Abstract
Small intestinal bacterial overgrowth (SIBO) contributes to the formation of an inflammatory environment in various intestinal and extraintestinal diseases. Cytokines that participate in these mechanisms are yet to be examined. Upper gastrointestinal endoscopy with duodenal aspiration was performed in 224 patients. Quantitative cultures of aerobic species were performed, concentrations of interleukin 1β (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNF-α) were measured, and loads of Escherichia coli, Klebsiella pneumoniae, Methanobevibacter smithii, and Aeromonas spp. were detected via real-time PCR in the duodenal fluid. Analysis showed that the odds ratio (OR) for elevated IL-1β levels was 2.61 (1.06–6.43, p = 0.037) among patients with SIBO compared to patients without SIBO, while there was no significant difference at elevated IL-6 and TNF-α levels between patients with and without SIBO, using ≥10³ cfu/mL as a cut-off. The presence of all three elevated cytokine levels has OR 3.47 (1.06–11.34, p = 0.030) among patients with SIBO. Klebsiella pneumoniae detection was positively related with IL-6 and TNF-α levels, when Methanobevibacter smithii was positively related with IL-1β levels. The presence of SIBO is associated with elevated IL-1β levels in the duodenal fluid. There is a high prevalence of all three proinflammatory cytokine levels elevated (IL-1β, IL-6, and TNF-α) in the duodenal fluid among patients with SIBO.
Keywords: small intestinal bacterial overgrowth, inflammation, cytokines, interleukine-1b
1. Introduction
Small intestine bacterial overgrowth (SIBO) represents the overgrowth of bacterial species, which usually predominate in the large bowel, in the proximal small intestine. Lactulose and hydrogen breath tests are often used as noninvasive diagnostic tests with questionable accuracy [1]. The gold-standard for diagnosis remains the culture of the fluid in the proximal part of the small intestine [2].
SIBO has been linked for many years to signs and symptoms such as bloating and diarrhea that occur due to the production of gas and other metabolites by the small intestine coliforms [3]. SIBO is more common in patients with inflammatory bowel disease (IBS) [4]. However, not all patients with SIBO present with IBS and vice versa, leading to the question if what is needed for SIBO to stimulate one IBS-like phenotype is the generation of one pro-inflammatory reaction at the level of the proximal small intestine.
Several years ago, we ran a prospective study where quantitative cultures were studied in the fluid collected from the third part of the duodenum in 897 patients who underwent upper GI tract endoscopy. In this manuscript, we present a sub-group analysis of 224 patients were the concentrations of the proinflammatory cytokines interleukin (IL)-1β, IL-6 (IL-6) and tumor necrosis factor alpha (TNF-α) were measured in the duodenal fluid.
2. Materials and Methods
2.1. Study Design
This was a prospective study conducted between September 2009 and March 2013. Study participants were patients that underwent upper gastrointestinal (GI) endoscopy in the Gastroenterology Department of Sismanogleion General Hospital of Athens, after they signed a written informed consent. Study protocol had the approval of Hospital’s Ethics Committee (Ethical Approval Number 296/2009). This study was performed in compliance with the Declaration of Helsinki and Good Clinical Practice principles.
Inclusion criteria were the following: (a) age ≥18 years; (b) written informed consent; and (c) clinical indication for upper GI endoscopy. Exclusion criteria were the following: (a) human immunodeficiency virus (HIV) infection; (b) chronic hepatitis B, hepatitis C, or hepatitis E infection; (c) Child Pugh liver cirrhosis stages B and C; (d) active upper gastrointestinal bleeding; (e) gastroesophageal reflux disease (GERD); (f) Helicobacter Pylori infection; (g) celiac disease; (h) enteric infections; (i) systemic sclerosis; (j) antibiotics consumption four weeks prior to endoscopy [5,6]; (k) inflammatory bowel disease; (l) microscopic colitis; (m) any gastric surgery; and (n) unstable thyroid disease.
Fluid from the third portion of the duodenum was collected during upper GI endoscopy. Water was not flushed in the duodenal lumen before the completion of aspiration. If there was inadequate volume of fluid in the duodenal lumen, there was an approach of the endoscope close to the intestinal wall, so that the biggest possible amount of fluid was aspirated. Aspirate was collected by placing a sterile suction catheter inside a sterile overtube, which was advanced through the suction channel of the endoscope. The fluid was placed immediately in transport vials and transferred to the laboratory. The samples were prospectively collected and samples were kept refrigerated until measurements were completed. Cultures of duodenal fluid and identification of bacteria were collected as described previously [7]. Briefly, an aliquot of 0.1 mL was plated onto MacConckey agar (Becton Dickinson, Conckeysville, MD, USA) and incubated for 18 h at 35 °C. Bacterial growth was determined after multiplying the number of isolated bacteria with the respective dilution factor. Concentrations of tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), and intereukin 1β (IL-1β) were measured in the duodenal fluid by an enzyme immunosorbent assay (R&D Inc., Minneapolis MN); the lower detection limit was 20 pg/mL for IL-1β and TNF-α and 60 pg/mL for IL-6. Quantitative real time PCR was performed in 125 patients for detecting the load of total prokaryotes, Escherichia coli (E. coli), Klebsiella pneumoniae, Aeromonas spp., and Methanobevibacter smithii (M. smithii). Quantitative PCR analyses of the above 125 patients have already been described previously [8].
For each patient enrolled the following information was recorded; age, gender, height, weight, reason for endoscopy, endoscopic findings, other diseases, and intake of any medication. All patients underwent colonoscopy in order to rule out colonic diseases such as IBD and microscopic colitis. The presence and classification of preexisting IBS was evaluated according to Rome IV criteria [9]. The presence of Helicobacter Pylori infection was evaluated with a Rapid Urease test (CLO test) during upper GI endoscopy; if positive, patient was not enrolled in the study. Furthermore, biopsies from duodenum were obtained during upper GI endoscopy in order to rule out small intestine comorbidities such as celiac disease or giardiasis.
2.2. Study Endpoints
The primary study endpoint was the relationship between SIBO and proinflammatory cytokines TNF-α, IL-1β, and IL-6 in the duodenal fluid. Three secondary study endpoints were set: (a) the impact of specific bacteria species that were detected via real-time PCR on the elevation of TNF-α, IL-1β, and IL-6 levels in the duodenal fluid; (b) possible risk factors that may be linked to SIBO.
2.3. Statistical Analysis
All statistical analyses were performed using SPSS version 20.0 for Windows software (SPSS Inc., Chicago, IL, USA). In order to explore the primary study endpoint, SIBO was defined in three different ways using three different cutoffs of concentrations of colonic type bacteria in the duodenal aspirate, i.e., >10³, >10⁴, and >10⁵ cfu/mL. Comparisons for qualitative variables between patients with SIBO and those without SIBO were calculated by the Chi-square test. Cytokines were expressed as % of patients with detectable concentrations. Variables with normal distribution of values were compared using Student’s t-test and variables with non-normal distribution of values were compared using Mann–Whitney U test. Odds ratios (OR) and 95% confidence intervals (CI) were calculated by Mantel–Haenszel’s statistics. Step-wise forward logistic regression analysis was performed with the presence of SIBO as a dependent variable and the presence of IBS or other co-morbidities, drug intake, findings from endoscopy, and cytokine levels as independent variables. p value less than 0.05 was considered significant.
3. Results
Demographic characteristics of enrolled patients are classified into those with and those without SIBO and are shown in Table 1. As a whole, patients with SIBO were older, had a higher frequency of type 2 diabetes mellitus and predominant-diarrhea IBS.
Table 1.
Demographic characteristic of enrolled patients.
| No SIBO (n = 156) |
SIBO (n = 68) | p | |
|---|---|---|---|
| Male (n, %) | 73 (46.8) | 36 (52.9) | 0.397 |
| Age ≥ 60 years (n, %) | 93 (59.6) | 55 (80.9) | 0.002 |
| BMI ≥ 22 kg/m2 (n, %) | 136 (87.2) | 52 (76.5) | 0.045 |
| Presence of IBS (n, %) | 75 (48.1) | 42 (61.8) | 0.059 |
| Type of IBS (n, %) | |||
| IBS-D | 23 (14.7) | 18 (26.5) | 0.037 |
| IBS-C | 13 (8.3) | 3 (4.4) | 0.295 |
| Mixed type IBS | 39 (25.0) | 21 (30.9) | 0.361 |
| Comorbidities (n, %) | |||
| T2DM | 28 (17.9) | 21 (30.9) | 0.036 |
| CHF | 36 (23.1) | 20 (29.4) | 0.314 |
| COPD | 16 (10.3) | 5 (7.5) | 0.513 |
| CRF | 7 (4.5) | 2 (2.9) | 0.588 |
| Solid tumor malignancy | 14 (9.0) | 4 (5.9) | 0.434 |
| History of drug intake (n, %) | |||
| PPIs | 38 (24.4) | 13 (19.1) | 0.390 |
| NSAIDs | 9 (5.8) | 2 (2.9) | 0.368 |
| Aspirin | 27 (17.3) | 15 (22.1) | 0.402 |
| Acenocumarone | 11 (7.1) | 7 (10.3) | 0.412 |
| H2-blockers | 2 (1.3) | 1 (1.5) | 0.910 |
| Antacids | 5 (3.2) | 1 (1.5) | 0.460 |
| Clinical reason for gastroscopy (n, %) | |||
| Dyspepsia | 94 (60.3) | 39 (57.4) | 0.684 |
| Anemia | 73 (46.8) | 36 (52.9) | 0.397 |
| Fever of unknown origin | 7 (4.5) | 2 (2.9) | 0.588 |
| Endoscopic findings (n, %) | |||
| Gastritis | 69 (44.2) | 25 (36.8) | 0.298 |
| Duodenal ulcer | 12 (7.7) | 8 (11.8) | 0.326 |
| Gastric ulcer | 2 (1.3) | 1 (1.5) | 0.910 |
3.1. Primary Endpoint
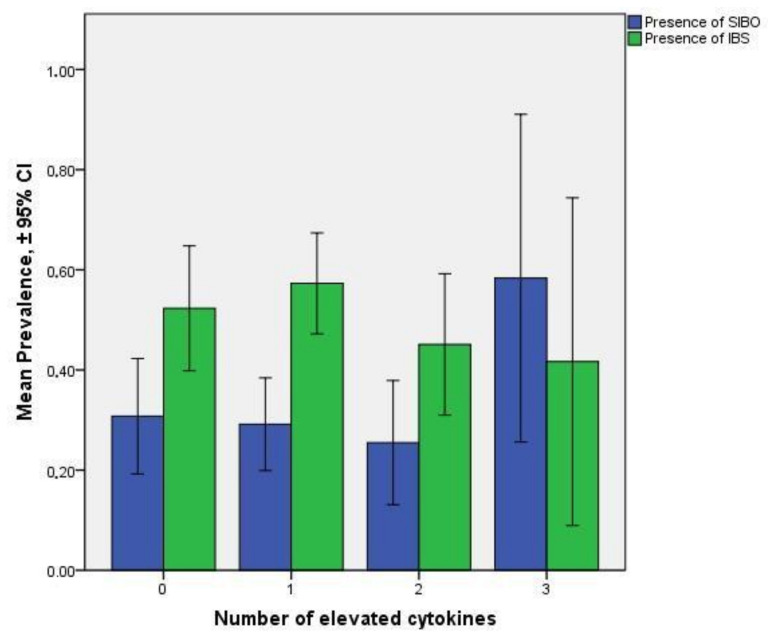
An analysis between the presence of SIBO and the presence of elevated proinflammatory cytokine levels in the duodenal fluid was conducted, where all diagnostic cut-offs of isolation of bacteria from the duodenal aspirate were considered as previously discussed [2], i.e., ≥10³ cfu/mL, ≥10⁴ cfu/mL, and ≥10⁵ cfu/mL. Regardless of the set cut-off, the OR for elevated IL-1β levels was greater among patients with SIBO compared to patients without SIBO. On the contrary, there was no significant difference at elevated IL-6 and TNF-α levels between patients with and without SIBO (Table 2 and Table 3). The presence of all three elevated cytokine levels has OR 3.47 (1.06–11.34, p = 0.03) among patients with SIBO (Figure 1). No significant association between the number of elevated cytokine levels and the presence of IBS was detected (Figure 1).
Table 2.
Univariate analysis of independent factors and their relationship with SIB.
| OR | 95% Cis | p | |
|---|---|---|---|
| Male gender | 0.504 | 0.167–1.522 | 0.224 |
| Age ≥ 60 years | 16.264 | 2.917–90.684 | 0.001 |
| BMI ≥ 22 | 0.269 | 0.059–1.232 | 0.091 |
| Obesity | 1.466 | 0.349–6.151 | 0.601 |
| IBS | 8.964 | 2.716–29.581 | <0.001 |
| T2DM | 2.927 | 0.832–10.295 | 0.094 |
| CHF | 0.961 | 0.238–3.869 | 0.955 |
| COPD | 0.312 | 0.060–1.632 | 0.168 |
| CRF | 0.596 | 0.043–8.243 | 0.700 |
| Solid Tumor Malignancy | 0.283 | 0.038–2.105 | 0.218 |
| PPIs | 0.382 | 0.098–1.498 | 0.168 |
| NSAIDs | 0.682 | 0.031–14.928 | 0.808 |
| Aspirin | 1.952 | 0.403–9.452 | 0.406 |
| Acenocumarone | 1.236 | 0.168–9.069 | 0.835 |
| H2 blockers | n/a | 0.000 | 1.000 |
| Antacids | 1.813 | 0.114–28.959 | 0.674 |
| Dyspepsia | 0.527 | 0.160–1.732 | 0.291 |
| Anemia | 0.641 | 0.160–2.568 | 0.530 |
| Fever of unkown origin | 1.354 | 0.109–16.771 | 0.813 |
| Gastritis | 1.527 | 0.515–4.526 | 0.445 |
| Duodenal Ulcer | 1.471 | 0.242–8.931 | 0.675 |
| Gastric Ulcer | <0.001 | 0.000 | 0.999 |
| Elevated IL-1β | 3.803 | 0.953–15.171 | 0.058 |
| Elevated IL-6 | 2.099 | 0.608–7.252 | 0.241 |
| Elevated TNFa | 0.381 | 0.094–1.542 | 0.176 |
Table 3.
Multivariate forward stepwise logistic regression analysis of statistically significant factors independently related with SIBO.
| OR | 95% Cis | p | |
|---|---|---|---|
| Age ≥ 60 years | 4.10 | 1.93–8.70 | <0.001 |
| BMI ≥ 22 | 0.37 | 0.17–0.83 | 0.016 |
| IBS | 2.10 | 1.12–3.96 | 0.021 |
| Elevated IL-1β | 2.61 | 1.06–6.43 | 0.037 |
Figure 1.
Prevalence of SIBO (using ≥10³ cfu/mL as a cut-off) (blue bars) and IBS (green bars) among patients with 0, 1, 2, and 3 elevated cytokine levels (TNF-α, IL-6, and IL-1β) detected in the duodenal fluid. Patients with all 3 cytokine levels elevated have OR 3.47 (1.06–11.34, p = 0.03) presenting with SIBO.
3.2. Secondary Endpoints
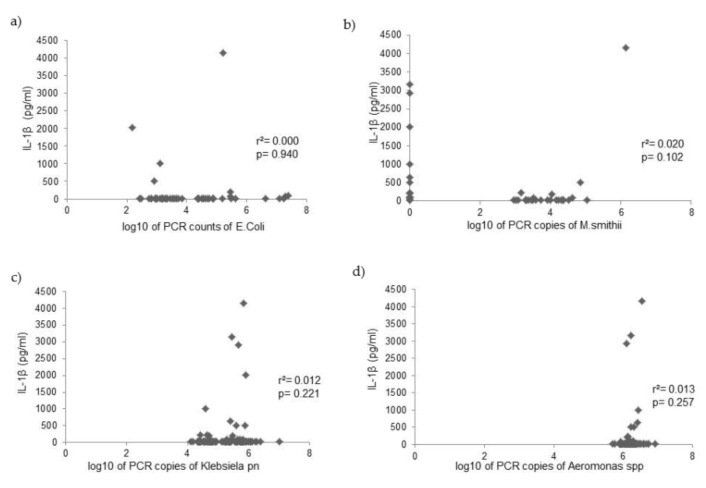
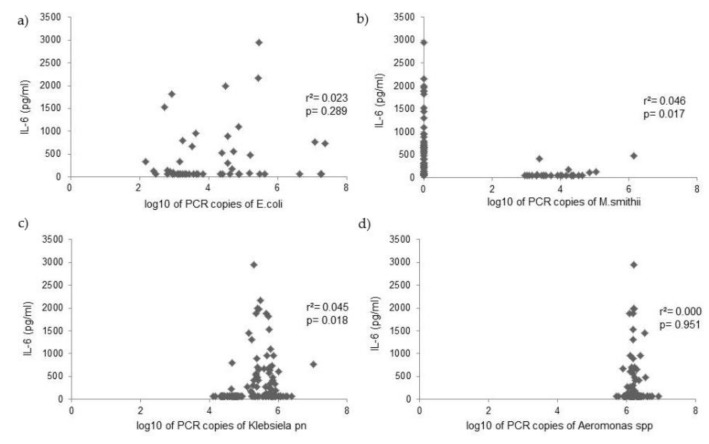
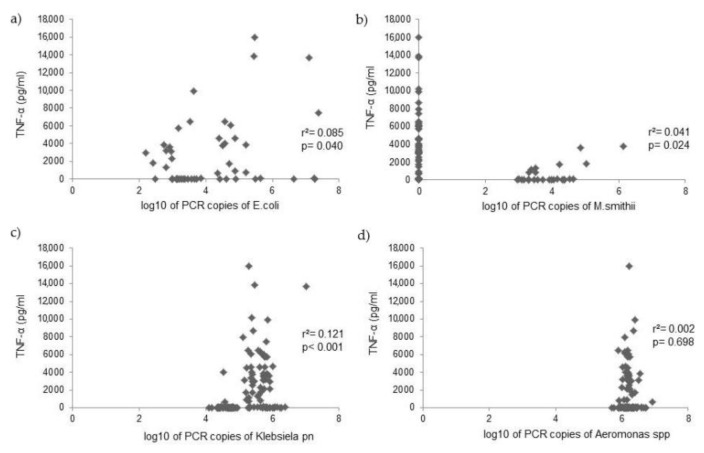
PCR counts of Escherichia coli, Klebsiella pneumoniae, Aeromonas spp., and Methanobevibacter smithii were measured in the duodenal fluid of 125 patients, and analysis investigated the relationship with cytokine levels. Analysis with the Mann–Whitney U test showed that Klebsiella pneumoniae PCR copies were positively related to IL-6 and TNF-α levels, while M. smithii PCR copies were positively related with IL-1β levels (Table 4). PCR copies of E. coli and Aeromonas spp. were not related to cytokine levels in the duodenal fluid (Figure 2, Figure 3 and Figure 4).
Table 4.
Linkage between elevated cytokine levels and PCR copies of bacterial species, and bacterial counts in aerobic cultures, using Mann–Whitney U test.
| IL-1β (−) |
IL-1β (+) |
p | IL-6 (−) |
IL-6 (+) |
p | TNF-α (−) |
TNF-α (+) |
p | |
|---|---|---|---|---|---|---|---|---|---|
| Mean Rank | Mean Rank | Mean Rank | Mean Rank | Mean Rank | Mean Rank | ||||
| E. coli | 62.4 | 66.5 | 0.603 | 59.2 | 68.3 | 0.118 | 62.4 | 63.3 | 0.883 |
| Klebsiella pn. | 62.1 | 68.0 | 0.511 | 54.1 | 75.6 | 0.001 | 50.1 | 68.4 | 0.010 |
| M. smithii | 61.0 | 74.0 | 0.044 | 66.7 | 57.7 | 0.053 | 67.4 | 61.2 | 0.217 |
| Aeromonas spp. | 63.3 | 61.1 | 0.801 | 65.6 | 59.4 | 0.110 | 67.6 | 61.1 | 0.353 |
|
Bacterial counts
in aerobic cultures |
110.4 | 129.0 | 0.099 | 112.1 | 113.8 | 0.829 | 111.0 | 113.2 | 0.768 |
Figure 2.
Correlation between IL-1β levels and PCR copies of (a) Escherichia coli, (b) Methanobevribacter smithii, (c) Klebsiella pneumoniae, and (d) Aeromonas spp. in the duodenal fluid.
Figure 3.
Correlation between IL-6 levels and PCR copies of (a) Escherichia coli, (b) Methanobevribacter smithii, (c) Klebsiella pneumoniae, and (d) Aeromonas spp. in the duodenal fluid.
Figure 4.
Correlation between TNF-α levels and PCR copies of (a) Escherichia coli, (b) Methanobevribacter smithii, (c) Klebsiella pneumoniae, and (d) Aeromonas spp. in the duodenal fluid.
4. Discussion
Our study investigates the connection between SIBO and proinflammatory cytokines’ levels in the duodenal fluid. The inflammatory substrate of SIBO and the contribution of cytokines in its interactions have been studied for decades, and there is an assumption that the presence of SIBO contributes towards the upregulation of inflammatory cytokines, mainly in the intestine, and systematically at a lesser point. Cytokine mRNA expression of German shepherd dogs with SIBO was investigated but no significant difference was detected [10]. IL-1β, IL-6, Toll-like receptor 4 (TLR-4), and interleukin 10 (IL-10) levels were measured in mucosal tissue of ileum in mice with post infectious IBS, and TLR-4 was detected at higher levels in mice with SIBO [11]. TLR-4 expression from liver biopsies in patients with metabolic associated fat liver disease (MAFLD) was also higher when SIBO was present, although no significant difference at serum TNF-α levels was detected [12]. Serum IL-6, IL-8, and TNF-α levels were significantly higher in SIBO positive ulcerative colitis patients as compared to SIBO negative patients [13]. SIBO rates were higher in patients with Crohn’s disease and led to elevated levels of fecal calprotectin, a well-known biomarker indicating local inflammation in small intestine [14]. Riordan et al. studied mucosal cytokine production in SIBO and also demonstrated no difference in TNF-α along with interferone-γ levels, while detected levels of IL-6 were higher in SIBO positive patients [15]. No differences in serum TNF-α, IL-6, and IL-8 levels were observed between SIBO positive and SIBO negative IBS patients, whereas levels of anti-inflammatory cytokine IL-10 were lower in the first group [16]. Similar proinflammatory patterns with elevated cytokines that also led to increased small bowel homing T-cells were detected in patients with predominant diarrhea IBS [17] and with Helicobacter pylori negative functional dyspepsia [18], both of the above mentioned conditions closely linked to SIBO. Our study detected a positive connection between SIBO per se and the presence of an indigenous proinflammatory environment in the small bowel.
Although IL-1β has an established role in pathophysiology of inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) [19], studies on changes in IL-1β levels in patients with SIBO are rare. Patients with IBS were proven to have higher small intestine mucosal IL-1β levels when they were SIBO positive [20]. In the same study, increased IL-1β level was predominantly associated with bloating and loose stools (Bristol type 6). In a recent study, also on patients with IBS, IL-1β levels in the peripheral blood were higher in the SIBO positive group compared to the SIBO negative group [21]. IL-1β levels decreased significantly after antibiotic treatment strengthening the hypothesis of SIBO’s contribution to elevated IL-1β. For the first time in our study the connection between SIBO and elevated intestinal IL-1β levels was examined, regardless of the presence of other small intestine disorders.
Recent studies suggest that distinct commensals such as Proteus mirabilis and Escherichia coli can cause IL-1β dependent intestinal inflammation via NLRP3 inflammasome activation in IBD models [22,23]. NLRP3 inflammasome is a well-defined intracellural multiprotein complex found in monocytes that plays an important role in intestinal homeostasis by regulating not only IL-1β activation, but also IL-18 activation and pyroptosis, among others [24]. In this context, SIBO presence could lead to NLRP3 inflammasome dysfunction and intestinal inflammation due to consequent IL-1β upregulation. Further research is needed to explore a possible dysfunction of NLRP3 inflammasome in patients with SIBO.
The proinflammatory pattern with all three cytokines (IL-1β, IL-6, and TNF-α) elevated in the duodenal fluid that was strongly connected with SIBO in our study may suggest an intestinal motility disruption mechanism. IL-1β is considered as a mediator of intestinal dysfunction as it causes a decrease in smooth muscle contractility in rat ileum [25]. Selective jejunal manipulation causes a decrease in smooth muscle contractility and significant delay in colonic transit is accompanied by upregulation of IL-6 and TNF-α [26]. TNF-α, in turn, directly induces motor dysfunctions by acting on the smooth muscle, as shown in a trinitrobenzenesulfonic acid (TNBS) induced colitis model in mice [27]. Small bowel motility is the most important mechanism preventing SIBO. Disruption of the enteric nervous system, visceral musculature, or both, can result in SIBO, and SIBO in turn can aggravate these impaired functions [28]. Indigenous proinflammatory cytokines can play an important factor in this bidirectional connection.
Klebsiella pneumoniae is a widely known pathogen that inhabits the large bowel and is shown to have high frequency in SIBO [7,29]. Our study shows that high PCR counts of Klebsiella pneumoniae in the duodenal fluid are connected with elevated IL-6 and TNF-α levels. Methanobevibacter smithii, an archaea that is considered to be the most abundant methanogen in the human gut, has been positively linked to predominant constipation IBS as excessive methane production in the small intestine leads to slower small bowel transit and increased contractile activity [30]. Our study shows that high PCR load of M. smithii is associated with elevated IL-1β levels in the duodenal fluid. Considering that IL-1β may be a contributing factor in intestinal motility dysfunction [18], M. smithii could possibly lead to local small intestine immune activation through IL-1β upregulation.
The presence of SIBO was significantly more frequent among patients with type 2 diabetes mellitus (T2DM) in our study. This finding comes in accordance to previous literature that shows a high prevalence of SIBO in patients with T2DM, together with delayed intestinal transit [31]. As T2DM can lead to visceral neuropathy and slowed orocecal transit [32], an assumption could be made that T2DM can lead to SIBO through impaired intestinal motility. However, a recent study indicates that SIBO positive patients with T2DM have elevated serum proinflammatory cytokine (TNF-α, IL-6, and IL-10) levels implying a bidirectional connection between T2DM and SIBO [33]. As previously discussed, SIBO may also promote local inflammation in patients with MALFD [12], another aspect of metabolic syndrome, strengthening the hypothesis of a linkage between SIBO and metabolic syndrome that can lead to both intestinal and systemic inflammation.
Furthermore, our study showed that patients with a BMI lower than 22 have a higher risk of SIBO, as shown in Table 2 and Table 3. Taking into consideration SIBO’s classic symptoms are malnutrition and weight loss, among others, it should come as no surprise that there is a higher prevalence of SIBO among underweight patients. Equivalent findings presented among patients with chronic pancreatitis and pancreatic exocrine insufficiency, where patients with weight loss were more likely to have SIBO [34]. On the other hand, no significant relationship between weight loss and SIBO was found in patients with Parkinson’s disease [35]. Further studies are needed to support this association. In addition, PPI intake did not affect SIBO prevalence among patients, which comes into accordance with a previous study of a population of 897 patients where neither the intake nor the duration of PPI consumption was found to have any impact on SIBO [4].
Our study presents certain limitations; first is the non-randomized design. Second is the lack of use of a method to prevent contamination during endoscopy, although there was a low probability of contamination, as the detected aerobe bacteria were usually inhabitants of the large bowel. Third is the absence of concomitant hydrogen breath test that would rule out the probability of possible contamination. Fourth is the lack of anaerobic cultures. Last is the relatively small number of SIBO positive patients.
5. Conclusions
Our study prospectively demonstrated, for the first time, the association of elevated IL-6, IL-1β, and TNF-α levels combined and IL-1β levels alone at a lesser point in the duodenal fluid with SIBO. The study also demonstrated that the presence of M. smithii is related with elevated IL-1β levels, while the presence of Klebsiella pneumoniae is related with high IL-6 and TNF-α levels in the duodenal fluid.
Author Contributions
E.R. wrote the manuscript, analyzed the data, reviewed the manuscript for intellectual content, and approved the final version to be submitted. E.P. participated in data collection, reviewed the manuscript for intellectual content, and approved the final version to be submitted. M.P. participated in data collection, reviewed the manuscript for intellectual content, and approved the final version to be submitted. K.T. participated in data collection, reviewed the manuscript for intellectual content, and approved the final version to be submitted. E.J.G.-B. conceived the study design, analyzed the data, participated in data collection, reviewed the manuscript for intellectual content, and approved the final version to be submitted. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Sismanogleion General Hospital of Athens (protocol code 296/2009).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data will be submitted upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sundin O.H., Mendoza-Ladd A., Morales E., Fagan B.M., Zeng M., Diaz-Arevalo D., Ordonez J., McCallum R.W. Does a glucose-based hydrogen and methane breath test detect bacterial overgrowth in the jejunum? Neurogastroenterol. Motil. 2018;30:e13350. doi: 10.1111/nmo.13350. [DOI] [PubMed] [Google Scholar]
- 2.Rezaie A., Buresi M., Lembo A., Lin H., McCallum R., Rao S., Schmulson M., Valdovinos M., Zakko S., Pimentel M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am. J. Gastroenterol. 2017;112:775–784. doi: 10.1038/ajg.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley E.M.M. The spectrum of small intestinal bacterial overgrowth (SIBO) Curr. Gastroenterol. Rep. 2019;21:3. doi: 10.1007/s11894-019-0671-z. [DOI] [PubMed] [Google Scholar]
- 4.Giamarellos-Bourboulis E.J., Pyleris E., Barbatzas C., Pistiki A., Pimentel M. Small intestinal bacterial overgrowth is associated with irritable bowel syndrome and is independent of proton pump inhibitor usage. BMC Gastroenterol. 2016;16:67. doi: 10.1186/s12876-016-0484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah S.C., Day L.W., Somsouk M., Sewell J.L. Meta-analysis: Antibiotic therapy for small intestinal bacterial overgrowth. Aliment. Pharmacol. Ther. 2013;38:925–934. doi: 10.1111/apt.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao S.S.C., Bhagatwala J. Small Intestinal Bacterial Overgrowth: Clinical Features and Therapeutic Management. Clin. Transl. Gastroenterol. 2019;10:e00078. doi: 10.14309/ctg.0000000000000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pyleris E., Giamarellos-Bourboulis E.J., Tzivras D., Koussoulas V., Barbatzas C., Pimentel M. The prevalence of overgrowth by aerobic bacteria in the small intestine by small bowel culture: Relationship with irritable bowel syndrome. Dig. Dis. Sci. 2012;57:1321–1329. doi: 10.1007/s10620-012-2033-7. [DOI] [PubMed] [Google Scholar]
- 8.Giamarellos-Bourboulis E., Tang J., Pyleris E., Pistiki A., Barbatzas C., Brown J., Lee C.C., Harkins T.T., Kim G., Weitsman S., et al. Molecular assessment of differences in the duodenal microbiome in subjects with irritable bowel syndrome. Scand. J. Gastroenterol. 2015;50:1076–1087. doi: 10.3109/00365521.2015.1027261. [DOI] [PubMed] [Google Scholar]
- 9.Mearin F., Lacy B.E., Chang L., Chey W.D., Lembo A.J., Simren M., Spiller R. Bowel Disorders. Gastroenterology. 2016;150:1393–1407. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 10.German A.J., Helps C.R., Hall E.J., Day M.J. Cytokine mRNA expression in mucosal biopsies from German shepherd dogs with small intestinal enteropathies. Dig. Dis. Sci. 2000;45:7–17. doi: 10.1023/A:1005436721798. [DOI] [PubMed] [Google Scholar]
- 11.Chen B., Zhu S., Du L., He H., Kim J.J., Dai N. Reduced interstitial cells of Cajal and increased intraepithelial lymphocytes are associated with development of small intestinal bacterial overgrowth in post-infectious IBS mouse model. Scand. J. Gastroenterol. 2017;52:1065–1071. doi: 10.1080/00365521.2017.1342141. [DOI] [PubMed] [Google Scholar]
- 12.Kapil S., Duseja A., Sharma B.K., Singla B., Chakraborti A., Das A., Ray P., Dhiman R.K., Chawla Y. Small intestinal bacterial overgrowth and toll-like receptor signaling in patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2016;31:213–221. doi: 10.1111/jgh.13058. [DOI] [PubMed] [Google Scholar]
- 13.Rana S.V., Sharma S., Kaur J., Prasad K.K., Sinha S.K., Kochhar R., Malik A., Morya R.K. Relationship of cytokines, oxidative stress and GI motility with bacterial overgrowth in ulcerative colitis patients. J. Crohn’s Colitis. 2014;8:859–865. doi: 10.1016/j.crohns.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Ricci J.E.R., Chebli L.A., Ribeiro T., Castro A.C.S., Gaburri P.D., Pace F., Barbosa K., Ferreira L., Passos M., Malaguti C., et al. Small-Intestinal Bacterial Overgrowth is Associated With Concurrent Intestinal Inflammation But Not With Systemic Inflammation in Crohn’s Disease Patients. J. Clin. Gastroenterol. 2018;52:530–536. doi: 10.1097/MCG.0000000000000803. [DOI] [PubMed] [Google Scholar]
- 15.Riordan S.M., McIver C.J., Wakefield D., Duncombe V.M., Bolin T.D., Thomas M.C. Mucosal cytokine production in small-intestinal bacterial overgrowth. Scand. J. Gastroenterol. 1996;31:977–984. doi: 10.3109/00365529609003117. [DOI] [PubMed] [Google Scholar]
- 16.Chu H., Fox M., Zheng X., Deng Y., Long Y., Huang Z., Du L., Xu F., Dai N. Small Intestinal Bacterial Overgrowth in Patients with Irritable Bowel Syndrome: Clinical Characteristics, Psychological Factors, and Peripheral Cytokines. Gastroenterol. Res. Pract. 2016;2016:3230859. doi: 10.1155/2016/3230859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao J. Correlation between anxiety-depression status and cytokines in diarrhea-predominant irritable bowel syndrome. Exp. Ther. Med. 2013;6:93–96. doi: 10.3892/etm.2013.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liebregts T., Adam B., Bredack C., Gururatsakul M., Pilkington K.R., Brierley S.M., Blackshaw L.A., Gerken G., Talley N.J., Holtmann G. Small bowel homing t cells are associated with symptoms and delayed gastric emptying in functional dyspepsia. Am. J. Gastroenterol. 2011;106:1089–1098. doi: 10.1038/ajg.2010.512. [DOI] [PubMed] [Google Scholar]
- 19.Liebregts T., Adam B., Bredack C., Roth A., Heinzel S., Lester S., Downie-Doyle S., Smith E., Drew P., Talley N.J., et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava D., Ghoshal U., Mittal R.D., Ghoshal U.C. Associations between IL-1RA polymorphisms and small intestinal bacterial overgrowth among patients with irritable bowel syndrome from India. Neurogastroenterol. Motil. 2014;26:1408–1416. doi: 10.1111/nmo.12399. [DOI] [PubMed] [Google Scholar]
- 21.Yu X., Li Y., Xiang F., Feng J. Correlation between small intestinal bacterial overgrowth and irritable bowel syndrome and the prognosis of treatment. Ann. Palliat. Med. 2021;10:3364–3370. doi: 10.21037/apm-21-427. [DOI] [PubMed] [Google Scholar]
- 22.Seo S.U., Kamada N., Munoz-Planillo R., Kim Y.G., Kim D., Koizumi Y., Hasegawa M., Himpsl S.D., Browne H.P., Lawley T.D., et al. Distinct Commensals Induce Interleukin-1beta via NLRP3 Inflammasome in Inflammatory Monocytes to Promote Intestinal Inflammation in Response to Injury. Immunity. 2015;42:744–755. doi: 10.1016/j.immuni.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De la Fuente M., Franchi L., Araya D., Diaz-Jimenez D., Olivares M., Alvarez-Lobos M., Golenbock D., Gonzalez M.J., Lopez-Kostner F., Quera R., et al. Escherichia coli isolates from inflammatory bowel diseases patients survive in macrophages and activate NLRP3 inflammasome. Int. J. Med. Microbiol. 2014;304:384–392. doi: 10.1016/j.ijmm.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Opipari A., Franchi L. Role of inflammasomes in intestinal inflammation and Crohn’s disease. Inflamm. Bowel Dis. 2015;21:173–181. doi: 10.1097/MIB.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 25.Ohama T., Hori M., Sato K., Ozaki H., Karaki H. Chronic treatment with interleukin-1beta attenuates contractions by decreasing the activities of CPI-17 and MYPT-1 in intestinal smooth muscle. J. Biol. Chem. 2003;278:48794–48804. doi: 10.1074/jbc.M310166200. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz N.T., Kalff J.C., Turler A., Speidel N., Grandis J.R., Billiar T.R., Bauer A.J. Selective jejunal manipulation causes postoperative pan-enteric inflammation and dysmotility. Gastroenterology. 2004;126:159–169. doi: 10.1053/j.gastro.2003.10.060. [DOI] [PubMed] [Google Scholar]
- 27.Kinoshita K., Hori M., Fujisawa M., Sato K., Ohama T., Momotani E., Ozaki H. Role of TNF-alpha in muscularis inflammation and motility disorder in a TNBS-induced colitis model: Clues from TNF-alpha-deficient mice. Neurogastroenterol. Motil. 2006;18:578–588. doi: 10.1111/j.1365-2982.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 28.Ghoshal U.C., Ghoshal U. Small intestinal bacterial overgrowth and other intestinal disorders. Gastroenterol. Clin. N. Am. 2017;46:103–120. doi: 10.1016/j.gtc.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs C., Coss Adame E., Attaluri A., Valestin J., Rao S.S. Dysmotility and proton pump inhibitor use are independent risk factors for small intestinal bacterial and/or fungal overgrowth. Aliment. Pharmacol. Ther. 2013;37:1103–1111. doi: 10.1111/apt.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pimentel M., Lin H.C., Enayati P., van den Burg B., Lee H.R., Chen J.H., Park S., Kong Y., Conklin J. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G1089–G1095. doi: 10.1152/ajpgi.00574.2004. [DOI] [PubMed] [Google Scholar]
- 31.Rana S.V., Malik A., Bhadada S.K., Sachdeva N., Morya R.K., Sharma G. Malabsorption, orocecal transit time and small intestinal bacterial overgrowth in type 2 diabetic patients: A connection. Indian J. Clin. Biochem. 2017;32:84–89. doi: 10.1007/s12291-016-0569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Triantafyllou K., Kalantzis C., Papadopoulos A.A., Apostolopoulos P., Rokkas T., Kalantzis N., Ladas S.D. Video-capsule endoscopy gastric and small bowel transit time and completeness of the examination in patients with diabetes mellitus. Dig. Liver Dis. 2007;39:575–580. doi: 10.1016/j.dld.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Malik A., Morya R.K., Saha S., Singh P.K., Bhadada S.K., Rana S.V. Oxidative stress and inflammatory markers in type 2 diabetic patients. Eur. J. Clin. Investig. 2020;50:e13238. doi: 10.1111/eci.13238. [DOI] [PubMed] [Google Scholar]
- 34.Ni Chonchubhair H.M., Bashir Y., Dobson M., Ryan B.M., Duggan S.N., Conlon K.C. The prevalence of small intestinal bacterial overgrowth in non-surgical patients with chronic pancreatitis and pancreatic exocrine insufficiency (PEI) Pancreatology. 2018;18:379–385. doi: 10.1016/j.pan.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 35.DiBaise J.K., Crowell M.D., Driver-Dunckley E., Mehta S.H., Hoffman-Snyder C., Lin T., Adler C.H. Weight Loss in Parkinson’s Disease: No Evidence for Role of Small Intestinal Bacterial Overgrowth. J. Parkinson’s Dis. 2018;8:571–581. doi: 10.3233/JPD-181386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be submitted upon request.