Abstract
Hydrophobic surfaces have aroused considerable attention because of their extensive potential applications. In this work, we developed a facile strategy for fabricating hydrophobic and anti-fouling surfaces on wood substrates. The modification was accomplished simply by immerging wood into the tetramethylcyclotetrasiloxane (D4H) modifier solution for 5 min. The D4H modified wood was characterized using Fourier transform infrared spectroscopy, X-ray photoelectron spectroscopy, scanning electron microscope, and energy dispersive spectrometer. The result shows that the D4H modified wood had good hydrophobicity, and the water contact angle of wood in the radial and cross sections reached 140.1° and 152°. In addition, the obtained hydrophobic wood surface also showed anti-fouling properties, UV resistance and could withstand the tape peel test and finger wiping.
Keywords: wood, hydrophobicity, surface modification, tetramethylcyclotetrasiloxane (D4H), anti-fouling
1. Introduction
Wood is the only renewable resource among the four internationally recognized raw materials. Due to its unique material properties and excellent environmental properties, wood is deeply loved by people [1,2,3,4]. However, wood has a rich pore structure and contains many hydrophilic hydroxyl groups [5,6,7]. Wood easily absorbs moisture in a humid environment. This can cause the wood to deform, discolor, rot and crack, thereby shortening the service life of the wood [8,9,10]. Therefore, in order to prolong the service life of wood, it is necessary to protect the wood.
In the past, chemical treatment of wood is a common method for enhancing water repellency and durability of wood. The chemical treatment of wood includes acetylation treatment [11,12,13], graft modification treatment [14,15,16,17,18], etc. These chemical treatments can effectively slow down the wood’s moisture absorption and extend its service. But, these modification methods cannot completely prevent moisture intrusion into the wood. Recently, constructing superhydrophobic surfaces on wood substrates is considered a promising approach to enhancing water repellency [19,20,21,22]. Numerous studies reported the successful construction of superhydrophobic coatings on wood substrates. Lin et al. obtained superhydrophobic wood using fumed silica and polymethylhydrosilane (PMHS) via a dehydrogenation reaction. The obtained modified wood has good superhydrophobic properties [5]. Yue et al. successfully deposited a superhydrophobic RTVSR coating onto the wood surface with SiO2 nanoparticles. The resulting superhydrophobic wood has good durability [23]. Jia et al., obtained remarkably robust superhydrophobic wood by using SiO2, commercial EP, and F13-TMS as a hydrophobic modifier. The resulting wood remains superhydrophobic after being subjected to long-term abrasion testing [24]. Yang et al., prepared superhydrophobic wood samples using polydopamine (PDA), 3-mercaptopropyltriethoxysilane (KH580) and nano-Al2O3 as the micro-nano structure maker. The obtained superhydrophobic wood showed excellent resistance to flow scouring, acid and base corrosion, and organic solvents [25]. However, these superhydrophobic wood surfaces have been prepared by complicated processing procedures and using expensive modifiers, which highly limit their industrial production. High hydrophobicity is sufficient to extend the service life of wood, and it does not need to be superhydrophobic.
Here, highly hydrophobic wood has been prepared by a simple, efficient, one-step method. Briefly, tetramethylcyclotetrasiloxane (D4H) was chosen from industrial raw materials to graft hydrophobic –CH3 groups on wood substrates. D4H is a reactive siloxane containing silicon-hydrogen (–Si–H) bonds that can undergo addition reactions with unsaturated olefins. Mainly used for the preparation of various polymethyl hydrogen siloxanes with specific hydrogen content and functional group-modified polysiloxanes. In this work, the modification process is very simple: by dipping the wood in D4H modification solution it can be obtained highly hydrophobic wood with a water contact angle (WCA) of 140.1° and withstand UV radiation and anti-fouling properties.
2. Materials and Methods
2.1. Materials
Tetramethylcyclotetrasiloxane (D4H) was obtained from Xiamen Guiyou New Material Technology Co., Ltd. (Fujian, China). Kastredt catalyst was provided by Dongguan Maiteng Rubber and Plastic Materials Co., Ltd. (Guangdong, China). N-hexane was obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). For this study, the Chinese fir (sapwood) with a density of 340 ± 40 kg/m3) was purchased from the Guangdong province of China. The dimensions of the wood sample are 20 mm × 20 mm × 10 mm. The moisture content of the wood was 12%.
2.2. Hydrophobic Modification of Wood Substrates
Modified fluid with D4H concentrations of 0%, 1%, 5%, 10%, 15%, 20%, and 25% were prepared by mixing the D4H, n-hexane, and catalyst together. The formula for D4H modifier solutions is shown in Table 1. The wood samples were immerged into the D4H solutions for 5 min and then withdrawn. Then, the D4H-treated wood specimens were finally washed with n-hexane to remove the D4H not covalently bonded onto the wood surface. Finally, the resulting wood sample was dried at 100 °C for 30 min.
Table 1.
Formula for D4H modifier solutions.
| D4H Contents | D4H /g | n-hexane/g | Kastredt Catalyst/mL |
|---|---|---|---|
| 0% | 0.0 | 50.0 | 0.15 |
| 1% | 0.5 | 49.5 | 0.15 |
| 5% | 2.5 | 47.5 | 0.15 |
| 10% | 5 | 45 | 0.15 |
| 15% | 7.5 | 42.5 | 0.15 |
| 20% | 10 | 40.0 | 0.15 |
| 25% | 12.5 | 37.5 | 0.15 |
2.3. Characterization
The surface morphology and microstructure of the samples were performed by scanning electron microscopy (SEM, S-4800, Hitachi, Japan). The elemental analysis of the wood surface was performed through an energy dispersive spectrometer (EDS, EX-250, Horiba, Japan). Infrared spectrometer (IRPrestige-21, Shimadzu, Japan) and X-ray photoelectron spectroscopy (XPS, Thermo Scientific Escalab 250Xi, Waltham, MA, USA) were tested to characterize the functional group composition and changes in the surface chemistry of the D4H modified wood. The woods’ water contact angle (WCA) was measured using a 5 μL distilled water droplet on a contact angle meter (Powereach JC2000D1; Shanghai, China). After the water droplet stayed on the wood surface for 30 s, the WCA was measured. The WCA was obtained as averages of measurements taken from at least five different positions on each sample’s surface. In addition, the 24-h water absorption test and abrasion resistance tests (finger wiping, and tape peeling tests) of the D4H modified wood samples were characterized according to the previous research reports [17,18,26,27].
3. Results and Discussion
3.1. The Mechanism of D4H Modified Wood
The mechanism of D4H modification to prepare hydrophobic wood is: the hydrophilic –OH groups on the wood surface will undergo a dehydrogenation reaction with the –Si–H bonds on the D4H structure in the presence of catalysts, so that the D4H with hydrophobic methyl (–CH3) groups will be grafted onto the wood surface to finally obtain hydrophobized wood. As shown in Figure 1a, D4H is a reactive siloxane containing four Si–H participates in various chemical reactions, especially with unsaturated olefins and –OH groups containing materials, such as wood with abundant hydroxyl groups. In addition, the methyl group in the D4H structure makes it have low surface energy. As shown in Figure 1b, the surface of unmodified wood is composed of a large number of hydrophilic –OH groups. In the presence of a Kastredt catalyst, the –OH groups on the wood surface undergo an ultrafast dehydrogenation reaction with D4H. As shown in Movie S1 (see Supplementary Materials), after immersing the wood samples in the D4H modifier solution, a large number of air bubbles was seen. As shown in Figure 1c, through this simple modification method, it can be speculated that D4H is covalently grafted onto the wood surface. Consequently, the resulting wood samples will have good hydrophobic properties.
Figure 1.
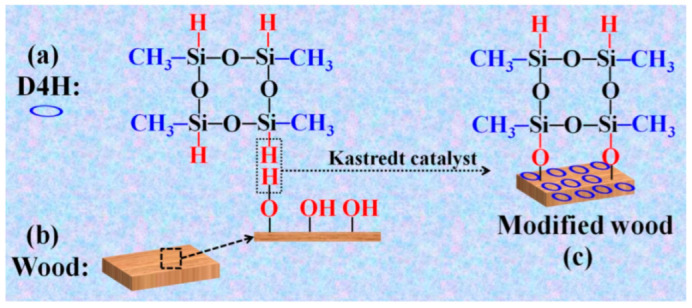
Schematic representation of the process for obtaining the D4H modified wood: (a) D4H structure; (b) Wood; (c) Modified wood.
3.2. Change in Chemical Properties of Wood
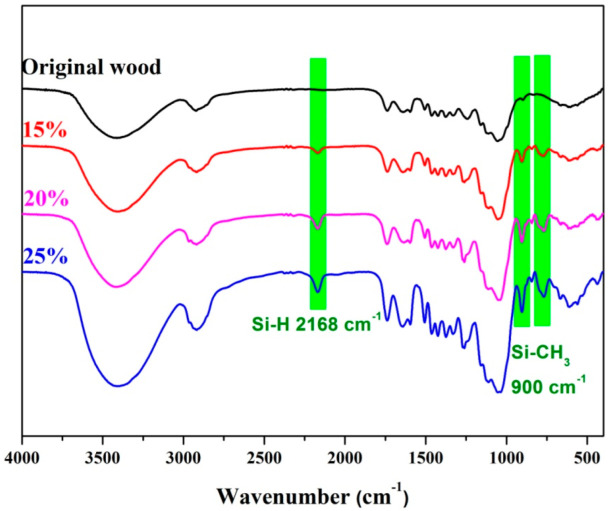
FTIR spectrum of wood before and after D4H modification is shown in Figure 2. By comparison, the samples before and after D4H modification have some same bands. Bands at 3411 cm−1 are attributed to the stretching vibration of the –OH group [1]. The absorption peak at 2916 cm−1 belongs to the stretching vibration of the –CH3 groups [18]. And the absorption peaks at 1743 cm−1 and 1465 cm−1 are assigned to the C=O stretching vibration and C=C asymmetric stretching vibration [2], respectively. In addition to the same absorption peaks, D4H-modified wood samples appeared with new absorption peaks. The absorption peak at 2168 cm−1 can be ascribed to the –Si–H of the D4H structure [28,29]. The band at 900 cm−1 is attributed to the –Si–CH3 absorption peaks [18]. The absorption peak at 762 cm−1 is assigned to the Si–O–Si bending mode from the D4H structure [1,30]. Combined with the experimental phenomenon of Movie S1 (see Supplementary Materials), it can be further speculated that D4H was grafted on the wood surface in the form of chemical bonds.
Figure 2.
FTIR spectra of wood before and after D4H modification.
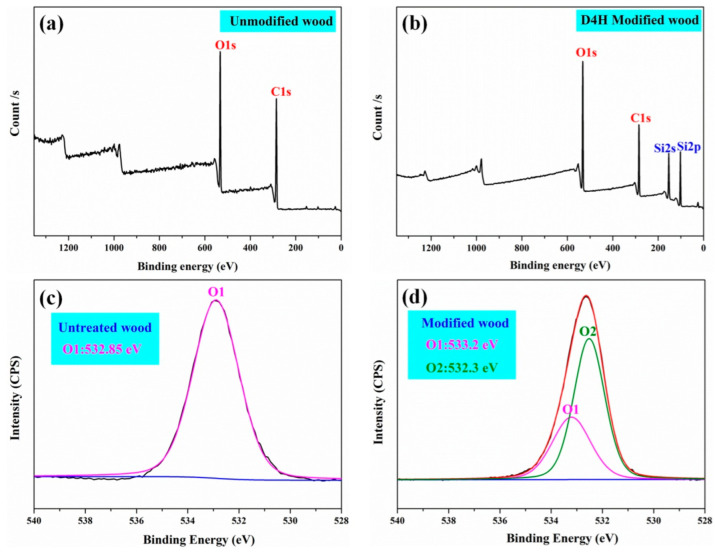
To further determine the surface chemistry of wood before and after D4H modification, an XPS test was carried out. As shown in Figure 3a,b, both unmodified and D4H-modified wood (25% D4H content) samples have C1s (285.17 eV) and O1s (531.18 eV) characteristic signal peaks [31]. In addition, D4H-modified wood samples appeared with characteristic signal peaks of Si, which were located in 103.28 eV (Si2p) and 154.28 eV (Si2s), respectively. This may be attributed to the grafting of D4H on the wood surface. To better research the surface functional group composition of D4H-modified wood and untreated wood, the high-resolution O1s spectra were resolved into spectra of various oxygen components. As shown in Figure 3c,d, compared to untreated wood, the D4H treated wood showed a new O2 (Si–O–Si or Si–O–C bonds) signal, which appeared at about 532.46 eV. The appearance of the O2 signal can further speculate that D4H was grafted on the wood surface in the form of chemical bonds [18].
Figure 3.
XPS spectra of untreated (a) and 25% D4H treated wood (b) and high-resolution spectra of O1s (c) and (d).
3.3. Morphological Observation and Elemental Composition Analysis
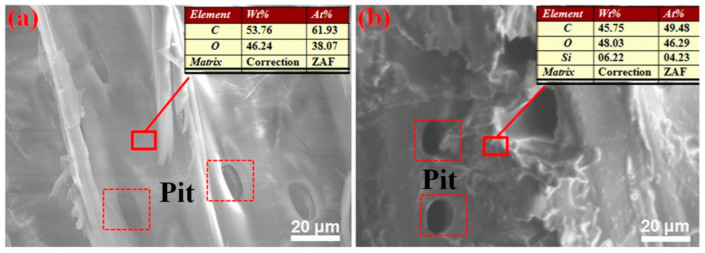
Changes in surface morphology and chemical composition of wood before and after D4H treatment were determined by the SEM-EDS test. As shown in Figure 4a, the surface of unmodified wood is smooth and rich in micron-sized pores. After surface modification with D4H (25% content), we can see that there is no obvious change in the surface morphology of the modified wood, which indicates that the D4H modification has no effect on the microstructure. The EDS results are presented as an inset in Figure 4a,b. The EDS analysis of the original wood reveals that it contains C (61.93%) and O (38.07%) atoms, and the Si element was not detected. In contrast, D4H wood samples exhibit the presence of the Si element. In a word, these results (FTIR, XPS and SEM-EDS) can confirm that D4H is covalently grafted on the wood surface.
Figure 4.
SEM images and EDS analysis of wood before (a) and after (b) 25% D4H modification.
3.4. Hydrophobicity of Wood
As shown in Figure 5, with the increase in D4H concentration, the WCA of modified wood also increased gradually. The temperature and humidity of the environment during the contact angle test were 25 °C and 60%, respectively. The WCA of the untreated wood at radial and cross-sections are only 27.8° and 0°, respectively, indicating that the unmodified wood is very hydrophilic. As the D4H concentration increased from 0% to 1%, the WCA of the treated wood in radial and cross-sections increased to 130.9° and 149.5°, respectively. This is due to the grafting of hydrophobic –CH3 groups (from D4H) on the surface of the modified wood. According to Figure 5, when the D4H concentration was increased from 10% to 25%, the WCA of the modified wood in the radial and cross-sections did not change significantly. This can be attributed to the fact that the wood surface has been completely covered by D4H with low surface energy, there is no site to continue grafting D4H, so the hydrophobicity will not change significantly. It should be noted here that D4H modification of wood surfaces is very efficient. This is mainly attributed to the high reactivity of –OH groups on the wood surface with D4H in the presence of catalysts. As shown in Movie S1 (see Supplementary Materials), when the unmodified wood sample was immersed in the D4H modifier solution, a large number of bubbles can be seen, which is due to the dehydrogenation reaction of the wood and D4H.
Figure 5.
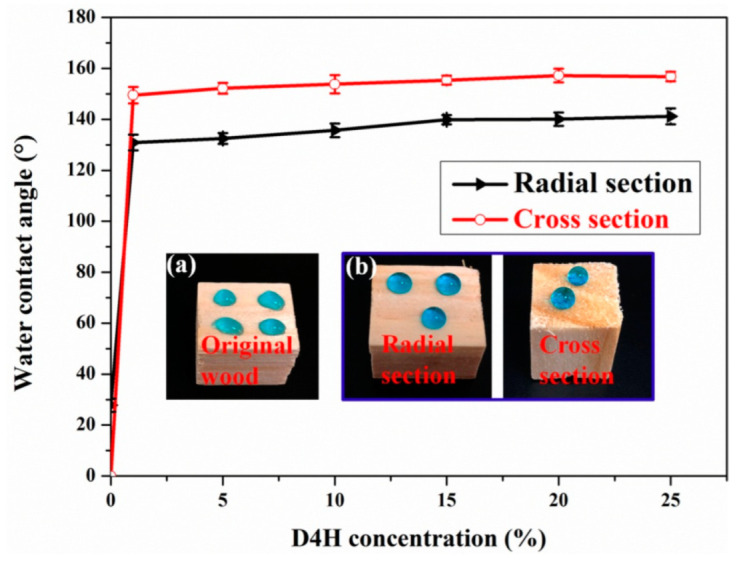
WCA on the surface of wood samples treated with different D4H concentrations. Photographs of water droplets (dyed with methylene blue) on the original (radial section) (a) and D4H modified wood (cross section and radial section) (b).
3.5. The Durability Test of D4H Modified Wood Surface
To demonstrate the practicality of D4H-modified wood, abrasion resistance tests were carried out. As shown in Figure 6a, the abrasion resistance performance of D4H-modified (25% content) wood samples were evaluated by a finger wiping test. Press a finger firmly on the surface of the D4H-modified wood, and then drop water droplets on the surface to observe the shape of the water droplets. Clearly, the D4H-modified wood samples still have good hydrophobic properties after wiping them with a finger. The water droplets dyed with methyl blue are spherical on the wiped surface of the D4H-modified wood, with a WCA of 139.4°. As shown in Figure 6b, abrasion resistance of D4H modified wood was further evaluated by a tape peel test. The tape was glued to the D4H modified (25% content) wood surface and tightly pressed several times, and then the tape was taken off. And then test the WCA of the wood surface after the tape is peeled off. The results show that the D4H-modified wood still has good hydrophobic properties. In addition, we also evaluated the chemical stability of D4H-modified wood (shown in Supplementary Materials).
Figure 6.
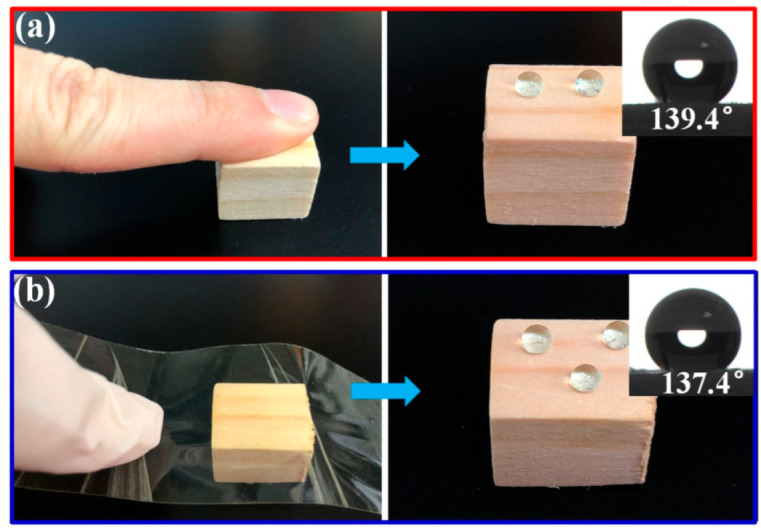
Images of finger wiping test (a) and tape peeling (b).
To further evaluate the durability of the hydrophobic layer on the surface of D4H-modified wood, a UV resistance test was carried out. As shown in Figure 7, the D4H modified (25% content) wood samples were placed under a UV lamp with a power of 36 W (wavelength: 356 nm) and irradiated for different times (0–72 h). As can be seen from the figure, after 72 h of UV irradiation, the D4H modified wood samples still have good water repellency, and the WCA at the cross-section and radial section was still greater than 149° and 136°. It means that the D4H-modified wood possessed good UV durability.
Figure 7.
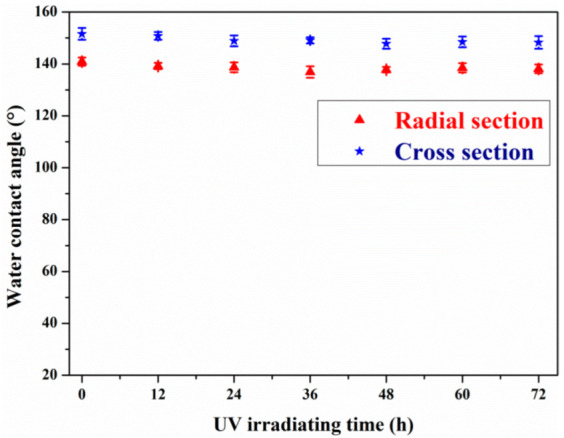
UV radiation resistance test of D4H modified wood.
3.6. Water Absorption
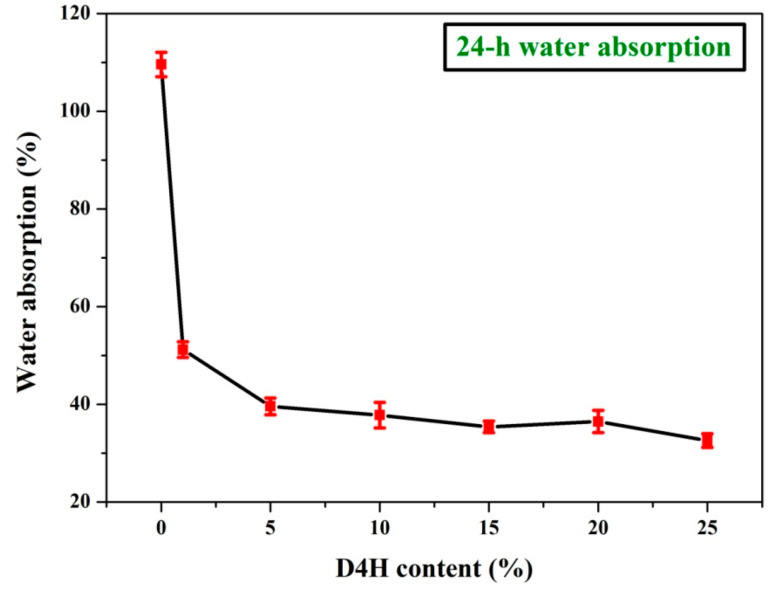
Figure 8 shows the water absorption test chart of unmodified wood and D4H-modified wood after soaking in water for 24 h. Obviously, the 24-h water absorption value of wood samples modified with D4H is much lower than that of unmodified wood (109.6%). When the concentration of the D4H modifier exceeds 15%, the modified wood has a lower water absorption value. And, when the D4H concentration reaches 25%, the water absorption value of modified wood reaches the lowest value (32.6%). The result shows that D4H modification can impart wood with good water repellency.
Figure 8.
24-h water absorption test before and after wood modification.
3.7. Anti-Fouling Performance Test
In this study, the D4H-modified wood did not achieve superhydrophobicity in the radial section. The radial section of wood is widely used in daily life. This means that the wood obtained by D4H treatment does not have self-cleaning properties. Nevertheless, a certain level of anti-fouling effect is enough for some wood products used indoors. After D4H modification, the surface of the resulting wood sample was grafted with hydrophobic –CH3 groups, which reduced the surface energy of the wood and made the wood have good anti-fouling performance. Figure 9 shows the anti-fouling resistance test images of unmodified wood and D4H modified. Put soy sauce, milk, and orange juice droplets on the surface of unmodified and modified wood, then use a paper towel to absorb the contamination to see if it will leave marks on the surface. Obviously, the unmodified wood is wetted by milk, soy sauce, and orange juice indicating that the unmodified wood is heavily polluted. As a comparison, the wood samples obtained by D4H modification have no residual contaminants on the surface. Therefore, it can be concluded that D4H-modified wood had good anti-fouling properties to common household pollutants.
Figure 9.
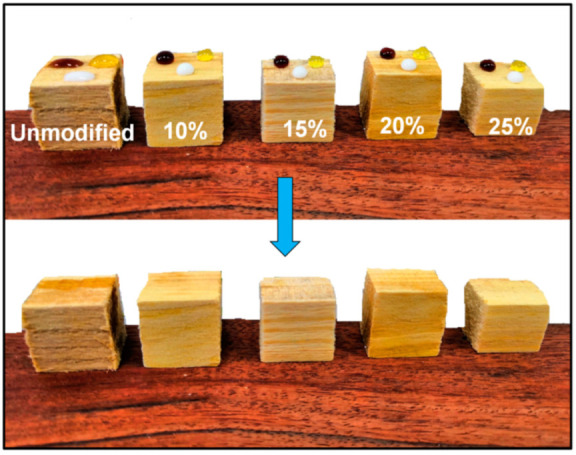
Anti-fouling property of untreated and D4H (different contents) treated woods to milk, soy sauce, and orange juice.
4. Conclusions
In summary, highly hydrophobic wood with good anti-fouling properties was successfully fabricated by simple soaking in a D4H modifier solution. The proposed formation mechanism of hydrophobicity on the wood surface resulted from D4H with low surface energy covalently grafting on the surface of the wood. The WCA of the D4H treated wood was increased significantly compared to that of untreated wood, and the water absorption of the D4H-treated (25% content) wood samples was lower than 40% after 24 h of water immersion. In addition, the obtained hydrophobic wood showed good UV resistance and wear resistance, which indicates promising application prospects in numerous fields.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/polym1723173/s1, Figure S1: the chemical corrosion resistance test; Movie S1.
Author Contributions
Conceptualization, H.W. and S.C.; data curation, M.X., B.L. and X.F.; writing—original draft, S.C. and M.T.; writing—review & editing, H.W. and S.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The authors gratefully acknowledge the financial support of this work by the National Natural Science Foundation of China (No.32001009) and the Foundation of Educational Commission of Hubei Province (Q20192602).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lu Y., Feng M., Zhan H. Preparation of SiO2-Wood Composites by an Ultrasonic-Assisted Sol-Gel Technique. Cellulose. 2014;21:4393–4403. doi: 10.1007/s10570-014-0437-6. [DOI] [Google Scholar]
- 2.Wang K., Dong Y., Yan Y., Zhang W., Qi C., Han C., Li J., Zhang S. Highly Hydrophobic and Self-Cleaning Bulk Wood Prepared by Grafting Long-Chain Alkyl onto Wood Cell Walls. Wood Sci. Technol. 2017;51:395–411. doi: 10.1007/s00226-016-0862-9. [DOI] [Google Scholar]
- 3.Sangregorio A., Muralidhara A., Guigo N., Thygesen L.G., Marlair G., Angelici C., Jong E.D., Sbirrazzuoli N. Humin Based Resin for Wood Modification and Property Improvement. Green Chem. 2020;22:235–243. doi: 10.1039/C9GC03620B. [DOI] [Google Scholar]
- 4.Wang S., Liu C., Liu G., Zhang M., Li J., Wang C. Fabrication of Superhydrophobic Wood Surface by a Sol-Gel Process. Appl. Surf. Sci. 2011;258:806–810. doi: 10.1016/j.apsusc.2011.08.100. [DOI] [Google Scholar]
- 5.Lin W., Zhang X., Cai Q., Yang W., Chen H. Dehydrogenation-Driven Assembly of Transparent and Durable Superhydrophobic ORMOSIL Coatings on Cellulose-Based Substrates. Cellulose. 2020;1:7805–7821. doi: 10.1007/s10570-020-03288-2. [DOI] [Google Scholar]
- 6.Jia S., Chen H., Luo S., Qing Y., Deng S., Yan N., Wu Y. One-Step Approach to Prepare Superhydrophobic Wood with Enhanced Mechanical and Chemical Durability: Driving of Alkali. Appl. Surf. Sci. 2018;455:115–122. doi: 10.1016/j.apsusc.2018.05.169. [DOI] [Google Scholar]
- 7.Jia S., Liu M., Wu Y., Luo S., Qing Y., Chen H. Facile and Scalable Preparation of Highly Wear-Resistance Superhydrophobic Surface on Wood Substrates Using Silica Nanoparticles Modified by VTES. Appl. Surf. Sci. 2016;386:115–124. doi: 10.1016/j.apsusc.2016.06.004. [DOI] [Google Scholar]
- 8.Li Y., Wu Q., Li J., Liu Y., Wang X., Liu Z. Improvement of Dimensional Stability of Wood via Combination Treatment: Swelling with Maleic Anhydride and Grafting with Glycidyl Methacrylate and Methyl Methacrylate. Holzforschung. 2012;66:59–66. doi: 10.1515/HF.2011.123. [DOI] [Google Scholar]
- 9.Wang S., Wang C., Liu C., Zhang M., Ma H., Li J. Fabrication of Superhydrophobic Spherical-like α-FeOOH Films on the Wood Surface by a Hydrothermal Method. Colloids Surf. A. 2012;403:29–34. doi: 10.1016/j.colsurfa.2012.03.051. [DOI] [Google Scholar]
- 10.Sun Q., Yu H., Liu Y., Li J., Lu Y., Hunt J.F. Improvement of Water Resistance and Dimensional Stability of Wood through Titanium Dioxide Coating. Holzforschung. 2010;64:757–761. doi: 10.1515/hf.2010.114. [DOI] [Google Scholar]
- 11.Matsunaga M., Hewage D.C., Kataoka Y., Ishikawa A., Kobayashi M., Kiguchi M. Acetylation of wood using supercritical carbon dioxide. J. Trop. for Sci. 2016;28:132–138. [Google Scholar]
- 12.Thygesen L.G., Elder T. Moisture in untreated, acetylated, and furfurylated Norway Spruce Studied during Drying using Time Domain NMR. Wood Fiber Sci. 2008;40:309–320. [Google Scholar]
- 13.Huang X., Kocaefe D., Kocaefe Y.S., Pichette A. Combined Effect of Acetylation and Heat Treatment on the Physical, Mechanical and Biological Behavior of Jack Pine (Pinus Banksiana) Wood. Eur. J. Wood Wood Prod. 2018;76:525–540. doi: 10.1007/s00107-017-1232-5. [DOI] [Google Scholar]
- 14.Han X., Yin Y., Zhang Q., Li R., Pu J. Improved Wood Properties via Two-Step Grafting with Itaconic Acid (IA) and Nano-SiO2. Holzforschung. 2018;72:499–506. doi: 10.1515/hf-2017-0117. [DOI] [Google Scholar]
- 15.Kumar A., Richter J., Tywoniak J., Hajek P., Adamopoulos S., Segedin U., Petric M. Surface Modification of Norway Spruce Wood by Octadecyltrichlorosilane (OTS) Nanosol by Dipping and Water Vapour Diffusion Properties of the OTS-Modified Wood. Holzforschung. 2017;72:45–56. doi: 10.1515/hf-2017-0087. [DOI] [Google Scholar]
- 16.Fu Y., Li G., Yu H., Liu Y. Hydrophobic Modification of Wood via Surface-Initiated ARGET ATRP of MMA. Appl. Surf. Sci. 2012;258:2529–2533. doi: 10.1016/j.apsusc.2011.10.087. [DOI] [Google Scholar]
- 17.Wang K., Dong Y., Yan Y., Zhang S., Li J. Improving Dimensional Stability and Durability of Wood Polymer Composites by Grafting Polystyrene onto Wood Cell Walls. Polym. Compos. 2018;39:119–125. doi: 10.1002/pc.23912. [DOI] [Google Scholar]
- 18.Lin W., Huang Y., Li J., Liu Z., Yang W., Li R., Chen H., Zhang X. Preparation of Highly Hydrophobic and Anti-Fouling Wood Using Poly(Methylhydrogen)Siloxane. Cellulose. 2018;25:7341–7353. doi: 10.1007/s10570-018-2074-y. [DOI] [Google Scholar]
- 19.Chang H., Tu K., Wang X., Liu J. Fabrication of Mechanically Durable Superhydrophobic Wood Surfaces Using Polydimethylsiloxane and Silica Nanoparticles. RSC Adv. 2015;5:30647–30653. doi: 10.1039/C5RA03070F. [DOI] [Google Scholar]
- 20.Gao L., Lu Y., Zhan X., Li J., Sun Q. A Robust, Anti-Acid, and High-Temperature-Humidity-Resistant Superhydrophobic Surface of Wood Based on a Modified TiO2 Film by Fluoroalkyl Silane. Surf. Coat. Technol. 2015;262:33–39. doi: 10.1016/j.surfcoat.2014.12.005. [DOI] [Google Scholar]
- 21.Tu K., Wang X., Kong L., Guan H. Facile Preparation of Mechanically Durable, Self-Healing and Multifunctional Superhydrophobic Surfaces on Solid Wood. Mater. Design. 2018;140:30–36. doi: 10.1016/j.matdes.2017.11.029. [DOI] [Google Scholar]
- 22.Wu Y., Jia S., Qing Y., Luo S., Liu M. A Versatile and Efficient Method to Fabricate Durable Superhydrophobic Surfaces on Wood, Lignocellulosic Fiber, Glass, and Metal Substrates. J. Mater. Chem. 2016;4:14111–14121. doi: 10.1039/C6TA05259B. [DOI] [Google Scholar]
- 23.Yue D., Lin S., Cao M., Lin W., Zhang X. Fabrication of Transparent and Durable Superhydrophobic Polysiloxane/SiO2 Coating on the Wood Surface. Cellulose. 2021;28:1–14. doi: 10.1007/s10570-021-03758-1. [DOI] [Google Scholar]
- 24.Jia S., Lu X., Luo S., Qing Y., Yan N., Wu Y. Efficiently Texturing Hierarchical Epoxy Layer for Smart Superhydrophobic Surfaces with Excellent Durability and Exceptional Stability Exposed to Fire. Chem. Eng. J. 2018;348:212–223. doi: 10.1016/j.cej.2018.04.195. [DOI] [Google Scholar]
- 25.Yang J., Li H., Yi Z., Liao M., Qin Z. Stable Superhydrophobic Wood Surface Constracting by KH580 and Nano-Al2O3 on Polydopamine Coating with Two Process Methods. Colloid Surf. A. 2022;637:128219–128229. doi: 10.1016/j.colsurfa.2021.128219. [DOI] [Google Scholar]
- 26.Lin W., Cao M., Olonisakin K., Li R., Zhang X., Yang W. Superhydrophobic Materials with Good Oil/Water Separation and Self-Cleaning Property. Cellulose. 2021;28:10425–10439. doi: 10.1007/s10570-021-04175-0. [DOI] [Google Scholar]
- 27.Tu K., Wang X., Kong L., Chang H., Liu J. Fabrication of robust, damage-tolerant superhydrophobic coatings on naturally micro-grooved wood surfaces. RSC Adv. 2016;6:701–707. doi: 10.1039/C5RA24407B. [DOI] [Google Scholar]
- 28.Brassard J., Sarkar D.K., Perron J. Synthesis of Monodisperse Fluorinated Silica Nanoparticles and Their Superhydrophobic Thin Films. ACS Appl. Mater. Inter. 2011;3:3583–3588. doi: 10.1021/am2007917. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X., Xia B., Ding B., Zhang Y., Luo J., Jiang B. Ultra-Fast Surface Hydrophobic Modification of Sol-Gel Silica Antireflective Coating with Enhanced Abrasion-Resistance. Mater. Lett. 2013;104:31–33. doi: 10.1016/j.matlet.2013.04.016. [DOI] [Google Scholar]
- 30.Latthe S.S., Liu S., Terashima C., Nakata K., Fujishima A. Transparent, Adherent, and Photocatalytic SiO2-TiO2 Coatings on Polycarbonate for Self-Cleaning Applications. Coatings. 2014;4:497–507. doi: 10.3390/coatings4030497. [DOI] [Google Scholar]
- 31.Poaty B., Riedl B., Blanchet P., Blanchard V., Stafford L. Improved Water Repellency of Black Spruce Wood Surfaces after Treatment in Carbon Tetrafluoride Plasmas. Wood Sci. Technol. 2013;47:411–422. doi: 10.1007/s00226-012-0505-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.