Abstract
The biotransformation of 2,4,6-trinitrotoluene (TNT) (175 μM) by Phanerochaete chrysosporium with molasses and citric acid at pH 4.5 was studied. In less than 2 weeks, TNT disappeared completely, but mineralization (liberated 14CO2) did not exceed 1%. A time study revealed the presence of several intermediates, marked by the initial formation of two monohydroxylaminodinitrotoluenes (2- and 4-HADNT) followed by their successive transformation to several other products, including monoaminodinitrotoluenes (ADNT). A group of nine acylated intermediates were also detected. They included 2-N-acetylamido-4,6-dinitrotoluene and its p isomer, 2-formylamido-4,6-dinitrotoluene and its p isomer (as acylated ADNT), 4-N-acetylamino-2-amino-6-nitrotoluene and 4-N-formylamido-2-amino-6-nitrotoluene (as acetylated DANT), 4-N-acetylhydroxy-2,6-dinitrotoluene and 4-N-acetoxy-2,6-dinitrotoluene (as acetylated HADNT), and finally 4-N-acetylamido-2-hydroxylamino-6-nitrotoluene. Furthermore, a fraction of HADNTs were found to rearrange to their corresponding phenolamines (Bamberger rearrangement), while another group dimerized to azoxytoluenes which in turn transformed to azo compounds and eventually to the corresponding hydrazo derivatives. After 30 days, all of these metabolites, except traces of 4-ADNT and the hydrazo derivatives, disappeared, but mineralization did not exceed 10% even after the incubation period was increased to 120 days. The biotransformation of TNT was accompanied by the appearance of manganese peroxidase (MnP) and lignin-dependent peroxidase (LiP) activities. MnP activity was observed almost immediately after TNT disappearance, which was the period marked by the appearance of the initial metabolites (HADNT and ADNT), whereas the LiP activity was observed after 8 days of incubation, corresponding to the appearance of the acyl derivatives. Both MnP and LiP activities reached their maximum levels (100 and 10 U/liter, respectively) within 10 to 15 days after inoculation.
Contamination of soils by explosives such as 2,4,6-trinitrotoluene (TNT), generated as waste from the munitions and defense industries, is a significant worldwide environmental problem. It is estimated that TNT alone is produced in amounts close to 2 million pounds a year (19) and threatens human life through the food chain (51). The compound is mutagenic and toxic and has a tendency to persist in the environment (34, 44, 49, 51). There have been several attempts to biodegrade TNT, but thus far the compound has been found to undergo biotransformation rather than mineralization (5, 9, 13, 16, 28, 41, 48), giving in most cases the initial products 4-amino-2,6-dinitrotoluene (4-ADNT), 2-amino-4,6-dinitrotoluene (2-ADNT), 2,4-diamino-6-nitrotoluene (2,4-DANT), and 2,6-diamino-4-nitrotoluene (2,6-DANT) (11, 24).
Several other studies on the degradation of TNT by Phanerochaete chrysosporium have been reported, and in most cases mineralization amounts larger than those normally obtained with bacteria were observed (8, 14, 29, 40, 41, 43). The degree of TNT mineralization varies and depends on whether ligninolytic (nitrogen-limiting) or nonligninolytic (nitrogen-sufficient) conditions are used in the culture medium. For example, Fernando et al. (14) reported 85% degradation of TNT in both water (100 ppm) and soil (10,000 ppm), with 18.4 and 19.6% mineralization in stationary ligninolytic culture medium, respectively. This suggested that TNT is not toxic to the fungi at high concentrations. No products were identified to account for the remaining TNT that was degraded. Spiker et al. (40) demonstrated that a TNT concentration of greater than 15 ppm inhibited mineralization, resulting in 1 to 3% of 14CO2 being liberated. Furthermore, Michels and Gottschalk (30) have reported that high concentrations of TNT inhibit lignin peroxidase (LiP) of the fungi.
The sluggish mineralization that is frequently observed for TNT despite its efficient transformation is attributed to the formation of dead-end products that act to deroute the process of mineralization. The identities of these transformed products remained, in most cases, unknown due to the absence of rapid and sensitive analytical techniques suitable for direct detection of transient species during the course of the reaction. We have recently reported that despite the almost complete disappearance of TNT with an anaerobic sludge, negligible amounts of 14CO2 were detected (20). Liquid chromatography-mass spectrometry (LC-MS) and SPME gas chromatography-MS studies revealed the predominant formation of triaminotoluene (80%), which subsequently polymerized or was transformed to other phenolic products. The latter compounds were formed through hydrolytic cleavage of the NH2 group (20) rather than through Bamberger rearrangement, which is encountered in the formation of phenolamines from hydroxylamino aromatic compounds (10, 15, 21).
One objective of the present study was to apply LC-MS in an attempt to identify all possible transformed products involved in the transformation of TNT with the fungus P. chrysosporium in agitated cultures at pH 4.5. A time course study to help understand the fate of these products and their effect on the mineralization process will also be discussed.
MATERIALS AND METHODS
Reagents and materials.
TNT was obtained from Centre de Recherche pour la Défense (Valcartier, Quebec, Canada) with a chemical purity, as measured by high-pressure liquid chromatography (HPLC), of 9.5%. [U-14C]TNT was synthesized to a radiochemical purity that exceeded 95%, as described by HPLC by Ampleman et al. (2). 2-ADNT, 4-ADNT, 2,4-DANT, 2,6-DANT, 2-hydroxylamino-4,6-dinitrotoluene (2-HADNT), 4-hydroxylamino-2,6-dinitrotoluene (4-HADNT), 2,2′,6,6′-tetranitro-4,4′-azoxytoluene (TN-4,4′-AzoxyT), 4,4′,6,6′-tetranitro-2,2′-azoxytoluene (TN-2,2′-AzoxyT), 2,2′,6,6′-tetranitro-4,4′-azotoluene (TN-4,4′-AzoT), and 4,4′,6,6′-tetranitro-2,2′-azotoluene (TN-2,2′-AzoT) were obtained from AccuStandard Inc. (New Haven, Conn.). The molasses used was a cane sugar which was analyzed by HPLC and found to contain 36% sucrose, 6% glucose, and 7% fructose. This type of molasses is also known to contain pantothenic acid (25 ppm) and only 0.1% nitrogen (25).
Microcosms for degradation of TNT.
In a typical setup, a serum bottle (100 ml) was charged with 40 ml of the mineral salt medium used in the procedure described by Greer et al. (18) [13 mM KH2PO4, 6.4 mM Na2HPO4, 0.395 mM MgSO4 · 7H2O, 1 μM AlK(SO4)2 · 12H2O, 2 μM FeSO4 · 7H2O, 10 μM ZnSO4 · 7H2O, 10 μM MnSO4 · H2O, 1 μM CuSO4 · 7H2O, 1 μM Co(NO3)2 · 6H2O, 10 μM Ca(NO3)2 · 2H2O, and 2 μM NaMoO4 · 2H2O], followed by the addition of molasses (2.65 g/liter) as a carbon source and citric acid (2.5 g/liter) to maintain a pH of 4.5. The mixture was then autoclaved at 120°C for 40 min. The fungal strain used in the study was P. chrysosporium BKM-F-1767 (ATCC 24725) and was kept on malt agar slants (20 g of agar, 20 g of malt extract, and 1 g of yeast extract/liter). Spore solution (1-ml aliquots; 5 × 106 spores/ml) was added to each microcosm; this was followed by the addition of TNT, taken from an acetone stock solution (39,380 ppm), to a final concentration of 40 ppm. The microcosms were then sealed with Teflon-coated serum caps for incubation at 37°C in a rotary shaker (Brunswick, Edison, N.J.) at 135 rpm. Some serum bottles (microcosms) were supplemented with [U-14C]TNT (100,000 dpm) and then fitted with a small test tube containing 1.0 ml of 0.5 M KOH to trap liberated carbon dioxide (14CO2). The headspace in each microcosm was flushed with oxygen gas to maintain aerobic conditions and then sealed with butyl rubber septa and aluminum crimp seals to prevent the loss of 14CO2 and other volatile metabolites. Control microcosms were prepared by using the fungus and culture medium without TNT, and a second control contained an autoclaved fungus medium to which TNT but no fungi were added. Each microcosm was wrapped with aluminum foil to protect the mixture against photolysis. Microcosms with 14C-labeled TNT were routinely sampled (daily or every 2 days) for the determination of 14CO2 in the KOH trap by using a Tri-Carb 4530 liquid scintillation counter (model 2100 TR; Packard Instrument Company, Meriden, Conn.). Microcosms that did not receive 14C-labeled TNT were reserved for LC-MS analysis of residual TNT and its metabolites in the aqueous phase after filtration. After LC-MS analysis, these filtrates were extracted with acetonitrile to account for any insoluble TNT metabolites. Certain microcosms were sacrificed to determine the metabolite concentrations in the mycelium mat. The separated mycelia were sonicated (Blackstone Ultrasonics, Jamestown, N.Y.) with acetonitrile (10 ml) at 10°C for 16 h. The decanted acetonitrile layer was filtered through a 0.45-μm-pore-size Millex-HV filter for subsequent LC-MS analysis.
Enzyme assays.
LiP activity was determined by monitoring the conversion of veratryl alcohol to veratryl aldehyde by hydrogen peroxide at 310 nm as described by Tien and Kirk (45). The Mn(II)-dependent peroxidase (MnP) activity was determined by monitoring the disappearance of vanillyl acetone at 334 nm as described by Paszcynski et al. (33).
LC-MS.
LC-MS was performed on a Micromass Platform II benchtop single-quadrupole mass detector fronted by a Hewlett-Packard 1100 series HPLC system. The chromatographic conditions used were a C8 LC column (25 cm by 4.6 mm; 5-μm-diameter particles) and acetonitrile-water gradient programmed from 30 to 80% (vol/vol), using a flow rate of 1 ml/min with a postcolumn split of 5:95. Analyte ionization, a process which produces mainly the deprotonated molecular mass ion M − H, was achieved in the negative electron spray ionization mode by using a probe tip potential of 3.0 kV and a skimmer voltage of 30 V. The temperature of the electron spray ionization capillary was maintained at 90°C. The mass spectrum was typically scanned at a rate of 1 s/100 Da. The total ion current was acquired between 40 and 500 Da, which was followed by extracting the deprotonated molecular mass ion [M − H]− of the suspected metabolite. In the case of DANT, analyte ionization was achieved by using positive electron spray ionization, a process which produces mainly the protonated molecular mass ion M + H.
Further confirmation of the identities of targeted metabolites was accomplished by comparison with commercially available reference compounds. Alternatively, in the case of acetylated metabolites, the standards were synthesized starting from the corresponding amine by using the acetic anhydride-bicarbonate method (4). Briefly, 1-ml aqueous aliquots (1 mM) of either the monoamine ADNT, the diamine DANT, or the hydroxylamine HADNT were treated with acetic anhydride (or formic acid) and stirred at room temperature for 30 min. The mixture was neutralized with sodium bicarbonate for subsequent direct analysis by LC-MS.
Analysis of nitrite and ammonium ions.
The aqueous layer was analyzed for NO2− ions with an SP 8100 HPLC with a 25- by 0.46-cm PRP-X 100 Hamilton column and a Waters 431 conductivity detector. Methanol (10%) buffered (pH 8.5) with a solution of p-hydroxybenzoic acid was used as the mobile phase at flow rate of 2 ml/min. Analytical grade sodium nitrite was used as the standard. Ammonium ions were analyzed by using the same system but with a PRP-X 200 Hamilton column with 30% methanol in a 6 mM nitric acid solution at a flow rate of 0.75 ml/min.
RESULTS AND DISCUSSION
Metabolite identification.
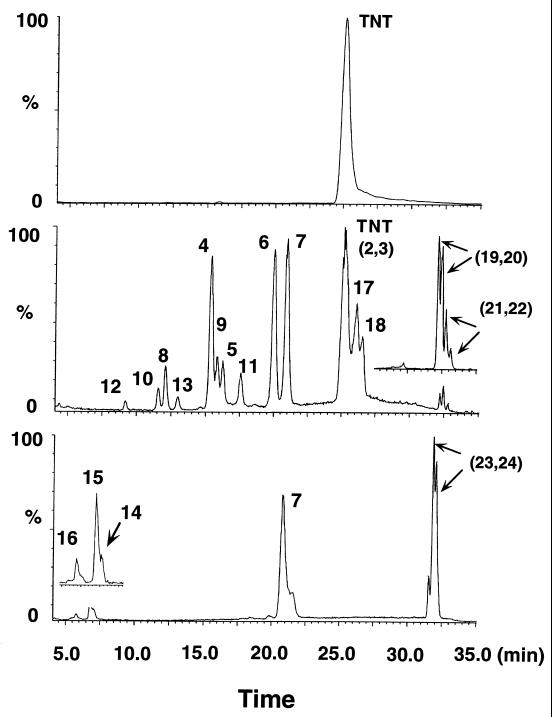
Figure 1 shows a typical representation of TNT (40 mg/liter) transformation profiles at three time points, i.e., after 0, 3, and 25 days of incubation with the fungus P. chrysosporium. Several LC-MS signals, representing TNT intermediate products, were detected during the first 3 days of incubation, which in turn, as the time progressed, transformed to other products. Despite TNT disappearance during the first 10 days of incubation, mineralization did not exceed 1% as measured by liberated 14CO2. After 30 days, 14CO2 liberation reached its maximum value of 10% of the original TNT amount, since no more mineralization was observed even after the incubation period was extended to 120 days. As mentioned earlier, high concentrations of TNT have been reported to be toxic to the fungus (40) and to inhibit LiP production (30). In the present study, we found that a minimum concentration of 50 mg of TNT per liter was needed before TNT’s toxic effect could be observed (data not shown).
FIG. 1.
Typical time course profile of the disappearance of TNT (40 mg/liter) and the appearance of its intermediate products after treatment with the fungus P. chrysosporium in an agitated culture with molasses at pH 4.5. Profiles were obtained at three different time points (top, 0 days; middle, 3 days; bottom, 25 days).
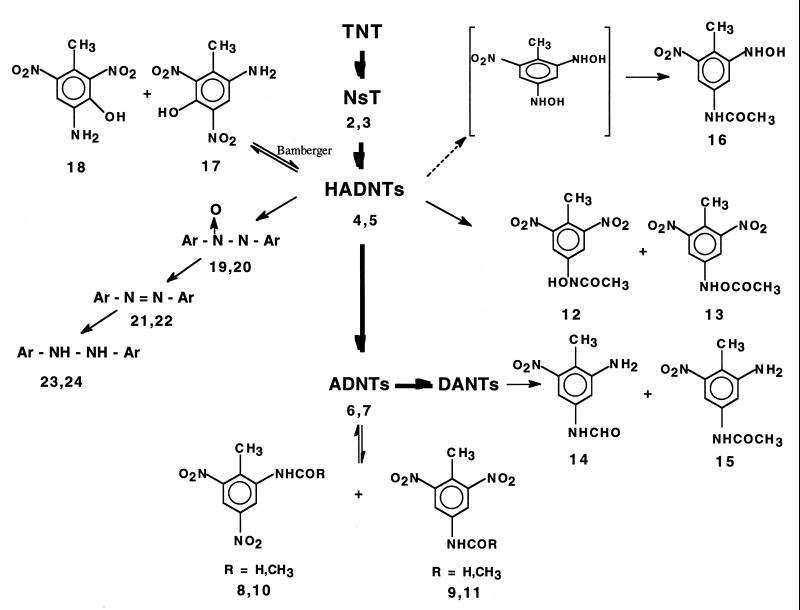
To help understand the fate of TNT, particularly that of the transformed fraction that escaped mineralization, key products were first identified, subgrouped according to their functional groups, and then fitted in a pathway by using a time course study. In most cases, the TNT metabolites were identified by using a combination of their mass data as represented by the deprotonated molecular mass ion and the retention time (M − H [in daltons], retention time [in minutes]) and, when available, by comparison with reference materials. In a few cases the identities of the products had to be predicted based on their mass data and their estimated location in the transformation process.
The first such group of LC-MS signals (peaks 4 to 7) were identified by comparison with commercially available reference materials as 2-HADNT (peak 4) (212, 15.3), 4-HADNT (peak 5) (212, 16.3), 2-ADNT (peak 6) (196, 19.8), and 4-ADNT (peak 7) (196, 20.6). The two suspected nitroso derivatives 2-nitroso-4,6-dinitrotoluene (2-NsT) (peak 2) (210, 25.1) and 4-nitroso-2,6-dinitrotoluene (4-NsT) (peak 3) (210, 25.1) severely overlapped with TNT and could be identified only by extracting their M − H at m/z 210 Da. The mass spectrum of TNT alone did not show this characteristic mass ion. These initial products have been frequently detected under both aerobic and anaerobic conditions (8, 12).
A second group of LC-MS peaks (peaks 8 to 16) were identified as the acyl (formyl and/or acetyl) derivatives of ADNTs (ortho and para), HADNTs (ortho and para), 2,4-DANT, and 2-hydroxylamino-4-amino-6-nitrotoluene. Peaks 8 (224, 12.0) and 9 (224, 15.7), matching a molecular formula of C8H6N3O5, were identified as 2-formamido-4,6-dinitrotoluene (2-N-FmDNT) and 4-formamido-2,6-dinitrotoluene (4-N-FmDNT), respectively. Their identities were confirmed by comparison with reference materials prepared by reacting 2- and 4-ADNT separately with formic acid and sodium bicarbonate. Likewise, peaks 10 and 11 both showed their [M − H]− at m/z 238 Da, which represented a molecular formula of C9H8N3O5, and were identified as ortho-acetylamido-4,6-dinitrotoluene (2-N-AcDNT) and para-acetylamido-2,6-dinitrotoluene (4-N-AcDNT), respectively. Their identities were confirmed by comparison with reference materials prepared by acetylating 2- and 4-ADNT separately with acetic anhydride and sodium bicarbonate.
Similarly, peaks 12 (254, 9.1) and 13 (254, 12.8) showed the same [M − H]− at m/z 254 Da, which matched a molecular formula of C9H8N3O6. The two peaks were tentatively identified as 2,6-dinitro-4-N-acetylamidohydroxytoluene (4-N-AcHDNT) (peak 12) and 2,6-dinitro-4-N-acetoxytoluene (4-N-AcoxyDNT) (peak 13). We presumed that isomer 12, which is expected to be more polar because of a free OH group, eluted before isomer 13. No acetyl derivatives of 2-HADNT were observed, presumably because their formation was inhibited by the steric effects of the ortho-CH3 group (10, 29). In an attempt to prepare the two suspected products by acetylating the HADNT, we obtained instead two products with [M − H]− also occurring at 254 Da but with retention times different from those for peaks 12 and 13. These two chemically generated products were presumed to be the acetyl derivatives of the phenolamines formed via the acid-catalyzed Bamberger rearrangement of HADNT. This result might be taken as indirect evidence that a selective enzymatic acetylation of 4-HADNT occurred to produce products 12 and 13 in the fungus-treated TNT culture.
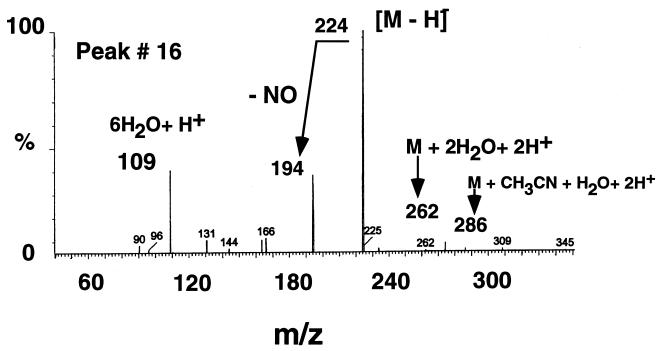
On the other hand, LC-MS peaks 14 to 16, which appeared between 5.0 and 7.0 min, became visible only after 20 days of incubation (Fig. 1). Peaks 14 (194 Da, 6.7 min) and 15 (208, 7.1) matched molecular formulas of C8H8N3O3 and C9H10N3O3, respectively. Reference materials prepared by acylating 2,4-ADNT with either formic acid or acetic anhydride showed LC-MS data similar to those obtained earlier for the two metabolites 14 and 15 (i.e., 194, 6.7 and 208, 7.1, respectively). Peaks 14 and 15 were eventually identified as 4-N-formamido-2-amino-6-nitrotoluene (4-N-FmANT) and 4-N-acetylamino-2-amino-6-nitrotoluene (4-N-AcANT), respectively. The third LC-MS peak, peak 16 (224, 5.52), which appeared at a retention time of 5.52 min and possessed an [M − H]− at m/z 224 Da, matched a molecular formula of C9H10N3O4. Another relevant mass ion at m/z 286 Da was also observed and was attributed to M− + CH3CN + H2O + 2H+. This peak was tentatively identified as 4-N-acetylamino-2-hydroxylamino-6-noitrotoluene (4-N-AcOHANT), since no reference materials could be obtained. Further details are shown in Fig. 2. The three acylated derivatives 14 to 16 have been observed recently by Bruns-Nagel et al. (6) during a coupled anaerobic-aerobic composting of TNT. In that case, however, a positive chemical ionization was used in the LC-MS, thus giving the protonated molecular mass ions [M + H]+ at m/z 196, 210, and 226 Da instead of the present deprotonated [M − H]− values at m/z 194, 208, and 224 Da, respectively.
FIG. 2.
Typical mass spectrum of the acylated TNT metabolite 4-N-AcHANT (peak 16).
Two more LC-MS peaks, designated 17 (212 Da, 25.9 min) and 18 (212, 26.1), both matched a molecular formula of C7H6N3O5, which was similar to that obtained earlier for the two HADNT isomers 4 and 5. However, when either 2-HADNT or 4-HADNT was treated with dilute hydrochloric acid (pH 4.5), a major LC-MS signal was detected in each case with the same [M − H] at m/z 212 Da but with the same retention times as those observed for peaks 17 and 18 (i.e., 25.9 and 26.1 min, respectively). These two peaks were eventually identified as the two phenolamines ortho-amino-5-hydroxy-4,6-dinitrotoluene (2-A-5-OH-4,6-DNT) (peak 17) and para-amino-5-hydroxy-2,6-dinitrotoluene (4-A-5-OH-2,6-DNT) (peak 18). It has been reported that when aromatic hydroxylamines are generated under acidic conditions the NHOH group rearranges to produce the corresponding phenolamine (3, 26, 37, 42, 50). This acid-catalyzed rearrangement, known as Bamberger rearrangement, has recently been found to occur enzymatically during TNT degradation under neutral conditions with the anaerobic microorganism Clostridium acetobutylicum (22).
The last group of peaks, designated 19 to 24, with retention times ranging from 30 to 33 min, were apparently related to a dimerization reaction that involved HADT. Peaks 19 and 20 were identified as the two azoxy isomers TN-2,2′-AzoxyT and TN-4,4′-AzoxyT, respectively, by comparison with commercially available reference materials, using their [M − H]− at m/z 405 and their retention times at 32.1 and 32.3 min, respectively. The formation of TNT azoxy products is frequently observed under both biotic and abiotic conditions, and their formation was attributed to a spontaneous condensation between 4-HADNT and 4-NsT (29). Likewise, the two peaks 21 and 22 also had different retention times (i.e., 33.5 and 34.1 min) but the same [M − H]− (m/z 389 Da). The two signals both had the [M − H]− at 16 mass units (1 O atom) lower than that of the corresponding azoxy derivative, indicating their presence as the corresponding reduced azoxy isomers. By comparison with commercially available reference materials, these two peaks were identified as TN-2,2′-AzoT (peak 21) and TN-4,4′-AzoT (peak 22). The remaining pair of LC-MS signals, peaks 23 and 24, which were not completely resolved, were also detected at two different retention times (i.e., 33.3 and 33.7 min) but once again with the same [M − H]− at m/z 391 Da, which was 2 mass units (2 H atoms) higher than that of the above-described azo dimers. Peaks 23 and 24 were tentatively identified as the reduced forms of the azo derivatives (i.e., 4′,6,6′-tetranitro-2,2′-hydrazotoluene [TN-2,2′-HydrazoT] and 2,2′,6,6′-tetranitro-4,4′-hydrazotoluene [TN-4,4′-HydrazoT], respectively.
Time course profiles of metabolites for mechanism elucidation.
As the preceding discussion indicated, there were several products formed during TNT degradation with P. chrysosporium. To help us to understand the various transformations among these intermediates, LC-MS time course studies were conducted. The LC-MS peak areas of TNT and/or its intermediate products were measured at various time points and graphed to produce profiles that can be used to monitor the appearance and disappearance of related intermediates. To measure mineralization (liberated 14CO2) a separate set of micrococosms containing [U-14C]TNT was used.
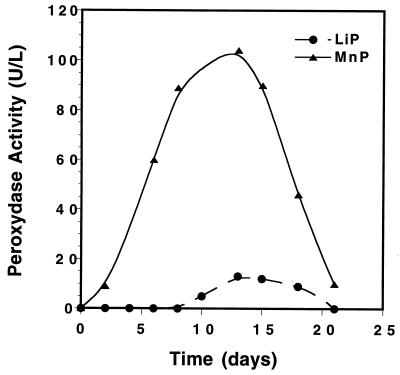
To help us to understand the physiological state of the fungus during TNT biotransformation, both LiP and MnP activities were measured at various time intervals, as shown in Fig. 3. The fungus was found to exhibit both MnP and LiP activities. However, the LiP activity was found to be lower than that of MnP by at least a factor of 10. Also, a lag period of 6 to 8 days was needed before LiP activity could be observed. In contrast, no such delay was observed in the case of MnP. Interestingly, both enzymes showed maximum activity at between 10 and 15 days, after which the activities of both enzymes declined until they approached zero after 20 days of incubation. Stahl and Aust (41) have reported similar physiological behavior from the same fungus spores. However, the MnP activity was found to be higher than the LiP activity by at least a factor of 5.
FIG. 3.
LiP and MnP activities of P. chrysosporium in spore culture medium supplemented with molasses.
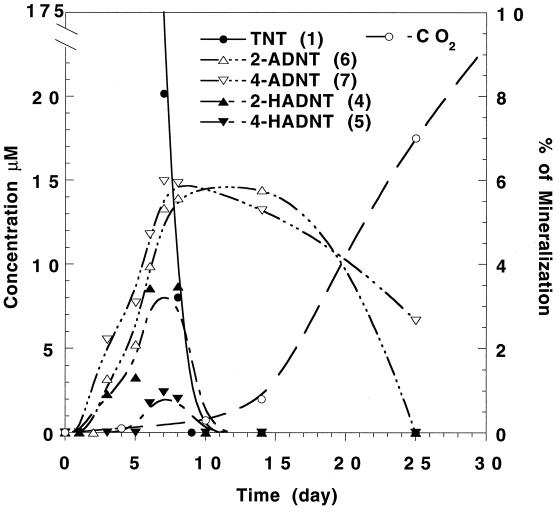
Figure 4 shows the disappearance of TNT together with the appearance of its two prime metabolites, 2- and 4-HADNT (metabolites 4 and 5), and their reduced monoamines (metabolites 6 and 7). Both HADNT prime products were transformed beyond detection after less than 10 days of incubation, which was also the time marked by TNT disappearance under nonligninolytic conditions (Fig. 3). The reduction in the concentrations of the two HADNTs was accompanied by a gradual buildup of the two monoamines 2-ADNT and 4-ADNT (metabolites 6 and 7). Although the regioselectivity of HADNT formation seemed to favor reduction at the ortho position by a factor of 2, para-ADNT (metabolite 7) was formed in a yield which was 25% higher than that of its ortho isomer (metabolite 6). This indicated that the rate of 2-HADNT transformation is higher than that for its para isomer.
FIG. 4.
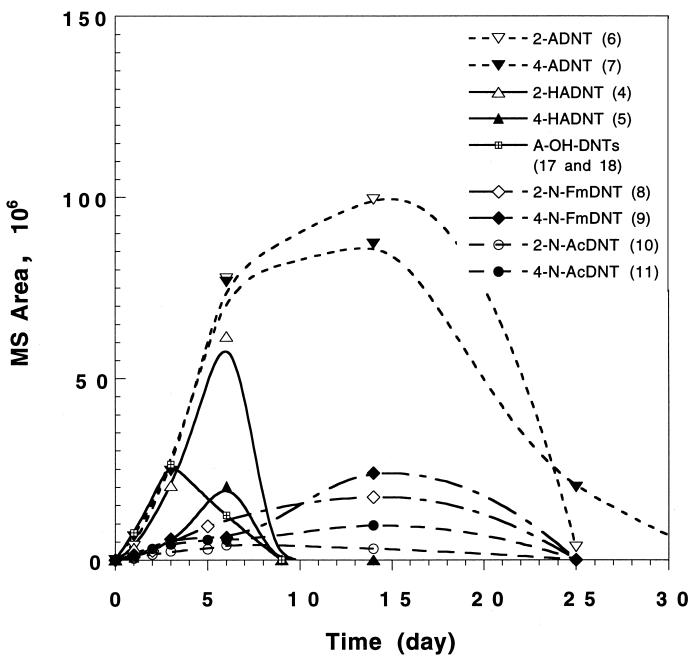
Time course profile for the disappearance and appearance of TNT prime metabolites HADNT and ADNT together with mineralization data following the treatment of TNT (40 mg/liter) with the fungus P. chrysosporium. A-OH-DNTs (metabolites 17 and 18) are the summed Bamberger-rearranged products of HADNTs (4 and 5).
Figure 4 shows that the two HADNTs could be observed only in the presence of the original substrate TNT and not necessarily in the presence of the monoamines, thereby implying the absence of any reversible connection between HADNTs (metabolites 4 and 5) and their reduced monoamines ADNTs (metabolites 6 and 7). The formation of the two monoamines peaked at between 10 and 15 days, but almost complete transformation occurred after 30 days. Interestingly, the highest ligninase activity was observed between 10 and 15 days. After 10 days of incubation, a period marked by the disappearance of TNT and its two prime products 4 and 5, roughly 30% of the transformed TNT could be accounted for by the formation of the two ADNTs 6 and 7. Despite the disappearance of TNT and its prime metabolites 4 to 7, less than 10% of the transformed amount of TNT was measured as 14CO2. As Fig. 4 shows, mineralization commenced after 6 days, which is the period marked by the appearance of ligninase activity (Fig. 3). In contrast, TNT disappearance and the subsequent formation of its prime metabolites HADNTs and ADNTs were obviously nonligninolytic processes.
Another time course study was thus conducted to monitor the formation and disappearance of the monoamine metabolites (metabolites 6 and 7) against those of other TNT intermediates, such as Bamberger-rearranged intermediates (2-A-5-OH-4,6-DNT [metabolite 17] and 4-A-5-OH-2,6-DNT [metabolite 18]) and the acyl derivatives (Fig. 5). By examining the time profiles of TNT biotransformation in Fig. 5, it can be seen that the metabolites can be classified into primary and secondary products. For example, the data in Fig. 5 clearly shows two parabolic curves: one to the left between 2 and 10 days and the second to the right between 3 and 30 days, representing the evolution of their secondary acyl (actyl and formyl) products (metabolites 8 to 11). Interestingly, the formation of the prime ADNT metabolites (6 and 7) started immediately after the disappearance of TNT under nonligninolytic conditions, and their presence was maintained into the ligninolytic state of the fungus, whereas the formation of the acyl secondary products (Fig. 5) correlated with the LiP and MnP enzymatic activity profiles of the fungus (Fig. 3), in which maximum amounts of these products were obtained after both enzymes achieved their maximum activity levels, i.e., after 10 to 15 days of incubation. Valli et al. (47) reported that the initial amine metabolites formed from the treatment of 2,4-dinitrotoluene with the same fungus undergo oxidation by MnP to produce quinones. These quinones are then reduced, methylated, and denitrated by either LiP and MnP. However, neither nitrire nor nitrate was found in the present study.
FIG. 5.
Time course study of the formation and disappearance of TNT prime metabolites (HADNT and ADNT) together with their secondary products the phenolamines (A-OH-DNTs) and the acylated derivatives during TNT degradation with the fungus P. chrysosporium. The cumulative amounts of the Bamberger-rearranged phenolamines (A-OH-DNTs) were plotted instead of the discrete isomeric ones. In the case of the acyl derivatives (8 to 11) the peak area counts instead of the actual amounts were used to draw the curves.
Figure 5 shows that the amounts of the formylated metabolites (Fm-N-DNT; metabolites 8 and 9) were always larger than those of the acetylated ones (Ac-N-DNT; metabolites 10 and 11) by at least a factor of 2. Also, the para isomer in each case was formed in a yield which is about 25% higher than that of its ortho counterpart, which was possibly caused by steric inhibitory effects from the ortho CH3 group. All acylated derivatives 8 to 11 could be detected only while their suspected precursors, the two monoamines, were still present in the system. After 30 days of incubation, both the monoamines (6 and 7) and their acylated derivatives (8 to 11) disappeared, but without causing a dramatic increase in mineralization (i.e., liberated 14CO2 did not exceed 10%). This observation might support the reversible connection between the acylated metabolites and their corresponding precursors, the monoamines. For instance, this hypothesis was recently supported by Bruns-Nagel et al. (6), who described the reversible formation of acetylated TNT intermediates (i.e., they do not form dead-end products in the degradation process). It has also been reported that formylation and acetylation processes could serve as detoxification mechanisms in soil, as is the case with aniline (46). In the present study, the presence of the amines and their acyl derivatives together may support the view of their coexistence in a reversible relationship.
The acylated derivatives 12 to 16 were not included in the time course study. However, both acetyl derivatives of HADNT, 12 and 13, could be detected only as long as HADNT was present in the system, also implying the presence of a reversible reaction between them. The acylated metabolites 4-N-FmANT (metabolite 14), 4-N-AcANT (metabolite 15), and 4-N-AcHANT (metabolite 16) were all detected in trace amounts and could not be quantified for inclusion in the time course study.
Since no significant increase in CO2 was observed and all of these acylated intermediates disappeared, then one may ask what became of them. Although we cannot provide an answer to this question at this time, we can presume that some of these acylated derivatives, particularly 12 and 13, are reduced to give ADNT in a reaction similar to the one that occurred for the reduction of HADNT to produce ADNT. For instance, by the end of the incubation period, which lasted 30 days, 4-ADNT was the only prime metabolite that could be detected (although in trace amounts). Nonetheless, none of the detected acylated TNT intermediates accumulated in the system.
It was also presumed that 4-N-AcANT (metabolite 15) was a derivative of 2,4-DANT and not the reverse. The 2,4-DANT itself was detected only occasionally and in trace amounts. Furthermore, 4-N-AcANT was encountered in soil samples that had been contaminated with 2,4-DANT and also in experiments designed to biodegrade the diamine (36). On the other hand, Gilcrease and Murphy (17) have reported that under nitrate-reducing conditions, TNT can be transformed by Pseudomonas fluorescens to 2,4-DANT, which subsequently is transformed to 4-N-AcANT as a dead-end product with no role in mineralization. In contrast, with the same bacterium, ethanol as the C source, and 2,4-DANT as the sole N source, Naumova et al. (31) reported the formation of phloroglucinol (1,3,5-trihydroxybenzene) and pyrogallol (1,2,3-trihydroxybenzene), both of which require nitrogen elimination.
The microbial acylation (acetylation and formylation) of aromatic amines has been previously described, although the mechanism for this remains unclear in most cases (1, 6, 7, 17). Also, reviews of the reactivities of TNT metabolites, particularly that of HADNT, and the formation of the corresponding acyl derivatives have recently been published (10, 29). In the case of the fungus P. chrysosporium, the formation of 4-N-FmDNT was suggested to act as an intermediate in the formation of 2,4-DANT (29).
The phenolamines 17 and 18, both of which are formed under nonligninolytic conditions, were observed only in the presence of HADNTs, suggesting their coexistence in a reversible manner. Furthermore, no products directly related to these acid-catalyzed Bamberger products could be identified. On the other hand the formation of 4-HADNT (metabolite 5) together with its Bamberger-rearranged phenolamine product (metabolite 17) has been reported to occur under anaerobic (C. acetobutylicum) and near-neutral conditions (21). Schenzle et al. (35) have shown that 3-nitrophenol transforms to 3-hydroxylaminophenol under anaerobic conditions, which in turn transforms to 3-aminohydroquinone in a reaction similar to that observed for the acid-catalyzed Bamberger rearrangement. In the present study, neither 2,4-dihydroxylamino-6-nitrotoluene nor its Bamberger-rearranged products were observed; this is possibly due to the aerobic and acidic conditions (pH 4.5) used. However, Fiorella and Spain (15) have observed 2,4-dihydroxylamino-6-nitrotoluene after treatment of TNT with Pseudomonas pseudoalcaligenes JS52, whereas Lewis et al. (27) reported the formation of 2,4-dihydroxylamino-6-nitrotoluene as an intermediate of TNT biotransformation by the obligate anaerobe Clostridium bifermentans. In the present study, the presence of metabolite 16 (4-N-AcAHNT) among the detected TNT metabolites could support the involvement of 2,4-dihydroxylaminonitrotoluene as an intermediate in the biotransformation process.
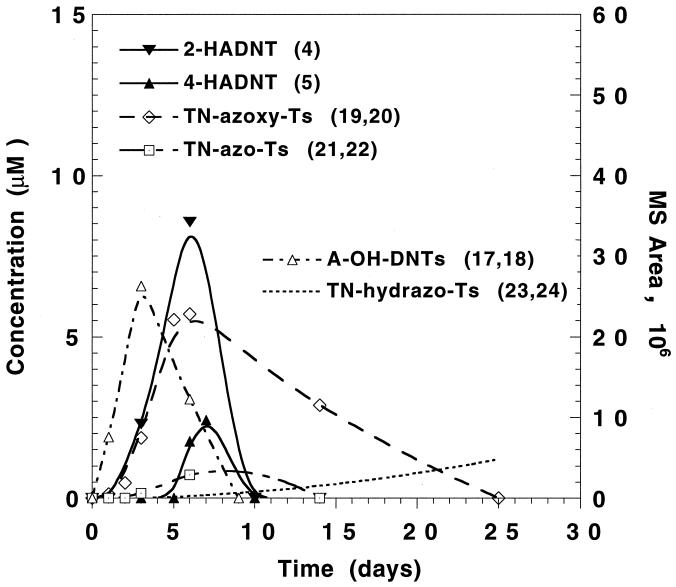
The last time course study was constructed to examine the formation and disappearance profiles of the azoxy intermediates and their related reduced products (LC-MS peaks 19 to 24) against those of the HADNT prime metabolites (peaks 4 and 5), as shown in Fig. 6. The azoxy products appeared with the formation of HADNT and disappeared with the disappearance of HADNT, although at a much lower rate. For instance, the disappearance of HADNT (10 days) was also marked by the disappearance of the azoxy intermediates. However, the disappearance of the two azoxy derivatives was accompanied by the appearance of its reduced products, the azo dimers (TN-2,2′-AzoT and TN-4,4′-AzoT), followed by the formation of the hydrazo derivatives (TN-2,2′-HydrazoT and TN-4,4′-HydrazoT). Figure 6 shows that the two azoxy derivatives 19 and 20 were clearly nonligninolytic, since maximum yields were obtained after only 5 days of incubation, long before the onset of the ligninolytic phase of the fungus (Fig. 3).
FIG. 6.
Typical time course profile representing the formation and disappearance of the azoxy products (metabolites 19 and 20) to produce the corresponding azo derivatives (21 and 22), which finally are reduced further to the hydrazines 23 and 24. For the TN-AzoT and the TN-HydrazoT compounds, the peak area counts were used to draw the curves.
Figure 6 also shows that another fraction of HADNT transformed reversibly to give the phenolamines, since the latter could be observed only as long as HADNT was present in the system. Other transformations for HADNT are shown in Fig. 5, where the prime products were found to transform to ADNT and several other acylated products. After 25 days of incubation, the only metabolites that were detected were traces of 4-ADNT and the hydrazo derivatives, suggesting the partial decomposition of these hydrazo compounds back into amines, possibly through abiotic means.
As far as azoxy derivatives are concerned, it would be difficult at present to determine whether these azoxy compounds are formed via enzymatic or chemical routes, since both routes have been reported for the dimerized coupling of HADNT and NsT to provide such adducts during TNT biotransformation (10, 29). Also, azoxy compounds have been reported to biotransform to the corresponding azo compounds (—N⩵N—) during their mineralization with the same fungus (29, 38). Transformation of azoxy dimers to the corresponding azo derivatives followed by mineralization has been reported earlier (32, 38).
Neither NO2− nor NH4+ ions were detected, indicating that inorganic nitrogen species expected from the small amount of mineralization (10%) observed might have ended up in the biomass. However, cases of denitration of polynitroorganics, such as that of 2,4,-dinitrotoluene with P. chrysosporium (41, 47), 2,4-dinitrotoluene with a Pseudomonas sp. (39), and TNT with a Bacillus sp. (23), have been reported. When the mycelia from these cultures were extracted with acetonitrile (12 h), only negligible amounts of 4-ADNT and the hydrazine dimer TN-4,4′-HydrazoT could be detected. In the present study, no other products from the mycelia were included in the time course study. The results of the time course study conducted in the liquid phase are summarized in the constructed pathway shown in Fig. 7.
FIG. 7.
Constructed pathway for TNT biotransformation during treatment with the fungus P. chrysosporium with citric acid and molasses in agitated cultures at pH 4.5. The dashed arrow indicates products that were expected but have not been detected.
Conclusion.
The present LC-MS study demonstrates the effectiveness of the fungus P. chrysosporium in transforming TNT into several primary and secondary products, although two significant changes were introduced in the normal protocol used for incubation of the fungus: (i) the use of agitated cultures instead of stationary ones and (ii) addition of TNT with the fungi as opposed to addition after 6 days. The coexistence of an unusually high number of intermediates indicates the complexities associated with TNT biotransformation, exemplified by a unique reactivity and fast transformation. The formation of several TNT products by one microorganism (P. chrysosporium) can be taken as proof of the involvement of several enzymes in the biotransformation process. We found that the disappearance of TNT and the formation of its prime metabolites (HADNT and ADNT) together with their Bamberger and azoxy products occurred prior to the onset of the ligninase activity by the fungus, while those of the secondary acyl, azo, and hydrazo products became noticeable during the ligninase state of the fungus, which started after 6 to 8 days of incubation. A positive practical conclusion from this work might arise from the formation of the acylated TNT intermediates, which did not accumulate in the system and may hold a key to an optimized TNT detoxification process.
ACKNOWLEDGMENTS
We thank the Department of National Defence, Canada, and the National Research Council Canada for supporting the work of A. Halasz.
We also thank A. Corriveau and S. Deschamps for their analytical support and J. Hodgson for the fungal strain.
Footnotes
National Research Council Canada publication number 41843.
REFERENCES
- 1.Alvarez M A, Kitts C L, Botsford J L, Unkefer P. Pseudomonas aeruginosa strain MA01 aerobically metabolizes the aminodinitrotoluenes produced by 2,4,6-trinitrotoluene nitro-group reduction. Can J Microbiol. 1995;41:984–991. doi: 10.1139/m95-137. [DOI] [PubMed] [Google Scholar]
- 2.Ampleman G, Thiboutot S, Lavigne J, Marois A, Hawari J, Jones A J, Rho D. Synthesis of 14C-labeled RDX, TNT, NC and GAP for use in assessing the biodegradation potential of these energetic chemicals. J Label Compd Radiopharm. 1995;36:559–577. [Google Scholar]
- 3.Bamberger E. Arylhydroxylamine und Arylazide. Leibigs Ann Chim. 1921;424:232–321. [Google Scholar]
- 4.Blau K, Halket J M, editors. Handbook of derivatives for chromatography. 2nd ed. London, England: J. Wiley & Sons Ltd; 1993. Acetylation in aqueous solution; pp. 38–39. [Google Scholar]
- 5.Boopathy R, Kulpa C F. Nitroaromatic compounds serve as nitrogen source for Desulfovibrio sp. (B strain) Can J Microbiol. 1993;39:430–433. doi: 10.1139/m93-062. [DOI] [PubMed] [Google Scholar]
- 6.Bruns-Nagel D, Drzyzga O, Steinbach K, Schmidt T C, Von Low E, Gorontzy T, Blotevogel K H, Gemsa D. Anaerobic/aerobic composting of 2,4,6-trinitrotoluene-contaminated soil in a reactor system. Environ Sci Technol. 1998;32:1676–1679. [Google Scholar]
- 7.Bruns-Nagel D, Breitung J, Von Low E, Steinbach K, Gorontzy T, Kahl M, Blotevogel K-H, Gemsa D. Microbial transformation of 2,4,6-trinitrotoluene in aerobic soil columns. Appl Environ Microbiol. 1996;62:2651–2656. doi: 10.1128/aem.62.7.2651-2656.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bumpus J A, Tatarko M. Biodegradation of 2,4,6-trinitrotoluene by Phanerochaete chrysosporium: identification of initial degradation products and the discovery of a TNT metabolite that inhibits lignin peroxidases. Curr Microbiol. 1994;28:185–190. [Google Scholar]
- 9.Carpenter D F, McCormick N G, Cornell J H, Kaplan A M. Microbial transformation of 14C-labeled 2,4,6-trinitrotoluene in an activated-sludge system. Appl Environ Microbiol. 1978;35:949–954. doi: 10.1128/aem.35.5.949-954.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corbett M D, Corbett B R. Bioorganic chemistry of the arylhydroxylamine and nitrosoarene functional groups. In: Spain J C, editor. Biodegradation of nitroaromatic compounds. New York, N.Y: Plenum Press; 1995. pp. 151–182. [Google Scholar]
- 11.Crawford R L. Biodegradation of nitrated munition compounds and herbicides by obligately anaerobic bacteria. In: Spain J C, editor. Biodegradation of nitroaromatic compounds. New York, N.Y: Plenum Press; 1995. pp. 87–98. [Google Scholar]
- 12.Donnelly K C, Chen J C, Huebner H J, Brown K W, Autenrieth R L, Bonner J S. Utility of four strains of white rot fungi for the detoxification of 2,4,6-trinitrotoluene in liquid cultures. Environ Tox Chem. 1997;16:1105–1110. [Google Scholar]
- 13.Duque E, Haidour A, Godoy F, Ramos J L. Construction of a Pseudomonas hybrid strain that mineralizes 2,4,6-trinitrotoluene. J Bacteriol. 1993;175:2278–2283. doi: 10.1128/jb.175.8.2278-2283.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernando T, Bumpus J A, Aust S D. Bioremediation of TNT (2,4,6-trinitrotoluene) by Phanerochaete chrysosporium. Appl Environ Microbiol. 1990;56:1666–1671. doi: 10.1128/aem.56.6.1666-1671.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiorella P D, Spain J C. Transformation of 2,4,6-trinitrotoluene by Pseudomonas pseudoalcaligenes JS52. Appl Environ Microbiol. 1997;63:2007–2015. doi: 10.1128/aem.63.5.2007-2015.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funk S B, Roberts D H, Crawford D L, Crawford L R. Initial-phase optimization for bioremediation of munition compound-contaminated soils. Appl Environ Microbiol. 1993;59:2171–2177. doi: 10.1128/aem.59.7.2171-2177.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilcrease P C, Murphy V G. Bioconversion of 2,4-diamino-6-nitrotoluene to a novel metabolite under anoxic and aerobic conditions. Appl Environ Microbiol. 1995;61:4209–4214. doi: 10.1128/aem.61.12.4209-4214.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greer C W, Hawari J, Samson R. Influence of environmental factors on 2,4-dichlorophenoxyacetic acid degradation by Pseudomonas cepacia isolated from peat. Arch Microbiol. 1990;154:317–322. doi: 10.1007/BF00276525. [DOI] [PubMed] [Google Scholar]
- 19.Harter D R. The use and importance of nitroaromatic chemicals in the chemical industry. In: Ricket D E, editor. Toxicity of nitroaromatic chemicals. Chemical Industry Institute of Toxicology Series. New York, N.Y.: Hemisphere Publishing Corp.; 1985. pp. 1–14. [Google Scholar]
- 20.Hawari J, Halasz A, Paquet L, Zhou E, Spencer B, Ampleman G, Thiboutot S. Characterization of metabolites in the biotransformation of 2,4,6-trinitrotoluene with anaerobic sludge: role of triaminotoluene. Appl Environ Microbiol. 1998;64:2200–2206. doi: 10.1128/aem.64.6.2200-2206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes J B, Wang C, Yesland K, Richardson A, Bhadra R, Bennett G, Rudolph F. Bamberger rearrangement during TNT Metabolism by Clostridium acetobutylicum. Environ Sci Technol. 1998;32:494–500. [Google Scholar]
- 22.Hughes J B, Wang C Y, Bhadra R, Richardson A, Bennett G, Rudolph F. Reduction of 2,4,6-trinitrotoluene by Clostridium acetobutylicum through hydroxylamino-nitrotoluene intermediates. Environ Toxicol Chem. 1998;17:343–348. [Google Scholar]
- 23.Kalafut T, Wales M E, Rastogi V K, Naumova R P, Zaripova S K, Wild J. Biotransformation patterns of 2,4,6-trinitrotoluene by aerobic bacteria. Curr Microbiol. 1998;36:45–54. doi: 10.1007/s002849900278. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan D L, Kaplan M A. Thermophilic biotransformation of 2,4,6-trinitrotoluene under simulated composting conditions. Appl Environ Microbiol. 1982;44:757–760. doi: 10.1128/aem.44.3.757-760.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirk Othmer F. Encyclopedia of chemical technology. 3rd ed. Vol. 21. New York, N.Y: Wiley Interscience Publishing; 1983. p. 878. [Google Scholar]
- 26.Kohnstam, G., W. A. Petch, and L. H. Williams. 1984. Kinetic substituent and isotope effects in the acid-catalysed rearrangement of N-phenylhydroxylamines. Are nitrenium ions involved? J. Chem. Soc. Perkin Trans. II, 423–427.
- 27.Lewis T A, Goszczynski S, Crawford R L, Korus R A, Admassu W. Products of anaerobic 2,4,6-triaminotoluene transformation by Clostridium bifermantans. Appl Environ Microbiol. 1996;62:4999–4674. doi: 10.1128/aem.62.12.4669-4674.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manning J F, Jr, Boopathy R, Kulpa C F. A laboratory study in support of the pilot demonstration of a biological soil slurry reactor. Report SFIM-AEC-TS-CR-94038. Argonne, Ill: Bioremediation Group, Environmental Research Division, Argonne National Laboratory; 1995. [Google Scholar]
- 29.Michels J, Gottschalk G. Pathway of 2,4,6-trinitrotoluene (TNT) degradation by Phanerochaete chrysosporium. In: Spain J C, editor. Biodegradation of nitroaromatic compounds. New York, N.Y: Plenum Press; 1995. pp. 135–139. [Google Scholar]
- 30.Michels J, Gottschalk G. Inhibition of the lignin peroxidase of Phanerochaete chrysosporium by hydroxylaminodinitrotoluene, an early intermediate in the degradation of 2,4,6-trinitrotoluene. Appl Environ Microbiol. 1994;60:187–194. doi: 10.1128/aem.60.1.187-194.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naumova R P, Amerkhanova N N, Shaikhutdinov A. Study of the first stage of the conversion of trinitrotoluene under the action of Pseudomonas denitrificans. Priklad Biokhim Mikrobiol. 1979;15:45–50. [Google Scholar]
- 32.Pasti-Grigsby M B, Paszczynski A, Goszczynski S, Crawford D L, Crawford R L. Influence of aromatic substitution patterns on azo dyes by Streptomyces spp. and Phanerochaete chrysosporium. Appl Environ Microbiol. 1992;58:3605–3613. doi: 10.1128/aem.58.11.3605-3613.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paszczynski A, Huynh V B, Crawford R. Comparison of ligninase-1 and peroxidase-M2 from the white-rot fungus Phanerochaete chrysosporium. Arch Biochem Biophys. 1986;244:750–765. doi: 10.1016/0003-9861(86)90644-2. [DOI] [PubMed] [Google Scholar]
- 34.Rieger P-G, Knackmuss H-J. Basic knowledge and perspectives on biodegradation of 2,4,6-trinitrotoluene and related nitroaromatic compounds in contaminated soil. In: Spain J C, editor. Biodegradation of nitroaromatic compounds. New York, N.Y: Plenum Press; 1995. pp. 1–18. [Google Scholar]
- 35.Schenzle A, Lenke H, Fischer P, Williams P A, Knackmuss H-J. Catabolism of 3-nitrophenol by Ralstonia eutropha JMP 134. Appl Environ Microbiol. 1997;63:1421–1427. doi: 10.1128/aem.63.4.1421-1427.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheremata, T., et al. Unpublished data.
- 37.Shine H J. Aromatic Rearrangement. Amsterdam, The Netherlands: Elseiver; 1967. pp. 124–271. [Google Scholar]
- 38.Spadaro J T, Gold M H, Renganathan V. Degradation of azo dyes by the lignin-degrading fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1992;58:2397–2401. doi: 10.1128/aem.58.8.2397-2401.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spanggord R J, Spain J C, Nishino S F, Mortelmans K E. Biodegradation of 2,4-dinitrotoluene by a Pseudomonas sp. Appl Environ Microbiol. 1991;57:3200–3205. doi: 10.1128/aem.57.11.3200-3205.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spiker J K, Crawford D L, Crawford R L. Influence of 2,4,6-trinitrotoluene (TNT) concentration on the degradation of TNT in explosive-contaminated soils by the white-rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1992;58:3199–3202. doi: 10.1128/aem.58.9.3199-3202.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stahl J D, Aust S D. Biodegradation of 2,4,6-trinitrotoluene by the white rot fungus Phanerochaete chrysosporium. In: Spain J C, editor. Biodegradation of nitroaromatic compounds. New York, N.Y: Plenum Press; 1995. pp. 117–133. [Google Scholar]
- 42.Stone, T., K. Hamamoto, Y. Seiji, S. Shinkai, and O. Manaba. 1981. Kinetics and mechanisms of the Bamberger rearrangement, part 4. Rearrangement of sterically hindered phenylhydroxylamines to 4-aminophenols in aqueous sulfuric acid solution, J. Chem. Soc. Perkin Trans. II, 1596–1598.
- 43.Sublette K L, Ganapathy E V, Schwartz S. Degradation of munition wastes by Phanerochaete chrysosporium. Appl Biochem Biotechnol. 1992;34/35:709–723. [Google Scholar]
- 44.Sunahara G I, Dodard S, Sarrazin M, Paquet L, Ampleman G, Thiboutot S, Hawari J, Renoux A Y. Development of a soil extraction procedure for ecotoxicity characterization of energetic compounds. Ecotoxicol Environ Safety. 1998;39:185–194. doi: 10.1006/eesa.1997.1624. [DOI] [PubMed] [Google Scholar]
- 45.Tien M, Kirk T K. Lignin-degrading enzyme from Phanerochaete chrysosporium: purification, characterization, and catalytic properties of a unique H2O2-requiring oxygenase. Proc Natl Acad Sci USA. 1984;81:2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tweedy B G, Loeppky C, Ross J A. Metobromuron: acetylation of the aniline moiety as a detoxification mechanism. Science. 1970;168:482–483. doi: 10.1126/science.168.3930.482. [DOI] [PubMed] [Google Scholar]
- 47.Valli K, Brock B J, Joshi D K, Gold M H. Degradation of 2,4-dinitrotoluene by the lignin-degrading fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1992;58:221–228. doi: 10.1128/aem.58.1.221-228.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vorbeck C V, Lenke H, Fischer P, Spain J C, Knackmuss H-J. Initial reductive reactions in aerobic microbial metabolisms of 2,4,6-trinitrotoluene. Appl Environ Microbiol. 1998;64:246–252. doi: 10.1128/aem.64.1.246-252.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker J E, Kaplan D L. Biological degradation of explosives and chemical agents. Biodegradation. 1992;3:369–385. [Google Scholar]
- 50.Williams D L H. Aromatic rearrangement. In: Bamford C H, Tipper C F H, editors. Comprehensive chemical kinetics. Vol. 13. Amsterdam, The Netherlands: Elseiver; 1972. pp. 433–466. [Google Scholar]
- 51.Won W D, Di Salvo L H, Ng J. Toxicity and mutagenecity of 2,4,6-trinitrotoluene and its microbial metabolites. Appl Environ Microbiol. 1976;31:575–580. doi: 10.1128/aem.31.4.576-580.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]