Abstract
Objective
There is insufficient research on digestive symptoms and outcomes following coronavirus disease (COVID-19) vaccination. We aimed to investigate digestive symptoms and related complications among South Koreans who were administered COVID-19 vaccines.
Methods
Forty-six patients (men: 22, women: 24) with a median age of 68 years (interquartile range:55.5, 73.8 years) who experienced digestive symptoms following COVID-19 vaccination between March 1 and July 30, 2021, were included. This retrospective single-center study collected information on clinical symptoms, laboratory tests, imaging results, comorbidities, complications, treatment type, and prognosis.
Results
Thirty-three (71.7%), nine (19.6%), and three (6.5%) patients were administered AZD1222 (AstraZeneca), BNT162b2 (Pfizer/BioNTech), and JNJ-78436735 (Johnson and Johnson) vaccines, respectively. Patients were classified with mild (25 patients, 54.3%), moderate (five patients, 10.9%), and severe (16 patients, 34.8%) based on disease severity. Digestive symptoms included abdominal pain, diarrhea, dyspepsia, and nausea, which usually developed within 1 day (78.3%) following the first vaccination. In total, 14 (30.4%) patients experienced only gastrointestinal symptoms, whereas 32 (69.6%) experienced non-gastrointestinal symptoms. Complications included enterocolitis (76%), acute kidney injury (9%), anaphylactoid reaction (2%), and duodenal perforation (2%).
Conclusions
COVID-19 vaccines caused digestive symptoms and other complications that ranged from mild to severe. While further validation is required, our results suggest that monitoring digestive symptoms following COVID-19 vaccination can help detect rather severe complications that require medical intervention.
Keywords: COVID-19, Vaccine, Adverse reaction, Digestive symptoms
1. Introduction
The World Health Organization declared the coronavirus disease (COVID-19) outbreak a global pandemic in March 2020 [1]. COVID-19 vaccines are the primary defense against the pandemic; they reduce disease severity and mortality [2]. Four COVID-19 vaccines have been approved for use [3] in South Korea: AZD1222 (AstraZeneca), BNT162b2 (Pfizer–BioNTech), JNJ-78436735 (Janssen), and mRNA-1273 (Moderna); they are currently in use as of August 20, 2021. These vaccines are effective in preventing COVID-19 infection and are generally safe to use with a low incidence of adverse events (AEs) [[4], [5], [6], [7], [8], [9],10]. However, rare and critical complications such as encephalomyelitis [11], anaphylaxis [12], vesiculo-bullous rash [13], acute kidney injury [14], intravascular thrombosis, and thrombocytopenia [[14], [15], [16]] have been reported following vaccine administration.
Most AEs following COVID-19 vaccination are mild and transient. The common side effects include headache, muscle pain, chills, diarrhea, and pain at the inoculation site. Previous studies reported that digestive symptoms following COVID-19 vaccination were not severe AEs, and they could be managed without medical treatment [17,18]. However, some critical complications were identified in patients with digestive symptoms in the emergency department (ED). There is insufficient information regarding the clinical characteristics of digestive symptoms following the vaccination. Therefore, we aimed to investigate the clinical characteristics and outcomes of patients experiencing digestive symptoms following COVID-19 vaccination.
2. Methods
2.1. Design
This retrospective single-center study was conducted at the Seoul National University Boramae Medical Center in Seoul, South Korea, from March 1 to July 30, 2021. The study protocol was approved by the Institutional Review Board of the Seoul National University Boramae Medical Center (approval number 30–2021-113). The requirement for informed consent was waived because of the retrospective nature of the study.
We collected cases of patients who visited the ED for digestive symptoms following COVID-19 vaccination. Only those that had an association between vaccination and digestive symptoms were included in the study. The inclusion criteria were as follows: (1) newly developed gastrointestinal symptoms after vaccination (symptom onset is measured after vaccination) and no gastrointestinal symptoms prior to vaccination; (2) gastrointestinal symptoms requiring medical treatment; (3) gastrointestinal symptoms result in a major portion of the patient's discomfort; (4) patients visiting the ED for gastrointestinal symptoms. The exclusion criteria were as follows: (1) gastrointestinal symptoms prior to vaccination; (2) no history of recent COVID-19 infection; (3) positive real-time polymerase chain reaction test results for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); and (4) no history of medications that can cause indigestion.
2.2. Data collection
Clinical data were collected from medical records by trained research assistants according to the stipulated guidelines [19]. Investigators retrieved data regarding comorbidities, clinical characteristics, laboratory test results, radiological findings, vaccine brand, and outcomes from electrical medical records. Reviewers who were clinical physicians reviewed clinical data from electronic medical records; discrepancies among them were resolved by the investigators. Diagnostic information was acquired sequentially based on medical history, physical examination, and categories according to the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10; Version 2019) [20].
2.3. Outcome measures
Digestive symptoms, including abdominal pain, diarrhea, and indigestion, were defined as the main symptoms. Other symptoms such as dyspnea, generalized weakness, headache, and decreased consciousness levels were considered additional symptoms. Patients were classified into three groups based on disease severity according to the Korean Food and Drug Administration guidelines regarding symptoms, laboratory results, medical treatment, hospitalization, and recovery period [21,22]. Patients in the mild group had stable vital signs and normal C-reactive protein (CRP) levels, whereas those in the moderate group had stable vital signs and abnormal CRP levels; they required medical treatment. Patients in the severe group had unstable vital signs, abnormal laboratory results, and an extended recovery period, requiring hospitalization.
2.4. Statistical analysis
Descriptive and categorical variables are reported as medians (interquartile ranges [IQRs]) and frequencies (percentages), respectively. Statistical significance was set at P < 0.05. Statistical analyses were performed using SPSS version 20 (IBM Corp, Armonk, NY, USA).
3. Results
3.1. Baseline characteristics
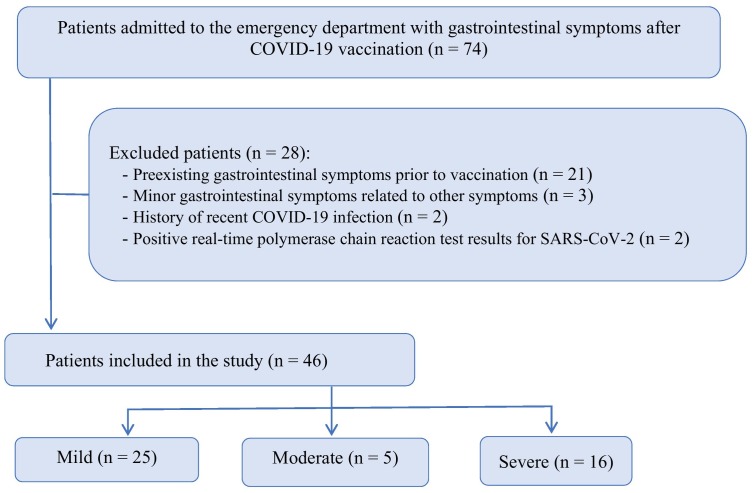
Seventy-four patients were preliminarily screened for digestive symptoms following COVID-19 vaccination. We excluded 28 patients whose digestive symptoms exhibited no definite causal relationship with COVID-19 vaccination (Fig. 1 ). Finally, we analyzed 46 patients (men: 22, women: 24). Fig. 1 shows the flowchart of patient inclusion. Table 1 presents the patients' baseline characteristics (median age: 68 years; IQR: 55.5, 73.8 years). COVID-19 vaccines from three different brands were used; 33 patients received AZD1222 (AstraZeneca), nine received BNT162b2 (Pfizer/BioNTech), and three received JNJ-78436735 (Johnson and Johnson). The median time for the onset of digestive symptoms after vaccination was 1 (1,1) days. Most digestive symptoms appeared following the administration of the first dose of the vaccine. Comorbidities included hypertension, diabetes mellitus, chronic obstructive pulmonary disease, and musculoskeletal disease (Table 1).
Fig. 1.
Patient inclusion flowchart.
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Table 1.
Baseline characteristics of the study population (n = 46)
| Characteristics | Mild n = 25 |
Moderate n = 5 |
Severe n = 16 |
Total N = 46 |
|---|---|---|---|---|
| Age (years), median [IQR] | 60 [50, 72] | 42 [37, 50] | 71 [62, 77] | 68 [56, 74] |
| ≤40 | 6 | 1 | 1 | 8 |
| 41–60 | 5 | 2 | 2 | 9 |
| 61–80 | 12 | 1 | 10 | 23 |
| 81–90 | 2 | 1 | 3 | 6 |
| Sex | ||||
| Male | 8 | 2 | 12 | 22 |
| Female | 17 | 3 | 4 | 24 |
| Comorbidities | ||||
| Cardiovascular disease | 5 | 2 | 9 | 16 |
| Endocrine disease | 4 | 2 | 5 | 11 |
| Digestive disease | 2 | 6 | 8 | |
| Respiratory disease | 1 | 2 | 3 | |
| Malignancy | 1 | 1 | 2 | |
| Allergy | 1 | 1 | ||
| Muscular skeletal disease | 3 | 1 | 3 | 7 |
| Vaccine brand | ||||
| AZD1222 | 16 | 3 | 14 | 33 |
| BNT162b2 | 6 | 2 | 1 | 9 |
| JNJ-78436735 | 2 | 1 | 3 | |
| AZD1222 + BNT162b2 | 1 | 1 | ||
| Number of vaccinations | ||||
| Dose 1 | 18 | 9 | 9 | 36 |
| Dose 2 | 7 | 1 | 2 | 10 |
| Symptom onset after vaccination (days), median [IQR] | 1 [1,1] | 1 [1,2] | 1 [1, 1] | 1 [1, 1] |
| 1 | 22 | 3 | 15 | 40 |
| 2 | 2 | 2 | 4 | |
| 3 | 1 | 1 | 2 |
IQR, interquartile range.
3.2. Symptoms and outcomes
In total, 14 (30%) patients experienced digestive symptoms only (Table 2 ). These included abdominal pain, diarrhea, dyspepsia, nausea, hematochezia, and melena. Patients (70%) experienced additional symptoms, including fever and poor oral intake (Table 2). Abdominal pain, diarrhea, fever, and inadequate oral intake were frequently observed in severe cases.
Table 2.
Symptoms and laboratory findings of the study population (n = 46)
| Clinical symptoms | Mild n = 25 |
Moderate n = 5 |
Severe n = 16 |
Total N = 46 |
|---|---|---|---|---|
| Gastrointestinal symptoms | ||||
| Dyspepsia | 4 | 2 | 4 | 10 |
| Abdominal pain | 15 | 1 | 10 | 28 |
| Nausea | 10 | 1 | 11 | |
| Diarrhea | 13 | 5 | 7 | 25 |
| Hematochezia | 1 | 1 | ||
| Melena | 1 | 1 | ||
| Non-gastrointestinal symptoms | ||||
| Fever | 4 | 4 | 5 | 13 |
| Headache | 11 | 1 | 1 | 13 |
| General myalgia | 1 | 2 | 3 | |
| General weakness | 2 | 3 | 2 | 7 |
| Poor oral intake | 6 | 4 | 10 | |
| Dizziness | 8 | 1 | 1 | 10 |
| Epistaxis | 1 | 1 | ||
| None | 8 | 3 | 11 | |
| Gastrointestinal symptoms only | 8 | 6 | 14 | |
| Non-gastrointestinal symptoms | 17 | 5 | 10 | 32 |
| Laboratory test results | ||||
| AST > 38 IU/L or ALT >45 IU/L | 1 | 1 | 3 | 5 |
| C-reactive protein >1 mg/dL | 3 | 16 | 19 | |
| White blood cell count >10,000 cells/μL | 3 | 3 | 16 | 22 |
| Segment neutrophils >70% | 9 | 4 | 16 | 28 |
| Lymphocyte count <1200 cells/μL | 9 | 4 | 16 | 29 |
| None | 5 | 3 | 8 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
The following abnormal laboratory findings were commensurate with symptom severity: aspartate aminotransferase >38 IU/L, alanine aminotransferase >45 IU/L, CRP > 1 mg/dL, white blood cell count >10,000 cells/μL, segmented neutrophils >70%, and lymphocyte count <1200 cells/μL (Table 2). Complications included enterocolitis, anaphylactoid reaction, acute kidney injury, and duodenal perforation (Table 3 ). Sixteen patients (35%) were critical cases. The median recovery period was 5.0 (3.0, 7.0) days. Medical treatment included intravenous fluid resuscitation, antibiotic agents, antiviral agents, and corticosteroids. Sixteen patients (35%) received antibiotic agents, and one received antiviral agents (Table 3).
Table 3.
Complications observed in the study population (n = 46)
| Characteristics | Mild (n = 25) |
Moderate (n = 5) |
Severe (n = 16) |
Total (N = 46) |
|---|---|---|---|---|
| Complications | ||||
| Enterocolitis | 25 | 5 | 5 | 35 |
| Anaphylactoid reaction | 1 | 1 | ||
| Acute kidney injury after enterocolitis | 4 | 4 | ||
| Duodenal perforation | 1 | 1 | ||
| Acute exacerbation of chronic obstructive pulmonary disease | 1 | 1 | ||
| Spontaneous bacterial peritonitis | 1 | 1 | ||
| Acute hepatitis | 1 | 1 | ||
| Acute cholangitis | 1 | 1 | ||
| Meningitis | 1 | 1 | ||
| Recovery period, days, median [IQR] | 3 [3, 5] | 5 [5, 5] | 7 [7, 8] | 5 [3, 7] |
| Medical treatment | ||||
| Intravenous fluid resuscitation | 25 | 5 | 16 | 46 |
| Antibiotic agent | 3 | 5 | 8 | 16 |
| Antiviral agent | 1 | 1 | ||
| Corticosteroids | 2 | 2 |
IQR, interquartile range.
4. Discussion
SARS-CoV-2 causes digestive complications and can invade several digestive organs by binding to angiotensin-converting enzyme-2 receptors, abundant in several gastrointestinal organs [23]. Patients with COVID-19 frequently experience digestive symptoms such as abdominal pain, vomiting, anorexia, diarrhea, and nausea [24,25]. Additionally, there are reports of various complications, including acute liver disease, acute acalculous cholecystitis, acute pancreatitis, intestinal obstruction, acute colon pseudoobstruction, and mesenteric ischemia [[26], [27], [28], [29], [30]].
Adverse reactions following COVID-19 vaccination are rather similar to complications in COVID-19 patients. These have been described as “Vaccine-Induced COVID-19 Mimicry” Syndrome, a condition caused by COVID-19 vaccines [31]. However, compared to patients with COVID-19 [[32], [33], [34]], vaccinated individuals had a faster onset of symptoms, a higher rate of asymptomatic infections, and lower severity and mortality [2].
In this study, the most common digestive symptoms were abdominal pain and diarrhea, whereas the most common complication was enterocolitis. Digestive symptoms mainly occurred following administration of the first vaccine dose, with symptom onset usually occurring within one day. Among the mild cases, the proportion of women was high, and the proportion of men was high among the severe cases. AZD122 resulted in more adverse reactions after vaccination than BNT162b2. These results were quite similar to those from our previous study [22].
There was a high incidence of complications in older patients with multiple comorbidities. Patients with prerenal acute kidney injury frequently experienced diarrhea and poor oral intake.
Patients with severe complications had to be hospitalized for appropriate treatment. Anaphylactoid reaction was treated with intravenous volume infusion and epinephrine. Enteritis generally improved with adequate intravenous fluid resuscitation. Spontaneous bowel peritonitis was treated with antibiotics. Acute kidney injuries improved with fluid resuscitation. Acute cholangitis was treated with antibiotics and endoscopic retrograde cholangiopancreatography. Patients with duodenal ulcer perforation underwent laparoscopic primary repair. Severe complications were more prevalent in patients with abdominal pain, diarrhea, old age, cardiovascular disease, endocrine disease, and elevated CRP levels than those without these factors. These results were similar to those of our previous study [35].
To the best of our knowledge, this is the first study focusing on digestive symptoms after COVID-19 vaccination. However, this study has some limitations. First, it was a retrospective single-center study with a small sample size. Considering the results of our previous study [22], actual adverse events may have a higher incidence. Second, there could be difficulties in defining the causal–temporal relationship between COVID-19 vaccination and several complications. Third, only the patients who visited the ED were considered, and outpatients were not included in this study. However, the study population was well-balanced in terms of age, comorbidities, symptoms, severity, and outcomes, which facilitates the appropriate evaluation of post-vaccination adverse reactions. Therefore, future large-scale studies are required to support our findings.
5. Conclusions
We found that digestive symptoms and complications after COVID-19 vaccination are similar to those faced by real COVID-19 patients. While further research is required, our results suggest that digestive symptoms following COVID-19 vaccination can help detect severe complications requiring medical treatment.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Source of support
None.
CRediT authorship contribution statement
Dong Seok Lee: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Ji Won Kim: Writing – review & editing, Writing – original draft, Validation, Supervision, Methodology, Formal analysis, Data curation, Conceptualization. Kook Lae Lee: Validation, Supervision, Methodology, Data curation. Yong Jin Jung: Validation, Supervision, Methodology, Data curation. Hyoun Woo Kang: Validation, Supervision, Methodology, Data curation.
Declaration of Competing Interest
None.
Acknowledgments
We would like to thank Editage (https://www.editage.co.kr) for the assistance in English language editing.
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboardhttps://covid19.who.int/, [accessed 21 August, 2021].
- 2.Swan D.A., Bracis C., Janes H., Moore M., Matrajt L., Reeves D.B., et al. COVID-19 vaccines that reduce symptoms but do not block infection need higher coverage and faster rollout to achieve population impact. Sci Rep. 2021;11(1):1–9. doi: 10.1038/s41598-021-94719-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Coronavirus disease (COVID-19): Vaccineshttps://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines, [accessed 10 September, 2021].
- 4.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein N.P., Lewis N., Goddard K., Fireman B., Zerbo O., Hanson K.E., et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021 doi: 10.1001/jama.2021.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliver S.E., Gargano J.W., Marin M., Wallace M., Curran K.G., Chamberland M., et al. The advisory committee on immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1922–1924. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paul-Ehrlich-Institut. Safety of COVID-19 Vaccineshttps://www.pei.de/EN/newsroom/dossier/coronavirus/medicine-safety.html, [accessed 21 August 2021].
- 9.The Norwegian Medicines Agency. Reported suspected adverse reactions of covid-19 vaccineshttps://legemiddelverket.no/english/covid-19-and-medicines/vaccines-against-covid-19/reported-suspected-adverse-reactions-of-covid-19-vaccines, [accessed 19 Aug, 2021].
- 10.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. The Lancet. 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goss A.L., Samudralwar R.D., Das R.R., Nath A. ANA investigates: neurological complications of COVID-19 vaccines. Ann Neurol. 2021;89(5):856. doi: 10.1002/ana.26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimabukuro T.T., Cole M., Su J.R. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US—December 14, 2020-January 18, 2021. JAMA. 2021;325(11):1101–1102. doi: 10.1001/jama.2021.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coto-Segura P., Fernández-Prada M., Mir-Bonafé M., García-García B., González-Iglesias I., Alonso-Penanes P., et al. Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: report of four cases and review of the literature. Clin Exp Dermatol. 2021 doi: 10.1111/ced.14835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebedev L., Sapojnikov M., Wechsler A., Varadi-Levi R., Zamir D., Tobar A., et al. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021 doi: 10.1053/j.ajkd.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cari L., Fiore P., Naghavi Alhosseini M., Sava G., Nocentini G. Blood clots and bleeding events following BNT162b2 and ChAdOx1 nCoV-19 vaccine: an analysis of European data. J Autoimmun. 2021;122 doi: 10.1016/j.jaut.2021.102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pottegård A., Lund L.C., Karlstad Ø., Dahl J., Andersen M., Hallas J., et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. Bmj. 2021;373 doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prevention CfDCa. Selected Adverse Events Reported after COVID-19 Vaccinationhttps://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html, [accessed 20 December, 2021].
- 18.Centers for Disease Control and Prevention. Different COVID-19 Vaccineshttps://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html, [accessed 3 August, 2021].
- 19.Gilbert E.H., Lowenstein S.R., Koziol-McLain J., Barta D.C., Steiner J. Chart reviews in emergency medicine research: where are the methods? Ann Emerg Med. 1996;27(3):305–308. doi: 10.1016/S0196-0644(96)70264-0. [DOI] [PubMed] [Google Scholar]
- 20.ICD-10International Statistical Classification of Diseases and Related Health Problems10th Revision (ICD-10 Version)https://icd.who.int/browse10/2019/en;, [accessed 21 August 2021]
- 21.Adverse reactions in vaccine clinical trials. Severity Assessment Guidelineshttps://www.mfds.go.kr/brd/m_1060/view.do?seq=14891, [accessed 30 August 2021].
- 22.Lee D.S., Kim J.W., Lee K.L., Jung Y.J., Kang H.W. Adverse events following coronavirus disease 2019 vaccination in South Korea between February 28 and August 21, 2021: a nationwide observational study. Int J Infect Dis. 2022 doi: 10.1016/j.ijid.2022.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ni W., Yang X., Yang D., Bao J., Li R., Xiao Y., et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24(1):422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva F., Brito B.B., Santos M.L.C., Marques H.S., Silva Júnior R.T.D., Carvalho L.S., et al. COVID-19 gastrointestinal manifestations: a systematic review. Rev Soc Bras Med Trop. 2020;53 doi: 10.1590/0037-8682-0714-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaafarani H.M.A., El Moheb M., Hwabejire J.O., Naar L., Christensen M.A., Breen K., et al. Gastrointestinal complications in critically ill patients with COVID-19. Ann Surg. 2020;272(2) doi: 10.1097/sla.0000000000004004. e61-e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao X., Lei Z., Gao F., Xie Q., Jang K., Gong J. The impact of coronavirus disease 2019 (COVID-19) on liver injury in China: a systematic review and meta-analysis. Medicine (Baltimore) 2021;100(4) doi: 10.1097/md.0000000000024369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thuluva S.K., Zhu H., Tan M.M.L., Gupta S., Yeong K.Y., Cheong Wah S.T., et al. A 29-year-old male construction worker from India who presented with left- sided abdominal pain due to isolated superior mesenteric vein thrombosis associated with SARS-CoV-2 infection. Am J Case Rep. 2020;21 doi: 10.12659/ajcr.926785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balaphas A., Gkoufa K., Meyer J., Peloso A., Bornand A., McKee T.A., et al. COVID-19 can mimic acute cholecystitis and is associated with the presence of viral RNA in the gallbladder wall. J Hepatol. 2020;73(6):1566–1568. doi: 10.1016/j.jhep.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schepis T., Larghi A., Papa A., Miele L., Panzuto F., De Biase L., et al. SARS-CoV2 RNA detection in a pancreatic pseudocyst sample. Pancreatology. 2020;20(5):1011–1012. doi: 10.1016/j.pan.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kowarz E., Krutzke L., Reis J., Bracharz S., Kochanek S., Marschalek R. “Vaccine-induced Covid-19 mimicry” syndrome: splice reactions within the SARS-CoV-2 spike open reading frame result in spike protein variants that may cause thromboembolic events in patients immunized with vector-based vaccines. Res Square. 2021 doi: 10.21203/rs.3.rs-558954/v1. [DOI] [Google Scholar]
- 32.Zayet S., Kadiane-Oussou N.J., Lepiller Q., Zahra H., Royer P.Y., Toko L., et al. Clinical features of COVID-19 and influenza: a comparative study on Nord Franche-Comte cluster. Microbes Infect. 2020;22(9):481–488. doi: 10.1016/j.micinf.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China Jama. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stokes E.K., Zambrano L.D., Anderson K.N., Marder E.P., Raz K.M., El Burai Felix S., et al. Coronavirus Disease 2019 Case Surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(24):759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bishehsari F., Adnan D., Deshmukh A., Khan S.R., Rempert T., Dhana K., et al. Gastrointestinal symptoms predict the outcomes from COVID-19 infection. J Clin Gastroenterol. 2022;56(2) doi: 10.1097/mcg.0000000000001513. e145-e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.