Abstract
Patients with stroke may experience a certain degree of cognitive decline during the period of recovery, and a considerable number of such patients have been reported to show permanent cognitive damage. Therefore, the period of recovery and rehabilitation following stroke is critical for rapid cognitive functional improvements. As dysfunctional breathing has been reported as one of the factors affecting the quality of life post stroke, a number of studies have focused on the need for improving the breathing function in these patients. Numerous breathing exercises have been reported to enhance the respiratory, pulmonary, cognitive, and psychological functions. However, scientific evidence on the underlying mechanisms by which these exercises improve cognitive function is scattered at best. Therefore, it has been difficult to establish a protocol of breathing exercises for patients with stroke. In this review, we summarize the psychological, vascular, sleep-related, and biochemical factors influencing cognition in patients and highlight the need for breathing exercises based on existing studies. Breathing exercises are expected to contribute to improvements in cognitive function in stroke based on a diverse array of supporting evidence. With relevant follow-up studies, a protocol of breathing exercises can be developed for improving the cognitive function in patients with stroke.
Keywords: cerebrovascular disease, hemiplegia, stroke, cognitive function, breathing exercise
1. Introduction
Stroke is caused by a fall in blood supply to the brain or due to cerebral hemorrhage and is the most common cerebrovascular condition [1]. With the increase in the average life expectancy as a result of lifestyle improvements and advances in health care, the number of patients with stroke has seen an upward trend [2]. Furthermore, studies have shown a possible association between the coronavirus disease (COVID-19) and acute cerebrovascular diseases such as ischemic stroke, hemorrhagic stroke, and cerebral venous thrombosis [3]. With the continuing COVID-19 pandemic, a steep increase in stroke incidence is therefore likely.
Health-related quality of life (HRQoL), comprising physical, psychological, and cognitive functions, is substantially low among stroke survivors [4,5,6]. In particular, decline in attention, memory, and executive function is a serious problem in patients who have had a stroke [7]. Indeed, as high as 80% of patients with stroke are presumed to have cognitive decline [8] with more than 1 in 3 of them showing permanent decline [9,10,11]. Since cognitive function plays a critical role in the performance of most activities of daily living [12,13], rapid cognitive functional improvement is recognized as an important component of recovery and rehabilitation after stroke.
The following mechanism has been proposed for the pathophysiological role of breathing in stroke—hemiplegia causes an asymmetry in the body, which leads to inefficient movements, with a direct or indirect impact on the activation of respiratory muscles, ultimately affecting the respiratory cycle [14]. Muscles such as the diaphragm, transverse abdominis, and pelvic floor perform local stabilization, with dual roles in breathing and trunk stabilization [15,16,17]. When stroke adversely affects the activation of those muscles, trunk stabilization is impacted [18,19], which affects the muscles related to breathing [20,21]. Such pathological progression may lead to a vicious cycle, wherein the abnormal function of the muscles related to breathing affects trunk stabilization, which in turn affects breathing in patients with stroke [22,23]. This could be one of the factors deteriorating the HRQoL in these patients [24,25].
In addition, pulmonary complications, such as respiratory failure, pneumonia, pleural effusion, acute respiratory distress syndrome, pulmonary edema, and pulmonary embolism from venous thromboembolism, are commonly observed in patients with stroke [26]. Pulmonary function has been demonstrated to be significantly decreased in patients with stroke compared to that in healthy individuals [27]. In particular, the activity of the diaphragm has been shown to be reduced in patients with stroke with hemiplegia [19], which suggests that the respiratory muscle has a critical role in the recovery of pulmonary dysfunction after stroke. Previous studies have attempted to test interventions that improve the respiratory and pulmonary function of patients with stroke [14,28,29,30]. Furthermore, a meta-analysis reported that respiratory muscle exercises enhanced muscular strength and reduced the risk of respiratory complications in patients with stroke [31]. Despite adequate levels of chest wall expansion, the tidal ventilation, lung capacity, and lung volume should be maintained to improve the efficiency of breathing in patients with stroke [32]. However, the exact mechanisms of such effects have not been sufficiently studied, with a consequent lack of a suitable protocol related to breathing exercises for stroke.
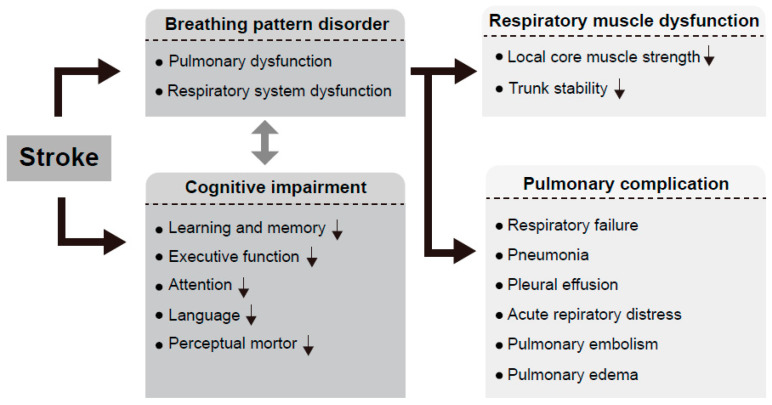
Furthermore, the specific mechanism by which enhanced breathing improves cognitive function in patients with stroke is yet to be elucidated. However, the positive effects of breathing exercises on cognitive functional improvement have been reported in studies although not on patients with stroke. Multiple studies on healthy adults have reported a positive effect of breathing exercises on cognitive functional improvement [33,34,35]. Considering the synchronization of natural breathing and neuronal activity, breathing activates the cortex, hippocampus, and amygdala that are related to memory performance [36]. Abnormal breathing, such as mouth breathing in children, has been reported to reduce academic achievement and memory [37]. These studies suggest an association between normal breathing and cognitive function. Another study on patients with stroke indicated a potential interaction between breathing and cognitive function—walking activated the cortex to increase the respiratory demand, and thus, walking ability was reduced upon engagement of the respiratory cortex [38]. Therefore, it is necessary to investigate the influence of respiratory function and breathing exercises on cognitive function in patients with stroke. Herein, we review previous studies on the factors related to cognitive function in patients with stroke and the association between those factors and breathing (Figure 1).
Figure 1.
Relationship between cognitive function and breathing disorders in patients with stroke. Breathing pattern disorder and pulmonary dysfunction appearing after stroke not only cause various pulmonary complications but also affect the function of local core muscles directly related to breathing. Breathing pattern disorder has a negative effect on trunk stability and is thought to be related to cognitive decline in patients with stroke.
2. Method
We performed the literature review using the terms “stroke”, “breathing pattern disorder”, “stroke vs. breathing pattern disorder”, “stroke vs. cognitive decline”, “stroke vs. depression vs. cognition”, “stroke vs. psychological factor vs. cognition”, “stroke vs. vascular factor vs. cognition”, “stroke vs. hypertension vs. cognition, “stroke vs. sleep factor vs. cognition”, “stroke vs. OSA vs. cognition”, “stroke vs. biochemical factor vs. cognition”, “stroke vs. IL-6 vs. cognition”, “psychological factor vs. breathing exercise”, “depression vs. breathing exercise”, “parasympathetic nerve vs. breathing exercise”, “vascular factor vs. breathing exercise”, “hypertension vs. breathing exercise”, “chemoreflex vs. breathing exercise”. “baroreflex vs. breathing exercise”, “sleep vs. breathing exercise”, “OSA vs. breathing exercise”, “biochemical factor vs. breathing exercise”, and “IL-6 vs. breathing exercise” along with the Medical Subject Headings (MESH) terms. The reference list of the articles was carefully reviewed as a potential source of information. The search was based on Pubmed, Scopus, and Google Scholar engines. Selected publications were analyzed, and their synthesis was used to write the review and support the hypothesis of the relationship between breathing exercise and stroke.
3. Common Factors of Cognitive Decline in Patients with Stroke
3.1. Psychological Factors Involved in Post-Stroke Cognitive Impairment
Cognitive function decline is a common occurrence in patients with stroke [8,39]. As a result of damage to the brain, approximately 80% of these patients exhibit a decline in cognitive function, and approximately 20–60% show depression [40,41]. The correlation between cognitive decline and post-stroke depression (PSD) is well-established [42]. The most consistent finding in systematic reviews, including the study by Mansur et al., on the predictive factors for depression after stroke is the association between PSD and post-stroke cognitive decline [43,44]. As such, numerous studies are in support of the association between cognitive decline in stroke and depression [45,46]. Of late, research interests surrounding this topic have increased remarkably.
3.2. Vascular Factors Involved in Post-Stroke Cognitive Impairment
Acute stroke and other types of vascular disease are well-known causes of cognitive impairment [47,48,49,50]. Asymptomatic cerebrovascular diseases, including silent brain infarcts and leukoaraiosis, manifest with cognitive impairment and are related to risk factors such as hypertension and atrial fibrillation [51,52,53,54,55]. Hypertension was observed in over 60% of patients with stroke [56], and approximately 25% of patients with stroke had atrial fibrillation [57]. Indeed, Waldstein et al. [58] reported that patients with peripheral arterial disease (PAD), as a common form of peripheral vascular disease, exhibited impairment of cognitive function. These previous studies suggest that vascular factors associated with cognitive impairment have been recognized as an important consequence of stroke [59,60].
3.3. Sleep-Related Factors Involved in Post-Stroke Cognitive Impairment
Sleep disorders occur frequently in patients with stroke. In a previous meta-analysis, over 50% of patients with stroke had a concomitant sleep disorder [61,62]. In addition, 68% of patients with acute ischemic stroke were estimated to have sleep disorders such as insomnia [63], and approximately 44% of patients with cerebral infarction were shown to have sleep disturbances at three months post-stroke [64]. Sleep disorder has a negative impact on various aspects of neurological recovery and quality of life [65,66,67]. Indeed, sleep disorder was shown to increase the risk of stroke recurrence [68] and had an undesirable effect on the cognitive function in patients with stroke [69].
Obstructive sleep apnea (OSA) is known to be an independent risk factor for stroke and is one of the common sleep disorders occurring in these patients. Approximately 30–70% of patients with stroke also have OSA [70]. In a recent study by Aaronson et al. [69], which was conducted on 80 patients with stroke with OSA and 67 without OSA, a higher decline in cognitive function was revealed in those with OSA. These results suggest that sleep disorder is a key factor influencing cognitive decline in patients with stroke as well as in healthy individuals [71,72,73].
3.4. Biochemical Factors Involved in Post-Stroke Cognitive Impairment
Blood lipid concentrations and abnormal neurotransmitter levels have been reported to be related to cognitive function decline in patients with stroke. In a study conducted on patients with acute ischemic stroke, the risk of cognitive decline was directly proportionate to non-high density lipoprotein cholesterol (non-HDL-C) levels [74]. Similarly, serum glutamate concentration was reported to be a critical predictor of the increased size of the cerebral infarct [75]. Zeydan et al. showed that a key factor in the cognitive decline in patients with stroke was the loss of glutamatergic neuron synaptic function [76]. Indeed, the preservation of glutamatergic neuron synaptic function was reported to help maintain the cognitive functions after stroke and prevent dementia [77,78]. The increase in the pleiotropic cytokine, interleukin-6 (IL-6), which induces an acute inflammatory response, was also reported to be related with cognitive decline in patients with stroke [79].
4. Associations between the Factors Influencing Cognitive Decline and Breathing in Stroke
Breathing exercises are known to improve the physiological, psychological, and cognitive function [80,81,82,83], and are currently suggested as a supplementary treatment for stress, anxiety, depression, asthma, and chronotropism [84,85,86,87,88]. Breathing can be either voluntary or involuntary. Voluntarily breathing is controlled by a complex feedback system involving the autonomous neural network, brain stem nucleus, limbic system, cortical areas, and the neuroendocrine system [80,84]. The voluntary control of breathing has been reported to exert a positive effect on autonomic nervous system functions, including variable heart rate, expiratory flow rate, and vagal tone [89,90,91]. Three months of slow breathing exercises have been shown to cause a decline in heart rate as well as an elevated sensitivity to cardiac response to standing in healthy individuals [92]. This study thus highlights the importance of breathing control as well as the method used for controlling one’s breathing. Furthermore, this study suggests future directions for research on breathing exercise methods to improve the breathing function in patients with stroke who frequently have breathing abnormalities.
4.1. Psychological Factors and Breathing in Stroke
PSD is the most common neuropsychiatric complication in patients with stroke [93,94]. Studies report that approximately one in three patients with stroke exhibit PSD [44,95,96,97]. In a study on healthy older adults, breathing exercises were shown to have a positive effect on psychological functions [98,99,100]. In a study by Brown and Gerbarg, breathing exercises were suggested to be beneficial for patients with PSD [80]. Breathing exercises restore the normal state of the autonomic system by regulating the movement of the respiratory system [92,101]. Moreover, enhanced parasympathetic nerve activity may lead to improvements in psychological as well as cognitive functions [101,102]. As the most significant positive effect of breathing exercises, enhanced parasympathetic nerve activity was shown to reduce the response to psychological stress and, consequently, exert positive effects on various domains across psychology, cognition, and behavior [80,84] (Table 1).
Table 1.
Effects of breathing exercises on psychological factors.
| Author (Year) | Participants | Time and Duration |
Type | Primary Outcome | Results |
|---|---|---|---|---|---|
| Subbalakshmi et al. (2014) [91] | Healthy adults, males and females | 20 min, 1 time | Acute, Nadi-shoidahana Pranayama |
Basal heart rate, systolic blood pressure, peak expiratory flow rate, respiratory, cardiovascular parameters | Reduced basal heart rate and systolic blood pressure, improved peak expiratory flow rate, no difference in respiratory and cardiovascular parameters |
| Udupa et al. (2003) [90] | Normal young adults, males and females | 20 min/d, 3 months | Long-term, Pranayama breathing |
Parasympathetic and sympathetic activity | Decreased sympathetic activity, increased parasympathetic activity |
| Klainin-Yobas et al. (2015) [100] | Normal young adults, males and females | 30 min 2 times/d, 3 months | Long-term, slow breathing |
Autonomic functions | Improved autonomic functions |
| Hyun et al. (2009) [98] | Older adults, males and females | 60 min/d, 3 times a week, 12 weeks | Long-term, Danjeon breathing |
Vital capacity, physical fitness, anxiety, depression | Reduced anxiety and depression, no difference in vital capacity and physical fitness |
| Krishnamurthy and Telles (2007) [99] | Older adults, males and females | 75 min/d, 6 times a week, 24 weeks | Long-term, Pranayama breathing with yoga training |
Depression | Reduced depression |
4.2. Vascular Factors and Breathing in Stroke
Stroke-induced damage to areas of the brain associated with autonomous function has a substantial influence on the blood pressure control and cardiac function during the period of recovery [103,104]. In addition, patients showing post-stroke hemiplegia exhibited a markedly low level of residual blood flow in the paretic lower limb [105,106,107]. Such problems of vascular function can negatively affect the performance of activities of daily living (ADL) and the quality of life [108,109]. Reduced physical activity can in turn affect the blood flow velocity, endothelial function, and arterial diameter through secondary reduction in blood flow [108,110].
No study has yet directly investigated the effects of breathing exercise and respiratory function on vascular function in patients with stroke; however, several studies have been conducted on patients with hypertension, which is known to be the most serious risk factor for stroke incidence.
Breathing exercises are widely acknowledged as a non-pharmaceutical intervention for the control of hypertension, a risk factor of stroke [111,112,113]. The mechanism of action is as follows: the pressor receptor stimulating the autonomic nervous system during prolonged inhalation and exhalation increases the baroreflex sensitivity (BRS) and decreases the sympathetic activity and chemoreflex activation [114,115]. In numerous studies, slow breathing exercises have shown positive effects on BRS, blood pressure, and autonomic nervous system function [114,116,117]. Hypertension is a particularly important risk factor for hemorrhagic stroke although it contributes to atherosclerotic disease that can lead to ischemic stroke as well, increasing the risk of stroke by approximately 2.87 times [118,119]. The prevalence of stroke in hypertension patients aged 50 years or above was 20% of the total population, and the prevalence continuously increased with increasing age [120]. Based on the correlation between hypertension and stroke, further studies should be conducted to determine the effects of breathing exercises on the vascular function, blood pressure, and autonomic nervous system in patients with stroke (Table 2).
Table 2.
Effects of breathing exercises on vascular factors.
| Author (Year) | Participants | Time and Duration |
Type | Primary Outcome | Results |
|---|---|---|---|---|---|
| Kalaivani et al. (2019) [111] | Hypertension patients, males and females | 10 min 2 times/d, 5 days | Short-term, alternate nostril breathing |
Hypertension | Reduced hypertension |
| Mourya et al. (2009) [112] | Hypertension patients, males and females | 15 min 2 times/d, 3 months | Long-term, slow breathing |
Hypertension, sympathetic and parasympathetic reactivity | Reduced hypertension, improved sympathetic and parasympathetic reactivity |
| Kaushik et al. (2006) [113] | Hypertension patients, males and females | 10 min, 1 time | Acute, slow breathing |
Hypertension | Reduced hypertension |
| Joseph et al. (2005) [114] | Hypertension patients, males and females | 2 min of controlled breathing at 6 cycles/min, 1 time | Acute, slow breathing |
Hypertension, baroreflex sensitivity | Reduced hypertension, enhanced baroreflex sensitivity |
| Bernardis et al. (2001) [115] | Healthy adults, males and females | 10–15 min, 1 time | Acute, slow breathing |
Hypoxic and hypercapnic chemoreflex, baroreflex sensitivity | Reduced chemoreflex, enhanced baroreflex sensitivity |
| Kalaivani et al. (2019) [111] | Hypertension patients, males and females | 10 min 2 times/d, 5 days | Alternate nostril breathing | Hypertension | Reduced hypertension |
4.3. Sleep and Breathing in Stroke
Sleep-related breathing disorders occur in more than half of patients with stroke [61,121]. They are also an independent risk factor for stroke [122,123,124] while being responsible for the risk of stroke recurrence, mortality, and deterioration of cognitive function [125,126]. Sleep-related breathing disorders include habitual snoring, upper respiratory tract resistance syndrome, aperiodic breathing, and sleep apnea syndrome. OSA refers to the partial or complete collapse of the upper airway during sleep, resulting in reduced or absent (or apnea) airflow lasting for 10 s [123]. Many OSA patients are highly likely to show cardiovascular or cerebrovascular diseases, as OSA is also associated with hypertension, a direct risk factor of stroke [120,127,128,129]. OSA is also associated with fibrinogen levels, a key independent risk factor for myocardial infarction and vascular diseases [130,131]. Elevated fibrinogen levels are correlated with increased risk of cardiovascular events in patients with stroke [130,131,132]. As OSA reduces the cerebral blood volume and decreases the blood supply to the brain, it is viewed as a risk factor for stroke [133].
In a study by Yaggi et al. conducted on 1022 adults with no history of stroke or myocardial infarction, the risk of stroke was shown to increase as the severity of OSA increased [134]. This result was verified in further follow-up studies and meta-analyses, where adults with OSA showed approximately a two-fold higher risk of stroke [135,136,137,138,139]. Additionally, untreated OSA after acute stroke increases long-term mortality and neurological outcomes [125,126], which highlights the importance of rapid treatment of post-stroke OSA.
The most well-known treatment of OSA in patients with stroke is continuous positive airway pressure (CPAP). While CPAP was shown to have positive effects on neuronal recovery, sleep, depression, and long-term survival [140,141,142,143], patients with stroke show a reduced long-term compliance to CPAP compared to healthy individuals [144]. This may be related to the difficulty in wearing and retaining the CPAP mask due to weak upper extremity and face as well as due to depression [144]. CPAP also has a role in causing phobia related to rhinocleisis [145], the arousal of the respiratory tract related to oral exposure and drying of mucous membranes [146]; therefore, there are challenges in its successful application in patients with stroke. This suggests the need for simple interventions or treatment strategies for OSA.
Among the interventions to improve OSA are a diversity of breathing re-education (BRE) approaches. These include the Buteyko method, inhalation resistance breathing training, and diaphragmatic breathing [147,148,149,150]. The BRE approach aims to improve the abnormal breathing pattern in patients with chronic hyperventilation. It involves exercises such as breath-holding and controlled breathing to restore the normal nasal/diaphragmatic pattern and treat dysfunctional breathing habits, such as abnormal mouth breathing and abnormal apical breathing or upper chest breathing [151].
Mouth breathing is associated with the severity of OSA [152,153], as it plays a part in snoring, OSA, apnea, and hypopnea [153]. In addition, patients with OSA were reported to show reduced strength of the diaphragm and muscles related to breathing compared with age- and sex-matched controls [147]. In a study by Courtney, the magnitude and stability of respiratory motor output for muscles of the upper airway was reported to be a key contributing factor across all forms of sleep apnea [147].
The BRE approach of Mckeown, which applies the Buteyko method, includes the conversion of mouth breathing to nasal breathing during rest, exercise, and sleep [151]. Such breathing exercises play a part in restoring nasal breathing, enhancing the function of the diaphragm, reducing breathing rate, and increasing the tolerance to changes in arterial carbon dioxide pressure [154]. Recent studies have shown that the BRE approach improves breathing patterns and can be beneficial for OSA patients [147,155]. However, no study has investigated the effects of breathing exercises or the re-education approaches for OSA and other sleep-related disorders in patients with stroke, suggesting the need for relevant further studies (Table 3).
Table 3.
Effects of breathing exercises on sleep factors.
| Author (Year) | Participants | Time and Duration |
Type | Primary Outcome | Results |
|---|---|---|---|---|---|
| Ojay and Ernst (2000) [156] | Chronic snorers | 20 min/d, 3 months | Long-term, diaphragmatic breathing and singing and exercises training |
Snoring, nasal problem | Reduced snoring |
| Vranish and Bailey (2016) [148] | OSA patients | 5 min/d, 6 weeks | Long-term, inspiratory muscle training |
Respiratory muscle strength, sleep, snoring, inflammation, metabolism | Improved respiratory muscle strength and improved sleep, reduced inflammation, improved metabolism |
| Birch (2021) [149] | Practitioners and OSA patients | 15 min 3 times/d, 2 weeks | Short-term, breathing retraining (Buteyko berating exercises) |
Sleep, breathing pattern, general health, quality of life | Improved sleep, improved breathing pattern, improved general health, improved quality of life |
| Birch (2004) [150] | 44-year-old male (with asthma, severe COPD, OSA) (Case study n = 1) | 15 min 3 times/d, 2 years | Long-term breathing retraining (Buteyko berating exercises) |
CPAP, OSA | Improved CPAP, improved OSA |
4.4. Biochemical Factors and Breathing in Stroke
The magnitude of cerebral infarction on CT increases with the level of IL-6. IL-6 is the most well-known biochemical marker of stroke and is associated with a poor 3-month and 12-month prognosis [157,158]. Respiratory failure caused by the SARS-CoV-2 virus or COVID-19 infection is correlated with the cytokine release syndrome that leads to the need for mechanical ventilation [159]. IL-6 is known as the main chemokine inducing the cytokine-release syndrome [159,160,161]. Moreover, COVID-19 severity was shown to be associated with the risk of acute stroke [162,163], suggesting that studies on the relationship between the level of IL-6 and breathing in patients with stroke are warranted.
In a recent study using a mouse model, the neurological damage in mice with lung damage could be reduced by suppressing levels of IL-6 [164]. Although only a few studies have reported on the relationship between breathing exercises and IL-6 in patients with stroke, several studies have been conducted on different sets of participants. In a study conducted on overweight or obese adult males, the practice of breathing exercises was reported to be associated with significantly reduced levels of plasma IL-6 [165]. Similarly, in another study conducted on healthy individuals, breathing exercises were reported to have reduced the levels of IL-6 [166].
In a study conducted on healthy mammals, the respiratory system was markedly affected by an increase in IL-6 levels, which increased the mechanical ventilation during exhalation [167]. These results suggest a possible correlation between the levels of IL-6 and breathing based on its role in the pathogenesis of respiratory diseases (Table 4).
Table 4.
Effects of breathing exercises on biochemistry factors.
| Author (Year) | Participants | Time and Duration |
Type | Primary Outcome | Results |
|---|---|---|---|---|---|
| Sudarku (2010) [166] | University students, males and females | 4 set 3 times/d, 7 weeks | Long-term, slow breathing |
IL-6, IL-4, IL-2, cortisol, beta endorphin, IgG | Decreased IL-6, decreased IL-4, increased beta endorphin |
| Sparrow et al. (2021) [164] | Adult mice (mechanical ventilation-induced lung injury) |
Mice were treated with anti-IL-6 antibody and anti-IL-6 receptor antibody | Neuronal injury, stress, inflammation | Reduced neuronal injury, reduced stress, reduced inflammation | |
| Sarvottam et al. (2012) [165] | Overweight and obese males | 50 min/d, 10 days | Short-term Pranayama breathing, Asanas (yoga postures) |
CVD risk, IL-6, adiponectin, endotheline-1 | Reduced risk of CVD, decreased IL-6, increased adiponectin |
5. Rationale for the Development of Breathing Exercise Protocols to Attenuate Cognitive Decline in Patients with Stroke
Breathing exercises can restore the normal state of the autonomic nervous system [91,101] and increase the parasympathetic nervous system activity with the anticipated psychological changes and improved cognitive functions [101,102]. However, further research into the influence of breathing exercises on the vascular function, blood pressure, and autonomic nervous system changes in patients with stroke is necessary.
The importance of rapid treatment for post-stroke OSA has been recognized, and studies have reported on the positive effect of BRE based on the Buteyko method on breathing patterns in OSA patients [147,155]. However, no study has yet reported on the effects of general breathing exercises and BRE on OSA or other sleep-related breathing disorders in patients with stroke, and this is another gap in the literature that ought to be addressed.
Through literature review, we examined the factors related to cognitive decline in patients with stroke and their associations with normal natural breathing and breathing exercises. Breathing exercises were found to contribute to cognitive functional improvement in patients with stroke. In particular, it can be inferred that slow breathing exercise is the most helpful breathing exercise for improving cognitive function in stroke patients. However, there is a limitation in explaining the time, period, and intensity for breathing exercises; accordingly, the development of breathing exercise protocols to improve cognitive functions in patients with stroke is necessary.
The global COVID-19 pandemic that began in early 2020 has prompted focused efforts on the development of pulmonary rehabilitation using breathing exercise programs worldwide [168]. A similar high-quality breathing exercise protocol to suit the characteristics of patients with stroke would be useful in the current era. Finally, studies on the correlation between the effects of such protocols and cognitive decline that is most detected in patients with stroke would be highly beneficial.
Author Contributions
Conceptualization, E.-S.K. and M.-S.H.; methodology, E.-S.K. and M.-S.H.; validation, M.-S.H.; data curation, E.-S.K.; writing—original draft preparation, E.-S.K. and M.-S.H.; writing—review and editing, J.S.Y. and M.-S.H.; visualization, J.S.Y.; supervision, M.-S.H.; project administration, M.-S.H.; funding acquisition, M.-S.H. All authors critically revised the manuscript for important intellectual content, and approved the final version for publication. All authors significantly contributed to the research. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2021S1A5A8069272).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adlard P.A., Perreau V.M., Cotman C.W. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol. Aging. 2005;26:511–520. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Kim J.Y., Kang K., Kang J., Koo J., Kim D.H., Kim B.J., Kim W.J., Kim E.G., Kim J.G., Kim J.M., et al. Executive summary of stroke statistics in Korea 2018: A report from the epidemiology research council of the korean stroke society. J. Stroke. 2019;21:42–59. doi: 10.5853/jos.2018.03125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghasemi M., Umeton R.P., Keyhanian K., Mohit B., Rahimian N., Eshaghhosseiny N., Davoudi V. SARS-CoV-2 and Acute Cerebrovascular Events: An Overview. J. Clin. Med. 2021;10:3349. doi: 10.3390/jcm10153349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wæhler I.S., Saltvedt I., Lydersen S., Fure B., Askim T., Einstad M.S., Thingstad P. Association between in-hospital frailty and health-related quality of life after stroke: The Nor-COAST study. BMC Neurol. 2021;21:100. doi: 10.1186/s12883-021-02128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tourani S., Behzadifar M., Martini M., Aryankhesal A., Taheri Mirghaed M., Salemi M., Behzadifar M., Bragazzi N.L. Health-related quality of life among healthy elderly Iranians: A systematic review and meta-analysis of the literature. Health Qual. Life Outcomes. 2018;16:18. doi: 10.1186/s12955-018-0845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haley W.E., Roth D.L., Kissela B., Perkins M., Howard G. Quality of life after stroke: A prospective longitudinal study. Qual. Life Res. 2011;20:799–806. doi: 10.1007/s11136-010-9810-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donovan N.J., Kendall D.L., Heaton S.C., Kwon S., Velozo C.A., Duncan P.W. Conceptualizing functional cognition in stroke. Neurorehabil. Neural Repair. 2008;22:122–135. doi: 10.1177/1545968307306239. [DOI] [PubMed] [Google Scholar]
- 8.Leśniak M., Bak T., Czepiel W., Seniów J., Członkowska A. Frequency and prognostic value of cognitive disorders in stroke patients. Dement. Geriatr. Cogn. Disord. 2008;26:356–363. doi: 10.1159/000162262. [DOI] [PubMed] [Google Scholar]
- 9.Jokinen H., Melkas S., Ylikoski R., Pohjasvaara T., Kaste M., Erkinjuntti T., Hietanen M. Post-stroke cognitive impairment is common even after successful clinical recovery. Eur. J. Neurol. 2015;22:1288–1294. doi: 10.1111/ene.12743. [DOI] [PubMed] [Google Scholar]
- 10.Jacquin A., Binquet C., Rouaud O., Graule-Petot A., Daubail B., Osseby G.-V., Bonithon-Kopp C., Giroud M., Béjot Y. Post-Stroke Cognitive Impairment: High Prevalence and Determining Factors in a Cohort of Mild Stroke. J. Alzheimer’s Dis. 2014;40:1029–1038. doi: 10.3233/JAD-131580. [DOI] [PubMed] [Google Scholar]
- 11.Mellon L., Brewer L., Hall P., Horgan F., Williams D., Hickey A., McGee H., Shelley E., Kelly P., Dolan E. Cognitive impairment six months after ischaemic stroke: A profile from the ASPIRE-S study. BMC Neurol. 2015;15:31. doi: 10.1186/s12883-015-0288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crescentini C., Seyed-Allaei S., Vallesi A., Shallice T. Two networks involved in producing and realizing plans. Neuropsychologia. 2012;50:1521–1535. doi: 10.1016/j.neuropsychologia.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Liu J., Wang Q., Liu F., Song H., Liang X., Lin Z., Hong W., Yang S., Huang J., Zheng G., et al. Altered functional connectivity in patients with post-stroke memory impairment: A resting fMRI study. Exp. Ther. Med. 2017;14:1919–1928. doi: 10.3892/etm.2017.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Britto R.R., Rezende N.R., Marinho K.C., Torres J.L., Parreira V.F., Teixeira-Salmela L.F. Inspiratory muscular training in chronic stroke survivors: A randomized controlled trial. Arch. Phys. Med. Rehabil. 2011;92:184–190. doi: 10.1016/j.apmr.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 15.Kawabata M., Shima N., Nishizono H. Regular change in spontaneous preparative behaviour on intra-abdominal pressure and breathing during dynamic lifting. Eur. J. Appl. Physiol. 2014;114:2233–2239. doi: 10.1007/s00421-014-2944-4. [DOI] [PubMed] [Google Scholar]
- 16.Emerich Gordon K., Reed O. The Role of the Pelvic Floor in Respiration: A Multidisciplinary Literature Review. J. Voice. 2020;34:243–249. doi: 10.1016/j.jvoice.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 17.Hodges P.W., Cresswell A.G., Daggfeldt K., Thorstensson A. In vivo measurement of the effect of intra-abdominal pressure on the human spine. J. Biomech. 2001;34:347–353. doi: 10.1016/S0021-9290(00)00206-2. [DOI] [PubMed] [Google Scholar]
- 18.Lanini B., Bianchi R., Romagnoli I., Coli C., Binazzi B., Gigliotti F., Pizzi A., Grippo A., Scano G. Chest wall kinematics in patients with hemiplegia. Am. J. Respir. Crit. Care Med. 2003;168:109–113. doi: 10.1164/rccm.200207-745OC. [DOI] [PubMed] [Google Scholar]
- 19.Jung K.J., Park J.Y., Hwang D.W., Kim J.H., Kim J.H. Ultrasonographic diaphragmatic motion analysis and its correlation with pulmonary function in hemiplegic stroke patients. Ann. Rehabil. Med. 2014;38:29–37. doi: 10.5535/arm.2014.38.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee K., Park D., Lee G.C. Progressive Respiratory Muscle Training for Improving Trunk Stability in Chronic Stroke Survivors: A Pilot Randomized Controlled Trial. J. Stroke Cerebrovasc. Dis. 2019;28:1200–1211. doi: 10.1016/j.jstrokecerebrovasdis.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 21.de Almeida I.C.L., Clementino A.C.C.R., Rocha E.H.T., Brandão D.C., De Andrade A.D. Effects of hemiplegy on pulmonary function and diaphragmatic dome displacement. Respir. Physiol. Neurobiol. 2011;178:196–201. doi: 10.1016/j.resp.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Lee K., Cho J.E., Hwang D.Y., Lee W. Decreased respiratory muscle function is associated with impaired trunk balance among chronic stroke patients: A cross-sectional study. Tohoku J. Exp. Med. 2018;245:79–88. doi: 10.1620/tjem.245.79. [DOI] [PubMed] [Google Scholar]
- 23.Jandt S.R., da Sil Caballero R.M., Junior L.A.F., Dias A.S. Correlation between trunk control, respiratory muscle strength and spirometry in patients with stroke: An observational study. Physiother. Res. Int. 2011;16:218–224. doi: 10.1002/pri.495. [DOI] [PubMed] [Google Scholar]
- 24.Patel M.D., Tilling K., Lawrence E., Rudd A.G., Wolfe C.D.A., McKevitt C. Relationships between long-term stroke disability, handicap and health-related quality of life. Age Ageing. 2006;35:273–279. doi: 10.1093/ageing/afj074. [DOI] [PubMed] [Google Scholar]
- 25.Pollock R.D., Rafferty G.F., Moxham J., Kalra L. Respiratory muscle strength and training in stroke and neurology: A systematic review. Int. J. Stroke. 2013;8:124–130. doi: 10.1111/j.1747-4949.2012.00811.x. [DOI] [PubMed] [Google Scholar]
- 26.Gao J., Zhou C., Zhang H. Mechanical ventilation in patients with acute ischemic stroke: From pathophysiology to clinical practice. Crit. Care. 2020;24:139. doi: 10.1186/s13054-020-2806-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ezeugwu V.E., Olaogun M., Mbada C.E., Adedoyin R. Comparative Lung function performance of stroke survivors and age-matched and sex-matched controls. Physiother. Res. Int. 2013;18:212–219. doi: 10.1002/pri.1547. [DOI] [PubMed] [Google Scholar]
- 28.Cho Y.-H., Lee S.-B. Impact of Respiratory Muscle Exercises on Pulmonary Function and Quality of Sleep among Stroke Patients. J. Korean Soc. Phys. Med. 2015;10:123–125. doi: 10.13066/kspm.2015.10.4.123. [DOI] [Google Scholar]
- 29.Kim B.-R., Kang J.-I., Kim Y.-N., Jeong D.-K. Effects of Respiratory Muscle Strengthening Exercise on Respiratory Function and Activities of Daily Living in Stroke Patients. J. Korean Phys. Ther. 2017;29:1–6. doi: 10.18857/jkpt.2017.29.1.1. [DOI] [Google Scholar]
- 30.Choi H.E., Jo G.Y., Do H.K., On C.W. Comprehensive Respiratory Muscle Training Improves Pulmonary Function and Respiratory Muscle Strength in Acute Stroke Patients. J. Cardiopulm. Rehabil. Prev. 2021;41:166–171. doi: 10.1097/HCR.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 31.Wu F., Liu Y., Ye G., Zhang Y. Respiratory Muscle Training Improves Strength and Decreases the Risk of Respiratory Complications in Stroke Survivors: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2020;101:1991–2001. doi: 10.1016/j.apmr.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Frownfelter D., Dean E. Cardiovascular and Pulmonary Physical Therapy-E-Book: Evidence to Practice. Elsevier Health Sciences; Amsterdam, The Netherlands: 2014. [Google Scholar]
- 33.Chambers R., Lo B.C.Y., Allen N.B. The impact of intensive mindfulness training on attentional control, cognitive style, and affect. Cognit. Ther. Res. 2008;32:303–322. doi: 10.1007/s10608-007-9119-0. [DOI] [Google Scholar]
- 34.Chiesa A., Calati R., Serretti A. Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clin. Psychol. Rev. 2011;31:449–464. doi: 10.1016/j.cpr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Yadav G., Mutha P.K. Deep Breathing Practice Facilitates Retention of Newly Learned Motor Skills. Sci. Rep. 2016;6:37069. doi: 10.1038/srep37069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zelano C., Jiang H., Zhou G., Arora N., Schuele S., Rosenow J., Gottfried J.A. Nasal respiration entrains human limbic oscillations and modulates cognitive function. J. Neurosci. 2016;36:12448–12467. doi: 10.1523/JNEUROSCI.2586-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuroishi R.C.S., Garcia R.B., Valera F.C.P., Anselmo-Lima W.T., Fukuda M.T.H. Déficits de memória operacional, compreensão de leitura e habilidades aritméticas em crianças com síndrome da respiração oral: Estudo transversal analítico. Sao Paulo Med. J. 2015;133:78–83. doi: 10.1590/1516-3180.2013.7630011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nierat M.C., Demiri S., Dupuis-Lozeron E., Allali G., Morélot-Panzini C., Similowski T., Adler D. When breathing interferes with cognition: Experimental inspiratory loading alters timed up-and-go test in normal humans. PLoS ONE. 2016;11:e0151625. doi: 10.1371/journal.pone.0151625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haring H.-P. Cognitive impairment after stroke. Curr. Opin. Neurol. 2002;15:79–84. doi: 10.1097/00019052-200202000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Robinson R.G., Jorge R.E. Post-stroke depression: A review. Am. J. Psychiatry. 2016;173:221–231. doi: 10.1176/appi.ajp.2015.15030363. [DOI] [PubMed] [Google Scholar]
- 41.Whyte E.M., Mulsant B.H. Post stroke depression: Epidemiology, pathophysiology, and biological treatment. Biol. Psychiatry. 2002;52:253–264. doi: 10.1016/S0006-3223(02)01424-5. [DOI] [PubMed] [Google Scholar]
- 42.Serrano S., Domingo J., Rodríguez-Garcia E., Castro M.D., Del Ser T. Frequency of cognitive impairment without dementia in patients with stroke: A two-year follow-up study. Stroke. 2007;38:105–110. doi: 10.1161/01.STR.0000251804.13102.c0. [DOI] [PubMed] [Google Scholar]
- 43.Kutlubaev M.A., Hackett M.L. Part II: Predictors of depression after stroke and impact of depression on stroke outcome: An updated systematic review of observational studies. Int. J. Stroke. 2014;9:1026–1036. doi: 10.1111/ijs.12356. [DOI] [PubMed] [Google Scholar]
- 44.Hackett M.L., Pickles K. Part I: Frequency of Depression after Stroke: An Updated Systematic Review and Meta-Analysis of Observational Studies. Int. J. Stroke. 2014;9:1017–1025. doi: 10.1111/ijs.12357. [DOI] [PubMed] [Google Scholar]
- 45.Terroni L., Sobreiro M.F.M., Conforto A.B., Adda C.C., Guajardo V.D., de Lucia M.C.S., Fráguas R. Association among depression, cognitive impairment and executive dysfunction after stroke. Dement. Neuropsychol. 2012;6:152–157. doi: 10.1590/S1980-57642012DN06030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allida S., Cox K.L., Hsieh C.-F., House A., Hackett M.L. Pharmacological, psychological and non-invasive brain stimulation interventions for preventing depression after stroke. Cochrane Database Syst. Rev. 2020;5:CD003689. doi: 10.1002/14651858.CD003689.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brainin M., Tuomilehto J., Heiss W.D., Bornstein N.M., Bath P.M.W., Teuschl Y., Richard E., Guekht A., Quinn T., Auer S., et al. Post-stroke cognitive decline: An update and perspectives for clinical research. Eur. J. Neurol. 2015;22:229–238. doi: 10.1111/ene.12626. [DOI] [PubMed] [Google Scholar]
- 48.Zhou D.H.D., Wang J.Y.J., Li J., Deng J., Gao C., Chen M. Frequency and risk factors of vascular cognitive impairment three months after ischemic stroke in China: The chongqing stroke study. Neuroepidemiology. 2005;24:87–95. doi: 10.1159/000081055. [DOI] [PubMed] [Google Scholar]
- 49.de Haan E.H., Nys G.M., Van Zandvoort M.J. Cognitive function following stroke and vascular cognitive impairment. Curr. Opin. Neurol. 2006;19:559–564. doi: 10.1097/01.wco.0000247612.21235.d9. [DOI] [PubMed] [Google Scholar]
- 50.Coco D.L., Lopez G., Corrao S. Cognitive impairment and stroke in elderly patients. Vasc. Health Risk Manag. 2016;12:105–116. doi: 10.2147/VHRM.S75306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conen D., Rodondi N., Müller A., Beer J.H., Ammann P., Moschovitis G., Auricchio A., Hayoz D., Kobza R., Shah D., et al. Relationships of Overt and Silent Brain Lesions With Cognitive Function in Patients With Atrial Fibrillation. J. Am. Coll. Cardiol. 2019;73:989–999. doi: 10.1016/j.jacc.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 52.Windham B.G., Griswold M.E., Wilkening S.R., Su D., Tingle J., Coker L.H., Knopman D., Gottesman R.F., Shibata D., Mosley T.H. Midlife Smaller and Larger Infarctions, White Matter Hyperintensities, and 20-Year Cognitive Decline. Ann. Intern. Med. 2019;171:389–396. doi: 10.7326/M18-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Debette S., Markus H.S. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta-analysis. BMJ. 2010;341:288. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knopman D., Boland L.L., Mosley T., Howard G., Liao D., Szklo M., McGovern P., Folsom A.R. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/WNL.56.1.42. [DOI] [PubMed] [Google Scholar]
- 55.Lu T., Wang Z., Cui Y., Zhou J., Wang Y., Ju S. Disrupted Structural Brain Connectome Is Related to Cognitive Impairment in Patients With Ischemic Leukoaraiosis. Front. Hum. Neurosci. 2021;15:268. doi: 10.3389/fnhum.2021.654750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qureshi A.I., Ezzeddine M.A., Nasar A., Suri M.F.K., Kirmani J.F., Hussein H.M., Divani A.A., Reddi A.S. Prevalence of elevated blood pressure in 563 704 adult patients with stroke presenting to the ED in the United States. Am. J. Emerg. Med. 2007;25:32–38. doi: 10.1016/j.ajem.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sposato L.A., Cipriano L.E., Saposnik G., Vargas E.R., Riccio P.M., Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: A systematic review and meta-analysis. Lancet Neurol. 2015;14:377–387. doi: 10.1016/S1474-4422(15)70027-X. [DOI] [PubMed] [Google Scholar]
- 58.Waldstein S.R., Tankard C.F., Maier K.J., Pelletier J.R., Snow J., Gardner A.W., Macko R., Katzel L.I. Peripheral Arterial Disease and Cognitive Function. Psychosom. Med. 2003;65:757–763. doi: 10.1097/01.PSY.0000088581.09495.5E. [DOI] [PubMed] [Google Scholar]
- 59.Bowler J.V. The concept of vascular cognitive impairment. J. Neurol. Sci. 2002;203–204:11–15. doi: 10.1016/S0022-510X(02)00253-8. [DOI] [PubMed] [Google Scholar]
- 60.Wang F., Hua S., Zhang Y., Yu H., Zhang Z., Zhu J., Liu R., Jiang Z. Association Between Small Vessel Disease Markers, Medial Temporal Lobe Atrophy and Cognitive Impairment After Stroke: A Systematic Review and Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2021;30:105460. doi: 10.1016/j.jstrokecerebrovasdis.2020.105460. [DOI] [PubMed] [Google Scholar]
- 61.Seiler A., Camilo M., Korostovtseva L., Haynes A.G., Brill A.-K., Horvath T., Egger M., Bassetti C.L. Prevalence of sleep-disordered breathing after stroke and TIA. Neurology. 2019;92:e648–e654. doi: 10.1212/WNL.0000000000006904. [DOI] [PubMed] [Google Scholar]
- 62.Brown D.L., Lisabeth L.D., Zupancic M.J., Concannon M., Martin C., Chervin R.D. High prevalence of supine sleep in ischemic stroke patients. Stroke. 2008;39:2511–2514. doi: 10.1161/STROKEAHA.107.513572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palomäki H., Berg A., Meririnne E., Kaste M., Lönnqvist R., Lehtihalmes M., Lönnqvist J. Complaints of poststroke insomnia and its treatment with mianserin. Cerebrovasc. Dis. 2003;15:56–62. doi: 10.1159/000067127. [DOI] [PubMed] [Google Scholar]
- 64.Suh M., Choi-Kwon S., Kim J.S. Sleep Disturbances at 3 Months after Cerebral Infarction. Eur. Neurol. 2016;75:75–81. doi: 10.1159/000443763. [DOI] [PubMed] [Google Scholar]
- 65.Hermann D.M., Siccoli M., Bassetti C.L. Sleep-wake disorders and stroke. Schweizer Arch. Neurol. Psychiatr. 2003;154:369–373. doi: 10.4414/sanp.2003.01416. [DOI] [Google Scholar]
- 66.Wondergem R., Pisters M.F., Wouters E.J., Olthof N., De Bie R.A., Visser-Meily J.M.A., Veenhof C. The course of activities in daily living: Who is at risk for decline after first ever stroke? Cerebrovasc. Dis. 2017;43:1–8. doi: 10.1159/000451034. [DOI] [PubMed] [Google Scholar]
- 67.Donnellan C., Hickey A., Hevey D., O’Neill D. Effect of mood symptoms on recovery one year after stroke. Int. J. Geriatr. Psychiatry. 2010;25:1288–1295. doi: 10.1002/gps.2482. [DOI] [PubMed] [Google Scholar]
- 68.Yu S., Arima H., Bertmar C., Hirakawa Y., Priglinger M., Evans K., Krause M. Depression but not anxiety predicts recurrent cerebrovascular events. Acta Neurol. Scand. 2016;134:29–34. doi: 10.1111/ane.12503. [DOI] [PubMed] [Google Scholar]
- 69.Aaronson J.A., Van Bennekom C.A.M., Hofman W.F., Van Bezeij T., Van Den Aardweg J.G., Groet E., Kylstra W.A., Schmand B. Obstructive sleep apnea is related to impaired cognitive and functional status after stroke. Sleep. 2015;38:1431–1437B. doi: 10.5665/sleep.4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yaggi H.K., Concato J., Kernan W.N., Lichtman J.H., Brass L.M., Mohsenin V. Obstructive Sleep Apnea as a Risk Factor for Stroke and Death. N. Engl. J. Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 71.Aloia M.S., Arnedt J.T., Davis J.D., Riggs R.L., Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: A critical review. J. Int. Neuropsychol. Soc. 2004;10:772–785. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 72.Beebe D.W., Groesz L., Wells C., Nichols A., McGee K. The neuropsychological effects of obstructive sleep apnea: A meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 73.Weihs A., Frenzel S., Grabe H.J. The Link Between Obstructive Sleep Apnoea and Neurodegeneration and Cognition. Curr. Sleep Med. Rep. 2021;7:87–96. doi: 10.1007/s40675-021-00210-5. [DOI] [Google Scholar]
- 74.Lu D., Li P., Zhou Y., Xu X., Zhang H., Liu L., Tian Z. Association between serum non-high-density lipoprotein cholesterol and cognitive impairment in patients with acute ischemic stroke. BMC Neurol. 2016;16:154. doi: 10.1186/s12883-016-0668-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Castellanos M., Sobrino T., Pedraza S., Moldes O., Pumar J.M., Silva Y., Serena J., García-Gil M., Castillo J., Dávalos A. High plasma glutamate concentrations are associated with infarct growth in acute ischemic stroke. Neurology. 2008;71:1862–1868. doi: 10.1212/01.wnl.0000326064.42186.7e. [DOI] [PubMed] [Google Scholar]
- 76.Zeydan B., Deelchand D.K., Tosakulwong N., Lesnick T.G., Kantarci O.H., Machulda M.M., Knopman D.S., Lowe V.J., Jack C.R., Petersen R.C., et al. Decreased Glutamate Levels in Patients with Amnestic Mild Cognitive Impairment: An sLASER Proton MR Spectroscopy and PiB-PET Study. J. Neuroimaging. 2017;27:630–636. doi: 10.1111/jon.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kirvell S.L., Elliott M.S., Kalaria R.N., Hortobágyi T., Ballard C.G., Francis P.T. Vesicular glutamate transporter and cognition in stroke. Neurology. 2010;75:1803–1809. doi: 10.1212/WNL.0b013e3181fd6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roalf D.R., Sydnor V.J., Woods M., Wolk D.A., Scott J.C., Reddy R., Moberg P.J. A quantitative meta-analysis of brain glutamate metabolites in aging. Neurobiol. Aging. 2020;95:240–249. doi: 10.1016/j.neurobiolaging.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spalletta G., Cravello L., Imperiale F., Salani F., Bossù P., Picchetto L., Cao M., Rasura M., Pazzelli F., Orzi F., et al. Neuropsychiatric symptoms and interleukin-6 serum levels in acute stroke. J. Neuropsychiatry Clin. Neurosci. 2013;25:255–263. doi: 10.1176/appi.neuropsych.12120399. [DOI] [PubMed] [Google Scholar]
- 80.Brown R.P., Gerbarg P.L. Sudarshan Kriya Yogic Breathing in the Treatment of Stress, Anxiety, and Depression: Part I—Neurophysiologic Model. J. Altern. Complement. Med. 2005;11:189–201. doi: 10.1089/acm.2005.11.189. [DOI] [PubMed] [Google Scholar]
- 81.Ma X., Yue Z.Q., Gong Z.Q., Zhang H., Duan N.Y., Shi Y.T., Wei G.X., Li Y.F. The effect of diaphragmatic breathing on attention, negative affect and stress in healthy adults. Front. Psychol. 2017;8:874. doi: 10.3389/fpsyg.2017.00874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zeidan F., Johnson S.K., Diamond B.J., David Z., Goolkasian P. Mindfulness meditation improves cognition: Evidence of brief mental training. Conscious. Cogn. 2010;19:597–605. doi: 10.1016/j.concog.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 83.Wei G.-X., Li Y.-F., Yue X.-L., Ma X., Chang Y.-K., Yi L.-Y., Li J.-C., Zuo X.-N. Tai Chi Chuan modulates heart rate variability during abdominal breathing in elderly adults. PsyCh J. 2016;5:69–77. doi: 10.1002/pchj.105. [DOI] [PubMed] [Google Scholar]
- 84.Brown R.P., Gerbarg P.L. Sudarshan Kriya Yogic Breathing in the Treatment of Stress, Anxiety, and Depression: Part II—Clinical Applications and Guidelines. J. Altern. Complement. Med. 2005;11:711–717. doi: 10.1089/acm.2005.11.711. [DOI] [PubMed] [Google Scholar]
- 85.Ainsworth B., Bruton A., Thomas M., Yardley L. One year later: Highlighting the challenges and opportunities in disseminating a breathing-retraining digital behaviour change intervention. Digit. Health. 2020;6:1–5. doi: 10.1177/2055207620936441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bruton A., Lee A., Yardley L., Raftery J., Arden-Close E., Kirby S., Zhu S., Thiruvothiyur M., Webley F., Taylor L., et al. Physiotherapy breathing retraining for asthma: A randomised controlled trial. Lancet Respir. Med. 2018;6:19–28. doi: 10.1016/S2213-2600(17)30474-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vagedes J., Helmert E., Kuderer S., Vagedes K., Wildhaber J., Andrasik F. The Buteyko breathing technique in children with asthma: A randomized controlled pilot study. Complement. Ther. Med. 2021;56:102582. doi: 10.1016/j.ctim.2020.102582. [DOI] [PubMed] [Google Scholar]
- 88.Agte V.V., Chiplonkar S.A. Sudarshan Kriya Yoga for improving antioxidant status and reducing anxiety in adults. Altern. Complement. Ther. 2008;14:96–100. doi: 10.1089/act.2008.14204. [DOI] [Google Scholar]
- 89.Sakakibara M., Hayano J. Effect of Slowed Respiration on Cardiac Parasympathetic Response to Threat. Psychosom. Med. 1996;58:32–37. doi: 10.1097/00006842-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 90.Udupa K., Madanmohan, Bhavanani A.B., Vijayalakshmi P., Krishnamurthy N. Effect of pranayam training on cardiac function in normal young volunteers. Indian J. Physiol. Pharmacol. 2003;47:27–33. [PubMed] [Google Scholar]
- 91.Subbalakshmi N.K., Saxena S.K., Urmimala, D’Souza U.J.A. Immediate effect of ‘Nadi -shodhana pranayama’ on some selected parameters of cardiovascular, pulmonary, and higher functions of brain. Thai J. Physiol. Sci. 2014;18:10–16. [Google Scholar]
- 92.Pal G.K., Velkumary S., Madanmohan A. Effect of short-term practice of breathing exercises on autonomic functions in normal human volunteers. Indian J. Med. Res. 2004;120:115–121. [PubMed] [Google Scholar]
- 93.Shi Y., Yang D., Zeng Y., Wu W. Risk factors for post-stroke depression: A meta-analysis. Front. Aging Neurosci. 2017;9:218. doi: 10.3389/fnagi.2017.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Taylor-Rowan M., Momoh O., Ayerbe L., Evans J.J., Stott D.J., Quinn T.J. Prevalence of pre-stroke depression and its association with post-stroke depression: A systematic review and meta-analysis. Psychol. Med. 2019;49:685–696. doi: 10.1017/S0033291718002003. [DOI] [PubMed] [Google Scholar]
- 95.Hackett M.L., Yapa C., Parag V., Anderson C.S. Frequency of depression after stroke: A systematic review of observational studies. Stroke. 2005;36:1330–1340. doi: 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- 96.Mitchell A.J., Sheth B., Gill J., Yadegarfar M., Stubbs B., Yadegarfar M., Meader N. Prevalence and predictors of post-stroke mood disorders: A meta-analysis and meta-regression of depression, anxiety and adjustment disorder. Gen. Hosp. Psychiatry. 2017;47:48–60. doi: 10.1016/j.genhosppsych.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 97.Jørgensen T.S.H., Wium-Andersen I.K., Wium-Andersen M.K., Jørgensen M.B., Prescott E., Maartensson S., Kragh-Andersen P., Osler M. Incidence of Depression After Stroke, and Associated Risk Factors and Mortality Outcomes, in a Large Cohort of Danish Patients. JAMA Psychiatry. 2016;73:1032–1040. doi: 10.1001/jamapsychiatry.2016.1932. [DOI] [PubMed] [Google Scholar]
- 98.Hyun K.-S., Won J.-S., Kim W.-O., Han S.-S., Lee J.-A. The effects of danjeon breathing exercise on vital capacity, physical fitness, anxiety and depression among older adults. J. Korean Acad. Community Health Nurs. 2009;20:474–482. [Google Scholar]
- 99.Krishnamurthy M.N., Telles S. Assessing depression following two ancient Indian interventions: Effects of yoga and ayurveda on older adults in a residential home. J. Gerontol. Nurs. 2007;33:17–23. doi: 10.3928/00989134-20070201-05. [DOI] [PubMed] [Google Scholar]
- 100.Klainin-Yobas P., Oo W.N., Suzanne Yew P.Y., Lau Y. Effects of relaxation interventions on depression and anxiety among older adults: A systematic review. Aging Ment. Health. 2015;19:1043–1055. doi: 10.1080/13607863.2014.997191. [DOI] [PubMed] [Google Scholar]
- 101.Russo M.A., Santarelli D.M., O’Rourke D. The physiological effects of slow breathing in the healthy human. Breathe. 2017;13:298–309. doi: 10.1183/20734735.009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brosschot J.F., Gerin W., Thayer J.F. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. J. Psychosom. Res. 2006;60:113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- 103.Henderson L.A., Richard C.A., Macey P.M., Runquist M.L., Yu P.L., Galons J.P., Harper R.M. Functional magnetic resonance signal changes in neural structures to baroreceptor reflex activation. J. Appl. Physiol. 2004;96:693–703. doi: 10.1152/japplphysiol.00852.2003. [DOI] [PubMed] [Google Scholar]
- 104.Rincon F., Dhamoon M., Moon Y., Paik M.C., Boden-Albala B., Homma S., Di Tullio M.R., Sacco R.L., Elkind M.S.V. Stroke location and association with fatal cardiac outcomes: Northern manhattan study (NOMAS) Stroke. 2008;39:2425–2431. doi: 10.1161/STROKEAHA.107.506055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ivey F.M., Gardner A.W., Dobrovolny C.L., Macko R.F. Unilateral impairment of leg blood flow in chronic stroke patients. Cerebrovasc. Dis. 2004;18:283–289. doi: 10.1159/000080353. [DOI] [PubMed] [Google Scholar]
- 106.Billinger S.A., Kluding P.M. Use of doppler ultrasound to assess femoral artery adaptations in the hemiparetic limb in people with stroke. Cerebrovasc. Dis. 2009;27:552–558. doi: 10.1159/000214218. [DOI] [PubMed] [Google Scholar]
- 107.Billinger S.A., Gajewski B.J., Guo L.X., Kluding P.M. Single limb exercise induces femoral artery remodeling and improves blood flow in the hemiparetic leg poststroke. Stroke. 2009;40:3086–3090. doi: 10.1161/STROKEAHA.109.550889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bleeker M.W.P., De Groot P.C.E., Poelkens F., Rongen G.A., Smits P., Hopman M.T.E. Vascular adaptation to 4 wk of deconditioning by unilateral lower limb suspension. Am. J. Physiol. -Heart Circ. Physiol. 2005;288:1747–1755. doi: 10.1152/ajpheart.00966.2004. [DOI] [PubMed] [Google Scholar]
- 109.Bleeker M.W.P., De Groot P.C.E., Rongen G.A., Rittweger J., Felsenberg D., Smits P., Hopman M.T.E. Vascular adaptation to deconditioning and the effect of an exercise countermeasure: Results of the Berlin Bed Rest study. J. Appl. Physiol. 2005;99:1293–1300. doi: 10.1152/japplphysiol.00118.2005. [DOI] [PubMed] [Google Scholar]
- 110.Dinenno F.A., Jones P.P., Seals D.R., Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: Relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100:164–170. doi: 10.1161/01.CIR.100.2.164. [DOI] [PubMed] [Google Scholar]
- 111.Kalaivani S., Kumari M., Pal G. Effect of alternate nostril breathing exercise on blood pressure, heart rate, and rate pressure product among patients with hypertension in JIPMER, Puducherry. J. Educ. Health Promot. 2019;8:145. doi: 10.4103/jehp.jehp_32_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mourya M., Mahajan A.S., Singh N.P., Jain A.K. Effect of slow- and fast-breathing exercises on autonomic functions in patients with essential hypertension. J. Altern. Complement. Med. 2009;15:711–717. doi: 10.1089/acm.2008.0609. [DOI] [PubMed] [Google Scholar]
- 113.Kaushik R.M., Kaushik R., Mahajan S.K., Rajesh V. Effects of mental relaxation and slow breathing in essential hypertension. Complement. Ther. Med. 2006;14:120–126. doi: 10.1016/j.ctim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 114.Joseph C.N., Porta C., Casucci G., Casiraghi N., Maffeis M., Rossi M., Bernardi L. Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension. 2005;46:714–718. doi: 10.1161/01.HYP.0000179581.68566.7d. [DOI] [PubMed] [Google Scholar]
- 115.Bernardi L., Gabutti A., Porta C., Spicuzza L. Slow breathing reduces chemoreflex response to hypoxia and hypercapnia, and increases baroreflex sensitivity. J. Hypertens. 2001;19:2221–2229. doi: 10.1097/00004872-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 116.Fonkoue I.T., Marvar P.J., Norrholm S.D., Kankam M.L., Li Y., DaCosta D., Rothbaum B.O., Park J. Acute effects of device-guided slow breathing on sympathetic nerve activity and baroreflex sensitivity in posttraumatic stress disorder. Am. J. Physiol. -Heart Circ. Physiol. 2018;315:H141–H149. doi: 10.1152/ajpheart.00098.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oneda B., Ortega K.C., Gusmão J.L., Araújo T.G., Mion D. Sympathetic nerve activity is decreased during device-guided slow breathing. Hypertens. Res. 2010;33:708–712. doi: 10.1038/hr.2010.74. [DOI] [PubMed] [Google Scholar]
- 118.Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Blaha M.J., Dai S., Ford E.S., Fox C.S., Franco S., et al. Heart Disease and Stroke Statistics—2014 Update: A Report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boehme A.K., Esenwa C., Elkind M.S.V. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017;120:472–495. doi: 10.1161/CIRCRESAHA.116.308398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goldstein L.B., Adams R., Alberts M.J., Appel L.J., Brass L.M., Bushnell C.D., Culebras A., DeGraba T.J., Gorelick P.B., Guyton J.R., et al. Primary prevention of ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council: Cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: The American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 121.Johnson K.G., Johnson D.C. Frequency of sleep apnea in stroke and TIA patients: A meta-analysis. J. Clin. Sleep Med. 2010;6:131–137. doi: 10.5664/jcsm.27760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang Y., Ding R., Hu D., Zhang F., Sheng L. Reliability and validity of a Chinese version of the HADS for screening depression and anxiety in psycho-cardiological outpatients. Compr. Psychiatry. 2014;55:215–220. doi: 10.1016/j.comppsych.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 123.Gottlieb D.J., Punjabi N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA. 2020;323:1380–1400. doi: 10.1001/jama.2020.3514. [DOI] [PubMed] [Google Scholar]
- 124.Javaheri S., Barbe F., Campos-Rodriguez F., Dempsey J.A., Khayat R., Javaheri S., Malhotra A., Martinez-Garcia M.A., Mehra R., Pack A.I., et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J. Am. Coll. Cardiol. 2017;69:841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xie W., Zheng F., Song X. Obstructive sleep apnea and serious adverse outcomes in patients with cardiovascular or cerebrovascular disease: A PRISMA-compliant systematic review and meta-analysis. Medicine. 2014;93:e336. doi: 10.1097/MD.0000000000000336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Birkbak J., Clark A.J., Rod N.H. The effect of sleep disordered breathing on the outcome of stroke and transient ischemic attack: A systematic review. J. Clin. Sleep Med. 2014;10:103–108. doi: 10.5664/jcsm.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Peppard P.E., Young T., Palta M., Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 128.Nieto F.J., Young T.B., Lind B.K., Redline S., Agostino R.B.D., Newman A.B., Lebowitz M.D., Pickering T.G. in a Large Community-Based Study for the Sleep Heart Health Study. JAMA. 2000;284:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 129.O’Donnell M., Hankey G.J., Rangarajan S., Chin S.L., Rao-Melacini P., Ferguson J., Xavier D., Lisheng L., Zhang H., Pais P., et al. Variations in knowledge, awareness and treatment of hypertension and stroke risk by country income level. Heart. 2021;107:282–289. doi: 10.1136/heartjnl-2019-316515. [DOI] [PubMed] [Google Scholar]
- 130.Wessendorf T.E., Thilmann A.F., Wang Y.M., Schreiber A., Konietzko N., Teschler H. Fibrinogen levels and obstructive sleep apnea in ischemic stroke. Am. J. Respir. Crit. Care Med. 2000;162:2039–2042. doi: 10.1164/ajrccm.162.6.2001048. [DOI] [PubMed] [Google Scholar]
- 131.Acharya P., Jakobleff W.A., Forest S.J., Chinnadurai T., Mellas N., Patel S.R., Kizer J.R., Billett H.H., Goldstein D.J., Jorde U.P., et al. Fibrinogen Albumin Ratio and Ischemic Stroke During Venoarterial Extracorporeal Membrane Oxygenation. ASAIO J. 2020;66:277–282. doi: 10.1097/MAT.0000000000000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ruan Y., Yuan C., Liu Y., Zeng Y., Cheng H., Cheng Q., Chen Y., Huang G., He W., He J. High fibrinogen-to-albumin ratio is associated with hemorrhagic transformation in acute ischemic stroke patients. Brain Behav. 2021;11:e01855. doi: 10.1002/brb3.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bagai K. Obstructive Sleep Apnea, Stroke, and Cardiovascular Diseases. Neurologist. 2010;16:129–139. doi: 10.1097/NRL.0b013e3181f097cb. [DOI] [PubMed] [Google Scholar]
- 134.Sahlin C., Sandberg O., Gustafson Y., Stenlund G.H., Franklin K.A. Obstructive sleep apnea is a risk factor for death in patients with stroke: A 10-year follow-up. Arch. Intern. Med. 2008;168:297–301. doi: 10.1001/archinternmed.2007.70. [DOI] [PubMed] [Google Scholar]
- 135.Dong J.Y., Zhang Y.H., Qin L.Q. Obstructive sleep apnea and cardiovascular risk: Meta-analysis ofprospective cohort studies. Atherosclerosis. 2013;229:489–495. doi: 10.1016/j.atherosclerosis.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 136.Wang X., Ouyang Y., Wang Z., Zhao G., Liu L., Bi Y. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: A meta-analysis of prospective cohort studies. Int. J. Cardiol. 2013;169:207–214. doi: 10.1016/j.ijcard.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 137.Li M., Hou W.S., Zhang X.W., Tang Z.Y. Obstructive sleep apnea and risk of stroke: A meta-analysis of prospective studies. Int. J. Cardiol. 2014;172:466–469. doi: 10.1016/j.ijcard.2013.12.230. [DOI] [PubMed] [Google Scholar]
- 138.Xie C., Zhu R., Tian Y., Wang K. Association of obstructive sleep apnoea with the risk of vascular outcomes and all-cause mortality: A meta-analysis. BMJ Open. 2017;7:e013983. doi: 10.1136/bmjopen-2016-013983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nathaniel T., Sanders C., Knisely K., Edrissi C., Rathfoot C., Poupore N., Wormack L. Obstructive sleep apnea and stroke severity: Impact of clinical risk factors. Brain Circ. 2021;7:92–103. doi: 10.4103/bc.bc_57_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bravata D.M., Sico J., Vaz Fragoso C.A., Miech E.J., Matthias M.S., Lampert R., Williams L.S., Concato J., Ivan C.S., Fleck J.D., et al. Diagnosing and treating sleep apnea in patients with acute cerebrovascular disease. J. Am. Heart Assoc. 2018;7:e008841. doi: 10.1161/JAHA.118.008841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Khot S.P., Davis A.P., Crane D.A., Tanzi P.M., Lue D.L., Claflin E.S., Becker K.J., Longstreth W.T., Watson N.F., Billings M.E. Effect of continuous positive airway pressure on stroke rehabilitation: A pilot randomized sham-controlled trial. J. Clin. Sleep Med. 2016;12:1019–1026. doi: 10.5664/jcsm.5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aaronson J.A., Hofman W.F., Van Bennekom C.A.M., Van Bezeij T., Van Den Aardweg J.G., Groet E., Kylstra W.A., Schmand B. Effects of continuous positive airway pressure on cognitive and functional outcome of stroke patients with obstructive sleep apnea: A randomized controlled trial. J. Clin. Sleep Med. 2016;12:533–541. doi: 10.5664/jcsm.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Parra O., Sánchez-Armengol Á., Capote F., Bonnin M., Arboix A., Campos-Rodríguez F., Pérez-Ronchel J., Durán-Cantolla J., Martínez-Null C., de la Peña M., et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: A randomized controlled trial. J. Sleep Res. 2015;24:47–53. doi: 10.1111/jsr.12181. [DOI] [PubMed] [Google Scholar]
- 144.Davis A.P., Billings M.E., Longstreth W.T., Khot S.P. Early diagnosis and treatment of obstructive sleep apnea after stroke Are we neglecting a modifiable stroke risk factor? Neurol. Clin. Pract. 2013;3:192–201. doi: 10.1212/CPJ.0b013e318296f274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Weaver T.E., Grunstein R.R. Adherence to continuous positive airway pressure therapy: The challenge to effective treatment. Proc. Am. Thorac. Soc. 2008;5:173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rowland S., Aiyappan V., Hennessy C., Catcheside P., Chai-Coezter C.L., McEvoy R.D., Antic N.A. Comparing the efficacy, mask leak, patient adherence, and patient preference of three different CPAP interfaces to treat moderate-severe obstructive sleep apnea. J. Clin. Sleep Med. 2018;14:101–108. doi: 10.5664/jcsm.6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Courtney R. Breathing retraining in sleep apnoea: A review of approaches and potential mechanisms. Sleep Breath. 2020;24:1315–1325. doi: 10.1007/s11325-020-02013-4. [DOI] [PubMed] [Google Scholar]
- 148.Vranish J.R., Bailey E.F. Inspiratory muscle training improves sleep and mitigates cardiovascular dysfunction in obstructive sleep apnea. Sleep. 2016;39:1179–1185. doi: 10.5665/sleep.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Birch M. Sleep apnoea: A survey of breathing retraining. Aust. Nurs. J. 2021;10:40–41. [PubMed] [Google Scholar]
- 150.Birch M. Obstructive sleep apnoea and breathing retraining. Aust. Nurs. J. 2004;12:27–29. [PubMed] [Google Scholar]
- 151.McKeown P. The Buteyko technique: News. J. Dent. Sleep Med. 2019;6:2. doi: 10.15331/jdsm.7078. [DOI] [Google Scholar]
- 152.Suzuki M., Tanuma T. The effect of nasal and oral breathing on airway collapsibility in patients with obstructive sleep apnea: Computational fluid dynamics analyses. PLoS ONE. 2020;15:e0231262. doi: 10.1371/journal.pone.0231262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hsu Y., Lan M., Huang Y., Kao M., Lan M. Association between breathing route, oxygen desaturation, and upper airway morphology. Laryngoscope. 2021;131:E659–E664. doi: 10.1002/lary.28774. [DOI] [PubMed] [Google Scholar]
- 154.Bachour A., Maasilta P. Mouth Breathing Compromises Adherence to Nasal Continuous Positive Airway Pressure Therapy. Chest. 2004;126:1248–1254. doi: 10.1378/chest.126.4.1248. [DOI] [PubMed] [Google Scholar]
- 155.McKeown P., O’Connor-Reina C., Plaza G. Breathing Re-Education and Phenotypes of Sleep Apnea: A Review. J. Clin. Med. 2021;10:471. doi: 10.3390/jcm10030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ojay A., Ernst E. Can singing exercises reduce snoring? A pilot study. Complement. Ther. Med. 2000;8:151–156. doi: 10.1054/ctim.2000.0376. [DOI] [PubMed] [Google Scholar]
- 157.Acalovschi D., Wiest T., Hartmann M., Farahmi M., Mansmann U., Auffarth G.U., Grau A.J., Green F.R., Grond-Ginsbach C., Schwaninger M. Multiple levels of regulation of the interleukin-6 system in stroke. Stroke. 2003;34:1864–1869. doi: 10.1161/01.STR.0000079815.38626.44. [DOI] [PubMed] [Google Scholar]
- 158.Waje-Andreassen U., Kråkenes J., Ulvestad E., Thomassen L., Myhr K.M., Aarseth J., Vedeler C.A. IL-6: An early marker for outcome in acute ischemic stroke. Acta Neurol. Scand. 2005;111:360–365. doi: 10.1111/j.1600-0404.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- 159.Aziz M., Fatima R., Assaly R. Elevated interleukin-6 and severe COVID-19: A meta-analysis. J. Med. Virol. 2020;92:2283–2285. doi: 10.1002/jmv.25948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020;111:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mastroianni F. First, Do No Harm: Caution Against Use of Tocilizumab in COVID-19. Chest. 2020;158:2233. doi: 10.1016/j.chest.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Siepmann T., Sedghi A., Simon E., Winzer S., Barlinn J., de With K., Mirow L., Wolz M., Gruenewald T., Schroettner P., et al. Increased risk of acute stroke among patients with severe COVID-19: A multicenter study and meta-analysis. Eur. J. Neurol. 2021;28:238–247. doi: 10.1111/ene.14535. [DOI] [PubMed] [Google Scholar]
- 163.Sashindranath M., Nandurkar H.H. Endothelial dysfunction in the brain: Setting the stage for stroke and other cerebrovascular complications of COVID-19. Stroke. 2021;52:1895–1904. doi: 10.1161/STROKEAHA.120.032711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sparrow N.A., Anwar F., Covarrubias A.E., Rajput P.S., Rashid M.H., Nisson P.L., Gezalian M.M., Toossi S., Ayodele M.O., Karumanchi S.A., et al. Interleukin-6 Inhibition Reduces Neuronal Injury In A Murine Model of Ventilator-Induced Lung Injury. Am. J. Respir. Cell Mol. Biol. 2021;65:403–412. doi: 10.1165/rcmb.2021-0072OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sarvottam K., Magan D., Yadav R.K., Mehta N., Mahapatra S.C. Adiponectin, Interleukin-6, and Cardiovascular Disease Risk Factors Are Modified by a Short-Term Yoga-Based Lifestyle Intervention in Overweight and Obese Men. J. Altern. Complement. Med. 2012;19:397–402. doi: 10.1089/acm.2012.0086. [DOI] [PubMed] [Google Scholar]
- 166.Sudarku R.A. The Changes in Level of 8-endorphin, Interleukin-2, Interleukin-4, Interleukin-6, Imunoglobulin and Cortisol Hormone on Practices of Brething Exercise. Bul. Penelit. Sist. Kesehat. 2010;13 [Google Scholar]
- 167.Rubini A. Interleukin-6 and lung inflammation: Evidence for a causative role in inducing respiratory system resistance increments. Inflamm. Allergy-Drug Targets. 2013;12:315–321. doi: 10.2174/1871528111312050003. [DOI] [PubMed] [Google Scholar]
- 168.Spruit M.A., Holland A.E., Singh S.J., Tonia T., Wilson K.C., Troosters T. COVID-19: Interim guidance on rehabilitation in the hospital and post-hospital phase from a European Respiratory Society- And American Thoracic Society-coordinated international task force. Eur. Respir. J. 2020;56:2002197. doi: 10.1183/13993003.02197-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]