Abstract
Invasive infections due to Trichosporon spp. are life-threatening opportunistic fungal infections that may affect a wide array of organs. Here, we described a case of pericardial effusion due to Trichosporon japonicum in a 42-year-old female after a heart transplantation. T. japonicum was isolated from the pericardial fluid, pericardial drain hole and the swab of the sternal surgery scar wound. The late mycological diagnosis due to blood culture negative, the ineffective control of pulmonary bacterial infection and the late start antifungal therapy were the contributing factors in the patient’s death.
Keywords: Trichosporon japonicum , yeast, pericarditis
1. Introduction
Trichosporon are emerging opportunistic basidiomycetous yeast-like organisms. Ubiquitous in the environment, they are occasionally involved in invasive fungal diseases [1]. Patients with hematological malignancies, persistent neutropenia, intravenous and urinary catheters, those who have had thoracic or abdominal surgery, and those who are immunosuppressed or pre-exposed to antifungal therapy, especially to echinocandins, are at risk of invasive trichosporonosis [2]. The most common species involved in clinical infections are Trichosporon asahii, followed by T. inkin, T. faecale, and T. asteroides [3].
In this report, we present a rare case of a Trichosporon japonicum infection in a 42 years old female following heart transplantation. We discussed the value of the different diagnostic tools available and performed a review of the literature on T. japonicum infections.
2. Case Presentation
2.1. Patient History
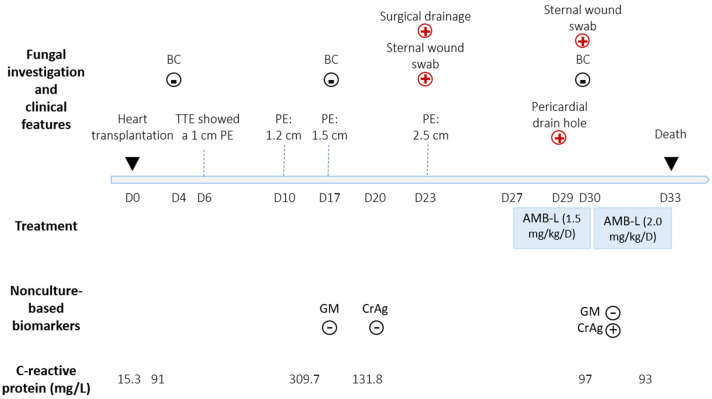
A 42-year-old female was admitted at La Timone hospital (Marseille, France) to undergo a heart transplantation. She had a history of congenital cardiopathy (single ventricle) with multiple cardiac decompensation episodes, severe left ventricular dysfunction (LVEF 25%) and New York Heart Association class III dyspnea. The patient was on mechanical ventilation throughout the duration of the hospitalization, and did not develop any fever. At day 6 post transplantation, a routine trans-thoracic echography showed a 1 cm pericardial effusion (Figure 1). The pericardial effusion progressively increased to 1.2 cm at day 10, and 1.5 cm at day 17. At day 13, the patient developed an acute respiratory distress syndrome, and HSV-1 PCR was positive (in blood and bronchoalveolar lavage fluid); the patient was treated with acyclovir. The following bacteria were repeatedly cultured from respiratory samples from day 6 post transplant: Pseudomonas aeruginosa (one bronchial aspirate and one bronchoalveolar lavage fluid), Stenotrophomonas maltophilia (three bronchial aspirate, one bronchoalveolar lavage fluid and one sputum) and Citrobacter freundii (one bronchial aspirate and one bronchoalveolar lavage fluid). Following that, the patient was treated with an adapted antibiotic therapy, which unfortunately does not enable the control of the infection. From the first day post transplant, the patient had a supranormal white blood cell count with an average of 28 × 109/L (range: 18 × 109–43 × 109).
Figure 1.
Timeline of Trichosporon japonicum pericarditis course. TTE: trans-thoracic echography; AMB-L: Amphotericin B liposomal; BC: Blood culture; PE: Pericardial effusion; CrAg: Cryptococcal antigen; GM: Aspergillus galactomannan; +: positive; −: negative;  : positive Trichosporon japonicum culture; ▪: negative Trichosporon japonicum culture.
: positive Trichosporon japonicum culture; ▪: negative Trichosporon japonicum culture.
2.2. Diagnostic Assessment and Therapeutic Intervention
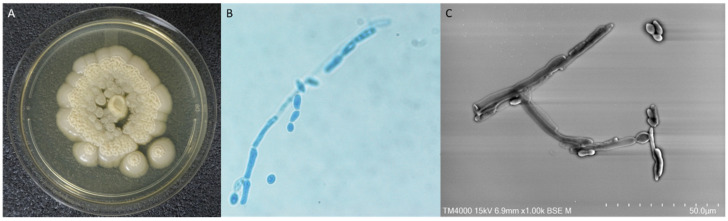
At day 23, the pericardial effusion became circumferential and measured 2.4 cm. The patient underwent a surgical drainage. The pericardial aspirate was purulent and grew cream-colored dry wrinkled colonies with irregular margins after 3 days (Figure 2A). Bacterial culture was negative. Fresh microscopic examination of the colonies revealed round yeast cells with blastoconidia and arthroconidia (Figure 2B,C). Trichosporon japonicum was identified by MALDI-TOF mass spectrometry (MicroflexLT, Bruker Daltonics GmbH, Bremen, Germany) with a Logscore value at 2.02 obtained from the standard manufacturer library, and it was deposited (strain number: IHEM 28563). DNA sequence-based identification was performed as previously described [4]. The 100% identity with the rRNA intergenic spacer IGS1 sequence (GenBank AB066426.1) of JCM8357, the type strain of T. japonicum, confirmed this identification. The other genomic regions rRNA Internal Transcribed Spacer 1 and 2, the D1/D2 domains of the rRNA large-subunit were less informative but in agreement with this identification. All nucleotide sequences of the present isolate were submitted to GenBank (Acc. No OM865139, OM865141 and OM897590). The NCBI BLASTn results are detailed in Supplementary Table S1. The same yeast was isolated from the swab of sternal surgery scar wound (day 23 and day 30) and pericardial drain hole (day 29). Blood culture (BacT/ALERT system, bioMerieux, Craponne, France) remain negative for the duration of the stay. Aspergillus galactomannan antigenaemia (Bio-Rad Laboratories, Marnes-La-Coquette, France) was negative, at day 17 and day 30. Cryptococcal serum antigen (CrAg® LFA kit, IMMY, Norman, OK, USA) was negative at day 20 but positive (1:80 titer) at day 30.
Figure 2.
Trichosporon japonicum morphological features. (A) Colony of Trichosporon japonicum on Sabouraud dextrose agar media. (B) Fresh microscopic examination of the colonies stained with Mycetblue® (Biosynex, Graffenstaden, France) ×1000 original magnification. (C) Scanning Electron Microscopy examination (15 KeV, lens mode 3, Scale bar 50 μm) of the colonies using the TM4000 PlusTM (Hitachi, Japan) instrument.
The patient was treated with liposomal amphotericin B (1.5 mg/kg/D) starting from day 27. The patient developed a multiple organ failure and died at day 33.
Antifungal susceptibility testing was performed on the strain cultured from the pericardial fluid by using the colorimetric broth microdilution Sensititre Yeast-OneTM YO10 (Thermo Fisher Diagnostics, Dardilly, France) and/or the agar diffusion EtestTM (BioMérieux, Craponne, France). The following minimum inhibitory concentration (MIC) values of T. japonicum isolate were found: amphotericin B (YO10: 0.5 mg/L; Etest: 0.5 mg/L), micafungin (YO10: >8 mg/L), caspofungin (YO10: >8 mg/L), anidulafungin (YO10: >8 mg/L), 5-fluorocytosine (YO10: 4 mg/L), voriconazole (YO10: 0.12 mg/L; Etest: 0.125 mg/L), posaconazole (YO10: 0.25 mg/L; Etest: 0.75 mg/L), itraconazole (YO10: 0.12 mg/L), fluconazole (YO10: 4 mg/L) and isavuconazole (Etest: 0.38 mg/L).
3. Discussion
Trichosporon japonicum was first isolated in 1998 from air collected in the house of a patient with summer-type hypersensitivity pneumonitis in Japan [5]. So far, T. japonicum infections have been scarcely reported in the literature. A review of the English language literature in Medline by using the following keywords: “Trichosporon japonicum” AND “Human” (Table 1) found only five references reporting a total of six human cases of T. japonicum infections. These infections were documented in blood (n = 2) [6,7], urinary tract (n = 2) [8] and respiratory tract (n = 2) from respiratory samples [9,10]. The mean age of reported cases was 24 years (range: 8–50), the sex ratio was 1.5 and three patients died. Regarding the patients’ risk factors, two patients were kidney transplant recipients, two patients had a hematologic malignancy (AML, ALL), and similarly to our patient, one underwent cardiac surgery. No data was provided about the remaining case in whom Trichosporon japonicum DNA was detected in lower respiratory specimens [10]. In the present case, our patient developed a pericardial effusion following heart transplantation. The heart preservation fluid culture remained sterile, which should rule out transmission by the graft. Finally, our patient is the second case described following heart surgery and the third after solid organ transplantation.
Table 1.
Cases report of Trichosporon japonicum infections, review of literature (including the present case).
| Age | Gender | Comorbidity Conditions |
Clinical Presentation |
Site of Positive Culture |
Treatment | Outcome | Reference | |
|---|---|---|---|---|---|---|---|---|
| Molecule | Duration | |||||||
| 8 | F | AML | Respiratory distress | Sputum | AMB-L (5 mg/kg/D) + ITRA (100 mg/D) | NS | Death | [9] |
| - | - | - | Hypersensitivity pneumonitis | BALF | - | - | - | [10] |
| 18 | F | Transcutaneous biventricular assist device | Fungemia | Blood, aortic cannula, removed left ventricular apex cuff | AMB-L + 5FC switch VORI |
11 days / 6 weeks |
Survival at 2 months | [6] |
| 36 | M | Kidney transplant recipient | Urinary tract infection | Urine | VORI + CASPO | NS | Survival | [8] |
| 50 | M | Kidney transplant recipient | Urinary tract infection | Urine | VORI + CASPO | 15 days | Death | [8] |
| 8 | M | ALL | Fungemia | Blood | AMB (3 mg/kg/D) + VORI (8 mg/kg twice a day) | 11 days | Death | [7] |
| 42 | F | Heart transplant recipient | Pericardial effusion | Pericardial fluid | AMB-L (1.5 mg/kg/D) | 6 days | Death | Present case |
AML: Acute Myeloid Leukemia; ALL: acute B cell lymphoblastic leukemia; F: Female; M: Male; D: day; BALF: Broncho alveolar lavage fluid; AMB-L: Liposomal amphotericin B; ITRA: itraconazole; 5FC: 5-fluorocytosine; CASPO: caspofungin; VORI: voriconazole; NS: Not Specified.
Prompt diagnosis and timely management of trichosporonosis are essential. The gold standard diagnosis is the culture with growth between 48 and 72 h on Sabouraud medium [11] and their ability to grow on non-specific media. In the present case, the repetitive blood cultures remained negative, but the samples grew rapidly (within 72 h). T. japonicum was identified by the MALDI TOF MS and DNA sequencing confirmed the identification. Whereas the ITS and D1/D2 domains of rDNA are considered as the gold standard for medically important yeasts identification [12], in this present case, they could not differentiate T. japonicum, T. asahii and T. asteroides. The IGS region is more discriminating and has confirmed the species (Table S1). Our results confirm previous reports that the rRNA IGS1 region nucleotide sequence is the most discriminating and relevant for the precise species identification within the Trichosporon genus [1,11,13,14,15].
Like Cryptococcus spp., Trichosporon spp. are Basidiomycota, and cross-reactions between cryptococcal polysaccharide antigen detection and Trichosporon species are known [16,17]. Until now, the reported cases of cross-reactions concerned the following species: Trichosporon cutaneum [18,19], Trichosporon beigelii [16,20,21,22,23], Trichosporon asahii [24,25] and Trichosporon dermatis [26]. We present the first case of a positive cryptococcal antigen detection during a Trichosporon japonicum infection. Interestingly, in the literature, only Bogomin et al. [6] performed a cryptococcal antigen detection in a T. japonicum fungemia in a patient with a transcutaneous biventricular assist device. The latex agglutination cryptococcal capsular polysaccharide antigen test returned negative, although performed concomitantly with positive blood cultures. In our patient, the serum cryptococcal antigen using CrAg® LFA kit (IMMY, Norman, OK, USA) was negative 3 days before the pericardial fluid puncture and positive 7 days after. Three situations have been reported regarding the time to serum cryptococcal antigen positivity in patients infected with the other Trichosporon species. Karigane et al. reported Trichosporon asahii fungemia in an AML patient with positive cryptococcal antigenemia 5 days before the isolation of the fungus [25], while others reported a positive cryptococcal antigen concomitantly [16] or after the yeast isolation [20,26]. Interestingly, in our patient, the cryptococcal antigen was positive in several biological fluids, such as urine or cerebrospinal fluid, all confirmed by a subsequent Trichosporon positive culture [19,22].
Cross-reaction between Aspergillus galactomannan detection and Trichosporon spp. have been reported [27,28]. A dual positivity of cryptococcal antigen and Aspergillus galactomannan has been described in case of disseminated Trichosporon dermatis infection [26]. In our patient, Aspergillus galactomannan assay in the serum at day 17 and day 30 was negative. We tested cryptococcal antigen (IMMY, Norman, OK, USA) and Platelia Aspergillus galactomanan (Bio-Rad Laboratories, Marnes-La-Coquette, France) in the supernatant from a T. japonicum culture. Both returned positive, suggesting that T. japonicum share common epitopes with Cryptococcus and Aspergillus cell wall components. As recommended in the ESCMID and ECMM joint clinical guidelines [28], in case of suspected invasive Trichosporon infection, the diagnosis should include the combined use of culture, Aspergillus galactomannan and cryptococcal antigen determination. It would be interesting to extend the use of these tools to the first-line diagnostic approach of invasive fungal infections.
There is no consensus on the treatment of trichosporonosis and data concerning MIC interpretation for all antifungal drugs are scarce [3]. Like in most basidiomycetes, the use of echinocandins is not recommended due to natural resistance [29]. Consistently, in our case, the isolates of T. japonicum showed high MIC for echinocandins and low MIC for azoles except for fluconazole. Despite data suggesting a discrepancy between in vitro and in vivo activity, susceptibility testing is still recommended for epidemiological knowledge [3,8]. Recent guidelines moderately recommend voriconazole for initial antifungal therapy, exhibiting excellent in vitro and in vivo activity against Trichosporon spp. [3,8]. In the present case, treatment with amphotericin B was initiated following sensitivity testing and late in the course of the disease. Therapeutic failure is thus difficult to assess.
4. Conclusions
Trichosporon japonicum infection is rare. In case of suspected invasive Trichosporon infection, prompt diagnosis, including the combined use of culture, Aspergillus galactomannan and cryptococcal antigen determination and the rapid initiation of antifungal treatment are essential.
Acknowledgments
We sincerely thank Takashi Irie, Kyoko Imai, Shigeki Matsubara, Taku Sakazume, Toshihide Agemura and the Hitachi team in Japan for the collaborative study conducted together with IHU Méditerranée Infection, and the installation of TM4000 Plus microscopes at the IHU Méditerranée Infection facility.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens11050598/s1, Table S1: Molecular identification of Trichosporon japonicum sequencing ITS, D1/D2 and IGS genetic regions.
Author Contributions
Resources, E.M., J.K., J.R. and C.L.; writing—original draft preparation, E.M., J.K. and C.L.; writing—review and editing, S.R. and C.L.; supervision, C.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Assistance Publique des Hôpitaux de Marseille (APHM) (protocol code 2019-73 on 29 May 2019).
Informed Consent Statement
Patient consent was waived due to her inability to sign a written consent (hospitalization in intensive care unit then death). This case, completely anonymized, reports the history of the patient with her management in accordance with ethical respect. No further analysis was performed.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the French Government under the Investissements d’avenir (Investments for the Future) program managed by the Agence Nationale de la Recherche (ANR, fr: National Agency for Research) (reference: Méditerranée Infection 10-IAHU-03) and by Région Provence Alpes Côte d’Azur and European funding FEDER PRIMI.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Colombo A.L., Padovan A.C.B., Chaves G.M. Current Knowledge of Trichosporon spp. and Trichosporonosis. Clin. Microbiol. Rev. 2011;24:682–700. doi: 10.1128/CMR.00003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bretagne S., Renaudat C., Desnos-Ollivier M., Sitbon K., Lortholary O., Dromer F. French Mycosis Study Group Predisposing Factors and Outcome of Uncommon Yeast Species-Related Fungaemia Based on an Exhaustive Surveillance Programme (2002-14) J. Antimicrob. Chemother. 2017;72:1784–1793. doi: 10.1093/jac/dkx045. [DOI] [PubMed] [Google Scholar]
- 3.Chen S.C.-A., Perfect J., Colombo A.L., Cornely O.A., Groll A.H., Seidel D., Albus K., de Almedia J.N., Garcia-Effron G., Gilroy N., et al. Global Guideline for the Diagnosis and Management of Rare Yeast Infections: An Initiative of the ECMM in Cooperation with ISHAM and ASM. Lancet Infect. Dis. 2021;21:e375–e386. doi: 10.1016/S1473-3099(21)00203-6. [DOI] [PubMed] [Google Scholar]
- 4.Kabtani J., Diongue K., Dione J.-N., Delmas A., L’Ollivier C., Amoureux M.-C., Ndiaye D., Ranque S. Real-Time PCR Assay for the Detection of Dermatophytes: Comparison between an In-House Method and a Commercial Kit for the Diagnosis of Dermatophytoses in Patients from Dakar, Senegal. J. Fungi. 2021;7:949. doi: 10.3390/jof7110949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugita T., Nakase T. Trichosporon Japonicum sp. Nov. Isolated from the Air. Pt 4Int. J. Syst. Bacteriol. 1998;48:1425–1429. doi: 10.1099/00207713-48-4-1425. [DOI] [PubMed] [Google Scholar]
- 6.Bongomin F., Otu A., Calisti G., Richardson M.D., Barnard J., Venkateswaran R., Vergidis P. Trichosporon Japonicum Fungemia and Ventricular Assist Device Infection in an Immunocompetent Patient. Open Forum Infect. Dis. 2019;6:ofz343. doi: 10.1093/ofid/ofz343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albitar-Nehme S., Agosta M., Kowalska A.H., Mancinelli L., Onori M., Lucignano B., Mattana G., Quagliarella F., Cefalo M.G., Merli P., et al. Case Report: Trichosporon Japonicum Fungemia in a Pediatric Patient with Refractory Acute B Cell Lymphoblastic Leukemia. Front. Pediatr. 2022;10:861476. doi: 10.3389/fped.2022.861476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li T., Huang Y., Chen X., Wang Z., Xu Y. Urinary Tract Infections Caused by Fluconazole-Resistant Trichosporon Japonicum in 2 Kidney Transplant Patients and Analysis of Their Homology. Open Forum Infect. Dis. 2020;7:ofaa365. doi: 10.1093/ofid/ofaa365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ağirbasli H., Bilgen H., Ozcan S.K., Otlu B., Sinik G., Cerikçioğlu N., Durmaz R., Can E., Yalman N., Gedikoğlu G., et al. Two Possible Cases of Trichosporon Infections in Bone-Marrow-Transplanted Children: The First Case of T. Japonicum Isolated from Clinical Specimens. Jpn. J. Infect. Dis. 2008;61:130–132. [PubMed] [Google Scholar]
- 10.Unoura K., Miyazaki Y., Sumi Y., Tamaoka M., Sugita T., Inase N. Identification of Fungal DNA in BALF from Patients with Home-Related Hypersensitivity Pneumonitis. Respir. Med. 2011;105:1696–1703. doi: 10.1016/j.rmed.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Lara B.R., de Camargo B.B., Paula C.R., Junior D.P.L., Garces H.G., Arnoni M.V., Silveira M., Gimenes V.M.F., Siqueira L.P.M., Takahashi J.P.F., et al. Comparing the Phenotypic, Genotypic, and Proteomic Identification of Trichosporon Species: A Globally Emerging Yeast of Medical Importance. Med. Mycol. 2021;59:1181–1190. doi: 10.1093/mmy/myab050. [DOI] [PubMed] [Google Scholar]
- 12.Aydin M., Kustimur S., Kalkanci A., Duran T. Identification of Medically Important Yeasts by Sequence Analysis of the Internal Transcribed Spacer and D1/D2 Region of the Large Ribosomal Subunit. Rev. Iberoam. Micol. 2019;36:129–138. doi: 10.1016/j.riam.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Sugita T., Nakajima M., Ikeda R., Matsushima T., Shinoda T. Sequence Analysis of the Ribosomal DNA Intergenic Spacer 1 Regions of Trichosporon Species. J. Clin. Microbiol. 2002;40:1826–1830. doi: 10.1128/JCM.40.5.1826-1830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz M.R., Fell J.W. High-Throughput Detection of Pathogenic Yeasts of the Genus Trichosporon. J. Clin. Microbiol. 2004;42:3696–3706. doi: 10.1128/JCM.42.8.3696-3706.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desnos-Ollivier M., Maufrais C., Pihet M., Aznar C., Dromer F. French Mycoses Study Group Epidemiological Investigation for Grouped Cases of Trichosporon Asahii Using Whole Genome and IGS1 Sequencing. Mycoses. 2020;63:942–951. doi: 10.1111/myc.13126. [DOI] [PubMed] [Google Scholar]
- 16.McManus E.J., Jones J.M. Detection of a Trichosporon beigelii Antigen Cross-Reactive with Cryptococcus neoformans Capsular Polysaccharide in Serum from a Patient with Disseminated Trichosporon Infection. J. Clin. Microbiol. 1985;21:681–685. doi: 10.1128/jcm.21.5.681-685.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melcher G.P., Reed K.D., Rinaldi M.G., Lee J.W., Pizzo P.A., Walsh T.J. Demonstration of a Cell Wall Antigen Cross-Reacting with Cryptococcal Polysaccharide in Experimental Disseminated Trichosporonosis. J. Clin. Microbiol. 1991;29:192–196. doi: 10.1128/jcm.29.1.192-196.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimazu K., Ando M., Sakata T., Yoshida K., Araki S. Hypersensitivity Pneumonitis Induced by Trichosporon cutaneum. Am. Rev. Respir. Dis. 1984;130:407–411. doi: 10.1164/arrd.1984.130.3.407. [DOI] [PubMed] [Google Scholar]
- 19.Gökahmetoğlu S., Nedret Koç A., Nas H. Case Reports. Isolation of Two Trichosporon cutaneum Strains from Urine. Mycoses. 2002;45:132–134. doi: 10.1046/j.1439-0507.2002.00740.x. [DOI] [PubMed] [Google Scholar]
- 20.Campbell C.K., Payne A.L., Teall A.J., Brownell A., Mackenzie D.W. Cryptococcal Latex Antigen Test Positive in Patient with Trichosporon Beigelii Infection. Lancet. 1985;2:43–44. doi: 10.1016/S0140-6736(85)90093-5. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi M., Kotani S., Fujishita M., Taguchi H., Moriki T., Enzan H., Miyoshi I. Immunohistochemical Identification of Trichosporon Beigelii in Histologic Section by Immunoperoxidase Method. Am. J. Clin. Pathol. 1988;89:100–105. doi: 10.1093/ajcp/89.1.100. [DOI] [PubMed] [Google Scholar]
- 22.Surmont I., Vergauwen B., Marcelis L., Verbist L., Verhoef G., Boogaerts M. First Report of Chronic Meningitis Caused by Trichosporon Beigelii. Eur. J. Clin. Microbiol. Infect. Dis. 1990;9:226–229. doi: 10.1007/BF01963845. [DOI] [PubMed] [Google Scholar]
- 23.Walsh T.J., Melcher G.P., Rinaldi M.G., Lecciones J., McGough D.A., Kelly P., Lee J., Callender D., Rubin M., Pizzo P.A. Trichosporon Beigelii, an Emerging Pathogen Resistant to Amphotericin B. J. Clin. Microbiol. 1990;28:1616–1622. doi: 10.1128/jcm.28.7.1616-1622.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng M.P., Nguyen T.T., Parkes L.O., Dufresne P.J., Sheppard D.C. Cross-Reacting Ustilago Maydis Causing False-Positive Cryptococcal Antigen Test Results. J. Clin. Microbiol. 2017;55:3135–3137. doi: 10.1128/JCM.00920-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karigane D., Sakurai M., Matsuyama E., Ide K., Yamamoto-Takeuchi S., Inazumi T., Kohashi S. Successful Treatment of Breakthrough Disseminated Trichosporon Asahii Fungemia in a Patient with Acute Myeloid Leukemia Receiving Itraconazole Prophylaxis. Med. Mycol. Case Rep. 2018;20:1–3. doi: 10.1016/j.mmcr.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fekkar A., Brun S., D’Ussel M., Uzunov M., Cracco C., Dhédin N., Buffet P., Mazier D., Datry A. Serum Cross-Reactivity with Aspergillus Galactomannan and Cryptococcal Antigen during Fatal Disseminated Trichosporon Dermatis Infection. Clin. Infect. Dis. 2009;49:1457–1458. doi: 10.1086/644499. [DOI] [PubMed] [Google Scholar]
- 27.Lyman C.A., Devi S.J., Nathanson J., Frasch C.E., Pizzo P.A., Walsh T.J. Detection and Quantitation of the Glucuronoxylomannan-like Polysaccharide Antigen from Clinical and Nonclinical Isolates of Trichosporon Beigelii and Implications for Pathogenicity. J. Clin. Microbiol. 1995;33:126–130. doi: 10.1128/jcm.33.1.126-130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arendrup M.C., Boekhout T., Akova M., Meis J.F., Cornely O.A., Lortholary O. European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group; European Confederation of Medical Mycology ESCMID and ECMM Joint Clinical Guidelines for the Diagnosis and Management of Rare Invasive Yeast Infections. Clin. Microbiol. Infect. 2014;20((Suppl. S3)):76–98. doi: 10.1111/1469-0691.12360. [DOI] [PubMed] [Google Scholar]
- 29.Pfaller M.A., Messer S.A., Woosley L.N., Jones R.N., Castanheira M. Echinocandin and Triazole Antifungal Susceptibility Profiles for Clinical Opportunistic Yeast and Mold Isolates Collected from 2010 to 2011: Application of New CLSI Clinical Breakpoints and Epidemiological Cutoff Values for Characterization of Geographic and Temporal Trends of Antifungal Resistance. J. Clin. Microbiol. 2013;51:2571–2581. doi: 10.1128/JCM.00308-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.