Summary:
A role for B cells in autoimmune diseases is now clearly established both in mouse models and humans by successful treatment of multiple sclerosis and rheumatoid arthritis with anti-CD20 monoclonal antibodies that eliminate B cells. However, the underlying mechanisms by which B cells promote the development of autoimmune diseases remain poorly understood. Here we review evidence that patients with autoimmune disease suffer from defects in early B cell tolerance checkpoints and therefore fail to counter-select developing autoreactive B cells. These B cell tolerance defects are primary to autoimmune diseases and may result from altered B cell receptor signaling and dysregulated T cell/regulatory T cell compartment. As a consequence, large numbers of autoreactive naïve B cells accumulate in the blood of patients with autoimmune diseases and may promote autoimmunity through the presentation of self-antigen to T cells. In addition, new evidence suggests that this reservoir of autoreactive naïve B cells contains clones that may develop into CD27-CD21-/lo B cells associated with increased disease severity and plasma cells secreting potentially pathogenic autoantibodies after the acquisition of somatic hypermutations that improve affinity for self-antigens.
Keywords: B cell development, immune tolerance checkpoint, autoimmune disease, autoantibodies
Introduction
Millions of individuals worldwide are affected by autoimmune disorders but their etiologies remain poorly understood. Defects in B cell tolerance are associated with most autoimmune diseases and are illustrated by the production of autoantibodies that target self-antigens. Some of these autoantibodies are pathogenic because they interfere with the function of the molecules they recognize, such as the acetylcholine receptor (AChR)/ muscle-specific tyrosine kinase (MuSK) in myasthenia gravis (MG) and aquaporin-4 water channel (AQP4) in neuromyelitis optica spectrum disease (NMOSD) 1,2. Others target nucleic acids or their associated proteins, allowing the formation of immune complexes that deposit in various organs of patients with systemic lupus erythematosus (SLE) and induce organ damage 3. These immune complexes also allow the activation of myeloid cells expressing both FcRs binding autoantibodies and Toll-like receptors (TLRs), such as TLR7, TLR8, and TLR9, that recognize autoantibody-bound nucleic acids and lead to cell activation and foster inflammation 4. However, the relevance of the various autoantibodies in the pathophysiology of type 1 diabetes (T1D) is unclear and their identification in patients with multiple sclerosis (MS) is elusive. While B cells have been shown to be essential for the development of diabetes in the NOD mouse model, additional investigations revealed that B cells promote diabetes by recognizing self-antigens with their autoreactive antibodies and presenting self-antigens via MHC class II molecules to T cells 5–12. Hence, these data suggest that self-antigen presentation by autoreactive B cells that escaped tolerance may initiate the development of autoimmune diseases. The identification of impaired B cell tolerance checkpoints in patients with autoimmune diseases and the recent identification of pathogenic anti-AQP4 clones originating from unmutated autoreactive naïve B cells in patients with NMOSD agree with this scenario and will be presented and discussed in this review.
Central and peripheral B cell tolerance checkpoints shape the human naïve B cell repertoire
Self-tolerance is achieved by silencing self-reactive lymphocytes that are generated during either B cell development in the bone marrow or B cell activation in the periphery 13. Engineered models using transgenic and knock-in mice have revealed that developing B cells expressing self-reactive receptors can be silenced by one of three mechanisms: 1. clonal deletion; 2. clonal unresponsiveness to antigen or anergy; 3. “receptor editing” or antigen receptor gene replacement by continued V(D)J recombination catalyzed by the recombinase-activating genes (RAGs) 13–16. However, the frequency of self-reactive antibodies that arise during unmanipulated B cell development could neither be assessed using these mice, nor could it be determined when such antibodies were actually removed from the repertoire under physiologic circumstances.
To determine the proportion of autoreactive B cells that were removed from the nascent repertoire and how central B cell tolerance was established in humans, we assessed the frequencies of autoreactive clones in sequential subsets of B cells during their early B cell development in the bone marrow and the blood of healthy donors 17. This approach was dependent on a method that allows Ig gene amplification, cloning, and expression in vitro of recombinant antibodies initially produced by single human B cells 17. By testing the reactivity of recombinant antibodies against double-stranded DNA, insulin, and LPS in ELISAs or immunofluorescence on slide-coated HEP-2 cells, we previously established that a first step for immature B cell selection removes the vast majority of developing B cells that express polyreactive and anti-nuclear antibodies in bone marrow and is referred to as the central B cell tolerance checkpoint 17,18. In addition, using a second ELISA test in which plates are coated with HEp-2 cell lysates, we found that a peripheral B cell tolerance checkpoint further eliminates autoreactive new emigrant/transitional B cells that escaped central tolerance before they entered the mature naïve B cell compartment 17. This bimodal removal of autoreactive clones from the initial B cell repertoire generated by random V(D)J recombination may result from B cell exposure to self-antigens first in the bone marrow where B cell production takes place in adults and then in the periphery when immature B cells migrate out of the bone marrow and encounter a new set of self-antigens. This model is supported by our recent observations showing that autoimmune regulator (AIRE)-deficient patients display specific defects in the peripheral B cell tolerance checkpoint caused by a failure of AIRE-mediated T cell/regulatory T cell (Treg) selection that normally prevents the expansion of autoreactive naïve B cells recognizing peripheral self-antigens 19. The mechanisms by which T cell/Tregs may control the peripheral selection of B cells continuously produced by the bone marrow are not well characterized at this point, but specific defects in this checkpoint have been observed in FOXP3-deficient, immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) patients who lack functional Tregs 20. In addition, decreased Treg frequencies and/or impaired Treg suppressive function in patients with ADA-, CD40L-, DOCK8-, MHC class II, and WASp-deficiency as well as the absence of T cells in either CD3D- or CD3E-deficient patients also resulted in the specific accumulation of autoreactive clones in the mature naïve B cell compartments of these patients, further suggesting the B cell extrinsic regulation of this peripheral checkpoint 19,21–24.
In contrast, central B cell tolerance is not affected by decreased Treg frequencies or suppressive function and appears instead to be regulated by intrinsic B cell pathways 21,25–28. Indeed, mutations in genes that encode molecules belonging to the BCR and TLR pathways such as Bruton’s tyrosine kinase (BTK), Adenosine deaminase (ADA), Wiskott-Aldrich syndrome protein (WASP), MYD88, IRAK-4, Transmembrane Activator and CAML Interactor (TACI), and Activation induced cytidine deaminase (AID) affected central B cell tolerance, thereby revealing a B cell intrinsic regulation of this checkpoint 21,24,26–29. These studies suggest that, in addition to BCRs, TLRs, especially those binding to nucleic acids and expressed in B cells such as TLR7 and TLR9, contribute to the induction of B cell tolerance as also demonstrated in mice 30. This may be especially important for membrane-bound self-antigens that may be co-expressed with nucleic acids at the surface of apoptotic cells or cellular debris and therefore co-engage BCR and TLRs in developing B cells and inducing tolerance mechanisms such as receptor editing and eventually lead to the expression of AID, which results in the deletion of autoreactive clones 30–33. Our data, consistent with this hypothesis, showed that p53 inhibition increased the proportion of AID-expressing B cells in the bone marrow of humanized mice and abrogated central B cell tolerance, likely by rescuing developing autoreactive B cells from apoptosis 34. These collective analyses of patients with primary immunodeficiencies revealed that the regulation of central B cell tolerance in humans requires proper BCR and TLR signaling and function, whereas the peripheral B cell tolerance checkpoint depends on T cells/Tregs to prevent the accumulation of autoreactive naïve B cells.
Defective early B cell tolerance checkpoints in patients with autoimmune diseases
Autoantibody production is a characteristic of most autoimmune diseases including SLE, T1D, MG, NMOSD, and rheumatoid arthritis (RA) 35,36. Autoantibodies appear in the serum many years before the onset of the clinical disease, which suggests an early break in B cell tolerance 37,38. The underlying mechanisms that account for autoreactive B cell and autoantibody production in patients with autoimmune diseases remain elusive. We sought to determine the stage of B cell development at which B cell tolerance was broken in patients with autoimmune diseases. Two non-exclusive scenarios could be considered: first, B cell tolerance may not be established properly during early B cell development, resulting in the accumulation of autoreactive naïve B cell clones in the periphery; alternatively, early B cell tolerance checkpoints may be functional and B cells present in a normal repertoire may receive signals from T cells to develop into clones secreting high affinity self-reactive antibodies.
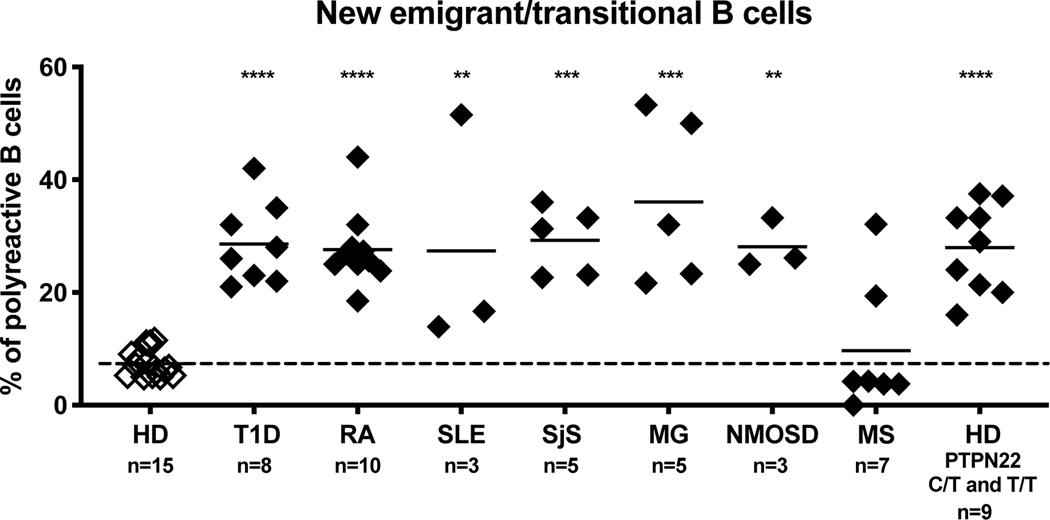
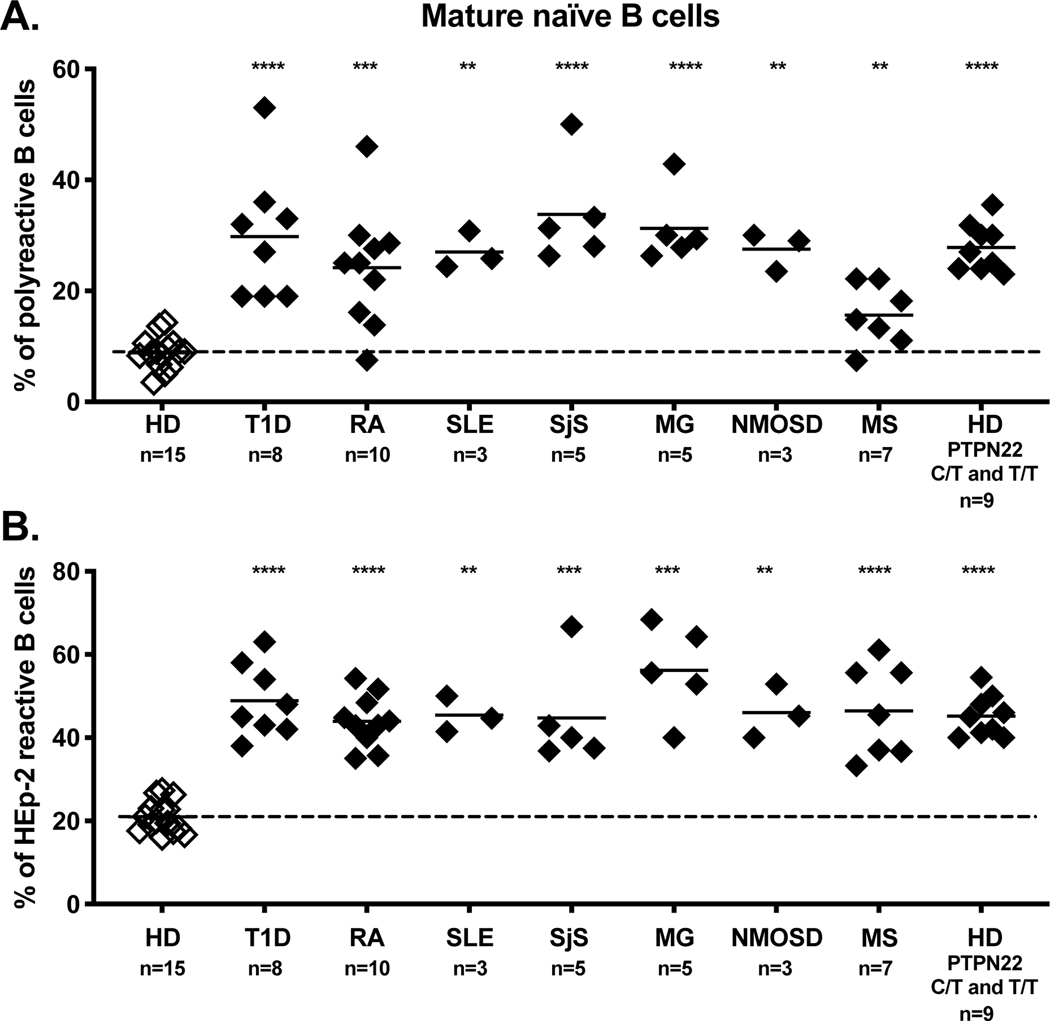
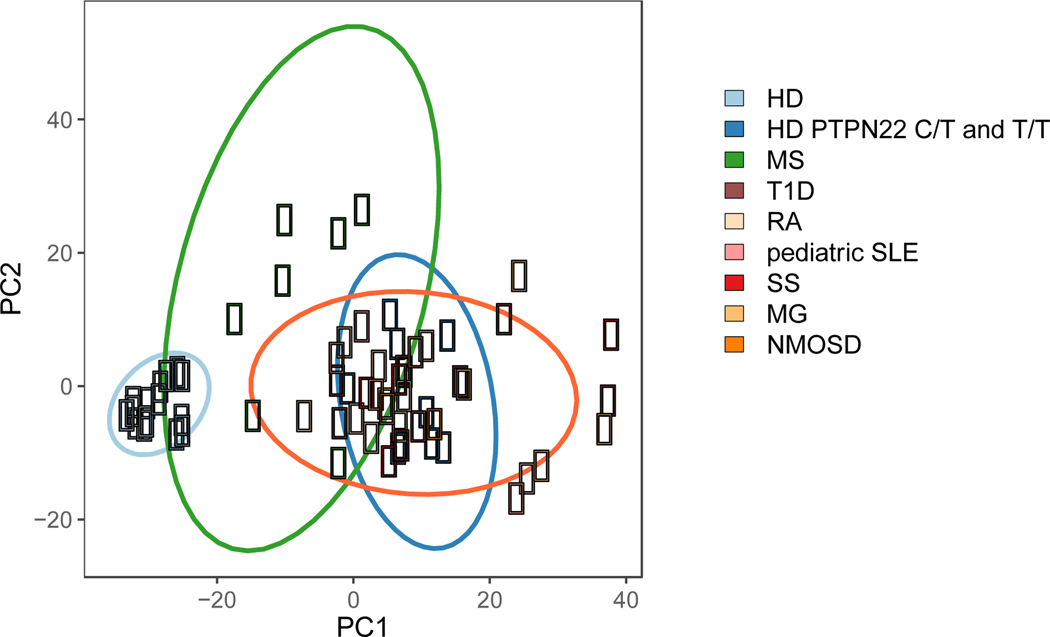
To determine whether early B cell tolerance checkpoints were functional in patients with autoimmune diseases, we assessed the frequencies of autoreactive clones in new emigrant and mature naïve B cells isolated from the blood of untreated patients with SLE, RA, T1D, Sjögren’s syndrome (SjS), MS, MG, and NMOSD. Despite the broad age range and duration of disease, we found that three out of three pediatric SLE patients, ten out of ten RA patients, six out of six T1D patients, five out of five SjS patients, three out of three MG patients, and three out of three NMOSD patients displayed elevated frequencies of new emigrant/transitional B cells expressing autoreactive/polyreactive antibodies when compared to healthy donors 39–45 (Fig. 1). These collective findings demonstrate that central B cell tolerance is not functional in these patients. In addition, these patients also displayed elevated frequencies of autoreactive and polyreactive mature naïve B cells that accumulated in their blood, suggesting that their peripheral B cell tolerance checkpoint may also not be functional 39–45 (Fig. 2). Indeed, we have previously reported that a functional peripheral B cell tolerance checkpoint can decrease the frequencies of autoreactive B cells in the mature naïve B cell compartment of asymptomatic subjects who display defective central B cell tolerance induced by heterozygous TNFRSF13B gene mutation that encodes TACI 29. A recent study utilizing an alternative method to assess the frequency of autoreactive clones confirmed the impairment of early B cell tolerance checkpoints in SLE patients 46. In contrast, the establishment of B cell tolerance in MS patients differs from other patients with autoimmune diseases in that five out of seven patients with MS displayed normal central B cell tolerance 45 (Fig. 1). However, seven out of seven MS patients displayed an impaired peripheral B cell tolerance checkpoint, which resulted in the peripheral accumulation of autoreactive and polyreactive mature naïve B cells 45 (Fig. 2). Principal component analysis of the collective checkpoint assay data demonstrates a conspicuously distinct grouping between healthy donors as a first cluster and patients with autoimmune disease and healthy donors carrying the 1858T PTPN22 risk allele associated with many autoimmune diseases as another big cluster (Fig. 3) 47. Interestingly, MS patients cluster into a third group distinct from both healthy donors and other autoimmune patients, demonstrating the unique B cell tolerance defect in this disease (Fig. 3).
Figure 1. Central B cell tolerance is compromised in patients with autoimmune disease and healthy donors carrying the 1858T PTPN22 polymorphism.
Frequencies of polyreactive new emigrant/transitional B cells in patients with type 1 diabetes (T1D), rheumatoid arthritis (RA), pediatric systemic lupus erythematosus (SLE), Sjögren’s syndrome (SjS), myasthenia gravis (MG), neuromyelitis optica spectrum disease (NMOSD), multiple sclerosis (MS) and healthy donors (HD) with either heterozygous (C/T) or homozygous (T/T) PTPN22 polymorphism were compared to the frequencies derived from a cohort of healthy donors who did not carry the 1858T PTPN22 risk allele. Proportions of polyreactive antibodies expressed by new emigrant/transitional B cells were plotted for each subject group along with the mean and standard deviation for each subject group. Statistical differences are shown when significant (****, p < 0.0001; ***, p < or = to 0.001; **, p < or = to 0.01).
Figure 2. The peripheral B cell tolerance checkpoint is compromised in patients with autoimmune disease and healthy donors carrying the 1858T PTPN22 polymorphism.
Frequencies of (A) polyreactive and (B) autoreactive (HEp-2 reactive) mature naïve B cells in seven distinct autoimmune diseases and healthy donors with either heterozygous (C/T) or homozygous (T/T) PTPN22 polymorphism were compared to the frequencies derived from a cohort of healthy donors who did not carry the 1858T PTPN22 risk allele. Proportions of (A) polyreactive antibodies or (B) autoreactive antibodies reactive toward a human epithelial type 2 (HEp-2) cell lysate expressed by mature naïve B cells were plotted for each subject group along with the mean and standard deviation for each subject group. Statistical differences are shown when significant (****, p < 0.0001; ***, p < or = to 0.001; **, p < or = to 0.01).
Figure 3. Principal component analysis of the frequency of polyreactive and autoreactive B cells in seven distinct autoimmune diseases, healthy donors carrying 1858T PTPN22 risk allele(s) and non-carrier healthy donors.
Proportions of polyreactive antibodies expressed by new emigrant/transitional B cells and proportions of both polyreactive and autoreactive antibodies expressed by mature naïve B cells were plotted for each subject group. Healthy donor (HD)-derived B cells populations (light blue) cluster together, reflecting the low frequencies of polyreactive and autoreactive clones correlating with functional early B cell tolerance checkpoints. Type 1 diabetes (T1D), rheumatoid arthritis (RA), pediatric systemic lupus erythematosus (SLE), Sjögren’s syndrome (SjS), myasthenia gravis (MG), and neuromyelitis optica spectrum disease (NMOSD) segregate away from healthy donors, illustrating the accumulation of polyreactive and autoreactive B cells due to impaired central and peripheral B cell tolerance checkpoints. Healthy donors with either a heterozygous (C/T) or homozygous (T/T) PTPN22 polymorphism (dark blue), who also display defective early B cell tolerance checkpoints, cluster with the autoimmune disease cohort. B cells from MS patients (green) display a heterogeneous pattern. While two patients with MS cluster with the autoimmune cohort and displayed impaired central and peripheral B cell tolerance checkpoints, most MS patients cluster as an independent group characterized by specific defects of the peripheral B cell tolerance checkpoint.
Some of these autoreactive naïve B cells and mostly polyreactive clones expressed unmutated antibodies that could recognize the RA-specific antigens IgG and cyclic citrullinated peptides, extracts of human brain white matter affected in MS and SjS-targeted Ro52/SS-A self-antigen with low affinity 40,41,45. However, somatic hypermutation during autoimmune B cell responses may increase their affinity for self-antigens (see below). We conclude that patients with autoimmune diseases suffer from defective early B cell tolerance checkpoints which increases the proportion of autoreactive naïve B cells in their blood and which may favor the development of autoimmunity by increasing the incidence of autoreactive B cells that can present self-antigens to T cells.
Origins of the impaired early B cell tolerance checkpoints in patients with autoimmune diseases
The analysis of patients with primary immunodeficiencies suggests that the impaired central B cell tolerance that characterizes most patients with autoimmune diseases—except MS—may result from defective/hyporesponsive BCR/TLR pathways. Hence, risk alleles associated with autoimmune diseases that affect BCR/TLR function may alter the establishment of B cell tolerance. Genome wide association studies (GWAS) have identified the 1858T polymorphism in the PTPN22 gene (PTPN22 T) to be one of the strongest genetic risk factors after the MHC, and is associated with the development of many autoimmune diseases including RA, T1D, and SLE, but not MS 47. This polymorphism encodes PTPN22 phosphatases with a tryptophan at position 620 (620W PTPN22) instead of an arginine in the common 620R PTPN22 variant. This missense mutation abrogates PTPN22 interaction with CSK tyrosine kinase and results in decreased T cell receptor (TCR) and B cell receptor (BCR) signaling 48–51.
In agreement with our observations in primary immunodeficiencies, in which defective BCR/TLR function induces a defective central B cell tolerance, we reported that the presence of a PTPN22 T allele is sufficient to induce central and peripheral B cell selection defects in healthy donors similar to those in patients with autoimmune diseases (Fig. 1, 2 and 3) 28. Hence, a single PTPN22 T risk allele has a dominant effect on impairing autoreactive B cell counterselection before onset of autoimmunity. In agreement with this hypothesis, we demonstrated that a lentiviral-driven expression of 620W PTPN22 variant in human B cells that developed in humanized mice was sufficient to abrogate central B cell tolerance, whereas the expression of the common 620R PTPN22 had no impact on the functionality of this tolerance checkpoint 52. In addition, the substitution of the conserved arginine by a tryptophan at the equivalent position, 619, in murine Ptpn22 (called PEP) also promotes autoimmunity in knock-in mice 53. Furthermore, the same study also showed that the restricted expression of 619W PEP to only B cells was sufficient to impair immune tolerance and to produce anti-dsDNA autoantibodies 53.
Collectively, these observations demonstrate an essential role for PTPN22 and its 620W variant in the development of autoimmunity by regulating autoreactive B cell selection, differentiation, and activation, which may explain why the PTPN22 T risk allele confers a high risk of developing many autoimmune diseases with the exception of MS 47. GWAS studies analyzing polymorphisms associated with MS identified gene variants that may affect more specifically APCs, such as monocytes, and T cells—notably Tregs—in addition to B cells 54,55. These findings are in agreement with the specific impairment in the peripheral B cell tolerance checkpoint in MS that resemble those associated with defective Treg function. Indeed, Tregs from MS patients display defective suppressive function and abnormally produce IFNγ 56,57. Many other risk alleles identified by GWAS and associated with autoimmune diseases such as BLK and BANK1 also belong to the BCR signaling pathway and may also alter the establishment of B cell tolerance 47. However, the analysis in our labs of more than 110 subjects who did not carry the PTPN22 T allele failed to identify a single individual with impaired central B cell tolerance, which suggests that other common variants associated with autoimmune diseases identified by GWAS and with a frequency greater than 5% are unlikely to interfere with the establishment of central B cell tolerance impaired in most patients with autoimmune diseases. What could then interfere with the removal of developing autoreactive B cells? We do not exclude the involvement of B cell extrinsic factor that may alter BCR/TLR function in patients with autoimmune diseases, but we previously reported that suppressing inflammation with either methotrexate or anti-TNFα reagents did not correct early B cell tolerance checkpoints in RA patients 42. In addition, the engraftment of humanized mice with hematopoietic stem cells (HSCs) isolated from an RA patient or a T1D patients led to the production of human B cells containing a high frequency of autoreactive clones that were similar to those in the patients 58. These findings point toward a genetic or epigenetic origin of early B cell tolerance checkpoint defects. In line with this hypothesis, B cell depletion therapy mediated by rituximab that eliminates B cells also failed to restore the functionality of early B cell tolerance checkpoints in early onset T1D patients 59. As a consequence, newly generated B cells after rituximab treatment contain many autoreactive clones, which may explain the relapse that occurs in many autoimmune patients after B cell depletion therapy.
In addition to altered BCR signaling induced by the PTPN22 T allele, we recently reported defects in TLR9 function in B cells from untreated SLE patients that may also account for the impaired central B cell tolerance in these patients 60,61. TLR9 exerts tolerogenic function as evidenced by lupus-prone Tlr9 KO mice which display exacerbated autoimmune disease 62. While TLR9 ligands activate B cells, co-crosslinking of BCR and TLR9 that mimics autoreactive B cell stimulation by dsDNA-containing self-antigen induces cell death by apoptosis after transient B cell expansion and therefore prevents anti-dsDNA autoantibody production 63,64. These data demonstrate that TLR9 plays an important role in limiting the peripheral activation of autoreactive B cells. Decreased TLR9 function in SLE B cells is associated with the downregulation of CD19/CD21 expression on the surface of these cells, but the molecular mechanisms associated with altered CD19/CD21 expression or TLR9 function remain unknown at this point. Decreased CD19 expression was also reported in mice humanized with T1D-derived and RA-derived HSCs and associated with the production of autoreactive B cells 58,65. Since CD19 is important for both BCR and TLR signaling and function, decreased CD19 expression may therefore affect the removal of developing autoreactive B cells 66,67. It also remains to be determined if TLR9 function may also be affected in B cells from patients with other autoimmune diseases in which tolerance to self-antigens interacts with dsDNA such as topoisomerase I and centromere proteins in systemic sclerosis (SSc). Altogether, our data suggest that patients with autoimmune diseases may often display altered BCR and TLR9 function in B cells associated with an impaired central B cell tolerance that may promote the emergence of autoimmune manifestations.
Tolerance checkpoint defects alter the naïve B cell repertoire
We showed that autoreactive naïve B cells escape counterselection by defective early B cell tolerance checkpoints in patients with autoimmune diseases. As a consequence, the naïve BCR repertoire includes clones that would otherwise have been eliminated. Thus, deviations from the repertoire established in healthy controls may be conspicuous. Earlier studies of single B cells isolated from the blood of RA and SLE patients, in which tolerance checkpoints are impaired, demonstrated that characteristics of the Ig kappa (Igκ) repertoire from new emigrant/transitional B cells that recently emigrated from the bone marrow, and to a lesser extent mature naïve B cells, differed from those in healthy control counterparts 39,40,68.
Light chain Igκ secondary recombination that may mediate receptor editing, which is the main mechanism of central B cell tolerance, occurs through secondary recombination in which incoming Vκ genes delete pre-existing VκJκ genes by recombining with downstream Jκs 16,69. Igκ secondary recombination therefore results in increased utilization of upstream Vκs and downstream Jκs. We found that about a third of RA patients (RA Group I) displayed new emigrant/transitional B cells that express BCRs with Igκ gene segments characterized by decreased secondary recombination events, whereas the rest of RA patients (RA Group II) did not show evidence of dysregulated secondary recombination 40,68. Indeed, new emigrant/transitional B cells from Group I RA patients expressed an Igκ repertoire with a dearth in upstream Vκ and downstream Jκ3–4-5 genes combined to increased Jκ1 usage and suggesting a lack of secondary recombination events in these immature B cells that exited the bone marrow 40,68. This pattern of Ig light chain antibody rearrangements, potentially characteristic of a defective regulation of secondary recombination, was also reported in B cells from SLE patients 70–72. In addition, inefficient receptor editing in T1D is also evidenced in NOD mice and patients 73–75. However, our recent studies revealed that the altered Igκ repertoire in B cells from Group I RA patients may originate from decreased or impaired ataxia-telangiectasia mutated (ATM) expression and function 68. Indeed, both AT patients and NSG humanized mice injected with an ATM inhibitor displayed an Igκ repertoire with a lack of breadth of Vκ-Jκ rearrangements similar to that in group I RA patients 68. Since ATM controls the repair of RAG-mediated DNA double-strand breaks and regulates cell-cycle checkpoints, novel RAG-mediated DNA lesions in immature B cells undergoing Igκ secondary recombination may fail to be properly repaired these when the ATM function is defective, leading to genomic instability and cell loss 76. The increased frequency of immature B cells undergoing apoptosis in the bone marrow of NSG humanized mice injected with ATMi supports this scenario 68. It remains to be determined if the altered B cell repertoire induced by decreased ATM function in some RA patients may contribute to disease pathogenesis or if it is solely a byproduct of improper ATM expression in developing B cells.
The restricted size of Ig gene sequencing of antibodies cloned from single B cells analyzed during B cell tolerance checkpoint studies, which can include up to several hundred unique BCR sequences, limited the identification of wider repertoire abnormalities between individuals with or without functioning B cell tolerance, especially for the heavy chains encoded by the recombination of numerous VH, D and JH gene segments. In contrast, adaptive immune receptor repertoire sequencing (AIRR-seq) generates considerably larger BCR libraries, often reaching over 1,000,000 unique sequences. Since the circulating peripheral repertoire in humans includes up to 1011 B cells 77, AIRR-seq provides the depth necessary to adequately depict such large populations and therefore affords a comprehensive evaluation of BCR repertoire properties. An increasing compilation of AIRR-seq studies including both total and sorted B cell populations from patients with autoimmunity revealed altered BCR repertoires in MS 78, SLE 79, and celiac disease 80.
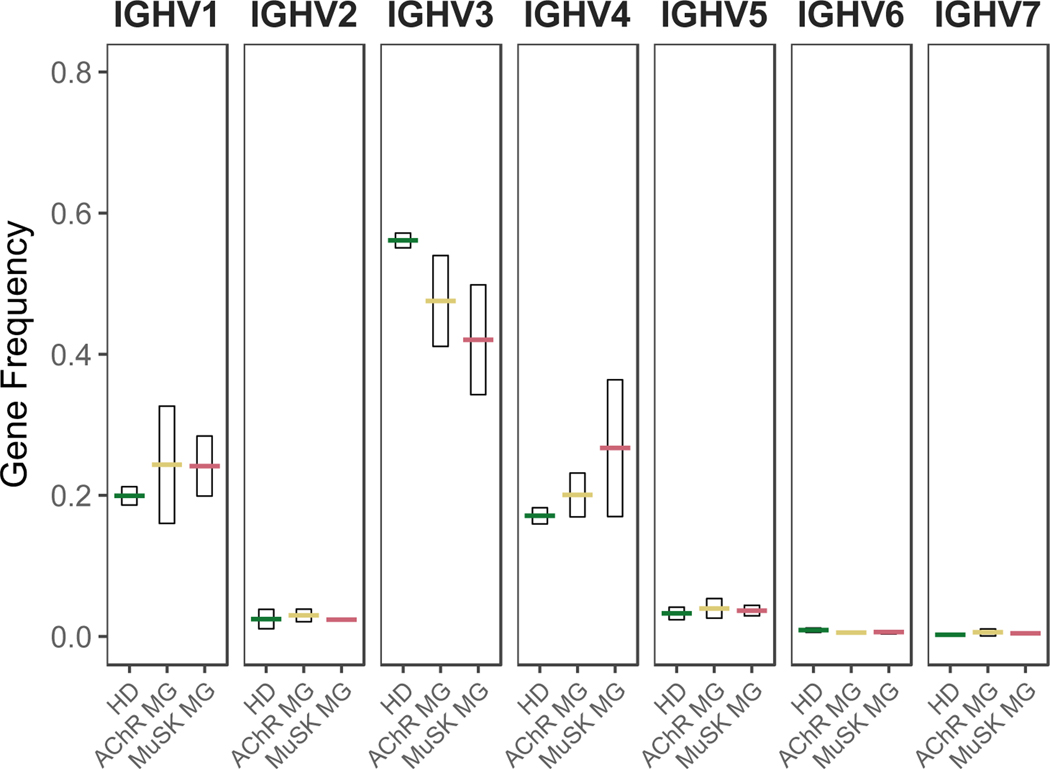
We more specifically applied AIRR-seq to analyze the BCR repertoire of naïve B cells from AChR and MuSK MG patients that are enriched in autoreactive clones due to impaired central and peripheral B cell tolerance checkpoints in MG and compared them to those of healthy donors (HD) to potentially identify naïve BCR repertoire features associated with autoimmunity 81. Our initial study focused on determining whether the naïve BCR repertoires in MG included skewed immunoglobulin gene usage and physiochemical properties 81. In agreement with a previous study, the naïve B cell repertoires obtained from multiple healthy control subjects displayed highly consistent IGH variable (IGHV) gene usage with limited variability 82. In contrast, naïve BCR repertoires from MG patients were highly diverse among individuals and may reflect dysregulation B cell development in these patients 81. Biased usage of IGHV gene segments were observed in naïve B cells from MG patients as illustrated by decreased usage of IGHV3 family gene segments and increased in IGHV1 and IGHV4 family gene usage 81 (Fig. 4). Other studies offer similar findings regarding differential variable region gene segment expression. Systemic sclerosis (SSc), or scleroderma, is a rare chronic autoimmune disease that leads to tissue fibrosis and vasculopathy 83. Antinuclear antibodies (ANA) are detectable in 90–95% of patients, indicative of immune, and particularly B cell, abnormalities. An AIRR-seq investigation revealed that differential expression of IGHV genes in the repertoires from SSc cohorts relative to a healthy cohort are present in the IgD/IgM compartment 84. These collective data therefore show that defective early B cell tolerance checkpoints in patients with autoimmune diseases alter the generation of the BCR repertoires of naïve B cells associated with inadequate counter-selection of developing autoreactive clones.
Figure 4. Variable region family usage is skewed in the naïve BCR repertoire of patients with myasthenia gravis who suffer from impaired early B cell tolerance checkpoints.
Antibody heavy chain variable region family usage for the naïve (IgM) B cell compartment. The analysis was performed with 114,296 unique sequences collected from four HDs, three AChR autoantibody positive MG patients (AChR), and three MuSK autoantibody positive MG patients (MuSK). Usage is shown as a frequency of the total unique IGHV sequences (y-axis) for each subject (symbols). Horizontal bars indicate the mean abundance over all subjects of a given status and vertical shading indicates +/− 1 SD about the mean. Data previously reported 81.
Impaired early B cell tolerance checkpoints likely favor the production of circulating atypical CD19hiCD27-CD21-/l0 B cells in some autoimmune diseases
Several groups have now reported that CD19hiCD27-CD21-/lo B cells that are scarce in the blood of healthy donors are enriched in various patients with autoimmune diseases including SLE, RA, and SjS 85–88. This circulating atypical CD19hiCD27-CD21-/lo B cell subset is also expanded in the blood of patients with chronic viral infection such as HIV and HCV and in patients with malaria, suggesting that ongoing immune responses driven by some self-antigens or chronic exposure to pathogens may favor the production of these B cells 89–91. Indeed, we found that CD19hiCD27-CD21-/lo B cells from RA and SjS patients often express autoreactive and polyreactive antibodies, which reveals that they are the product of autoimmune responses 85–87. The precursors of these B cells in some autoimmune diseases are currently unknown, but experiments in which the somatic hypermutations were reverted to germline counterparts in recombinant antibodies expressed by expanded CD19hiCD27-CD21-/lo B cells in SjS suggest that their unmutated precursors were already autoreactive 87,92. Hence, the accumulation of CD19hiCD27-CD21-/lo B cells in some autoimmune diseases may result from the activation of autoreactive naïve B cells that escaped the impaired early B cell tolerance in RA, SjS, and SLE. Of note, we did not observe an increase in CD19hiCD27-CD21-/lo B cells in MS or T1D patients, suggesting that self-antigens targeted in these autoimmune diseases may not trigger the expansion of these B cells or that their expansion is not sufficient to be detected systemically in patient’s blood 45 (E. Meffre, personal communication). CD19hiCD27-CD21-/lo B cells are characterized by the expression of a specific set of molecules compared to other B cell subsets and which include CD11c and T-bet induced by IFNγ 85,86,93,94. Hence, the production of these B cells may occur in an inflammatory environment.
In agreement with this hypothesis, the mechanisms responsible for the generation of these B cells involve dysregulated IL-21/IL-4/ IFNγ production during B cell responses in germinal centers 93–96. A recent study from our group shows that CD19hiCD27-CD21-/lo B cells are specifically enriched in RA patients with defective ATM activation and correlate with a high prevalence of erosive joint disease, likely through their increased expression of pro-osteoclastic RANKL and IL-6 cytokines upon activation 68. Thus, elevated frequencies of circulating CD19hiCD27-CD21-/lo B cells in newly diagnosed RA patients may represent a valuable biomarker to identify patients with a high risk for developing erosive joint damage. In line with this observation, increase in circulating CD19hiCD27-CD21-/lo B cells in SLE patients is also associated with more severe autoimmune disease 93. We conclude that the impaired selection of developing autoreactive B cells in patients with autoimmune diseases likely favors the production of circulating CD19hiCD27-CD21-/lo B cells that express autoreactive antibodies, produce pro-inflammatory cytokines, and thereby foster severe disease manifestation.
B cell tolerance checkpoint defects promote autoantibody production
While early B cell tolerance checkpoint defects in autoimmune diseases result in the accumulation of autoreactive naïve B cells with an altered BCR repertoire in patient’s blood, it remained to be determined whether these naïve autoreactive clones may favor the production of autoantibodies in their sera. A direct method to test this hypothesis consists of measuring the affinity of recombinant monoclonal antibodies (mAbs) cloned from naïve B cells for self-antigens targeted by autoimmune responses. We recently evaluated by Surface Plasmon Resonance the affinity of mAbs expressed by mature naïve B cells from AIRE-deficient patients who display autoimmune manifestations including T1D and present a whole series of anti-cytokine autoantibodies in their serum, which target various IFNs and IL-17 family members 19,97–99. We found that the impaired peripheral B cell tolerance checkpoint induced by AIRE deficiency results in the production of autoreactive mature naïve B cells that express antibodies devoid of somatic hypermutations that recognized IFNs, IL-17A, IL-17F and insulin with micromolar affinity and that were initially identified as polyreactive clones using our reference assay 17,19. Thus, anti-cytokine and anti-insulin clones are already present in the naïve B cell compartments of AIRE-deficient patients and their affinity for self is further increased by somatic hypermutation 100. It remains to be determined if patients with classical autoimmune diseases such as AIRE-deficient patients harbor autoreactive clones in their naïve B cell compartments with measurable affinity for self-antigens recognized by serum autoantibodies.
A second indirect approach to assess if impaired early B cell tolerance checkpoints contribute to autoantibody production consisted of artificially removing somatic hypermutation from mutated recombinant autoreactive clones with known self-antigen specificity and then testing the specificity of the unmutated revertants that may correspond to the naïve germline-encoded precursors activated by the autoimmune response. This process involves the replacement of somatically mutated bases that result in an amino acid replacement back to the bases present in the corresponding germline sequence of Ig gene segment 44,101. Because it is impossible to determine whether somatic mutations have been introduced in regions containing non-template N nucleotides that do not match germline Ig gene segments at V-D and D-J junctions, reversion accuracy is unclear. For instance, unmutated revertants from anti-cytokine autoantibodies in AIRE-deficient patients did not bind to their respective self-antigen, suggesting that these specificities emerged from unreactive clones through acquisition of somatic hypermutations, whereas we showed instead that anti-cytokine reactivity could be easily detected in the unmutated naïve B cell compartment of these patients as presented above 19,100. In pemphigus vulgaris, somatically hypermutated pathogenic autoantibodies recognize desmoglein-3, whereas the vast majority of unmutated revertants did not bind this self-antigen 102. Both of these studies did not include reversion of the IgH CDR3 region which can include identifiable mutations in the D and JH gene segments. In contrast, the germline-versions of human recombinant mAbs that recognize peptidylarginine deiminase (PAD) retained their anti-PAD binding although it was decreased compared to the mutated counterparts 103,104. In addition, unmutated revertants of mAbs targeting infection-derived influenza antigens were also able to recognize the targeted antigen, suggesting that reactive clones from immune responses may emerge from the activation of clones with relative initial affinity for the antigen improved by somatic hypermutations 105–107.
To establish a potential link between early B cell tolerance checkpoint defects and autoantibody production, we examined the reactivity of a set of unmutated revertants corresponding to pathogenic anti-AQP4 autoantibodies cloned from NMOSD patient cerebrospinal fluid (CSF) plasmablasts and plasma cells 108,109. We increased reversion accuracy by only selecting autoantibody sequences that displayed short (less than ten) non template N-nucleotide additions in heavy chain CDR3s 44. In addition we reverted any mutations that were identified in the D and JH gene segments. The affinity of the mature anti-AQP4 mAbs ranged from modest to strong (Kd 15.2–559 nM), but none of the germline revertants bound to AQP4, suggesting that somatic hypermutation is required for the generation of NMOSD-specific anti-AQP4 autoantibodies 44. However, several germline autoantibody revertants were found to be both polyreactive and autoreactive, suggesting that mutated anti-AQP4 autoantibodies may originate from the pool of autoreactive mature naïve B cells that escape deletion at B cell counterslection steps in these patients. Similarly, a fraction of mutated clones expressing autoantibodies directed towards the extractable nuclear antigens (ENA) Ro52 and La in SLE patients were found to be polyreactive or self-reactive when their sequence was reverted to germline 110. Thus, impaired B cell tolerance checkpoints in patients with autoimmune diseases result in the production of autoreactive clones that may be activated by T cells and increase their specificity for self-antigens by acquiring somatic mutations, which may lead to subsequent disease manifestation when pathogenic.
Concluding remarks
We have presented data that support the important role of impaired central and peripheral B cell tolerance checkpoints in autoimmune diseases by producing autoreactive naïve B cells with altered BCR repertoire that may present self-antigens to T cells, become activated to produce autoreactive and potentially pathogenic autoantibodies, as well as pro-inflammatory cytokines favoring disease severity. While these autoreactive B cell selection defects pre-exist the onset of autoimmunity, environmental factors play an important role in triggering the events that lead to a break in tolerance and autoimmune diseases. For instance, viral infection by EBV has been associated with many autoimmune diseases including SLE, SjS, and RA and coxsackievirus B with T1D 111–117. It is unknown if the altered naïve B cell repertoire in patients with autoimmune diseases may affect their anti-viral immune response. While some studies suggest that SLE patients may be more susceptible to infections, their anti-influenza responses appeared as good if not even better than those in healthy donors, suggesting that impaired early B cell tolerance checkpoints may actually favor anti-viral responses 118. Similarly, anti-HIV responses may benefit from an increase in autoreactive naïve B cells since polyreactivity increases the affinity of anti-HIV antibodies by heteroligation 119. However, anti-bacterial responses and especially the production of IgA targeting commensal bacteria may be altered in some patients with autoimmune diseases, resulting in a failure to maintain gut microbiota. For instance, monocytes from SLE patients shows a transcriptional signature characteristic of chronic endotoxin exposure that could indicate a breach in the gut barrier, allowing the escape of commensal bacteria or antigens in periphery 120. In agreement with this hypothesis, SLE patients display autoreactive VH4–34-expressing IgG+ memory B cells that may be produced by systemic anti-commensal bacteria responses and expand during flares 79,121. In addition, Kriegel and colleagues recently reported that the translocation of commensal bacteria and especially Enterococcus gallinarum from the gut to the liver promotes the development of SLE, further suggesting that commensal bacteria fail to be restrained in the gut of these patients 122,123. Finally, altered gut microbiota have been associated with the development of autoimmunity 124–127. It remains to be determined whether the impaired early B cell tolerance checkpoints and the altered naïve B cell repertoire that they induce may favor intestinal dysbiosis in patients with autoimmune diseases.
Finally, anti-CTLA-4 and anti-PD-1/anti-PD-L1 “checkpoint inhibitors” (CPIs) that block immune inhibitory ligands have revolutionized the treatment of cancers 128–132. However, CPI regimens also induce serious immune and autoimmune related adverse events (IrAEs), which may be life-threatening. Thyroiditis and hypophysitis with secondary or primary adrenal insufficiency, as well as gonadal deficiency, have been reported in 40% and 13% of patients treated with anti-CTLA-4 or anti-PD-1 mAbs but can involve nearly half of patients treated with the combination 130,132–137. In addition, the development of insulin dependent diabetes may also occur in patients who were treated with anti-PD-1 or anti-PD-L1 mAbs with or without anti-CTLA-4 138. Since classical, spontanous T1D is characterized by early B cell tolerance checkpoint defects, it is tempting to speculate that CPI may interefere with the establishment of B cell tolerance and potentially result in the accumulation of large numbers of autoreactive B cells in the blood of CPI-treated cancer patients, thereby rendering them susceptible to developing autoimmune manifestation. As a consequence, B cell depletion may represent a therapeutic strategy to prevent the development of IrAEs in CPI-treated cancer patients without interfering with CPI anti-tumor responses 139.
Acknowledgments
We thank Karen Boss for editorial assistance and Roy Jiang of the Yale University School of Medicine MD/PhD program for assistance with data presentation. EM is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers AI061093 and AI071087 and TIL award from the Lupus Research Alliance. KCO is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health through grant award numbers AI114780 and AI142198, the National Institute of Neurological Diseases and Stroke under award number NS115054, and a Neuromuscular Disease Research program award from the Muscular Dystrophy Association (MDA) under award number MDA575198.
References
- 1.Yi JS, Guptill JT, Stathopoulos P, Nowak RJ, O’Connor KC. B cells in the pathophysiology of myasthenia gravis. Muscle Nerve. 2018;57(2):172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett JL, O’Connor KC, Bar-Or A, et al. B lymphocytes in neuromyelitis optica. Neurol Neuroimmunol Neuroinflam. 2015;2(3):e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsokos GC, Lo MS, Costa Reis P, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12(12):716–730. [DOI] [PubMed] [Google Scholar]
- 4.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serreze DV, Chapman HD, Varnum DS, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Igmunull mice. J Exp Med. 1996;184:2049–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu CY, Rodriguez-Pinto D, Du W, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117(12):3857–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serreze DV, Fleming SA, Chapman HD, Richard SD, Leiter EH, Tisch RM. B lymphocytes are critical antigen-presenting cells for the initiation of T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 1998;161:3912–3918. [PubMed] [Google Scholar]
- 8.Falcone M, Lee J, Patstone G, Yeung B, Sarvetnick N. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J Immunol. 1998;161(3):1163–1168. [PubMed] [Google Scholar]
- 9.Akashi T, Nagafuchi S, Anzai K, et al. Direct evidence for the contribution of B cells to the progression of insulitis and the development of diabetes in non-obese diabetic mice. Int Immunol. 1997;9(8):1159–1164. [DOI] [PubMed] [Google Scholar]
- 10.Hulbert C, Riseili B, Rojas M, Thomas JW. B cell specificity contributes to the outcome of diabetes in nonobese diabetic mice. J Immunol. 2001;167(10):5535–5538. [DOI] [PubMed] [Google Scholar]
- 11.Felton JL, Maseda D, Bonami RH, Hulbert C, Thomas JW. Anti-Insulin B Cells Are Poised for Antigen Presentation in Type 1 Diabetes. J Immunol. 2018;201(3):861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noorchashm H, Lieu YK, Noorchashm N, et al. I-Ag7-mediated antigen presentation by B lymphocytes is critical in overcoming a checkpoint in T cell tolerance to islet beta cells of nonobese diabetic mice. J Immunol. 1999;163(2):743–750. [PubMed] [Google Scholar]
- 13.Goodnow CC. Balancing immunity and tolerance: deleting and tuning lymphocyte repertoires. Proc Natl Acad Sci USA. 1996;93(6):2264–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemazee D. Does immunological tolerance explain the waste in the B-lymphocyte immune system? Experiment and theory. Ann N Y Acad Sci. 1995;764:397–401. [DOI] [PubMed] [Google Scholar]
- 15.Radic MZ, Weigert M. Origins of anti-DNA antibodies and their implications for B-cell tolerance. Ann N Y Acad Sci. 1995;764:384–396. [DOI] [PubMed] [Google Scholar]
- 16.Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat Immunol. 2004;6:645–650. [DOI] [PubMed] [Google Scholar]
- 17.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. [DOI] [PubMed] [Google Scholar]
- 18.Meffre E. The establishment of early B cell tolerance in humans: lessons from primary immunodeficiency diseases. Ann N Y Acad Sci. 2011;1246:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sng J, Ayoglu B, Chen JW, et al. AIRE expression controls the peripheral selection of autoreactive B cells. Sci Immunol. 2019;4(34). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinnunen T, Chamberlain N, Morbach H, et al. Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood. 2013;121(9):1595–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sauer AV, Morbach H, Brigida I, Ng YS, Aiuti A, Meffre E. Defective B cell tolerance in adenosine deaminase deficiency is corrected by gene therapy. J Clin Invest. 2012;122(6):2141–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hervé M, Isnardi I, Ng YS, et al. CD40 ligand and MHC class II expression are essential for human peripheral B cell tolerance. J Exp Med. 2007;204(7):1583–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssen E, Morbach H, Ullas S, et al. Dedicator of cytokinesis 8-deficient patients have a breakdown in peripheral B-cell tolerance and defective regulatory T cells. J Allergy Clin Immunol. 2014;134(6):1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castiello MC, Bosticardo M, Pala F, et al. Wiskott-Aldrich Syndrome protein deficiency perturbs the homeostasis of B-cell compartment in humans. J Autoimmun. 2014;50:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol. 2008;20(6):632–638. [DOI] [PubMed] [Google Scholar]
- 26.Ng Y-S, Wardemann H, Chelnis J, Cunningham-Rundles C, Meffre E. Bruton’s tyrosine kinase (Btk) is essential for human B cell tolerance. J Exp Med. 2004;200:927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isnardi I, Ng YS, Srdanovic I, et al. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29(5):746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menard L, Saadoun D, Isnardi I, et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest. 2011;121(9):3635–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romberg N, Chamberlain N, Saadoun D, et al. CVID-associated TACI mutations affect autoreactive B cell selection and activation. J Clin Invest. 2013;123(10):4283–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuraoka M, Snowden PB, Nojima T, et al. BCR and Endosomal TLR Signals Synergize to Increase AID Expression and Establish Central B Cell Tolerance. Cell Rep. 2017;18(7):1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyers G, Ng YS, Bannock JM, et al. Activation-induced cytidine deaminase (AID) is required for B-cell tolerance in humans. Proc Natl Acad Sci USA. 2011;108(28):11554–11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuraoka M, Holl TM, Liao D, et al. Activation-induced cytidine deaminase mediates central tolerance in B cells. Proc Natl Acad Sci U S A. 2011;108(28):11560–11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuraoka M, Meffre E, Kelsoe G. The First B-Cell Tolerance Checkpoint in Mice and Humans: Control by AID. Adv Immunol. 2018;139:51–92. [DOI] [PubMed] [Google Scholar]
- 34.Cantaert T, Schickel JN, Bannock JM, et al. Activation-Induced Cytidine Deaminase Expression in Human B Cell Precursors Is Essential for Central B Cell Tolerance. Immunity. 2015;43(5):884–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85(3):307–310. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278(4):369–395. [DOI] [PubMed] [Google Scholar]
- 37.Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. [DOI] [PubMed] [Google Scholar]
- 38.Rantapaa-Dahlqvist S, de Jong BAW, Berglin E, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. [DOI] [PubMed] [Google Scholar]
- 39.Yurasov S, Wardemann H, Hammersen J, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201(5):703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp Med. 2005;201(10):1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glauzy S, Sng J, Bannock JM, et al. Defective Early B Cell Tolerance Checkpoints in Sjogren’s Syndrome Patients. Arthritis Rheumatol. 2017;69(11):2203–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menard L, Samuels J, Ng YS, Meffre E. Inflammation-independent defective early B cell tolerance checkpoints in rheumatoid arthritis. Arthritis Rheum. 2011;63(5):1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JY, Stathopoulos P, Gupta S, et al. Compromised fidelity of B-cell tolerance checkpoints in AChR and MuSK myasthenia gravis. Ann Clin Transl Neurol. 2016;3(6):443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cotzomi E, Stathopoulos P, Lee CS, et al. Early B cell tolerance defects in neuromyelitis optica favour anti-AQP4 autoantibody production. Brain. 2019;142(6):1598–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinnunen T, Chamberlain N, Morbach H, et al. Specific peripheral B cell tolerance defects in patients with multiple sclerosis. J Clin Invest. 2013;123(6):2737–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe A, Su KY, Kuraoka M, et al. Self-tolerance curtails the B cell repertoire to microbial epitopes. JCI Insight. 2019;4(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho JH, Gregersen PK. Genomics and the multifactorial nature of human autoimmune disease. New Engl J Med. 2011;365(17):1612–1623. [DOI] [PubMed] [Google Scholar]
- 48.Begovich AB, Carlton VEH, Honigberg LA, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004;75(2):330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rieck M, Arechiga A, Onengut-Gumuscu S, Greenbaum C, Concannon P, Buckner JH. Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes. J Immunol. 2007;179(7):4704–4710. [DOI] [PubMed] [Google Scholar]
- 50.Salmond RJ, Brownlie RJ, Morrison VL, Zamoyska R. The tyrosine phosphatase PTPN22 discriminates weak self peptides from strong agonist TCR signals. Nat Immunol. 2014;15(9):875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arechiga AF, Habib T, He Y, et al. Cutting edge: The PTPN22 allelic variant associated with autoimmunity impairs B cell signaling. J Immunol. 2009;182(6):3343–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schickel JN, Kuhny M, Baldo A, et al. PTPN22 inhibition resets defective human central B cell tolerance. Sci Immunol. 2016;1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dai X, James RG, Habib T, et al. A disease-associated PTPN22 variant promotes systemic autoimmunity in murine models. J Clin Invest. 2013;123(5):2024–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.International Multiple Sclerosis Genetics C. A systems biology approach uncovers cell-specific gene regulatory effects of genetic associations in multiple sclerosis. Nat Commun. 2019;10(1):2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.International Multiple Sclerosis Genetics Consortium. Electronic address ccye, International Multiple Sclerosis Genetics C. Low-Frequency and Rare-Coding Variation Contributes to Multiple Sclerosis Risk. Cell. 2018;175(6):1679–1687 e1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17(6):673–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of Functional Suppression by CD4+CD25+ Regulatory T Cells in Patients with Multiple Sclerosis. J Exp Med. 2004;199(7):971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borsotti C, Danzl NM, Nauman G, et al. HSC extrinsic sex-related and intrinsic autoimmune disease-related human B-cell variation is recapitulated in humanized mice. Blood Adv. 2017;1(23):2007–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chamberlain N, Massad C, Oe T, Cantaert T, Herold KC, Meffre E. Rituximab does not reset defective early B cell tolerance checkpoints. J Clin Invest. 2016;126(1):282–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gies V, Schickel JN, Jung S, et al. Impaired TLR9 responses in B cells from patients with systemic lupus erythematosus. JCI Insight. 2018;3(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dieudonne Y, Gies V, Guffroy A, et al. Transitional B cells in quiescent SLE: An early checkpoint imprinted by IFN. J Autoimmun. 2019;102:150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25(3):417–428. [DOI] [PubMed] [Google Scholar]
- 63.Sindhava VJ, Oropallo MA, Moody K, et al. A TLR9-dependent checkpoint governs B cell responses to DNA-containing antigens. J Clin Invest. 2017;127(5):1651–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nundel K, Green NM, Shaffer AL, et al. Cell-intrinsic expression of TLR9 in autoreactive B cells constrains BCR/TLR7-dependent responses. J Immunol. 2015;194(6):2504–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lang J, Ota T, Kelly M, et al. Receptor editing and genetic variability in human autoreactive B cells. J Exp Med. 2016;213(1):93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tedder TF, Inaoki M, Sato S. The CD19-CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity. 1997;6:107–118. [DOI] [PubMed] [Google Scholar]
- 67.Morbach H, Schickel JN, Cunningham-Rundles C, et al. CD19 controls Toll-like receptor 9 responses in human B cells. J Allergy Clin Immunol. 2016;137(3):889–898 e886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mensah KA, Chen JW, Schickel JN, et al. Impaired ATM activation in B cells is associated with bone resorption in rheumatoid arthritis. Sci Transl Med. 2019;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prak EL, Weigert M. Light chain replacement: a new model for antibody gene rearrangement. J Exp Med. 1995;182(2):541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bensimon C, Chastagner P, Zouali M. Human lupus anti-DNA autoantibodies undergo essentially primary V kappa gene rearrangements. EMBO J. 1994;13:2951–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suzuki N, Harada T, Mihara S, Sakane T. Characterization of a germline Vk gene encoding cationic anti-DNA antibody and role of receptor editing for development of the autoantibody in patients with systemic lupus erythematosus. J Clin Invest. 1996;98:1843–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dorner T, Foster SJ, Farner NL, Lipsky PE. Immunoglobulin kappa chain receptor editing in systemic lupus erythematosus. J Clin Invest. 1998;102(4):688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silveira PA, Dombrowsky J, Johnson E, Chapman HD, Nemazee D, Serreze DV. B cell selection defects underlie the development of diabetogenic APCs in nonobese diabetic mice. J Immunol. 2004;172(8):5086–5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bonami RH, Thomas JW. Targeting Anti-Insulin B Cell Receptors Improves Receptor Editing in Type 1 Diabetes-Prone Mice. J Immunol. 2015;195(10):4730–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Panigrahi AK, Goodman NG, Eisenberg RA, Rickels MR, Naji A, Luning Prak ET. RS rearrangement frequency as a marker of receptor editing in lupus and type 1 diabetes. J Exp Med. 2008;205(13):2985–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bredemeyer AL, Sharma GG, Huang CY, et al. ATM stabilizes DNA double-strand-break complexes during V(D)J recombination. Nature. 2006;442(7101):466–470. [DOI] [PubMed] [Google Scholar]
- 77.Glanville J, Zhai W, Berka J, et al. Precise determination of the diversity of a combinatorial antibody library gives insight into the human immunoglobulin repertoire. Proc Natl Acad Sci U S A. 2009;106(48):20216–20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Palanichamy A, Apeltsin L, Kuo TC, et al. Immunoglobulin class-switched B cells form an active immune axis between CNS and periphery in multiple sclerosis. Sci Transl Med. 2014;6(248):248ra106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tipton CM, Fucile CF, Darce J, et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol. 2015;16(7):755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roy B, Neumann RS, Snir O, et al. High-Throughput Single-Cell Analysis of B Cell Receptor Usage among Autoantigen-Specific Plasma Cells in Celiac Disease. J Immunol. 2017;199(2):782–791. [DOI] [PubMed] [Google Scholar]
- 81.Vander Heiden JA, Stathopoulos P, Zhou JQ, et al. Dysregulation of B Cell Repertoire Formation in Myasthenia Gravis Patients Revealed through Deep Sequencing. J Immunol. 2017;198(4):1460–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rubelt F, Bolen CR, McGuire HM, et al. Individual heritable differences result in unique cell lymphocyte receptor repertoires of naive and antigen-experienced cells. Nat Commun. 2016;7:11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685–1699. [DOI] [PubMed] [Google Scholar]
- 84.de Bourcy CFA, Dekker CL, Davis MM, Nicolls MR, Quake SR. Dynamics of the human antibody repertoire after B cell depletion in systemic sclerosis. Sci Immunol. 2017;2(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Isnardi I, Ng YS, Menard L, et al. Complement receptor 2/CD21- human naive B cells contain mostly autoreactive unresponsive clones. Blood. 2010;115(24):5026–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saadoun D, Terrier B, Bannock J, et al. Expansion of autoreactive unresponsive CD21-/low B cells in Sjogren’s syndrome-associated lymphoproliferation. Arthritis Rheum. 2013;65(4):1085–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Glauzy S, Boccitto M, Bannock JM, et al. Accumulation of Antigen-Driven Lymphoproliferations in Complement Receptor 2/CD21(-/low) B Cells From Patients With Sjogren’s Syndrome. Arthritis Rheumatol. 2018;70(2):298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rubtsov AV, Rubtsova K, Fischer A, et al. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood. 2011;118(5):1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moir S, Ho J, Malaspina A, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205(8):1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weiss GE, Crompton PD, Li S, et al. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol. 2009;183(3):2176–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Charles ED, Brunetti C, Marukian S, et al. Clonal B cells in patients with hepatitis C virus-associated mixed cryoglobulinemia contain an expanded anergic CD21low B-cell subset. Blood. 2011;117(20):5425–5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bende RJ, van Noesel CJM. Rheumatoid Factor Reactivity of Expanded CD21(-/low) B Cells in Patients With Sjogren’s Syndrome: Comment on the Article by Glauzy et al et al. Arthritis Rheumatol. 2019;71(1):169–170. [DOI] [PubMed] [Google Scholar]
- 93.Jenks SA, Cashman KS, Zumaquero E, et al. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity. 2018;49(4):725–739 e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zumaquero E, Stone SL, Scharer CD, et al. IFNgamma induces epigenetic programming of human T-bet(hi) B cells and promotes TLR7/8 and IL-21 induced differentiation. Elife. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Naradikian MS, Myles A, Beiting DP, et al. Cutting Edge: IL-4, IL-21, and IFN-gamma Interact To Govern T-bet and CD11c Expression in TLR-Activated B Cells. J Immunol. 2016;197(4):1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meffre E, Louie A, Bannock J, et al. Maturational characteristics of HIV-specific antibodies in viremic individuals. JCI Insight. 2016;1(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kisand K, Boe Wolff AS, Podkrajsek KT, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207(2):299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Puel A, Doffinger R, Natividad A, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207(2):291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ferre EM, Rose SR, Rosenzweig SD, et al. Redefined clinical features and diagnostic criteria in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. JCI Insight. 2016;1(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Meyer S, Woodward M, Hertel C, et al. AIRE-Deficient Patients Harbor Unique High-Affinity Disease-Ameliorating Autoantibodies. Cell. 2016;166(3):582–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Herve M, Xu K, Ng YS, et al. Unmutated and mutated chronic lymphocytic leukemias derive from self-reactive B cell precursors despite expressing different antibody reactivity. J Clin Invest. 2005;115(6):1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Di Zenzo G, Di Lullo G, Corti D, et al. Pemphigus autoantibodies generated through somatic mutations target the desmoglein-3 cis-interface. J Clin Invest. 2012;122(10):3781–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harris ML, Darrah E, Lam GK, et al. Association of autoimmunity to peptidyl arginine deiminase type 4 with genotype and disease severity in rheumatoid arthritis. Arthritis Rheum. 2008;58(7):1958–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shi J, Darrah E, Sims GP, et al. Affinity maturation shapes the function of agonistic antibodies to peptidylarginine deiminase type 4 in rheumatoid arthritis. Ann Rheum Dis. 2018;77(1):141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pappas L, Foglierini M, Piccoli L, et al. Rapid development of broadly influenza neutralizing antibodies through redundant mutations. Nature. 2014;516(7531):418–422. [DOI] [PubMed] [Google Scholar]
- 106.Corti D, Suguitan AL Jr., Pinna D, et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J Clin Invest. 2010;120(5):1663–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Corti D, Voss J, Gamblin SJ, et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333(6044):850–856. [DOI] [PubMed] [Google Scholar]
- 108.Bennett JL, Lam C, Kalluri SR, et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol. 2009;66(5):617–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Owens GP, Ritchie A, Rossi A, et al. Mutagenesis of the aquaporin 4 extracellular domains defines restricted binding patterns of pathogenic neuromyelitis optica IgG. J Biol Chem. 2015;290(19):12123–12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mietzner B, Tsuiji M, Scheid J, et al. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci U S A. 2008;105(28):9727–9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.James JA, Kaufman KM, Farris AD, Taylor-Albert E, Lehman TJ, Harley JB. An increased prevalence of Epstein-Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J Clin Invest. 1997;100(12):3019–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gross AJ, Hochberg D, Rand WM, Thorley-Lawson DA. EBV and systemic lupus erythematosus: a new perspective. J Immunol. 2005;174(11):6599–6607. [DOI] [PubMed] [Google Scholar]
- 113.Saal JG, Krimmel M, Steidle M, et al. Synovial Epstein-Barr virus infection increases the risk of rheumatoid arthritis in individuals with the shared HLA-DR4 epitope. Arthritis Rheum. 1999;42(7):1485–1496. [DOI] [PubMed] [Google Scholar]
- 114.Alspaugh MA, Henle G, Lennette ET, Henle W. Elevated levels of antibodies to Epstein-Barr virus antigens in sera and synovial fluids of patients with rheumatoid arthritis. J Clin Invest. 1981;67(4):1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Scotet E, David-Ameline J, Peyrat MA, et al. T cell response to Epstein-Barr virus transactivators in chronic rheumatoid arthritis. J Exp Med. 1996;184(5):1791–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saito I, Servenius B, Compton T, Fox RI. Detection of Epstein-Barr virus DNA by polymerase chain reaction in blood and tissue biopsies from patients with Sjogren’s syndrome. J Exp Med. 1989;169(6):2191–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tracy S, Drescher KM, Chapman NM, et al. Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. J Virol. 2002;76(23):12097–12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kaur K, Zheng NY, Smith K, et al. High Affinity Antibodies against Influenza Characterize the Plasmablast Response in SLE Patients After Vaccination. PLoS One. 2015;10(5):e0125618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mouquet H, Scheid JF, Zoller MJ, et al. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467(7315):591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shi L, Zhang Z, Yu AM, et al. The SLE transcriptome exhibits evidence of chronic endotoxin exposure and has widespread dysregulation of non-coding and coding RNAs. PLoS One. 2014;9(5):e93846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schickel JN, Glauzy S, Ng YS, et al. Self-reactive VH4–34-expressing IgG B cells recognize commensal bacteria. J Exp Med. 2017;214(7):1991–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Manfredo Vieira S, Hiltensperger M, Kumar V, et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359(6380):1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Greiling TM, Dehner C, Chen X, et al. Commensal orthologs of the human autoantigen Ro60 as triggers of autoimmunity in lupus. Sci Transl Med. 2018;10(434). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hevia A, Milani C, Lopez P, et al. Intestinal dysbiosis associated with systemic lupus erythematosus. MBio. 2014;5(5):e01548–01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Berer K, Mues M, Koutrolos M, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479(7374):538–541. [DOI] [PubMed] [Google Scholar]
- 126.Markle JG, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339(6123):1084–1088. [DOI] [PubMed] [Google Scholar]
- 127.Proal AD, Albert PJ, Marshall TG. The human microbiome and autoimmunity. Curr Opin Rheumatol. 2013;25(2):234–240. [DOI] [PubMed] [Google Scholar]
- 128.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. [DOI] [PubMed] [Google Scholar]
- 132.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. Cancer immunotherapy - immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol. 2017;13(4):195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Horvat TZ, Adel NG, Dang TO, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Min L, Ibrahim N. Ipilimumab-induced autoimmune adrenalitis. Lancet Diabetes Endocrinol. 2013;1(3):e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hughes J, Vudattu N, Sznol M, et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38(4):e55–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Damsky W, Jilaveanu L, Turner N, et al. B cell depletion or absence does not impede anti-tumor activity of PD-1 inhibitors. J Immunother Cancer. 2019;7(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]