Abstract
Smoking traditionally has not been considered as a cause of bronchiectasis. However, few studies have evaluated the association between smoking and bronchiectasis. This study aimed to investigate the association between smoking status and bronchiectasis development in young adults. This study included 6,861,282 adults aged 20–39 years from the Korean National Health Insurance Service database 2009–2012 who were followed-up until the date of development of bronchiectasis, death, or 31 December 2018. We evaluated the incidence of bronchiectasis according to smoking status. During a mean of 7.4 years of follow-up, 23,609 (0.3%) participants developed bronchiectasis. In multivariable Cox regression analysis, ex-smokers (adjusted hazard ratio (aHR) = 1.07, 95% confidence interval (CI) = 1.03–1.13) and current-smokers (aHR = 1.06, 95% CI = 1.02–1.10) were associated with incident bronchiectasis, with the highest HR in ≥ 10 pack-years current smokers (aHR = 1.12, 95% CI = 1.06–1.16). The association of smoking with bronchiectasis was more profound in females than in males (p for interaction < 0.001), in younger than in older participants (p for interaction = 0.036), and in the overweight and obese than in the normal weight or underweight (p for interaction = 0.023). In conclusion, our study shows that smoking is associated with incident bronchiectasis in young adults. The association of smoking with bronchiectasis development was stronger in females, 20–29 year-olds, and the overweight and obese than in males, 30–40-year-olds, and the normal weight or underweight, respectively.
Keywords: bronchiectasis, smoking, risk factor, epidemiology
1. Introduction
Non-cystic fibrosis bronchiectasis (hereafter referred to as bronchiectasis), a chronic lung disease characterized by irreversible dilatation and destruction of airways, has been considered an orphan disease [1]. However, in terms of medical cost and mortality, the prevalence and disease burden of bronchiectasis have been increasing worldwide [1,2,3,4], indicating that this disease is an orphan no longer [5,6]. Thus, global strategies are needed to reduce the disease burden of bronchiectasis, which requires exploration of associated factors of bronchiectasis.
Current guidelines for diagnosis of bronchiectasis recommend comprehensive work-up to evaluate the etiology of bronchiectasis [7,8], which includes asthma, chronic obstructive pulmonary disease (COPD), immunodeficiencies, autoimmune diseases, alpha one antitrypsin deficiency, primary ciliary dyskinesia, and previous respiratory infections. Despite these workups, many cases of bronchiectasis are idiopathic [9], suggesting the presence of risk factors associated with personal habits and social, regional, and environmental factors. Smoking is one of the most important factors affecting the development of lung diseases such as COPD, lung cancer, and pulmonary tuberculosis (TB) [10,11,12,13]. Regarding bronchiectasis and smoking, several registry studies have suggested smoke exposure as a risk factor in pediatric patients with bronchiectasis [14,15,16]. However, it is largely unknown whether smoking is related to development of bronchiectasis in adults. Considering the importance of prevention of chronic disease at an earlier stage, the association of smoking with bronchiectasis in young adults could provide clinically relevant information.
Hence, this study aimed to investigate the association of smoking status with development of bronchiectasis in young adults using a nation-wide cohort of young adults.
2. Methods
2.1. Study Population
We used the Korean National Health Insurance Service (NHIS) database, the single-payer universal health system of Republic of Korea. The NHIS maintains claims data on all reimbursed inpatient and outpatient visits, procedures, and prescriptions. Additionally, the NHIS database includes data from annual or biennial health screening exams provided free of charge by the Ministry of Health and Welfare. Regardless of age, employees that pay insurance premiums, including the self-employed, are eligible for health examination every two years; employees engaged in manual labour receive health examination every year. For those who do not pay insurance premiums, health examination is provided to individuals ≥ 40 years of age every two years. Approximately 70–80% of all eligible persons underwent screening [17].
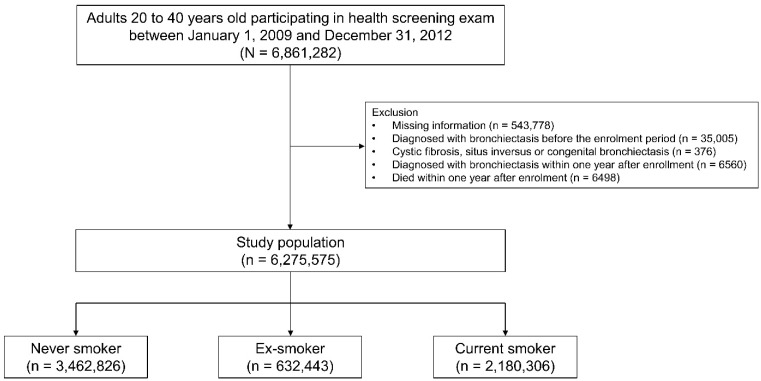
This study initially included 6,861,282 individuals, aged 20–39 years, who participated in a health screening exam between 1 January 2009 and 31 December 2012. We excluded the participants who had missing information for at least one of variables analyzed in this study (n = 543,778), those diagnosed with bronchiectasis before the enrollment period (n = 35,005), those diagnosed with cystic fibrosis, situs inversus, or congenital bronchiectasis before the enrolment period (n = 376), those diagnosed with bronchiectasis within one year after enrollment (n = 6560), and those who died within one year after enrollment (n = 6498). Finally, a total of 6,275,575 participants were included in this study (Figure 1). The cohort was followed from the enrollment date to the date of bronchiectasis development, death, or the end of the study period (31 December 2018), whichever came first (Supplemental Figure S1).
Figure 1.
Flow chart of the study population.
The study protocol was approved by the Institutional Review Board of Chungbuk National University Hospital (No. 2022-01-009). The requirement for informed consent was waived because the NHIS database was constructed after anonymization of patient data.
2.2. Exposure: Smoking Status
We obtained information on smoking status from the health examination database. Participants were asked to complete a self-administered questionnaire and to provide categorical responses to questions on smoking status (i.e., never smoker, ex-smoker, or current smoker). Current smokers recorded the total duration of smoking (years) and the average number of cigarettes smoked daily. The cumulative smoking exposure was reported as pack-years (PY) by multiplying the average cigarette consumption per day (packs) and the smoking period (years). We divided current smokers into two groups according to cumulative smoking exposure: less than 10 PY and more than 10 PY.
2.3. Outcome: Incident Bronchiectasis
The main study outcome was incidence of bronchiectasis according to smoking status. Bronchiectasis was defined by the following criteria: (1) at least one claim under the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) diagnosis code J47, and (2) exclusion of individuals with cystic fibrosis, situs inversus or congenital bronchiectasis [18,19,20,21].
2.4. Covariates
Covariates were determined based on the literature and data availability and included socioeconomic status, lifestyle factors, and comorbidities. Body mass index (BMI) was calculated as weight in kilograms divided by square of height in meters and was categorized according to Asian-specific criteria [22]. Data on alcohol consumption and regular physical activity were collected from self-administered questionnaires. Categories for alcohol consumption were none (0 g/day), mild (<30 g/day), and heavy (≥30 g/day) [19,23,24]. Categories for exercise were regular (>30 min of moderate physical at least 5 times per week or >20 min of strenuous physical activity at least 3 times per week) and non-regular [19,23,24]. Income level was dichotomized at the lowest 20%; low-income category also included Medicaid beneficiaries [19,23,24]. The residential area was stratified into urban and rural areas. Number of hospital visits counted the number of outpatient visits and the number of hospitalizations.
Comorbidities were assessed during the enrollment period and defined using the following ICD-10 codes: asthma (J45–J46), pulmonary TB (A15–A19), non-tuberculous mycobacterial infection (A310), diabetes mellitus (E11–E14), chronic kidney disease or end stage of renal disease (N18.1–N18.5 and N18.9), gastroesophageal reflux (K21), solid cancer (C00–C97), connective tissue disease (M05, M06, M32, M35, and M45), hematologic malignancy (C90, C910, C920, C921, C922, C924–926, C928, and C930), transplantation status (Z940, Z944, Z941, and Z942), human immunodeficiency virus (HIV) infection and acquired immune deficiency syndrome (AIDS) (B20–B24), and inflammatory bowel disease (K50–K51) [5,19,23,25].
2.5. Statistical Analysis
Baseline characteristics of participants are presented as means (standard deviations) or numbers (%) according to smoking status. The incidence rate of bronchiectasis was calculated by dividing the number of events by 1000 person-years (PY). Cox proportional hazards regression analyses were conducted to obtain the hazard ratios (HRs) and 95% confidence intervals (CIs) for the occurrence of bronchiectasis based on smoking status. Model 1 was unadjusted. In multivariable analyses, age, sex, BMI, alcohol consumption, regular exercise, low income, area of residence (rural or urban), and the number of hospital visits were adjusted in Model 2; comorbidities that might be associated with the occurrence of bronchiectasis (respiratory disease, connective tissue disease, solid cancer, hematologic malignancy, transplantation status, immunodeficiency, inflammatory bowel disease, and HIV and AIDS) were further adjusted in Model 3. Statistical analyses were conducted using SAS software (version 9.4; SAS Institute, Cary, NC, USA), and statistical significance was defined as a two-sided p value < 0.05.
3. Results
3.1. Baseline Characteristics
Table 1 summarized the baseline characteristics of the study population according to smoking status. The never-smokers were the youngest (30.1 ± 5.1 years), had the lowest BMI (22.2 ± 3.5 kg/m2), and had the smallest proportion of regular exercisers (11.3%) among the groups (p < 0.001 for all). However, the proportion of urban residents (48.9%) was highest in never-smokers (p < 0.001). On the other hand, current smokers had the highest proportions of males (93.2%) and of heavy alcohol consumption (17.3%) across smoking status categories (p < 0.001 for both).
Table 1.
Baseline characteristics of the study population according to smoking status.
| Smoking Status | |||||
|---|---|---|---|---|---|
| Total (N = 6,275,575) |
Never Smoker (n = 3,462,826) |
Ex-Smoker (n = 632,443) |
Current Smoker (n = 2,180,306) |
p-Value | |
| Age, years | 30.8 ± 4.9 | 30.1 ± 5.1 | 32.5 ± 4.64 | 31.4 ± 4.74 | <0.001 |
| <30 | 2,655,026 (42.4) | 1,675,479 (48.4) | 175,548 (27.7) | 803,999 (36.8) | <0.001 |
| ≥30 | 3,620,549 (57.6) | 1,787,347 (51.6) | 456,895 (72.3) | 1,376,307 (63.2) | |
| Sex | <0.001 | ||||
| Male | 3,712,379 (59.2) | 1,135,390 (32.8) | 546,321 (86.4) | 2,030,668 (93.2) | |
| Female | 2,563,196 (40.8) | 2,327,436 (67.2) | 86,122 (13.6) | 149,638 (6.8) | |
| BMI (kg/m2) | 23.0 ± 3.6 | 22.2 ± 3.5 | 24.0 ± 3.3 | 24.0 ± 3.6 | <0.001 |
| <18.5 kg/m2 | 476,686 (7.6) | 371,396 (10.7) | 19,801 (3.1) | 85,489 (3.9) | <0.001 |
| 18.5–22.9 kg/m2 | 2,931,905 (46.7) | 1,885,080 (54.4) | 227,953 (36.0) | 818,872 (37.5) | |
| 23–24.9 kg/m2 | 1,203,883 (19.2) | 557,097 (16.1) | 158,130 (25.0) | 488,656 (22.4) | |
| ≥25 kg/m2 | 1,663,101 (26.5) | 649,253 (18.8) | 226,559 (35.8) | 787,289 (36.1) | |
| Alcohol consumption | <0.001 | ||||
| None | 2,367,631 (37.7) | 1,811,004 (52.3) | 141,565 (22.4) | 415,062 (19.1) | |
| Mild | 3,353,737 (53.5) | 1,557,776 (45.0) | 408,841 (64.6) | 1,387,120 (63.6) | |
| Heavy | 554,207 (8.8) | 94,046(2.7) | 82,037 (13.0) | 378,124 (17.3) | |
| Regular exercise | <0.001 | ||||
| No | 5,470,001 (87.2) | 3,069,862 (88.7) | 518,788 (82.0) | 1,881,351 (86.3) | |
| Yes | 805,574 (12.8) | 392,964 (11.3) | 113,655 (18.0) | 298,955 (13.7) | |
| Low income | <0.001 | ||||
| No | 5,276,741 (84.1) | 2,812,455 (81.2) | 567,943 (89.8) | 1,896,343 (87.0) | |
| Yes | 998,834 (15.9) | 650,371 (18.8) | 64,500 (10.2) | 283,963 (13.0) | |
| Residence | <0.001 | ||||
| Rural | 3,276,315 (52.2) | 1,767,983 (51.1) | 330,394 (52.2) | 1,177,938 (54.0) | |
| Urban | 2,999,260 (47.8) | 1,694,843 (48.9) | 302,049 (47.8) | 1,002,368 (46.0) | |
| Number of hospital visits | 3.6 ± 6.5 | 3.9 ± 6.9 | 4.2 ± 7.2 | 2.9 ± 5.7 | <0.001 |
| Admission | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.1 ± 0.3 | <0.001 |
| Outpatient | 3.6 ± 6.5 | 3.9 ± 6.8 | 4.1 ± 7.1 | 2.9 ± 5.6 | <0.001 |
| Comorbidities | |||||
| DM | 122,007 (1.9) | 44,005 (1.2) | 15,258 (2.4) | 62,744 (2.9) | <0.001 |
| CKD or ESRD | 2317 (0.1) | 1280 (0.1) | 483 (0.1) | 554 (0.1) | <0.001 |
| GERD | 674,566 (10.8) | 414,329 (11.9) | 74,335 (11.7) | 185,902 (8.5) | <0.001 |
| Respiratory disease | 328,703 (5.3) | 208,670 (6.1) | 34,419 (5.5) | 85,614 (4.0) | <0.001 |
| Asthma | 326,557 (5.2) | 207,418 (6.0) | 34,133 (5.4) | 85,006 (3.9) | <0.001 |
| TB | 2418 (0.1) | 1437 (0.1) | 320 (0.1) | 661 (0.1) | <0.001 |
| NTM disease | 104 (<0.1) | 63 (<0.1) | 17 (<0.1) | 24 (<0.1) | 0.013 |
| Others | |||||
| Connective tissue disease | 44,670 (0.7) | 27,211 (0.8) | 4878 (0.8) | 12,581 (0.6) | <0.001 |
| Solid cancer | 12,563 (0.2) | 9782 (0.3) | 1685 (0.3) | 1096 (0.1) | <0.001 |
| Hematologic malignancy | 14 (<0.1) | 8 (<0.1) | 6 (<0.1) | 0 (<0.1) | <0.001 |
| Transplantation status | 53 (<0.1) | 25 (<0.1) | 19 (<0.1) | 9 (<0.1) | <0.001 |
| HIV/AIDS | 441 (<0.1) | 176 (<0.1) | 60 (<0.1) | 205 (<0.1) | <0.001 |
| Immunodeficiency | 295 (<0.1) | 210 (<0.1) | 26 (<0.1) | 59 (<0.1) | <0.001 |
| Inflammatory bowel disease | 7906 (0.2) | 4539 (0.1) | 1275 (0.2) | 2092 (0.1) | <0.001 |
Abbreviations: BMI, body mass index; DM, diabetes mellitus; CKD, chronic kidney disease; ESRD, end-stage renal disease; GERD, gastroesophageal reflux disease; TB, tuberculosis; NTM, nontuberculous mycobacteria; HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome.
Regarding comorbidities, current smokers had the largest proportion of diabetes mellitus (2.9%), whereas never smokers had the largest proportions of gastroesophageal reflux (11.9%) and respiratory disease (6.1%), most of which was asthma (6.0%), compared with other smoking status categories (p < 0.001 for all).
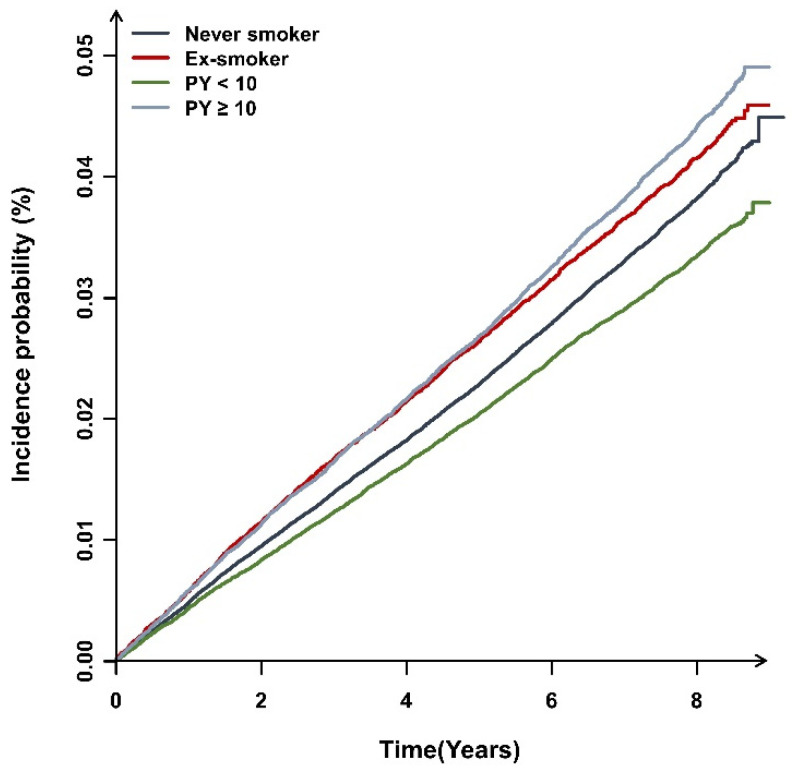
3.2. The Incidence of Bronchiectasis and Smoking
During a mean follow-up period of 7.4 (±1.1) years, 23,609 (0.3%) participants developed bronchiectasis. As shown in Figure 2, the cumulative incidence of bronchiectasis was significantly different according to smoking status (log-rank test, p < 0.001). As shown in Table 2, the HR of bronchiectasis development was significantly increased in ex-smokers (adjusted hazard ratio (aHR) in the Model 3 = 1.07, 95% CI 1.03–1.13) and current-smokers (aHR = 1.06 in the Model 3, 95% CI = 1.02–1.10), with the highest HR in ≥10 PY smokers (aHR in the Model 3 = 1.12, 95% CI = 1.06–1.16). However, the HR was not increased in <10 PY current smokers when compared to never-smokers (aHR in the Model 3 = 1.03, 95% CI = 0.98–1.07).
Figure 2.
Cumulative incidence of bronchiectasis according to smoking status. Abbreviation: PY, pack-years.
Table 2.
Association of smoking status with the incidence of bronchiectasis.
| HR (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Smoking Status | N | Bronchiectasis | Duration (PY) | IR per 1000 | Model 1 | Model 2 | Model 3 | |
| Overall | Never smoker | 3,462,826 | 12,884 | 25,333,8814 | 0.508 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 632,443 | 2639 | 4,731,442 | 0.557 | 1.09 (1.05,1.14) | 1.08 (1.03,1.13) | 1.07 (1.03,1.13) | |
| Current smoker | 2,180,306 | 8086 | 16,133,463 | 0.501 | 0.98 (0.95,1.01) | 1.05 (1.01,1.09) | 1.06 (1.02,1.10) | |
| Pack years < 10 | 1,358,100 | 4453 | 9,963,491 | 0.446 | 0.87 (0.84,0.91) | 1.03 (0.98,1.06) | 1.03 (0.98,1.07) | |
| Pack years ≥ 10 | 822,206 | 3633 | 6,169,972 | 0.588 | 1.15 (1.11,1.19) | 1.11 (1.05,1.15) | 1.12 (1.06,1.16) | |
| p for trend | 0.035 | <0.001 | <0.001 | |||||
| Sex | ||||||||
| Male | Never smoker | 1,135,390 | 4029 | 8,376,524 | 0.480 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 546,321 | 2321 | 4,119,567 | 0.563 | 1.17 (1.11,1.23) | 1.07 (1.01,1.12) | 1.06 (1.01,1.12) | |
| Current smoker | 2,030,668 | 7494 | 15,077,177 | 0.497 | 1.03 (0.99,1.07) | 1.03 (0.99,1.07) | 1.038(0.99,1.08) | |
| Pack years < 10 | 1,216,241 | 3911 | 8,960,985 | 0.436 | 0.90 (0.86,0.94) | 0.98 (0.94,1.03) | 0.99 (0.94,1.03) | |
| Pack years ≥ 10 | 814,427 | 3583 | 6,116,192 | 0.585 | 1.21 (1.16,1.27) | 1.09 (1.04,1.14) | 1.10 (1.05,1.15) | |
| p for trend | <0.001 | 0.018 | 0.005 | |||||
| Female | Never smoker | 2,327,436 | 8855 | 16,957,357 | 0.522 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 86,122 | 318 | 611,874 | 0.519 | 0.99 (0.89,1.12) | 1.07 (0.96,1.20) | 1.06 (0.95,1.19) | |
| Current smoker | 149,638 | 592 | 1,056,285 | 0.560 | 1.07 (0.99,1.17) | 1.21 (1.11,1.31) | 1.20 (1.10,1.31) | |
| Pack years < 10 | 141,859 | 542 | 1,002,505 | 0.540 | 1.04 (0.95,1.13) | 1.18 (1.08,1.29) | 1.18 (1.08,1.29) | |
| Pack years ≥ 10 | 7779 | 50 | 53,780 | 0.929 | 1.79 (1.36,2.37) | 1.51 (1.14,2.00) | 1.49 (1.12,1.97) | |
| p for trend | 0.041 | <0.001 | <0.001 | |||||
| p value | <0.001 | <0.001 | <0.001 | |||||
| p for interaction | <0.001 | <0.001 | <0.001 | |||||
| Age, years | ||||||||
| <30 | Never smoker | 1,675,479 | 4484 | 12,126,911 | 0.369 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 175,548 | 505 | 1,276,722 | 0.395 | 1.06 (0.97,1.17) | 1.13 (1.02,1.24) | 1.12 (1.02,1.24) | |
| Current smoker | 803,999 | 2127 | 5,826,907 | 0.365 | 0.98 (0.93,1.03) | 1.10 (1.03,1.17) | 1.10 (1.03,1.17) | |
| Pack years < 10 | 672,670 | 1730 | 4,855,497 | 0.356 | 0.96 (0.91,1.01) | 1.09 (1.02,1.16) | 1.09 (1.01,1.16) | |
| Pack years ≥ 10 | 131,329 | 397 | 971,410 | 0.408 | 1.10 (0.99,1.22) | 1.18 (1.05,1.32) | 1.18 (1.05,1.32) | |
| p for trend | 0.904 | 0.001 | 0.001 | |||||
| ≥30 | Never smoker | 1,787,347 | 8400 | 13,206,970 | 0.636 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 456,895 | 2134 | 3,454,719 | 0.617 | 0.97 (0.92,1.01) | 1.06 (1.01,1.12) | 1.05 (1.01,1.11) | |
| Current smoker | 1,376,307 | 5959 | 10,306,556 | 0.578 | 0.91 (0.87,0.93) | 1.03 (0.98,1.07) | 1.03 (0.99,1.08) | |
| Pack years < 10 | 685,430 | 2723 | 5,107,993 | 0.533 | 0.83 (0.80,0.87) | 0.98 (0.93,1.03) | 0.98 (0.94,1.04) | |
| Pack years ≥ 10 | 690,877 | 3236 | 5,198,562 | 0.622 | 0.97 (0.93,1.01) | 1.08 (1.03,1.14) | 1.09 (1.04,1.15) | |
| p for trend | <0.001 | 0.029 | 0.001 | |||||
| p for interaction | <0.001 | 0.022 | 0.036 | |||||
| BMI (kg/m2) | ||||||||
| <18.5 | Never smoker | 371,396 | 1716 | 2,709,061 | 0.633 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 19,801 | 111 | 144,234 | 0.769 | 1.21 (1.01,1.47) | 0.98 (0.80,1.21) | 0.98 (0.80,1.20) | |
| Current smoker | 85,489 | 443 | 623,242 | 0.710 | 1.12 (1.01,1.24) | 0.85 (0.74,0.98) | 0.86 (0.75,0.99) | |
| Pack years < 10 | 64,695 | 292 | 468,357 | 0.623 | 0.98 (0.87,1.11) | 0.86 (0.74,1.01) | 0.86 (0.74,1.01) | |
| Pack years ≥ 10 | 20,794 | 151 | 154,884 | 0.974 | 1.53 (1.29,1.81) | 0.85 (0.69,1.04) | 0.85 (0.69,1.05) | |
| p for trend | 0.001 | 0.038 | 0.046 | |||||
| 18.5–22.9 | Never smoker | 1,885,080 | 7056 | 13,792,785 | 0.511 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 227,953 | 1080 | 1,698,419 | 0.635 | 1.24 (1.16,1.32) | 1.16 (1.08,1.24) | 1.15 (1.07,1.23) | |
| Current smoker | 818,872 | 3209 | 6,054,509 | 0.530 | 1.03 (0.99,1.07) | 1.04 (0.98,1.09) | 1.04 (0.99,1.10) | |
| Pack years < 10 | 552,628 | 1917 | 4,050,839 | 0.473 | 0.92 (0.87,0.97) | 1.02 (0.96,1.08) | 1.02 (0.96,1.09) | |
| Pack years ≥ 10 | 266,244 | 1292 | 2,003,669 | 0.644 | 1.25 (1.18,1.33) | 1.07 (1.01,1.15) | 1.08 (1.01,1.16) | |
| p for trend | <0.001 | 0.163 | 0.099 | |||||
| 23–24.9 | Never smoker | 557,097 | 1877 | 4,088,205 | 0.459 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 158,130 | 625 | 1,192,235 | 0.524 | 1.14 (1.04,1.24) | 1.08 (0.98,1.19) | 1.07 (0.97,1.19) | |
| Current smoker | 488,656 | 1680 | 3,633,804 | 0.462 | 1.01 (0.94,1.07) | 1.04 (0.96,1.12) | 1.04 (0.96,1.13) | |
| Pack years < 10 | 298,815 | 887 | 2,203,629 | 0.402 | 0.87 (0.80,0.94) | 0.99 (0.90,1.08) | 0.99 (0.90,1.08) | |
| Pack years ≥ 10 | 189,841 | 793 | 1,430,175 | 0.554 | 1.20 (1.11,1.31) | 1.11 (1.01,1.22) | 1.12 (1.01,1.23) | |
| p for trend | 0.064 | 0.151 | 0.110 | |||||
| ≥25 | Never smoker | 649,253 | 2235 | 4,743,830 | 0.471 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 226,559 | 823 | 1,696,552 | 0.485 | 1.02 (0.94,1.11) | 1.03 (0.94,1.12) | 1.02 (0.94,1.12) | |
| Current smoker | 787,289 | 2754 | 5,821,907 | 0.473 | 1.00 (0.94,1.06) | 1.10 (1.03,1.17) | 1.10 (1.03,1.18) | |
| Pack years < 10 | 441,962 | 1357 | 3,240,663 | 0.418 | 0.88 (0.83,0.95) | 1.05 (0.97,1.13) | 1.05 (0.97,1.13) | |
| Pack years ≥ 10 | 345,327 | 1397 | 2,581,243 | 0.541 | 1.14 (1.07,1.22) | 1.16 (1.07,1.25) | 1.17 (1.08,1.26) | |
| p for trend | 0.046 | <0.001 | <0.001 | |||||
| p for interaction | 0.064 | 0.015 | 0.023 | |||||
Model 1 was unadjusted; Model 2 was adjusted for age, sex, body mass index, alcohol consumption, regular exercise, low income, residential area, and number of hospital visits; Model 3 was additionally adjusted for respiratory disease, connective tissue disease, solid cancer, hematologic malignancy, transplantation status, immunodeficiency, inflammatory bowel disease, and HIV or AIDS. Abbreviations: HR, hazard ratio; CI, confidence interval; PY, person-years; IR, incidence rate; BMI, body mass index; HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome.
The association of smoking with incident bronchiectasis was more profound in females than in males (p for interaction < 0.001), in the younger than in the older (p for interaction = 0.036), and in the overweight and obese than in the normal weight or underweight (p for interaction = 0.023).
3.3. Effect of Comorbid Profiles on the Relationship between Smoking Status and Incident Bronchiectasis
We further investigated the impact of comorbid profiles on the association of bronchiectasis with smoking status, which are related to the development of bronchiectasis (Table 3). Comorbid profiles (respiratory diseases, connective tissue diseases, inflammatory bowel diseases, and immunocompromised diseases) did not have a significant impact on the association between smoking status and incident bronchiectasis (p for interaction > 0.05 for all comorbidities).
Table 3.
Impact of comorbidities on the relationship between smoking status and incident bronchiectasis.
| HR (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Smoking Status | N | Bronchiectasis | Duration (PY) | IR per 1000 | Model 1 | Model 2 | Model 3 | |
| Respiratory disease | ||||||||
| No | Never smoker | 3,254,156 | 11,471 | 23,832,643 | 0.481 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 598,024 | 2380 | 4,477,618 | 0.531 | 1.10 (1.05,1.15) | 1.08 (1.03,1.14) | 1.08 (1.03,1.13) | |
| Current smoker | 2,094,692 | 7490 | 15,510,580 | 0.482 | 1.02 (0.93,1.03) | 1.06 (1.02,1.10) | 1.06 (1.02,1.10) | |
| Pack years < 10 | 1,304,433 | 4127 | 9,577,112 | 0.430 | 0.89 (0.86,0.92) | 1.03 (0.99,1.07) | 1.03 (0.99,1.07) | |
| Pack years ≥ 10 | 790,259 | 3363 | 5,933,468 | 0.566 | 1.17 (1.13,1.22) | 1.11 (1.06,1.17) | 1.11 (1.06,1.17) | |
| p for trend | 0.001 | <0.001 | <0.001 | |||||
| Yes | Never smoker | 208,670 | 1413 | 1,501,238 | 0.941 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 34,419 | 259 | 253,823 | 1.020 | 1.08 (0.95,1.23) | 1.01 (0.87,1.17) | 1.01 (0.87,1.17) | |
| Current smoker | 85,614 | 596 | 622,883 | 0.956 | 1.01 (0.92,1.12) | 1.02 (0.91,1.15) | 1.03 (0.93,1.14) | |
| Pack years < 10 | 53,667 | 326 | 386,379 | 0.843 | 0.89 (0.79,1.01) | 0.98 (0.85,1.12) | 0.98 (0.85,1.12) | |
| Pack years ≥ 10 | 31,947 | 270 | 236,504 | 1.141 | 1.21 (1.06,1.38) | 1.09 (0.93,1.28) | 1.09 (0.93,1.27) | |
| p for trend | 0.197 | 0.435 | 0.448 | |||||
| p for interaction | 0.957 | 0.877 | 0.891 | |||||
| Connective tissue disease | ||||||||
| No | Never smoker | 3,435,615 | 12,688 | 25,135,861 | 0.504 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 627,565 | 2597 | 4,695,583 | 0.553 | 1.09 (1.04,1.14) | 1.07 (1.02,1.13) | 1.07 (1.02,1.12) | |
| Current smoker | 2,167,725 | 7995 | 16,041,918 | 0.498 | 0.98 (0.95,1.01) | 1.05 (1.02,1.09) | 1.05 (1.02,1.09) | |
| Pack years < 10 | 1,350,665 | 4411 | 9,909,679 | 0.445 | 0.88 (0.85,0.91) | 1.02 (0.98,1.06) | 1.02 (0.98,1.07) | |
| Pack years ≥ 10 | 817,060 | 3584 | 6,132,238 | 0.584 | 1.15 (1.11,1.19) | 1.10 (1.05,1.15) | 1.11 (1.06,1.16) | |
| p for trend | 0.029 | <0.001 | <0.001 | |||||
| Yes | Never smoker | 27,211 | 196 | 198,020 | 0.989 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 4878 | 42 | 35,858 | 1.171 | 1.18 (0.85,1.65) | 1.35 (0.92,1.98) | 1.34 (0.92,1.96) | |
| Current smoker | 12,581 | 91 | 91,545 | 0.994 | 1.01 (0.78,1.29) | 1.19 (0.86,1.64) | 1.19 (0.86,1.63) | |
| Pack years < 10 | 7435 | 42 | 53,811 | 0.780 | 0.78 (0.56,1.10) | 0.97 (0.66,1.42) | 0.97 (0.66,1.42) | |
| Pack years ≥ 10 | 5146 | 49 | 37,733 | 1.298 | 1.31 (0.96,1.80) | 1.57 (1.06,2.34) | 1.58 (1.06,2.35) | |
| p for trend | 0.483 | 0.114 | 0.111 | |||||
| p for interaction | 0.690 | 0.495 | 0.508 | |||||
| Inflammatory bowel disease | ||||||||
| No | Never smoker | 3,458,287 | 12,862 | 25,300,827 | 0.508 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 631,168 | 2625 | 4,721,944 | 0.555 | 1.09 (1.04,1.13) | 1.08 (1.03,1.13) | 1.07 (1.02,1.12) | |
| Current smoker | 2,178,214 | 8067 | 16,118,195 | 0.500 | 0.98 (0.95,1.01) | 1.06 (1.01,1.09) | 1.06 (1.02,1.09) | |
| Pack years < 10 | 1,356,726 | 4442 | 9,953,533 | 0.446 | 0.87 (0.84,0.90) | 1.02 (0.98,1.06) | 1.02 (0.98,1.06) | |
| Pack years ≥ 10 | 821,488 | 3625 | 6,164,661 | 0.588 | 1.15 (1.11,1.19) | 1.10 (1.05,1.15) | 1.11 (1.06,1.16) | |
| p for trend | 0.042 | <0.001 | <0.001 | |||||
| Yes | Never smoker | 4539 | 22 | 33,054 | 0.665 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 1275 | 14 | 9497 | 1.474 | 2.21 (1.13,4.33) | 1.80 (0.86,3.78) | 1.80 (0.85,3.78) | |
| Current smoker | 2092 | 19 | 15,268 | 1.244 | 1.86 (1.01,3.45) | 1.59 (0.79,3.19) | 1.55 (0.77,3.11) | |
| Pack years < 10 | 1374 | 11 | 9957 | 1.104 | 1.65 (0.80,3.42) | 1.46 (0.66,3.21) | 1.42 (0.64,3.14) | |
| Pack years ≥ 10 | 718 | 8 | 5310 | 1.506 | 2.26 (1.01,5.08) | 1.84 (0.74,4.55) | 1.83 (0.74,4.54) | |
| p for trend | 0.029 | 0.195 | 0.208 | |||||
| p for interaction | 0.110 | 0.138 | 0.149 | |||||
| Solid cancer or Hematologic malignancy | ||||||||
| No | Never smoker | 3,453,036 | 12,830 | 25,263,418 | 0.507 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 630,754 | 2631 | 4,718,957 | 0.557 | 1.09 (1.05,1.14) | 1.08 (1.03,1.13) | 1.07 (1.02,1.12) | |
| Current smoker | 2,179,210 | 8081 | 16,125,496 | 0.501 | 0.98 (0.95,1.01) | 1.05 (1.02,1.09) | 1.06 (1.02,1.09) | |
| Pack years < 10 | 1,357,444 | 4448 | 9,958,767 | 0.446 | 0.87 (0.85,0.91) | 1.02 (0.98,1.06) | 1.02 (0.98,1.06) | |
| Pack years ≥ 10 | 821,766 | 3633 | 6,166,728 | 0.589 | 1.15 (1.11,1.20) | 1.10 (1.05,1.15) | 1.11 (1.06,1.16) | |
| p for trend | 0.027 | <0.001 | <0.001 | |||||
| Yes | Never smoker | 9790 | 54 | 70,462 | 0.766 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 1689 | 8 | 12,484 | 0.640 | 0.82 (0.39,1.72) | 1.26 (0.49,3.18) | 1.26 (0.49,3.19) | |
| Current smoker | 1096 | 5 | 7967 | 0.627 | 0.81 (0.32,2.02) | 1.44 (0.48,4.28) | 1.44 (0.48,4.28) | |
| Pack years < 10 | 656 | 5 | 4724 | 1.058 | 1.36 (0.54,3.41) | 2.19 (0.77,6.23) | 2.18 (0.76,6.20) | |
| Pack years ≥ 10 | 440 | 0 | 3243 | 0 | NA | NA | NA | |
| p for trend | 0.362 | 0.807 | 0.814 | |||||
| p for interaction | 0.637 | 0.657 | 0.651 | |||||
| Immunocompromised disease * | ||||||||
| No | Never smoker | 3,462,415 | 12,880 | 25,330,891 | 0.508 | 1 (reference) | 1 (reference) | 1 (reference)z |
| Ex-smoker | 632,338 | 2638 | 4,730,674 | 0.557 | 1.09 (1.05,1.14) | 1.08 (1.03,1.13) | 1.07 (1.02,1.12) | |
| Current smoker | 2,180,033 | 8085 | 16,131,533 | 0.501 | 0.98 (0.95,1.01) | 1.05 (1.01,1.09) | 1.06 (1.02,1.09) | |
| Pack years < 10 | 1,357,914 | 4453 | 9,962,181 | 0.446 | 0.87 (0.84,0.90) | 1.02 (0.98,1.06) | 1.02 (0.98,1.07) | |
| Pack years ≥ 10 | 822,119 | 3632 | 6,169,352 | 0.588 | 1.15 (1.11,1.19) | 1.10 (1.05,1.15) | 1.11 (1.06,1.16) | |
| p for trend | 0.034 | <0.001 | <0.001 | |||||
| Yes | Never smoker | 411 | 4 | 2989 | 1.337 | 1 (reference) | 1 (reference) | 1 (reference) |
| Ex-smoker | 105 | 1 | 767 | 1.303 | 0.98 (0.11,8.78) | 2.24 (0.15,32.63) | 1.50 (0.07,28.54) | |
| Current smoker | 273 | 1 | 1929 | 0.518 | 0.38 (0.04,3.41) | 1.31 (0.08,20.82) | 2.18 (0.09,51.96) | |
| Pack years < 10 | 186 | 0 | 1309 | 0 | NA | NA | NA | |
| Pack years ≥ 10 | 87 | 1 | 620 | 1.612 | 1.19 (0.13,10.72) | 6.93 (0.24,199.33) | 9.62 (0.22,416.78) | |
| p for trend | 0.560 | 0.511 | 0.351 | |||||
| p for interaction | 0.992 | 0.987 | 0.863 | |||||
Model 1 was unadjusted; Model 2 was adjusted for age, sex, BMI, alcohol consumption, regular exercise, low income, residential area, and number of hospital visits; Model 3 was additionally adjusted for respiratory disease, connective tissue disease, solid cancer, hematologic malignancy, transplantation status, immunodeficiency, inflammatory bowel disease, and HIV or AIDS. * Immunocompromised disease comprised immunodeficiency, organ transplantation, and HIV or AIDS. Abbreviations: HR, hazard ratio; CI, confidence interval; PY, person-years; IR, incidence rate; BMI, body mass index; HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; NA, not applicable.
4. Discussion
This population-based longitudinal cohort study assessed the association of smoking status with incident bronchiectasis among young adults. Smokers were more likely to be diagnosed with bronchiectasis than never-smokers, which was especially high in ≥10 PY current smokers. The association of smoking with bronchiectasis was more profound in females than in males, in the younger (20–29 years) than in the older (30–39 years), and in the overweight and obese than in the normal weight or underweight. On the other hand, comorbid profiles (respiratory diseases, connective tissue diseases, inflammatory bowel diseases, and immunocompromised diseases) did not have a significant impact on the association between smoking status and bronchiectasis.
Smoking is one of the most important factors influencing the development of chronic lung diseases such as COPD, lung cancer, and pulmonary TB [10,11,12,13]. However, the association between smoking and incident bronchiectasis has not been well elucidated. Studies so far have focused only on the association between smoking and the worse clinical outcomes of patients with bronchiectasis [25,26]. To the best of our knowledge, this study is the first to evaluate the association between smoking status and incident bronchiectasis in young adults. Our study also has the advantage of considering personal habits, socioeconomic factors, and comorbidities that might affect the development of bronchiectasis.
One possible explanation for the higher association with bronchiectasis in smokers when compared to never-smokers might be related to smoking-related impaired lung defense mechanisms, which increase the risk of respiratory infections. Chronic infection is a major component of the pathophysiologic mechanism of bronchiectasis [27]. Smoking facilitates recurrent respiratory infection through alterations in mechanisms of the host defense system including structural changes in the respiratory tract, a decrease in mucociliary clearance, and a decrease in immune response such as decreased secretary immunoglobulin [28,29,30]. Furthermore, it is also well demonstrated that respiratory infection can be a key player in the development of bronchiectasis in patients with COPD [28]. Another possible explanation is that smoking increases the inflammatory activities of the airways. Oxides in cigarette smoke can cause airway inflammation and tissue damage through various kinds of protease including neutrophil elastase and matrix metalloproteinase [31], which are known to play a critical role in the development and progression of bronchiectasis. For example, neutrophil elastase destroys the extracellular matrix, increases mucous gland proliferation and mucus production, reduces ciliary body beat rate, and damages airway epithelium directly [32,33,34]. Furthermore, the effect of smoking on incident bronchiectasis was significant in ≥10 PY smokers as compared to never smokers, although the effect was insignificant in <10 PY smokers. The phenomenon might be explained by the dose–response relationships; smoking amounts of <10 PY might not be enough to result in incident bronchiectasis.
Interestingly, our subgroup analyses showed that the association of smoking history with incident bronchiectasis was greater in females, the younger (20–29 years), and the overweight and obese than in males, the older (30–39 years), and the normal weight or underweight, respectively. The risk of developing a chronic respiratory disease associated with smoking, such as lung cancer and COPD, is greater in females than in males, and it is thought to be related to sex disparity [35]. Supporting this, it was found that smoking affects the development of COPD and lung cancer by increasing the expression of the estrogen receptor. Smoking aggravates the effects of estrogen and endocrine disruptive chemicals that target the estrogen receptor to contribute to lung carcinogenesis [36]. In addition, a previous study showed that the expression of estrogen receptors in lung tissue in a mouse model of COPD was associated with increased small airway remodeling in females when compared to male mice with chronic smoke exposure [37]. The excess risk of small airway disease in female mice after chronic smoke exposure was associated with increased oxidative stress and TGF-β1 signaling, and estrogen might regulate this oxidant/TGF-β signaling axis. Although little information is available about the mechanism of how smoking influences the development of bronchiectasis, these previous findings suggest that similar pathophysiology might be applicable in bronchiectasis [37,38].
Obesity can play a significant role in the pathogenesis of pulmonary diseases through mechanisms that involve the release of proinflammatory mediators by adipose tissue [39]. Inflammatory responses in the lungs are influenced by adipocytokines, leptin and adiponectin, cytokines, acute phase proteins, and other mediators produced by adipose tissue [40,41,42]. These findings support the notion that obesity increases lung inflammation. Smoking has been reported to exacerbate systemic inflammation caused by obesity and is believed to increase lung inflammation. Because of the free radicals present in smoking, and the inflammatory response they induce, obesity-induced oxidative stress (production of reactive oxygen species) is enhanced, which increases systemic inflammation [43]. These mechanisms suggest that smoking has a greater effect on the obese than on those of normal weight or underweight in the development of bronchiectasis. Our study also showed that the effect of smoking on the development of bronchiectasis is greater with younger than older. This finding renders an important clinical implication emphasizing that quitting smoking might be more effective in younger participants than in older participants to prevent bronchiectasis. However, since the mechanism explaining this phenomenon is not clear, thus, future studies are needed on this issue.
There are several limitations to this study. First, the diagnosis of bronchiectasis was based on the ICD-10 diagnosis code and not on chest computed tomography results. Thus, there could be misclassification of the diagnosis of bronchiectasis. Second, there might be a bias that smokers underwent more chest computed tomography than did non-smokers, which might have led to more diagnoses of bronchiectasis in smokers than in never-smokers. Third, due to the lack of data on childhood respiratory infection, an important risk factor of bronchiectasis in young adults, our study could not consider this in our analyses. Fourth, as we solely focused on the smoking amount and the risk of bronchiectasis in current smokers; the association between smoking amount and the risk of bronchiectasis in ex-smokers should be investigated in future studies.
5. Conclusions
This large, nationwide, longitudinal study demonstrated that smoking history is associated with incident bronchiectasis development in young adults. The association of smoking with incident bronchiectasis was more prominent in females, younger individuals (20–29 years), and overweight and obese participants than in their respective counterparts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jpm12050691/s1, Figure S1: Study periods and follow up.
Author Contributions
Literature search—All authors; Study design—B.Y., K.H., H.C. and H.L.; Data analysis—K.H. and B.K.; Data interpretation—All authors; Writing—B.Y., H.K.K., J.S.K., E.-G.K., H.C. and H.L.; Tables and figures—B.Y., K.H., H.C. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation (NRF) of Korea grant funded by the Ministry of Science, Information and Communications Technologies (MSIT) (No. 2020R1F1A1070468 to H.L.), the Bio & Medical Technology Development Program of the NRF of Korea funded by the MSIT (No. NRF-2021M3E5D1A01015176 to H.L.), and the Korean Ministry of Education (No. 2021R1I1A3052416 to H.C.). This work was also supported by the grant from the NRF of Korea (2020R1A5A2017476 to E.-G.K.).
Institutional Review Board Statement
This study protocol was approved by the Institutional Review Board of Chungbuk National University Hospital (No. 2022-01-009).
Informed Consent Statement
The requirement for informed consent was waived because the NHIS database was constructed after the anonymization of patient data.
Data Availability Statement
Data is available from the National Health Insurance Service database, the single-payer universal health system of Republic of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Imam J.S., Duarte A.G. Non-CF bronchiectasis: Orphan disease no longer. Respir. Med. 2020;166:105940. doi: 10.1016/j.rmed.2020.105940. [DOI] [PubMed] [Google Scholar]
- 2.King P.T. The pathophysiology of bronchiectasis. Int. J. Chronic Obstr. Pulm. Dis. 2009;4:411–419. doi: 10.2147/COPD.S6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quint J.K., Millett E.R., Joshi M., Navaratnam V., Thomas S.L., Hurst J.R., Smeeth L., Brown J.S. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: A population-based cohort study. Eur. Respir. J. 2016;47:186–193. doi: 10.1183/13993003.01033-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang B., Choi H., Lim J.H., Park H.Y., Kang D., Cho J., Lee J.S., Lee S.W., Oh Y.M., Moon J.Y., et al. The disease burden of bronchiectasis in comparison with chronic obstructive pulmonary disease: A national database study in Korea. Ann. Transl. Med. 2019;7:770. doi: 10.21037/atm.2019.11.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi H., Yang B., Kim Y.J., Sin S., Jo Y.S., Kim Y., Park H.Y., Ra S.W., Oh Y.M., Chung S.J., et al. Increased mortality in patients with non cystic fibrosis bronchiectasis with respiratory comorbidities. Sci. Rep. 2021;11:7126. doi: 10.1038/s41598-021-86407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diel R., Chalmers J.D., Rabe K.F., Nienhaus A., Loddenkemper R., Ringshausen F.C. Economic burden of bronchiectasis in Germany. Eur. Respir. J. 2019;53:1802033. doi: 10.1183/13993003.02033-2018. [DOI] [PubMed] [Google Scholar]
- 7.Polverino E., Goeminne P.C., McDonnell M.J., Aliberti S., Marshall S.E., Loebinger M.R., Murris M., Cantón R., Torres A., Dimakou K., et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017;50:1700629. doi: 10.1183/13993003.00629-2017. [DOI] [PubMed] [Google Scholar]
- 8.Hill T.A., Sullivan L.A., Chalmers D.J., De Soyza A., Stuart Elborn J., Andres Floto R., Grillo L., Gruffydd-Jones K., Harvey A., Haworth S.C., et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax. 2019;74:1–69. doi: 10.1136/thoraxjnl-2018-212463. [DOI] [PubMed] [Google Scholar]
- 9.Lee H., Choi H., Chalmers J.D., Dhar R., Nguyen T.Q., Visser S.K., Morgan L.C., Oh Y.-M. Characteristics of bronchiectasis in Korea: First data from the Korean Multicentre Bronchiectasis Audit and Research Collaboration registry and comparison with other international registries. Respirology. 2021;26:619–621. doi: 10.1111/resp.14059. [DOI] [PubMed] [Google Scholar]
- 10.Salvi S. Tobacco smoking and environmental risk factors for chronic obstructive pulmonary disease. Clin. Chest Med. 2014;35:17–27. doi: 10.1016/j.ccm.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Gupta N., Malhotra N., Ish P. GOLD 2021 guidelines for COPD—What’s new and why. Adv. Respir. Med. 2021;89:344–346. doi: 10.5603/ARM.a2021.0015. [DOI] [PubMed] [Google Scholar]
- 12.Silva D.R., Muñoz-Torrico M., Duarte R., Galvão T., Bonini E.H., Arbex F.F., Arbex M.A., Augusto V.M., Rabahi M.F., Mello F.C.Q. Risk factors for tuberculosis: Diabetes, smoking, alcohol use, and the use of other drugs. J. Bras. Pneumol. Publ. Soc. Bras. Pneumol. Tisilogia. 2018;44:145–152. doi: 10.1590/s1806-37562017000000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loeb L.A., Ernster V.L., Warner K.E., Abbotts J., Laszlo J. Smoking and lung cancer: An overview. Cancer Res. 1984;44:5940–5958. [PubMed] [Google Scholar]
- 14.Singleton R., Morris A., Redding G., Poll J., Holck P., Martinez P., Kruse D., Bulkow L.R., Petersen K.M., Lewis C. Bronchiectasis in Alaska Native children: Causes and clinical courses. Pediatric Pulmonol. 2000;29:182–187. doi: 10.1002/(SICI)1099-0496(200003)29:3<182::AID-PPUL5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 15.Edwards E.A., Asher M.I., Byrnes C.A. Paediatric bronchiectasis in the twenty-first century: Experience of a tertiary children’s hospital in New Zealand. J. Paediatr. Child Health. 2003;39:111–117. doi: 10.1046/j.1440-1754.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 16.Das L., Kovesi T.A. Bronchiectasis in children from Qikiqtani (Baffin) Region, Nunavut, Canada. Ann. Am. Thorac. Soc. 2015;12:96–100. doi: 10.1513/AnnalsATS.201406-257OC. [DOI] [PubMed] [Google Scholar]
- 17.Song S.O., Jung C.H., Song Y.D., Park C.Y., Kwon H.S., Cha B.S., Park J.Y., Lee K.U., Ko K.S., Lee B.W. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab. J. 2014;38:395–403. doi: 10.4093/dmj.2014.38.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi H., Yang B., Nam H., Kyoung D.-S., Sim Y.S., Park H.Y., Lee J.S., Lee S.W., Oh Y.-M., Ra S.W., et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur. Respir. J. 2019;54:1900194. doi: 10.1183/13993003.00194-2019. [DOI] [PubMed] [Google Scholar]
- 19.Yang B., Han K., Kim S.H., Lee D.H., Park S.H., Yoo J.E., Shin D.W., Choi H., Lee H. Being Underweight Increases the Risk of Non-Cystic Fibrosis Bronchiectasis in the Young Population: A Nationwide Population-Based Study. Nutrients. 2021;13:3206. doi: 10.3390/nu13093206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi H., Lee H., Ryu J., Chung S.J., Park D.W., Sohn J.W., Yoon H.J., Kim S.H. Bronchiectasis and increased mortality in patients with corticosteroid-dependent severe asthma: A nationwide population study. Ther. Adv. Respir. Dis. 2020;14:1753466620963030. doi: 10.1177/1753466620963030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang B., Lee D.-H., Han K., Choi H., Kang H.K., Shin D.W., Lee H. Female Reproductive Factors and the Risk of Bronchiectasis: A Nationwide Population-Based Longitudinal Study. Biomedicines. 2022;10:303. doi: 10.3390/biomedicines10020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim M.K., Lee W.Y., Kang J.H., Kang J.H., Kim B.T., Kim S.M., Kim E.M., Suh S.H., Shin H.J., Lee K.R., et al. 2014 clinical practice guidelines for overweight and obesity in Korea. Endocrinol. Metab. 2014;29:405–409. doi: 10.3803/EnM.2014.29.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoo J.E., Kim D., Han K., Rhee S.Y., Shin D.W., Lee H. Diabetes Status and Association With Risk of Tuberculosis Among Korean Adults. JAMA Netw. Open. 2021;4:e2126099. doi: 10.1001/jamanetworkopen.2021.26099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi H., Han K., Yang B., Shin D.W., Sohn J.W., Lee H. Female reproductive factors and incidence of non-tuberculous mycobacterial pulmonary disease among postmenopausal women in Korea. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022:ciac134. doi: 10.1093/cid/ciac134. [DOI] [PubMed] [Google Scholar]
- 25.Sin S., Yun S.Y., Kim J.M., Park C.M., Cho J., Choi S.M., Lee J., Park Y.S., Lee S.M., Yoo C.G., et al. Mortality risk and causes of death in patients with non-cystic fibrosis bronchiectasis. Respir. Res. 2019;20:271. doi: 10.1186/s12931-019-1243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goeminne P.C., Nawrot T.S., Ruttens D., Seys S., Dupont L.J. Mortality in non-cystic fibrosis bronchiectasis: A prospective cohort analysis. Respir. Med. 2014;108:287–296. doi: 10.1016/j.rmed.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Flume P.A., Chalmers J.D., Olivier K.N. Advances in bronchiectasis: Endotyping, genetics, microbiome, and disease heterogeneity. Lancet. 2018;392:880–890. doi: 10.1016/S0140-6736(18)31767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez-García M., de la Rosa-Carrillo D., Soler-Cataluña J.J., Catalan-Serra P., Ballester M., Roca Vanaclocha Y., Agramunt M., Ballestin J., Garcia-Ortega A., Oscullo G., et al. Bronchial Infection and Temporal Evolution of Bronchiectasis in Patients With Chronic Obstructive Pulmonary Disease. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021;72:403–410. doi: 10.1093/cid/ciaa069. [DOI] [PubMed] [Google Scholar]
- 29.Arcavi L., Benowitz N.L. Cigarette smoking and infection. Arch. Intern. Med. 2004;164:2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 30.Marcy T.W., Merrill W.W. Cigarette smoking and respiratory tract infection. Clin. Chest Med. 1987;8:381–391. doi: 10.1016/S0272-5231(21)01035-2. [DOI] [PubMed] [Google Scholar]
- 31.Ishii Y. Smoking and respiratory diseases. Nihon Rinsho. Jpn. J. Clin. Med. 2013;71:416–420. [PubMed] [Google Scholar]
- 32.Gramegna A., Amati F., Terranova L., Sotgiu G., Tarsia P., Miglietta D., Calderazzo M.A., Aliberti S., Blasi F. Neutrophil elastase in bronchiectasis. Respir. Res. 2017;18:211. doi: 10.1186/s12931-017-0691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sagel S.D., Wagner B.D., Anthony M.M., Emmett P., Zemanick E.T. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2012;186:857–865. doi: 10.1164/rccm.201203-0507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chalmers J.D., Finch S. Sputum colour in non-CF bronchiectasis: The original neutrophil biomarker. Respirology. 2014;19:153–154. doi: 10.1111/resp.12228. [DOI] [PubMed] [Google Scholar]
- 35.Vidaillac C., Yong V.F.L., Jaggi T.K., Soh M.M., Chotirmall S.H. Gender differences in bronchiectasis: A real issue? Breathe. 2018;14:108–121. doi: 10.1183/20734735.000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stabile L.P., Siegfried J.M. Estrogen receptor pathways in lung cancer. Curr. Oncol. Rep. 2004;6:259–267. doi: 10.1007/s11912-004-0033-2. [DOI] [PubMed] [Google Scholar]
- 37.Tam A., Churg A., Wright J.L., Zhou S., Kirby M., Coxson H.O., Lam S., Man S.F., Sin D.D. Sex Differences in Airway Remodeling in a Mouse Model of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2016;193:825–834. doi: 10.1164/rccm.201503-0487OC. [DOI] [PubMed] [Google Scholar]
- 38.Wang R.D., Tai H., Xie C., Wang X., Wright J.L., Churg A. Cigarette smoke produces airway wall remodeling in rat tracheal explants. Am. J. Respir. Crit. Care Med. 2003;168:1232–1236. doi: 10.1164/rccm.200307-1006OC. [DOI] [PubMed] [Google Scholar]
- 39.Mancuso P. Obesity and lung inflammation. J. Appl. Physiol. 2010;108:722–728. doi: 10.1152/japplphysiol.00781.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexeeff S.E., Litonjua A.A., Suh H., Sparrow D., Vokonas P.S., Schwartz J. Ozone exposure and lung function: Effect modified by obesity and airways hyperresponsiveness in the VA normative aging study. Chest. 2007;132:1890–1897. doi: 10.1378/chest.07-1126. [DOI] [PubMed] [Google Scholar]
- 41.Bellmeyer A., Martino J.M., Chandel N.S., Scott Budinger G.R., Dean D.A., Mutlu G.M. Leptin resistance protects mice from hyperoxia-induced acute lung injury. Am. J. Respir. Crit. Care Med. 2007;175:587–594. doi: 10.1164/rccm.200603-312OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett W.D., Hazucha M.J., Folinsbee L.J., Bromberg P.A., Kissling G.E., London S.J. Acute pulmonary function response to ozone in young adults as a function of body mass index. Inhal. Toxicol. 2007;19:1147–1154. doi: 10.1080/08958370701665475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Vaart H., Postma D.S., Timens W., Ten Hacken N.H. Acute effects of cigarette smoke on inflammation and oxidative stress: A review. Thorax. 2004;59:713–721. doi: 10.1136/thx.2003.012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available from the National Health Insurance Service database, the single-payer universal health system of Republic of Korea.