Abstract
Apart from autopsy, tissue correlates of coronavirus disease 2019 (COVID-19) clinical stage are lacking. In the current study, cutaneous punch biopsy specimens of 15 individuals with severe/critical COVID-19 and six with mild/moderate COVID-19 were examined. Evidence for arterial and venous microthrombi, deposition of C5b-9 and MASP2 (representative of alternative and lectin complement pathways, respectively), and differential expression of interferon type I–driven antiviral protein MxA (myxovirus resistance A) versus SIN3A, a promoter of interferon type I–based proinflammatory signaling, were assessed. Control subjects included nine patients with sepsis-related acute respiratory distress syndrome (ARDS) and/or acute kidney injury (AKI) pre–COVID-19. Microthrombi were detected in 13 (87%) of 15 patients with severe/critical COVID-19 versus zero of six patients with mild/moderate COVID-19 (P < 0.001) and none of the nine patients with pre–COVID-19 ARDS/AKI (P < 0.001). Cells lining the microvasculature staining for spike protein of severe acute respiratory syndrome coronavirus 2, the etiologic agent of COVID-19, also expressed tissue factor. C5b-9 deposition occurred in 13 (87%) of 15 patients with severe/critical COVID-19 versus zero of six patients with mild/moderate COVID-19 (P < 0.001) and none of the nine patients with pre–COVID-19 ARDS/AKI (P < 0.001). MASP2 deposition was also restricted to severe/critical COVID-19 cases. MxA expression occurred in all six mild/moderate versus two (15%) of 13 severe/critical cases (P < 0.001) of COVID-19. In contrast, SIN3A was restricted to severe/critical COVID-19 cases co-localizing with severe acute respiratory syndrome coronavirus 2 spike protein. SIN3A was also elevated in plasma of patients with severe/critical COVID-19 versus control subjects (P ≤ 0.02). In conclusion, the study identified premortem tissue correlates of COVID-19 clinical stage using skin. If validated in a longitudinal cohort, this approach could identify individuals at risk for disease progression and enable targeted interventions.
Most adults infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) develop minimal symptoms.1 However, following an approximately 8-day interval, a subset of individuals with mild to moderate symptoms advance to severe/critical coronavirus disease 2019 (COVID-19).1, 2, 3 Progressive respiratory failure with development of acute respiratory distress syndrome (ARDS) and acute kidney injury (AKI) characterize the highest risk for mortality.3, 4, 5 Autopsy-based studies document a strong correlation of organ dysfunction and death with systemic microvascular injury and thrombosis.2,6, 7, 8, 9, 10 These lesions occur in the context of complement activation and a proinflammatory state.2,11 Deep immune profiling suggests an “immunologic signature” linked to disease trajectory, with interferon type I (IFN-I) a prominent component.12,13 Impaired IFN-I antiviral responses, including down-regulation of the IFN-I–driven antiviral gene MX1 (myxovirus resistance 1), which encodes MxA (myxovirus resistance A), is associated with SARS-CoV-2 persistence.14 In contrast, up-regulation of MxA characterizes the limited disease progression seen in SARS-CoV-2–infected non-human primates.15
Indeed, IFN-I is a significant driver of pathology in a mouse model of SARS-CoV-2 infection,16 and SARS-CoV-1–induced respiratory failure in mice is mitigated by knockout of complement C3, despite equivalent viral loads in the lungs of wild-type and mutant mice.17 These preclinical models, along with transcriptomic studies of peripheral blood mononuclear cells and pulmonary tissue at autopsy, suggest primacy for similar postinfection events in the pathophysiology of advancing COVID-19. However, there is no direct, tissue-based premortem evidence for correlation of complement and IFN-I antiviral and regulatory signals with COVID-19 clinical stage or progression. We hypothesized that interrogation of the microvasculature from an easily accessible tissue, deltoid skin, would exhibit differential regulation of IFN-I–driven antiviral versus proinflammatory pathways, that this distinction is directly linked to SARS-CoV-2 expression, and that it will correlate with the presence of microvascular thrombi, all linked to COVID-19 clinical stage.
Materials and Methods
Study Design and Patients
This investigator-initiated, prospective case series involved a single institution. All individuals in the COVID-19 cohorts had skin and/or plasma samples collected between April 1, 2020, and December 31, 2020. Subjects were not randomly selected but referred to our group by treating physicians for evaluation of a severe/critical COVID-19 case with or without cutaneous lesions, or a mild or moderate COVID-19 case with cutaneous lesions. Clinical definitions of COVID-19 stage were adapted by the CDC from Guan et al.18 The severe/critical cases represent the first 15 SARS-CoV-2–infected patients (Cases 1 to 15) for which Hematology in-patient consult services at Weill Cornell Medicine (WCM) were approached by each patient’s treating physician to consider recommendation of additional diagnostic procedures in the setting of deteriorating clinical status. An additional patient with critical COVID-19 (Case A) was referred within the same time frame but was distinct from this case series as the patient's admission RT-PCR assay result for SARS-CoV-2 was negative but the patient was found to be COVID-19 positive 1 day later. (The patient is included for purposes of certain assays because, apart from most of our other cases, multiple premortem skin samples were available.) All patients with severe/critical COVID-19, including Case A, were in an intensive care unit setting when skin biopsies were requested. Specifically, based on a report from our group regarding microthrombi accompanied by complement deposition in the normal-appearing skin (n = 3) or lung (n = 2) of five patients with severe/critical COVID-19,2 and a study by another group showing similar complement deposition in the renal microvasculature of patients with severe/critical COVID-19,19 the treating physician requested skin biopsies to seek evidence for possible complement activation in tissue, in consideration of initiating anticomplement therapy. Deposition of complement membrane attack complex C5b-9 had previously been documented by our group in the normal-appearing deltoid skin of patients with thrombotic microangiopathies linked to dysregulation of complement activation apart from COVID-19.20,21
Clinical, laboratory, and pathologic characteristics of our three patient groups are summarized in Table 1, Table 2, Table 3, Table 4. All patients with severe and critical COVID-19 (Tables 1 and 2) had an admission respiratory tract sample positive for SARS-CoV-2 according to RT-PCR. “Severe” COVID-19 involved tachypnea plus hypoxemia (oxygen <92% in ambient air) not requiring mechanical ventilation, as represented by Cases 8 and 12. “Critical” patients, those requiring invasive mechanical ventilation, characterized the remaining 13 cases; 85% (11 of 13) of the critical cases also had an AKI (Table 1), defined as an increase in serum creatinine by ≥0.3 mg/dL within 48 hours, or an increase in serum creatinine levels to ≥1.5 times baseline within 7 days. All received hydroxychloroquine, 600 mg every 12 hours for 1 day, followed by 400 mg every 12 hours for 4 days. Cases 5, 6, 8, and 9 also received the SARS-CoV-2 antiviral drug remdesivir, 5 mg/kg intravenously daily for 10 days, and cases 3, 5, 6, 9, 11, and 14 received at least one dose of the anti–IL-6 receptor antibody tocilizumab, 400 mg or 800 mg, based on body weight. Glucocorticoids were used at variable intervals in all severe/critical patients, along with a variety of prophylactic or therapeutic anticoagulation regimens. Cases 5, 6, and 9 received the anti-C5 complement antibody eculizumab after their biopsy. Their outcomes have been reported elsewhere.22
Table 1.
Patients with Severe/Critical COVID-19: Comorbidities and Clinical Laboratory Results
| Code | Sex | Age, y | Outcome | Co-morbidities/thrombotic complications | AKI/dialysis | d-dimer, ng/mL | Fibrinogen, mg/dL | AST, U/L | ANC, /mm3 | CK U/L | LDH, U/L |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 73 | Died | Class I obesity, HTN | +/No | 40,134 | 983 | 658 | 12.6 | 1157 | 893 |
| 2 | Female | 66 | Died | None/ischemic stroke | +/Yes | 12,528 | 638 | 105 | 26.5 | 313 | 1768 |
| 3 | Female | 36 | Hospitalized >10 wk | Fibrosing mediastinitis, history of hemophagocytic lymphohistiocytosis |
– | 9307 | 971 | 58 | 34.1 | 401 | 432 |
| 4 | Male | 70 | Hospitalized >10 wk |
DM2, HTN, HLD | +/No | 1026 | 518 | 49 | 13.7 | 113 | 616 |
| 5 | F | 40 | Discharged | Overweight | – | 1196 | 699 | 161 | 12.7 | 478 | 663 |
| 6 | M | 28 | Died | Class III obesity/DVT | +/No | 12,184 | 528 | 40 | 21.3 | 912 | 891 |
| 7 | M | 52 | Discharged | Class I obesity, sickle cell anemia, pulmonary hypertension/DVT | +/Yes | 3084 | ND | 20 | 14.5 | 40 | 600 |
| 9 | M | 62 | Hospitalized >10 wk |
Class I obesity, atrial fibrillation/circuit thrombi | +/Yes | 16,585 | 908 | 204 | 22.4 | 381 | 475 |
| 10 | F | 30 | Died | Class I obesity, DM2, pulmonary atresia/circuit thrombi | +/No | 4105 | 313 | 401 | 21.2 | 895 | 1282 |
| 11 | M | 72 | Died | Class I obesity, prostate cancer | +/No | 3192 | ND | 124 | 14.7 | 203 | 600 |
| 13 | M | 43 | Hospitalized >10 wk |
Class I obesity/DVT | +/Yes | 4740 | 693 | 1474 | 17.3 | 70 | 3199 |
| 14 | M | 71 | Died | Overweight, HTN, ulcerative colitis | +/No | 7975 | ND | 72 | 16.0 | 1286 | 505 |
| 15 | F | 79 | Died | HTN, HLD, hypothyroidism | +/No | 15,500 | 610 | >6000 | 10.4 | ND | >4200 |
| A | M | 86 | Died | Non-Hodgkin lymphoma, sepsis | +/No | ND | 311 | 264 | 0.3 | ND | 2751 |
| 8 | F | 33 | Discharged | Class II obesity | – | 505 | 638 | 86 | 9.1 | 508 | 791 |
| 12 | F | 73 | Discharged | Class I obesity, DM2, HTN, HLD, atrial fibrillation | – | 18,572 | 829 | 56 | 9.9 | 308 | 512 |
Cases 8 and 12 are classified as clinically severe, and the other 13 cases as clinically critical, per CDC guidelines. Case A was also a critical coronavirus disease 2019 (COVID-19) patient. Laboratory values represent peak values. d-dimer, normal range (nl.) 0 to 229 ng/mL; fibrinogen, nl. 180 to 400 mg/dL. Obesity: overweight, body mass index (BMI) >25 kg/m2 but <30 kg/m2; class I obesity, BMI >30 kg/m2 but <35 kg/m2; class II obesity, BMI >35 kg/m2 but <40 kg/m2; and class III obesity, BMI >40 kg/m2.
F, female; M, male; +, presence of acute kidney injury (AKI); –, absence of AKI; ANC, absolute neutrophil count; AST, aspartate transaminase (nl. ≤34 U/L); CK, creatine kinase (nl. 34 to 145 U/L); DM2, type 2 diabetes; DVT, deep vein thrombosis; HLD, hyperlipidemia; HTN, hypertension; LDH, lactate dehydrogenase (nl. 118 to 230 U/L); ND, not done.
Table 2.
Patients with Severe/Critical COVID-19: Inflammatory Markers and Skin Biopsy Findings
| Code | Livedo rash, areas | CRP, mg/dL | IL-6, pg/mL | Ferritin, ng/mL | C3, mg/dL | C4, mg/dL | CH50, HU | C5b-9+ MVEC | MASP2+ MVEC | Microthrombi | MxA+ MVEC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Acral | 44.2 | 181 | 6057 | ND | ND | ND | + | + | + | – |
| 2 | Acral | 21.0 | ND | 2418 | 115 | 27.8 | ND | + | + | + | – |
| 3 | Acral | >200 | 72 | 8891 | 137 | 29.6 | 324 | + | + | – | – |
| 4 | Forearm | 12.2 | 63 | 702 | 87 | 15.7 | 106 | + | – | + | – |
| 5 | Multiple | 5.5 | ND | 3169 | 150 | 37.9 | 0 | + | + | + | + |
| 6 | – | 32.4 | ND | 4576 | 112 | 22.2 | 87 | + | ND | + | – |
| 7 | – | 144.1 | ND | 1987 | 82 | 33.4 | 88 | + | + | + | – |
| 9 | Acral | 29.1 | 428 | 3507 | 180 | 32.4 | 192 | + | ND | + | – |
| 10 | – | 176.9 | 5 | 1343 | 82 | 27.3 | 123 | + | ND | + | – |
| 11 | – | 27.4 | 14 | 4651 | 107 | 15 | 148 | + | ND | + | + |
| 13 | – | 16.1 | 53 | 2434 | 122 | 32 | 158 | + | ND | + | ND |
| 14 | – | 16.4 | 30 | ND | 113 | 43.7 | 162 | + | ND | + | – |
| 15 | – | 11.6 | ND | 2858 | 52 | 2.8 | ND | + | + | + | ND |
| 8 | – | >200 | ND | 3122 | ND | ND | ND | – | + | + | – |
| 12 | – | 186.6 | ND | 829 | 152 | 49.8 | 129 | – | ND | – | – |
+ and – symbols indicate the presence or absence, respectively, of a particular lesion, complement or myxovirus resistance A (MxA) staining, or microthrombus. IL-6, normal range (nl.) ≤5 pg/mL; ferritin, nl. 10 to 291 ng/mL. Complement factors: C3, nl. 90 to 180 mg/dL; C4, nl. 12 to 36 mg/dL; CH50, nl. 60 to 144 hemolytic units (HU). Patients 8 and 12 were severe, the rest critical.
COVID-19, coronavirus disease 2019; CRP, C-reactive protein (nl. ≤0.9 mg/dL); ND, not done.
Table 3.
Severe Disease in Non–COVID-19 Control Patients
| Code | Sex | Age, y | Primary Diagnoses | Outcome | Co-morbidities/thrombotic complications | Fibrinogen, mg/dL | AKI/dialysis | AST, U/L | ANC, /mm3 | LDH, U/L |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 65 | CMMoL, ARDS | Discharged | Allogeneic stem cell transplant, AIHA, CMV viremia, parainfluenza pneumonia | 310 | – | 154 | 2.9 | 375 |
| 2 | F | 37 | HELLP syndrome | Discharged | C-section Gestational diabetes |
176 | +/No | 266 | 16.7 | 513 |
| 3 | F | 38 | ARDS | Discharged | Pneumonia, seizures | ND | +/No | 108 | 15.2 | 1574 |
| 4 | F | 56 | AML, ARDS | Died | Allogeneic stem cell transplant, cardiogenic shock, pneumonia, coronavirus infection (not CoV-1,2), DVT, PE | 391 | +/Yes | 88 | 14.3 | 977 |
| 5 | F | 81 | IgGκ multiple myeloma, ARDS | Died | DM2, pancreatitis, atrial fibrillation, influenza A pneumonia | 525 | +/No | 94 | 11.0 | 1555 |
| 6 | F | 45 | Angioimmunoblastic T-cell lymphoma | Died | Allogeneic stem cell transplant, Pseudomonas aeruginosa sepsis, HHV6 viremia, Coombs-negative hemolytic anemia, GvHD | ND | +/Yes | 33 | 2.0 | 1549 |
| 7 | F | 72 | r/o TTP | Discharged | HTN, HLD, atrial fibrillation/ischemic stroke | ND | – | 52 | 5.1 | ND |
| 8 | M | 76 | Cryptogenic cirrhosis | Discharged | Coombs-negative hemolytic anemia | ND | – | 87 | 1.6 | 1479 |
| 9 | M | 86 | Non-Hodgkin lymphoma, recurrent | Died | Sepsis, Coombs-negative hemolytic anemia | 311 | +/No | 264 | 0.3 | 2751 |
F, female; M, male; +, presence of acute kidney injury (AKI); –, absence of AKI; AIHA, autoimmune hemolytic anemia; AML, acute myelogenous leukemia; ANC, absolute neutrophil count; ARDS, acute respiratory distress syndrome; AST, aspartate transaminase; CMMoL, chronic myelomonocytic leukemia; CMV, cytomegalovirus; CoV-1,2, coronavirus 1,2; COVID-19, coronavirus disease 2019; DM2, type 2 diabetes mellitus; DVT, deep vein thrombosis; GvHD, graft-versus-host disease; HELLP, hemolysis/elevated liver enzymes/low platelets; HHV6, human herpesvirus 6; HLD, hyperlipidemia; HTN, hypertension; LDH, lactate dehydrogenase; ND, not done; PE, pulmonary embolism; r/o, rule out; TTP, thrombotic thrombocytopenic purpura.
Table 4.
Patients with Mild or Moderate COVID-19: Comorbidities and Clinical Laboratory Results
| Code | Sex | Age, y | COVID-19 stage | CoV-2 RT-PCR/risk factor for COVID-19 | Co-morbidities | Rash, clinical description | ANC, /mm3 | d-dimer, ng/mL | C5b-9+ MVEC | Microthrombi | MxA+MVEC |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 65 | Moderate | + | HTN, HLD, DM2, ESRD, rheumatoid arthritis | Vasculitic | 14.7 | 1976 | – | – | + |
| 2 | F | 72 | Moderate | + | HTN, CAD, atrial fibrillation, systemic lupus erythematosus | Vasculitic | 3.8 | 672 | – | – | + |
| 3 | F | 58 | Moderate | + | None | Vasculitic | ND | ND | – | – | + |
| 4 | F | 48 | Mild | – | HLD, CAD | Toes, chilblains | 2.2 | ND | – | – | + |
| 5 | M | 16 | Mild | – Live-in sibling with moderate COVID-19 |
None | Toes, chilblains | ND | ND | – | – | + |
| 6 | F | 65 | Mild | Declined; exposure to meat-packing plant cases | HTN, COPD | Fingers, chilblains | ND | ND | – | – | + |
+ and – symbols indicate the presence or absence, respectively, of a particular lesion, complement or myxovirus resistance A (MxA) staining, or microthrombus.
F, female; M, male; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CoV-2, coronavirus 2; COVID-19, coronavirus disease 2019; DM2, type 2 diabetes mellitus; ESRD, end-stage renal disease; HLD, hyperlipidemia; HTN, hypertension; ND, not done.
Six mild or moderate cases of COVID-19 were referred to our Dermatology outpatient clinic because of a skin rash in the setting of symptoms consistent with SARS-CoV-2 infection (Table 4). “Mild” cases had one or more symptoms, including fever, chills, myalgia, arthralgia, headache, fatigue, cough, sore throat, nasal congestion, nausea, emesis, and diarrhea; “moderate” cases also had tachypnea and/or dyspnea. The three patients with moderate cases had lesions consistent with lymphocytic vasculitis or palpable purpura and positive SARS-CoV-2 RT-PCR findings. The three mild cases had acral chilblains in the absence of a positive SARS-CoV-2 RT-PCR result but with risk factors for SARS-CoV-2 acquisition. The absence of a positive RT-PCR test result for COVID-19 in the latter has been previously reported in individuals with respiratory symptoms, risk factors for SARS-CoV-2 exposure, and chilblains-like lesions.23
For analysis of plasma IFN-α and SIN3A (a co-repressor protein that enhances proinflammatory signaling linked to IFN-I24), samples from 12 of the patients with critical COVID-19 having undergone skin biopsy, plus an additional 20 individuals with critical COVID-19 from whom plasma but not skin biopsy samples were available, were used (n = 32; 13 female subjects, 19 male subjects). For the group with moderate COVID-19, two individuals providing skin samples, plus an additional 11 individuals from whom only plasma samples were available, were tested (n = 13; six female subjects, seven male subjects). Control subjects included 13 age-matched healthy female subjects and nine healthy male subjects.
The cohort of hospitalized patients with severe/critical non–COVID-19 represents cases from WCM Pathology Archives. Forty-four percent (four of nine) had ARDS requiring prolonged mechanical ventilation, and 67% (six of nine) had an AKI (Table 3).
Microscopic and Immunohistochemistry Studies
Routine hematoxylin and eosin staining with light microscopy, and immunohistochemistry (IHC) to assess for deposition of C5b-9 and MASP2 via a diaminobenzidine technique, were conducted on 4-mm cutaneous punch biopsy specimens, as described in detail by Magro et al.2,20,21 SIN3A deposition was explored by using a rabbit anti-human mSIN3A polyclonal IgG (ab3479, 1:750 dilution; Abcam, Cambridge, United Kingdom). Staining for SARS-CoV-2 spike protein and tissue factor (TF) expression used a rabbit polyclonal IgG (no. 3525; ProSci, Poway, CA) or goat anti-human TF antibody (R&D Systems, Minneapolis, MN) together with Nuance software (Nuance Communications, Burlington, MA) to co-localize expression of these proteins with SIN3A or cell markers. The latter involved co-staining with anti–monocyte/macrophage (CD68) and anti-endothelial cell (CD31/PECAM-1) polyclonal antibodies. Evidence for thrombosis was sought by direct visualization and staining for platelet microthrombi by using an anti-CD61 antibody. An Agilent reagent (M077701-5; Agilent Technologies, Santa Clara, CA) and paraffin-embedded tissue sections with a modified Leica protocol (Leica Microsystems, Buffalo Grove, IL) were used for IHC; this involved heat-mediated antigen retrieval with Tris-EDTA buffer (pH = 9, epitope retrieval solution 2) for 20 minutes, followed by incubation with each antibody for 15 minutes. Hematoxylin and eosin counterstain was mounted with Leica Micromount. Assessment of MxA protein involved a Vision BioSystems Define Kit (Vision BioSystems, Hingham, MA) and dilution of primary antibody to 1:1600.
C5b-9 deposition was defined as a granular and/or homogeneous pattern above background, typically endothelial and/or subendothelial based, and affecting smaller caliber vessels throughout the dermis and subcutaneous fat. An evidence-based cutoff of ≥10 C5b-9–staining vessels was considered positive, as reported by our group.25 C5b-9 deposits along the dermal–epidermal junction, basement membrane zones of the eccrine coil, or within the pilar erector muscle, dermal elastic fibers, elastic lamina of vessels, or elastic tissue surrounding lymphatics were considered nondiagnostic. For MASP-2 IHC, any positive staining of microvessels above background was recorded.
Circulating IFN-α and SIN3A Levels
IFN-α and SIN3A concentrations in plasma were assessed via ELISA [R&D Systems and BioSource (ThermoFisher Scientific, Waltham, MA), respectively] using peripheral blood collected in acid-citrate-dextrose vacutainer tubes.
Statistical Analysis
Comparison of differences in plasma-based biomarkers was calculated by using a two-tailed t-test in Prism 7 software (GraphPad, San Diego, CA). Differences in C5b-9, MASP2, MxA, and microthrombi on tissue biopsy involved χ2 analysis.
Study Approval
Use of archival pathology specimens and related case histories was covered under WCM Institutional Review Board–approved protocol 20-02021524. Clinical laboratory data were obtained through the WCM COVID-19 Institutional Data Repository. Case reports lacking identifiers are considered exempt by the WCM Institutional Review Board.
Results
Baseline Characteristics of the Study Subgroups
The severe/critical COVID-19 cohort, including Case A, had clinical features and laboratory findings consistent with established factors for advanced SARS-CoV-2 infection and a heightened risk for mortality. Specifically, co-morbidities for COVID-19 progression, including advanced age, obesity, diabetes mellitus, malignancy, hypertension, and atrial fibrillation, characterized 93% (14 of 15) of the severe/critical COVID-19 cases (Tables 1 and 2), as well as 100% (nine of nine) of the hospitalized non–COVID-19 control cases (Table 3). Sixty percent (nine of 15) were aged ≥50 years in the COVID-19 group, similar to the 67% (six of nine) aged ≥50 years among the hospitalized control subjects. Equity across the sexes was noted for the severe/critical COVID-19 group, with 53% male. This is important as there is a sexual dimorphism, at least in mice, in SIN3A expression, with normal male mice having much higher tissue levels than their female counterparts.26 Four (67%) of the six mild/moderate cases had co-morbidities linked to COVID-19 progression (Table 4). During a 6-month period of observation, seven (47%) of the 15 patients with severe/critical COVID-19 died, and an additional four patients remained hospitalized for >10 weeks (Table 1). None of the patients with mild or moderate disease progressed clinically during that period.
Biochemical and hematologic laboratory values consistent with disease severity, as established in large clinical cohorts, was also similar between the severe/critical COVID-19 cohort and the hospitalized control subjects. Specifically, peak lactate dehydrogenase levels ≥550 U/L and absolute neutrophil count ≥11.6 correlate with prolonged intensive care unit stays and mortality in COVID-1927; these values were met by 67% (10 of 15) and 80% (12 of 15) of the severe/critical COVID-19 cases, respectively (Table 1). Sixty-seven percent (six of nine tested) of the non–COVID-19 hospitalized patients also had this level of lactate dehydrogenase elevation (Table 3). None of the mild/moderate individuals tested had such abnormalities (data not shown).
Clinical thrombosis was recognized in six (40%) of 15 severe/critical COVID-19 cases during their hospitalization, three with deep vein thrombosis (Cases 6, 7, and 13), two with continuous renal replacement therapy circuit thrombosis (Cases 9 and 10), and one with stroke (Case 2) (Table 1). Organ and tissue-based abnormalities indicative of a hypercoagulable state in COVID-19, including elevation of aspartate transaminase and creatine kinase ≥2 times the upper limit of normal, were observed in 67% (10 of 15) and 71% (10 of 14 tested) of the severe/critical COVID-19 cases, respectively (Table 1). Aspartate transaminase elevations were noted in 67% (six of nine) of the hospitalized non–COVID-19 control subjects, 22% of whom (two of nine) had clinically recognized thrombosis, one with deep vein thrombosis and pulmonary embolism (Case 4) and one with stroke (Case 7) (Table 3).
Biomarkers indicative of a coagulopathy included d-dimer and fibrinogen levels. d-dimer levels were elevated to high in the severe/critical COVID-19 cohort, with a mean of 10,042 ng/mL (range, 505 to 18,572 ng/mL; normal range, 0 to 229 ng/mL) (Table 1). D-dimer information was not available for the hospitalized non–COVID-19 control subjects. However, fibrinogen levels correlate with incidence of thrombosis in both COVID-19–related and non–COVID-19 ARDS,27 and fibrinogen concentrations exceeded the upper limit of normal (400 mg/dL) for 92% (11 of 12) of the severe/critical COVID-19 cases tested (Table 1) but only 20% (one of five tested) of the hospitalized control patients (Table 3). Two of the six patients with mild/moderate COVID-19 had elevated d-dimer levels, and both had moderate symptoms (Table 4).
Biomarkers of a proinflammatory state, including C-reactive protein, IL-6, and ferritin, were elevated in all patients with severe/critical COVID-19 who were tested (Table 2). Levels of complement components C3 and C4 were within the normal range for most of these patients, but total hemolytic complement levels (CH50) were elevated in five (45%) of the 11 patients tested (Table 2). They were not evaluated in the non–COVID-19 hospitalized group or in the group with mild/moderate COVID-19.
Microscopic and IHC Analysis of Skin Biopsy Specimens
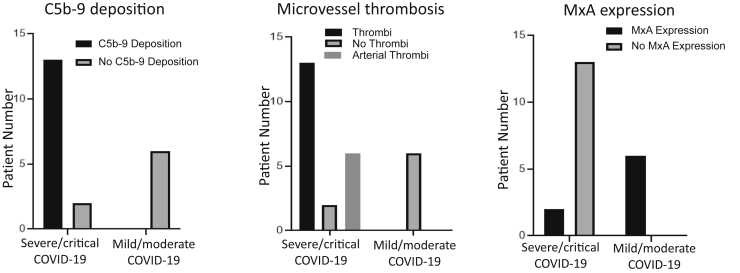
Livedo rashes, indicative of a systemic microvascular thrombotic process,25 were recognized in 40% (six of 15) of the severe/critical cases (Table 2) but in none of the mild/moderate cases or the hospitalized pre–COVID-19 control subjects. In terms of normal-appearing deltoid skin, 87% (13 of 15) of the severe/critical COVID-19 cases exhibited C5b-9 deposition in the microvasculature (Figures 1A and 2 and Table 2). Case A had similar C5b-9 deposition in normal-appearing deltoid skin. In addition, all six cases of critical COVID-19 with visible skin lesions had C5b-9 deposition in lesional skin (data not shown), as previously documented by our group for identical SARS-CoV-2–related livedo rashes.25 None of the six mild/moderate cases exhibited C5b-9 deposition in skin associated with rashes (P < 0.01) (Figure 2), nor did any of the biopsy specimens of normal-appearing deltoid skin in the nine hospitalized non–COVID-19 control subjects (data not shown; P < 0.001). Similarly, MASP2 deposition on microvasculature, in a pattern resembling that of C5b-9, was restricted to the severe/critical COVID-19 group, present in seven of eight cases examined (Table 2).
Figure 1.
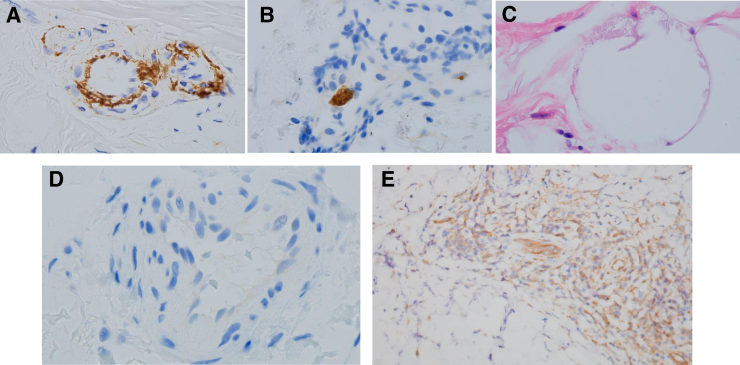
Representative examples of C5b-9 deposition, platelet microthrombi, lipomembranous fat necrosis, and myxovirus resistance A (MxA) expression in biopsy specimens of normal-appearing deltoid skin from patients with critical coronavirus disease 2019 (COVID-19). A: Granular endothelial and subendothelial deposits of C5b-9 were present in venules, capillaries, and small arteries. Illustrated is granular deposition in a capillary and an arteriole. B: Staining for the platelet marker CD61 highlights an incipient platelet thrombus in an arteriole. C: Lipomembranous fat necrosis is a cardinal hallmark of incipient ischemic alterations within the fat. It is characterized by a fern-like alteration of the adipocyte membrane. D: Interferon-I–induced MxA expression is absent from cutaneous arterioles of a representative patient with critical COVID-19. Lesional skin from this patient showed a similar lack of MxA expression (not shown). E: MxA expression is prominent in lesional skin of a patient with moderate COVID-19. Original magnification: diaminobenzidine (DAB) stain, ×1000 (A, B, and D); hematoxylin and eosin stain, ×1000 (C); DAB stain, ×400 (E).
Figure 2.
Pathophysiological correlates of coronavirus disease 2019 (COVID-19) disease stage. Fifteen individuals with severe (n = 2) or critical (n = 13) COVID-19 and six individuals with mild/moderate COVID-19 were studied. C5b-9 deposition, microthrombus formation, and myxovirus resistance A (MxA) expression differed significantly in skin biopsy specimens from patients with severe/critical versus those with mild/moderate COVID-19 (P < 0.001 for group comparisons in all three graphs).
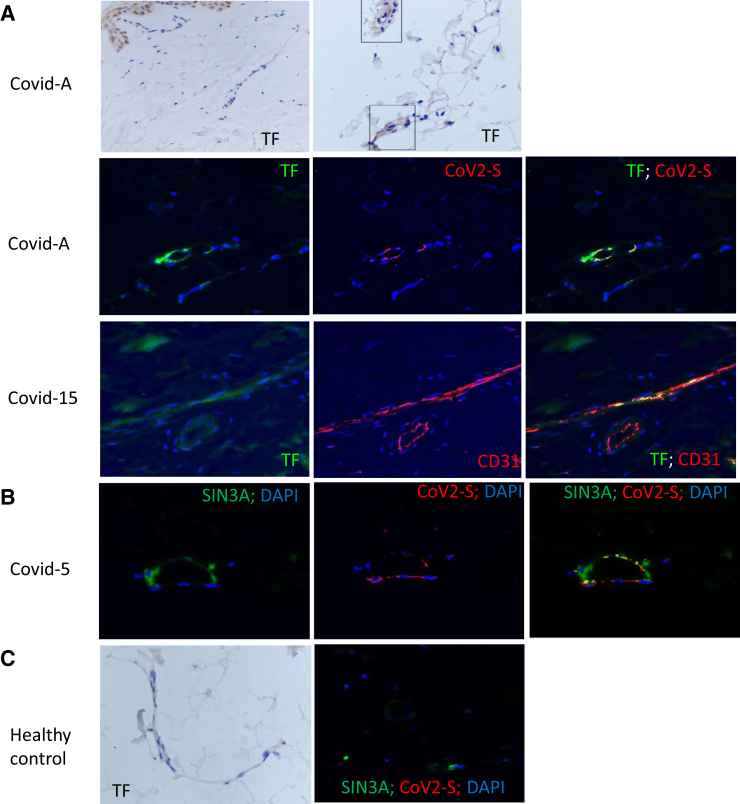
These differences in complement deposition were reflected by three additional histopathologic findings. First, there was a striking incidence of microthrombi in normal-appearing skin of the severe/critical cases [87% (13 of 15)], including six (40%) of 15 with arteriolar thrombi (Cases 2, 4, 5, 6, 9, and15) versus zero of nine hospitalized control subjects and zero of six in the lesional skin of the mild/moderate cases (P < 0.001) (Figures 1 and 2). The microthrombi varied from platelet aggregates highlighted by CD61+ deposits adherent to endothelium (Figure 1B) to fully formed clots. Both venous and arterial microthrombi were similarly present in the normal-appearing deltoid skin of Case A. There was a clear association between complement C5b-9 and/or MASP2 deposits with the presence of microthrombi in the severe/critical COVID-19 group for 100% (13 of 13) with observed microthrombi (Table 2). In contrast, only one of the two individuals without microthrombi had such deposits (Table 2), and none of six individuals with mild/moderate COVID-19, all lacking microthrombi, had complement expression (Figure 2). Second, lipomembranous fat necrosis, a manifestation of local hypoxia,28 was present in five of 15 severe/critical COVID-19 cases (33%) (Figure 1C). Such necrosis has been recognized in association with other microthrombotic conditions linked to tissue hypoxia. Third, there was staining for TF in the microvasculature of a patient with severe COVID-19 that was not apparent in the healthy control subject (Figure 3A). Co-localization of TF in areas staining for clustered SARS-CoV-2 spike protein and CD31 (Figure 3A), but not in areas of CD68+ staining (not shown), was recognized. However, resolution of TF staining did not permit definitive localization to a CD31+ cell versus a peri-endothelial distribution.
Figure 3.
Representative examples of tissue factor (TF) and SIN3A protein showing co-localization with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein (CoV2-S) deposition on CD31+ cells of normal-appearing deltoid skin from patients with coronavirus disease 2019 (COVID-19) acute respiratory distress syndrome versus non-COVID acute respiratory distress syndrome. As described in Materials and Methods, immunohistochemistry was used to detect all proteins illustrated here. The different colors used to define co-localization of these IHC-labeled proteins were generated by using Nuance software, as described in the text. A: No staining for TF is evident in biopsy specimen of normal-appearing deltoid skin from a healthy control versus abundant staining for TF in the microvasculature of both superficial (left panel of first row) and deep cutaneous (boxed areas in right panel of first row) vessels in a patient with critical COVID-19 (Covid-A). Using Nuance software in analysis of critical COVID-19 Case 15 (Covid-15), the TF image appears green, and the CD31 endothelial marker appears red. A merged image shows expression of TF in areas of CD31 expression, as revealed by intense yellow staining. This analysis was repeated for TF (green) and CoV2-S protein (red), showing co-localization (yellow stain). B: Using Nuance software to analyze critical COVID-19 Case 5 (Covid-5), the SIN3A image appears green, and the CoV2-S protein appears red. A merged image shows a significant degree of SIN3A and S protein co-localization, as revealed by intense yellow staining. C: A deltoid skin biopsy specimen of a healthy control stained for TF (first panel) and with anti-SIN3A (green) and anti–SARS-CoV-2 (red) reagents (second panel). No expression of TF and SARS-CoV-2 and minimal expression of SIN3A is apparent. Original magnification, ×400 (A-C).
In terms of IFN-I–associated signals, IFN-I–inducible antiviral protein MxA was present in 100% (six of six) of the mild/moderate cases (Table 4) but only in 15% (two of 13) of normal-appearing skin biopsy samples tested, and none of the six livedo rashes in the patients with severe/critical COVID-19 (P < 0.001) (Figure 1, D and E, Figure 2, and Table 2). Several reports20, 29 have documented the lack of MxA expression in microvessels of normal donors and individuals without active viral infections. MxA staining was observed in only one of the eight patients tested from the hospitalized control group; this patient (Case 4) had ARDS complicated by a non–SARS-CoV-1,-2 infection (Table 3). In contrast, SIN3A expression was prominent in the microvasculature of normal-appearing deltoid skin from patients with severe/critical COVID-19 (Figure 3B) but not in similar sections from normal control subjects (Figure 3C). SIN3A co-localized with areas of SARS-CoV-2 expression (Figure 3C).
Plasma SIN3A and IFN-α Levels
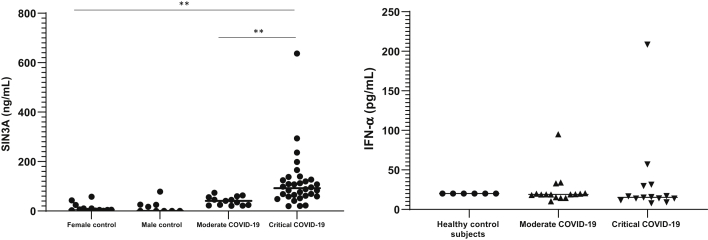
There was no statistically significant difference in SIN3A levels between healthy female and male normal control subjects (Figure 4). The mean level of plasma SIN3A was 136.6 ng/mL in the critical COVID-19 group (n = 32; 13 female subjects, 19 male subjects) versus 17.8 ng/mL in healthy female control subjects (n = 13), an 8.1-fold increase, and 16.2 ng/mL in healthy male control subjects (n = 9), an 8.4-fold increase (P ≤ 0.02 for both sexes) (Figure 4). Patients with moderate COVID-19 (n = 13; six female subjects, seven male subjects) had a mean SIN3A level of 34.6 ng/mL, which was 3.9-fold lower than that in the critical group and not statistically different from healthy control subjects (P = 0.06) (Figure 4). In contrast, IFN-α levels were not statistically different between healthy control subjects (mean, 20.1 pg/mL) and patients with moderate (mean, 19.1 pg/mL) or critical (mean, 15.4 pg/mL) COVID-19 (Figure 4).
Figure 4.
Plasma levels of interferon type I (IFN-I) co-repressor protein SIN3A, but not IFN-α, correlate with coronavirus disease 2019 (COVID-19) stage. Levels of SIN3A were assessed by using ELISA in plasma of individuals with moderate (n = 13; six female subjects, seven male subjects) or severe/critical (n = 32; 13 female subjects, 19 male subjects) COVID-19 and compared with healthy age-matched control subjects (female subjects, n = 13; male subjects, n = 9). Levels of IFN-α were assessed by using ELISA and plasma of individuals with moderate (n = 16) or severe/critical (n = 14) COVID-19 and compared with healthy age-matched control subjects (female subjects, n = 6). ∗∗P ≤ 0.01.
Discussion
The clinical-pathologic associations explored in this study using skin, a tissue that is readily accessible by simple punch biopsy, and standard IHC techniques established three key points. First, the study provides new evidence for both venous and arterial microthrombosis in the normal-appearing skin of patients with severe/critical COVID-19, but not in the skin of SARS-CoV-2–infected individuals with self-limited disease of mild/moderate intensity. This included 40% of COVID-19 patients with ARDS having arteriolar thrombi versus none of nine critically ill pre–COVID-19 control subjects, four of whom also had sepsis-associated ARDS. It is consistent with the autopsy-based observation that arteriolar thrombi are ninefold more prevalent in the lungs of patients with severe/critical COVID-19 versus the lungs of those with other forms of ARDS,6 and microthrombi are fivefold more prevalent in the hearts of patients dying of COVID-19 versus specimens from control patients, with or without cardiac disease.30 It also supports the hypothesis, based on autopsy of multiple organ systems in patients with COVID-19,9 that numerous microthrombi in both venous and arterial vessels may be a unique characteristic of COVID-19 ARDS versus other forms of ARDS and, as shown here, is a systemic phenomenon that can be documented by biopsy of normal-appearing skin.
The demonstration of co-staining of TF with SARS-CoV-2 spike protein in the microvasculature in association with microthrombi provides premortem documentation of what had been observed by our group at autopsy in the lungs of patients with critical COVID-19.31 In the earlier study, TF protein expression was 2.1-fold higher in COVID-19 versus non–COVID-19 ARDS lungs (P < 0.005) and 11-fold (P < 0.001) higher than in control lungs, with fibrin thrombi and thrombi positive for platelet factor 4 found in close proximity to regions expressing TF in COVID-19 ARDS lungs. Further studies are required to determine the precise identity of the cells staining for TF and SARS-CoV-2 products. However, our current findings suggest that the higher levels of TF in cells of skin microvessels could similarly initiate thrombus formation by thrombin generation, platelet activation, and TF-mediated signaling for inflammation,32 and thus TF could be a potential therapeutic target for SARS-CoV-2–induced thrombosis.
Second, the study confirms our initial anecdotal observation, based on only three cases of severe/critical COVID-19,2 of the presence of terminal complement complex C5b-9 and lectin pathway of complement MASP2 deposition in the microvasculature of normal-appearing skin. This study includes 15 additional patients with severe/critical COVID-19 and, for the first time, appropriate control subjects. It also documents that such complement deposition is not a feature of skin obtained before the COVID-19 pandemic from critically ill patients with ARDS and/or AKI and co-morbidities similar to those of patients with COVID-19.
Third, the study found a marked dichotomy in the expression of the IFN-I–inducible protein MxA in both normal-appearing and lesional skin of patients with severe/critical COVID-19 versus patients with mild/moderate COVID-19 (15% versus 100%). IFN-I may have a key modulatory role in COVID-19 progression. MxA is up-regulated as a first line of innate immune defense in many viral disorders24,33 but is suppressed in advanced SARS-CoV disease,34 as the study now shows in vivo for SARS-CoV-2 infection. Not only did patients with severe/critical COVID-19 have a deficit in the IFN-I–associated MxA antiviral response, but they had a parallel excess, in skin and plasma, of SIN3A, which can perpetuate IFN-I–linked inflammation.24 The latter is consistent with the fact that although plasma IFN-I levels are often low in patients with critical COVID-19,35 IFN-I–driven inflammatory pathways may be highly elevated.36 The recent demonstration of STAT3 up-regulation in the lungs of COVID-19 patients at autopsy37 is consistent with our observation of SIN3A elevation in the cutaneous microvasculature of patients with severe/critical COVID-19, as SIN3A is the primary suppressor of major inhibitory pathways for IFN-I–driven inflammation, acting via binding to STAT3 homodimers.24 It offers tissue-based support for the concept of a novel biology specific to COVID-19 within the framework of what otherwise appears as largely identical transcriptional signals seen in comparisons of SARS-CoV-2 infections versus other respiratory viral infections.38 It also parallels the finding that a major category of differentially expressed genes in human lung cells infected with SARS-CoV-2 in vitro involves down-regulation of negative regulators of proinflammatory cytokine biosynthesis.39
The documentation of co-staining of SARS-CoV-2 spike protein with SIN3A on the microvasculature suggests direct activation of this pathway by the virus in vivo, but it does not presuppose infection of endothelial cells. The ability of SARS-CoV-2 to directly infect endothelial cells remains controversial.40, 41, 42, 43 A recent autopsy-based study from the NIH showed SARS-CoV-2 RNA in skin from 68% of severe/critical patients, with in situ hybridization suggesting evidence of virus replication at multiple additional extrapulmonary sites, including endothelium.42 This parallels an earlier autopsy study documenting SARS-CoV-2 protein in multiple organs.44 However, these observations are also consistent with in vitro systems documenting induction of IFN-I–responsive genes and coagulation factors by isolated SARS-CoV-2 spike and nucleocapsid proteins, in the absence of direct infection.45,46
This work is limited by its cross-sectional nature and nonrandomized subject referral process. The patients with severe/critical COVID-19 had progressed to a point at which intervention with anticomplement therapies was under consideration. Similarly, patients with mild/moderate COVID-19 were referred because of a cutaneous lesion. Before the clinical significance of these findings as actionable correlates of disease progression can be established, a prospective study is required using serial biopsy specimens of normal-appearing skin.
The current study does have several strengths. Longitudinal, tissue-based studies should be possible because of our demonstration of the utility of simple punch skin biopsies. They enable tissue-based premortem assessment for microvascular thrombosis, complement deposition, and IFN-I–based responses at different time points. In addition, such information may help inform treatment decisions. For example, deposition of both MASP2 and the MASP2-binding protein MBL has been found in lungs of patients with COVID-19 and ARDS at autopsy, findings not seen in H1N1 influenza–linked ARDS.2,47 Patients with severe/critical COVID-19 refractory to current therapeutic interventions may be candidates for use of the anti-MASP2 antibody narsoplimab48 and other anticomplement agents, including the anti-C5 agent eculizumab,22 as well as selective blockade of C5a, recently shown to be a driver of microvascular platelet aggregation in in vitro models for COVID-19 plasma-associated vascular injury.49 Defibrotide is an additional possible intervention. The latter is approved by the US Food and Drug Administration for the treatment of veno-occlusive disease/sinusoidal obstruction syndrome.50 It has antiplatelet activity, and several studies suggest similarities between the endotheliopathy of veno-occlusive disease/sinusoidal obstruction syndrome and SARS-CoV-2 infection.51,52 Cutaneous biopsy may also permit exploration of the “long COVID” syndrome, postacute sequelae of SARS-CoV-2 infection, which involves pulmonary and extrapulmonary organ systems.53 For example, in terms of chronic neurologic sequelae of SARS-CoV-2, magnetic resonance imaging scans, cerebral blood flow studies, and cerebrospinal fluid sampling implicate microvascular and IFN-I abnormalities similar to those seen at autopsy in the brain of patients with severe/critical COVID-1954,55 and in our cutaneous biopsy specimens of patients with severe/critical COVID-19.
Conclusions
This study provides the first premortem evidence for systemic venous and arteriolar microthrombosis in patients with severe/critical COVID-19 versus those with mild/moderate COVID-19. It confirms the original observation, based on only three severe/critical COVID-19 cases, of terminal complement complex C5b-9 and lectin pathway of complement MASP2 deposition in the cutaneous microvasculature, and documents that such complement involvement is not a feature of pre–COVID-19 ARDS. It supports the hypothesis that these microvascular changes may be a unique characteristic of COVID-19 ARDS versus other forms of ARDS.
These observations have clinical implications. First, although anticoagulants were used in the pre–COVID-19 era in sepsis-associated pneumonias to reduce macrovessel thromboembolism, most randomized trials to date have not shown benefits of add-on or escalated antithrombotic therapy over usual standard of care in hospitalized patients critically ill with COVID-19 ARDS.56 Standard antithrombotic agents may not be capable of reducing the microvessel thrombosis of SARS-CoV-2 infection. Second, the study found a marked dichotomy in the expression of IFN-I–inducible antiviral protein MxA versus IFN-I proinflammatory regulator SIN3A in patients with severe/critical COVID-19 versus those with mild/moderate COVID-19. Increased SIN3A levels in plasma and expression in skin microvasculature were associated with the severity of COVID-19. These results indicate that SIN3A along with coagulation and complement pathway markers may refine the approach to predicting COVID-19 progression and intervening in its advance.
Footnotes
Supported by grants from Omeros, Inc., Jazz Pharmaceuticals, and the Angelo Donghia Foundation (J.L.), and partly supported by National Heart, Lung, and Blood Institute grant HL148123 (J.A.). This work was made possible through data provided by the Cornell COVID-19 Registry, led by Drs. Parag Goyal, Justin Choi, Laura Pinheiro, and Monika Safford of Weill Cornell Medicine.
Disclosures: J.L. has received grants and honoraria from Alexion, Inc. and Omeros, Inc., manufacturers of anticomplement drugs, and Jazz Pharmaceuticals, manufacturer of defibrotide.
Author Contributions
All authors were involved in the analysis and interpretation of data, and all approved the final version of the manuscript. J.L., J.J.M., J.A., and C.M. conceived and designed the study, and drafted the manuscript; A.N.C. provided some cutaneous samples; J.H. performed or supervised the cutaneous biopsies; C.M. conducted and interpreted skin histopathology and IHC; G.N. performed SIN3A and SARS-CoV-2 expression studies; M.S. compiled clinical data from record reviews; S.E.R.-B. acquired and categorized plasma samples; S.E. performed the ELISA assays and statistical analysis.
References
- 1.Pedersen S.F., Ho Y.-C. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202–2205. doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S., Hisch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., the Northwell COVID-19 Research Consortium, Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., Cookingham J., Coppa K., Diefenbach M.A., Dominello A.J., Duer-Hefele J., Falzon L., Gitlin J., Hajizadeh N., Harvin T.G., Hirschwerk D.A., Kim E.J., Kozel Z.M., Marrast L.M., Mogavero J.N., Osorio G.A., Qiu M., Zanos T.P. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Docherty A.B., Harrision E.M., Green C.A., Hardwick H.E., Pius R., Norman L., Holden K.A., Read J.M., Dondelinger F., Carson G., Merson L., Lee J., Plotkin D., Sigfrid L., Halpin S., Jackson C., Gamble C., Horby P.W., Nguyen-Van-Tam J.S., Ho A., Russell C.D., Dunning J., Openshaw P.J., Baillie J.K., Semple M.G., ISARIC4C Investigators Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., Li J., Yao Y., Ge S., Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Intl. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falasca L., Nardacci R., Colombo J., Lalle E., Di Caro A., Nicastri E., Antinori A., Petrosillo N., Marchioni L., Biava G., D’Offizi G., Palmieri F., Goletti D., Zumla A., Ippolito G., Piacentini M., Del Nonno F. Postmortem findings in Italian patients with COVID-19: a descriptive full autopsy study of cases with and without comorbidities. J Infect Dis. 2020;222:1807–1815. doi: 10.1093/infdis/jiaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bussani R., Schneider E., Zentilin C., Collesi C., Ali H., Braga L., Volpe M.C., Colliva A., Zanconati F., Berlot G., Silvestri F., Zacchigna S., Giacca M. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine. 2020;61:103104. doi: 10.1016/j.ebiom.2020.103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryce C., Grimes Z., Pujadas E., Ahuja S., Beasley M.B., Albrecht R., et al. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod Pathol. 2021;34:1456–1467. doi: 10.1038/s41379-021-00793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W., Pan J.Y. Anatomical and pathological observation and analysis of SARS and COVID-19: microthrombosis is the main cause of death. Biol Proced Online. 2021;23:4. doi: 10.1186/s12575-021-00142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holter J.C., Pischke S.E., de Boer E., Lind A., Jenum S., Holten A.R., Tonby K., Barratt-Due A., Sokolova M., Schjalm C., Chaban V., Kolderup A., Tran T., Tollefsrud Gjølberg T., Skeie L.G., Hesstvedt L., Ormåsen V., Fevang B., Austad C., Müller K.E., Fladeby C., Holberg-Petersen M., Halvorsen B., Müller F., Aukrust P., Dudman S., Ueland T., Andersen J.T., Lund-Johansen F., Heggelund L., Dyrhol-Riise A.M., Mollnes T.E. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc Natl Acad Sci U S A. 2020;117:25018–25025. doi: 10.1073/pnas.2010540117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathew D., Giles J.R., Baxter A.E., Oldridge D.A., Greenplate A.R., Wu J.E., et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369:eabc8511. doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin-Sancho L., Lewinski M.K., Pache L., Stoneham C.A., Yin X., Pratt D., Churas C., Rosenthal S.B., Liu S., De Jesus P.D., O'Neill A.M., Gounder A.P., Nguyen C., Pu Y., Oom A.L., Miorin L., Rodriguez-Frandsen A., Urbanowski M., Shaw M.L., Chang M.W., Benner C., Frieman M.B., García-Sastre A., Ideker T., Hultquist J.F., Guatelli J., Chanda S.K. Functional landscape of SARS-CoV-2 cellular restriction. Mol Cell. 2021;81:2656–2668. doi: 10.1016/j.molcel.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., Péré H., Charbit B., Bondet V., Chenevier-Gobeaux C., Breillat P., Carlier N., Gauzit R., Morbieu C., Pène F., Marin N., Roche N., Szwebel T.A., Merkling S.H., Treluyer J.M., Veyer D., Mouthon L., Blanc C., Tharaux P.L., Rozenberg F., Fischer A., Duffy D., Rieux-Laucat F., Kernéis S., Terrier B. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369:718–724. doi: 10.1126/science.abc6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aid M., Busman-Sahay K., Vidal S.J., Maliga Z., Bondoc S., Starke C., Terry M., Jacobson C.A., Wrijil L., Ducat S., Brook O.R., Miller A.D., Porto M., Pellegrini K.L., Pino M., Hoang T.N., Chandrashekar A., Patel S., Stephenson K., Bosinger S.E., Andersen H., Lewis M.G., Hecht J.L., Sorger P.K., Martinot A.J., Estes J.D., Barouch D.H. Vascular disease and thrombosis in SARS-CoV-2 infected rhesus macaques. Cell. 2020;183:1354–1366.e13. doi: 10.1016/j.cell.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Israelow B., Song E., Mao T., Lu P., Meir A., Liu F., Alfajaro M.M., Wei J., Dong H., Homer R.J., Ring A., Wilen C.B., Iwasaki A. Mouse model of SARS-CoV-2 reveals inflammatory role of type I interferon signaling. J Exp Med. 2020;217:e20201241. doi: 10.1084/jem.20201241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gralinski L.E., Sheahan T.P., Morrison T.E., Menachery V.D., Jensen K., Leist S.R., Whitmore A., Heise M.T., Baric R.S. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018;9:e01753-18. doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diao B., Wang C., Wang R., Feng Z., Zhang J., Yang H., Tan Y., Wang H., Wang C., Liu L., Liu Y., Liu Y., Wang G., Yuan Z., Hou X., Ren L., Wu Y., Chen Y. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. Nat Commun. 2021;12:2506. doi: 10.1038/s41467-021-22781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magro C.M., Poe J.C., Kim C., Shapiro L., Nuovo G., Crow M.K., Crow Y.J. Degos disease: a C5b-9/interferon-[alpha]-mediated endotheliopathy syndrome. Am J Clin Pathol. 2011;135:599–610. doi: 10.1309/AJCP66QIMFARLZKI. [DOI] [PubMed] [Google Scholar]
- 21.Magro C.M., Momtahen S., Mulvey J.J., Yassin A.H., Kaplan R.B., Laurence J.C. Role of the skin biopsy in the diagnosis of atypical hemolytic uremic syndrome. Am J Dermatopathol. 2015;37:349–356. doi: 10.1097/DAD.0000000000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laurence J., Mulvey J.J., Seshadri M., Racanelli A., Harp J., Schenck E.J., Zappetti D., Horn E.M., Magro C.M. Anti-complement C5 therapy with eculizumab in three cases of critical COVID-19. Clin Immunol. 2020;219:108555. doi: 10.1016/j.clim.2020.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andina D., Noguera-Morel L., Bascuas-Arribus M., Gaitero-Tristán J., Alonso-Cadenas J.A., Escalada-Pellitero S., Hernández-Martín Á., de la Torre-Espi M., Colmenero I., Torrelo A. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;33:406–411. doi: 10.1111/pde.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ivashkiv L.B., Donlin L.T. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magro C.M., Mulvey J.J., Laurence J., Sanders S., Crowson A.N., Grossman M., Harp J., Nuovo G. The differing pathophysiologies that underlie COVID-19-associated perniosis and thrombotic retiform purpura: a case series. Br J Dermatol. 2021;184:141–150. doi: 10.1111/bjd.19415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J., Arnold A.P. Sexually dimorphic expression of co-repressor SIN3A in mouse kidney. Endocr Res. 2005;31:111–119. doi: 10.1080/07435800500229243. [DOI] [PubMed] [Google Scholar]
- 27.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Fagot Gandet F., Fafi-Kremer S., Castelain V., Schneider F., Grunebaum L., Anglés-Cano E., Sattler L., Mertes P.-M., Meziani F., CRIS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) High risk of thrombosis in severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahn S., Yoo M., Lee S., Choi E. A clinical and histopathological study of 22 patients with membranous lipodystrophy. Clin Exp Dermatol. 1996;21:269–272. doi: 10.1111/j.1365-2230.1996.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 29.Manzano G.S., Woods J.K., Amato A.A. Covid-19-associated myopathy caused by type I interferonopathy. N Engl J Med. 2020;383:2389–2390. doi: 10.1056/NEJMc2031085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson J.E., McGuone D., Xu M.L., Jane-Wit D., Mitchell R.N., Libby P., Pober J.S. Coronavirus disease 2019 (COVID-19) coronary vascular thrombosis: correlation with neutrophil but not endothelial activation. Am J Pathol. 2022;192:112–120. doi: 10.1016/j.ajpath.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subrahmanian S., Borczuk A., Salvatore S., Fung K.-M., Merrill J.T., Laurence J., Ahamed J. Tissue factor upregulation is associated with SARS-CoV-2 in the lungs of COVID-19 patients. J Thromb Haemost. 2021;19:2268–2274. doi: 10.1111/jth.15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahamed J., Versteeg H.H., Kerver M., Chen V.M., Mueller B.M., Hogg P.J., Ruf W. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci U S A. 2006;103:13932–13937. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bailey C.C., Zhong G., Huang I.-C., Farzan M. IFITM-family proteins: the cell’s first line of antiviral defense. Annu Rev Virol. 2014;1:261–283. doi: 10.1146/annurev-virology-031413-085537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ching J.C.-Y., Chan K.Y.K., Lee E.H.L., Xu M.-S., Ting C.K.P., So T.M.K., Sham P.C., Leung G.M., Peiris J.S.M., Khoo U.-S. Significance of the myxovirus resistance A (MxA) gene -123C>A single-nucleotide polymorphism in suppressed interferon beta induction of severe acute respiratory syndrome coronavirus infection. J Infect Dis. 2010;201:1899–1908. doi: 10.1086/652799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trouillet-Assant S., Viel S., Gaymard A., Pons S., Richard J.-C., Perret M., Villard M., Brengel-Pesce K., Lina B., Mezidi M., Bitker L., Belot A., COVID HCL Study Group Type I IFN immunoprofiling in COVID-19 patients. J Allergy Clin Immunol. 2020;146:206–208.e2. doi: 10.1016/j.jaci.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Z., Ren L., Zhang L., Zhong J., Xiao Y., Jia Z., Guo L., Yang J., Wang C., Jiang S., Yang D., Zhang G., Li H., Chen F., Xu Y., Chen M., Gao Z., Yang J., Dong J., Liu B., Zhang X., Wang W., He K., Jin Q., Li M., Wang J. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27:883–890.e2. doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rendeiro A.F., Ravichandran H., Bram Y., Chandar V., Kim J., Meydan C., Park J., Foox J., Hether T., Warren S., Kim Y., Reeves J., Salvatore S., Mason C.E., Swanson E.C., Borczuk A.C., Elemento O., Schwartz R.E. The spatial landscape of lung pathology during COVID-19 progression. Nature. 2021;593:564–569. doi: 10.1038/s41586-021-03475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thair S.A., He Y.D., Hasin-Brumshtein Y., Sakaram S., Pandya R., Toh J., Rawling D., Remmel M., Coyle S., Dalekos G.N., Koutsodimitropoulos I., Vlachogianni G., Gkeka E., Karakike E., Damoraki G., Antonakos N., Khatri P., Giamarellos-Bourboulis E.J., Sweeney T.E. Transcriptomic similarities and differences in host response between SARS-CoV-2 and other viral infections. iScience. 2021;24:101947. doi: 10.1016/j.isci.2020.101947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim D., Kim S., Park J., Chang H.R., Chang J., Ahn J., Park H., Park J., Son N., Kang G., Kim J., Kim K., Park M.-S., Kim Y.K., Baek D. A high-resolution temporal atlas of the SARS-CoV-2 translatome and transcriptome. Nat Commun. 2021;12:5120. doi: 10.1038/s41467-021-25361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado Del Pozo C., Prosper F., Romero J.P., Wirnsberger G., Zhang H., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicosia R.F., Ligresti G., Caporarello N., Akliesh S., Ribatti D. COVID-19 vasculopathy: mounting evidence for an indirect mechanism of endothelial injury. Am J Pathol. 2021;191:1374–1384. doi: 10.1016/j.ajpath.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chertow D.S., Stein S., Ramelli S., Grazioli A., Chung J.-Y., Singh M., Yinda C.K., Winkler C.W., Dickey J.M., Ylaya K., Ko S.K., Platt A., Burbelo P.D., Quezado M., Pittaluga S., Purcell M., Munster V.J., Belinky F., Ramos-Benitez M.J., Boritz E.A., Herr D.L., Rabin J., Saharia K.K., Madathil R.J., Tabatabai A., Soherwardi S., McCurdy M.T., Peterson K.E., Cohen J.L., de Wit E., Vannella K.M., Hewitt S.M., Kleiner D.E. SARS-CoV-2 infection and persistence throughout the human body and brain. 10.21203/rs.3.rs-1139035/v1 Research Square [Preprint], doi:
- 43.Bailey AL Dmytrenko O., Greenberg L., Bredemeyer A.L., Ma P., Liu J., Penna V., Winkler E.S., Sviben S., Brooks E., Nair A.P., Heck K.A., Rali A.S., Simpson L., Saririan M., Hobohm D., Stump W.T., Fitzpatrick J.A., Xie X., Zhang X., Shi P.Y., Hinson J.T., Gi W.T., Schmidt C., Leuschner F., Lin C.Y., Diamond M.S., Greenberg M.J., Lavine K.J. SARS-CoV-2 infects human engineered heart tissues and models COVID-19 myocarditis. JACC Basic Transl Sci. 2021;6:331–345. doi: 10.1016/j.jacbts.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao X.-H., Luo T., Shi Y., He Z.-C., Tang R., Zhang P.-P., et al. A cohort autopsy study defines COVID-19 systemic pathogenesis. Cell Res. 2021;31:836–846. doi: 10.1038/s41422-021-00523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cascarina S.M., Ross E.D. A proposed role for the SARS-CoV-2 nucleocapsid protein in the formation and regulation of biomolecular condensates. FASEB J. 2020;34:9832–9842. doi: 10.1096/fj.202001351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu J., Yuan X., Chen H., Chaturvedi S., Braunstein E.M., Brodsky R.A. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136:2080–2089. doi: 10.1182/blood.2020008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malaquias M.A.S., Gardotti A.C., da Silva Motta J., Jr., Martins A.P.C., Azevedo M.L.V., Benevides A.P.K., Cézar-Neto P., Panini do Carmo L.A., Zeni R.C., Raboni S.M., Fonseca A.S., Machado-Souza C., Moreno-Amaral A.N., de Noronha L. The role of the lectin pathway of the complement system in SARS-CoV-2 lung injury. Transl Res. 2021;231:55–63. doi: 10.1016/j.trsl.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rambaldi A., Gritti G., Mico M.C., Frigeni M., Borleri G., Salvi A., Landi F., Pavoni C., Sonzogni A., Gianatti A., Binda F., Fagiuoli S., Di Marco F., Lorini L., Remuzzi G., Whitaker S., Demopulos G. Endothelial injury and thrombotic microangiopathy in COVID-19: treatment with the lectin-pathway inhibitor narsoplimab. Immunobiolgy. 2020;225:152001. doi: 10.1016/j.imbio.2020.152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aiello S., Gastoldi S., Galbusera M., Ruggenenti P., Portalupi V., Rota S., Rubis N., Liguori L., Conti S., Tironi M., Gamba S., Santarsiero D., Benigni A., Remuzzi G., Noris M. C5a and C5aR1 are key drivers of microvascular platelet aggregation in clinical entities spanning from aHUS to COVID-19. Blood Adv. 2022;6:866–881. doi: 10.1182/bloodadvances.2021005246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richardson P., Aggarwal S., Topaloglu O., Villa K.F., Corbacioglu S. Systematic review of defibrotide studies in the treatment of veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) Bone Marrow Transpl. 2019;54:1951–1962. doi: 10.1038/s41409-019-0474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frame D, Scappaticci GB, Braun TM, Maliarik M, Sisson TH, Pipe SW, Lawrence DA, Richardson PG, Hollinstat M, Hyzy RC, Kaul DR, Gregg KS, Lama VN, Yanik GA. Defibrotide therapy for SARS-CoV-2 ARDS. Chest. 2022 doi: 10.1016/j.chest.2022.03.046. 162:346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahamed J., Laurence J. Long COVID endotheliopathy: hypothesized mechanisms and potential therapeutic approaches. J Clin Invest. 2022;132:e161167. doi: 10.1172/JCI161167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 54.Qin Y., Wu J., Chen T., Li J., Zhang G., Wu D., Zhou Y., Zheng N., Cai A., Ning Q., Manyande A., Xu F., Wang J., Zhu W. Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J Clin Invest. 2021;131:e147329. doi: 10.1172/JCI147329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Remrik J., Wilcox J.A., Babady N.E., McMillen T.A., Vachha B.A., Halpern N.A., Dhawan V., Rosenblum M., Iacobuzio-Donahue C.A., Avila E.K., Santomasso B., Boire A. Inflammatory leptomeningeal cytokines mediate COVID-19 neurologic symptoms in cancer patients. Cancer Cell. 2021;39:276–283.e3. doi: 10.1016/j.ccell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spyropoulos A.C., Bonaca M.P. Studying the coagulopathy of COVID-19. Lancet. 2022;399:118–119. doi: 10.1016/S0140-6736(21)01906-1. [DOI] [PMC free article] [PubMed] [Google Scholar]