Abstract
Gestational diabetes mellitus (GDM) is one of the most common complications of pregnancy, affecting up to 14% of pregnant women. The population of patients with risk factors of GDM is increasing; thus, it is essential to improve management of this condition. One of the key factors affecting perinatal outcomes in GDM is glycaemic control. Until recently, glucose monitoring was only available with self-monitoring of blood glucose (SMBG). However, nowadays, there is a new method, continuous glucose monitoring (CGM), which has been shown to be safe in pregnancy. Since proper glycaemia assessment has been shown to affect perinatal outcomes, we decided to perform a systematic review to analyse the role of CGM in glycaemic control in GDM. We conducted a web search of the MEDLINE, EMBASE, Cochrane Library, Scopus, and Web of Science databases according to the PRISMA guidelines. The web search was performed by two independent researchers and resulted in 14 articles included in the systematic review. The study protocol was registered in the PROSPERO database with registration number CRD42021289883. The main outcome of the systematic review was determining that, when compared, CGM played an important role in better glycaemic control than SMBG. Furthermore, glycaemic control with CGM improved qualification for insulin therapy. However, most of the articles did not reveal CGM’s role in improving neonatal outcomes. Therefore, more studies are needed to analyse the role of CGM in affecting perinatal outcomes in GDM.
Keywords: gestational diabetes mellitus, continuous glucose monitoring, self-monitoring of blood glucose, hyperglycaemia, hypoglycaemia
1. Introduction
Gestational diabetes mellitus (GDM) is the most common complication of pregnancy, with an incidence rate of up to 14% of all pregnant women [1]. Over a long period of time, it was defined as any degree of glucose intolerance with the onset or first recognition during pregnancy [2,3]. However, now it is debated whether this definition is appropriate due to its limitations, including imprecise information about diagnostic thresholds for GDM [4]. The population of patients with risk factors for GDM is continuously increasing, thus, it is essential to improve the management of GDM [5]. It is believed that glycaemic control plays a major role in the proper treatment of GDM [6,7]. Until recently glycaemic control in GDM was mainly based on the self-monitoring of blood glucose (SMBG) [8]. However, the main inconveniences of this method are multiple finger-pricking for a single glycaemia measurement and intermittent checking of glucose levels, which might lead to poor patient compliance [9]. Recently, a new method for glycaemic control was introduced, namely, continuous glucose monitoring (CGM) [10]. The method uses a subcutaneous sensor to collect the glycaemia results. The main benefit of CGM is that, after insertion, the system analyses the actual glycaemia constantly without any additional invasive procedure [11]. An important advantage of CGM is the evaluation of time the patient spends in normoglycaemia. It is called time-in-range, and it is defined as the percentage of time in which glycaemia is in reference range [12]. It is believed that time-in-range is a more accurate outcome to assess the patient’s compliance.
There are ongoing debates about what type of glycaemia measurement method is the most effective for pregnant women diagnosed with GDM. It is hypothesized that CGM is superior to SMBG, but due to the high price of the device and a lack of reimbursement for GDM in many countries, it is not used as the method of choice [7,8].
The aim of this systematic review is to assess the efficacy of continuous glucose monitoring on glycaemic control in pregnant women with GDM. In addition, this review will focus on the need for pharmacological treatment and perinatal outcomes in the population of patients using CGM.
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
We conducted a systematic web search in the MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), Scopus, and Web of Science databases according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The systematic review has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) registry (CRD42021289883). The keywords utilized for the research were: continuous glucose monitoring, flash glucose monitoring, and gestational diabetes mellitus. The time frame of the research was from database inception date to November 2021. The inclusion criteria were: randomized controlled trials and observational studies, and human studies in English. The exclusion criteria were types of studies other than the inclusion criteria, animal studies, and studies in different languages than English (Table 1).
Table 1.
Inclusion and exclusion criteria for the systematic review.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Randomized controlled trials and observational studies |
Case reports, review articles, editorial comments |
| Human studies Studies in English |
Animal studies Studies in different languages than English |
Following the initial screening, publications were analysed further by title and abstract to exclude studies that did not meet the inclusion criteria. After initial selection, the remaining full articles were screened to assess the final number of eligible publications included to the systematic review. Two of the authors independently evaluated all retrieved studies against the eligibility criteria and, in cases of differing opinion, the publication was discussed with the third author.
Due to heterogeneity in terms of continuous glucose monitoring devices, study duration, and number of patients among the included articles, no meta-analysis was performed.
2.2. Data Analysis
Data were extracted independently by two researchers. The following data were extracted: type of article, year of publication, type of continuous glucose monitoring, number of patients included in the study, fasting, postprandial and nocturnal glycaemia, time in range, qualification for insulin therapy, incidence of severe nocturnal hypoglycaemia, glycosylated haemoglobin concentration (HbA1c), gestational weight gain, newborn birth weight, and other neonatal outcomes.
2.3. Outcomes
The main outcome was glycaemic control (fasting, postprandial and nocturnal glycaemia). Several secondary outcomes were also investigated, including: qualification to insulin therapy, incidence of severe nocturnal hypoglycaemia, HbA1c, gestational weight gain, newborn birth weight, and other neonatal outcomes.
3. Results
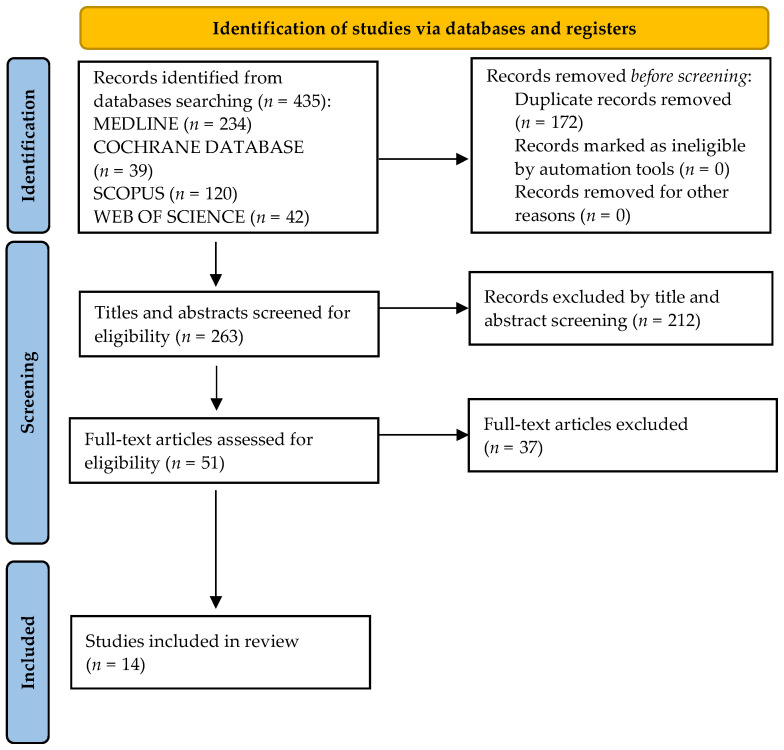
A total of 435 articles were identified through a systematic review of the literature (Figure 1).
Figure 1.
PRISMA flow diagram.
After initial screening, 172 duplicates were excluded and 263 titles and abstracts were screened further for eligibility criteria, leaving a total of 51 full-text publications. Review of the full-text articles resulted in 37 studies being excluded from further assessment. A total of 14 remaining publications were included in the final analysis of this systematic review (Table 2).
Table 2.
Characteristics of studies included in the systematic review.
| Study ID | Study Design |
Study Population |
Type of CGM | Duration of CGM Usage | Outcome | Results |
|---|---|---|---|---|---|---|
| Paramasivam S et al. [6] | RCT * | 57 GDM patients | iPro™ 2 Medtronic | 6 days | Incidence of hypoglycaemia, insulin therapy, maternal and neonatal outcomes | Higher detection of hypoglycaemia in CGM group; no difference in other outcomes |
| Afandi B et al. [7] | Prospective observational study | 25 GDM patients | iPro™ 2 Medtronic | 5 days | Incidence of hyper- and hypoglycaemia, HbA1c level, qualification to insulin therapy | Lower incidence of hyperglycaemia and higher detection of hypoglycaemia in CGM group |
| Márquez-Pardo S et al. [8] | Prospective observational study | 77 GDM patients | iPro™ 2 Medtronic | 6 days | Incidence of hyperglycaemia, qualification to insulin therapy | Higher detection of hyperglycaemia, more qualification to insulin therapy in CGM group |
| Chen R et al. [9] | Prospective observational study | 57 GDM patients | Medtronic MiniMed | 72 h | Incidence of postprandial hyperglycaemia and nocturnal hypoglycaemia; HbA1c level | Higher detection of nocturnal hypoglycaemia and postprandial hyperglycaemia in CGM group, no difference in HbA1c level between the groups |
| Lane AF et al. [11] | RCT | 40 GDM patients | Medtronic MiniMed/iPro™ 2 Medtronic | 28 days | Incidence of hyper- and hypoglycaemia, time in range, HbA1c level, maternal and neonatal outcomes | No difference between the groups |
| Yu F et al. [12] | Prospective cohort study | 340 GDM patients | Medtronic MiniMed | 72 h a week for 5 weeks | Glycaemia control, insulin therapy, maternal and neonatal outcomes | Shorter durations of hyper- and hypoglycaemia, more patients qualified to insulin therapy in CGM group; less incidence of LGA *, neonatal hypoglycaemia and hyperbilirubinemia in CGM group |
| Cypryk K et al. [13] | Prospective observational study | 12 GDM patients, 7 patients non-GDM | Medtronic MiniMed | 72 h | Glycaemia control | No difference between the groups |
| Zhang X et al. [14] | RCT | 110 GDM patients | ISGMS * (Abbott Diabetes Care) | 14 days | Incidence of hypoglycaemia, gestational weight gain, health behaviour patterns | Lower gestational weight gain, better health behaviour patterns and lower incidence of hypoglycaemia in CGM group |
| Buhling KJ et al. [15] | Prospective observational study | 63 GDM, 17 IGT, 24 non-GDM, 9 non-pregnant patients | Medtronic MiniMed | 72 h | Glycaemia control, neonatal outcomes | Higher detection of hyperglycaemia in CGM group, no difference in other outcomes between the groups |
| Zaharieva D et al. [16] | Prospective Observational Study | 90 GDM patients | iPRO Medtronic | 7 days | Incidence of hyperglycaemia | Higher detection of hyperglycaemia in CGM group |
| Alfadhli E et al. [17] | RCT | 130 GDM patients | Guardian® RT-CGMS MiniMed |
3–7 days | Fasting and postprandial glycaemia, HbA1c level, insulin therapy, maternal and neonatal outcomes | No difference between the groups |
| Kestila K et al. [18] | RCT | 73 GDM patients | Medtronic MiniMed | Mean 47.4 h | Insulin therapy, maternal and neonatal outcomes | Higher number of patients qualified for insulin therapy in CGM group; no difference in maternal and neonatal outcomes between the groups |
| Yogev Y et al. [19] | Prospective observational study | 6 PGDM, 2 GDM patients, |
Medtronic MiniMed | 72 h | Glycaemia, HbA1c level, insulin therapy, maternal and neonatal outcomes | Higher detection of nocturnal hypoglycaemia and postprandial hyperglycaemia, better modification of insulin therapy in CGM group; no difference in other outcomes between the groups |
| Wei Q et al. [20] | RCT | 106 GDM patients | Medtronic MiniMed | 48–72 h | Glycaemia, HbA1c level, insulin therapy, maternal and neonatal outcomes | Higher number of patients qualified to insulin therapy, better detection of nocturnal hypoglycaemia and postprandial hyperglycaemia, less gestational weight gain in CGM group; No difference in other outcomes between the groups |
* RCT = Randomised controlled trial; LGA = large for gestational age; ISGMS: instantaneous scanning glucose monitoring system.
3.1. Glycaemic Control
3.1.1. Hyperglycaemia
In five studies, it was found that CGM is better at detecting episodes of hyperglycaemia as compared to SMBG [7,9,12,15,16]. In two studies, it was found that CGM detected more hyperglycaemic events than SMBG [9,15]. However, Afandi et al. demonstrated that the incidence rate of hyperglycaemia in all patients included in the study reached 5.65% using CGM versus 14.2% using SMBG (p < 0.05) [7]. The incidence of hyperglycaemia above 180 mg/dL in the CGM and SMBG groups was estimated to be <1.0% and 2% of all readings, respectively (p < 0.05). In another prospective study, hyperglycaemic events were analysed further, and the result was that, in the CGM group, the duration of time spent in hyperglycaemia was shorter than in the SMBG group [12]. One study found that CGM is a better detector of nocturnal hyperglycaemia than SMBG [16]. On the other hand, three studies described no statistical difference between the SMBG and CGM groups in detecting glycaemia above the reference range [11,13,17].
3.1.2. Hypoglycaemia
We found eight articles about incidences of hypoglycaemia [6,7,9,11,12,13,14,15]. In most studies, the outcome was that CGM detects a higher number of hypoglycaemia episodes than SMBG [6,7,9,15]. It played an especially significant role in pregnant women qualified for insulin therapy [9,19]. Chen et al. underlined CGM’s role in especially detecting nocturnal hypoglycaemia in patients requiring pharmacological treatment [9].
There was only one study, by Zhang et al., that calculated a significantly lower number of patients with hypoglycaemic events in the CGM group (overall, 3 patients with hypoglyceamic episodes (5.45%) in CGM versus 12 patients (21.82%) in SMBG group; χ2 = 6.253, p = 0.012) [13]. Yu at el analysed hypoglycaemia further and showed significant a difference in the duration of time spent in hypoglycaemia, with lower results in the CGM group [12].
3.2. Insulin Therapy
Five studies analysed how qualification to insulin therapy differs between the CGM and SMBG groups [6,8,18,19,20]. In three of them, it was noted that CGM is a better predictor for the initiation of antihyperglycaemic treatment [8,18,20]. Kestilä et al. found that using SMBG only leads to underestimation of the actual number of patients requiring insulin therapy [18]. In another study, it was also confirmed that CGM detects a higher number of patients who should be qualified for pharmacological treatment [8].
Two studies analysed whether CGM has an impact on insulin dosage. Paramasivan et al. conducted a randomised, controlled trial and revealed that the total insulin requirement was higher in the CGM group throughout pregnancy; however, there was no significant difference in the insulin dosage between the groups (CGM vs. control: 16.2 ± 6.4 vs. 11.8 ± 13.6 units, p = 0.314) [6]. An interesting outcome was demonstrated in the study by Yogev et al.; namely, the CGM group demanded 33% less long and intermediate-acting insulin, while, simultaneously, having higher (mean 20%) postprandial morning and afternoon insulin doses than the SMBG group [19].
3.3. HBA1c
HBA1c levels were analysed in six studies [5,8,10,16,18,19]. A randomized, controlled trial assessing HbA1c results in patients with GDM treated with insulin revealed significantly lower HbA1c concentration in the CGM group (CGM group: 5.2 ± 0.4% vs. SMBG group: 5.6 ± 0.6%, p < 0.006) [6]. Furthermore, in the CGM group, HbA1c remained unchanged, in contrast to SMBG group, in which HbA1c levels increased over the course of pregnancy. Despite the above-mentioned results in five other studies, no significant differences in HbA1c concentration between CGM and SMBG groups were observed [9,11,17,19,20].
3.4. Gestational Weight Gain
Gestational weight gain was analysed in three publications [14,18,20]. Two of them revealed a significantly lower increase in weight gain in the CGM group [14,20]. In addition, there was less incidence of excessive weight gain in the group using continuous glucose monitoring [14,20]. Nevertheless, the third publication, by Kestila et al., did not confirm the impact of CGM on gestational weight gain [18].
3.5. Neonatal Outcomes
Seven studies compared neonatal outcomes, and the results are not conclusive [6,11,12,13,17,18,20]. In the study by Paramasivan et al., no significant difference in newborn weight between the CGM and SMBG groups was noted (CGM: 2842.4 g ± 448.6 vs. SMBG: 2976.0 g ± 473.5; p = 0.311) [6]. Another two prospective studies confirmed their result [18,20]. In the study by Kestilla et al., the incidence of macrosomia was similar in both groups (p = 0.33) [18]. In contrast, Yu F et al. observed significantly lower neonatal weight in the CGM group (an average difference of 207 g; p < 0.001) and higher incidence of macrosomia or LGA in the SMBG group (p < 0.05) [12]. The authors also analysed other neonatal outcomes, but the results were inconclusive. There was a significantly lower incidence of neonatal hypoglycaemia and hyperbilirubinemia in the CGM group; however, NICU admission rates did not differ between the groups [12]. In four other studies, the authors showed no differences in any analysed neonatal outcomes [11,17,18,20].
4. Discussion
In this systematic review, we aimed to assess the efficacy of CGM on glycaemic control in GDM. Overall, the results of our review provide clear evidence for the superiority of CGM over SMBG in dysglycaemia assessment. In the majority of studies, it was shown that, in the CGM group, there was a better detection of dysglycaemia than in the SMBG group [6,7,9,12,15]. However, few studies did not confirm the statistical difference between those two methods [11,13,17]. The difference in outcomes might be the consequence of different methodologies used in the studies, including the number of patients recruited or study duration (for example, too short a period of time to reveal a statistical difference between the groups).
An interesting outcome analysed in the review was the role of CGM in detecting nocturnal hypoglycaemia. In four studies, CGM performed better in the assessment of hypoglycaemic events [6,7,9,16]. Yu et al. revealed that CGM shortens the time spent in hypoglycaemia as compared to SMBG [12]. Moreover, the authors observed that CGM had an impact on diet control, weight monitoring and appropriate exercise. Thus, shorter time spent in hypoglycaemia was correlated with better health behaviour patterns and patient compliance. Overall, it is believed that improved nocturnal hypoglycaemia detection by CGM might have implications for better modification of GDM treatment, not only better qualification for insulin therapy, but also diet modifications [6,12]. It might play a particular role for patients requiring pharmacological treatment.
HbA1c levels, widely used as an assessment tool for patients with diabetes compliance, did not differ between the groups in almost all analysed articles [11,19,20]. Only one study noted the role of CGM in improving HbA1c levels throughout pregnancy [6]. Consequently, these outcomes might confirm that HbA1c is not the most reliable parameter used for gestational diabetes management.
Regarding insulin therapy, almost all studies demonstrated CGM’s superiority over SMBG in predicting adequate antihyperglycaemic treatment [8,18,19,20]. Continuous glucose monitoring not only enabled better qualifications of patients for insulin therapy, but also had an impact on dose modification. CGM improved adjustments in the insulin dosage that, in consequence, could minimalize complications associated with improper treatment [20].
Regarding neonatal outcomes, in most of the included studies, there was no statistical difference between the CGM and SMBG groups [6,18,20]. Only one study, including over 300 patients, revealed a significantly lower incidence of LGA and lower birth weight in the CGM than in the SMBG group [12]. As a result, further studies need to be conducted to elucidate whether lack of differences in neonatal outcomes may be a consequence of methodological shortcomings. It seems likely that if continuous glucose monitoring better detects dysglycaemia and improves pharmacological treatment, it should have an impact on neonatal outcomes.
A few studies revealed the role of CGM in improving health behaviour patterns [14,20]. Zhang et al. noted its role, especially with regard to lower gestational weight gain compared to the SMBG group [14]. However, there is limited data available in other analysed studies about this maternal outcome. The possible cause of this ambiguous result might be a short period of CGM usage in the majority of the included articles (less than 7 days of measurements per patient).
Several methodological flaws limit the internal validity of this systematic review. First, the main limitation of the analysed studies is that they included small study groups (there was only one study including >150 patients). For example, Paramasivan et al. studied the impact of CGM on maternal and neonatal outcomes with a relatively small group of patients (n = 25 in CGM and n = 25 in SMBG group) [6]. Hence, some of their results do not merge together—the study revealed the impact of continuous glucose monitoring on improving glycaemia control, but it revealed no significant differences in neonatal outcomes. Secondly, not all of the studies were randomized, controlled trials; therefore, some of the results might have been prone to recall bias. Thirdly, the periods planned for conducting the study were relatively short (median: 5 days), which may not allow the demonstration of significant differences in certain perinatal outcomes. Furthermore, there were not many studies that analysed additional maternal outcomes.
The strengths of this systematic review include study selection from five major databases and their further analysis based on clearly defined inclusion and exclusion criteria.
5. Conclusions
This systematic review supports the thesis that CGM is superior to SMBG in the management of dysglycaemia in GDM. Our findings suggest that CGM better detects hyper- and hypolgycaemic events and is a more appropriate predictor of qualification for insulin therapy. Therefore, these results provide justification for the idea that CGM plays an important role in glycaemia management in GDM. CGM improves the detection of fasting and postprandial hyperglycaemia. Additionally, it better assesses nocturnal hypoglycemia episodes. Improved identification of dysglycaemia allows for better patient compliance as well as decreases the rate of unnecessary interventions, including qualification for insulin therapy and further improper dose adjustment.
On the other hand, the results for neonatal outcomes, including LGA incidence in both methods of measurement, were inconclusive. There is limited evidence that CGM improves any of the analysed neonatal outcomes. Furthermore, it will be essential to elucidate the role of CGM in changing patient health behaviour patterns.
To conclude, more prospective studies focusing on maternal and neonatal outcomes in GDM-complicated pregnancies monitored by CGM need to be conducted to support the existing evidence and to solve the inconclusive findings.
Author Contributions
A.M. and P.J.S. drafted the manuscript. P.J.S., M.W., and D.B.-O. participated in the revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request. The data are not publicly available due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ogurtsova K., da Rocha Fernandes J.D., Huang Y., Linnenkamp U., Guariguata L., Cho N.H., Cavan D., Shaw J.E., Makaroff L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 2.International Association of Diabetes. Pregnancy Study Groups Consensus Panel International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32((Suppl. 1)):S62–S67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44((Suppl. 1)):S15–S33. doi: 10.2337/dc21-S002. [DOI] [PubMed] [Google Scholar]
- 5.Deputy N.P., Kim S.Y., Conrey E.J., Bullard K.M. Prevalence and Changes in Preexisting Diabetes and Gestational Diabetes Among Women Who Had a Live Birth—United States, 2012–2016. MMWR Morb. Mortal. Wkly. Rep. 2018;67:1201–1207. doi: 10.15585/mmwr.mm6743a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paramasivam S.S., Chinna K., Singh A.K.K., Ratnasingam J., Ibrahim L., Lim L.L., Tan A.T.B., Chan S.P., Tan P.C., Omar S.Z., et al. Continuous glucose monitoring results in lower HbA1c in Malaysian women with insulin-treated gestational diabetes: A randomized controlled trial. Diabet. Med. 2018;35:1118–1129. doi: 10.1111/dme.13649. [DOI] [PubMed] [Google Scholar]
- 7.Afandi B., Hassanein M., Roubi S., Nagelkerke N. The value of Continuous Glucose Monitoring and Self-Monitoring of Blood Glucose in patients with Gestational Diabetes Mellitus during Ramadan fasting. Diabetes Res. Clin. Pract. 2019;151:260–264. doi: 10.1016/j.diabres.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 8.Marquez-Pardo R., Torres-Barea I., Cordoba-Dona J.A., Cruzado-Begines C., Garcia-Garcia-Doncel L., Aguilar-Diosdado M., Baena-Nieto M.G. Continuous Glucose Monitoring and Glycemic Patterns in Pregnant Women with Gestational Diabetes Mellitus. Diabetes Technol. Ther. 2020;22:271–277. doi: 10.1089/dia.2019.0319. [DOI] [PubMed] [Google Scholar]
- 9.Chen R., Yogev Y., Ben-Haroush A., Jovanovic L., Hod M., Phillip M. Continuous glucose monitoring for the evaluation and improved control of gestational diabetes mellitus. J. Matern. Fetal Neonatal. Med. 2003;14:256–260. doi: 10.1080/jmf.14.4.256.260. [DOI] [PubMed] [Google Scholar]
- 10.Murphy H.R., Rayman G., Lewis K., Kelly S., Johal B., Duffield K., Fowler D., Campbell P.J., Temple R.C. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: Randomised clinical trial. BMJ. 2008;337:a1680. doi: 10.1136/bmj.a1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane A.S., Mlynarczyk M.A., de Veciana M., Green L.M., Baraki D.I., Abuhamad A.Z. Real-Time Continuous Glucose Monitoring in Gestational Diabetes: A Randomized Controlled Trial. Am. J. Perinatol. 2019;36:891–897. doi: 10.1055/s-0039-1678733. [DOI] [PubMed] [Google Scholar]
- 12.Yu F., Lv L., Liang Z., Wang Y., Wen J., Lin X., Zhou Y., Mai C., Niu J. Continuous glucose monitoring effects on maternal glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus: A prospective cohort study. J. Clin. Endocrinol. Metab. 2014;99:4674–4682. doi: 10.1210/jc.2013-4332. [DOI] [PubMed] [Google Scholar]
- 13.Cypryk K., Pertynska-Marczewska M., Szymczak W., Wilcynski J., Lewinski A. Evaluation of metabolic control in women with gestational diabetes mellitus by the continuous glucose monitoring system: A pilot study. Endocr. Pract. 2006;12:245–250. doi: 10.4158/EP.12.3.245. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X., Jiang D., Wang X. The effects of the instantaneous scanning glucose monitoring system on hypoglycemia, weight gain, and health behaviors in patients with gestational diabetes: A randomised trial. Ann. Palliat. Med. 2021;10:5714–5720. doi: 10.21037/apm-21-439. [DOI] [PubMed] [Google Scholar]
- 15.Buhling K.J., Kurzidim B., Wolf C., Wohlfarth K., Mahmoudi M., Wascher C., Siebert G., Dudenhausen J.W. Introductory experience with the continuous glucose monitoring system (CGMS; Medtronic Minimed) in detecting hyperglycemia by comparing the self-monitoring of blood glucose (SMBG) in non-pregnant women and in pregnant women with impaired glucose tolerance and gestational diabetes. Exp. Clin. Endocrinol. Diabetes. 2004;112:556–560. doi: 10.1055/s-2004-830399. [DOI] [PubMed] [Google Scholar]
- 16.Zaharieva D.P., Teng J.H., Ong M.L., Lee M.H., Paldus B., Jackson L., Houlihan C., Shub A., Tipnis S., Cohen O., et al. Continuous Glucose Monitoring Versus Self-Monitoring of Blood Glucose to Assess Glycemia in Gestational Diabetes. Diabetes Technol. Ther. 2020;22:822–827. doi: 10.1089/dia.2020.0073. [DOI] [PubMed] [Google Scholar]
- 17.Alfadhli E., Osman E., Basri T. Use of a real time continuous glucose monitoring system as an educational tool for patients with gestational diabetes. Diabetol. Metab. Syndr. 2016;8:48. doi: 10.1186/s13098-016-0161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kestila K.K., Ekblad U.U., Ronnemaa T. Continuous glucose monitoring versus self-monitoring of blood glucose in the treatment of gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2007;77:174–179. doi: 10.1016/j.diabres.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Yogev Y., Ben-Haroush A., Chen R., Kaplan B., Phillip M., Hod M. Continuous glucose monitoring for treatment adjustment in diabetic pregnancies—A pilot study. Diabet. Med. 2003;20:558–562. doi: 10.1046/j.1464-5491.2003.00959.x. [DOI] [PubMed] [Google Scholar]
- 20.Wei Q., Sun Z., Yang Y., Yu H., Ding H., Wang S. Effect of a CGMS and SMBG on Maternal and Neonatal Outcomes in Gestational Diabetes Mellitus: A Randomized Controlled Trial. Sci. Rep. 2016;6:19920. doi: 10.1038/srep19920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request. The data are not publicly available due to privacy or ethical restrictions.