Abstract
Background
Scleractinian corals of the genus Montipora (Anthozoa, Cnidaria) possess some unusual biological traits, such as vertical transmission of algal symbionts; however, the genetic bases for those traits remain unknown. We performed extensive comparative genomic analyses among members of the family Acroporidae (Montipora, Acropora, and Astreopora) to explore genomic novelties that might explain unique biological traits of Montipora using improved genome assemblies and gene predictions for M. cactus, M. efflorescens and Astreopora myriophthalma.
Results
We obtained genomic data for the three species of comparable high quality to other published coral genomes. Comparative genomic analyses revealed that the gene families restricted to Montipora are significantly more numerous than those of Acropora and Astreopora, but their functions are largely unknown. The number of gene families specifically expanded in Montipora was much lower than the number specifically expanded in Acropora. In addition, we found that evolutionary rates of the Montipora-specific gene families were significantly higher than other gene families shared with Acropora and/or Astreopora. Of 40 gene families under positive selection (Ka/Ks ratio > 1) in Montipora, 30 were specifically detected in Montipora-specific gene families. Comparative transcriptomic analysis of early life stages of Montipora, which possesses maternally inherited symbionts, and Acropora, which lacks them, revealed that most gene families continuously expressed in Montipora, but not expressed in Acropora do not have orthologs in Acropora. Among the 30 Montipora-specific gene families under positive selection, 27 are expressed in early life stages.
Conclusions
Lineage-specific gene families were important to establish the genus Montipora, particularly genes expressed throughout early life stages, which under positive selection, gave rise to biological traits unique to Montipora. Our findings highlight evolutionarily acquired genomic bases that may support symbiosis in these stony corals and provide novel insights into mechanisms of coral-algal symbiosis, the physiological foundation of coral reefs.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12862-022-02023-8.
Keywords: Corals, Montipora, Comparative genomics, Evolution, Rapidly evolving genes, Symbiosis, Transcriptome, Vertical transmission of algal symbionts
Background
Coral reefs are the most biologically diverse shallow water marine ecosystems [1]. Reef-building corals and endosymbiotic algae of the family Symbiodiniaceae, photosynthetic products of which provide host corals with energy and nutrients, establish mutualistic relationships that are fundamental to coral reefs [2–4]. However, reef-building corals have declined in recent decades due to a variety of anthropogenic stresses, including ocean warming associated with climate change [5–7]. These stresses result in coral bleaching (the breakdown of the symbiosis between corals and their algal endosymbionts [8]), which ultimately leads to loss of habitat for numerous marine species and can precipitate the collapse of entire coral reef ecosystems [9].
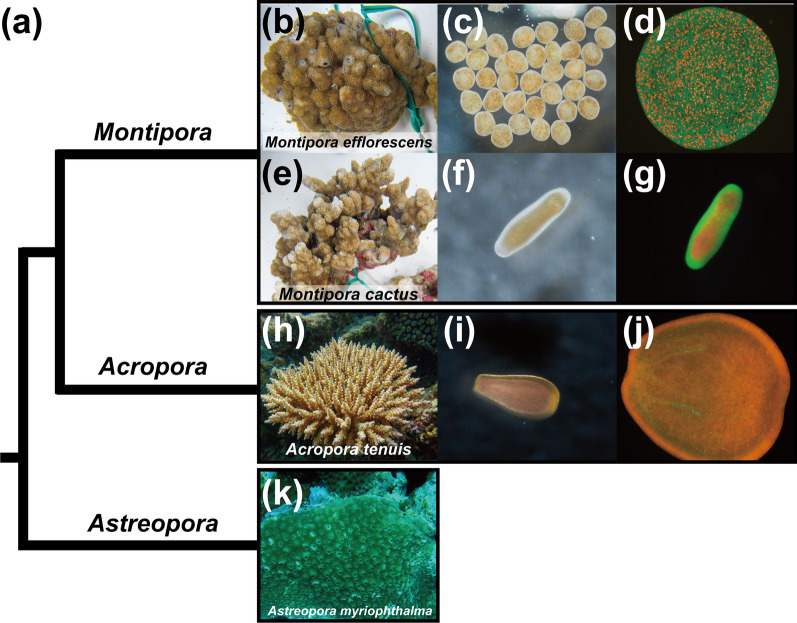
The genus Montipora (family Acroporidae; Fig. 1) is one of the most widespread genera of reef-building corals in the Indo-Pacific [10]. Colony morphology in the genus varies from submassive to laminar, encrusting, and branching colonies [10, 11]. Montipora has some unusual and interesting biological traits among acroporid corals, such as maternal transmission of symbionts and higher stress tolerance. Symbiont transmission maintains symbioses across generations and strongly influences host evolution and adaptation to environments [12–14]. Two fundamental symbiont transmission modes predominate in nature (reviewed in [14]): horizontal transmission (symbionts acquired from the environment) and vertical transmission (symbionts acquired maternally). While most coral species (~ 71%), including Acropora, acquire symbionts from the ocean in each generation [15], all known Montipora species acquire algal symbionts vertically [16, 17] (Fig. 1). Offspring of horizontal recipients generally associate with a broad range of symbiont types and acquire optimal symbionts from new environments [18, 19]; however, there is no guarantee that optimal symbionts will be available. By contrast, offspring of vertical recipients inherit symbionts that are suitable for their physiology [20], but if they encounter an environment that differs significantly from that of their parents, or if the environment changes too drastically, the inherited symbionts may be disadvantageous. Montipora also exhibits low sensitivity to ocean acidification and thermal stressors compared to other coral species [21, 22]. These distinct differences between Montipora and its close relative, Acropora, may have occurred after their divergence (approx. 125 Mya [23]).
Fig. 1.
Phylogenetic relationships in the Acroporidae and acroporid morphology. a Schematic phylogenetic tree representing evolutionary relationships within the Acroporidae. b and e Colonies of Montipora efflorescens (b) and M. cactus (e). c and d Eggs of M. efflorescens with algal symbionts photographed under visible light (c) and blue light (d). f and g A planula larva of M. efflorescens with algal symbionts, photographed under visible light (f) and blue light (g). h A colony of Acropora tenuis. i and j A planula larva of A. tenuis without algal symbionts photographed under visible light (i) and blue light (j). k A colony of Astreopora myriophthalma. Algal symbionts (brown dots) in eggs and planula larvae of Montipora (c and f). Green fluorescence was from fluorescent proteins of Montipora and red fluorescence was from chlorophyll in algal symbionts (d and g). Orange and cyan-green fluorescence were from fluorescent proteins of Acropora (j)
In the family Acroporidae, whole-genome data are becoming more readily available, now including 16 species of Acropora [23–26], 3 species of Montipora [23, 27, 28] and 1 Astreopora species [23], the latter being the sister taxon of the remainder of the Acroporidae [29] (Fig. 1). Recently, Shinzato et al. [23] performed a large-scale genomic comparison of acroporids (using genomes of Acropora, Montipora, and Astreopora) and proposed that the evolutionary success of Acropora may have resulted from gene duplications. Although some studies have performed genome-wide analysis using Montipora genomes [27, 28], the genomic basis for their unique biological traits remains unknown. Exploiting abundant acroporid genomic resources, we performed comparative genomic analyses using improved genomic data of Montipora and Astreopora. We further identified genes with high evolutionary rates in Montipora that may be associated with adaptive evolution, and we specifically attempted to identify genes related to maintenance of maternally inherited symbionts by comparing gene expression during early life stages of Montipora and Acropora.
Results
Improvement of genome assemblies and gene predictions for Montipora and Astreopora
Assembly error, including retention of allelic contigs in haploid assemblies, is problematic for downstream analyses, mainly due to redundant genome sequences (alleles from the same genetic locus). We curated scaffold sequences of M. cactus and M. efflorescens by removing scaffold sequences with high or low coverage and those that may have originated from one of two allelic copies in heterozygous regions. Numbers of scaffold sequences were significantly reduced from the previous version, from 4925 to 3521 in M. cactus and from 5162 to 3589 in M. efflorescens (Table 1). For Astreopora, possible allelic scaffold sequences were removed from the genome assembly during the previous study [23]. The previous version of gene models for M. cactus, M. efflorescens, and Astreopora were predicted using AUGUSTUS, based solely on a training set built for Acropora or for protein homology with gene models of other corals [23]. Thus, it was highly possible that lineage-specific genes were missed in the previous version. In this study, we performed gene prediction for M. cactus, M. efflorescens, and Astreopora myriophthalma using a combination of ab initio and RNA-seq evidence-based prediction. We predicted 29,158 protein-coding genes for M. cactus, 29,424 for M. efflorescens and 25,406 for Astreopora myriophthalma (Table 1). Benchmarking universal single-copy orthologs (BUSCO) completeness scores were 93.3% (of which 0.8% were duplicated) for M. cactus, 91.2% (of which 1.4% were duplicated) for M. efflorescens and 94.5% (of which 1.3% were duplicated) for Astreopora myriophthalma, which were considerably better scores than the previous version (Table 1). In comparison to other Montipora gene models, gene models reported by Shumaker et al. [28] may have contained a higher fraction of diploid copies (93.4% complete BUSCO, with 18.3% duplicated; Table1). Completeness of gene models reported by Helmkampf et al. [27] was lower than that reported by Shumaker et al. [28] (64.2%, with 0.5% duplicated; Table1). Thus, the gene models reported by Shumaker et al. [28] contained many duplicates, but those reported by Helmkampf et al. [27] lacked many genes. In contrast, BUSCO completeness scores of M. cactus, M. efflorescens and Astreopora myriophthalma reported in this study were comparable to published gene models of other coral species, including A. millepora, predicted using the NCBI annotation pipeline (Table 1). These improvements to the Montipora and Astreopora genomes enabled more accurate comparative genomics among acroporids.
Table 1.
Statistics for gene prediction and assembly of Montipora, Astreopora and other coral genomes
| Genus | Montipora | Astreopora | Acropora | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | M. cactus | M. cactus | M. efflorescens | M. efflorescens | M. capitata | M. capitata | A. myriophthalma | A. myriophthalma | A. tenuis | A. digitifera | A. milleporaa |
| References | This study | Shinzato et al. [23] | This study | Shinzato et al. [23] | Shumaker et al. [28] | Helmkampf et al. [27] | This study | Shinzato et al. [23] | Shinzato et al. [23] | Shinzato et al. [23] | Ying et al. [25] |
| Genome assembly | |||||||||||
| Assembly length | 640,828,888 | 65,27,28,006 | 63,05,35,801 | 64,33,12,941 | 88,57,04,498 | 61,45,09,607 | 37,34,03,447 | 37,34,03,447 | 40,30,90,051 | 41,58,26,927 | 38,65,99,652 |
| No. of scaffolds | 3532 | 4925 | 3589 | 5162 | 3043 | 27,865 | 1149 | 1149 | 1538 | 955 | 3869 |
| N50 | 9,38,091 | 8,98,511 | 11,68,433 | 11,32,316 | 5,40,623 | 1,85,537 | 16,34,277 | 16,34,277 | 11,65,818 | 18,56,312 | 4,94,532 |
| Genes | |||||||||||
| No. of protein coding genes | 29,158 | 21,983 | 29,424 | 21,370 | 63,227 | 36,691 | 25,406 | 27,576 | 22,905 | 22,327 | 23,710 |
| Avg. length | 12,905 | 15,343 | 12,546 | 15,531 | 6507b | 4048b | 9550 | 5503b | 10,298 | 10,445 | 9294 |
| Transcripts | |||||||||||
| Avg. no. of CDS (exon) per gene | 6.6 (7.2) | 7.7 (7.9) | 6.5 (7.1) | 7.8 (8.0) | 4.5 (NA) | 3.62 (NA) | 6.0 (6.4) | 5.0 (NA) | 7.2 (8.1) | 6.6 (8,5) | 7.2 (7.5) |
| Avg. CDS (exon) length per gene (bp) | 1411 (2,033) | 1871 (2139) | 1390 (1994) | 1876 (2143) | 1162 (NA) | 1103 (NA) | 1647 (2132) | 1426 (NA) | 1562 (2383) | 1435 (2415) | 1600 (2404) |
| Avg. CDS (exon) length | 215 (280) | 244 (271) | 215 (280) | 241 (270) | 259 (NA) | 305 (NA) | 230 (279) | 283 (NA) | 216 (294) | 217 (285) | 312 (317) |
| Tortal CDS (exon) length (Mbp) | 41 (59) | 41 (45) | 41 (59) | 40 (44) | 73 (NA) | 40 (NA) | 42 (54) | 41 (NA) | 36 (55) | 32 (54) | 38 (57) |
| BUSCO completeness (v5.0) | |||||||||||
| Complete | 93.3% | 87.9% | 91.2% | 84.1% | 93.4%b | 64.2%b | 94.5% | 78.4%b | 92.9% | 89.3% | 94.7% |
| Single-copy | 92.5% | 87.3% | 89.8% | 83.6% | 75.1%b | 63.7%b | 93.2% | 77.7%b | 92.2% | 88.6% | 93.7% |
| Duplicated | 0.8% | 0.6% | 1.4% | 0.5% | 18.3%b | 0.5%b | 1.3% | 0.7%b | 0.7% | 0.7% | 1.0% |
| Fragmented | 4.1% | 5.7% | 4.5% | 7.2% | 4.1%b | 13.5%b | 3.1% | 7.7%b | 2.9% | 4.7% | 2.7% |
| Missing | 2.6% | 6.4% | 4.3% | 8.7% | 2.5%b | 2.5%b | 2.4% | 13.9%b | 4.2% | 6.0% | 2.6% |
aRefSeq assembly (GCF_004143615.1) was used for the analysis
bCDS were used for the calculation
Comparison of gene families within the Acroporidae
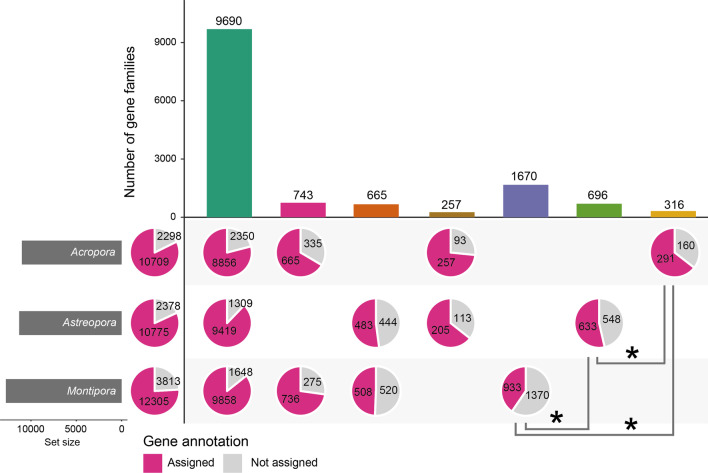
Identifying orthologous relationships between sequences is fundamental for comparative genomic analyses. To obtain orthologous relationships among acroporid genomes, we used three Acropora species (A. digitifera, A. millepora, and A. tenuis), two Montipora species (M. cactus and M. efflorescens), and Astreopora myriophthalma. We obtained 12,769 gene families for Montipora, 11,007 for Acropora and 11,309 for Astreopora (Fig. 2). We then categorized each gene family into seven groups, (1) shared by all three genera (9690 gene families), (2) shared by Montipora and Acropora (743 gene families), (3) shared by Montipora and Astreopora (665 gene families), (4) shared by Acropora and Astreopora (257 gene families), (5) restricted to Montipora (1670 gene families), (6) restricted to Astreopora (696 gene families) and (7) restricted to Acropora (316 gene families) (Fig. 2). 75.8% (9690/12,769) of the gene families in Montipora, 88% (9690/11,007) in Acropora, and 85.7% (9690/11,309) in Astreopora were shared among all three genera (Fig. 2), indicating that a large number (~ 88%) of gene families are shared throughout the Acroporidae, and these are likely to be the core-gene families among acroporids (Additional file 9: Data S1).
Fig. 2.
Gene family composition in acroporid genomes and the higher proportion of genes in Montipora having unknown functions. Left horizontal bars indicate numbers of gene families in each genus. Vertical bars indicate numbers of gene families conserved among genera. Pie charts indicate the generic composition in a given number of gene families (vertical bars). Gene annotation was performed using BLAST searches against the Swiss-Prot database (BLASTP, E-value cutoff: 1e−5) and protein domain search with hidden Markov models against the Pfam database (InterProScan, E-value cutoff: 1e−3). Numbers in pie charts indicate the number of genes with similarity to either Swiss-Prot or Pfam or neither. Proportions of gene annotations were compared among gene families specific to each lineage and asterisks indicate statistical significance (Pairwise proportion test: p < 0.05). Upset plot was produced using the “UpSetR” package [79]
The two major clades of reef-building corals, known as Robusta and Complexa [30], possess different metabolic pathways [31]. From the six species, we compared 303 functional modules comprising ten categories in the Kyoto Encyclopedia of Genes and Genomes (KEGG) metabolic pathways and found that metabolic pathways were basically conserved in the three genera (Additional file 1: Table S1). An enzyme involved in cysteine biosynthesis (KEGG module ID: M00338) and methionine degradation (KEGG module ID: M00035) was not detected among the six species (Additional file 1: Table S1), as reported in Shinzato et al. [23, 24]. Although one gene (KEGG entry ID: K04486) involved in the histidine biosynthetic pathway (KEGG module ID: M00026) was detected in acroporid corals used in this study, the remaining genes required to complete the pathway were not detected (Additional file 1: Table S1), as reported in Ying et al. [31]. Taken together, gene families involved in common features, such as amino acid synthesis, are widely conserved in the three genera.
While we identified 696 lineage-specific gene families in Astreopora and 316 in Acropora, we identified 1670 gene families restricted to Montipora (2307 genes in M. cactus and 2303 in M. efflorescens) (Fig. 2). The proportion of lineage-specific gene families in Montipora (13.07%) was significantly larger than that in Acropora (2.87%) and Astreopora (6.15%) (Pairwise proportion test: p < 0.05). Although we performed gene annotation with BLAST searches against the Swiss-Prot database (BLASTP, E-value cutoff: 1e−5) and hidden Markov models against the Pfam database (InterProScan, E-value cutoff: 1e−3), the proportion of Montipora-specific gene families with gene annotation was significantly lower than in Acropora and Astreopora (Pairwise proportion test: p < 0.05 for all combinations; Fig. 2). This indicates that functions of gene families restricted to Montipora are largely unknown.
Gene expansions in Montipora and comparisons among acroporids
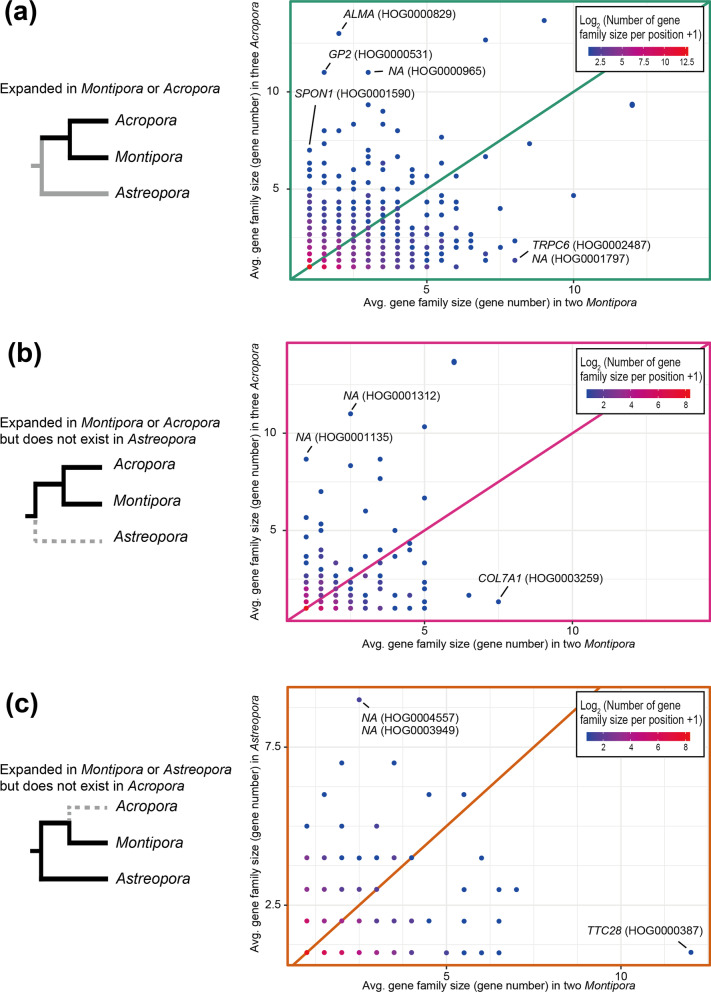
Gene duplication has contributed to acquisition of new gene functions during evolution [32, 33]. To explore gene families that underwent expansions, we first compared gene numbers of 9,690 gene families shared by the three genera and 743 gene families shared by Montipora and Acropora (Fig. 2). In these two groups, genes in families that underwent gene expansions in either Montipora or Acropora might have been duplicated after Montipora and Acropora diverged from their last common ancestor. Three gene families, homologous dimethylsulfoniopropionate (DMSP) lyase (ALMA; HOG0000829), Endonuclease-reverse transcriptase (GP1; HOG0000531), and Spondin (SPON1; HOG0001590), and three non-annotated gene families (NA; HOG0000965, HOG0001135, and HOG0001312), were significantly expanded in Acropora (Fisher’s exact test: p < 0.05; Fig. 3a and b). Recently, it was reported that DMSP lyase is the most expanded gene family in Acropora [28], and our result is consistent with that report, supporting the accuracy of this analysis. We found that three gene families, transient receptor potential protein (TRPC; HOG0002487), collagen alpha-1 (VII) chain (COL7A1; HOG0003259) and non-annotated gene family (NA; HOG0001797) are significantly expanded in Montipora compared with Acropora (Fisher’s exact test: p < 0.05; Fig. 3a and 3b).
Fig. 3.
Gene family expansions in Montipora. a Numbers of genes in Montipora and Acropora in each gene family shared by the three genera. b Numbers of genes in Montipora and Acropora in each gene family shared by Montipora and Acropora. c Numbers of genes in Montipora and Astreopora in each gene family shared by Montipora and Astreopora. The diagonal solid line indicates 1:1 numbers of genes in orthologous families. Possible gene names and gene family IDs are shown for significantly expanded gene families (Fisher’s exact test: p < 0.05). Scatter plot was produced using the “ggplot2” package [80]
Next, we compared gene numbers of 665 gene families shared by Montipora and Astreopora (Fig. 2), in which gene duplication may have occurred after divergence of Montipora or Astreopora. These genes may have been lost in Acropora. Two gene families (HOG0003949 and HOG0004557) lacking Swiss-Prot annotation were significantly expanded in Astreopora (Fisher’s exact test: p < 0.05; Fig. 3c), whereas one other gene family, tetratricopeptide repeat protein 28 (TTC28; HOG0000387), which is involved in the cell cycle in humans [34], was significantly expanded in Montipora compared with Astreopora (Fisher’s exact test: p < 0.05; Fig. 3c).
Estimation of evolutionary rate in each Montipora gene family group
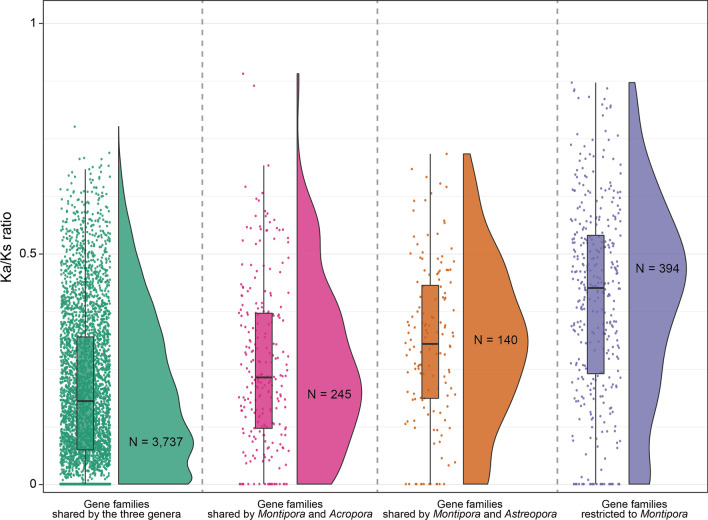
The ratio of nonsynonymous (Ka) to synonymous substitutions (Ks) reflects the strength of selective pressure on protein sequences [35]. For example, when Ka is less than Ks (Ka/Ks < 1), selection has occurred to eliminate mutations of protein sequences (purifying selection). In contrast, when Ka is larger than Ks (Ka/Ks > 1), selection has occurred to mutate the protein sequences (positive selection). In order to evaluate the strength of selective pressure acting on protein sequences in each Montipora gene family, we calculated pairwise Ka/Ks ratios between Montipora single-copy orthologous gene pairs (M. cactus versus M. efflorescens) for each of the four groups: (1) gene families shared by the three Acroporidae genera, (2) gene families shared by Montipora and Acropora, (3) gene families shared by Montipora and Astreopora, and (4) gene families restricted to Montipora (Fig. 4). When we compared Ka/Ks ratio between groups, gene families restricted to Montipora showed the highest Ka/Ks ratio (Wilcoxon rank sum test: p < 0.05; Fig. 4), indicating that this gene family group has undergone a relaxation of purifying selection, and that functional constraints on this gene family group are relaxed. This could explain why the deduced gene functions of gene families restricted to Montipora are largely unknown.
Fig. 4.
Relaxed purifying selection in Montipora-specific gene families. The y-axis represents the ratio of nonsynonymous (Ka) to synonymous amino acid substitutions (Ks). Orthologous gene pairs in two Montipora species (M. cactus and M. efflorescens) are used for calculation of pairwise Ka/Ks ratios. Ka/Ks ratios were compared among gene families and significant differences were observed in all pairwise combinations (Wilcoxon rank sum test: p < 0.05). A raincloud plot was produced using the “raincloudplots” package [81]. Details of 40 gene families exhibiting Ka/Ks > 1 are shown in Table 2
Positive selection specific to Montipora
To identify genes with fast evolutionary rates that may be associated with adaptive evolution in Montipora, we focused on gene families exhibiting Ka/Ks > 1. We found evidence of positive selection in 40 gene families (rapidly evolving gene families) (Table 2). Of those, 10 families are shared by the three genera or shared by Montipora and Acropora, while the remaining 30 families are restricted to Montipora (Table 2), suggesting that these 30 gene families arose specifically in that lineage and likely contribute to biological traits unique to Montipora. Although 28 of the 30 gene families restricted to Montipora were without annotation, their possible subcellular localization ranging from membrane to organelle was predicted by DeepLoc, a deep learning neural networks model (Table 2).
Table 2.
Gene families under positive selection (Ka/Ks ratio > 1) in Montipora
| Gene family group | Gene family ID | Expressed in early life stages of | Swiss-Prot annotation (BLASTP, e-value cutoff: 1e−5) | Pfam domain (InterProScan, e-value cutoff: 1e−3) | Subcellular localization | Ka | Ks | Ka/Ks ratio |
|---|---|---|---|---|---|---|---|---|
| Common to the three genera | HOG0011297 | Montipora and Acropora | G patch domain and ankyrin repeat-containing protein 1 |
Ankyrin repeats (3 copies) (PF12796) G-patch domain (PF01585) |
Nucleus | 1.02946 | 0.868207 | 1.18573 |
| HOG0013033 | Montipora and Acropora | NA | NA | Nucleus | 0.047466 | 0.016942 | 2.80166 | |
| HOG0013161 | Montipora and Acropora | NA | NA | Extracellular | 0.067747 | 0.011507 | 5.88723 | |
| HOG0013504 | Montipora and Acropora | NA | NA | Extracellular | 0.250122 | 0.146258 | 1.71014 | |
| HOG0016452 | Montipora and Acropora | Nucleoredoxin-like protein 2 | Thioredoxin-like (PF13905) | Cytoplasm | 1.04061 | 0.866303 | 1.2012 | |
| Common to Montipora and Acropora | HOG0000399 | - | NA | NA | Mitochondrion | 1.04494 | 0.829832 | 1.25922 |
| HOG0007354 | Montipora and Acropora | NA | NA | Cytoplasm | 0.043171 | 0.01538 | 2.80698 | |
| HOG0008211 | Montipora and Acropora | E3 ubiquitin-protein ligase TRIM71 |
RING-type zinc-finger (PF13445) B-box zinc finger (PF00643) |
Nucleus | 1.02988 | 0.901833 | 1.14199 | |
| HOG0008701 | Montipora and Acropora | Collagen alpha-1(V) chain | Fibrillar collagen C-terminal domain (PF01410) | Extracellular | 0.086894 | 0.031594 | 2.75035 | |
| HOG0018764 | Montipora and Acropora | Histamine H2 receptor | 7 transmembrane receptor (rhodopsin family) (PF00001) | Cell membrane | 1.03386 | 0.896216 | 1.15358 | |
| Restricted to Montipora | HOG0000410 | Montiporaa | NA | NA | Cytoplasm | 1.0489 | 0.858182 | 1.22224 |
| HOG0006069 | Montiporaa | NA | NA | Cytoplasm | 1.06207 | 0.789879 | 1.34459 | |
| HOG0025744 | Montiporaa | NA | NA | Cytoplasm | 1.04814 | 0.846526 | 1.23816 | |
| HOG0025248 | Montiporaa | NA | NA | Golgi apparatus | 1.06112 | 0.837214 | 1.26744 | |
| HOG0025123 | Montiporaa | NA | NA | Mitochondrion | 1.03568 | 0.871894 | 1.18785 | |
| HOG0025167 | Montiporaa | NA | NA | Mitochondrion | 1.05341 | 0.81252 | 1.29647 | |
| HOG0025503 | Montiporaa | NA | NA | Mitochondrion | 1.06094 | 0.794999 | 1.33451 | |
| HOG0025176 | Montiporaa | NA | NA | Plastid | 1.07662 | 0.736763 | 1.46128 | |
| HOG0025663 | Montiporaa | NA | NA | Lysosome/Vacuole | 1.0435 | 0.883964 | 1.18048 | |
| HOG0023383 | Montiporab | NA | NA | Cytoplasm | 1.0506 | 0.86614 | 1.21297 | |
| HOG0024846 | Montiporab | NA | NA | Cytoplasm | 0.157342 | 0.054572 | 2.8832 | |
| HOG0025044 | Montiporab | NA | NA | Cytoplasm | 1.04722 | 0.8635 | 1.21276 | |
| HOG0025199 | Montiporab | NA | NA | Cytoplasm | 1.04978 | 0.833375 | 1.25967 | |
| HOG0024900 | Montiporab | NA | NA | Endoplasmic reticulum | 0.072466 | 0.030348 | 2.38782 | |
| HOG0025608 | Montiporab | NA | NA | Endoplasmic reticulum | 1.05623 | 0.836453 | 1.26275 | |
| HOG0024879 | Montiporab | NA | NA | Extracellular | 1.04481 | 0.820572 | 1.27328 | |
| HOG0024911 | Montiporab | NA | NA | Extracellular | 1.08591 | 0.764205 | 1.42097 | |
| HOG0024765 | Montiporab | NA | NA | Mitochondrion | 1.06916 | 0.745005 | 1.43511 | |
| HOG0025496 | Montiporab | NA | NA | Mitochondrion | 1.03657 | 0.886546 | 1.16922 | |
| HOG0025423 | Montiporab | NA | NA | Mitochondrion | 1.05678 | 0.799324 | 1.32209 | |
| HOG0025535 | Montiporab | Integrase/recombinase xerD homolog | NA | Mitochondrion | 1.04223 | 0.880029 | 1.18431 | |
| HOG0025606 | Montiporab | NA | NA | Mitochondrion | 1.05418 | 0.833955 | 1.26408 | |
| HOG0024806 | Montiporab | NA | Zinc knuckle (PF00098) | Nucleus | 1.0438 | 0.876216 | 1.19126 | |
| HOG0024834 | Montiporab | NA | NA | Nucleus | 1.04457 | 0.853951 | 1.22323 | |
| HOG0024972 | Montiporab | NA | NA | Nucleus | 1.06879 | 0.737384 | 1.44943 | |
| HOG0025048 | Montiporab | NA | NA | Nucleus | 1.0465 | 0.828206 | 1.26357 | |
| HOG0025616 | Montiporab | NA | NA | Nucleus | 1.07293 | 0.838674 | 1.27932 | |
| HOG0024996 | - | NA | NA | Cell membrane | 0.827813 | 0.407974 | 2.02908 | |
| HOG0024975 | - | NA | NA | Endoplasmic reticulum | 1.02887 | 0.884795 | 1.16284 | |
| HOG0025445 | - | TNF receptor-associated factor 6 | TRAF-type zinc finger (PF02176) | Nucleus | 0.094242 | 0.019425 | 4.85163 |
aExpressed at least one early life stages
bExpressed at all three early life stages
Gene expression unique to early life stages of Montipora
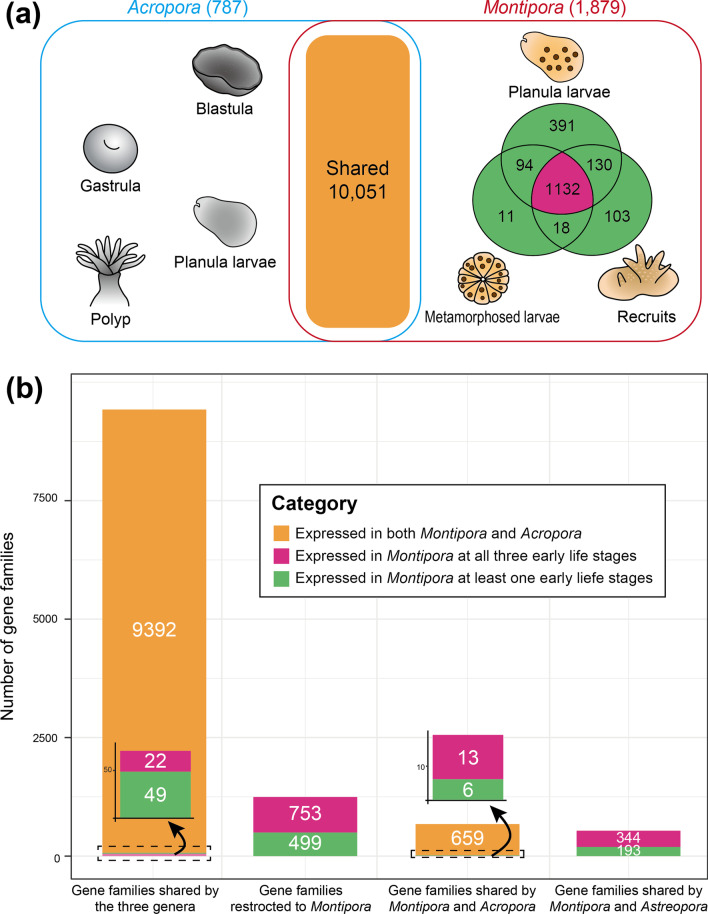
Presence of maternally inherited algal symbionts at an early life stage is the most obvious difference between vertical and horizontal transmitters (Fig. 1). In order to identify gene families specifically involved in symbiosis in vertical transmitters, we compared the repertoire of expressed genes in early life stages of Montipora with those expressed in Acropora. In this analysis, a gene family was considered expressed even if only one gene in that family was expressed (Transcripts per million (TPM) > 1). We confirmed that 11,930 and 10,838 gene families were expressed during early life stages of Montipora and Acropora, respectively (Fig. 5a). Of these, 10,051 gene families (84% in Montipora and 93% in Acropora) were shared by both during early life stages (Fig. 5a), suggesting that these are essential for early development of acroporid corals; thus, we did not focus on these in the present study. We identified 1,879 gene families that were exclusively expressed in Montipora (Fig. 5b). Among those, 60% (1132 gene families) were expressed in planula larvae, metamorphosed larvae, and recruit stages (Fig. 5a), suggesting that these genes may be related to maintenance of algal symbionts in Montipora. Interestingly, 97% of these gene families ((753 + 344) / 1132, Fig. 5b) that were expressed throughout the three life stages were specific to Montipora or shared by Astreopora (Additional file 2: Table S2). In contrast, the remaining 3% of gene families ((22 + 13) / 1,132, Fig. 5b) have orthologs in Acropora, but were not expressed in Acropora. Nonetheless, they were expressed throughout early life stages of Montipora Additional file 3: Table S3). Within gene families containing gene duplications in the Montipora genomes above, two gene families (HOG0001797 and HOG0000387) were exclusively expressed in at least one early life stage in Montipora, and one of them (HOG0000387) was expressed throughout all three early life stages (Additional file 2: Table S2). Among the identified 30 rapidly evolving gene families restricted to Montipora, we detected gene expression of 90% of these families. Expression of nine families was detected in at least one early life stage of Montipora, and the remaining 18 gene families were continuously expressed throughout all three early life stages (Table 2).
Fig. 5.
Expression patterns of gene families during early life stages of Montipora and Acropora. a Numbers of gene families that are commonly or exclusively expressed in early life stages of Montipora and Acropora. Numbers of gene families that are exclusively expressed in each genus are shown in parentheses after generic names. Gene families that are commonly expressed in both Montipora and Acropora early life stages are shown in an orange box. Gene families that are exclusively expressed in early life stages of Montipora were further classified according to whether they are expressed in one of the stages (green), or throughout all stages (red). b Gene families expressed in early Montipora life stages. For Montipora, RNA-seq data from planula larvae, metamorphosed larvae and recruits were used. For Acropora, RNA-seq data from blastulae, gastrulae, planula larvae and polyps were used. SRA accession numbers for the RNA-seq data are provided in Additional file 7: Table S7
Discussion
Improved genome information for the genera Montipora and Astreopora
BUSCO completeness scores for improved gene models of M. cactus, M. efflorescens, and Astreopora myriophthalma were 93.3% (0.8% duplicates), 91.2% (1.4% duplicates), and 94.5% (1.3% duplicates), respectively (Table 1). They are considerably better than those of M. capitata (93.4% (18.3% duplicates) from Shumaker et al. [28] and 64.2% (0.5% duplicates) from Helmkampf et al. [27]; Table 1), and were comparable to those of other coral species (Table 1). These numbers indicate that we successfully obtained high-quality gene models from Montipora and Astreopora species. Numbers of genes in M. cactus and M. efflorescens genomes were not quite as large as those of M. capitata reported by Shumaker et al. [28]. Previously, it was reported that M. capitata has fewer exons and shorter coding regions per gene than other corals [27, 28]; however, this was not the case with M. cactus and M. efflorescens (Table 1). Fewer exons and shorter coding regions per gene could be an unusual feature of the M. capitata genome or could reflect the quality of the genome assembly. Indeed, the N50 size, one of the indices to evaluate the quality of genome assembly, was larger for both M. cactus and M. efflorescens genome assemblies than for M. capitata (Table 1).
Possible genomic evolutionary strategy unique to Montipora
Recent large-scale genome comparisons of acroporid genomes showed that 28 gene families were specifically expanded in Acropora, but none in Montipora [23]. Nonetheless, we identified four expanded gene families in Montipora (Fig. 3). Although the number of gene families in Montipora is not much different from those of Acropora and Astreopora, the proportion of lineage-specific gene families in Montipora was significantly larger (Fig. 2). Montipora does not appear to have duplicated existing gene families, as has Acropora. Lineage-specific gene families contribute to larger gene numbers in Montipora genomes, and emergence of lineage-specific genes may have helped to establish maternal transmission of symbionts in Montipora corals. In particular, Montipora-specific gene families under positive selection may be major contributors.
Three gene families, homologous to TRPC6, TTC28, and COL7A1, and one gene family without annotation were significantly expanded in Montipora compared with Acropora or Astreopora (Fig. 3). Known functions of transient receptor potential (TRP) proteins encoded by TRPC are diverse (reviewed in [36]). For example, TRP proteins respond to hypertonicity in yeasts [37, 38], detect and avoid noxious chemicals in nematodes [39], and discriminate warmth, heat, and cold in humans [36]. In each case, TRP proteins mediate sensory transduction in cells [36]. In corals, expression levels of TRP-like genes change when the concentration of CO2 in seawater changes [40]. They also change diurnally [41, 42] or when exposed to symbiotic algae [43, 44]. The TRPC6-like gene family, specifically expanded in Montipora, may also be involved in sensory transduction during environmental transitions. The TTC28-like gene family has tetratricopeptide repeats (PF12176 and/or PF13424) and caspase HetF associated with Tprs (CHAT) domains (PF12770) (Additional file 4: Table S4). Canonical TTC28 is composed of tetratricopeptide repeats and CHAT domains (Q96AY4: TTC28_HUMAN [34]) and genes in the gene family (HOG0000387) are also composed of tetratricopeptide repeats and CHAT domains, indicating that this gene family may have been duplicated from canonical TTC28, which is conserved in all acroporids examined in this study (HOG0016559 in Additional file 9: Data S1). TTC28 is required for the cell cycle in humans [34]. The expanded TTC28-like gene family may also be involved in cell cycle in Montipora. Collagen is expressed in gastrodermis at a specific developmental stage of cnidarian larvae [45–47] and the expanded collagen-like gene family may function in early development of Montipora.
In this study, we identified 40 genes under positive selection in Montipora (Table 2). Positive selection has often been detected in genes involved in immunity in vertebrates [48]. In corals, genes related to immunity, such as lectins and antimicrobial peptides, are also under positive selection [23, 49, 50]. In the 40 rapidly evolving gene families found in this study, with one exception, no genes appeared homologous to immune-related genes (Table 2). In addition, 28 of 30 rapidly evolving gene families restricted to Montipora have no annotated function (Table 2). Generally, genes with no homology to genes of other lineages are called orphan genes [51]. They are thought to arise principally by two processes: gene duplication or de novo evolution from non-coding regions [51]. If a gene originates by duplication, the protein domains tend to be conserved in the new genes, since a functional protein domain cannot easily be changed by mutations [52], suggesting that the 28 rapidly evolving gene families originated by de novo evolution from non-coding regions. Orphan genes are expected to interact specifically with the environment as a consequence of lineage-specific adaptation [51]. Therefore, orphan genes may contribute to adaptive evolution in Montipora. In particular, 18 rapidly evolving gene families with expression throughout the three early life stages, planula larvae, metamorphosed larvae, and recruits, may have important functions in symbiosis during early life stages of Montipora.
Conclusions
In this study, we highlighted possible genomic underpinnings of maternal transmission of symbionts in Montipora using high-quality genomic information of Montipora and Astreopora. We found that the driving force behind evolution of Montipora was lineage-specific gene families, rather than gene duplication, as among Acropora corals. The importance of rapidly evolving gene families in Montipora for maternal transmission of symbionts is inferred. Our dataset and findings offer novel insights into mechanisms of coral-algal symbiosis. Although genetic tools for manipulating corals have been established [53, 54], development of more efficient methods to deliver gene-knockdown or -knockout reagents into large numbers of zygotes will facilitate rapid screening for relevant phenotypes of candidate genes. In addition, coral cell lines which have the capacity to incorporate algal symbionts has been developed [55], allowing us to observe ongoing symbiosis at the single cell level. Together, these advances will facilitate a deeper understanding of cellular and molecular mechanisms of coral-algal symbiosis.
Methods
Sample preparation, RNA extraction, and RNA-Seq
Colonies of M. cactus, M. efflorescens, and Astreopora myriophthalma were collected in Sekisei Lagoon, Okinawa, Japan in May 2015, and were maintained in aquaria at the Research Center for Subtropical Fisheries, Seikai National Fisheries Research Institute, until spawning. Permits for coral collection were kindly provided by the Okinawa Prefectural Government for research use (Permits #29-74). Coral fragments (~ 3 cm diameter) from adult colonies of M. cactus, M. efflorescens, and Astreopora myriophthalma were snap frozen in liquid nitrogen and stored at -80℃ until use. Fragments were then crushed in liquid nitrogen with an iron and hammer into powder. Total RNA was extracted from the powder using an RNeasy Plant Mini Kit (QIAGEN). A TruSeq Stranded mRNA Library Kit (Illumina) was used for mRNA sequencing library preparation, and each library was sequenced from 100-bp paired-end libraries using a NovaSeq 6000 (Illumina). For Montipora, eggs, sperm, planula larvae (1 and 4 d post-fertilization) were collected and preserved with TRIzol reagent (Thermo Fisher Scientific) at -80℃ until use. Total RNA was extracted from preserved eggs, sperm, and planula larvae as in Yoshioka et al. [56]. KAPA RNA HyperPrep Kits (Kapa Biosystems) and MGIEasy RNA Directional Library Prep Sets (MGI) were used for total RNA and mRNA sequencing library preparation, and each library was sequenced on a NovaSeq 6000 in 150-bp paired-end and a DNBSEQ-G400RS (MGI) in 100-bp paired-end mode. This information is summarized in Additional file 5: Table S5.
Curating scaffold sequences of M. cactus and M. efflorescens and gene prediction
We downloaded scaffold sequences of M. cactus and M. efflorescens, assembled from DNA sequences extracted from symbiotic algae-free coral sperm [23], from the genome browser of the OIST Marine Genomics Unit (https://marinegenomics.oist.jp). We identified scaffold sequences with high or low coverage or those that may have originated from one of two allelic copies of heterozygous regions, using Purge Haplotigs v1.1.1 [57] with default settings. These were excluded from subsequent analyses.
In addition to the above RNA samples, we used publicly available RNA-seq data from NCBI SRA for gene prediction (Additional file 6: Table S6). Low-quality reads (quality score < 20 and length < 20 bp) and sequence adaptors were trimmed using CUTADAPT v1.18 [58]. A total of 31 and 2 RNA-seq libraries were used for Montipora and Astreopora gene prediction, respectively. Repetitive elements in the scaffolds were identified de novo with RepeatScout v1.0.6 [59] and RepeatMasker v4.1.0 (http://www.repeatmasker.org). Repetitive elements were filtered out by length (> 50 bp) and occurrence (more than 10 times for Montipora, more than 60 times for Astreopora). Gene prediction was first executed with the BRAKER pipeline v2.1.2 [60], with AUGUSTUS v3.3.3. RNA-seq reads were aligned to each genome sequence with HISAT v2.1.0 [61]. Then, the alignment information was used for BRAKER gene prediction with options “UTR = on”, “softmasking”, and “AUGUSTUS_ab_initio.” To improve gene prediction, we further executed genome-guided transcriptome assembly using StringTie [62] with option “-m 500.” Genome-based transcript structure was predicted with TransDecoder (https://github.com/TransDecoder/TransDecoder/wiki). During read alignment, we used soft-masked repeats for genome-guided transcriptome assembly and hard-masked repeats for BRAKER gene prediction. Finally, genes that were present in genome-guided assembly or ab initio prediction, but absent in predictions from the hint file were added to the prediction from the hint file using GffCompare [63], as summarized in Additional file 8: Figure S1. To evaluate the completeness of predicted genes, we used BUSCO v5.0 [64] with Metazoa OrthoDB10 dataset (2021–02-24, n = 954).
Gene annotation, orthology inference within the Acroporidae
We used publicly available gene models for A. digitifera [23, 24], A. tenuis [23], and A. millepora [25] in addition to Montipora and Astreopora gene models. For A. millopora, we downloaded gene models from NCBI RefSeq (RefSeq assembly accession: GCF_004143615.1). We downloaded gene models of v2.0 for A. digitifera and v1.0 for A. tenuis from the genome browser of the OIST Marine Genomics Unit, respectively. We selected three Acropora species for the following reasons. A. digitifera genome sequences were assembled with PacBio long-reads [23]. A. millepora genome assembly and gene models were curated by NCBI. A. tenuis had the second highest gene model completeness (BUSCO completeness scores) among Acropora genomes following A. millepora [23] and represents a distinct phylogenetic clade from A. digitifera and A. millepora [23]. We selected the longest transcript variants from each gene and translated them into protein sequences. All proteomes were annotated with BLASTP [65] (E-value cut off: 1e−5) against the Swiss-Prot database (8 January 2021). In addition, domains in protein sequences were searched using hidden Markov models against the Pfam database with InterProScan v5.31–70.0 (E-value cutoff: 1e−3) [66]. In addition, putative transposable elements in gene models were identified with TransposonPSI (http://transposonpsi.sourceforge.net/), Dfam scan (release 3.3; Storer et al. [67]), and Pfam keyword (“Reverse transcriptase” and “Integrase”). All protein sequences were also annotated with KEGG [68] in all eukaryote genes using GenoMaple v2.3.2 [69] with the GHOSTX search engine and the bi-directional best hit method. Module completion ratio (MCR) was calculated in each functional module defined by KEGG, also using GenoMaple v2.3.2. For clustering of orthologous genes (herein gene families) of the Acroporidae, we used OrthoFinder v2.4.0 [70] and Porites australiensis gene models [71] were also included as an outgroup for the Acroporidae. In this study, we used phylogenetic hierarchical orthogroups (HOG) as gene families. Gene families shared by the three Acropora species were defined as Acropora gene families. Gene families shared by the two Montipora species were defined as Montipora gene families. Gene families containing transposon-like genes were excluded from subsequent analyses.
Transcriptomic comparisons between Montipora and Acropora
We used RNA-seq data of M. efflorescens (planula larvae), A. tenuis (blastula, gastrula, planula larvae and polyps) and A. digitifera (blastula, gastrula, planula larvae and polyps) (Additional file 7: Table S7). In addition, publicly available RNA-seq data of M. capitata (planula larvae, metamorphosed larvae, and recruits) were also used in this study Additional file 7: Table S7). Low-quality reads (quality score < 20 and length < 20 bp) and sequence adaptors were trimmed using CUTADAPT v1.18. Cleaned RNA-seq reads were mapped to each organism's gene models (For M. capitata RNA-seq data, we used M. efflorescens gene models as a reference) using SALMON v1.0.0 [72]. Expression levels were quantified using SALMON v1.0.0. Genes with TPM > 1 were considered expressed. Then expressed genes were classified into corresponding gene families based on the above gene family inference.
Estimation of the ratio of nonsynonymous to synonymous substitutions
Protein sequences of putative single-copy orthologs between M. cactus and M. efflorescens were aligned pairwise with MAFFT [73]. Aligned nucleotide codon sequences without alignment gaps were retrieved using the PAL2NAL script [74]. Genes with nucleotide alignment lengths longer than 120 bp were used for further analysis. We calculated pairwise nonsynonymous (Ka) and synonymous (Ks) substitution ratios of single-copy genes between M. cactus and M. efflorescens using KaKs_Calculator 2.0 [75] with option “-MA”. Following Villanueva-Canas et al. [76], we discarded gene families showing Ks < 0.01, as such low Ks values may result in inaccurate Ka/Ks estimates, and gene families showing Ks or Ka > 2 indicating saturation of substitutions. Genes exhibiting Ka/Ks ratios with p < 0.05 (Fisher’s exact test) were used for further analysis. Subcellular localization of gene families showing Ka/Ks > 1 was predicted using the DeepLoc-1.0 online server [77].
Statistical analysis
Pairwise proportion tests were conducted to compare lineage-specific gene families (“number of lineage-specific gene families” / “number of gene families in lineage”) and gene annotation proportions of lineage-specific gene families (“number of genes with annotation” / “number of genes without annotation”). Fisher’s exact test was conducted to identify expanded gene families in each group (“number of genes in one gene family in species A” / “number of genes in the rest of the gene family in species A” versus “number of genes in one gene family in species B” / “number of genes in the rest of the gene family in species B”). We considered a p < 0.05 as significantly expanded. The Wilcoxon rank sum test was conducted to compare median Ka/Ks values between gene family groups. All statistical tests were performed in R v4.0.3 [78].
Supplementary Information
Additional file 1: Table S1. Summary of comparison of KEGG metabolic pathways among acroporid genomes. MCR indicates the module completion ratio, calculated by GenoMaple.
Additional file 2: Table S2. IDs of gene families common to Montipora and Astreopora or restricted to Montipora, that are expressed during three life stages (planula larvae, metamorphosed larvae, and recruits).
Additional file 3: Table S3. IDs of gene families common to three genera (Montipora, Acropora and Astreopora) or common to Montipora and Acropora, that are expressed during three life stages (planula larvae, metamorphosed larvae, and recruits).
Additional file 4: Table S4. Summary of gene families significantly (Fisher’s exact test: p < 0.05) expanded in the Montipora genome compared to those of Acropora or Astreopora.
Additional file 5: Table S5. Summary of samples used for RNA-seq.
Additional file 6: Table S6. Summary of publicly available RNA-seq samples that were included for gene prediction in Montipora and Astreopora.
Additional file 7: Table S7. Summary of RNA-seq samples used for comparative transcriptomic analyses.
Additional file 8: Figure S1. Summary of gene predictions for M. cactus, M. efflorescens, and Astreopora myriophthalma.
Additional file 9: Data S1. Result of orthologous clustering in Acroporidae genomes using OrthoFinder.
Additional file 10: Data S2. Summary of retained scaffolds after genome assembly curation for Montipora cactus and M. efflorescens.
Additional file 11: Data S3. Gene models for M. cactus in GTF format.
Additional file 12: Data S4. Gene models for M. efflorescens in GTF format.
Additional file 13: Data S5. Gene models for Astreopora myriophthalma in GTF format.
Acknowledgements
Computations were partially performed on the NIG supercomputer at ROIS National Institute of Genetics. We thank Dr. Steven D. Aird for carefully reviewing our manuscript and for helpful comments.
Abbreviations
- BUSCO
Benchmarking universal single-copy orthologs
- KEGG
Kyoto encyclopedia of genes and genomes
- DMSP
Dimethylsulfoniopropionate
- Ka
Nonsynonymous substitutions
- Ks
Synonymous substitutions
- TPM
Transcript per million
- TRP
Transient receptor potential protein
- TTC
Tetratricopeptide repeat protein
- CHAT
Caspase HetF associated with Tprs
- HOG
Phylogenetic hierarchical orthogroups
- MCR
Module completion ratio
Author contributions
CS and GS conceptualized, and CS supervised the project. GS, CS and YZ performed coral sampling. HY helped with sampling of Montipora larvae. YY performed molecular biological experiments and bioinformatic analyses. YY and CS wrote main manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by JSPS KAKENHI Grants (20H03235 and 20K21860 for CS, 21H04742 for HY, 19K15902 for YZ and 20H03066 for GS) and Grant-in-Aid for JSPS Fellows to YY (20J21301).
Availability of data and materials
Raw RNA-sequencing data have been deposited in the DDBJ/EMBL/GenBank databases under accession number DRA011820 (BioProject ID: PRJDB11460). A genome browser for M. cactus, M. efflorescens and Astreopora myriophthalma is available from the Marine Genomics Unit web site (https://marinegenomics.oist.jp/gallery). Sequence IDs of retained scaffolds were prepared as Additional file 10: Data S2. Gene models in GTF format for M. cactus, M. efflorescens and Astreopora myriophthalma are provided as Additional file 11: Data S3, Additional file 12: Data S4, Additional file 13: Data S5. For M. capitata, we downloaded genome assembly and gene models reported from Shumaker et al. [28] (URL: http://cyanophora.rutgers.edu/montipora/) and Helmkampf et al. [27] (Data set https://doi.org/10.15482/USDA.ADC/1503958).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Chuya Shinzato is an editorial board member for BMC Ecology and Evolution. All other authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roberts CM, McClean CJ, Veron JEN, Hawkins JP, Allen GR, McAllister DE, Mittermeier CG, Schueler FW, Spalding M, Wells F, Vynne C, Werner TB. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science. 2002;295(5558):1280–1284. doi: 10.1126/science.1067728. [DOI] [PubMed] [Google Scholar]
- 2.Muscatine L, Porter JW. Reef corals: mutualistic symbioses adapted to nutrient-poor environments. Bioscience. 1977;27(7):454–460. doi: 10.2307/1297526. [DOI] [Google Scholar]
- 3.Muscatine L, McCloskey LR, Marian RE. Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration 1. Limnol Oceanogr. 1981;26(4):601–611. doi: 10.4319/lo.1981.26.4.0601. [DOI] [Google Scholar]
- 4.Falkowski PG, Dubinsky Z, Muscatine L, Porter JW. Light and the bioenergetics of a symbiotic coral. Bioscience. 1984;34(11):705–709. doi: 10.2307/1309663. [DOI] [Google Scholar]
- 5.Carpenter KE, Abrar M, Aeby G, Aronson RB, Banks S, Bruckner A, Chiriboga A, Cortés J, Delbeek JC, DeVantier L. One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science. 2008;321(5888):560–563. doi: 10.1126/science.1159196. [DOI] [PubMed] [Google Scholar]
- 6.Hoegh-Guldberg O, Poloczanska ES, Skirving W, Dove S. Coral reef ecosystems under climate change and ocean acidification. Front Mar Sci. 2017;4:158. doi: 10.3389/fmars.2017.00158. [DOI] [Google Scholar]
- 7.Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science. 2018;359(6371):80–83. doi: 10.1126/science.aan8048. [DOI] [PubMed] [Google Scholar]
- 8.Brown BE. Coral bleaching: causes and consequences. Coral Reefs. 1997;16(S1):S129–S138. doi: 10.1007/s003380050249. [DOI] [Google Scholar]
- 9.Bellwood DR, Hughes TP, Folke C, Nystrom M. Confronting the coral reef crisis. Nature. 2004;429(6994):827–833. doi: 10.1038/nature02691. [DOI] [PubMed] [Google Scholar]
- 10.Veron JEN, Stafford-Smith M. Corals of the world. 1. Townsville: Australian Inst. of Marine Science; 2000. [Google Scholar]
- 11.Veron J. Scleractinia of Eastern Australia, Part 5: Acroporidae. Aust Inst Mar Sci Monogr Ser. 1984;6:485. [Google Scholar]
- 12.Yamamura N. Vertical transmission and evolution of mutualism from parasitism. Theor Popul Biol. 1993;44(1):95–109. doi: 10.1006/tpbi.1993.1020. [DOI] [Google Scholar]
- 13.Yamamura N. Evolution of mutualistic symbiosis: a differential equation model. Res Popul Ecol. 1996;38(2):211–218. doi: 10.1007/BF02515729. [DOI] [Google Scholar]
- 14.Bright M, Bulgheresi S. A complex journey: transmission of microbial symbionts. Nat Rev Microbiol. 2010;8(3):218–230. doi: 10.1038/nrmicro2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baird AH, Guest JR, Willis BL. Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu Rev Ecol Evol Syst. 2009;40:551–571. doi: 10.1146/annurev.ecolsys.110308.120220. [DOI] [Google Scholar]
- 16.Richmond RH, Hunter CL. Reproduction and recruitment of corals: comparisons among the Caribbean, the tropical Pacific, and the Red Sea. Mar Ecol Prog Ser. 1990;60(1):185–203. doi: 10.3354/meps060185. [DOI] [Google Scholar]
- 17.Fadlallah YH. Sexual reproduction, development and larval biology in scleractinian corals. Coral Reefs. 1983;2(3):129–150. doi: 10.1007/BF00336720. [DOI] [Google Scholar]
- 18.Douglas AE. Host benefit and the evolution of specialization in symbiosis. Heredity. 1998;81(6):599–603. doi: 10.1046/j.1365-2540.1998.00455.x. [DOI] [Google Scholar]
- 19.Rowan R, Knowlton N. Intraspecific diversity and ecological zonation in coral-algal symbiosis. Proc Natl Acad Sci. 1995;92(7):2850–2853. doi: 10.1073/pnas.92.7.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byler KA, Carmi-Veal M, Fine M, Goulet TL. Multiple symbiont acquisition strategies as an adaptive mechanism in the coral Stylophora pistillata. PLoS ONE. 2013;8(3):e59596. doi: 10.1371/journal.pone.0059596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Putnam HM, Davidson JM, Gates RD. Ocean acidification influences host DNA methylation and phenotypic plasticity in environmentally susceptible corals. Evol Appl. 2016;9(9):1165–1178. doi: 10.1111/eva.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibbin EM, Putnam HM, Gates RD, Nitschke MR, Davy SK. Species-specific differences in thermal tolerance may define susceptibility to intracellular acidosis in reef corals. Mar Biol. 2015;162(3):717–723. doi: 10.1007/s00227-015-2617-9. [DOI] [Google Scholar]
- 23.Shinzato C, Khalturin K, Inoue J, Zayasu Y, Kanda M, Kawamitsu M, Yoshioka Y, Yamashita H, Suzuki G, Satoh N. Eighteen coral genomes reveal the evolutionary origin of Acropora strategies to accommodate environmental changes. Mol Biol Evol. 2021;38(1):16–30. doi: 10.1093/molbev/msaa216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinzato C, Shoguchi E, Kawashima T, Hamada M, Hisata K, Tanaka M, Fujie M, Fujiwara M, Koyanagi R, Ikuta T. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature. 2011;476(7360):320–323. doi: 10.1038/nature10249. [DOI] [PubMed] [Google Scholar]
- 25.Ying H, Hayward DC, Cooke I, Wang W, Moya A, Siemering KR, Sprungala S, Ball EE, Forêt S, Miller DJ. The whole-genome sequence of the coral Acropora millepora. Genome Biol Evol. 2019;11(5):1374–1379. doi: 10.1093/gbe/evz077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuller ZL, Mocellin VJ, Morris LA, Cantin N, Shepherd J, Sarre L, Peng J, Liao Y, Pickrell J, Andolfatto P. Population genetics of the coral Acropora millepora: toward genomic prediction of bleaching. Science. 2020 doi: 10.1126/science.aba4674. [DOI] [PubMed] [Google Scholar]
- 27.Helmkampf M, Bellinger MR, Geib SM, Sim SB, Takabayashi M. Draft genome of the rice coral Montipora capitata obtained from linked-read sequencing. Genome Biol Evol. 2019;11(7):2045–2054. doi: 10.1093/gbe/evz135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shumaker A, Putnam HM, Qiu H, Price DC, Zelzion E, Harel A, Wagner NE, Gates RD, Yoon HS, Bhattacharya D. Genome analysis of the rice coral Montipora capitata. Sci Rep. 2019;9(1):1–16. doi: 10.1038/s41598-019-39274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukami H, Omori M, Hatta M. Phylogenetic relationships in the coral family Acroporidae, reassessed by inference from mitochondrial genes. Zool Sci. 2000;17(5):689–696. doi: 10.2108/zsj.17.689. [DOI] [PubMed] [Google Scholar]
- 30.Romano SL, Palumbi SR. Evolution of scleractinian corals inferred from molecular systematics. Science. 1996;271(5249):640–642. doi: 10.1126/science.271.5249.640. [DOI] [Google Scholar]
- 31.Ying H, Cooke I, Sprungala S, Wang W, Hayward DC, Tang Y, Huttley G, Ball EE, Forêt S, Miller DJ. Comparative genomics reveals the distinct evolutionary trajectories of the robust and complex coral lineages. Genome Biol. 2018;19(1):1–24. doi: 10.1186/s13059-018-1552-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohno S. Evolution by gene duplication. London: George Allen & Unwin; 1970. [Google Scholar]
- 33.Conant GC, Wolfe KH. Turning a hobby into a job: how duplicated genes find new functions. Nat Rev Genet. 2008;9(12):938–950. doi: 10.1038/nrg2482. [DOI] [PubMed] [Google Scholar]
- 34.Izumiyama T, Minoshima S, Yoshida T, Shimizu N. A novel big protein TPRBK possessing 25 units of TPR motif is essential for the progress of mitosis and cytokinesis. Gene. 2012;511(2):202–217. doi: 10.1016/j.gene.2012.09.061. [DOI] [PubMed] [Google Scholar]
- 35.Hurst LD. The Ka/Ks ratio: diagnosing the form of sequence evolution. Trends Genet TIG. 2002;18(9):486. doi: 10.1016/S0168-9525(02)02722-1. [DOI] [PubMed] [Google Scholar]
- 36.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426(6966):517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 37.Denis V, Cyert MS. Internal Ca2 release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J Cell Biol. 2002;156(1):29–34. doi: 10.1083/jcb.200111004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou X, Batiza AF, Loukin SH, Palmer CP, Kung C, Saimi Y. The transient receptor potential channel on the yeast vacuole is mechanosensitive. Proc Natl Acad Sci. 2003;100(12):7105–7110. doi: 10.1073/pnas.1230540100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Bono M, Tobin DM, Davis MW, Avery L, Bargmann CI. Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature. 2002;419(6910):899–903. doi: 10.1038/nature01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moya A, Huisman L, Ball EE, Hayward DC, Grasso LC, Chua CM, Woo HN, Gattuso J, Foret S, Miller DJ. Whole transcriptome analysis of the coral Acropora millepora reveals complex responses to CO2-driven acidification during the initiation of calcification. Mol Ecol. 2012;21(10):2440–2454. doi: 10.1111/j.1365-294X.2012.05554.x. [DOI] [PubMed] [Google Scholar]
- 41.Bertucci A, Forêt S, Ball EE, Miller DJ. Transcriptomic differences between day and night in Acropora millepora provide new insights into metabolite exchange and light-enhanced calcification in corals. Mol Ecol. 2015;24(17):4489–4504. doi: 10.1111/mec.13328. [DOI] [PubMed] [Google Scholar]
- 42.Oldach MJ, Workentine M, Matz MV, Fan T, Vize PD. Transcriptome dynamics over a lunar month in a broadcast spawning acroporid coral. Mol Ecol. 2017;26(9):2514–2526. doi: 10.1111/mec.14043. [DOI] [PubMed] [Google Scholar]
- 43.Mohamed AR, Cumbo V, Harii S, Shinzato C, Chan CX, Ragan MA, Bourne DG, Willis BL, Ball EE, Satoh N, Miller DJ. The transcriptomic response of the coral Acropora digitifera to a competent Symbiodinium strain: the symbiosome as an arrested early phagosome. Mol Ecol. 2016;25(13):3127–3141. doi: 10.1111/mec.13659. [DOI] [PubMed] [Google Scholar]
- 44.Yoshioka Y, Yamashita H, Suzuki G, Zayasu Y, Tada I, Kanda M, Satoh N, Shoguchi E, Shinzato C. Whole-genome transcriptome analyses of native symbionts reveal host coral genomic novelties for establishing coral-algae symbioses. Genome Biol Evol. 2021;13(1):evaa240. doi: 10.1093/gbe/evaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sebé-Pedrós A, Saudemont B, Chomsky E, Plessier F, Mailhé M, Renno J, Loe-Mie Y, Lifshitz A, Mukamel Z, Schmutz S. Cnidarian cell type diversity and regulation revealed by whole-organism single-cell RNA-Seq. Cell. 2018;173(6):1520–1534. e20. doi: 10.1016/j.cell.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 46.Levy S, Elek A, Grau-Bové X, Menéndez-Bravo S, Iglesias M, Tanay A, Mass T, Sebé-Pedrós A. A stony coral cell atlas illuminates the molecular and cellular basis of coral symbiosis, calcification, and immunity. Cell. 2021;184(11):2973–2987. e18. doi: 10.1016/j.cell.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu M, Zheng X, Fan C, Zheng Y. Lineage dynamics of the endosymbiotic cell type in the soft coral Xenia. Nature. 2020;582(7813):534–538. doi: 10.1038/s41586-020-2385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shultz AJ, Sackton TB. Immune genes are hotspots of shared positive selection across birds and mammals. Elife. 2019;8:e41815. doi: 10.7554/eLife.41815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iguchi A, Shinzato C, Foreˆt S, Miller DJ. Identification of fast-evolving genes in the scleractinian coral Acropora using comparative EST analysis. PLoS ONE. 2011;6(6):e20140. doi: 10.1371/journal.pone.0020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayes ML, Eytan RI, Hellberg ME. High amino acid diversity and positive selection at a putative coral immunity gene (tachylectin-2) BMC Evol Biol. 2010;10(1):1–15. doi: 10.1186/1471-2148-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tautz D, Domazet-Lošo T. The evolutionary origin of orphan genes. Nat Rev Genet. 2011;12(10):692–702. doi: 10.1038/nrg3053. [DOI] [PubMed] [Google Scholar]
- 52.Albà MM, Castresana J. On homology searches by protein Blast and the characterization of the age of genes. BMC Evol Biol. 2007;7(1):1–8. doi: 10.1186/1471-2148-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yasuoka Y, Shinzato C, Satoh N. The mesoderm-forming gene brachyury regulates ectoderm-endoderm demarcation in the coral Acropora digitifera. Curr Biol. 2016;26(21):2885–2892. doi: 10.1016/j.cub.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 54.Cleves PA, Strader ME, Bay LK, Pringle JR, Matz MV. CRISPR/Cas9-mediated genome editing in a reef-building coral. Proc Natl Acad Sci. 2018;115(20):5235–5240. doi: 10.1073/pnas.1722151115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawamura K, Sekida S, Nishitsuji K, Shoguchi E, Hisata K, Fujiwara S, Satoh N. In vitro symbiosis of reef-building coral cells with photosynthetic dinoflagellates. Front Mar Sci. 2021 doi: 10.3389/fmars.2021.706308. [DOI] [Google Scholar]
- 56.Yoshioka Y, Yamashita H, Suzuki G, Shinzato C. Larval transcriptomic responses of a stony coral, Acropora tenuis, during initial contact with the native symbiont, Symbiodinium microadriaticum. Sci Rep. 2022;12(1):1–11. doi: 10.1038/s41598-021-99269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roach MJ, Schmidt SA, Borneman AR. Purge Haplotigs: allelic contig reassignment for third-gen diploid genome assemblies. BMC Bioinf. 2018;19(1):460. doi: 10.1186/s12859-018-2485-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.J. 2011;17(1):10. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 59.Price AL, Jones NC, Pevzner PA. De novo identification of repeat families in large genomes. Bioinformatics. 2005;21(suppl1):i351–i358. doi: 10.1093/bioinformatics/bti1018. [DOI] [PubMed] [Google Scholar]
- 60.Brůna T, Hoff KJ, Lomsadze A, Stanke M, Borodovsky M. BRAKER2: automatic eukaryotic genome annotation with GeneMark-EP and AUGUSTUS supported by a protein database. NAR Genomics Bioinf. 2021;3(1):108. doi: 10.1093/nargab/lqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pertea M, Pertea GM, Antonescu CM, Chang T, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33(3):290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pertea G, Pertea M. GFF utilities: GffRead and GffCompare. F1000Research. 2020 doi: 10.12688/f1000research.23297.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 65.Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST plus : architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jones P, Binns D, Chang H, Fraser M, Li W, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, Pesseat S, Quinn AF, Sangrador-Vegas A, Scheremetjew M, Yong S, Lopez R, Hunter S. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30(9):1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Storer J, Hubley R, Rosen J, Wheeler TJ, Smit AF. The Dfam community resource of transposable element families, sequence models, and genome annotations. Mob DNA. 2021;12(1):1–14. doi: 10.1186/s13100-020-00230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kanehisa M, Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arai W, Taniguchi T, Goto S, Moriya Y, Uehara H, Takemoto K, Ogata H, Takami H: MAPLE 2.3. 0: an improved system for evaluating the functionomes of genomes and metagenomes. Biosci Biotechnol Biochem 2018, 82(9):1515–1517. [DOI] [PubMed]
- 70.Emms DM, Kelly S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 2019;20(1):1–14. doi: 10.1186/s13059-019-1832-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shinzato C, Takeuchi T, Yoshioka Y, Tada I, Kanda M, Broussard C, Iguchi A, Kusakabe M, Marin F, Satoh N: Whole-genome sequencing highlights conservative genomic strategies of a stress-tolerant, long-lived scleractinian coral, Porites australiensis Vaughan, 1918. Genome Biology and Evolution 2021, 10.1093/gbe/evab270. [DOI] [PMC free article] [PubMed]
- 72.Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 2017;14(4):417–419. doi: 10.1038/nmeth.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Katoh K, Standley DM. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol Biol Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suyama M, Torrents D, Bork P: PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res 2006, 34(suppl_2):W609-W612. [DOI] [PMC free article] [PubMed]
- 75.Wang D, Zhang Y, Zhang Z, Zhu J, Yu J: KaKs_Calculator 2.0: a toolkit incorporating gamma-series methods and sliding window strategies. Genomics, proteomics & bioinformatics 2010, 8(1):77–80. [DOI] [PMC free article] [PubMed]
- 76.Villanueva-Canas JL, Laurie S, Alba MM. Improving genome-wide scans of positive selection by using protein isoforms of similar length. Genome Biol Evol. 2013;5(2):457–467. doi: 10.1093/gbe/evt017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Almagro Armenteros JJ, Sønderby CK, Sønderby SK, Nielsen H, Winther O. DeepLoc: prediction of protein subcellular localization using deep learning. Bioinformatics. 2017;33(21):3387–3395. doi: 10.1093/bioinformatics/btx431. [DOI] [PubMed] [Google Scholar]
- 78.R core team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2020.
- 79.Conway JR, Lex A, Gehlenborg N. UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics. 2017. [DOI] [PMC free article] [PubMed]
- 80.Wickham H. Elegant graphics for data analysis. Media. 2009;35(211):10.1007. [Google Scholar]
- 81.Allen M, Poggiali D, Whitaker K, Marshall TR, Kievit RA. Raincloud plots: a multi-platform tool for robust data visualization. Wellcome Open Res. 2019 doi: 10.12688/wellcomeopenres.15191.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Summary of comparison of KEGG metabolic pathways among acroporid genomes. MCR indicates the module completion ratio, calculated by GenoMaple.
Additional file 2: Table S2. IDs of gene families common to Montipora and Astreopora or restricted to Montipora, that are expressed during three life stages (planula larvae, metamorphosed larvae, and recruits).
Additional file 3: Table S3. IDs of gene families common to three genera (Montipora, Acropora and Astreopora) or common to Montipora and Acropora, that are expressed during three life stages (planula larvae, metamorphosed larvae, and recruits).
Additional file 4: Table S4. Summary of gene families significantly (Fisher’s exact test: p < 0.05) expanded in the Montipora genome compared to those of Acropora or Astreopora.
Additional file 5: Table S5. Summary of samples used for RNA-seq.
Additional file 6: Table S6. Summary of publicly available RNA-seq samples that were included for gene prediction in Montipora and Astreopora.
Additional file 7: Table S7. Summary of RNA-seq samples used for comparative transcriptomic analyses.
Additional file 8: Figure S1. Summary of gene predictions for M. cactus, M. efflorescens, and Astreopora myriophthalma.
Additional file 9: Data S1. Result of orthologous clustering in Acroporidae genomes using OrthoFinder.
Additional file 10: Data S2. Summary of retained scaffolds after genome assembly curation for Montipora cactus and M. efflorescens.
Additional file 11: Data S3. Gene models for M. cactus in GTF format.
Additional file 12: Data S4. Gene models for M. efflorescens in GTF format.
Additional file 13: Data S5. Gene models for Astreopora myriophthalma in GTF format.
Data Availability Statement
Raw RNA-sequencing data have been deposited in the DDBJ/EMBL/GenBank databases under accession number DRA011820 (BioProject ID: PRJDB11460). A genome browser for M. cactus, M. efflorescens and Astreopora myriophthalma is available from the Marine Genomics Unit web site (https://marinegenomics.oist.jp/gallery). Sequence IDs of retained scaffolds were prepared as Additional file 10: Data S2. Gene models in GTF format for M. cactus, M. efflorescens and Astreopora myriophthalma are provided as Additional file 11: Data S3, Additional file 12: Data S4, Additional file 13: Data S5. For M. capitata, we downloaded genome assembly and gene models reported from Shumaker et al. [28] (URL: http://cyanophora.rutgers.edu/montipora/) and Helmkampf et al. [27] (Data set https://doi.org/10.15482/USDA.ADC/1503958).