Abstract
Objectives
The aim of this study was to assess the immunogenicity of SARS-CoV-2 available vaccines among people living with HIV (PLWH) after a complete vaccination scheme, and determine predictors of seroconversion.
Methods
This multicentre prospective cohort study included 420 PLWH who had received a standard immunization, either with mRNA or adenoviral-vectored COVID-19 vaccines. Antibody response was evaluated within 1 to 2 months after the last dose of the vaccine with a quantitative determination of antitrimeric spike protein-specific IgG antibodies and IgG neutralizing antibodies.
Results
Overall, 384 of 420 PLWH (91%) showed antibody response to vaccination. Seroconversion was observed in 308 of 326 individuals with cluster of differentiation 4 (CD4) counts ≥350 cells/mm3 (95%), 55 of 61 PLWH with 200 to 349 cells/mm3 (90%), and 21 of 33 PLWH with CD4 counts <200 cells/mm3 (64%; p < 0.001). The median log10 IgG neutralization levels were 2.4 IU/mL (Q1–Q3, 1.0–3.1) among PLWH with CD4 counts <200 cells/mm3, 3.1 IU/mL (Q1–Q3, 2.8–3.4) for the 200 to 349 cells/mm3 group, and 3.1 IU/mL (Q1–Q3, 2.7–3.4) for PLWH with CD4 counts ≥350 cells/mm3 (p = 0.016). In the multivariate analysis, CD4 counts ≥350 cells/mm3 (OR: 7.10; 95% CI, 1.91–26.46; p = 0.004) and receiving mRNA-vectored COVID-19 vaccines (OR: 8.19; 95% CI, 3.24–20.70; p ≤ 0.001) were independently associated with a higher probability of response to vaccination.
Discussion
HIV-related immunosuppression impairs the antibody response to SARS-CoV-2 vaccines. Specific vaccination schemes should be urgently tailored in this setting, particularly in patients with CD4 cell counts <200 cells/μL. Adenoviral-vectored vaccines should be avoided in PLWH whenever possible.
Keywords: CD4 T-cell counts, Humoral response, People living with HIV, SARS-CoV-2, Vaccine
Introduction
Since the onset of the SARS-CoV-2 pandemic in December 2019, more than 332.6 million cumulative cases and 5.55 million deaths worldwide have been reported, including people living with HIV (PLWH) [1]. Despite the availability of several vaccines, the pandemic remains difficult to control. Currently available SARS-CoV-2 vaccines show high efficacy in terms of prevention of severe COVID-19, hospitalization, and COVID-19-related death [[2], [3], [4]]. However, the efficacy of these vaccines in PLWH has not been entirely well-established to date. Only a few clinical trials have included PLWH, mostly with high cluster of differentiation 4 (CD4) cell counts and suppressed HIV viremia achieved with antiretroviral therapy (ART) [3,4]. More recently, preliminary observational studies have suggested that, in well-controlled PLWH, immunization rates after mRNA vaccines delivery were similar to those observed in the general population [5,6]. However, the results of these studies may be limited because of their low sample size and, especially, because PLWH with low CD4 cell counts were not included. Therefore, the available data are insufficient and may not be accurate.
Likewise, there is no available information on how specific factors may affect the performance of vaccination in PLWH. Despite ART, the immune dysfunction associated with HIV infection may not be completely reversed [7]. For this reason, SARS-CoV-2 vaccination might result in immune responses of lesser magnitude and/or persistence among PLWH, as seen in the setting of other vaccine-eligible diseases, especially in individuals with severe immunosuppression [8]. In addition, the type of vaccine and the presence of specific comorbidities might preclude the development of a protective immunological response. Hence, there is an urgent need to characterize the immune response and correlates of vaccine efficacy in PLWH, and more specifically in those with a more advanced immune deficiency.
Thus, the aim of this study was to assess the immunogenicity of available SARS-CoV-2 vaccines among PLWH after a complete vaccination scheme, and determine factors associated with seroconversion.
Methods
Study design and patients
This multicentre prospective cohort study of PLWH was conducted at the units of infectious diseases of three university hospitals in Southern Spain from January to December 2021. PLWH were invited to participate if they met the following inclusion criteria: Age >18 years and complete immunization scheme, either with mRNA or adenovirus-vectored COVID-19 vaccines. Blood samples were obtained from all participants between 4 and 8 weeks after the last dose of the COVID-19 vaccine. Patients with documented prior SARS-CoV-2 natural infection diagnosed by PCR, antigen detection, or serology were excluded.
Vaccination schemes
Immunization was carried out according to the national recommendations in force [9]. Vaccination schemes were considered complete when patients received either two doses of the Pfizer-BioNTech mRNA vaccine (BNT162b2), Moderna (mRNA-1273 Spikevax), or adenovirus-vectored Oxford-AstraZeneca vaccine (ChAdOx1 nCoV-19; AZD1222), or one dose of the adenovirus-vectored COVID-19 Janssen vaccine (Ad26.COV2.S).
Outcomes and definitions
The main outcome of this study was the presence of specific IgG antibodies against the spike protein (anti-S) of SARS-CoV-2 ≥33.8 binding antibody units per mL (BAU/mL) [10]. Seroconversion was defined as the detection of anti-S levels above this cut-off point. All patients who did not reach this anti-S level after a complete immunization scheme were considered nonresponders to vaccination. Additionally, levels of anti-S and IgG neutralization antibodies within the spike protein encoded by vaccines after vaccination were determined. PLWH were stratified according to CD4 cell counts, evaluated within 3 months before vaccination, in three groups: <200 cells/m3, 200 to 349 cells/m3, and ≥350 cells/m3. Comorbidities were evaluated from patients' electronic clinical records at each centre. Chronic kidney disease was defined as glomerular filtration rate <35 mL/min/1.73 m2 for ≥3 months, irrespective of cause.
Laboratory procedures
To rule out natural infection, all patients were tested every 6 months since the onset of the COVID-19 pandemic for SARS-CoV-2 total antibodies (including IgG) against the nucleocapsid protein (anti-N) by electrochemiluminescence immunoassay (ELECSYST Anti-SARS-CoV-2; Roche Diagnostic International) as routine clinical follow up. Measurement of anti-N was also performed at the time of vaccination response assessment. Titres of SARS-CoV-2 spike binding antibodies against the subunits S1 and S2 of the virus spike protein were measured with chemiluminescent immunoassay automated equipment (LIAISONSARS-CoV-2 IgG kit; DiaSorin, Saluggia, Italy) on a LIAISON XL analyzer in accordance with the manufacturer's instructions. The immunogenicity results are reported as an international standard unit (BAU/mL for binding assay formats).
Specificity and sensitivity were 100% (95% CI, 95%–100%) and 91% (95% CI, 79%–96%) using the cut-off point provided by the manufacturer [10]. Serum IgG neutralizing antibodies were analyzed using a COVID-19 Spike Quantitative Virclia IgG Monotest (VIRCELL, S.L., Granada, Spain). The assay is an indirect chemiluminescent immunoassay to test IgG antibodies in SARS-CoV-2 Spike, used as a surrogate marker of neutralization, with a specificity and sensitivity of 100% (95% CI, 96–100%) 99% (95% CI, 92–100%) against a neutralization assay [11].
Statistical analysis
First, the descriptive analyses were performed. Continuous variables were expressed as median (Q1–Q3) and categorical variables as frequencies (percentage). The rate of COVID-19 vaccine responders was calculated by dividing the number of responders by the total number of PLWH included in the study. Continuous variables were categorized by the median value or clinically relevant cut-off points. Anti-S and neutralization IgG level values were log transformed for the analysis. Frequencies were compared with the ꭓ2 or Fisher's test when there was at least one cell with an expected frequency lower than five. The Mann–Whitney U test was used to compare continuous variables. A one-way analysis of variance was used for comparisons among three or more groups. In addition, a post hoc analysis was performed for pairwise group comparisons. A multiple-comparisons correction was performed with the Bonferroni or Tamhane method. The correlation between anti-S and neutralization IgG was assessed with a Spearman correlation analysis. Differences were considered statistically significant for p values ≤ 0.05.
A multivariable binary logistic regression was used to assess risk factors associated with no response to COVID-19 vaccination. Baseline variables that were significantly different among the CD4 cell counts strata, along with factors associated with no response to COVID-19 vaccination, in the bivariable analysis with a p value < 0.2 were included in the multivariable model, along with age and sex.
All data analyses were performed using the SPSS statistical software package, version 25.0 (IBM, Armonk, NY) and Stata 15.0 Statistics/Data Analysis (StataCorp, College Station, TX).
Ethics
This study was conducted according to the Helsinki declaration, and approved by the ethics committee of the Hospital Universitario Virgen de Valme. All patients gave written informed consent before being recruited in this study.
Results
Characteristics of the study population
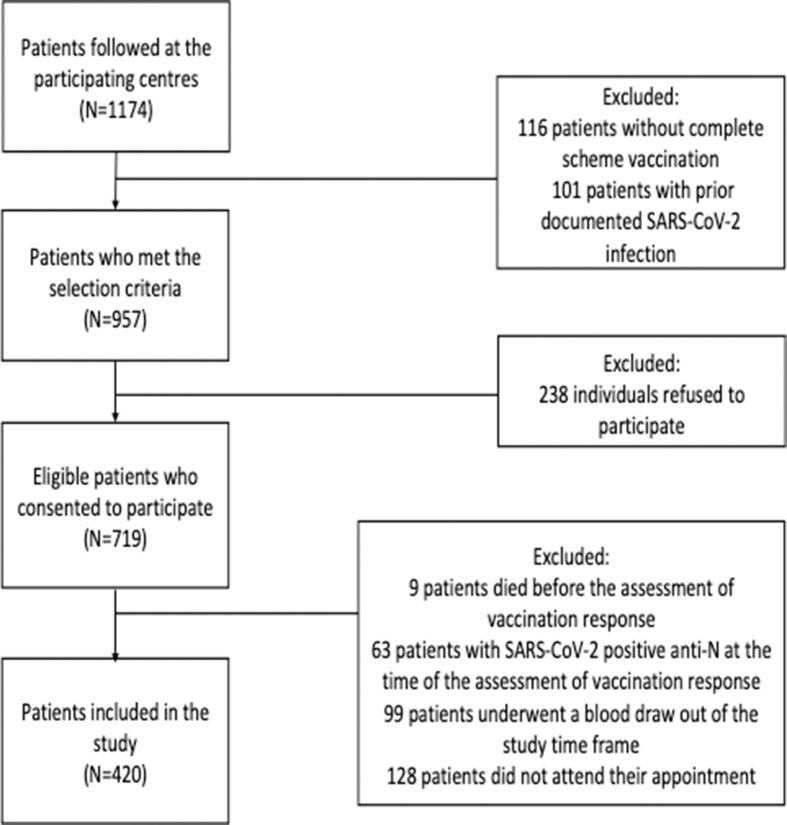
Supplementary Fig. 1 depicts the study flowchart. A total of 420 PLWH were included in this study. The main features of the study population are displayed in Table 1 . All participants were receiving ART at the time of SARS-CoV-2 vaccination, and 362 PLWH (86%) had a plasma HIV-RNA load of <50 cp/ml. The median CD4 cell count was 586 cells/mm3 (Q1–Q3, 380–786). Thirty-three PLWH (8%) showed CD4 cell counts <200 cells/mm3, and 114 PLWH (27%) had CD4 cell counts <350 cel/mm3. PLWH with CD4 cell counts <200 cells/mm3 more frequently had chronic kidney disease, lower nadir CD4 cell counts, and detectable HIV viremia (Table 1).
Table 1.
Characteristics of the study population (N = 420)
| Parameter, n (%) | CD4 cell counts <200 cells/mm3 (n = 33) | CD4 cell counts 200-349 cells/mm3 (n = 61) | CD4 cell counts ≥350 cells/mm3 (n = 326) | Overall (N = 420) | Univariate p value |
|---|---|---|---|---|---|
| Male sex | 28 (85) | 49 (80) | 266 (82) | 343 (82) | 0.862 |
| Age, ya | 56 (51–61) | 56 (52–62) | 55 (48–60) | 55 (49–60) | 0.466 |
| Injection drug use | 15 (47) | 37 (61) | 107 (33) | 159 (38) | <0.001 |
| Cirrhosis | 6 (18) | 17 (28) | 24 (7) | 47 (11) | <0.001 |
| Chronic kidney disease | 4 (12) | 1 (2) | 5 (2) | 10 (2) | <0.001 |
| Immunosuppressive therapy | 1 (3) | 3 (5) | 9 (3) | 13 (3) | 0.672 |
| Charlson indexa | 4 (2–7) | 2 (1–4) | 2 (0–3) | 2 (1–3) | <0.001 |
| CDC clinical category A | 4 (14) | 28 (48) | 196 (62) | 228 (57) | <0.001 |
| Nadir CD4 cell counts, cells/mm3a | 30 (9–71) | 127 (47–253) | 270 (112–421) | 223 (70–376) | <0.001 |
| Plasma HIV-RNA <50 c/mL | 23 (70) | 52 (87) | 289 (93) | 362 (87) | <0.001 |
Data are number (%) of participants. CD4, cluster of differentiation 4.
Median.
SARS-CoV-2 vaccination and antibody response in PLWH
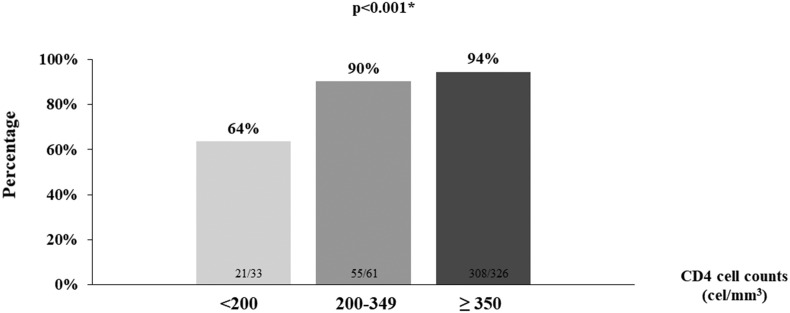
Most patients were immunized with mRNA vaccines (Table 2 ). The proportion of individuals who received SARS-CoV-2 mRNA vaccination was higher among PLWH with CD4 cell counts <200 cells/mm3 than in patients with CD4 cell counts above this cutoff point (Table 2). Overall, 351 PLWH (91.2%) showed seroconversion after a complete vaccination scheme. When accounting for type of vaccine, a greater proportion of PLWH seroconverted after receiving mRNA vaccination compared with those who were immunized with the adenovirus-vectored COVID-19 vaccine (Table 3 ). Seroconversion was more common in individuals with CD4 cell count >350 cells/mm3, followed by patients with cell counts of 200 to 349 cells/mm3, than among PLWH with CD4 cell counts <200 cells/mm3 (Fig. 1 ).
Table 2.
Distribution of SARS-CoV-2 vaccines by CD4 cell counts (N = 420)
| Parameter, n (%) | CD4 cell counts <200 cells/mm3 (n = 33) | CD4 cell counts 200-349 cells/mm3 (n = 61) | CD4 cell counts ≥350 cells/mm3 (n = 326) | Overall (N = 420) | p valuea |
|---|---|---|---|---|---|
| mRNA vaccines (BNT162b2 COMIRNATY & mRNA-1273 Spikevax) | 28 (90) | 43 (74) | 239 (79) | 310 (79) | 0.198 |
| Adenovirus vaccines (ChAdOx1 nCoV-19 & Ad26.COV2.S) | 3 (10) | 15 (26) | 63 (21) | 81 (21) |
Data are number (%) of participants. CD4, cluster of differentiation 4.
Comparison of frequencies among CD4 cell count groups.
Table 3.
Predictors of antibody response to vaccination among people living with HIV (N = 420)
| Variables | Categories | Response to vaccination, n (%) | Bivariable p-value | OR (95% CI) | Multivariable p-value |
|---|---|---|---|---|---|
| Sex | Male; female | 316 (92); 68 (88) | 0.280 | Ref; 0.64 (0.24–1.75) | 0.383 |
| Age, years | < 55; ≥ 55 | 178 (90); 206 (93) | 0.290 | 1.04 (1.00–1.08)d | 0.040 |
| HIV infection way | Injection; noninjection drug user | 144 (91); 240 (92) | 0.622 | 1.47 (0.55–3.90); Ref | 0.441 |
| CDC clinical category | A, B, or C | 220 (97); 154 (88) | <0.001 | 2.56 (0.98–6.65); Ref | 0.054 |
| Nadir CD4 cell counts (cells/mm3)a | <200; ≥200 | 143 (88); 188 (97) | 0.001 | ―b | — |
| Charlson index | <2; ≥2 | 170 (93); 214 (90) | 0.345 | — | — |
| Cirrhosis | No; yes | 343 (89); 41 (87) | 0.270 | 1.59 (0.43–5.86); Ref | 0.489 |
| Chronic kidney disease | No; yes | 375 (98); 9 (90) | 0.596 | ―c | — |
| Immunosuppressive therapy | No; yes | 373 (92); 11 (85) | 0.297 | — | — |
| CD4 cell counts, cells/mm3 | <200; 200–349; ≥350 | 21 (64); 55(90); 308 (95) | <0.001 | Ref; 3.94 (0.84–18.53) 7.10 (1.91–26.46) | 0.084; 0.004 |
| Plasma HIV-RNA, c/mL | <50; ≥50 | 336 (92); 33 (81) | 0.019 | 0.98 (0.27–3.52); Ref | 0.973 |
| Vaccine | mRNA; adenovirus | 292 (94); 63 (78) | <0.001 | 8.19 (3.24–20.70); Ref | <0.001 |
Table shows patient characteristics associated with a greater probability of seroconverting after a complete immunization scheme against SARS-CoV-2. For the bivariate analysis, continuous variables were categorized according to the median value or using clinically significant cut-off points. Variables associated with the main endpoint in the bivariate analysis with p < 0.05, along with baseline characteristics that showed significant differential distribution among CD4 strata, were entered in a multivariate analysis, and a logistic binary regression model was conducted. Age was entered as a continuous variable, and all other parameters were entered as categorical variables. The model was built using an automatic procedure. The validity of the final model was assessed by estimating goodness of fit with the Hosmer–Lemeshow test. Results are expressed as OR and their 95% CI. In total, six variables, along with age and sex, were included in the final model. The Hosmer–Lemeshow test was used for goodness of fit for logistic regression with p = 0.676. Ref, reference.
Available for 357 patients.
The parameter nadir CD4 cell count was not entered in the model to avoid overfitting. Instead, CD4 cell count at the time of vaccination was selected, because this parameter is a strong predictor of response in the setting of other vaccines in people living with HIV.
Not entered in the model because of the small number of cases.
Per 1 year increase (included as continuous variables in multivariate model).
Fig. 1.
Proportion of people living with HIV who seroconvert after COVID vaccination, by cluster of differentiation 4+ cell count (N = 420).
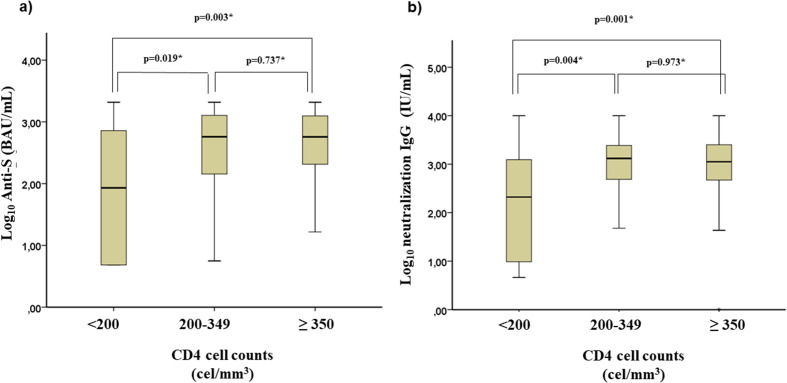
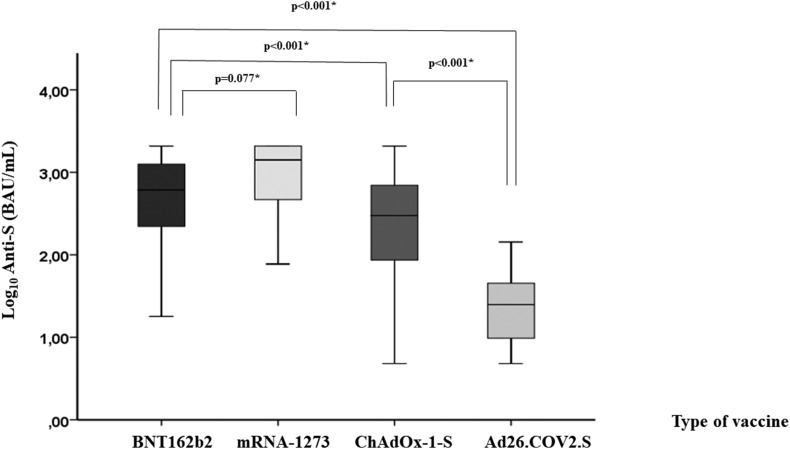
The level of serum antibody reached was associated with CD4 cell counts (Fig. 2 ). Hence, the group with the lowest CD4 cell counts had reduced IgG anti-S levels compared with those in the other two groups (median log10 IgG anti-S: 1.9 BAU/mL (Q1–Q3, 0.7–3.1) vs. 2.8 BAU/mL (Q1–Q3, 2.2–3.1; p = 0.041) and 2.7 BAU/mL (Q1–Q3, 2.3–3.1; p = 0.008) for the 200 to 349 cells/mm3 and ≥350 cells/mm3 groups, respectively; Fig. 2A). Likewise, neutralization IgG levels were lower among PLWH with severe immunosuppression (median log10 neutralization IgG: 2.4 IU/mL (Q1–Q3, 1.0–3.1) vs. 3.1 IU/mL (Q1–Q3, 2.8–3.4; p = 0.013) and 3.1 IU/mL (Q1–Q3, 2.7–3.4; p = 0.003) for the 200 to 349 cells/mm3 and ≥350 cells/mm3 groups, respectively; Fig. 2B). A high correlation was observed between anti-S and neutralization IgG levels (Spearman's rho: 0.844; p < 0.001). Finally, IgG anti-S levels were higher after an mRNA-based vaccination scheme compared with those reached with adenovirus-vector COVID-19 vaccines (median log10 IgG anti-S: 2.9 BAU/mL (Q1–Q3, 2.4–3.2) vs. 2.2 BAU/mL (Q1–Q3, 1.6–2.7; p < 0.001; Fig. 3 ).
Fig. 2.
Levels of A) IgG antibodies against the spike protein by cluster of differentiation 4+ cell count (N = 420); and B) neutralization IgG by cluster of differentiation 4+ cell count (N = 420).
Fig. 3.
Levels of IgG antibodies against the spike protein by type of vaccine received (N = 420).
Predictors of seroconversion after SARS-CoV-2 vaccination among PLWH
In the bivariate analysis, predictors of seroconversion after SARS-CoV-2 immunization were CDC clinical category, nadir CD4 cell counts, CD4 cell counts, and plasma HIV RNA before vaccination, as well as type of vaccine (Table 3). In the multivariate analysis, age, type of vaccine, and CD4 cell counts before vaccination were predictors of seroconversion after SARS-CoV-2 complete vaccination scheme (Table 3).
Discussion
This study suggests that, among PLWH with low CD4 cell counts, COVID-19 vaccine humoral responses is reduced with standard immunization schemes. In contrast, among individuals with CD4 counts >200 cells/mm3, the rates of seroconversion after COVID-19 immunization are high. Additionally, vaccination elicited robust humoral response in this subset. Finally, mRNA vaccines seem to be more immunogenic than adenovirus-vectored COVID-19 vaccines, also in PLWH.
Characterizing the response to SARS-CoV-2 vaccination among PLWH is essential to design effective vaccination programs. PLWH have an elevated risk of severe COVID-19 disease, which is related to CD4 cell counts and HIV viral load [12]. For this reason, PLWH, and particularly those with severe immunosuppression, are prioritized for SARS-CoV-2 vaccination [9]. Despite efficient virological suppression and immune recovery with normalization of CD4 cell counts on ART, immune dysfunction, chronic HIV-related inflammation, and T-cell senescence may persist [7]. Particularly, among PLWH, low CD4 cell counts are commonly associated with a poorer response to vaccination against other preventable diseases [8], and immunization schemes must be boosted in this population to achieve immune protection. To date, information on COVID-19 vaccination response in this subset is scant. Several studies have examined the ability of the Pfizer-BNT162b2 mRNA or ChAdOx1 nCoV-19 vaccine to elicit an immune response to SARS-CoV-2 in PLWH. Most studies concluded that well-controlled PLWH mount similar immune responses as HIV-negative controls individuals [5,6,[13], [14], [15]].
Nevertheless, there is some controversy regarding the effect of CD4 cell counts on the response induced by those vaccines. One study found a similar anti-SARS-CoV-2 binding antibody response in PLWH with CD4 counts <300 cells/μL compared with HIV-negative individuals [15], but other studies showed a considerably weaker response among PLWH with some degree of immunosuppression [[16], [17], [18], [19], [20]]. The main limitation of these studies was the small number of participants with low CD4 cell counts. In the present study, the frequency of nonseroconverters was slightly higher among PLWH with CD4 cell counts <350 cells/mm3 and dramatically increased among those with CD4 cell counts <200 cells/mm3. In addition, levels of anti-S and neutralization IgG were significantly lower in the group of PLWH with CD4 <200 cells/mm3. Conversely, no significant differences were found when comparing individuals with 200 to 350 cells/mm3 with patients with ≥350 cells/mm3. Our results are in agreement with those of previous studies of PLWH with high CD4 cell counts. In addition, we provide data on the poorer response to vaccination of PLWH with CD4 cell counts <200 cells/mm3.
The effect of HIV status on the protection mediated by the adenovirus-vectored vaccine is not fully known. Despite promising data from a phase 2/3 clinical trial [13], results should be interpreted with caution because the sample size was small and PLWH with severe immunosuppression were exclude from the study. In the present work, PLWH who received adenovirus-vectored vaccines were less likely to seroconvert after a full immunization scheme. Again, because of the observational study design, we must be cautious in the interpretation of these data. Nonetheless, a recent meta-analysis revealed that, among the general population, BNT162b2 and mRNA-1273 vaccines were associated with the highest efficacy to prevent symptomatic COVID-19 compared with other vaccines [21].
There is a substantial body of evidence for a strong humoral and cellular response after SARS-COV-2 vaccination that may persist over many months [22], which also seems to occur in well-controlled PLWH [23]. Besides, high antibody levels were recently demonstrated to correlate with strengthened vaccine efficacy and a decreased risk of COVID-19, mainly mediated by neutralizing antibodies [24]. In that study, IgG anti-S and neutralization IgG levels were also strongly correlated. In the present work, a high correlation was also observed between anti-S and neutralization IgG levels. Consequently, in many viral diseases [25], antibody determination could be an accessible biomarker to assess individual risks. Thus, such determinations could easily allow for the identification of individuals who may require tailored immunization strategies to achieve long-term protection. These individuals might benefit from receiving additional vaccine doses, which has demonstrated in a preliminary study to strongly boost humoral response in PLWH with advanced disease [26]. In addition, antibody determination may be helpful to establish immunization priorities, particularly in subsets with supply shortage of COVID-19 vaccine.
This study has some limitations. First, because of its observational nature, there might be a risk of unbalanced groups for comparison. However, after adjusting for variables associated with seroconversion and those different among CD4 cell counts strata, CD4 cell counts were independent predictors of seroconversion after vaccination. Second, cellular immune response was not assessed. For SARS-CoV-2, as well as other respiratory viruses, coordination between humoral and cellular responses is pivotal to control and clear SARS-CoV-2 [22]. In this sense, antibody titres might be underestimating the potential breadth of the immune response to SARS-CoV-2 [27]. Nevertheless, in PLWH, a correlation has been observed between the CD4/CD8 ratio and the magnitude of T-cell responses against SARS-CoV-2 [28]. Additionally, a preliminary study has shown that cellular-mediated response after COVID-19 immunization in PLWH with CD4 cell counts <200 cells/mm3 is poorer than in PLWH with CD4 cell counts >500 cells/mm3 [23]. Finally, the size of the group with CD4 cell counts <200 cells/mm3 was small. However, this is fortunately the current scenario among PLWH under ART [29]. In this sense, to our knowledge, this is the first study to include a high number of PLWH with current low CD4 cell counts who were vaccinated with available mRNA or adenoviral-vectored COVID-19 vaccines. Additionally, prior SARS-CoV-2 infection was ruled out by testing the entire cohort every 6 months for anti-N. Given that PLWH have a lower probability of showing detectable anti-N than people without HIV infection after COVID-19 [30], performing a single anti-N test may lead to an underestimation of prior COVID-19. These are strengths of this study.
Conclusions
Available COVID-19 vaccines elicit robust humoral responses in well-controlled PLWH, especially those who have received mRNA vaccines. However, individuals with CD4 cell counts <200 cells/mm3 are less likely to respond to a complete immunization scheme, which might put them at a higher risk of a COVID-19 breakthrough infection and worse clinical outcomes. This finding supports the urgency of developing improved SARS-CoV-2 vaccination strategies for PLWH who are deeply immunosuppressed. PLWH with low CD4 cell counts could benefit from monitoring seroconversion and antibody titres to tailor vaccination schemes.
Research ethics statement
This study was conducted according to the Helsinki declaration, and approved by the ethics committee of the Hospital Universitario Virgen de Valme. All patients gave written informed consent before being recruited in this study.
Transparency declaration
The authors report no conflict of interest. This work was supported, in part, by the Spanish Network for AIDS Investigation (www.red.es/redes/inicio; RD16/0025/0040) as part of the Nacional I + D + I, ISCIII Subdirección General de Evaluación and the European Fund for Development of Regions. Juan Antonio Pineda received a research extension grant from the Programa de Intensificación de la Actividad de Investigación del Servicio Nacional de Salud Carlos III. Anaïs Corma-Gómez received a Río Hortega grant from the Instituto de Salud Carlos III (grant number CM19/00251) and research extension grant, Acción B, from the Acción para el Refuerzo de la Actividad Investigadora en las Unidades Clínicas del Servicio Andaluz de Salud 2021, Clínicos Investigadores (grant number B-0061-2021). Juan Macías received a research extension grant, Acción A, from the Acción para el Refuerzo de la Actividad Investigadora en las Unidades Clínicas del Servicio Andaluz de Salud 2021, grant number A1-0060-2021). Marta Santos received a Río Hortega grant from the Instituto de Salud Carlos III (grant number CM21/00263). Antonio Rivero-Juárez is the recipient of a Miguel Servet Research Contract awarded by the Ministerio de Ciencia, Promoción y Universidades of Spain (CP18/00111). Antionio Rivero is the beneficiary of contracts for the intensification of research activity in the public health system awarded by the Ministerio de Ciencia, Promoción y Universidades of Spain (INT20-00028).
Author contributions
Conceptualization by ACG, LMR, JM, JAP, and FG. Methodology by ACG, LMR, JM, JAP, and FG. Formal analysis by ACG, LMR, JM, and JAP. Investigation by ACG, MFF, EG, AFL, LV, and FG. Resources by ACG, MFF, EG, AFL, LV, CGA, ARJ, CD, MS, RP, AR, LMR, JM, JAP, and FG. Data curation by ACG and MFF. Writing of the original draft by ACG and JM. Review and editing by ACG, MFF, EG, AFL, LV, CGA, ARJ, CD, MS, RP, AR, LMR, JM, JAP, and FG. Visualization by ACG, MFF, and JM. Supervision by JM, JAP, LMR, and FG. Project administration by ACG, MFF, and JM. Funding acquisition by JM, JAP, and FG.
Editor: R. Chemaly
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.05.018.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Fig. S1.
References
- 1.Geretti A.M., Stockdale A.J., Kelly S.H., Cevik M., Collins S., Waters L., et al. Outcomes of coronavirus disease 2019 (COVID-19) related hospitalization among people with human immunodeficiency virus (HIV) in the ISARIC World Health Organization (WHO) Clinical Characterization Protocol (UK): a prospective observational study. Clin Infect Dis. 2021;73:e2095–e2106. doi: 10.1093/cid/ciaa1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.20900/agmr20210002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1016/j.euo.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy I., Wieder-Finesod A., Litchevsky V., Biber A., Indenbaum V., Olmer L., et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin Microbiol Infect. 2021;27:1851–1855. doi: 10.1016/j.cmi.2021.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woldemeskel B.A., Karaba A.H., Garliss C.C., Beck E.J., Wang K.H., Laeyendecker O., et al. The BNT162b2 mRNA vaccine elicits robust humoral and cellular immune responses in people living with human immunodeficiency virus (HIV) Clin Infect Dis. 2022;74:1268–1270. doi: 10.1093/cid/ciab648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paiardini M., Müller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev. 2013;254:78–101. doi: 10.1111/imr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kernéis S., Launay O., Turbelin C., Batteux F., Hanslik T., Boëlle P.Y. Long-term immune responses to vaccination in HIV-infected patients: a systematic review and meta-analysis. Clin Infect Dis. 2014;58:1130–1139. doi: 10.1093/cid/cit937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Sanidad Ministerio. Estrategia de vacunación COVID-19 en España. https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/vacunaCovid19.htm Available at:
- 10.Tré-Hardy M., Wilmet A., Beukinga I., Dogné J.M., Douxfils J., Blairon L. Validation of a chemiluminescent assay for specific SARS-CoV-2 antibody. Clin Chem Lab Med. 2020;58:1357–1364. doi: 10.1515/cclm-2020-0594. [DOI] [PubMed] [Google Scholar]
- 11.Roche Diagnostics GmbH. Elecsys anti-SARS-CoV-2 S assay. 2021. [Google Scholar]
- 12.Sun J, Patel RC, Zheng Q, Madhira V, Olex AL, Islam JY, et al. COVID-19 disease severity among people with HIV infection or solid organ transplant in the United States: a nationally-representative, multicenter, observational cohort study. medRxiv. In press. 10.1101/2021.07.26.21261028. [DOI]
- 13.Frater J., Ewer K.J., Ogbe A., Pace M., Adele S., Adland E., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV. 2021;8:e474–e485. doi: 10.1016/S2352-3018(21)00103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruddy J.A., Boyarsky B.J., Werbel W.A., Bailey J.R., Karaba A.H., Garonzik-Wang J.M., et al. Safety and antibody response to the first dose of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccine in persons with HIV. AIDS. 2021;35:1872–1874. doi: 10.1097/QAD.0000000000002945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergman P., Blennow O., Hansson L., Mielke S., Nowak P., Chen P., et al. Safety and efficacy of the mRNA BNT162b2 vaccine against SARS-CoV-2 in five groups of immunocompromised patients and healthy controls in a prospective open-label clinical trial. EBioMedicine. 2021;74:103705. doi: 10.1016/j.ebiom.2021.103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nault L., Marchitto L., Goyette G., Tremblay-Sher D., Fortin C., Martel-Laferrière V., et al. COVID-19 vaccine immunogenicity in people living with HIV-1. Vaccine. 2022;40:3633–3637. doi: 10.1016/j.vaccine.2022.04.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heftdal L.D., Knudsen A.D., Hamm S.R., Hansen C.B., Møller D.L., Pries-Heje M., et al. Humoral response to two doses of BNT162b2 vaccination in people with HIV. J Intern Med. 2022;291:513–518. doi: 10.1111/joim.13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antinori A, Cicalini S, Meschi S, Bordoni V, Lorenzini P, Vergori A et al. Immunogenicity of mRNA vaccination against SARS-CoV-2 in persons living with HIV (PLWHs) with low CD4 count or previous AIDS. Presented at: European AIDS Clinical Society 18th European AIDS Conference. London, UK; October 27–30, 2021.
- 19.Touizer E., Alrubayyi A., Rees-Spear C., Fisher-Pearson N., Griffith S.A., Muir L., et al. Failure to seroconvert after two doses of BNT162b2 SARS-CoV-2 vaccine in a patient with uncontrolled HIV. Lancet HIV. 2021;8:e317–e318. doi: 10.1016/S2352-3018(21)00099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassold N., Brichler S., Ouedraogo E., Leclerc D., Carroue S., Gater Y., et al. Impaired antibody response to COVID-19 vaccination in advanced HIV infection. AIDS. 2022;36:F1–F5. doi: 10.1097/QAD.0000000000003166. [DOI] [PubMed] [Google Scholar]
- 21.Rotshild V., Hirsh-Raccah B., Miskin I., Muszkat M., Matok I. Comparing the clinical efficacy of COVID-19 vaccines: a systematic review and network meta-analysis. Sci Rep. 2021;11:22777. doi: 10.1038/s41598-021-02321-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Milne G., Hames T., Scotton C., Gent N., Johnsen A., Anderson R.M., et al. Does infection with or vaccination against SARS-CoV-2 lead to lasting immunity? Lancet Respir Med. 2021;9:1450–1466. doi: 10.1016/S2213-2600(21)00407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cicalini S., Vergori A., Cozzi-Lepri A., Meschi S., Bordoni V., Lanini S., et al. Durability of SARS-CoV-2 mRNA vaccine immune response in PLWH with advanced disease. CROI. 2022;2:291. [Google Scholar]
- 24.Gilbert P.B., Montefiori D.C., McDermott A.B., Fong Y., Benkeser D., Deng W., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plotkin S.A. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vergori A., Cicalini S., Cozzi-Lepri A., Matusali G., Bordoni V., Lanini S., et al. Immunogenicity and reactogenicity to CVOID-19 mRNA vaccine additional dose in PLWH. CROI. 2022;2:291. [Google Scholar]
- 27.Reynolds C.J., Swadling L., Gibbons J.M., Pade C., Jensen M.P., Diniz M.O., et al. Discordant neutralizing antibody and T cell responses in asymptomatic and mild SARS-CoV-2 infection. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abf3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alrubayyi A., Gea-Mallorquí E., Touizer E., Hameiri-Bowen D., Kopycinski J., Charlton B., et al. Characterization of humoral and SARS-CoV-2 specific T cell responses in people living with HIV. Nat Commun. 2021;12:5839. doi: 10.1038/s41467-021-26137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berenguer J., Díez C., Martín-Vicente M., Micán R., Pérez-Elías M.J., García-Fraile L.J., et al. Prevalence and factors associated with SARS-CoV-2 seropositivity in the Spanish HIV research network cohort. Clin Microbiol Infect. 2021;27:1678–1684. doi: 10.1016/j.cmi.2021.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macías J., Fernández-Fuertes M., Oliver N., Corma-Gómez A., Real L.M., Pineda J.A. Lower probability of persistence of total anti-SARS-CoV-2 antibodies after COVID-19 among people living with HIV. Clin Microbiol Infect. 2022;28:755–756. doi: 10.1016/j.cmi.2022.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]