Abstract
Background:
Autologous blood or marrow transplantation (aBMT) is considered standard-of-care for multiple-myeloma (MM). Significantly improved survival necessitates an understanding of the morbidity burden borne by the growing survivor population.
Methods:
We evaluated severe/ life-threatening chronic health conditions (CHCs) and subsequent neoplasms (SNs) in MM patients treated with aBMT using BMTSS. Study participants (n=630) had undergone aBMT for MM at one of 3 BMT centers, had survived ≥2y after aBMT, and were ≥18y of age at survey completion. aBMT survivors identified 289 nearest-age siblings to constitute an unaffected comparison group. Scoring of CHCs was based on CTCAE v5.0 to determine severity (grade 3: serious; grade 4: life-threatening).
Results:
The 10y cumulative incidence of any grade 3–4 CHC in aBMT survivors was 57.6±3.2%. MM survivors were at 40% higher odds of developing grades 3–4 CHCs when compared with siblings (95%CI, 1.0–1.9). Amongst SNs, 96% were solid tumors, yielding a 10y cumulative incidence of 13.6±2.5%. Pre-aBMT exposure to cyclophosphamide (HR=3.5, 95%CI, 1.5–8.1) and immunomodulatory drugs (IMiDs, HR=3.9, 95%CI, 1.5–10.1) were associated with increased risk of solid tumors. Melanoma (10y cumulative incidence: 3.3%±1.2%), and squamous cell carcinoma (SCC: 10y cumulative incidence: 5.1%±1.8%), were the most common SNs. Pre-aBMT exposure to cyclophosphamide (HR=6.02, 95%CI, 1.4–26.1) and IMiDs (HR=7.9, 95%CI, 0.9–68.5) were associated with increased melanoma risk.
Conclusions:
The 10y cumulative incidence of severe/life-threatening CHCs approaches 60% in long-term survivors of MM; solid SNs constitute a large burden of the morbidity. This study provides evidence for close monitoring of the survivors to manage morbidity.
Keywords: Hematopoietic stem cell transplant, multiple myeloma, autografts, second neoplasms, long-term survivors
Precis for use in the Table of Contents:
Cumulative incidence of grade 3–4 toxicities in multiple myeloma autologous transplant survivors approached 60% at 10 years
Cumulative incidence of subsequent neoplasms in multiple myeloma autologous transplant survivors was 13.9%±2.5%
Introduction
Autologous blood or marrow transplantation (aBMT), either after initial treatment or as salvage for relapsed/refractory disease is accepted as standard-of-care for multiple myeloma (MM). 1 Over past two decades, advances in therapeutic strategies for MM have led to significant improvement in overall and progression-free survival (PFS), 2 necessitating evaluation of the health and well-being of these survivors. A few studies have described PFS3–6, and subsequent neoplasms post aBMT7–11 in MM patients, but these have been limited by smaller sample sizes or brief post-aBMT follow-up. aBMT remains the cornerstone treatment for patients with MM, leading to higher complete response rates (59% vs. 48%) and improved PFS (median PFS 50 vs. 36 mo.). 12 MM is the most common indication for aBMT, almost 9,000 patients with MM underwent aBMT in the US in 2018. 13 With the growing number of patients living several years after a diagnosis of MM, there is a need to evaluate the health and well-being of these survivors, in order to develop evidence for risk-based anticipatory care. Using the BMT Survivor Study (BMTSS), we examined the overall burden of morbidity and the burden of subsequent neoplasms reported by MM patients who were alive two or more years from aBMT.
Methods
Bone Marrow Transplant Survivor Study (BMTSS) is a collaborative effort between University of Alabama at Birmingham, City of Hope, and University of Minnesota, examining the long-term outcomes of individuals who have survived 2 or more years after aBMT performed at the participating institutions. The Human Subjects Committee at the participating institutions approved the study; informed consent was provided according to the Declaration of Helsinki. For this report, patients were eligible if they had undergone an aBMT for MM between 1974 and 2014, had survived for 2 or more years after aBMT, and were ≥18 years of age and alive at study participation. Of the 1,171 eligible patients, 55 (4.7%) were lost to follow-up. Among the 1116 patients approached, 630 (56.5%) participated. Participants were more likely than non-participants to be white (72% vs. 54.3%), were older at aBMT (mean age: 57.7 vs. 56.2 years, p=0.007), and less likely to have undergone aBMT in recent years (median year of aBMT: 2009 vs. 2012, p <0.0001). Participating aBMT survivors identified a nearest-age sibling to constitute an unaffected comparison group. Siblings provide the ability to make direct comparisons with survivors and provide data on outcomes in a non-cancer population not available from other sources (vital statistics, NHIS etc.) and serve as an additional group for consistency of findings between data sources. 14,15 Siblings in the BMTSS16–18 and another large childhood cancer survivor study (CCSS) 19 cohort have served as an effective comparison group, associated with high participation rates, ease of access, and uniformity of socio-economic status and health awareness.
The 630 MM survivors and the 289 unaffected siblings completed a 255-item BMTSS survey that included questions regarding sociodemographic characteristics (race/ethnicity, education, marital status, employment, household income, and insurance status), diagnosis by a healthcare provider of specific chronic health conditions (CHCs) along with the age at onset of CHCs. We used the common terminology criteria for adverse events (CTCAE version 5.0) to assign a level of severity to each CHC type in both the survivors and siblings. CTCAE have been used to grade health conditions in cancer survivors18 and distinguish grades 1 through 4 for each event (grade 1, mild; grade 2, moderate; grade 3, severe; grade 4, life-threatening/disabling). Details related to MM, pre-aBMT treatment, priming for stem cell mobilization, conditioning therapy, and post-aBMT treatment were obtained from each center’s BMT database, supplemented by medical record abstraction.
Statistical Analysis
Descriptive statistics were used to characterize the study population and compare between groups with χ2 tests, exact tests, and t-tests as appropriate. The prevalence of severe (grade 3) or life-threatening (grade 4) CHCs was determined for participating aBMT survivors and siblings. The cumulative incidence of grades 3 or 4 CHCs was calculated among aBMT recipients.
Risk of CHCs in aBMT survivors compared with siblings
The magnitude of risk of any grades 3 or 4 CHCs in aBMT survivors when compared with siblings was determined using logistic regression techniques. The model was adjusted for sex, age at study participation (≥60 years vs. <60 years), race/ethnicity (non-Hispanic whites vs. others), education (<high-school, high school/some college, college graduate/post-graduate), annual household income (<$50,000, $50,000-$100,000, >$100,000), and health insurance status (yes vs. no). Odds ratios (ORs) with 95% confidence intervals (95%CIs) were reported.
Risk factors associated with CHCs in aBMT survivors
Cox regression analysis techniques were used to determine the factors associated with CHCs in aBMT survivors; the magnitude of association was reported as relative risk (RR) and 95%CI. Explanatory variables were selected a priori and were used to assess their simultaneous impact on the risk of CHCs. These variables included age at aBMT (≥60 years vs. <60 years), sex, race/ethnicity (non-Hispanic whites vs. others), education (<high-school, high school/some college, college graduate/post-graduate), health insurance coverage (yes vs. no), annual household income (<$50,000, $50,000-$100,000, >$100,000), therapeutic exposures used prior to aBMT [cyclophosphamide (yes/no); vincristine, doxorubicin and dexamethasone (VAD) (yes/no); immunomodulatory drugs (IMiDs: thalidomide, lenalidomide) (yes/no); melphalan (yes/no); and radiation (yes/no)], conditioning (total body irradiation [TBI] with or without melphalan; melphalan alone; others), and transplanting institution.
Risk of subsequent neoplasms
We also examined the risk of subsequent neoplasms in the MM cohort. Using the analytic approaches described above, we calculated the cumulative incidence of all subsequent neoplasms taken together, as well as for individual types of the more prevalent subsequent neoplasms. Using Cox regression analytic methods, we examined the demographic factors and therapeutic exposures associated with an increased risk of subsequent neoplasms.
Results
Demographics and clinical characteristics of study participants
The demographic characteristics of aBMT survivors and siblings are presented in Table 1. Mean age at study participation (64.2 years vs. 64.0 years, p=0.7), sex (female: 42.1% vs. 42.2%, p=0.9) and health insurance coverage (99.2% vs. 99.7%, p=0.4) were comparable between aBMT survivors and siblings. Siblings were more likely to be non-Hispanic white (84.4% vs. 62.2% p<0.001), college graduates (56.4% vs. 43.8%, p<0.0001), and more likely to have an annual household income >$100,000 (41.5% vs. 23.5%, p<0.001) (Table 1).
Table 1:
Demographic and Clinical Characteristics of the Study Participants
| Characteristics | Survivor (n=630) | Sibling (n=289) | P-value |
|---|---|---|---|
| Age at aBMT (years, mean ± SD) | 57.6 ± 8.5 | NA | |
| Interval between aBMT and survey (mean, years± SD) | 6.6 ± 3.7 | NA | |
| Age at survey (mean, years± SD) | 64.2 ± 7.9 | 64.0 ± 8.1 | 0.7 |
| Sex n (%) | |||
| Female | 265 (42.1) | 122 (42.2) | 1.0 |
| Race and Ethnicity n (%) | |||
| White | 393 (62.2) | 244 (84.4) | <0.001 |
| Hispanic | 109 (17.3) | 18 (6.2) | |
| Black | 87 (13.8) | 9 (3.1) | |
| Asian | 21 (3.3) | 14 (4.8) | |
| Mixed/other/unknown | 21 (3.3) | 4 (1.4) | |
| Education n (%) | |||
| <High school | 42 (6.7) | 5 (1.7) | 0.0001 |
| High school and some college | 311 (49.4) | 121 (41.9) | |
| College graduate and post-graduate | 276 (43.8) | 163 (56.4) | |
| Missing | 1 (0.16) | 0 (0.00) | |
| Annual household income n (%) | |||
| < $50,000 | 188 (29.8) | 49 (16.7) | <0.001 |
| $50,000-$100,000 | 205 (32.5) | 89 (30.8) | |
| >$100,000 | 148 (23.5) | 120 (41.5) | |
| Missing | 89 (14.1) | 31 (10.7) | |
| Current insurance n (%) | |||
| Yes | 625 (99.2) | 288 (99.7) | 0.4 |
| Conditioning n (%) | |||
| Cyclophosphamide | 40 (7.60) | NA | |
| Melphalan | 517 (98.3) | NA | |
| TBI | 29 (5.5) | NA | |
| Other | 38 (7.2) | NA | |
| Missing | 104 | NA | |
| Therapeutic exposures pre-aBMT n (%) | |||
| Cyclophosphamide | 78 (15.1) | NA | |
| Melphalan | 25 (4.8) | NA | |
| Steroids | 512 (99) | NA | |
| Doxorubicin | 113 (21.9) | NA | |
| Bortezomib | 270 (52.2) | NA | |
| Vincristine | 93 (18) | NA | |
| IMiD (Thalidomide or lenalidomide) | 330 (63.8) | NA | |
| Radiation therapy | 120 (23.2) | NA | |
| Other | 20 (3.9) | NA | |
| Missing | 113 | NA | |
| Grade 3–4 Chronic Health Conditions | |||
| Yes | 273 (43.3) | 107 (37) | 0.07 |
Abbreviations: TBI: total body radiation, IMiD: Immunomodulatory drugs
Disease and transplant characteristics of the aBMT survivors are also presented in Table 1. Mean age (±SD) at aBMT was 57.6 years (±8.5), and mean interval (±SD) between aBMT and study participation was 6.6 years (±3.7). Pre-aBMT exposures included IMiDs in 63.8%, radiation in 23.2%, VAD in 17%, cyclophosphamide in 15.1% and melphalan in 4.8% of the study participants. aBMT conditioning included melphalan in 98.3% of the participants and TBI in 5.5% of the study participants (Table 1).
Burden of morbidity in aBMT survivors vs. siblings
As shown in Table 2, the prevalence of any grades 3 or 4 CHCs among aBMT survivors was 43.3%. Specific grade 3 or 4 CHCs are also detailed in Table 2. The prevalence of cataract (17.6% vs, 8.6%, p=0.0003), venous thrombo-embolism (8.1% vs 4.2%, p=0.04) and subsequent neoplasms (6.7% vs. 2.1%, p=0.004) were noted to be higher in aBMT survivors vs. siblings. In an analysis adjusted for age at questionnaire, sex, race/ethnicity, education, annual household income, and current insurance status, the aBMT survivors were at a 1.4-fold higher odds of developing a grade 3 or 4 CHC as compared to siblings (95%CI: 1.0–1.9, p=0.03).
Table 2:
Prevalence of Grade 3–4 Chronic Health Conditions in aBMT Survivors and Siblings
| Chronic Health Conditions | Survivor n (%) | Sibling n (%) | P-value |
|---|---|---|---|
| Any grades 3–4 CHC | 273 (43.3%) | 107 (37.0%) | 0.07 |
| Auditory | |||
| Hearing loss | 38 (6.0%) | 27 (9.3%) | 0.07 |
| Ocular | |||
| Legally blind | 5 (0.8%) | 5 (1.7%) | 0.3 |
| Cataract | 111 (17.6%) | 25 (8.7%) | 0.0003 |
| Renal | |||
| Renal failure requiring dialysis | 0 (0%) | 1 (0.4%) | 0.3 |
| Endocrine | |||
| Thyroid dysfunction | 6 (1%) | 9 (3.1%) | 0.02 |
| Diabetes mellitus | 12 (1.9%) | 8 (2.8%) | 0.5 |
| Musculoskeletal | |||
| Joint replacement | 33 (5.2%) | 25 (8.7%) | 0.06 |
| Cardiovascular disease | |||
| Coronary artery disease | 22 (3.5%) | 19 (6.6%) | 0.04 |
| Congestive heart failure | 10 (1.6%) | 6 (2.1%) | 0.6 |
| Arrhythmia | 11 (1.8%) | 3 (1.0%) | 0.6 |
| Valvular heart disease | 1 (0.2%) | 1 (0.4%) | 0.5 |
| Cerebrovascular disease | 14 (2.2%) | 8 (2.8%) | 0.6 |
| Venous thrombo-embolism | 51 (8.1%) | 12 (4.2%) | 0.03 |
| Pulmonary disease | |||
| Any pulmonary condition | 5 (0.8%) | 0 (0%) | 0.3 |
| Gastrointestinal disease | |||
| Liver disease | 2 (0.3%) | 1 (0.4%) | 1.0 |
| Gastro-intestinal disease | 7 (1.1%) | 1 (0.4%) | 1.0 |
| Neurological disease | |||
| Any neurological condition | 5 (0.8%) | 3 (1.0%) | 0.7 |
| Subsequent neoplasms | 42 (6.7%) | 6 (2.1%) | 0.004 |
Burden of morbidity in aBMT survivors
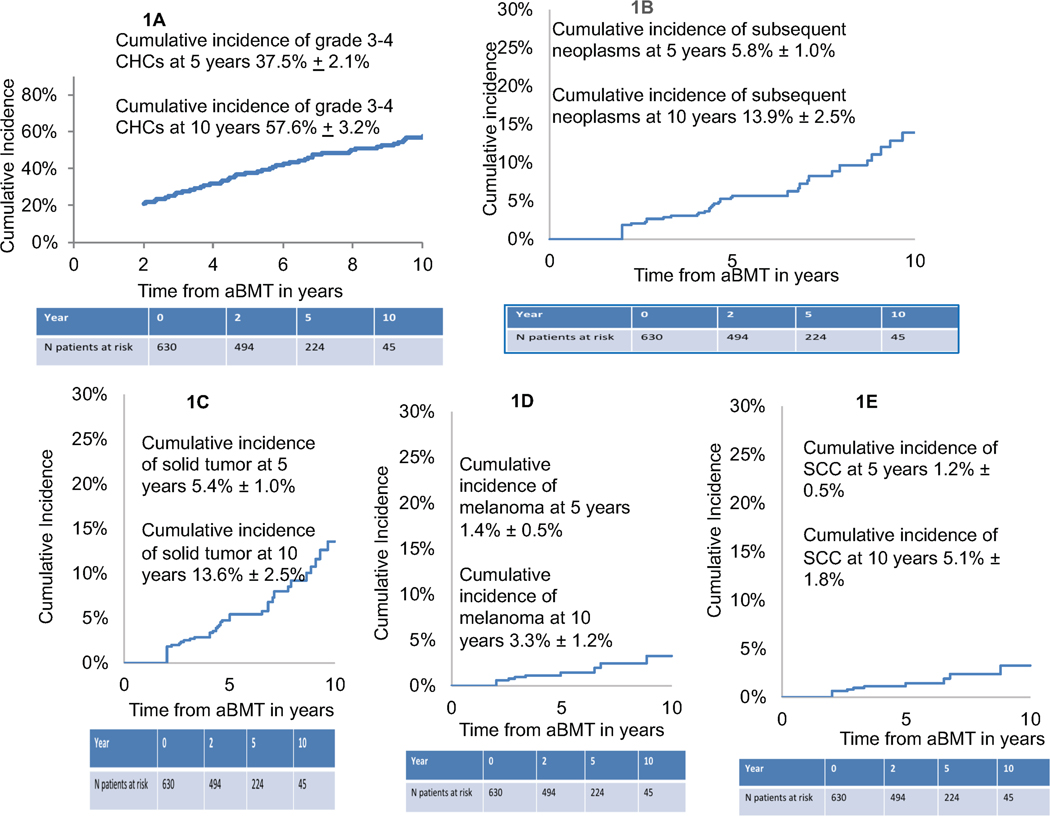
The 10-year cumulative incidence of any grade 3 or 4 CHC in aBMT survivors was 57.6%±3.2% (Figure 1A). Multivariable regression analyses identified that patients with older age at BMT (≥60 years) had a 2.2-fold (95%CI: 1.7–2.9, p <0.0001) higher risk of any subsequent grade 3 or 4 CHC (Table 3), when compared to the younger patients. Those receiving a TBI-based conditioning regimen showed a trend towards a higher risk for any subsequent grade 3 or 4 CHC when compared to those not receiving TBI (RR=1.6, 95%CI: 0.95–2.7, p=0.08) (Table 3).
Figure 1.
Figure 1A: Cumulative incidence of any grade 3–4 Chronic Health Condition in aBMT survivors
Figure 1B: Cumulative incidence of any subsequent malignant neoplasms in aBMT survivors
Figure 1C: Cumulative incidence of Solid Tumors in aBMT survivors
Figure 1D: Cumulative incidence of Melanoma in aBMT survivors
Figure 1E: Cumulative incidence of SCC in aBMT survivors
Abbreviations: aBMT: Autologous blood or marrow transplantation, SCC: squamous cell carcinoma, CHC: chronic health condition
Table 3:
Cox Regression Analysis of Specific Chronic Health Conditions in Multiple Myeloma Patients Treated with aBMT
| Variable | Any grades 3–4 CHC | Subsequent neoplasms | Solid tumor | Melanoma | SCC | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95% CI) | p-value | HR (95%CI) | p-value | HR (95%CI) | p-value | HR (95%CI) | p-value | |
| Age at aBMT (referent group <60y) | ||||||||||
| Age at aBMT ≥60y | 2.24 (1.7–2.9) | <0.0001 | 2.12(1.12–4.01) | 0.02 | 1.90(1.0–3.6) | 0.05 | 3.07(0.9–10.6) | 0.08 | ||
| Race/ethnicity (referent group: non-white) | ||||||||||
| Non-Hispanic white | 1.26 (0.98–1.6) | 0.08 | 2.38(1.1–5.17) | 0.03 | 2.23(1.0–4.9) | 0.04 | ||||
| Conditioning | ||||||||||
| TBI ± other | 1.59 (0.95–2.7) | 0.08 | ||||||||
| Pre-aBMT therapeutic exposures | ||||||||||
| Cyclophosphamide | 3.36(1.44–7.84) | 0.005 | 3.46(1.5–8.1) | 0.004 | 6.02(1.4–26.1) | 0.02 | ||||
| IMiD | 3.86(1.49–9.94) | 0.005 | 3.89(1.5–10.1) | 0.005 | 7.9(0.9–68.5) | 0.06 | ||||
Abbreviations: aBMT: Autologous hematopoietic cell transplant, HR: hazard ratio, CI: confidence interval, IMiD: Immunomodulatory drug (Thalidomide or Lenalidomide), TBI: total body irradiation, SCC: squamous cell carcinoma
Subsequent neoplasms
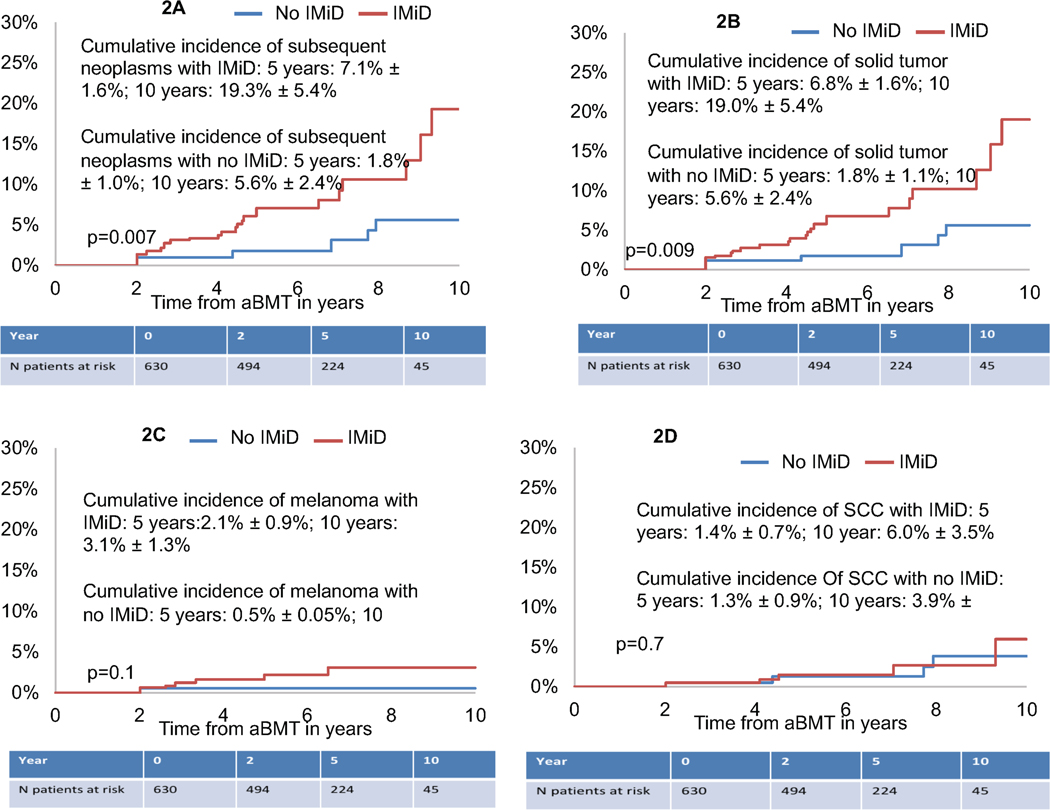
Forty-two aBMT survivors developed 52 subsequent neoplasms. These included solid tumors (n=50), acute lymphocytic leukemia (ALL, n=1) and myelodysplastic syndrome (MDS, n=1). The solid tumors included melanoma (n=11), squamous cell cancer of the skin (n=11), basal cell carcinoma (n=6), breast cancer (n=6), prostate cancer (n=6), thyroid cancer (n=4), and 6 other miscellaneous subsequent neoplasms. The 10-year cumulative incidence of subsequent neoplasms was 13.9%±2.5% (Figure 1B). Older age at aBMT (≥60y: HR=2.1; 95%CI, 1.1–4.0, p=0.02), non-Hispanic white race/ethnicity (HR=2.4; 95%CI, 1.1–5.2, p=0.03), and pre-aBMT exposure to cyclophosphamide (HR=3.4, 95%CI,1.4–7.8, p=0.005) and IMiDs (HR=3.9, 95%CI, 1.5–9.9, p=0.005) were associated with an increased risk of subsequent neoplasms (Table 3). aBMT survivors with pre-aBMT exposure to IMiDs had a significantly higher cumulative incidence of any subsequent neoplasms when compared with those not exposed to IMiDs (19.3%±5.4% vs. 5.6%±2.4., p=0.007, Figure 2A).
Figure 2.
Figure 2A: Cumulative incidence of any subsequent malignant neoplasms in aBMT survivors by IMiD exposure
Figure 2B: Cumulative incidence of Solid Tumors in aBMT survivors by IMiD exposure
Figure 2C: Cumulative incidence of Melanoma in aBMT survivors by IMiD exposure
Figure 2D: Cumulative incidence of SCC in aBMT survivors by IMiD exposure
Abbreviations: aBMT: Autologous blood or marrow transplantation, SCC: squamous cell carcinoma, IMiD: Immunomodulatory drugs
We also evaluated risk factors associated with development of solid tumors, melanoma, and squamous cell carcinoma. The cumulative incidence of developing a solid tumor was 13.6±2.5% at 10 years (Figure 1C). aBMT survivors with pre-aBMT exposure to IMiDs had a significantly higher 10-year cumulative incidence of solid tumors when compared with those without pre-aBMT IMiD exposure (19%±5.4% vs. 5.6%±2.4%, p=0.009) (Figure 2B). Multivariable regression analysis (Table 3) revealed that non-Hispanic white race/ethnicity (HR=2.2; 95%CI, 1.0–4.9, p=0.04) and pre-aBMT exposure to cyclophosphamide (HR=3.5, 95%CI, 1.5–8.1, p=0.004) and IMiDs (HR=3.9, 95%CI, 1.5–10.1, p=0.005) were associated with an increased risk of solid tumors. A trend towards a higher risk of solid tumors was also seen with older age at aBMT (≥60y: HR=1.9; 95%CI, 1.0–3.6, p=0.05) (Table 3).
The cumulative incidence of developing a melanoma was 3.3%±1.2% at 10 years (Figure 1D). Pre-aBMT exposure to cyclophosphamide (HR=6.0, 95%CI, 1.4–26.0, p=0.02) was associated with a higher risk of melanoma. Pre-aBMT exposure to IMiDs (HR=7.9, 95%CI, 1.0–68.5, p=0.06) showed a trend towards a higher risk of melanoma (Table 3, Figure 2C). The cumulative incidence of developing a SCC was 5.1%±1.8% at 10 years (Figure 1E). Older age at BMT (≥60 years) was associated with a trend towards a higher risk of SCC (HR=3.1, 95%CI,0.9–10.6, p=0.07) (Table 3). Prior IMiD exposure did not impact risk of SCC (Figure 2D, Table 3).
Discussion
We found a significant burden of morbidity borne by MM survivors, such that the cumulative incidence of severe/life-threatening CHCs approached 60% at 10 years after aBMT. This represented a 40% higher burden of severe/life threatening morbidity when compared with siblings. In particular, we found that the 10-year cumulative incidence of solid subsequent neoplasms among those exposed to IMiDs approached 20%.
The 5-year cumulative incidence of any severe/life-threatening CHCs was 37.5% and increased to 57.6% by 10 years from aBMT in this cohort of MM patients treated with aBMT and followed for an average of 6.6 years. The incidence continues to climb, with no evidence of a plateau. We found older age at aBMT (≥60 years) and TBI-based conditioning to be associated with a higher burden of grades 3–4 toxicities, thus identifying vulnerable populations that need close long-term follow-up. The most prevalent morbidities were subsequent neoplasms, venous thrombo-embolism, and cataracts. Venous thrombo-embolism is reported to occur at a higher frequency in patients with MM. 27 Cataracts are a well-described complication after BMT, and similar to prior studies evaluating aBMT survivors of lymphoma24,26, we noted a higher frequency of cataracts in long term survivors of aBMT for MM. However, the burden of morbidity due to other grade 3–4 CHCs was comparable between the survivors and the sibling comparison group, with the exception of coronary artery disease and thyroid dysfunction (lower in survivors). This was likely due to the older age of both the survivors and siblings, contributing to the presence of comorbidities in both groups. Nonetheless, this study provides us with an opportunity to provide risk-based surveillance.
Previous studies have suggested an increased risk of subsequent neoplasms in patients with MM; 7,9,10,28,29 ranging between 3% and 20%28, depending on the methodology for ascertainment of subsequent neoplasms, and the length of follow-up (≤5 years in most studies9,10,29). Studies have reported exposure to alkylating agents, lenalidomide, melphalan, TBI, and older age at exposure to be associated with subsequent neoplasms. 28 In our cohort, we found the cumulative incidence of subsequent neoplasms to approach 14% at 10 years post-aBMT. We identified older age, non-Hispanic white race/ethnicity, and pre-aBMT exposure to cyclophosphamide and IMiDs to be associated with a higher risk of subsequent neoplasms. We found a higher risk of subsequent neoplasms in non-Hispanic whites as compared to other groups. This may be in part due to the higher number of skin cancers in our study. A previous SEER-based analysis also demonstrated an increased risk of developing melanomas, non-Hodgkin lymphoma, and acute myeloid leukemia in non-Hispanic whites28,30, when compared with the general population.
Unlike previous studies, solid tumors constituted 96% of subsequent neoplasms in our study; among the solid tumors, melanoma and squamous cell carcinoma of the skin were the most prevalent. When evaluating risk factors for development of these tumors, older age at aBMT, non-Hispanic white race and ethnicity and prior exposure to cyclophosphamide and IMiDs were associated with higher risk of solid tumors overall, and especially of melanomas. The pathogenesis of subsequent neoplasms among MM patients is not well understood. Genetic factors31, interacting with therapeutic exposures, especially with immune-modulating agents32, could play a role and need to be examined in detail. A large analysis from CIBMTR found a higher incidence of acute myeloid leukemia and melanoma after autologous transplant for MM11. A similar increase has been reported in other transplant settings and in persons with immunesuppression33. Prior studies including a large meta-analysis evaluated development of subsequent neoplasms in patients exposed to lenalidomide and found a higher incidence of hematologic malignancies. 29 In our study, two patients developed hematologic malignancies, 1 each with ALL and MDS. Our study also demonstrated an association of solid malignancies with cyclophosphamide. Studies have reported an association of leukemia34, bladder cancer35, and bone cancer36 with higher cumulative doses of cyclophosphamide. Pathogenetic mechanisms postulated include DNA double-strand break-induced gene translocations and genomic instability due to loss of DNA repair37–39. The presence of inherited genetic polymorphisms may also modulate the risks of solid cancers after alkylator-based chemotherapy40.
Our results differ from prior studies29 perhaps due to differences in the study population. Inclusion of ≥2 year survivors in our cohort could possibly result in an under-estimation of MDS because of the short latency accompanied by the high fatality rate. Further, our study had longer follow-up, allowing for emergence of solid tumors with longer latency.
We acknowledge the limitations in our study, particularly the lack of information regarding dose and duration of IMiD exposure, the lack of information regarding post-aBMT therapeutic exposures, and reliance on self-report. The reliability and validity of the BMTSS questionnaire have been tested, and the responses have demonstrated a high level of sensitivity and specificity, 41 confirming that survivors are able to report the occurrence of adverse medical conditions with accuracy. Further, the subsequent neoplasms in our study were confirmed with pathology reports and/or clinician reports. These limitations notwithstanding, in this large cohort of MM patients followed long-term, this study describes the overall burden of severe/life-threatening morbidity, with emphasis on the risk of subsequent neoplasms after exposure to therapeutic agents.
In conclusion, the burden of severe/life-threatening CHCs approaches 60% in MM patients treated with aBMT. Subsequent neoplasms constitute a significant burden of morbidity. This study identifies demographic factors and treatment exposures associated with increased risk of CHCs and subsequent neoplasms, and provides evidence for close monitoring of these survivors to anticipate and manage morbidity.
Acknowledgements:
This study was supported by grants from the National Cancer Institute (R01 CA078938), and the Leukemia and Lymphoma Society (R6502-16) (SB)
Footnotes
Conflicts of interest: The authors declare no competing financial interests.
References
- 1.Hari P. Recent advances in understanding multiple myeloma. Hematol Oncol Stem Cell Ther. 2017;10(4):267–271. [DOI] [PubMed] [Google Scholar]
- 2.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldschmidt H, Lokhorst HM, Mai EK, et al. Bortezomib before and after high-dose therapy in myeloma: long-term results from the phase III HOVON-65/GMMG-HD4 trial. Leukemia. 2018;32(2):383–390. [DOI] [PubMed] [Google Scholar]
- 4.Biran N, Jacobus S, Vesole DH, et al. Outcome with lenalidomide plus dexamethasone followed by early autologous stem cell transplantation in patients with newly diagnosed multiple myeloma on the ECOG-ACRIN E4A03 randomized clinical trial: long-term follow-up. Blood Cancer J. 2016;6(9):e466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrero S, Ladetto M, Drandi D, et al. Long-term results of the GIMEMA VEL-03–096 trial in MM patients receiving VTD consolidation after ASCT: MRD kinetics’ impact on survival. Leukemia. 2015;29(3):689–695. [DOI] [PubMed] [Google Scholar]
- 6.Turesson I, Velez R, Kristinsson SY, Landgren O. Patterns of improved survival in patients with multiple myeloma in the twenty-first century: a population-based study. J Clin Oncol. 2010;28(5):830–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa LJ, Godby KN, Chhabra S, Cornell RF, Hari P, Bhatia S. Second primary malignancy after multiple myeloma-population trends and cause-specific mortality. Br J Haematol. 2018;182(4):513–520. [DOI] [PubMed] [Google Scholar]
- 8.Fenk R, Neubauer F, Bruns I, et al. Secondary primary malignancies in patients with multiple myeloma treated with high-dose chemotherapy and autologous blood stem cell transplantation. Br J Haematol. 2012;156(5):683–686. [DOI] [PubMed] [Google Scholar]
- 9.Attal M, Lauwers-Cances V, Marit G, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1782–1791. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy PL, Owzar K, Hofmeister CC, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366(19):1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahindra A, Raval G, Mehta P, et al. New cancers after autotransplantations for multiple myeloma. Biol Blood Marrow Transplant. 2015;21(4):738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N Engl J Med. 2017;376(14):1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D”Souza A FC. Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR Summary Slides, 2019. Available at https://wwwcibmtrorg. 2019.
- 14.Dai YX, Tai YH, Chen CC, Chang YT, Chen TJ, Chen MH. Bidirectional association between alopecia areata and major depressive disorder among probands and unaffected siblings: A nationwide population-based study. J Am Acad Dermatol. 2020. [DOI] [PubMed] [Google Scholar]
- 15.Milne RL, John EM, Knight JA, et al. The potential value of sibling controls compared with population controls for association studies of lifestyle-related risk factors: an example from the Breast Cancer Family Registry. Int J Epidemiol. 2011;40(5):1342–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arora M, Sun CL, Ness KK, et al. Physiologic Frailty in Nonelderly Hematopoietic Cell Transplantation Patients: Results From the Bone Marrow Transplant Survivor Study. JAMA Oncol. 2016;2(10):1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armenian SH, Sun CL, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood. 2011;118(5):1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. 2010;116(17):3129–3139; quiz 3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. [DOI] [PubMed] [Google Scholar]
- 20.Bhatia S, Robison LL, Francisco L, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105(11):4215–4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czyz A, Lojko-Dankowska A, Dytfeld D, et al. Prognostic factors and long-term outcome of autologous haematopoietic stem cell transplantation following a uniform-modified BEAM-conditioning regimen for patients with refractory or relapsed Hodgkin lymphoma: a single-center experience. Med Oncol. 2013;30(3):611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodman KA, Riedel E, Serrano V, Gulati S, Moskowitz CH, Yahalom J. Long-term effects of high-dose chemotherapy and radiation for relapsed and refractory Hodgkin’s lymphoma. J Clin Oncol. 2008;26(32):5240–5247. [DOI] [PubMed] [Google Scholar]
- 23.Hill BT, Rybicki L, Bolwell BJ, et al. The non-relapse mortality rate for patients with diffuse large B-cell lymphoma is greater than relapse mortality 8 years after autologous stem cell transplantation and is significantly higher than mortality rates of population controls. Br J Haematol. 2011;152(5):561–569. [DOI] [PubMed] [Google Scholar]
- 24.Majhail NS, Ness KK, Burns LJ, et al. Late effects in survivors of Hodgkin and non-Hodgkin lymphoma treated with autologous hematopoietic cell transplantation: a report from the bone marrow transplant survivor study. Biol Blood Marrow Transplant. 2007;13(10):1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majhail NS, Weisdorf DJ, Defor TE, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin’s lymphoma. Biol Blood Marrow Transplant. 2006;12(10):1065–1072. [DOI] [PubMed] [Google Scholar]
- 26.Myers RM, Hill BT, Shaw BE, et al. Long-term outcomes among 2-year survivors of autologous hematopoietic cell transplantation for Hodgkin and diffuse large b-cell lymphoma. Cancer. 2018;124(4):816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Stefano V, Za T, Rossi E. Venous thromboembolism in multiple myeloma. Semin Thromb Hemost. 2014;40(3):338–347. [DOI] [PubMed] [Google Scholar]
- 28.Musto P, Anderson KC, Attal M, et al. Second primary malignancies in multiple myeloma: an overview and IMWG consensus. Ann Oncol. 2017;28(2):228–245. [DOI] [PubMed] [Google Scholar]
- 29.Palumbo A, Bringhen S, Kumar SK, et al. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data. Lancet Oncol. 2014;15(3):333–342. [DOI] [PubMed] [Google Scholar]
- 30.Ailawadhi S, Swaika A, Razavi P, Yang D, Chanan-Khan A. Variable risk of second primary malignancy in multiple myeloma patients of different ethnic subgroups. Blood Cancer J. 2014;4:e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Landgren O, Ma W, Kyle RA, Rajkumar SV, Korde N, Albitar M. Polymorphism of the erythropoietin gene promotor and the development of myelodysplastic syndromes subsequent to multiple myeloma. Leukemia. 2012;26(4):844–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas A, Mailankody S, Korde N, Kristinsson SY, Turesson I, Landgren O. Second malignancies after multiple myeloma: from 1960s to 2010s. Blood. 2012;119(12):2731–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen P, Hansen S, Moller B, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 1999;40(2 Pt 1):177–186. [DOI] [PubMed] [Google Scholar]
- 34.Zou D, An G, Zhu G, et al. Secondary monoclonal gammopathy of undetermined significance is frequently associated with high response rate and superior survival in patients with plasma cell dyscrasias. Biol Blood Marrow Transplant. 2014;20(3):319–325. [DOI] [PubMed] [Google Scholar]
- 35.Travis LB, Curtis RE, Glimelius B, et al. Bladder and kidney cancer following cyclophosphamide therapy for non-Hodgkin’s lymphoma. J Natl Cancer Inst. 1995;87(7):524–530. [DOI] [PubMed] [Google Scholar]
- 36.Hawkins MM, Wilson LM, Burton HS, et al. Radiotherapy, alkylating agents, and risk of bone cancer after childhood cancer. J Natl Cancer Inst. 1996;88(5):270–278. [DOI] [PubMed] [Google Scholar]
- 37.Allan JM, Travis LB. Mechanisms of therapy-related carcinogenesis. Nat Rev Cancer. 2005;5(12):943–955. [DOI] [PubMed] [Google Scholar]
- 38.Chakraborty S, Sun CL, Francisco L, et al. Accelerated telomere shortening precedes development of therapy-related myelodysplasia or acute myelogenous leukemia after autologous transplantation for lymphoma. J Clin Oncol. 2009;27(5):791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wynn RF, Cross MA, Hatton C, et al. Accelerated telomere shortening in young recipients of allogeneic bone-marrow transplants. Lancet. 1998;351(9097):178–181. [DOI] [PubMed] [Google Scholar]
- 40.Fenske TS, McMahon C, Edwin D, et al. Identification of candidate alkylator-induced cancer susceptibility genes by whole genome scanning in mice. Cancer Res. 2006;66(10):5029–5038. [DOI] [PubMed] [Google Scholar]
- 41.Louie AD, Robison LL, Bogue M, Hyde S, Forman SJ, Bhatia S. Validation of self-reported complications by bone marrow transplantation survivors. Bone Marrow Transplant. 2000;25(11):1191–1196. [DOI] [PubMed] [Google Scholar]