Abstract
In spite of the environmental and commercial interests in the bacterial leaching of pyrite, two central questions have not been answered after more than 35 years of research: does Thiobacillus ferrooxidans enhance the rate of leaching above that achieved by ferric sulfate solutions under the same conditions, and if so, how do the bacteria affect such an enhancement? Experimental conditions of previous studies were such that the concentrations of ferric and ferrous ions changed substantially throughout the course of the experiments. This has made it difficult to interpret the data obtained from these previous works. The aim of this work was to answer these two questions by employing an experimental apparatus designed to maintain the concentrations in solution at a constant value. This was achieved by using the constant redox potential apparatus described previously (P. I. Harvey, and F. K. Crundwell, Appl. Environ. Microbiol. 63:2586–2592, 1997; T. A. Fowler, and F. K. Crundwell, Appl. Environ. Microbiol. 64:3570–3575, 1998). Experiments were conducted in both the presence and absence of T. ferrooxidans, maintaining the same conditions in solution. The rate of dissolution of pyrite with bacteria was higher than that without bacteria at the same concentrations of ferrous and ferric ions in solution. Analysis of the dependence of the rate of leaching on the concentration of ferric ions and on the pH, together with results obtained from electrochemical measurements, provided clear evidence that the higher rate of leaching with bacteria is due to the bacteria increasing the pH at the surface of the pyrite.
The oxidation of pyrite (FeS2) in the presence of Thiobacillus ferrooxidans is a significant factor in the formation of acid mine drainage, an environmental problem of considerable concern (7). However, T. ferrooxidans is used in commercial processes to extract gold from pyrite and arsenopyrite. The processing plant at Sansu, Ghana, treats more than 960 tons of pyrite concentrate per day with bacteria, while other processing plants have been commissioned in Australia, Brazil, and South Africa (5).
The mechanisms of bacterial interaction with pyrite are the subject of much debate and controversy, despite the commercial and environmental interests in the process. It is well known that pyrite is dissolved by ferric ions, forming ferrous ions, and that T. ferrooxidans catalyzes the oxidation of ferrous ions to regenerate the ferric ions. This set of reactions is as follows (24):
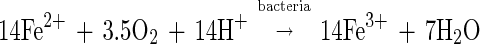 |
1 |
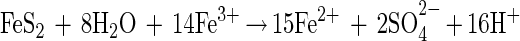 |
2 |
In this set of reactions, the only role of the bacteria is to regenerate the ferric ions that are consumed in the oxidation of pyrite. In this mechanism, the rate of dissolution of pyrite is dependent on the concentrations in solution, in particular the concentrations of ferric and ferrous ions, and the pH (15, 30). In this mechanism, the bacteria do not directly affect the rate of dissolution of pyrite.
However, in 1964, Silverman and Ehrlich (24) proposed that this is not the only role that is played by T. ferrooxidans. They suggested that T. ferrooxidans enhances the rate of pyrite oxidation above that achieved by chemical reaction with ferric ions under the same conditions. They proposed that the bacteria interacted directly with the mineral, possibly by the extracellular secretion of an enzyme or by oxidation with an enzyme specific to sulfide minerals present on the cell wall. The mechanism of Silverman and Ehrlich (24) includes the following reaction:
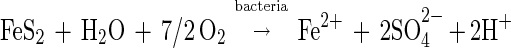 |
3 |
together with the set of reactions shown in equations 1 and 2.
Since this proposal, different authors have made opposing claims concerning the role of T. ferrooxidans in the leaching of pyrite (1–4, 6, 10, 18, 19, 21, 28). A common feature of previous work is that the concentrations of ferric and ferrous ions were substantially different in the experiments with bacteria and those without bacteria (8). This makes the resolution of this controversy very difficult and renders many of the previous claims debatable.
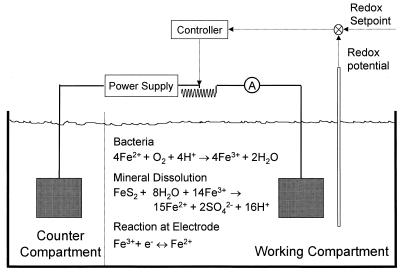
Members of our group designed a novel experiment to overcome this problem (8, 11). The concentrations of ferrous and ferric ions in solution were maintained at the initial value for the duration of the experiment. Therefore, experiments could be conducted with and without bacteria under the same solution conditions. The experimental apparatus was an electrolytic cell separated into two compartments by an ion-exchange membrane. The leaching experiment was performed in one compartment. The redox potential, which is a direct measure of the concentrations of ferrous and ferric ions in solution, was maintained at a constant value by the automatic manipulation of the electrolytic current to the cell. The apparatus is shown in Fig. 1. In principle, this reactor is a fed-batch reactor in which the concentration of substrate is replenished by the electrolytic current.
FIG. 1.
Schematic diagram of the experimental apparatus. Leaching experiments are conducted in the working compartment. The flow of current is regulated by adjusting the variable resistor so that the redox potential remains at the set-point value.
Since the solution conditions were controlled throughout the course of the experiment, the effect of T. ferrooxidans on the dissolution reaction could be determined directly by comparing the extents of dissolution of pyrite with and without bacteria.
Previous experiments using this apparatus have shown that the rate of growth of T. ferrooxidans is unaffected by the small electrolytic current (11) and that the only role of T. ferrooxidans in the dissolution of sphalerite (ZnS) is the oxidation of ferrous ions under the conditions of those experiments (8).
Therefore, in spite of much interest in the leaching of pyrite, two questions central to the debate on the mechanism of the bacterial interaction with pyrite remain unanswered: does T. ferrooxidans enhance the rate of dissolution of pyrite above that achieved by chemical reaction with ferric sulfate at the same concentrations in solution, and if so, by what mechanism does this occur? In this paper, we report the results of highly controlled experiments performed with and without bacteria under the same solution conditions that clearly answer these questions.
MATERIALS AND METHODS
Apparatus.
The electrolysis cell was made of Plexiglas and was divided into two sections by an anion-exchange membrane (Sybron Chemicals Inc., Birmingham, N.J.). The electrolysis cell was fitted with a Plexiglas lid to minimize evaporation of the solution. The working volume of the cell was 2 liters. The contents of the working compartment were stirred by a three-bladed impeller driven by an overhead motor, and the compartment was sparged with air. Electrodes for measuring the redox potential and the concentration of oxygen were suspended in the solution in the working compartment.
Redox potential measurements were made with a platinum electrode and a Ag/AgCl reference electrode by using a high-impedance galvanically isolated differential amplifier and an analog digital control card (type PC30; Eagle Technology, Cape Town, South Africa) recorded by computer. A computer program determined the values of the output signals from the PC30 card to the relay switch and the variable resistor, which varied the direction and magnitude of the current. The current was measured by the potential difference across a precision resistor.
The redox potential was controlled within 1.0 mV (0.1%) of the set point for the duration of the experiment. All the solution samples were analyzed for ferrous ions in order to confirm that the control of the redox potential maintained the concentration of ferrous ions at a constant value. Typical results showed that the concentration of the ferrous ions differed from the initial value by less than 0.025 g/liter (2.5%) over the course of the 100-h experiments.
The pH was measured throughout each experiment and maintained to within 0.05 pH unit of the initial value by the manual addition of 0.5 M sodium hydroxide solution or 98% sulfuric acid. The concentration of dissolved oxygen was measured (apparatus from Hanna Instruments) and kept constant at 5.9 mg/liter. The electrolysis cell was placed in a water bath, and the temperature was maintained at 35 ± 0.1°C.
Bacterial culture.
A pure strain of T. ferrooxidans (FC1) was used. This organism, which was supplied by D. Rawlings of the University of Cape Town, Cape Town, South Africa, has been thoroughly characterized (20). The bacteria were cultured on a medium which contained (per liter) 1.5 g of (NH4)2SO4, 0.5 g of K2HPO4, 0.5 g of MgSO4 · 7H2O, and 45 g of FeSO4 · 7H2O (25). The pH of the medium was adjusted by adding H2SO4. The bacteria were maintained in the exponential growth phase by subculturing a third of the culture volume on a daily basis. The leaching of zinc sulfide at high concentrations of ferrous ions in solution showed that the bacteria oxidized elemental sulfur within 10 h (9). These results showed that the capacity of the bacteria for the oxidation of sulfur was not affected by maintaining them on ferrous sulfate.
Preparation and characterization of the ore.
The pyrite concentrate, from the Kasese deposit in Uganda, was supplied by D. Morin of Bureau de Recherches Géologiques et Minières (BGRM), Orleans, France. This sample was milled and wet-screened to a size fraction of −75 + 63 μm. The ore contained 1.4% Co and 41.8% Fe. Powder X-ray diffraction indicated that the sample contained no mineral phases other than pyrite. The cobalt in the pyrite was evenly distributed throughout the particle. Cobalt substitutes for iron in the crystal structure. The amount of iron (in milligrams) dissolved from the pyrite in aqua regia was given by the following: Fe = 29.9 Co (9 points; R2 = 0.999), where Co is the amount (in milligrams) of Co dissolved (0.05 − 3.0 mg/liter). The direct proportionality between the cobalt and the iron dissolved from the pyrite indicates that the cobalt is a suitable tracer for determining the amount of iron released into solution by pyrite dissolution in a high background concentration of iron. This enabled the design of experimental conditions in which the total concentration of dissolved iron changed by less than 1.0% over the duration of the experiment (100 h).
Reagents.
Analytical-grade reagents were used throughout this work.
Analytical techniques.
The bacterial cell number in solution was determined by counting with a hemacytometer (depth, 0.1 mm; area, 0.0025 mm2). The cells were stained with crystal violet in a citric acid solution. The standard deviation for the cell number was 1.2% of the mean (10 replicates).
The concentration of ferrous ions in solution was determined by titration with potassium dichromate by using sodium diphenylamine sulfonate as the indicator (29). The standard deviation for the determination of the concentration of ferrous ions was 1.1% of the mean (10 replicates). The total concentration of iron in solution was determined by using the titration for ferrous ions once the iron had been reduced to the ferrous state with stannous chloride. The concentration of ferric ions was calculated by determining the difference. (Note that measurements of the redox potential were used for control purposes only; they were not used for chemical analysis.)
The concentration of cobalt in solution was analyzed by atomic absorption spectrophotometry (Varian Spectra AA30 spectrophotometer). The standard deviation for the determination of the concentration of cobalt in solution was 0.2% of the mean (10 replicates).
Procedure.
All experiments were conducted in the same medium but with different concentrations of ferric ions and different pH values. The concentration of ferrous ions was 1.00 g/liter for all experiments. The loading concentration for solids was 10.00 g of pyrite per liter. The preparations for bacterial leaching experiments were inoculated with inocula equivalent to 10% (by volume) of the reactor size. Samples were drawn from the working compartment at the same time intervals for every leaching experiment performed.
A part of each sample was used to determine the bacterial cell population in suspension. The remaining part of each sample was immediately filtered with a Millipore filter (Sterifil aseptic system with a sterile, individually sealed 0.45-μm-pore-size filter membrane). The filtrate was used to determine the concentrations of ferrous, ferric, and cobalt ions in solution. The solid residues on the filter paper were immersed in a 95% solution of ethanol to fix the bacteria (in bacterial leaching experiments) on the surfaces of the mineral particles. These were critical point dried and coated for investigation with a scanning electron microscope. Samples were withdrawn at regular intervals from the countercompartment. These were also analyzed to determine the concentrations of iron and cobalt ions in solution in the countercompartment. Analyses revealed that neither iron nor cobalt was transferred from the working compartment to the countercompartment through the anion-exchange membrane.
The solution samples obtained from the sterile (chemical leaching) experiments were checked for contamination by T. ferrooxidans. No bacteria were detected by microscopic investigation. Between each successive experiment, the electrolysis cell was soaked in hydrochloric acid, rinsed with water, cleaned with an ammonia-based solution (pH 10), and finally rinsed with distilled water.
RESULTS
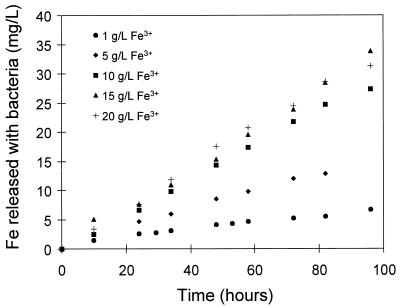
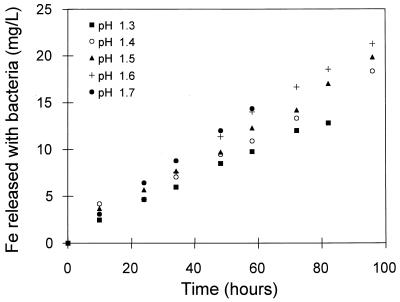
The results for the dissolution of pyrite with bacteria at controlled redox potentials are shown in Fig. 2 and 3. The rate of dissolution of pyrite in the presence of bacteria increases with increases in the concentration of ferric ions and the pH (Fig. 2 and 3). These effects of the concentration of the ferric ions and the pH are similar to those reported for the dissolution of pyrite without bacteria (15, 30).
FIG. 2.
Effect of the concentration of ferric ions in solution on the bacterial leaching of pyrite. The solution conditions were as follows: 10% (vol/vol) T. ferrooxidans inoculum; Fe2+ concentration, 1.0 g/liter; density of solids, 10 g/liter; temperature, 35°C; pH 1.3; O2 concentration, 5.9 mg/liter; and redox potential range, 540 to 467 mV (versus Ag/AgCl).
FIG. 3.
Effect of the pH of the solution on the bacterial leaching of pyrite. The solution conditions were as follows: 10% (vol/vol) T. ferrooxidans inoculum; Fe3+ concentration, 5.0 g/liter; Fe2+ concentration, 1.0 g/liter; density of solids, 10 g/liter; temperature, 35°C; O2 concentration, 5.9 mg/liter; and redox potential range, 506 to 502 mV (versus Ag/AgCl).
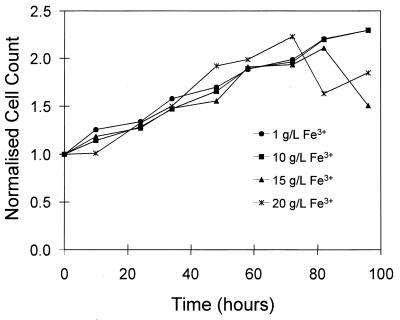
Figure 4 is a plot of the normalized number of bacteria in suspension for four of the experiments shown in Fig. 2. The normalized bacterial cell number is the bacterial cell count divided by the initial bacterial count. The number of bacteria in solution increased steadily for the first 80 h of the experiment. In two of the experiments, the number dropped after 80 h, probably due to attachment to the pyrite particles. The number of bacteria in suspension was an underestimate of the total number of bacteria present, since bacteria attach to the surfaces of the pyrite particles.
FIG. 4.
Effect of the concentration of the ferric ions in solution on the normalized bacterial cell number in suspension. The initial cell numbers were as follows: 3.1 × 108 cells/ml (1 g/liter), 3.6 × 108 cells/ml (10 g/liter), 3.6 × 108 cells/ml (15 g/liter), and 3.4 × 108 cells/ml (20 g/liter).
The bacteria on the surface were examined by a scanning electron microscope. Samples taken after 10 h of leaching revealed the presence of individually attached bacteria on the mineral surfaces. At longer reaction times, the mineral surfaces were covered with large amounts of extracellular polymeric substances and bacteria, indicating that significant numbers of bacteria were attached to the surfaces.
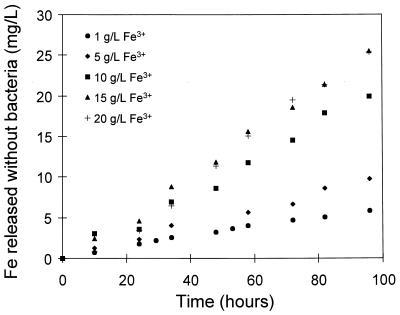
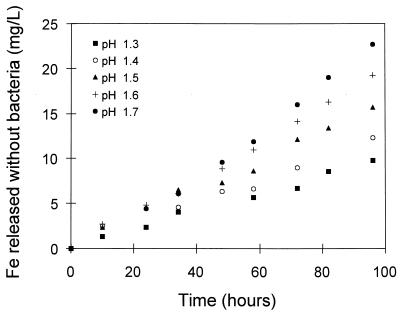
The effects of the concentration of ferric ions and the pH on the rate of the dissolution of pyrite without bacteria are shown in Fig. 5 and 6. These results are similar to those presented in Fig. 2 and 3 with respect to the effects of ferric ions and pH. However, it is clear that the rate of dissolution obtained with bacteria is higher than that obtained without bacteria.
FIG. 5.
Effect of the concentration of ferric ions in solution on the chemical leaching of pyrite. The solution conditions were as follows: Fe2+ concentration, 1.0 g/liter; density of solids, 10 g/liter; temperature, 35°C; pH 1.3; and O2 concentration, 5.9 mg/liter.
FIG. 6.
Effect of the pH of the solution on the chemical leaching of pyrite. The solution conditions were as follows: Fe3+ concentration, 5.0 g/liter; Fe2+ concentration, 1.0 g/liter; density of solids, 10 g/liter; temperature, 35°C; and O2 concentration, 5.9 mg/liter.
Dissolution reactions are dependent on the amount of surface area that is available. However, as dissolution proceeds, both the particle size and the surface area are reduced. The shrinking-particle theory (14) accounts for this change in size. This theory predicts that if the concentration of the reactant in solution is constant for the duration of the reaction and if the particles are of uniform size, then the conversion, X, is given by the following (14):
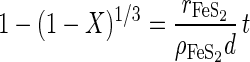 |
4 |
where rFeS2 is the intrinsic rate of dissolution (in moles per square meter per minute), d is the particle size (68.7 × 10−6 m), ρFeS2 is the molar density (41,900 mol/m3), and t is the reaction time (in minutes). The conversion is the amount of pyrite dissolved divided by the total amount of pyrite initially in the reactor.
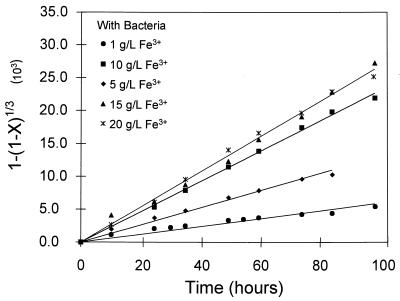
The leaching experiments presented in this study meet the criteria for the application of equation 4, namely, that the concentrations in solution are constant and that the particles are of uniform size. Equation 4 indicates that if the shrinking-particle theory holds true, then a plot of 1 − (1 − X)1/3 against t should be a straight line through the origin with a slope proportional to the intrinsic rate of dissolution. Such plots are shown in Fig. 7 and 8. These plots are straight lines through the origin, indicating that reactions both with and without bacteria result in a uniform decrease in particle size. The rate of reaction, rFeS2, was evaluated from the slope of the lines in Fig. 7 and 8. The rate of leaching of the cobalt-containing pyrite without bacteria was between two and five times higher than that obtained by Williamson and Rimstidt (30) for the chemical leaching of pyrite from three different sources.
FIG. 7.
Plot of 1 − (1 − X)1/3 versus the time needed to determine the rate of dissolution from the shrinking-particle model for the effect of the concentration of ferric ions in the presence of bacteria. The conversion, X, is calculated from the data presented in Fig. 2.
FIG. 8.
Plot of 1 − (1 − X)1/3 versus the time needed to determine the rate of dissolution from the shrinking-particle model for the effect of the concentration of ferric ions in the absence of bacteria. The conversion, X, is calculated from the data presented in Fig. 5.
DISCUSSION
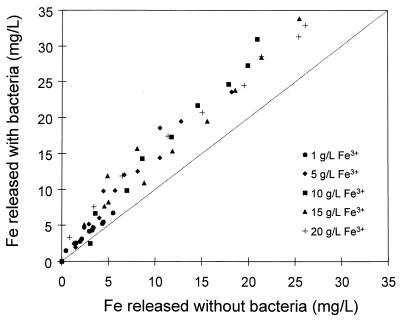
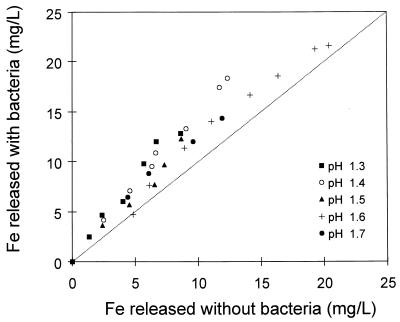
Since the solution conditions were the same in the experiments with and without bacteria, the effect of the bacteria on the dissolution of pyrite could be determined directly by comparing the amounts of iron released with and without bacteria. Figure 9 compares the amounts of iron dissolved with and without bacteria for leaching experiments conducted at various concentrations of ferric ions. These results clearly show that the presence of bacteria increases the rate of dissolution of pyrite. Figure 10 compares the amounts of iron dissolved with and without bacteria for experiments conducted at different pH values. The rate of dissolution of pyrite is enhanced by the bacteria above that achieved by chemical dissolution under the same solution conditions.
FIG. 9.
Comparison of the conversion of pyrite obtained in a bacterial leaching experiment and the conversion of pyrite obtained in a chemical leaching experiment at different concentrations of ferric ions. The same solution conditions were used for both experiments.
FIG. 10.
Comparison of the conversion of pyrite obtained in a bacterial leaching experiment and the conversion of pyrite obtained in a chemical leaching experiment at different pH values. The same solution conditions were used for both experiments.
The results in Fig. 9 indicate that the degree to which the bacteria enhance the rate of leaching is not dependent on the concentration of ferric ions. However, the results shown in Fig. 10 indicate that as the pH of the solution is decreased, the bacteria have a greater effect on the rate of dissolution of pyrite.
This is the first work that has answered the first of the long-standing questions that arose from the proposal made by Silverman and Ehrlich (24). However, these results were unexpected in the light of our previous findings on sphalerite (8). In that study, we found that the presence of bacteria does not increase the rate of dissolution of sphalerite. In order to explore the reasons for the increase in the rate of leaching of pyrite in the presence of bacteria, we analyzed the dependence of the rate of dissolution on the concentration of ferric ions and on the pH.
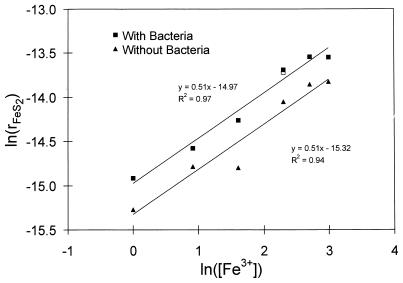
The rate of reaction was evaluated by using the shrinking-particle theory discussed above. The dependence of the rate of dissolution on the concentration of ferric ions and on the pH is shown in Fig. 11 and 12. The results shown in Fig. 11 emphasize that the degree to which the bacteria increase the rate of dissolution is not affected by the concentration of ferric ions. However, the results shown in Fig. 12 indicate that the degree of enhancement of the rate of dissolution in the presence of bacteria is affected by the pH. The order of reaction with respect to ferric ions is 0.51 both with bacteria and without bacteria. The order of reaction with respect to H+ is −0.39 with bacteria, and it is −0.50 without bacteria. The results shown in Fig. 12 suggest that rates of dissolution of pyrite with and without bacteria would be equal at a pH of approximately 2.1.
FIG. 11.
Plot of the natural logarithm of the rate of dissolution against the natural logarithm of ferric ion concentration to determine the order of reaction with respect to Fe3+.
FIG. 12.
Plot of the logarithm (log10) of the rate of dissolution against the pH of the solution to determine the order of reaction with respect to H+. (Note that pH = −log10 [H+].)
The dissolution of pyrite is dependent on the concentrations of ferric and ferrous ions and on the pH (15, 30). The orders of reaction found here agree with those published for the chemical leaching of pyrite. For example, McKibben and Barnes (15) obtained orders of reaction of 0.5 and −0.5 with respect to ferric ions and H+, respectively.
A significant amount of research has shown that the rate of reaction of mineral sulfides is controlled by the electrochemistry occurring at the mineral-solution interface (17). The mixed-potential model of leaching assumes that the charge transfer processes occurring at the mineral surface are those that control the rate of dissolution (16). An expression for the rate of the dissolution of pyrite can be derived from the mixed-potential model of leaching (13, 16). This expression is given by the following equation (13):
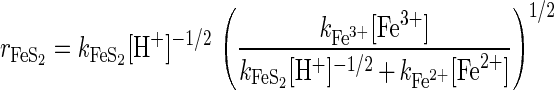 |
5 |
where rFeS2 is the rate of dissolution (in moles per square meter per second) and k is the rate constant.
Equation 5 can be reconciled with the empirical expressions found by McKibben and Barnes (15) and Williamson and Rimstidt (30). The rate of dissolution increases with increasing concentrations of ferric ions and increasing values of pH. The order of reaction with respect to ferric ions predicted by equation 5 is 0.5, which is in agreement with the results for the leaching experiments both with and without bacteria.
If kFeS2[H+]−1/2 ≪ kFe2+[Fe2+], that is, at relatively low values of pH and high concentrations of ferrous ions, the order of reaction with respect to H+ predicted by equation 5 is −0.5. This is in agreement with the results obtained for the leaching experiments without bacteria. As the relative contribution of the term kFeS2[H+]−1/2 increases (the pH increases or the concentration of ferrous ions decreases), the order of reaction with respect to H+ increases. In the limit that kFeS2[H+]−1/2 ≫ kFe2+[Fe2+], the order of reaction with respect to H+ is −0.25. The order of reaction with respect to H+ in the experiments with bacteria is −0.39, in agreement with equation 5.
Thus, the electrochemical mechanism describes the kinetics of dissolution reported here and in the literature (14, 26). Schippers and Sand (22) recently presented a mechanism, derived from work by Stuedel (26) on the homogeneous catalytic oxidation of H2S, that argues that the formation of various sulfur compounds controls the rate of dissolution. However, since their mechanism does not explain the kinetics of dissolution, they have not successfully identified the rate-determining process. Thus, while the reactions they propose may occur, they do not influence the rate of dissolution.
The electrochemical analysis presented above suggests that the mechanism by which bacteria increase the rate of reaction is by increasing the relative contribution of the term kFeS2[H+]−1/2 in equation 5. This may be achieved by increasing the pH or by decreasing the concentration of ferrous ions at the surfaces of the mineral particles. The difference in order of reaction with respect to the pH values shown in Fig. 12 suggests that the bacteria attach to the surface of the pyrite and create a local environment which has a higher pH value than that of the bulk solution. Since the rate of leaching of pyrite is dependent on the pH, this change in the pH of the local environment at the surface is sufficient to increase the rate of leaching. Figure 12 also suggests that the pH of the local environment at the surface is approximately 2.1.
In order to further explore the factor responsible for the increase in the rate of dissolution, experiments were conducted in which the mixed potential of a pyrite electrode was measured in solutions at a constant redox potential of 600 mV versus Ag/AgCl (12, 13). At this value of redox potential, the concentration of ferrous ions in solution was less than 0.01 g/liter. A change in the concentration of ferrous ions at this low concentration will not affect the rate of leaching.
The mixed potential of the electrode exposed to solutions without bacteria was constant for more than 7 days. The mixed potential of the electrode exposed to solutions with bacteria at a controlled redox potential decreased steadily over the 7-day period (13). A possible explanation for the increase in the rate of dissolution in the presence of bacteria shown in Fig. 9 and 10 is the secretion of an enzymatic oxidant or the presence of an enzyme on the cell wall that is capable of oxidizing the pyrite. However, these mechanisms should give rise to an increase in the mixed potential, which was not observed (13). For this reason, an explanation based on an oxidant produced by the bacteria was rejected.
The expression for the mixed potential, Em, corresponding to the rate given in equation 5 is as follows (13):
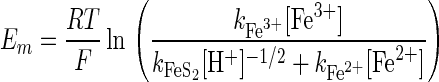 |
6 |
where R is the gas constant, T is the temperature, and F is the faraday constant.
Equations 5 and 6 indicate that an increase in the pH results in a decrease in the mixed potential and an increase in the rate of dissolution. This is the only possible explanation for both the leaching results and the mixed-potential results. Furthermore, this mechanism is consistent with the previous finding that the presence of T. ferrooxidans did not increase the rate of dissolution of sphalerite (8), since the dissolution of sphalerite by ferric ions is not dependent on the pH of the solution in this range of pH values (23, 27).
The pH at the surface may be raised by the bacterial consumption of H+ in the oxidation of ferrous ions or by the pH buffering action of the exopolysaccharides deposited on the pyrite particles by the bacteria. Further work is required to determine how the bacteria achieve this.
We have presented unique data which unambiguously shows that T. ferrooxidans enhances the rate of leaching of pyrite above that achieved without bacteria under the same solution conditions. This is the first data to be presented in which the experiments with and without bacteria were performed under the same conditions in solution. The analysis of this data and the electrochemical results (13) show that the only explanation for this phenomenon is that the bacteria increase the pH at the mineral surface. This explanation means that attached bacteria enhance the rate of leaching, but not by the action of a biological or enzymatic oxidant. Thus, this work has answered both of the long-standing questions concerning the bacterial leaching of pyrite.
ACKNOWLEDGMENTS
We thank Billiton Process Research and the Foundation for Research Development for funding this project.
We also thank D. Rawlings (University of Cape Town) and E. Lawson (University of the Witwatersrand, Johannesburg, South Africa) for supplying the bacterial culture and valuable assistance. We are grateful to D. Morin (BRGM) for supplying the pyrite sample.
REFERENCES
- 1.Boon M, Heijnen J J. Chemical oxidation kinetics of pyrite in bioleaching processes. Hydrometallurgy. 1998;48:27–41. [Google Scholar]
- 2.Boon M, Snijder M, Hansford G S, Heijnen J J. The oxidation kinetics of zinc sulphide with Thiobacillus ferrooxidans. Hydrometallurgy. 1998;48:171–186. [Google Scholar]
- 3.Choi W K, Torma A E, Ohline R W, Ghali E. Electrochemical aspects of zinc sulphide leaching by Thiobacillus ferrooxidans. Hydrometallurgy. 1993;33:137–152. [Google Scholar]
- 4.Corrans I J, Harris B, Ralph B J. Bacterial leaching: an introduction to its application and theory and a study on its mechanism of operation. J S Afr Inst Mining Metallurgy. 1972;72:221–230. [Google Scholar]
- 5.Dew D W, Lawson E N, Broadhurst J L. The BIOX® process for biooxidation of gold-bearing ore or concentrates. In: Rawlings D E, editor. Biomining: theory, microbes and industrial processes. Berlin, Germany: Springer-Verlag; 1998. pp. 45–80. [Google Scholar]
- 6.Duncan D W, Landesman J, Walden C C. Role of Thiobacillus ferrooxidans in the oxidation of sulfide minerals. Can J Microbiol. 1967;13:397–403. doi: 10.1139/m67-052. [DOI] [PubMed] [Google Scholar]
- 7.Evangelou V P. Pyrite oxidation and its control: solution chemistry, surface chemistry, acid mine drainage (AMD), molecular oxidation mechanisms. Boca Raton, Fla: CRC Press; 1995. p. 1. [Google Scholar]
- 8.Fowler T A, Crundwell F K. Leaching of zinc sulfide by Thiobacillus ferrooxidans: experiments with a controlled redox potential indicate no direct bacterial mechanism. Appl Environ Microbiol. 1998;64:3570–3575. doi: 10.1128/aem.64.10.3570-3575.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fowler, T. A., and F. K. Crundwell. Unpublished data.
- 10.Free M L, Oolman T, Nagpal S, Dahlstroom D A. Bioleaching of sulphide ores—distinguishing between indirect and direct mechanisms. In: Smith R W, Misra M, editors. Mineral bioprocessing. Warrendale, Pa: Minerals, Metals and Materials Society; 1991. pp. 485–495. [Google Scholar]
- 11.Harvey P I, Crundwell F K. Growth of Thiobacillus ferrooxidans: a novel experimental design for batch growth and bacterial leaching studies. Appl Environ Microbiol. 1997;63:2586–2592. doi: 10.1128/aem.63.7.2586-2592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holmes P R. An electrochemical study of the anodic, oxidative and bacterial dissolution of pyrite. Ph.D. thesis. Johannesburg, South Africa: University of the Witwatersrand; 1998. [Google Scholar]
- 13.Holmes, P. R., T. A. Fowler, and F. K. Crundwell. The mechanism of bacterial action in the leaching of pyrite by Thiobacillus ferrooxidans: an electrochemical study. J. Electrochem. Soc., in press.
- 14.Levenspiel O. Chemical reaction engineering. 2nd ed. New York, N.Y: John Wiley and Sons; 1972. [Google Scholar]
- 15.McKibben M A, Barnes H L. Oxidation of pyrite in low temperature acidic solution: rate laws and surface textures. Geochim Cosmochim Acta. 1986;50:1509–1520. [Google Scholar]
- 16.Nicol M J, Needes C S R, Finkelstein N P. Electrochemical model for the leaching of uranium dioxide. In: Burkin A R, editor. Leaching and reduction in hydrometallurgy. London, United Kingdom: Institute of Mining and Metallurgy; 1975. pp. 1–11. [Google Scholar]
- 17.Nicol M J. Plenary lecture: the role of electrochemistry in hydrometallurgy. In: Hiskey J B, Warren G W, editors. Hydrometallurgy. Fundamentals, technology and innovation. Littleton, Colo: Society for Mining, Metallurgy and Exploration, Inc.; 1993. pp. 43–62. [Google Scholar]
- 18.Nyavor K, Egiebor N O, Fedorak P M. Bacteria oxidation of sulphides during acid mine drainage formation: a mechanistic study. In: Warren G W, editor. EPD Congress, 1996. Warrendale, Pa: Minerals, Metals and Materials Society; 1996. pp. 269–287. [Google Scholar]
- 19.Porro S, Ramirez S, Reche C, Curutchet G, Alonso-Romanowski S, Donati E. Bacterial attachment—its role in bioleaching processes. Process Biochem. 1997;32:573–578. [Google Scholar]
- 20.Rawlings D E. Restriction enzyme analysis of 16s rRNA genes for the rapid identification of Thiobacillus ferrooxidans, Thiobacillus thiooxidans and Leptospirillum ferrooxidans strains in leaching environments. In: Jerez C A, Vargas T, Toledo H, Wiertz J V, editors. Biohydrometallurgical processing. II. Santiago, Chile: University of Chile Press; 1995. pp. 9–17. [Google Scholar]
- 21.Sand W, Gerke T, Hallmann R, Schippers A. Sulfur chemistry, biofilm, and the (in)direct attack mechanism—a critical evaluation of bioleaching. Appl Microbiol Biotechnol. 1995;43:961–966. [Google Scholar]
- 22.Schippers A, Sand W. Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl Environ Microbiol. 1999;65:319–321. doi: 10.1128/aem.65.1.319-321.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott P D, Nicol M J. The kinetics of the leaching of zinc sulphide concentrates in acid solutions containing ferric sulphate. Report no. 1949. Randburg, South Africa: National Institute of Metallurgy; 1978. [Google Scholar]
- 24.Silverman M P, Ehrlich H L. Microbial formation and degradation of minerals. Adv Appl Microbiol. 1964;6:181–183. [Google Scholar]
- 25.Silverman M P, Lundgren D G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959;77:642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stuedel R. Mechanism for the formation of elemental sulfur from aqueous sulphide in chemical and microbiological desulfurization processes. Ind Eng Chem Res. 1996;35:1417–1423. [Google Scholar]
- 27.Verbaan B, Crundwell F K. An electrochemical model for the leaching of a sphalerite concentrate. Hydrometallurgy. 1986;16:345–359. [Google Scholar]
- 28.Verbaan B, Huberts R. An electrochemical study of the bacterial leaching of synthetic Ni3S2. Int J Miner Process. 1988;24:185–202. [Google Scholar]
- 29.Vogel A I. A textbook of quantitative inorganic analysis. 1962. p. 309. , 319. Longman, London, United Kingdom. [Google Scholar]
- 30.Williamson M A, Rimstidt J D. The kinetics and electrochemical rate-determining step of aqueous pyrite oxidation. Geochim Cosmochim Acta. 1994;58:5443–5454. [Google Scholar]