Abstract
Background: Due to findings on adverse reactions and clinical efficacy of different vaccinations against SARS-CoV-2, the administration of vaccination regimens containing both adenoviral vector vaccines and mRNA-based vaccines has become common. Data are still needed on the direct comparison of immunogenicity for these different regimens. Methods: We compared markers for immunogenicity (anti-S1 IgG/IgA, neutralizing antibodies, and T-cell response) with three different vaccination regimens (homologous ChAdOx1 nCoV-19 (n = 103), or mixture of ChAdOx1 nCoV-19 with mRNA-1273 (n = 116) or BNT162b2 (n = 105)) at two time points: the day of the second vaccination as a baseline and 14 days later. Results: All examined vaccination regimens elicited measurable immune responses that were significantly enhanced after the second dose. Homologous ChAdOx1 nCoV-19 was markedly inferior in immunogenicity to all other examined regimens after administration of the second dose. Between the heterologous regimens, mRNA-1273 as second dose induced greater antibody responses than BNT162b2, with no difference found for neutralizing antibodies and T-cell response. Discussion: While these findings allow no prediction about clinical protection, from an immunological point of view, vaccination against SARS-CoV-2 with an mRNA-based vaccine at one or both time points appears preferable to homologous vaccination with ChAdOx1 nCoV-19. Whether or not the demonstrated differences between the heterologous regimens are of clinical significance will be subject to further research.
Keywords: SARS-CoV-2, vaccination, immunogenicity, serology
1. Introduction
Since its inception in late 2019 [1], various measures have been employed in the attempt to contain the pandemic of Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The most promising of these measures certainly is the vaccination against SARS-CoV-2. Since the end of 2020, several vaccines, using different modes of action on the molecular level, have been approved and administered in most parts of the world, among them the adenoviral vector vaccine ChAdOx1 nCoV-19 (ChAdOx, AstraZeneca, Cambridge, UK) [2], and the two mRNA vaccines BNT162b2 (BNT, BioNTech/Pfizer, Mainz/New York, NY, Germany/USA) [3] and mRNA-1273 (Mod, Moderna, Cambridge, MA, USA) [4]. As more data concerning efficacy and reactogenicity of these vaccines emerge [5,6,7,8], recommendations regarding the administration of these vaccines change. As of July 2021, the German permanent commission on vaccination (Ständige Impfkommission) recommends for all vaccinees, who have received a first dose of the vaccination with ChAdOx, to receive a second dose of either BNT or Mod (heterologous vaccination). However, recipients can also make the informed decision to receive a second dose of ChAdOx (homologous vaccination). Generally, mainly due to reports of vaccine-induced immune thrombotic thrombocytopenia (VITT) in predominantly younger (<60 years) female recipients of ChAdOx [8], in Germany, the use of ChAdOx is recommended only for recipients ≥ 60 years. To date, several studies have shown that heterologous vaccinations are non-inferior to homologous vaccinations with ChAdOx. On the contrary, data have shown that some immune responses to heterologous vaccinations may be even greater than those to homologous vaccinations [9,10,11,12].
During a previous study, examining homologous mRNA-based vaccination regimens, we saw that vaccination with mRNA-1273 was associated with stronger immune responses, compared with BNT162b2, while there was an inverse correlation between age and the examined immune responses [13]. In another study, we examined the kinetics of the B-cell response to a second dose of three different vaccines against SARS-CoV-2 in a cohort of participants who had previously received a first dose of ChAdOx. We found that within the first seven days after the second vaccination, a second dose of Mod or BNT elicits earlier and stronger B-cell responses than a second dose of ChAdOx, while a trend showed stronger reactions after a second vaccination with Mod compared to BNT [14].
This study aims to expand the current knowledge on heterologous vaccinations against SARS-CoV-2 by comparing both regimens of heterologous vaccination currently practiced in Germany with the homologous vaccination with ChAdOx. To this end, we examined several markers for the immune response to the different vaccination regimens in different cohorts of vaccinees. Drawing on available data, we hypothesized that:
In continuation of the trend already seen within the first seven days after the second vaccination, heterologous vaccinations induce an immune response comparable or superior in size to that induced by homologous vaccination with ChAdOx.
Among heterologous vaccinations, a second dose of Mod induces a greater immune response than a second dose of BNT (in analogy to the corresponding homologous vaccination regimens [13] and the trend we have seen within the first seven days [14]).
Age might be a general influencing factor, with older age possibly being associated with weaker immune responses.
2. Methods
2.1. Study Population
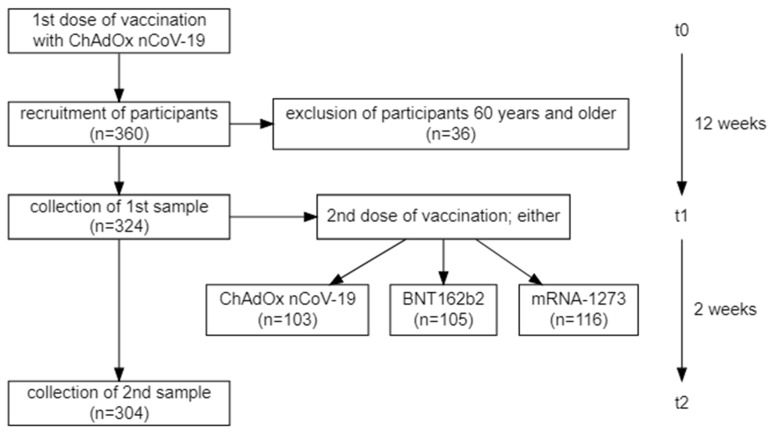
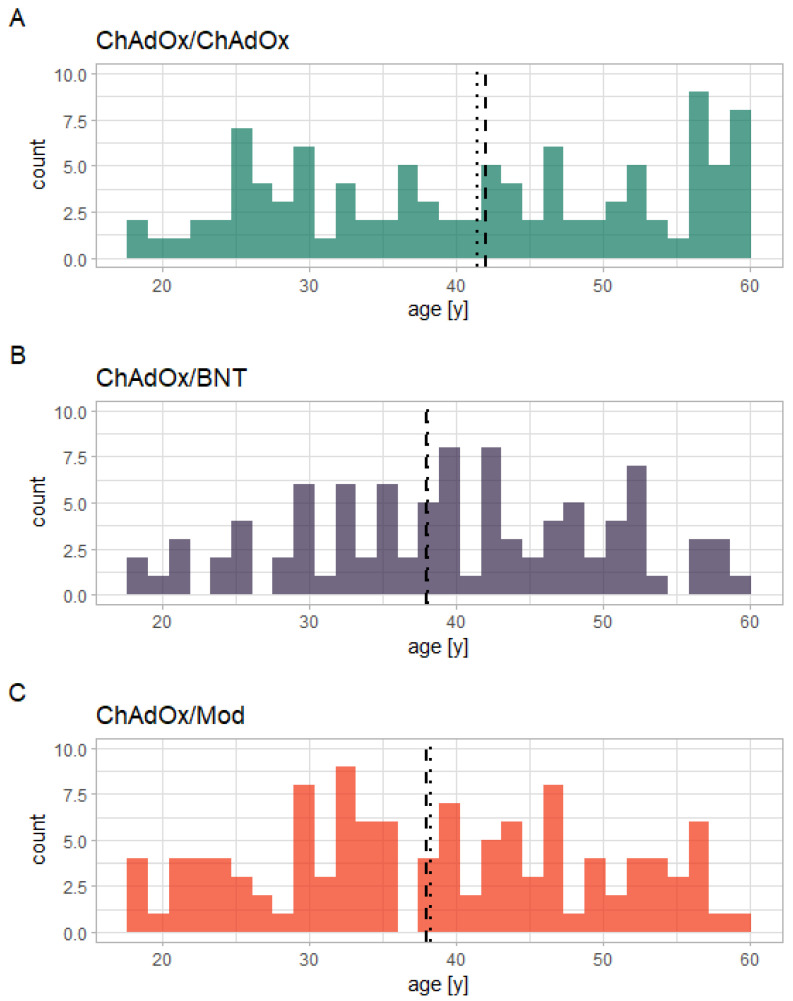
For this observational (i.e., non-randomized) study, participants (all in all 360) were recruited from individuals receiving their vaccination against SARS-CoV-2 at the University Hospital Schleswig-Holstein (Lübeck, Germany). The study population represents a sample of convenience and no statistical sample size considerations were performed beforehand. Three cohorts were selected: the first cohort consisted of vaccinees who received two doses of ChAdOx (ChAdOx/ChAdOx); the second cohort of those who received a first dose of ChAdOx and a second one of Mod (ChAdOx/Mod); and the third cohort of those who received a first dose of ChAdOx and a second one of BNT (ChAdOx/BNT). More information on the vaccination rollout and the recruitment of participants can be found in the supplement. Figure 1 represents a visualization of the process for vaccination and recruitment. As was expected due to the abovementioned recommendation by Germany health authorities, the age distribution among participants receiving ChAdOx/ChAdOx included more persons 60 years of age or older (see Figure 2).
Figure 1.
Flow diagram showing the vaccination program and the recruitment of participants for the current study and the respective time intervals in between the different time points.
Figure 2.
Histogram showing the different age distributions among participants receiving the three different vaccination regimens in the current study: (A) ChAdOx/ChAdOx; (B) ChAdOx/BNT; (C) ChAdOx/Mod). The dashed vertical lines mark the respective median, the dotted lines the mean.
The interval between both doses was 12 weeks for all three cohorts. The participants were asked to disclose any immunosuppressive medication; five participants confirmed this (the respective substances being: Budesonid (per inhalationem), Rituximab, Infliximab, Ciclosporin + Dupilumab, and Methotrexate. Due to German recommendations concerning the vaccination regimen in different age groups, there was a slightly unequal age distribution among the three cohorts for participants 60 years and older. No information on adverse events was solicited from the participants and none was received unsolicited. In order to ensure an equal distribution among age groups and sexes among the three cohorts, only participants younger than 60 years of age (n = 324) were included in the statistical analysis of differences between the cohorts. The analysis of the data of the 36 participants 60 years of age and older can nonetheless be found in the Supplementary Materials.
Prior to participation, all participants gave written informed consent to all procedures they underwent. The study was approved by the University of Kiel institutional review board (AZ: D642/20) and performed in accordance with the declaration of Helsinki [15].
2.2. Sample Characteristics
Blood samples were collected from the participants at two different time points: immediately prior to receiving the second dose of the vaccination against SARS-CoV-2 (t1; in order to assess the serological status after having received one dose of ChAdOx) and two weeks after this (t2). At each of these two time points, a serum and a lithium–heparinate blood sample were collected from each participant. The samples were subsequently pseudonymized before tests were performed.
2.3. Anti-SARS-CoV-2 Antibodies
Antibodies of the classes IgA and IgG against the S1 subunit of the Spike protein of SARS-CoV-2 (anti-S1 IgG) as well as IgG against the nucleocapsid (NCP) antigen were measured from the serum samples using the Anti-SARS-CoV-2-ELISA IgA and Anti-SARS-CoV-2-QuantiVac-ELISA (IgG) test kits by EUROIMMUN (Lübeck, Germany) according to the manufacturers’ instructions. While the IgA test kit yields a semiquantitative test result reported as the optical density (OD) of the sample compared to that of a control, the IgG test kit yields a quantitative result reported in binding antibody units per milliliter (BAU/mL), ensuring international comparability of the results. For anti-S1 IgA and anti-NCP IgG, values of >1.1 OD were considered as reactive, values of <0.8 OD as negative and all values in between as borderline. For anti-S1 IgG, values of >35.2 BAU/mL were considered as reactive, values of <25.6 BAU/mL as negative and all values in between as borderline.
2.4. Surrogate Neutralization Assay
The capacity of the anti-S1 IgG to potentially neutralize SARS-CoV-2 was tested via a surrogate neutralization assay (NeutraLISA, EUROIMMUN, Lübeck, Germany). The surrogate neutralization assay (NeutraLISA, EUROIMMUN, Lübeck, Germany) is a competitive ELISA in which soluble biotinylated ACE2, contained in a buffer, competes with anti-S1 IgG in the sample for the binding at the recombinant S1 subunit of the Spike protein of SARS-CoV-2 that is attached to a solid phase. The amount of ACE2 that is detected after a washing step via an enzymatic reaction, employing streptavidin-coupled peroxidase, is inversely proportional to the concentration of neutralizing antibodies. The signal detected in samples is compared to the mean signal of a duplicate measurement of the buffer alone (blank), for which the highest possible signal is expected. The result is reported as inhibition [%], which is calculated via the following formula: . Of note, by design, this assay has an upper limit of quantification at 100%. Differences in concentrations of neutralizing antibodies beyond this point are not quantifiable via this assay. Values < 20% are considered non-reactive, values ≥ 35 are considered reactive and values between 20 and 35% are considered to be borderline. According to the manufacturer, there is a concordance of 98.6% between this method and the examination of neutralizing antibodies via plaque reduction neutralization test (PRNT50) [16].
2.5. Interferon-γ Release Assay
An Interferon-γ-release assay (IGRA) was performed as a marker for the recipients’ T-cell response to the vaccination against SARS-CoV-2. This was carried out using assays by EUROIMMUN (SARS-CoV-2 IGRA stimulation tube set, Lübeck, Germany). For the Interferon-γ-release assay (IGRA), lithium–heparinate anticoagulated whole blood, collected from the participants, was divided into three aliquots undergoing different stimulation protocols for 20–24 h: one aliquot being stimulated with SARS-CoV-2-specific antigens (a mixture of antigens based on the n-terminal domain of the spike protein including the RBD region); another being stimulated with a lectin-based mitogen; and a third aliquot (blank) undergoing no stimulation at all (as a baseline control). Subsequent to these stimulation steps, all aliquots were centrifuged and interferon-γ was measured in the supernatant plasma using an ELISA by EUROIMMUN (Lübeck, Germany). The IGRA yields a quantitative level of measured Interferon-γ, reported in mIU/mL. Interferon-γ values of >200 mIU/mL were considered as reactive, 100–200 mIU/mL are considered borderline and <100 mIU/mL as negative. Apart from the IGRA, we did not perform further analyses of the T-cell response quantifying the contribution for different subsets of T-cells (such as CD4+ of CD8+ T-cells) to the detected Interferon-γ release. However, previous studies have shown that stimulation with SARS-CoV-2 peptide pools elicits a broad T-cell responses encompassing both CD4+ and CD8+ T-cells (among others) [17,18,19].
2.6. Statistical Analysis
As a first step, normality of the data was assessed via the Shapiro–Wilk test. As all examined variables were non-normally distributed, non-parametric testing was applied for further statistical analysis. For the analysis of differences in continuous variables between multiple groups, the Kruskal–Wallis test was calculated. If the influence of more than one categorical independent variable was examined, p-values were adjusted for multiple comparisons using the method described by Benjamini and Yekutieli [20]. If this exploratory analysis showed significant main or interaction effects for the examined factors, post-hoc testing via Dunn’s test, a pairwise Mann–Whitney test with Benjamini–Yekutieli correction for multiple comparisons, was applied. For differences between two groups, the Mann–Whitney U test was used. For the analysis of the distribution among a categorically scaled variable between different groups, Pearson’s chi-squared test was used. For the analysis of the association between two continuous variables, correlations using Spearman’s rho were calculated. This test was chosen due to its ability to identify non-linear correlations, as were detected during the previous study [13]. Statistical significance was assumed for p-values < 0.05. Wherever average values with a measure of dispersion are reported, these represent the medians and the median absolute deviation (MAD), unless otherwise stated. All statistical analyses were performed using the open-source software for statistical computing and graphics R (version 4.1.0; R Core Team; Vienna, Austria) with the integrated development environment RStudio (Version 1.4.1717; R Core Team; Vienna, Austria) [21].
3. Results
3.1. Study Population
Overall, 324 participants were included in the statistical analysis, of whom 178 (54.9%) were female. Of these 324, 103 participants (31.8%) received ChAdOx/ChAdOx, 116 (35.8%) received ChAdOx/Mod, and 105 (32.4%) received ChAdOx/BNT. The participants’ mean age was 39.3 (sd: ±11.7; range: 18–59; see Figure 2 for a histogram of participants’ ages for each different vaccination regimen). For details on the demographical data of the participants, see Table S1. The t1 data from all 324 participants is available for all biomarkers. Due to loss to follow-up, at t2, data are available for 304 participants (ChAdOx/ChAdOx: 95; ChAdOx/Mod: 109; ChAdOx/BNT: 100). All participants showed sufficient cellular responses to the control mitogens in the IGRA. A Kruskal–Wallis test with the factor vaccination regimen revealed no statistically significant effect on participants’ ages (p = 0.07), as did a Mann–Whitney U test with the factor sex (p = 0.572). Pearson’s chi-squared test revealed that there was no statistically significant difference in gender distribution between the different vaccination regimens (chi-squared = 0.30179, df = 2, p-value = 0.8599).
3.2. Effect of the Second Dose
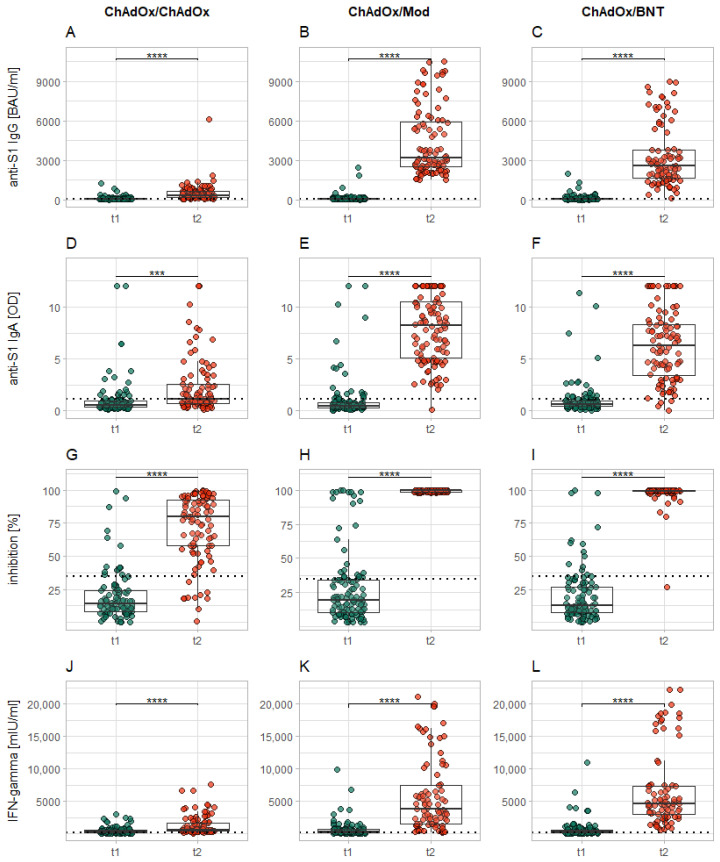
The comparison among all examined markers across all vaccination regimens of the current study revealed a significant increase in these markers from t1 to t2 (p < 0.001–0.0001; see Figure 3A–L). For average results of all markers at both time points and for all subgroups, please see Table 1.
Figure 3.
Increase in levels of all examined markers from immediately before the second vaccination (t1) to 14 days after it (t2). Each column of plots represents the reactions to a specific vaccination regimen (ChAdOx/ChAdOx: (A,D,G,J); ChAdOx/Mod: (B,E,H,K); ChAdOx/BNT: (C,F,I,L)), while each row represents the different examined markers (anti-S1 IgG: (A–C); anti-S1 IgA: (D–F); neutralizing antibodies: (G–I); IGRA: (J–L)). The dotted horizontal lines represent the cutoff for reactivity of the different assays. Levels of significance: * = p < 0.05; ** = p < 0.01; *** = p < 0.001; **** = p < 0.0001.
Table 1.
Average values (reported via medians ± median absolute deviation) for all measured markers at all time points, including the respective group sizes, for the whole cohort and the following subgroups: women, men, participants having received ChAdOx/ChAdOx, ChAdOx/Mod, ChAdOx/BNT, and participants who have tested anti-NCP IgG positive at the first time point.
| Subgroup | Age (years) | 12 Weeks after First Dose | 14 Days after Second Dose | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Anti-S1 IgG (BAU/mL) | Anti-S1 IgA (OD Ratio) | Neutralizing Antibodies (%) | IGRA (mIU/mL) | Anti-S1 IgG (BAU/mL) | Anti-S1 IgA (OD Ratio) | Neutralizing Antibodies (%) | IGRA (mIU/mL) | ||
| Whole cohort | 39 ± 13.3 | 63.9 ± 51 | 0.57 ± 0.36 | 16 ± 13.3 | 258 ± 262 | 2314 ± 2365 | 5.1 ± 4.9 | 99 ± 1.5 | 3289 ± 4028 |
| females | 39 ± 13.3 | 65.2 ± 53 | 0.5 ± 0.33 | 16 ± 13.3 | 280 ± 270 | 2557 ± 2961 | 4.7 ± 4.7 | 99 ± 1.5 | 3698 ± 4151 |
| males | 39.5 ± 15.6 | 63.0 ± 49.4 | 0.68 ± 0.44 | 14 ± 10.4 | 235 ± 251 | 2066 ± 2126 | 6.5 ± 5.0 | 99 ± 1.5 | 3025 ± 3747 |
| ChAdOx/ChAdOx | 42 ± 17.8 | 60.5 ± 49.6 | 0.56 ± 0.34 | 13 ± 8.9 | 307 ± 302 | 358 ± 289 | 1.3 ± 1 | 80 ± 23.7 | 564 ± 465 |
| ChAdOx/Mod | 38 ± 12.6 | 69.1 ± 48.6 | 0.49 ± 0.31 | 19 ± 16.3 | 286 ± 289 | 3747 ± 2492 | 8.2 ± 4.1 | 100 ± 0 | 5324 ± 6073 |
| ChAdOx/BNT | 38 ± 13.3 | 66.4 ± 58.6 | 0.66 ± 0.37 | 13 ± 11.9 | 235 ± 222 | 2595 ± 1548 | 6.3 ± 3.4 | 99 ± 1.5 | 4872 ± 3639 |
| Anti-NCP IgG pos. | 34 ± 8.9 | 841 ± 953 | 3.9 ± 4.6 | 94 ± 7.4 | 978 ± 1119 | 2771 ± 2540 | 8.3 ± 5.6 | 99 ± 1.5 | 7313 ± 8597 |
3.3. Influence of Sex on the Immune Response
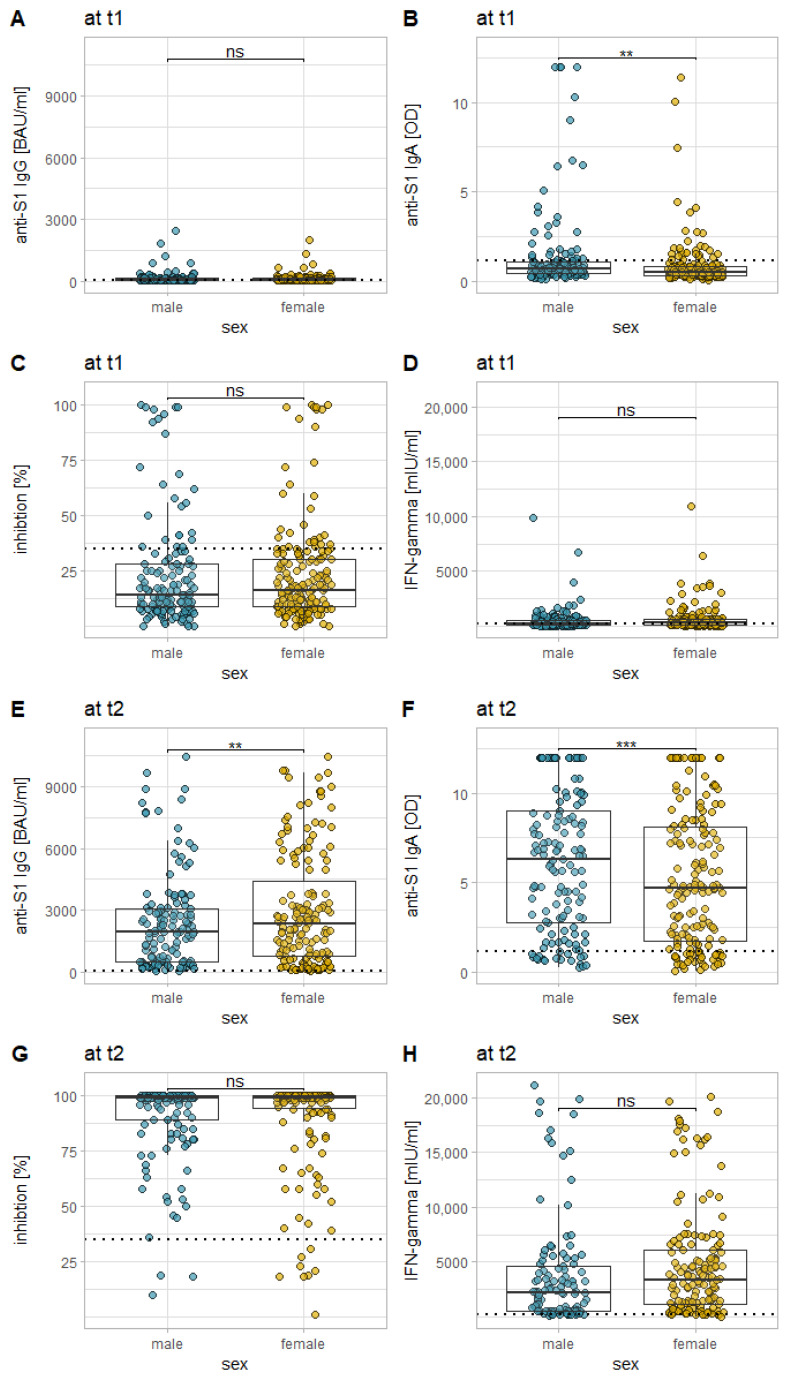
Mann–Whitney U tests revealed that there were differences between the two sexes in both IgG and IgA response, whereas for anti-S1 IgG, there were slightly stronger responses for female participants at t2 (p = 0.0442), for anti-S1 IgA, there were slightly stronger responses for male participants, both at t1 (p = 0.0001) and at t2 (p = 0.0331). For both neutralizing antibodies and for the IGRA, no statistically significant difference could be found between the two sexes (see Figure 4).
Figure 4.
Comparison of the examined immune responses, both 12 weeks after the first vaccination (A–D), and 14 days after the second vaccination (E–H) in between the sexes (for all vaccination regimes of the current study cumulatively). Each plot represents a different marker: anti-S1 IgG (A,E), anti-S1 IgA (B,F), the inhibition by neutralizing antibodies (C,G), and T-cell response as measured via IGRA (D,H). The dotted horizontal lines represent the cutoff for reactivity of the different assays. * = p < 0.05; ** = p < 0.01; *** = p < 0.001; **** = p < 0.0001; ns = not statistically significant.
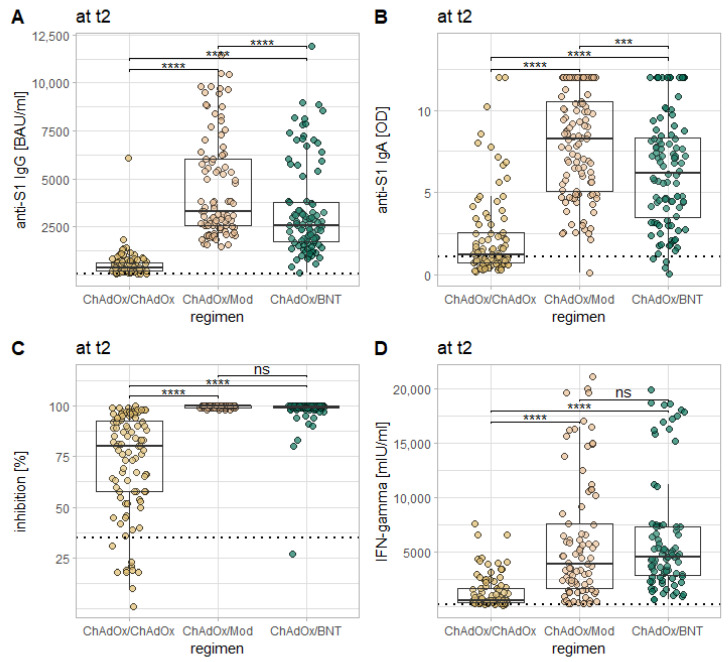
3.4. Influence of the Vaccination Regimen on the Immune Response
Kruskal–Wallis tests with the factor vaccination regimen revealed a statistically significant effect on the levels of all examined markers at t2 (p < 0.0001 in all cases). Post-hoc testing via Dunn’s test revealed that for anti-S1 IgG and IgA, as well as for neutralizing antibodies, comparisons of all three vaccination regimens in the current study with one another yield statistically significant differences (p < 0.0001 for all comparisons between ChAdOx/ChAdOx and both heterologous regimens; the comparison between ChAdOx/Mod and ChAdOx/BNT yielding somewhat smaller levels of significance at t2: anti-S1 IgG: p = 0.001; anti-S1 IgA: p = 0.006; neutralizing antibodies: p = 0.041). For all of these three markers, levels were highest after ChAdOx/Mod, followed by ChAdOx/BNT and ChAdOx/ChAdOx. For the IGRA, comparisons between ChAdOx/ChAdOx and both heterologous regimens also revealed highly significant differences (p < 0.0001) with stronger results for the heterologous regimens, but no statistically significant difference between both heterologous regimens (p = 0.812; see Figure 5).
Figure 5.
Comparison of the examined immune responses 14 days after the second vaccination in between the three vaccination regimes in the current study. Each plot represents a different marker: anti-S1 IgG (A), anti-S1 IgA (B), the inhibition by neutralizing antibodies (C), and T-cell response as measured via IGRA (D). The dotted horizontal lines represent the cutoff for reactivity of the different assays. Levels of significance: * = p < 0.05; ** = p < 0.01; *** = p < 0.001; **** = p < 0.0001; ns = not statistically significant.
At t1, Kruskal–Wallis tests showed a statistically significant effect for the vaccination regimen (i.e., for the type of second vaccine, which the participants had not yet received) only for anti-S1 IgA (p = 0.033) and neutralizing antibodies (p = 0.018). Post-hoc testing via Dunn’s test revealed no statistically significant difference between any of the regimens for anti-S1 IgA, while it revealed a small but statistically significant difference in neutralizing antibodies between participants who would later receive Mod or BNT, with somewhat stronger reaction for the former (19 ± 16.3% vs. 13 ± 11.9%; p = 0.044).
3.5. Influence of Age on the Immune Response
There was no correlation in meaningful effect size between any of the examined markers and the participants’ age, neither after the first, nor after the second dose of the vaccination (absolute rho ≤ 0.2 for all calculated correlations). In analogy, when participants are grouped together by life decade, there are only a few comparisons that reveal statistically significant differences in the results of the different markers for each life decade (specifically: the comparisons between results for participants in the 2nd life decade with those for the 3rd, 4th, and 5th life decade reveal higher levels of anti-S1 IgG for the 2nd life decade at t1 (p-values: 0.007, 0.014, and 0.007, respectively), while the same is true for the comparison between the 3rd and the 5th life decade at t2, revealing higher levels of neutralizing antibodies for the former group (p = 0.042).
3.6. Influence of Prior Infection on the Immune Response
At t1, there were nine participants who tested positive for anti-NCP IgG, suggesting that they had been exposed to SARS-CoV-2 at some earlier point in time. All of these nine participants tested positive again at t2; two received ChAdOx/ChAdOx, three received ChAdOx/BNT, and four received ChAdOx/Mod. There were no participants who tested positive at t2 after testing negative at t1, suggesting that none of the participants had contracted SARS-CoV-2 in the meantime.
Despite the very low number of previously exposed individuals, Mann–Whitney U tests showed that these had significantly higher levels of anti-S1 IgG (p = 0.0004), anti-S1 IgA (p = 0.0002), inhibition (via surrogate neutralization assay; p = 0.002), and IFN-γ (via IGRA; p = 0.003) at t1 than participants who tested anti-NCP negative (although with great interindividual fluctuations). At t2, none of these comparisons revealed a statistically significant difference. When comparing the results for previously infected participants after the first dose with those of unexposed individuals after the second dose (in order to see if one dose of ChAdOx and a previous or following COVID-infection result in a similar immune response as two doses of vaccines for unexposed individuals), we found that both anti-S1 IgG and neutralizing antibodies were significantly lower (p = 0.008 and p = 0.004, respectively) for previously infected participants at t1 than for unexposed participants at t2, with no significant difference for anti-S1 IgA and IFN-γ. When the same comparison was made with only those unexposed participants who received ChAdOx as a second dose, no significant differences could be found, while the comparison with those uninfected participants who had received either BNT or Mod showed significantly stronger reactions for the unexposed individuals for all comparisons.
3.7. Borderline or Non-Reactive Samples
For all examined markers as well as at both time points, we observed samples that returned only borderline or negative results. A detailed analysis of these samples can be found in the Supplementary Materials. In short, participants who tested borderline or negative at t2 already had on average lower levels of the same markers at t1 when compared to participants who donated reactive samples at t2. This difference was apparently not mediated by age.
4. Discussion
Our results show that all examined vaccination regimens are immunogenic and that the second dose of the vaccine acts as a booster with significant increases in the levels of all examined markers after the second vaccination. However, the results also reveal significant differences between the different vaccination regimens: no matter which marker is examined, the heterologous vaccination regimens with an mRNA-based vaccine as second dose induce significantly greater reactions than ChAdOx/ChAdOx. The median levels of anti-S1 IgG and Interferon-γ (via IGRA) are on average tenfold higher for the heterologous vaccination regimens than for ChAdOx/ChAdOx, while levels of antibody-induced inhibition are considerably lower and more dispersed for the latter. In addition, a small, but non-negligible number of participants, especially receiving ChAdOx/ChAdOx, did not develop levels of the examined markers considered to be reactive. This is a clear continuation in the results we saw within the first 7 days after the second vaccination [14], with the exception that after 14 days, we were able to detect significant increases in B- and T-cell responses after a second vaccination with ChAdOx, while we were not able to detect significant increases for most markers within the first 7 days.
It has been shown in the course of several studies to date that heterologous vaccination regimens against SARS-CoV-2 are safe and immunogenic [9,10,11,12,22,23,24,25]. All available studies support our finding that heterologous vaccination regimens (ChAdOx/BNT or ChAdOx/Mod) elicit a stronger immune response than ChAdOx/ChAdOx. Where this was examined [9,10,11,12,22], heterologous regimens (mostly ChAdOx/BNT) were also found to elicit immune responses comparable to those elicited by BNT/BNT. Interestingly several of these other studies demonstrated at least partially stronger immune responses to heterologous regimens compared to homologous ones [9,10,11,12,26]. A weakness of all previous studies is that none of them examined the regimens ChAdOx/Mod or Mod/Mod separately. Those who included participants receiving Mod rather pooled the results with those of the corresponding BNT regimen, which might skew the resulting data, as we could show significant differences between ChAdOx/Mod and ChAdOx/BNT, with the stronger responses, at least for anti-S1 IgA and IgG, for the former. This difference between ChAdOx/Mod and ChAdOx/BNT might be a result of a higher mRNA concentration in the former (100 µg vs. 30 µg, according to the manufacturers).
When discussing these data, it is important to bear in mind that they only represent measures for vaccinees’ immune responses to the different vaccination regimens and that it is difficult at this point in time to infer conclusions about a recipient’s protection from SARS-CoV-2 from these data. While it is convenient to assume that higher levels, especially of anti-S1 IgG and antibody-induced inhibition, confer a higher degree of clinical protection from SARS-CoV-2, evidence to support this claim is still scarce. As of yet, levels of neutralizing antibodies likely permit the best prediction of protective immunity from SARS-CoV-2 [27,28], while there is also evidence that higher levels of anti-S1 IgG and T-cell responses are associated with protection from SARS-CoV-2 [29]. Furthermore, several studies have shown that anti-S1 IgG levels correlate well with the antibodies’ ability to neutralize the virus [30]. As there is evidence that antibodies against SARS-CoV-2 tend to wane over time, both after vaccination [31] and infection with SARS-CoV-2 [32,33], higher initial levels of anti-SARS-CoV-2 antibodies in all likelihood provide protection over a longer period of time than do lower levels. Compounding this, antibody levels induced by ChAdOx wane at a higher pace than those induced by BNT [31]. The fact that ChAdOx/ChAdOx induces lower levels of anti-SARS-CoV-2 antibodies than do mRNA-based vaccines, and that these levels furthermore appear to wane faster might explain why clinical efficacy is higher for Mod/Mod and BNT/BNT than for ChAdOx/ChAdOx [2,3,4]. All this suggests that vaccination regimens, either homologous or heterologous, employing mRNA-based vaccines against SARS-CoV-2, are more effective and likely provide better protection from SARS-CoV-2 than ChAdOx/ChAdOx.
As mentioned, we could show stronger immune responses at t2 for ChAdOx/Mod, compared to ChAdOx/BNT for all examined markers except for IFN-γ, probably as a consequence of the higher dose of mRNA in Mod. Whether or not this translates to better clinical protection from SARS-CoV-2 remains questionable, as the differences are not as pronounced. The same is true for the differences we previously found between the homologous mRNA-based regimens (Mod/Mod and BNT/BNT) and their heterologous counterparts [13]. In this study, there is also a possible bias towards Mod, as we could show slightly higher levels of neutralizing antibodies at t1 already in participants who would later receive Mod, compared to those who would receive BNT.
Interestingly, contrary to findings regarding homologous mRNA-based regimens [34], including our own [13], we could find no relevant influence of age on any part of the immune response in the current study. It is possible that age is a more important influencing factor for homologous vaccinations than for heterologous vaccinations, but it is also possible that the influences of age on the immune response to vaccination against SARS-CoV-2 decreases over time, which is supported by the fact that the negative correlation between the examined markers and age was stronger two weeks after the first vaccination than after the second vaccination in the previous study [13]. However, it is also possible that we missed the potential effects of very old age, as the oldest participant recruited was 70 years old and a previous study saw a marked drop in antibody levels for vaccines older than 80 years old [34].
The analysis of non-reactive or borderline results for any marker shows that, for most participants, two effects must interact to lead to these results: certain persons (independent of their age) appear to show generally weaker responses to the vaccination (which is apparent from them already showing weaker responses after the first vaccination than the rest of the cohort). However, for most, it is unclear what exactly causes these weaker responses, apart from a small number of participants who reported receiving immunosuppressive medication. In some of these persons, a second dose of ChAdOx (as opposed to Mod or BNT) might not be immunogenic enough to elicit significant immune responses. The fact that there was no difference in immune responses between the ChAdOx/ChAdOx sub-cohort and the other two sub-cohorts after the first dose (except for the small difference in neutralizing antibodies between ChAdOx/ChAdOx and ChAdOx/Mod) suggests that the general distribution among potential weak responders was not skewed towards ChAdOx/ChAdOx, but that they were present in recipients of the other two regimens as well.
We saw those participants likely to have been exposed to SARS-CoV-2 as indicated by anti-NCP IgG positivity developed higher levels of most examined markers for immunity already at t1. In the previous study, we observed a similar effect [13] and it is generally known that persons who have been exposed to SARS-CoV-2 develop much higher titers of anti-SARS-CoV-2 antibodies after the first dose of the vaccination than unexposed individuals [35,36,37,38]. The comparison of results for previously infected participants after the first dose and unexposed participants after the second dose shows that the combination of one dose of ChAdOx and an infection has a similar boostering effect as a second dose of ChAdOx has for unexposed individuals who have already received one dose of ChAdOx. However, we could also observe that heterologous vaccination (ChAdOx/BNT or ChAdOx/Mod) in unexposed individuals leads to stronger responses of all examined markers compared to the combined effect of one dose of ChAdOx and an infection. Therefore, it seems advisable that previously infected individuals who have received one dose of ChAdOx and individuals infected after the first ChAdOx dose should also receive a second dose (preferably BNT or Mod) as a boost in their immune response.
Finally, there is the difference in anti-S1 IgG and IgA levels between the sexes. However, this difference can only be found for all regimens cumulatively with no significant differences for each regimen separately, and it is not uniform for IgG (which is higher in women only at t2) and IgA (which is higher in men at both t1 and t2). There is evidence for other vaccines that women generally tend to develop stronger humoral reactions, especially IgG, in response to vaccinations [39,40], while there is some evidence that men generally have higher levels of serum IgA than women [41]. Therefore, the reported differences, while statistically significant, might rather be a physiological phenomenon than a SARS-CoV-2 specific effect, and are of questionable clinical significance. Of note, we did not find any sex-associated difference in immune responses to Mod/Mod or BNT/BNT in the previous study [13].
Generally, the role of IgA in respiratory infections and especially the role of the IgA responses in the serum after vaccination is not completely understood [42]. While the early IgA response after the first dose of a vaccination against SARS-CoV-2 (as we have seen in the previous study [13]) might represent a recall response of mucosal B-memory cells formed in response to previous pulmonary coronavirus infections, the response to the second dose is more likely to be dominated by naïve B-cells recruited by the first dose [43]. Whether serum anti-S1 IgA can be used as a marker for mucosal immunity against SARS-CoV-2 (which is important as a first line defense) is therefore unclear and warrants further investigation.
While we did not include recipients of homologous mRNA-based vaccination regimens in the current study, comparisons can be made with the results for the previous study [13], always keeping in mind some methodological differences between the studies (such as different inter-dose intervals): while the antibody-responses (anti-S1 IgG and IgA as well as neutralizing antibodies) appear to be within the same order of magnitude for the heterologous regimens of the current study and the homologous mRNA-based regimens of the previous study (compare the respective tables 1), homologous ChAdOx also elicits markedly weaker responses compared to homologous mRNA-based regimens. This supports findings by others [9,12,22]. Furthermore, while the homologous mRNA-bases vaccines of the previous study appear to have some edge over the heterologous regimens of the current study, this, as well as its clinical significance, would have to be examined further in upcoming studies. A comparison of IGRA-results between the two studies is not possible due to methodological differences.
In conclusion, judging from the markers for assumed immunity against SARS-CoV-2 that we examined, vaccination regimens that employ an mRNA-based vaccine against SARS-CoV-2, either as second dose or for both doses, appear to be preferable to ChAdOx/ChAdOx. Our data do not support superiority of heterologous vaccination regimens over homologous mRNA-based regimens. Rather, the comparison with the previous study [14] shows that, if anything, homologous mRNA-based regimens are likely superior to their heterologous counterparts in the induction of anti-S1 IgG and IgA. However, a detailed analysis of different mRNA-based homo- and heterologous regimens is still lacking. Therefore, while heterologous vaccination regimens might be preferable to ChAdOx/ChAdOx, from our data, it is at best uncertain whether they have an advantage over Mod/Mod or BNT/BNT.
Our study has several limitations. First, no markers for clinical efficacy were examined, but only markers for immunogenicity. The degree of clinical protection conferred by the examined immunological markers cannot be judged from our data. Further, we did not perform a neutralization assay sensu strictu but a surrogate neutralization assay with an upper limit of detection. This hampers comparisons between all vaccination regimens employing mRNA-based vaccines as they elicit very strong reactions in this assay. This is also the cause of the phenomenon that the dispersion of the results for this assay was much greater for ChAdOx/ChAdOx at t2 than for the two heterologous regimens, since the former regimens induced significantly weaker results and therefore did not reach the upper limit of detection as the latter two regimens did. Moreover, the period of observation was still short with the last follow-up being 14 days after the second vaccination. Thus, no prediction for the endurance of the markers examined can be made by us. Furthermore, it is possible that immune responses to ChAdOx/ChAdOx are more protracted than responses to mRNA-based regimens. Therefore, comparing all vaccination regimens at the same time point might underestimate the immune responses to ChAdOx/ChAdOx. However, given the size of the differences between ChAdOx/ChAdOx and the other regimens, it is likely that they remain statistically significant independent of the time point for the comparison. Another limitation of our study is that we did perform pre-pandemic serum negative controls in the current study. However, in our previous study, we performed a pre-vaccination serological status, using the very same methods as in the current study, establishing that the status of being non-vaccinated and non-previously infected is reliably associated with non-reactive results in all assays used in the current study [13]. Finally, we did not examine anti-viral vector immunity in our study. This phenomenon might have contributed to the weaker responses to ChAdOx/ChAdOx in comparison with the other regimens and must be explored further in upcoming research.
Acknowledgments
The authors want to express their gratitude towards all colleagues and coworkers who have made this study possible, with special thanks to Ingrid Ascha, Franziska Peters, Dorothea Bachmann, Sarah Schultz, Silke Lipke-Reinke, Astrid Messall, Kathrin Johannsen, Michaela Seidel, Alexandra Jurat, Grazyna Wardzinski, and Jana Eicke-Metzenthin, without whom this project would not have been possible.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines10050649/s1, Figure S1: Analysis of data from participants 60 years of age and older; Table S1: numerical makeup of the cohort, whose data went into statistical analysis; Table S2: Differences in immunogenicity of three different homo- and heterologous vaccination regimens against SARS-CoV-2.
Author Contributions
R.D.H.M., M.Z., D.J., D.P., S.R.S., K.-P.W., B.S., F.L., and R.J. designed the study. R.D.H.M., M.Z., D.J., L.S.N., J.D., S.E., J.R., and S.G. contributed to recruitment of participants. The described vaccinations were performed under the direction of S.G., M.Z., and D.J. The assays were performed by R.D.H.M., M.Z., D.J., C.B., D.P., K.S., V.H., D.Z., and C.K. Data analysis was performed by R.D.H.M., M.Z., D.J., K.S., V.H., and D.Z. The manuscript was written by R.D.H.M., with support from all authors. All authors have read and agreed to the published version of the manuscript.
Funding
No external funding was received for this study.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the Christian-Albrechts-University Kiel (protocol code AZ: D642/20; date of approval: 5 March 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
K.S., V.H., D.Z., and C.K. currently are employees of the EUROIMMUN AG (Lübeck, Germany). All other coauthors have no conflict of interest to declare.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., Hernán M.A., Lipsitch M., Reis B., Balicer R.D. BNT162b2 MRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson M.G., Burgess J.L., Naleway A.L., Tyner H.L., Yoon S.K., Meece J., Olsho L.E.W., Caban-Martinez A.J., Fowlkes A., Lutrick K., et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and MRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers—Eight U.S. Locations, December 2020–March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021;70:495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniel W., Nivet M., Warner J., Podolsky D.K. Early Evidence of the Effect of SARS-CoV-2 Vaccine at One Medical Center. N. Engl. J. Med. 2021 doi: 10.1056/NEJMc2102153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 NCov-19 Vaccination. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillus D., Schwarz T., Tober-Lau P., Vanshylla K., Hastor H., Thibeault C., Jentzsch S., Helbig E.T., Lippert L.J., Tscheak P., et al. Safety, Reactogenicity, and Immunogenicity of Homologous and Heterologous Prime-Boost Immunisation with ChAdOx1 NCoV-19 and BNT162b2: A Prospective Cohort Study. Lancet Respir. Med. 2021 doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt T., Klemis V., Schub D., Mihm J., Hielscher F., Marx S., Abu-Omar A., Ziegler L., Guckelmus C., Urschel R., et al. Immunogenicity and Reactogenicity of Heterologous ChAdOx1 NCoV-19/MRNA Vaccination. Nat. Med. 2021:1–6. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rose R., Neumann F., Grobe O., Lorentz T., Fickenscher H., Krumbholz A. Humoral Immune Response after Different SARS-CoV-2 Vaccination Regimens. BMC Med. 2022;20:31. doi: 10.1186/s12916-021-02231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenbusch M., Schumacher S., Vogel E., Priller A., Held J., Steininger P., Beileke S., Irrgang P., Brockhoff R., Salmanton-García J., et al. Heterologous Prime-Boost Vaccination with ChAdOx1 NCoV-19 and BNT162b2. Lancet Infect. Dis. 2021 doi: 10.1016/S1473-3099(21)00420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markewitz R., Pauli D., Dargvainiene J., Steinhagen K., Engel S., Herbst V., Zapf D., Krüger C., Sharifzadeh S., Schomburg B., et al. The Temporal Course of T- and B-Cell-Responses to Vaccination with BNT162b2 and MRNA-1273. Clin. Microbiol. Infect. 2021 doi: 10.1016/j.cmi.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markewitz R., Juhl D., Pauli D., Görg S., Junker R., Rupp J., Engel S., Steinhagen K., Herbst V., Zapf D., et al. Kinetics of the Antibody Response to Boostering with Three Different Vaccines Against SARS-CoV-2. Front. Immunol. 2022;13:26. doi: 10.3389/fimmu.2022.811020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Medical Association World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 16.Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., et al. Virological Assessment of Hospitalized Patients with COVID-2019. Nature. 2020 doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 17.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., Hippenstiel S., Dingeldey M., Kruse B., Fauchere F., et al. SARS-CoV-2-Reactive T Cells in Healthy Donors and Patients with COVID-19. Nature. 2020;587:270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 19.Giménez E., Albert E., Torres I., Remigia M.J., Alcaraz M.J., Galindo M.J., Blasco M.L., Solano C., Forner M.J., Redón J., et al. SARS-CoV-2-Reactive Interferon-γ-Producing CD8+ T Cells in Patients Hospitalized with Coronavirus Disease 2019. J. Med. Virol. 2021;93:375–382. doi: 10.1002/jmv.26213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamini Y., Yekutieli D. The Control of the False Discovery Rate in Multiple Testing under Dependency. Ann. Stat. 2001;29:1165–1188. doi: 10.1214/aos/1013699998. [DOI] [Google Scholar]
- 21.R Core Team . R: A Language and Environment for Statistical Computing. R Core Team; Vienna, Austria: 2020. [Google Scholar]
- 22.Liu X., Shaw R.H., Stuart A.S., Greenland M., Dinesh T., Provstgaard-Morys S., Clutterbuck E., Ramasamy M.N., Aley P.K., Farooq Mujadidi Y., et al. Safety and Immunogenicity Report from the Com-COV Study—A Single-Blind Randomised Non-Inferiority Trial Comparing Heterologous And Homologous Prime-Boost Schedules with An Adenoviral Vectored and MRNA COVID-19 Vaccine. Social Science Research Network; Rochester, NY, USA: 2021. [Google Scholar]
- 23.Borobia A.M., Carcas A.J., Pérez-Olmeda M., Castaño L., Bertran M.J., García-Pérez J., Campins M., Portolés A., González-Pérez M., Morales M.T.G., et al. Immunogenicity and Reactogenicity of BNT162b2 Booster in ChAdOx1-S-Primed Participants (CombiVacS): A Multicentre, Open-Label, Randomised, Controlled, Phase 2 Trial. Lancet. 2021;398:121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Normark J., Vikström L., Gwon Y.-D., Persson I.-L., Edin A., Björsell T., Dernstedt A., Christ W., Tevell S., Evander M., et al. Heterologous ChAdOx1 NCoV-19 and MRNA-1273 Vaccination. N. Engl. J. Med. 2021 doi: 10.1056/NEJMc2110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groß R., Zanoni M., Seidel A., Conzelmann C., Gilg A., Krnavek D., Erdemci-Evin S., Mayer B., Hoffmann M., Pöhlmann S., et al. Heterologous ChAdOx1 NCoV-19 and BNT162b2 Prime-Boost Vaccination Elicits Potent Neutralizing Antibody Responses and T Cell Reactivity against Prevalent SARS-CoV-2 Variants. eBioMedicine. 2022;75 doi: 10.1016/j.ebiom.2021.103761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pozzetto B., Legros V., Djebali S., Barateau V., Guibert N., Villard M., Peyrot L., Allatif O., Fassier J.-B., Massardier-Pilonchéry A., et al. Immunogenicity and Efficacy of Heterologous ChadOx1/BNT162b2 Vaccination. Nature. 2021 doi: 10.1038/s41586-021-04120-y. [DOI] [PubMed] [Google Scholar]
- 27.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021:1–7. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 28.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., Mandelboim M., Gal Levin E., Rubin C., Indenbaum V., et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wyllie D., Jones H.E., Mulchandani R., Trickey A., Taylor-Phillips S., Brooks T., Charlett A., Ades A.E., Investigators E.-H., Moore P., et al. SARS-CoV-2 Responsive T Cell Numbers and Anti-Spike IgG Levels Are Both Associated with Protection from COVID-19: A Prospective Cohort Study in Keyworkers. medRxiv. 2021 doi: 10.1101/2020.11.02.20222778. [DOI] [Google Scholar]
- 30.Post N., Eddy D., Huntley C., van Schalkwyk M.C.I., Shrotri M., Leeman D., Rigby S., Williams S.V., Bermingham W.H., Kellam P., et al. Antibody Response to SARS-CoV-2 Infection in Humans: A Systematic Review. PLoS ONE. 2020;15:e0244126. doi: 10.1371/journal.pone.0244126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shrotri M., Navaratnam A.M.D., Nguyen V., Byrne T., Geismar C., Fragaszy E., Beale S., Fong W.L.E., Patel P., Kovar J., et al. Spike-Antibody Waning after Second Dose of BNT162b2 or ChAdOx1. Lancet. 2021 doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wheatley A.K., Juno J.A., Wang J.J., Selva K.J., Reynaldi A., Tan H.-X., Lee W.S., Wragg K.M., Kelly H.G., Esterbauer R., et al. Evolution of Immune Responses to SARS-CoV-2 in Mild-Moderate COVID-19. Nat. Commun. 2021;12:1162. doi: 10.1038/s41467-021-21444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaebler C., Wang Z., Lorenzi J.C.C., Muecksch F., Finkin S., Tokuyama M., Cho A., Jankovic M., Schaefer-Babajew D., Oliveira T.Y., et al. Evolution of Antibody Immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collier D.A., Ferreira I.A.T.M., Kotagiri P., Datir R.P., Lim E.Y., Touizer E., Meng B., Abdullahi A., CITIID-NIHR BioResource COVID-19 Collaboration. Elmer A., et al. Age-Related Immune Response Heterogeneity to SARS-CoV-2 Vaccine BNT162b2. Nature. 2021 doi: 10.1038/s41586-021-03739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., Bermúdez-González M.C., Bielak D.A., Carreño J.M., Chernet R.L., et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 MRNA Vaccine. N. Engl. J. Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manisty C., Otter A.D., Treibel T.A., McKnight Á., Altmann D.M., Brooks T., Noursadeghi M., Boyton R.J., Semper A., Moon J.C. Antibody Response to First BNT162b2 Dose in Previously SARS-CoV-2-Infected Individuals. Lancet Lond. Engl. 2021;397:1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradley T., Grundberg E., Selvarangan R., LeMaster C., Fraley E., Banerjee D., Belden B., Louiselle D., Nolte N., Biswell R., et al. Antibody Responses after a Single Dose of SARS-CoV-2 MRNA Vaccine. N. Engl. J. Med. 2021 doi: 10.1056/NEJMc2102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saadat S., Tehrani Z.R., Logue J., Newman M., Frieman M.B., Harris A.D., Sajadi M.M. Binding and Neutralization Antibody Titers After a Single Vaccine Dose in Health Care Workers Previously Infected With SARS-CoV-2. JAMA. 2021 doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Green M.S., Shohat T., Lerman Y., Cohen D., Slepon R., Duvdevani P., Varsano N., Dagan R., Mendelson E. Sex Differences in the Humoral Antibody Response to Live Measles Vaccine in Young Adults. Int. J. Epidemiol. 1994;23:1078–1081. doi: 10.1093/ije/23.5.1078. [DOI] [PubMed] [Google Scholar]
- 40.Fischinger S., Boudreau C.M., Butler A.L., Streeck H., Alter G. Sex Differences in Vaccine-Induced Humoral Immunity. Semin. Immunopathol. 2019;41:239–249. doi: 10.1007/s00281-018-0726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoica G., Macarie E., Michiu V., Stoica R.C. Biologic Variation of Human Immunoglobulin Concentration. I. Sex-Age Specific Effects on Serum Levels of IgG, IgA, IgM and IgD. Med. Interne. 1980;18:323–332. [PubMed] [Google Scholar]
- 42.Metzger D.W. IgA and Respiratory Immunity. In: Kaetzel C.S., editor. Mucosal Immune Defense: Immunoglobulin A. Springer; Boston, MA, USA: 2007. pp. 269–290. [Google Scholar]
- 43.Brewer R.C., Ramadoss N.S., Lahey L.J., Jahanbani S., Robinson W.H., Lanz T.V. BNT162b2 Vaccine Induces Divergent B Cell Responses to SARS-CoV-2 S1 and S2. Nat. Immunol. 2021:1–7. doi: 10.1038/s41590-021-01088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.