Abstract
The major soil-transmitted helminths that infect humans are the roundworms, whipworms and hookworms. Soil-transmitted helminth infections rank among the most important neglected tropical diseases in terms of morbidity, and almost one billion people are still infected with at least one species. While anthelmintic drugs are available, they do not offer long term protection against reinfection, precipitating the need for vaccines that provide long-term immunologic defense. Vaccine discovery and development is in advanced clinical development for hookworm infection, with a bivalent human hookworm vaccine in clinical trials in Brazil and Africa, but is in its infancy for both roundworm (ascariasis) and whipworm (trichuriasis) infections. One of the greatest hurdles to developing soil-transmitted helminth vaccines is the potent immunoregulatory properties of these helminths, creating a barrier to the induction of meaningful long-term protective immunity. While challenging for vaccinologists, this phenomenon presents unique opportunities to develop an entirely new class of anti-inflammatory drugs that capitalise on these immunomodulatory strategies. Epidemiologic studies and clinical trials employing experimental soil-transmitted helminth challenge models, when coupled with findings from animal models, show that at least some soil-transmitted helminth-derived molecules can protect against the onset of autoimmune, allergic and metabolic disorders, and several natural products with the desired bioactivity have been isolated and tested in pre-clinical settings. The yin and yang of soil-transmitted helminth infections reflect both the urgency for effective vaccines and the potential for new immunoregulatory molecules from parasite products.
Keywords: Soil-transmitted helminth, Hookworm, Whipworm, Ascaris, Vaccine, Immunomodulation, Inflammation
1. Biology and epidemiology
The soil-transmitted helminths (STHs) is a group of parasitic nematodes of humans that are mostly restricted to the world’s tropical and subtropical climates, especially in low- and middle-income countries (LMICs), where they cause infection through contact with parasite eggs or infective larval stages (Bethony et al., 2006). The adult developmental stages of STHs are found in the gastrointestinal (GI) tract where they establish chronic infections in which individual worms can live for 1–10 years. The STHs of particular worldwide importance that we will address herein are the roundworms, whipworms and hookworms.
Of the roundworms, the major species to infect humans is Ascaris lumbricoides, the largest (up to 400 mm in length) and most prevalent of the STHs (Elkins et al., 1986). Humans become infected after ingesting embryonated eggs, which hatch to release larvae that penetrate the intestinal mucosa to commence an obligatory extra-intestinal migratory phase in the liver and lungs, whereupon they migrate up the trachea and are swallowed, thus re-entering the GI tract. In the small intestine they develop into dioecious adult worms which mate and the female releases hundreds of thousands of eggs per day.
The major whipworm to infect humans is Trichuris trichiura (Else et al., 2020). People become infected by ingesting eggs, whereupon first stage larvae (L1) hatch and penetrate the epithelial cells at the base of the crypts of the large intestine (colon) where they create a multicellular epithelial tunnel. Larvae grow and moult multiple times before becoming 30–50 mm adult males and females with the characteristic whip like appearance. Zoonotic infections with other Trichuris spp. occur, primarily Trichuris suis from pigs and Trichuris vulpis from dogs, but neither species completes its development in humans nor are they considered to be pathogenic. Indeed, experimental human infection with T. suis has been reported in numerous clinical trials due to the immunoregulatory nature of the infection (see subsequent sections).
The third major group within the STHs is the human hookworms (Hotez et al., 2005). Adult hookworms reside for many years in the small intestine where they feed on blood and can cause iron deficiency anaemia (IDA) when present in high numbers, particularly in individuals who are malnourished or have compromised iron stores. Children and women of reproductive age (especially pregnant women) with low underlying iron stores are especially prone to develop hookworm IDA. The three main hookworm species to infect humans are Necator americanus, Ancylostoma duodenale and Ancylostoma ceylanicum. The most predominant human hookworm is N. americanus, which is particularly common in southern China, Southeast Asia, the Americas and most of the African continent. Ancylostoma ceylanicum was thought to be primarily a parasite of dogs, but has recently been identified as a significant human species throughout the Asia-Pacific region where it is sometimes co-endemic with N. americanus (Inpankaew et al., 2014; Bradbury et al., 2017; Colella et al., 2021). Humans become infected with N. americanus through percutaneous penetration of infective third-stage larvae (L3) whereas the ancylostomatids can infect via both percutaneous and oral (and even trans-colostral) routes. A human experimental challenge model has been established for N. americanus (Loukas et al., 2016; Diemert et al., 2018a; Hoogerwerf et al., 2021) and it has been shown to be safe and well tolerated in doses exceeding 100 topically applied L3 (Hoogerwerf et al., 2021). This has enabled the establishment of a platform to test anthelmintic drugs and vaccines as well as assessing the therapeutic potential of experimental hookworm infections for treating diseases that result from a dysregulated immune system (Ryan et al., 2020).
Human strongyloidiasis is caused primarily by Strongyloides stercoralis, a STH with a unique life cycle that entails alternative free-living and parasitic developmental pathways in response to environmental stimuli (reviewed in Krolewiecki and Nutman, 2019). Unlike the other STHs, eggs shed by the female worm hatch in the small intestine and it is the L1 rhabditiform larvae that are passed in the faeces and are the diagnostic stage detected by microscopy. Due to the presence of larvae in the duodenum, autoinfection is a risk if larvae reach the infective L3 stage before being shed from the host. This can lead to hyperinfection syndrome where larvae are mostly restricted to the gut and lungs, or disseminated strongyloidiasis where larvae can invade other organs. Both conditions are common with subjects receiving high doses of corticosteroids, such as post-organ transplant patients, and can be fatal (Cappello and Hotez, 1993). Human to human infection is typically via percutaneous penetration by L3s but oral transmission is thought to be possible. Dogs can also harbour S. stercoralis infection and are thought to be a source of human infection in some areas (Jaleta et al., 2017). Once larvae penetrate the skin they rapidly migrate through subcutaneous tissue and sometimes leave pruritic linear streaks along the lower trunk and buttocks, often referred to as larva currens.
Increasing attention is also being paid to the zoonotic roundworms, Toxocara canis and Toxcara cati, and their importance to public health (Ma et al., 2018). Toxocara spp. are primarily parasites of dogs and cats, but are thought to infect tens of millions of people based on seropositivity rates. Toxocara does not fully mature in humans but undergoes various forms of larva migrans, where larvae migrate through the liver and lungs (visceral larva migrans), eyes (ocular larva migrans) or neurological tissues (neurotoxocariasis). The term covert toxocariasis has been used to report on the asymptomatic or low-grade Toxocara infection, which can result in chronic eosinophilia, asthma, or developmental delays.
2. Disease burden, pathogenesis, and current control efforts
The STH infections rank among the most common neglected tropical diseases. Practically all children and many adults (including millions of pregnant women) who live in extreme poverty are affected by at least one of these infections. However, while STH infections are typically chronic and debilitating conditions, they are not considered major causes of global mortality. We therefore assess their public health impact through global and regional prevalence estimates or a metric used by the Global Burden of Disease Study (GBD) and known as the disability-adjusted life year (DALY).
The latest iteration for the year 2019 (GBD 2019) has determined that 909 million people suffer from these infections (IHME, 2020a). Ascariasis is the most common STH infection with 446 million prevalent infections (IHME, 2020b), followed by trichuriasis, currently estimated at 360 million prevalent infections (IHME, 2020c), and hookworm infection at 173 million (IHME, 2020d). Together these STH infections result in approximately 2,000 deaths annually, and almost 2 million DALYs (IHME, 2020a). However, some investigators feel that even these numbers may fail to fully account for all of the chronic morbidities (Herricks et al., 2017). Currently, the GBD2019 estimates that hookworm infection accounts for almost one-half of the DALYs lost (Fig. 1), mostly due to moderate and severe anaemia in children and pregnant women (IHME, 2020d).
Fig. 1.
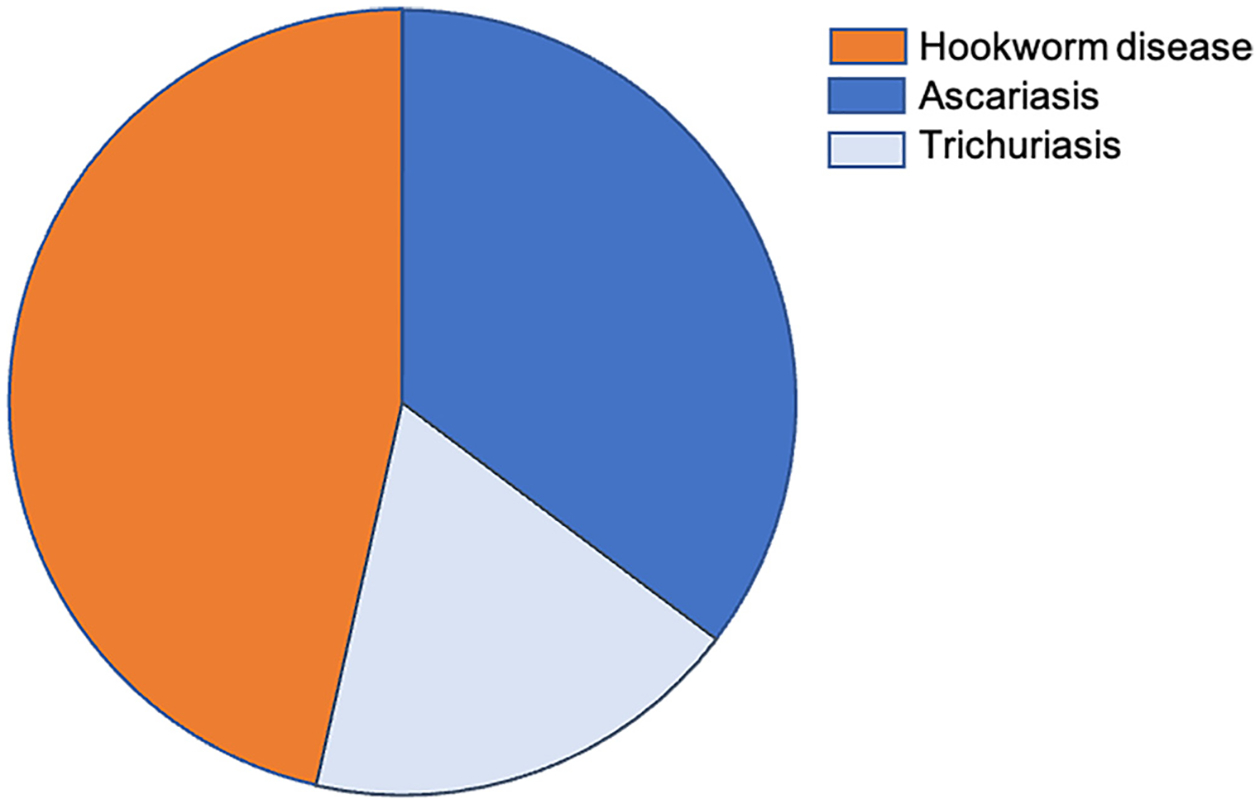
Composition of disability adjusted life years by constituent Level 4 causes for both sexes combined, 2019. Adapted from IHME, 2020a.
Although the GBD2019 does not provide estimates for other STH infections, both strongyloidiasis and toxocariasis are highly prevalent diseases. One independent estimate reported 386 million people with strongyloidiasis in 2020 (Fleitas et al., 2020), although their method relied on hookworm infection prevalence estimates that far exceeded those released by the GBD2019. An earlier estimate for strongyloidiasis from 2017 was higher still (Buonfrate et al., 2020). In some estimations, human toxocariasis has emerged as one of the most prevalent STH infections. However, human toxocariasis is assessed by measuring antibodies in patient sera rather than by faecal examination. A 2019 systematic review and meta-analysis estimate puts the global seroprevalence of toxocariasis at 19%, but with far higher estimates in tropical regions, especially Africa and South-East Asia (Rostami et al., 2019).
The pathogenesis of disease caused by STH infections is often considered in aggregate, although in fact, each type of infection is unique in terms of pathogenesis and clinical outcome. However, there are some common features. For ascariasis, trichuriasis, and toxocariasis, typically the severest illness occurs in children because they harbour higher worm loads than adults (Else et al., 2020). Hookworms are an important exception as high hookworm burdens are found in both children and adults, including pregnant women (Ness et al., 2020). It has been estimated that approximately 7 million pregnancies are complicated by hookworm infection in Sub-Saharan Africa (Brooker et al., 2008). In general, heavier worm burdens in children lead to chronic impairments in physical growth and cognitive and intellectual development through underlying mechanisms that may be linked to malnutrition and/or host inflammatory responses (Else et al., 2020).
Each type of STH also induces unique pathologic effects. For example, large Ascaris roundworms cause intestinal obstruction, especially in the ileum of small children (Else et al., 2020), and this finding accounts for a high percentage of the 2,000 or more annual deaths. In addition, Ascaris roundworms can mechanically obstruct the bile and pancreatic ducts, especially in adults. Still another aspect is the finding that in their migration through the lungs, Ascaris larvae can induce severe allergic responses that produce a mixed picture of eosinophilic asthma and chronic obstructive lung disease (Weatherhead et al., 2018). In contrast, Trichuris whipworms inhabit the large intestine where they cause colitis and inflammatory bowel disease, and in heavy infections, a Trichuris dysentery syndrome which can lead to rectal prolapse (Else et al., 2020). The mechanisms of colitis are due primarily to the ability of adult Trichuris whipworms to release chemically-active compounds from their stichosome, a unique parasite organ located in the anterior portion of the worm embedded in the host gut mucosa. The release of small and macro-molecules from the parasite stichosome causes biochemical changes to host epithelia, allowing the anterior end of the parasite to burrow in cellular tunnels to induce inflammation and intestinal blood loss (Else et al., 2020). However, hookworms cause far greater blood loss due to the ability of the adult stage parasites to ingest a plug of host tissue causing direct mechanical and enzymatic damage (Loukas et al., 2016). The red blood cells are ingested and lysed by the adult hookworm. The gut of the adult hookworm is lined with proteolytic enzymes, many of which are specific for cleaving haemoglobin and operate in a cascade of hydrolytic cleavages and protein breakdown (Ranjit et al., 2009). This allows the adult hookworm to digest haemoglobin and absorb host peptides and amino acids. For that reason, the major consequence of heavy hookworm blood ingestion is moderate and severe anaemia, as well as protein malnutrition and hypoalbuminaemia (Loukas et al., 2016). Severe hookworm anaemia together with the foetal iron demands in pregnancy can produce serious deleterious effects for both mothers and newborns.
Strongyloidiasis produces its most severe form of illness in patients who receive high doses of corticosteroids, or in some cases other immunosuppressive therapies or co-infection with HTLV-1 (Mejia and Nutman, 2012). This results in a severe hyperinfection syndrome associated with gut microperforation, bacteraemia, and even bacterial meningitis (Mejia and Nutman, 2012). Human toxocariasis results from larval migration through host tissues, eliciting eosinophilia and lung allergic responses similar to those observed for larval Ascaris infection in the lungs, or in the liver leading to hepatitis (Ma et al., 2018). In addition, Toxocara larval migration through the brain may produce a subclinical cerebritis leading to intellectual declines and developmental delays (Hotez, 2014; Ma et al., 2018).
Such widespread pathology caused by STH has demanded much greater efforts to control infections. In 2001, the World Health Assembly pledged to deworm up to 100% of all school-aged children by the year 2010. The approach relied on studies conducted during the 1990s that found a single dose of an anthelminthic drug, typically a single dose of albendazole or mebendazole, could reduce the bioburden of STH infections. This allowed paediatric catch-up growth and improvements in childhood cognition and intellectual capacity (Savioli et al., 1992). Indeed, early on, there was evidence of not only improvements in child development, but also in educational attainment and long-term economic returns (Migel and Kremer, 2004). Countering this were systematic reviews and Cochrane analyses that questioned these benefits (Taylor-Robinson et al., 2009, 2015), which led to a vigorous disagreement in the medical parasitology community. One analysis suggested that such “worm wars” might be resolved by recognising the differences between each of the STH rather than lumping them together as a single STH entity, in which the significant effects in one species would be negated by lack of effects in the others. In addition, it is important to control for the differential effects of drug treatment on each of the major helminth species, and compare light versus moderate or severe helminth infections of children, particularly with respect to cognitive improvements (Majid et al., 2019).
Currently, hundreds of millions of both school-aged children and pre-school children receive annual deworming in low- and middle-income countries. Less clear is whether this approach actually can lead to the elimination of STH infections, or whether additional selective pressure should be applied in the form of simultaneously treating adults on an annual basis (Ásbjörnsdóttir et al., 2018). A “deworm3” project is underway to assess whether more aggressive deworming approaches could lead to gradual declines in parasite prevalence and intensity (Ásbjörnsdóttir et al., 2018) or whether new and improved technologies might be required, such as evaluating existing anthelmintic drugs in combination, for example albendazole combined with ivermectin (Palmeirim et al., 2018; Moser et al., 2019). In addition, efforts are in progress to explore new chemical entities (NCEs) and new classes of anthelmintic drugs (Elfawal et al., 2019), as well as anthelmintic vaccines. With regards to the former, a novel screening pipeline has been proposed and is starting to yield promising NCEs, while anthelmintic vaccines are discussed in more depth below. In parallel, expanded studies are in progress to evaluate whether mass drug administration could induce parasite drug resistance to albendazole or mebendazole (Vlaminck et al., 2018). This new Starworms project examines single nucleotide polymorphisms in the genes encoding β-tubulin, the major drug target of albendazole and mebendazole (Vlaminck et al., 2018).
3. Vaccines for STHs – human trials, animal models and challenges to development
There is a general absence of reliable protective immunity to STH infections among those living in endemic areas of poverty. That said, a status quo is achieved in many infected subjects, where worm burdens that contribute to morbidity (particularly in the young and malnourished) are frequently encountered, but uncontrolled heavy intensity infections are less common. Age- and exposure-acquired immunity to hookworms (reviewed in (Loukas et al., 2016a) and whipworms (reviewed in (Else et al., 2020)) is slow to develop, if at all, however Ascaris burdens tend to peak in early adolescence and decrease with age along with a robust T helper 2 (Th2) immune response (Turner et al., 2003).
Given the absence of naturally acquired sterilizing immunity in STH infections, the goal of current vaccination strategies is to attain partial immunity that minimises the impact of moderate to heavy intensity infections. To date, the most compelling strategy for developing helminth vaccines (not just STH) is the irradiated parasite approach. Many studies have shown in animal models of human helminths as well as with helminths of livestock and companion animals that vaccination with live radiation-attenuated larvae confers strong protection against challenge infection. Indeed, in the 1960s Miller showed that subcutaneous vaccination of dogs with infective larvae of the canine hookworm Ancylostoma caninum that were attenuated with X-rays resulted in protection of pups against challenge infection (Miller, 1964). Protection was attributed to distinct but interrelated factors including reduction in larval infectivity, reduced pathogenicity, and the sterilising effect of radiation on female worm fecundity (manifesting as reduced egg burdens in vaccinees). Subsequent studies showed that gamma radiation was successful, and these criteria guided the development, manufacture and licensing of a gamma-irradiated A. caninum L3 vaccine in the early 1970s (Miller, 1978). Despite high efficacy, animals showed residual levels of infection that dented confidence in its value, and the vaccine was removed from the market. Irradiated STH vaccines require a ready supply and industrial scale methods for producing the attenuated immunogen (larvae), and thus pose a number of challenges for regulatory approval and wide-scale implementation. As such, there were no efforts to develop and clinically test an attenuated larval vaccine for any human STH infection until recently when Chapman and colleagues undertook a phase 1 randomised, double-blind, placebo-controlled, challenge study to assess the safety and tolerability of ultraviolet light C (UVC)-irradiated N. americanus in healthy hookworm-naive adults in Australia (Chapman et al., 2021). The vaccine was well tolerated, safe and despite the small cohort sizes, significantly reduced larval burdens were recovered from hatched eggs of vaccinated participants compared with controls after challenge infection with non-attenuated larvae. While an ultimate hookworm vaccine is unlikely to take the form of irradiated larvae due to the logistical challenges outlined above, this trial nonetheless set a benchmark against which to compare subunit vaccines that are currently in development (see below), and was the first study to prove that partially protective immunity could be induced in humans by vaccination. Similar studies with attenuated forms of other STHs have yet to be undertaken, but we urge researchers to perform these trials as both proof-of-concept studies and to generate valuable reagents (eg. sera) that can be utilised in the search for protective antigens.
STHs produce a diverse array of secreted molecules and vesicles that interact with surrounding host tissues where they orchestrate various parasitism processes such as tissue penetration, somatic migration, feeding and immune modulation. Proteins involved in these processes are prime candidate antigens to target with subunit vaccines. Along those lines, a Human Hookworm Vaccine Initiative was established in 2000 to reproduce the effects of irradiated larvae but using recombinant protein subunit vaccines (Hotez et al., 2003). Based on earlier findings of two predominant larval secreted proteins known as Ancylostoma secreted protein-1 (ASP-1) (Hawdon et al., 1996) and ASP-2 (Hawdon et al., 1999) released upon host stimulation, and which were also found in N. americanus (Na-ASP-2) (Goud et al., 2005), the HHVI focused on these two molecules. It was subsequently found that ASP-2 was an immunodominant macromolecule associated with irradiated vaccines (Bethony et al., 2005). Although an alum formulation of yeast-expressed recombinant ASP-2 was shown to be safe and immunogenic in helminth-naïve human volunteers (Bethony et al., 2008), follow up phase 1 studies in an endemic area of Brazil revealed that the vaccine was allergenic due to the presence of pre-vaccination host IgE among those chronically exposed to the parasite (Diemert et al., 2012). For that reason, larval antigen vaccines were abandoned in favour of adult hookworm antigens.
As a second and more successful strategy for human hookworm vaccines (Hotez et al., 2010), recombinant forms of two parasite-derived enzymes involved in blood-feeding and detoxification are in clinical development - the haemoglobin-degrading aspartic protease, Na-APR-1 (Pearson et al., 2009), and the haem-detoxifying glutathione-S-transferase, Na-GST-1 (Asojo et al., 2007; Hotez et al., 2010). Both vaccines have been tested independently in phase 1a safety trials (https://clinicaltrials.gov/ct2/show/NCT01717950) (Diemert et al., 2017), and more recently in a N. americanus endemic region of Gabon as a combination of co-administered vaccines adjuvanted with alhydrogel (Adegnika et al., 2021). Both vaccines were shown to be immunogenic and safe, and now await efficacy testing using the controlled hookworm infection model. Both Na-APR-1 and Na-GST-1 are expressed in the gut of the adult stage parasite. Moreover, both of these adult hookworm antigens appear to circumvent past issues with inducing allergic responses. In general, STH and other helminth vaccine antigen selection approaches now have rigorous criteria for down-selecting proteins that drive IgE responses in infected individuals (Diemert et al., 2018b).
There is an equally real need for a vaccine that reduces infection intensity and transmission in trichuriasis. Despite mass anthelmintic drug administration programs to school-age children, T. trichiura infection continues to be a burden, notably due to the low efficacy of current drugs and high rates of post-treatment re-infection. To our knowledge, there has never been a clinical trial with a Trichuris vaccine of any sort. However, a comprehensive review on trichuriasis vaccines with a major emphasis on candidate antigens tested in the Trichuris muris mouse model was recently published (Hayon et al., 2021). Vaccination of susceptible mice with Freund’s adjuvanted T. muris excretory/secretory (ES) products conferred complete protection against challenge infection (Dixon et al., 2010). Many of these ES antigens originate from the Trichuris stichosome organ which is embedded in the host colonic mucosa (Briggs et al., 2018). While this highlights the value of the murine model for pre-clinical discovery and development of a subunit vaccine for human whipworm infection, it should be noted that susceptibility and immunologic resistance/clearance of the infection is mouse strain- (median histocompatibility complex) and sex-dependent, and is influenced by the nature of exposure, notably trickle versus bolus infection (Yousefi et al., 2021). One subunit vaccine that is showing particular promise is the Trichuris stichosome secreted antigen known as whey acidic protein (WAP) (Briggs et al., 2018). Still another contains a fragment spanning the catalytic domain of serine/threonine phosphatase 2A fused to a self-adjuvanting synthetic oleic-vinyl sulfone, which when administered to mice intra-nasally provided almost complete protection against T. muris challenge infection. Indeed, similar self-adjuvanting mucosally-delivered vaccines based on peptides/subunits of protective protein antigens from hookworms also confer high protection in animal models (Bartlett et al., 2020). These findings, while preliminary in nature, highlight the potential of this vaccine platform and the advantages it confers for distribution of vaccines to remote areas.
Vaccines against ascariasis are proposed to reduce the parasite burden and, consequently, infection-induced morbidity and transmission (Else et al., 2020). Mouse models, while not allowing complete development of these large parasites in a small animal, have proven useful in assessing anti-larval responses in the lungs. Multiple exposures of mice to eggs of the pig whipworm Ascaris suum conferred high levels of protection against accumulation of larvae in the lungs (Nogueira et al., 2016). Moreover, vaccination of mice with different A. suum extracts derived from adult and larval parasites provided partial protection against larvae reaching the lungs, and passive transfer of IgG from vaccinated mice conferred similar levels of protection (Gazzinelli-Guimarães et al., 2018). Infection of pigs with A. suum has been used as a permissive model of human ascariasis, and oral vaccination of pigs with radiation-attenuated eggs conferred 94% protection against challenge infection (Urban and Tromba, 1984). While ascariasis vaccines have not yet entered clinical development or testing, a number of subunit vaccines have been assessed in the mouse model, including As37 which is conserved amongst STH and represents a potential pan-STH vaccine candidate (Versteeg et al., 2020), as well as another antigen known as As16 (Wei et al., 2017). A chimeric antigen consisting of peptides from multiple antigens was recently shown to confer robust protection against establishment of larvae in the lungs (de Castro et al., 2021). In parallel with each STH vaccine are efforts to combine these antigens in a universal or multivalent pananthelmintic vaccine, in addition to studies to identify common consensus antigens against roundworms, whipworms and hookworms (Zhan et al., 2014).
Although very high levels of protection have been observed in rodent models of STH infections, a lower bar has been set for efficacy of human vaccines due to the limitations of these animal models. Moreover, modelling studies have shown that such high levels of protection are not required to substantially reduce morbidity due to the correlation between infection intensity and pathogenesis in hookworm infection at least (Bartsch et al., 2016). A caveat that must be considered with all helminth vaccine programs is the issue of administering vaccines to individuals who are already infected, often chronically so, and therefore under the influence of helminthiasis-driven immunoregulation (see next section). At the very least, subjects should be dewormed with an anthelmintic drug prior to vaccination, but the long-lasting immunomodulatory effects of STH infections are well documented (Loukas et al., 2016; Else et al., 2020), and could result in reduced vaccine efficacy in affected individuals. Finally, such vaccines are predicted to be highly cost-effective and cost-saving due to the poverty promoting effects of these parasites (Bartsch et al., 2016). For that reason, they are sometimes referred to as ‘antipoverty vaccines’ (Hotez et al., 2011).
4. Next generation integrated control and elimination
The current approaches emphasizing mass treatment or deworming have so far not succeeded in promoting STH elimination unless there is a commensurate rise in living standards and economic development. Efforts to optimize mass treatment by expanding access to anthelmintics for the entire community and combining traditional and new anthelmintics drugs – including ivermectin or moxidectin (where these drugs are already deployed for onchocerciasis and lymphatic filariasis control programs) – to create added synergies for trichuriasis and hookworm, will help. But even these measures may be insufficient. For instance, anthelmintic drug resistance monitoring needs to be better and fully integrated into global deworming programs. We also have an opportunity to introduce new anthelmintic vaccines for STH infections and potentially integrate them in vaccine-linked chemotherapy approaches (Zawawi and Else, 2020). Adding vaccinations to deworming could reduce the amplitude of post-treatment reinfections to a point in which STH transmission is no longer sustained, a goal aided by the absence of significant animal reservoirs for these infections (except possibly for ascariasis in pigs). A similar approach has been proposed for schistosomiasis in Africa and the Americas. Currently, the global policymakers have not prioritized vaccine-linked chemotherapy approaches, choosing instead to rely exclusively on deworming despite major questions regarding its sustainability (Lin and Addiss, 2018).
5. Immunoregulatory strategies of STH
Protective immunity to STH infections takes on different forms depending on the individual species and the route of infection. Necator americanus for example first enters the human host via the skin, whereas the whipworms and ascarids are orally infective. While we have a general understanding of the protective mechanisms at play from rodent models, such as N. brasiliensis and T. muris (Allen and Sutherland, 2014), the protective response in humans is less well understood, and indeed for hookworms at least, there is no clear-cut evidence of protective immunity (Loukas et al., 2016). Nonetheless, a robust type 2 response is initiated early with IgE-armed basophils trapping larvae in the skin, and further clearance of STH larvae occurs in the lungs, where M2 macrophages damage and orchestrate the clearance of parasites (Loukas et al., 2016; Else et al., 2020). Once adult worms are lodged in the gut, secretion of type 2 cytokines and IL-22 in intestinal mucosal tissue of experimentally infected subjects has been detected, and induction of goblet cell hyperplasia and mucus production (Broadhurst et al., 2010; Gaze et al., 2012), all of which culminate in worm expulsion. Moreover, the production of IL-25 by intestinal tuft cells has been shown to be a key early event in triggering protective TH2 responses in animal models (Gerbe et al., 2016).
In the face of this robust modified type 2 response, it is remarkable how ineffective our natural defenses against STH are, reflecting the ability of these parasites to modulate and disable host immune mechanisms (McSorley and Maizels, 2012), and contributing to the difficulty in developing highly efficacious subunit vaccines. In particular, acquired immunity to repeated STH infections is poorly expressed, with rapid re-infection following anthelmintic chemotherapy (Jia et al., 2012), and no obvious decline in worm burdens (notably for hookworm infections) observed as populations age (Anderson and May, 1982). STHs combine short-term tactics to minimise immune stimulation with a longer-term strategy to exploit host immune regulatory networks, in order to mute host reactivity and inactivate expulsion mechanisms.
In the first instance, parasites that enter through the skin (eg hookworms, Strongyloides) encounter different barriers to those taking the oral route (Ascaris, Trichuris, Toxocara). Barrier tissues release alarmins, such as IL-33 and TSLP, to alert the immune system and prime antigen-presenting cells (dendritic cells, DCs); in animal models at least, helminths release products that block the IL-33 pathway (Osbourn et al., 2017; Vacca et al., 2020), thus neutralising the ability of epithelial cells to kick-start the immune response. Similarly, secretions of Trichuris suis reduce TSLP release by intestinal epithelial cells (Hiemstra et al., 2014), as well as interfere with DC activity as discussed below.
Live larvae are generally sheathed with a redundant cuticle that can be rapidly discarded on entry into the host (Kumar and Pritchard, 1992). The sheath is now recognised not only as a protective layer, but as a decoy. Thus, skin-penetrating N. americanus larvae attract lectin-dependent DC adherence to their sheath, allowing the parasites to migrate away from immune cells immobilised on the cast material (Hassan et al., 2018). Similarly, Toxocara larvae which can migrate through somatic tissues express a surface coat that attracts antibody and granulocyte binding, but is readily shed to facilitate immune evasion (Fattah et al., 1986; Page et al., 1992).
While in the first instance, STH evasion mechanisms vary widely according to their route of entry and subsequent migratory tropism, all helminth species exert profound effects on DCs (White and Artavanis-Tsakonas, 2012). DCs are the pivotal population which presents parasite antigens to T cells, triggers the activation of these cells, and drives them toward the Th2 mode that is necessary for parasite destruction. Thus, blocking DC function is of critical importance in STH infection. For STHs with limited options for in vivo models, investigations have primarily been conducted in vitro, using ES products from congeners (eg A. suum and T. suis for their human counterparts). These ES products abrogate DC responses to bacterial stimuli (eg lipopolysaccharide), minimise inflammatory cytokine production, and reduce their ability to stimulate T cells. In the case of Ascaris, an abundant body constituent (pseudocoelomic fluid, PCF) was found to mediate these effects (McConchie et al., 2006). More recently, Ascaris PCF has been shown to strongly interfere with the central pathway of toll like receptor (TLR) signaling in DCs through MyD88 (Arora et al., 2020). A close parallel exists with the ES products of T. suis which not only down-modulate human DCs in a similar manner (Klaver et al., 2013), but are able to interfere with macrophage TLR signaling (Ottow et al., 2014).
Having negotiated the innate immune system, STHs then need to create a new balance in the immune system to minimise local inflammation and permit their continued tenure. If this balance is not struck, parasites may be expelled but the host may also suffer collateral immunopathology from an over-zealous immune response. To some extent, this new host–parasite accord resembles a form of immunological tolerance mediated at two levels. First, immune cells may be intrinsically hyporesponsive or down-regulated, as occurs in the modified type 2 response (Maizels and Yazdanbakhsh, 2003). Secondly, immune reactivity to STHs may be actively suppressed by the regulatory T cell (Treg) population that normally protects the body from autoimmunity and food allergy through soluble regulatory cytokines (IL-10 and TGFβ) and surface inhibitory receptors such as CTLA-4 (White et al., 2020). Consistent with this concept, children exposed to high levels of Ascaris and Trichuris showed poor immune reactivity (IL-4 and IFNγ production) and high levels of regulatory cytokines in in vitro lymphocyte assays (Turner et al., 2008; Figueiredo et al., 2010).
Effector T cell hyporesponsiveness is commonly observed across many human helminth infections, including STHs (Figueiredo et al., 2010), and may result from aberrent DC signalling, Treg modulation, or both. Paradoxically, hyporesponsive individuals can display very high levels of IgG4 isotype antibodies rather than IgE; indeed, in human ascariasis, the IgG4:IgE ratio correlated with intensity of infection (Turner et al., 2005). An interesting parallel was drawn with the “Modified Type 2” phenotype observed during desensitization of allergic individuals, in which the IgE isotype is displaced by a dominant IgG4 antibody response (Platts-Mills et al., 2004). Subsequently, IgG4 was found to be promoted by IL-10 and TGF-β (Satoguina et al., 2008), suggesting that this isotype may be a reflection of potent Treg activation during STH infection.
The Treg pathway is emerging as a central feature in human STH infections, and is tracked by expression of the canonical transcription factor FOXP3 (Logan et al., 2018; de Ruiter et al., 2020). In some animal models of STH, parasites secrete proteins that induce Foxp3 expression and functional Tregs (Johnston et al., 2017; White et al., 2021), including in human peripheral blood T cells (Cook et al., 2021). Direct evidence for Treg expansion, or Treg-driving secreted products, in human STH infections is relatively limited, although in natural N. americanus infections there are greater frequencies of FOXP3+ Tregs, associated with elevated CTLA-4 as well as IL-17 (Ricci et al., 2011). A similar rise in FOXP3+ Tregs was observed in coeliac disease patients given experimental therapy with live hookworms (Croese et al., 2015). In Strongyloides patients, elevated Tregs are associated with suppression of the Th2 effector cytokine IL-5 (Montes et al., 2009), while in a remarkable study in Argentina, multiple sclerosis patients acquiring intestinal helminth infections (not exclusively STHs) showed raised FOXP3+ Treg numbers and were protected from disease relapse (Correale and Farez, 2007). As well as Treg frequency, their function may also be altered in STH infection, as following albendazole treatment of children in Indonesia, Treg numbers did not decline but their CTLA-4 expression was abated (Wammes et al., 2016).
6. Use of STH and their molecular derivatives to treat inflammatory diseases
The safety and tolerability of experimental human helminth infections have resulted in their use as a novel therapeutic modality for the treatment of a range of diseases that result from a dysregulated immune response, notably allergic and autoimmune diseases (Garg et al., 2014; Zuo et al., 2018; Ryan et al., 2020). Two helminths, both STHs, have been used – the zoonotic T. suis and the anthropophilic N. americanus. The therapeutic efficacy and tolerability of orally administered T. suis ova (TSO) has been assessed in phase 1 trials in patients with IBD – Crohn’s disease and ulcerative colitis - and despite promising phase 1 trials (Summers, 2005), subsequent phase 2 trials failed to reach their clinical endpoints in both IBD (Schölmerich et al., 2016) and multiple sclerosis (Voldsgaard et al., 2015; Fleming et al., 2019).
Trichuris suis is a parasite of pigs and is expelled from the human body rapidly, thereby requiring frequent dosing. Necator. americanus however is primarily anthropophilic and survives for many years in infected people (Loukas et al., 2016). Experimental N. americanus infection in human volunteers is safe and well-tolerated (Blount et al., 2009; Feary et al., 2009; Daveson et al., 2011). Croese et al. assessed the safety of low-dose percutaneously administered N. americanus L3 in patients with Crohn’s disease (one of the two major forms of IBD), and found the infection to be well tolerated; moreover, despite being on open label trial, all patients who remained in the trial for 1 year were in disease remission (Croese et al., 2006). A randomised controlled trial (RCT) is underway in New Zealand to comprehensively assess the efficacy of N. americanus as a maintenance therapy in patients with ulcerative colitis (Australian New Zealand Clinical Trials Registry ACTRN12620000956909). Necator americanus has also been assessed for efficacy in a RCT in coeliac disease, where subjects on a gluten-free diet received N. americanus L3 or topical chilli sauce (placebo) (Croese et al., 2020). Hookworm infection did not restore tolerance to sustained moderate consumption of gluten but was associated with improved symptom scores after intermittent consumption of lower gluten doses, and infection has been shown to promote Treg responses in the gut of human subjects (Croese et al., 2015).
An increasing body of literature supports a role for helminths in combatting inflammation in type 2 diabetes (de Ruiter et al., 2017; Gao et al., 2021). Animal studies with model STHs have shown that hookworms can prevent diet-induced obesity and insulin resistance in mice (Yang et al., 2013; Khudhair et al., 2021). Epidemiologic studies have revealed a negative association between STH infections and metabolic syndrome, particularly for Strongyloides infection (Tracey et al., 2016). Following on from this review, the first clinical trial assessing experimental helminth infection (N. americanus) in metabolic syndrome is underway in Australia (Pierce et al., 2019). Most of these early-phase clinical trials with experimental STH infections were impaired by the absence of current good laboratory/manufacturing protocols for infective stage parasites. Methods for the production of cGMP hookworms were recently reported (Diemert et al., 2018) and are essential if helminth therapy is to be widely adopted and commercially developed in the future.
A rapidly growing array of helminth-derived immunomodulatory molecules is being defined and evaluated in pre-clinical models of inflammatory diseases (Maizels et al., 2018; Ryan et al., 2020). ES products from STHs, as frequently found across the helminth groups, can dampen inflammation in mouse models (Ebner et al., 2014). To date, relatively few individual mediators have been defined from STHs (Fig. 2), but notable among them are members of the TIMP-like hookworm Anti-Inflammatory Protein family, AIP-1 (Buitrago et al., 2021) and AIP-2 (Navarro et al., 2016), the latter of which acts through DCs to expand Tregs and protect against inducible asthma and colitis. The cystatin family of cysteine protease inhibitors are abundantly secreted by parasitic nematodes and have been shown to have anti-inflammatory properties (Schierack et al., 2003), including those secreted by Ascaris (Coronado et al., 2019) and the rodent hookworm N. brasiliensis (Dainichi et al., 2001). These proteins suppress antigen-specific responses by inhibiting the catalytic activity of proteases involved in antigen processing, and the former has proven efficacious in a mouse model of house dust mite airway allergy. STH secrete immunoregulatory molecules that act directly on T cells, typified by the hookworm homologues of sea anemone ShK toxins that block Kv1.3 voltage-gated potassium channels and are crucial for the activation of effector memory T cells (Smallwood et al., 2021). STH also secrete proteins that mimic cytokines and cytokine receptors, including the major secreted protein of T. muris, p43, which has homology to the IL-13 receptor α2, and binds to IL-13, inhibiting its function in vitro and in vivo (Bancroft et al., 2019). Finally, the SCP/TAPS family of cysteine-rich proteins is massively expanded in the genomes and secretomes of STHs, particularly hookworms (Cantacessi et al., 2009; Tang et al., 2014), and two members of this family have been shown to have immunomodulatory properties. Neutrophil Inhibitory Factor (NIF) from A. caninum binds to CD11b/CD18 and inhibits neutrophil accumulation at sites of tissue injury (Moyle et al., 1994), and progressed to phase 2 clinical trials for treating ischemic stroke (Krams et al., 2003). The major larval secreted protein released upon tissue invasion by N. americanus, Na-ASP-2, is a SCP/TAPS which binds to CD79A on B cells and suppresses expression of genes that regulate B cell signalling (Tribolet et al., 2014).
Fig. 2.
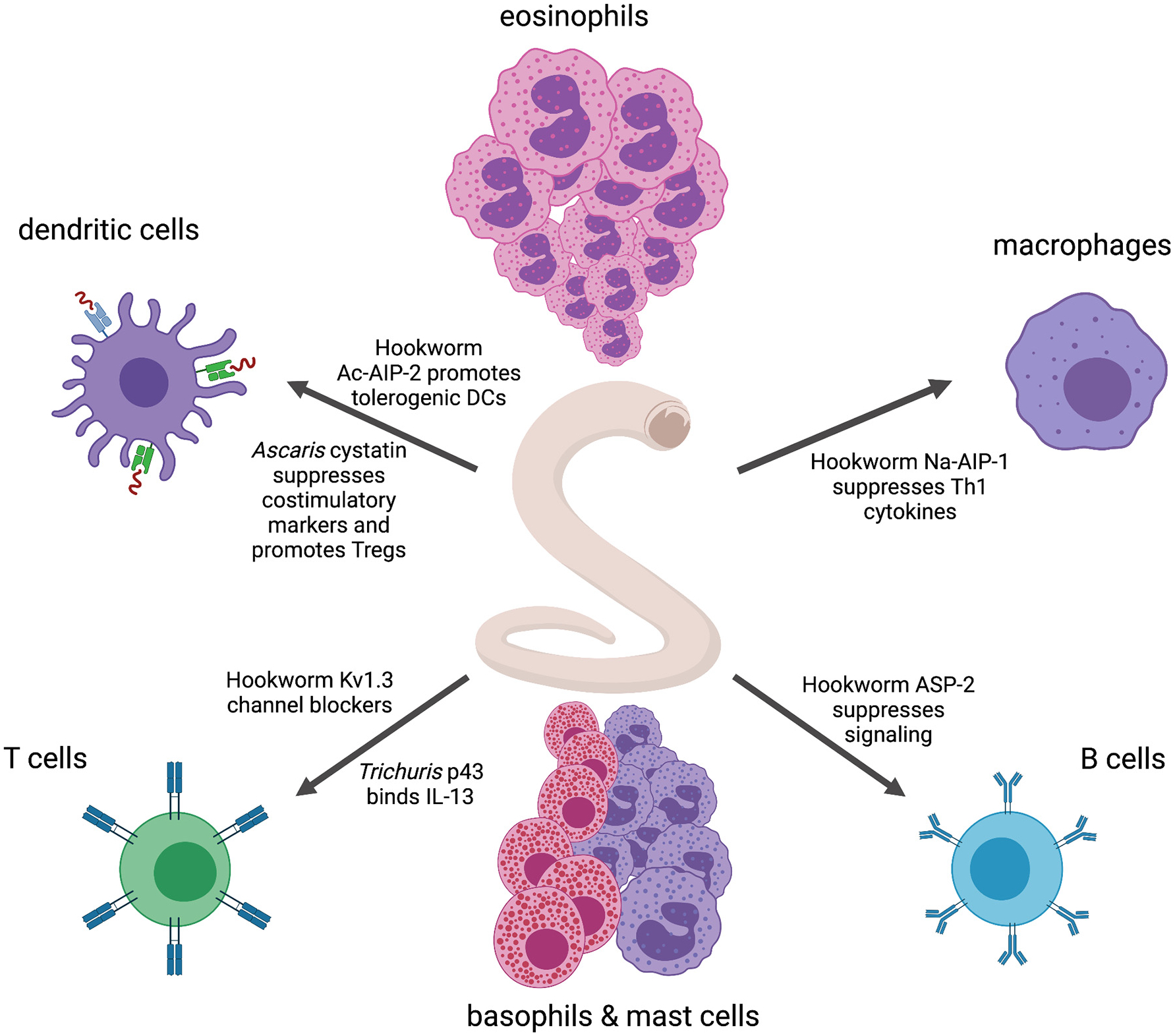
Mechanisms by which soil transmitted helminth defined recombinant proteins modulate the host immune system.
In addition to proteins, STHs secrete small molecules (Wangchuk et al., 2019a, 2019b; Yeshi et al., 2020). Ancylostoma caninum metabolites can ameliorate colitis in mice and suppress inflammatory cytokine secretion from human peripheral blood mononuclear cells (Wangchuk et al., 2019a, 2019b). There is also an emerging role for microRNAs contained within extracellular vesicles of related animal parasites in suppressing inflammation (Coakley et al., 2017; White et al., 2020), including prevention of inducible colitis (Eichenberger et al., 2018), and this is an exciting field that is rapidly gaining momentum (Drurey and Maizels, 2021). Finally, STHs have been shown to modulate inflammation indirectly through manipulation of the host microbiome, a topic that has been extensively reviewed elsewhere (Giacomin et al., 2015; Brosschot and Reynolds, 2018).
7. Concluding remarks
The morbidity attributable to STH infections is substantial, and uncontested. Vaccines are sorely needed in the absence of major progress towards elimination based on mass drug administration alone. The absence of vaccines for any human helminth infection can be attributed to a lack of financial investment in this area, but it is not just a money problem. Helminths deploy a sophisticated array of strategies to divert and subvert the human host’s best attempts to immunologically terminate them. While frustrating to researchers and devastating to infected subjects who suffer the pathologic sequelae, the immunomodulatory properties of these helminths have revealed hitherto unexplored pathways to suppress inflammation and other physiological processes. Coevolution of vertebrates with their invertebrate parasites has resulted in an arms race that must hold a treasure trove of untapped opportunities for modern medicine. The safety and tolerability profile of relatively high numbers of N. americanus in healthy volunteers (Hoogerwerf et al., 2019) and the tantalising pre-clinical studies revealing therapeutic moieties in animal models of inflammatory disorders set the scene for exciting and fruitful endeavours ahead. We look forward to the next major breakthroughs in the field, whether they be aimed at eliminating or promoting these infections under the right circumstances. Of course, there remains the question that in a worm-free world, will we suffer from an even greater burden of non-communicable diseases of modernity? That subject poses moral and ethical dilemmas, and as such warrants an entire article unto itself.
Acknowledgements
AL is the recipient of a Senior Principal Research Fellowship (1117504) and program grant (1132975) from the National Health and Medical Research Council, Australia. PJH is the recipient of a R21 grant for trichuriasis vaccines from the National Institutes of Health, USA, NIH R21 AI 144555. RMM gratefully acknowledges funding from the Wellcome Trust, UK, through an Investigator Award (Ref 219530) and the core-funded Wellcome Centre for Integrative Parasitology, UK (Ref: 104111).
References
- Adegnika AA, de Vries SG, Zinsou FJ, Honkepehedji YJ, Dejon Agobé J-C, Vodonou KG, Bikangui R, Bouyoukou Hounkpatin A, Bache EB, Massinga Loembe M, van Leeuwen R, Molemans M, Kremsner PG, Yazdanbakhsh M, Hotez PJ, Bottazzi ME, Li G, Bethony JM, Diemert DJ, Grobusch MP, Mouwenda YD, Betouke Ongwe E, Nkoma Mouima A-M, Nouatin OP, Edoa JR, Manouana PG, Pinto de Jesus S, Kühne V, Mordmueller B, Lell B, Agnandji ST, Koehler C, 2021. Safety and immunogenicity of co-administered hookworm vaccine candidates Na-GST-1 and Na-APR-1 in Gabonese adults: a randomised, controlled, double-blind, phase 1 dose-escalation trial. Lancet Infect. Dis 21, 275–285. 10.1016/S1473-3099(20)30288-7. [DOI] [PubMed] [Google Scholar]
- Allen JE, Sutherland TE, 2014. Host protective roles of type 2 immunity: Parasite killing and tissue repair, flip sides of the same coin. Sem. Immunol 26, 329–340. 10.1016/j.smim.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, May RM, 1982. Population dynamics of human helminth infections: control by chemotherapy. Nature 297, 557–563. 10.1038/297557a0. [DOI] [PubMed] [Google Scholar]
- Arora P, Moll JM, Andersen D, Workman CT, Williams AR, Kristiansen K, Brix S, 2020. Body fluid from the parasitic worm Ascaris suum inhibits broad-acting pro-inflammatory programs in dendritic cells. Immunol 159, 322–334. 10.1111/imm.v159.310.1111/imm.13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ásbjörnsdóttir KH, Ajjampur SSR, Anderson RM, Bailey R, Gardiner I, Halliday KE, Ibikounle M, Kalua K, Kang G, Littlewood DTJ, Luty AJF, Means AR, Oswald W, Pullan RL, Sarkar R, Schär F, Szpiro A, Truscott JE, Werkman M, Yard E, Walson JL, Garba A, 2018. Assessing the feasibility of interrupting the transmission of soil-transmitted helminths through mass drug administration: The DeWorm3 cluster randomized trial protocol. PLOS Neglect. Trop. Dis 12, e0006166. 10.1371/journal.pntd.0006166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asojo OA, Homma K, Sedlacek M, Ngamelue M, Goud GN, Zhan B, Deumic V, Asojo O, Hotez PJ, 2007. X-ray structures of Na-GST-1 and Na-GST-2 two glutathione s-transferase from the human hookworm Necator americanus. BMC Struct. Biol 7, 42. 10.1186/1472-6807-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft AJ, Levy CW, Jowitt TA, Hayes KS, Thompson S, Mckenzie EA, Ball MD, Dubaissi E, France AP, Bellina B, Sharpe C, Mironov A, Brown SL, Cook PC, MacDonald AS, Thornton DJ, Grencis RK, 2019. The major secreted protein of the whipworm parasite tethers to matrix and inhibits interleukin-13 function. Nature Commun 10. 10.1038/s41467-019-09996-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett S, Eichenberger RM, Nevagi RJ, Ghaffar KA, Marasini N, Dai Y, Loukas A, Toth I, Skwarczynski M, 2020. Lipopeptide-based oral vaccine against hookworm infection. J. Infect. Dis 221, 934–942. 10.1093/infdis/jiz528. [DOI] [PubMed] [Google Scholar]
- Bartsch SM, Hotez PJ, Hertenstein DL, Diemert DJ, Zapf KM, Bottazzi ME, Bethony JM, Brown ST, Lee BY, 2016. Modeling the economic and epidemiologic impact of hookworm vaccine and mass drug administration (MDA) in Brazil, a high transmission setting. Vaccine 34, 2197–2206. 10.1016/j.vaccine.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ, 2006. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367, 1521–1532. 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- Bethony J, Loukas A, Smout M, Brooker S, Mendez S, Plieskatt J, Goud G, Bottazzi ME, Zhan B, Wang Y, Williamson A, Lustigman S, Correa-Oliveira R, Xiao S, Hotez PJ, 2005. Antibodies against a secreted protein from hookworm larvae reduce the intensity of hookworm infection in humans and vaccinated laboratory animals. FASEB J 19, 1743–1745. 10.1096/fj.05-3936fje. [DOI] [PubMed] [Google Scholar]
- Bethony JM, Simon G, Diemert DJ, Parenti D, Desrosiers A, Schuck S, Fujiwara R, Santiago H, Hotez PJ, 2008. Randomized, placebo-controlled, double-blind trial of the Na-ASP-2 Hookworm Vaccine in unexposed adults. Vaccine 26, 2408–2417. 10.1016/j.vaccine.2008.02.049. [DOI] [PubMed] [Google Scholar]
- Blount D, Hooi D, Feary J, Venn A, Telford G, Brown A, Britton J, Pritchard D, 2009. Immunologic profiles of persons recruited for a randomized, placebo-controlled clinical trial of Hookworm infection. Am. J. Trop. Med. Hyg 81, 911–916. 10.4269/ajtmh.2009.09-0237. [DOI] [PubMed] [Google Scholar]
- Bradbury RS, Hii SF, Harrington H, Speare R, Traub R, 2017. Ancylostoma ceylanicum Hookworm in the Solomon Islands. Emerg. Infect. Dis 23, 252–257. 10.3201/eid2302.160822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs N, Wei J, Versteeg L, Zhan B, Keegan B, Damania A, Pollet J, Hayes KS, Beaumier C, Seid CA, Leong J, Grencis RK, Bottazzi ME, Sastry KJ, Hotez PJ, Mitre E, 2018. Trichuris muris whey acidic protein induces type 2 protective immunity against whipworm. PLOS Path 14, e1007273. 10.1371/journal.ppat.1007273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadhurst MJ, Leung JM, Kashyap V, McCune JM, Mahadevan U, McKerrow JH, Loke P, 2010. IL-22+ CD4+ T cells are associated with therapeutic Trichuris trichiura infection in an ulcerative colitis patient. Sci. Transl. Med 2, 60ra88. 10.1126/scitranslmed.3001500. [DOI] [PubMed] [Google Scholar]
- Brooker S, Hotez PJ, Bundy DAP, Raso G, 2008. Hookworm-related anaemia among pregnant women: A systematic review. PLoS Neglect. Trop. Dis 2, e291. 10.1371/journal.pntd.0000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosschot TP, Reynolds LA, 2018. The impact of a helminth-modified microbiome on host immunity. Mucosal Immunol 11, 1039–1046. 10.1038/s41385-018-0008-5. [DOI] [PubMed] [Google Scholar]
- Buitrago G, Pickering D, Ruscher R, Cobos Caceres C, Jones L, Cooper M, van Waardenberg A, Ryan S, Miles K, Field M, Dredge K, Daly NL, Giacomin PR, Loukas A, 2021. A netrin domain-containing protein secreted by the human hookworm Necator americanus protects against CD4 T cell transfer colitis. Transl. Res 232, 88–102. 10.1016/j.trsl.2021.02.012. [DOI] [PubMed] [Google Scholar]
- Buonfrate D, Bisanzio D, Giorli G, Odermatt P, Fürst T, Greenaway C, French M, Reithinger R, Gobbi F, Montresor A, Bisoffi Z, 2020. The global prevalence of Strongyloides stercoralis infection. Pathogens 9, 468. 10.3390/pathogens9060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantacessi C, Campbell BE, Visser A, Geldhof P, Nolan MJ, Nisbet AJ, Matthews JB, Loukas A, Hofmann A, Otranto D, Sternberg PW, Gasser RB, 2009. A portrait of the “SCP/TAPS” proteins of eukaryotes — Developing a framework for fundamental research and biotechnological outcomes. Biotech. Adv 27, 376–388. 10.1016/j.biotechadv.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Cappello M, Hotez P, 1993. Disseminated strongyloidiasis. Semin. Neurol 13, 169–174. 10.1055/s-2008-1041122. [DOI] [PubMed] [Google Scholar]
- Chapman PR, Webster R, Giacomin P, Llewellyn S, Becker L, Pearson MS, de Labastida Rivera F, O’Rourke P, Engwerda CR, Loukas A, McCarthy JS, 2021. Vaccination of human participants with attenuated Necator americanus hookworm larvae and human challenge in Australia: a dose-finding study and randomised, placebo-controlled, phase 1 trial S1473–3099(21)00153–5 Lancet Infect. Dis 10.1016/S1473-3099(21)00153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coakley G, Mccaskill JL, Borger JG, Mcsorley HJ, Maizels RM, Buck AH, Coakley G, Mccaskill JL, Borger JG, Simbari F, Robertson E, Millar M, Harcus Y, Mcsorley HJ, Maizels RM, Buck AH, 2017. Extracellular vesicles from a helminth parasite suppress macrophage activation and constitute an effective vaccine for protective immunity. Cell Rep 19, 1545–1557. 10.1016/j.celrep.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella V, Bradbury R, Traub R, 2021. Ancylostoma ceylanicum. Trends Parasitol 37, 844–845. 10.1016/j.pt.2021.04.013. [DOI] [PubMed] [Google Scholar]
- Cook L, Reid KT, Häkkinen E, Bie B, Tanaka S, Smyth DJ, White MPJ, Wong MQ, Huang Q, Gillies JK, Ziegler SF, Maizels RM, Levings MK, 2021. Induction of stable human FOXP3 + Tregs by a parasite-derived TGF-β mimic. Immunol. Cell Biol 99, 833–847. 10.1111/imcb.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado S, Zakzuk J, Regino R, Ahumada V, Benedetti I, Angelina A, Palomares O, Caraballo L, 2019. Ascaris lumbricoides cystatin prevents development of allergic airway inflammation in a mouse model. Front. Immunol 10, 2280. 10.3389/fimmu.2019.02280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correale J, Farez M, 2007. Association between parasite infection and immune responses in multiple sclerosis. Ann. Neurol 61, 97–108. 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- Croese J, Giacomin P, Navarro S, Clouston A, McCann L, Dougall A, Ferreira I, Susianto A, O’Rourke P, Howlett M, McCarthy J, Engwerda C, Jones D, Loukas, 2015. Experimental hookworm infection and gluten microchallenge promote tolerance in celiac disease. J. Allergy Clin. Immunol 135, 508–516.e5. 10.1016/j.jaci.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Croese J, Miller GC, Marquart L, Llewellyn S, Gupta R, Becker L, Clouston AD, Welch C, Sidorenko J, Wallace L, Visscher PM, Remedios ML, McCarthy JS, O’Rourke P, Radford-Smith G, Loukas A, Norrie M, Masson JW, Gearry RB, Rahman T, Giacomin PR, 2020. Randomized, placebo controlled trial of experimental Hookworm infection for improving gluten tolerance in celiac disease. Clin. Transl. Gastroenterol 11, e00274. 10.14309/ctg.0000000000000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croese J, O’Neil J, Masson J, Cooke S, Melrose W, Pritchard D, Speare R, 2006. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut 55, 136–137. 10.1136/gut.2005.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri WA, Dainichi T, Maekawa Y, Ishii K, Zhang T, Nashed BF, Sakai T, Takashima M, Himeno K, 2001. Nippocystatin, a cysteine protease inhibitor from Nippostrongylus brasiliensis, inhibits antigen processing and modulates antigen-specific immune response. Infect. Immun 69, 7380–7386. 10.1128/IAI.69.12.7380-7386.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daveson AJ, Jones DM, Gaze S, McSorley H, Clouston A, Pascoe A, Cooke S, Speare R, Macdonald GA, Anderson R, McCarthy JS, Loukas A, Croese J, Gluud LL, 2011. Effect of hookworm infection on wheat challenge in celiac disease - a randomised double-blinded placebo controlled trial. PLoS ONE 6, e17366. 10.1371/journal.pone.0017366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro JC, de Almeida LV, Cardoso MS, Oliveira FMS, Nogueira DS, Reis-Cunha JL, Magalhaes LMD, Zhan B, Bottazzi ME, Hotez PJ, Bueno LL, Bartholomeu DC, Fujiwara RT, 2021. Vaccination with chimeric protein induces protection in murine model against ascariasis. Vaccine 39, 394–401. 10.1016/j.vaccine.2020.11.046. [DOI] [PubMed] [Google Scholar]
- de Ruiter K, Jochems SP, Tahapary DL, Stam KA, König M, van Unen V, Laban S, Höllt T, Mbow M, Lelieveldt BPF, Koning F, Sartono E, Smit JWA, Supali T, Yazdanbakhsh M, 2020. Helminth infections drive heterogeneity in human type 2 and regulatory cells. Sci. Transl. Med 12, eaaw3703. 10.1126/scitranslmed.aaw3703. [DOI] [PubMed] [Google Scholar]
- de Ruiter K, Tahapary DL, Sartono E, Soewondo P, Supali T, Smit JWA, Yazdanbakhsh M, 2017. Helminths, hygiene hypothesis and type 2 diabetes. Parasite Immunol 39,. 10.1111/pim.12404 e12404. [DOI] [PubMed] [Google Scholar]
- Diemert D, Campbell D, Brelsford J, Leasure C, Li G, Peng J, Zumer M, Younes N, Bottazzi ME, Mejia R, Pritchard DI, Hawdon JM, Bethony JM, 2018a. Controlled human Hookworm infection: accelerating human hookworm vaccine development. open forum. Infect. Dis 5, ofy083. 10.1093/ofid/ofy083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diemert DJ, Bottazzi ME, Plieskatt J, Hotez PJ, Bethony JM, 2018b. Lessons along the critical path: developing vaccines against human helminths. Trends Parasitol 34, 747–758. 10.1016/j.pt.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Diemert DJ, Freire J, Valente V, Fraga CG, Talles F, Grahek S, Campbell D, Jariwala A, Periago MV, Enk M, Gazzinelli MF, Bottazzi ME, Hamilton R, Brelsford J, Yakovleva A, Li G, Peng J, Correa-Oliveira R, Hotez P, Bethony J, McCarthy JS, 2017. Safety and immunogenicity of the Na-GST-1 hookworm vaccine in Brazilian and American adults. PLOS Neglect. Trop. Dis 11, e0005574. 10.1371/journal.pntd.0005574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diemert DJ, Pinto AG, Freire J, Jariwala A, Santiago H, Hamilton RG, Periago MV, Loukas A, Tribolet L, Mulvenna J, Correa-Oliveira R, Hotez PJ, Bethony JM, 2012. Generalized urticaria induced by the Na-ASP-2 hookworm vaccine: Implications for the development of vaccines against helminths. J. Allergy Clin. Immunol 130, 169–176.e6. 10.1016/j.jaci.2012.04.027. [DOI] [PubMed] [Google Scholar]
- Dixon H, Little MC, Else KJ, 2010. Characterisation of the protective immune response following subcutaneous vaccination of susceptible mice against Trichuris muris. Int. J. Parasitol 40, 683–693. 10.1016/j.ijpara.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drurey C, Maizels RM, 2021. Helminth extracellular vesicles: Interactions with the host immune system. Mol. Immunol 137, 124–133. 10.1016/j.molimm.2021.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner F, Hepworth MR, Rausch S, Janek K, Niewienda A, Kühl A, Henklein P, Lucius R, Hamelmann E, Hartmann S, 2014. Therapeutic potential of larval excretory/secretory proteins of the pig whipworm Trichuris suis in allergic disease. Allergy 69, 1489–1497. 10.1111/all.12496. [DOI] [PubMed] [Google Scholar]
- Eichenberger RM, Ryan S, Jones L, Buitrago G, Polster R, Montes de Oca M, Zuvelek J, Giacomin PR, Dent LA, Engwerda CR, Field MA, Sotillo J, Loukas A, 2018. Hookworm secreted extracellular vesicles interact with host cells and prevent inducible colitis in mice. Front. Immunol 9. 10.3389/fimmu.2018.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfawal MA, Savinov SN, Aroian R, v.,, 2019. Drug screening for discovery of broad-spectrum agents for soil-transmitted nematodes. Sci. Rep 9, 12347. 10.1038/s41598-019-48720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins DB, Haswell-Elkins M, Anderson RM, 1986. The epidemiology and control of intestinal helminths in the Pulicat Lake region of Southern India. I. Study design and pre- and post-treatment observations on Ascaris lumbricoides infection. Trans. R. Soc. Trop. Med. Hyg 80, 774–792. 10.1016/0035-9203(86)90384-6. [DOI] [PubMed] [Google Scholar]
- Else KJ, Keiser J, Holland CV, Grencis RK, Sattelle DB, Fujiwara RT, Bueno LL, Asaolu SO, Sowemimo OA, Cooper PJ, 2020. Whipworm and roundworm infections. Nature Rev. Dis. Primers 6. 10.1038/s41572-020-0171-3. [DOI] [PubMed] [Google Scholar]
- Fattah DI, Maizels RM, McLaren DJ, Spry CJF, 1986. Toxocara canis: Interaction of human blood eosinophils with the infective larvae. Exp. Parasitol 61, 421–431. 10.1016/0014-4894(86)90198-0. [DOI] [PubMed] [Google Scholar]
- Feary J, Venn A, Brown A, Hooi D, Falcone FH, Mortimer K, Pritchard DI, Britton J, 2009. Safety of hookworm infection in individuals with measurable airway responsiveness : a randomized placebo-controlled feasibility study. Clin. Exp. Allergy 39, 1060–1068. 10.1111/j.1365-2222.2009.03187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo CA, Barreto ML, Rodrigues LC, Cooper PJ, Silva Nívea.B., Amorim LD, Alcantara-Neves NM, 2010. Chronic intestinal helminth infections are associated with immune hyporesponsiveness and induction of a regulatory network. Infect. Immun 78, 3160–3167. 10.1128/IAI.01228-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleitas PE, Travacio M, Martí-Soler H, Socías ME, Lopez WR, Krolewiecki AJ, Cappello M, 2020. The Strongyloides stercoralis-hookworms association as a path to the estimation of the global burden of strongyloidiasis: A systematic review. PLOS Neglect. Trop. Dis 14, e0008184. 10.1371/journal.pntd.0008184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J, Hernandez G, Hartman L, Maksimovic J, Nace S, Lawler B, Risa T, Cook T, Agni R, Reichelderfer M, Luzzio C, Rolak L, Field A, Fabry Z, 2019. Safety and efficacy of helminth treatment in relapsing-remitting multiple sclerosis: Results of the HINT 2 clinical trial. Multiple Sclerosis J 25, 81–91. 10.1177/1352458517736377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhang R, Li R, Tang C, Pan Q, Pen P, 2021. The effects of helminth infections against type 2 diabetes. Parasitol. Res 120, 1935–1942. 10.1007/s00436-021-07189-6. [DOI] [PubMed] [Google Scholar]
- Garg SK, Croft AM, Bager P, 2014. Helminth therapy (worms) for induction of remission in inflammatory bowel disease. Cochrane Database Syst. Rev 1, CD009400. 10.1002/14651858.CD009400.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaze S, McSorley HJ, Daveson J, Jones D, Bethony JM, Oliveira LM, Speare R, McCarthy JS, Engwerda CR, Croese J, Loukas A, Grencis RK, 2012. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Path 8, e1002520. 10.1371/journal.ppat.1002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzinelli-Guimarães AC, Gazzinelli-Guimarães PH, Nogueira DS, Oliveira FMS, Barbosa FS, Amorim CCO, Cardoso MS, Kraemer L, Caliari MV, Akamatsu MA, Ho PL, Jones KM, Weatherhead J, Bottazzi ME, Hotez PJ, Zhan B, Bartholomeu DC, Russo RC, Bueno LL, Fujiwara RT, 2018. IgG induced by vaccination with Ascaris suum extracts is protective against infection. Front. Immunol 9, 2535. 10.3389/fimmu.2018.02535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbe F, Sidot E, Smyth DJ, Ohmoto M, Matsumoto I, Dardalhon V, Cesses P, Garnier L, Pouzolles M, Brulin B, Bruschi M, Harcus Y, Zimmermann Valérie.S., Taylor N, Maizels RM, Jay P, 2016. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529, 226–230. 10.1038/nature16527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomin P, Croese J, Krause L, Loukas A, Cantacessi C, 2015. Suppression of inflammation by helminths: a role for the gut microbiota? Phil. Trans. R. Soc. Series B, Biol. Sci 370, 20140296. 10.1098/rstb.2014.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goud GN, Bottazzi ME, Zhan B, Mendez S, Deumic V, Plieskatt J, Liu S, Wang Y, Bueno L, Fujiwara R, Samuel A, Ahn SY, Solanki M, Asojo OA, Wang J, Bethony JM, Loukas A, Roy M, Hotez PJ, 2005. Expression of the Necator americanus hookworm larval antigen Na-ASP-2 in Pichia pastoris and purification of the recombinant protein for use in human clinical trials. Vaccine 23, 4754–4764. 10.1016/j.vaccine.2005.04.040. [DOI] [PubMed] [Google Scholar]
- Hassan A, Pritchard DI, Ghaemmaghami AM, 2018. Human dendritic cell sequestration onto the Necator americanus larval sheath during ex-sheathing: a possible mechanism for immune privilege. Parasitology 145, 1183–1190. 10.1017/S0031182018000136. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Jones BF, Hoffman DR, Hotez PJ, 1996. Cloning and characterization of ancylostoma-secreted protein. J. Biol. Chem 271, 6672–6678. 10.1074/jbc.271.12.6672. [DOI] [PubMed] [Google Scholar]
- Hawdon JM, Narasimhan S, Hotez PJ, 1999. Ancylostoma secreted protein 2: cloning and characterization of a second member of a family of nematode secreted proteins from Ancylostoma caninum. Mol. Biochem. Parasitol 99, 149–165. 10.1016/S0166-6851(99)00011-0. [DOI] [PubMed] [Google Scholar]
- Hayon J, Weatherhead J, Hotez PJ, Bottazzi ME, Zhan B, 2021. Advances in vaccine development for human trichuriasis. Parasitol 24;1–12. 10.1017/S0031182021000500. [DOI] [PubMed] [Google Scholar]
- Herricks JR, Hotez PJ, Wanga V, Coffeng LE, Haagsma JA, Basáñez M-G, Buckle G, Budke CM, Carabin H, Fèvre EM, Fürst T, Halasa YA, King CH, Murdoch ME, Ramaiah KD, Shepard DS, Stolk WA, Undurraga EA, Stanaway JD, Naghavi M, Murray CJL, Zhou X-N, 2017. The global burden of disease study 2013: What does it mean for the NTDs? PLOS Neglect. Trop. Dis 11, e0005424. 10.1371/journal.pntd.0005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiemstra IH, Klaver EJ, Vrijland K, Kringel H, Andreasen A, Bouma G, Kraal G, van Die I, den Haan JMM, 2014. Excreted/secreted Trichuris suis products reduce barrier function and suppress inflammatory cytokine production of intestinal epithelial cells. Mol. Immunol 60, 1–7. 10.1016/j.molimm.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Hoogerwerf M-A, Coffeng LE, Brienen EAT, Janse JJ, Langenberg MCC, Kruize YCM, Gootjes C, Manurung MD, Dekker M, Becker L, Erkens MAA, van der Beek MT, Ganesh MS, Feijt C, Winkel BMF, Westra IM, Meij P, Loukas A, Visser LG, de Vlas SJ, Yazdanbakhsh M, van Lieshout L, Roestenberg M, 2019. New insights into the kinetics and variability of egg excretion in controlled human hookworm infections. J. Infect. Dis 220, 1044–1048. 10.1093/infdis/jiz218. [DOI] [PubMed] [Google Scholar]
- Hoogerwerf M-A, Koopman JPR, Janse JJ, Langenberg MCC, van Schuijlenburg R, Kruize YCM, Brienen EAT, Manurung MD, Verbeek-Menken P, van der Beek MT, Westra IM, Meij P, Visser LG, van Lieshout L, de Vlas SJ, Yazdanbakhsh M, Coffeng LE, Roestenberg M, 2021. A randomized controlled trial to investigate safety and variability of egg excretion after repeated controlled human hookworm infection. J. Infect. Dis 223, 905–913. 10.1093/infdis/jiaa414. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, 2014. Neglected infections of poverty in the United States and their effects on the brain. JAMA Psychol 71, 1099–1100. 10.1001/jamapsychiatry.2014.1045. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Bethony J, Bottazzi ME, Brooker S, Buss P, 2005. Hookworm: “The great infection of mankind”. PLoS Med 2, e67. 10.1371/journal.pmed.0020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez PJ, Bethony JM, Diemert DJ, Pearson M, Loukas A, 2010. Developing vaccines to combat hookworm infection and intestinal schistosomiasis. Nat. Rev. Microbiol 8, 814–826. 10.1038/nrmicro2438. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Zhan B, Bethony JM, Loukas A, Williamson A, Goud GN, Hawdon JM, Dobardzic A, Dobardzic R, Ghosh K, Bottazzi ME, Mendez S, Zook B, Wang Y, Liu S, Essiet-Gibson I, Chung-Debose S, Xiao S, Knox D, Meagher M, Inan M, Correa-Oliveira R, Vilk P, Shepherd HR, Brandt W, Russell PK, 2003. Progress in the development of a recombinant vaccine for human hookworm disease: The Human Hookworm Vaccine Initiative. Int. J. Parasitol 33, 1245–1258. 10.1016/S0020-7519(03)00158-9. [DOI] [PubMed] [Google Scholar]
- IHME, 2020a. Institute for Health Metrics and Evaluation. Intestinal nematode infections — Level 3 cause http://www.healthdata.org/results/gbd_summaries/2019/intestinal-nematode-infections-level-3-cause. http://www.healthdata.org/results/gbd_summaries/2019/intestinal-nematode-infections-level-3-cause (accessed 10.6.21).
- IHME, 2020b. Institute for Health Metrics and Evaluation - Ascariasis level 4 accessed 10.6.21 http://www.healthdata.org/results/gbd_summaries/2019/ascariasis-level-4-cause,.
- IHME, 2020c. Institute for Health Metrics and Evaluation - Trichuriasis, level 4 http://www.healthdata.org/results/gbd_summaries/2019/trichuriasis-level-4-cause.
- IHME, 2020d. Institute of Health Metrics and Evaulation. Hookworm disease, level 4 http://www.healthdata.org/results/gbd_summaries/2019/hookworm-disease-level-4-cause.
- Inpankaew T, Schär F, Dalsgaard A, Khieu V, Chimnoi W, Chhoun C, Sok D, Marti H, Muth S, Odermatt P, Traub RJ, 2014. High prevalence of Ancylostoma ceylanicum hookworm infections in humans, Cambodia, 2012. Emerg. Infect. Dis 20, 976–982. 10.3201/eid2006.131770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaleta TG, Zhou S, Bemm FM, Schär F, Khieu V, Muth S, Odermatt P, Lok JB, Streit A, Fuehrer H-P, 2017. Different but overlapping populations of Strongyloides stercoralis in dogs and humans—Dogs as a possible source for zoonotic strongyloidiasis. PLOS Neglect. Trop. Dis 11, e0005752. 10.1371/journal.pntd.0005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia TW, Melville S, Utzinger J, King CH, Zhou XN, Cooper PJ, 2012. Soil-transmitted helminth reinfection after drug treatment: a systematic review and meta-analysis. PLoS Neglect. Trop. Dis 6, e1621. 10.1371/journal.pntd.0001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CJC, Smyth DJ, Kodali RB, White MPJ, Harcus Y, Filbey KJ, Hewitson JP, Hinck CS, Ivens A, Kemter AM, Kildemoes AO, Le Bihan T, Soares DC, Anderton SM, Brenn T, Wigmore SJ, Woodcock HV, Chambers RC, Hinck AP, McSorley HJ, Maizels RM, 2017. A structurally distinct TGF-β mimic from an intestinal helminth parasite potently induces regulatory T cells. Nature Commun 8. 10.1038/s41467-017-01886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khudhair Z, Alhallaf R, Eichenberger RM, Whan J, Kupz A, Field M, Krause L, Wilson DT, Daly NL, Giacomin P, Sotillo J, Loukas A, 2021. Gastrointestinal helminth infection improves insulin sensitivity, decreases systemic inflammation, and alters the composition of gut microbiota in distinct mouse models of type 2 diabetes. Front. Endocrinol 11, 1132. 10.3389/fendo.2020.606530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaver EJ, Kuijk LM, Laan LC, Kringel H, van Vliet SJ, Bouma G, Cummings RD, Kraal G, van Die I, 2013. Trichuris suis-induced modulation of human dendritic cell function is glycan-mediated. Int. J. Parasitol 43, 191–200. 10.1016/j.ijpara.2012.10.021. [DOI] [PubMed] [Google Scholar]
- Krams M, Lees KR, Hacke W, Grieve AP, Orgogozo J-M, Ford GA, 2003. Acute stroke therapy by inhibition of neutrophils (ASTIN). Stroke 34, 2543–2548. 10.1161/01.STR.0000092527.33910.89. [DOI] [PubMed] [Google Scholar]
- Krolewiecki A, Nutman TB, 2019. Strongyloidiasis. Infect. Dis. Clin. N. Am 33, 135–151. 10.1016/j.idc.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Pritchard DI, 1992. Skin penetration by ensheathed third-stage infective larvae of Necator americanus, and the host’s immune response to larval antigens. Int. J. Parasitol 22, 573–579. 10.1016/0020-7519(92)90004-5. [DOI] [PubMed] [Google Scholar]
- Lin WM, Addiss DG, 2018. Sustainable access to deworming drugs in a changing landscape. Lancet Infect. Dis 18, e395–e398. 10.1016/S1473-3099(18)30351-7. [DOI] [PubMed] [Google Scholar]
- Logan J, Navarro S, Loukas A, Giacomin P, 2018. Helminth-induced regulatory T cells and suppression of allergic responses. Curr. Opin. Immunol 54, 1–6. 10.1016/j.coi.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Loukas A, Hotez PJ, Diemert D, Yazdanbakhsh M, McCarthy JS, Correa-Oliveira R, Croese J, Bethony JM, 2016. Hookworm infection. Nat. Rev. Dis. Primers 2 10.1038/nrdp.2016.88. [DOI] [PubMed] [Google Scholar]
- Ma G, Holland CV, Wang T, Hofmann A, Fan C-K, Maizels RM, Hotez PJ, Gasser RB, 2018. Human toxocariasis. Lancet Infect. Dis 18, e14–e24. 10.1016/S1473-3099(17)30331-6. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Smits HH, McSorley HJ, 2018. Modulation of host immunity by helminths: the expanding repertoire of parasite effector molecules. Immunity 49, 801–818. 10.1016/j.immuni.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels RM, Yazdanbakhsh M, 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nature Rev. Immunol 3, 733–744. 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- Majid MF, Kang SJ, Hotez PJ, Mc Farland D, 2019. Resolving “worm wars”: An extended comparison review of findings from key economics and epidemiological studies. PLOS Neglect. Trop. Dis 13, e0006940. 10.1371/journal.pntd.0006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConchie BW, Norris HH, Bundoc VG, Trivedi S, Boesen A, Urban JF, Keane-Myers AM, 2006. Ascaris suum-derived products suppress mucosal allergic inflammation in an interleukin-10-independent manner via interference with dendritic cell function. Infect. Immun 74, 6632–6641. 10.1128/IAI.00720-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSorley HJ, Maizels RM, 2012. Helminth Infections and Host Immune Regulation. Clin. Microbiol. Rev 25, 585–608. 10.1128/CMR.05040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia R, Nutman TB, 2012. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Curr. Opin. Infect. Dis 25, 458–463. 10.1097/QCO.0b013e3283551dbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migel E, Kremer M, 2004. Worms: identifying impacts on education and health in the presence of treatment externalities. Econometrica 72, 159–217. [Google Scholar]
- Miller T, 1978. Industrial development and field use of the canine hookworm vaccine. Adv. Parasitol 16, 333–342. [DOI] [PubMed] [Google Scholar]
- Miller TA, 1964. Effect of x-irradiation upon the infective larvae of Ancylostoma caninum and the immunogenic effect in dogs of a single infection with 40 kR-irradiated larvae. J. Parasitol 50, 735. 10.2307/3276194. [DOI] [PubMed] [Google Scholar]
- Montes M, Sanchez C, Verdonck K, Lake JE, Gonzalez E, Lopez G, Terashima A, Nolan T, Lewis DE, Gotuzzo E, White AC, Steinmann P, 2009. Regulatory T cell expansion in HTLV-1 and strongyloidiasis co-infection is associated with reduced IL-5 responses to Strongyloides stercoralis antigen. PLoS Neglect. Trop. Dis 3, e456. 10.1371/journal.pntd.0000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser W, Schindler C, Keiser J, 2019. Drug combinations against soil-transmitted helminth infections 103, 91–115. 10.1016/bs.apar.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Moyle M, Foster DL, McGrath DE, Brown SM, Laroche Y, De Meutter J, Stanssens P, Bogowitz CA, Fried VA, Ely JA, 1994. A hookworm glycoprotein that inhibits neutrophil function is a ligand of the integrin CD11b/CD18. J. Biol. Chem 269, 10008–10015. [PubMed] [Google Scholar]
- Navarro S, Pickering DA, Ferreira IB, Jones L, Ryan S, Troy S, Leech A, Hotez PJ, Zhan B, Laha T, Prentice R, Sparwasser T, Croese J, Engwerda CR, Upham JW, Julia V, Giacomin PR, Loukas A, 2016. Hookworm recombinant protein promotes regulatory T cell responses that suppress experimental asthma. Sci. Transl. Med 8, 362ra143. 10.1126/scitranslmed.aaf8807. [DOI] [PubMed] [Google Scholar]
- Ness TE, Agrawal V, Bedard K, Ouellette L, Erickson TA, Hotez P, Weatherhead JE, 2020. Maternal hookworm infection and its effects on maternal health: a systematic review and meta-analysis. Am. J. Trop. Med. Hyg 103, 1958–1968. 10.4269/ajtmh.20-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira DS, Gazzinelli-Guimarães PH, Barbosa FS, Resende NM, Silva CC, de Oliveira LM, Amorim CCO, Oliveira FMS, Mattos MS, Kraemer LR, Caliari MV, Gaze S, Bueno LL, Russo RC, Fujiwara RT, Doenhoff M, 2016. Multiple exposures to ascaris suum induce tissue injury and mixed Th2/Th17 immune response in mice. PLOS Neglect. Trop. Dis 10, e0004382. 10.1371/journal.pntd.0004382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osbourn M, Soares DC, Vacca F, Cohen ES, Scott IC, Gregory WF, Smyth DJ, Toivakka M, Kemter AM, le Bihan T, Wear M, Hoving D, Filbey KJ, Hewitson JP, Henderson H, Gonzàlez-Cìscar A, Errington C, Vermeren S, Astier AL, Wallace WA, Schwarze J, Ivens AC, Maizels RM, McSorley HJ, 2017. HpARI protein secreted by a helminth parasite suppresses interleukin-33. Immunity 47, 739–751.e5. 10.1016/j.immuni.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottow MK, Klaver EJ, van der Pouw Kraan TCTM, Heijnen PD, Laan LC, Kringel H, Vogel DYS, Dijkstra CD, Kooij G, van Die I, 2014. The helminth Trichuris suis suppresses TLR4-induced inflammatory responses in human macrophages. Genes Immun 15, 477–486. 10.1038/gene.2014.38. [DOI] [PubMed] [Google Scholar]
- Page AP, Rudin W, Fluri E, Blaxter ML, Maizels RM, 1992. Toxocara canis: A labile antigenic surface coat overlying the epicuticle of infective larvae. Exp. Parasitol 75, 72–86. 10.1016/0014-4894(92)90123-R. [DOI] [PubMed] [Google Scholar]
- Palmeirim MS, Hürlimann E, Knopp S, Speich B, Belizario V, Joseph SA, Vaillant M, Olliaro P, Keiser J, Stolk WA, 2018. Efficacy and safety of co-administered ivermectin plus albendazole for treating soil-transmitted helminths: A systematic review, meta-analysis and individual patient data analysis. PLOS Neglect. Trop. Dis 12, e0006458. 10.1371/journal.pntd.0006458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson MS, Bethony JM, Pickering DA, Oliveira LM, Jariwala A, Santiago H, Miles AP, Zhan B, Jiang D, Ranjit N, Mulvenna J, Tribolet L, Plieskatt J, Smith T, Bottazzi ME, Jones K, Keegan B, Hotez PJ, Loukas A, 2009. An enzymatically inactivated hemoglobinase from Necator americanus induces neutralizing antibodies against multiple hookworm species and protects dogs against heterologous hookworm infection. FASEB J 23, 3007–3019. 10.1096/fj.09-131433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce D, Merone L, Lewis C, Rahman T, Croese J, Loukas A, McDonald M, Giacomin P, McDermott R, 2019. Safety and tolerability of experimental hookworm infection in humans with metabolic disease: Study protocol for a phase 1b randomised controlled clinical trial. BMC Endocr. Disord 19, 136. 10.1186/s12902-019-0461-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platts-Mills TAE, Woodfolk JA, Erwin EA, Aalberse R, 2004. Mechanisms of tolerance to inhalant allergens: the relevance of a modified Th2 response to allergens from domestic animals. Springer Sem. Immunopathol 25, 271–279. 10.1007/s00281-003-0149-8. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Zhan B, Hamilton B, Stenzel D, Lowther J, Pearson M, Gorman J, Hotez P, Loukas A, 2009. Proteolytic degradation of hemoglobin in the intestine of the human hookworm Necator americanus. J. Infect. Dis 199, 904–912. 10.1086/59867910.1086/597048. [DOI] [PubMed] [Google Scholar]
- Ricci ND, Fiúza JA, Bueno LL, Cançado GGL, Gazzinelli-Guimarães PH, Martins VG, Matoso LF, Miranda R.R.C.de., Geiger SM, Correa-Oliveira R, Gazzinelli A, Bartholomeu DC, Fujiwara RT, Yazdanbakhsh M, 2011. Induction of CD4+CD25+FOXP3+ regulatory T cells during human hookworm infection modulates antigen-mediated lymphocyte proliferation. PLoS Neglect. Trop. Dis 5, e1383. 10.1371/journal.pntd.0001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami A, Riahi SM, Holland CV, Taghipour A, Khalili-Fomeshi M, Fakhri Y, Omrani VF, Hotez PJ, Gasser RB, Nicoletti A, 2019. Seroprevalence estimates for toxocariasis in people worldwide: A systematic review and meta-analysis. PLOS Neglect. Trop. Dis 13, e0007809. 10.1371/journal.pntd.0007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SM, Eichenberger RM, Ruscher R, Giacomin PR, Loukas A, Lok JB, 2020. Harnessing helminth-driven immunoregulation in the search for novel therapeutic modalities. PLOS Path 16, e1008508. 10.1371/journal.ppat.1008508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoguina JS, Adjobimey T, Arndts K, Hoch J, Oldenburg J, Layland LE, Hoerauf A, 2008. Tr1 and naturally occurring regulatory T cells induce IgG4 in B cells through GITR/GITR-L interaction, IL-10 and TGF-β. Eur. J. Immunol 38, 3101–3113. 10.1002/eji.200838193. [DOI] [PubMed] [Google Scholar]
- Savioli L, Bundy D, Tomkins A, 1992. Intestinal parasitic infections: a soluble public health problem. Trans. R. Soc. Trop. Med. Hyg 86, 353–354. 10.1016/0035-9203(92)90215-X. [DOI] [PubMed] [Google Scholar]
- Schierack P, Lucius R, Sonnenburg B, Schilling K, Hartmann S, 2003. Parasite-specific immunomodulatory functions of filarial cystatin. Infect. Immun 71, 2422–2429. 10.1128/IAI.71.5.2422-2429.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schölmerich J, Fellermann K, Seibold FW, Rogler G, Langhorst J, Howaldt S, Novacek G, Petersen AM, Bachmann O, Matthes H, Hesselbarth N, Teich N, Wehkamp J, Klaus J, Ott C, Dilger K, Greinwald R, Mueller R, 2016. A randomised, double-blind, placebo-controlled trial of Trichuris suis ova in active Crohn’s disease. J. Crohns Colitis 11, 390–399. 10.1093/ecco-jcc/jjw184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood TB, Navarro S, Cristofori-Armstrong B, Watkins TS, Tungatt K, Ryan RYM, Haigh OL, Lutzky VP, Mulvenna JP, Rosengren KJ, Loukas A, Miles JJ, Clark RJ, 2021. Synthetic hookworm-derived peptides are potent modulators of primary human immune cell function that protect against experimental colitis in vivo. J. Biol. Chem 297, 100834. 10.1016/j.jbc.2021.100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers RW, 2005. Trichuris suis therapy in Crohn’s disease. Gut 54, 87–90. 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YT, Gao X, Rosa BA, Abubucker S, Hallsworth-Pepin K, Martin J, Tyagi R, Heizer E, Zhang X, Bhonagiri-Palsikar V, Minx P, Warren WC, Wang Q, Zhan B, Hotez PJ, Sternberg PW, Dougall A, Gaze ST, Mulvenna J, Sotillo J, Ranganathan S, Rabelo EM, Wilson RK, Felgner PL, Bethony J, Hawdon JM, Gasser RB, Loukas A, Mitreva M, 2014. Genome of the human hookworm Necator americanus. Nature Genet 46, 261–269. 10.1038/ng.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D, Jones A, Garner P, Yamey G, 2009. Does deworming improve growth and school performance in children? PLoS Neglect. Trop. Dis 3, e358. 10.1371/journal.pntd.0000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson DC, Maayan N, Soares-Weiser K, Donegan S, Garner P, 2015. Deworming drugs for soil-transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin, and school performance. Cochrane Database Syst. Rev 7, CD000371. 10.1002/14651858.CD000371.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey EF, McDermott RA, McDonald MI, 2016. Do worms protect against the metabolic syndrome? A systematic review and meta-analysis. Diabetes Res. Clin. Practice 120, 209–220. 10.1016/j.diabres.2016.08.014. [DOI] [PubMed] [Google Scholar]
- Tribolet L, Cantacessi C, Pickering DA, Navarro S, Doolan DL, Trieu A, Fei H, Chao Y, Hofmann A, Gasser RB, Giacomin PR, Loukas A, 2014. Probing of a human proteome microarray with a recombinant pathogen protein reveals a novel mechanism by which hookworms suppress B-cell receptor signaling. J. Infect. Dis 211, 416–425. 10.1093/infdis/jiu451. [DOI] [PubMed] [Google Scholar]
- Turner JD, Faulkner H, Kamgno J, Cormont F, Van Snick J, Else KJ, Grencis RK, Behnke JM, Boussinesq M, Bradley JE, 2003. Th2 cytokines are associated with reduced worm burdens in a human intestinal helminth infection. J. Infect. Dis 188, 1768–1775. 10.1086/jid.2003.188.issue-1110.1086/379370. [DOI] [PubMed] [Google Scholar]
- Turner JD, Faulkner H, Kamgno J, Kennedy MW, Behnke J, Boussinesq M, Bradley JE, 2005. Allergen-specific IgE and IgG4 are markers of resistance and susceptibility in a human intestinal nematode infection. Microbes Infect 7, 990–996. 10.1016/j.micinf.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Turner JD, Jackson JA, Faulkner H, Behnke J, Else KJ, Kamgno J, Boussinesq M, Bradley JE, 2008. Intensity of intestinal infection with multiple worm species is related to regulatory cytokine output and immune hyporesponsiveness. J. Infect. Dis 197, 1204–1212. 10.1086/58875810.1086/586717. [DOI] [PubMed] [Google Scholar]
- Urban JJ, Tromba F, 1984. An ultraviolet-attenuated egg vaccine for swine ascariasis: parameters affecting the development of protective immunity. Am. J. Vet. Res 45, 2104–2108. [PubMed] [Google Scholar]
- Vacca F, Chauché C, Jamwal A, Hinchy EC, Heieis G, Webster H, Ogunkanbi A, Sekne Z, Gregory WF, Wear M, Perona-Wright G, Higgins MK, Nys JA, Cohen ES, McSorley HJ, 2020. A helminth-derived suppressor of ST2 blocks allergic responses. eLife 9,. 10.7554/eLife.54017 e54017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg L, Wei J, Liu Z, Keegan B, Fujiwara RT, Jones KM, Asojo O, Strych U, Bottazzi ME, Hotez PJ, Zhan B, Diemert DJ, 2020. Protective immunity elicited by the nematode-conserved As37 recombinant protein against Ascaris suum infection. PLOS Neglect. Trop. Dis 14, e0008057. 10.1371/journal.pntd.0008057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaminck J, Cools P, Albonico M, Ame S, Ayana M, Bethony J, Cringoli G, Dana D, Keiser J, Maurelli MP, Montresor A, Mekonnen Z, Mirams G, Corrêa-Oliveira R, Prichard R, Rashwan N, Rinaldi L, Sayasone S, Thomas E, Verweij JJ, Vercruysse J, Levecke B, Cantacessi C, 2018. Comprehensive evaluation of stool-based diagnostic methods and benzimidazole resistance markers to assess drug efficacy and detect the emergence of anthelmintic resistance: A Starworms study protocol. PLOS Neglect. Trop. Dis 12, e0006912. 10.1371/journal.pntd.0006912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voldsgaard A, Bager P, Garde E, Åkeson P, Leffers AM, Madsen CG, Kapel C, Roepstorff A, Thamsborg SM, Melbye M, Siebner H, Søndergaard HB, Sellebjerg F, Sørensen PS, 2015. Trichuris suis ova therapy in relapsing multiple sclerosis is safe but without signals of beneficial effect. Multiple Sclerosis J 21, 1723–1729. 10.1177/1352458514568173. [DOI] [PubMed] [Google Scholar]
- Wammes LJ, Hamid F, Wiria AE, May L, Kaisar MMM, Prasetyani-Gieseler MA, Djuardi Y, Wibowo H, Kruize YCM, Verweij JJ, de Jong SE, Tsonaka R, Houwing-Duistermaat JJ, Sartono E, Luty AJF, Supali T, Yazdanbakhsh M, 2016. Community deworming alleviates geohelminth-induced immune hyporesponsiveness. Proc. Natl. Acad. Sci 113, 12526–12531. 10.1073/pnas.1604570113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangchuk P, Kouremenos K, Eichenberger RM, Pearson M, Susianto A, Wishart DS, McConville MJ, Loukas A, 2019a. Metabolomic profiling of the excretory–secretory products of hookworm and whipworm. Metabolomics 15, 101. 10.1007/s11306-019-1561-y. [DOI] [PubMed] [Google Scholar]
- Wangchuk P, Shepherd C, Constantinoiu C, Ryan RYM, Kouremenos KA, Becker L, Jones L, Buitrago G, Giacomin P, Wilson D, Daly N, McConville MJ, Miles JJ, Loukas A, Herbert DR, 2019b. Hookworm-derived metabolites suppress pathology in a mouse model of colitis and inhibit secretion of key inflammatory cytokines in primary human leukocytes. Infect. Immun 87. 10.1128/IAI.00851-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherhead JE, Porter P, Coffey A, Haydel D, Versteeg L, Zhan B, Gazzinelli Guimarães AC, Fujiwara R, Jaramillo AM, Bottazzi ME, Hotez PJ, Corry DB, Beaumier CM, Adams JH, 2018. Ascaris larval infection and lung invasion directly induce severe allergic airway disease in mice. Infect. Immun 86. 10.1128/IAI.00533-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Versteeg L, Liu Z, Keegan B, Gazzinelli-Guimarães AC, Fujiwara RT, Briggs N, Jones KM, Strych U, Beaumier CM, Bottazzi ME, Hotez PJ, Zhan B, Dalton JP, 2017. Yeast-expressed recombinant As16 protects mice against Ascaris suum infection through induction of a Th2-skewed immune response. PLOS Neglect. Trop. Dis 11, e0005769. 10.1371/journal.pntd.0005769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MPJ, McManus CM, Maizels RM, 2020. Regulatory T-cells in helminth infection: induction, function and therapeutic potential. Immunology 160, 248–260. 10.1111/imm.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MPJ, Smyth DJ, Cook L, Ziegler SF, Levings MK, Maizels RM, 2021. The parasite cytokine mimic Hp -TGM potently replicates the regulatory effects of TGF-β on murine CD4 + T cells. Immunol. Cell Biol 99, 848–864. 10.1111/imcb.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RR, Artavanis-Tsakonas K, 2012. How helminths use excretory secretory fractions to modulate dendritic cells. Virulence 3, 668–677. 10.4161/viru.22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Grinchuk V, Smith A, Qin B, Bohl JA, Sun R, Notari L, Zhang Z, Sesaki H, Urban JF, Shea-Donohue T, Zhao A, Adams JH, 2013. Parasitic nematode-induced modulation of body weight and associated metabolic dysfunction in mouse models of obesity. Infect. Immun 81, 1905–1914. 10.1128/IAI.00053-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshi K, Creek DJ, Anderson D, Ritmejerytė E, Becker L, Loukas A, Wangchuk P, 2020. Metabolomes and lipidomes of the infective stages of the gastrointestinal nematodes, Nippostrongylus brasiliensis and Trichuris muris. Metabolites 10, 446. 10.3390/metabo10110446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi Y, Haq S, Banskota S, Kwon YH, Khan WI, 2021. Trichuris muris model: role in understanding intestinal immune response, inflammation and host defense. Pathogens 10, 925. 10.3390/pathogens10080925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawawi A, Else KJ, 2020. Soil-transmitted helminth vaccines: are we getting closer? Front. Immunol 11, 2426. 10.3389/fimmu.2020.576748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan B, Beaumier CM, Briggs N, Jones KM, Keegan BP, Bottazzi ME, Hotez PJ, 2014. Advancing a multivalent ‘Pan-anthelmintic’ vaccine against soil-transmitted nematode infections. Exp. Rev. Vaccines 13, 321–331. 10.1586/14760584.2014.872035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T, Kamm MA, Colombel J-F, Ng SC, 2018. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nature Rev. Gastroenterol. Hepatol 15, 440–452. 10.1038/s41575-018-0003-z. [DOI] [PubMed] [Google Scholar]


