Abstract
Background: Various synthetic and biological wound dressings are available for the treatment of superficial burns, and standard care differs among hospitals. Nevertheless, the search for an ideal wound dressing offering a safe healing environment as well as optimal scar quality while being economically attractive is a continuing process. In recent years, Dressilk®, which consists of pure silk, has become the standard of care for the treatment of superficial burns in our hospital. However, no long-term scar-evaluation studies have been performed to compare Dressilk® with the often-used and more expensive Suprathel® in the treatment of superficial burns. Methods: Subjective and objective scar evaluations were performed three, six, and twelve months after treatment in patients who received simultaneous treatment of 20 superficial burn wounds with both Suprathel® and Dressilk®. The evaluations were performed using the Vancouver Scar Scale, the Cutometer®, Mexameter®, Tewameter®, and the O2C®. Results: Both dressings showed mostly equivalent results in subjective scar evaluations. In the objective scar evaluations, the wounds treated with Dressilk® showed a faster return to the qualities of non-injured skin. Wound areas treated with the two dressings showed no significant differences in elasticity and transepidermal water loss after 12 months. Only oxygen saturation was significantly lower in wound areas treated with Suprathel® (p = 0.008). Subjectively, wound areas treated with Dressilk® showed significantly higher pigmentation after six months, which was not apparent after 12 months. Conclusion: Both wound dressings led to esthetically satisfying scar recovery without significant differences from normal uninjured skin after 12 months. Therefore, Dressilk® remains an economically and clinically interesting alternative to Suprathel® for the treatment of superficial burns.
Keywords: superficial burns, silk, Suprathel, Dressilk, wound healing
1. Introduction
The appearance and quality of burn scars are of major importance for the affected patients [1]. With advancements in medical treatments and reductions in mortality rates after burn injuries [2], the long-term consequences of scar quality, such as textural or pigmentation problems, are now receiving more attention from surgeons. Thus, an optimal wound dressing that can minimize patient requests to improve scar quality is needed [1]. To this end, objective and reproducible scar evaluations are necessary to measure not only the quality of the different treatments, but also the efficacy of anti-scar techniques [3].
Currently, numerous wound dressings are available for the treatment of superficial burns [4,5,6,7]. In particular, synthetic and commercially available materials that can accelerate wound healing and reduce scarring are the main focus of current research in burn medicine [6,8,9,10,11,12,13]. Different materials have been established for the treatment of burns in recent years. Suprathel®, a biosynthetic copolymer wound dressing, was originally developed to improve wound closure, especially of split-skin graft donor sites, and in the conservative treatment of second-degree burns. In recent studies, it was shown to promote wound healing and reduce wound infection [14]. As an alternative material, silk spun by silkworm consists of the protein fibroin and shows many promising characteristics such as biocompatibility, tunable mechanical properties, minimal inflammation in host tissue, low cost and ease of use [6,15]. Due to these qualities, Dressilk® has become the standard of care (SOC) for the treatment of superficial partial-thickness burn wounds at our burn center in recent years.
Since many burn centers prefer using Suprathel® for these wounds, we had previously compared wound healing and patient satisfaction after the treatment of superficial burns with Dressilk® and Suprathel® [16]. However, to our knowledge, no study has attempted a direct comparison of scarring after burn wound treatment with Suprathel® and Dressilk®. Therefore, we aimed to subjectively and objectively evaluate long-term scar quality after wound treatment with both wound dressings.
2. Materials and Methods
Study design: The present study evaluated the scar quality of superficial partial-thickness burn wounds after simultaneous treatment with Suprathel® and Dressilk®. The study protocol had been reviewed and approved in advance by the Ethical Review Committee of the University of Witten Herdecke, Germany (number 5/2017), and the experimental procedures were conducted in accordance with the Declaration of Helsinki. Complete informed consent was obtained from all patients.
A total of 20 patients with superficial partial-thickness burns had previously been treated with Suprathel® and Dressilk® in an intra-individual study design, with half of the burned areas receiving Suprathel® and the other half being treated with Dressilk® (Figure 1) [16]. All of the treated patients were at least 18 years old, had a superficial partial-thickness burn caused by contact with a hot surface, flames, or a hot liquid and a wound area ≥0.3% of the total burned surface area (TBSA). For this study, all 20 patients were invited for follow-up examinations to evaluate scar quality after three, six, and twelve months (Figure 2). In the follow-up assessments, all study areas were photo-documented by standardized means and the scars assessed subjectively and objectively. We excluded patients who did not provide consent for the follow-up assessments and those who did not comply with the protocol for the examinations.
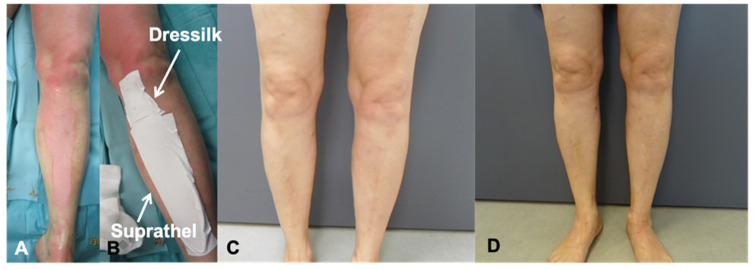
Figure 1.
Partial thickness burn of the left leg; (A,B) before and after debridement; (C) 6-month follow-up; (D) 12-month follow-up.
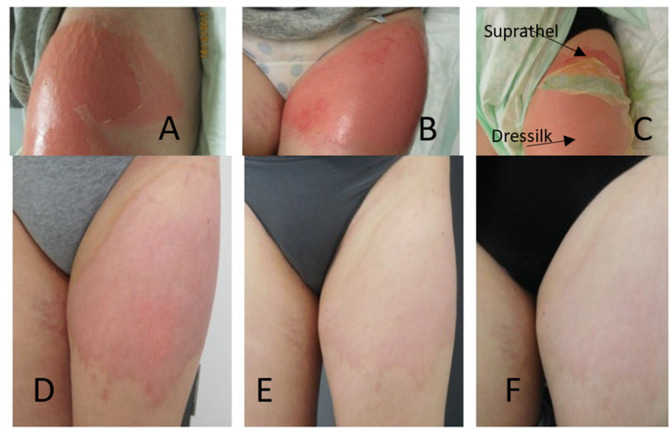
Figure 2.
Partial thickness burn of the left leg; (A,B) before and after debridement; (C) application of Dressilk and Suprathel; (D) 3-month follow-up; (E) 6-month follow-up; (F) 12-month follow up.
Subjective scar assessment was performed using the Vancouver Scar Scale (VSS). The traditional VSS is a validated subjective scale for scar assessment that has been described in detail in previous studies [17,18,19,20].
Objective scar assessment was performed with established objective scar-assessment tools such as the Cutometer®, Mexameter®, Tewameter® (Courage + Khazala Electronic GmbH, Cologne, Germany), and the O2C® (Oxygen to see; LEA Medizintechnik, Giessen, Germany). More details regarding these tools are provided in the following paragraphs:
The Cutometer® is used to determine the viscoelasticity and pliability of skin. The measurement principle of this tool is based on the concept of negative pressure, which is produced with a pump in the device and draws the skin into the opening of the measuring probe [21]. Three different measurement variables were taken into account for this study: (1) R0, the maximum suction depth of the skin, which represents the firmness of the skin; (2) R2, the difference between suction and retraction, i.e., elasticity, and (3) F1, the area under the curve up to the maximum amplitude [21].
The Mexameter® is used to assess skin discoloration, which is evaluated measuring vascularization (erythema) and pigmentation (Melanin). The device emits light of three different wavelengths and then evaluates the light reflected by the skin in order to calculate the amount of light absorbed [22].
The Tewameter® is a modern digital evaporimeter that non-invasively measures the evaporative water loss (EWL) of the skin. The EWL is defined as the quantity of water in grams that passes through the skin to the surrounding atmosphere per hour and area (m2) [23]. Two sensors for temperature and relative humidity measure the EWL indirectly with the help of a formula [24]. The transepidermal water loss (TEWL), an essential parameter indicating the efficiency of the skin barrier, and the skin surface water loss (SSWL), which indicates the water-binding capacity, were evaluated [25].
The O2C® assesses perfusion by combining photo-spectroscopy and Laser Doppler to evaluate superficial oxygen saturation (sO2), hemoglobin concentration (rHb), and flow [26,27].
Statistical analysis: We used Microsoft Excel (2017, Microsoft, USA) to manage data and create the charts. The data evaluated in this study were collected prospectively. After a thorough review of the data, SPSS (Version 21, IBM, USA) was used for final statistical analysis. Statistical significance was considered at p ≤ 0.05. The Friedman and Wilcoxon test was performed to identify statistically significant differences between the subgroups.
3. Results
Twenty patients (12 males and 8 females) were treated with both wound dressings between May 2017 and May 2018 and asked to participate in this follow-up study for scar evaluation. The patient characteristics are presented in Table 1.
Table 1.
Patient characteristics (age, sex and location of treated injury).
| Patient ID | Sex | Age | Area Treated with Dresssilk® | Area Treated with Suprathel® |
|---|---|---|---|---|
| 1 | M | >40–50 | Right forearm | Right hand |
| 2 | M | >60 | Left thigh | Right thigh |
| 3 | M | >30–40 | Left thigh | Right thigh |
| 4 | M | >50–60 | Right hand and forearm | Right forearm |
| 5 | M | >40–50 | Right forearm | Left forearm |
| 6 | M | >40–50 | Left D1 + D2 | Left D3–D5 |
| 7 | F | >50–60 | Right forearm | Left upper arm |
| 8 | F | >40–50 | Left hand | Right hand |
| 9 | F | <20 | Left thigh distal | Left thigh proximal |
| 10 | M | >30–40 | Left upper arm | Left forearm |
| 11 | F | >20–30 | Right thigh distal | Right thigh proximal |
| 12 | F | >40–50 | Right breast | Abdomen |
| 13 | M | >30–40 | Left hand and forearm | Right hand and forearm |
| 14 | M | >20–30 | Right proximal forearm | Right hand and forearm |
| 15 | M | >20–30 | Right hip | Right hand |
| 16 | M | >30–40 | Left forearm proximal | Right forearm distal |
| 17 | M | >60 | Right upper arm | Right forearm |
| 18 | F | >20–30 | Abdomen | Abdomen |
| 19 | F | >50–60 | Right thigh | Right shank |
| 20 | F | >50–60 | Left foot and upper leg | Left shank and forearm |
3.1. Subjective Scar Evaluation
The VSS evaluations showed no significant differences in blood circulation, pigmentation, elasticity and skin thickness between the wound areas treated after 12 months. A significant difference in blood flow (p = 0.005) was only observed after six months, when the burn wound treated with Dressilk® was less red and significantly more pigmented (p = 0.02) than the second-degree burn treated with Suprathel®.
3.2. Objective Scar Evaluations
Subsequently, scarring was objectively evaluated in comparison with the selected healthy skin areas in the follow-up examinations performed after three, six, and twelve months and compared to the selected healthy skin areas.
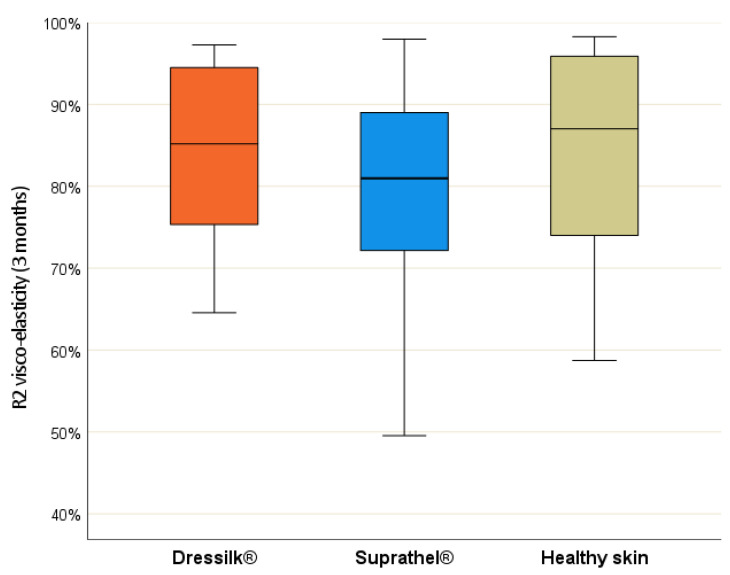
Cutometer®—In assessments of the firmness of the skin (R0 value), significant differences could be seen between the two dressings after three months (p = 0.013). Suprathel® showed a significant difference in comparison with the healthy skin (p = 0.024). At this point, the elasticity (R2 value) of the wound treated with Suprathel® (p = 0.017) differed significantly from the standard value (Figure 3).
Figure 3.
R2-values (visco-elasticity) after 3 months of areas treated initially with Suprathel (blue), Dressilk(orange) and the uninjured control area (green).
However, the R2 values for both materials were similar (p = 0.057). For the F1 value, a significant difference between the two dressings could only be detected after three months (p = 0.01). After six and twelve months, no further statistical differences were detected in the measured values (Table 2).
Table 2.
Cutometer R0-values (stretchability/firmness) in mm and R2-values (visco-elasticity) in %, F1-values (elasticity) in mm2 after 3, 6 and 12 months of areas treated initially with Suprathel, Dressilk and the uninjured control area.
| Cutometer-Measurement | Month | Dressilk® | Suprathel® | Healthy Skin | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| R0 | 3 | 0.57 | 0.45 | 0.44 | 0.36 | 0.58 | 0.38 |
| 6 | 0.83 | 0.54 | 0.79 | 0.53 | 0.79 | 0.59 | |
| 12 | 0.83 | 0.43 | 0.75 | 0.39 | 0.82 | 0.4 | |
| R2 | 3 | 84.02% | 0.11 | 79.44% | 0.13 | 84.87% | 0.12 |
| 6 | 85.63% | 0.07 | 81.24% | 0.12 | 83.18% | 0.11 | |
| 12 | 82.32% | 0.13 | 81.84% | 0.11 | 82.36% | 0.13 | |
| F1 | 3 | 0.09 | 0.08 | 0.07 | 0.06 | 0.07 | 0.05 |
| 6 | 0.11 | 0.08 | 0.15 | 0.15 | 0.11 | 0.08 | |
| 12 | 0.17 | 0.12 | 0.15 | 0.07 | 0.16 | 0.09 | |
Mexameter®—No significant differences were observed in pigmentation between the dressings after three and six months.
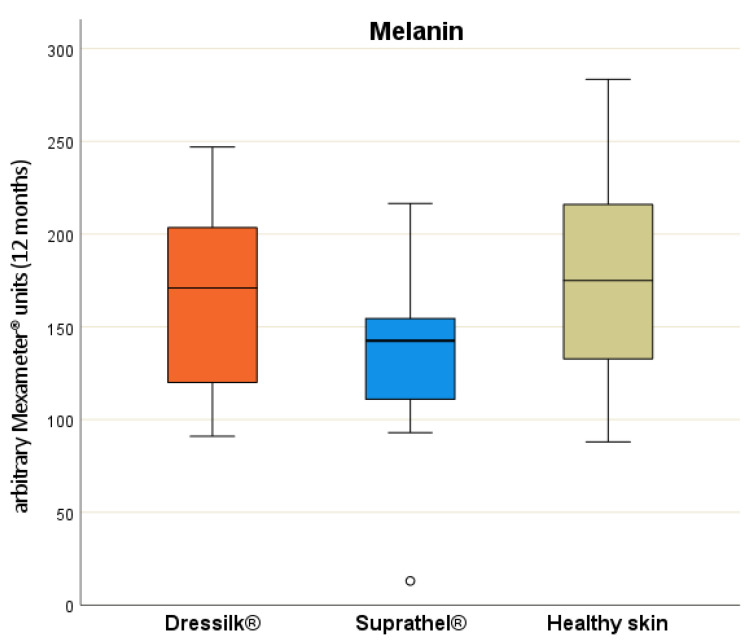
A significant difference in comparison with the normal skin could be detected at six months after Suprathel® treatment (p = 0.021). After 12 months, a significant difference in pigmentation (p = 0.009) could be observed between both dressings (Figure 4).
Figure 4.
Level of melanin in arbitrary Mexameter® units after 12 months of areas treated initially with Suprathel (blue), Dressilk (orange) and the uninjured control area (green).
Thus, the wound areas treated with Dressilk® showed no significant difference in pigmentation in comparison with normal skin, whereas those treated with Suprathel® showed a significant difference (p = 0.004). In assessments of erythema, both dressings showed significantly higher values than those for normal skin after three, six, and twelve months, whereas the inter-therapeutic difference was not significant (Table 3).
Table 3.
Level of melanin/erythema in arbitrary Mexameter® units after 3, 6 and 12 months of areas treated initially with Suprathel, Dressilk and the uninjured control area.
| Mexameter Measurements |
Month | Dressilk® | Suprathel® | Healthy Skin | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Melanin | 3 | 100 | 56 | 91 | 48 | 123 | 57 |
| 6 | 118 | 43 | 106 | 58 | 138 | 53 | |
| 12 | 163 | 5 | 135 | 45 | 175 | 59 | |
| Erythema | 3 | 429 | 11 | 441 | 141 | 310 | 120 |
| 6 | 354 | 119 | 335 | 116 | 268 | 98 | |
| 12 | 288 | 97 | 280 | 114 | 237 | 100 | |
Tewameter®—The transepidermal water loss (TEWL) values for the wounds treated with Dressilk® did not differ significantly from those for the wounds treated with Suprathel® after months three and twelve (see Table 4). During both measurements, the TEWL was significantly lower than the values for healthy skin.
Table 4.
Transepidermal water loss (TEWL) in g/h/m2 after 3, 6 and 12 months of areas treated initially with Suprathel, Dressilk and the uninjured control area.
| Tewameter Measurement | Month | Dressilk® | Suprathel® | Healthy Skin | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| TEWL | 3 | 18 | 11 | 22 | 13 | 13 | 9 |
| 6 | 16 | 14 | 20 | 16 | 14 | 13 | |
| 12 | 15 | 11 | 15 | 10 | 12 | 7 | |
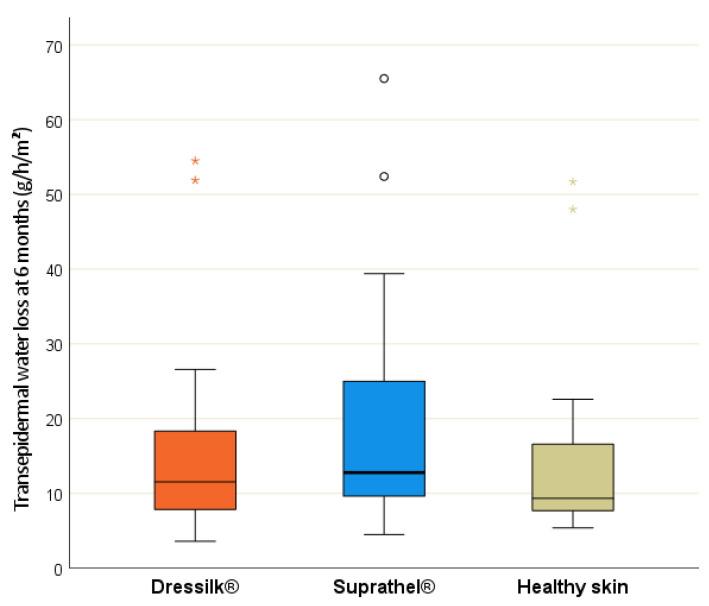
Interestingly, a significant difference was observed in the TEWL values between the two wound dressings after 6 months (p = 0.017; Figure 5), with the wounds treated with Dressilk® showing no significant difference to normal skin, unlike Suprathel® (p = 0.044).
Figure 5.
Transepidermal water loss (TEWL) in g/h/m2 after 6 months of areas treated initially with Suprathel (blue), Dressilk (orange) and the uninjured control area (green), significant differences marked.
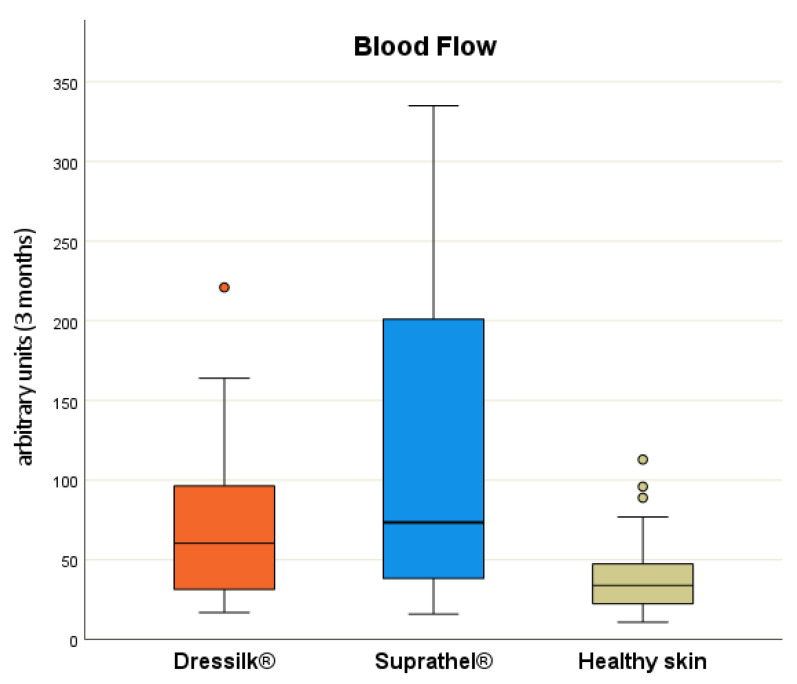
Oxygen to see (O2C)®—After three months, there were no significant differences between the two dressings in terms of sO2 and rHb values. However, the blood flow rate differed at this point and was significantly higher in the wound areas treated with Suprathel® (p = 0.04). In comparison with normal skin, the wound areas treated with both dressings showed significantly higher values for all measured parameters after 3 months (Figure 6).
Figure 6.
Flow in arbitrary units after 3 months of areas treated initially with Suprathel (blue), Dressilk (orange) and the uninjured control area (green), significant differences marked.
In assessments of sO2, the wounds treated with Dressilk® did not differ from the healthy skin after month six (p = 0.053). However, after 12 months, the sO2 values of the differently treated wounds differed significantly (p = 0.004), and the sO2 of the burn wounds treated with Dressilk® was significantly lower. Similar to the findings after month six, the wound treated with Dressilk® no longer differed from normal skin, in contrast to the wound treated with Suprathel® (p = 0.008). The rHb and flow rate showed no significant difference between the therapies at this point. However, Suprathel® (p = 0.002) and Dressilk® (p = 0.007) still showed significant differences in rHb in comparison with normal skin, whereas the flow rates in wounds treated with both dressings (p > 0.05) were consistent with that of normal skin (Table 5). Altogether, after 12 months, significant differences between the areas treated with the two dressings were solely detected with the O2C regarding the sO2 value. All other values showed no significant difference between the two treatments at this point.
Table 5.
Oxygen levels in %, rHb/Flow in AU after 3, 6 and 12 months of areas treated initially with Suprathel, Dressilk and the uninjured control area.
| O2C Measurements |
Month | Dressilk® | Suprathel® | Healthy Skin | |||
|---|---|---|---|---|---|---|---|
| MW | SD | MW | SD | MW | SD | ||
| sO2 | 3 | 65 | 22 | 69 | 18 | 47 | 16 |
| 6 | 56 | 22 | 62 | 22 | 50 | 17 | |
| 12 | 49 | 22 | 59 | 24 | 49 | 17 | |
| rHb | 3 | 93 | 13 | 93 | 14 | 74 | 10 |
| 6 | 87 | 14 | 87 | 13 | 76 | 12 | |
| 12 | 83 | 13 | 86 | 17 | 77 | 10 | |
| Flow | 3 | 72 | 53 | 108 | 93 | 42 | 29 |
| 6 | 79 | 53 | 73 | 57 | 48 | 35 | |
| 12 | 47 | 32 | 56 | 57 | 44 | 21 | |
Overall, after 12 months, only a few differences between the two differently treated areas could be detected. The VSS, Cutometer and Tewameter showed no differences; only the melanin values from the Mexameter and the sO2 value from the O2C were significantly different.
4. Discussion
To the best of our knowledge, this is the first study using standardized and reproducible scar-evaluation tools to compare the scar quality after treatment with the wound dressings Dressilk® and Suprathel®.
4.1. Scar Assessment
4.1.1. Subjective Scar Assessment
In burn rehabilitation, patient satisfaction with the appearance of their scar is of high importance [28]. The outcome of the treatment procedure is heavily influenced by the patients’ opinion about the scarring [29,30]. With a focus on patient satisfaction, we performed VSS assessments in the follow-up examinations performed after three, six and twelve months.
As already described in the results of our previous study [16], both wound dressings appear to be subjectively equal in terms of patient satisfaction. However, the VSS [31,32,33] evaluations showed some subjective differences, consistent with the results of our previous study comparing Dressilk® to Biobrane® [6]. We were able to detect favorable results for Dressilk® in the VSS, especially in terms of blood flow (month 6, p = 0.005). Similarly, as shown in another study, this was also found in the POSAS Observer scale [16].
4.1.2. Objective Scar Assessment
Additionally, consistent with our previous studies [6,7,34], we evaluated the scars by using objective scar-assessment tools. As outlined in the literature, these tools are especially necessary to minimize inter-examiner variability and maximize the reliability of the results [35].
As a tool to detect elasticity, the Cutometer® has already been validated as a reliable scar-assessment tool [36,37,38]. In this study, a significant difference in skin firmness (R0, p = 0.013) was observed only within three months. Interestingly, the scar previously treated with Dressilk® did not show a difference in firmness and elasticity in comparison with normal skin from the three-month measurement onward. Although scar elasticity has been suggested to not reach the elasticity of normal skin, as shown in the measurements reported by Anthonissen et al. [39], this was not consistent with our findings. Nevertheless, it should be emphasized that scarring depends on the initial burn depth. Superficial burns usually heal without scarring, whereas the deep partial burns involving the dermal layer beneath the papillary layer cause scarring [40,41]. Moreover, the dermis containing the protein elastin might not be severely damaged in superficial-thickness burns, leading to our results. Interestingly, the data reported by Busche et al. suggest that even two years after superficial burn injury, the scars of superficial burn injuries solely treated with fatty gaze did not show elasticity similar to that of normal skin [29].
The Mexameter® allows evaluation of skin vascularization (erythema) and pigmentation (melanin) [22]. External evaluation in previous studies has also been shown to be reliable in comparison with subjective scar-assessment tools such as the VSS [42,43,44]. Superficial partial-thickness burns are known to lead to alterations of skin pigmentation [34,45,46]. Inter-individual influences in factors such as sun or UV protection may also influence objective assessments. In our objective scar assessment, we showed that the wound areas were redder with higher erythema than normal skin after 12 months, consistent with the findings of a previous study [34]. Nonetheless, the pigmentation after Dressilk® application seemed to be superior, showing no difference to normal skin at this point. In contrast, subjective scar assessments, as mentioned above and shown in the literature [47], can indicate non-apparent differences even sooner. Fortunately for burn surgeons, recent studies have shown that the highest patient satisfaction is related to coloration of the scar in conservatively treated superficial burn injuries [29]. For cases involving unsatisfactory hypopigmentation of burn scars, Busch et al. showed promising results by examining medical needling in combination with the ReCell Technique in 20 patients. In these cases, the melanin level increased significantly after 12 months while also showing subjective individual improvement of the scars [1].
The Tewameter® is a modern digital evaporimeter that has been used in different studies and has been shown to be a reliable tool [23,25,48]. The TEWL is an essential parameter to determine the efficiency of the skin barrier [25]. Interestingly, we were able to show that, after six months, the initial wound area treated with Dressilk® did not differ from normal skin, compared to the initial wound area treated with Suprathel®. Although this difference was not verifiable after 12 months, it may indicate slightly faster skin barrier normalization in wounds treated with Dressilk®. Consistent with this finding, the mean TEWL for Dressilk® was lower than for Biobrane® after 6 months in our previous study, although the difference was not statistically significant [34]. Even more, this finding indicates that the stratum corneum can restore its impaired function within months after the burn injury [25,39]. Consistent with our results, studies have shown that the primarily higher scar TEWL values gradually disappear over time [25]. However, some parameters can also influence these differences in TEWL, e.g., individual differences in skin treatments such as consequent moisturizing. We aimed to avoid the effects of these phenomena by choosing an intra-individual study design.
The O2C® device assesses perfusion by a combination of photo-spectroscopy and Laser Doppler. Measurements of sO2, rHb and flow can be obtained using this approach [27]. This device has gained interest as a monitoring device after reconstructive surgery, especially for the evaluation of postoperative recovery and noninvasive perfusion control [27,49,50]. Moreover, the remaining blood flow can be used as a parameter to express the healing potential of burn-injured areas [51]. In this study, the scar tissue formerly treated with Suprathel® showed both a significantly higher flow rate than that treated with Dressilk ® after 3 months (p = 0.041) and a higher sO2 after 12 months (p = 0.004). Although these measurements may indicate a possible beneficial wound-healing potential for Suprathel®, Dressilk®, contrastingly, did not show any significant difference in oxygen saturation compared to the healthy skin from the six-month measurement onward. In contrast, our prior study results did not show significant differences between treated (Dressilk® vs. Biobrane®) and non-treated areas after 6 and 12 months, regardless of the applied dressing [34]. The informative value of these parameters in scar evaluation remains unclear, since the blood flow and oxygen saturation are also related to the initial burn depth [51].
4.2. Limitations
In this context, the influences of various conditions that cannot be controlled must be reviewed critically. For example, we could not exclude the potential influences of differences in outside temperatures, increased perspiration, or very dry, brittle skin on the measurement results. Strong exposure to the sun due to insufficient sun protection or visits to a tanning bed could also have led to different long-term results. Nevertheless, by performing exact photo-documentation of the measurement points and ensuring that the assessments were performed by the same investigator and in the same examination room, we were able to ensure maximal accuracy in the comparisons.
5. Conclusions
Objective and repeatable scar-assessment tools allow quantification of the benefits of wound dressings in relation to scar quality. Both evaluated dressing materials showed similarly good results in the long-term objective and subjective scar assessments after 12 months. Nevertheless, Dressilk® showed slight benefits in different categories and an overall faster return to the characteristics of non-injured skin areas. Since Dressilk® has already been implemented in the SOC for superficial burns at our clinic, our study results underline that Dressilk® remains an interesting alternative to Suprathel® for the treatment of superficial partial-thickness burns. Further research on a larger variety of scars is needed for implementing and finding ideal objective scar-evaluation methods and facilitating repeatability.
Author Contributions
Conceptualization, J.L.S. and A.S.; methodology, J.L.S.; software, J.L.S., R.L. and J.A.; validation, J.L.S. and R.L.; formal analysis, J.L.S., J.A. and R.L.; investigation, J.L.S. and J.A.; data curation, J.L.S. and J.A.; writing—original draft preparation, M.B. and J.L.S.; writing—review and editing J.L.S., P.I.H. and P.C.F.; visualization, J.L.S.; supervision, J.L.S.; project administration, J.L.S. and A.S.; funding acquisition, J.L.S. and A.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study protocol was reviewed and approved in advance by the Ethical Review Committee of the University of Witten Herdecke, Germany (number 5/2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data can be obtained through the authors.
Conflicts of Interest
The authors disclose a conflict of interest in connection with the submitted study since it was partly supported by Prevor (France), Nevertheless, Prevor had no influence in planning and conducting the study. Furthermore, Prevor had no role in the data analysis and the submitted manuscript.
Funding Statement
This research was supported, in part, by Prevor (France).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Busch K.H., Bender R., Walezko N., Aziz H., Altintas M.A., Aust M.C. Combination of medical needling and non-cultured autologous skin cell transplantation (ReNovaCell) for repigmentation of hypopigmented burn scars. Burns. 2016;42:1556–1566. doi: 10.1016/j.burns.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 2.McGwin G., Cross J.M., Ford J.W., Rue L.W. Long-term trends in mortality according to age among adult burn patients. J. Burn Care Rehabil. 2003;24:21–25. doi: 10.1097/00004630-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Baumann M.E., DeBruler D.M., Blackstone B.N., Coffey R.A., Boyce S.T., Supp D.M., Bailey J.K., Powell H.M. Direct comparison of reproducibility and reliability in quantitative assessments of burn scar properties. Burns. 2021;47:466–478. doi: 10.1016/j.burns.2020.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Kee E.G., Kimble R.M., Cuttle L., Khan A., Stockton K.A. Randomized controlled trial of three burns dressings for partial thickness burns in children. Burns. 2015;41:946–955. doi: 10.1016/j.burns.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Saeidinia A., Keihanian F., Lashkari A.P., Lahiji H.G., Mobayyen M., Heidarzade A., Golchai J. Partial-thickness burn wounds healing by topical treatment: A randomized controlled comparison between silver sulfadiazine and centiderm. Medicine. 2017;96:e6168. doi: 10.1097/MD.0000000000006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiefer J.L., Arens E., Grigutsch D., Rath R., Hoffmann A., Fuchs P.C., Schulz A. A prospective intra-individual evaluation of silk compared to Biobrane for the treatment of superficial burns of the hand and face. Burns. 2017;43:539–548. doi: 10.1016/j.burns.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Schulz A., Depner C., Lefering R., Kricheldorff J., Kästner S., Fuchs P.C., Demir E. A prospective clinical trial comparing Biobrane® Dressilk® and PolyMem® dressings on partial-thickness skin graft donor sites. Burns. 2016;42:345–355. doi: 10.1016/j.burns.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Balasubramani M., Kumar T.R., Babu M. Skin substitutes: A review. Burns. 2001;27:534–544. doi: 10.1016/S0305-4179(01)00018-3. [DOI] [PubMed] [Google Scholar]
- 9.Kagan R.J., Peck M.D., Ahrenholz D.H., Hickerson W.L., Holmes I.V.J., Korentager R., Kraatz J., Pollock K., Kotoski G. Surgical management of the burn wound and use of skin substitutes: An expert panel white paper. J. Burn Care Res. 2013;34:e60–e79. doi: 10.1097/BCR.0b013e31827039a6. [DOI] [PubMed] [Google Scholar]
- 10.Baron H. Textile wound coverings. Langenbecks Arch. Klin Chir Dtsch Chir. 1953;274:510–533. [PubMed] [Google Scholar]
- 11.Baron H. Standardization of wound textiles. Nature. 1955;175:760–763. doi: 10.1038/175760b0. [DOI] [PubMed] [Google Scholar]
- 12.Boateng J.S., Matthews K.H., Stevens H.N., Eccleston G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008;97:2892–2923. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- 13.Agren M.S., Werthen M. The extracellular matrix in wound healing: A closer look at therapeutics for chronic wounds. Int. J. Low Extrem. Wounds. 2007;6:82–97. doi: 10.1177/1534734607301394. [DOI] [PubMed] [Google Scholar]
- 14.Uhlig C., Rapp M., Hartmann B., Hierlemann H., Planck H., Dittel K.K. Suprathel-an innovative, resorbable skin substitute for the treatment of burn victims. Burns. 2007;33:221–229. doi: 10.1016/j.burns.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Gholipourmalekabadi M., Sapru S., Samadikuchaksaraei A., Reis R.L., Kaplan D.L., Kundu S.C. Silk fibroin for skin injury repair: Where do things stand? Adv. Drug Deliv. Rev. 2019;153:28–53. doi: 10.1016/j.addr.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Schiefer J.L., Andreae J., Bagheri M., Fuchs P.C., Lefering R., Heitzmann W., Schulz A. A clinical comparison of pure knitted silk and a complex synthetic skin substitute for the treatment of partial thickness burns. Int. Wound J. 2021;19:178–187. doi: 10.1111/iwj.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nedelec B., Shankowsky H.A., Tredget E.E. Rating the resolving hypertrophic scar: Comparison of the Vancouver Scar Scale and scar volume. J. Burn Care Rehabil. 2000;21:205–212. doi: 10.1097/00004630-200021030-00005. [DOI] [PubMed] [Google Scholar]
- 18.Forbes-Duchart L., Marshall S., Strock A., Cooper J.E. Determination of inter-rater reliability in pediatric burn scar assessment using a modified version of the Vancouver Scar Scale. J. Burn Care Res. 2007;28:460–467. doi: 10.1097/BCR.0b013E318053D3BB. [DOI] [PubMed] [Google Scholar]
- 19.Rennekampff H.O., Rabbels J., Reinhard V., Becker S.T., Schaller H.E. Comparing the Vancouver Scar Scale with the cutometer in the assessment of donor site wounds treated with various dressings in a randomized trial. J. Burn Care Res. 2006;27:345–351. doi: 10.1097/01.BCR.0000216311.61266.00. [DOI] [PubMed] [Google Scholar]
- 20.Bae S.H., Bae Y.C. Analysis of frequency of use of different scar assessment scales based on the scar condition and treatment method. Arch. Plast. Surg. 2014;41:111–115. doi: 10.5999/aps.2014.41.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Courage + Khazaka Electronic GmbH Cutometer® Dual MPA 580—Literature List Cutometer®. [(accessed on 7 April 2022)]. Available online: https://www.courage-khazaka.de/images/Downloads/Studien/Studies_Cutometer.pdf.
- 22.Courage + Khazaka Electronic GmbH Mexameter® MX 18—Literature List Mexameter®. [(accessed on 7 April 2022)]. Available online: https://www.courage-khazaka.de/images/Downloads/Studien/Studies_Mexameter.pdf.
- 23.Busche M.N., Roettger A., Herold C., Vogt P.M., Rennekampff H.O. Evaporative water loss in superficial to full thickness burns. Ann. Plast. Surg. 2016;77:401–405. doi: 10.1097/SAP.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 24.Courage + Khazaka Electronic GmbH Tewameter® TM 300—Literature List Tewameter®. [(accessed on 7 April 2022)]. Available online: https://www.courage-khazaka.de/images/Downloads/Studien/Studies_Tewameter.pdf.
- 25.Gardien K.L., Baas D.C., de Vet H.C., Middelkoop E. Transepidermal water loss measured with the Tewameter TM300 in burn scars. Burns. 2016;42:1455–1462. doi: 10.1016/j.burns.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 26.GmbH, L.M O2C (Oxygen to See) 2013. [(accessed on 7 April 2022)]. Available online: http://www.lea.de/deu/indexd.html.
- 27.Henton J.M., Simmons J.M., Hettiaratchy S., Jain A. Perfusion dynamics in lower limb reconstruction: Investigating postoperative recovery and training using combined white light photospectroscopy and laser Doppler (O2C((R))) J. Plast. Reconstr. Aesthet. Surg. 2015;68:1286–1292. doi: 10.1016/j.bjps.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Ren Z., Chang W.C., Zhou Q., Wang Y., Wang H., Hu D. Recovery of lost face of burn patients, perceived changes, and coping strategies in the rehabilitation stage. Burns. 2015;41:1855–1861. doi: 10.1016/j.burns.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 29.Busche M.N., Thraen A.C., Gohritz A., Rennekampff H.O., Vogt P.M. Burn scar evaluation using the cutometer® MPA 580 in comparison to "Patient and Observer Scar Assessment Scale" and "Vancouver Scar Scale". J. Burn Care Res. 2018;39:516–526. doi: 10.1093/jbcr/irx009. [DOI] [PubMed] [Google Scholar]
- 30.Draaijers L.J., Tempelman F.R., Botman Y.A., Tuinebreijer W.E., Middelkoop E., Kreis R.W., Van Zuijlen P.P. The patient and observer scar assessment scale: A reliable and feasible tool for scar evaluation. Plast. Reconstr. Surg. 2004;113:1960–1965. doi: 10.1097/01.PRS.0000122207.28773.56. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan T.A., Smith J., Kermode J., Mclver E., Courtemanche D.J. Rating the burn scar. J. Burn Care Rehabil. 1990;11:256–260. doi: 10.1097/00004630-199005000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Chipp E., Charles L., Thomas C., Whiting K., Moiemen N., Wilson Y. A prospective study of time to healing and hypertrophic scarring in paediatric burns: Every day counts. Burn. Trauma. 2017;5:3. doi: 10.1186/s41038-016-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klosova H., Klein L., Blaha J. Analysis of a retrospective double-centre data-collection for the treatment of burns using biological cover Xe-derma(R) Ann. Burn. Fire Disasters. 2014;27:171–175. [PMC free article] [PubMed] [Google Scholar]
- 34.Schiefer J.L., Rath R., Ahrens E., Grigutsch D., Gräff I., Stromps J.P., Fuchs P.C., Schulz A. Evaluation of scar quality after treatment of superficial burns of the hands and face with Dressilk or Biobrane—An intra-individual comparison. Burns. 2018;44:305–317. doi: 10.1016/j.burns.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 35.Lee K.C., Bamford A., Gardiner F., Agovino A., ter Horst B., Bishop J., Sitch A., Grover L., Logan A., Moiemen N.S. Investigating the intra- and inter-rater reliability of a panel of subjective and objective burn scar measurement tools. Burns. 2019;45:1311–1324. doi: 10.1016/j.burns.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poetschke J., Dornseifer U., Clementoni M.T., Reinholz M., Schwaiger H., Steckmeier S., Ruzicka T., Gauglitz G.G. Ultrapulsed fractional ablative carbon dioxide laser treatment of hypertrophic burn scars: Evaluation of an in-patient controlled, standardized treatment approach. Lasers Med. Sci. 2017;32:1031–1040. doi: 10.1007/s10103-017-2204-z. [DOI] [PubMed] [Google Scholar]
- 37.Goei H., van der Vlies C., Tuinebreijer W., van Zuijlen P., Middelkoop E., van Baar M. Predictive validity of short term scar quality on final burn scar outcome using the Patient and Observer Scar Assessment Scale in patients with minor to moderate burn severity. Burns. 2017;43:715–723. doi: 10.1016/j.burns.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Held M., Engelke A.-S., Tolzmann D.S., Rahmanian-Schwarz A., Schaller H.-E., Rothenberger J. Biomechanical skin property evaluation for wounds treated with synthetic and biosynthetic wound dressings and a newly developed collagen matrix during healing of superficial skin defects in a rat models. Wounds. 2016;28:334–340. [PubMed] [Google Scholar]
- 39.Anthonissen M., Daly D., Fieuws S., Massagé P., Van Brussel M., Vranckx J., Van den Kerckhove E. Measurement of elasticity and transepidermal water loss rate of burn scars with the Dermalab((R)) Burns. 2013;39:420–428. doi: 10.1016/j.burns.2012.07.026. [DOI] [PubMed] [Google Scholar]
- 40.Brusselaers N., Monstrey S., Vogelaers D., Hoste E., Blot S. Severe burn injury in Europe: A systematic review of the incidence, etiology, morbidity, and mortality. Crit. Care. 2010;14:R188. doi: 10.1186/cc9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu P.X., Diao W.Q., Qi Z.L., Cai J.L. Effect of Dermabrasion and ReCell® on large superficial facial scars caused by burn, trauma and acnes. Chin. Med. Sci. J. 2016;31:173–179. doi: 10.1016/S1001-9294(16)30047-5. [DOI] [PubMed] [Google Scholar]
- 42.Nedelec B., Correa J.A., de Oliveira A., LaSalle L., Perrault I. Longitudinal burn scar quantification. Burns. 2014;40:1504–1512. doi: 10.1016/j.burns.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Nedelec B., Correa J.A., Rachelska G., Armour A., LaSalle L. Quantitative measurement of hypertrophic scar: Interrater reliability and concurrent validity. J. Burn Care Res. 2008;29:501–511. doi: 10.1097/BCR.0b013e3181710881. [DOI] [PubMed] [Google Scholar]
- 44.van der Wal M., Bloemen M., Verhaegen P., Tuinebreijer W., de Vet H., van Zuijlen P., Middelkoop E. Objective color measurements: Clinimetric performance of three devices on normal skin and scar tissue. J. Burn Care Res. 2013;34:e187-94. doi: 10.1097/BCR.0b013e318264bf7d. [DOI] [PubMed] [Google Scholar]
- 45.Danielsen P., Jorgensen L., Jørgensen B., Karlsmark T., Ågren M. Erythema persists longer than one year in split-thickness skin graft donor sites. Acta Derm. Venereol. 2013;93:281–285. doi: 10.2340/00015555-1455. [DOI] [PubMed] [Google Scholar]
- 46.Evers L.H., Bhavsar D., Mailänder P. The biology of burn injury. Exp. Dermatol. 2010;19:777–783. doi: 10.1111/j.1600-0625.2010.01105.x. [DOI] [PubMed] [Google Scholar]
- 47.Bond J.S., Duncan J.A., Mason T., Sattar A., Boanas A., O’Kane S., Ferguson M.W. Scar redness in humans: How long does it persist after incisional and excisional wounding? Plast. Reconstr. Surg. 2008;121:487–496. doi: 10.1097/01.prs.0000299183.88334.37. [DOI] [PubMed] [Google Scholar]
- 48.Cho Y.S., Jeon J.H., Hong A., Yang H.T., Yim H., Cho Y.S., Kim D.H., Hur J., Kim J.H., Chun W., et al. The effect of burn rehabilitation massage therapy on hypertrophic scar after burn: A randomized controlled trial. Burns. 2014;40:1513–1520. doi: 10.1016/j.burns.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Medved F., Medesan R., Rothenberger J.M., Schaller H.-E., Schoeller T., Manoli T., Weitgasser L., Naumann A., Weitgasser L. Analysis of the microcirculation after soft tissue reconstruction of the outer ear with burns in patients with severe burn injuries. J. Plast. Reconstr. Aesthet. Surg. 2016;69:988–993. doi: 10.1016/j.bjps.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 50.Rothenberger J., Amr A., Schaller H.-E., Rahmanian-Schwarz A. Evaluation of a non-invasive monitoring method for free flap breast reconstruction using laser doppler flowmetrie and tissue spectrophotometry. Microsurgery. 2013;33:350–357. doi: 10.1002/micr.22096. [DOI] [PubMed] [Google Scholar]
- 51.Jaspers M.E., van Haasterecht L., van Zuijlen P., Mokkink L.B. A systematic review on the quality of measurement techniques for the assessment of burn wound depth or healing potential. Burns. 2019;45:261–281. doi: 10.1016/j.burns.2018.05.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be obtained through the authors.