Abstract
The deregulation of energetic and cellular metabolism is a signature of cancer cells. Thus, drugs targeting cancer cell metabolism may have promising therapeutic potential. Previous reports demonstrate that the widely used normoglycemic agent, metformin, can decrease the risk of cancer in type 2 diabetics and inhibit cell growth in various cancers, including pancreatic, colon, prostate, ovarian, and breast cancer. While metformin is a known adenosine monophosphate-activated protein kinase (AMPK) agonist and an inhibitor of the electron transport chain complex I, its mechanism of action in cancer cells as well as its effect on cancer metabolism is not clearly established. In this review, we will give an update on the role of metformin as an antitumoral agent and detail relevant evidence on the potential use and mechanisms of action of metformin in cancer. Analyzing antitumoral, signaling, and metabolic impacts of metformin on cancer cells may provide promising new therapeutic strategies in oncology.
Keywords: metformin, diabetes, cancer metabolism, AMPK, PI3K, therapeutics, drug repurposing
1. Introduction
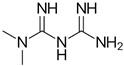
The history of the biguanide, metformin (molecular formula C4-H11-N5, Table 1), is linked to Galega officinalis and is also known as French lilac or Italian fitch. The Galega officinalis represents a traditional herbal medicine that was found to lower blood glucose in 1918 [1]. Guanidine derivatives were used to treat diabetes mellitus (DM) in the 1920s and 1930s but with the availability of insulin were discontinued due to their toxicity [2]. During World War II and throughout the search for antimalarial agents, metformin was re-discovered and determined to lower blood glucose levels [3,4]. The French physician-scientist Jean Sterne was the first to report the use of metformin to treat DM in 1957 and named the compound Glucophage, which means glucose eater [5]. Since its introduction, metformin has become the most prescribed glucose-lowering drug worldwide [2].
Table 1.
Metformin’s biometric information.
| Characteristics | Metformin |
|---|---|
| Structural name | 3-(diaminomethylidene)-1,1-dimethylguanidine |
| Structure |
|
| Formula | C4-H11-N5 |
| Molecular weight | 129.16 g/mol |
| Density | 1.3 g/cm3 |
| Melting point | 223–226 °C |
| Boiling point | 224.1 °C at 760 mmHg |
| Color | White |
| CAS number | 657-24-9 |
| PubChem Substance ID | 4091 |
In 1998, the UK Prospective Diabetes Study (UKPDS), a prospective randomized trial of 5100 type 2 DM patients who received glucose-lowering treatment for more than a decade showed reduced cancer risk [6]. Subsequent large database analyses have reported lower incidence of certain types of cancer among diabetic populations taking metformin despite data indicating that these diabetic populations were overall more prone to developing cancer. This has led to a deeper investigation into the role of metformin in cancer [7,8]. Here, we review five years of updated literature on metformin’s antineoplastic activity, its mechanisms of action, as well as current limitations and future directions for the repurposing of metformin in the treatment of cancer.
2. Metformin in Cancer
To date, there are over 50 recent or active clinical trials investigating the use of metformin in human malignancies (Table 2). Total daily dose of oral metformin in these clinical trials ranges from 500 to 3000 mg. This range reflects the previously established dosing strategy used to treat patients with type 2 DM, with gastrointestinal (GI) toxicity limiting use beyond 2500 mg per day [9]. In future clinical trials, we suggest aiming to achieve the maximum tolerated dose of 2500 mg per day given the majority of preclinical studies required high concentrations of metformin to achieve anti-cancer activity [10]. Furthermore, we recommend planned dose escalation to allow for GI habituation as well as allowance of dose interruptions and reductions for drug toxicity to reflect real-world practices.
Table 2.
Recent clinical trials investigating oral metformin use in cancers [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. N/A: not applicable.
| Tumor Location |
Trial Reference |
ID/ Phase |
Tumor/Patient Characteristics | Number of Participants | Treatment | Result | Other Comments |
|---|---|---|---|---|---|---|---|
| Various Solid Tumors |
[38] | Phase Ib | Variety of advanced solid tumors refractory to standard therapies | 9 | Everolimus + metformin (n = 9; metformin 500 mg twice daily) | Combination therapy was poorly tolerated | Open-label, prospective, single-center, dose-escalation study, The Netherlands |
| [39] | -- | Variety of advanced solid tumors (metastatic or unresectable) | 24 | Sirolimus + metformin (n = 11; maintenance on 1000 mg once daily) vs. sirolimus (n = 13) |
Combination therapy did not improve mTOR inhibition | Open-label, randomized | |
| [40] | NCT01442870 Phase I | Variety of solid tumors (nondiabetic, histologically confirmed solid tumors receiving adjuvant or systemic chemotherapy) | 100 | Concurrent chemotherapy + metformin (n = 49; 500 mg twice daily) vs. delayed chemotherapy + metformin (n = 51; 500 mg twice daily) |
Metformin is safe to use in combination with a wide range of chemotherapy regimens | Delayed-start, randomized | |
| [41] | NCT02496741 Phase Ib | IDH1-mutated solid tumors including chondrosarcoma (refractory grade II-III), glioma (WHO grade II-IV), and intrahepatic cholangiocarcinoma | 17 | Chloroquine + metformin (n = 17; maximum of 1500 mg twice daily) | Combination treatment with chloroquine and metformin did not induce clinical response | Prospective, open-label, dose-escalation, The Netherlands | |
| Glioma | N/A |
NCT04945148 Phase II |
Glioblastoma, IDH-wildtype | 640 | Metformin (1500–3000 mg daily) plus radiation and temozolomide | No results available | Open-label, prospective, single-center, France |
| N/A |
NCT02149459 Phase I |
Brain neoplasms | 18 | Metformin (dose not specified), radiation, and low carbohydrate diet | No results available | Open-label, prospective, single-center, Israel | |
| N/A |
NCT02780024 Phase II |
Glioblastoma | 50 | Metformin (dose not specified) and neoadjuvant temozolomide followed by combined radiation and temozolomide | No results available | Open-label, prospective, single-center, Canada | |
| N/A |
NCT03243851 Phase II |
Recurrent or refractory glioblastoma | 81 | Metformin (ramp up to 2000 mg daily) and low dose temozolomide | No results available | Open-label, prospective, single-center, South Korea | |
| N/A |
NCT03151772 Phase I |
Glioblastoma | 3 | Metformin (850 mg daily) and disulfiram for 3 days preoperatively | No results available, study was terminated for low enrollment | Open-label, prospective, single-center, Sweden | |
| N/A |
NCT04691960 Phase II |
Glioblastoma | 36 | Metformin (ramp up to 850 mg three times daily) and ketogenic diet | No results available | Open-label, prospective, single-center, US | |
| N/A |
NCT05183204 Phase II |
Glioblastoma | 33 | Metformin (ramp up to 850 mg three times daily as tolerated), ketogenic diet and Paxalisib| | No results available | Open-label, prospective, single-center, US | |
| N/A |
NCT01430351 Phase I |
Glioblastoma and gliosarcoma | 144 | Metformin (dose not specified), mefloquine, memantine, hydrochloride, hydrochloride, and temozolomide | No results available | Open-label, prospective, single-center, US | |
| Bladder Tumors |
[26] | NCT03379909 Phase II | Non-muscle-invasive bladder cancer (intermediate-risk) | 49 (target) | Metformin (maximum of 3000 mg daily) | Ongoing | Multicenter, open-label |
| Breast Tumors |
[16] | NCT00490139 Phase III | HER2-positive primary breast cancer | 8381 | Substudy analysis of diabetic study participants on/off metformin therapy (dose not specified; all patients previously taking for DM) in patients receiving relevant anti-HER2 therapies, described elsewhere | Diabetic patients with HER2-positive breast cancer demonstrated better outcomes when treated with metformin compared to diabetic breast cancer patients not on metformin, whereas outcomes of patients with HR-negative status were not affected by diabetes treatment status | Randomized, adjuvant trial |
| [11] | NCT01654185 Phase II | Hormone receptor positive locally advanced or metastatic breast cancer | 60 | Aromatase inhibitor (exemestane or letrozole) + metformin (n = 30; maintenance on 500 mg daily) vs. aromatase inhibitor (exemestane or letrozole) + placebo (n = 30) |
No improved efficacy was observed in the addition of metformin to aromatase inhibitor treatment | Randomized, China | |
| [42] | NCT01266486 Phase I | Treatment-naïve primary breast cancer | 40 | Metformin (n = 40; maintenance on 1500 mg daily) | Metformin treatment precipitated two distinct metabolic responses in tumors | Window study design, UK | |
| [14] | NCT01310231 Phase II | Metastatic breast cancer (nondiabetic) | 40 | Chemotherapy + metformin (n = 22; maintenance on 850 mg daily) vs. chemotherapy + placebo (n = 18) |
Combined chemotherapy with metformin had no demonstrated effect on PFS, OS, or RR | Randomized, double-blind, Canada | |
| [12] | NCT01885013 Phase II | Metastatic breast cancer (HER2-negative, nondiabetic) | 122 | Chemotherapy (doxorubicin + cyclophosphamide) + metformin (n = 57; maintenance on 2000 mg daily) vs. chemotherapy (doxorubicin + cyclophosphamide) (n = 65) |
The addition of metformin did not provide a meaningful clinical benefit to PFS or OS but was found to decrease the incidence of severe neutropenia | Open-label, multicenter, randomized | |
| [13] | NCT01650506 Phase I | Metastatic triple negative breast cancer who had received at least one prior therapy | 8 | Erlotinib + metformin (n = 8; maximum dose was 850 mg thrice daily) | Combination therapy was well-tolerated but did not result in objective tumor response | USA | |
| [15] | IRCT20100706004329N7 | Breast fibroadenoma (nondiabetic) | 175 | Metformin (n = 83; maximum dose was 1000 mg daily) vs. placebo (n = 92) |
The effect of metformin is most obvious in smaller masses and appears to have a favorable effect compared to placebo in terms of reducing chances of significant enlargement of tumors | Iran | |
| [17] | NCT01627067 Phase II | Metastatic, hormone receptor-positive, HER2-negative breast cancer (obese or overweight, postmenopausal) | 22 | Everolimus + exemestane + metformin (n = 22; 1000 mg twice daily) | This treatment combination had moderate clinical benefit | USA | |
| Colorectal Tumors | [24] | -- | Stage II-III colon cancer | 120 out of total 3759 enrolled in TOSCA | Goal of original TOSCA study was to compare 3- vs. 6-month treatment with fluoropyrimidine-oxaliplatin adjuvant chemotherapy (post-resection)
|
Neither metformin use, nor DM, nor metformin dosage were associated with OR/RFS | Subanalysis |
| [23] | NCT01312467 Phase IIa | Nondiabetic, obese patients with recent history of colorectal adenoma | 32 | Metformin (n = 32; maintenance on 1000 mg twice daily) | Metformin intervention did not reduce rectal mucosa pS6 (marker of polyp suppression) or Ki-67 (marker of proliferation) levels | USA | |
| [25] | Phase II | Refractory colon cancer | 41 | Irinotecan + metformin (n = 41; maintenance on 2500 mg daily) | Irinotecan/metformin was able to provide disease control, with diarrhea as a significant side effect | Single-center | |
| Lung Tumors | [18] | NCT01864681 Phase II | Non-small cell lung cancer (locally advanced, stage IIIb-IV, EGFR mutated, treatment-naïve, nondiabetic) | 224 | Gefitinib + metformin (n = 100; maintenance on 1000 mg twice daily) vs. gefitinib + placebo (n = 100) |
Combination treatment resulted in non-significantly worse outcomes and was accompanied by more side effects (diarrhea) | Multicenter, double-blind, China |
| [22] | NCT01578551 Phase II | Chemo-naïve or metastatic nonsquamous NSCLC (stage IIIB or IV; nondiabetic) | 25 | Carboplatin + paclitaxel + bevacizumab + metformin (n = 19; 1000 mg twice daily) vs. carboplatin + paclitaxel + bevacizumab (n = 6) |
The metformin combination treatment group experienced increased PF | Single center, open-label, USA | |
| [19] | NCT03071705 Phase II | Lung adenocarcinoma (EGFR-mutated, stage IIIb-IV) | 139 | EGFR-TKI (erlotinib, afatinib, or gefitinib) + metformin (n = 69; 500 mg twice daily) vs. EGFR-TKI (erlotinib, afatinib, or gefitinib) (n = 70) |
The addition of metformin to EGFR-TKI standard therapy significantly improved PFS and OS in advanced lung adenocarcinoma patients | Randomized, open-label, prospective, Mexico | |
| [20] | NCT02186847 Phase II | NSCLC (unresectable, stage III; nondiabetic) | 167 | Chemoradiation + metformin (n = 86; maintained on 2000 mg daily) vs. chemoradiation (n = 81) |
There was no survival benefit associated with metformin addition to traditional chemoradiation therapy | Randomized, open-label, multicenter, international | |
| [21] | NCT02115464 Phase II | Locally advanced NSCLC (nondiabetic) | 54 | Chemoradiation (platinum-based) + metformin (n = 26; maintained on 2000 mg daily) vs. chemoradiation (platinum-based) (n = 28) |
Trial was stopped early due to low accrual; the addition of metformin to chemoradiotherapy was associated with a worse treatment outcome and increased toxicity | Randomized, open-label, multicenter, Canada | |
| Ovarian Tumors | [27] | ChiCTR-IOR-17011859 | Epithelial ovarian cancer (nondiabetic) | 47 | Debulking + paclitaxel/carboplatin + metformin (n = 20; 850 mg daily) Debulking + paclitaxel/carboplatin (n = 24) |
There was no evidence of metformin effect on PFS | China |
| [29] | NCT02312661 Phase I | Advanced epithelial ovarian cancer (FIGO III-IV) | 15 | Paclitaxel/carboplatin + metformin (n = 15; maximum dose of 1000 mg thrice daily) | The recommended phase II dose is 1000 mg thrice daily and there is a potential pharmacokinetic interaction between metformin and carboplatin, though the combination is well-tolerated | Dose escalation study, the Netherlands | |
| [28] | NCT01579812 Phase II | Advanced-stage (IIC/III/IV) epithelial ovarian cancer (nondiabetic) | 38 evaluable | Neoadjuvant metformin + debulking surgery + adjuvant chemotherapy plus metformin (n = 23; maintenance on 1000 mg twice daily) vs. neoadjuvant chemotherapy and metformin + interval debulking surgery + adjuvant chemotherapy plus metformin (n = 15) |
Addition of metformin is associated with better OS and a significant cancer stem cell population reduction | USA | |
| Prostate Tumors | [43] | EudraCT number 2014–005193-11 | Prostate cancer (newly diagnosed, localized, scheduled for radical prostatectomy) | 100 | Metformin (n = 50; maintenance on 1000 mg twice daily) vs. placebo (n = 50) |
Ongoing | Randomized, placebo-controlled, double-blind, window of opportunity, UK |
| [30] | NCT01677897 Phase II | Prostate cancer (metastatic, castration-resistant, with PSA progression while on abiraterone therapy) | 25 | Abiraterone + metformin (n = 25; 1000 mg twice daily) | Combination therapy resulted in no clinical benefit and did not affect progression; higher-than-expected gastrointestinal toxicity was also reported | Pilot study, Switzerland | |
| [31] | NCT01796028 Phase II | Prostate cancer (metastatic, castration-resistant, nondiabetic) | 99 | Docetaxel + metformin (n = 50; 850 mg twice daily) vs. docetaxel + placebo (n = 49) |
No improvement was observed in metformin group vs. placebo | French, prospective, multicenter, randomized, placebo-controlled | |
| [32] | NCT02614859 Phase II | Prostate cancer (nondiabetic, recurrent PC, overweight or obese with BMI > 25) | 29 | Bicalutamide + metformin (n = 20; 1000 mg twice daily) vs. bicalutamide (n = 9) | This study was ended early due to predicted inability to reach its primary endpoint (achievement of undetectable PSA at 32 weeks) | Randomized, open-label, USA | |
| Skin Tumors | [44] | NCT02325401 | HNSCC | 39 | Metformin (n = 39; maintenance on 2000 mg daily) | Metformin is capable of modulating the HNSCC microenvironment | Window of opportunity (post-biopsy, pre-resection) |
| [33] | NCT01840007 Phase I | Metastatic melanoma (patients who progressed after first-line treatment and were not eligible or did not respond to ipilimumab) | 17 | Metformin (n = 17; 1000 mg thrice daily) | Metformin shows no efficacy and poor safety in treating metastatic melanoma | Multicenter, pilot, prospective, open-label, France | |
| [45] | NCT02083692 | HNSCC (nondiabetics) | 50 | Metformin (n = 49; maintenance on 1000 mg twice daily) | Metformin treatment alters the immune tumor microenvironment, regardless of HPV status | Non-randomized | |
| [46] | NCT02325401 Phase I | Locally advanced HNSCC (nondiabetic, stage III-IV) | 20 | Cisplatin + radiotherapy + metformin (n = 20; maximum dose was 3000 mg daily) | Cisplatin did not appear to affect metformin pharmacokinetics | USA | |
| [47] | NCT02581137 Phase IIa | Oral premalignant lesions (nondiabetic) | 26 | Metformin (n = 26; maintenance on 2000 mg daily) | Metformin treatment was associated with good histological response and decreased mTOR activity | Open-label | |
| [48] | NCT02083692 | HNSCC | 50 | Metformin (n = 39 completed; maintenance on 1000 mg twice daily) | Metformin treatment alters the immune tumor microenvironment and results in increased apoptosis in HPV-, tobacco+ HNSCC patients compared to HPV+ HNSCC patients | USA | |
| Uterine Tumors | [34] | Phase III | Endometrioid endometrial cancer or atypical endometrial hyperplasia (pre-surgery) | 88 | Metformin (n = 45; maintenance on 850 mg twice daily) vs. placebo (n = 43) |
Pre-surgical treatment with metformin does not reduce tumor proliferation | Multicenter, randomized, double-blind, pre-surgical window study design, UK |
| [36] | NCTO1877564 | Endometrial cancer (nondiabetic, obese, pre-surgery) | 13 | Metformin (maintenance at 850 mg twice daily) | Pre-surgical treatment with metformin alters steroid receptor signaling of EC cells | Window design | |
| [37] | jRCT2031190065 | Endometrial cancer | 120 (target) | Medroxyprogesterone acetate vs. medroxyprogesterone acetate + metformin (750 mg daily) vs. medroxyprogesterone acetate + metformin (1500 mg daily) |
Ongoing | Prospective, randomized, open, blinded-endpoint, dose–response, multicenter, Japan | |
| [35] | NCT03618472 | Endometrial cancer (nondiabetic) | 49 | Metformin (n = 25; 850 mg daily) vs. placebo (n = 24) |
Pre-surgical metformin treatment significantly decreased proliferative tissue marker Ki-67 | Randomized, double-blind, placebo-controlled, Thailand | |
| Leukemia | N/A |
NCT01324180 Phase I |
Relapsed acute lymphoblastic leukemia | 14 | Metformin (twice daily in dose escalation schema) in combination with vincristine, dexamethasone, PEG-asparaginase, doxorubicin, and intrathecal cytarabine | Completed | Single group assignment, interventional, dose-escalating, open-label |
| N/A |
NCT01849276 Phase I |
Relapsed/refractory acute myeloid leukemia | 2 | Metformin (twice daily in dose escalation schema on days 1–15) + intravenous cytarabine | Terminated (due to slow accrual) | Single group assignment, interventional, open-label | |
| Lymphoma | N/A |
NCT03200015 Phase II |
Diffuse large B-cell lymphoma (DLBCL) | 15 | Metformin (ramp up to 850 mg thrice daily) + rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone | Unknown | Single group assignment, interventional, open-label |
| N/A |
NCT02531308 Phase II |
DLBCL | 5 | Metformin (ramp up to 850 mg twice daily) + rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone, pegfilgrastim | Terminated (slow accrual) | Single group assignment, interventional, open-label | |
| Myeloma | N/A |
NCT03829020 Phase I |
Recurrent plasma cell myeloma and refractory plasma cell myeloma | 36 | Metformin (dose escalation schema) + bortezomib, nelfinavir | Recruiting | Single group assignment, interventional |
| N/A |
NCT02948283 Phase I |
Recurrent plasma cell myeloma and refractory plasma cell myeloma | 3 | Metformin (twice daily in dose escalation schema) + ritonavir | Completed | Single group assignment, interventional |
2.1. Glioma
While there remains a lack of high-level evidence describing the specific role of metformin in patients with brain tumors, available literature has reported several advantages of repurposing metformin to be used in the management of glioma. Systemically administered drugs must be able to cross the blood–brain barrier (BBB) to effectively treat brain tumors. Using a rat model, orally administered metformin was found to penetrate the BBB at a high rate with biodistribution throughout the central nervous system [49]. Furthermore, metformin reduces vasogenic brain edema and the neurological symptoms that accompany brain tumors [50]. There has also been recent effort to characterize the subpopulations of glioma patients that would benefit most from metformin. A recent retrospective study of 1093 patients with high-grade glioma from a population-based clinical cancer registry in Germany reported a survival benefit from metformin in patients with World Health Organization (WHO) grade III glioma [51]. The benefit in WHO grade III glioma is attributed to the high frequency of isocitrate dehydrogenase (IDH) mutations, which can increase the vulnerability of tumor cells to therapeutic interventions targeting glutamine and mitochondrial metabolism [52].
2.2. Breast Cancer
Despite promising preclinical studies demonstrating the synergistic effects of metformin and breast cancer chemotherapeutics [53], several clinical trials investigating the addition of metformin to traditional treatment regimens did not result in improved efficacy. Negative results were seen with trials using metformin and aromatase inhibitors in hormone receptor (HR)-positive breast cancer [11], metformin/doxorubicin/cyclophosphamide in human epidermal growth factor receptor 2 (HER2)-negative breast cancer [12], and metformin and erlotinib in patients with metastatic triple negative breast cancer [13]. Another trial of nondiabetic patients receiving several different chemotherapeutic agents for metastatic breast cancer found that the addition of metformin had no effect on progression free survival (PFS) or overall survival (OS) [14]. However, there have been some positive results using metformin to treat breast tumors. Metformin monotherapy has been found to reduce the likelihood of significant tumor enlargement in women with breast fibroadenomas [15]. Interestingly, subanalysis of a trial featuring HER2-positive breast cancer patients revealed that metformin-treated DM participants had better prognoses compared to patients not treated with metformin, whereas the outcomes of patients with HR-negative cancers were not affected by DM status [16]. Furthermore, combined therapy with everolimus, exemestane, and metformin provided moderate clinical benefit in overweight and obese patients with metastatic, HR-positive, HER2-negative breast cancer [17].
2.3. Lung Cancer
The use of metformin in non-small cell lung cancer (NSCLC) is the focus of many conflicting clinical trial results. Based on preclinical studies indicating that metformin can sensitize lung cancer cells to tyrosine kinase inhibitors (TKIs), a combination of gefitinib, a TKI-targeting mutant epidermal growth factor receptor (EGFR), and metformin was tested in nondiabetic NSCLC patients. However, co-treatment resulted in non-significantly worse outcomes for NSCLC patients in terms of PFS and OS [18,54]. In contrast, a trial comparing EGFR-TKI combination treatment with metformin versus EGFR-TKI monotherapy in advanced NSCLC found that there was a significant survival benefit to the addition of metformin [19]. It is possible that the synergistic effect of metformin and EGFR-TKIs is only observable in patients with higher body mass index (BMI), thereby resulting in conflicting phase II trial results [55]. These mixed results extend beyond that of EGFR-TKI combination therapies. Two studies examining the impact of combining metformin with chemoradiation found that metformin resulted in either no survival benefit [20] or worse outcomes, potentially due to drug–drug interactions [21]. Others have reported PFS and/or survival benefits in diabetic NSCLC patients treated with metformin in combination with chemotherapy [22,56,57]. A recent meta-analysis concluded that more randomized clinical trials, particularly those incorporating time-dependent analyses in nondiabetic patients, are necessary to determine the association between metformin and OS in NSCLC [58].
2.4. Colorectal Cancer
Clinical use of metformin to suppress polyp formation and proliferation in the rectal mucosa of nondiabetic, obese patients with a history of colorectal adenoma has been unsuccessful to date [23]. Furthermore, a subanalysis from the large scale Three or Six Colon Adjuvant (TOSCA) trial found that neither metformin use nor DM status were associated with survival outcomes in colorectal patients receiving adjuvant chemotherapy post-resection [24]. Despite these negative findings, a recent study suggests the potential use of metformin alongside irinotecan for disease control in refractory colorectal patients [25].
2.5. Esophageal Cancers
Metformin dosing below the anti-cancer threshold may still activate the tumor immune microenvironment in animal models and patients with esophageal squamous cell carcinoma [59], which in turn may be beneficial for priming patients for subsequent immune checkpoint inhibitor treatment.
2.6. Kidney Cancer
Retrospective analysis of clinical trials involving metastatic renal cell carcinoma (mRCC) patients found that the addition of metformin to the TKI, sunitinib, in DM patients was associated with an improved OS compared to use of other diabetic agents [60]. Another retrospective study found that, regardless of diabetic status, the addition of metformin to sunitinib or an alternative TKI, pazopanib, in mRCC patients resulted in a PFS and OS benefit [61].
2.7. Liver Cancer
A large, retrospective study comparing diabetic patients receiving sulfonylureas versus metformin revealed a strong inverse correlation between metformin use and incidence of hepatocellular carcinoma (HCC) (56% risk reduction), indicating the potential use of metformin as a preventative agent for liver cancer. No association was observed for several other solid tumors after adjusting for BMI and level of glycemic control [62]. Metformin treatment may enhance the benefit of certain interventions, as was demonstrated in a retrospective analysis of patients undergoing Yttrium-90 radioembolization segmentectomy for non-resectable HCC [63]. However, metformin use does not appear to affect HCC recurrence in diabetic patients following initial resection [64].
2.8. Bladder Cancer
A retrospective analysis of diabetic patients with Bacillus Calmette–Guerin (BCG)-treated, non-muscle-invasive bladder cancer (NMIBC) found that metformin use was associated with increased disease-specific survival and OS [65]. Exploiting the fact that metformin accumulates in the urine prior to excretion, an ongoing trial is testing oral metformin treatment in patients with NMIBC [26]. The high upper limit on metformin dosing in this study (3000 mg daily) may allow for observation of tumor effects not seen in studies using lower doses.
2.9. Ovarian Cancer
The effect of metformin on epithelial ovarian cancer (EOC) patient outcomes is ambiguous. A clinical trial in China found that addition of metformin to the traditional therapy for EOC had no impact on PFS [27]. However, a US trial in nondiabetic EOC patients found that neoadjuvant metformin treatment resulted in better-than-expected OS as well as a significant reduction in cancer stem cells [28]. A recent dose escalation study demonstrated that the combination of metformin and paclitaxel/carboplatin is well-tolerated [29].
2.10. Pancreatic Cancer
A meta-analysis of 21 studies found that metformin treatment was associated with a survival benefit in patients with concurrent DM and pancreatic cancer (PC), specifically for patients at early and intermediate PC disease stages [66], suggesting its potential as an adjuvant chemotherapeutic.
2.11. Prostate Cancer
Clinical studies in metastatic, castration-resistant prostate cancer patients show that the addition of metformin is not able to rescue resistance to anti-androgen agent, abiraterone [30], nor is it able to improve survival or response outcomes when combined with a chemotherapy agent, docetaxel [31]. A recent trial combining metformin with a different anti-androgen agent, bicalutamide, in overweight and obese prostate cancer patients found that this paired treatment had no effect on PSA levels compared to bicalutamide alone [32].
2.12. Skin Cancer
In the treatment of metastatic melanoma, neither metformin monotherapy [33] nor combination with immune checkpoint inhibitors (anti-PD-1 and anti-PD-1/anti-CTLA-4) [67] has been shown to improve patient outcomes.
2.13. Uterine Cancer
Pre-hysterectomy metformin treatment in women with endometrial cancer (EC) has yielded mixed results; one study found no anti-cancer effects [34], while others suggest that metformin reduces tumor proliferation [35] and promotes anti-tumor effects by altering EC steroid receptor signaling [36]. These pre-surgical study designs are limited due to the short treatment period and small number of patients enrolled. A recent meta-analysis concluded that metformin does not function as an anti-proliferative agent in EC and is not a beneficial adjunct therapy to progesterone therapy for EC patients seeking to spare their fertility [68], though this latter point is still being investigated in an ongoing clinical trial in Japan [37].
2.14. Acute Myeloid Leukemia
A retrospective hospital cohort study found that though metformin users did not fare better than non-users in OS and disease-free state, they did far better than insulin users. Insulin users were found to have a two-fold increase in the risk of death and an 85% greater risk of relapse [69].
2.15. Chronic Myeloid Leukemia
In a single center observation study, metformin use in combination with a TKI was associated with 100% cytogenetic response (CCyR) compared to only 73.6% of single agent TKI [70]. Patients receiving a TKI with or without metformin were able to achieve major molecular response (MMR) as well as complete molecular response (CMR), however, metformin users achieved this within a shorter period of time with a median time to response of 11.1 months and 37.4 months, respectively, compared to 19.5 months and not reached in the control group [70]. Furthermore, CML leukemic stem cells (LSCs) have been shown to have increased mitochondrial oxygen consumption compared to hematopoietic stem cells (HSCs) [71], which could be specifically targeted by metformin [70].
2.16. Acute Lymphoblastic Leukemia
In a prospective study of 102 patients with de novo Philadelphia-negative B-cell ALL, metformin use was associated with a lower risk of therapeutic failure (odds ratio (OR) 0.07, 95% confidence interval (CI) 0.0037–1.53) and early relapse (OR 0.05, 95% CI 0.0028–1.153) [72]. Furthermore, the patients who benefited most were those with high expression of multi-drug resistant protein, ATP binding cassette subfamily B member 1 (ABCB1) [72]. In a small phase I clinical trial of ALL patients, the addition of metformin to standard chemotherapy was well-tolerated and yielded responses in a heavily pretreated population, with 56% achieving a complete response (CR) [73].
2.17. Myelodysplastic Syndrome
In a single prospective study, no mortality benefit was detected among myelodysplastic syndrome patients receiving metformin or sulfonylureas [74].
2.18. Lymphoma
In a population-based case-control study and two large, retrospective analyses, there were no significant correlations between metformin use and disease progression or survival in patients with non-Hodgkin lymphoma (NHL) [75,76,77]. However, in a Taiwanese study using a database of over 600,000 newly diagnosed DM patients enrolled in the National Health Insurance database, metformin initiators consistently had a lower risk of NHL [78]. Furthermore, in a retrospective case-control study of DM patients with diffuse large B-cell lymphoma (DLBCL) treated with or without metformin, metformin was associated with improved response to immunochemotherapy [79]. The metformin group had CR and objective response rates (ORR) of 84% and 88%, respectively, compared to control groups, which had rates of 48% and 68% [79]. Additionally, a retrospective case–control study found that CR was achieved in 92% of DLBCL patients on metformin, compared to 54% of control subjects [80]. This data was corroborated by a retrospective study of DLBCL patients with diabetes in which metformin use was associated with improved PFS from 60 to 90 months and OS from 71 to 100 compared to diabetic patients not on metformin [81].
2.19. Multiple Myeloma
High levels of insulin and a history of DM are poor prognostic indicators for patients with multiple myeloma (MM) [82]. However, within the DM population, metformin was associated with a decreased incidence of death from MM [82]. Metformin use has also been associated with decreased progression of monoclonal gammopathy of unknown significance (MGUS) to MM [83,84]. The current risk of progression to MM is 1% per year in MGUS patients [85]. In a retrospective cohort study from the US Veterans Health Administration database that followed patients diagnosed with MGUS for a total of 10 years, 3% of metformin users progressed to MM compared with 5% of non-users [83]. Among those who did progress to MM, the individuals on metformin progressed in an average of 71 months compared to 47 months in non-users. A similar benefit was found in a matched case–control study from a population-representative database of 11,000,000 individuals treated over an 18-year period in the United Kingdom, but only for those who had received metformin for at least two years [83].
3. Metformin, Mechanism of Action
3.1. Anti-Cancer Activity of Metformin
In multiple malignancies, metformin has been shown to exert anti-cancer properties, such as decreased proliferation, cell cycle arrest, and induction of apoptosis and/or autophagy [86,87,88]. More recently, it has also been established that metformin can induce alternative forms of cell death, such as pyroptosis, which involves an inflammatory, caspase 1-dependent programmed cell death. Metformin has been shown to induce pyroptosis through adenosine monophosphate-activated protein kinase (AMPK)-dependent activation of sirtuin 1, a nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase, and downstream nuclear factor kappa B (NF-kB) expression [89]. In breast cancer cell lines, metformin has also been shown to induce oxidative stress-dependent necroptosis, which was rescued with necroptosis inhibitors [90]. In in vitro and in vivo models of breast cancer, metformin was found to induce ferroptosis, which is a non-apoptotic form of cell death that involves iron-dependent accumulation of lipid oxidation and depletion of plasma membrane polyunsaturated fatty acids [91,92]. Ferroptosis was induced by upregulation of miRNA-324-3p expression and subsequent downregulation of glutathione peroxidase 4, which is a glutathione-dependent antioxidant enzyme that prevents ferroptosis [91]. Finally, metformin can also induce mitophagy in a cervical cancer cell line [93].
In addition to tumor-killing properties, cancer drug development has also focused on decreasing metastatic spread as well as recurrence post-treatment. Metformin has recently been found to decrease cell motility and invasion while increasing cellular adhesion in multiple solid tumor models [94,95,96,97,98,99]. Furthermore, metformin could specifically target cancer stem cells [96,100,101,102,103,104,105,106,107,108,109]. The mechanisms by which cancer stem cells were targeted varied but included targeting of mitochondrial respiration in osteosarcoma stem cells [100], inhibition of stem cell markers, specifically CD133 in HCC and oral cancer cell lines [102,104] and CD47 in breast cancer [105], and regulation of crucial transcription factors [103,110].
3.2. Mechanisms of Metformin’s Anti-Cancer Activity
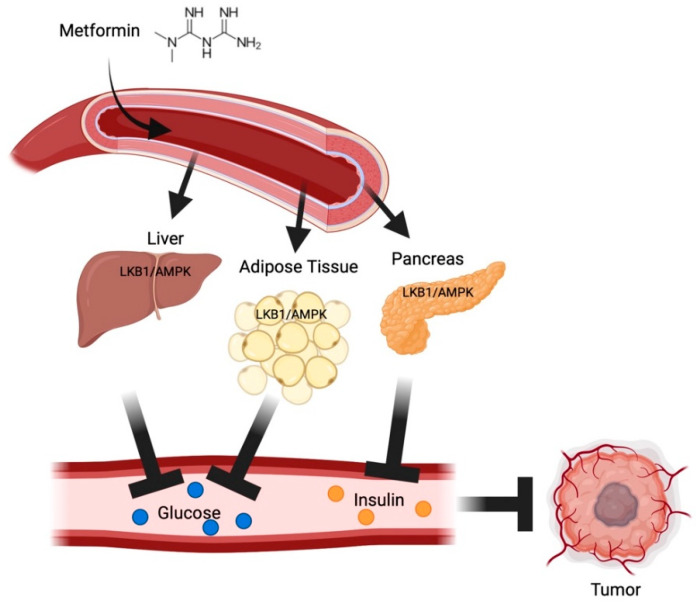
Nearly 25 years of literature consistently demonstrates that there is no single unifying mechanism of action of metformin in cancer. As a normoglycemic agent for type 2 DM, metformin decreases hepatic gluconeogenesis and lipid synthesis, decreases adipose tissue fatty acid synthesis and lipolysis, decreases pancreatic insulin secretion, and increases muscle glucose uptake [111,112] (Figure 1). This can occur either through liver kinase B1 (LKB1)/AMPK activation in target tissues or a direct inhibition of insulin signaling.
Figure 1.
Overview of metformin’s systemic effects on tumor growth. Metformin’s activation of the LKB1/AMPK pathway in hepatocytes and adipocytes, and in the pancreas, leads to reduced blood glucose and insulin availability, respectively. Decreased glucose and insulin availability can slow tumor growth and progression. LKB1: Liver Kinase B1, AMPK: AMP-Activated Protein Kinase. Created in BioRender.
Next, we will briefly summarize the well-established activity of metformin in cancer that has been recently reviewed [48,86,87] and as summarized in Figure 2. We will then shift to novel mechanisms of action established over the last five years, including immunomodulatory and epigenetic effects of metformin.
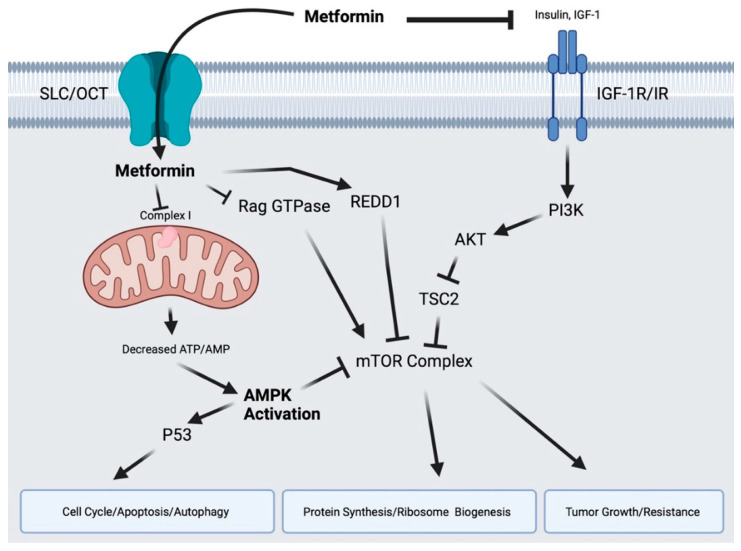
Figure 2.
Molecular effects of metformin in cancer cells. Metformin directly inhibits complex I of the electron transport chain in the mitochondria resulting in decreased ATP/AMP ratio and activation of AMPK. AMPK activation inhibits mTOR and activates P53 to impact subsequent cellular processes. Metformin also inhibits mTOR in an AMPK-independent manner, through Rag GTPases and REDD1. Reduced insulin availability through metformin’s systemic effects indirectly modulates the proliferative pathway, PI3K/AKT. AMP: Adenosine Monophosphate; AMPK: AMP-Activated Protein Kinase; ATP: Adenosine Triphosphate; IGF: Insulin-like Growth Factors; IGF-R: Insulin-like Growth Factor Receptor; mTOR: Mammalian Target of Rapamycin; OTC: Organic Cation Transporter; PI3K: Phosphoinositide 3-kinase; REDD1: Regulated in Development and DNA damage responses 1; SLC: Solute Carrier Transporter; TSC2: Tuberous Sclerosis Complex 2. Created in BioRender.
Metformin’s well-established anti-cancer mechanisms involve direct and indirect, AMPK-dependent and -independent inhibition of mammalian target of rapamycin (mTOR), which plays a significant role in promoting tumor proliferation as well as inhibiting apoptosis and autophagy. The indirect, AMPK-independent inhibition of mTOR stems from metformin’s ability to decrease systemic insulin [113,114,115,116]. Decreased insulin leads to decreased signaling through the phosphoinositide 3-kinase (PI3K)-protein kinase B (AKT) pathway, subsequently allowing tuberous sclerosis complex 2 (TSC2) to inhibit mTOR [117]. Metformin is also taken up by cancer cells through organic cation transporters [118] and subsequently inhibits complex I of the mitochondrial electron transport chain leading to decreased oxidative phosphorylation [119]. The decreased ratio of adenosine triphosphate (ATP) to adenosine monophosphate (AMP) leads to cellular stress, activation of AMPK [120,121,122,123], and downstream inhibition of mTOR kinase activity, which results in a decrease in protein synthesis, cell growth, and proliferation [117,124,125,126,127]. Early on, metformin’s role in cancer clearly showed that AMPK-dependent inhibition of mTOR is required for multiple anti-cancer effects, as the phenotype can be rescued by targeting AMPK with siRNA or Compound C as well as constitutive activation of mTOR and short hairpin RNA targeting TSC2 [117,124,125,128,129]. Furthermore, multiple reports have demonstrated that metformin can also activate AMPK indirectly through activation of upstream energy sensor, LKB1, or via ataxia telangiectasia mutated (ATM) [130,131,132]. Metformin also inhibits mTOR independently of AMPK through activation of DNA-damage-inducible transcript 4 (REDD1), which inhibits mTOR via TSC2 activation [133], or via inhibition of Rag GTPases [134]. Metformin’s inhibition of Rag GTPases was independent of amino acid levels, which have previously been shown to control Rag GTPases and downstream mTOR activity [135,136].
Metformin’s mechanism of action also involves regulation of additional transcription factors, such forkhead box O3a (FOXO3a), mitogen-activated protein kinase (MAPK), Sonic hedgehog, Wnt, Notch, and Kruppel-like factor 5 [103,110,137,138]. FOXO3a upregulation by metformin is particularly interesting given FOXO3a’s ability to induce MAPK-dependent expression of the mitochondrial genome to support mitochondrial metabolism. In fact, activation of FOXO3a has been shown to be necessary for metformin’s pro-apoptotic and chemosensitizing effects in multiple tumor models by allowing metformin to promote mitochondrial biogenesis while simultaneously inhibiting complex I activity [137,138]. It is these multifaceted aspects of metformin that make it a unique drug and encourages further elucidation of its anti-cancer mechanism of action to identify optimal drug combinations to effectively target cancer cells.
3.3. Immunomodulatory Effects of Metformin
More recently, metformin has been found to exhibit antitumor activity through regulation of the immune response to cancer. Multiple studies have found metformin can decrease programmed death-ligand 1 (PD-L1) on tumor cells through both AMPK-dependent [139,140,141] and AMPK-independent [142,143] mechanisms, resulting in enhanced cytotoxic T lymphocyte activity. However, this anti-PD-L1 activity may be tissue-dependent. In a NSCLC model, the inverse was found to be true in which LKB1-overexpression actually increased PD-L1 in an AMPK-dependent fashion [144]. As a result, LKB1-intact NSCLC tumors could be sensitized to anti-PD-1 antibodies with metformin whereas no obvious suppression from metformin was observed in LKB1-deficient tumors [144].
Metformin may also act directly on cytotoxic T cells to augment their anti-cancer activity. Metformin administration induces interferon-gamma (IFN-γ) production in CD8+ tumor infiltrating lymphocytes in multiple solid tumor models [145,146,147]. Furthermore, metformin inhibited accumulation and suppressive activity of myeloid-derived suppressor cells, which are a major immunosuppressive cell type that inhibits T-cells and promotes tumor immune escape [145,148]. Interestingly, metformin is detrimental to CD19-chimeric antigen receptor-modified T cells as it inhibits proliferation and cytotoxicity while inducing apoptosis via AMPK activation and downstream suppression of mTOR [149]. Thus, the T-cell targeting properties of metformin may be context- and cancer subtype-dependent.
In addition to T cell regulation, metformin can enhance natural killer (NK) cell cytotoxicity of human cervical cancer cells by altering tumor cell surface expression of NK-cell ligands via the PI3K/AKT pathway, leading to increased NK cell activation [106]. Furthermore, direct exposure of NK cells to metformin enhances their cytolytic activity and increases NK cell tumor infiltration independently of AMPK [150]. Metformin also directly and indirectly modulates macrophage-targeting of tumor cells. Metformin represses CD47 gene expression in a miRNA-708-dependent manner to allow macrophage phagocytosis of breast cancer stem cells [105]. Furthermore, metformin modulates expression of macrophage-related cytokines, thereby suppressing the ability of cancer cells to promote the protective macrophage 2 phenotype and promoting the anti-cancer macrophage 1 phenotype in an AMPK/NF-κB-dependent manner [151,152].
3.4. Epigenetic Regulation of Metformin
Epigenetic mechanisms, such as hypermethylation of tumor suppressor genes, general hypomethylation of the genome, and alterations in histone posttranslational modifications, play a role in tumorigenesis and therapy resistance [153]. Recent studies indicate that metformin can target cancer cells through epigenetic modifications. Metformin-activated AMPK has been demonstrated to increase global DNA methylation in colon, breast, and endometrial cancer cells [154,155,156]. Altered DNA methyltransferase (DNMT) activity by metformin also contributed to anti-cancer activity by regulating long non-coding RNAs [157,158]. In two studies, metformin has been found to regulate epigenetics specifically through targeting the oncometabolite 2-hydroxyglutarate (2HG) [159,160]. Interestingly, in one study this was through the traditional route of targeting IDH1/2 mutations in endometrial cancer [160]. In another study, the 2HG oncometabolite was found to be elevated in breast cancer in vitro and in vivo in the absence of IDH1/2 mutations [159]. Metformin specifically inhibited 2HG production in this model through knockdown of phosphoglycerate dehydrogenase in an AMPK-dependent manner leading to anti-cancer activity [159]. Additional work has demonstrated that metformin can also suppress epigenetic modifier, enhancer of zeste homolog 2 (EZH2), in its anti-cancer activity in prostate adenocarcinoma and neuroendocrine tumors [161,162]. Metformin can also target histone acetylation to antagonize melanoma progression [163].
4. Conclusive Remarks
Preclinical studies have consistently demonstrated antineoplastic effects of metformin. Additionally, observational and epidemiological studies have reported lower incidence and mortality rates of cancer in patients taking metformin. However, these results have translated to modest benefits in clinical trials, which may be attributed to several hypotheses that can guide future research. The inherent limitations of observational and retrospective study designs can be a source of potential bias leading to an overestimation of the benefits of metformin in patients. Moreover, while preclinical models have been key in characterizing the antineoplastic mechanisms of metformin, they suffer from several limitations that impact their translation to the clinic. Some authors have argued that metformin concentrations used in preclinical studies were significantly higher than the plasma concentrations reached in clinical trials [10]. Additionally, in vivo models require optimization to recapitulate tumor heterogeneity, including cancer stem cells [164], and the immuno- and micro-environments to better predict clinical results [165].
Of note, many of the relevant clinical trials either recruited a small number of patients or enrolled patients with an advanced cancer stage, both of which can confound results. To optimize the design of clinical trials, additional research is required to identify key factors (both patient- and tumor-related) that affect metformin sensitivity. For example, the insulin-lowering effect of metformin is thought to contribute to its anti-cancer activity, which suggests that patients with hyperinsulinemia or tumors expressing the insulin receptor, LKB1, or TSC2 may benefit most from metformin [166].
Acknowledgments
We thank the Hunterian Neurosurgical Research Laboratory. Figures were created with BioRender.com (accessed on 28 March 2022).
Author Contributions
S.J.S., S.A., H.G., A.B., N.S. and B.M.T., writing—original draft preparation, S.J.S., S.A., H.G., N.S. and B.M.T., writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
S.J.S. is funded by the National Institute of Health T32 HL0439.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bailey C.J., Turner R.C. Metformin. N. Engl. J. Med. 1996;334:574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 2.Bailey C.J. Metformin: Historical overview. Diabetologia. 2017;60:1566–1576. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- 3.Curd F.H.S., Davey D.G., Rose F.L. Studies on synthetic antimalarial drugs; Some biguanide derivatives as new types of antimalarial substances with both therapeutic and causal prophylactic activity. Ann. Trop. Med. Parasitol. 1945;39:208–216. doi: 10.1080/00034983.1945.11685237. [DOI] [PubMed] [Google Scholar]
- 4.Chen K.K., Anderson R.C. The toxicity and general pharmacology of N1-p-chlorophenyl-N5-isopropyl biguanide. J. Pharmacol. Exp. Ther. 1947;91:157–160. [PubMed] [Google Scholar]
- 5.Sterne J. Traits and portraits. Maroc. Medical. 1957;36:593–618. [PubMed] [Google Scholar]
- 6.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. doi: 10.1016/S0140-6736(98)07037-8. [DOI] [PubMed] [Google Scholar]
- 7.Evans J.M.M., Donnelly L.A., Emslie-Smith A.M., Alessi D.R., Morris A.D. Metformin and reduced risk of cancer in diabetic patients. Br. Med. J. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Currie C.J., Poole C.D., Gale E.A.M. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 9.Nathan D.M., Buse J.B., Davidson M.B., Ferrannini E., Holman R.R., Sherwin R., Zinman B. Medical Management of Hyperglycemia in Type 2 Diabetes: A Consensus Algorithm for the Initiation and Adjustment of Therapy—A Consensus Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Volume 27. American Diabetes Association; Alexandria, VI, USA: 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saengboonmee C., Sanlung T., Wongkham S. Repurposing metformin for cancer treatment: A great challenge of a promising drug. Anticancer. Res. 2021;41:5913–5918. doi: 10.21873/anticanres.15410. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Y., Gong C., Wang Z., Zhang J., Wang L., Zhang S., Cao J., Tao Z., Li T., Wang B., et al. A randomized phase II study of aromatase inhibitors plus metformin in pre-treated postmenopausal patients with hormone receptor positive metastatic breast cancer. Oncotarget. 2017;8:84224. doi: 10.18632/oncotarget.20478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nanni O., Amadori D., De Censi A., Rocca A., Freschi A., Bologna A., Gianni L., Rosetti F., Amaducci L., Cavanna L., et al. Metformin plus chemotherapy versus chemotherapy alone in the first-line treatment of HER2-negative metastatic breast cancer. The MYME randomized, phase 2 clinical trial. Breast Cancer Res. Treat. 2019;174:433–442. doi: 10.1007/s10549-018-05070-2. [DOI] [PubMed] [Google Scholar]
- 13.Fenn K., Maurer M., Lee S.M., Crew K.D., Trivedi M.S., Accordino M.K., Hershman D.L., Kalinsky K. Phase 1 study of erlotinib and metformin in metastatic triple-negative breast cancer. Clin. Breast Cancer. 2020;20:80–86. doi: 10.1016/j.clbc.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pimentel I., Lohmann A.E., Ennis M., Dowling R.J.O., Cescon D., Elser C., Potvin K.R., Haq R., Hamm C., Chang M.C., et al. A phase II randomized clinical trial of the effect of metformin versus placebo on progression-free survival in women with metastatic breast cancer receiving standard chemotherapy. Breast. 2019;48:17–23. doi: 10.1016/j.breast.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Alipour S., Abedi M., Saberi A., Maleki-Hajiagha A., Faiz F., Shahsavari S., Eslami B. Metformin as a new option in the medical management of breast fibroadenoma; A randomized clinical trial. BMC Endocr. Disord. 2021;21:1–10. doi: 10.1186/s12902-021-00824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonnenblick A., Agbor-Tarh D., Bradbury I., Di Cosimo S., Azim H.A., Fumagalli D., Sarp S., Wolff A.C., Andersson M., Kroep J., et al. Impact of diabetes, insulin, and metformin use on the outcome of patients with human epidermal growth factor receptor 2-positive primary breast cancer: Analysis from the ALTTO phase III randomized trial. J. Clin. Oncol. 2017;35:1421. doi: 10.1200/JCO.2016.69.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yam C., Esteva F.J., Patel M.M., Raghavendra A.S., Ueno N.T., Moulder S.L., Hess K.R., Shroff G.S., Hodge S., Koenig K.H., et al. Efficacy and safety of the combination of metformin, everolimus and exemestane in overweight and obese postmenopausal patients with metastatic, hormone receptor-positive, HER2-negative breast cancer: A phase II study. Investig. New Drugs. 2019;37:345–351. doi: 10.1007/s10637-018-0700-z. [DOI] [PubMed] [Google Scholar]
- 18.Li K., Li L., Zhang P., Kang J., Wang Y.B., Chen H.Y., He Y. A multicenter double-blind phase II study of metformin with gefitinib as first-line therapy of locally advanced non–small-cell lung cancer. Clin. Lung Cancer. 2017;18:340–343. doi: 10.1016/j.cllc.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Arrieta O., Barrón F., Padilla M.Á.S., Avilés-Salas A., Ramírez-Tirado L.A., Arguelles Jiménez M.J., Vergara E., Zatarain-Barrón Z.L., Hernández-Pedro N., Cardona A.F., et al. Effect of metformin plus tyrosine kinase inhibitors compared with tyrosine kinase inhibitors alone in patients with epidermal growth factor receptor-mutated lung adenocarcinoma: A phase 2 randomized clinical trial. JAMA Oncol. 2019;5:e192553. doi: 10.1001/jamaoncol.2019.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skinner H., Hu C., Tsakiridis T., Santana-Davila R., Lu B., Erasmus J.J., Doemer A.J., Videtic G.M.M., Coster J., Yang A.X., et al. Addition of metformin to concurrent chemoradiation in patients with locally advanced non-small cell lung cancer: The NRG-LU001 phase 2 randomized clinical trial. JAMA Oncol. 2021;7:1324–1332. doi: 10.1001/jamaoncol.2021.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsakiridis T., Pond G.R., Wright J., Ellis P.M., Ahmed N., Abdulkarim B., Roa W., Robinson A., Swaminath A., Okawara G., et al. Metformin in combination with chemoradiotherapy in locally advanced non-small cell lung cancer: The OCOG-ALMERA randomized clinical trial. JAMA Oncol. 2021;7:1333–1341. doi: 10.1001/jamaoncol.2021.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marrone K.A., Zhou X., Forde P.M., Purtell M., Brahmer J.R., Hann C.L., Kelly R.J., Coleman B., Gabrielson E., Rosner G.L., et al. A randomized phase II study of metformin plus paclitaxel/carboplatin/bevacizumab in patients with chemotherapy-naïve advanced or metastatic nonsquamous non-small cell lung cancer. Oncologist. 2018;23:859–865. doi: 10.1634/theoncologist.2017-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zell J.A., McLaren C.E., Morgan T.R., Lawson M.J., Rezk S., Albers C.G., Chen W.P., Carmichael J.C., Chung J., Richmond E., et al. A phase IIa trial of metformin for colorectal cancer risk reduction among individuals with history of colorectal adenomas and elevated body mass index. Cancer Prev. Res. 2020;13:203–212. doi: 10.1158/1940-6207.CAPR-18-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vernieri C., Galli F., Ferrari L., Marchetti P., Lonardi S., Maiello E., Iaffaioli R.V., Zampino M.G., Zaniboni A., De Placido S., et al. Impact of metformin use and diabetic status during adjuvant fluoropyrimidine-oxaliplatin chemotherapy on the outcome of patients with resected colon cancer: A TOSCA study subanalysis. Oncologist. 2019;24:385–393. doi: 10.1634/theoncologist.2018-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bragagnoli A.C., Araujo R.L.C., Ferraz M.W., Dos Santos L.V., Abdalla K.C., Comar F., Santos F.A., Oliveira M.A., Carvalheira J.B.C., Cárcano F.M., et al. Metformin plus lrinotecan in patients with refractory colorectal cancer: A phase 2 clinical trial. Br. J. Cancer. 2021;124:1072–1078. doi: 10.1038/s41416-020-01208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molenaar R.J., Van Hattum J.W., Brummelhuis I.S., Oddens J.R., Savci-Heijink C.D., Boevé E.R., Van Der Meer S.A., Witjes J.F., Pollak M.N., De Reijke T.M., et al. Study protocol of a phase II clinical trial of oral metformin for the intravesical treatment of non-muscle invasive bladder cancer. BMC Cancer. 2019;19:1–9. doi: 10.1186/s12885-019-6346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Y., Zhu J., Zhang H., Liu Y., Sun H. Metformin plus first-line chemotherapy versus chemotherapy alone in the treatment of epithelial ovarian cancer: A prospective open-label pilot trial. Cancer Chemother. Pharmacol. 2019;84:1349–1357. doi: 10.1007/s00280-019-03963-7. [DOI] [PubMed] [Google Scholar]
- 28.Brown J.R., Chan D.K., Shank J.J., Griffith K.A., Fan H., Szulawski R., Yang K., Reynolds R.K., Johnston C., McLean K., et al. Phase II clinical trial of metformin as a cancer stem cell–targeting agent in ovarian cancer. JCI Insight. 2020;5:e133247. doi: 10.1172/jci.insight.133247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Broekman K.E., Hof M.A.J., Touw D.J., Gietema J.A., Nijman H.W., Lefrandt J.D., Reyners A.K.L., Jalving M. Phase I study of metformin in combination with carboplatin/paclitaxel chemotherapy in patients with advanced epithelial ovarian cancer. Investig. New Drugs. 2020;38:1454–1462. doi: 10.1007/s10637-020-00920-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mark M., Klingbiel D., Mey U., Winterhalder R., Rothermundt C., Gillessen S., Von Moos R., Pollak M., Manetsch G., Strebel R., et al. Impact of addition of metformin to abiraterone in metastatic castration-resistant prostate cancer patients with disease progressing while receiving abiraterone treatment (metAb-pro): Phase 2 pilot study. Clin. Genitourin. Cancer. 2019;17:e323–e328. doi: 10.1016/j.clgc.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Pujalte Martin M., Borchiellini D., Thamphya B., Guillot A., Paoli J.B., Besson D., Hilgers W., Priou F., el Kouri C., Hoch B., et al. TAXOMET: A french prospective multicentric randomized phase II study of docetaxel plus metformin versus docetaxel plus placebo in metastatic castration-resistant prostate cancer. Clin. Genitourin. Cancer. 2021;19:501–509. doi: 10.1016/j.clgc.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Bilusic M., Toney N.J., Donahue R.N., Wroblewski S., Zibelman M., Ghatalia P., Ross E.A., Karzai F., Madan R.A., Dahut W.L., et al. A randomized phase 2 study of bicalutamide with or without metformin for biochemical recurrence in overweight or obese prostate cancer patients (BIMET-1) Prostate Cancer Prostatic Dis. 2022:1–6. doi: 10.1038/s41391-022-00492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montaudié H., Cerezo M., Bahadoran P., Roger C., Passeron T., Machet L., Arnault J.P., Verneuil L., Maubec E., Aubin F., et al. Metformin monotherapy in melanoma: A pilot, open-label, prospective, and multicentric study indicates no benefit. Pigment. Cell Melanoma Res. 2017;30:378–380. doi: 10.1111/pcmr.12576. [DOI] [PubMed] [Google Scholar]
- 34.Kitson S.J., Maskell Z., Sivalingam V.N., Allen J.L., Ali S., Burns S., Gilmour K., Latheef R., Slade R.J., Pemberton P.W., et al. PRE-surgical metformin in uterine malignancy (PREMIUM): A multi-center, randomized double-blind, placebo-controlled phase III trial. Clin. Cancer Res. 2019;25:2424–2432. doi: 10.1158/1078-0432.CCR-18-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petchsila K., Prueksaritanond N., Insin P., Yanaranop M., Chotikawichean N. Effect of metformin for decreasing proliferative marker in women with endometrial cancer: A randomized double-blind placebo-controlled trial. Asian Pac. J. Cancer Prev. 2020;21:733. doi: 10.31557/APJCP.2020.21.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pabona J.M.P., Burnett A.F., Brown D.M., Quick C.M., Simmen F.A., Montales M.T.E., Liu S.J., Rose T., Alhallak I., Siegel E.R., et al. Metformin promotes anti-tumor biomarkers in human endometrial cancer cells. Reprod. Sci. 2020;27:267–277. doi: 10.1007/s43032-019-00019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitsuhashi A., Kawasaki Y., Hori M., Fujiwara T., Hanaoka H., Shozu M. Medroxyprogesterone acetate plus metformin for fertility-sparing treatment of atypical endometrial hyperplasia and endometrial carcinoma: Trial protocol for a prospective, randomised, open, blinded-endpoint design, dose-response trial (FELICIA trial) BMJ Open. 2020;10:e035416. doi: 10.1136/bmjopen-2019-035416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molenaar R.J., Van De Venne T., Weterman M.J., Mathot R.A., Klümpen H.J., Richel D.J., Wilmink J.W. A Phase Ib Study of Everolimus Combined with Metformin for Patients with Advanced Cancer. Investig. New Drugs. 2018;36:53–61. doi: 10.1007/s10637-017-0478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sehdev A., Karrison T., Zha Y., Janisch L., Turcich M., Cohen E.E.W., Maitland M., Polite B.N., Gajewski T.F., Salgia R., et al. A Pharmacodynamic Study of Sirolimus and Metformin in Patients with Advanced Solid Tumors. Cancer Chemother. Pharmacol. 2018;82:309–317. doi: 10.1007/s00280-018-3619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saif M.W., Rajagopal S., Caplain J., Grimm E., Serebrennikova O., Das M., Tsichlis P.N., Martell R. A Phase I Delayed-Start, Randomized and Pharmacodynamic Study of Metformin and Chemotherapy in Patients with Solid Tumors. Cancer Chemother. Pharmacol. 2019;84:1323–1331. doi: 10.1007/s00280-019-03967-3. [DOI] [PubMed] [Google Scholar]
- 41.Khurshed M., Molenaar R.J., Van Linde M.E., Mathôt R.A., Struys E.A., Van Wezel T., Van Noorden C.J.F., Klümpen H.-J., Bovée J.V.M.G., Wilmink J.W. A Phase Ib Clinical Trial of Metformin and Chloroquine in Patients with IDH1-Mutated Solid Tumors. Cancers. 2021;13:2474. doi: 10.3390/cancers13102474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lord S.R., Cheng W.C., Liu D., Gaude E., Haider S., Metcalf T., Patel N., Teoh E.J., Gleeson F., Bradley K., et al. Integrated Pharmacodynamic Analysis Identifies Two Metabolic Adaption Pathways to Metformin in Breast Cancer. Cell Metab. 2018;28:679–688. doi: 10.1016/j.cmet.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crawley D., Chandra A., Loda M., Gillett C., Cathcart P., Challacombe B., Cook G., Cahill D., Santa Olalla A., Cahill F., et al. Metformin and Longevity (METAL): A Window of Opportunity Study Investigating the Biological Effects of Metformin in Localised Prostate Cancer. BMC Cancer. 2017;17:1–12. doi: 10.1186/s12885-017-3458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curry J., Johnson J., Tassone P., Vidal M.D., Menezes D.W., Sprandio J., Mollaee M., Cotzia P., Birbe R., Lin Z., et al. Metformin Effects on Head and Neck Squamous Carcinoma Microenvironment: Window of Opportunity Trial. Laryngoscope. 2017;127:1808–1815. doi: 10.1002/lary.26489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amin D., Richa T., Mollaee M., Zhan T., Tassone P., Johnson J., Luginbuhl A., Cognetti D., Martinez-Outschoorn U., Stapp R., et al. Metformin Effects on FOXP3+ and CD8+ T Cell Infiltrates of Head and Neck Squamous Cell Carcinoma. Laryngoscope. 2020;130:E490–E498. doi: 10.1002/lary.28336. [DOI] [PubMed] [Google Scholar]
- 46.Gulati S., Desai J., Palackdharry S.M., Morris J.C., Zhu Z., Jandarov R., Riaz M.K., Takiar V., Mierzwa M., Gutkind J.S., et al. Phase 1 Dose-Finding Study of Metformin in Combination with Concurrent Cisplatin and Radiotherapy in Patients with Locally Advanced Head and Neck Squamous Cell Cancer. Cancer. 2020;126:354–362. doi: 10.1002/cncr.32539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gutkind J.S., Molinolo A.A., Wu X., Wang Z., Nachmanson D., Harismendy O., Alexandrov L.B., Wuertz B.R., Ondrey F.G., Laronde D., et al. Inhibition of MTOR Signaling and Clinical Activity of Metformin in Oral Premalignant Lesions. JCI Insight. 2021;6:e147096. doi: 10.1172/jci.insight.147096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Curry J.M., Johnson J., Mollaee M., Tassone P., Amin D., Knops A., Whitaker-Menezes D., Mahoney M.G., South A., Rodeck U., et al. Metformin Clinical Trial in HPV+ and HPV-Head and Neck Squamous Cell Carcinoma: Impact on Cancer Cell Apoptosis and Immune Infiltrate. Front. Oncol. 2018;8:436. doi: 10.3389/fonc.2018.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Łabuzek K., Suchy D., Gabryel B., Bielecka A., Liber S., Okopień B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 2010;62:956–965. doi: 10.1016/S1734-1140(10)70357-1. [DOI] [PubMed] [Google Scholar]
- 50.Takata F., Dohgu S., Matsumoto J., Machida T., Kaneshima S., Matsuo M., Sakaguchi S., Takeshige Y., Yamauchi A., Kataoka Y. Metformin induces up-regulation of blood—Brain barrier functions by activating AMP-activated protein kinase in rat brain microvascular endothelial cells. Biochem. Biophys. Res. Commun. 2013;433:586–590. doi: 10.1016/j.bbrc.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 51.Seliger C., Luber C., Gerken M., Schaertl J., Proescholdt M., Riemenschneider M.J., Meier C.R., Bogdahn U., Leitzmann M.F., Klinkhammer-Schalke M., et al. Use of metformin and survival of patients with high-grade glioma. Int. J. Cancer. 2019;144:273–280. doi: 10.1002/ijc.31783. [DOI] [PubMed] [Google Scholar]
- 52.Cuyàs E., Fernández-Arroyo S., Corominas-Faja B., Rodríguez-Gallego E., Bosch-Barrera J., Martin-Castillo B., De Llorens R., Joven J., Menendez J.A. Oncometabolic mutation IDH1 R132H confers a metformin-hypersensitive phenotype. Oncotarget. 2015;6:12279–12296. doi: 10.18632/oncotarget.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Ashmawy N.E., Khedr N.F., El-Bahrawy H.A., Abo Mansour H.E. Metformin augments doxorubicin cytotoxicity in mammary carcinoma through activation of adenosine monophosphate protein kinase pathway. Tumor Biology. 2017;39:1–9. doi: 10.1177/1010428317692235. [DOI] [PubMed] [Google Scholar]
- 54.Li L., Jiang L., Wang Y., Zhao Y., Zhang X.J., Wu G., Zhou X., Sun J., Bai J., Ren B., et al. Combination of metformin and gefitinib as first-line therapy for nondiabetic advanced NSCLC Ppatients with EGFR mutations: A randomized, double-blind phase II trial. Clin. Cancer Res. 2019;25:6967–6975. doi: 10.1158/1078-0432.CCR-19-0437. [DOI] [PubMed] [Google Scholar]
- 55.Arrieta O., Zatarain-Barron Z.L., Turcott J.G., Barron F., Yundamuri S., Cardona A.F., Rosell R. Association of BMI with benefit of metformin plus epidermal growth factor receptor-tyrosine kinase inhibitors in patients with advanced lung adenocarcinoma: A secondary analysis of a phase 2 randomized clinical trial. JAMA Oncol. 2022;8:477–479. doi: 10.1001/jamaoncol.2021.7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu T., Li D., He Y., Zhang F., Qiao M., Chen Y. Prognostic value of metformin for non-small cell lung cancer patients with diabetes. World J. Surg. Oncol. 2018;16:1–5. doi: 10.1186/s12957-018-1362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J.L., Tsai Y.T., Lin C.H., Cidem A., Staniczek T., Chang G.R.L., Yen C.C., Chen W., Chong K.Y., Chen C.M. Benefits of metformin combined with pemetrexed-based platinum doublets as a first-line therapy for advanced lung adenocarcinoma patients with diabetes. Biomolecules. 2021;11:1252. doi: 10.3390/biom11081252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brancher S., Ribeiro A.E., Toporcov T.N., Weiderpass E. The role of metformin on lung cancer survival: The first systematic review and meta-analysis of observational studies and randomized clinical trials. J. Cancer Res. Clin. Oncol. 2021;147:2819–2836. doi: 10.1007/s00432-021-03728-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang S., Lin Y., Xiong X., Wang L., Guo Y., Chen Y., Chen S., Wang G., Lin P., Chen H., et al. Low-dose metformin reprograms the tumor immune microenvironment in human esophageal cancer: Results of a phase II clinical trial. Clin. Cancer Res. 2020;26:4921–4932. doi: 10.1158/1078-0432.CCR-20-0113. [DOI] [PubMed] [Google Scholar]
- 60.Hamieh L., McKay R.R., Lin X., Moreira R.B., Simantov R., Choueiri T.K. Effect of metformin use on survival outcomes in patients with metastatic renal cell carcinoma. Clin. Genitourin. Cancer. 2017;15:221–229. doi: 10.1016/j.clgc.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 61.Fiala O., Ostašov P., Rozsypalová A., Hora M., Šorejs O., Šustr J., Bendová B., Trávníček I., Filipovský J., Fínek J., et al. Metformin use and the outcome of metastatic renal cell carcinoma treated with sunitinib or pazopanib. Cancer Manag. Res. 2021;13:4077. doi: 10.2147/CMAR.S305321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murff H.J., Roumie C.L., Greevy R.A., Hackstadt A.J., McGowan L.E.A., Hung A.M., Grijalva C.G., Griffin M.R. Metformin use and incidence cancer risk: Evidence for a selective protective effect against liver cancer. Cancer Causes Control. 2018;29:823–832. doi: 10.1007/s10552-018-1058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elsayed M., Wagstaff W., Behbahani K., Villalobos A., Bercu Z., Majdalany B.S., Akce M., Schuster D.M., Mao H., Kokabi N. Improved tumor response in patients on metformin undergoing yttrium-90 radioembolization segmentectomy for hepatocellular carcinoma. CardioVascular Interv. Radiol. 2021;44:1937–1944. doi: 10.1007/s00270-021-02916-z. [DOI] [PubMed] [Google Scholar]
- 64.Cho W.R., Wang C.C., Tsai M.Y., Chou C.K., Liu Y.W., Wu Y.J., Lin M.T., Chen K.D., Chuang C.H., Huang P.Y., et al. Impact of metformin use on the recurrence of hepatocellular carcinoma after initial liver resection in diabetic patients. PLoS ONE. 2021;16:e0247231. doi: 10.1371/journal.pone.0247231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Z., Ong W., Shen T., Sng J., Lata R., Mahendran R., Kesavan E., Chiong E. Beyond diabetes mellitus: Role of metformin in non-muscle invasive bladder cancer. Singap. Med. J. 2020 doi: 10.11622/smedj.2020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi Y.Q., Zhou X.C., Du P., Yin M.Y., Xu L., Chen W.J., Xu C.F. Relationships are between metformin use and survival in pancreatic cancer patients concurrent with diabetes: A systematic review and meta-analysis. Medicine. 2020;99:e21687. doi: 10.1097/MD.0000000000021687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Afzal M.Z., Mercado R.R., Shirai K. Efficacy of metformin in combination with immune checkpoint inhibitors (anti-PD-1/anti-CTLA-4) in metastatic malignant melanoma. J. ImmunoTherapy Cancer. 2018;6:1–10. doi: 10.1186/s40425-018-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prodromidou A., Lekka S., Fotiou A., Psomiadou V., Iavazzo C. The evolving role of targeted metformin administration for the prevention and treatment of endometrial cancer: A systematic review and meta-analysis of randomized controlled trials. J. Gynecol. Obstet. Hum. Reprod. 2021;50:102164. doi: 10.1016/j.jogoh.2021.102164. [DOI] [PubMed] [Google Scholar]
- 69.Ceacareanu A., Nimako G., Wintrob Z.A. Missing the benefit of metformin in acute myeloid leukemia: A problem of contrast? J. Res. Pharm. Pract. 2017;6:145. doi: 10.4103/jrpp.JRPP_17_37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pokorny R., Stenehjem D.D., Gilreath J.A. Impact of metformin on tyrosine kinase inhibitor response in chronic myeloid leukemia. J. Oncol. Pharm. Pract. 2022;28:10781552221077254. doi: 10.1177/10781552221077254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kuntz E.M., Baquero P., Michie A.M., Dunn K., Tardito S., Holyoake T.L., Helgason G.V., Gottlieb E. Targeting mitochondrial oxidative phosphorylation eradicates therapy-resistant chronic myeloid leukemia stem cells. Nat. Med. 2017;23:1234–1240. doi: 10.1038/nm.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramos-Peñafiel C., Olarte-Carrillo I., Cerón-Maldonado R., Rozen-Fuller E., Kassack-Ipiña J.J., Meléndez-Mier G., Collazo-Jaloma J., Martínez-Tovar A. Effect of metformin on the survival of patients with ALL who express high levels of the ABCB1 drug resistance gene. J. Transl. Med. 2018;16:1–9. doi: 10.1186/s12967-018-1620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Trucco M., Barredo J.C., Goldberg J., Leclerc G.M., Hale G.A., Gill J., Setty B., Smith T., Lush R., Lee J.K., et al. A phase I window, dose escalating and safety trial of metformin in combination with induction chemotherapy in relapsed refractory acute lymphoblastic leukemia: Metformin with induction chemotherapy of vincristine, dexamethasone, PEG-asparaginase, and doxorubicin. Pediatric Blood Cancer. 2018;65:e27224. doi: 10.1002/pbc.27224. [DOI] [PubMed] [Google Scholar]
- 74.Brailovski E., Li Q., Liu N., Leber B., Khalaf D., Sabloff M., Christou G., Yee K., Chodirker L., Parmentier A., et al. The Impact of Oral Hypoglycemics and Statins on Outcomes in Myelodysplastic Syndromes. Blood. 2021;138:3064. doi: 10.1182/blood-2021-149855. [DOI] [PubMed] [Google Scholar]
- 75.Ye X., Zhang G., Righolt C., Johnston J.B., Banerji V., Gibson S.B., Mahmud S.M. Metformin is not associated with incidence risk of non-hodgkin lymphomas among diabetic patients. Cancer Epidemiol. Biomark. Prev. 2018;27:610–612. doi: 10.1158/1055-9965.EPI-18-0012. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y., Maurer M.J., Larson M.C., Allmer C., Feldman A.L., Bennani N.N., Thompson C.A., Porrata L.F., Habermann T.M., Witzig T.E., et al. Impact of metformin use on the outcomes of newly diagnosed diffuse large B-cell lymphoma and follicular lymphoma. Br. J. Haematol. 2019;186:820–828. doi: 10.1111/bjh.15997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smyth L., Blunt D.N., Gatov E., Nagamuthu C., Croxford R., Mozessohn L., Cheung M.C. Statin and Cyclooxygenase-2 Inhibitors Improve Survival in Newly Diagnosed Diffuse Large B-Cell Lymphoma: A Large Population-Based Study of 4913 Subjects. Br. J. Haematol. 2020;191:396–404. doi: 10.1111/bjh.16635. [DOI] [PubMed] [Google Scholar]
- 78.Tseng C.H. Metformin Is Associated with a Lower Risk of Non-Hodgkin Lymphoma in Patients with Type 2 Diabetes. Diabetes Metab. 2019;45:458–464. doi: 10.1016/j.diabet.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 79.Jiang X.N., Zhang Y., Wang W.G., Sheng D., Zhou X.Y., Li X.Q. Alteration of cholesterol metabolism by metformin is associated with improved outcome in type II diabetic patients with diffuse large B-cell lymphoma. Front. Oncol. 2021;11:1632. doi: 10.3389/fonc.2021.608238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alkhatib Y., Abdel Rahman Z., Kuriakose P. Clinical Impact of Metformin in Diabetic Diffuse Large B-Cell Lymphoma Patients: A Case-Control Study. Leuk. Lymphoma. 2017;58:1130–1134. doi: 10.1080/10428194.2016.1239822. [DOI] [PubMed] [Google Scholar]
- 81.Singh A.R., Gu J.J., Zhang Q., Torka P., Sundaram S., Mavis C., Hernandez-Ilizaliturri F.J. Metformin Sensitizes Therapeutic Agents and Improves Outcome in Pre-Clinical and Clinical Diffuse Large B-Cell Lymphoma. Cancer Metab. 2020;8:1–13. doi: 10.1186/s40170-020-00213-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu W., Merriman K., Nabaah A., Seval N., Seval D., Lin H., Wang M., Qazilbash M.H., Baladandayuthapani V., Berry D., et al. The association of diabetes and anti-diabetic medications with clinical outcomes in multiple myeloma. Br. J. Cancer. 2014;111:628–636. doi: 10.1038/bjc.2014.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boursi B., Mamtani R., Yang Y.X., Weiss B.M. Impact of Metformin on the Progression of MGUS to Multiple Myeloma. Leuk. Lymphoma. 2017;58:1265–1267. doi: 10.1080/10428194.2016.1236375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chang S.-H., Luo S., O’Brian K.K., Thomas T.S., Colditz G.A., Carlsson N.P., Carson K.R. Association between Metformin Use and Progression of Monoclonal Gammopathy of Undetermined Significance to Multiple Myeloma in US Veterans with Diabetes Mellitus: A Population-Based Retrospective Cohort Study. Lancet Haematol. 2015;2:e30–e36. doi: 10.1016/S2352-3026(14)00037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kyle R.A., Therneau T.M., Rajkumar S.V., Offord J.R., Larson D.R., Plevak M.F., Melton L.J. A Long-Term Study of Prognosis in Monoclonal Gammopathy of Undetermined Significance. N. Engl. J. Med. 2002;346:564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 86.Misirkic Marjanovic M.S., Vucicevic L.M., Despotovic A.R., Stamenkovic M.M., Janjetovic K.D. Dual Anticancer Role of Metformin: An Old Drug Regulating AMPK Dependent/Independent Pathways in Metabolic, Oncogenic/Tumorsuppresing and Immunity Context. Am. J. Cancer Res. 2021;11:5625–5643. [PMC free article] [PubMed] [Google Scholar]
- 87.Saraei P., Asadi I., Kakar M.A., Moradi-Kor N. The Beneficial Effects of Metformin on Cancer Prevention and Therapy: A Comprehensive Review of Recent Advances. Cancer Manag. Res. 2019;11:3295–3313. doi: 10.2147/CMAR.S200059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chomanicova N., Gazova A., Adamickova A., Valaskova S., Kyselovic J. The Role of AMPK/MTOR Signaling Pathway in Anticancer Activity of Metformin. Physiol. Res. 2021;70:501–508. doi: 10.33549/physiolres.934618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng Z., Bian Y., Zhang Y., Ren G., Li G. Metformin Activates AMPK/SIRT1/NF-ΚB Pathway and Induces Mitochondrial Dysfunction to Drive Caspase3/GSDME-Mediated Cancer Cell Pyroptosis. Cell Cycle. 2020;19:1089–1104. doi: 10.1080/15384101.2020.1743911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dias Lopes N.M., Marinello P.C., Sanches L.J., Da Silva Brito W.A., Lovo-Martins M.I., Pinge-Filho P., Luiz R.C., Cecchini R., Cecchini A.L. Patterns of Cell Death Induced by Metformin in Human MCF-7 Breast Cancer Cells. Pathol. Res. Pract. 2020;216:153199. doi: 10.1016/j.prp.2020.153199. [DOI] [PubMed] [Google Scholar]
- 91.Hou Y., Cai S., Yu S., Lin H. Metformin Induces Ferroptosis by Targeting MiR-324-3p/GPX4 Axis in Breast Cancer. Acta Biochim. Biophys. Sin. 2021;53:333–341. doi: 10.1093/abbs/gmaa180. [DOI] [PubMed] [Google Scholar]
- 92.Chen J., Qin C., Zhou Y., Chen Y., Mao M., Yang J. Metformin May Induce Ferroptosis by Inhibiting Autophagy via LncRNA H19 in Breast Cancer. FEBS Open Bio. 2022;12:146–153. doi: 10.1002/2211-5463.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen J., Zhou C., Yi J., Sun J., Xie B., Zhang Z., Wang Q., Chen G., Jin S., Hou J., et al. Metformin and Arsenic Trioxide Synergize to Trigger Parkin/Pink1-Dependent Mitophagic Cell Death in Human Cervical Cancer HeLa Cells. J. Cancer. 2021;12:6310. doi: 10.7150/jca.61299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hassan M.A., Fakhoury I., Masri Z.E., Ghazale N., Dennaoui R., Atat O.E., Kanaan A., El-Sibai M. Metformin Treatment Inhibits Motility and Invasion of Glioblastoma Cancer Cells. Anal. Cell. Pathol. 2018;2018:5917470. doi: 10.1155/2018/5917470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Athreya A.P., Kalari K.R., Cairns J., Gaglio A.J., Wills Q.F., Niu N., Weinshilboum R., Iyer R.K., Wang L. Model-Based Unsupervised Learning Informs Metformininduced Cell-Migration Inhibition through an AMPK-Independent Mechanism in Breast Cancer. Oncotarget. 2017;8:27199. doi: 10.18632/oncotarget.16109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bocci F., Jolly M.K., George J.T., Levine H., Onuchic J.N. A Mechanism-Based Computational Model to Capture the Interconnections among Epithelial-Mesenchymal Transition, Cancer Stem Cells and Notch-Jagged Signaling. Oncotarget. 2018;9:29906. doi: 10.18632/oncotarget.25692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duan W., Qian W., Zhou C., Cao J., Qin T., Xiao Y., Cheng L., Li J., Chen K., Li X., et al. Metformin Suppresses the Invasive Ability of Pancreatic Cancer Cells by Blocking Autocrine TGF-SS1 Signaling. Oncol. Rep. 2018;40:1495–1502. doi: 10.3892/or.2018.6518. [DOI] [PubMed] [Google Scholar]
- 98.Guo Z., Zhao M., Howard E.W., Zhao Q., Parris A.B., Ma Z., Yang X. Phenformin Inhibits Growth and Epithelial-Mesenchymal Transition of ErbB2—Overexpressing Breast Cancer Cells through Targeting the IGF1R Pathway. Oncotarget. 2017;8:60342. doi: 10.18632/oncotarget.19466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kang S., Kim B.R., Kang M.H., Kim D.Y., Lee D.H., Oh S.C., Min B.W., Um J.W. Anti-Metastatic Effect of Metformin via Repression of Interleukin 6-Induced Epithelial—Mesenchymal Transition in Human Colon Cancer Cells. PLoS ONE. 2018;13:e0205449. doi: 10.1371/journal.pone.0205449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deguchi T., Hosoya K., Kim S., Murase Y., Yamamoto K., Bo T., Yasui H., Inanami O., Okumura M. Metformin Preferentially Enhances the Radio-Sensitivity of Cancer Stem-like Cells with Highly Mitochondrial Respiration Ability in HMPOS. Mol. Ther. Oncolytics. 2021;22:143–151. doi: 10.1016/j.omto.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kwon Y.S., Chun S.Y., Nan H.Y., Nam K.S., Lee C., Kim S. Metformin Selectively Targets 4T1 Tumorspheres and Enhances the Antitumor Effects of Doxorubicin by Downregulating the AKT and STAT3 Signaling Pathways. Oncol. Lett. 2019;17:2523–2530. doi: 10.3892/ol.2018.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maehara O., Ohnishi S., Asano A., Suda G., Natsuizaka M., Nakagawa K., Kobayashi M., Sakamoto N., Takeda H. Metformin Regulates the Expression of CD133 Through the AMPK-CEBPβ Pathway in Hepatocellular Carcinoma Cell Lines. Neoplasia. 2019;21:545–556. doi: 10.1016/j.neo.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shi P., Liu W., Tala T., Wang H., Li F., Zhang H., Wu Y., Kong Y., Zhou Z., Wang C., et al. Metformin Suppresses Triple-Negative Breast Cancer Stem Cells by Targeting KLF5 for Degradation. Cell Discov. 2017;3:1–13. doi: 10.1038/celldisc.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Siddappa G., Kulsum S., Ravindra D.R., Kumar V.V., Raju N., Raghavan N., Sudheendra H.V., Sharma A., Sunny S.P., Jacob T., et al. Curcumin and Metformin-Mediated Chemoprevention of Oral Cancer Is Associated with Inhibition of Cancer Stem Cells. Mol. Carcinog. 2017;56:2446–2460. doi: 10.1002/mc.22692. [DOI] [PubMed] [Google Scholar]
- 105.Tan W., Tang H., Jiang X., Ye F., Huang L., Shi D., Li L., Huang X., Li L., Xie X., et al. Metformin Mediates Induction of MiR-708 to Inhibit Self-Renewal and Chemoresistance of Breast Cancer Stem Cells through Targeting CD47. J. Cell. Mol. Med. 2019;23:5994–6004. doi: 10.1111/jcmm.14462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xia C., Yang F., He Z., Cai Y. ITRAQ-Based Quantitative Proteomic Analysis of the Inhibition of Cervical Cancer Cell Invasion and Migration by Metformin. Biomed. Pharmacother. 2020;123:109762. doi: 10.1016/j.biopha.2019.109762. [DOI] [PubMed] [Google Scholar]
- 107.Singh S.K., Clarke I.D., Hide T., Dirks P.B. Cancer Stem Cells in Nervous System Tumors. Oncogene. 2004;23:7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 108.Singh S.K., Hawkins C., Clarke I.D., Squire J.A., Bayani J., Hide T., Henkelman R.M., Cusimano M.D., Dirks P.B. Identification of Human Brain Tumour Initiating Cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 109.Najbauer J., Kraljik N., Németh P. Glioma Stem Cells: Markers, Hallmarks and Therapeutic Targeting by Metformin. Pathol. Oncol. Res. 2014;20:789–797. doi: 10.1007/s12253-014-9837-z. [DOI] [PubMed] [Google Scholar]
- 110.Schulten H.J. Pleiotropic Effects of Metformin on Cancer. Int. J. Mol. Sci. 2018;19:2850. doi: 10.3390/ijms19102850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stumvoll M., Nurjhan N., Perriello G., Dailey G., Gerich J.E. Metabolic Effects of Metformin in Non-Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1995;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 112.Jalving M., Gietema J.A., Lefrandt J.D., Jong S.D., Reyners A.K.L., Gans R.O.B., Vries E.G.E.D. Metformin: Taking Away the Candy for Cancer? Eur. J. Cancer. 2010;46:2369–2380. doi: 10.1016/j.ejca.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 113.Memmott R.M., Mercado J.R., Maier C.R., Kawabata S., Fox S.D., Dennis P.A. Metformin Prevents Tobacco Carcinogen-Induced Lung Tumorigenesis. Cancer Prev. Res. 2010;3:1066–1076. doi: 10.1158/1940-6207.CAPR-10-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu B., Fan Z., Edgerton S.M., Yang X., Lind S.E., Thor A.D. Potent Anti-Proliferative Effects of Metformin on Trastuzumab-Resistant Breast Cancer Cells via Inhibition of ErbB2/IGF-1 Receptor Interactions. Cell Cycle. 2011;10:2959–2966. doi: 10.4161/cc.10.17.16359. [DOI] [PubMed] [Google Scholar]
- 115.Chaudhary S.C., Kurundkar D., Elmets C.A., Kopelovich L., Athar M. Metformin, an antidiabetic agent reduces growth of cutaneous squamous cell carcinoma by targeting MTOR signaling pathway. Photochem. Photobiol. 2012;88:1–72. doi: 10.1111/j.1751-1097.2012.01165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Würth R., Pattarozzi A., Gatti M., Bajetto A., Corsaro A., Parodi A., Sirito R., Massollo M., Marini C., Zona G., et al. Metformin Selectively Affects Human Glioblastoma Tumor-Initiating Cell Viability: A Role for Metformin-Induced Inhibition of Akt. Cell Cycle. 2013;12:145–156. doi: 10.4161/cc.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zakikhani M., Blouin M.J., Piura E., Pollak M.N. Metformin and Rapamycin Have Distinct Effects on the AKT Pathway and Proliferation in Breast Cancer Cells. Breast Cancer Res. Treat. 2010;123:271–279. doi: 10.1007/s10549-010-0763-9. [DOI] [PubMed] [Google Scholar]
- 118.Jonker J.W., Schinkel A.H. Pharmacological and Physiological Functions of the Polyspecific Organic Cation Transporters: OCT1, 2, and 3 (SLC22A1-3) J. Pharmacol. Exp. Ther. 2004;308:2–9. doi: 10.1124/jpet.103.053298. [DOI] [PubMed] [Google Scholar]
- 119.Hardie D.G., Hawley S.A., Scott J.W. AMP—Activated Protein Kinase—Development of the Energy Sensor Concept. J. Physiol. 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., et al. Role of AMP-Activated Protein Kinase in Mechanism of Metformin Action. J. Clin. Investig. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Doran E., Halestrap A.P. Cytochrome c Release from Isolated Rat Liver Mitochondria Can Occur Independently of Outer-Membrane Rupture: Possible Role of Contact Sites. Biochem. J. 2000;348:343–350. doi: 10.1042/bj3480343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.El-Mir M.Y., Nogueira V., Fontaine E., Avéret N., Rigoulet M., Leverve X. Dimethylbiguanide Inhibits Cell Respiration via an Indirect Effect Targeted on the Respiratory Chain Complex I. J. Biol. Chem. 2000;275:12590–12597. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 123.Scotland S., Saland E., Skuli N., de Toni F., Boutzen H., Micklow E., Sénégas I., Peyraud R., Peyriga L., Théodoro F., et al. Mitochondrial Energetic and AKT Status Mediate Metabolic Effects and Apoptosis of Metformin in Human Leukemic Cells. Leukemia. 2013;27:2129–2138. doi: 10.1038/leu.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zakikhani M., Dowling R.J.O., Sonenberg N., Pollak M.N. The Effects of Adiponectin and Metformin on Prostate and Colon Neoplasia Involve Activation of AMP—Activated Protein Kinase. Cancer Prev. Res. 2008;1:369–375. doi: 10.1158/1940-6207.CAPR-08-0081. [DOI] [PubMed] [Google Scholar]
- 125.Zakikhani M., Dowling R., Fantus I.G., Sonenberg N., Pollak M. Metformin Is an AMP Kinase-Dependent Growth Inhibitor for Breast Cancer Cells. Cancer Res. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 126.Gotlieb W.H., Saumet J., Beauchamp M.C., Gu J., Lau S., Pollak M.N., Bruchim I. In Vitro Metformin Anti-Neoplastic Activity in Epithelial Ovarian Cancer. Gynecol. Oncol. 2008;110:246–250. doi: 10.1016/j.ygyno.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 127.Song C.W., Lee H., Dings R.P.M., Williams B., Powers J., dos Santos T., Choi B.H., Park H.J. Metformin Kills and Radiosensitizes Cancer Cells and Preferentially Kills Cancer Stem Cells. Sci. Rep. 2012;2:1–9. doi: 10.1038/srep00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Green A.S., Chapuis N., Maciel T.T., Willems L., Lambert M., Arnoult C., Boyer O., Bardet V., Park S., Foretz M., et al. The LKB1/AMPK Signaling Pathway Has Tumor Suppressor Activity in Acute Myeloid Leukemia through the Repression of MTOR-Dependent Oncogenic MRNA Translation. Blood. 2010;116:4262–4273. doi: 10.1182/blood-2010-02-269837. [DOI] [PubMed] [Google Scholar]
- 129.Shi W.-Y., Xiao D., Wang L., Dong L.-H., Yan Z.-X., Shen Z.-X., Chen S.-J., Chen Y., Zhao W.-L. Therapeutic Metformin/AMPK Activation Blocked Lymphoma Cell Growth via Inhibition of MTOR Pathway and Induction of Autophagy. Cell Death Dis. 2012;3:e275. doi: 10.1038/cddis.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Suzuki A., Kusakai G., Kishimoto A., Shimojo Y., Ogura T., Lavin M.F., Esumi H. IGF-1 Phosphorylates AMPK-Alpha Subunit in ATM-Dependent and LKB1-Independent Manner. Biochem. Biophys. Res. Commun. 2004;324:986–992. doi: 10.1016/j.bbrc.2004.09.145. [DOI] [PubMed] [Google Scholar]
- 131.Sun Y., Connors K.E., Yang D.Q. AICAR Induces Phosphorylation of AMPK in an ATM-Dependent, LKB1-Independent Manner. Mol. Cell. Biochem. 2007;306:239–245. doi: 10.1007/s11010-007-9575-6. [DOI] [PubMed] [Google Scholar]
- 132.Alexander A., Cai S.-L., Kim J., Nanez A., Sahin M., MacLean K.H., Inoki K., Guan K.-L., Shen J., Person M.D., et al. ATM Signals to TSC2 in the Cytoplasm to Regulate MTORC1 in Response to ROS. Proc. Natl. Acad. Sci. USA. 2010;107:4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ben Sahra I., Regazzetti C., Robert G., Laurent K., Le Marchand-Brustel Y., Auberger P., Tanti J.-F., Giorgetti-Peraldi S., Bost F. Metformin, Independent of AMPK, Induces MTOR Inhibition and Cell-Cycle Arrest through REDD1. Cancer Res. 2011;71:4366–4372. doi: 10.1158/0008-5472.CAN-10-1769. [DOI] [PubMed] [Google Scholar]
- 134.Kalender A., Selvaraj A., Kim S.Y., Gulati P., Brûlé S., Viollet B., Kemp B.E., Bardeesy N., Dennis P., Schlager J.J., et al. Metformin, Independent of AMPK, Inhibits MTORC1 in a Rag GTPase-Dependent Manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim E., Goraksha-Hicks P., Li L., Neufeld T.P., Guan K.L. Regulation of TORC1 by Rag GTPases in Nutrient Response. Nat. Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sancak Y., Peterson T.R., Shaul Y.D., Lindquist R.A., Thoreen C.C., Bar-Peled L., Sabatini D.M. The Rag GTPases Bind Raptor and Mediate Amino Acid Signaling to MTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Celestini V., Tezil T., Russo L., Fasano C., Sanese P., Forte G., Peserico A., Lepore Signorile M., Longo G., De Rasmo D., et al. Uncoupling FoxO3A Mitochondrial and Nuclear Functions in Cancer Cells Undergoing Metabolic Stress and Chemotherapy Article. Cell Death Dis. 2018;9:1–20. doi: 10.1038/s41419-018-0336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Grossi V., Fasano C., Celestini V., Signorile M.L., Sanese P., Simone C. Chasing the Foxo3: Insights into Its New Mitochondrial Lair in Colorectal Cancer Landscape. Cancers. 2019;11:414. doi: 10.3390/cancers11030414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cha J.H., Yang W.H., Xia W., Wei Y., Chan L.C., Lim S.O., Li C.W., Kim T., Chang S.S., Lee H.H., et al. Metformin Promotes Antitumor Immunity via Endoplasmic-Reticulum-Associated Degradation of PD-L1. Mol. Cell. 2018;71:606–620. doi: 10.1016/j.molcel.2018.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xue J., Li L., Li N., Li F., Qin X., Li T., Liu M. Metformin Suppresses Cancer Cell Growth in Endometrial Carcinoma by Inhibiting PD-L1. Eur. J. Pharmacol. 2019;859:172541. doi: 10.1016/j.ejphar.2019.172541. [DOI] [PubMed] [Google Scholar]
- 141.Munoz L.E., Huang L., Bommireddy R., Sharma R., Monterroza L., Guin R.N., Samaranayake S.G., Pack C.D., Ramachandiran S., Reddy S.J.C., et al. Metformin Reduces PD-L1 on Tumor Cells and Enhances the Anti-Tumor Immune Response Generated by Vaccine Immunotherapy. J. ImmunoTherapy Cancer. 2021;9:e002614. doi: 10.1136/jitc-2021-002614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lu Y., Xin D., Guan L., Xu M., Yang Y., Chen Y., Yang Y., Wang-Gillam A., Wang L., Zong S., et al. Metformin Downregulates PD-L1 Expression in Esophageal Squamous Cell Catrcinoma by Inhibiting IL-6 Signaling Pathway. Front Oncol. 2021;11:762523. doi: 10.3389/fonc.2021.762523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang J.-J., Zhang Q.-S., Li Z.-Q., Zhou J.-W., Du J. Metformin Attenuates PD-L1 Expression through Activating Hippo Signaling Pathway in Colorectal Cancer Cells. Am. J. Transl. Res. 2019;11:6965. [PMC free article] [PubMed] [Google Scholar]
- 144.Shen X., Zhao Y., Liu G., Zhou H.-L., Fan J., Zhang L., Li Y.-L., Wang Y., Liang J., Xu Z.-X. Upregulation of Programmed Death Ligand 1 by Liver Kinase B1 and Its Implication in Programmed Death 1 Blockade Therapy in Non-Small Cell Lung Cancer. Life Sci. 2020;256:117923. doi: 10.1016/j.lfs.2020.117923. [DOI] [PubMed] [Google Scholar]
- 145.Xu P., Yin K., Tang X., Tian J., Zhang Y., Ma J., Xu H., Xu Q., Wang S. Metformin Inhibits the Function of Granulocytic Myeloid-Derived Suppressor Cells in Tumor-Bearing Mice. Biomed. Pharmacother. 2019;120:108800. doi: 10.1016/j.biopha.2019.109458. [DOI] [PubMed] [Google Scholar]
- 146.Nishida M., Yamashita N., Ogawa T., Koseki K., Warabi E., Ohue T., Komatsu M., Matsushita H., Kakimi K., Kawakami E., et al. Mitochondrial Reactive Oxygen Species Trigger Metformin-Dependent Antitumor Immunity via Activation of Nrf2/MTORC1/P62 Axis in Tumor-Infiltrating CD8T Lymphocytes. J. ImmunoTherapy Cancer. 2021;9:e002954. doi: 10.1136/jitc-2021-002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Taghipour F., Oladpour O., Rezayati M.T., Khorramdelazad H., Nemati M., Taghipour Z., Masoumi J., Hassan Z.M., Jafarzadeh A. Modulatory Effects of Metformin Alone and in Combination with Cimetidine and Ibuprofen on T Cell-Related Parameters in a Breast Cancer Model. Iran J. Allergy Asthma Immunol. 2021;20:600. doi: 10.18502/ijaai.v20i5.7410. [DOI] [PubMed] [Google Scholar]
- 148.Veeramachaneni R., Yu W., Newton J.M., Kemnade J.O., Skinner H.D., Sikora A.G., Sandulache V.C. Metformin Generates Profound Alterations in Systemic and Tumor Immunity with Associated Antitumor Effects. J. ImmunoTherapy Cancer. 2021;9 doi: 10.1136/jitc-2021-002773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mu Q., Jiang M., Zhang Y., Wu F., Li H., Zhang W., Wang F., Liu J., Li L., Wang D., et al. Metformin Inhibits Proliferation and Cytotoxicity and Induces Apoptosis via AMPK Pathway in CD19-Chimeric Antigen Receptor-Modified T Cells. OncoTargets Ther. 2018;11:1767. doi: 10.2147/OTT.S154853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xia W., Qi X., Li M., Wu Y., Sun L., Fan X., Yuan Y., Li J. Metformin Promotes Anticancer Activity of NK Cells in a P38 MAPK Dependent Manner. OncoImmunology. 2021;10:1995999. doi: 10.1080/2162402X.2021.1995999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chiang C.F., Chao T.T., Su Y.F., Hsu C.C., Chien C.Y., Chiu K.C., Shiah S.G., Lee C.H., Liu S.Y., Shieh Y.S. Metformin-Treated Cancer Cells Modulate Macrophage Polarization through AMPK-NF-ΚB Signaling. Oncotarget. 2017;8:20706. doi: 10.18632/oncotarget.14982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu Q., Tong D., Liu G., Gao J., Wang L.A., Xu J., Yang X., Xie Q., Huang Y., Pang J., et al. Metformin Inhibits Prostate Cancer Progression by Targeting Tumor-Associated Inflammatory Infiltration. Clin. Cancer Res. 2018;24:5622–5634. doi: 10.1158/1078-0432.CCR-18-0420. [DOI] [PubMed] [Google Scholar]
- 153.Pei H., Guo W., Peng Y., Xiong H., Chen Y. Targeting Key Proteins Involved in Transcriptional Regulation for Cancer Therapy: Current Strategies and Future Prospective. Med. Res. Rev. 2022 doi: 10.1002/med.21886. [DOI] [PubMed] [Google Scholar]
- 154.Zhong T., Men Y., Lu L., Geng T., Zhou J., Mitsuhashi A., Shozu M., Maihle N.J., Carmichael G.G., Taylor H.S., et al. Metformin Alters DNA Methylation Genome-Wide via the H19/SAHH Axis. Oncogene. 2017;36:2345–2354. doi: 10.1038/onc.2016.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cuyàs E., Fernández-Arroyo S., Verdura S., García R.Á.-F., Stursa J., Werner L., Blanco-González E., Montes-Bayón M., Joven J., Viollet B., et al. Metformin Regulates Global DNA Methylation via Mitochondrial One-Carbon Metabolism. Oncogene. 2018;37:963–970. doi: 10.1038/onc.2017.367. [DOI] [PubMed] [Google Scholar]
- 156.Davies G., Lobanova L., Dawicki W., Groot G., Gordon J.R., Bowen M., Harkness T., Arnason T. Metformin Inhibits the Development, and Promotes the Resensitization, of Treatment-Resistant Breast Cancer. PLoS ONE. 2017;12:e0187191. doi: 10.1371/journal.pone.0187191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu P., Tang Y., Fang X., Xie C., Zeng J., Wang W., Zhao S. Metformin Suppresses Hypopharyngeal Cancer Growth by Epigenetically Silencing Long Non-Coding RNA SNHG7 in FaDu Cells. Front. Pharmacol. 2019;10:143. doi: 10.3389/fphar.2019.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li K., Gao S., Ma L., Sun Y., Peng Z.-Y., Wu J., Du N., Ren H., Tang S.-C., Sun X. Stimulation of Let-7 Maturation by Metformin Improved the Response to Tyrosine Kinase Inhibitor Therapy in an M6A Dependent Manner. Front. Oncol. 2021;11:731561. doi: 10.3389/fonc.2021.731561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Oh S., Cho Y.U., Chang M., Park S., Kwon H. Metformin Decreases 2-HG Production through the MYC-PHGDH Pathway in Suppressing Breast Cancer Cell Proliferation. Metabolites. 2021;11:480. doi: 10.3390/metabo11080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bai M., Yang L., Liao H., Liang X., Xie B., Xiong J., Tao X., Chen X., Cheng Y., Chen X., et al. Metformin Sensitizes Endometrial Cancer Cells to Chemotherapy through IDH1-Induced Nrf2 Expression via an Epigenetic Mechanism. Oncogene. 2018;37:5666–5681. doi: 10.1038/s41388-018-0360-7. [DOI] [PubMed] [Google Scholar]
- 161.Tyagi M., Cheema M.S., Dryhurst D., Eskiw C.H., Ausió J. Metformin Alters H2A.Z Dynamics and Regulates Androgen Dependent Prostate Cancer Progression. Oncotarget. 2018;9:37054. doi: 10.18632/oncotarget.26457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Barazeghi E., Hellman P., Norlén O., Westin G., Stålberg P. EZH2 Presents a Therapeutic Target for Neuroendocrine Tumors of the Small Intestine. Sci. Rep. 2021;11:1–13. doi: 10.1038/s41598-021-02181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li K., Zhang T.T., Wang F., Cui B., Zhao C.X., Yu J.J., Lv X.X., Zhang X.W., Yang Z.N., Huang B., et al. Metformin Suppresses Melanoma Progression by Inhibiting KAT5-Mediated SMAD3 Acetylation, Transcriptional Activity and TRIB3 Expression. Oncogene. 2018;37:2967–2981. doi: 10.1038/s41388-018-0172-9. [DOI] [PubMed] [Google Scholar]
- 164.Carmignani M., Volpe A.R., Aldea M., Soritau O., Irimie A., Florian I.S., Tomuleasa C., Baritchii A., Petrushev B., Crisan G., et al. Glioblastoma Stem Cells: A New Target for Metformin and Arsenic Trioxide. J. Biol. Regul. Homeost. Agents. 2014;28:1–15. [PubMed] [Google Scholar]
- 165.Saito A., Kitayama J., Horie H., Koinuma K., Ohzawa H., Yamaguchi H., Kawahira H., Mimura T., Lefor A.K., Sata N. Metformin Changes the Immune Microenvironment of Colorectal Cancer in Patients with Type 2 Diabetes Mellitus. Cancer Sci. 2020;111:4012–4020. doi: 10.1111/cas.14615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Belfiore A., Frasca F. IGF and Insulin Receptor Signaling in Breast Cancer. J. Mammary Gland. Biol. Neoplasia. 2008;13:381–406. doi: 10.1007/s10911-008-9099-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.