Abstract
A tetrazolium dye reduction assay was used to study factors governing the killing of bacteria by oyster hemocytes. In vitro tests were performed on bacterial strains by using hemocytes from oysters collected from the same location in winter and summer. Vibrio parahaemolyticus strains, altered in motility or colonial morphology (opaque and translucent), and Listeria monocytogenes mutants lacking catalase, superoxide dismutase, hemolysin, and phospholipase activities were examined in winter and summer. Vibrio vulnificus strains, opaque and translucent (with and without capsules), were examined only in summer. Among V. parahaemolyticus and L. monocytogenes, significantly (P < 0.05) higher levels of killing by hemocytes were observed in summer than in winter. L. monocytogenes was more resistant than V. parahaemolyticus or V. vulnificus to the bactericidal activity of hemocytes. In winter, both translucent strains of V. parahaemolyticus showed significantly (P < 0.05) higher susceptibility to killing by hemocytes than did the wild-type opaque strain. In summer, only one of the V. parahaemolyticus translucent strains showed significantly (P < 0.05) higher susceptibility to killing by hemocytes than did the wild-type opaque strain. No significant differences (P > 0.05) in killing by hemocytes were observed between opaque (encapsulated) and translucent (nonencapsulated) pairs of V. vulnificus. Activities of 19 hydrolytic enzymes were measured in oyster hemolymph collected in winter and summer. Only one enzyme, esterase (C4), showed a seasonal difference in activity (higher in winter than in summer). These results suggest that differences existed between bacterial genera in their ability to evade killing by oyster hemocytes, that a trait(s) associated with the opaque phenotype may have enabled V. parahaemolyticus to evade killing by the oyster’s cellular defense, and that bactericidal activity of hemocytes was greater in summer than in winter.
Eastern oysters, Crassostrea virginica, are found in coastal waters of the Atlantic and Gulf coasts of the United States. Of economic importance, the value of the oyster harvest from United States waters was over $100 million in 1995 (24). Ecologically important as well, oysters possess the capacity of filtering up to 34 liters of water per h, thereby removing particulates and pollutants (20). This filter feeding behavior exposes these benthic invertebrates to a constant challenge by invasive and pathogenic microbes. To cope with this challenge, oysters possess humoral (9) and cellular (13, 14) defense mechanisms. Hemocytes form the cellular defense arsenal against infectious microbes by their ability to phagocytize, encapsulate, and kill microbes (43). Microbial killing may result from the combined action of humoral defense factors such as agglutinins and lysosomal enzymes plus toxic reactive oxygen intermediates formed during a respiratory burst associated with the hemocyte phagocytic process (2, 13, 43). Oyster agglutinins are thought to enhance phagocytosis (opsonization) by facilitating bacterial aggregation or binding of bacteria to hemocytes (15, 36). Lysosomal enzymes may act on the surface of microbes, contributing to their recognition and/or destruction by hemocytes (2).
Frequently eaten raw, oysters are often implicated as the source of human vibrio and other food-borne diseases (33). Some oyster-associated human pathogens, such as enteric bacteria, are transients whose presence is largely due to contamination of the water by human fecal wastes. Others, like Vibrio vulnificus and Vibrio parahaemolyticus, are of nonfecal origin and occur naturally in most shellfish harvesting areas of the United States (38). The effect of environmental salinity and temperature on the occurrence of vibrios in oysters has been studied in an effort to predict their densities in shellfish (32, 42). These vibrios, which persist and replicate in oysters (17, 41), were selected for the current study to identify additional factors that modulate their densities in oysters.
Although reports of the isolation of Listeria monocytogenes from oysters are lacking, this facultative, gram-positive bacterial pathogen was isolated from blue crab (11) and shrimp (23). Since microscopic studies have revealed a fascinating relationship between L. monocytogenes and its host (25) and since mutants with defects in their primary virulence factors were available (34), this bacterium was selected as an additional test microbe.
Characterizing bacterial interactions with cellular defenses may help explain the persistence of bacteria in oyster tissues. Few studies have examined the direct interaction of hemocytes and bacteria (18, 21). Comprehensive analyses of the colonization potential of specific bacteria in oysters are also lacking. In this study, bactericidal activity of oyster hemocytes was measured seasonally. Different bacterial mutants were used to identify potential strategies used by bacteria to reduce susceptibility to hemocyte killing. In mammalian systems, these strategies, aimed at blocking one or more steps in phagocytosis, include avoiding contact with phagocytes, inhibition of engulfment, and survival inside of phagocytes (12). Test bacteria that included parental strains and corresponding mutants of V. parahaemolyticus, V. vulnificus, and L. monocytogenes, lacking various putative virulence factors, were chosen with these avoidance strategies in mind. To quantify the bactericidal activity of oyster hemocytes, a recently developed in vitro colorimetric method was used (44).
MATERIALS AND METHODS
Oyster harvesting and handling.
Oysters (C. virginica) were collected in winter (30 January 1998) and again in summer (9 June 1998) from Bayou Texar, an inlet of Escambia Bay, Fla. Temperatures and ambient salinities at the collection site were 14°C and 5‰ in January and 30°C and 16‰ in June, respectively. Oysters were immediately transported to the U.S. Environmental Protection Agency’s Gulf Ecology Division laboratory and were held in a 1,099-liter holding tank equipped with a flow-through unfiltered seawater delivery system for 2 to 14 days prior to experimentation. The flow rate of seawater in this holding tank was approximately 90 liters/h.
Collecting hemolymph.
Hemolymph was withdrawn from the sinus of the adductor muscle through a notch in the oyster shell by using a syringe fitted with a 23-gauge needle. To reduce cell clumping, hemolymph samples were placed on ice. For each trial, an equal volume of hemolymph collected from each of 10 oysters was pooled to yield sufficient numbers of hemocytes for multiple simultaneous tests. The killing index (KI) of each bacterial species was assessed by using three separate pools of hemolymph.
Bacteria and culture conditions.
Strains used are listed in Table 1. The V. parahaemolyticus strains included the type strain (American Type Culture Collection), wild-type opaque (OP) and translucent (Tra) strains, a transposon mutant unable to switch to the OP parent (Fix Tra), and three motility mutants. One of the motility mutants was defective in swarming (Laf−), and two were defective in both swimming and swarming (Fla− Laf− and ParaFla− Laf−). Three different isogenic pairs of V. vulnificus strains were also tested. One member of each pair produced opaque colonies (O) and possessed capsules, and the other member produced translucent colonies (T) and lacked capsules. The L. monocytogenes strains included a parental wild-type (Wild) strain and mutants defective in the hemolysin, listeriolysin O, phospholipase C, superoxide dismutase activity, and catalase activity.
TABLE 1.
Bacterial strains used in this study
| Bacterial strain | Relevant characteristics | Reference or source |
|---|---|---|
| Vibrio parahaemo-lyticus | ||
| 17802 | Type strain | 19, American Type Culture Collection |
| BB22OP | Wild-type; opaque | 3, L. McCarter |
| BB22TR | Wild-type; translucent | L. McCarter |
| LM4462 | Transposon mutant unable to switch to OP; parent, BB22OP | 28 |
| LM1017 | Lacking lateral flagella (swarm−); parent, BB22TR | 31 |
| LM4170 | Paralyzed polar flagellum and lacking lateral flagella (swim− and swarm−) | 26 |
| ML120 | Lacking polar and lateral flagella (swim− and swarm−) | 29 |
| Vibrio vulnificus | ||
| M06-24 | Biotype 1; clinical isolate; opaque colonies; encapsulated cells | 45 |
| CDV752 | Translucent colonies; acapsular cells; derivative of M06-24 | 45 |
| 938 | Biotype 2; isolated from an eel in Denmark; opaque colonies; encapsulated cells | 39 |
| 938 | Translucent colonies; acapsular cells; derivative of 938 | 39 |
| 30249 | Biotype 1; clinical isolate; opaque colonies; encapsulated cells | 39 |
| ABZ1 | Translucent colonies; acapsular cells; derivative of 1003 | 47 |
| Listeria mono-cytogenes | ||
| 10403S | Wild-type; parental strain | 5, D. A. Portnoy |
| DP-L2161 | Hemolysin− listeriolysin− mutant of 10403S | D. A. Portnoy |
| DP-L1936 | Mutant of 10403S defective in phospholipase(s) | 40, D. A. Portnoy |
| DHL1 | Superoxide dismutase− mutant of 10403S | S. E. Martin |
| 1370 | Catalase− mutant of 10403S | S. E. Martin |
Vibrio spp. were cultured in nutrient broth (Difco Laboratories) supplemented with 2% NaCl (NBS), and L. monocytogenes was cultured in Trypticase soy broth (TSB) (Difco). Agar (1.5%) was added for culture on solid media. Filtered sea water (FSW) for diluent and testing was pumped from Santa Rosa Sound, near Gulf Breeze, Fla., diluted to a salinity of 20‰, sterilized by filtration (pore size, 0.22 μm), and maintained at 25°C.
Bacteria were grown to late-logarithmic or early-stationary phase by incubation for 18 h (25°C) with shaking (200 rpm) in 125-ml Erlenmeyer flasks containing 10 ml of culture broth. Bacteria were harvested by centrifugation (12,000 × g; 10 min) and suspended in an equal volume of FSW. Appropriate dilutions were made in FSW for use in the killing assay. Numbers of culturable bacteria were determined by spreading 0.1-ml aliquots of diluted bacterial suspension in triplicate on NBS or TSB agar plates and counting colonies arising after 18 to 36 h at 25°C.
Killing assay.
The percentage of bacteria killed (KI) was determined in flat-bottomed 96-well microtiter plates as described by Volety et al. (44), except streptomycin was not used.
Essential steps in the procedure of Volety et al. (44) were performed as follows. First, bacteria suspended in FSW were incubated in the presence and absence of plasma-free hemocytes at a bacteria/hemocyte ratio of approximately 10:1. To prepare plasma-free hemocytes, aliquots of hemolymph containing 105 hemocytes were placed in two sets of wells containing 100 μl of FSW. Microtiter plates containing hemolymph were centrifuged (160 × g; 10 min) to promote hemocyte adhesion. The plasma-FSW supernatant was removed with a multichannel pipette. The first two sets of wells contained the hemocytes. One of these sets of wells received FSW, and the other set of wells received FSW plus bacteria. The last two sets of wells did not contain hemocytes. One of these sets of wells contained FSW only (blank control), and the other set of wells received FSW plus bacteria. Microtiter plates were then centrifuged (160 × g; 10 min) to encourage bacterial contact with hemocyte monolayers and were incubated in a moist chamber (3 h at 17°C) to allow hemocyte killing.
In the second step, a recovery-growout period for surviving bacteria was started by adding the appropriate culture broth and incubating for 2 h at 25°C for the vibrios and 2.5 h at 37°C for L. monocytogenes strains.
In the final step, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium and phenylmethasulfazone (MTS-PMS) reagents (20 μl) (Promega Corporation, Madison, Wis., and Sigma Chemical Company, St. Louis, Mo., respectively) were added, and incubation was continued for an additional 30 min. Numbers of viable bacteria were determined colorimetrically by measurement of formazan, the soluble reduction product of MTS-PMS, at 490 nm with an enzyme-linked immunosorbent assay microplate reader (model 311-SX; Bio-Tek Instruments, Inc.). Eight replicate wells were used for each treatment. Absorbance (A) values were corrected by subtracting the background absorbances of the FSW only. The percentage of bacteria killed (KI) was calculated from the A values of the reduced MTS-PMS as follows:
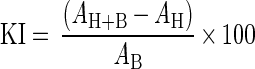 |
where H stands for hemocytes and B stands for bacteria. In cases where the calculated KI was <0, the KI was set at a value of 0.00 for statistical analyses.
In preliminary experiments, the relationship between numbers of bacteria and absorbance of the formazan was examined. Serial dilutions (1:2) of each bacterial strain minus hemocytes were loaded into 96-well microtiter plates (eight replicates per bacterial dilution), and the assay was performed. A values of reduced dye were regressed against dilutions of bacteria to verify a linear relationship and confirm the KI calculation procedure.
Enzyme assays.
Activities of 19 hydrolytic enzymes were measured in hemolymph by using the API ZYM system (BioMerieux Vitek, Inc., Hazelwood, Mo.). In six replicate determinations conducted in both winter and summer sampling periods, 65 μl of freshly collected, pooled hemolymph (3 × 106 cells · ml−1) was added to each microtube in the API ZYM test strip. Strips were incubated for 4 h at 37°C and developed, and their color reactions were recorded according to the manufacturer’s instructions.
Statistical analyses.
Two-factor analysis of variance (ANOVA) was performed separately on V. parahaemolyticus and L. monocytogenes KI data from all sampling periods to elucidate differences in mean KI due to the main effects, strain and season. No significant interaction between bacterial strain and season was found for either V. parahaemolyticus or L. monocytogenes, so one-way ANOVA was employed for each species at each sampling period to test for differences in KI due to the effect of strain. Data collected in summer from V. vulnificus were also analyzed by one-way ANOVA to test for differences in KI due to strain. Where significant differences were found, Tukey’s multiple comparison test was employed to resolve significant differences between means. A Student’s t test was used to assess differences in the profiles of the measured hemocytic enzymes in summer versus winter. Results were deemed significant at P ≤ 0.05.
RESULTS
The potential of oyster hemocytes to kill V. parahaemolyticus was significantly greater in summer than winter. In summer, the mean KI ± standard deviation for all V. parahaemolyticus strains was 49 ± 16 compared to 19 ± 16 in winter.
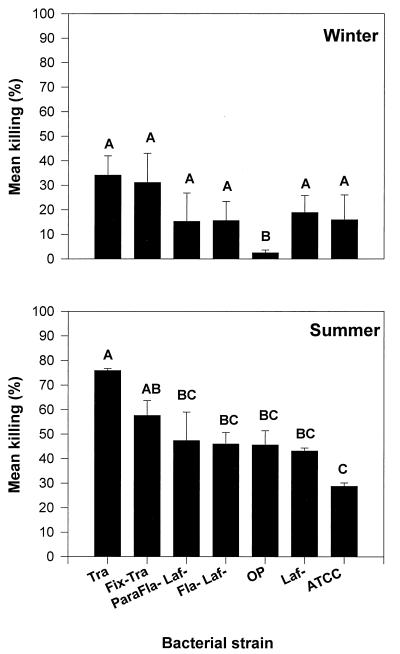
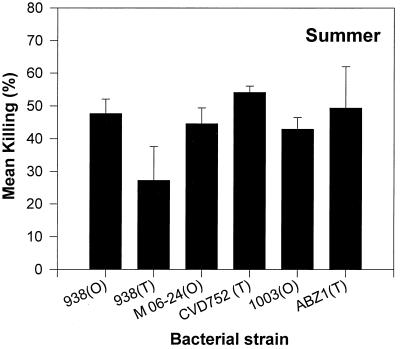
Mean ± standard error (SE) KIs of hemocytes from oysters collected in summer and winter and incubated with seven strains of V. parahaemolyticus varied significantly (Fig. 1). Translucent strains of V. parahaemolyticus, the Tra and Fix Tra strains, were more susceptible to killing by oyster hemocytes than opaque strains. In both summer and winter, these two strains exhibited the highest KIs of all V. parahaemolyticus strains tested. In winter, both the Tra and Fix Tra strains yielded significantly higher KIs than the wild-type opaque strain, BB220P (Wild). In summer, the KI obtained with strain BB22TR (Tra) was significantly higher than any of the opaque V. parahaemolyticus strains tested. The KI ± SE of the fixed translucent strain LM4462 (Fix Tra) (58 ± 11) was again higher than that of the wild-type opaque strain (46 ± 10), but the difference was not statistically significant. Contrary to the responses of V. parahaemolyticus strains, the translucent phenotype (corresponding to lack of a capsule) did not appear to render strains of V. vulnificus more susceptible to killing by oyster hemocytes. No significant differences in KIs of any of the three wild-type V. vulnificus strains and their corresponding acapsular derivatives were obtained (Fig. 2).
FIG. 1.
Mean ± SE KIs of hemocytes incubated with eight strains of V. parahaemolyticus in winter and summer. Bars with the same letter(s) were not significantly different according to the Tukey test. Tra, wild-type translucent; Fix− Tra, transposon mutant unable to switch to opaque; ParaFla− Laf−, paralyzed polar flagellum and lacking lateral flagella (swim− and swarm−); Fla− Laf, lacking polar and lateral flagella (swim− and swarm−); OP, wild-type opaque; Laf−, lacking lateral flagella (swarm−).
FIG. 2.
Mean ± SE KIs of hemocytes incubated with six strains of V. vulnificus in summer. Differences in KIs shown here were not significantly different according to the Tukey test. O, opaque colonies possessing encapsulated cells; T, translucent colonies possessing acapsular cells.
Defects in motility did not appear to affect the ability of V. parahaemolyticus to avoid killing by oyster hemocytes. In winter, all strains, including those defective in motility, yielded significantly higher KIs than the wild-type opaque strain (OP). This trend was not repeated in summer, when none of the strains defective in motility yielded KIs significantly higher than that of the OP strain (Fig. 1).
Colonies produced on NBS after 24 h at 25°C by translucent and opaque strains of V. vulnificus were more similar to each other than colonies produced by translucent and opaque strains of V. parahaemolyticus. Opaque strains of both V. parahaemolyticus and V. vulnificus produced circular colonies between 0.5 and 1 mm in diameter that had entire edges and were convex in elevation. In contrast, the translucent colonies of V. parahaemolyticus strains were much larger (3 to 6 mm in diameter) and irregular in shape, possessing lobate edges and raised elevations. No differences in colonial morphologies among L. monocytogenes strains were observed.
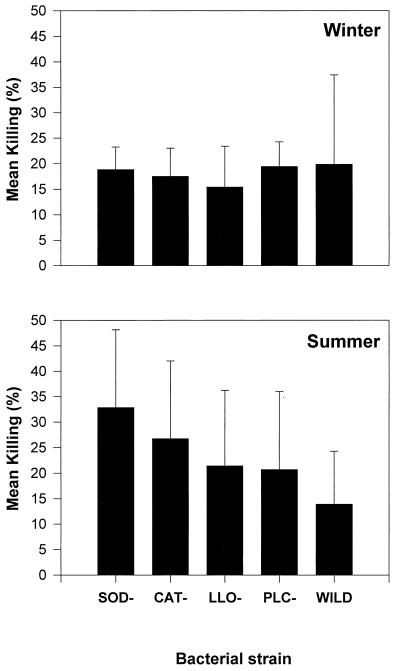
As with V. parahaemolyticus, the mean KI ± SE of all L. monocytogenes strains was higher in summer (23 ± 22) than in winter (18 ± 14); this difference, however, was not significant. L. monocytogenes was, on average, more resistant to killing by oyster hemocytes than either V. parahaemolyticus or V. vulnificus (Fig. 1, 2, and 3). In summer, the mean KIs ± SEs derived from all V. parahaemolyticus and V. vulnificus strains were 49.0 ± 16 and 44.3 ± 9.3, respectively. Both KI averages were significantly higher than the mean KI derived from L. monocytogenes strains in summer, 23.4 ± 6.5. In addition, L. monocytogenes strains lacking the potential virulence factors catalase (strain 1370), superoxide dismutase (strain DHL1), listeriolysin O (strain DP-L2161), and two secreted phospholipases C (strain DP-L1936) were not significantly more susceptible to killing by oyster hemocytes than their parental wild-type strains in either the winter or summer trials. In the summer trial, however, there was a nonsignificant trend that showed all L. monocytogenes strains lacking a putative virulence factor more susceptible to killing than their parental wild-type strain (Fig. 3).
FIG. 3.
Mean ± SE KIs of hemocytes incubated with five strains of L. monocytogenes in winter and summer. Differences in KIs shown here were not significantly different according to the Tukey test. SOD, superoxide dismutase; CAT, catalase; LLO, listeriolysin O; PLC, phospholipase C; WILD, parental wild-type.
Using the API ZYM strips, activities of individual hydrolytic enzymes were measured in 65-μl aliquots of each pooled hemolymph sample. As shown in Table 2, only 6 of 19 enzymes (C4 esterase, C8 esterase lipase, leucine arylamidase, phosphatase acid, naphthol-AS-BI-phosphohydrolase, and β-galactosidase) assayed in hemolymph showed activities that averaged ≥5 nmol of cleaved substrate. Of these six, C4 esterase was the only enzyme that showed a significant seasonal difference in activity (higher in winter than summer). In both summer and winter, the aminopeptidase leucine arylamidase displayed the highest activity (22 nmol of cleaved substrate from 65 μl of hemolymph) (Table 2).
TABLE 2.
Enzyme activities in oyster (C. virginica) hemolymph
| Enzyme | Activitya
|
|
|---|---|---|
| Winter | Summer | |
| Esterase (C4)b | 15 ± 5.5 | 8.3 ± 2.6 |
| Esterase lipase (C8) | 5.8 ± 2.0 | 6.7 ± 2.6 |
| Leucine arylamidase | 22 ± 6.6 | 22 ± 4.9 |
| Acid phosphatase | 10 ± 0.0 | 13 ± 4.1 |
| Naphthol-AS-BI-phosphohydrolase | 5.0 ± 0.0 | 5.0 ± 4.8 |
| β-Galactosidase | 10 ± 0.0 | 8.3 ± 2.6 |
Activity, measured semiquantitatively as nanomoles of cleaved substrate, was determined by using the API ZYM system. Activities are the means of six separate determinations ± standard deviations using pooled hemolymph from 10 oysters per pool. With the exception of esterase (C4), no significant differences (P > 0.05) in enzymatic activity were detected between values for winter and summer. Only those enzymes whose mean activities reached ≥5 nmol of cleaved substrate are presented here.
Activity of esterase (C4) was significantly higher in the winter than summer.
DISCUSSION
Research on cellular immune response in oysters has relied mainly on indirect measurements (e.g., chemiluminescence, agglutination, cell migration, and association of bacteria with hemocytes) to quantify different phases of the phagocytic process (2). Although these approaches have yielded a great deal of information regarding oyster immune function, direct methods, like dye reduction (used here) or plate count, not only yield comparable results (44) but also may better represent the cellular defense capability by directly measuring bacterial death.
Using the plate count to measure phagocytic activity and bacterial degradation by oyster hemocytes, Harris-Young et al. (22) found less killing of an opaque than a translucent morphotype of V. vulnificus biotype 1. In agreement with the results of Harris-Young et al. (22), we observed higher, although not significantly higher, KIs with translucent than with opaque V. vulnificus variants in two of the three isogenic pairs examined. We obtained contrary results with the only biotype 2 pair, 938 (O) and 938 (T), tested (Fig. 2). Strains belonging to this biotype are eel pathogens (1) and, unlike biotype 1 strains, the presence of a capsule is not required for the development of vibriosis in its host (4). It is possible that biotype 1 translucent variants did not completely lack capsules but rather possessed incomplete capsular materials (46), rendering them slightly more resistant to killing than a translucent variant completely lacking capsular material. Also in agreement with Harris-Young et al. (22) was our observation of higher KIs with Tra and Fix Tra V. parahaemolyticus strains than with the wild-type opaque variant (Fig. 1).
Colonial morphologies between the wild-type opaque (OP) and translucent variants (Tra and Fix Tra strains) of V. parahaemolyticus were more strikingly different than those between the opaque and translucent variants of V. vulnificus. Although the difference between the V. vulnificus forms was shown to be due to encapsulation (46), this has not been documented for V. parahaemolyticus. Moreover, OP and Tra phenotypes of V. parahaemolyticus involve multiple traits and may not be as simple as the presence or absence of capsules (28). Electron micrographs revealed a dense ruthenium red staining layer on the surface of plate-grown OP cells and a thin layer on plate-grown Tra cells (27).
Motility may play a role in the ability of some bacteria to evade killing by oyster hemocytes. Cells of V. parahaemolyticus possess two types of locomotion, swimming and swarming (30). Swimming cells, adapted for locomotion in liquid medium, are propelled by a single flagellum (Fla) located at one pole. When a swimming cell contacts and adheres to a surface, it becomes a swarmer cell through a series of changes that includes the production of numerous lateral flagella (Laf). This differentiation is an adaption for colonization of surfaces. From our results, it appears that motility may not be important in the cell’s ability to evade killing by oyster hemocytes. However, it must be noted that in our tests, V. parahaemolyticus strains were cultured in broth where the production of lateral flagella (swarming motility) was repressed.
L. monocytogenes is a human, intracellular pathogen (35) known to be associated with shellfish (23). The KIs for all L. monocytogenes variants in both winter and summer were low, with a high degree of variability among hemocyte pools. This suggests that this pathogen was not easily killed by oyster hemocytes, and no single virulence factor tested rendered the cells more susceptible to hemocyte killing.
Both L. monocytogenes and V. parahaemolyticus KIs were higher in summer than in winter. Reasons for this are not entirely clear. Phagocytic killing is partially mediated through the action of digestive enzymes (9). Various hydrolase activities were identified in hemolymph of C. virginica (8); no seasonal differences in enzymatic activity were studied. Chu and La Peyre (10) found higher levels of hemolymph lysozyme in winter than summer months in Chesapeake Bay oyster hemolymph, but Fisher et al. (16) found the opposite for oysters from Apalachicola Bay, Fla. It is likely that one or more of the four phases of phagocytosis (attraction, attachment, internalization, and intracellular degradation) is more active because of a higher metabolic rate during the warmer summer months.
This study utilized several bacterial mutants defective in a variety of potential virulence factors and their corresponding wild-type parental strains to determine whether microbial factors were protective against hemocyte killing. It appeared the opaque phenotype of V. parahaemolyticus offered some protection against killing by oyster hemocytes. Although the absence of multiple traits characterizing the translucent phenotype of V. parahaemolyticus rendered this microbe more susceptible to killing by oyster hemocytes, the absence of single enzymatic virulence factors known to enable L. monocytogenes to become an intracellular human pathogen (34) did not increase oyster hemocyte killing of this bacterium. Perhaps this bacterium, like Staphylococcus aureus, is resistant to the action of oyster lysosomal hydrolases (37) and not reliant on other virulence factors to protect from oyster hemocyte killing.
It is not fully understood why certain bacteria were more susceptible to killing by oyster hemocytes. Cheng (7) hypothesized that surfaces of resistant cells lack substrates susceptible to humoral response molecules, including lysosomal enzymes, and that resistant cells lack a triggering mechanism for the release of lysosomal enzymes from hemocytes. Cheng (6) also postulated that substances that inactivate hydrolases are elaborated by resistant cells. Any combination of these and other factors may govern the capacity of a bacterium to evade killing by oyster hemocytes. Even without knowing the specific mechanisms, the results from this study clearly demonstrate a differential ability of oyster hemocytes to kill various bacteria.
Although the potential ramifications of these results for human health and ecological conditions remain to be explored, the significance of this work is multifaceted. Consumption of raw oysters has been implicated in numerous food poisoning outbreaks. Thus, their microbial flora is of great concern to public health. The ability of oysters to eliminate pathogenic bacteria from their systems may partially be determined by the ability of hemocytes to recognize, bind, and phagocytose these microbes. Virulence factors may also play a role in the ability of bacteria to survive molluscan cellular defense mechanisms. Thus, an understanding of the interactions between pathogenic bacteria and oyster hemocytes is important in elucidating mechanisms responsible for bacterial persistence in oyster tissues. Second, the ability of the oyster to defend against invasive bacteria may be an inherent requirement of a healthy oyster population, and a healthy oyster population is important to most estuarine ecosystems.
ACKNOWLEDGMENTS
We thank A. DePaola, S. E. Martin, L. L. McCarter, D. A. Portnoy, and A. B. Zuppardo for providing bacterial strains, and J. T. Winstead for collecting oysters. We appreciate very helpful comments from L. L. McCarter.
Footnotes
Contribution no. 1063 of the U.S. EPA’s National Health and Environmental Effects Research Laboratory and Gulf Ecology Division, Gulf Breeze, Fla.
REFERENCES
- 1.Amaro C, Biosca E G. Vibrio vulnificus biotype 2, pathogenic for eels, is also an opportunistic pathogen for humans. Appl Environ Microbiol. 1996;62:1454–1457. doi: 10.1128/aem.62.4.1454-1457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson R S. Interactions of Perkinsus marinus with humoral factors and hemocytes of Crassostrea virginica. J Shellfish Res. 1996;15:127–134. [Google Scholar]
- 3.Belas R, Simon M, Silverman M. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J Bacteriol. 1986;167:210–218. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biosca E G, Llorens H, Garay E. Presence of a capsule in Vibrio vulnificus biotype 2 and its relationship to virulence for eels. Infect Immun. 1993;61:1611–1618. doi: 10.1128/iai.61.5.1611-1618.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop D K, Hinrichs D J. Adaptive transfer of immunity to Listeria monocytogenes: the influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987;139:2005–2009. [PubMed] [Google Scholar]
- 6.Cheng T C. The role of hemocytic hydrolases in the defense of molluscs against invading parasites. Haliotis. 1977;8:193–209. [Google Scholar]
- 7.Cheng T C. Hemocytes: forms and functions. In: Kennedy V S, Newell R I E, Eble A F, editors. The eastern oyster. College Park, Md: Maryland Sea Grant College, University of Maryland System; 1996. pp. 299–333. [Google Scholar]
- 8.Cheng T C, Rodrick G E. Lysosomal and other enzymes in the hemolymph of Crassostrea virginica and Mercenaria mercenaria. Comp Biochem Physiol. 1975;52B:443–447. doi: 10.1016/0305-0491(75)90159-5. [DOI] [PubMed] [Google Scholar]
- 9.Chu F L E. Humoral defense factors in marine bivalves. Am Fish Soc Spec Publ. 1988;18:178–188. [Google Scholar]
- 10.Chu F E, La Peyre J F. Effect of environmental factors and parasitism on hemolymph lysozyme and protein in American oysters (Crassostrea virginica) J Invertebr Pathol. 1989;54:224–232. [Google Scholar]
- 11.Degnan A J, Kaspar C W, Otwell W S, Tamplin M L, Luchansky J B. Evaluation of lactic acid bacterium fermentation products and food-grade chemicals to control Listeria monocytogenes in blue crab (Callinectes sapidus) meat. Appl Environ Microbiol. 1994;60:3198–3203. doi: 10.1128/aem.60.9.3198-3203.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deitsch K W, Moxon E R, Wellems T E. Shared themes of antigenic variation and virulence in bacterial, protozoal, and fungal infections. Microbiol Mol Biol Rev. 1997;61:281–293. doi: 10.1128/mmbr.61.3.281-293.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng S Y. Cellular defense mechanisms of oysters and mussels. Am Fish Soc Spec Publ. 1988;18:153–168. [Google Scholar]
- 14.Fisher W S. Environmental influence on bivalve hemocyte function. Am Fish Soc Spec Publ. 1988;18:178–188. [Google Scholar]
- 15.Fisher W S, DiNuzzo A R. Agglutination of bacteria and erythrocytes by serum factors from six species of marine molluscs. J Invertebr Pathol. 1991;57:380–394. doi: 10.1016/0022-2011(91)90142-d. [DOI] [PubMed] [Google Scholar]
- 16.Fisher W S, Oliver L M, Edwards P E. Hematologic and serologic variability of eastern oysters from Apalachicola Bay, Florida. J Shellfish Res. 1996;15:555–564. [Google Scholar]
- 17.Fletcher G C, Scott P D, Hay B E. The depuration of pacific oysters (Crassostrea gigas) In: Otwell W S, Rodrick G E, Martin R E, editors. Molluscan shellfish depuration. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 227–238. [Google Scholar]
- 18.Foley D A, Cheng T C. A quantitative study of phagocytosis by hemolymph cells of the pelecypods Crassostrea virginica and Mercenaria mercenaria. J Invertebr Pathol. 1975;25:189–197. doi: 10.1016/0022-2011(75)90068-3. [DOI] [PubMed] [Google Scholar]
- 19.Fujino T, Sakazaki R, Tamura K. Designation of the type strain of Vibrio parahaemolyticus and description of 200 strains of the species. Int J Syst Bacteriol. 1974;24:447–449. [Google Scholar]
- 20.Galtsoff P S. The American oyster Crassostrea virginica. Gmelin Fish Bull (Washington, DC) 1964;64:185–218. [Google Scholar]
- 21.Harris-Young L, Tamplin M L, Fisher W S, Mason J W. Effects of physicochemical factors and bacterial colony morphotype on association of Vibrio vulnificus with hemocytes of Crassostrea virginica. Appl Environ Microbiol. 1993;59:1012–1017. doi: 10.1128/aem.59.4.1012-1017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris-Young L, Tamplin M L, Mason J W, Aldrich H C, Jackson J K. Viability of Vibrio vulnificus in association with hemocytes of the American oyster (Crassostrea virginica) Appl Environ Microbiol. 1995;61:52–57. doi: 10.1128/aem.61.1.52-57.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartemink R, Georgsson F. Incidence of Listeria species in seafood and seafood salads. Int J Food Microbiol. 1991;12:189–195. doi: 10.1016/0168-1605(91)90069-2. [DOI] [PubMed] [Google Scholar]
- 24.MacKenzie C L. History of oystering in the United States and Canada, featuring the eight greatest oyster estuaries. Mar Fish Res. 1996;58:1–78. [Google Scholar]
- 25.Marquis H, Goldfine H, Portnoy D A. Proteolytic pathways of activation and degradation of a bacterial phospholipase C during infection by Listeria monocytogenes. J Cell Biol. 1997;137:1381–1392. doi: 10.1083/jcb.137.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarter L L. MotX, the channel component of the sodium-type flagellar motor. J Bacteriol. 1994;176:5988–5998. doi: 10.1128/jb.176.19.5988-5998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarter, L. L. 1998. Personal communication.
- 28.McCarter L L. OpaR, a homolog of Vibrio harveyi LuxR, controls opacity of Vibrio parahaemolyticus. J Bacteriol. 1998;180:3166–3173. doi: 10.1128/jb.180.12.3166-3173.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarter L, Hilmen M, Silverman M. Flagellar dynamometer controls swarmer cell differentiation of Vibrio parahaemolyticus. Cell. 1988;54:345–351. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- 30.McCarter L, Silverman M. Surface induced swarmer cell differentiation of Vibrio parahaemolyticus. Mol Microbiol. 1990;4:1057–1062. doi: 10.1111/j.1365-2958.1990.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 31.McCarter L L, Wright M E. Identification of genes encoding components of the swarmer cell flagellar motor and propeller and a sigma factor controlling differentiation of Vibrio parahaemolyticus. J Bacteriol. 1993;175:3361–3371. doi: 10.1128/jb.175.11.3361-3371.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motes M L, DePaola A, Cook D W, Veazey J E, Hunsucker J C, Garthright W E, Blodgett R J, Chirtel S J. Influence of water temperature and salinity on Vibrio vulnificus in northern gulf and Atlantic coast oysters (Crassostrea virginica) Appl Environ Microbiol. 1998;64:1459–1465. doi: 10.1128/aem.64.4.1459-1465.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Academy of Science. Seafood safety report. Microbiological and parasitic exposures and health effects. Washington, D.C: National Academy Press; 1991. pp. 33–94. [Google Scholar]
- 34.Portnoy D A, Charkraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992;60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Portnoy D A, Jacks P S, Hinrichs D J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Renwratz L. Involvement of agglutinins (lectins) in invertebrate defense reactions. The immunobiological importance of carbohydrate-specific binding molecules. Dev Comp Immunol. 1983;7:603–608. [Google Scholar]
- 37.Rodrick G E, Cheng T C. Kinetic properties of lysozyme from the hemolymph of Crassostrea virginica. J Invertebr Pathol. 1974;24:41–48. doi: 10.1016/0022-2011(74)90162-1. [DOI] [PubMed] [Google Scholar]
- 38.Rodrick G E, Schneider K R. Vibrios in depuration. In: Otwell W S, Rodrick G E, Martin R E, editors. Molluscan shellfish depuration. Boca Raton, Fla: CRC Press, Inc.; 1991. pp. 115–128. [Google Scholar]
- 39.Simonson J G, Siebeling R J. Immunogenicity of Vibrio vulnificus capsular polysaccharides and polysaccharide-protein conjugates. Infect Immun. 1993;61:2053–2058. doi: 10.1128/iai.61.5.2053-2058.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith G A, Marquis H, Jones S, Johnston N C, Portnoy D A, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamplin M L, Capers G. Persistence of Vibrio vulnificus in tissues of Gulf Coast oysters, Crassostrea virginica, exposed to seawater disinfected with UV light. Appl Environ Microbiol. 1992;58:1506–1510. doi: 10.1128/aem.58.5.1506-1510.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tamplin M. The ecology of Vibrio vulnificus. In: Watkins W, McCarthy S, editors. Proceedings of the 1994 Workshop. Washington, D.C: Office of Seafood; 1995. pp. 75–86. [Google Scholar]
- 43.Volety A K, Chu F L E. Suppression of chemiluminescence of eastern oyster (Crassostrea virginica) hemocytes by the protozoan parasite Perkinsus marinus. Dev Comp Immunol. 1995;19:135–142. doi: 10.1016/0145-305x(94)00059-o. [DOI] [PubMed] [Google Scholar]
- 44.Volety A K, Oliver L M, Genthner F J, Fisher W S. A novel and rapid assay to assess the bactericidal activity of oyster (Crassostrea virginica) hemocytes against Vibrio parahaemolyticus, a pathogenic bacterium: standardization and optimization using tetrazolium dye reduction. Aquaculture. 1999;172:205–222. [Google Scholar]
- 45.Wright A C, Simpson L M, Oliver J D, Morris J G., Jr Phenotypic evaluation of acapsular transposon mutants of Vibrio vulnificus. Infect Immun. 1990;58:192–197. doi: 10.1128/iai.58.6.1769-1773.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida S, Ogawa M, Mizuguchi Y. Relation of capsular materials and colony opacity to virulence of Vibrio vulnificus. Infect Immun. 1985;47:446–451. doi: 10.1128/iai.47.2.446-451.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zuppardo A B, Siebeling R J. An epimerase gene essential for capsule synthesis in Vibrio vulnificus. Infect Immun. 1998;66:2601–2606. doi: 10.1128/iai.66.6.2601-2606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]