Abstract
During Epstein–Barr virus (EBV) lytic replication, viral DNA synthesis is carried out in viral replication factories called replication compartments (RCs), which are located at discrete sites in the nucleus. Viral proteins constituting the viral replication machinery are accumulated in the RCs to amplify viral genomes. Newly synthesized viral DNA is stored in a subdomain of the RC termed the BMRF1-core, matured by host factors, and finally packed into assembled viral capsids. Late (L) genes are transcribed from DNA stored in the BMRF1-core through a process that is mainly dependent on the viral pre-initiation complex (vPIC). RC formation is a well-regulated system and strongly advantageous for EBV survival because of the following aspects: (1) RCs enable the spatial separation of newly synthesized viral DNA from the cellular chromosome for protection and maturation of viral DNA; (2) EBV-coded proteins and their interaction partners are recruited to RCs, which enhances the interactions among viral proteins, cellular proteins, and viral DNA; (3) the formation of RCs benefits continuous replication, leading to L gene transcription; and (4) DNA storage and maturation leads to efficient progeny viral production. Here, we review the state of knowledge of this important viral structure and discuss its roles in EBV survival.
Keywords: Epstein–Barr virus, lytic replication, replication compartment, replication, transcription, DNA damage response, viral pre-initiation complex (vPIC)
1. Introduction
Epstein–Barr virus (EBV) is a Gammaherpesvirinae family member and a human lymphotropic herpesvirus. EBV is widely spread among humans and transmitted via saliva, mainly during childhood. EBV is associated with various tumors, such as Hodgkin’s lymphoma, diffuse large B-cell lymphoma, Burkitt lymphoma, and NK/T-cell lymphoma [1,2,3,4,5]. Furthermore, it is closely linked to gastric cancer, nasopharyngeal carcinoma, and breast cancer, as it can also infect epithelial cells [6,7,8]. Epidemiologically, human tumors associated with EBV are now estimated at around 200,000 cases per year [1]. Upon primary infection, EBV infects resting B lymphocytes to establish a lifelong continuous latent infection without the production of progeny viruses, while expressing a limited set of genes (latent genes) [9,10,11]. On the other hand, productive replication is initiated by the expression of BZLF1, an EBV-coding lytic switch gene that induces reactivation [12,13]. Lytic reactivation occurs spontaneously and requires the EBV origin of lytic replication (oriLyt) that lies on the viral genome [14,15]. During lytic replication, large numbers of lytic genes are sequentially expressed. Depending on the stage of lytic replication, these genes are classified into immediate early (IE), early (E), and late (L) genes. The EBV genome is amplified 100- to 1000-fold by rolling circle replication using the EBV replication machinery. This machinery consists of EBV-coding replication proteins, which are E gene products [16]. The synthesized viral DNA is produced as DNA concatemers consisting of multiple head-to-tail repeats of single EBV genome units [17]. This concatemeric DNA is cleaved, and a single unit of viral DNA is packed into assembled capsids using capsid packaging proteins, thus producing viral particles [18].
The major feature of EBV lytic replication is the formation of a replication compartment (RC) [19,20,21]. RCs are viral replication factories composed of viral replication proteins that appear at discrete sites in the nuclei during lytic replication [22]. Since viral genomes are synthesized in RCs, RCs enlarge and appear as large globular nuclear subdomains at the late stage of the lytic phase [19]. In addition to DNA synthesis, RCs are sites of viral gene transcription, capsid assembly, and other regulations required to carry out lytic replication. In the RC, a sub-domain called the BMRF1-core assembles and stores the viral DNA [23]. Inhibition of RC formation by drugs such as phosphonoacetic acid (PAA) prevents the production of progeny viruses [19]. In this review, we focus on RCs and their sub-domain the BMRF1-core during the lytic phase and discuss this highly regulated system and the strong advantages of RC formation as a strategy for EBV production, including efficient genome replication, maintenance of genome stability by modification of host factors, and regulation of viral gene transcription.
2. RC Characterization
Various microscopic analyses have revealed that most viruses, including DNA and RNA viruses, amplify their own genomes at the site of replication [24,25,26,27]. In particular, double-stranded (ds) DNA viruses, such as Herpesviridae, Adenoviridae, Polyomaviridae, and Papillomviridae, replicate within RCs at discrete sites in the nuclei (also known as “replication centers” or “replication foci”), which are not covered by the cellular membrane. RCs may form through liquid–liquid phase separation (LLPS), like other membraneless nuclear bodies [28,29,30]. Once viral productive replication is induced, the size of RCs increases over time because viral and host replication proteins are recruited, and newly synthesized viral genomes are stored. Some inhibitors of viral replication, such as PAA, prevent RC formation [19,31]. In most cases, RCs are also the sites of viral capsid or virion assembly. This allows immediate packaging of the synthesized and stored genomes into capsids or virions [32]. Such efficient production of virions leads to the infection of more cells, thereby elevating tumorigenic potential [33]. Hence, RCs are thought to be the center of dsDNA viral productive replication and have been well-studied as therapeutic targets of infectious diseases and tumors caused by these dsDNA viruses [28,33].
In particular, the RCs of human herpesviruses have been extensively studied. Herpesviruses are widespread, adapted to human life, and are associated with various diseases and tumors. RCs are closely linked to the unique life cycles and pathogenesis of these viruses [34,35]. The life cycle of herpesviruses has two types of phases: a latent phase and a lytic phase. EBV is a Gammaherpesvirinae family member strongly associated with various human cancers [36,37]. EBV establishes lifelong latency after primary infection and immortalizes infected cells. During the latent phase, EBV genomes form episomal structures expressing a limited set of viral genes, called latent genes, in the absence of productive replication or RC formation. The switch from latency to lytic replication (termed “reactivation”) occurs spontaneously, starting with the expression of BZLF1, an EBV IE gene. During the lytic phase, herpesviruses compose their own replication machinery, the RCs, which mainly consist of herpesvirus replication proteins [22]. In the case of EBV, BMRF1 (DNA polymerase processivity factor and dsDNA binding protein), BALF2 (single-stranded (ss) DNA binding protein), BALF5 (DNA polymerase), and BBLF4/BSLF1/BBLF2/3 (the helicase–primase complex) have been identified as replication proteins and are known as the main components of RCs. These proteins are well-conserved among human herpesviruses [22]. Although BZLF1 acts as a lytic switch mediator at the early stage of the lytic phase and is distributed diffusely throughout the nuclei, BZLF1 moves to the RCs to associate with viral DNA and BBLF4 [21,34]. In addition to replication proteins and lytic regulators, viral factors, such as BKRF3 (uracil DNA glycosylase), BGLF4 (protein kinase), and BPLF1 (deubiquitinase), also accumulate in RCs [38,39,40] (listed in Table 1). BKRF3 is required for viral DNA synthesis, along with BALF5 and BMRF1 interaction [38]. The advantages of BGLF4 and BPLF1 accumulation to RCs are meticulously described in Section 3 and Section 5. EBV infects both B lymphocytes and epithelial cells, and RCs can be observed in both types of cell lines upon artificial lytic induction. Similar to what happens with other dsDNA viruses, host factors are also recruited to RCs to participate in viral replication, transcription, or capsid assembly; their details will be described later.
Table 1.
Viral proteins accumulate to RCs. “Microscopic analysis” means that the localization to RCs of the viral protein was improved by IFA. “N/A” indicates that the localization to RCs of the viral protein was not improved by IFA but that it was estimated to accumulate in RCs by co-immunoprecipitation analysis with viral proteins recruited to RCs or other functional assays.
| Viral Protein | Functions | Roles in RCs | Microscopic Analysis | Refs. |
|---|---|---|---|---|
| EBNA1 | Bridge between chromosome and viral episome | Scaffold for newly synthesized DNA | Yes | [19] |
| BZLF1 | Lytic cycle switch | Activates and modulates DDR L gene transcription |
Yes | [19] |
| Rta | Transcriptional activator | L gene transcription | Yes | [19] |
| BALF2 | ssDNA binding protein | Consists of viral replication forks and synthesizes viral DNA | Yes | [19] |
| BALF5 | DNA polymerase | Consists of viral replication forks and synthesizes viral DNA | Yes | [19] |
| BMRF1 | dsDNA binding protein polymerase processsivity factor |
Consists of viral replication forks and synthesizes viral DNA Binds to dsDNA and composes the BMRF1-core Mediates DNA repair L gene transcription |
Yes | [19] |
| BBLF2/3 | Helicase–primase complex | Consists of viral replication forks and synthesizes viral DNA | Yes | [19] |
| BBLF4 | Helicase–primase complex | Consists of viral replication forks and synthesizes viral DNA | Yes | [19] |
| BSLF1 | Helicase–primase complex | Consists of viral replication forks and synthesizes viral DNA | Yes | [19] |
| BKRF3 | Uracil DNA glycosylase | Assists in the synthesis of viral DNA | Yes | [38] |
| BGLF4 | Protein kinase | Conducts chromosomal condensationActivates DDR | Yes | [39] |
| BPLF1 | Deubiquitinase | Mediates DNA repair | Yes | [40] |
| BGLF5 | Alkaline nuclease | Viral mRNA export | Yes | [41] |
| BMLF1 | Transcriptional activator | Viral mRNA export | Yes | [41] |
| BFRF3 | Small capsid protein | Consists of viral capsids | Yes | [42] |
| BVRF1 | Minor capsid protein/Capsid packaging protein | Packages viral DNA into capsids | Yes | [42] |
| BGLF1 | Minor capsid protein/Capsid packaging protein | Packages viral DNA into capsids | Yes | [42] |
| BFLF1 | Capsid packaging protein | Packages viral DNA into capsids | Yes | [42] |
| BDRF1 | Minor capsid protein/Capsid packaging protein | Packages viral DNA into capsids | N/A | [43] |
| BVRF2 | Capsid protease | Consists of viral capsids | Yes | [42] |
| BDLF1 | Capsid Triplex 2 | Consists of viral capsids | Yes | [42] |
| BORF1 | Capsid Triplex 1 | Consists of viral capsids | Yes | [42] |
| BBRF1 | Capsid portal protein | Consists of viral capsids | Yes | [42] |
| BdRF1 | Capsid scaffold protein | Consists of viral capsids and is cleaved | N/A | [44] |
| BcLF1 | Major capsid protein | Consists of viral capsids | N/A | [44] |
| BDLF3.5 | vPIC component | L gene transcription | N/A | [45] |
| BDLF4 | vPIC component | L gene transcription | N/A | [45] |
| BVLF1 | vPIC component | L gene transcription | N/A | [45] |
| BGLF3 | vPIC component | L gene transcription | N/A | [45] |
| BFRF2 | vPIC component | L gene transcription | N/A | [45] |
| BcRF1 | vPIC component | L gene transcription | Yes | [46,47] |
3. RC Growth and Maintenance
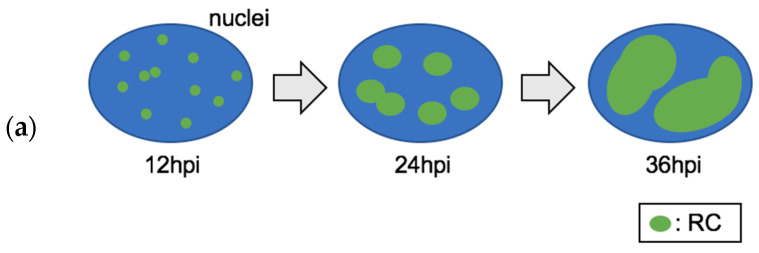
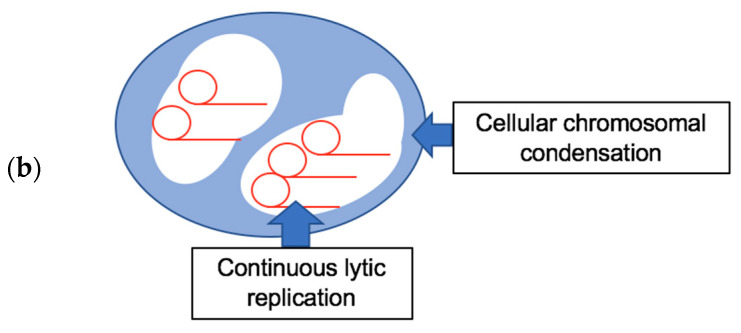
Fluorescence in situ hybridization (FISH) analysis using EBV latently infected cell lines has visualized the EBV genome, which appears as an enormous number of small dots. Until EBV establishes latency from primary infection, EBV amplifies its genome, and each single genome forms a circular episome that binds to AT-rich and gene-poor regions of human chromosomes via EBV nuclear antigen (EBNA1), a latent EBV gene [48,49]. EBNA1 bridges the EBV episome and cellular chromosomes, binding EBV to the latent origin of replication (ori-P). On the other hand, in the early phase of lytic replication, several medium-sized dots, so to say primary RCs, can be observed by FISH analysis or immunofluorescence analysis (IFA) using specific antibodies for viral replication proteins (Figure 1a, 12 h post induction (hpi)). These small RCs grow bigger and seem to fuse with each other as lytic replication proceeds (Figure 1a, 24 hpi). Finally, at the late stage of lytic replication, RCs appear as one or two large globular nuclear subdomains and occupy 30–35% of the nucleus (Figure 1a, 36 hpi) [50]. As mentioned before, RCs are generally characterised by FISH analysis using probes specific to EBV genomes or by IFA using specific antibodies for BMRF1, BALF5, or BALF2 with fixed cells [19,23]. These methods cannot monitor RC growth in a single cell over time. The question is, however, how individual episomes grow into such an enormous nuclear subdomain. Sugden’s group had developed the visible replicon system, which encodes the EBV ori-P, oriLyt, and LacI-LacO targeting system fused tdTomato fluorescence protein containing a nuclear localization signal to monitor viral DNA synthesis by time-lapse imaging [51,52]. Nagaraju et al. explored the question by using this EBV replicon system to visualize the development of RCs in live cells. Surprisingly, each developing RC contains similar levels of viral DNA during the same stage of lytic replication. This suggests that each developing RC synchronises with other RCs. Moreover, the nuclear volumes are enlarged at the late stage of lytic replication [50]. It has also been observed that 4′,6-diamidino-2-phenylindole (DAPI) does not stain the sites of RCs, and electron microscopic analysis has shown that host chromatin is shoved by RCs and condensed [42]. Thus, for RC development and successful viral production, the extrachromosomal space is essential (Figure 1b). BGLF4 EBV-coded protein kinase, which is expressed in the early stage of lytic replication and is mostly located in RCs, interacts with the condensin complex and stimulates topoisomerase II, which conducts chromosomal condensation similar to a premature mitotic event and provides extra space for RCs [53]. BGLF4 kinase inactivates MCM4-MCM6-MCM7 helicase activity by phosphorylating MCM4, which blocks host chromosomal DNA replication and allows RCs to occupy more space [54]. Even in the late stage of lytic replication, DNA synthesis occurs continuously, maintaining the morphology of the RCs [55]. Some BZLF1-inducible host factors, such as target-of-rapamycin complex 2 (TORC2), localize to RCs [56]. These factors may contribute to the continuous DNA replication in RCs. EBNA1, an EBV latent protein, is also expressed during the lytic phase and accumulates in RCs. EBNA1 is thought to bind to the ori-P region on newly synthesized DNA stored in RCs, which functions as a scaffold to maintain the RC structure in the nuclei [57]. Interestingly, even the exogenous gene coding ori-Lyt is amplified in RCs coordinated with RCs [58]. This evidence allows us to imagine RCs, which successfully gain enough space and separate the nucleus spatiotemporally, benefiting the protection of both newly synthesized EBV genomes and assembled viral capsids and the coordination among single episomes.
Figure 1.
(a) The formation and growth of RCs over time. These small RCs, seem at 12 hpi, grow bigger and seem to fuse with each other as lytic replication proceeds (24 hpi). At 36 hpi, RCs appear as one or two large globular nuclear subdomains. (b) Model showing the development of RCs and their occupation of extrachromosomal space.
4. The DNA Storage Subdomain, the BMRF1-Core
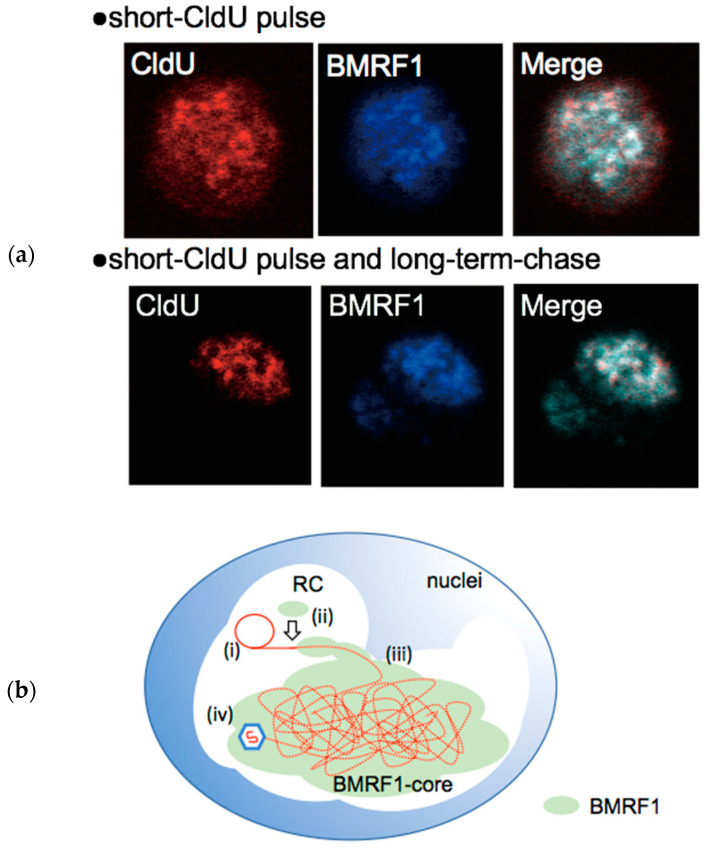
As mentioned before, viral replication proteins are recruited to the RC and compose replication forks in the RCs. BMRF1 is a multifunctional EBV replication protein that plays a central role in lytic replication via RCs. It acts as a DNA polymerase processivity factor, a dsDNA-binding protein, and a lytic gene transcription factor. BMRF1 is considered an E gene and is highly expressed through the lytic phase. Electrophoresis, sedimentation assay, and blue native PAGE analysis have revealed that BMRF1 mostly exists as a C-shaped head-to-head homodimer connecting the βI1 strands and requires C95. Such dimeric forms possess dsDNA binding activity [59]. However, some BMRF1 molecules form a ring-shaped tetramer via tail-to-tail contact, which contributes to viral replication [60]. Mutations lacking the dsDNA binding capacity fail to form RCs. Therefore, in RCs, it is suspected that newly synthesized viral dsDNA is covered by BMRF1. We previously discovered that BMRF1 binding to dsDNA occurs at discrete areas of the RCs, and, using confocal microscopic analysis and 3D reconstruction, found that BMRF1 seems to be surrounded by other viral replication proteins such as BALF2 or BALF5 [23]. This evidence suggests that there are BMRF subdomains in RCs, which we named the “BMRF1-core”. The BMRF-core has been observed at 24 h post-lytic induction when using the B95.8 cell line. Viral DNA labelling experiments using thymidine analogues have revealed short- 5-chloro-2’-deoxyuridine (CldU) pulse-labeled viral DNA outside the BMRF1-core, where BALF2 or BALF5 are also located (Figure 2a, upper panels). In contrast, short-CldU pulse and long-term-chase-treated viral DNA move to the inside of the BMRF1-core (Figure 2a, lower panels). Hence, these observations suggest that during lytic replication, newly synthesized viral DNA is bound by BMRF1, which possesses dsDNA binding capacity, folded to form the BMRF1-core, and stored until packed into the viral capsid (Figure 2b). In fact, our previous study indicated that the BMRF1-core is the site of capsid assembly. We observed that EBV capsid packaging proteins are localized inside the BMRF1-core, although EBV capsid proteins are located outside and inside. These results suggest that EBV viral empty capsids are assembled in RCs and transported to the BMRF1-core, and that the packed BMRF1-core stores viral DNA into the capsids [42]. The BMRF1-core divides the area of the RCs spatiotemporally, which allows for efficient capsid assembly.
Figure 2.
(a) Pulse-chase analysis revealing the existence of the RC subdomain, the BMRF1-core. The lytic phase was induced in Tet-Z/B95.8 cells by Dox treatment. At 24 h post-induction, the cells were treated with CldU for 10 min (short-CldU pulse). Then, cells were washed and incubated for 1 h to allow labelled DNA to move to DNA storage sites (short-CldU pulse and long-term chase). Approximately 40 cells were analyzed and images were captured. (b) Model of BMRF1-core formation. (i) The newly synthesized viral DNA mainly at the outside of the BMRF1-core in the RC. (ii) BMRF1 binds to synthesized viral DNA and folds to form the BMRF1-core that seems to be accumulated in the BMRF1-core. (iii) Viral DNA is stored until packed into the viral capsid. (iv) Finally, viral DNA is packed into the self-assembled capsid.
5. The Contribution of DNA Damage Responses to Viral DNA at RCs
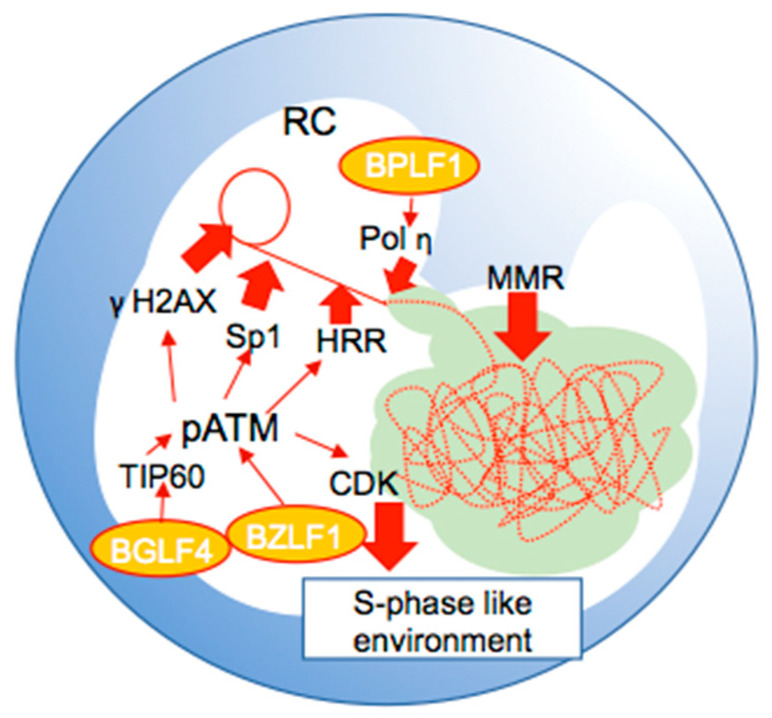
Genomes packed into capsids should maintain high fidelity to ensure EBV survival. Herpesviruses hijack and/or utilize the host DNA damage response (DDR) and DNA repair system to assist their replication (Figure 3). Some lytic genes are DDR inducers, and their main target is ataxia telangiectasia-mutated (ATM), the central kinase of the DDR. ATM normally exists as a homodimer or as a multimer inactive form. Once DNA damage is sensed, ATM rapidly autophosphorylates at serine 1981, which causes dimer dissociation [61]. The activation of ATM by autophosphorylation initiates ATM kinase activity. BGLF4 phosphorylates and activates TIP60 histone acetyltransferase, promoting DDR through the acetylation of ATM, which causes the phosphorylation of ATM, H2AX, and downstream factors [62]. BZLF also induces phosphorylation of ATM, H2AX, and 53BP1 independently of other lytic proteins [63]. BGLF4- and BZLF1-DDR induction activities are modulated by SUMO binding [64]. Most activated DDR factors, such as phosphorylated ATM (pATM) and phosphorylated H2AX (γH2AX), are recruited to RCs upon lytic infection [63,65,66]. S-phase cyclin-dependent kinases (CDKs) are highly activated via ATM checkpoint signaling, one of the downstream pathways of ATM that creates an S-phase-like intracellular environment [65]. The phosphorylation of Sp1, a host transcriptional factor, by ATM is also important for viral DNA synthesis and RC maintenance [66]. ATM also facilitates DNA repair in a manner similar to that of homologous recombination repair (HHR). As newly replicated viral DNA possesses double-strand breaks (DSBs), the DSB sensor activated by ATM (the Mre11–Rad50–Nbs1 (MRN) complex) binds to the DSB region. The MRN complex promotes sequential HHR to repair DSBs in newly synthesized viral DNA, cooperating with viral lytic replication [67]. Proliferating cell nuclear antigen (PCNA), a DNA sliding clamp, is loaded onto newly synthesized viral DNA, and a series of mismatch repair (MMR) factors are recruited to viral DNA in RCs [68]. Factors belonging to the MMR pathway have been observed inside the BMRF1-core, although HHR factors are located both outside and inside the BMRF1-core [23]. This suggests that MMR is involved in the maturation of the newly synthesized viral DNA. The BMRF1-core plays a part in spatiotemporally dividing the different pathways. Studies on the accumulation of such DDR factors to herpesviral RCs have been conducted in other herpesviruses, such as herpes simplex virus type 1 (HSV-1) and human cytomegalovirus (HCMV). RCs of HSV-1 or HCMV are also formed; HRR factors, including the MRN complex and Rad51, are recruited to the RCs [69,70,71,72,73,74]. In addition, BPLF1, an EBV-coded deubiquitinase, recruits polymerase eta (pol η), which is the host polymerase that specializes in DNA repair, onto newly synthesized viral DNA during the lytic phase to mature but not synthesize viral DNA [75]. Furthermore, BPLF1 enhances pol η expression, likely by inhibiting proteasomal degradation through its deubiquitinase activity.
Figure 3.
Model of the interactions among DNA damage response factors which enhance EBV DNA synthesis and viral proteins at RCs. The central kinase of the DDR, ATM, is activated by BGLF4 and BZLF1. Phosphorylated ATM (pATM) activates H2AX (γH2AX), S-phase cyclin-dependent kinases (CDKs), Sp1, and homologous recombinational repair (HRR) enhance viral DNA synthesis. BPLF1 recruits pol η onto newly synthesized viral DNA to mature. Mismatch repair (MMR) factors are also involved in the maturation of viral DNA stored at the BMRF1-core.
On the other hand, EBV lytic replication triggers and utilizes the DDR for genome integrity and fidelity. Recent studies have shown that EBV also interferes with some part of the DDR to avoid apoptosis. BZLF1 causes mislocalization of 53BP1 and RNF8, the latter being a ubiquitin–protease ligase that acts in the DNA repair pathway, but not those of pATM, MDC1, or γH2AX from DSBs [76]. BMRF1 interacts with the nucleosome remodeling and deacetylation (NuRD) complex at RCs, which prevents the accumulation of the essential RING finger ubiquitin ligase for DNA repair, RNF168, in sites of DBSs, mainly in the host genome [77]. Additionally, the BKRF4 EBV tegument protein, which is expressed at the late stage of the lytic phase and is not located in RCs, interferes with histone ubiquitination at DSBs by inhibiting the recruitment of RNF168 [78,79]. Likewise, DDR factors, such as γH2AX, Mre11, and RPA32, are recruited to Kaposi’s sarcoma-associated herpesvirus (KSHV) RCs and not 53BP1 [80]. Taken together, these phenomena may contribute to the herpesvirus-specialized forms of DDR and DNA repair, and indirectly protect newly synthesized viral DNA stored in RCs. Mislocalization of DNA repair molecules from DSBs in host DNA induces cellular genome instability [81,82,83]. The formation of RCs and the specialization of a set of DDR factors at RCs indirectly contribute to EBV-driven oncogenesis by promoting viral genome stability and host genomic instability, even during the lytic phase [33].
6. The Regulation of L Gene Transcription in RCs
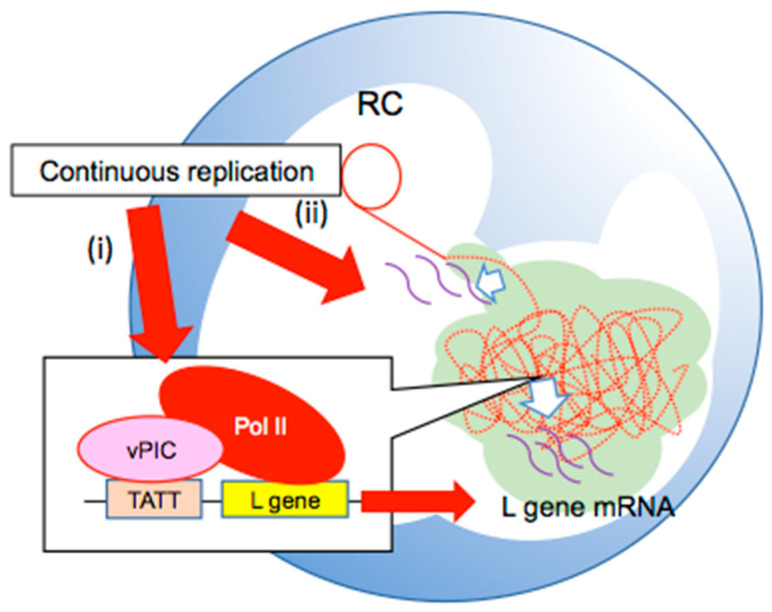
RCs are also transcription sites. The major breakthrough regarding EBV transcription during the lytic phase was the discovery of the viral pre-initiation complex (vPIC). In the EBV L promoter region, a TATT motif is present instead of the TATA box that is normally found in eukaryotic promoters [84,85,86]. BcRF1 has been identified as a TATA-binding protein (TBP)-like protein that interacts with the TATT motif on the L promoter region and is essential for L gene transcription [46]. Six viral proteins (BDLF3.5, BDLF4, BVLF1, BGLF3, BFRF2, and BcRF1) compose the vPIC, all of which are necessary for L gene transcription [45]. This transcriptional system via vPIC is conserved among beta- and gamma- but not alpha-herpesviruses. Some set of vPIC components are stabilized by host factors such as CDK2, an S-phase-like CDK that is activated during the lytic phase through the phosphorylation of BDLF4 [87]. Herpesviral TBP-like proteins, such as BcRF1, can directly bind to host RNA polymerase II (Pol II) via the N-terminal domain, although cellular TBP does not normally bind to Pol II [88]. Confocal microscopy has revealed that Pol II accumulates inside the BMRF1-core at the late stage of the lytic cycle, while at the early stage Pol II is located outside the BMRF1-core [47]. BcRF1, which is considered to bind to Pol II, and transcribed mRNAs of L genes are localised inside the BMRF1-core, whereas the mRNAs of E genes are located outside the BMRF1-core [47]. vPIC-mediated L gene transcription requires continuous viral lytic replication [89] and seems to template mature and stored newly synthesized viral DNA in the BMRF1-core (Figure 4) [90].
Figure 4.
The interaction between continuous replication and vPIC-dependent or -independent L gene transcription in RCs. (i) Continuous replication is required by vPIC-dependent L gene transcription. vPIC, including viral TBP-like protein, interacts with the TATT motif on the L promoter region of viral DNA stored in the BMRF1-core. (ii) Some sets of L genes are transcribed independently of vPIC. Viral DNA replication stimulates such gene transcription.
In contrast, although vPIC is essential for almost all L genes, some sets of L genes, such as BCRF1 (vIL10) and BPLF1, do not require vPIC [91,92]. The “leaky” transcription of these genes is activated in a DNA replication-dependent manner and is caused by superimposition of both early and late transcription at the same promoter [90,91]. BGLF4 kinase activity elevates both vPIC-dependent and -independent L-gene transcription [92,93]. Therefore, continuous lytic DNA synthesis at RCs is required for both “true” and “leaky” L gene transcription (Figure 4) [55].
Additionally, the EBV-coded transcriptional factors BZLF1, Rta, and BMRF1 act as mediators of L gene transcription. Rta interacts with host factors such as TSG101 at RCs and upregulates L gene transcription [94]. The region encoding the transcription activity of the L gene in BZLF1 is involved in RC formation [95]. A subset of L genes classified as “true late” are partially transcribed in response to cellular transcriptional factors, such as AP-1 proteins with alanine-to-serine mutations, independently of viral DNA replication [96,97]. The interaction of BMRF1 with the SWI/SNF chromatin modifier subset BRG1 also upregulates L gene transcription [98]. Taken together, the roles of RCs in L gene transcription are: (1) acting as sites of vPIC-mediated transcription for storing viral DNA in the BMRF1-core, (2) allowing continuous replication, which leads to L gene transcription in vPIC-dependent and -independent manners, and (3) recruiting most EBV-coded transcription mediators and their interaction partners. RC formation may provide advantages for the interactions among viral transcriptional factors, cellular factors, and viral DNA.
7. The Indirect Maintenance of RCs
Viral and cellular factors, even those that do not localize to RCs, partly assist the formation and maintenance of RCs. One of the heat shock proteins (Hsps), Hsp90, is a well-conserved molecular chaperone that mediates molecule maturation, stabilization, and intracellular trafficking [99]. Since BALF5 does not possess nuclear localization signals (NLSs), Hsp90 beta, which is localized in the cytoplasm, mediates the interaction between BALF5 and BMRF1, facilitating the nuclear translocation of BALF5 [100].
APOBEC3B (A3B) is a member of the APOBEC family of ssDNA cytosine deaminases, which participate in antiviral innate immunity, and seems to be antagonistic to EBV viral gene stability, as A3B catalytic activity mediates deamination of cytosine to uracil in ssDNA. BORF2, an EBV ribonucleotide reductase, interacts with A3B and is transported to the cytoplasm from the nucleus, although deletion of BORF2 leads to the localization of A3B to RCs and reduces viral production [101]. Such protection systems by viral ribonucleotide reductases are highly conserved among alpha- and gamma-herpesviruses [102]. This shows that viral DNA stored in the RC is well protected from the cellular antiviral innate immune response.
8. Conclusions and Future Directions
Once EBV reactivation occurs, RC formation is observed. The four reasons for RC formation are: (1) RCs enable the spatial separation of newly synthesized viral DNA from the cellular chromosome for viral DNA protection and maturation; (2) EBV-coded proteins and their interaction partners are recruited to RCs, which enhances interactions among viral proteins, cellular proteins, and viral DNA; (3) RC formation facilitates continuous replication, leading to L gene transcription; and (4) DNA storage and maturation leads to efficient progeny viral production. These aspects benefit EBV, since viral genomes can be amplified rapidly and immediately, in addition to maintaining stability by interacting with host factors, such as DNA repair factors, and evasion of some DDR factors or A3B. Most viral genes that encode structural proteins are classified as L genes [9]. Both efficient L gene transcription and genome stability are essential for virion production. RCs play an important role in almost all phases of lytic replication.
Recently, the period from primary infection to the establishment of latency, termed the pre-latent phase, has attracted great interest because early events are directly linked to EBV-driven transformation [103,104]. During the pre-latent phase, latent genes, as well as some sets of lytic genes, are expressed temporarily [103,105]. DDR is also temporarily induced and attenuated until latency is established [106]. However, unlike lytic replication, cellular DNA synthesis and cell division occur several days after primary infection, progeny viruses are not produced, and BZLF1 is not necessary for infection during the pre-latent phase [107,108]. Thus, because cellular aspects during the pre-latent phase differ from those of the lytic phase, little is known about how viral DNAs are synthesized, including whether RCs are formed, although viral DNA is abundantly amplified during the pre-latent phase. Moreover, recently it has been suggested that cells during the “abortive lytic phase” exist [109]. The “abortive lytic phase” is, so to speak, an “incomplete lytic cycle”, wherein several early lytic genes are expressed without progeny viral production like the pre-latent phase [108,110]. Studying these “incomplete lytic phases”, including whether RCs are assembled or not, might unveil the novel significance of RC formation.
Funding
This work was supported by grants-in-aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (20K18911 and 21J40038).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shannon-Lowe C., Rickinson A. The Global Landscape of EBV-Associated Tumors. Front. Oncol. 2019;9:713. doi: 10.3389/fonc.2019.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epstein M.A., Achong B.G., Barr Y.M. Virus Particles in Cultured Lymphoblasts from Burkitt’s Lymphoma. Lancet. 1964;1:702–703. doi: 10.1016/S0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 3.Oyama T., Ichimura K., Suzuki R., Suzumiya J., Ohshima K., Yatabe Y., Yokoi T., Kojima M., Kamiya Y., Taji H., et al. Senile EBV + B-cell lymphoproliferative disorders: A clinicopathologic study of 22 patients. Am. J. Surg. Pathol. 2003;27:16–26. doi: 10.1097/00000478-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Levine P.H., Ablashi D.V., Berard C.W., Carbone P.P., Waggoner D.E., Malan L. Elevated antibody titers to Epstein-Barr virus in Hodgkin’s disease. Cancer. 1971;27:416–421. doi: 10.1002/1097-0142(197102)27:2<416::AID-CNCR2820270227>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 5.Kimura H. EBV in T-/NK-Cell Tumorigenesis. Adv. Exp. Med. Biol. 2018;1045:459–475. doi: 10.1007/978-981-10-7230-7_21. [DOI] [PubMed] [Google Scholar]
- 6.Camargo M.C., Murphy G., Koriyama C., Pfeiffer R.M., Kim W.H., Herrera-Goepfert R., Corvalan A.H., Carrascal E., Abdirad A., Anwar M., et al. Determinants of Epstein-Barr virus-positive gastric cancer: An international pooled analysis. Br. J. Cancer. 2011;105:38–43. doi: 10.1038/bjc.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Png Y.T., Yang A.Z.Y., Lee M.Y., Chua M.J.M., Lim C.M. The Role of NK Cells in EBV Infection and EBV-Associated NPC. Viruses. 2021;13:300. doi: 10.3390/v13020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinclair A.J., Moalwi M.H., Amoaten T. Is EBV Associated with Breast Cancer in Specific Geographic Locations? Cancers. 2021;13:819. doi: 10.3390/cancers13040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young L.S., Rickinson A.B. Epstein-Barr virus: 40 years on. Nat. Rev. Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 10.Henle W. Role of Epstein-Barr virus in infectious mononucleosis and malignant lymphomas in man. Fed. Proc. 1972;31:1674. [PubMed] [Google Scholar]
- 11.Babcock G.J., Decker L.L., Volk M., Thorley-Lawson D.A. EBV persistence in memory B cells in vivo. Immunity. 1998;9:395–404. doi: 10.1016/S1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Sasson S.A., Klein G. Activation of the Epstein-Barr virus genome by 5-aza-cytidine in latently infected human lymphoid lines. Int. J. Cancer. 1981;28:131–135. doi: 10.1002/ijc.2910280204. [DOI] [PubMed] [Google Scholar]
- 13.Fixman E.D., Hayward G.S., Hayward S.D. Replication of Epstein-Barr virus oriLyt: Lack of a dedicated virally encoded origin-binding protein and dependence on Zta in cotransfection assays. J. Virol. 1995;69:2998–3006. doi: 10.1128/jvi.69.5.2998-3006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammerschmidt W., Sugden B. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell. 1988;55:427–433. doi: 10.1016/0092-8674(88)90028-1. [DOI] [PubMed] [Google Scholar]
- 15.Fixman E.D., Hayward G.S., Hayward S.D. Trans-acting requirements for replication of Epstein-Barr virus ori-Lyt. J. Virol. 1992;66:5030–5039. doi: 10.1128/jvi.66.8.5030-5039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furnari F.B., Adams M.D., Pagano J.S. Regulation of the Epstein-Barr virus DNA polymerase gene. J. Virol. 1992;66:2837–2845. doi: 10.1128/jvi.66.5.2837-2845.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmermann J., Hammerschmidt W. Structure and role of the terminal repeats of Epstein-Barr virus in processing and packaging of virion DNA. J. Virol. 1995;69:3147–3155. doi: 10.1128/jvi.69.5.3147-3155.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conway J.F., Homa F.L. Nucleocapsid Structure, Assembly and DNA Packaging of Herpes Simplex Virus. Caister Academic Press; Norwich, UK: 2011. [Google Scholar]
- 19.Daikoku T., Kudoh A., Fujita M., Sugaya Y., Isomura H., Shirata N., Tsurumi T. Architecture of replication compartments formed during Epstein-Barr virus lytic replication. J. Virol. 2005;79:3409–3418. doi: 10.1128/JVI.79.6.3409-3418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsurumi T., Fujita M., Kudoh A. Latent and lytic Epstein-Barr virus replication strategies. Rev. Med. Virol. 2005;15:3–15. doi: 10.1002/rmv.441. [DOI] [PubMed] [Google Scholar]
- 21.Takagi S., Takada K., Sairenji T. Formation of intranuclear replication compartments of Epstein-Barr virus with redistribution of BZLF1 and BMRF1 gene products. Virology. 1991;185:309–315. doi: 10.1016/0042-6822(91)90778-A. [DOI] [PubMed] [Google Scholar]
- 22.Weller S.K., Coen D.M. Herpes simplex viruses: Mechanisms of DNA replication. Cold Spring Harb. Perspect. Biol. 2012;4:a013011. doi: 10.1101/cshperspect.a013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugimoto A., Kanda T., Yamashita Y., Murata T., Saito S., Kawashima D., Isomura H., Nishiyama Y., Tsurumi T. Spatiotemporally different DNA repair systems participate in Epstein-Barr virus genome maturation. J. Virol. 2011;85:6127–6135. doi: 10.1128/JVI.00258-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novoa R.R., Calderita G., Arranz R., Fontana J., Granzow H., Risco C. Virus factories: Associations of cell organelles for viral replication and morphogenesis. Biol. Cell. 2005;97:147–172. doi: 10.1042/BC20040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Netherton C.L., Wileman T. Virus factories, double membrane vesicles and viroplasm generated in animal cells. Curr. Opin. Virol. 2011;1:381–387. doi: 10.1016/j.coviro.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul D., Bartenschlager R. Architecture and biogenesis of plus-strand RNA virus replication factories. World J. Virol. 2013;2:32–48. doi: 10.5501/wjv.v2.i2.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Netherton C., Moffat K., Brooks E., Wileman T. A guide to viral inclusions, membrane rearrangements, factories, and viroplasm produced during virus replication. Adv. Virus Res. 2007;70:101–182. doi: 10.1016/S0065-3527(07)70004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmid M., Speiseder T., Dobner T., Gonzalez R.A. DNA virus replication compartments. J. Virol. 2014;88:1404–1420. doi: 10.1128/JVI.02046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seyffert M., Georgi F., Tobler K., Bourqui L., Anfossi M., Michaelsen K., Vogt B., Greber U.F., Fraefel C. The HSV-1 Transcription Factor ICP4 Confers Liquid-Like Properties to Viral Replication Compartments. Int. J. Mol. Sci. 2021;22:4447. doi: 10.3390/ijms22094447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hidalgo P., Pimentel A., Mojica-Santamaria D., von Stromberg K., Hofmann-Sieber H., Lona-Arrona C., Dobner T., Gonzalez R.A. Evidence That the Adenovirus Single-Stranded DNA Binding Protein Mediates the Assembly of Biomolecular Condensates to Form Viral Replication Compartments. Viruses. 2021;13:1778. doi: 10.3390/v13091778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong L., Hayward G.S. Assembly of complete, functionally active herpes simplex virus DNA replication compartments and recruitment of associated viral and cellular proteins in transient cotransfection assays. J. Virol. 1997;71:3146–3160. doi: 10.1128/jvi.71.4.3146-3160.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamberti C., Weller S.K. The herpes simplex virus type 1 cleavage/packaging protein, UL32, is involved in efficient localization of capsids to replication compartments. J. Virol. 1998;72:2463–2473. doi: 10.1128/JVI.72.3.2463-2473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosemarie Q., Sugden B. Epstein-Barr Virus: How Its Lytic Phase Contributes to Oncogenesis. Microorganisms. 2020;8:1824. doi: 10.3390/microorganisms8111824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liao G., Wu F.Y., Hayward S.D. Interaction with the Epstein-Barr virus helicase targets Zta to DNA replication compartments. J. Virol. 2001;75:8792–8802. doi: 10.1128/JVI.75.18.8792-8802.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobiler O., Weitzman M.D. Herpes simplex virus replication compartments: From naked release to recombining together. PLoS Pathog. 2019;15:e1007714. doi: 10.1371/journal.ppat.1007714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shannon-Lowe C., Rickinson A.B., Bell A.I. Epstein-Barr virus-associated lymphomas. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017;372:20160271. doi: 10.1098/rstb.2016.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wen K.W., Wang L., Menke J.R., Damania B. Cancers associated with human gammaherpesviruses. FEBS J. 2021 doi: 10.1111/febs.16206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su M.T., Liu I.H., Wu C.W., Chang S.M., Tsai C.H., Yang P.W., Chuang Y.C., Lee C.P., Chen M.R. Uracil DNA glycosylase BKRF3 contributes to Epstein-Barr virus DNA replication through physical interactions with proteins in viral DNA replication complex. J. Virol. 2014;88:8883–8899. doi: 10.1128/JVI.00950-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J.T., Yang P.W., Lee C.P., Han C.H., Tsai C.H., Chen M.R. Detection of Epstein-Barr virus BGLF4 protein kinase in virus replication compartments and virus particles. Pt 12J. Gen. Virol. 2005;86:3215–3225. doi: 10.1099/vir.0.81313-0. [DOI] [PubMed] [Google Scholar]
- 40.Whitehurst C.B., Vaziri C., Shackelford J., Pagano J.S. Epstein-Barr virus BPLF1 deubiquitinates PCNA and attenuates polymerase eta recruitment to DNA damage sites. J. Virol. 2012;86:8097–8106. doi: 10.1128/JVI.00588-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park R., Miller G. Epstein-Barr Virus-Induced Nodules on Viral Replication Compartments Contain RNA Processing Proteins and a Viral Long Noncoding RNA. J. Virol. 2018;92:e01254-18. doi: 10.1128/JVI.01254-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugimoto A., Yamashita Y., Kanda T., Murata T., Tsurumi T. Epstein-Barr virus genome packaging factors accumulate in BMRF1-cores within viral replication compartments. PLoS ONE. 2019;14:e0222519. doi: 10.1371/journal.pone.0222519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Visalli R.J., Schwartz A.M., Patel S., Visalli M.A. Identification of the Epstein Barr Virus portal. Virology. 2019;529:152–159. doi: 10.1016/j.virol.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Henson B.W., Perkins E.M., Cothran J.E., Desai P. Self-assembly of Epstein-Barr virus capsids. J. Virol. 2009;83:3877–3890. doi: 10.1128/JVI.01733-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aubry V., Mure F., Mariame B., Deschamps T., Wyrwicz L.S., Manet E., Gruffat H. Epstein-Barr virus late gene transcription depends on the assembly of a virus-specific preinitiation complex. J. Virol. 2014;88:12825–12838. doi: 10.1128/JVI.02139-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gruffat H., Kadjouf F., Mariame B., Manet E. The Epstein-Barr virus BcRF1 gene product is a TBP-like protein with an essential role in late gene expression. J. Virol. 2012;86:6023–6032. doi: 10.1128/JVI.00159-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugimoto A., Sato Y., Kanda T., Murata T., Narita Y., Kawashima D., Kimura H., Tsurumi T. Different distributions of Epstein-Barr virus early and late gene transcripts within viral replication compartments. J. Virol. 2013;87:6693–6699. doi: 10.1128/JVI.00219-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moquin S.A., Thomas S., Whalen S., Warburton A., Fernandez S.G., McBride A.A., Pollard K.S., Miranda J.L. The Epstein-Barr Virus Episome Maneuvers between Nuclear Chromatin Compartments during Reactivation. J. Virol. 2018;92:e01413-17. doi: 10.1128/JVI.01413-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chakravorty A., Sugden B. The AT-hook DNA binding ability of the Epstein Barr virus EBNA1 protein is necessary for the maintenance of viral genomes in latently infected cells. Virology. 2015;484:251–258. doi: 10.1016/j.virol.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagaraju T., Sugden A.U., Sugden B. Four-dimensional analyses show that replication compartments are clonal factories in which Epstein-Barr viral DNA amplification is coordinated. Proc. Natl. Acad. Sci. USA. 2019;116:24630–24638. doi: 10.1073/pnas.1913992116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiu Y.F., Sugden A.U., Sugden B. Epstein-Barr viral productive amplification reprograms nuclear architecture, DNA replication, and histone deposition. Cell Host Microbe. 2013;14:607–618. doi: 10.1016/j.chom.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nanbo A., Sugden A., Sugden B. The coupling of synthesis and partitioning of EBV’s plasmid replicon is revealed in live cells. EMBO J. 2007;26:4252–4262. doi: 10.1038/sj.emboj.7601853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee C.P., Chen J.Y., Wang J.T., Kimura K., Takemoto A., Lu C.C., Chen M.R. Epstein-Barr virus BGLF4 kinase induces premature chromosome condensation through activation of condensin and topoisomerase II. J. Virol. 2007;81:5166–5180. doi: 10.1128/JVI.00120-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kudoh A., Daikoku T., Ishimi Y., Kawaguchi Y., Shirata N., Iwahori S., Isomura H., Tsurumi T. Phosphorylation of MCM4 at sites inactivating DNA helicase activity of the MCM4-MCM6-MCM7 complex during Epstein-Barr virus productive replication. J. Virol. 2006;80:10064–10072. doi: 10.1128/JVI.00678-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li D., Fu W., Swaminathan S. Continuous DNA replication is required for late gene transcription and maintenance of replication compartments in gammaherpesviruses. PLoS Pathog. 2018;14:e1007070. doi: 10.1371/journal.ppat.1007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murata T., Sato Y., Nakayama S., Kudoh A., Iwahori S., Isomura H., Tajima M., Hishiki T., Ohshima T., Hijikata M., et al. TORC2, a coactivator of cAMP-response element-binding protein, promotes Epstein-Barr virus reactivation from latency through interaction with viral BZLF1 protein. J. Biol. Chem. 2009;284:8033–8041. doi: 10.1074/jbc.M808466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daikoku T., Kudoh A., Fujita M., Sugaya Y., Isomura H., Tsurumi T. In vivo dynamics of EBNA1-oriP interaction during latent and lytic replication of Epstein-Barr virus. J. Biol. Chem. 2004;279:54817–54825. doi: 10.1074/jbc.M405911200. [DOI] [PubMed] [Google Scholar]
- 58.Amon W., White R.E., Farrell P.J. Epstein-Barr virus origin of lytic replication mediates association of replicating episomes with promyelocytic leukaemia protein nuclear bodies and replication compartments. Pt 5J. Gen. Virol. 2006;87:1133–1137. doi: 10.1099/vir.0.81589-0. [DOI] [PubMed] [Google Scholar]
- 59.Murayama K., Nakayama S., Kato-Murayama M., Akasaka R., Ohbayashi N., Kamewari-Hayami Y., Terada T., Shirouzu M., Tsurumi T., Yokoyama S. Crystal structure of epstein-barr virus DNA polymerase processivity factor BMRF1. J. Biol. Chem. 2009;284:35896–35905. doi: 10.1074/jbc.M109.051581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakayama S., Murata T., Yasui Y., Murayama K., Isomura H., Kanda T., Tsurumi T. Tetrameric ring formation of Epstein-Barr virus polymerase processivity factor is crucial for viral replication. J. Virol. 2010;84:12589–12598. doi: 10.1128/JVI.01394-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bakkenist C.J., Kastan M.B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 62.Li R., Zhu J., Xie Z., Liao G., Liu J., Chen M.R., Hu S., Woodard C., Lin J., Taverna S.D., et al. Conserved herpesvirus kinases target the DNA damage response pathway and TIP60 histone acetyltransferase to promote virus replication. Cell Host Microbe. 2011;10:390–400. doi: 10.1016/j.chom.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang’ondu R., Teal S., Park R., Heston L., Delecluse H., Miller G. DNA Damage Signaling Is Induced in the Absence of Epstein-Barr Virus (EBV) Lytic DNA Replication and in Response to Expression of ZEBRA. PLoS ONE. 2015;10:e0126088. doi: 10.1371/journal.pone.0126088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li R., Wang L., Liao G., Guzzo C.M., Matunis M.J., Zhu H., Hayward S.D. SUMO binding by the Epstein-Barr virus protein kinase BGLF4 is crucial for BGLF4 function. J. Virol. 2012;86:5412–5421. doi: 10.1128/JVI.00314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kudoh A., Fujita M., Zhang L., Shirata N., Daikoku T., Sugaya Y., Isomura H., Nishiyama Y., Tsurumi T. Epstein-Barr virus lytic replication elicits ATM checkpoint signal transduction while providing an S-phase-like cellular environment. J. Biol. Chem. 2005;280:8156–8163. doi: 10.1074/jbc.M411405200. [DOI] [PubMed] [Google Scholar]
- 66.Hau P.M., Deng W., Jia L., Yang J., Tsurumi T., Chiang A.K., Huen M.S., Tsao S.W. Role of ATM in the formation of the replication compartment during lytic replication of Epstein-Barr virus in nasopharyngeal epithelial cells. J. Virol. 2015;89:652–668. doi: 10.1128/JVI.01437-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kudoh A., Iwahori S., Sato Y., Nakayama S., Isomura H., Murata T., Tsurumi T. Homologous recombinational repair factors are recruited and loaded onto the viral DNA genome in Epstein-Barr virus replication compartments. J. Virol. 2009;83:6641–6651. doi: 10.1128/JVI.00049-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daikoku T., Kudoh A., Sugaya Y., Iwahori S., Shirata N., Isomura H., Tsurumi T. Postreplicative mismatch repair factors are recruited to Epstein-Barr virus replication compartments. J. Biol. Chem. 2006;281:11422–11430. doi: 10.1074/jbc.M510314200. [DOI] [PubMed] [Google Scholar]
- 69.Lilley C.E., Carson C.T., Muotri A.R., Gage F.H., Weitzman M.D. DNA repair proteins affect the lifecycle of herpes simplex virus 1. Proc. Natl. Acad. Sci. USA. 2005;102:5844–5849. doi: 10.1073/pnas.0501916102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luo M.H., Rosenke K., Czornak K., Fortunato E.A. Human cytomegalovirus disrupts both ataxia telangiectasia mutated protein (ATM)- and ATM-Rad3-related kinase-mediated DNA damage responses during lytic infection. J. Virol. 2007;81:1934–1950. doi: 10.1128/JVI.01670-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shirata N., Kudoh A., Daikoku T., Tatsumi Y., Fujita M., Kiyono T., Sugaya Y., Isomura H., Ishizaki K., Tsurumi T. Activation of ataxia telangiectasia-mutated DNA damage checkpoint signal transduction elicited by herpes simplex virus infection. J. Biol. Chem. 2005;280:30336–30341. doi: 10.1074/jbc.M500976200. [DOI] [PubMed] [Google Scholar]
- 72.Taylor T.J., Knipe D.M. Proteomics of herpes simplex virus replication compartments: Association of cellular DNA replication, repair, recombination, and chromatin remodeling proteins with ICP8. J. Virol. 2004;78:5856–5866. doi: 10.1128/JVI.78.11.5856-5866.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilkinson D.E., Weller S.K. Recruitment of cellular recombination and repair proteins to sites of herpes simplex virus type 1 DNA replication is dependent on the composition of viral proteins within prereplicative sites and correlates with the induction of the DNA damage response. J. Virol. 2004;78:4783–4796. doi: 10.1128/JVI.78.9.4783-4796.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dembowski J.A., Dremel S.E., DeLuca N.A. Replication-Coupled Recruitment of Viral and Cellular Factors to Herpes Simplex Virus Type 1 Replication Forks for the Maintenance and Expression of Viral Genomes. PLoS Pathog. 2017;13:e1006166. doi: 10.1371/journal.ppat.1006166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dyson O.F., Pagano J.S., Whitehurst C.B. The Translesion Polymerase Pol eta Is Required for Efficient Epstein-Barr Virus Infectivity and Is Regulated by the Viral Deubiquitinating Enzyme BPLF1. J. Virol. 2017;91:e00600-17. doi: 10.1128/JVI.00600-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang J., Deng W., Hau P.M., Liu J., Lau V.M., Cheung A.L., Huen M.S., Tsao S.W. Epstein-Barr virus BZLF1 protein impairs accumulation of host DNA damage proteins at damage sites in response to DNA damage. Lab. Investig. 2015;95:937–950. doi: 10.1038/labinvest.2015.69. [DOI] [PubMed] [Google Scholar]
- 77.Salamun S.G., Sitz J., De La Cruz-Herrera C.F., Yockteng-Melgar J., Marcon E., Greenblatt J., Fradet-Turcotte A., Frappier L. The Epstein-Barr Virus BMRF1 Protein Activates Transcription and Inhibits the DNA Damage Response by Binding NuRD. J. Virol. 2019;93:e01070-19. doi: 10.1128/JVI.01070-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ho T.H., Sitz J., Shen Q., Leblanc-Lacroix A., Campos E.I., Borozan I., Marcon E., Greenblatt J., Fradet-Turcotte A., Jin D.Y., et al. A Screen for Epstein-Barr Virus Proteins That Inhibit the DNA Damage Response Reveals a Novel Histone Binding Protein. J. Virol. 2018;92:e00262-18. doi: 10.1128/JVI.00262-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Masud H., Watanabe T., Yoshida M., Sato Y., Goshima F., Kimura H., Murata T. Epstein-Barr Virus BKRF4 Gene Product Is Required for Efficient Progeny Production. J. Virol. 2017;91:e00975-17. doi: 10.1128/JVI.00975-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hollingworth R., Skalka G.L., Stewart G.S., Hislop A.D., Blackbourn D.J., Grand R.J. Activation of DNA Damage Response Pathways during Lytic Replication of KSHV. Viruses. 2015;7:2908–2927. doi: 10.3390/v7062752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ahmad A., Enzlin J.H., Bhagwat N.R., Wijgers N., Raams A., Appledoorn E., Theil A.F., Hoeijmakers J.H.J., Vermeulen W., Jaspers N.G.J., et al. Mislocalization of XPF-ERCC1 nuclease contributes to reduced DNA repair in XP-F patients. PLoS Genet. 2010;6:e1000871. doi: 10.1371/journal.pgen.1000871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khanal S., Galloway D.A. High-risk human papillomavirus oncogenes disrupt the Fanconi anemia DNA repair pathway by impairing localization and de-ubiquitination of FancD2. PLoS Pathog. 2019;15:e1007442. doi: 10.1371/journal.ppat.1007442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gruhne B., Sompallae R., Masucci M.G. Three Epstein-Barr virus latency proteins independently promote genomic instability by inducing DNA damage, inhibiting DNA repair and inactivating cell cycle checkpoints. Oncogene. 2009;28:3997–4008. doi: 10.1038/onc.2009.258. [DOI] [PubMed] [Google Scholar]
- 84.Amon W., Binne U.K., Bryant H., Jenkins P.J., Karstegl C.E., Farrell P.J. Lytic cycle gene regulation of Epstein-Barr virus. J. Virol. 2004;78:13460–13469. doi: 10.1128/JVI.78.24.13460-13469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Serio T.R., Cahill N., Prout M.E., Miller G. A functionally distinct TATA box required for late progression through the Epstein-Barr virus life cycle. J. Virol. 1998;72:8338–8343. doi: 10.1128/JVI.72.10.8338-8343.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wyrwicz L.S., Rychlewski L. Identification of Herpes TATT-binding protein. Antivir. Res. 2007;75:167–172. doi: 10.1016/j.antiviral.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 87.Sato Y., Watanabe T., Suzuki C., Abe Y., Masud H., Inagaki T., Yoshida M., Suzuki T., Goshima F., Adachi J., et al. S-Like-Phase Cyclin-Dependent Kinases Stabilize the Epstein-Barr Virus BDLF4 Protein To Temporally Control Late Gene Transcription. J. Virol. 2019;93:e01707-18. doi: 10.1128/JVI.01707-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Castaneda A.F., Didychuk A.L., Louder R.K., McCollum C.O., Davis Z.H., Nogales E., Glaunsinger B.A. The gammaherpesviral TATA-box-binding protein directly interacts with the CTD of host RNA Pol II to direct late gene transcription. PLoS Pathog. 2020;16:e1008843. doi: 10.1371/journal.ppat.1008843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Djavadian R., Chiu Y.F., Johannsen E. An Epstein-Barr Virus-Encoded Protein Complex Requires an Origin of Lytic Replication In Cis to Mediate Late Gene Transcription. PLoS Pathog. 2016;12:e1005718. doi: 10.1371/journal.ppat.1005718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chakravorty A., Sugden B., Johannsen E.C. An Epigenetic Journey: Epstein-Barr Virus Transcribes Chromatinized and Subsequently Unchromatinized Templates during Its Lytic Cycle. J. Virol. 2019;93:e02247-18. doi: 10.1128/JVI.02247-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Djavadian R., Hayes M., Johannsen E. CAGE-seq analysis of Epstein-Barr virus lytic gene transcription: 3 kinetic classes from 2 mechanisms. PLoS Pathog. 2018;14:e1007114. doi: 10.1371/journal.ppat.1007114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McKenzie J., Lopez-Giraldez F., Delecluse H.J., Walsh A., El-Guindy A. The Epstein-Barr Virus Immunoevasins BCRF1 and BPLF1 Are Expressed by a Mechanism Independent of the Canonical Late Pre-initiation Complex. PLoS Pathog. 2016;12:e1006008. doi: 10.1371/journal.ppat.1006008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.El-Guindy A., Lopez-Giraldez F., Delecluse H.J., McKenzie J., Miller G. A locus encompassing the Epstein-Barr virus bglf4 kinase regulates expression of genes encoding viral structural proteins. PLoS Pathog. 2014;10:e1004307. doi: 10.1371/journal.ppat.1004307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chua H.H., Lee H.H., Chang S.S., Lu C.C., Yeh T.H., Hsu T.Y., Cheng T.H., Cheng J.T., Chen M.R., Tsai C.H. Role of the TSG101 gene in Epstein-Barr virus late gene transcription. J. Virol. 2007;81:2459–2471. doi: 10.1128/JVI.02289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park R., Heston L., Shedd D., Delecluse H.J., Miller G. Mutations of amino acids in the DNA-recognition domain of Epstein-Barr virus ZEBRA protein alter its sub-nuclear localization and affect formation of replication compartments. Virology. 2008;382:145–162. doi: 10.1016/j.virol.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lyons D.E., Yu K.P., Vander Heiden J.A., Heston L., Dittmer D.P., El-Guindy A., Miller G. Mutant Cellular AP-1 Proteins Promote Expression of a Subset of Epstein-Barr Virus Late Genes in the Absence of Lytic Viral DNA Replication. J. Virol. 2018;92:e01062-18. doi: 10.1128/JVI.01062-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu K.P., Heston L., Park R., Ding Z., Wang’ondu R., Delecluse H.J., Miller G. Latency of Epstein-Barr virus is disrupted by gain-of-function mutant cellular AP-1 proteins that preferentially bind methylated DNA. Proc. Natl. Acad. Sci. USA. 2013;110:8176–8181. doi: 10.1073/pnas.1301577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Su M.T., Wang Y.T., Chen Y.J., Lin S.F., Tsai C.H., Chen M.R. The SWI/SNF Chromatin Regulator BRG1 Modulates the Transcriptional Regulatory Activity of the Epstein-Barr Virus DNA Polymerase Processivity Factor BMRF1. J. Virol. 2017;91:e02114-16. doi: 10.1128/JVI.02114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Whitesell L., Lindquist S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 100.Kawashima D., Kanda T., Murata T., Saito S., Sugimoto A., Narita Y., Tsurumi T. Nuclear transport of Epstein-Barr virus DNA polymerase is dependent on the BMRF1 polymerase processivity factor and molecular chaperone Hsp90. J. Virol. 2013;87:6482–6491. doi: 10.1128/JVI.03428-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cheng A.Z., Yockteng-Melgar J., Jarvis M.C., Malik-Soni N., Borozan I., Carpenter M.A., McCann J.L., Ebrahimi D., Shaban N.M., Marcon E., et al. Epstein-Barr virus BORF2 inhibits cellular APOBEC3B to preserve viral genome integrity. Nat. Microbiol. 2019;4:78–88. doi: 10.1038/s41564-018-0284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheng A.Z., Moraes S.N., Attarian C., Yockteng-Melgar J., Jarvis M.C., Biolatti M., Galitska G., Dell’Oste V., Frappier L., Bierle C.J., et al. A Conserved Mechanism of APOBEC3 Relocalization by Herpesviral Ribonucleotide Reductase Large Subunits. J. Virol. 2019;93:e01539-19. doi: 10.1128/JVI.01539-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jochum S., Moosmann A., Lang S., Hammerschmidt W., Zeidler R. The EBV immunoevasins vIL-10 and BNLF2a protect newly infected B cells from immune recognition and elimination. PLoS Pathog. 2012;8:e1002704. doi: 10.1371/journal.ppat.1002704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mrozek-Gorska P., Buschle A., Pich D., Schwarzmayr T., Fechtner R., Scialdone A., Hammerschmidt W. Epstein-Barr virus reprograms human B lymphocytes immediately in the prelatent phase of infection. Proc. Natl. Acad. Sci. USA. 2019;116:16046–16055. doi: 10.1073/pnas.1901314116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kalla M., Hammerschmidt W. Human B cells on their route to latent infection--early but transient expression of lytic genes of Epstein-Barr virus. Eur. J. Cell Biol. 2012;91:65–69. doi: 10.1016/j.ejcb.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 106.Nikitin P.A., Yan C.M., Forte E., Bocedi A., Tourigny J.P., White R.E., Allday M.J., Patel A., Dave S.S., Kim W., et al. An ATM/Chk2-mediated DNA damage-responsive signaling pathway suppresses Epstein-Barr virus transformation of primary human B cells. Cell Host Microbe. 2010;8:510–522. doi: 10.1016/j.chom.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pich D., Mrozek-Gorska P., Bouvet M., Sugimoto A., Akidil E., Grundhoff A., Hamperl S., Ling P.D., Hammerschmidt W. First Days in the Life of Naive Human B Lymphocytes Infected with Epstein-Barr Virus. MBio. 2019;10:e01723-19. doi: 10.1128/mBio.01723-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Inagaki T., Sato Y., Ito J., Takaki M., Okuno Y., Yaguchi M., Masud H., Watanabe T., Sato K., Iwami S., et al. Direct Evidence of Abortive Lytic Infection-Mediated Establishment of Epstein-Barr Virus Latency During B-Cell Infection. Front. Microbiol. 2020;11:575255. doi: 10.3389/fmicb.2020.575255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Murata T., Sato Y., Kimura H. Modes of infection and oncogenesis by the Epstein-Barr virus. Rev. Med. Virol. 2014;24:242–253. doi: 10.1002/rmv.1786. [DOI] [PubMed] [Google Scholar]
- 110.Okuno Y., Murata T., Sato Y., Muramatsu H., Ito Y., Watanabe T., Okuno T., Murakami N., Yoshida K., Sawada A., et al. Defective Epstein-Barr virus in chronic active infection and haematological malignancy. Nat. Microbiol. 2019;4:404–413. doi: 10.1038/s41564-018-0334-0. [DOI] [PubMed] [Google Scholar]