Abstract
(1) Background: Dyes play an important role in food, medicine, textile, and other industries, which make human life more colorful. With the increasing demand for food safety, the development of natural dyes becomes more and more attractive. (2) Methods: The literature was searched using the electronic databases PubMed, Web of Science, and SciFinder and this scoping review was carried out following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). (3) Results: 248 articles were included in this review. This review summarizes the research progress on natural dyes in the last ten years. According to structural features, natural dyes mainly include carotenoids, polyphenols, porphyrins, and alkaloids, and some of the newest dyes are summarized. Some pharmacological activities of carotenoids, anthocyanin, curcumin, and betalains in the last 10 years are summarized, and the biological effects of dyes regarding illumination conditions. The disadvantages of natural dyes, including sources, cost, stability, and poor bioavailability, limit their application. Here, some feasible strategies (potential resources, biotechnology, new extraction and separation strategies, strategies for improving stability) are described, which will contribute to the development and utilization of natural dyes. (4) Conclusion: Natural dyes show health benefits and potential in food additives. However, it is necessary for natural dyes to pass toxicity tests and quality tests and receive many regulatory approvals before their final entry into the market as food colorants or as drugs.
Keywords: natural dyes, structure features, pharmacological activities, development strategies
1. Introduction
Dyes play an important role in food, medicine, textile, and other industries, which make human life more colorful. Dyes are divided into natural and synthetic dyes according to their source. However, many synthetic colorants have environmental toxicity and threaten human health. These adverse effects of synthetic colors have made the scientific community skewed toward natural colors [1]. With the increase in the demand for natural dyes in food, cosmetics, and other fields, it is of important value to develop natural dyes, especially in the food field.
Natural dyes are widely found on land and in the sea, and can be extracted from plants, animals, microorganisms, minerals, and some other materials. Most mineral dyes cannot be used in the food industry because they are harmful to humans. Most plant dyes, animal dyes, and microbial dyes are not only safe and reliable, but also have functions of nutrition and pharmacological activities such as antioxidant, anti-inflammatory, anti-cancer, anti-obesity, anti-microbial, and anti-viral effects. The use of natural dyes has a long history, for example, indigo, which is extracted from plants, has been used for thousands of years [2]. In addition to being classified by source and color [3], natural colorants are divided into the major categories by chemical structure, such as indole derivatives (quinones and violacein), alkaloids, polyenes, macrolides, peptides, or terpenoids [4]. In recent years, some new natural dyes have been isolated, and new activities, mechanisms, and new applications have been explored.
This review summarizes the research progress on natural dyes in the last ten years. Information in the last 10 years (from 2012 to 2022) was searched using the databases PubMed and Web of Science, structures of compounds were checked with the database SciFinder, and the review was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [5,6]. The category of natural dyes and main compounds are listed according to the literature, and different categories of dyes were used as terms to retrieve related bioactivities (Table 1). This review involves some new natural dyes and development strategies of natural dyes, which provide insight for further development and potential applications of the natural dyes.
Table 1.
Terms used in the search strategy.
| Electronic Database | Search and Terms |
|---|---|
| Web of Science PubMed |
#1 (“Natural dye” OR “Natural pigment” OR “Natural colorants”) AND (“Carotenoids” OR “Anthocyanins” OR “Curcumin” OR “Chlorophylls” OR “Alkaloid” OR “Quinone”) #2 “New” And “Pigment” AND (“Carotenoids” OR “Anthocyanins” OR “Curcumin” OR “Alkaloid” OR “Quinone”) AND “Isolated” #3 (“Carotenoids” OR “Anthocyanins” OR “Curcumin” OR “Betalain”) AND (“Antioxidant” OR “Inflammatory” OR “Anti-cancer” OR “Cancer” OR “Anti-bacterial” OR “Antimicrobial” OR “Obesity” OR “Anti-obesity” OR “Diabetes” OR “Cardiovascular” OR “Anti-viral” OR “Neuroprotective” OR “Alzheimer’s disease”) #4 (“Extraction” OR “Isolation” OR “Extracted” OR “Isolated”) AND (“Carotenoids” OR “Anthocyanins”) AND “New method” |
2. Results
2.1. Literature Search Results
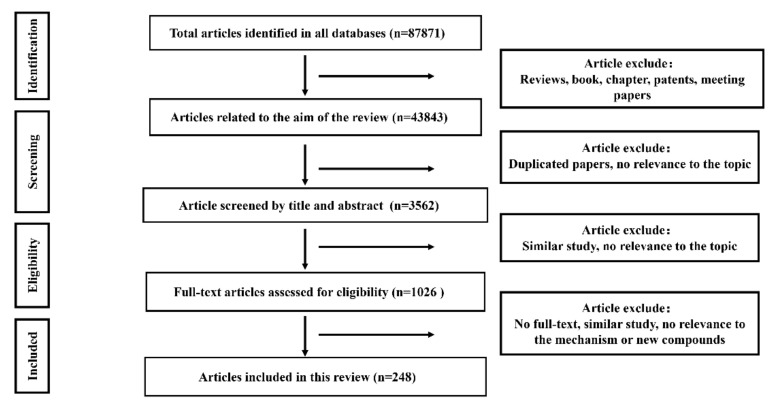
The flowchart of the literature search and selection of this review is shown in Figure 1. Overall, 87,871 studies were identified. Then, 44,028 records were removed for the following reasons: reviews, book chapters, letters, news, patents, meeting papers, reports, etc. Duplicated papers and records that are not relevant to the topic were excluded after the database screening, and 3562 studies were identified. By screening the titles and abstracts, 2536 records were excluded because they were similar and had no relevance to the scope of this review. Then, the full text of 1026 papers was reviewed and assessed to check if they were eligible for this scoping review. As a result, 248 studies were included in this review.
Figure 1.
The flowchart of the selection process of literature based on PRISMA.
2.2. Resources
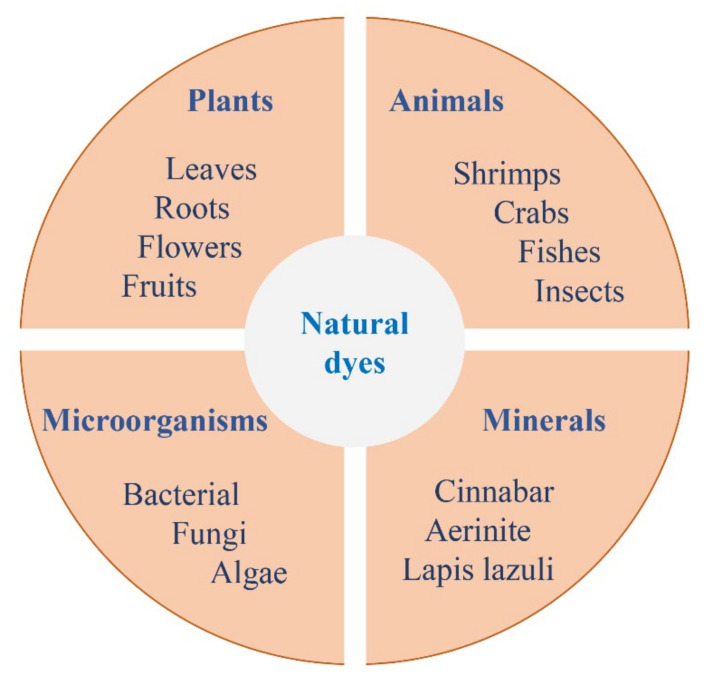
Natural dyes can be extracted from plants, animals, microorganisms, minerals, and some other materials (Figure 2).
Figure 2.
Resources of natural dyes including plants, animals, microorganisms, and minerals.
2.2.1. Plants
In nature, the leaves, roots, flowers, and fruits of plants are all important sources of natural dyes [7,8,9] and these natural dyes determine the color of different parts of plants. For example, chlorophylls are responsible for the green color of leaves, carotenoids are responsible for yellow and red flowers, vegetables, and fruits, while anthocyanins determine the color of flowers and fruits from orange to dark blue [10]. The color of mangoes and tomatoes is associated with carotenes and lycopene, respectively [11,12]. Cornflowers, blueberries, mulberries, and strawberries are rich in anthocyanins. Betalains present in vacuoles of plants are responsible for beetroot’s deep red or yellow color [13]. Seasons change, plant leaves change from green to yellow or red, and the changes in leaves’ color during leaf senescence depend on the different combinations of chlorophylls, carotenoids, and anthocyanins [14,15]. Most of the sources of plant dyes are leaves, flowers, fruits, and roots, which are renewable resources and can be used as a source of natural dyes.
2.2.2. Animals
Animals are also sources of natural dyes. The most common ones are carmine acid, astaxanthin, and lac dyes. Carmine acid, varying from pink to reddish-purple, is a natural dye extracted from the dried bodies of females of the insect species Dactylopius coccus Costa (cochineal). Carmine acid has been used in food, cosmetics, medicine. and textile production and is allowed by the food laws in different countries [16,17]. Astaxanthin is widely found in nature, especially in aquaculture products such as shrimp, crab, and fish [18]. Astaxanthin, as a red–orange ketocarotenoid, has excellent antioxidant activity [19] and it is widely used as a color additive in production [20]. Lac dyes are pink–red–purple organic colourants derived from an insect and contain several components, such as laccaic acid A, laccaic acid B, laccaic acid C, laccaic acid D, and laccaic acid E [21]. Lac dyes have been used for textiles and painting for thousands of years [22]. Recently, some new quinone dyes were isolated from the deep-sea crinoid Hypalocrinus naresianus [23,24]. With the development of marine resources, deep-sea animals are expected to become potential new sources of natural dyes.
2.2.3. Microorganisms
Microorganism communities are the most widely distributed living organisms. They are closely connected with animals, plants, and other microorganisms in the form of saprophytic states, symbiosis, and parasitism, and constitute an important part of the biosphere and ecosystem [25]. Microorganisms, including bacteria, fungi, and some algae, are an important source of natural dyes [26,27]. At the moment, an extraordinary range of microbial pigments in various environments is available, such as carotenoids (β-carotene, canthaxanthin, astaxanthin), bacteriochlorophylls, flavins, indigoids, melanins, pheomelanin, prodigiosin, violacein, glaukothalin, phycocyanin, xanthomonadin [28,29,30]. Fungi have been identified as potential dye producers, and some pigment-producing fungi are as follows: Aspergillus niger, Aspergillus versicolor, Monascus sp., Trichoderma viride, Penicillium purpurogenum [31], Aspergillus sydowii, Aspergillus aureolatus, Aspergillus keveii, Penicillium flavigenum, Penicillium chermesinum, Epicoccum nigrum, Lecanicillium aphanocladii, and Fusarium sp. [32]. Some of the fungal dyes have already been used as food colorants in the market, such as Monascus pigments, arpink red from Penicillium oxalicum, riboflavin from Ashbyagossypii, and β-carotene from Blakeslea trispora [33,34]. Since the 1880s, Monascus pigment has been widely used as food coloring throughout the world [35]. In Asia, Monascus pigment has been widely utilized in food industries, especially in China and Japan [36]. Compared to plants and animals, fungi show immense advantages in production and cost. For example, fungal dyes are season-independent and can grow in a cheap culture medium easily and fast. In particular, fungal dyes show good stability, solution, different color shades, and easy processing [37]. Microbial dyes are expected to replace synthetic colorings and, since most of them are eco-friendly and nontoxic to humans, they can be used for application as food additives and in the medicinal field. Fungi could be a good and readily available source of natural dyes.
2.2.4. Minerals
Mineral dyes are refined from natural minerals and are mainly used for painting, handicrafts, antiques, and restoration of cultural relics. Since ancient times, cinnabar (HgS) has been used as a red dye, widely used in the art of ancient Rome, for adornment, and in medieval manuscripts of colored drawing or patterns [38]. Aerinite is a light-blue mineral, which comes from local ores in the southern Pyrenees. Blue dye can be created from aerinite and was used in Romanesque wall paintings in Andorra and Catalonia, Spain [39]. Natural ultramarine (UMB) dyes from lapis lazuli have been used in the past [40]. The UMB dyes, as one group of mineral dyes, are characterized by sodalite structure and colored sulfur species as chromophores are encapsulated inside. The general formula of UMB dyes is Na8[AlSiO4]6[S2S3]2 [41]. Ancient painters, potters, and craftsmen obtained blue, green, red, and black colors from rocks or minerals. Therefore, minerals dyes are more suitable for use in art and architecture than food additives.
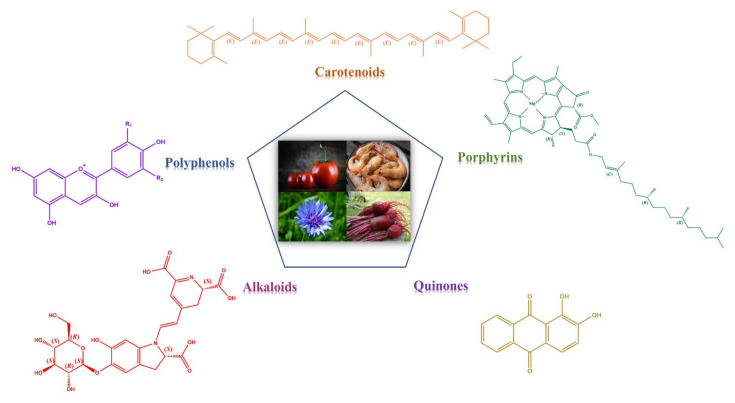
2.3. Structural Features of Natural Dyes
Natural dyes can be divided into the major categories by chemical structure. They mainly include carotenoids, polyphenols, porphyrins, alkaloids, and quinones (Figure 3).
Figure 3.
The major categories of natural dyes divided by chemical structure.
2.3.1. Carotenoids
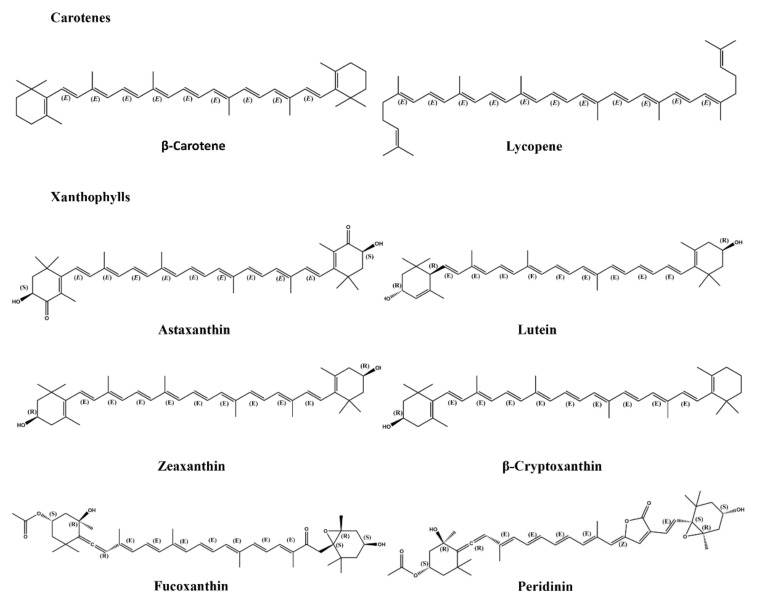
Carotenoids are tetraterpene, liposoluble, and yellowish-orange dyes, and the difference in colors depends on the specific conjugated double-bond structure of molecules [42]. Carotenoids, as the most widely distributed dyes in nature, are found in microorganisms (photosynthetic bacteria, some species of archaea), plants (leaves, fruits, flowers), and animals (birds, insects, fish, and crustaceans) [8]. According to the length of their carbon backbone, carotenoids have been classified as C30, C40, and C50 carotenoids [43]. C40 carotenoids have eight isoprene molecules and the yellow, orange, and red hues depend on an extensively conjugated polyene chain. This characteristic chemical structure is responsible for their physiological function as an antioxidant and provitamin A nutrient, as well as their ability to protect from UV radiation [44]. Carotenoids are divided into two groups: carotenes and xanthophylls (Figure 4). Carotenes, such as α-carotene, β-carotene, γ-carotene, and lycopene, are hydrocarbons. About 50 kinds of carotenes have been found in nature [45]. β-carotene can be converted into vitamin A after entering the body, which is an important vitamin for humans and it can help prevent eye damage and protect skin [46]. Xanthophylls are carotenoids containing oxygen atoms, such as astaxanthin, lutein, zeaxanthin, β-cryptoxanthin, fucoxanthin, and peridinin. Structures of xanthophylls show marked diversity and about 800 kinds of xanthophylls have been reported in nature up until 2018 [8]. Those molecules of xanthophylls include hydroxy, carbonyl, aldehyde, carboxylic, epoxide, and furanoxide groups. They assist in photoprotection and light harvesting, and play potential roles in the photosynthetic system. In fact, some xanthophylls have been widely used in various fields. For example, natural astaxanthin has been approved as a food colorant in fish and animal feed by the FDA [47]. Canthaxanthin is also widely applied in food, cosmeceutical, pharmaceutical, fishery, poultry, and other industries [48]. Canthaxanthin as a food additive in fisheries could improve the color of shrimp and salmonid fish [49]. In addition, zeaxanthin and lutein are considered to play a potential role in maintaining eye health [50]. In recent years, several carotenoids have been isolated (Table 2), but more attention has been paid to screening strains from nature that can produce carotenoids. Carotenoids are widely produced by microorganisms and plants. Carotenoids as essential components of the human body have great development value. The health benefits of carotenoids have also been widely studied.
Figure 4.
The structures of carotenoids including carotenes and xanthophylls.
Table 2.
Several newly isolated and identified natural dyes in the last 10 years.
| Category | Compounds | Source | Ref. |
|---|---|---|---|
| Carotenoids | 6′-Epimonadoxanthin | Rosary goby (Gymnogobius castaneus) | [51] |
| 3′-Deoxycapsorubin | Red mamey (Pouteria sapota) | [52] | |
| 3,3′-Dideoxycapsorubin | Red mamey (Pouteria sapota) | [52] | |
| Methyl 5-glucosyl-5,6-dihydro-apo-4,4′-lycopenoate | Planococcus maritimus strain iso-3 | [53] | |
| Diapolycopenedioc Acid Xylosylesters A/B/C | Rubritalea squalenifaciens | [53] | |
| 13Z-zeaxanthin dipalmitate | Wolfberry | [54] | |
| Anthocyanins | Malvidin-3-(p-coumaroyl)-rutinoside-5-glucoside | Transgenic Del/Ros1 tomato fruit | [55] |
| Malvidin-3-(feruloyl)-rutinoside-5-glucoside | Transgenic Del/Ros1 tomato fruit | [55] | |
| Petunidin-3-(cis-p-coumaroyl)-rutinoside-5-glucoside | Tomato cultivar Indigo Rose | [56] | |
| Malvidin-3-(cis-p-coumaroyl)-rutinoside-5-glucoside | Tomato cultivar Indigo Rose | [56] | |
| Petunidin-3-(trans-p-coumaroyl-rhamonside)-glucoside-5-glucoside | Tomato cultivar Indigo Rose | [56] | |
| Malvidin-3-(p-methoxy-trans-coumaroyl)-rutinoside-5-glucosid | Tomato cultivar Indigo Rose | [56] | |
| Delphinidin 3-O-a-l-rhamnopyranosyl-(1→6)-b-d-glucopyranoside-30-O-b-d-glucopyranoside | Tamarillo fruit | [57] | |
| Cyanidin 3-[2′’-(6′’’-coumaroyl)-glucosyl]-glucoside | Nitraria tangutorum | [58] | |
| Pelargonidin-3-O-coumaroylglucoside | Mulberry (Morus moraceae) juice | [59] | |
| Delphinidin-3-O-coumaroylglucoside | Mulberry (Morus moraceae) juice | [59] | |
| Cyanidin 3-O-[2-O-(2-O-(4-O-(6-O-(4-O-(β-glucopyranosyl)-trans-caffeoyl)-β-glucopyranosyl)-trans-caffeoyl)-β-glucopyranosyl)-6-O-(trans-sinapoyl)-β-glucopyranoside]-5-O-[6-O-(malonyl)- glucopyranoside] | Purple-violet flowers of Moricandia arvensis | [60] | |
| 5,7-Dimethylmalvidin 3-O-β-galactopyranoside | Blue Plumbago flower | [61] | |
| 5,7-Di-methylpetunidin 3-O-β-galactopyranoside | Blue Plumbago flower | [61] | |
| 5,7-Di-methyldelphinidin 3-O-β-galactopyranoside | Blue Plumbago flower | [56] | |
| 5,7-Dimethylmalvidin 3-O-α-rhamnopyranoside | Blue Plumbago flower | [61] | |
| 5,7-Dimethyldelphinidin 3-O-α- rhamnopyranoside | Blue Plumbago flower | [61] | |
| 5,7-Dimethylpetunidin 3-O-α-rhamnopyranoside | Blue Plumbago flower | [61] | |
| petunidin 3-O-[6-O-(4-O-(4-O-cis-(β-d-glucopyranoside)-p-coumaroyl)-α-l-rhamnopyranosyl)-β-d-glucopyranoside] -5-O-[β-d-glucopyranoside] | Wild Lycium ruthenicum Murr. | [62] | |
| 3-O-(6-O-α-l-Rhamnopyranosyl-β-d-glucopyranosyl)-7-O-(6-O-(4-O-(6-O-(E)-caffeoyl-β-d-glucopyranosyl)-(E)-caffeoyl)-β-d-glucopyranosyl) del-phinidin | Bluish-purple petals of Chinese bellflower (Platycodon grandifloru) | [63] | |
| 3-O-(6-O-α-l-Rhamnopyranosyl-β-d-glucopyranosyl)-7-O-(6-O-(4-O-(6-O-(4-O-β-d-glucopyranosyl-(E)-p-coumaroyl)-β-d-glucopyranosyl)-(E)-caffeoyl)-β-d-glucopyranosyl) delphinidin | Bluish-purple petals of Chinese bellflower (Platycodon grandifloru) | [63] | |
| 3-O-(6-O-α-l-Rhamnopyranosyl-β-d-glucopyranosyl)-7-O-(6-O-(4-O-(6-O-(4-O-β-d-glucopyranosyl-(E)-caffeoyl)-β-d-glucopyranosyl)-(E)-p-coumaroyl)-β-d-glucopyranosyl) delphinidin | Bluish-purple petals of Chinese bellflower (Platycodon grandifloru) | [63] | |
| Alatanin D | Purple yam (Dioscorea alata L.) | [64] | |
| Alatanin E | Purple yam (Dioscorea alata L.) | [64] | |
| Alatanin F | Purple yam (Dioscorea alata L.) | [64] | |
| Alatanin G | Purple yam (Dioscorea alata L.) | [64] | |
| Panaxidin A (pelaragonidin-4-vinylcatechol) | Panax quinquefolius L. | [65] | |
| Panaxidin B (pelargonidin-4-vinylphenol) | Panax quinquefolius L. | [65] | |
| Alkaloid | Alstoscholarisine F/G | Alstonia scholaris | [66] |
| Oryzadiamine C | Oryza sativa mutant | [67] | |
| Oryzadiamine A | Oryza sativa with yellow grain | [68] | |
| Rosellin A | Mushroom Mycena rosella | [69] | |
| Rosellin B | Mushroom Mycena rosella | [69] | |
| Ergopigment 8/9/10 | Claviceps purpurea | [70] | |
| Katorazone | Streptomyces sp. IFM 11299 | [71] | |
| 2-(4-((3E,5E)-14-Aminotetradeca-3,5-dienyloxy) butyl)-1,2,3,4-tetrahydroisoquinolin-4-ol | Fusarium moniliforme KUMBF1201 | [72] | |
| 6′-O-malonyl-amaranthin | Callus culture of Celosia cristata L. | [73] | |
| Quinone | Hypalocrinins A/B/C/D/E/F/G | Deep-sea crinoid Hypalocrinus naresianus | [24] |
| 5′-Hydroxytrypethelone | The mycobiont of lichen Trypethelium eluteriae Sprengel | [74] | |
| Gymnochrome A/H | Deep-sea crinoid Hypalocrinus naresianus | [23] | |
| 1,4,6b,7,10-Pentahydroxy-1,2,6b,7,8,12b-hexahydroperylene-3,9-dione | Endophytic fungus Alternaria tenuissima SS77 | [75] | |
| 1,4,9,12a-Tetrahydroxy-12-methoxy-1,2,11,12,12a,12b-Hexahydroperylene-3,10-dione | Endophytic fungus Alternaria tenuissima SS77 | [75] | |
| 1,4,9-tri-hydroxy-1,2-Dihydroperylene-3,10-dione | Endophytic fungus Alternaria tenuissima SS77 | [75] | |
| Alaternosides A/C | Rhamnus alaternus L | [76] | |
| 6-Methoxy-rhodocomatulin 7-methyl ether | Australian sponge Clathria hirsuta | [77] | |
| 3-Bromo-6-methoxy-12-desethyl- rhodocomatulin 7-methyl ether | Australian sponge Clathria hirsuta | [77] | |
| 3-Bromo-6-methoxy-rhodocomatulin 7-methyl ether | Australian sponge Clathria hirsuta | [77] | |
| 3-Bromorhodocomatulin 7-methyl ether | Australian sponge Clathria hirsuta | [77] | |
| Grandiquinone A | Leaves of Tectona grandis | [78] | |
| Phomopsanthraquinone | Fungus Phomopsis sp. PSU-MA214 | [79] |
2.3.2. Polyphenols
Anthocyanin is one of the most representative polyphenol natural dyes. Anthocyanins and their glycosides, as species of water-soluble pigments, are ubiquitous in the plant world. More than 250 kinds of anthocyanin molecules have been found from agricultural products and they are responsible for the colors of products such as blueberry, black wolfberry, cherry, black raspberry, strawberry, grape, and purple and red corn [80]. The color of anthocyanin depends on the pH of the solution, which might be due to the transformation of anthocyanin structure in different pH conditions [81]. For example, roselle anthocyanins became red at pH 2.0–3.0 because the anthocyanins mainly were present in the form of yellow salt ions; the red decreased at pH 4.6–6.0 with the structures transforming into blue quinonoidal base; at pH 7.0–9.0 the structures transformed to colorless pseudo-base and the color gradually turned to blue. When the pH value was greater than 9.0, anthocyanins turned to yellow-green due to the degradation in the strongly alkaline conditions [9,82]. Based on this characteristic, anthocyanins can be developed as indicators to monitor the freshness of food and have great potential in intelligent packaging.
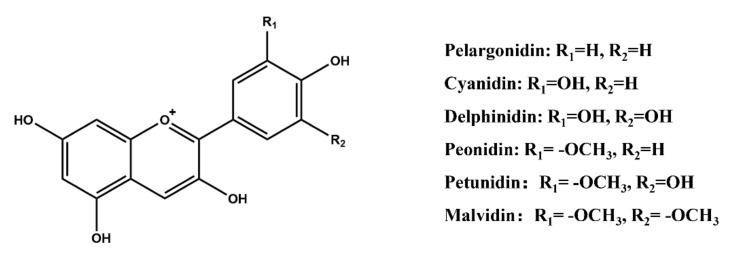
Pelargonidin, cyanidin, delphinidin, peonidin, petunidin, and malvidin are the six main anthocyanin compounds (Figure 5), and they differ in the number and positions of the substituents in the benzene ring [83]. However, anthocyanins are present in nature in the form of glycosides because free anthocyanins are unstable. Anthocyanins often form 3-glycoside or 3,5-diglycoside compounds with one or more glucose (the most common), rhamnose, galactose, xylose, arabinose, etc. through glycosidic bonds [84,85]. The application of anthocyanins in foodstuffs has been approved in many countries, including in Europe (EU E No. E163), the United States, and Japan, and they are mainly used in beverages, confectionery, baking, frozen snacks, and dairy and fruit products [86]. Some new anthocyanins have been isolated and identified in recent years (Table 2), and anthocyanin synthesis-related genes in different fruits and plants have also been extensively studied, which provide a new strategy for anthocyanin development.
Figure 5.
Molecular structures of the main anthocyanins.
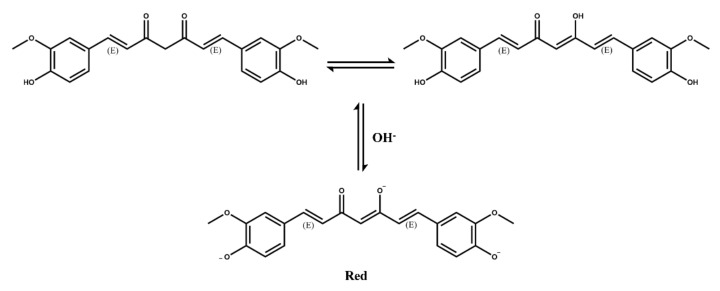
Curcumin is a polyphenolic compound extracted from Curcuma longa L., which has been generally recognized as safe [87]. Curcumin has also been approved by the FDA as a natural food additive. Curcumin can be used as an acid–base indicator because it changes from yellow to red at pH greater than 8 (Figure 6). Anthocyanin and curcumin are the main phenolic natural dyes and promising natural dyes because they have been shown to have many health benefits. Curcumin also plays a potential role in intelligent packaging [88].
Figure 6.
Molecular structures of curcumin and the color changes in acid–base conditions.
2.3.3. Porphyrins
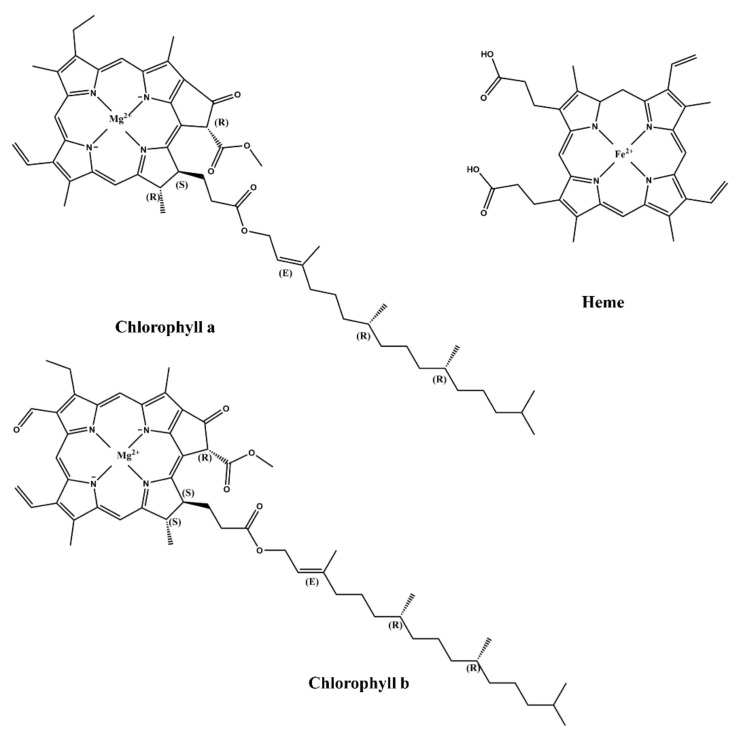
Porphyrin dyes are also known as tetrapyrrole derivatives and mainly include heme and chlorophylls (Figure 7). Chlorophylls are magnesium-tetrapyrrole molecules and the major photosynthetic greenish pigments found in algae, plants, and cyanobacteria that play essential roles in photosynthesis [89,90]. Chlorophylls mainly include five types: chlorophyll a, chlorophyll b, chlorophyll c, chlorophyll d, and chlorophyll f. Chlorophyll a and b play a dominant role in photosynthetic organisms, and chlorophyll a is essential in photochemistry. Chlorophyll a as a blue/green pigment with maximum absorbance from 660 to 665 nm and is the main pigment of phytoplankton; it is considered to indicate the rhythm of marine ecosystems [91]. Chlorophyll b is converted to chlorophyll a via 7-hydroxymethyl chlorophyll a [92]. Chlorophyll d is found in marine cyanobacteria and red algae, and chlorophyll f, found in various genera of cyanobacteria, co-occurs with chlorophyll a [90,93]. Chlorophyll d and chlorophyll f show red-shifted absorption features compared to chlorophyll a and chlorophyll b because of the position of formyl substitutions. The maximal absorptions of chlorophyll d and chlorophyll f are 697 nm and 707 nm in methanol, respectively [93]. Sodium copper chlorophyllin, a green colorant, has been approved by the USA FDA for use only in “citrus-based dry beverage mixes in an amount not exceeding 0.2 percent of the dry mix” (http://www.ecfr.gov/cgibin/textidx?SID=6ffd146f772f44d1b7b76af13be18518&node=21:1.0.1.1.27.1.31.12&rgn=div8 (accessed on 5 May 2020)).
Figure 7.
Molecular structures of heme, chlorophyll a, and chlorophyll b.
2.3.4. Alkaloids
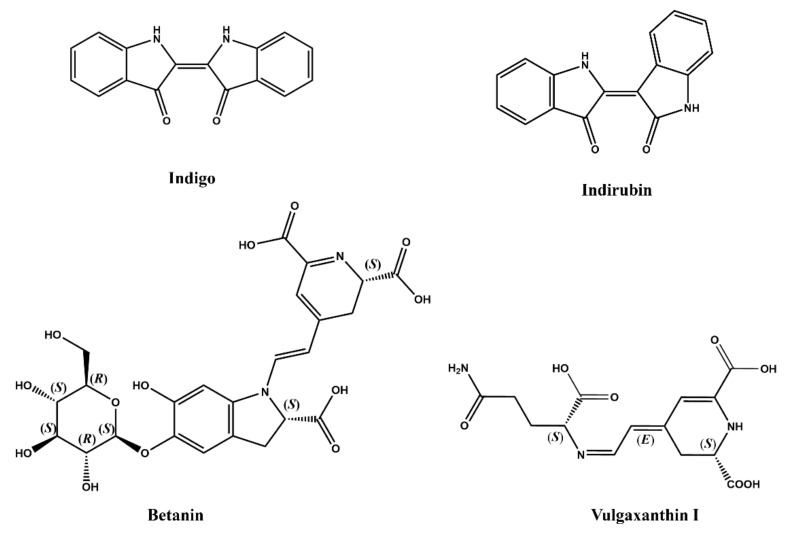
Betalains as water-soluble natural plant dyes have been recognized as red food-coloring agents due to no toxicity and health benefits [94]. Betalains contain several dye compounds and those compounds contain nitrogen. The dye compounds in betalains mainly include violet betalains such as betanin and yellow betaxanthins [95], and betanin and vulgaxanthin I as representative structures of betalains are shown in Figure 8. Betalains are red at pH 6–7, but instability with light and heat limits the application of betalains. Therefore, they can be used as a colorant for food products with a short shelf life and food stored at low temperature in opaque packings, such as ice cream, frozen desserts, and yogurts [96]. Betanin (E-number E162, CI Natural Red 33) as the main betalain component has been widely used as a colorant for food products, such as ice creams, yogurts, cake mixes, soft drinks, and gummy candies [97,98]. In the application of betanin as a food colorant, a content of less than 50 mg/kg can provide the most ideal color [97].
Figure 8.
Structure of alkaloid pigments including indigo, indirubin, and betalains.
Indigo as an ancient natural dye is extracted from tropical plants, woad (Isatis tinctoria), and true indigo (Indigofera tinctoria) [99,100], and it is a remarkably stable blue dye and has a long history of use in textiles. The bis(indole) indigotin is responsible for the blue color of indigo-based dyes [101] and a new class of indigoids has been discovered [102]. FD&C blue no. 2 is its disulfonate sodium salt and has been used in food and cosmetic industries [103]. Synthetic indigo dyes contain only one pigment, indigo, and natural indigo dyes also contain indirubin, which have anti-inflammatory and anti-tumor effects [104]. Additionally, betalains and indigo dyes are widely used in medicine and other fields because of their extensive biological activities. In addition to these typical alkaloid dyes, some potential new alkaloid dyes have been isolated from microorganisms and plants in recent years (Table 2). Even though studies have shown that they show some pharmacological activities, their safety still needs further study.
2.3.5. Quinones
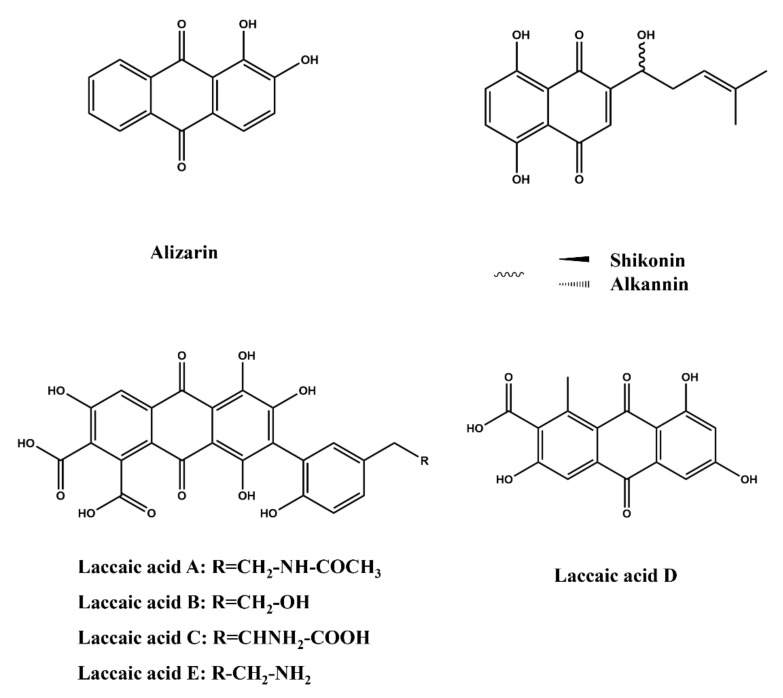
Alizarin, 1,2-dihydroxy-9,10-anthraquinone (Figure 9), is the principal component of the natural dye extracted from madder root (Rubia tinctorum) [105]. It is a type of anthraquinone fluorescent dye [106]. Because of their structural features, the anthraquinone derivatives can easily and closely interact with DNA molecules [107]. Therefore, they confer potential anti-tumor capabilities to these tricyclic planiform compounds because of the function of inducing cell apoptosis [108]. Alizarin has been applied in colorimetric indicators of PH, glucose, and others, for example, alizarin complexone functionalized mesoporous silica nanoparticles as smart nanoformulations which could be used in optical diagnosis, individualized treatment, and noninvasive monitoring of diabetes management [109].
Figure 9.
Structure of quinone dyes including alizarin, skikonin/alkannin, laccaic acid A/B/C/E, and laccaic acid D.
Alkannin and its enantiomer shikonin are valuable natural dyes found in purple-colored roots of red gromwell (Lithospermum erythrorhizon) [110]. Recently, shikonins/alkannins have been discovered to exhibit a range of pharmacological properties and could be used as drug scaffolds to design a set of derivatives [111], which has excellent prospects in drug development. Furthermore, the components of lac dyes laccaic acid A, laccaic acid B, laccaic acid C, laccaic acid D, and laccaic acid E are also quinone dyes (Figure 9). In the last decade, marine resources and microorganism resources were the main sources of new quinone dyes, which suggested new directions and strategies for developing natural dyes. However, the toxicity and pharmacological activities of these new natural dyes need to be further studied so that they can be applied in the market.
2.4. Pharmacological Activities of Natural Dyes and Related Mechanisms
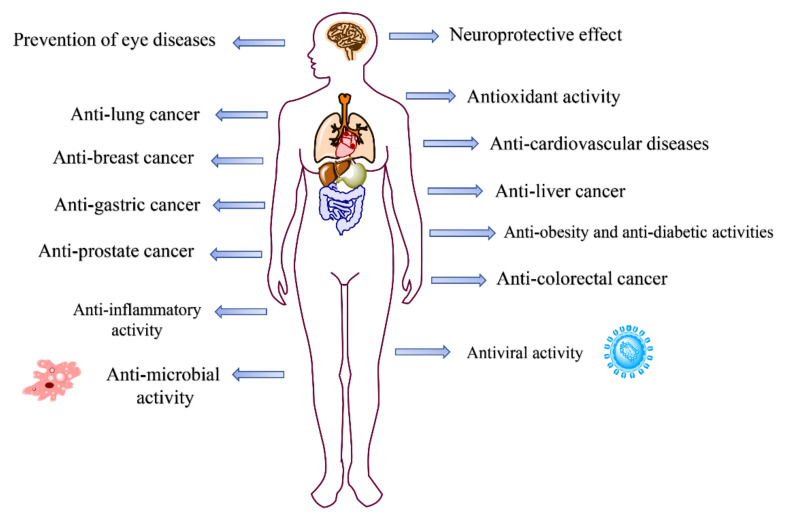
The pharmacological activities and related mechanisms of natural dyes have been studied extensively. The health benefits of carotenoids, anthocyanins, curcumins, and betalains were mainly discussed in recent years, including antioxidant activity, anti-inflammatory activity, anti-cancer activity, anti-cardiovascular disease activity, anti-obesity and anti-diabetic activity, anti-microbial activity, anti-viral activity, and neuroprotective effect (Figure 10).
Figure 10.
The pharmacological activities of natural dyes.
2.4.1. Antioxidant activities
Oxidative stress is caused by excessive production of reactive oxygen species (ROS), which leads to damage to cellular proteins, lipids, and DNA, and cell necrosis and apoptosis occur when the antioxidant defense system cannot eliminate ROS [112]. Oxidative stress might lead to many diseases including cardiovascular diseases, neurodegenerative diseases, obesity/diabetes, and cancer, and even be associated with human lifespan [113,114,115]. Nowadays, natural dyes such as carotenoid and polyphenol dyes are considered excellent antioxidants in nutraceutical and pharmaceutical fields. Carotenoids with provitamin activity can effectively scavenge ROS and reduce oxidative stress in the human body. In particular, astaxanthin showed excellent antioxidant activity in free radical scavenging, singlet oxygen quenching, inducing the antioxidant enzymeparaoxonase-1, enhancing glutathione concentrations, and preventing lipid peroxidation [116]. In the human umbilical vein endothelial cell (HUVEC) model, astaxanthin generated small amounts of ROS to activate the Nrf-2/HO-1 antioxidant pathway. However, it has been demonstrated that β-carotene at high doses shows pro-oxidant effects because it could produce radical ions that might damage cells [117]. In cellular and animal models, anthocyanins have been shown to reduce the generation of ROS and protect from oxidative damage, thereby preventing other diseases [118,119]. Anthocyanins as antioxidants could scavenge free radicals, enhance antioxidant enzyme activity, suppress oxidative stress via clearance of ROS, and sustain the level of GSH and the glutathione antioxidant defense system [120,121,122,123]. The antioxidant activity of curcumin was realized by inhibition of serum malondialdehyde, up-regulating transcription and expression levels of antioxidant enzymes and improving mitochondrial function [124,125]. Some mechanisms of antioxidant activity are shown in Table 3, showing that natural dyes can scavenge free radicals, reduce the generation of ROS, inhibit lipid peroxidation and malondialdehyde (MDA), and up-regulate transcription and expression levels of antioxidant enzymes including total superoxide dismutase (SOD), total catalase (CAT), and glutathione peroxidase (GPx). Antioxidant activity is considered to be an important mechanism for the treatment and prevention of other diseases, so it will be mentioned for other diseases.
Table 3.
Mechanisms of some natural dyes for antioxidant activity.
| Category | Compounds Name | Mechanism | Refs. |
|---|---|---|---|
| Carotenoids | Astaxanthin | Scavenged free radicals, quenched singlet oxygen, ↑ antioxidant enzyme paroxoanase-1, ↑ glutathione concentrations, ↓ lipid peroxidation. | [116] |
| Activated the Nrf-2/HO-1 antioxidant pathway by generating small amounts of ROS in HUVEC model. | [126] | ||
| ↓ Oxidative stress, ↓ MDA content, ↑ SOD | [127] | ||
| Lycopene | ↓ NADPH oxidase, ↓ ROS production | [128] | |
| Lutein | ↑ SOD, ↓ ROS level, ↑ CAT, ↑ GPx, ↓ GR, ↓ MDA, ↑ reduced glutathione level | [129] | |
| Zeaxanthin | ↓ Myeloperoxidase, ↓ MDA, ↑ SOD, ↑ CAT, ↑ glutathione level | [130] | |
| Polyphenols | Anthocyanins | Scavenged free radicals, ↑ SOD, ↑ total antioxidant activity | [120] |
| Cyanidin-3-arabinoside | ↓ Renal oxidative stress (↑ SOD, ↑ CAT), ↓ lipid peroxidation (↓ TBARS and ↓ MDA) | [121] | |
| Gy3G, Mv3G | ↓ ROS, sustained the level of GSH and glutathione antioxidant defense system | [122] | |
| Petunidin-3,5-O-diglucoside | Scavenged free radicals, ↓ ROS, ↓ MDA level and GSH consumption | [123] | |
| Anthocyanin extract from purple highland barley | Scavenged free radicals, ↓ ROS, ↑ SOD, ↑ CAT | [131] | |
| Curcumin | ↓ Serum MDA, ↑ total antioxidant activity, ↑ transcription and expression levels of antioxidant enzymes, ↑ mitochondrial function | [124,125] | |
| Alkaloids | Betalain | ↓ MDA, ↑ CAT, ↑ SOD, ↑ GPx, ↑ xanthine oxidase | [132] |
| Betanin | Scavenged free radicals, ↓ MDA, ↑ total antioxidant activity | [133] |
2.4.2. Anti-Inflammatory Activities
Growing evidence suggests that inflammatory responses play a critical role in the development and progression of major human diseases [134]. Oral administration of zeaxanthin could ameliorate acetic acid-induced colitis by antioxidative effects and modulation of pro-inflammatory cytokines. Zeaxanthin suppressed tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), interleukin-6 (IL-6), interleukin-1 beta (IL-1β), and nuclear transcription factor kappa B (NF-κB) levels, and inhibited nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) protein expression [130]. Oral administration with β-carotenoid ameliorated ulcerative colitis-associated local and systemic damage in mice by acting on multiple targets such as NF-κB, COX-2, STAT3 IL-17, nuclear erythroid 2 (NF-E2)-related factor 2 (Nrf2), matrix metalloproteinase-9 (MMP-9), and connective tissue growth factor [135]. Studies have found that the anti-inflammatory mechanisms of astaxanthin involve multiple signaling pathways including PI3K/AKT, Nrf2, NF-κB, ERK1/2, JNK, p38 MAPK, and JAK-2/STAT-3, and the anti-inflammatory effects showed preventive effects on a variety of diseases [136]. Malvidin 3,5-diglucosid as an anthocyanin could reduce inflammation symptoms through reducing NO production and reducing the induction of pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6 in lipopolysaccharide (LPS)-induced RAW264.7 macrophages [137]. The anti-inflammatory action effect of anthocyanins can be attributed primarily to their antioxidant properties. Anthocyanins extracted from Trifolium pratense (red clover) inhibited the expression of genes such as TNF-α, IL-1β, iNOS, COX-2, and monocyte chemoattractant protein (MCP-1) and translocation of the p65 subunit of NF-κB into the nucleus [138]. In addition to down-regulation of the redox-sensitive nuclear NF-κB signaling pathway, the mitogen-activated protein kinase pathways also appeared to play a role [139]. Curcumin exerts anti-inflammatory effects by regulating inflammatory signaling pathways and inhibiting the production of inflammatory mediators. Curcumin exerted its anti-inflammatory effect by inhibiting TLR4 expression, the phosphorylation of ERK, JNK, p38, and NF-κB in macrophages and TLR4-MAPK/NF-κB pathways were involved [140]. The NF-κB signal pathway is the principal inflammatory signaling pathway, and it reduces the expression of inflammatory cytokines including IL-1β, IL-6, TNF-α, COX-2. Carotenoids, anthocyanins, and curcumin all regulate this pathway to achieve anti-inflammatory effects, and natural dyes can be used as food supplements to treat inflammation and a variety of inflammation-related diseases.
2.4.3. Anti-Cancer Activities
The incidence of cancers continuously increased in the last few years and cancers have overtaken cardiovascular diseases as the leading cause of death in some high-income countries [141]. Cancer of the endocrine, digestive, urinary, and immune systems includes breast cancer, liver cancer, gastric cancer, colorectal cancer, prostate cancer, lung cancer, skin cancer, etc. [142]. Natural dyes have shown anti-cancer activities against several types of cancer (Table 4). Carotenoids including β-carotene, lycopene, crocin, astaxanthin, and fucoxanthin have shown anti-proliferative and pro-apoptotic actions against various cancers. For example, astaxanthin has been reported to inhibit the proliferation of breast cancer cells by modulating different signaling pathways and molecular targets such as inhibition of cellular migration and cell number, suppressing expression levels of pontin, mutp53, Oct4, and Nanog, and activation of Bax/Bcl2, cleaved caspase-3, and cleaved caspase-9 as well as the phosphorylation of ERK1/2, JNK, and p38. Anthocyanins play a potential role in preventing and treating cancer [143,144,145]. Anthocyanin extracts and anthocyanins, such as cyanidin-3-glucoside (C3G), peonidin-3-glucoside (Pn-3-G), malvidin-3-glucoside (M3G), delphinidin-3,5-O-diglucoside (D-3-5-D), cyaniding-3-rutinoside (C3R), pelargonidin-3-glucoside (Pg-3-G), cyanidin-3-xylosylutinoside (C3XR), and proanthocyanidins, have been reported have inhibitory effects on breast cancer, colorectal cancer, liver cancer, lung cancer, and prostate cancer. For example, anthocyanin extracts from purple potato can suppress colon tumorigenesis by suppressing the Wnt/β-catenin signaling pathway and enhancing mitochondrion-mediated apoptosis [146]. M3G as an adjuvant ingredient or nutritional supplement can prevent liver cancer by inhibiting proliferation, migration, and invasion-related pathways and promoting the apoptosis of liver tumor cells [147]. In addition, grape seed proanthocyanidins showed a radioprotective effect on normal lung cells and were considered an ideal radioprotective drug for lung cancer patients treated with radiotherapy [148]. Curcumin is a promising candidate for prevention and treatment of several cancers. The anti-cancer activities of curcumin involve different signaling pathways and molecular targets including modulation of growth factors, enzymes, transcription factors, kinases, and inflammatory cytokines, and up-regulating pro-apoptotic and down-regulating anti-apoptotic proteins [149]. The anti-lung cancer activity of curcumin has been reported and curcumin could suppress cell proliferation and induce apoptosis via modulating the JAK2/STAT3 signaling pathway, PI3K/Akt signaling pathway, and Wnt/β-catenin pathway [150,151,152,153]. Therefore, natural pigments can inhibit the proliferation of cancer cells and induce apoptosis in colon cancer, breast cancer, lung cancer, liver cancer, gastric cancer, prostate cancer, etc., and can be considered nutritional supplements and food additives.
Table 4.
The mechanism of some natural pigments for anti-cancer activity.
| Cancers | Compounds Name |
Category | Mechanism | Refs. |
|---|---|---|---|---|
| Breast cancer | Lycopene | Carotenoids | Activation of ERK1/2, ↓ cyclin D1 ↑ p21 ↓ phosphorylation of Akt and its downstream molecule mTOR ↑ Bax | [154] |
| β-Carotene | Carotenoids | ↑ Apoptosis ↓ cell cycle ↓ PI3K/Akt ↓ ERK |
[155,156] | |
| Lutein | Carotenoids | ↓ Breast cancer cell proliferation, ↑ expression of cellular antioxidant enzymes, ↓ ROS, ↑ NrF2/ARE pathway, ↓ NF-κB signaling pathway ↑ p53, ↓ HSP60 |
[157,158] | |
| Crocin | Carotenoids | ↑ Disrupting the microtubule network ↓ Wnt/β-catenin target genes |
[159,160] | |
| Astaxanthin | Carotenoids | ↓ Cellular migration, ↓ cell number ↓ Expression levels of pontin, mutp53, Oct4, and Nanog, ↓ proliferation Activation of Bax/Bcl2, cleaved caspase-3, and cleaved caspase-9 as well as the phosphorylation of ERK1/2, JNK, and p38 |
[143,144,145] | |
| D-3-5-D, C3R | Polyphenols | ↑ Intracellular reactive oxygen, ↑ apoptosis ↓ MCF-7 cells in the G2/M phases |
[161] | |
| C3G, Pg-3-G | Polyphenols | ↓ AMPK, ↑ apoptosis ↑ Oxidative stress |
[162] | |
| Curcumin | Polyphenols | ↓ NF-κB signaling pathway ↓ HER2-TK ↓ Akt protein, ↓ ubiquitin-proteasome pathway ↓ PI3K/Akt signaling pathway ↓ EGFR signaling |
[163,164,165,166,167] | |
| Betanin | Alkaloids | ↑ Apoptosis-related proteins (Bad, TRAILR4, FAS, p53) | [168] | |
| Colorectal Cancer | Astaxanthin | Carotenoids | ↓ Invadopodia, ↓ EMT, ↑ E-cadherin, ↓ vimentin, ↓ cortactin, ↓ MMP2, ↑ miR-29a-3p, ↓ ZEB1, ↓ MYC ↑ Apoptosis, ↑ Bax, ↑ caspase-3, ↓ Bcl2 |
[169,170] |
| Fucoxanthin | Carotenoids | ↓ Proliferation ↑ DNA damage |
[171,172] | |
| Crocin | Carotenoids | ↑ Caspase-3 and -7, ↓ proliferation | [173] | |
| C3G, C3XR, C3R | Polyphenols | ↑ Probiotics, ↓ inflammation ↓ Pathogenic bacteria |
[174] | |
| C3G, C3XR, C3R | Polyphenols | ↑ MiR-24-1-5p, ↓ β-catenin | [175] | |
| Pg-3-G | Polyphenols | ↓ HT-29 colon cancer cells | [176] | |
| Anthocyanin extract | Polyphenols | ↓ Wnt/β-catenin ↓ Mitochondrion-mediated apoptosis |
[146] | |
| Curcumin | Polyphenols | ↓ NF-κB pathway, ↓ cell cycle ↑ Cytochrome c, ↑ Bax and p53, ↓ Bcl-2 |
[177,178] | |
| Betaxanthin and betacyanin | Alkaloids | ↓ Bcl2-like protein 4, ↓ cleaved poly ADP-ribosyl polymerase 1, ↓ cleaved caspase-3 ↓ Anti-apoptotic protein B-cell leukemia/lymphoma 2 levels |
[179] | |
| Gastric cancer | Crocin | Carotenoids | ↓ KLF5 HIF-1, ↑ miR-320, ↓ epithelial–mesenchymal transition, ↓ migration | [180] |
| β-Carotene | Carotenoids | ↓ Cell viability, ↑ DNA damage, ↑ apoptotic indices, ↑ caspase-3, ↓ Ku70/80 | [181] | |
| Fucoxanthin | Carotenoids | ↑ Beclin-1, ↑ LC3, ↑ cleaved caspase-3 (CC3), ↓ Bcl-2, ↓ cell cycle, ↑ apoptosis, ↓ Mcl-1, STAT3, and p-STAT3 |
[182,183] | |
| Astaxanthin | Carotenoids | ↓ Cell cycle ↑ NADPH oxidase activity, ↑ ROS levels, ↑ LDH release, ↑ the number of propidium iodide-positive cells ↑ RIP1/RIP3/MLKL signaling pathway |
[184,185] | |
| Curcumin | Polyphenols | ↓ STAT3 pathway | [178] | |
| Liver cancer | Astaxanthin | Carotenoids | ↑ Cell number in G2 phase ↑ Cell number in G2/M phase ↑ Apoptosis ↑ Oxidative stress, ↑ adiponectin |
[186,187,188] |
| Crocin | Carotenoids | ↓ NF-κB, ↓ inflammation, ↓ cell cycle, ↑ apoptosis | [189] | |
| Fucoxanthin | Carotenoids | ↓ Glutathione (GSH) content, ↓ proliferation Reverting body weight, serum albumin, antioxidant enzymes, all the liver enzymes, serum bilirubin, and stress markers to normal levels in hepatocellular carcinoma rats |
[190,191] | |
| C3G, Pn-3-G | Polyphenols | ↓ TNF-α, iNOS, NF-κB ↓ Cell proliferation |
[192] | |
| C3G, C3R | Polyphenols | ↓ Lipid peroxidation, ↓ COX-2 ↑ Nrf2-mediated antioxidant enzymes |
[193] | |
| M3G | Polyphenols | ↓ Proliferation, ↑ apoptosis, ↓ ROS, ↑ JNK/p38 MAPK pathways, ↓ AKT phosphorylation, ↓ migration, ↓ invasion | [147] | |
| Curcumin | Polyphenols | ↓ Migration, ↓ invasion, ↓ epithelial–mesenchymal transition, ↓ aryl hydrocarbon receptor/ERK/SK1/S1P3 signaling pathway | [194] | |
| Betanin | Alkaloids | ↑ Nrf2, ↑ mitogen-activated protein kinases | [195] | |
| Lung cancer | Astaxanthin | Carotenoids | ↑ Cell number in G0/G1 phase ↑ p38 MAPK ↑ Apoptosis |
[196] |
| Crocin | Carotenoids | ↑ G0/G1 arrest, ↑ mRNA levels of p53 and Bax, ↓ Bcl-2, ↑ apoptosis | [197] | |
| Lutein | Carotenoids | ↓ PI3K/AKT, ↑ apoptosis | [198] | |
| C3G | Polyphenols | ↓ Lung tumor multiplicity and tumor area, ↓ expression of proliferative cell nuclear antigen (PCNA) and Ki-67 | [199] | |
| Curcumin | Polyphenols | ↓ NF-κB, ↓ JAK2/STAT3 signaling pathway, ↓ JAK2 ↓ Cell proliferation, ↑ apoptosis ↑ microRNA-192-5p, ↓ PI3K/Akt signaling pathway ↓ Wnt/β-catenin pathway |
[150,151,152,153] | |
| Betalain | Alkaloids | ↑ Proliferation, ↓ cell cycles, ↑ p53/p21, ↓ levels of cyclin-D1 complex, ↓ levels of p-PI3K, ↓ p-Akt, ↓ mammalian target of rapamycin | [200] | |
| Prostate cancer | Astaxanthin | Carotenoids | ↑ Apoptosis, ↑ cleaved caspase-3; ↑ miR-375 and miR-487b |
[201] |
| Crocin | Carotenoids | ↓ Proliferation, ↓ cell cycle, ↑ apoptosis ↓ Bcl-2, ↓ Bax |
[202] | |
| Proanthocyanidins | Polyphenols | ↓ Notch1 pathway | [203] | |
| C3G | Polyphenols | ↓ Epithelial–mesenchymal transition | [204] | |
| Curcumin | Polyphenols | ↓ Expression of CYP11A1 and HSD3B2, ↑ AKR1C2, ↓ dihydrotestos terone ↑ miR-34a, ↓ β-catenin, ↓ c-myc |
[205,206] |
2.4.4. Anti-Obesity and Anti-Diabetic Activities
The WHO estimates that by 2025, approximately 167 million people will become less healthy because they are overweight or obese (https://www.who.int/news/item/04-03-2022-world-obesity-day-2022-accelerating-action-to-stop-obesity (accessed on 1 April 2022)). Obesity occurs when excess adipose tissue accumulates in the body, which can result in metabolic syndrome, including type 2 diabetes, hypertension, and dyslipidemia [207]. (3R,3′R)-Astaxanthin could be a supplement to prevent weight gain, reduce plasma and liver triacylglycerol, and decrease plasma and liver total cholesterol [208]. At the same time, carotenoids could regulate gut microflora to reduce the incidence of obesity [208,209]. Lutein showed a preventive effect against cardiac and renal injury in STZ-induced hyperglycemic rats by altering antioxidant enzyme activities [129]. In anti-diabetic activity, astaxanthin could attenuate STZ-induced diabetes by decreasing blood glucose and total cholesterol levels, and increasing blood levels of high-density lipoprotein cholesterol (HDL-C) in a dose-dependent manner [210]. Anthocyanins have shown anti-obesity effects through multiple mechanisms including inhibiting lipid absorption, regulating lipid metabolism, increasing energy expenditure, suppressing food intake, and regulating gut microflora [211]. Anthocyanins could suppress lipid accumulation in adipocytes [212] and reduce high-fat diet-induced metabolic damage [213]. Body mass index and body weight were reduced when anthocyanin supplementation was 300 mg/d or less for 4 weeks [214]. Cyanidin 3-caffeoyl-p-hydroxybenzoylsophoroside-5-glucoside as an anthocyanin isolated from purple-fleshed sweet potato showed hypoglycemic effects by specifically suppressing hepatic glucose output [215]. C3G from black soybeans showed anti-diabetes effects by inducing the differentiation of 3T3-L1 preadipocytes into smaller and insulin-sensitive adipocytes [216]. Curcumin has been used as a pharmacological traditional medicinal agent in Ayurvedic medicine for about 6000 years. The anti-obesity mechanisms of curcumin are associated with the enzymes, energy expenditure, adipocyte differentiation, lipid metabolism, gut microflora, and anti-inflammatory potential [217]. Betacyanins purified from Hylocereus undatus peel could ameliorate obesity and insulin resistance in high-fat diet-fed mice [218]. Natural pigments could be considered as dietary supplements to prevent and ameliorate obesity and type 2 diabetes.
2.4.5. Anti-Cardiovascular Disease Effects
Cardiovascular diseases (CVDs) are a group of disorders that affect the heart and blood vessels and represent the leading cause of morbidity and mortality worldwide [219]. Oxidative stress and inflammation play an important role in CVDs. It has been suggested that carotenoids with antioxidant activity could prevent and ameliorate CVDs by suppressing oxidative stress and mitigating inflammatory responses [220]. Lutein, a major carotenoid, showed a protective effect in a cardiac failure rat model by improving cardiac morphology, antioxidant status via positively regulating the Nrf2/HO-1 signaling pathway, and reducing inflammatory markers (IL-1β, IL-6, TNF-α, NF-κB, p65) and apoptotic markers (caspase-3 and caspase-9) [221]. Data from several epidemiological studies have reported an inverse correlation between anthocyanin intake and risk of CVDs or CVD-related mortality. Higher habitual anthocyanin intake was also inversely associated with a risk of total myocardial infarction in premenopausal women [222] and nonfatal myocardial infarction in men [223]. Molecular mechanisms of action of anthocyanins are complex and include modulation of gene expression, cell signaling, and miRNA expression [224]. Curcumin has been found to ameliorate various CVDs such as atherosclerosis, cardiac hypertrophy, cardiac fibrosis, heart failure, myocardial infarction, and ischemia by multiple mechanisms and modulating multiple signaling pathways [225]. For example, recent research has found that curcumin shows a significant protective effect in myocardial ischemia–reperfusion by activating the PI3K/AKT/mTOR signaling pathway and inhibiting inflammation, apoptosis, and oxidative stress [226]. Betalain treatment protected hearts from failing via microRNA-mediated activation of the anti-inflammatory signaling and restoring the matrix protein modulation [132].
2.4.6. Anti-Microbial Activity
Red cabbage and sour cherry pomace anthocyanin extracts show anti-microbial effects on Escherichia coli, Staphylococcus aureus, Listeria monocytogenes, Salmonella Typhimurium, and Bacillus cereus [227]. Curcumin has a broad spectrum of anti-bacterial actions against a wide range of bacteria [228]. Curcumin and its derivatives (curcumin monoglucoside, curcumin diglucoside) possess strong anti-microbial properties against Streptococcus pneumoniae, even in penicillin-resistant strains. Curcumin showed anti-bacterial activity against tested strains of methicillin-resistant S. aureus because of increased membrane permeability and DNA fragmentation [229]. The efficacy of curcumin against Helicobacter pylori has been studied and the potential mechanism is down-regulation of IL-17 through the induction of indoleamine 2,3-dioxygenase in H. pylori-infected human gastric mucosa [230]. The anti-fungal activity of curcumin has also been reported and curcumin could inhibit biofilm formation and filamentation of Candida albicans. In anti-microbial photodynamic therapy, chlorophyll a and chlorophyll b exhibited high anti-microbial activity under irradiation [231]. Additionally, betalains have shown inhibitory effects against Gram-negative bacteria such as Pseudomonas aeruginosa and S. Typhimurium, among others, and Gram-positive bacteria, such as S. aureus, Enterococcus faecalis, and L. monocytogenes [232,233]. Dyes extracted from Rhodotorula glutinis could effectively inhibit the growth of B. cereus, Salmonella enteritidis, and E. coli [234]. Chaetoviridide A and Chaetoviridide B are new dye compounds isolated from the deep-sea fungus Chaetomium sp. NA-S01-R1, and they showed anti-bacterial activities against Vibrio rotiferianus and Vibrio vulnificus [235]. In addition, the applications of natural dyes isolated from fungi in healthcare have been explored, and silk sutures treated with an optimum concentration of natural fungal dye could inhibit the growth of S. aureus and E. coli [236]. Therefore, natural dyes play a potential role in anti-microbial activity against different pathogenic bacteria and have a bright prospect in healthcare applications.
2.4.7. Anti-Viral Activities
The carotenoids isolated from haloalkaliphilic archaeon Natrialba sp. M6 exhibited significantly stronger activity in eliminating hepatitis C virus (HCV) and hepatitis B virus (HBV) in infected human blood mononuclear cells than currently used drugs. This anti-viral activity may be attributed to its inhibitory potential against HCV RNA and HBV DNA polymerases, which thereby suppresses HCV and HBV replication, as indicated by a high viral clearance % in the treated cells [237]. A marine carotenoid, siphonaxanthin from Codium fragile, showed significant anti-viral activity with an IC50 of 87.4 µM against SARS-CoV-2 pseudovirus entry, and was predicted to have relatively low acute toxicities [238]. Anthocyanin fractions of strawberry, raspberry, bilberry, and lingonberry showed strongly anti-viral effects against influenza virus A/H3N2 through inhibiting the replication of the virus [239]. Delphinidin, belonging to the anthocyanin family, has shown inhibitory effect against HCV by a new mechanism, alteration of the viral particle structure, that impairs its attachment to the cell surface [240]. The anti-viral activity of curcumin has been widely studied, and the main mechanisms include direct interference with viral replication machinery and suppression of cellular signaling pathways essential for cellular replication, such as PI3K/Akt, NF-κB [241]. In Vero cells infected with EV71, the addition of curcumin significantly suppressed the synthesis of viral RNA, the expression of viral protein, and the overall production of viral progeny [242]. A recent study showed that curcumin inhibited in vitro SARS-CoV-2 infection in Vero E6 cells by affecting the SARS-CoV-2 replicative cycle and curcumin exhibited a virucidal effect with a variant/strain-independent anti-viral effect and immune-modulatory properties [243]. Natural dyes with anti-viral activity might play a potential role in the development and progression of COVID-19, which should be explored further.
2.4.8. Neuroprotective Effect
Altered amyloid precursor protein (APP) processing potentiates the aggregation of glycation products, and amyloid-β (Aβ) toxicity is a key pathogenic feature of Alzheimer’s disease (AD) [244]. Carotenoids including cryptocapsin, cryptocapsin-5,6-epoxide, and zeaxanthin showed anti-amyloidogenic potential by preventing the formation of the fibril and through disruption of the Aβ aggregates [245]. Lutein protected dopaminergic neurons by enhancing antioxidant defense and diminishing mitochondrial dysfunction and apoptotic death, suggesting the potential benefits of lutein for Parkinson’s disease treatment [246]. Natural dietary supplementation of anthocyanins could ameliorate neurodegeneration and memory impairment in a mouse model of Alzheimer’s disease. Anthocyanins as a potent antioxidant neuroprotective agent reduced AβO-induced neurotoxicity in HT22 cells via the PI3K/Akt/Nrf2 signaling pathway and improved memory-related pre- and postsynaptic protein markers and memory functions in APP/PS1 mice [247]. Bilberry anthocyanin consumption was considered to reverse AD-induced cognitive disfunction, decrease hippocampal neuroinflammatory responses, and induce phagocytosis of microglia to beta-amyloid protein plaques by regulating the CD33/TREM2/TYROBP signaling pathway in microglia [248]. Pelargonidin belongs to the anthocyanins and has been found to improve Aβ (25–35)-induced memory deficit through mitigation of oxidative stress, cholinergic dysfunction, and astrocyte reaction [249]. The effect of curcumin on AD involves multiple signaling pathways such as anti-amyloid and metal iron chelating properties, antioxidation and anti-inflammatory activities [250], and curcumin treatment protected rat PC12 cells from Aβ (25–35)-induced reduction in cell viability, apoptosis, the release of LDH, and MDA production [251]. In AlCl3-induced AD rats, betalain ameliorated AD by modulating oxidative stress and the NF-κB signaling pathway [252]. Therefore, natural dyes could be a potent dietary supplement with antioxidant and neuroprotective effects.
2.4.9. Biological Effects of Dyes Regarding Illumination Conditions
Different from other compounds, dyes have the unique feature that the biological effects are affected by illumination conditions (strict dark, ambient light, or controlled illumination). Photodynamic therapy (PDT) is a promising new treatment which uses suitable photosensitizers, appropriate wavelengths of light, and oxygen to kill cancer cells and microorganisms [90]. In recent years, the application of natural dyes as photosensitizers in PDT has been studied. Erythrosine combined with C3G as photosensitizers in PDT could eliminate Porphyromonas gingivalis biofilms [253]. PDT using purpurin (an anthraquinone pigment) could effectively inhibit the growth of triple negative breast cancer cells both in vitro and in vivo [254]. Blue light-activated curcumin markedly damaged membrane permeability, resulting in cell death of S. aureus [255]. A clinical trial suggested oral curcumin together with visible light might be a new therapeutic method for moderate to severe plaque psoriasis [256]. Chlorophylls and derivatives as photosensitizers in PDT could be used to treat acne vulgaris and microbial infection [231,257,258,259]. In therapeutic PDT, the photosensitizers should absorb between 600 and 800 nm, and natural dyes might show potential as photosensitizers, which is worthy of further study.
In fact, there are some debates about the biological activity of curcumin because it is an unstable, reactive, nonbioavailable compound, which is also considered a PAINS medication [260]. Some studies have found no significant difference between curcumin and placebo [261], but some pharmacological activities of curcumin have been validated in animal experiments and clinical trials in recent years [262,263,264,265]. Many factors affect the activity of curcumin. The biggest problem is its poor water solubility, low absorption, and fast metabolization and clearance. Therefore, curcumin is not necessarily ineffective, which might be due to the low bioavailability. Recently, the interaction between gut microbiota and curcumin was hypothesized to explain how curcumin directly exerts its regulatory effects on the gut microbiota, thus explaining the paradox between its low systemic bioavailability and its wide pharmacological activities [266]. Interestingly, light irradiation could enhance the biological effects of curcumin. Low-dose curcumin plus visible light exposure could significantly inhibit metastatic processes of renal cell carcinoma [267], and light exposure might also enhance its efficacy in bladder cancer cell lines [268]. Exposure to light also enhanced its anti-microbial capacity because of curcumin phototoxicity in bacterial cells [269]. Therefore, although light affects the stability of some dyes, whether light exposure can enhance the pharmacological activities of other dyes is still a problem. The biological effects of the dyes regarding the illumination conditions should be studied. Even so, it is necessary to improve the stability and bioavailability of natural dyes, and nanoscale formulations are effective strategies and discussed in the next section.
2.5. Challenges and Potential of Natural Dyes
Although a variety of natural dyes have shown preventive and therapeutic effects on a variety of diseases, there are still some challenges in practical application. (1) Stability: Usage of this colorant in other products is limited by its poor stability to heat, light, and pH conditions. (2) Water solubility and bioaccessibility: Some natural dyes including carotenoids and curcumin have poor water solubility that limits their oral administration and decreases their bioavailability. (3) Resource constraints and extensive use: Some natural plant dyes will be limited by seasons and resources. In order to solve those problems, some strategies have been put forward.
2.5.1. Resources
According to the published literature, the study of marine natural products has increasingly captured the attention of scientists in recent years. Some new natural dyes including carotenoids and quinone dyes have been isolated from marine resources [23,24,51,77]. Additionally, new sources of natural dyes have been found in marine environments, such as a fast-growing strain of Chlorella saccharophila and a new variety of the ridgetail white prawn [270,271]. Marine resources show great potential for screening for new natural dyes and high-yield dye sources. In contrast to other resources, microorganisms have enormous advantages including rapid growth, easy processing, and independence from weather conditions. Apart from colorants, some bacterial, fungal, and microalgal dyes possess many biological properties such as antioxidant, anti-microbial, and anti-cancer activities [90,272]. Microorganisms are an abundant source of novel bioactive compounds and, unlike higher organisms, they are a source of easily renewable resources that give rise to production with a potentially greater yield [27,273]. Some pigment-producing microorganisms are easy to culture and have low production cost, so they are becoming more and more important application objects in the production of natural dyes, and have very broad application prospects. The optimized culture conditions could accelerate biosynthesis of the dyes and enhance dye production [272,274]. Therefore, exploration of marine resources and microorganism dyes is necessary.
2.5.2. Biotechnology
As described above, microorganisms have great potential in the development of natural dyes. In recent years, metabolic and genetic engineering approaches have been made to modify or introduce particular pathway genes into microorganisms to increase production of natural dyes, especially carotenoids and anthocyanins [275,276]. In recent years, it has been reported that heterologous genes were transferred into E. coli to synthesize carotenoids. E. coli has been engineered to produce various carotenoids, including lycopene, carotene, astaxanthin, and crocin [277]. Park et al. introduced heterologous crt genes (crtE, crtY, crtI, crtB, and crtZ) from Pantoea ananatis and the truncated BKT gene (trCrBKT) from Chlamydomonas reinhardtii to construct the astaxanthin biosynthetic pathway, and enhanced production of astaxanthin [278]. E. coli has been considered the most suitable for anthocyanin production in previous studies, and some anthocyanins were obtained from this microbial cell factory, such as cyanidin 3-O-glucoside and 3′-O-methylated and peonidin 3-O-glucoside [279,280]. Saccharomyces cerevisiae has also been used to produce anthocyanins in recent years. Eichenberger et al. engineered S. cerevisiae for de novo production of the three basic anthocyanins, pelargonidin-3-O-glucoside, cyanidin-3-O-glucoside, and delphinidin-3-O-glucoside [281]. An S. cerevisiae–S. cerevisiae co-culture platform was designed to manufacture two anthocyanidins in flask-scale culture [282]. Biotechnology makes the production of anthocyanin no longer dependent on plants alone. With optimizing the culture and advances in biotechnology, microbial cell factories are the most promising system to increase the yield of natural dyes for commercial and industrial purposes.
2.5.3. Efficient Extraction and Separation Strategy
In the actual development and application of natural dye, high-yield, lower-cost, and environment-friendly technology is also very important. In recent years, some novel extraction techniques have shown these advantages.
Ultrasound-assisted extraction is an effective method to extract natural dyes, and it has been widely used in the extraction of anthocyanins and carotenoids because of shorter extraction time, higher efficiency, and low solvent volumes [283,284]. The microwave-assisted extraction process is economical due to shorter extraction time and less solvent consumption. This method has been used to extract natural dyes from plants [285,286]. Supercritical fluid extraction is not only an advanced extraction technology for natural products, but also an environmentally friendly technology. Recent studies used supercritical CO2 to extract carotenoids and anthocyanins [287]. Regardless of the extraction method used, the optimized conditions should be screened and used to improve yield. In the separation of natural dyes, high-speed countercurrent chromatography (HSCCC) as a chromatographic technique of the liquid-liquid type with large sample recovery and low loss showed a bright prospect to separate natural dyes [288,289]. In fact, those techniques can be used in combination. In order to extract anthocyanins more efficiently, a novel procedure of ultrasound-assisted deep eutectic solvent extraction was proposed, and the HSCCC method was involved in the separation and purification of anthocyanins [289].
2.5.4. Improvement of Dye Stability
Co-Pigmentation
Co-pigments form noncovalent complexes with anthocyanins and have been used to enhance color and stability. Different co-pigments could change the properties of pigments by hyperchromic and bathochromic shifts [290]. Co-pigmentation of anthocyanins and phenolic compounds and plant extracts has been reported. Rosmarinic acid, syringic acid, and catechin showed significant hyperchromic effects for black chokeberry (Aronia melanocarpa) anthocyanin [291]. Molecular modeling results showed that multiple co-pigments may intensify the color of anthocyanins more than individual ligands, and phenolic acid–flavonol–anthocyanin could be used as promising food red-colorants [292]. Blueberry wines showed higher alcohol and titratable acidity, and lower sugar content by addition of caffeic acid, syringic acid, and quercetin co-pigments during fermentation [293]. Additionally, herbal extracts and plant extracts improved the stability of anthocyanins, hyperchromic effect, and color density [291,294]. Piperine could be used to increase the bioavailability of curcumin because of the molecular interactions of curcumin and piperine [295]. However, co-pigmentation depends on the intermolecular interaction between co-pigments and anthocyanins, and different co-pigments should be selected for different dyes.
Encapsulation
Encapsulation systems have been used to increase the solubility, chemical stability, pharmacological activity, and bioavailability of natural dyes. Maltodextrin, lipid-based nanocarriers including nanoliposomes, nanoemulsions and solid lipid nanoparticles [296], biopolymer-based nanocarriers such as proteins [297], carbohydrates, and chitosan [298], gold nanoparticles [299], and clay minerals [293] have been studied.
Liposomes have shown many advantages and attracted extensive attention because of their good stability, nontoxicity, water-solubility, good cell compatibility, and targeted delivery [300]. Based on the above advantages and colorless liposomes and the instability of natural dyes, liposomes are widely used to encapsulate natural dyes to improve their stability and targeting delivery. Carotenoids including lutein, β-carotene, lycopene, and canthaxanthin were encapsulated in liposomes and the delivery systems improved the antioxidant activity of carotenoids in a DPPH-scavenging assay and ferric reduction capacity assay [301]. A study demonstrated that propolis–lycopene nanoemulsions could protect skin against UVA radiation and confer better therapeutic effects [302]. In another study, lycopene was loaded on nanostructured lipid carriers and solid lipid nanoparticles [303], and they had a significantly improved effect on neuronal protection [296]. To protect the anthocyanin from adverse external conditions, liposomes were prepared with a supercritical carbon dioxide process and had improved efficacy and potential application in food and nutraceuticals [304]. In addition, anthocyanin-loaded liposomes could effectively enhance the stability of anthocyanin, antioxidant activity, and skin permeability [305]. Shikonin-loaded liposomes were prepared with 1,2-dipalmitoyl-phosphatidylcholine and egg phosphatidylcholine lipids, which could reduce the side effects, enhance selectivity to cancer cells and solubility, and protect shikonin from internal biotransformations and oxidization [306]. Curcumin-loaded liposomes have been extensively studied to solve the disadvantages including poor aqueous solubility and low bioavailability [307]. Betanin-nanoliposomes could improve the stability and antioxidant activity of betanin. Gummy candies using betanin-nanoliposomes showed no differences in the sensory properties [308].
Therefore, liposomes are considered to be ideal models for encapsulating natural dyes, which can improve stability, enhance the bioavailability, and achieve targeted delivery.
3. Conclusions and Future Prospects
Natural dyes widely exist in plants, anmimals and microorganisms, and show multiple healthy effects including antioxidant, anti-inflammatory, anti-cancer, anti-microbial, and anti-viral effects, and prevention and treatment of a variety of diseases, including diabetes, obesity, cardiovascular and cerebrovascular diseases, eye diseases, and nervous system diseases. Many natural dyes have been developed as drugs and functional foods. Some natural dyes have been approved by the FDA as food additives, such as curcumin and phycocyanin. Natural dyes show great advantages in safety, but there are still problems such as stability, solubility, and economic applicability. Furthermore, the unique feature that the biological effects of dyes are affected the illumination conditions (strict dark, or ambient light, or controlled illumination) should be paid attention. Some new strategies for these problems have been proposed. Natural dyes have great potential for the discovery of new drugs and functional food products. In terms of cost, marine resources and microorganisms are considered potential resources of natural dyes in the future. Some new technologies could be used for the production, extraction, and separation of natural dyes to improve the yield of natural dyes, such as metabolic and genetic engineering approaches, ultrasound-assisted extraction, and HSCCC. Co-pigments and encapsulation systems have been studied extensively and proven to improve the hyperchromic effect, stability, solubility, and bioavailability. Red, yellow, and blue dyes can be screened or modified from natural dyes, and other colors can be deployed in different proportions.
In conclusion, natural dyes play an important role in food, medicine, textile, and other industries, which make human life more colorful. This review classifies natural dyes by structural features and summarizes the research progress on natural dyes in the last ten years, including some of the newest dyes, pharmacological activities, and promising strategies for developing natural dyes. The review provides new insight for further development and potential applications of natural dyes.
Author Contributions
Conceptualization, H.C.; writing—original draft preparation, N.L.; investigation, Q.W.; formal analysis, S.L.; investigation, N.L.; data curation, J.Z.; writing—review and editing, N.L.; visualization, J.L.; supervision, H.C.; pfunding acquisition, H.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
There are no conflict of interest to declare.
Funding Statement
This research was funded by a grant from the National Key Research and Development Program of China, grant number 2021YFE0110000.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reyes F.G., Valim M.F., Vercesi A.E. Effect of Organic Synthetic Food Colours on Mitochondrial Respiration. Food Addit. Contam. 1996;13:5–11. doi: 10.1080/02652039609374376. [DOI] [PubMed] [Google Scholar]
- 2.Giulieri F., Ovarlez S., Chaze A.M. Indigo/Sepiolite Nanohybrids: Stability of Natural Pigments Inspired by Maya Blue. Int. J. Nanotechnol. 2012;9:605–617. doi: 10.1504/IJNT.2012.045334. [DOI] [Google Scholar]
- 3.Wrolstad R.E., Culver C.A. Alternatives to Those Artificial FD&C Food Colorants. Annu. Rev. Food Sci. Technol. 2012;3:59–77. doi: 10.1146/annurev-food-022811-101118. [DOI] [PubMed] [Google Scholar]
- 4.Newsome A.G., Culver C.A., Van Breemen R.B. Nature’s Palette: The Search for Natural Blue Colorants. J. Agric. Food Chem. 2014;62:6498–6511. doi: 10.1021/jf501419q. [DOI] [PubMed] [Google Scholar]
- 5.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2020;372:n71. doi: 10.1136/bmj.n71.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. J. Clin. Epidemiol. 2021;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Tian L., Tan Y., Chen G., Wang G., Sun J., Ou S., Chen W., Bai W. Metabolism of Anthocyanins and Consequent Effects on the Gut Microbiota. Crit. Rev. Food Sci. Nutr. 2019;59:982–991. doi: 10.1080/10408398.2018.1533517. [DOI] [PubMed] [Google Scholar]
- 8.Maoka T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020;74:1–16. doi: 10.1007/s11418-019-01364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S., Mu B., Wang X., Wang A. Recent Researches on Natural Pigments Stabilized by Clay Minerals: A Review. Dye. Pigment. 2021;190:109322. doi: 10.1016/j.dyepig.2021.109322. [DOI] [Google Scholar]
- 10.Bayer E., Egeter H., Fink A., Nether K., Wegmann K. Complex Formation and Flower Colors. Angew. Chemie Int. Ed. Engl. 1966;5:791–798. doi: 10.1002/anie.196607911. [DOI] [Google Scholar]
- 11.Yungyuen W., Vo T.T., Uthairatanakij A., Ma G., Zhang L.C., Tatmala N., Kaewsuksaeng S., Jitareerat P., Kato M. Carotenoid Accumulation and the Expression of Carotenoid Metabolic Genes in Mango during Fruit Development and Ripening. Appl. Sci. 2021;11:4249. doi: 10.3390/app11094249. [DOI] [Google Scholar]
- 12.Yu J.H., Gleize B., Zhang L.F., Caris-Veyrat C., Renard C. A D-Optimal Mixture Design of Tomato-Based Sauce Formulations: Effects of Onion and EVOO on Lycopene Isomerization and Bioaccessibility. Food Funct. 2019;10:3589–3602. doi: 10.1039/C9FO00208A. [DOI] [PubMed] [Google Scholar]
- 13.Li G., Meng X., Zhu M., Li Z. Research Progress of Betalain in Response to Adverse Stresses and Evolutionary Relationship Compared with Anthocyanin. Molecules. 2019;119:3078. doi: 10.3390/molecules24173078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landi M., Tattini M., Gould K.S. Multiple Functional Roles of Anthocyanins in Plant-Environment Interactions. Environ. Exp. Bot. 2015;119:4–17. doi: 10.1016/j.envexpbot.2015.05.012. [DOI] [Google Scholar]
- 15.Gong K., Pan Y., Rather L.J., Wang W., Zhou Q., Zhang T., Li Q. Natural Pigment during Flora Leaf Senescence and Its Application in Dyeing and UV Protection Finish of Silk and Wool—A Case Study of Cinnamomum Camphora. Dye. Pigment. 2019;166:114–121. doi: 10.1016/j.dyepig.2019.03.037. [DOI] [Google Scholar]
- 16.Borges M.E., Tejera R.L., Díaz L., Esparza P., Ibáñez E. Natural Dyes Extraction from Cochineal (Dactylopius Coccus). New Extraction Methods. Food Chem. 2012;132:1855–1860. doi: 10.1016/j.foodchem.2011.12.018. [DOI] [Google Scholar]
- 17.Fernández-López J.A., Angosto J.M., Giménez P.J., León G. Thermal Stability of Selected Natural Red Extracts Used as Food Colorants. Plant Foods Hum. Nutr. 2013;68:11–17. doi: 10.1007/s11130-013-0337-1. [DOI] [PubMed] [Google Scholar]
- 18.Scurria A., Tixier A.S.F., Lino C., Pagliaro M., D’Agostino F., Avellone G., Chemat F., Ciriminna R. High Yields of Shrimp Oil Rich in Omega-3 and Natural Astaxanthin from Shrimp Waste. ACS OMEGA. 2020;5:17500–17505. doi: 10.1021/acsomega.0c01978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tizkar B., Seidavi A., Ponce-Palafox J.T., Pourashoori P. The Effect of Astaxanthin on Resistance of Juvenile Prawns Macrobrachium Nipponense (Decapoda: Palaemonidae) to Physical and Chemical Stress. Rev. Biol. Trop. 2014;62:1331–1341. doi: 10.15517/rbt.v62i4.13057. [DOI] [PubMed] [Google Scholar]
- 20.Calvo N.S., Reynoso C.M., Resnik S., Cortés-Jacinto E., Collins P. Thermal Stability of Astaxanthin in Oils for Its Use in Fish Food Technology. Anim. Feed Sci. Technol. 2020;270:114668. doi: 10.1016/j.anifeedsci.2020.114668. [DOI] [Google Scholar]
- 21.Santos R., Hallett J., Oliveira M.C., Sousa M.M., Sarraguça J., Simmonds M.S.J., Nesbitt M. HPLC-DAD-MS Analysis of Colorant and Resinous Components of Lac-Dye: A Comparison between Kerria and Paratachardina Genera. Dye. Pigment. 2015;118:129–136. doi: 10.1016/j.dyepig.2015.02.024. [DOI] [Google Scholar]
- 22.Berbers S.V.J., Tamburini D., van Bommel M.R., Dyer J. Historical Formulations of Lake Pigments and Dyes Derived from Lac: A Study of Compositional Variability. Dye. Pigment. 2019;170:107579. doi: 10.1016/j.dyepig.2019.107579. [DOI] [Google Scholar]
- 23.Vemulapalli S.P.B., Fuentes-Monteverde J.C., Karschin N., Oji T., Griesinger C., Wolkenstein K. Structure and Absolute Configuration of Phenanthro-Perylene Quinone Pigments from the Deep-Sea Crinoid Hypalocrinus Naresianus. Mar. Drugs. 2021;19:445. doi: 10.3390/md19080445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolkenstein K., Fuentes-Monteverde J.C., Nath N., Oji T., Griesinger C. Hypalocrinins, Taurine-Conjugated Anthraquinone and Biaryl Pigments from the Deep Sea Crinoid Hypalocrinus Naresianus. J. Nat. Prod. 2019;82:163–167. doi: 10.1021/acs.jnatprod.8b00803. [DOI] [PubMed] [Google Scholar]
- 25.Aylward F.O., Eppley J.M., Smith J.M., Chavez F.P., Scholin C.A., DeLong E.F. Microbial Community Transcriptional Networks Are Conserved in Three Domains at Ocean Basin Scales. Proc. Natl. Acad. Sci. USA. 2015;112:5443–5448. doi: 10.1073/pnas.1502883112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soliev A.B., Hosokawa K., Enomoto K. Bioactive Pigments from Marine Bacteria: Applications and Physiological Roles. Evid.-Based Complement. Altern. Med. 2011;2011:670349. doi: 10.1155/2011/670349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Numan M., Bashir S., Mumtaz R., Tayyab S., Rehman N.U., Khan A.L., Shinwari Z.K., Al-Harrasi A. Therapeutic Applications of Bacterial Pigments: A Review of Current Status and Future Opportunities. 3 Biotech. 2018;8:207. doi: 10.1007/s13205-018-1227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venil C.K., Zakaria Z.A., Ahmad W.A. Bacterial Pigments and Their Applications. Process Biochem. 2013;48:1065–1079. doi: 10.1016/j.procbio.2013.06.006. [DOI] [Google Scholar]
- 29.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H.G., Prinsep M.R. Marine Natural Products. Nat. Prod. Rep. 2013;30:237–323. doi: 10.1039/C2NP20112G. [DOI] [PubMed] [Google Scholar]
- 30.Ramesh C., Vinithkumar N.V., Kirubagaran R., Venil C.K., Dufossé L. Multifaceted Applications of Microbial Pigments: Current Knowledge, Challenges and Future Directions for Public Health Implications. Microorganisms. 2019;7:186. doi: 10.3390/microorganisms7070186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gmoser R., Ferreira J.A., Lennartsson P.R., Taherzadeh M.J. Filamentous Ascomycetes Fungi as a Source of Natural Pigments. Fungal Biol. Biotechnol. 2017;4:4. doi: 10.1186/s40694-017-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Souza P.N.D., Grigoletto T.L.B., de Moraes L.A.B., Abreu L.M., Guimaraes L.H.S., Santos C., Galvao L.R., Cardoso P.G. Production and Chemical Characterization of Pigments in Filamentous Fungi. Microbiology. 2016;162:12–22. doi: 10.1099/mic.0.000168. [DOI] [PubMed] [Google Scholar]
- 33.Dufosse L. Microbial Production of Food Grade Pigments. Food Technol. Biotechnol. 2006;44:313–321. [Google Scholar]
- 34.Kim D., Ku S. Beneficial Effects of Monascus Sp. KCCM 10093 Pigments and Derivatives: A Mini Review. Molecules. 2018;23:98. doi: 10.3390/molecules23010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joshi V.K., Attri D., Bala A., Bhushan S. Microbial Pigments. Indian J. Biotechnol. 2003;2:362–369. [Google Scholar]
- 36.Feng Y., Shao Y., Chen F. Monascus Pigments. Appl. Microbiol. Biotechnol. 2012;96:1421–1440. doi: 10.1007/s00253-012-4504-3. [DOI] [PubMed] [Google Scholar]
- 37.Manikprabhu D., Lingappa K. Gamma Actinorhodin a Natural and Attorney Source for Synthetic Dye to Detect Acid Production of Fungi. Saudi J. Biol. Sci. 2013;20:163–168. doi: 10.1016/j.sjbs.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neiman M.K., Balonis M., Kakoulli I. Cinnabar Alteration in Archaeological Wall Paintings: An Experimental and Theoretical Approach. Appl. Phys. A Mater. Sci. Process. 2015;121:915–938. doi: 10.1007/s00339-015-9456-x. [DOI] [Google Scholar]
- 39.Pérez-Arantegui J., Pardos C., Abad J.L., García J.R. Microcharacterization of a Natural Blue Pigment Used in Wall Paintings during the Romanesque Period in Northern Spain. Microsc. Microanal. 2013;19:1645–1652. doi: 10.1017/S1431927613013391. [DOI] [PubMed] [Google Scholar]
- 40.Škvarlová A., Kaňuchová M., Kozáková Ľ., Valušová E., Holub M., Škvarla J. Preparation and Characterization of Ultramarine Blue Pigments from Fly Ash by Using the X-Ray Photoelectron Spectroscopy (XPS) for the Determination of Chemical States of Sulphur in Chromophores. Microporous Mesoporous Mater. 2019;284:283–288. doi: 10.1016/j.micromeso.2019.04.039. [DOI] [Google Scholar]
- 41.Borhade A.V., Kshirsagar T.A., Dholi A.G. Novel Synthesis of Ultramarine Blue from Waste Coal Fly Ash via Thiocyanate Aluminosilicate Sodalite. J. Sulfur Chem. 2016;37:632–645. doi: 10.1080/17415993.2016.1173215. [DOI] [Google Scholar]
- 42.Grabowska M., Wawrzyniak D., Rolle K., Chomczyński P., Oziewicz S., Jurga S., Barciszewski J. Let Food Be Your Medicine: Nutraceutical Properties of Lycopene. Food Funct. 2019;10:3090–3102. doi: 10.1039/C9FO00580C. [DOI] [PubMed] [Google Scholar]
- 43.Heider S.A.E., Peters-Wendisch P., Wendisch V.F., Beekwilder J., Brautaset T. Metabolic Engineering for the Microbial Production of Carotenoids and Related Products with a Focus on the Rare C50 Carotenoids. Appl. Microbiol. Biotechnol. 2014;98:4355–4368. doi: 10.1007/s00253-014-5693-8. [DOI] [PubMed] [Google Scholar]
- 44.Avalos J., Limon M.C. Biological Roles of Fungal Carotenoids. Curr. Genet. 2015;61:309–324. doi: 10.1007/s00294-014-0454-x. [DOI] [PubMed] [Google Scholar]
- 45.Britton G., Liaanen-Jensen S., Pfander H. Carotenoids Handbook. Birkhäuser; Basel, Switzerland: 2004. Abstract. [Google Scholar]
- 46.Grune T., Lietz G., Palou A., Ross A.C., Stahl W., Tang G.W., Thurnham D., Yin S.A., Biesalski H.K. Beta-Carotene is an Important Vitamin A Source for Humans. J. Nutr. 2010;140:2268S–2285S. doi: 10.3945/jn.109.119024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pashkow F.J., Watumull D.G., Campbell C.L. Astaxanthin: A Novel Potential Treatment for Oxidative Stress and Inflammation in Cardiovascular Disease. Am. J. Cardiol. 2008;101:S58–S68. doi: 10.1016/j.amjcard.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Hojjati M., Razavi S.H., Rezaei K., Gilani K. Stabilization of Canthaxanthin Produced by Dietzia Natronolimnaea HS-1 with Spray Drying Microencapsulation. J. Food Sci. Technol. 2014;51:2134–2140. doi: 10.1007/s13197-012-0713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Das A., Yoon S.H., Lee S.H., Kim J.Y., Oh D.K., Kim S.W. An Update on Microbial Carotenoid Production: Application of Recent Metabolic Engineering Tools. Appl. Microbiol. Biotechnol. 2007;77:505–512. doi: 10.1007/s00253-007-1206-3. [DOI] [PubMed] [Google Scholar]
- 50.Gammone M.A., Riccioni G., D’Orazio N. Marine Carotenoids against Oxidative Stress: Effects on Human Health. Mar. Drugs. 2015;13:6226–6246. doi: 10.3390/md13106226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maoka T. New Acetylenic Carotenoid 6′-Epimonadoxanthin from the Rosary Goby Gymnogobius Castaneus. J. Oleo Sci. 2018;67:1259–1263. doi: 10.5650/jos.ess18153. [DOI] [PubMed] [Google Scholar]
- 52.Murillo E., Mosquera Y., Kurtán T., Gulyás-Fekete G., Nagy V., Deli J. Isolation and Characterization of Novel Capsorubin-like Carotenoids from the Red Mamey (Pouteria sapota) Helv. Chim. Acta. 2012;95:983–988. doi: 10.1002/hlca.201100493. [DOI] [Google Scholar]
- 53.Shindo K., Misawa N. New and Rare Carotenoids Isolated from Marine Bacteria and Their Antioxidant Activities. Mar. Drugs. 2014;12:1690–1698. doi: 10.3390/md12031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Long J.T., Fan H.X., Zhou Z.Q., Sun W.Y., Li Q.W., Wang Y., Ma M., Gao H., Zhi H. The Major Zeaxanthin Dipalmitate Derivatives from Wolfberry. J. Asian Nat. Prod. Res. 2020;22:746–753. doi: 10.1080/10286020.2019.1621855. [DOI] [PubMed] [Google Scholar]
- 55.Su X., Xu J., Rhodes D., Shen Y., Song W., Katz B., Tomich J., Wang W. Identification and Quantification of Anthocyanins in Transgenic Purple Tomato. Food Chem. 2016;202:184–188. doi: 10.1016/j.foodchem.2016.01.128. [DOI] [PubMed] [Google Scholar]
- 56.Wang H., Sun S., Zhou Z., Qiu Z., Cui X. Rapid Analysis of Anthocyanin and Its Structural Modi Fi Cations in Fresh Tomato Fruit. Food Chem. 2020;333:127439. doi: 10.1016/j.foodchem.2020.127439. [DOI] [PubMed] [Google Scholar]
- 57.Osorio C., Hurtado N., Dawid C., Hofmann T., Heredia-Mira F.J., Morales A.L. Chemical Characterisation of Anthocyanins in Tamarillo (Solanum betaceum Cav.) and Andes Berry (Rubus glaucus Benth.) Fruits. Food Chem. 2012;132:1915–1921. doi: 10.1016/j.foodchem.2011.12.026. [DOI] [Google Scholar]
- 58.Wang Y.M., Yu C.H., Zhao X.J., Zhao J.Q. A Rapid High-Performance Liquid Chromatography Separation of a New Anthocyanin from Nitraria Tangutorum. J. Asian Nat. Prod. Res. 2020;22:503–507. doi: 10.1080/10286020.2019.1593968. [DOI] [PubMed] [Google Scholar]
- 59.Engmann N.F., Ma Y.K., Ying X., Qing Y. Investigating the Effect of High Hydrostatic Pressure Processing on Anthocyanins Composition of Mulberry (Morus moraceae) Juice. Czech J. Food Sci. 2013;31:72–80. doi: 10.17221/530/2011-CJFS. [DOI] [Google Scholar]
- 60.Tatsuzawa F., Kato K., Sato M., Ito S., Muraoka H., Takahata Y., Ogawa S. Acylated Cyanidin 3-Sophoroside-5-Glucoside in Purple-Violet Flowers of Moricandia Arvensis (Brassicaceae) Nat. Prod. Commun. 2015;10:457–459. doi: 10.1177/1934578X1501000321. [DOI] [PubMed] [Google Scholar]
- 61.Skaar I., Jordheim M., Byamukama R., Mbabazi A., Wubshet S.G., Kiremire B., Andersen Ø.M. New Anthocyanidin and Anthocyanin Pigments from Blue Plumbago. J. Agric. Food Chem. 2012;60:1510–1515. doi: 10.1021/jf2048004. [DOI] [PubMed] [Google Scholar]
- 62.Jin H., Liu Y., Guo Z., Yang F., Wang J., Li X., Peng X., Liang X. High-Performance Liquid Chromatography Separation of Cis—Trans Anthocyanin Isomers from Wild Lycium Ruthenicum Murr. Employing a Mixed-Mode Reversed-Phase/Strong Anion-Exchange Stationary Phase. J. Agric. Food Chem. 2015;63:500–508. doi: 10.1021/jf504525w. [DOI] [PubMed] [Google Scholar]
- 63.Kondo T., Hagihara S., Takaya Y., Yoshida K. Polyacylated Anthocyanins in Bluish-Purple Petals of Chinese Bellflower, Platycodon Grandiflorum. Int. J. Mol. Sci. 2021;22:4044. doi: 10.3390/ijms22084044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moriya C., Hosoya T., Agawa S., Sugiyama Y., Kozone I., Shin-Ya K., Terahara N., Kumazawa S. New Acylated Anthocyanins from Purple Yam and Their Antioxidant Activity. Biosci. Biotechnol. Biochem. 2015;79:1484–1492. doi: 10.1080/09168451.2015.1027652. [DOI] [PubMed] [Google Scholar]
- 65.Nabuurs M.H., McCallum J.L., Brown D.C., Kirby C.W. NMR Characterization of Novel Pyranoanthocyanins Derived from the Pulp of Panax Quinquefolius L. (North American Ginseng) Magn. Reson. Chem. 2017;55:177–182. doi: 10.1002/mrc.4366. [DOI] [PubMed] [Google Scholar]
- 66.Yang X.W., Song C.W., Zhang Y., Khan A., Jiang L.P., Chen Y.B., Liu Y.P., Luo X.D. Alstoscholarisines F and G, Two Unusual Monoterpenoid Indole Alkaloids from the Leaves of Alstonia Scholaris. Tetrahedron Lett. 2015;56:6715–6718. doi: 10.1016/j.tetlet.2015.10.051. [DOI] [Google Scholar]
- 67.Nakano H., Yoshida M., Kaji R., Sakai M., Doi S., Kosemura S. Oryzamutaic Acid K and Oryzadiamine C, Alkaloids from an Oryza Sativa Mutant with Yellow Endosperm. Tetrahedron Lett. 2020;61:152381. doi: 10.1016/j.tetlet.2020.152381. [DOI] [Google Scholar]
- 68.Nakano H., Ono H., Kaji R., Sakai M., Doi S., Kosemura S. Oryzadiamines A and B, Alkaloids from Oryza Sativa with Yellow Grain. Tetrahedron Lett. 2020;61:151519. doi: 10.1016/j.tetlet.2019.151519. [DOI] [Google Scholar]
- 69.Lohmann J.S., von Nussbaum M., Brandt W., Mülbradt J., Steglich W., Spiteller P. Rosellin A and B, Two Red Diketopiperazine Alkaloids from the Mushroom Mycena Rosella. Tetrahedron. 2018;74:5113–5118. doi: 10.1016/j.tet.2018.06.049. [DOI] [Google Scholar]
- 70.Lünne F., Köhler J., Stroh C., Müller L., Daniliuc C.G., Mück-Lichtenfeld C., Würthwein E.U., Esselen M., Humpf H.U., Kalinina S.A. Insights into Ergochromes of the Plant Pathogen Claviceps Purpurea. J. Nat. Prod. 2021;84:2630–2643. doi: 10.1021/acs.jnatprod.1c00264. [DOI] [PubMed] [Google Scholar]
- 71.Abdelfattah M.S., Toume K., Arai M.A., Masu H., Ishibashi M. Katorazone, a New Yellow Pigment with a 2-Azaquinone-Phenylhydrazone Structure Produced by Streptomyces Sp. IFM 11299. Tetrahedron Lett. 2012;53:3346–3348. doi: 10.1016/j.tetlet.2012.04.073. [DOI] [Google Scholar]
- 72.Pradeep F.S., Palaniswamy M., Ravi S., Thangamani A., Pradeep B.V. Larvicidal Activity of a Novel Isoquinoline Type Pigment from Fusarium Moniliforme KUMBF1201 against Aedes Aegypti and Anopheles Stephensi. Process Biochem. 2015;50:1479–1486. doi: 10.1016/j.procbio.2015.05.022. [DOI] [Google Scholar]
- 73.Lystvan K., Kumorkiewicz A., Szneler E., Wybraniec S. Study on Betalains in Celosia Cristata Linn. Callus Culture and Identification of New Malonylated Amaranthins. J. Agric. Food Chem. 2018;66:3870–3879. doi: 10.1021/acs.jafc.8b01014. [DOI] [PubMed] [Google Scholar]
- 74.Basnet B.B., Liu L., Zhao W., Liu R., Ma K., Bao L., Ren J., Wei X., Yu H., Wei J., et al. New 1, 2-Naphthoquinone-Derived Pigments from the Mycobiont of Lichen Trypethelium Eluteriae Sprengel. Nat. Prod. Res. 2019;33:2044–2050. doi: 10.1080/14786419.2018.1484458. [DOI] [PubMed] [Google Scholar]
- 75.Chagas F.O., Dias L.G., Pupo M.T. New Perylenequinone Derivatives from the Endophytic Fungus Alternaria Tenuissima SS77. Tetrahedron Lett. 2016;57:3185–3189. doi: 10.1016/j.tetlet.2016.06.035. [DOI] [Google Scholar]
- 76.Ben Ammar R., Miyamoto T., Chekir-Ghedira L., Ghedira K., Lacaille-Dubois M.A. Isolation and Identification of New Anthraquinones from Rhamnus Alaternus L and Evaluation of Their Free Radical Scavenging Activity. Nat. Prod. Res. 2019;33:280–286. doi: 10.1080/14786419.2018.1446135. [DOI] [PubMed] [Google Scholar]
- 77.Khokhar S., Pierens G.K., Hooper J.N.A., Ekins M.G., Feng Y., Davis R.A. Rhodocomatulin-Type Anthraquinones from the Australian Marine Invertebrates Clathria Hirsuta and Comatula Rotalaria. J. Nat. Prod. 2016;79:946–953. doi: 10.1021/acs.jnatprod.5b01029. [DOI] [PubMed] [Google Scholar]
- 78.Kopa T.K., Tchinda A.T., Tala M.F., Zofou D., Jumbam R., Wabo H.K., Titanji V.P.K., Frédérich M., Tan N.H., Tane P. Antiplasmodial Anthraquinones and Hemisynthetic Derivatives from the Leaves of Tectona Grandis (Verbenaceae) Phytochem. Lett. 2014;8:41–45. doi: 10.1016/j.phytol.2014.01.010. [DOI] [Google Scholar]
- 79.Klaiklay S., Rukachaisirikul V., Phongpaichit S., Pakawatchai C., Saithong S., Buatong J., Preedanon S., Sakayaroj J. Anthraquinone Derivatives from the Mangrove-Derived Fungus Phomopsis Sp. PSU-MA214. Phytochem. Lett. 2012;5:738–742. doi: 10.1016/j.phytol.2012.08.003. [DOI] [Google Scholar]
- 80.Chen J., Xu B., Sun J., Jiang X., Bai W. Anthocyanin Supplement as a Dietary Strategy in Cancer Prevention and Management: A Comprehensive Review. Crit. Rev. Food. Sci. 2021:1–13. doi: 10.1080/10408398.2021.1913092. [DOI] [PubMed] [Google Scholar]
- 81.Kang S., Wang H., Guo M., Zhang L., Chen M., Jiang S., Li X., Jiang S. Ethylene-Vinyl Alcohol Copolymer-Montmorillonite Multilayer Barrier Film Coated with Mulberry Anthocyanin for Freshness Monitoring. J. Agric. Food Chem. 2018;66:13268–13276. doi: 10.1021/acs.jafc.8b05189. [DOI] [PubMed] [Google Scholar]
- 82.Zhang J., Zou X., Zhai X., Huang X.W., Jiang C., Holmes M. Preparation of an Intelligent PH Film Based on Biodegradable Polymers and Roselle Anthocyanins for Monitoring Pork Freshness. Food Chem. 2019;272:306–312. doi: 10.1016/j.foodchem.2018.08.041. [DOI] [PubMed] [Google Scholar]
- 83.Bueno J.M., Saez-Plaza P., Ramos-Escudero F., Jimenez A.M., Fett R., Asuero A.G. Analysis and Antioxidant Capacity of Anthocyanin Pigments. Part II: Chemical Structure, Color, and Intake of Anthocyanins. Crit. Rev. Anal. Chem. 2012;42:126–151. doi: 10.1080/10408347.2011.632314. [DOI] [Google Scholar]
- 84.De Freitas V., Mateus N. Chemical Transformations of Anthocyanins Yielding a Variety of Colours (Review) Environ. Chem. Lett. 2006;4:175–183. doi: 10.1007/s10311-006-0060-3. [DOI] [Google Scholar]
- 85.Pereira D.M., Valentao P., Pereira J.A., Andrade P.B. Phenolics: From Chemistry to Biology. Molecules. 2009;14:2202–2211. doi: 10.3390/molecules14062202. [DOI] [Google Scholar]
- 86.Cardoso A.L., Di Pietro P.F., Vieira F.G.K., Boaventura B.C.B., de Liz S., Borges G.D.C., Fett R., de Andrade D.F., da Silva E.L. Acute Consumption of Jucara Juice (Euterpe Edulis) and Antioxidant Activity in Healthy Individuals. J. Funct. Foods. 2015;17:152–162. doi: 10.1016/j.jff.2015.05.014. [DOI] [Google Scholar]
- 87.Tsuda T. Curcumin as a Functional Food-Derived Factor: Degradation Products, Metabolites, Bioactivity, and Future Perspectives. Food Funct. 2018;9:705–714. doi: 10.1039/C7FO01242J. [DOI] [PubMed] [Google Scholar]
- 88.Aliabbasi N., Fathi M., Emam-Djomeh Z. Curcumin: A Promising Bioactive Agent for Application in Food Packaging Systems. J. Environ. Chem. Eng. 2021;9:105520. doi: 10.1016/j.jece.2021.105520. [DOI] [Google Scholar]
- 89.Tezcan A., Aslan G.E., Kaman H. Evaluation of drought stress on the chlorophyll content of the plants: A review of the solanaceae family. Fresenius Environ. Bull. 2019;28:4636–4641. [Google Scholar]
- 90.Manivasagan P., Bharathiraja S., Moorthy M.S., Mondal S., Seo H., Lee K.D., Oh J. Marine Natural Pigments as Potential Sources for Therapeutic Applications. Crit. Rev. Biotechnol. 2018;38:745–761. doi: 10.1080/07388551.2017.1398713. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y.Q., Gao Z.Q. Contrasting Chlorophyll-a Seasonal Patterns between Nearshore and Offshore Waters in the Bohai and Yellow Seas, China: A New Analysis Using Improved Satellite Data. Cont. Shelf Res. 2020;203:104173. doi: 10.1016/j.csr.2020.104173. [DOI] [Google Scholar]
- 92.Ito H., Ohtsuka T., Tanaka A. Conversion of Chlorophyll b to Chlorophyll a via 7-Hydroxymethyl Chlorophyll. J. Biol. Chem. 1996;271:1475–1479. doi: 10.1074/jbc.271.3.1475. [DOI] [PubMed] [Google Scholar]
- 93.Chen M. Chapter Four—Chlorophylls d and f: Synthesis, Occurrence, Light-Harvesting, and Pigment Organization in Chlorophyll-Binding Protein Complexes. In: Grimm B., editor. Metabolism, Structure and Function of Plant Tetrapyrroles: Introduction, Microbial and Eukaryotic Chlorophyll Synthesis and Catabolism. Volume 90. Academic Press; Cambridge, MA, USA: 2019. pp. 121–139. [Google Scholar]
- 94.Tutunchi P., Roufegarinejad L., Hamishehkar H., Alizadeh A. Extraction of Red Beet Extract with β-Cyclodextrin-Enhanced Ultrasound Assisted Extraction: A Strategy for Enhancing the Extraction Efficacy of Bioactive Compounds and Their Stability in Food Models. Food Chem. 2019;297:124994. doi: 10.1016/j.foodchem.2019.124994. [DOI] [PubMed] [Google Scholar]
- 95.Khan M.I. Plant Betalains: Safety, Antioxidant Activity, Clinical Efficacy, and Bioavailability. Compr. Rev. Food Sci. Food Saf. 2016;15:316–330. doi: 10.1111/1541-4337.12185. [DOI] [PubMed] [Google Scholar]
- 96.Roriz C.L., Barreira J.C.M., Morales P., Barros L., Ferreira I.C.F.R. Gomphrena Globosa L. as a Novel Source of Food-Grade Betacyanins: Incorporation in Ice-Cream and Comparison with Beet-Root Extracts and Commercial Betalains. LWT. 2018;92:101–107. doi: 10.1016/j.lwt.2018.02.009. [DOI] [Google Scholar]
- 97.Deladino L., Alvarez I., De Ancos B., Sánchez-Moreno C., Molina-García A.D., Schneider Teixeira A. Betalains and Phenolic Compounds of Leaves and Stems of Alternanthera Brasiliana and Alternanthera Tenella. Food Res. Int. 2017;97:240–249. doi: 10.1016/j.foodres.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 98.Escribano J., Cabanes J., Jiménez-Atiénzar M., Ibañez-Tremolada M., Gómez-Pando L.R., García-Carmona F., Gandía-Herrero F. Characterization of Betalains, Saponins and Antioxidant Power in Differently Colored Quinoa (Chenopodium quinoa) Varieties. Food Chem. 2017;234:285–294. doi: 10.1016/j.foodchem.2017.04.187. [DOI] [PubMed] [Google Scholar]
- 99.Ferreira E.S.B., Hulme A.N., McNab H., Quye A. The Natural Constituents of Historical Textile Dyes. Chem. Soc. Rev. 2004;33:329–336. doi: 10.1039/b305697j. [DOI] [PubMed] [Google Scholar]
- 100.Clark R.J.H., Cooksey C.J., Daniels M.A.M., Withnall R. Indigo, Woad, and Tyrian Purple: Important Vat Dyes from Antiquity to the Present. Endeavour. 1993;17:191–199. doi: 10.1016/0160-9327(93)90062-8. [DOI] [Google Scholar]
- 101.De Melo J.S., Moura A.P., Melo M.J. Photophysical and Spectroscopic Studies of Indigo Derivatives in Their Keto and Leuco Forms. J. Phys. Chem. A. 2004;108:6975–6981. doi: 10.1021/jp049076y. [DOI] [Google Scholar]
- 102.Hong H., Conover C.M., Hofsommer D.T., Sanz C.A., Hicks R.G. Discovery and Properties of a New Indigoid Structure Type Based on Dimeric: Cis -Indigos. Org. Biomol. Chem. 2020;18:5838–5842. doi: 10.1039/D0OB01368D. [DOI] [PubMed] [Google Scholar]
- 103.Sharma V., McKone H.T., Markow P.G. A Global Perspective on the History, Use, and Identification of Synthetic Food Dyes. J. Chem. Educ. 2011;88:24–28. doi: 10.1021/ed100545v. [DOI] [Google Scholar]
- 104.Lai J.L., Liu Y.H., Liu C., Qi M.P., Liu R.N., Zhu X.F., Zhou Q.G., Chen Y.Y., Guo A.Z., Hu C.M. Indirubin Inhibits LPS-Induced Inflammation via TLR4 Abrogation Mediated by the NF-KB and MAPK Signaling Pathways. Inflammation. 2017;40:1–12. doi: 10.1007/s10753-016-0447-7. [DOI] [PubMed] [Google Scholar]
- 105.Jiang Y., Niu T.C., Wang Z.H., Tan W.S., Liu F., Kong Y. Electrochemical Polymerization of Alizarin and the Electrochemical Properties of Poly (Alizarin) Ionics. 2018;24:1391–1397. doi: 10.1007/s11581-017-2288-2. [DOI] [Google Scholar]
- 106.Gao J.Q., Guo Y.W., Wang J., Jin X.D., Wang Z.Q., Fan T.T., Li K., Xu Y.N. Spectroscopic Analysis of the Interactions of Anthraquinone Derivatives (Alizarin, Alizarin-DA and Alizarin-DA-Fe) with Bovine Serum Albumin (BSA) J. Solut. Chem. 2011;40:876–888. doi: 10.1007/s10953-011-9692-4. [DOI] [Google Scholar]
- 107.Liu J.M., Gao H., Li F.M., Liu Y.L., Liu J.Q., Ou-yang M.L., Wang H.X., Lin S.Q., Lin C.Q., Li Z.M. Determination of trace deoxyribonucleic acid based on a room temperature phosphorescent probe of alizarin red-piperidine self-ordered ring. Anal. Lett. 2010;43:300–311. doi: 10.1080/00032710903325864. [DOI] [Google Scholar]
- 108.Yao G.Y., Dai W.L., Ye M.Y., Huang R.Z., Pan Y.M., Liao Z.X., Wang H.S. Synthesis and Antitumor Properties of Novel Alizarin Analogs. Med. Chem. Res. 2014;23:5031–5042. doi: 10.1007/s00044-014-1062-5. [DOI] [Google Scholar]
- 109.Zou Z., He D.G., Cai L.L., He X.X., Wang K.M., Yang X., Li L.L., Li S.Q., Su X.Y. Alizarin Complexone Functionalized Mesoporous Silica Nanoparticles: A Smart System Integrating Glucose-Responsive Double-Drugs Release and Real-Time Monitoring Capabilities. ACS Appl. Mater. Interfaces. 2016;8:8358–8366. doi: 10.1021/acsami.5b12576. [DOI] [PubMed] [Google Scholar]
- 110.Auber R.P., Suttiyut T., McCoy R.M., Ghaste M., Crook J.W., Pendleton A.L., Widhalm J.R., Wisecaver J.H. Hybrid de Novo Genome Assembly of Red Gromwell (Lithospermum Erythrorhizon) Reveals Evolutionary Insight into Shikonin Biosynthesis. Hortic. Res. 2020;7:82. doi: 10.1038/s41438-020-0301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang R., Zhang X., Song H., Zhou S., Li S. Synthesis and Evaluation of Novel Alkannin and Shikonin Oxime Derivatives as Potent Antitumor Agents. Bioorganic Med. Chem. Lett. 2014;24:4304–4307. doi: 10.1016/j.bmcl.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 112.Ho X.L., Liu J.J.H., Loke W.M. Plant Sterol-Enriched Soy Milk Consumption Modulates 5-Lipoxygenase, 12-Lipoxygenase, and Myeloperoxidase Activities in Healthy Adults—A Randomized-Controlled Trial. Free Radic. Res. 2016;50:1396–1407. doi: 10.1080/10715762.2016.1252839. [DOI] [PubMed] [Google Scholar]
- 113.Kim S., Sieburth D. Sphingosine Kinase Regulates Neuropeptide Secretion During the Oxidative Stress-Response Through Intertissue Signaling. J. Neurosci. 2018;38:8160–8176. doi: 10.1523/JNEUROSCI.0536-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Patel R.S., Ghasemzadeh N., Eapen D.J., Sher S., Arshad S., Ko Y.A., Veledar E., Samady H., Zafari A.M., Sperling L., et al. Novel Biomarker of Oxidative Stress Is Associated with Risk of Death in Patients with Coronary Artery Disease. Circulation. 2016;133:361–369. doi: 10.1161/CIRCULATIONAHA.115.019790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bohn T., Bonet M.L., Borel P., Keijer J., Landrier J.F., Milisav I., Ribot J., Riso P., Winklhofer-Roob B., Sharoni Y., et al. Mechanistic Aspects of Carotenoid Health Benefits—Where Are We Now? Nutr. Res. Rev. 2021;34:276–302. doi: 10.1017/S0954422421000147. [DOI] [PubMed] [Google Scholar]
- 116.Dose J., Matsugo S., Yokokawa H., Koshida Y., Okazaki S., Seidel U., Eggersdorfer M., Rimbach G., Esatbeyoglu T. Free Radical Scavenging and Cellular Antioxidant Properties of Astaxanthin. Int. J. Mol. Sci. 2016;17:103. doi: 10.3390/ijms17010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ribeiro D., Sousa A., Nicola P., de Oliveira J., Rufino A.T., Silva M., Freitas M., Carvalho F., Fernandes E. Beta-Carotene and Its Physiological Metabolites: Effects on Oxidative Status Regulation and Genotoxicity in in Vitro Models. Food Chem. Toxicol. 2020;141:111392. doi: 10.1016/j.fct.2020.111392. [DOI] [PubMed] [Google Scholar]
- 118.Neves D., Valentao P., Bernardo J., Oliveira M.C., Ferreira J.M.G., Pereira D.M., Andrade P.B., Videira R.A. A New Insight on Elderberry Anthocyanins Bioactivity: Modulation of Mitochondrial Redox Chain Functionality and Cell Redox State. J. Funct. Foods. 2019;56:145–155. doi: 10.1016/j.jff.2019.03.019. [DOI] [Google Scholar]
- 119.Shen Y.B., Zhang H., Cheng L.L., Wang L., Qian H.F., Qi X.G. In Vitro and in Vivo Antioxidant Activity of Polyphenols Extracted from Black Highland Barley. Food Chem. 2016;194:1003–1012. doi: 10.1016/j.foodchem.2015.08.083. [DOI] [PubMed] [Google Scholar]
- 120.Ma T., Hu N., Ding C., Zhang Q., Li W., Suo Y., Wang H., Bai B., Ding C. In Vitro and in Vivo Biological Activities of Anthocyanins from Nitraria Tangutorun Bobr. Fruits. Food Chem. 2016;194:296–303. doi: 10.1016/j.foodchem.2015.07.110. [DOI] [PubMed] [Google Scholar]
- 121.Li L., Li J., Xu H., Zhu F., Li Z., Lu H., Zhang J., Yang Z., Liu Y. The Protective Effect of Anthocyanins Extracted from Aronia Melanocarpa Berry in Renal Ischemia-Reperfusion Injury in Mice. Mediators Inflamm. 2021;2021:7372893. doi: 10.1155/2021/7372893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ereminas G., Majiene D., Sidlauskas K., Jakstas V., Ivanauskas L., Vaitiekaitis G., Liobikas J. Neuroprotective Properties of Anthocyanidin Glycosides against H2O2-Induced Glial Cell Death Are Modulated by Their Different Stability and Antioxidant Activity in Vitro. Biomed. Pharmacother. 2017;94:188–196. doi: 10.1016/j.biopha.2017.07.077. [DOI] [PubMed] [Google Scholar]
- 123.Chen S., Hu N., Wang H., Wu Y., Li G. Bioactivity-Guided Isolation of the Major Anthocyanin from Lycium Ruthenicum Murr. Fruit and Its Antioxidant Activity and Neuroprotective Effects in Vitro and in Vivo. Food Funct. 2022;13:3247–3257. doi: 10.1039/D1FO04095B. [DOI] [PubMed] [Google Scholar]
- 124.Momeni H.R., Eskandari N. Effect of Curcumin on Kidney Histopathological Changes, Lipid Peroxidation and Total Antioxidant Capacity of Serum in Sodium Arsenite-Treated Mice. Exp. Toxicol. Pathol. 2017;69:93–97. doi: 10.1016/j.etp.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 125.Hassanzadeh S., Read M.I., Bland A.R., Majeed M., Jamialahmadi T., Sahebkar A. Curcumin: An Inflammasome Silencer. Pharmacol. Res. 2020;159:104921. doi: 10.1016/j.phrs.2020.104921. [DOI] [PubMed] [Google Scholar]
- 126.Niu T., Xuan R., Jiang L., Wu W., Zhen Z., Song Y., Hong L., Zheng K., Zhang J., Xu Q., et al. Astaxanthin Induces the Nrf2/HO-1 Antioxidant Pathway in Human Umbilical Vein Endothelial Cells by Generating Trace Amounts of ROS. J. Agric. Food Chem. 2018;66:1551–1559. doi: 10.1021/acs.jafc.7b05493. [DOI] [PubMed] [Google Scholar]
- 127.Xie S., Yin P., Tian L., Yu Y., Liu Y., Niu J. Dietary Supplementation of Astaxanthin Improved the Growth Performance, Antioxidant Ability and Immune Response of Juvenile Largemouth Bass (Micropterus salmoides) Fed High-Fat Diet. Mar. Drugs. 2020;18:642. doi: 10.3390/md18120642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bandeira A.C.B., da Silva T.P., de Araujo G.R., Araujo C.M., da Silva R.C., Lima W.G., Bezerra F.S., Costa D.C. Lycopene Inhibits Reactive Oxygen Species Production in SK-Hep-1 Cells and Attenuates Acetaminophen-Induced Liver Injury in C57BL/6 Mice. Chem. Biol. Interact. 2017;263:7–17. doi: 10.1016/j.cbi.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 129.Sharavana G., Joseph G.S., Baskaran V. Lutein Attenuates Oxidative Stress Markers and Ameliorates Glucose Homeostasis through Polyol Pathway in Heart and Kidney of STZ-Induced Hyperglycemic Rat Model. Eur. J. Nutr. 2017;56:2475–2485. doi: 10.1007/s00394-016-1283-0. [DOI] [PubMed] [Google Scholar]
- 130.El-Akabawy G., El-Sherif N.M. Zeaxanthin Exerts Protective Effects on Acetic Acid-Induced Colitis in Rats via Modulation of pro-Inflammatory Cytokines and Oxidative Stress. Biomed. Pharmacother. 2019;111:841–851. doi: 10.1016/j.biopha.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 131.Zhang Y., Yin L., Huang L., Tekliye M., Xia X., Li J., Dong M. Composition, Antioxidant Activity, and Neuroprotective Effects of Anthocyanin-Rich Extract from Purple Highland Barley Bran and Its Promotion on Autophagy. Food Chem. 2021;339:127849. doi: 10.1016/j.foodchem.2020.127849. [DOI] [PubMed] [Google Scholar]
- 132.Gao Y., Liang X., Tian Z., Ma Y., Sun C. Betalain Exerts Cardioprotective and Anti-Inflammatory Effects against the Experimental Model of Heart Failure. Hum. Exp. Toxicol. 2021;40:S16–S28. doi: 10.1177/09603271211027933. [DOI] [PubMed] [Google Scholar]
- 133.Da Silva D.V.T., dos Santos Baião D., de Oliveira Silva F., Alves G., Perrone D., Del Aguila E.M., Flosi Paschoalin V.M. Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments. Molecules. 2019;24:458. doi: 10.3390/molecules24030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pagliaro P., Penna C. Editorial: Alteration of Redox Equilibrium, Inflammation and Progression of Disease. Curr. Med. Chem. 2018;25:1272–1274. doi: 10.2174/092986732511180417115122. [DOI] [PubMed] [Google Scholar]
- 135.Trivedi P.P., Jena G.B. Mechanistic Insight into Beta-Carotene-Mediated Protection against Ulcerative Colitis-Associated Local and Systemic Damage in Mice. Eur. J. Nutr. 2015;54:639–652. doi: 10.1007/s00394-014-0745-5. [DOI] [PubMed] [Google Scholar]
- 136.Chang M.X., Xiong F. Astaxanthin and Its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules. 2020;25:5342. doi: 10.3390/molecules25225342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Abdin M., Hamed Y.S., Akhtar H.M.S., Chen D., Chen G., Wan P., Zeng X. Antioxidant and Anti-Inflammatory Activities of Target Anthocyanins Di-Glucosides Isolated from Syzygium Cumini Pulp by High Speed Counter-Current Chromatography. J. Food Biochem. 2020;44:1050–1062. doi: 10.1111/jfbc.13209. [DOI] [PubMed] [Google Scholar]
- 138.Lee S.G., Brownmiller C.R., Lee S. Anti-Inflammatory and Antioxidant Effects of Anthocyanins of Trifolium Pratense (Red Clover) in Lipopolysaccharide-Stimulated RAW-267.4 Macrophages. Nutrients. 2020;12:1089. doi: 10.3390/nu12041089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vendrame S., Klimis-Zacas D. Anti-Inflammatory Effect of Anthocyanins via Modulation of Nuclear Factor-ΚB and Mitogen-Activated Protein Kinase Signaling Cascades. Nutr. Rev. 2015;73:348–358. doi: 10.1093/nutrit/nuu066. [DOI] [PubMed] [Google Scholar]
- 140.Zhou Y., Zhang T., Wang X., Wei X., Chen Y., Guo L., Zhang J., Wang C. Curcumin Modulates Macrophage Polarization through the Inhibition of the Toll-like Receptor 4 Expression and Its Signaling Pathways. Cell. Physiol. Biochem. 2015;36:631–641. doi: 10.1159/000430126. [DOI] [PubMed] [Google Scholar]
- 141.Gyamfi J., Kim J., Choi J. Cancer as a Metabolic Disorder. Int. J. Mol. Sci. 2022;23:1155. doi: 10.3390/ijms23031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu J., Zhou H., Song L., Yang Z., Qiu M., Wang J., Shi S. Anthocyanins: Promising Natural Products with Diverse Pharmacological Activities. Molecules. 2021;26:3807. doi: 10.3390/molecules26133807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McCall B., McPartland C.K., Moore R., Frank-Kamenetskii A., Booth B.W. Effects of Astaxanthin on the Proliferation and Migration of Breast Cancer Cells in Vitro. Antioxidants. 2018;7:135. doi: 10.3390/antiox7100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ahn Y.T., Kim M.S., Kim Y.S., An W.G. Astaxanthin Reduces Stemness Markers in BT20 and T47D Breast Cancer Stem Cells by Inhibiting Expression of Pontin and Mutant P53. Mar. Drugs. 2020;18:577. doi: 10.3390/md18110577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim M.S., Ahn Y.T., Lee C.W., Kim H., An W.G. Astaxanthin Modulates Apoptotic Molecules to Induce Death of SKBR3 Breast Cancer Cells. Mar. Drugs. 2020;18:266. doi: 10.3390/md18050266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Charepalli V., Reddivari L., Radhakrishnan S., Vadde R., Agarwal R., Vanamala J.K.P. Anthocyanin-Containing Purple-Fleshed Potatoes Suppress Colon Tumorigenesis via Elimination of Colon Cancer Stem Cells. J. Nutr. Biochem. 2015;26:1641–1649. doi: 10.1016/j.jnutbio.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 147.Wang Y., Lin J., Tian J., Si X., Jiao X., Zhang W., Gong E., Li B. Blueberry Malvidin-3-Galactoside Suppresses Hepatocellular Carcinoma by Regulating Apoptosis, Proliferation, and Metastasis Pathways in Vivo and in Vitro. J. Agric. Food Chem. 2019;67:625–636. doi: 10.1021/acs.jafc.8b06209. [DOI] [PubMed] [Google Scholar]
- 148.Xu Y., Huang Y., Chen Y., Cao K., Liu Z., Wan Z., Liao Z., Li B., Cui J., Yang Y., et al. Grape Seed Proanthocyanidins Play the Roles of Radioprotection on Normal Lung and Radiosensitization on Lung Cancer via Differential Regulation of the MAPK Signaling Pathway. J. Cancer. 2021;12:2844–2854. doi: 10.7150/jca.49987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Giordano A., Tommonaro G. Curcumin and Cancer. Nutrients. 2019;11:2376. doi: 10.3390/nu11102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu L., Guo L., Liang Y., Liu X., Jiang L., Wang L. Curcumin Suppresses Stem-like Traits of Lung Cancer Cells via Inhibiting the JAK2/STAT3 Signaling Pathway. Oncol. Rep. 2015;34:3311–3317. doi: 10.3892/or.2015.4279. [DOI] [PubMed] [Google Scholar]
- 151.Zhang B.Y., Shi Y.Q., Chen X., Dai J., Jiang Z.F., Li N., Zhang Z.B. Protective Effect of Curcumin against Formaldehyde-Induced Genotoxicity in A549 Cell Lines. J. Appl. Toxicol. 2013;33:1468–1473. doi: 10.1002/jat.2814. [DOI] [PubMed] [Google Scholar]
- 152.Jin H., Qiao F., Wang Y., Xu Y., Shang Y. Curcumin Inhibits Cell Proliferation and Induces Apoptosis of Human Non-Small Cell Lung Cancer Cells through the Upregulation of MiR-192-5p and Suppression of PI3K/Akt Signaling Pathway. Oncol. Rep. 2015;34:2782–2789. doi: 10.3892/or.2015.4258. [DOI] [PubMed] [Google Scholar]
- 153.Lu Y., Wei C., Xi Z. Curcumin Suppresses Proliferation and Invasion in Non-Small Cell Lung Cancer by Modulation of MTA1-Mediated Wnt/β-Catenin Pathway. Vitr. Cell. Dev. Biol. Anim. 2014;50:840–850. doi: 10.1007/s11626-014-9779-5. [DOI] [PubMed] [Google Scholar]
- 154.Takeshima M., Ono M., Higuchi T., Chen C., Hara T., Nakano S. Anti-Proliferative and Apoptosis-Inducing Activity of Lycopene against Three Subtypes of Human Breast Cancer Cell Lines. Cancer Sci. 2014;105:252–257. doi: 10.1111/cas.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shree G.S., Prasad K.Y., Arpitha H.S., Deepika U.R., Kumar K.N., Mondal P., Ganesan P. Beta-Carotene at Physiologically Attainable Concentration Induces Apoptosis and down-Regulates Cell Survival and Antioxidant Markers in Human Breast Cancer (MCF-7) Cells. Mol. Cell. Biochem. 2017;436:1–12. doi: 10.1007/s11010-017-3071-4. [DOI] [PubMed] [Google Scholar]
- 156.Gloria N.F., Soares N., Brand C., Oliveira F.L., Borojevic R., Teodoro A.J. Lycopene and Beta-Carotene Induce Cell-Cycle Arrest and Apoptosis in Human Breast Cancer Cell Lines. Anticancer Res. 2014;34:1377–1386. [PubMed] [Google Scholar]
- 157.Chang J., Zhang Y., Li Y., Lu K., Shen Y., Guo Y., Qi Q., Wang M., Zhang S. NrF2/ARE and NF-ΚB Pathway Regulation May Be the Mechanism for Lutein Inhibition of Human Breast Cancer Cell. Future Oncol. 2018;14:719–726. doi: 10.2217/fon-2017-0584. [DOI] [PubMed] [Google Scholar]
- 158.Gong X., Smith J.R., Swanson H.M., Rubin L.P. Carotenoid Lutein Selectively Inhibits Breast Cancer Cell Growth and Potentiates the Effect of Chemotherapeutic Agents through ROS-Mediated Mechanisms. Molecules. 2018;23:905. doi: 10.3390/molecules23040905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hire R.R., Srivastava S., Davis M.B., Kumar Konreddy A., Panda D. Antiproliferative Activity of Crocin Involves Targeting of Microtubules in Breast Cancer Cells. Sci. Rep. 2017;7:44984. doi: 10.1038/srep44984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Arzi L., Farahi A., Jafarzadeh N., Riazi G., Sadeghizadeh M., Hoshyar R. Inhibitory Effect of Crocin on Metastasis of Triple-Negative Breast Cancer by Interfering with Wnt/β-Catenin Pathway in Murine Model. DNA Cell Biol. 2018;37:1068–1075. doi: 10.1089/dna.2018.4351. [DOI] [PubMed] [Google Scholar]
- 161.Tan J., Li Q., Xue H., Tang J. Ultrasound-Assisted Enzymatic Extraction of Anthocyanins from Grape Skins: Optimization, Identification, and Antitumor Activity. J. Food Sci. 2020;85:3731–3744. doi: 10.1111/1750-3841.15497. [DOI] [PubMed] [Google Scholar]
- 162.Mazzoni L., Giampieri F., Alvarez Suarez J.M., Gasparrini M., Mezzetti B., Forbes Hernandez T.Y., Battino M.A. Isolation of Strawberry Anthocyanin-Rich Fractions and Their Mechanisms of Action against Murine Breast Cancer Cell Lines. Food Funct. 2019;10:7103–7120. doi: 10.1039/C9FO01721F. [DOI] [PubMed] [Google Scholar]
- 163.Kim J.M., Noh E.M., Kwon K.B., Kim J.S., You Y.O., Hwang J.K., Hwang B.M., Kim B.S., Lee S.H., Lee S.J., et al. Curcumin Suppresses the TPA-Induced Invasion through Inhibition of PKCα-Dependent MMP-Expression in MCF-7 Human Breast Cancer Cells. Phytomedicine. 2012;19:1085–1092. doi: 10.1016/j.phymed.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 164.Yim-Im W., Sawatdichaikul O., Semsri S., Horata N., Mokmak W., Tongsima S., Suksamrarn A., Choowongkomon K. Computational Analyses of Curcuminoid Analogs against Kinase Domain of HER2. BMC Bioinformatics. 2014;15:261. doi: 10.1186/1471-2105-15-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Guan F., Ding Y., Zhang Y., Zhou Y., Li M., Wang C. Curcumin Suppresses Proliferation and Migration of MDA-MB-231 Breast Cancer Cells through Autophagy-Dependent Akt Degradation. PLoS ONE. 2016;11:e0146553. doi: 10.1371/journal.pone.0146553. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 166.Akkoç Y., Berrak Ö., Arisan E.D., Obakan P., Çoker-Gürkan A., Palavan-Ünsal N. Inhibition of PI3K Signaling Triggered Apoptotic Potential of Curcumin Which Is Hindered by Bcl-2 through Activation of Autophagy in MCF-7 Cells. Biomed. Pharmacother. 2015;71:161–171. doi: 10.1016/j.biopha.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 167.Sun X.D., Liu X.E., Huang D.S. Curcumin Induces Apoptosis of Triple-Negative Breast Cancer Cells by Inhibition of EGFR Expression. Mol. Med. Rep. 2012;6:1267–1270. doi: 10.3892/mmr.2012.1103. [DOI] [PubMed] [Google Scholar]
- 168.Nowacki L., Vigneron P., Rotellini L., Cazzola H., Merlier F., Prost E., Ralanairina R., Gadonna J.P., Rossi C., Vayssade M. Betanin-Enriched Red Beetroot (Beta Vulgaris L.) Extract Induces Apoptosis and Autophagic Cell Death in MCF-7 Cells. Phytother. Res. 2015;29:1964–1973. doi: 10.1002/ptr.5491. [DOI] [PubMed] [Google Scholar]
- 169.Kim H.Y., Kim Y.M., Hong S. Astaxanthin Suppresses the Metastasis of Colon Cancer by Inhibiting the MYC-Mediated Downregulation of MicroRNA-29a-3p and MicroRNA-200a. Sci. Rep. 2019;9:9457. doi: 10.1038/s41598-019-45924-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hormozi M., Ghoreishi S., Baharvand P. Astaxanthin Induces Apoptosis and Increases Activity of Antioxidant Enzymes in LS-180 Cells. Artif. Cells Nanomed. Biotechnol. 2019;47:891–895. doi: 10.1080/21691401.2019.1580286. [DOI] [PubMed] [Google Scholar]
- 171.Neumann U., Derwenskus F., Flister V.F., Schmid-Staiger U., Hirth T., Bischoff S.C. Fucoxanthin, a Carotenoid Derived from Phaeodactylum Tricornutum Exerts Antiproliferative and Antioxidant Activities in Vitro. Antioxidants. 2019;8:183. doi: 10.3390/antiox8060183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lopes-Costa E., Abreu M., Gargiulo D., Rocha E., Ramos A.A. Anticancer Effects of Seaweed Compounds Fucoxanthin and Phloroglucinol, Alone and in Combination with 5-Fluorouracil in Colon Cells. J. Toxicol. Environ. Health A Curr. Issues. 2017;80:776–787. doi: 10.1080/15287394.2017.1357297. [DOI] [PubMed] [Google Scholar]
- 173.Amin A., Farrukh A., Murali C., Soleimani A., Praz F., Graziani G., Brim H., Ashktorab H. Saffron and Its Major Ingredients’ Effect on Colon Cancer Cells with Mismatch Repair Deficiency and Microsatellite Instability. Molecules. 2021;26:3855. doi: 10.3390/molecules26133855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chen L., Jiang B., Zhong C., Guo J., Zhang L., Mu T., Zhang Q., Bi X. Chemoprevention of Colorectal Cancer by Black Raspberry Anthocyanins Involved the Modulation of Gut Microbiota and SFRP2 Demethylation. Carcinogenesis. 2018;39:471–481. doi: 10.1093/carcin/bgy009. [DOI] [PubMed] [Google Scholar]
- 175.Zhang H., Guo J., Mao L., Li Q., Guo M., Mu T., Zhang Q., Bi X. Up-Regulation of MIR-24-1-5p Is Involved in the Chemoprevention of Colorectal Cancer by Black Raspberry Anthocyanins. Br. J. Nutr. 2019;122:518–526. doi: 10.1017/S0007114518003136. [DOI] [PubMed] [Google Scholar]
- 176.López De Las Hazas M.C., Mosele J.I., Macià A., Ludwig I.A., Motilva M.J. Exploring the Colonic Metabolism of Grape and Strawberry Anthocyanins and Their in Vitro Apoptotic Effects in HT-29 Colon Cancer Cells. J. Agric. Food Chem. 2017;65:6477–6487. doi: 10.1021/acs.jafc.6b04096. [DOI] [PubMed] [Google Scholar]
- 177.Rajitha B., Belalcazar A., Nagaraju G.P., Shaib W.L., Snyder J.P., Shoji M., Pattnaik S., Alam A., El-Rayes B.F. Inhibition of NF-ΚB Translocation by Curcumin Analogs Induces G0/G1 Arrest and Downregulates Thymidylate Synthase in Colorectal Cancer. Cancer Lett. 2016;373:227–233. doi: 10.1016/j.canlet.2016.01.052. [DOI] [PubMed] [Google Scholar]
- 178.Guo L.D., Chen X.J., Hu Y.H., Yu Z.J., Wang D., Liu J.Z. Curcumin Inhibits Proliferation and Induces Apoptosis of Human Colorectal Cancer Cells by Activating the Mitochondria Apoptotic Pathway. Phytother. Res. 2013;27:422–430. doi: 10.1002/ptr.4731. [DOI] [PubMed] [Google Scholar]
- 179.Farabegoli F., Scarpa E.S., Frati A., Serafini G., Papi A., Spisni E., Antonini E., Benedetti S., Ninfali P. Betalains Increase Vitexin-2-O-Xyloside Cytotoxicity in CaCo-2 Cancer Cells. Food Chem. 2017;218:356–364. doi: 10.1016/j.foodchem.2016.09.112. [DOI] [PubMed] [Google Scholar]
- 180.Zhou Y., Xu Q., Shang J., Lu L., Chen G. Crocin Inhibits the Migration, Invasion, and Epithelial-Mesenchymal Transition of Gastric Cancer Cells via MiR-320/KLF5/HIF-1α Signaling. J. Cell. Physiol. 2019;234:17876–17885. doi: 10.1002/jcp.28418. [DOI] [PubMed] [Google Scholar]
- 181.Park Y., Choi J., Lim J.W., Kim H. Beta-Carotene-Induced Apoptosis Is Mediated with Loss of Ku Proteins in Gastric Cancer AGS Cells. Genes Nutr. 2015;10:467. doi: 10.1007/s12263-015-0467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhu Y., Cheng J., Min Z., Yin T., Zhang R., Zhang W., Hu L., Cui Z., Gao C., Xu S., et al. Effects of Fucoxanthin on Autophagy and Apoptosis in SGC-7901cells and the Mechanism. J. Cell. Biochem. 2018;119:7274–7284. doi: 10.1002/jcb.27022. [DOI] [PubMed] [Google Scholar]
- 183.Yu R.-X., Yu R.-T., Liu Z. Inhibition of Two Gastric Cancer Cell Lines Induced by Fucoxanthin Involves Downregulation of Mcl-1 and STAT3. Hum. Cell. 2018;31:50–63. doi: 10.1007/s13577-017-0188-4. [DOI] [PubMed] [Google Scholar]
- 184.Kim J.H., Park J.J., Lee B.J., Joo M.K., Chun H.J., Lee S.W., Bak Y.T. Astaxanthin Inhibits Proliferation of Human Gastric Cancer Cell Lines by Interrupting Cell Cycle Progression. Gut Liver. 2016;10:369–374. doi: 10.5009/gnl15208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim S., Lee H., Lim J.W., Kim H. Astaxanthin Induces NADPH Oxidase Activation and Receptor-interacting Protein Kinase 1-mediated Necroptosis in Gastric Cancer AGS Cells. Mol. Med. Rep. 2021;24:837. doi: 10.3892/mmr.2021.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shao Y., Ni Y., Yang J., Lin X., Li J., Zhang L. Astaxanthin Inhibits Proliferation and Induces Apoptosis and Cell Cycle Arrest of Mice H22 Hepatoma Cells. Med. Sci. Monit. 2016;22:2152–2160. doi: 10.12659/MSM.899419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li J., Dai W., Xia Y., Chen K., Li S., Liu T., Zhang R., Wang J., Lu W., Zhou Y., et al. Astaxanthin Inhibits Proliferation and Induces Apoptosis of Human Hepatocellular Carcinoma Cells via Inhibition of Nf-Κb P65 and Wnt/B-Catenin in Vitro. Mar. Drugs. 2015;13:6064–6081. doi: 10.3390/md13106064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ohno T., Shimizu M., Shirakami Y., Miyazaki T., Ideta T., Kochi T., Kubota M., Sakai H., Tanaka T., Moriwaki H. Preventive Effects of Astaxanthin on Diethylnitrosamine-Induced Liver Tumorigenesis in C57/BL/KsJ-Db/Db Obese Mice. Hepatol. Res. 2016;46:E201–E209. doi: 10.1111/hepr.12550. [DOI] [PubMed] [Google Scholar]
- 189.Amin A., Hamza A.A., Daoud S., Khazanehdari K., Al Hrout A., Baig B., Chaiboonchoe A., Adrian T.E., Zaki N., Salehi-Ashtiani K. Saffron-Based Crocin Prevents Early Lesions of Liver Cancer: In Vivo, In Vitro and Network Analyses. Recent Pat. Anticancer. Drug Discov. 2015;11:121–133. doi: 10.2174/1574892810666151102110248. [DOI] [PubMed] [Google Scholar]
- 190.Sangavi P., Langeswaran K., Kumar S.G. Anticarcinogenic Efficacy of Fucoxanthin on HepG2 Cell Lines. J. Clin. Diagn. Res. 2022;16:5–9. doi: 10.7860/JCDR/2022/49462.16007. [DOI] [Google Scholar]
- 191.Jin X., Zhao T.T., Shi D., Ye M.B., Yi Q. Protective Role of Fucoxanthin in Diethylnitrosamine-Induced Hepatocarcinogenesis in Experimental Adult Rats. Drug Dev. Res. 2019;80:209–217. doi: 10.1002/ddr.21451. [DOI] [PubMed] [Google Scholar]
- 192.Dokkaew A., Punvittayagul C., Insuan O., Limtrakul P., Wongpoomchai R. Protective Effects of Defatted Sticky Rice Bran Extracts on the Early Stages of Hepatocarcinogenesis in Rats. Molecules. 2019;24:2142. doi: 10.3390/molecules24112142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liao S., Liu J., Xu M., Zheng J. Evaluation of the Liver Cancer Prevention of Anthocyanin Extracts from Mulberry (Morus alba L.) Variety PR-01. Adv. Biosci. Biotechnol. 2018;9:423–442. doi: 10.4236/abb.2018.99030. [DOI] [Google Scholar]
- 194.Tsai C.F., Hsieh T.H., Lee J.N., Hsu C.Y., Wang Y.C., Kuo K.K., Wu H.L., Chiu C.C., Tsai E.M., Kuo P.L. Curcumin Suppresses Phthalate-Induced Metastasis and the Proportion of Cancer Stem Cell (CSC)-like Cells via the Inhibition of AhR/ERK/SK1 Signaling in Hepatocellular Carcinoma. J. Agric. Food Chem. 2015;63:10388–10398. doi: 10.1021/acs.jafc.5b04415. [DOI] [PubMed] [Google Scholar]
- 195.Krajka-Kuźniak V., Paluszczak J., Szaefer H., Baer-Dubowska W. Betanin, a Beetroot Component, Induces Nuclear Factor Erythroid-2-Related Factor 2-Mediated Expression of Detoxifying/Antioxidant Enzymes in Human Liver Cell Lines. Br. J. Nutr. 2013;110:2138–2149. doi: 10.1017/S0007114513001645. [DOI] [PubMed] [Google Scholar]
- 196.Ko J.C., Chen J.C., Wang T.J., Zheng H.Y., Chen W.C., Chang P.Y., Lin Y.W. Astaxanthin Down-Regulates Rad51 Expression via Inactivation of AKT Kinase to Enhance Mitomycin C-Induced Cytotoxicity in Human Non-Small Cell Lung Cancer Cells. Biochem. Pharmacol. 2016;105:91–100. doi: 10.1016/j.bcp.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 197.Chen S., Zhao S., Wang X., Zhang L., Jiang E., Gu Y., Shangguan A.J., Zhao H., Lv T., Yu Z. Crocin Inhibits Cell Proliferation and Enhances Cisplatin and Pemetrexed Chemosensitivity in Lung Cancer Cells. Transl. Lung Cancer Res. 2015;4:775–783. doi: 10.3978/j.issn.2218-6751.2015.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang W.L., Zhao Y.N., Shi Z.Z., Cong D., Bai Y.S. Lutein Inhibits Cell Growth and Activates Apoptosis via the PI3K/AKT/MTOR Signaling Pathway in A549 Human Non-Small-Cell Lung Cancer Cells. J. Environ. Pathol. Toxicol. Oncol. 2018;37:341–350. doi: 10.1615/JEnvironPatholToxicolOncol.2018027418. [DOI] [PubMed] [Google Scholar]
- 199.Amararathna M., Hoskin D.W., Rupasinghe H.P.V. Cyanidin-3-O-Glucoside-Rich Haskap Berry Administration Suppresses Carcinogen-Induced Lung Tumorigenesis in A/JCr Mice. Molecules. 2020;25:3823. doi: 10.3390/molecules25173823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yin Z., Yang Y., Guo T., Veeraraghavan V.P., Wang X. Potential Chemotherapeutic Effect of Betalain against Human Non-Small Cell Lung Cancer through PI3K/Akt/MTOR Signaling Pathway. Environ. Toxicol. 2021;36:1011–1020. doi: 10.1002/tox.23100. [DOI] [PubMed] [Google Scholar]
- 201.Ni X., Yu H., Wang S., Zhang C., Shen S. Astaxanthin Inhibits PC-3 Xenograft Prostate Tumor Growth in Nude Mice. Mar. Drugs. 2017;15:66. doi: 10.3390/md15030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.D’Alessandro A.M., Mancini A., Lizzi A.R., De Simone A., Marroccella C.E., Gravina G.L., Tatone C., Festuccia C. Crocus Sativus Stigma Extract and Its Major Constituent Crocin Possess Significant Antiproliferative Properties against Human Prostate Cancer. Nutr. Cancer. 2013;65:930–942. doi: 10.1080/01635581.2013.767368. [DOI] [PubMed] [Google Scholar]
- 203.Tyagi A., Kumar S., Raina K., Wempe M.F., Maroni P.D., Agarwal R., Agarwal C. Differential Effect of Grape Seed Extract and Its Active Constituent Procyanidin B2 3,3″-Di-O-Gallate against Prostate Cancer Stem Cells. Mol. Carcinog. 2019;58:1105–1117. doi: 10.1002/mc.22995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jongsomchai K., Leardkamolkarn V., Mahatheeranont S. A Rice Bran Phytochemical, Cyanidin 3-Glucoside, Inhibits the Progression of PC3 Prostate Cancer Cell. Anat. Cell Biol. 2020;53:481–492. doi: 10.5115/acb.20.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ide H., Lu Y., Noguchi T., Muto S., Okada H., Kawato S., Horie S. Modulation of AKR1C2 by Curcumin Decreases Testosterone Production in Prostate Cancer. Cancer Sci. 2018;109:1230–1238. doi: 10.1111/cas.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhu M., Zheng Z., Huang J., Ma X., Huang C., Wu R., Li X., Liang Z., Deng F., Wu J., et al. Modulation of MiR-34a in Curcumin-Induced Antiproliferation of Prostate Cancer Cells. J. Cell. Biochem. 2019;120:15616–15624. doi: 10.1002/jcb.28828. [DOI] [PubMed] [Google Scholar]
- 207.Gopal S.S., Maradgi T., Ponesakki G. 3-Antiobese Properties of Carotenoids: An Overview of Underlying Molecular Mechanisms. In: Charis M.G., editor. Carotenoids: Properties, Processing and Applications. Academic Press; Cambridge, MA, USA: 2020. pp. 75–105. [Google Scholar]
- 208.Wang J., Liu S., Wang H., Xiao S., Li C., Li Y., Liu B. Xanthophyllomyces Dendrorhous-Derived Astaxanthin Regulates Lipid Metabolism and Gut Microbiota in Obese Mice Induced by a High-Fat Diet. Mar. Drugs. 2019;17:337. doi: 10.3390/md17060337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wu T., Gao Y.F., Hao J.Y., Geng J.T., Zhang J.J., Yin J.J., Liu R., Sui W.J., Gong L.X., Zhang M. Capsanthin Extract Prevents Obesity, Reduces Serum TMAO Levels and Modulates the Gut Microbiota Composition in High-Fat-Diet Induced Obese C57BL/6J Mice. Food Res. Int. 2020;128:108774. doi: 10.1016/j.foodres.2019.108774. [DOI] [PubMed] [Google Scholar]
- 210.Fen Z.G., Ni Y.H., Wan C.Y., Liu F., Fu Z.W. Anti-Diabetic Effects of Astaxanthin on an STZ-Induced Diabetic Model in Rats. Endocr. J. 2021;68:451–459. doi: 10.1507/endocrj.EJ20-0699. [DOI] [PubMed] [Google Scholar]
- 211.Xie L., Su H., Sun C., Zheng X., Chen W. Recent Advances in Understanding the Anti-Obesity Activity of Anthocyanins and Their Biosynthesis in Microorganisms. Trends Food Sci. Technol. 2018;72:13–24. doi: 10.1016/j.tifs.2017.12.002. [DOI] [Google Scholar]
- 212.Lee B., Lee M., Lefevre M., Kim H.R. Anthocyanins Inhibit Lipogenesis During Adipocyte Differentiation of 3T3-L1 Preadipocytes. Plant Foods Hum. Nutr. 2014;69:137–141. doi: 10.1007/s11130-014-0407-z. [DOI] [PubMed] [Google Scholar]
- 213.Skates E., Overall J., DeZego K., Wilson M., Esposito D., Lila M.A., Komarnytsky S. Berries Containing Anthocyanins with Enhanced Methylation Profiles Are More Effective at Ameliorating High Fat Diet-Induced Metabolic Damage. Food Chem. Toxicol. 2018;111:445–453. doi: 10.1016/j.fct.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 214.Li Q., Yang G., Xu H., Tang S., Lee W.Y. Effects of Resveratrol Supplementation on Bone Quality: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. BMC Complement. Med. Ther. 2021;21:214. doi: 10.1186/s12906-021-03381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jang H.H., Kim H.W., Kim S.Y., Kim S.M., Kim J.B., Lee Y.M. In Vitro and in Vivo Hypoglycemic Effects of Cyanidin 3-Caffeoyl-p-Hydroxybenzoylsophoroside-5-Glucoside, an Anthocyanin Isolated from Purple-Fleshed Sweet Potato. Food Chem. 2019;272:688–693. doi: 10.1016/j.foodchem.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 216.Matsukawa T., Inaguma T., Han J., Villareal M.O., Isoda H. Cyanidin-3-Glucoside Derived from Black Soybeans Ameliorate Type 2 Diabetes through the Induction of Differentiation of Preadipocytes into Smaller and Insulin-Sensitive Adipocytes. J. Nutr. Biochem. 2015;26:860–867. doi: 10.1016/j.jnutbio.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 217.Kasprzak-Drozd K., Oniszczuk T., Gancarz M., Kondracka A., Rusinek R., Oniszczuk A. Curcumin and Weight Loss: Does It Work? Int. J. Mol. Sci. 2022;23:639. doi: 10.3390/ijms23020639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Song H.Z., Chu Q., Xu D.D., Xu Y., Zheng X.D. Purified Betacyanins from Hylocereus Undatus Peel Ameliorate Obesity and Insulin Resistance in High-Fat-Diet-Fed Mice. J. Agric. Food Chem. 2016;64:236–244. doi: 10.1021/acs.jafc.5b05177. [DOI] [PubMed] [Google Scholar]
- 219.Libby P., Ridker P.M., Hansson G.K. Progress and Challenges in Translating the Biology of Atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 220.Yao Y., Goh H.M., Kim J.E. The Roles of Carotenoid Consumption and Bioavailability in Cardiovascular Health. Antioxidants. 2021;10:1978. doi: 10.3390/antiox10121978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ouyang B., Li Z.L., Ji X.Y., Huang J.W., Zhang H.S., Jiang C.R. The Protective Role of Lutein on Isoproterenol-Induced Cardiac Failure Rat Model through Improving Cardiac Morphology, Antioxidant Status via Positively Regulating Nrf2/HO-1 Signalling Pathway. Pharm. Biol. 2019;57:529–535. doi: 10.1080/13880209.2019.1649436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Cassidy A., Mukamal K.J., Liu L., Franz M., Eliassen A.H., Rimm E.B. High Anthocyanin Intake is Associated with a Reduced Risk of Myocardial Infarction in Young and Middle-Aged Women. Circulation. 2013;127:188–196. doi: 10.1161/CIRCULATIONAHA.112.122408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cassidy A., Bertoia M., Chiuve S., Flint A., Forman J., Rimm E.B. Habitual Intake of Anthocyanins and Flavanones and Risk of Cardiovascular Disease in Men. Am. J. Clin. Nutr. 2016;104:587–594. doi: 10.3945/ajcn.116.133132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Krga I., Milenkovic D. Anthocyanins: From Sources and Bioavailability to Cardiovascular-Health Benefits and Molecular Mechanisms of Action. J. Agric. Food Chem. 2019;67:1771–1783. doi: 10.1021/acs.jafc.8b06737. [DOI] [PubMed] [Google Scholar]
- 225.Pourbagher-Shahri A.M., Farkhondeh T., Ashrafizadeh M., Talebi M., Samargahndian S. Curcumin and Cardiovascular Diseases: Focus on Cellular Targets and Cascades. Biomed. Pharmacother. 2021;136:111214. doi: 10.1016/j.biopha.2020.111214. [DOI] [PubMed] [Google Scholar]
- 226.Wu H.J., Zhang K., Ma J.J., Wang L., Zhuang Y. Mechanism of Curcumin against Myocardial Ischaemia-Reperfusion Injury Based on the P13K/Akt/MTOR Signalling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2021;25:5490–5499. doi: 10.26355/eurrev_202109_26658. [DOI] [PubMed] [Google Scholar]
- 227.Demirdöven A., Karabiyikli Ş., Tokatli K., Öncül N. Inhibitory Effects of Red Cabbage and Sour Cherry Pomace Anthocyanin Extracts on Food Borne Pathogens and Their Antioxidant Properties. LWT. 2015;63:8–13. doi: 10.1016/j.lwt.2015.03.101. [DOI] [Google Scholar]
- 228.Moghadamtousi S.Z., Kadir H.A., Hassandarvish P., Tajik H., Abubakar S., Zandi K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. Biomed Res. Int. 2014;2014:186864. doi: 10.1155/2014/186864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Batista de Andrade Neto J., Pessoa de Farias Cabral V., Brito Nogueira L.F., Rocha da Silva C., Gurgel do Amaral Valente Sá L., Ramos da Silva A., Barbosa da Silva W.M., Silva J., Marinho E.S., Cavalcanti B.C., et al. Anti-MRSA Activity of Curcumin in Planktonic Cells and Biofilms and Determination of Possible Action Mechanisms. Microb. Pathog. 2021;155:104892. doi: 10.1016/j.micpath.2021.104892. [DOI] [PubMed] [Google Scholar]
- 230.Larussa T., Gervasi S., Liparoti R., Suraci E., Marasco R., Imeneo M., Luzza F. Downregulation of Interleukin- (IL-) 17 through Enhanced Indoleamine 2,3-Dioxygenase (IDO) Induction by Curcumin: A Potential Mechanism of Tolerance towards Helicobacter Pylori. J. Immunol. Res. 2018;2018:3739593. doi: 10.1155/2018/3739593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Da Silva Souza Campanholi K., Jaski J.M., da Silva Junior R.C., Zanqui A.B., Lazarin-Bidóia D., da Silva C.M., da Silva E.A., Hioka N., Nakamura C.V., Cardozo-Filho L., et al. Photodamage on Staphylococcus Aureus by Natural Extract from Tetragonia Tetragonoides (Pall.) Kuntze: Clean Method of Extraction, Characterization and Photophysical Studies. J. Photochem. Photobiol. B Biol. 2020;203:111763. doi: 10.1016/j.jphotobiol.2019.111763. [DOI] [PubMed] [Google Scholar]
- 232.Canadanovic-Brunet J.M., Savatovic S.S., Cetkovic G.S., Vulic J.J., Djilas S.M., Markov S.L., Cvetkovic D.D. Antioxidant and Antimicrobial Activities of Beet Root Pomace Extracts. Czech J. Food Sci. 2011;29:575–585. doi: 10.17221/210/2010-CJFS. [DOI] [Google Scholar]
- 233.Tenore G.C., Novellino E., Basile A. Nutraceutical Potential and Antioxidant Benefits of Red Pitaya (Hylocereus polyrhizus) Extracts. J. Funct. Foods. 2012;4:129–136. doi: 10.1016/j.jff.2011.09.003. [DOI] [Google Scholar]
- 234.Yolmeh M., Hamedi H., Khomeiri M. Antimicrobial Activity of Pigments Extracted from Rhodotorula Glutinis Against Some Bacteria and Fungi. Zahedan J. Res. Med. Sci. 2016 doi: 10.17795/zjrms-4954. in press . [DOI] [Google Scholar]
- 235.Wang W.Y., Liao Y.Y., Chen R.X., Hou Y.P., Ke W.Q., Zhang B.B., Gao M.L., Shao Z.Z., Chen J.M., Li F. Chlorinated Azaphilone Pigments with Antimicrobial and Cytotoxic Activities Isolated from the Deep Sea Derived Fungus Chaetomium Sp NA-S01-R1. Mar. Drugs. 2018;16:61. doi: 10.3390/md16020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Manickam P., Thilagavathi G. Development of Antibacterial Silk Sutures Using Natural Fungal Extract for Healthcare Applications. J. Text. Sci. Eng. 2016;6:249. doi: 10.4172/2165-8064.1000249. [DOI] [Google Scholar]
- 237.Hegazy G.E., Abu-Serie M.M., Abo-Elela G.M., Ghozlan H., Sabry S.A., Soliman N.A., Abdel-Fattah Y.R. In Vitro Dual (Anticancer and Antiviral) Activity of the Carotenoids Produced by Haloalkaliphilic Archaeon Natrialba Sp. M6. Sci. Rep. 2020;10:5986. doi: 10.1038/s41598-020-62663-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yim S.K., Kim I., Warren B., Kim J., Jung K., Ku B. Antiviral Activity of Two Marine Carotenoids against Sars-Cov-2 Virus Entry in Silico and in Vitro. Int. J. Mol. Sci. 2021;22:6481. doi: 10.3390/ijms22126481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Nikolaeva-Glomb L., Mukova L., Nikolova N., Badjakov I., Dincheva I., Kondakova V., Doumanova L., Galabov A.S. In Vitro Antiviral Activity of a Series of Wild Berry Fruit Extracts against Representatives of Picorna-, Orthomyxo- and Paramyxoviridae. Nat. Prod. Commun. 2014;9:51–54. doi: 10.1177/1934578X1400900116. [DOI] [PubMed] [Google Scholar]
- 240.Calland N., Sahuc M.E., Belouzard S., Pene V., Bonnafous P., Mesalam A.A., Deloison G., Descamps V., Sahpaz S., Wychowski C., et al. Polyphenols Inhibit Hepatitis C Virus Entry by a New Mechanism of Action. J. Virol. 2015;89:10053–10063. doi: 10.1128/JVI.01473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mathew D., Hsu W.L. Antiviral Potential of Curcumin. J. Funct. Foods. 2018;40:692–699. doi: 10.1016/j.jff.2017.12.017. [DOI] [Google Scholar]
- 242.Qin Y., Lin L., Chen Y., Wu S., Si X., Wu H., Zhai X., Wang Y., Tong L., Pan B., et al. Curcumin Inhibits the Replication of Enterovirus 71 in Vitro. Acta Pharm. Sin. B. 2014;4:284–294. doi: 10.1016/j.apsb.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Marín-Palma D., Tabares-Guevara J.H., Zapata-Cardona M.I., Flórez-álvarez L., Yepes L.M., Rugeles M.T., Zapata-Builes W., Hernandez J.C., Taborda N.A. Curcumin Inhibits in Vitro Sars-Cov-2 Infection in Vero E6 Cells through Multiple Antiviral Mechanisms. Molecules. 2021;26:6900. doi: 10.3390/molecules26226900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Vicente Miranda H., El-Agnaf O.M.A., Outeiro T.F. Glycation in Parkinson’s Disease and Alzheimer’s Disease. Mov. Disord. 2016;31:782–790. doi: 10.1002/mds.26566. [DOI] [PubMed] [Google Scholar]
- 245.Lakey-Beitia J., Doens D., Jagadeesh Kumar D., Murillo E., Fernandez P.L., Rao K.S., Durant-Archibold A.A. Anti-Amyloid Aggregation Activity of Novel Carotenoids: Implications for Alzheimer’s Drug Discovery. Clin. Interv. Aging. 2017;12:815–822. doi: 10.2147/CIA.S134605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Nataraj J., Manivasagam T., Justin A., Essa M.M. Lutein Protects Dopaminergic Neurons against MPTP-Induced Apoptotic Death and Motor Dysfunction by Ameliorating Mitochondrial Disruption and Oxidative Stress. Nutr. Neurosci. 2015;19:237–246. doi: 10.1179/1476830515Y.0000000010. [DOI] [PubMed] [Google Scholar]
- 247.Ali T., Kim T., Rehman S.U., Khan M.S., Amin F.U., Khan M., Ikram M., Kim M.O. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018;55:6076–6093. doi: 10.1007/s12035-017-0798-6. [DOI] [PubMed] [Google Scholar]
- 248.Li J., Zhao R., Jiang Y., Xu Y., Zhao H., Lyu X., Wu T. Bilberry Anthocyanins Improve Neuroinflammation and Cognitive Dysfunction in APP/PSEN1 Mice: Via the CD33/TREM2/TYROBP Signaling Pathway in Microglia. Food Funct. 2020;11:1572–1584. doi: 10.1039/C9FO02103E. [DOI] [PubMed] [Google Scholar]
- 249.Sohanaki H., Baluchnejadmojarad T., Nikbakht F., Roghani M. Pelargonidin Improves Memory Deficit in Amyloid Β25-35 Rat Model of Alzheimer’s Disease by Inhibition of Glial Activation, Cholinesterase, and Oxidative Stress. Biomed. Pharmacother. 2016;83:85–91. doi: 10.1016/j.biopha.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 250.Chen M., Du Z.Y., Zheng X., Li D.L., Zhou R.P., Zhang K. Use of Curcumin in Diagnosis, Prevention, and Treatment of Alzheimer’s Disease. Neural Regen. Res. 2018;13:742–752. doi: 10.4103/1673-5374.230303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Qian W., Li H., Pan N., Zhang C. Curcumin Treatment Is Associated with Increased Expression of the N-Methyl-D-Aspartate Receptor (NMDAR) Subunit, NR2A, in a Rat PC12 Cell Line Model of Alzheimer’s Disease Treated with the Acetyl Amyloid-β Peptide, Aβ(25–35) Med. Sci. Monit. 2018;24:2693–2699. doi: 10.12659/MSM.906933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Shunan D., Yu M., Guan H., Zhou Y. Neuroprotective Effect of Betalain against AlCl3-Induced Alzheimer’s Disease in Sprague Dawley Rats via Putative Modulation of Oxidative Stress and Nuclear Factor Kappa B (NF-ΚB) Signaling Pathway. Biomed. Pharmacother. 2021;137:111369. doi: 10.1016/j.biopha.2021.111369. [DOI] [PubMed] [Google Scholar]
- 253.Teerakapong A., Damrongrungruang T., Sattayut S., Morales N.P., Tantananugool S. Efficacy of Erythrosine and Cyanidin-3-Glucoside Mediated Photodynamic Therapy on Porphyromonas Gingivalis Biofilms Using Green Light Laser. Photodiagn. Photodyn. Ther. 2017;20:154–158. doi: 10.1016/j.pdpdt.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 254.Huang P., Zhang B., Yuan Q., Zhang X., Leung W., Xu C. Photodynamic Treatment with Purpurin 18 Effectively Inhibits Triple Negative Breast Cancer by Inducing Cell Apoptosis. Lasers Med. Sci. 2021;36:339–347. doi: 10.1007/s10103-020-03035-w. [DOI] [PubMed] [Google Scholar]
- 255.Jiang Y., Leung A.W., Hua H., Rao X., Xu C. Photodynamic Action of LED-Activated Curcumin against Staphylococcus Aureus Involving Intracellular ROS Increase and Membrane Damage. Int. J. Photoenergy. 2014;2014:9–11. doi: 10.1155/2014/637601. [DOI] [Google Scholar]
- 256.Carrion-Gutierrez M., Ramirez-Bosca A., Navarro-Lopez V., Martinez-Andres A., Asín-Llorca M., Bernd A., Horga De La Parte J.F. Effects of Curcuma Extract and Visible Light on Adults with Plaque Psoriasis. Eur. J. Dermatol. 2015;25:240–246. doi: 10.1684/ejd.2015.2584. [DOI] [PubMed] [Google Scholar]
- 257.Song B.H., Lee D.H., Kim B.C., Ku S.H., Park E.J., Kwon I.H., Kim K.H., Kim K.J. Photodynamic Therapy Using Chlorophyll-a in the Treatment of Acne Vulgaris: A Randomized, Single-Blind, Split-Face Study. J. Am. Acad. Dermatol. 2014;71:764–771. doi: 10.1016/j.jaad.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 258.Suvorov N., Pogorilyy V., Diachkova E., Vasil’ev Y., Mironov A., Grin M. Derivatives of Natural Chlorophylls as Agents for Antimicrobial Photodynamic Therapy. Int. J. Mol. Sci. 2021;22:6392. doi: 10.3390/ijms22126392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Saide A., Lauritano C., Ianora A. Pheophorbide A: State of the Art. Mar. Drugs. 2020;18:257. doi: 10.3390/md18050257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Nelson K.M., Dahlin J.L., Bisson J., Graham J., Pauli G.F., Walters M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017;60:1620–1637. doi: 10.1021/acs.jmedchem.6b00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hejazi J., Rastmanesh R., Taleban F.A., Molana S.H., Hejazi E., Ehtejab G., Hara N. Effect of Curcumin Supplementation During Radiotherapy on Oxidative Status of Patients with Prostate Cancer: A Double Blinded, Randomized, Placebo-Controlled Study. Nutr. Cancer Int. J. 2016;68:77–85. doi: 10.1080/01635581.2016.1115527. [DOI] [PubMed] [Google Scholar]
- 262.Sadraei M.R., Tavalaee M., Forouzanfar M., Nasr-Esfahani M.H. Effect of Curcumin, and Nano-Curcumin on Sperm Function in Varicocele Rat Model. Andrologia. 2022;54:e14282. doi: 10.1111/and.14282. [DOI] [PubMed] [Google Scholar]
- 263.Kumar V., Prakash C., Singh R., Sharma D. Curcumin’s Antiepileptic Effect, and Alterations in Na v 1.1 and Na v 1.6 Expression in Iron-Induced Epilepsy. Epilepsy Res. 2019;150:7–16. doi: 10.1016/j.eplepsyres.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 264.Akomolafe S.F., Olasehinde T.A., Oyeleye S.I., Aluko T.B., Adewale O.O., Ijomone O.M. Curcumin Administration Mitigates Cyclophosphamide-Induced Oxidative Damage and Restores Alteration of Enzymes Associated with Cognitive Function in Rats’ Brain. Neurotox. Res. 2020;38:199–210. doi: 10.1007/s12640-020-00205-0. [DOI] [PubMed] [Google Scholar]
- 265.Mahammedi H., Planchat E., Pouget M., Durando X., Cure H., Guy L., Van-Praagh I., Savareux L., Atger M., Bayet-Robert M., et al. The New Combination Docetaxel, Prednisone and Curcumin in Patients with Castration-Resistant Prostate Cancer: A Pilot Phase II Study. Oncology. 2016;90:69–78. doi: 10.1159/000441148. [DOI] [PubMed] [Google Scholar]
- 266.Scazzocchio B., Minghetti L., D’Archivio M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients. 2020;12:2499. doi: 10.3390/nu12092499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Rutz J., Maxeiner S., Justin S., Bachmeier B., Bernd A., Kippenberger S., Zöller N., Chun F.K.H., Blaheta R.A. Low Dosed Curcumin Combined with Visible Light Exposure Inhibits Renal Cell Carcinoma Metastatic Behavior in Vitro. Cancers. 2020;12:302. doi: 10.3390/cancers12020302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Roos F., Binder K., Rutz J., Maxeiner S., Bernd A., Kippenberger S., Zöller N., Chun F.K.H., Juengel E., Blaheta R.A. The Antitumor Effect of Curcumin in Urothelial Cancer Cells Is Enhanced by Light Exposure in Vitro. Evid. Based Complement. Altern. Med. 2019;2019:6374940. doi: 10.1155/2019/6374940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Shlar I., Droby S., Rodov V. Modes of Antibacterial Action of Curcumin under Dark and Light Conditions: A Toxicoproteomics Approach. J. Proteomics. 2017;160:8–20. doi: 10.1016/j.jprot.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 270.Zhang C., Su F., Li S., Yu Y., Xiang J., Liu J., Li F. Isolation and Identification of the Main Carotenoid Pigment from a New Variety of the Ridgetail White Prawn Exopalaemon Carinicauda. Food Chem. 2018;269:450–454. doi: 10.1016/j.foodchem.2018.06.143. [DOI] [PubMed] [Google Scholar]
- 271.Singh D., Puri M., Wilkens S., Mathur A.S., Tuli D.K., Barrow C.J. Characterization of a New Zeaxanthin Producing Strain of Chlorella Saccharophila Isolated from New Zealand Marine Waters. Bioresour. Technol. 2013;143:308–314. doi: 10.1016/j.biortech.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 272.Hu C.W., Chuang L.T., Yu P.C., Chen C.N.N. Pigment Production by a New Thermotolerant Microalga Coelastrella Sp. F50. Food Chem. 2013;138:2071–2078. doi: 10.1016/j.foodchem.2012.11.133. [DOI] [PubMed] [Google Scholar]
- 273.Igreja W.S., Maia F.d.A., Lopes A.S., Chisté R.C. Biotechnological Production of Carotenoids Using Low Cost-Substrates Is Influenced by Cultivation Parameters: A Review. Int. J. Mol. Sci. 2021;22:8819. doi: 10.3390/ijms22168819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Darwesh O.M., Matter I.A., Almoallim H.S., Alharbi S.A., Oh Y.K. Isolation and Optimization of Monascus Ruber OMNRC45 for Red Pigment Production and Evaluation of the Pigment as a Food Colorant. Appl. Sci. 2020;10:8867. doi: 10.3390/app10248867. [DOI] [Google Scholar]
- 275.Sunil L., Shetty N.P. Biosynthesis and Regulation of Anthocyanin Pathway Genes. Appl. Microbiol. Biotechnol. 2022;106:1783–1798. doi: 10.1007/s00253-022-11835-z. [DOI] [PubMed] [Google Scholar]
- 276.Rapoport A., Guzhova I., Bernetti L., Buzzini P., Kieliszek M., Kot A.M. Carotenoids and Some Other Pigments from Fungi and Yeasts. Metabolites. 2021;11:92. doi: 10.3390/metabo11020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Wang W., He P., Zhao D., Ye L., Dai L., Zhang X., Sun Y., Zheng J., Bi C. Construction of Escherichia Coli Cell Factories for Crocin Biosynthesis. Microb. Cell Fact. 2019;18:120. doi: 10.1186/s12934-019-1166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Park S.Y., Binkley R.M., Kim W.J., Lee M.H., Lee S.Y. Metabolic Engineering of Escherichia Coli for High-Level Astaxanthin Production with High Productivity. Metab. Eng. 2018;49:105–115. doi: 10.1016/j.ymben.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 279.Shrestha B., Pandey R.P., Darsandhari S., Parajuli P., Sohng J.K. Combinatorial Approach for Improved Cyanidin 3-O-Glucoside Production in Escherichia Coli. Microb. Cell Fact. 2019;18:7. doi: 10.1186/s12934-019-1056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Cress B.F., Leitz Q.D., Kim D.C., Amore T.D., Suzuki J.Y., Linhardt R.J., Koffas M.A.G. CRISPRi-Mediated Metabolic Engineering of E. Coli for O-Methylated Anthocyanin Production. Microb. Cell Fact. 2017;16:10. doi: 10.1186/s12934-016-0623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Eichenberger M., Hansson A., Fischer D., Dürr L., Naesby M. De Novo Biosynthesis of Anthocyanins in Saccharomyces Cerevisiae. FEMS Yeast Res. 2018;18:foy046. doi: 10.1093/femsyr/foy046. [DOI] [PubMed] [Google Scholar]
- 282.Du Y., Yang B., Yi Z., Hu L., Li M. Engineering Saccharomyces Cerevisiae Coculture Platform for the Production of Flavonoids. J. Agric. Food Chem. 2020;68:2146–2154. doi: 10.1021/acs.jafc.9b07916. [DOI] [PubMed] [Google Scholar]
- 283.Espada-Bellido E., Ferreiro-González M., Carrera C., Palma M., Barroso C.G., Barbero G.F. Optimization of the Ultrasound-Assisted Extraction of Anthocyanins and Total Phenolic Compounds in Mulberry (Morus Nigra) Pulp. Food Chem. 2017;219:23–32. doi: 10.1016/j.foodchem.2016.09.122. [DOI] [PubMed] [Google Scholar]
- 284.Tiwari S., Upadhyay N., Singh A.K., Meena G.S., Arora S. Organic Solvent-Free Extraction of Carotenoids from Carrot Bio-Waste and Its Physico-Chemical Properties. J. Food Sci. Technol. 2019;56:4678–4687. doi: 10.1007/s13197-019-03920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Elik A., Yanık D.K., Göğüş F. Microwave-Assisted Extraction of Carotenoids from Carrot Juice Processing Waste Using Flaxseed Oil as a Solvent. Lwt. 2020;123:109100. doi: 10.1016/j.lwt.2020.109100. [DOI] [Google Scholar]
- 286.Elez Garofulić I., Dragović-Uzelac V., Režek Jambrak A., Jukić M. The Effect of Microwave Assisted Extraction on the Isolation of Anthocyanins and Phenolic Acids from Sour Cherry Marasca (Prunus Cerasus Var. Marasca) J. Food Eng. 2013;117:437–442. doi: 10.1016/j.jfoodeng.2012.12.043. [DOI] [Google Scholar]
- 287.De Andrade Lima M., Kestekoglou I., Charalampopoulos D., Chatzifragkou A. Supercritical Fluid Extraction of Carotenoids from Vegetable Waste Matrices. Molecules. 2019;24:466. doi: 10.3390/molecules24030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Gong Y., Huang X.Y., Liu J.F., Pei D., Sun X., Di D.L. Development of an Effective Method Based upon Second-Order Overlapping Repeated Sample Injections for Isolation of Carotenoids from Lycium Barbarum L. Fruits with Elution-Extrusion Counter-Current Chromatography. J. Chromatogr. A. 2021;1645:462026. doi: 10.1016/j.chroma.2021.462026. [DOI] [PubMed] [Google Scholar]
- 289.Xue H., Tan J., Li Q., Tang J., Cai X. Optimization Ultrasound-Assisted Deep Eutectic Solvent Extraction of Anthocyanins from Raspberry Using Response Surface Methodology Coupled with Genetic Algorithm. Foods. 2020;9:1409. doi: 10.3390/foods9101409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Trouillas P., Sancho-Garcia J.C., De Freitas V., Gierschner J., Otyepka M., Dangles O. Stabilizing and Modulating Color by Copigmentation: Insights from Review Theory and Experiment. Chem. Rev. 2016;116:4937–4982. doi: 10.1021/acs.chemrev.5b00507. [DOI] [PubMed] [Google Scholar]
- 291.Klisurova D., Petrova I., Ognyanov M., Georgiev Y., Kratchanova M., Denev P. Co-Pigmentation of Black Chokeberry (Aronia Melanocarpa) Anthocyanins with Phenolic Co-Pigments and Herbal Extracts. Food Chem. 2019;279:162–170. doi: 10.1016/j.foodchem.2018.11.125. [DOI] [PubMed] [Google Scholar]
- 292.Khalifa I., Du J., Nawaz A., Li C. Multiple Co-Pigments of Quercetin and Chlorogenic Acid Blends Intensify the Color of Mulberry Anthocyanins: Insights from Hyperchromicity, Kinetics, and Molecular Modeling Investigations. J. Sci. Food Agric. 2021;101:1579–1588. doi: 10.1002/jsfa.10777. [DOI] [PubMed] [Google Scholar]
- 293.Sun X., Shokri S., Gao B., Xu Z., Li B., Zhu T., Wang Y., Zhu J. Improving Effects of Three Selected Co-Pigments on Fermentation, Color Stability, and Anthocyanins Content of Blueberry Wine. Lwt. 2022;156:113070. doi: 10.1016/j.lwt.2022.113070. [DOI] [Google Scholar]
- 294.Ertan K., Türkyılmaz M., Özkan M. Color and Stability of Anthocyanins in Strawberry Nectars Containing Various Co-Pigment Sources and Sweeteners. Food Chem. 2020;310:125856. doi: 10.1016/j.foodchem.2019.125856. [DOI] [PubMed] [Google Scholar]
- 295.Patil V.M., Das S., Balasubramanian K. Quantum Chemical and Docking Insights into Bioavailability Enhancement of Curcumin by Piperine in Pepper. J. Phys. Chem. A. 2016;120:3643–3653. doi: 10.1021/acs.jpca.6b01434. [DOI] [PubMed] [Google Scholar]
- 296.Zhao Y.S., Xin Z., Li N.N., Chang S.Y., Chen Y.D., Geng L.N., Chang H.R., Shi H.L., Chang Y.Z. Nano-Liposomes of Lycopene Reduces Ischemic Brain Damage in Rodents by Regulating Iron Metabolism. Free Radic. Biol. Med. 2018;124:1–11. doi: 10.1016/j.freeradbiomed.2018.05.082. [DOI] [PubMed] [Google Scholar]
- 297.Mao L.K., Wang D., Liu F.G., Gao Y.X. Emulsion Design for the Delivery of Beta-Carotene in Complex Food Systems. Crit. Rev. Food Sci. Nutr. 2018;58:770–784. doi: 10.1080/10408398.2016.1223599. [DOI] [PubMed] [Google Scholar]
- 298.Toragall V., Jayapala N., Vallikannan B. Chitosan-Oleic Acid-Sodium Alginate a Hybrid Nanocarrier as an Efficient Delivery System for Enhancement of Lutein Stability and Bioavailability. Int. J. Biol. Macromol. 2020;150:578–594. doi: 10.1016/j.ijbiomac.2020.02.104. [DOI] [PubMed] [Google Scholar]
- 299.Hoshyar R., Khayati G.R., Poorgholami M., Kaykhaii M. A Novel Green One-Step Synthesis of Gold Nanoparticles Using Crocin and Their Anti-Cancer Activities. J. Photochem. Photobiol. B Biol. 2016;159:237–242. doi: 10.1016/j.jphotobiol.2016.03.056. [DOI] [PubMed] [Google Scholar]
- 300.Pattni B.S., Chupin V.V., Torchilin V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015;115:10938–10966. doi: 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- 301.Tan C., Xue J., Abbas S., Feng B., Zhang X., Xia S. Liposome as a Delivery System for Carotenoids: Comparative Antioxidant Activity of Carotenoids as Measured by Ferric Reducing Antioxidant Power, DPPH Assay and Lipid Peroxidation. J. Agric. Food Chem. 2014;62:6726–6735. doi: 10.1021/jf405622f. [DOI] [PubMed] [Google Scholar]
- 302.Butnariu M.V., Giuchici C.V. The Use of Some Nanoemulsions Based on Aqueous Propolis and Lycopene Extract in the Skin’s Protective Mechanisms against UVA Radiation. J. Nanobiotechnol. 2011;9:3. doi: 10.1186/1477-3155-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Zardini A.A., Mohebbi M., Farhoosh R., Bolurian S. Production and Characterization of Nanostructured Lipid Carriers and Solid Lipid Nanoparticles Containing Lycopene for Food Fortification. J. Food Sci. Technol. 2018;55:287–298. doi: 10.1007/s13197-017-2937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zhao L.S., Temelli F. Preparation of Anthocyanin-Loaded Liposomes Using an Improved Supercritical Carbon Dioxide Method. Innov. Food Sci. Emerg. Technol. 2017;39:119–128. doi: 10.1016/j.ifset.2016.11.013. [DOI] [Google Scholar]
- 305.Lee C., Na K. Anthocyanin-Loaded Liposomes Prepared by the PH-Gradient Loading Method to Enhance the Anthocyanin Stability, Antioxidation Effect and Skin Permeability. Macromol. Res. 2020;28:289–297. doi: 10.1007/s13233-020-8039-7. [DOI] [Google Scholar]
- 306.Kontogiannopoulos K.N., Assimopoulou A.N., Dimas K., Papageorgiou V.P. Shikonin-Loaded Liposomes as a New Drug Delivery System: Physicochemical Characterization and in Vitro Cytotoxicity. Eur. J. Lipid Sci. Technol. 2011;113:1113–1123. doi: 10.1002/ejlt.201100104. [DOI] [Google Scholar]
- 307.Feng T., Wei Y., Lee R.J., Zhao L. Liposomal Curcumin and Its Application in Cancer. Int. J. Nanomed. 2017;12:6027–6044. doi: 10.2147/IJN.S132434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Amjadi S., Ghorbani M., Hamishehkar H., Roufegarinejad L. Improvement in the Stability of Betanin by Liposomal Nanocarriers: Its Application in Gummy Candy as a Food Model. Food Chem. 2018;256:156–162. doi: 10.1016/j.foodchem.2018.02.114. [DOI] [PubMed] [Google Scholar]