis, is a CB1R partial agonist, similar to the endogenous cannabinoid ligands N-arachidonoylethanolamine (anandamide) and 2-arachidonoylglycerol (68). Sex differences in the eCB system are well documented, with sex hormones modulating eCB activity (69). The eCB system has also been hypothesized to play a role in the pathophysiology of various psychiatric disorders including mood disorders (70) and schizophrenia (71). Thus, eCB dysfunction may be associated with CUD and comorbid MIs in a sex-dependent manner.
Neurodevelopmental trajectories are sexually dimorphic with females undergoing neural maturation earlier (~10 years old) than males (~14 years old) (72). This period is characterized by dramatic changes in brain growth and connectivity orchestrated by the eCB system (73). Importantly, for males, these changes coincide with cannabis initiation given that cannabis experimentation often occurs in mid-to-late adolescence (irrespective of sex) (74). Cannabinoid-induced changes in eCB activity at this sensitive time may lead to critical neurobiological aberrations that alter brain function and behavior (75). Importantly, these changes have been implicated in the pathophysiology of schizophrenia (71). Furthermore, the same genetic variants (e.g., single nucleotide polymorphisms) that confer risk for schizophrenia in adolescence have been found to increase the risk of CUD (76). For example, evidence suggests an increased probability of early psychosis among individuals with polymorphisms in the catechol-O-methyltransferase (COMT) gene who use cannabis during adolescence (77). Notably, the prevalence of this polymorphism has been more strongly associated with the development of schizophrenia in males than females (78). Converging lines of evidence also suggest that heavier exposure to cannabis is associated with higher risk for psychotic outcomes (79,80), which is more common among males than females (81). Males are less sensitive to the effects of cannabis and thus may consume more cannabis than females in an effort to achieve similar euphoric and mood-enhancing effects (82). Because males consume more cannabis than females and at a higher frequency (81), they may be more susceptible to the negative neurobiological consequences that prime the brain for the development of an MI. This has been repeatedly shown for schizophrenia (83), and similar mechanisms may be at play for the development of depression in males (18). In contrast to males, females are less likely to consume cannabis (84), and use lower quantities of cannabis (81) and their earlier neurodevelopmental window may ‘protect’ them against some of the neurobiological consequences associated with cannabis exposure in adolescence. As such, sex-specific cannabis-onset and consumption patterns may underlie the greater odds of CUD and comorbid schizophrenia and psychotic disorders, and to a lesser extent depression, among males than females.
Reduced CB1R availability has been observed in several cortical and subcortical regions (e.g., hippocampus, insula) in males with schizophrenia (with no CUD/minimal cannabis exposure) compared to healthy control males (85) and in cannabis users without schizophrenia (86). CB1R downregulation may underlie increased tolerance to the effects of cannabis in heavy cannabis smokers (87), including the rewarding effects of THC (88). Thus, reduced CB1R availability may contribute to a greater likelihood of males having a CUD comorbid with schizophrenia/psychosis compared to females. However, at this point, there are no data related to CB1R levels in female schizophrenia patients. Sex-dependent research in the area of CBIRs is fundamental to our understanding of the role of the eCB system and its activity in CUD and comorbid MIs and should be urgently addressed.
Preclinical studies demonstrate that hormonal factors, specifically estradiol, strongly influence the functioning of the eCB system (89). Estradiol regulates CB1R expression in a region-dependent manner rendering sex differences in various brain structures including the amygdala (90). Given the amygdala’s role in mediating anxiety-like responses (91), higher CB1R density in the amygdala of females, compared to males, may be one mechanism contributing to their greater sensitivity to the anxiogenic effects of cannabis (90). In addition, anandamide levels are lower in females than males (92), which may reflect higher concentrations of fatty acid amide hydrolase in females (93), the metabolic enzyme responsible for degrading anandamide. Decreased whole-brain anandamide levels are predictive of anxiety-like behaviors (94) and are evident in female patients with anxiety disorders (92). Notably, lower levels of anandamide have also been documented in the cerebrospinal fluid of chronic cannabis users compared to infrequent cannabis users (95). Consistent with this reduction in anandamide levels in CUD, there is increased in vivo fatty acid amide hydrolase (FAAH, the degradative enzyme for anandamide) binding in humans with CUD measured with the PET tracer [11 C]CURB (96). Taken together, this suggests that a putative pathway may be implicated in both the development of CUD and anxiety disorders in females.
Age of onset of MIs
Conceivably, MIs that are diagnosed early in adolescence coinciding with initiation and peak cannabis use may potentiate the risk of developing comorbid CUD. Indeed, females tend to have an early onset of certain SMIs. For example, clinical studies report that the onset of generalized anxiety disorder in females occurs around age 15 (97) and increased rates of social anxiety in females are associated with transitioning to adolescence (98). Early onset of anxiety disorders may motivate and drive cannabis use in females, given their lower life experience and maturity, and lack of coping skills at this young age. As a result, females may use cannabis to manage stress and other negative symptoms associated with their primary disorder (99). While at low doses cannabis exerts anxiolytic-type effects, at high doses cannabis has the opposite effect and may exacerbate anxiety (100), leading to escalating cannabis use.
Further, adolescents with early exposure to addictive substances are also more likely to have conduct problems (101) and notably, ASPD onset is around age 13. In line with this, strong associations have been found between conduct problems in adolescence (e.g., verbal bullying (101), antisocial behavior (103), norm-violating behavior, aggression, and alcohol drinking (103)) and early cannabis initiation (101) as well as progression to daily use (103).
Thus, there is evidence that early onset of some MIs (anxiety disorders, SUDs, ASPD) in females is associated with cannabis use. Since females progress quickly from use to CUD (i.e., telescoping) (104), the likelihood of comorbidity among them is high and thus lends support for our finding of females at higher odds for CUD comorbid with selected SMIs compared to females with out SMI.
Limitations
This systematic review and meta-analysis have several limitations. The severity of CUD may have varied between studies (e.g., mild, moderate, severe, not specified). The severity of MI may have also varied between studies given both inpatient and outpatient populations were studied (40). Studies did not assess the potency of cannabis (i.e., THC or cannabidiol content), which may have differential effects on clinical outcomes (105). Many MIs are comorbid with each other (106); thus, it is possible that individuals with CUD and comorbid MI also met criteria for another MI that was not reported (e.g., TUD). In addition, assumptions were made for 14/37 studies where we were not able to procure the sex distribution data for MI or a discrepancy existed in sample size (see Supplementary Table 1, Table 4). Finally, we were not able to quantitate sex differences in CUD across psychiatric disorders using a meta-analytic approach due to the high heterogeneity of patient characteristics between studies.
Conclusions
We found that the odds of having CUD differ by sex, and the magnitude of this effect varies by MI. We found that males were more likely than females to have CUD with all MIs, except for anxiety disorders, ASPD, and conduct disorder. Importantly, females with selected MIs were found to be at increased odds of having CUD compared to females without an MI. To date, treatments (pharmacological, behavioral, and combined) for CUD and comorbid MI have not addressed the importance of sex as a factor in disease trajectory or clinical care. Our results suggest that females may benefit from increased cannabis screening when presenting with MI. For males, psychoeducation focusing on the consequences of early and heavy use may help attenuate or at least delay use to a time when they are less neurobiologically sensitive. Our meta-analysis was inconclusive due to high levels of heterogeneity between studies, indicating that future studies should attempt to recruit more homogeneous samples with explicit inclusion and exclusion criteria. The underrepresentation of females inclinical studies may be hampering our full understanding of sex effects associated with CUD and MI. Future research needs to be mindful of potential sex differences in the rates of CUD comorbid with MIs, which may ultimately aid in developing novel, personalized, and sex-specific treatments for these comorbid disorders.
Supplementary Material
Funding
This manuscript was supported in part by a CIHR Pre-Doctoral Award (to Ms Kozak), the Canada First Research Excellence Fund awarded to the Healthy Brains for Healthy Lives Initiative at McGill University (to Dr Rabin), NIDA grant R21-DA-043949 (to Dr George), NIDA grant R01-DA-047296 (to Dr Cooper), and the Semel Charitable Foundation (to Dr Cooper).
Footnotes
Disclosure statement
Dr George reports that he is a consultant for Frutarom, Novartis, and the Canadian Centre for Substance Use and Addiction in the last 12 months. Dr Cooper reports that she served on the scientific advisory board of FSD Pharma in the last 12 months.
References
- 1.Maxwell JC, Mendelson B. What do we know now about the impact of the laws related to Marijuana? J Addict Med 2016;10:3–12. doi: 10.1097/ADM.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Management of substance abuse - Cannabis Geneva: Switzerland; 2020. https://www.who.int/substance_abuse/facts/cannabis/en/: [Google Scholar]
- 3.Degenhardt L, Ferrari AJ, Calabria B, Hall WD, Norman RE, McGrath J, Flaxman AD, Engell RE, Freedman GD, Whiteford HA, et al. The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: results from the GBD 2010 study. PLoS One 2013;8:e76635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Compton WM, Han B, Jones CM, Blanco C. Cannabis use disorders among adults in the United States during a time of increasing use of cannabis. Drug Alcohol Depend 2019;204:107468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leyton M Cannabis legalization: did we make a mistake? Update 2019. J Psychiatry Neurosci 2019;44:291–93. doi: 10.1503/jpn,190136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canada S National Cannabis Survey, first quarter 2019 2019; Available from: https://wwwl50.statcan.gc.ca/nl/en/daily-quotidien/190502/dql90502a-eng.pdf?st=TQEzeqzW [last accessed 29 Jun 2021].
- 7.WHO. The Health and social effects of nonmedical cannabis use Geneva: Switzerland; 2016. [Google Scholar]
- 8.Sorkhou M, Bedder RH, George TP. The behavioural sequelae of cannabis use in healthy people. Front Psychiatr 2021;12:630247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gryczynski J, Schwartz RP, O’Gradt KE, Restivo L, Mitchell SG, Jaffe JH. Understanding patterns of high-cost health care use across different substance user groups. Health Aff (Millwood) 2016;35:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan SS, Secades-Ville R, Okuda M, Wang S, Perez-Fuentes G, Kerridge BT, Blanco C. Gender differences in cannabis use disorders: results from the national epidemiologic survey of alcohol and related conditions. Drug Alcohol Depend 2013;130:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sherman BJ, Baker NL, McRae-Clark AL. Gender differences in cannabis use disorder treatment: change readiness and taking steps predict worse cannabis out comes for women. Addict Behav 2016;60:197–202. doi: 10.1016/j.addbeh.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kandel DB, Chen K. Types of marijuana users by longitudinal course. J Stud Alcohol 2000;61:367–78. doi: 10.15288/jsa.2000.61.367. [DOI] [PubMed] [Google Scholar]
- 13.Wagner FA, Anthony JC. Male-female differences in the risk of progression from first use to dependence upon cannabis, cocaine, and alcohol. Drug Alcohol Depend 2007;86:191–98. doi: 10.1016/j.drugalcdep.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 14.StatisticsCanada. National Cannabis Survey, second quarter 2019 2019; Available from: https://wwwl50.statcan.gc.ca/nl/daily-quotidien/190815/dql90815a-eng.htm [last accessed 29 Jun 2021].
- 15.Hasin DS, Kerridge BT, Saha TD, Huang B, Pickering R, Smith SM, Jung J, et al. Prevalence and correlates of DSM-5 cannabis use disorder, 2012–2013: findings from the national epidemiologic survey on alcohol and related conditions-III. Am J Psychiatry 2016;173:588–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez-Quintero C, Perez de los Cabos J, Hasin DS, Okuda M, Wang S, Grant BF, Blanco C. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the national epidemiologic survey on alcohol and related conditions (NESARC). Drug Alcohol Depend 2011;115:120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Assari S, Mistry R, Howard Caldwell C, Zimmerman MA. Marijuana use and depressive symptoms; gender differences in African American adolescents. Front Psychol 2018:9:2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crane NA, Langenecker SA, Mermelstein RJ. Gender differences in the associations among marijuana use, cigarette use, and symptoms of depression during adolescence and young adulthood. Addict Behav 2015;49:33–39. doi: 10.1016/j.addbeh.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al Azizi SA, Omer AA, Mufaddel AA. cannabis use among people with mental illness: clinical and socio-demographic characteristics. Open J Psychiatry 2018;8:244–52. doi: 10.4236/ojpsych.2018.83021. [DOI] [Google Scholar]
- 20.SAMHSA. 2018 National survey on drug use and health: women; 2018. https://www.samhsa.gov/data/sites/default/files/reports/rpt23250/5_Women_2020_01_14_508.pdf [last accessed 29 Jun 2021].
- 21.Cooper ZD, Craft RM. Sex-dependent effects of cannabis and cannabinoids: a translational perspective. Neuropsychopharmacology 2018;43:34–51. doi: 10.1038/npp.2017.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agosti V, Nunes E, Levin F. Rates of psychiatric comorbidity among U.S. residents with lifetime cannabis dependence. Am J Drug Alcohol Abuse 2002;28:643–52. doi: 10.1081/ADA-120015873. [DOI] [PubMed] [Google Scholar]
- 23.Brabete AC, Greaves L, Hemsing N, Stinson J. Sex- and gender-based analysis in cannabis treatment outcomes: a systematic review. Int J Environ Res Public Health 2020; 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerda M, Mauro C, Hamilton A, Levy NS, Santaella-Tenorio J, Hasin D, Wall MM, et al. Association between recreational marijuana legalization in the United States and changes in Marijuana use and cannabis use disorder from 2008 to 2016. JAMA Psychiatry 2019;77:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camors C, Chavez S, Romi A. The cannabis industry within the USA: the influence of gender on cannabis policy and sales. Sustain Accounting Manage Policy Journal 2020;11: 1095–126. doi: 10.1108/SAMPJ-12-2018-0330. Ahead-of-print. [DOI] [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viechtbauer W Conducting Meta-Analyses in R with the metafor package. J Stat Software 2010;36:48. [Google Scholar]
- 28.Hunter JE, Schmidt FL. Fixed effects vs. random effects meta-analysis models: implications for cumulative research knowledge. Int J Sel Assess 2000;8:275–92. doi: 10.1111/1468-2389.00156. [DOI] [Google Scholar]
- 29.Van Den Noortgate W, Onghena P. Multilevel meta-analysis: a comparison with traditional meta-analytical procedures. Educ Psychol Meas 2003;63:765–90. doi: 10.1177/0013164403251027. [DOI] [Google Scholar]
- 30.Huedo-Medina TB, Sanchez-Meca J, Marrn-Martrnez F, Botella J. Assessing heterogeneity in meta-analysis: q statistic or I2 index? Psychol Methods 2006;11:193. [DOI] [PubMed] [Google Scholar]
- 31.Stinson FS, Ruan WJ, Pickering R, Grant BF. Cannabis use disorders in the USA: prevalence, correlates and co-morbidity. Psychol Med 2006;36:1447–1460. [DOI] [PubMed] [Google Scholar]
- 32.Wu LT, Brandy KT, Mannelli P, Killeen TK. Cannabis use disorders are comparatively prevalent among nonwhite racial/ethnic groups and adolescents: a national study. J Psychiatr Res 2014;50:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerridge BT, Pickering R, Chou P, Saha TD, Hasin DS. DSM-5 cannabis use disorder in the national epidemiologic survey on alcohol and related conditions-III: gender-specific profiles. Addict Behav 2018;76:52–60. [DOI] [PubMed] [Google Scholar]
- 34.Farmer RF, Kosty DB, Seeley JR, Gau JM, Duncan SC, Walker DD, Lewinsohn PM. Association of comorbid psychopathology with the duration of cannabis use disorders. Psychol Addict Behav 2016;30:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haberstick BC, Young SE, Zeiger JS, Hewitt JK, Hopfer CJ. Prevalence and correlates of alcohol and cannabis use disorders in the United States: results from the national longitudinal study of adolescent health. Drug Alcohol Depend 2014;136:158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai HM, Sitharthan T. Exploration of the comorbidity of cannabis use disorders and mental health disorders among inpatients presenting to all hospitals in New South Wales, Australia. Am J Drug Alcohol Abuse 2012;38:567–74. doi: 10.3109/00952990.2012.694523. [DOI] [PubMed] [Google Scholar]
- 37.Teesson M, Slade T, Swift W, Mills K, Memedovic S, Mewton L, Grove R, et al. Prevalence, correlates and comorbidity of DSM-IV Cannabis Use and Cannabis Use Disorders in Australia. Aust N Z J Psychiatry 2012;46:1182–1192. [DOI] [PubMed] [Google Scholar]
- 38.Swift W, Hall W, Teesson M. Cannabis use and dependence among Australian adults: results from the national survey of mental health and wellbeing. Addiction 2001;96:737–48. doi: 10.1046/j.1360-0443.2001.9657379.x. [DOI] [PubMed] [Google Scholar]
- 39.Mueser KT, Yarnold PR, Rosenberg SD, Swett C Jr, Miles KM, Hill D. Substance use disorder in hospitalized severely mentally ill psychiatric patients: prevalence, correlates, and subgroups. Schizophr Bull 2000;26:179–192. [DOI] [PubMed] [Google Scholar]
- 40.Zhu H, Wu LT. Sex differences in cannabis use disorder diagnosis involved hospitalizations in the United States. J Addict Med 2017;11:357–67. doi: 10.1097/ADM.0000000000000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karam EG, Yabroudi PF, Melhem NM. Comorbidity of substance abuse and other psychiatric disorders in acute general psychiatric admissions: a study from Lebanon. Compr Psychiatry 2002;43:463–68. doi: 10.1053/comp.2002.35910. [DOI] [PubMed] [Google Scholar]
- 42.Machielsen M, van der Sluis S, de Haan L. Cannabis use in patients with a first psychotic episode and subjects at ultra high risk of psychosis: impact on psychotic- and pre-psychotic symptoms. Aust N Z J Psychiatry 2010;44:721–28. doi: 10.3109/00048671003689710. [DOI] [PubMed] [Google Scholar]
- 43.Schimmelmann BG, Conus P, Cotton S, Kupferschmid S, McGorry PD, Lambert, M. Prevalence and impact of cannabis use disorders in adolescents with early onset first episode psychosis. Eur Psychiatry 2012;27:463–469. [DOI] [PubMed] [Google Scholar]
- 44.Lange EH, Nesvag R, Ringen PA, Hartberg CB, Haukvik UK, Andreassen OA, et al. One year follow-up of alcohol and illicit substance use in first-episode psychosis: does gender matter? Compr Psychiatry 2014;55:274–282. [DOI] [PubMed] [Google Scholar]
- 45.Brunette MF, Mueser KT, Babbin S, Meyer-Kalos P, Rosenheck R, Correll CU, Cather C, Robinson DG, Schooler NR, Penn DL, et al. Demographic and clinical correlates of substance use disorders in first episode psychosis. Schizophr Res 2018;194:4–12. [DOI] [PubMed] [Google Scholar]
- 46.Kavanagh DJ, Waghorn G, Jenner L, Chant DC, Carr V, Evans M, et al. Demographic and clinical correlates of comorbid substance use disorders in psychosis: multivariate analyses from an epidemiological sample. Schizophr Res 2004;66:115–124. [DOI] [PubMed] [Google Scholar]
- 47.Rabinowitz J, Bromet EJ, Lavelle J, Carlson G, Kovasznay B, Schwartz JE. Prevalence and severity of substance use disorders and onset of psychosis in first-admission psychotic patients. Psychol Med 1998;28:1411–1419. [DOI] [PubMed] [Google Scholar]
- 48.DeRosse P, Kaplan A, Burdick KE, Lencz T, Malhotra AK. Cannabis use disorders in schizophrenia: effects on cognition and symptoms. Schizophr Res 2010;120:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nesvag R, Knudsen GP, Bakken IJ, Hoye A, Ystrom E, Suren P, et al. Substance use disorders in schizophrenia, bipolar disorder, and depressive illness: a registry-based study. Soc Psychiatry Psychiatr Epidemiol 2015;50:1267–1276. [DOI] [PubMed] [Google Scholar]
- 50.Libuy N, de Angel V, Ibanez C, Murray RM, Mundt AP. The relative prevalence of schizophrenia among cannabis and cocaine users attending addiction services. Schizophr Res 2018;194:13–17. [DOI] [PubMed] [Google Scholar]
- 51.Dubertret C, Bidard I, Ades J, Gorwood P. Lifetime positive symptoms in patients with schizophrenia and cannabis abuse are partially explained by co-morbid addiction. Schizophr Res 2006;86:284–290. [DOI] [PubMed] [Google Scholar]
- 52.Harder VS, Stuart EA, Anthony JC. Adolescent cannabis problems and young adult depression: male-female stratified propensity score analyses. Am J Epidemiol 2008;168:592–601. doi: 10.1093/aje/kwnl84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feingold D, Rehm J, Lev-Ran S. Cannabis use and the course and outcome of major depressive disorder: a population based longitudinal study. Psychiatry Res 2017;251:225–34. doi: 10.1016/j.psychres.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 54.Pacek LR, Martins SS, Crum RM. The bidirectional relationships between alcohol, cannabis, co-occurring alcohol and cannabis use disorders with major depressive disorder: results from a national sample. J Affect Disord 2013;148:188–95. doi: 10.1016/j.jad.2012.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hunt GE, Malhi GS, Cleary M, Lai HM, Sitharthan T. Prevalence of comorbid bipolar and substance use disorders in clinical settings, 1990–2015: systematic review and meta-analysis. J Affect Disord 2016;206:331–349. [DOI] [PubMed] [Google Scholar]
- 56.Cassidy F, Aheam EP, Carroll BJ. Substance abuse in bipolar disorder. Bipolar Disord 2001;3:181–88. doi: 10.1034/j,1399-5618.2001.30403.x. [DOI] [PubMed] [Google Scholar]
- 57.Kawa I, Carter JD, Joyce PR, Doughty CJ, Frampton CM, Wells JE, et al. Gender differences in bipolar disorder: age of onset, course, comorbidity, and symptom presentation. Bipolar Disord 2005;7:119–125. [DOI] [PubMed] [Google Scholar]
- 58.Morgan VA, Mitchell PB, Jablensky AV. The epidemiology of bipolar disorder: sociodemographic, disability and service utilization data from the Australian national study of low prevalence (Psychotic) Disorders. Bipolar Disord 2005;7:326–37. doi: 10.1111/j.l399-5618.2005.00229.x. [DOI] [PubMed] [Google Scholar]
- 59.Strakowski SM, DelBello MP, Fleck DE, Adler CM, Anthenelli RM, Keck PE Jr, et al. Effects of co-occurring cannabis use disorders on the course of bipolar disorder after a first hospitalization for mania. Arch Gen Psychiatry 2007;64:57–64. [DOI] [PubMed] [Google Scholar]
- 60.van Rossum I, Boomsma M, Tenback D, Reed C. Does cannabis use affect treatment outcome in bipolar disorder? A longitudinal analysis. J Nerv Ment Dis 2009;197:35–40. [DOI] [PubMed] [Google Scholar]
- 61.Altshuler LL, Kupka RW, Hdlemann G, Frye MA, Sugar CA, McElroy SL, Nolen WA, Grunze H, Leverich GS, Keck PE, et al. Gender and depressive symptoms in 711 patients with bipolar disorder evaluated prospectively in the Stanley Foundation bipolar treatment outcome network. Am J Psychiatry 2010;167:708–15. [DOI] [PubMed] [Google Scholar]
- 62.Braga RJ, Burdick KE, Derosse P, Malhotra AK. Cognitive and clinical outcomes associated with cannabis use in patients with bipolar I disorder. Psychiatry Res 2012;200:242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lev-Ran S, Le Foil B, McKenzie K, George TP, Rehm J. Bipolar disorder and co-occurring cannabis use disorders: characteristics, co-morbidities and clinical correlates. Psychiatry Res 2013;209:459–465. [DOI] [PubMed] [Google Scholar]
- 64.Weinstock LM, Gaudiano BA, Wenze SJ, Epstein-Lubow G, Miller IW. Demographic and clinical characteristics associated with comorbid cannabis use disorders (CUDs) in hospitalized patients with bipolar I disorder. Compr Psychiatry 2016;65:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cotton SM, Lambert M, Berk M, Schimmelmann BG, Butselaar FJ, MoGorry PD, Conus P. Gender differences in first episode psychotic mania. BMC Psychiatry 2013;13:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shand FL, Degenhardt L, Slade T, Nelson EC. Sex differences amongst dependent heroin users: histories, clinical characteristics and predictors of other substance dependence. Addict Behav 2011;36:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mackie K Cannabinoid receptors: where they are and what they do. J Neuroendocrinol 2008;20:10–14. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 68.D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT. The psychotomimetic effects of intravenous ddta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsy chopharmacology 2004;29:1558–1572. [DOI] [PubMed] [Google Scholar]
- 69.Craft RM, Marusich JA, Wiley JL Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sd 2013;92:476–81. doi: 10.1016/j.lfs.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ashton CH, Moore PB, Gallagher P, Young AH. Cannabinoids in bipolar affective disorder: a review and discussion of their therapeutic potential. J Psychopharmacol 2005;19:293–300. [DOI] [PubMed] [Google Scholar]
- 71.Muller-Vahl KR, Emrich HM. Cannabis and schizophrenia: towards a cannabinoid hypothesis of schizophrenia. Expert Rev Neurother 2008;8:1037–48. doi: 10.1586/14737175.8.7.1037. [DOI] [PubMed] [Google Scholar]
- 72.Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 2007;36:1065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Realini N, Rubino T, Parolaro D. Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacol Res 2009;60:132–38. doi: 10.1016/j.phrs.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 74.Richmond-Rakerd LS, Slutske WS, Wood PK. Age of initiation and substance use progression: a multivariate latent growth analysis. Psychol Addict Behav 2017;31:664–75. doi: 10.1037/adb0000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malone DT, Hill MN, Rubino T. Adolescent cannabis use and psychosis: epidemiology and neurodevdopmental models. Br J Pharmacol 2010;160:511–22. doi: 10.1111/j.l476-5381.2010.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pasman JA, Verweij KJH, Gerring Z, Stringer S, Sanchez-Roige S, Treur JL, Abdellaoui A, Nivard MG, Baselmans BML, Ong J-S, et al. GW AS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal influence of schizophrenia. Nat Neurosci 2018;21:1161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry 2005;57:1117–27. [DOI] [PubMed] [Google Scholar]
- 78.Hoenicka J, Garrido E, Ponce G, Rodriguez-Jimenez R, Martinez I, Rubio G, et al. Sexually dimorphic interaction between the DRD1 and COMT genes in schizophrenia. Am J Med Genet B Neuropsychiatr Genet 2010;153B:948–954. [DOI] [PubMed] [Google Scholar]
- 79.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ 2002;325:1212–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moore TH, Zammit S, Lingford-Hughes A, Barnes TRE, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 2007;370:319–328. [DOI] [PubMed] [Google Scholar]
- 81.Tu AW, Ratner PA, Johnson JL. Gender differences in the correlates of adolescents’ cannabis use. Subst Use Misuse 2008;43:1438–63. doi: 10.1080/10826080802238140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matheson J, Sproule B, Di Ciano P, Fares A, Le Foil B, Mann RE, Brands B. Sex differences in the acute effects of smoked cannabis: evidence from a human laboratory study of young adults. Psychopharmacology (Berl) 2020;237:305–316. [DOI] [PubMed] [Google Scholar]
- 83.Bossong MG, Niesink RJ. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol 2010;92:370–85. [DOI] [PubMed] [Google Scholar]
- 84.Agrawal A, Lynskey MT. Does gender contribute to heterogeneity in criteria for cannabis abuse and dependence? Results from the national epidemiological survey on alcohol and related conditions. Drug Alcohol Depend 2007;88:300–07. doi: 10.1016/j.drugalcdep.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ranganathan M, Cortes-Briones J, Radhakrishnan R, Thumauer H, Planeta B, Skosnik P, Gao H, Labaree D, Neumeister A, Pittman B, et al. Reduced Brain Cannabinoid Receptor Availability in Schizophrenia. Biol Psychiatry 2016;79:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hirvonen J, Goodwin RS, Li C-T, Terry GE, Zoghbi SS, Morse C, Pike VW, Volkow ND, Huestis MA, Innis RB, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry 2012;17:642–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.D’Souza DC, Ranganathan M, Braky G, Gueorguieva R, Zimdo Z, Cooper T, et al. Blunted psychotomimetic and amnestic effects of ddta-9-tetrahydrocannabind in frequent users of cannabis. Neuropsychopharmacology 2008232505–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Colizzi M, McGuire P, Pertwee RG, Bhattacharyya S. Effect of cannabis on glutamate signalling in the brain: a systematic review of human and animal evidence. Neurosci Biobehav Rev 2016;64:359–381. [DOI] [PubMed] [Google Scholar]
- 89.Lopez HH. Cannabinoid-hormone interactions in the regulation of motivational processes. Horm Behav 2010;58:100–10. doi: 10.1016/j.yhbeh.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 90.Riebe CJ, Hill MN, Lee TT, Hillard CJ, Gorzalka BB. Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology 2010;35:1265–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Costaffeda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev 2008;58:57–70. [DOI] [PubMed] [Google Scholar]
- 92.Neumeister A, Normandin MD, Pietrzak RH, Piomelli D, Zheng MQ, Gujarro-Anton A, Potenza MN, Bailey CR, Lin SF, Najafzadeh S, et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol Psychiatry 2013;18:1034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Krebs-Kraft DL, Hill MN, Hillard CJ, McCarthy MM. Sex difference in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior. Proc Natl Acad Sci USA 2010;107:20535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gunduz-Cinar O, Hill MN, McEwen BS, Holmes A. Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends Pharmacol Sci 2013;34:637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morgan CJ, Page E, Schaefer C, Chatten K, Manocha A, Gulati S, et al. Cerebrospinal fluid anandamide levels, cannabis use and psychotic-like symptoms. Br J Psychiatry 2013;202:381–382. [DOI] [PubMed] [Google Scholar]
- 96.Boileau I, Mansouri E, Williams B, Le Foil B, Rusjan P, Mizrahi R, Tyndale RF, Huestis MA, Payer DE, Wilson AA, et al. Fatty acid amide hydrolase binding in brain of cannabis users: imaging with the novel radiotracer [11C] CURB. Biol Psychiatry 2016;80:691–701. doi: 10.1016/j.biopsych.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Simon NM, Zalta AK, Worthingston JJ 3rd, Hoge EA, Christian KM, Stevens JC, Pollack MH. Preliminary support for gender differences in response to fluoxetine for generalized anxiety disorder. Depress Anxiety 2006;23:373–376. [DOI] [PubMed] [Google Scholar]
- 98.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry 2003;60:837–844. [DOI] [PubMed] [Google Scholar]
- 99.Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. BMJ 2002;325:1195–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rubino T, Sala M, Vigano D, Braida D. Cellular mechanisms underlying the anxiolytic effect of low doses of peripheral Delta9-tetrahydrocannabinol in rats. Neuropsychopharmacology 2007;32:2036–2045. [DOI] [PubMed] [Google Scholar]
- 101.Pedersen W, Mastekaasa A, Wichstrom L. Conduct problems and early cannabis initiation: a longitudinal study of gender differences. Addiction 2001;96:415–31. doi: 10.1046/j.l360-0443.2001.9634156.x. [DOI] [PubMed] [Google Scholar]
- 102.Alegria AA, Petry NM, Liu SM, Blanco C, Skodol AE, Grant B, Hasin D. Sex differences in antisocial personality disorder: results from the national epidemiological survey on alcohol and related conditions. Personal Disord 2013;4:214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Coffey C, Lynskey M, Wolfe R, Patton GC. Initiation and progression of cannabis use in a population-based Australian adolescent longitudinal study. Addiction 2000;95:1679–1690. [DOI] [PubMed] [Google Scholar]
- 104.Fonseca F, Robles-Martfnez M, Tirado-Munoz J, Allas-Ferri M, Mestre-Pinto JI, et al. A gender perspective of addictive disorders. Curr Addict 2021;89–99:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hindley G, Beck K, Borgan F, Ginestet CE, McCutcheon R, Kleinloog D, Ganesh S, Radhakrishnan R, D’Souza DC, Howes OD, et al. Psychiatric symptoms caused by cannabis constituents: a systematic review and meta-analysis. Lancet Psychiatry 2020;7:344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kessler RC, Berglund P, Dernier O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch Gen Psychiatry 2005;62:593–602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


