Abstract
Prototheca microalgae were only recognized as pathogens of both humans and animals in the 1960s; however, since then, these microbes have been drawing increasing interest in both human and veterinary medicine. The first human outbreak of protothecosis in a tertiary care chemotherapy ward in 2018 further highlighted the need to understand in more depth and detail their ecology, etiology, pathogenesis and routes of transmission between different hosts, environments and habitats from a One Health perspective. Protothecal infections have been reported in a growing number of cattle herds around the world in recent decades, and Prototheca has become an important bovine mastitis pathogen in certain countries and regions. The survival of Prototheca in the environment and its ability to spread in the herd pose a serious challenge to the management of infected dairy farms. Prevention of the disease is particularly important, as there is no effective and reliable treatment for it and the chances of self-healing are minimal. Therefore, the development of more effective drugs is needed for the treatment of human and animal protothecosis. The prudent use of antibiotics and their replacement by alternative or preventive measures, when possible, may further contribute to the control of protothecal infections.
Keywords: Prototheca, protothecosis, mastitis, ecology, food safety, One Health
1. Introduction
Prototheca spp. are unicellular, achlorophyllous microalgae that occur in a wide range of natural habitats, occupying mostly aqueous environments with high organic matter content. Prototheca spp. are opportunistic pathogens of both humans and animals, where the precise pathogenic mechanisms of protothecal infections are still mainly unclear. Several Prototheca species, namely P. ciferrii (formerly P. zopfii genotype 1), P. bovis (formerly P. zopfii genotype 2), P. wickerhamii, P. blaschkeae, P. cutis and P. miyajii cause infections both in humans and in animals [1,2]. These observations highlight the importance and validity of One Health principles for current and future studies of Prototheca microalgae. Bovine mastitis is the predominant form of protothecal infections in animals; however, protothecosis has also been described in dogs, cats and goats, and sporadic cases have been reported in a number of other vertebrates [3].
Although protothecal disease is still considered uncommon, an increasing number of cases are identified globally, and Prototheca has gained a growing interest in human and veterinary medicine. Prototheca microalgae were first studied in 1894 by Krüger, who identified them as fungi on account of their cultural similarity to yeasts. The pathogenic potential of Prototheca at that time was not known [4]. The first animal and human infections by Prototheca were reported in 1952 [5] and in 1964 [6,7], respectively. Prototheca is now recognized in several countries or geographical regions as a major pathogen of bovine mastitis, and its growing importance was also highlighted by the first human outbreak of protothecosis in a tertiary care chemotherapy ward in 2018 [8,9,10].
The One Health principle recognizes relationships between the health of humans, animals and the ecosystem and encourages interdisciplinary approaches to address complex health issues [11]. Prototheca microalgae are adapted to natural environments, such as slime flux, and to man-made environments such as municipal sewage digesters that contain suitable nutrients. From these and from other environmental sources, Prototheca microalgae may disseminate into aquatic systems, from where they may enter and pass through the human and animal gastrointestinal tract and enter sewage. Under certain circumstances, such as by traumatic or other types of inoculation, these microalgae can cause opportunistic infections in humans and in domestic or wild animals [12].
The One Health approach can thus be indeed applied to better understand the relatively unexamined and unexplored characteristics of these opportunistic pathogenic microalgae. An analysis of available data concerning their occurrence in various habitats and environments and their role as causative agents of human and animal infections may identify current knowledge gaps regarding their epidemiology and routes of dissemination. Such an analysis could clearly benefit from applying the One Health approach to address one of today’s emerging health challenges.
2. The Prototheca Genus
Microalgae from the genus Prototheca are closely related to the green algae Chlorella spp., and both genera are placed within the family Chlorellaceae, order Chlorellales, class Trebouxiophyceae. However, Prototheca has lost its ability to synthesize chlorophyll and adopted a heterotrophic nutrition [10,13]. Prototheca species are distinguished from other algae, such as Chlorella, by their lack of chloroplasts and the presence of a two-layered, instead of a three-layered, cell wall [14]. Comparative analyses of rRNA sequences from various organisms revealed that Prototheca has closer relationships with plants than with fungi [15].
The most important animal protothecosis, bovine mastitis, is predominantly caused by P. bovis, while P. wickerhamii is the most common human pathogenic species [4,6]. Two other species, P. stagnora and P. moriformis, are commonly isolated from natural environments and are generally considered non-pathogenic. These two species can produce capsule, while this feature is not characteristic for clinical isolates of P. wickerhamii and P. bovis [1,4,6,16].
Initially, the rRNA gene cluster was the main target for Prototheca molecular taxonomic studies. The recently proposed use of the cytb gene offered advantages over the previously used rDNA markers because of its high discriminatory capacity, intra-strain sequence homogeneity and technical feasibility, including a reproducible amplification assay [1]. By applying cytb gene analyses, genotypes 1 and 2 of P. zopfii were elevated to species status in the forms of P. ciferrii and P. bovis, respectively. Furthermore, five new species were proposed: P. cerasi, P. cookei, P. pringsheimii, P. xanthoriae and the re-defined P. zopfii. In addition, a new species, P. paracutis, has also been recently described [17]. Of the 15 Prototheca species, only six (P. blaschkeae, P. bovis, P. cutis, P. miyajii, P. ciferrii and P. wickerhamii) have been clearly implicated as human and animal pathogens [1,8].
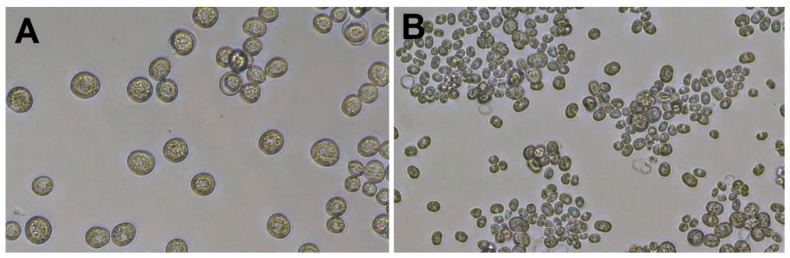
Prototheca cells (Figure 1) are non-motile and are much larger than bacteria. As an example, P. bovis can be up to 25 μm in diameter [1,6]. The Prototheca cell wall does not contain cellulose and chitin, which are constituents of plant and fungal cell walls, respectively. Prototheca have a characteristic cell wall of two layers: a thin outer layer and a translucent and thicker inner layer. The membrane-bound plastids of Prototheca cells may contain starch deposits and were proposed to represent vestigial chloroplasts of green algae [15].
Figure 1.
Cells of P. blaschkeae (A) and P. bovis (B) microalgae strains grown in RPMI 1640 medium. Images were taken at 200× magnification using a Leica DM IL LED Microscope (Leica Microsystems CMS GmbH, Wetzlar, Germany).
Prototheca cells undergo asexual reproduction through endospore formation subsequent to cytoplasm cleavage. About 2–20 endospores are formed, whose shape, number and size vary among Prototheca species. The sporangia (mother cells) break up under pressure from the enlarging spores, and the release of the spores takes place about every 5–6 h in the presence of adequate nutrients [10,18,19]. The sporangia of P. wickerhamii (of 3–10 μm size) are generally smaller than the sporangia of P. bovis (with a globose to ellipsoidal shape, on average 15 × 13 μm and 9 × 7 μm, respectively) [1,14]. P. wickerhamii tends to form symmetrical morula-like shaped sporangia (with endospores arranged symmetrically like a daisy), while P. bovis displays more random internal segmentation [20]. In Gram-stained preparations, Gram-positive spores and Gram-negative empty sporangia can be detected [4].
The number of daughter cells increases with the growth rate. The different growth rates also determine particular physiological states that are characterized by a specific cell size and relative DNA, RNA and proteins contents [15,16]. A thick cell wall and no cell divisions are characteristic of a resting cell stage.
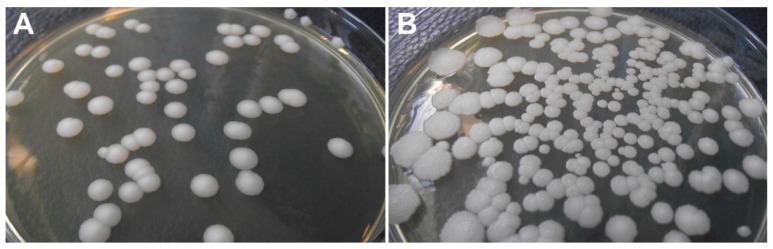
Prototheca cells settle rapidly in aqueous environments because of their sufficiently higher density compared to bacteria. Laboratory suspensions and likely the natural populations of Prototheca are also hydrophobic, contributing to their affinity for sediments and surface films [12]. Culturing at 30 °C for 72 h is appropriate for most Prototheca species, and the mastitis-causing Prototheca strains can be cultured at about 35 °C. However, certain strains show a preference for 25 °C, and some slow-growing isolates may require up to 7 days for the formation of visible colonies [6,21]. Culture media containing glucose (such as Sabouraud’s dextrose agar, Figure 2) are suitable for growing Prototheca, where 100 μg/mL chloramphenicol can be added to suppress bacterial growth [16]. Prototheca microalgae may not survive freezing for longer periods of time, and fresh samples are therefore recommended for culturing in situations when a culture-based analysis is required [22].
Figure 2.
Colonies of P. blaschkeae (A) and P. bovis (B) microalgae strains grown on Sabouraud agar medium.
3. Prototheca and Bovine Mastitis
P. zopfii was initially identified as the causative agent of bovine mastitis in Germany in 1952 [5,13]. Mastitis caused by Prototheca species represents the most important form of protothecosis in animals because of the associated economic losses (reduced milk production, premature culling of affected animals and veterinary expenses) [23,24].
Protothecal mastitis has been reported worldwide, including in the USA, Canada, Belgium, Denmark, Germany, Italy, Poland, Hungary, Brazil, Taiwan, Korea, China, Japan and in other countries [16,23]. It was proposed that endemic Prototheca mastitis with high numbers of affected cows could be identified mainly from geographical regions with a relatively high humidity and/or temperature, supporting the increased growth of these microalgae in the environment [25]. During a P. zopfii outbreak of clinical bovine mastitis in the State of São Paulo, Brazil, 77/211 (36.49%) milk samples proved positive for P. zopfii, as well as fecal samples of 11 calves and two cows and two swabs from teat cup rubbers. The microalgae could be also isolated from two water samples and one soil sample collected from the dry cow pasture [26]. A similar level of prevalence for P. zopfii (34.1%, in 14/41 lactating cows) was found during an outbreak of protothecal mastitis in Taiwan [27]. In Canada, the herd-level prevalence of Prototheca spp. was found to be around 6% based on bulk tank milk examination using a commercial polymerase chain reaction (PCR) assay [28]; in Poland, P. bovis was isolated from quarter milk samples on eight farms out of 16 examined [29].
Protothecal mastitis is usually recognized as a chronic and asymptomatic disease which causes increased somatic cell counts and reduced milk production due to the damage inflicted on the udder [30]. The clinical form appears much less frequently and is characterized by milk alterations (watery appearance and the presence of white flakes) and changes in the udder (swelling, hard tissue consistency, tenderness). However, Prototheca spp. as a causative agent of mastitis are often neglected, and protothecal mastitis is often suspected when resistance to antibiotic therapy is observed [31]. Out of the Prototheca spp., P. bovis is the most common cause of clinical or subclinical mastitis in cattle. Sporadically, P. blaschkeae has been documented [32]. P. wickerhamii, the main causative agent in human infections, has rarely been described in mastitis milk [33].
During the development of bovine mastitis, Prototheca cells probably enter the udder through the teat orifice, originating, for example, from contaminated milking equipment or from various environmental sources [34]. The reported level of infection within dairy herds is variable. In some herds, only a few animals are infected, but in other herds, P. bovis was detected in up to 30% of mastitis milk samples or more [35]. The mean prevalence in a herd is usually about 10% [29,36]. Although it is a persistent infection, unfortunately it is not always possible to detect all infected individuals in the herd because of intermittent shedding. Therefore, the re-examination of suspected cows is recommended to reveal all infected cows in the herd [37].
The infection is usually confined to the mammary gland and regional lymph nodes exhibiting granulomatous inflammation [4,23]. P. bovis may survive in the mammary gland during the dry period and be released back into the milk during subsequent lactation. However, in addition to the excretion of Prototheca spp. in milk, Prototheca spp. are also spread into the environment through feces [38]. Calves fed with Prototheca-containing mastitic milk may be a further source of environmental contamination on impacted farms [16]. Prototheca can be also isolated from various specimens (feces, mouth, nose, rectum and vagina) from cows whose milk was negative for Prototheca spp., suggesting that Prototheca spp. can also colonize the bovine gastrointestinal and urogenital tract without causing harm to the host [23]. Milk samples from 25 cows with mastitis and 24 samples of bulk-tank milk were examined for the presence of Prototheca in a study conducted in Japan [39], together with 360 specimens from cow-barn surroundings (drinking water, sewage, feces and sawdust). All 67 mastitis isolates were identified as P. bovis (i.e., P. zopfii genotype 2). However, among 32 isolates recovered from cow-barn surroundings only three isolates were identified as P. bovis, while the other 29 isolates were P. ciferrii, indicating that both Prototheca species could exist in the cow-barn surroundings, but no sites were frequent sources of P. bovis. It was therefore presumed that the route of dissemination for P. bovis was probably through milking equipment contaminated by protothecal mastitis [39].
Analyses of risk factors for Prototheca mastitis on dairy farms in Ontario, Canada indicated that the off-label intramammary administration of injectable drugs, antimicrobial treatment and multiple and possibly unsanitary intramammary infusions were associated with an increased risk of Prototheca mastitis [40]. The results of a cross-country study performed in Poland suggested that the size of the farm (in acreage) and the number of farm employees positively correlated with the incidence of Prototheca mastitis [29]. A study examining five dairy herds in the Apulia Region in Italy indicated that the presence of Prototheca spp. in dairy herds seemed to correlate with the hygienic conditions of the milking equipment and with the proper management of animal feeding and drinking water [41].
4. Protothecosis in Other Animals
Besides bovine mastitis, protothecosis has also been reported as an uncommon disease in a number of other animal species, including dogs (Canis lupus familiaris), cats (Felis catus), roe deer (Capreolus capreolus), goats (Capra hircus), horses (Equus caballus), a beaver (Castor canadensis), a cape hyrax (Procavia capensis), Atlantic salmon (Salmo salar), carp (Cyprinus carpio), fruit bat (Rousettus lanosus), a flying fox (Pteropus lylei) and a corn snake (Elaphe guttata guttata) [2,42,43,44,45,46].
While mastitis is the predominant form of protothecal infection in cattle, dogs typically suffer from disseminated infections where the outcome is usually fatal. Until recently, 59 infected dogs had been reported worldwide [8,47]. Dogs acquire Prototheca after the ingestion of large numbers of microalgae [8]. Therefore, infections often begin with chronic bloody diarrhea, followed by neurologic symptoms such as ataxia, blindness, deafness or seizure, with P. zopfii genotype 2 (that is, P. bovis) being most frequently isolated from canine protothecosis and, in rare cases, P. wickerhamii [4,48].
Protothecosis in cats has rarely been reported and usually causes nodular skin lesions in sites of penetrating injury, for example on the face, head or the distal limbs. The predominant species isolated from feline protothecosis is P. wickerhamii [8,49,50], while Huth et al. [51] reported a case of P. bovis-induced inflammation of the nasal skin and cutaneous mucosa of the right nostril in a 14-year-old cat. Protothecal infections have also been described in goats with nasal and cutaneous forms caused by P. wickerhamii [43]. Protothecosis of a fruit bat resulted in lesions in the lymphatic system, spleen, the central nervous system, heart, muscle and kidneys, causing severe granulomatous lymphadenitis and splenitis and a widespread granulomatous meningoencephalitis [42]. P. wickerhamii was isolated from a carp (Cyprinus carpio) which was underweight for its age, sluggish and displayed erratic swimming behavior (ataxia). Skin erosions and ulcerative and nodular lesions were spread over its whole-body surface. Similar lesions could be observed on several of its internal organs, including the liver and the intestinal mucosa [2].
During the course of a mouse protothecal mastitis model experiment [52], 6–8 weeks old lactating female mice were inoculated intramammarily with either P. bovis (50 µL containing 1 × 105 CFU/mL) or an equal volume of phosphate buffered saline (the control group). P. bovis induced acute mastitis with the infiltration of leukocytes throughout the parenchyma and in the lumen of alveoli. P. bovis cells were present both free within alveolar lumen and throughout the interstitium of the mammary tissue. Macrophages were found in the mammary interstitium and neutrophils were diffusely distributed in P. bovis-infected mice. The presence of P. bovis upregulated gene activity and protein production of pro-inflammatory TNF-α, IL-1β and Cxcl-1 in the mammary tissue at four days post inoculation [52]. P. bovis caused model mastitis in mice that manifested in the severe red swelling of the mice mammary glands as well as necrosis and nodules lesions in the infected mice mammary tissue, accompanied by macrophage and neutrophil infiltration [53].
In addition to the various types of infections caused by Prototheca, several reports also described the asymptomatic colonization of animals by the microalgae. As an example, 11/15 pig and 3/11 dog feces samples, respectively, examined in the Philippines were colonized by Prototheca, predominately P. zopfii, while other sampled animals proved negative [12]. P. wickerhamii was also recovered from a pigeon crop sample, but not from cloaca or droppings. It was proposed that the isolation of P. wickerhamii from the pigeon crop might be explained by the pigeon swallowing contaminated water [54]. P. zopfii was detected in 3/14 (21.4%) fecal samples of wild boars by using selective Prototheca isolation medium (PIM) [21,55]. Fecal samples of rats trapped on a small rural pig farm tested positive for P. zopfii, where contaminated feed may have been the source of the algae and the animals did not sustain their intestinal colonization when their feed was P. zopfii free [56]. P. zopfii was also isolated in monoculture from 9/146 (6.2%) horse fecal samples [57], indicating that a range of wild and domestic animals may harbour Prototheca in their gastrointestinal tract and disseminate Prototheca spp.
5. Prototheca in Natural, Agricultural and Other Human-Impacted Environments
Only few targeted environmental studies have been performed for Prototheca, and in several cases these investigated the environments of farm animals. Our understanding of the ecology of Prototheca microalgae is therefore rather limited. Species formerly designated as P. zopfii (either P. bovis or P. ciferrii) tended to be the most abundant in the environments of cattle farms [34]. Watering troughs, manure, feed and mud were Prototheca-positive environmental samples at agricultural farms in one study [23], where drinking water and manure were the major environmental reservoirs, similar to cases before. Wet feeds rich in starch and oligosaccharides, such as potato pulp, may also serve as a medium for Prototheca. The microalgae have been detected in milking equipment, in its pipelines, on teat cup rubbers and may even survive routine disinfection procedures with chlorine solution [16]. In a cross-country study conducted in Poland, bedding was the most Prototheca-abundant sample type among environmental sources, followed by barn walls, feed and drinking water. Environmental samples were most commonly positive for P. bovis (47.6%), followed by P. ciferrii (33.3%) and P. blaschkeae (19.1%) [29]. P. blaschkeae was also cultured from fecal and environmental samples of pig farms [58]. Furthermore, P. zopfii and P. wickerhamii were detected in freshwater aquariums and aquarium filters [12].
In terms of natural environments, the slime flux of certain deciduous trees is a habitat for Prototheca spp., such as in elm trees (Ulmus americana, U. carpinifolia), Japanese elm (Zelkova serrata), lime trees (Tilia spp.), Mizunara (Quercus crispula) and white mulberry (Morus alba). P. stagnora was found to be a habitant of older harvested banana (Musa sapientum) and plantain (M. paradisiaea) stumps, while P. wickerhamii colonized fresh Musa sp. stumps and the flower bract water of Heliconia sp. [59]. P. zopfii was also isolated from broccoli leaves [60], and two P. paracutis strains were isolated from the water and soil of a mangrove forest in Thailand [17]. Prototheca microalgae are not known as pathogens of plants. It is possible, however, that they play a certain role in slime flux formation, probably together with other microbes. Prototheca spp. were also present in soil specimens from beneath trees producing slime flux and from stream banks and in pasture soil [12].
The microalgae were cultured from sewage of different geographical locations, including Ohio, France, Spain and the Philippines. P. zopfii and P. wickerhamii were isolated from raw sewage and sludge from both aerobic and anaerobic wastewater treatment plants. P. wickerhamii is likely the most abundant species in human sewage [34], and it was suggested that the primary and secondary sewer system is a place of growth and reproduction for Prototheca. Prototheca spp. are generally killed by chlorination in the liquid effluent from settling ponds, but in certain cases of inadequate treatment they may escape into rivers [12]. These earlier observations led us to initiate a pilot bioinformatic analysis of shotgun metagenomic sequencing data for wastewater samples available in the National Center for Biotechnology Information Sequence Read Archive (NCBI SRA) database. The initial results detected P. zopfii/P. bovis microalgae in two wastewater metagenomic datasets from the USA and Pakistan, respectively (Libisch et al., unpublished data, Supplementary Table S1), indicating the potential utility of applying metagenomic methods to gain further insights into the ecology of Prototheca spp.
Concerning occurrences in food, Bacova and colleagues [61] monitored Prototheca spp. in milk samples obtained from supermarkets in the Czech Republic from different producers. P. bovis was found in 13 out of 16 milk samples at concentrations ranging from 1.50 × 100 gene copies/mL up to 1.18 × 104 gene copies/mL using real-time PCR. However, all samples were culture negative, confirming pasteurization effectivity in these milk samples [61]. On the other hand, in a study performed in Brazil Prototheca was detected by plate count in a cheese sample produced using Prototheca-contaminated milk [62]. The Prototheca spp. counts in bulk milk samples ranged from 1–3 × 104 colony forming units/mL (CFU/mL), while the examined cheese sample produced from contaminated milk contained Prototheca spp. at 7.5 × 10 CFU/mL [62]. In another study, raw milk and locally manufactured cheese samples were collected and analyzed from city markets in Qena Governorate, Egypt [63]. P. bovis was detected by PCR and had the highest incidence among Prototheca spp. in both the examined raw milk and cheese samples. The isolation of Prototheca from the examined cheese samples might have been due to the use of raw milk for cheese production, simple processing methods or alternatively contamination after heat treatment or the during production and handling steps of the cheese [63].
Prototheca microalgae were also found in other food products, such as in beef, pork, clams and crabs. Although there is a variety of sources from which they can be isolated, sewage water and animal waste probably represent their main environmental reservoir [15]. In a study conducted in China in Hubei province, a warm and humid region, P. bovis was found in brewer’s grains (an important feed additive for dairy cattle in China) and in fresh cow feces from a dairy farm affected by P. bovis infections. Moreover, P. bovis was also isolated from the truck transporting the brewer’s grains to the dairy farm, suggesting that such vehicles may also possibly disseminate P. bovis [53].
6. Prototheca in Human Disease
P. wickerhamii, the most common human pathogenic species, was described as a new species by Japanese scientists in 1959 [6]. Davies and colleagues published the first description of a human infection attributed to Prototheca, which concerned a 31-year-old rice farmer from Sierra Leone, in 1964 [7]. The patient had a papular, hypopigmented small lesion on his right foot that had gradually encircled the foot and spread up the leg. The patient was lost to follow-up after 1965 and presumably died with or because of his infection [7,14].
Human protothecosis has been classified in three main clinical forms, namely cutaneous lesions, olecranon bursitis and disseminated or systemic infections [20,64], and most infections are probably caused by a traumatic inoculation into subcutaneous tissues. In an analysis of 160 cases of human protothecal infections published before June 2011, more than half of the cases (93/160, 58.1%) were of the cutaneous form [6]. The incubation period for protothecal infections is not well documented; however, periods of weeks to months were suggested. A local or systemic immunosuppressive factor was identified in about half of the human cases. P. bovis appears to be the main Prototheca species in systemic protothecal infections, while P. wickerhamii is involved to a greater extent in cutaneous human infections. Workers, for example, in rice paddies, fishermen, farmers, handlers of raw seafood and aquarium staff were considered to have a higher risk of exposure to Prototheca species [20].
The incidence of protothecosis was roughly equal among males and females. The patients’ ages ranged from 78 days to 88 years, but it was skewed toward older ages. Skin infections comprised over 50% of the cases, and other infection types included disseminated infections, olecranon bursitis, wounds, septicaemia, nail lesions, peritonitis and miscellaneous other cases. Skin infections had the best prognosis, with a cure or improvement rate of about 78% and a mortality of only 1%, while disseminated cases had the worst prognosis, with about a 33% cure or improvement rate and a 56% mortality rate [6]. Overall, human protothecosis appears to be a rare disease with an increasing incidence in recent decades, with the increase being mostly attributed to patients with immunosuppression, corticosteroid treatment or both [6]. Hospital-acquired cases were mostly associated with surgery or orthopaedic procedures. Infections may occur when skin injuries come into contact with microalgae-contaminated water, while colonized patients with predisposing factors may develop endogenous protothecal infections [14].
The carriage of Prototheca in the human gastrointestinal tract has also been described, although in an earlier study it occurred sparingly in human feces (three positives out of 300 samples) [65]. In another report, a 2-month-old infant diagnosed with treatment-resistant gastroenteritis provided P. wickerhamii-positive stool cultures in the context of an otherwise usual intestinal flora [66]. In a recent survey in a rural area in Thailand [67], P. bovis was detected in the fecal samples of four out of 98 healthy volunteers exhibiting no diarrhea. Participants of this study spent extended periods of time on rice fields (similar to the first human case described by Davies [7]), and had frequent contact with ruminants and poultry, whose manure was used to fertilize local vegetable gardens. Since it was not possible to confidently identify the source of protothecal colonization, the authors highlighted the need to apply a comprehensive One Health approach that includes humans, animals and the environment in such future surveys [67].
7. Diagnosis and Treatment of Prototheca Infections
Prototheca infections can be diagnosed by histopathological examination and/or by the isolation of the causative agent [20]. Traditionally, Prototheca isolates were identified by their macro- and micromorphology and biochemical profiling. The creamy-white colonies can be mistaken for yeasts; however, Prototheca endospores are characteristic features for identification by microscopy [4,15]. Matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry proteomic analysis [6,10,58] and various molecular methods have also been developed for the identification and detection of Prototheca spp. [9,34,35,36,39,51,58,61]. Furthermore, enzyme-linked immunosorbent assay (ELISA) may be applied for discrimination between infected and non-infected dairy cows, and the detection of anti-protothecal antibodies in serum and whey provided sufficient specificity and sensitivity for the diagnosis of protothecal mastitis [9,66].
Definitive treatment guidelines for protothecal infections have not yet been established. This is because the algae often display high levels of resistance to a variety of antimicrobial agents and there is a lower correlation between in vivo clinical response and in vitro susceptibility results [20]. This low correlation is probably linked to the absence of guidelines for in vitro susceptibility testing for Prototheca microalgae and to the absence of established clinical breakpoints. Many aspects of in vitro susceptibility testing of Prototheca species are similar (or even identical) to those of the Clinical and Laboratory Standards Institute (CLSI) or European Committee on Antimicrobial Susceptibility Testing (EUCAST) procedures recommended for yeasts. However, specific conditions for protothecal growth should be considered when optimizing antimicrobial susceptibility testing for these organisms in future [20,68].
Standard treatment guidelines have not yet been established for human or for veterinary protothecal infections [69,70]. Prototheca spp. share some similar features with yeasts, specifically the presence of ergosterol in the cell membrane. For this reason, treatment of human protothecosis most commonly includes antifungal agents plus surgical approaches [20]. The surgical treatment should constitute complete excision because drainage might fail, provided the human protothecosis was proven before the intervention [71]. Wide surgical excision has been indicated for protothecal cutaneous lesions of dogs and cats, although a substantial number of animals subjected to excisional procedure or biopsy of solitary nodules developed systemic infection following the surgery [72].
Intravenous amphotericin B is currently considered the most effective treatment for human protothecal infections, with a cure or improvement rate of 72%. Itraconazole and fluconazole had cure or improvement rates of 71% and 65%, respectively. For relatively mild cases, it was recommended to begin with oral itraconazole or fluconazole and to use intravenous amphotericin B for serious infections and for infections that have failed azole treatment [6]. In addition, in vitro testing has shown that ravuconazole has a higher algaecide effect than other azoles tested against Prototheca species. This new azole agent, available since 2018 in Japan, may also be considered for the treatment of human and animal protothecosis [8].
In general, Prototheca spp. tested resistant to 5-flucytosine, and P. wickerhamii and P. bovis isolates showed variable resistance against fluconazole and itraconazole or voriconazole. Currently, little information is available about the resistance mechanisms responsible for the observed species- or strain-specific in vitro antimicrobial susceptibility patterns of Prototheca algae. Most polysaccharides in the Prototheca cell wall are β-1, 4-bonded rather than β-1, 3; thus, the echinocandins which inhibit β-1, 3 glucan synthesis are generally ineffective [8].
In the course of a chronic human meningitis case caused by P. wickerhamii in Japan, during a 3-year period of therapy, the induction of secondary drug resistance was reported. The minimum inhibitory concentrations (MICs) for amphotericin B and fluconazole increased from 0.39 to 3.13 μg/mL and from 50 to 200 μg/mL, respectively. This extent of clinically significant acquired resistance was regarded to be an uncommon phenomenon [73]. To date, no effective therapies against protothecal mastitis have been developed or routinely applied. Liposomal amphotericin B (with reduced nephrotoxicity, applied in human therapy) is highly expensive and thus unsuitable for routine use in cattle farming. Furthermore, in the European Union (EU), while amphotericin B is authorized in human medicines, there are no such authorized veterinary medicines in the EU. Nonetheless, textbooks suggest that amphotericin B has been used to treat systemic fungal infections in companion animals and certain fungal diseases in horses [74]. Amphotericin B cannot be used in food-producing animals in the EU, as it is not included in the Annex to Regulation (EU) No 37/2010 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin [74].
When protothecal infection is detected in a cow, the elimination of the animal from the herd is usually necessary. To avoid problems associated with protothecosis, the farmers are advised to follow preventive measurements against protothecosis. Excessive empirical use of antimicrobials should be avoided whenever possible, and the therapy should be based on the results of the laboratory identification of the causative agent of mastitis and its in vitro antimicrobial susceptibility pattern. Good hygienic conditions with respect to milking equipment should be maintained and the proper management of animal feeding and drinking water is advised [13,39,41].
Taken together, the development of novel and more effective agents is needed for the treatment of human and animal protothecosis [10], together with the establishment of standardized procedures for in vitro susceptibility testing. Some recent in vitro studies have shown various promising, novel algicidal treatments, such as silver nanoparticles [75] or guanidine [76], but their in vivo efficacy has not yet been demonstrated [34]. The expanding knowledge based on the sequencing of Prototheca genomes may contribute to a better understanding of their pathogenesis and pathways of infection and to developing specific immunisation procedures or algicidal substances against protothecal infections [13].
8. Resistance of Prototheca spp. against Environmental Conditions
Prototheca microalgae are likely protected from digestion in the gastrointestinal tract due to their sporopollenin cell wall. Sporopollenin is a natural organic polymer resistant to mechanical stress, physical treatment, degradation by acidic and alkaline hydrolysis or enzymatic breakdown. Laboratory experiments indicated that Prototheca spp. were unharmed by passing through rodent and primate intestinal tracts [6,12,48]. P. bovis strains were shown to tolerate pH 2.1 and 6.0% NaCl concentration, while P. blaschkeae strains had a lower salt and pH tolerance of pH 4.0 and 4% NaCl, respectively [24].
One of the important mechanisms that enable Prototheca spp. to persist in the environment is the formation of biofilms, which contributes to the resistance of Prototheca microalgae against various sanitizers on farms. Prototheca biofilms also displayed an increased resistance to antimicrobial agents [34,77]. The chlorination of effluents from wastewater treatment was variably effective in reducing the numbers of Prototheca. It was proposed that inadequate or no chlorination, heavy organic loading or high effluent volumes may result in the discharge of large numbers of Prototheca spp. into rivers and lakes [65].
The susceptibility of Prototheca algae to pasteurization has, thus far, only been examined in a small number of studies, with variable results [78,79,80,81]. Forty P. zopfii strains isolated from cow milk were subjected to different heat-treatments (72–75 °C for 15 s, 72–75 °C for 20 s and 62–65 °C for 30 min), where the resistance of 34 strains was reported in at least one of the conditions [4,78]. In another study, the effect of eight different temperature/time ratios on P. bovis and P. blaschkeae isolates was tested in the range of 62 °C for 15 min to 100 °C for 1 s [79]. Total growth inhibition was achieved only by using the 100 °C for 1 s treatment, suggesting that ultrapasteurization was the only procedure capable of ensuring that the milk becomes free of algae. A significant difference was found between the susceptibilities of the two species: P. blaschkeae displayed higher resistance to heat treatment than P. bovis, with an adjusted logCFU/mL mean count value 1.3-fold higher than that of P. bovis [79]. Lassa and colleagues reported that 13/50 (26%) of tested P. zopfii isolates demonstrated resistance to 72 °C for 15 s treatment, providing a concentration of 5–29 CFU/mL of milk [80]. Another recent work examined the susceptibility of five P. bovis and one P. blaschkeae isolates in the temperature range of 47–60 °C for 1–90 min [81]. In this study, all the Prototheca strains survived the 50 °C for 90 min treatment, but exposure to 60 °C for one minute was effective. Therefore, it was proposed that the commonly used pasteurization temperature of 72 °C for 15–20 s during the manufacturing of dairy products would eliminate Prototheca spp. and would ensure that the health and safe status of the product was not be compromised [81]. This latter finding agrees with the views of other authors [22], suggesting that the human health risk from the consumption of processed milk from sub-clinically infected cows is low, as pasteurization is usually effective against Prototheca. On the other hand, the consumption of raw milk from Prototheca-infected cows may still pose a potential human health risk [22]. Taken together, the experimental results available in the cited reports are not yet conclusive. One possible explanation for the observed variable heat-treatment susceptibility may be the tendency of these microalgae to form cell clumps, thus preventing adequate exposure of the algal cells in the middle of clumps to elevated temperatures. In this case, the homogenization of the milk during heat treatment may also potentially contribute to a more effective pasteurization process [79].
When Prototheca microalgae have been introduced into a farm, their eradication may be laborious. Due to dust or feces contamination on the teat skin before milking and fat and protein contamination after milking, respectively, dipping teats through the use of effective disinfectants before and after milking is recommended to prevent infections by environmental and contagious pathogens. Chlorhexidine and iodine are both suitable for the disinfection of teats and milking equipment under normal circumstances or during outbreaks of protothecal infections [82,83,84,85]. Minimal algaecide concentrations (MAC) of chlorhexidine and povidone-iodine were obtained for 16 P. zopfii isolates from Taiwanese dairy farms by the microdilution method, where the best concentrations of chlorhexidine and iodine for in vitro algaecide efficacy were 3.13 μg/mL and 390.63 μg/mL, respectively. These identified disinfectant concentrations were lower than that of the manufacturer’s recommendations [86].
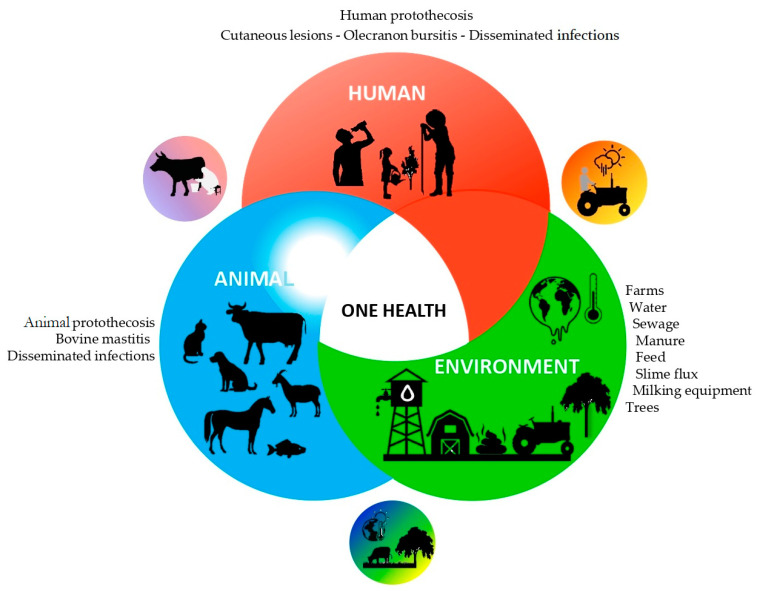
9. One Health Perspective
The culture-based studies performed by Pore and colleagues to screen for and to isolate Prototheca microalgae from a wide range of environmental samples and food products using PIM medium [21] provided highly valuable findings on the ecology of Prototheca microalgae and their potential routes of dissemination between different natural and human-impacted environments and habitats (Figure 3) [12,56,59,65]. In these studies, Prototheca spp. were also recovered from drinking water and various food products in the Philippines, and P. wickerhamii was isolated from the drinking water samples. Salads were contaminated predominately by P. zopfii, probably due to the ice used to chill the lettuce. Prototheca were detected from pork products, beef and carabao products, clams, crabs and from raw pork sourced in the markets. It was assumed that meat products were probably contaminated by pig intestinal contents because of the applied local abattoir practices. Effluents of slaughterhouse wastes in West Virginia, USA, were also found to be rich in Prototheca algae in another study [12].
Figure 3.
Prototheca infections and ecology from a One Health perspective: a schematic diagram summarizing the diverse habitats, human and animal hosts and possible routes of transmission for Prototheca microalgae.
An analysis of protothecal canine infections in Australia found that the cases occurred generally in medium to large breeds, likely living outdoors [47]. It remained unclear exactly how and why the microalgae initiated these infections. However, it was proposed that the first step could have been the colonization of the colon after the ingestion of high numbers of Prototheca cells from an environmental source, such as from dam water or stagnant pools. This would explain the observed overrepresentation of larger dogs with usually outdoors domiciles. Presumably, the next step of infection might have involved a disruption of the epithelial barrier of the bowel wall, thus permitting access for Prototheca to the submucosa [47]. A portal for internal inoculation may also have been provided by trauma, surgery or possibly through the bites of insects that populate the slime flux of trees [15].
Other observations pointed to the assumption that under certain circumstances Prototheca may also disseminate via the air, aerosols or by droplet infection. P. zopfii was identified in air samples from semi-closed pig farms evaluated in Korea, suggesting that Prototheca spp. may also be airborne [87,88]. In a report describing pyogranulomatous rhinitis and necrotizing sinusitis associated with P. bovis infection in two horses [44], it was noted that both the horses lesions developed only in the upper respiratory tract and that the infection therefore likely occurred via inhalation. The source of infections remained uncertain, although in one of the cases the pasture was located adjacent to that of dairy cows, and P. bovis is predominant in bovine protothecal mastitis [44].
Prototheca spp. can colonize the human skin, fingernails, the respiratory tract and digestive system. Infections may develop after the traumatic inoculation of contaminated soil, water or other materials [10,20]. Molecular typing of strains from environmental samples together with those causing the infection could contribute significantly to uncover the etiology of protothecal infections, if they can be closely linked to each other by genotyping. If the environment is the main source of protothecal infections, disease prevention may involve reducing the environmental load by microalgae (for example by chemical treatment of contaminated water), protecting subjects (such as by separating contaminated areas) or reducing pathogen load at the entry site through topical treatment [6].
The detection of Prototheca in pigeon crop and rat feces [54,56] point to the possibility of their role as vectors in the spread of microalgae between various environments or groups of animals [56]. However, neither animal-to-human nor human-to-human Prototheca transmission has been proven to date [9,15]; thus, to investigate the possible modes of transmission would require further targeted studies in the future.
Milk and dairy products contaminated with Prototheca algae have been proposed to be potential sources of human infection or colonization [4,41,79,89,90]. Costa and colleagues reported a relevant observation, when the occurrence of human enteritis was associated with and followed the consumption of cheese contaminated with Prototheca sp., and the algae were subsequently isolated from this patient’s feces [56,89,90]. The National Center for Epidemiology in Hungary examined the case of a 10-year-old girl suffering from bloody diarrhea on a rural farm [91]. Negative stool cultures were obtained on selective media for enteric bacterial pathogens, and the patient’s parasitological examination was also negative. However, mycological culture yielded a Prototheca sp. strain. It was not established that the bloody diarrhea was actually caused by the Prototheca strain, but the circumstances (farm lifestyle) made it conceivable that the consumption of raw milk was a possible source of infection. During repeated tests, her stool culture became Prototheca-negative along with the cessation of diarrheal symptoms. No information is available whether any therapy was applied during this case, and, if so, what medication was administered [91]. Similarly, a study of Joerger and colleagues [92] described a case of chronic meningitis due to P. zopfii in an adolescent girl who lived on a farm in rural Pennsylvania with her family. The source of P. zopfii infection was not identified; however, it was noted by the authors that she not only swam in freshwater ponds, but also had frequent exposure to soil on her family’s farm and frequently milked cows [92].
The consumption of milk and/or dairy products contaminated with Prototheca spp. [93] may represent one potential route of transmission. Although direct human infection through dairy products has not been never confirmed, it was confirmed that Prototheca spp. could be extracted to milk in relatively high quantities [61]. Prototheca cells do not usually survive pasteurization temperatures [61], although in a few studies the survival of Prototheca spp. was documented, with the same being true in the case in non-pasteurized cheese [62,63]. These observations support the necessity to implement effective control measures on dairy farms and appropriate quality control practices for milk products [79]. Further studies are therefore needed regarding the effect of common pasteurization regimens on Prototheca contaminated dairy products and regarding the potential public health risks associated with the consumption of raw milk from Prototheca-infected cows.
The prudent use of antibiotics is one of the key objectives of the One Health principles. In this respect, it is of specific relevance that antimicrobial treatment and multiple and possibly unsanitary intramammary infusions were identified as factors associated with an increased risk of Prototheca mastitis [40]. An analysis performed in Germany also suggested that antibiotic treatment could promote protothecal infections through inhibiting the competitive natural udder bacterium flora [16,66,94,95]. Pieper and colleagues recommended that a farmer–veterinarian relationship should be established, and treatment options discussed to avoid excessive, unsuccessful and extra-label antibiotic use for mastitis because Prototheca microalgae might act as opportunistic pathogens and may be promoted by antibiotic-induced suppression of the natural udder flora [96]. Consequently, excessive empirical use of antibiotics was also suggested by other authors to be avoided whenever possible, as the administration of antibiotics was considered useless, or even counter-productive, in the case of confirmed protothecal or Candida mastitis [13].
The effects of global climate change should also be considered when assessing the medium to long-term pathogenic potential of Prototheca microalgae from a One Health perspective. Higher temperatures often combined with high humidity might enhance the multiplication of the microalgae in the environment [4,13]. In Australia, although overall case numbers were low, canine protothecosis was found to be more common in South-East Queensland, a warm sub-tropical region of the continent. Furthermore, no canine protothecosis cases were identified from Tasmania and New Zealand during the study period, in two regions with cooler climates [47]. It was also reported that the occurrence of environmental mastitis may be influenced by the season of the year, with the rate of new infections being highest during the summer and periods of rainy weather, probably due to an increased number of microbes in the environment. In line with these reports, a statistically significant (p < 0.05) increase of environmental mastitis was detected during hot and wet weather, that is, in September to February, in a study conducted in Brazil [25].
10. Conclusions
Prototheca microalgae were recognized as pathogens for both humans and animals only in the 1960s; however, since then, these microbes have been drawing increasing interest in human and veterinary medicine. The first human outbreak of protothecosis, reported in a tertiary care chemotherapy ward in 2018, further highlighted the need to thoroughly understand in detail the ecology, pathogenesis and routes of transmission of Prototheca spp. between different hosts, environments and habitats from a One Health perspective. Several Prototheca species, namely P. ciferrii, P. bovis, P. wickerhamii, P. blaschkeae, P. cutis and P. miyajii, were shown to cause infections in both humans and animals.
Prototheca spp. have been also isolated from a wide range of environmental and other sources, including drinking water, raw milk, certain types of cheese, salads, lettuce and broccoli, raw meat and meat products, farm environments, animal feed, gastrointestinal and fecal samples from domestic and wild animals, a truck transporting cattle feed, farm air samples, sewage and sludge from wastewater treatment plants.
These observations indicate that, in addition to environmental reservoirs, a range of wild and domestic animals may potentially harbor Prototheca in their gastrointestinal tract and disseminate Prototheca spp. Prototheca microalgae can be isolated from milk in relatively high quantities; thus, the consumption of contaminated milk and/or dairy products may also represent a potential route of transmission. The actual source of protothecal infections remained uncertain in a number of cases. Furthermore, neither animal-to-human nor human-to-human Prototheca transmission has been proven unambiguously. Therefore, the molecular genotyping of Prototheca strains cultured from the environment together with those causing the infection would be necessary in future studies. A comprehensive One Health approach must include concurrent testing of human, animal and environmental samples involving the soil, water, air and aerosols.
The available experimental data regarding the susceptibility of Prototheca algae to milk pasteurization are not yet conclusive. Further and larger-scale studies are needed to address this issue, considering the propensity of these microalgae to form cell clumps, thus preventing adequate exposure of the cells in the middle of clumps to elevated temperatures.
The variable in vitro antimicrobial susceptibility pattern of Prototheca strains and species may be one of the causes of antifungal drug treatment failures, and sensitivity of Prototheca spp. in vitro does not necessarily correlate with its efficacy in vivo. Therefore, the development of new and effective agents is required for treating human and animal protothecal infections. The prudent use of antibiotics and their replacement with other alternative and preventive measures may potentially further contribute to the control of protothecosis cases.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/microorganisms10050938/s1, Table S1: Detection of P. zopfii/P. bovis microalgae in two wastewater metagenomic datasets by bioinformatic methods
Author Contributions
Conceptualization, P.L.P.; formal analysis, B.L.; investigation, B.L., C.P., A.C.-G., M.M., M.K., G.T., S.-T.C. and P.L.P.; writing—original draft preparation, B.L., M.M., M.K., G.T., S.-T.C. and P.L.P.; writing—review and editing, C.P. and A.C.-G.; supervision, P.L.P. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the French Government through the 101770R Campus France Scientific Research Stay Scholarship at the University of Nantes, France (B.L.) and by the Ministry of Agriculture of the Czech Republic, institutional support MZE-RO0518 and MZE-RO1421 (M.M. and M.K).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jagielski T., Bakuła Z., Gawor J., Maciszewski K., Kusber W.H., Dyląg M., Nowakowska J., Gromadka R., Karnkowska A. The Genus Prototheca (Trebouxiophyceae, Chlorophyta) Revisited: Implications from Molecular Taxonomic Studies. Algal Res. 2019;43:101639. doi: 10.1016/j.algal.2019.101639. [DOI] [Google Scholar]
- 2.Jagielski T., Dylag M., Roesler U., Murugaiyan J. Isolation of Infectious Microalga Prototheca wickerhamii from a Carp (Cyprinus carpio)—A First Confirmed Case Report of Protothecosis in a Fish. J. Fish Dis. 2017;40:1417–1421. doi: 10.1111/jfd.12614. [DOI] [PubMed] [Google Scholar]
- 3.Dos Anjos C., Sellera F.P., Gargano R.G., Lincopan N., Pogliani F.C., Ribeiro M.G., Jagielski T., Sabino C.P. Algicidal Effect of Blue Light on Pathogenic Prototheca Species. Photodiagnosis Photodyn. Ther. 2019;26:210–213. doi: 10.1016/j.pdpdt.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Milanov D.S., Suvajdžić L.D. Characteristics and Importance of the Genus Prototheca in Human and Veterinary Medicine. Proc. Nat. Sci. Matica. Srpska Novi. Sad. 2006;111:15–27. doi: 10.2298/ZMSPN0611015M. [DOI] [Google Scholar]
- 5.Kessell A.E., McNair D., Munday J.S., Savory R., Halliday C., Malik R. Successful treatment of multifocal pedal Prototheca wickerhamii infection in a feline immunodeficiency virus-positive cat with multiple Bowenoid in situ carcinomas containing papillomaviral DNA sequences. JFMS Open Rep. 2017;3:2055116916688590. doi: 10.1177/2055116916688590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todd J.R., Matsumoto T., Ueno R., Murugaiyan J., Britten A., King J.W., Odaka Y., Oberle A., Weise C., Roesler U., et al. Medical Phycology 2017. Med. Mycol. 2018;56:S188–S204. doi: 10.1093/mmy/myx162. [DOI] [PubMed] [Google Scholar]
- 7.Ollhoff R.D., Sellera F.P., Pogliani F.C. Etymologia: Prototheca. Emerg. Infect. Dis. 2021;27:2891. doi: 10.3201/eid2711.211554. [DOI] [Google Scholar]
- 8.Masuda M., Jagielski T., Danesi P., Falcaro C., Bertola M., Krockenberger M., Malik R., Kano R. Protothecosis in Dogs and Cats-New Research Directions. Mycopathologia. 2021;186:143–152. doi: 10.1007/s11046-020-00508-y. [DOI] [PubMed] [Google Scholar]
- 9.Khan I.D., Sahni A.K., Sen S., Gupta R.M., Basu A. Outbreak of Prototheca wickerhamii Algaemia and Sepsis in a Tertiary Care Chemotherapy Oncology Unit. Med. J. Armed. Forces India. 2018;74:358–364. doi: 10.1016/j.mjafi.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kano R. Emergence of Fungal-like Organisms: Prototheca. Mycopathologia. 2020;185:747–754. doi: 10.1007/s11046-019-00365-4. [DOI] [PubMed] [Google Scholar]
- 11.Conrad P.A., Meek L.A., Dumit J. Operationalizing a One Health Approach to Global Health Challenges. Comp. Immunol. Microbiol. Infect. Dis. 2013;36:211–216. doi: 10.1016/j.cimid.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Pore R.S., Barnett E.A., Barnes W.C.J., Walker J.D. Prototheca Ecology. Mycopathologia. 1983;81:49–62. doi: 10.1007/BF00443909. [DOI] [PubMed] [Google Scholar]
- 13.Milanov D., Petrović T., Polaček V., Suvajdzić L., Bojkovski J. Mastitis Associated with Prototheca zopfii-an Emerging Health and Economic Problem on Dairy Farms. J. Vet. Res. 2016;60:373–378. doi: 10.1515/jvetres-2016-0054. [DOI] [Google Scholar]
- 14.Nelson A.M., Neafie R.C., Connor D.H. Cutaneous Protothecosis and Chlorellosis, Extraordinary “Aquatic-Borne” Algal Infections. Clin. Dermatol. 1987;5:76–87. doi: 10.1016/S0738-081X(87)80012-3. [DOI] [PubMed] [Google Scholar]
- 15.Jagielski T., Lagneau P.E. Protothecosis. A Pseudofungal Infection. J. Mycol. Med. 2007;17:261–270. doi: 10.1016/j.mycmed.2007.08.003. [DOI] [Google Scholar]
- 16.Janosi S., Ratz F., Szigeti G., Kulcsar M., Kerenyi J., Lauko T., Katona F., Huszenicza G. Review of the Microbiological, Pathological, and Clinical Aspects of Bovine Mastitis Caused by the Alga Prototheca Zopfii. Vet. Q. 2001;23:58–61. doi: 10.1080/01652176.2001.9695082. [DOI] [PubMed] [Google Scholar]
- 17.Kunthiphun S., Endoh R., Takashima M., Ohkuma M., Tanasupawat S., Savarajara A. Prototheca paracutis sp. nov., a Novel Oleaginous Achlorophyllous Microalga Isolated from a Mangrove Forest. Mycoscience. 2019;60:165–169. doi: 10.1016/j.myc.2019.02.003. [DOI] [Google Scholar]
- 18.Consuelo Quinet Leimann B., Cezar Fialho Monteiro P., Lazéra M., Ulloa Candanoza E.R., Wanke B. Protothecosis. Med. Mycol. 2004;42:95–106. doi: 10.1080/13695780310001653653. [DOI] [PubMed] [Google Scholar]
- 19.Marques S., Silva E., Carvalheira J., Thompson G. Phenotypic characterization of mastitic Prototheca spp. isolates. Res. Vet. Sci. 2010;89:5–9. doi: 10.1016/j.rvsc.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 20.Lass-Florl C., Mayr A. Human Protothecosis. Clin. Microbiol. Rev. 2007;20:230–242. doi: 10.1128/CMR.00032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pore R.S. Selective Medium for the Isolation of Prototheca. Appl. Microbiol. 1973;26:648–649. doi: 10.1128/am.26.4.648-649.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cranefield S., Numan R., Mcdermott J. Prototheca mastitis: An Emerging Issue and a Challenge to Manage; Proceedings of the Society of Dairy Cattle Veterinarians of the NZVA Annual Conference; Wellington, New Zealand. 6–8 December 2017; pp. 71–73. [Google Scholar]
- 23.Jagielski T., Roeske K., Bakula Z., Piech T., Wlazlo L., Bochniarz M., Woch P., Krukowski H. A Survey on the Incidence of Prototheca Mastitis in Dairy Herds in Lublin Province, Poland. J. Dairy Sci. 2019;102:619–628. doi: 10.3168/jds.2018-15495. [DOI] [PubMed] [Google Scholar]
- 24.Jánosi S., Szigeti G., Ratz F., Lauko T., Kerenyi J., Tenk M., Katona F., Huszenicza A., Kulcsar M., Huszenicza G. Prototheca Zopfii Mastitis in Dairy Herds under Continental Climatic Conditions. Vet. Q. 2001;23:80–83. doi: 10.1080/01652176.2001.9695087. [DOI] [PubMed] [Google Scholar]
- 25.Costa E.O., Ribeiro A.R., Watanabe E.T., Melville P.A. Infectious Bovine Mastitis Caused by Environmental Organisms. Zentralbl. Veterinarmed. B. 1998;45:65–71. doi: 10.1111/j.1439-0450.1998.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 26.Costa E.O., Melville P.A., Ribeiro A.R., Watanabe E.T., Parolari M.C.F.F. Epidemiologic study of environmental sources in a Prototheca zopfii outbreak of bovine mastitis. Mycopathologia. 1997;137:33–36. doi: 10.1023/A:1006871213521. [DOI] [PubMed] [Google Scholar]
- 27.Chuang S.T., Shyu C.L., Chan J.P., Fung H.P. Isolation and identification of Prototheca zopfii from bovine mastitis in Taiwan. Taiwan Vet. J. 2002;28:94–98. [Google Scholar]
- 28.Bauman C.A., Barkema H.W., Dubuc J., Keefe G.P., Kelton D.F. Canadian National Dairy Study: Herd-Level Milk Quality. J. Dairy Sci. 2018;101:2679–2691. doi: 10.3168/jds.2017-13336. [DOI] [PubMed] [Google Scholar]
- 29.Jagielski T., Krukowski H., Bochniarz M., Piech T., Roeske K., Bakuła Z., Wlazło Ł., Woch P. Prevalence of Prototheca spp. on Dairy Farms in Poland—A Cross-Country Study. Microb. Biotechnol. 2019;12:556–566. doi: 10.1111/1751-7915.13394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suvajdzić B., Vasilev D., Karabasil N., Vučurović I., Čobanović N., Babić M., Katić V. Molecular Identification of Prototheca zopfii Genotype 2 Mastitis Isolates and their Influence on the Milk Somatic Cell Count. Vet. Arhiv. 2017;87:249–258. doi: 10.24099/vet.arhiv.151219. [DOI] [Google Scholar]
- 31.Wawron W., Bochniarz M., Piech T., Łopuszyński W., Wysocki J. Outbreak of Protothecal Mastitis in a Herd of Dairy Cows in Poland. Bull. Vet. Inst. Pulawy. 2013;57:335–339. doi: 10.2478/bvip-2013-0058. [DOI] [Google Scholar]
- 32.Ricchi M., De Cicco C., Buzzini P., Cammi G., Arrigoni N., Cammi M., Garbarino C. First Outbreak of Bovine Mastitis Caused by Prototheca blaschkeae. Vet. Microbiol. 2013;162:997–999. doi: 10.1016/j.vetmic.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Marques S., Silva E., Carvalheira J., Thompson G. Short Communication: In vitro Antimicrobial Susceptibility of Prototheca wickerhamii and Prototheca zopfii Isolated from Bovine Mastitis. J. Dairy Sci. 2006;89:4202–4204. doi: 10.3168/jds.S0022-0302(06)72465-1. [DOI] [PubMed] [Google Scholar]
- 34.Shave C.D., Millyard L., May R.C. Now for Something Completely Different: Prototheca, Pathogenic Algae. PLoS Pathog. 2021;17:e1009362. doi: 10.1371/journal.ppat.1009362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricchi M., Goretti M., Branda E., Cammi G., Garbarino C.A., Turchetti B., Moroni P., Arrigoni N., Buzzini P. Molecular Characterization of Prototheca Strains Isolated from Italian Dairy Herds. J. Dairy Sci. 2010;93:4625–4631. doi: 10.3168/jds.2010-3178. [DOI] [PubMed] [Google Scholar]
- 36.Shahid M., Ali T., Zhang L., Hou R., Zhang S., Ding L., Han D., Deng Z., Rahman A., Han B. Characterization of Prototheca zopfii Genotypes Isolated from Cases of Bovine Mastitis and Cow Barns in China. Mycopathologia. 2016;181:185–195. doi: 10.1007/s11046-015-9951-9. [DOI] [PubMed] [Google Scholar]
- 37.Park H.S., Moon D.C., Hyun B.H., Lim S.K. Short Communication: Occurrence and Persistence of Prototheca zopfii in Dairy Herds of Korea. J. Dairy Sci. 2019;102:2539–2543. doi: 10.3168/jds.2018-14979. [DOI] [PubMed] [Google Scholar]
- 38.Scaccabarozzi L., Turchetti B., Buzzini P., Pisoni G., Bertocchi L., Arrigoni N., Boettcher P., Bronzo V., Moroni P. Short Communication: Isolation of Prototheca Species Strains from Environmental Sources in Dairy Herds. J. Dairy Sci. 2008;91:3474–3477. doi: 10.3168/jds.2008-1115. [DOI] [PubMed] [Google Scholar]
- 39.Osumi T., Kishimoto Y., Kano R., Maruyama H., Onozaki M., Makimura K., Ito T., Matsubara K., Hasegawa A. Prototheca zopfii genotypes isolated from cow barns and bovine mastitis in Japan. Vet. Microbiol. 2008;131:419–423. doi: 10.1016/j.vetmic.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Pieper L., Godkin A., Kelton D. Risk Factors for Prototheca Mastitis on Ontario Dairy Farms. In: Smith R.A., editor. Proceedings of the Forty-Fifth Annual Conference; Montreal, QC, Canada. 20–22 September 2012; Ashland, OH, USA: American Association of Bovine Practitioners; p. 185. [DOI] [Google Scholar]
- 41.Bozzo G., Bonerba E., Di Pinto A., Bolzoni G., Ceci E., Mottola A., Tantillo G., Terio V. Occurrence of Prototheca Spp. in Cow Milk Samples. New Microbiol. 2014;37:459–464. [PubMed] [Google Scholar]
- 42.Stockinger B.G., Doster A.R. Disseminated Protothecosis in a Ruwenzori Long-haired Fruit Bat (Rousettus lanosus) J. Zoo Wildl. Med. 2017;48:1260–1263. doi: 10.1638/2017-0070R.1. [DOI] [PubMed] [Google Scholar]
- 43.Camboim E.K.A., Garino F.J., Dantas A.F.M., Simoes S.V.D., Melo M.A., Azevedo E.O., Mota R.A., Riet-Correa F. Protothecosis by Prototheca Wickerhamii in Goats. Mycoses. 2011;54:e196–e200. doi: 10.1111/j.1439-0507.2010.01864.x. [DOI] [PubMed] [Google Scholar]
- 44.Schöniger S., Roschanski N., Rosler U., Vidovic A., Nowak M., Dietz O., Wittenbrink M.M., Schoon H.-A. Prototheca Species and Pithomyces Chartarum as Causative Agents of Rhinitis and/or Sinusitis in Horses. J. Comp. Pathol. 2016;155:121–125. doi: 10.1016/j.jcpa.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Crispens C.G.J., Marion K.R. Algal Infection in a Corn Snake (Elaphe Guttata Guttata) Lab. Anim. Sci. 1975;25:788–789. [PubMed] [Google Scholar]
- 46.Macedo J.T.S.A., Riet-Correa F., Dantas A.F.M., Simoes S.V.D. Cutaneous and nasal protothecosis in a goat. Vet. Pathol. 2008;45:352–354. doi: 10.1354/vp.45-3-352. [DOI] [PubMed] [Google Scholar]
- 47.Stenner V.J., Mackay B., King T., Barrs V.R.D., Irwin P., Abraham L., Swift N., Langer N., Bernays M., Hampson E., et al. Protothecosis in 17 Australian Dogs and a Review of the Canine Literature. Med. Mycol. 2007;45:249–266. doi: 10.1080/13693780601187158. [DOI] [PubMed] [Google Scholar]
- 48.Irrgang A., Murugaiyan J., Weise C., Azab W., Roesler U. Well-Known Surface and Extracellular Antigens of Pathogenic Microorganisms among the Immunodominant Proteins of the Infectious Microalgae Prototheca Zopfii. Front. Cell Infect. Microbiol. 2015;5:67. doi: 10.3389/fcimb.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Roux A., Gumber S., Bauer R.W., Rademacher N., Gaschen L. Algal Meningoencephalitis due to Prototheca spp. in a Dog. Case Rep. Vet. Med. 2013;2013:474731. doi: 10.1155/2013/474731. [DOI] [Google Scholar]
- 50.Endo S., Sekiguchi M., Kishimoto Y., Kano R., Aoki S., Sichinohe T., Hasegawa A. The first case of feline Prototheca wickerhamii infection in Japan. J. Vet. Med. Sci. 2010;72:1351–1353. doi: 10.1292/jvms.09-0504. [DOI] [PubMed] [Google Scholar]
- 51.Huth N., Wenkel R.F., Roschanski N., Rösler U., Plagge L., Schöniger S. Prototheca zopfii genotype 2 induced nasal dermatitis in a cat. J. Compar. Pathol. 2015;152:287–290. doi: 10.1016/j.jcpa.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Shahid M., Cobo E.R., Chen L., Cavalcante P.A., Barkema H.W., Gao J., Xu S., Liu Y., Knight C.G., Kastelic J.P., et al. Prototheca zopfii genotype II induces mitochondrial apoptosis in models of bovine mastitis. Sci. Rep. 2020;10:698. doi: 10.1038/s41598-020-57645-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J., Chen X., Jin E., Wang G., Wu L., Shao Z., Wan P., Hu C., Li J., Chen J., et al. A Survey of Prototheca Bovis Infection in Dairy Farms of the Hubei Province, China. J. Vet. Med. Sci. 2021;83:1248–1255. doi: 10.1292/jvms.21-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosario Medina I., Roman Fuentes L., Batista Arteaga M., Real Valcarcel F., Acosta Arbelo F., Padilla Del Castillo D., Deniz Suarez S., Ferrer Quintana O., Vega Gutierrez B., Silva Sergent F., et al. Pigeons and Their Droppings as Reservoirs of Candida and Other Zoonotic Yeasts. Rev. Iberoam. Micol. 2017;34:211–214. doi: 10.1016/j.riam.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Uzal F.A., Plattner B.L., Hostetter J.M. Alimentary system. In: Maxie M., Kennedy J., editors. Palmer’s Pathology of Domestic Animals: Volume 2. 6th ed. Elsevier, Inc.; Amsterdam, The Netherlands: 2016. [DOI] [Google Scholar]
- 56.Camboim E.K., Neves P.B., Garino Júnior F., Medeiros J.M., Riet-Correa F. Protothecosis: An emergent disease. Pesq. Vet. Bras. 2010;30:94–101. doi: 10.1590/S0100-736X2010000100015. [DOI] [Google Scholar]
- 57.Enders F., Weber A. Pilot Study of the Occurrence of Prototheca in Fecal Samples of Horses. Berl. Munch Tierarztl. Wochenschr. 1993;106:264–265. [PubMed] [Google Scholar]
- 58.Ahrholdt J., Murugaiyan J., Straubinger R.K., Jagielski T., Roesler U. Epidemiological Analysis of Worldwide Bovine, Canine and Human Clinical Prototheca isolates by PCR Genotyping and MALDI-TOF Mass Spectrometry Proteomic Phenotyping. Med. Mycol. 2012;50:234–243. doi: 10.3109/13693786.2011.597445. [DOI] [PubMed] [Google Scholar]
- 59.Rapuntean S., Rapuntean G., FIT N.I., Cosmina C.U.C., Nadas G.C. Morphological and cultural characterization of some strains of unicellular algae of the genus Prototheca sampled from mastitic cow milk. Not. Bot. Hort. Agrobot. Cluj. 2009;37:31–40. [Google Scholar]
- 60.Utama G.L., Kurnani T.B.A., Sunardi R.L., Balia R.L. Waste Minimization of Cheese-making By-product Disposal Through Ethanol Fermentation and the Utilization of Distillery Wastes for Fertilizer; Proceedings of the Second International Conference on Science, Engineering & Environment; Osaka City, Japan. 21–23 November 2016; pp. 1–4. [Google Scholar]
- 61.Bacova R., Kralik P., Kucharovicova I., Seydlova R., Moravkova M. A Novel TaqMan qPCR Assay for Rapid Detection and Quantification of Pro-inflammatory Microalgae Prototheca spp. in Milk Samples. Med. Mycol. 2021;59:784–792. doi: 10.1093/mmy/myaa120. [DOI] [PubMed] [Google Scholar]
- 62.Costa E.O., Melville P.A., Ribeiro A.R., Watanabe E. Toxic Plants and Other Natural Toxicants. CABI Publishing; Wallingford, UK: 1998. Evaluation of the Occurrence of Algae of the Genus Prototheca in Cheese and Milk from Brazilian Dairy Herds; pp. 373–376. [Google Scholar]
- 63.AbdelHameed K.G. Detection of Prototheca zopfii in Raw Milk and Cheese with Special Reference to Their Antibiogram. J. Food Saf. 2016;36:214–219. doi: 10.1111/jfs.12233. [DOI] [Google Scholar]
- 64.Mayorga J., Barba-Gómez J.F., Verduzco-Martínez A.P., Munoz-Estrada V.F., Welsh O. Protothecosis. Clin. Dermatol. 2012;30:432–436. doi: 10.1016/j.clindermatol.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 65.Pore R.S., Boehm D.F., Barnett E.A. Prototheca (Achloric alga) in Wastewater. Water Air Soil. Pollut. 1986;27:355–362. doi: 10.1007/BF00649417. [DOI] [Google Scholar]
- 66.Pore R.S. Protothecosis. In: Mahy B.J.W., ter Meulen V., Borriello S.P., editors. Topley and Wilson’s Microbiology and Microbial Infections. Wiley; Hoboken, NJ, USA: 2010. [DOI] [Google Scholar]
- 67.Jinatham V., Cantoni D.M., Brown I.R., Vichaslip T., Suwannahitatorn P., Popluechai S., Tsaousis A.D., Gentekaki E. Prototheca bovis, a Unicellular Achlorophyllous Trebouxiophyte Green Alga in the Healthy Human Intestine. J. Med. Microbiol. 2021;70:001415. doi: 10.1099/jmm.0.001415. [DOI] [PubMed] [Google Scholar]
- 68.Linares M.J., Solís F., Casal M. In Vitro Activity of Voriconazole against Prototheca wickerhamii: Comparative Evaluation of Sensititre and NCCLS M27-A2 Methods of Detection. J. Clin. Microbiol. 2005;43:2520–2522. doi: 10.1128/JCM.43.5.2520-2522.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamashita M., Ikeda M., Kato I., Ohama Y., Ando M., Ikemura M., Jubishi D., Kanno Y., Okamoto K., Umeyama T., et al. Protothecosis in the mucosa of the pharynx mimicking pharyngeal cancer in an immunocompetent individual: A case report. Ann. Clin. Microbiol. Antimicrob. 2022;21:5. doi: 10.1186/s12941-022-00495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bochniarz M., Piech T., Kocki T., Iskra M., Krukowski H., Jagielski T. Tryptophan, Kynurenine and Kynurenic Acid Concentrations in Milk and Serum of Dairy Cows with Prototheca Mastitis. Animals. 2021;11:3608. doi: 10.3390/ani11123608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Telkes G., Dezsö K., Doros A., Mathe Z. Successful treatment of the gastrointestinal manifestation of prototheca in a kidney transplant recipient: A case report. Transplant. Proc. 2018;50:3928–3931. doi: 10.1016/j.transproceed.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 72.Ribeiro M.G. Protothecosis in Animals. In: Abuelo Á., Brutlag A., Carter K.K., Dart A., Davidson G., Davis J.L., Quesenberry K.E., Ramirez A., Swayne D.E., editors. MSD Veterinary Manual. MSD Publishing Group, Merck & Co., Inc.; Kenilworth, NJ, USA: 2021. [(accessed on 21 March 2022)]. Available online: https://www.msdvetmanual.com. [Google Scholar]
- 73.Takaki K., Okada K., Umeno M., Tanaka M., Takeda T., Ohsaki K., Takaki Y., Sawae Y. Chronic Prototheca Meningitis. Scand J. Infect. Dis. 1996;28:321–323. doi: 10.3109/00365549609027183. [DOI] [PubMed] [Google Scholar]
- 74.European Medicines Agency, Committee for Veterinary Medicinal Products (CVMP) Advice on the Designation of Antimicrobials or Groups of Antimicrobials Reserved for Treatment of Certain Infections in Humans—In Relation to Implementing Measures under Article 37 of Regulation (EU) 2019/6 on Veterinary Medicinal Products, EMA/CVMP/678496/2021. European Medicines Agency; Amsterdam, The Netherlands: 2022. [Google Scholar]
- 75.Jagielski T., Bakuła Z., Pleń M., Kamiński M., Nowakowska J., Bielecki J., Wolska K.I., Grudniak A.M. The Activity of Silver Nanoparticles against Microalgae of the Prototheca Genus. Nanomedicine. 2018;13:1025–1036. doi: 10.2217/nnm-2017-0370. [DOI] [PubMed] [Google Scholar]
- 76.Alves A.C., Capra E., Morandi S., Cremonesi P., Pantoja J., Langoni H., de Vargas A., da Costa M.M., Jagielski T., Bolaños C., et al. In Vitro Algicidal Effect of Guanidine on Prototheca zopfii Genotype 2 Strains Isolated from Clinical and Subclinical Bovine Mastitis. Lett. Appl. Microbiol. 2017;64:419–423. doi: 10.1111/lam.12737. [DOI] [PubMed] [Google Scholar]
- 77.Kwiecinski J. Biofilm Formation by Pathogenic Prototheca algae. Lett. Appl. Microbiol. 2015;61:511–517. doi: 10.1111/lam.12497. [DOI] [PubMed] [Google Scholar]
- 78.Melville P.A., Watanabe E.T., Benites N.R., Ribeiro A.R., Silva J.A., Garino Junior F., Costa E.O. Evaluation of the Susceptibility of Prototheca Zopfii to Milk Pasteurization. Mycopathologia. 1999;146:79–82. doi: 10.1023/A:1007005729711. [DOI] [PubMed] [Google Scholar]
- 79.Marques S., Silva E., Carvalheira J., Thompson G. Short Communication: Temperature Sensibility of Prototheca Blaschkeae Strains Isolated from Bovine Mastitic Milk. J. Dairy Sci. 2010;93:5110–5113. doi: 10.3168/jds.2010-3249. [DOI] [PubMed] [Google Scholar]
- 80.Lassa H., Jagielski T., Malinowski E. Effect of different heat treatments and disinfectants on the survival of Prototheca zopfii. Mycopathologia. 2011;171:177–182. doi: 10.1007/s11046-010-9365-7. [DOI] [PubMed] [Google Scholar]
- 81.Klimešová M., Kucharovičová I., Morávková M., Bačová R., Roubal P., Seydlová R., Nejeschlebová L. Monitoring of Biofilm Production and Thermoresistance of Prototheca spp. Isolated from Bulk Milk (in Czech) Mlékařské Listy Newsl. 2020;31:13–18. [Google Scholar]
- 82.Krukowski H., Lisowski A., Nowakowicz-Debek B., Wlazlo L. Susceptibility of Prototheca zopfii strains isolated from cows with mastitis to chlorhexidine and iodine. Turk. J. Vet. Anim. Sci. 2013;37:106–108. doi: 10.3906/vet-1110-43. [DOI] [Google Scholar]
- 83.Melville P.A., Benites N.R., Sinhorini I.L., Costa E.O. Susceptibility and features of the ultrastructure of Prototheca zopfii following exposure to copper sulphate, silver nitrate and chlorhexidine. Mycopathologia. 2002;156:1–7. doi: 10.1023/A:1021313118632. [DOI] [PubMed] [Google Scholar]
- 84.Salerno T., Ribeiro M.G., Langoni H., Siqueira A.K., da Costa E.O., Melville P.A., Bueno V.F.F., Yamamura A.A.M., Roesler U., da Silva A.V. In vitro algaecide effect of sodium hypochlorite and iodine based antiseptics on Prototheca zopfii strains isolated from bovine milk. Res. Vet. Sci. 2010;88:211–213. doi: 10.1016/j.rvsc.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 85.Sobukawa H., Watanabe M., Kano R., Ito T., Onozaki M., Hasegawa A., Kamata H. In vitro algaecide effect of disinfectants on Prototheca zopfii genotypes 1 and 2. J. Vet. Med. Sci. 2011;73:1527–1529. doi: 10.1292/jvms.11-0256. [DOI] [PubMed] [Google Scholar]
- 86.Hsieh J.C., Hsieh Y.F., Chuang S.H. Prototheca from bovine milk and associated minimal algaecide concentration of chlorhexidine and povidone-iodine in Taiwan. Tierarztl Prax Ausg G Grosstiere Nutztiere. 2020;48:1–6. doi: 10.1055/a-1274-9023. [DOI] [PubMed] [Google Scholar]
- 87.Roque K., Lim G.-D., Jo J.-H., Shin K.-M., Song E.-S., Gautam R., Kim C.-Y., Lee K., Shin S., Yoo H.-S., et al. Epizootiological Characteristics of Viable Bacteria and Fungi in Indoor Air from Porcine, Chicken, or Bovine Husbandry Confinement Buildings. J. Vet. Sci. 2016;17:531–538. doi: 10.4142/jvs.2016.17.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hofbauer W.K. Toxic or Otherwise Harmful Algae and the Built Environment. Toxins. 2021;13:465. doi: 10.3390/toxins13070465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Costa E.O., Melville P.A., Ribeiro A.R., Watanabe E.T. Relato de um Caso de Consumo de Queijo Fresco Contaminado com Prototheca spp. Napgama. 1998;1:9–10. [Google Scholar]
- 90.Langoni H., Troncarelli M.Z., Wanderley G.G., Salina A. Mammary protothecosis. A serious problem in dairy cattle. Veterinária E Zootec. 2013;20:552–566. [Google Scholar]
- 91.Zala J., Darvas E., Nagy T. Prototheca a Kórokozó Alga. Mikrobiológiai Körlevél. 2010;10:27–31. (In Hungarian) [Google Scholar]
- 92.Joerger T., Sulieman S., Carson V.J., Fox M.D. Chronic Meningitis Due to Prototheca zopfii in an Adolescent Girl. J. Pediatric. Infect. Dis. Soc. 2021;10:370–372. doi: 10.1093/jpids/piaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Buzzini P., Turchetti B., Facelli R., Baudino R., Cavarero F., Mattalia L., Mosso P., Martini A. First large-scale isolation of Prototheca zopfii from milk produced by dairy herds in Italy. Mycopathologia. 2004;158:427–430. doi: 10.1007/s11046-004-1819-3. [DOI] [PubMed] [Google Scholar]
- 94.Tenhagen B.A., Kalbe P., Klunder G., & Heuwieser W., Baumgartner B. Tierindividuelle Risikofaktoren für die Protothekenmastitis des Rindes Cow Specific Risk Factors for Mastitis Caused by Prototheca spp. DTW Dtsch. Tierarztl. Wochenschr. 1999;106:376–380. (In German) [PubMed] [Google Scholar]
- 95.Ranjan R., Swarup D., Patra R.C., Nandi D. Bovine protothecal mastitis: A review. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2006;1:1–7. doi: 10.1079/PAVSNNR20061017. [DOI] [Google Scholar]
- 96.Pieper L., Godkin A., Roesler U., Polleichtner A., Slavic D., Leslie K.E., Kelton D.F. Herd Characteristics and Cow-level Factors Associated with Prototheca Mastitis on Dairy Farms in Ontario, Canada. J. Dairy Sci. 2012;95:5635–5644. doi: 10.3168/jds.2011-5106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.