Table 1.
In vitro AChE inhibitory activity of compounds 10a–10s and 13a–13p.
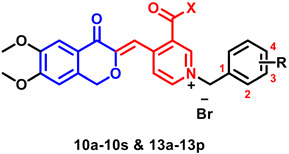
| |||||||
|---|---|---|---|---|---|---|---|
| Compd. | X | R | IC50 a (nM) | Compd. | X | R | IC50 a (nM) |
| AChE | AChE | ||||||
| 10a | Me | H | 1.61 ± 0.02 | 10s | Me | 4-CF3 | 325.94 ± 9.01 |
| 10b | Me | 2-F | 3.32 ± 0.21 | 13a | NH2 | H | 8.63 ± 0.06 |
| 10c | Me | 3-F | 4.03 ± 0.23 | 13b | NH2 | 2-F | 3.54 ± 0.05 |
| 10d | Me | 4-F | 7.69 ± 0.35 | 13c | NH2 | 3-F | 6.02 ± 0.08 |
| 10e | Me | 2-Cl | 5.86 ± 0.86 | 13d | NH2 | 4-F | 8.25 ± 0.13 |
| 10f | Me | 3-Cl | 10.26 ± 1.08 | 13e | NH2 | 2-Cl | 17.58 ± 0.30 |
| 10g | Me | 4-Cl | 67.32 ± 2.68 | 13f | NH2 | 3-Cl | 26.74 ± 0.28 |
| 10h | Me | 2-Br | 6.22 ± 0.05 | 13g | NH2 | 4-Cl | 188.34 ± 4.78 |
| 10i | Me | 3-Br | 10.98 ± 0.82 | 13h | NH2 | 2-Br | 21.71 ± 0.32 |
| 10j | Me | 4-Br | 75.72 ± 3.18 | 13i | NH2 | 3-Br | 38.14 ± 0.92 |
| 10k | Me | 2-Me | 4.80 ± 0.26 | 13j | NH2 | 4-Br | 421.76 ± 5.92 |
| 10l | Me | 3-Me | 6.61 ± 0.81 | 13k | NH2 | 2-Me | 12.14 ± 0.90 |
| 10m | Me | 4-Me | 54.10 ± 3.22 | 13l | NH2 | 3-Me | 16.26 ± 0.12 |
| 10n | Me | 2-NO2 | 13.58 ± 0.72 | 13m | NH2 | 4-Me | 42.47 ± 0.25 |
| 10o | Me | 3-NO2 | 22.45 ± 1.25 | 13n | NH2 | 2-CF3 | 30.71 ± 0.71 |
| 10p | Me | 4-NO2 | 128.86 ± 2.68 | 13o | NH2 | 3-CF3 | 43.05 ± 0.44 |
| 10q | Me | 2-CF3 | 18.87 ± 1.16 | 13p | NH2 | 4-CF3 | 441.18 ± 7.82 |
| 10r | Me | 3-CF3 | 25.32 ± 2.20 | Donepezil | 12.06 ± 0.01 | ||
a All values are expressed as mean ± SEM from three independent experiments.
