Abstract
Primary hypercholesterolemia is characterized by elevated LDL-cholesterol (LDL-C) levels isolated in autosomal dominant hypercholesterolemia (ADH) or associated with elevated triglyceride levels in familial combined hyperlipidemia (FCHL). Rare APOE variants are known in ADH and FCHL. We explored the APOE molecular spectrum in a French ADH/FCHL cohort of 5743 unrelated probands. The sequencing of LDLR, PCSK9, APOB, and APOE revealed 76 carriers of a rare APOE variant, with no mutation in LDLR, PCSK9, or APOB. Among the 31 APOE variants identified here, 15 are described in ADH, 10 in FCHL, and 6 in both probands. Five were previously reported with dyslipidemia and 26 are novel, including 12 missense, 5 synonymous, 2 intronic, and 7 variants in regulatory regions. Sixteen variants were predicted as pathogenic or likely pathogenic, and their carriers had significantly lower polygenic risk scores (wPRS) than carriers of predicted benign variants. We observed no correlation between LDL-C levels and wPRS, suggesting a major effect of APOE variants. Carriers of p.Leu167del were associated with a severe phenotype. The analysis of 11 probands suggests that carriers of an APOE variant respond better to statins than carriers of a LDLR mutation. Altogether, we show that the APOE variants account for a significant contribution to ADH and FCHL.
Keywords: hypercholesterolemia, ADH, FCHL, apolipoprotein E, APOE gene, mutation, variant
1. Introduction
Autosomal dominant hypercholesterolemia (ADH) is a major cause of premature atherosclerosis with a risk 13 times greater than all other coronary heart diseases (CHD) risk factors [1]. ADH is characterized by a selective increase in circulating low-density lipoproteins (LDL) due to reduced catabolism [2]. This increased level of LDL-cholesterol (LDL-C) in plasma since birth gives rise to tendon and skin xanthomas, arcus cornea, and vascular deposits, leading to premature CHD and death [3]. ADH is one of the most frequent monogenic diseases with a prevalence of one in 313 according to a recent meta-analysis [4]. The main ADH genes are encoding the LDL receptor (LDLR), apolipoprotein B (APOB) which is the LDL receptor protein-ligand, and proprotein convertase subtilisin/kexin type 9 (PCSK9) which enhances the intracellular degradation of LDL receptor [5]. The respective contributions of these three ADH-genes in 2054 French ADH patients are: LDLR 52%, APOB 3%, PCSK9 1%, whereas the remaining 44% of the probands had no ADH-mutation identified [6]. A polygenic origin is suggested in 36% of non-mutated patients [6,7]. These observations provide evidence for a greater level of genetic heterogeneity in ADH and the involvement of unknown genes [8]. In search of these new ADH-genes, a large ADH-affected French family with the APOE p.Leu167del mutation revealed it to be the fourth ADH-gene [9]. The study of 229 French ADH patients showed 1.3% likely pathogenic APOE variants indicating that the APOE gene significantly contributes to ADH [10]. Most ADH patients are treated with high-dose statins with an established efficacy for heterozygous carriers of LDLR, APOB, and PCSK9 mutations [11]. In these cases, APOE p.Leu167del carriers respond better to statins, with or without ezetimibe, than ADH subjects with a LDLR mutation [12].
Familial combined hyperlipidemia (FCHL) is a common disorder of lipid metabolism that leads to elevated levels of very-low-density lipoprotein (VLDL), low-density lipoprotein (LDL), or both in the plasma, leading to mixed hyperlipidemia with increased total cholesterol and triglyceride levels. FCHL occurs in up to 3% of the general population and may account for one-third to one-half of familial causes of early CHD [13]. The phenotype of FCHL is highly variable among family members depending on genetic and environmental factors and may present itself as mixed hyperlipidemia, isolated hypercholesterolemia, and hypertriglyceridemia. The phenotype may also present itself as a normal serum lipid profile in combination with abnormally elevated levels of apoB. FCHL is genetically complex with variable penetrance [13]. Most cases of FCHL are considered polygenic [13], and several genes are described in FCHL [14]. LDLR gene mutations are reported in 19.6% of FCHL patients [15]. Some of these mutations are identified as causal to ADH indicating that ADH patients with hypertriglyceridemia may be misdiagnosed with FCHL [15]. Thus, there is a phenotypic and genetic overlap between ADH and FCHL. Variants in APOE are also reported in FCHL and are responsible for 3.5% of FCHL cases in a Spanish population, of which 1.4% are carriers of the APOE p.Leu167del variant [16].
Apolipoprotein E (apoE) is a major apolipoprotein that is synthesized primarily in the liver and controls lipoprotein metabolism. The APOE gene (NM_000041.4) is composed of four exons and encodes the 317 amino acid apoE precursor that matures to a 299 amino acid protein with a molecular mass of 34 kDa. ApoE is a component of chylomicrons, VLDL, the triglyceride-rich remnants of chylomicrons and VLDL, and high-density lipoprotein (HDL). It is also a co-factor for the lipoprotein lipase responsible for triglyceride hydrolysis in VLDL which enables the formation of IDL and LDL. ApoE is also present on a subset of lipoprotein (a), IDL, and LDL [17]. Additionally, it is a key factor in the regulation of lipoprotein clearance through its binding to cell-surface receptors, including LDL receptor family members such as the LDL receptor, the VLDL receptor, and the LDL receptor-related protein 1 (LRP1). ApoE also binds to cell-surface heparan sulfate proteoglycans (HSPGs) [18]. An alteration of either the structure or the function of apoE could impact the metabolism and clearance of triglyceride-rich lipoproteins and plasma lipids [17,18].
Although LDL carries few apoE proteins, the concentration and size of LDL are influenced by the common apoE isoforms E2, E3, and E4 which differ within the mature protein at amino acid positions 112 and 158. ApoE3 is considered the normal isoform and contains a cysteine residue at position 112 and an arginine residue at 158. ApoE2 with a cysteine residue at both positions is defective in LDL receptor binding and is associated with the recessive form of type III hyperlipoproteinemia [19]. ApoE4 has an arginine residue at both positions 112 and 158 and is associated with increased levels of plasma LDL-C [20]. Polymorphisms in APOE are associated with LDL levels in genome-wide association studies [21] and are included in the wPRS calculation [7]. Variants that give rise to apoE isoforms are APOE4 rs429358, p.Cys130Arg and APOE2 rs7412, p.Arg176Cys. According to frequencies given by the Genome Aggregation Database (GnomAd), sequencing of about 100,000 subjects from various disease-specific and population genetic studies results in an APOE4 rs429358 allele frequency of 14.25% and an APOE2 rs7412 allele frequency of 6.542% in the total GnomAd population. Thus, the approximate prevalence for the APOE genotypes E2/E4, E3/E3, E3/E4, and E4/E4 are 0.9%, 75.9%, 14.3%, and 2.0%, respectively.
Beyond the common APOE variants, rare APOE variants are associated with different lipid pathologies including ADH and FCHL. Therefore, we aimed to explore the molecular spectrum of APOE variants in a French ADH/FCHL cohort.
2. Results
Among 5743 probands diagnosed with primary dyslipidemia (58% ADH and 42% FCHL), we identified a total of 76 carriers of a rare APOE variant (53% women, 48 ± 15 years old, LDL-MoM = 1.91 ± 0.56, TG-MoM = 2.10 ± 1.65) (Table 1). None of these 76 probands carried a LDLR, APOB, or PCSK9 variant with a deleterious or probably deleterious effect. Forty-nine patients (65%) diagnosed with ADH (55% women, 47±16 years old, LDL-MoM = 1.90 ± 0.50, TG-MoM = 1.28 ± 0.38) (Table 1) carried 21 different APOE variants (Figure 1, Table 2). Among the 21 variants, 3 were localized to the APOE promoter, 14 to exons, 2 to introns, and 2 to the 3’UTR region. Among the exonic variants, 10 were novel and not associated previously with dyslipidemia, whereas 4 were already associated with either ADH or type III hyperlipoproteinemia. Twenty-seven patients (35%) were diagnosed with FCHL (48% women, 51 ± 13 years old, LDL-MoM = 1.93 ± 0.66, TG-MoM = 3.54 ± 2.02) (Table 1) with 16 different variants (Figure 1, Table 2). Among the 16 variants, 6 were also carried by ADH probands but 10 were specific to FCHL. Only one was previously associated with primary dyslipidemia.
Table 1.
Description of the 76 probands with dyslipidemia.
| APOE Variant | LDL-MoM | TC-MoM | TG-MoM | Clinical Signs | Family History | Hyperlipidemia | ApoE Isoforms | 12-SNP wPRS | wPRS Decile b | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| rs1038445539 | c.-380A > G | p.? | 1.67 | 1.44 | 2.07 | Xanthelasma | Yes | FCHL | E3E4 | 0.743 | III |
| rs1038445539 | c.-380A > G | p.? | 2.52 | 1.91 | 6.1 | Corneal arcus | Yes | FCHL | E3E4 | 1.021 | VIII |
| c.-279G > A | p.? | 1.4 | 1.41 | 5.71 | Yes | FCHL | E3E4 | 1.207 | X | ||
| - | c.-233G > C | p.? | 2.00 | 1.71 | 1.30 | Yes | ADH | E3E4 | 1.296 | X | |
| c.-105A > G | p.? | 1.9 | 1.78 | 2.56 | Yes | FCHL | E3E3 | 1.164 | X | ||
| rs766215051 | c.-81G > A | p.? | 2.40 | 1.76 | 1.31 | ADH | E3E4 | 1.116 | IX | ||
| rs750782549 | c.-78C > G | p.? | 1.57 | 1.41 | 2.55 a | Yes | FCHL | E3E4 | 0.824 | IV | |
| rs750782549 | c.-78C > G | p.? | 1.59 | 1.60 | 2.04 | FCHL | E3E4 | 1.164 | X | ||
| rs750782549 | c.-78C > G | p.? | 1.52 | 1.33 | 1.65 | Xanthelasma | ADH | E3E4 | 0.950 | VI | |
| - | c.43+11G > A | p.? | 1.47 | 1.41 | 1.90 | ADH | E3E4 | 1.173 | X | ||
| rs770658351 | c.44-1G > C | p.? | 2.17 | 2.44 | na | ADH | E3E4 | 0.722 | II | ||
| rs144354013 | c.31A > G | p.Thr11Ala | 4.12 | na | 3.12 a | FCHL | E3E4 | 0.950 | VI | ||
| rs111833428 | c.69G > A | p.Ala23= | 1.41 | na | 5.89 a | FCHL | E3E3 | 1.097 | IX | ||
| rs776242156 | c.68C > T | p.Ala23Val | 1.48 | 1.29 | 0.57 | CVD | ADH | E3E4 | 1.136 | IX | |
| rs769452 | c.137T > C | p.Leu46Pro | 1.59 | 1.48 | 0.96 | ADH | E3E4 | 1.137 | IX | ||
| rs769452 | c.137T > C | p.Leu46Pro | 1.46 | 1.36 | 0.58 | Xanthoma | ADH | E4E4 | 1.136 | IX | |
| rs769452 | c.137T > C | p.Leu46Pro | 1.33 | 1.25 | 1.65 | ADH | E3E4 | 1.012 | VII | ||
| rs769452 | c.137T > C | p.Leu46Pro | 2.18 | 1.75 | 1.56 | ADH | E3E4 | 1.365 | X | ||
| rs769452 | c.137T > C | p.Leu46Pro | 1.62 | 1.41 | 0.75 | Yes | ADH | E3E4 | 1.149 | IX | |
| rs769452 | c.137T > C | p.Leu46Pro | 1.40 | 1.25 | 1.70 | Yes | ADH | E3E4 | 1.117 | IX | |
| rs769452 | c.137T > C | p.Leu46Pro | 1.62 | 1.38 | 1.56 | ADH | E3E4 | 1.240 | X | ||
| rs769452 | c.137T > C | p.Leu46Pro | 1.65 | 1.50 | 1.34 | ADH | E3E4 | 0.919 | V | ||
| rs769452 | c.137T > C | p.Leu46Pro | 1.53 | 1.86 | 0.77 | Yes | ADH | E3E4 | 1.049 | VIII | |
| rs769452 | c.137T > C | p.Leu46Pro | 2.25 | 1.90 | 0.75 | Yes | ADH | E3E4 | 1.045 | VIII | |
| rs769452 | c.137T > C | p.Leu46Pro | 1.62 | 1.43 | 1.09 | No | ADH | E3E4 | 1.068 | VIII | |
| rs769452 c | c.137T > C c | p.Leu46Pro c | 1.75 | 1.46 | 1.42 | Corneal arcus | ADH | E4E4 | 1.169 | X | |
| rs769452 | c.137T > C | p.Leu46Pro | 1.46 | 1.36 | 2.36 | Yes | FCHL | E2E4 | 1.128 | IX | |
| rs769452 | c.137T > C | p.Leu46Pro | 1.72 | 1.61 | 2.86 | CVD | FCHL | E3E4 | 1.133 | IX | |
| rs769452 | c.137T > C | p.Leu46Pro | 1.58 | 1.43 | 4.04 a | CVD | FCHL | E3E4 | 0.891 | V | |
| rs769452 | c.137T > C | p.Leu46Pro | 1.74 | 1.63 | 2.17 | Yes | FCHL | E3E4 | 1.120 | IX | |
| rs769452 | c.137T > C | p.Leu46Pro | 1.96 | 1.70 | 7.95 | Yes | FCHL | E3E4 | 0.727 | II | |
| rs767980905 | c.249C > T | p.Asp83= | 1.76 | 1.46 | 1.32 | ADH | E3E4 | 1.047 | VIII | ||
| rs11083750 | c.305C > T | p.Pro102Leu | 2.09 | 1.61 | 1.22 | ADH | E3E4 | 1.307 | X | ||
| rs573658040 | c.409C > T | p.Arg137Cys | 1.61 | 1.43 | 1.21 | ADH | E3E4 | 0.945 | VI | ||
| rs11542035 | c.410G > A | p.Arg137His | 1.70 | 1.51 | 1.40 | ADH | E3E3 | 0.912 | V | ||
| rs267606664 | c.434G > A | p.Gly145Asp | 1.4 | 1.43 | 3.03 | FCHL | E2E4 | 0.856 | IV | ||
| rs267606664 | c.434G > A | p.Gly145Asp | 2.21 | 2.72 | 5.5 a | Yes | FCHL | E3E4 | 0.480 | I | |
| rs1018669382 | c.463 C > T | p.Leu155Phe | 1.50 |
1.34 | 1.98 | ADH | E3E3 | 1.162 | X | ||
| rs769455 | c.487C > T | p.Arg163Cys | 1.68 | 1.54 | 1.60 | CVD | ADH | E3E3 | 0.948 | VI | |
| rs769455 | c.487C > T | p.Arg163Cys | 2.47 | na | 10 | Yes | FCHL | E3E3 | 0.625 | II | |
| rs769455 | c.487C > T | p.Arg163Cys | 1.41 | 1.23 | 2.52 | FCHL | E3E4 | 1.129 | IX | ||
| rs155726148 | c.500_502delTCC | p.Leu167del | 2.54 | 2.12 | 1.88 | CVD | ADH | E3E3 | 1.280 | X | |
| rs155726148 | c.500_502delTCC | p.Leu167del | 2.01 | 1.61 | 1.25 | ADH | E3E3 | 1.028 | VIII | ||
| rs155726148 | c.500_502delTCC | p.Leu167del | 2.33 | 2.23 | 1.47 | ADH | E3E3 | 1.030 | VIII | ||
| rs155726148 | c.500_502delTCC | p.Leu167del | 3.51 | 2.66 | 1.16 | Corneal arcus | ADH | E3E3 | 0.680 | II | |
| rs155726148 | c.500_502delTCC | p.Leu167del | 2.15 | 1.84 | 1.11 | ADH | E3E3 | 0.747 | III | ||
| rs155726148 | c.500_502delTCC | p.Leu167del | 3.55 | 2.52 | 1.03 | ADH | E3E3 | 1.098 | IX | ||
| rs155726148 | c.500_502delTCC | p.Leu167del | 2.43 | 2.03 | 1.11 | Yes | ADH | E3E3 | 0.985 | VII | |
| rs155726148 | c.500_502delTCC | p.Leu167del | 2.79 | 2.09 | 1.37 | ADH | E3E3 | 1.076 | VIII | ||
| rs155726148 | c.500_502delTCC | p.Leu167del | 1.33 | 1.21 | 0.66 | ADH | E3E3 | 0.920 | V | ||
| rs155726148 | c.500_502delTCC | p.Leu167del | 2.09 | 1.36 | 1.37 | Yes | ADH | E3E4 | 0.824 | IV | |
| rs155726148 | c.500_502delTCC | p.Leu167del | 2.47 | 2.12 | 0.88 | ADH | E3E4 | 0.983 | VII | ||
| rs155726148 | c.500_502delTCC | p.Leu167del | 1.93 | 1.61 | 1.05 | Corneal arcus | Yes | ADH | E3E4 | 1.190 | X |
| rs155726148 | c.500_502delTCC | p.Leu167del | 1.77 | 1.44 | 0.62 | Yes | ADH | E3E4 | 1.035 | VIII | |
| rs155726148 | c.500_502delTCC | p.Leu167del | 1.31 | 2.16 | 1.69 | Yes | ADH | E3E3 | 0.698 | II | |
| rs155726148 | c.500_502delTCC | p.Leu167del | 2.37 | 1.49 | 2.68 | FCHL | E3E4 | 0.832 | IV | ||
| rs155726148 | c.500_502delTCC | p.Leu167del | 1.58 | 1.47 | 2.02 | CVD | FCHL | E3E3 | 0.683 | II | |
| rs155726148 | c.500_502delTCC | p.Leu167del | 1.66 | 1.50 | 2.07 | Yes | FCHL | E3E4 | 0.921 | V | |
| rs155726148 | c.500_502delTCC | p.Leu167del | 3.51 | 2.61 | 3.23 | FCHL | E3E3 | 0.952 | VI | ||
| rs1239911444 | c.517C > T | p.Leu173= | 1.74 | 1.70 | 3.22 | Yes | FCHL | E3E3 | 0.918 | V | |
| rs1421977676 | c.536C > T | p.Val179Ala | 1.65 | 1.57 | 3.05 | CVD | FCHL | E3E3 | na | na | |
| rs781722239 | c.555C > T | p.Arg185= | 2.17 | 1.86 | 0.66 | Corneal arcus | ADH | E3E3 | 0.689 | II | |
| - | c.638T > A d | p.Val213Glu d | 2.51 | 1.99 | 1.07 | ADH | E3E3 | 0.896 | V | ||
| rs72654468 | c.651C > T | p.Ala217= | 1.54 | 1.42 | 1.13 | ADH | E3E3 | 1.243 | X | ||
| rs72654468 | c.651C > T | p.Ala217= | 2.08 | 1.69 | 1.85 | ADH | E3E3 | 1.020 | VIII | ||
| rs72654468 | c.651C > T | p.Ala217= | 1.55 | 1.40 | 1.30 | ADH | E3E3 | 1.130 | IX | ||
| - | c.652G > T | p.Gly218Cys | 1.76 | 1.48 | 1.24 | ADH | E3E4 | 0.945 | VI | ||
| rs762906934 | c.745G > A | p.Glu249Lys | Na | 1.62 | 2.19 | CVD | FCHL | E3E3 | 0.962 | VI | |
| - | c.754G > A | p.Glu252Lys | 1.61 | 1.45 | 2.53 | Yes | FCHL | E3E4 | 0.897 | V | |
| rs267606661 | c.805C > G | p.Arg269Gly | 2.42 | 1.99 | 2.13 | CVD | FCHL | E3E4 | 1.243 | X | |
| rs267606661 | c.805C > G | p.Arg269Gly | 1.55 | 1.30 | 1.95 | CVD | ADH | E4E4 | 0.855 | IV | |
| rs267606661 | c.805C > G | p.Arg269Gly | 1.81 | 1.50 | 1.51 | ADH | E3E4 | 1.067 | VIII | ||
| rs267606661 | c.805C > G | p.Arg269Gly | 1.71 | 1.52 | 1.43 | ADH | E3E4 | 0.933 | V | ||
| rs374329439 | c.*25C > T | 3’UTR variant | 1.53 | 1.42 | 1.54 | ADH | E3E3 | 1.083 | IX | ||
| rs374329439 | c.*25C > T | 3’UTR variant | 1.46 | 1.41 | 2.1 | FCHL | E3E3 | 1.099 | IX | ||
| - | c.*36C > G | 3’UTR variant | 1.50 | 1.32 | 1.52 | ADH | E3E4 | 1.344 | X | ||
| Median [First quartile–third quartile] |
1.71 [1.54–2.17] |
1.50 [1.41–1.81] |
1.56 [1.21–2.36] |
||||||||
na: non-available. a Triglyceride values under statin treatment. b Scores in deciles I–III have a strong probability of monogenic ADH, whereas scores in deciles VIII–X have a strong probability of polygenic hypercholesterolemia. c Homozygous carrier. d Homozygous carrier of the p.(Leu21dup) variant in PCSK9 is known to be associated with reduced LDL-C [26].
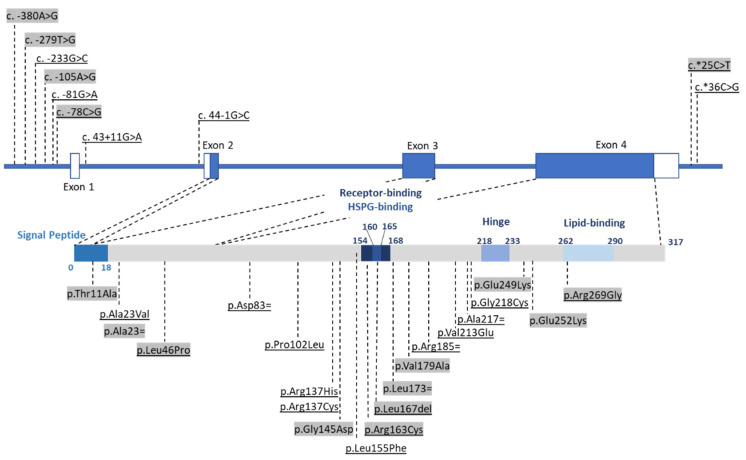
Figure 1.
Rare APOE variants identified in the French ADH/FCHL cohort. Three of the four APOE exons encode the 317 amino acid apoE precursor. The binding site for the LDL receptor is at residues 154–168. The lipid-binding site is at residues 262–290. Between the two sites, the hinge domain is at residues 218–233. Variants are distributed on coding, intronic, promoter, and 3’UTR regions, including missense, synonymous, splicing, or regulatory variants. Variants only present in FCHL patients are highlighted in grey, and variants present in both ADH and FCHL patients are highlighted in grey and underlined.
Table 2.
Description of the 31 APOE variants.
| rs Number | cDNA Position (NM_000041.4) | Protein Position (NP_000032.1) | Hyperlipidemia | AF a in the ADH/FCHL Cohort | FREX Total AF a | GnomAD Total AF a | PolyPhen 2 b | SIFT c | Mutation Taster d | CADD e | Provean f | Splice Site Affected g | ACMG (Varsome) h | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs1038445539 | c.-380A > G | 5’UTR variant | FCHL | 0.017 (2/11,486) | 0 | 0.005 (7/152,092) | na | na | na | 7.106 | na | no | na | |
| - | c.-279G > A | 5’UTR variant | FCHL | 0.009 (1/11,486) | 0 | 0 | na | na | na | 5.676 | na | no | na | |
| - | c.-233G > C | 5’UTR variant | ADH | 0.009 (1/11,486) | 0 | 0 | na | na | na | 10.31 (top 10%) | na | no | na | |
| - | c.-105A > G | 5’UTR variant | FCHL | 0.009 (1/11,486) | 0 | 0 | na | na | DC | 22.7 (top 1%) | na | no | VUS | |
| rs766215051 | c.-81G > A | 5’UTR variant | ADH | 0.009 (1/11,486) | 0 | 0.003 (5/152,130) | na | na | DC | 14.13 (top 10%) | na | no | VUS | |
| rs750782549 | c.-78C > G | 5’UTR variant | ADH, FCHL | 0.026 (3/11,486) i | 0 | 0.001 (2/152,116) | na | na | DC | 14.91 (top 10%) | na | no | VUS | |
| rs770658351 | c.43+11G > A | p.? | ADH | 0.009 (1/11,486) | 0 | 0 | na | na | SNP | 13.12 (top 10%) | na | no | VUS | |
| - | c.44-1G > C | p.? | ADH | 0.009 (1/11,486) | 0 | 0 | na | na | DC | 33 (top 0.1%) | na | Yes | P | |
| rs144354013 | c.31A > G | p.Thr11Ala | FCHL | 0.009 (1/11,486) | 0 | 0.009 (13/151,914) | B | T | SNP | 0.294 | N (0.8) | no | VUS/P | |
| rs776242156 | c.68C > T | p.Ala23Val | ADH | 0.009 (1/11,486) | 0 | 0.001 (1/152,206) | B | T | SNP | 0.047 | N (−0.2) | no | VUS/LP | |
| rs111833428 | c.69G > A | p.Ala23= | FCHL | 0.009 (1/11,486) | 0 | 0.023 (35/152,212) | na | na | SNP | 5.195 | N (0) | no | LB | |
| rs769452 | c.137T > C | p.Leu46Pro | ADH, FCHL | 0.157 (18/11,486) | 0.174 (2/1148) | 0.193 (293/152,188) | P | T | DC | 0.72 | N (−1.1) | no | LB | [10] |
| rs767980905 | c.249C > T | p.Asp83= | ADH | 0.009 (1/11,486) | 0 | 0.003 (4/152,218) | na | na | DC | 0.615 | N (0) | no | LB | |
| rs11083750 | c.305C > T | p.Pro102Leu | ADH | 0.009 (1/11,486) | 0 | 0 | PD | D | DC | 23.4 (top 1%) | D (−8.7) | no | LP | |
| rs573658040 | c.409C > T | p.Arg137Cys | ADH | 0.009 (1/11,486) | 0 | 0.002 (3/152,132) | PD | T | DC | 25.8 (top 1%) | N (−2.4) | no | VUS/P | |
| rs11542035 | c.410G > A | p.Arg137His | ADH | 0.009 (1/11,486) | 0 | 0.003(5/152,112) | P | T | SNP | 22.1 (top 1%) | N (−1.0) | no | VUS/P | |
| rs267606664 | c.434G > A | p.Gly145Asp | FCHL | 0.017 (2/11,486) | 0.087 (1/1148) | 0.015 (22/152,152) | PD | T | DC | 24.5 (top 1%) | N (0.656) | no | VUS/P | [27] |
| rs1018669382 | c.463 C > T | p.Leu155Phe | ADH | 0.009 (1/11,486) i | 0 | 0.001 (2/152,148) | B | T | SNP | 5.538 | N (−1.6) | no | VUS/P | |
| rs769455 | c.487C > T | p.Arg163Cys | ADH, FCHL | 0.026 (3/11,486) j | 0 | 0.643 (978/152,126) | PD | D | DC | 28.4 (top 1%) | D (−4.9) | no | VUS/P | [10] |
| rs515726148 | c.500_502delTCC | p.Leu167del | ADH, FCHL | 0.157 (18/11,486) i | 0 | 0.003 (4/152,132) | na | na | SNP | na | D (−7.4) | no | LP | [9,10,16,28,29,30] |
| rs1239911444 | c.517C > T | p.Leu173= | FCHL | 0.009 (1/11,486) | 0 | 0 | na | na | DC | 7.641 | N (0) | no | LB | |
| rs1421977676 | c.536T > C | p.Val179Ala | FCHL | 0.009 (1/11,486) | 0 | 0 | PD | T | SNP | 23.5 (top 1%) | N (−1.0) | no | VUS/P | |
| rs781722239 | c.555C > T | p.Arg185= | ADH | 0.009 (1/11,486) | 0 | 0.009 (13/151,932) | na | na | SNP | 7.192 | N (0) | no | LB | |
| - | c.638T > A | p.Val213Glu | ADH | 0.009 (1/11,486) | 0 | 0 | P | D | SNP | 11.3 (top 10%) | N (−0.6) | no | VUS/P | |
| rs72654468 | c.651C > T | p.Ala217= | ADH | 0.026 (3/11,486) j | 0.182 (2/1,094) | 0.089 (135/151,926) | na | na | SNP | 6.242 | N (0) | no | LB | |
| - | c.652G > T | p.Gly218Cys | ADH | 0.009 (1/11,486) | 0 | 0 | PD | T | SNP | 6.506 | N (−1.4) | no | VUS/P | |
| rs762906934 | c.745G > A | p.Glu249Lys | FCHL | 0.009 (1/11,486) | 0 | 0.001 (1/152,172) | B | T | SNP | 19.7 (top 10%) | N (−1.4) | no | VUS/P | |
| - | c.754G > A | p.Glu252Lys | FCHL | 0.009 (1/11,486) | 0 | 0 | P | D | SNP | 22.2 (top 1%) | D (−2.9) | no | VUS/P | |
| rs267606661 | c.805C > G | p.Arg269Gly | ADH, FCHL | 0.035 (4/11,486) | 0.087 (1/1148) | 0.030 (46/152,200) | P | D | DC | 23.3 (top 1%) | D (−2.9) | no | VUS/P | [10] |
| rs374329439 | c.*25C > T | 3’UTR variant | ADH, FCHL | 0.017 (2/11,486) | 0 | 0.071 (108/152,194) | na | na | SNP | 5.508 | na | no | VUS | |
| - | c.*36C > G | 3’UTR variant | ADH | 0.009 (1/11,486) | 0 | 0 | na | na | SNP | 6.597 | na | no | VUS |
a AF: allele frequency in % (allele count/number), na: not available. b B: benign; PD: probably damaging; P: possibly damaging. c T: tolerated; D: deleterious. d DC: disease-causing; SNP: single nucleotide polymorphism. e Variant with a score ≥ 20 is predicted to be among the top 1% of the most deleterious substitutions in the human genome; a score ≥ 10, among the top 10%. f Variant with a score ≤ −2.5 is considered ‘deleterious’ (D) and a score ≥ 2.5 is considered “neutral” (N). g Potential effect on splicing assessed with Alamut and Human Splicing Finder; Yes: Loss of intron 2 acceptor site. h P: pathogenic; LP: likely pathogenic; VUS: variant of uncertain significance; LB: likely benign. i AF significantly higher in this ADH/FCHL cohort than in GnomAD total population. j AF significantly lower in the studied cohort than in the GnomAD total population.
2.1. New APOE Variants in Primary Dyslipidemia
Of the 26 novel APOE variants (not previously reported with dyslipidemia), 12 were missense variants, 5 were synonymous substitutions, 2 were intronic, and 7 were in regulatory regions (Table 2). A large majority (21/26) were present at a higher frequency in the ADH/FCHL cohort compared to the 1148 alleles sequenced in the FREX control group that is representative of the French population, or the 152,200 alleles sequenced in GnomAD that are representative of the general population. Only two variants, c.-78C > G and p.Leu155Phe, were present at a significantly higher frequency in the ADH/FCHL cohort than in GnomAD. Moreover, the c.-78C > G variant was significantly more frequent in the ADH/FCHL cohort than in the GnomAD African/African-American population which has the highest allele frequency (Table S1). We added these data in Varsome through the activation of the PS4 ACMG criterion which identifies the prevalence of a variant in affected individuals that is significantly increased compared with the prevalence in controls. Based upon this criterion, the pathogenic prediction of c.-78C > G changed from variant of uncertain significance (VUS) to VUS/likely pathogenic (LP), and p.Leu155Phe changed from VUS/LP to LP. The c.44-1G > C variant was predicted as pathogenic because it destroyed the intron 2 acceptor splice site which may have led to the whole skipping of exon 2 or resulted in a cryptic splice site. The p.Pro102Leu variant was predicted as LP because it affected a well-conserved amino acid residue. The five synonymous variants were predicted as likely benign (LB) because they did not affect any splice site and thus might not be causative. The three 5’UTR variants nearest the gene from −78 to −105 were predicted as VUS, whereas the three farthest from the gene at −233 to −380 could not be analyzed by Varsome. In the 3’UTR, c.*25C > T was predicted to be within the miR-7704 target sequence known to be involved in tumorigenesis but not CVD [22].
2.2. Recurrent APOE Variants in ADH/FCHL Patients
The most frequent variant of the ADH/FCHL cohort, p.Leu167del, was carried by 14 ADH and four FCHL probands (Table 1). It was present at a significantly higher frequency in the ADH/FCHL cohort compared to the GnomAD total population (Table 2) as well as the GnomAD population with the highest allele frequency, the Latino/Admixed American population (Table S1). By adding this information in Varsome using the PS4 ACMG criterion, the pathogenic prediction changed from LP to pathogenic (P). The p.Leu46Pro variant that was previously reported in a French ADH proband [10] was carried by 12 ADH (11 heterozygotes and one homozygote) and 5 FHCL probands (Table 1). Interestingly, all carriers of the p.Leu46Pro variant were also carriers of the E4 allele due to the linkage disequilibrium between the two variants (D’ = 1.0, r2 = 0.266; Table S2). This variant was also reported in a dementia cohort [23]. A unique molecular event that probably occurred in the past in the E4 allele was transmitted through generations and is now reported as “ApoE4 Freiburg” [24]. The homozygote ApoE4 Freiburg carrier did not present a more severe phenotype (Table 1); thus, the transmission mode seemed to be dominant rather than semi-dominant [25]. The p.Arg163Cys variant that was previously reported in a French ADH family and two probands [10] was carried by one ADH subject who suffered from myocardial infarction at 40 years old and two FCHL subjects (Table 1). The p.Arg269Gly variant that was previously reported in one case of type IIa hyperlipidemia [9] was carried by three unrelated ADH probands and one FCHL proband (Table 1). The p.Gly145Asp variant that was previously described in a 43-year-old French patient presenting severe mixed dyslipidemia [9] was carried by two unrelated FCHL men (Table 1). It was not clear whether the variant p.Gly145Asp had an impact on the structure of apoE. The variation modified the protein net charge and thus may have altered the affinity of apoE for its receptor [10]. Interestingly, the p.Gly145Asp variant is in linkage disequilibrium with the E2 allele (D’ = 1.0, r2 = 0.240, Table S2).
2.3. Monogenic or Polygenic Dyslipidemia?
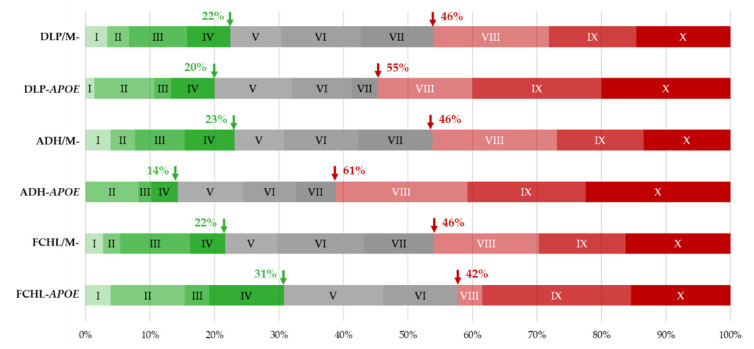
A substantial proportion of ADH/FCHL probands with no detectable mutations in LDLR, APOB, or PCSK9 have increased LDL-C concentrations that are explainable by co-inheritance of common LDL-C-raising alleles and which are therefore of polygenic origin (19). APOE carriers in the ADH/FCHL cohort may have thus also presented increased LDL-C due to a polygenic origin rather than a real effect of a defective apoE. We compared the distribution of the weighted polygenic risk score (wPRS) in the ADH/FCHL, ADH, and FCHL cohorts between probands with an apoE rare variant and probands with no LDLR, APOB, PCSK9, or APOE variant (Figure 2). The proportion of probands with a high probability of polygenic dyslipidemia was increased in the cohort of APOE variant carriers (55% vs. 46%), whereas the probability of monogenic dyslipidemia was similar (20% vs. 22%). The difference in the proportion of probands with a high probability of polygenic dyslipidemia was more marked in the ADH cohort (61% for APOE variant carriers vs. 46%). This was reversed in the FCHL cohort (42% APOE variant carriers vs. 46%) (Figure 2).
Figure 2.
Distribution of the 12-SNP weighted polygenic risk score (wPRS) within the deciles of the Whitehall II control cohort [7]. Comparison between dyslipidemic (DLP = ADH/FCHL), ADH, or FCHL probands carrying an APOE rare variant or without any ADH/FCHL causative mutation (/M-). Green arrows indicate the percentage of probands with a low wPRS and a probability of monogenic DLP that gradually increases under decile V. Red arrows indicate the percentage of probands with a wPRS in the top three deciles with a high probability of polygenic DLP.
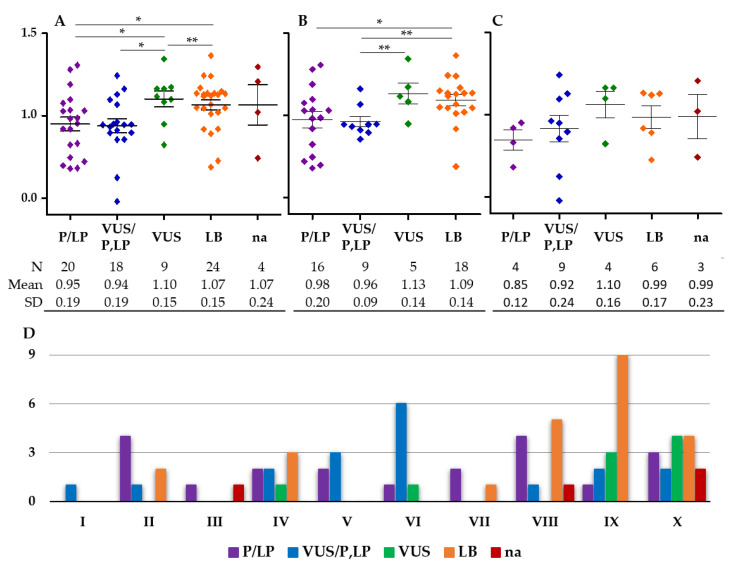
Conversely, the proportion of probands with a high probability of monogenic dyslipidemia was reduced in the cohort of ADH-APOE variant carriers (14% vs. 23%) and increased in the cohort of FCHL-APOE variant carriers (31% vs. 22%) (Figure 2). These observations suggest that a larger proportion of ADH cases were of polygenic origin among carriers of an APOE variant compared to non-carriers. Consequently, a substantial proportion of the APOE variants may not have been the major cause of ADH. To identify these variants, we compared the wPRS between carriers of variants grouped in the different pathogenicity groups according to Varsome classification: P/LP, VUS, and BL (Figure 3). The wPRS was significantly different among the five pathogenicity groups in the whole cohort (p = 0.025, Kruskal–Wallis test) (Figure 3A) and the ADH cohort (p = 0.022, Kruskal–Wallis test) (Figure 3B). No significant differences were observed in the FCHL cohort (Figure 3C).
Figure 3.
Weighted polygenic risk score (wPRS) in carriers of APOE variants grouped in different pathogenicity groups. The five pathogenicity groups predicted by Varsome according to the ACMG criterion are: pathogenic/likely pathogenic (P/LP); variant of uncertain significance (VUS); benign/likely benign (B/BL); predicted pathogenicity not available (na). The ADH/FCHL (A), ADH (B), and FCHL (C) cohorts are indicated above their respective plots. (D) Distribution of the variants from the five pathogenicity groups within the wPRS deciles of the Whitehall II control cohort [7]. * p < 0.05, ** p < 0.005, non-parametric Mann–Whitney test.
In the ADH/FCHL cohort, carriers of a VUS variant presented a significantly greater mean wPRS than carriers of a P/LP, VUS/P, LP, or LB variant. Carriers of a LB variant presented a significantly greater mean wPRS than carriers of a P/LP variant (Figure 3A). In the ADH cohort, carriers of a LB variant presented a significantly higher mean wPRS than carriers of a P/LP or VUS/P,LP variant, and carriers of a VUS presented a significantly greater mean wPRS than carriers of a VUS/P,LP variant (Figure 3B). These results indicated that among carriers of VUS and LB APOE variants, the proportion of polygenic ADH was greater than among carriers of P/LP and VUS/P,LP variants. Thus, six VUS and six LB APOE variants reported here may not have been the major cause of ADH (Table 2).
The distribution of variants from the five pathogenicity groups within the wPRS deciles of the Whitehall II control cohort was significantly different between the groups (p = 0.003, Kruskal–Wallis test) in the ADH/FCHL cohort (Figure 3D). The VUS and LB APOE variants were observed more frequently in probands with a high probability of polygenic dyslipidemia compared to the P/LP and VUS/P,LP variants. Altogether, the data suggested that patients with a VUS or LB variant probably had polygenic ADH, whereas carriers of a P/LP or VUS/P,LP variant suffered from monogenic ADH due to a major effect of the APOE variant.
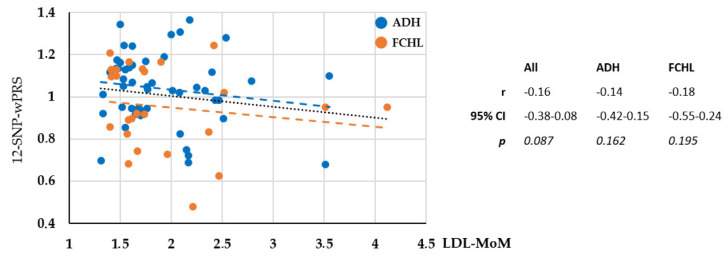
We did not detect any correlation between the LDL-MoM values and the 12-SNP wPRS for the 49 ADH patients, the 27 FCHL patients, or the full cohort (Figure 4). The E4 allele accounting for a large proportion of the 12-SNP wPRS and being present at a high frequency of 33.55% in the ADH/FCHL cohort compared to 14.25% in the 200,920 alleles of the GnomAD dataset. Thus, we calculated the 10-SNP wPRS but did not detect any correlation between the LDL-MoM values and the 10-SNP wPRS. These results suggested that in the ADH/FCHL cohort, the 12 genotyped alleles that increased LDL-C, and are incorporated into the wPRS, had no significant effect on the individual level of LDL-C. Elevated LDL-C may thus have been due to a major effect of the inherited pathogenic APOE variant or a variant in an unidentified gene linked to dyslipidemia.
Figure 4.
Correlation between the 12-SNP weighted polygenic risk score (wPRS) and the severity of the phenotype measured by the LDL-C. Multiple of median for LDL-C level (LDL-MoM). Non-parametric Spearman test.
2.4. Genotype–Phenotype Correlation
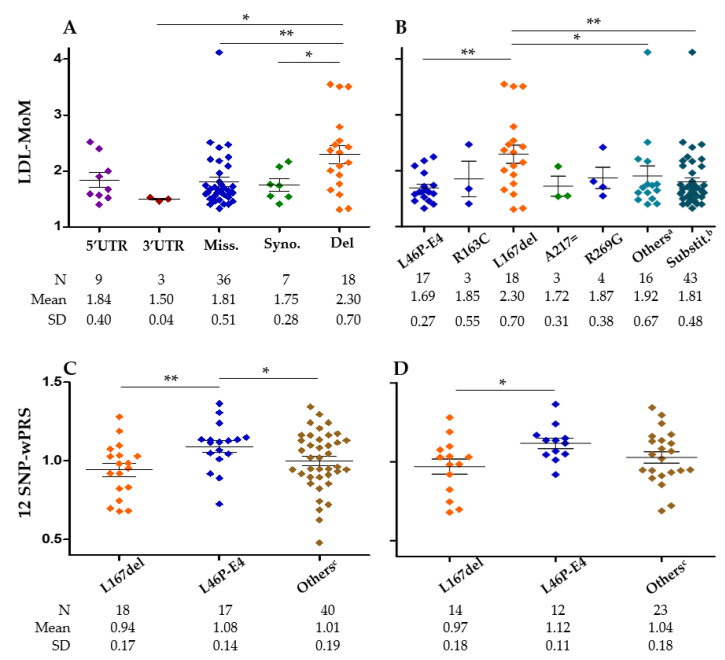
To our knowledge, no genotype–phenotype correlation has been reported among carriers of different causative variants within the APOE gene. The mean LDL-MoM was compared among different variants groups (Figure 5A,B). In the whole cohort, carriers of APOE p.Leu167del presented a significantly greater LDL-MoM than carriers of 3’UTR, missense, or synonymous variants (Figure 5A). This was also true when the p.Leu167del was compared to p.Leu46Pro/E4, other exonic variants or all the substitutions (Figure 5B). In the ADH cohort, carriers of p.Leu167del presented a significantly greater LDL-MoM than carriers of missense variants (p = 0.002), p.Leu46Pro/E4 (p = 0.008), p.Arg269Gly (p = 0.050), other exonic variants (p = 0.040), or all the substitutions (p = 0.002). No significant differences were observed in the FCHL cohort. Interestingly, the p.Leu46Pro/E4 carriers presented a significantly greater wPRS than p.Leu167del or all other variants combined in the whole cohort (Figure 5C). Nevertheless, no differences were observed between the three same variants groups with the 10-SNP wPRS that lacked the apoE isoform alleles: 0.99 ± 1.7, 0.91 ± 1.8, and 0.95 ± 2.0, respectively. This suggested that the E4 allele in linkage disequilibrium with the p.Leu46Pro variant supported the different 12-SNP wPRS values between the p.Leu46Pro/E4 carriers and the other variant carriers. The same observation was made in the ADH cohort (Figure 5D) but not in the FCHL cohort.
Figure 5.
Multiple of median for LDL-C (LDL-MoM) and weighted polygenic risk score (wPRS) among carriers of different variants. (A) LDL-MoM in carriers of 5’UTR, 3’UTR, missense, synonymous, and deletion variants for the whole cohort. (B) LDL-MoM in carriers of exonic variants for the whole cohort. a Other exonic variants than the five shown in the graph because with at least three carriers, b All exonic substitutions. (C) wPRS in carriers of p.Leu167del, p.Leu46Pro/E4, and other variants for the whole cohort. (D) wPRS in the carriers of p.Leu167del, p.Leu46Pro/E4, and other variants in the ADH cohort. c Variants other than p.Leu46Pro-E4 and p.Leu167del (exonic, intronic, 5’ and 3’ UTR). * p < 0.05, ** p < 0.005, non-parametric Mann–Whitney test.
The mean TG-MoM compared among the different molecular groups showed that p.Leu167del carriers presented a significantly lesser mean TG-MoM value than all the other APOE variant carriers in the whole cohort (1.48 ± 0.68 vs. 2.30 ± 1.83; p = 0.02). However, this was not the case in the ADH or FCHL cohort. Altogether, these results suggest that the p.Leu167del APOE variant was associated with a monogenic form of hypercholesterolemia, increased LDL-C levels, and reduced TG levels compared to other APOE variants.
2.5. Lipid-Lowering Treatment Response
LDL-C levels with and without statin treatment were available for 11 probands of the ADH/FCHL cohort (Table 3). The observed fold-reduction of LDL-C was significantly more than estimated for FH patients carrying ADH with a mutation within the LDLR gene (Table 3). Most of the variants were predicted LP, VUS/P, or VUS/LP but only the p.Arg185 silent variant was predicted to be LB. Thus, it was possible that the hypercholesterolemia of the carrier of the p.Arg185 silent variant was not due to this APOE rare variant. Nevertheless, with the 10 other APOE variants, the observed fold LDL-C reduction was significantly more than expected for FH patients (2.45 ± 0. 75 vs. 1.91 ± 0.29, p = 0.0426). Interestingly, the only patient not presenting the expected LDL-C reduction (1.2 vs. 2.2) was the only FCHL carrier of the p.Leu167del variant and the E3E4 apoE genotype.
Table 3.
LDL-C reduction under statins.
| APOE Variant | Pathogenic Prediction a | Gender | Age b | LDL-C without Preatment c | Treatment | Age d | LDL-C under Preatment c | Estimated Reduction e | Observed Reduction | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| rs776242156 | c.68C > T | p.Ala23Val | VUS/LP | M | 43 | 5.17 | Atorvastatin 20 | 46 | 1.42 | 1.8 | 3.6 |
| rs11542035 | c.410G > A | p.Arg137His | VUS/P | F | 61 | 6.45 | Simvastatin 20 | 62 | 3.06 | 1.6 | 2.1 |
| rs769455 | c.487C > T | p.Arg163Cys | VUS/P | M | 40 | 5.88 | Atorvastatin 80 Ezetimibe 10 | 41 | 1.69 | 2.5 | 3.5 |
| rs155726148 | c.500_502delTCC | p.Leu167del | LP | M | 69 | 7.24 | Atorvastatin 20 | 70 | 2.74 | 1.8 | 2.6 |
| rs155726148 | c.500_502delTCC | p.Leu167del | LP | F | 38 | 9.44 | Atorvastatin 80 | 56 | 3.59 | 2.2 | 2.6 |
| rs155726148 | c.500_502delTCC | p.Leu167del | LP | F | 31 | 6.18 | Atorvastatin 80 | 32 | 5.20 | 2.2 | 1.2 |
| rs155726148 | c.500_502delTCC | p.Leu167del | LP | M | 31 | 7.55 | Simvastatin 20 Ezetimibe 10 | 38 | 2.87 | 1.8 | 2.6 |
| rs155726148 | c.500_502delTTC | p.Leu167del | LP | M | 20 | 6.99 | Rosuvastatin 5 | 27 | 3.74 | 1.8 | 1.9 |
| rs781722239 | c.555C > T | p.Arg185= | LB | M | 65 | 7.81 | Atorvastatin 20 | 65 | 4.29 | 1.8 | 1.8 |
| rs267606661 | c.805C > G | p.Arg269Gly | VUS/P | M | 51 | 5.73 | Atorvastatin 20 | 58 | 3.18 | 1.8 | 1.8 |
| rs267606661 | c.805C > G | p.Arg269Gly | VUS/P | M | 59 | 6.33 | Atorvastatin 10 | 59 | 2.49 | 1.6 | 2.5 |
| Mean | 1.90 | 2.39 | |||||||||
| SD | 0.28 | 0.74 | |||||||||
| Wilcoxon matched-pairs test | p = 0.0426 | ||||||||||
a ACMG criteria from Varsome (Table 2); P: pathogenic; LP: likely pathogenic; VUS: variant of uncertain significance; LB: likely benign. b Age at lipid measurement without treatment. c mmol/L. d Age at lipid measurement under treatment. e Correction factors were obtained by the meta-analysis of 71 studies [34].
3. Discussion
In the French ADH/FCHL cohort studied here, 21 rare APOE variants in 49 ADH probands and 16 rare variants in 27 FCHL probands were identified, six of them being common to the two disease groups (Table 2, Figure 1). Sixteen of these rare APOE variants are very likely to be the major cause of the ADH/FCHL phenotype based on (1) their frequency in controls and the French ADH/FCHL cohort, (2) pathogenic prediction tools and three diagnostic lab classifications, and (3) assessment of their polygenic contribution. Although LDLR is still the main gene associated with primary hypercholesterolemia, our work shows that APOE contributes significantly, and it provides an updated full APOE molecular spectrum in a French ADH/FCHL cohort previously classified as mutation-negative.
In patients with ADH, triglyceride-increasing factors such as genetic and metabolic factors, diet, and APOE genotype could lead to the development of FCHL. Variants in the APOE gene may amplify the effect of these factors. Thus, according to the number or the nature of these factors, APOE variants could be associated with the overlapping phenotypes of FCHL, ADH, and sometimes familial dysbetalipoproteinemia when the subject is E2/E2 [31]. We, therefore, included subjects diagnosed with ADH and FCHL.
The most frequent variant in this cohort, p.Leu167del, is known as a causative mutation in ADH [9] and is the one associated with the more severe phenotype (Figure 5). The p.Leu167del variant is known to cause hypercholesterolemia in 3.1% of ADH subjects without LDL, APOB, and PCSK9 mutations in Spain [28] and a French patient among a cohort of 229 ADH subjects [10]. The hyperLDLemia observed in the French ADH family with p.Leu167del carriers was explained by an increased LDL pool, which was the consequence of an increase in VLDL production rate and a decrease in LDL catabolism [9]. Another study showed that VLDL carrying the p.Leu167del variant produces LDL receptor downregulation resulting in increased plasma LDL-C [28]. We find in this ADH/FCHL cohort that p.Leu167del carriers are characterized by significantly higher LDL levels compared to all other APOE variant carriers. This is mainly due to lower LDL levels of p.Leu46Pro/E4 carriers (Figure 5B).
The variant p.Leu46Pro is the second most frequent APOE variant in the cohort (Table 1). When associated with the E4 isoform (ApoE Freiburg), p.Leu46Pro affects the structure and stabilization of the apoE protein [32]. Since the homozygote carrier of the ApoE Freiburg did not present a phenotype more severe than heterozygote carriers, we suggest that the disease is dominant rather than semi-dominant. This is similar to the APOB p.Arg3527Gln mutation for which homozygotes are reported to have cholesterol concentrations in the range of heterozygotes carriers [33]. However, they are different from carriers of LDLR gene mutations which present a semi-dominant disease because each allele contributes to the phenotype (OMIM nos. 143890 and 606945). However, the p.Leu46Pro variant is predicted to be benign mostly due to its relatively high frequency, 0.77% in the European Finnish population (Table S1), whereas ApoE Freiburg (p.Leu46Pro/ApoE4) is atherogenic and significantly more common among CHD patients. ApoE Freiberg is reported to be likely pathogenic in ClinVar [24] and less frequent. Its greatest allele frequency is 0.15% in the European Finnish population (Table S1).
In addition to these well-characterized variants, we identified 16 exonic missense variants, among which p.Pro102Leu and p.Val213Glu were very rare. Although the substitution p.Pro102Leu is not reported in GnomAD, p.Pro102Arg at the same position is described in a subject with hypercholesterolemia in association with the ApoE4 isoform [35]. The p.Val213Glu carrier being homozygous for the hypocholesterolemic PCSK9 L10 polymorphism (Table 1) argues for the pathogenicity of APOE p.Val213Glu. The p.Gly145Asp variant is associated with dyslipidemia [10,36] and modifies ApoE towards a more negative isoelectric point that may alter its affinity for the receptor. Four of the exonic variants identified in the ADH/FCHL cohort affect positively charged arginine residues. The p.Arg137Cys and p.Arg137His variants localize within the receptor-binding domain of the protein, but additional studies are needed to characterize their effects on apoE function.
The known p.Arg163Cys variant [10] is predicted to be deleterious by all tools (Table 2) and is thus classified as a pathogenic variant in Lyon’s diagnostic lab as well as in one ClinVar report. However, this variant is very frequent at 2% in the African/African-American population. This is higher than its threshold filter allele frequency by “Popmax Filtering AF” [37] of 1.98% at 95% CI. The p.Arg163Cys variant is thus classified as a benign variant in Boulogne-Billancourt’s diagnostic lab (Table S1). However, this “Popmax Filtering AF” criteria does not always seem reliable. Indeed, the p.Pro685Leu FH-causing mutation in the LDLR gene is recognized as pathogenic, whereas a frequency of 0.072% in the African/African-American population is greater than its “Popmax Filtering AF” of 0.019% at 95% CI.
The p.Arg269Gly variant probably changes the properties of the C-terminal helical domain of apoE resulting in altered receptor interaction with lipoproteins [9]. The variation is predicted to be deleterious by all tools (Table 2) and classified as a VUS/pathogenic variant by Varsome despite its high frequency of 0.048% in the Non-Finnish European population of GnomAD (Table S1). The allele frequency observed in the ADH/FCHL cohort for c.-78C > G and p.Leu155Phe allows a change in the pathogenic prediction from VUS and VUS/P (Table 2) to VUS/LP and LP, respectively, as in Lyon’s diagnostic lab (Table S1). These classification differences illustrate the need for additional cohort analyses and functional studies, as highlighted by Chora et al. [38]. In addition, better clinical diagnoses as proposed by Masana et al. for ADH in Spain [39] will help to build a universal consensus.
Of the 10 variants in APOE non-coding regions only the variant c.44-1G > C is predicted as pathogenic through possible aberrant splicing of APOE mRNA. Its absence in control cohorts (Table 2) and the low wPRS observed for the carrier of this variant (Table 1) are further arguments for the pathogenicity of c.44-1G > C. The variant c.*25C > T is predicted to be located within a miRNA target. Variants in the 3’UTR of cholesterol homeostasis regulatory genes such as PCSK9 [40] are associated with modifications in cholesterol levels by miRNA regulation. However, additional studies are needed to explore if c.*25C > T affects APOE expression. Future functional studies in cell models expressing identified variants and RNA sequencing may be of great interest in evaluating the pathogenicity of each.
With the objective of evaluating the polygenic contribution in the ADH/FCHL cohort, we report that a greater proportion of ADH cases are polygenic among carriers of an APOE variant compared to ADH non-APOE-carriers (Figure 2). This result indicates that some APOE variants may not be the major cause of ADH. Furthermore, carriers of an APOE VUS or LB variant probably have polygenic ADH (Figure 3) and are probably not the major cause of ADH. In the APOE-ADH/FCHL cohort, the 12 common genotyped alleles that increase LDL-C in the weighted polygenic score (wPRS) have no significant effect on the individual level of LDL-C (Figure 4). This suggests a major effect due to the pathogenic APOE variant or a variant in another unidentified dyslipidemic gene.
Statins are the most used cholesterol-lowering drugs worldwide. In a small subgroup of 11 APOE-ADH/FCHL unrelated probands, including five p.Leu167del carriers, we report a significantly greater fold-reduction of LDL-C than estimated for FH patients who present ADH due to a mutation within the LDLR gene (Table 3). This improved response to statins is described in a cohort of 22 p.Leu167del Spanish carriers [12]. Our results argue for the screening of APOE variants in the dyslipidemia diagnosis, not only for the p.Leu167del but also for other rare variants throughout the APOE gene.
The main limitations of this study are the lack of functional validations and family studies to follow the segregation of the identified variants. In addition, statistical analyses are limited by the small sample size. Finally, a polygenic origin of the disease cannot be excluded in patients with a high wPRS.
4. Materials and Methods
4.1. Proband Inclusion
ADH and FCHL probands of European origin were recruited between 2012 and 2020 through the French National Research Network on Hypercholesterolemia which includes 38 clinicians from all over France. The ADH inclusion criterion was total and LDL-C values above the 90th percentile when compared to sex- and age-matched European populations [20,21]. This corresponded to a TC-MoM (see below) above 1.2 and a LDL-MoM (see below) above 1.3. The FCHL inclusion criteria were: total cholesterol and TG values above the 90th percentile when compared to sex- and age-matched European populations [41,42]. This corresponded to a TC-MoM (see below) above 1.2, and a TG-MoM (see below) above 2.0. For patients on regular treatment for whom pre-treatment values were not available, the untreated LDL-C value was estimated using the correction factors for statins ± ezetimibe medication given by a meta-analysis of 71 reports [34].
4.2. Molecular Analysis
DNA from peripheral blood leucocytes was amplified using the Multiplicom ADH MASTR assay v2.0 multiplexing kit (Agilent, Santa Clara, CA, USA) or libraries were prepared using Ampliseq, a SeqCapEZ Solution-Based Enrichment strategy (Roche NimbleGen Madison, WI, USA). Sequencing was performed on coding DNA sequences and flanking introns (exon padding +/−30 bp) of the LDLR, PCSK9, APOB, and APOE genes and SNPs included in the wPRS as described [43,44].
4.3. Variant Nomenclature
Variants were designated according to the Human Genome Variation Society recommendations (HGVS; https://www.hgvs.org/mutnomen, accessed on 6 November 2021). cDNA was numbered from +1 for A in the ATG translation initiation codon of the reference sequence (NM_000041.4). Amino acid residues were numbered from +1 for the initiating methionine of the protein sequence (NP_000032.1). Hence, 18 was added to the original numbering for ApoE corresponding to the 18 residues forming the signal peptide.
4.4. In Silico Variant Analyses
The causal effect of each variant was estimated with in silico prediction tools included in Alamut Visual version 2.15 (PolyPhen-2, SIFT, Mutation taster) (https://www.sophiagenetics.com/platform/alamut-visual-plus/, accessed on 6 November 2021) in addition to Provean (https://provean.jcvi.org, accessed on 6 November 2021) and CADD score (https://cadd.gs.washington.edu/snv, accessed on 6 November 2021). The potential effect of variants on splicing was assessed using Alamut Visual version 2.15 (MaxEntScan, NNSPLICE, GeneSplicer, ESE tools) and Human Splicing Finder (http://www.umd.be/hsf/, accessed on 6 November 2021). The frequency of variants in a control group representative of the French population was taken from the French Exome Project database (FREX; https://www.france-genomique.org/bases-de-donnees/frex-the-french-exome-project-database/, accessed on 6 November 2021). Variant frequencies in the general population were taken from the Genome Aggregation Database (gnomAD-v3.1.1; https://gnomad.broadinstitute.org/, accessed on 6 November 2021). ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/, accessed on 6 November 2021), the Leiden Open Variation Database (LOVD; https://www.lovd.nl/, accessed on 6 November 2021), and the Human Gene Mutation Database (HGMD; http://www.hgmd.cf.ac.uk/, accessed on 6 November 2021) were used to search for variants previously reported in human diseases. The MicroRNA Target Prediction Database was also used (miRDB; http://mirdb.org/, accessed on 6 November 2021).
4.5. Variant Classifications
Variants were classified according to the American College of Medical Genetics and the Association of Medical Pathologists (ACMG) guidelines [45] given by Varsome (https://varsome.com, accessed on 6 November 2021). This was applied to segregation and allelic in-house data of each diagnostic center (Lyon, Boulogne-Billancourt, Paris, France) and population allelic frequencies in GnomAD (http://gnomad.broadinstitute.org/, accessed on 6 November 2021).
4.6. Multiple of Median for Total Cholesterol, LDL-C, and Triglyceride Level Calculation
The multiple of median (MoM) for the total cholesterol (TC-MoM), LDL-C (LDL-MoM), and triglyceride (TG-MoM) values measured the deviation from the mean of a reference population of individual values. It allowed the comparison of lipid levels adjusted for age and gender using data from a French population of children [41] and a Dutch population of adults [42]. The MoMs are a ratio determined by the following: LDL-/TC-/TG-MoM = (LDL-C/TC/TG of the patient)/(LDL-C/TC/TG of the 50th percentile of his sex and age class)
4.7. Weighted Polygenic Risk Score (wPRS)
For each individual, the wPRS was calculated using the weighted sum of the risk allele for the 12 SNPs (alleles increasing LDL-C) and compared to those of 3020 normocholesterolemic men and women of European ancestry from the UK Whitehall II (WHII) cohort study [7]. The 10-SNP wPRS excluded the contribution of the ApoE isoform alleles: 12-SNP wPRS −0.2 for E4E4, −0.1 for E3E4, and +0.2 for E2E4.
4.8. Statistics
Statistical analyses were performed with JMP software (SAS Institute Inc., Cary, NC, USA) and GraphPad Prism® software. The non-parametric Mann–Whitney U test assessed differences between two groups. The non-parametric Kruskal–Wallis test assessed differences among more than two groups. The Spearman r test assessed the correlation between two variables. The non-parametric Wilcoxon matched-pairs test evaluated differences between the observed reduction of LDL-C levels after treatment and the expected reduction. p values ≤ 0.05 were considered statistically significant. Pairwise linkage disequilibria between the most frequent APOE variants having minor allele frequencies > 0.01% in the 76 index cases from the cohort were estimated by using Haploview 4.2 [46] and PLINK [47].
5. Conclusions
Through the sequencing of APOE in patients diagnosed with primary dyslipidemias without a mutation in the LDLR, APOB, or PCSK9 genes, we report a substantial number of rare variant carriers. However, the complex role of the ApoE in lipid homeostasis and the limited number of subjects make the interpretation of variant pathogenicity difficult. Although additional factors such as family segregation and functional studies may influence our interpretation, we conclude that screening of APOE should be included in routine diagnoses for ADH and FCHL to improve the prognosis and care management of patients and their families.
Acknowledgments
We thank all the French clinicians who provided biological and clinical data of probands: Sophie BELIARD (CHU Marseille), Caroline DOURMAP (CHU Pontchaillou, Rennes, France), Sophie GONBERT (Hôpitaux Universitaires Pitié Salpêtrière, Paris, France), Philippe MOULIN (Hospices Civils de Lyon), Matthieu WARGNY (CHU Nantes), Boris HANSEL (CHU Bichat, Paris, France), Dorota FERRIERES (CHU Toulouse), Francis DJIAN (Hôpitaux Universitaires Pitié Salpêtrière, Paris, France), Hélène DOLLFUS (CHU Strasbourg), Bertrand DUCORNET (CHU Ambroise Paré, Boulogne-Billancourt), Assie ESLAMI (Hôpital Européen Georges-Pompidou, Paris, France), François SCHIELE (CHU Besançon), Laure GROISNE (Hospices Civils de Lyon), Olivier HINSCHBERGER (CHU Sud Alsace, Mulhouse), Jean-Michel LECERF (Institut Pasteur de Lille), Rhyme JOUINI-BOUHAMRI (Hospices Civils de Lyon), Olga KALMYKOVA (Hôpitaux Universitaires Pitié Salpêtrière, Paris, France), Michel FARNIER (CHU Dijon), Sylvie MARSOT-FRELON (Hospices Civils de Lyon), Florian MORIN (Hôpital des Charpennes, Villeurbanne, France), Sophie NAMBOT (CHU Dijon), Noel PERETTI (Hospices Civils de Lyon), Linda PIVOIS (Clinique Saint Germain, Brive, France), Anne Laurence POUZOULET (Hôpitaux Universitaires Pitié Salpêtrière, Paris, France), Barbara ROHMER (ENDOC PED GHE), Laurène SCHOUMACKER (CHU Metz), Ariane SULTAN (CHU Lapeyronie, Montpellier), Vanina BONGARD (CHU Toulouse), and Frederic VILLENEUVE (Hôpitaux Universitaires Pitié Salpêtrière, Paris, France).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms23105792/s1.
Author Contributions
Funding acquisition: C.B., M.A. and M.V.; Conception of the work: M.D.-F., A.C., J.-P.R. and M.V.; Acquisition and analysis of data for Proband Recruitments: J.F., F.P., C.Y., V.C., S.C., E.B., A.G. and P.G.; Data collection and NGS: O.M., O.B., M.D.-F., A.C. and J.-P.R.; Data analysis: Y.A.K., A.P. and M.V.; Drafting the work: Y.A.K. and M.V.; Revising the work critically: All. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was performed in accordance with French bioethics regulations with adherence to the Declaration of Helsinki principles. The research project received IRB approval (research project trial no. 05-07-06 approved by the French Consultative Committee for the Protection of Person in Biomedical Research, Paris, Necker).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available on request to the corresponding author.
Conflicts of Interest
A.G. has received honoraria for public speaking or consultancy support from Akcea Therapeutics, AMGEN, Mylan, Novartis, Sanofi, Regeneron, Unilever, and MSD. C.B. and M.A.F. have received honoraria for consultancy support from AMGEN, Sanofi, and Regeneron. M.A.F. has received honoraria for consultancy support from Institut Mérieux. E.B. and M.V. has received honoraria for public speaking from Servier. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This work was supported by grants from the national project CHOPIN (CHolesterol Personalized Innovation) granted by the National Research Agency (ANR-16-RHUS-0007), INSERM (Institut National de la Santé et de la Recherche Médicale). YAK was supported by a grant from Ministère de l’Education Nationale et de la Technologie (France), a grant from Nouvelle Société Francophone de l’Athérosclérose (France), and grants from the Lebanese National Council for Scientific Research (CNRS-L) and the Council of Research of Saint-Joseph University of Beirut, Lebanon.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Do R., Stitziel N.O., Won H.-H., Jørgensen A.B., Duga S., Angelica Merlini P., Kiezun A., Farrall M., Goel A., Zuk O., et al. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature. 2015;518:102–106. doi: 10.1038/nature13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chemello K., García-Nafría J., Gallo A., Martín C., Lambert G., Blom D. Lipoprotein metabolism in familial hypercholesterolemia. J. Lipid Res. 2021;62:100062. doi: 10.1016/j.jlr.2021.100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berberich A.J., Hegele R.A. The complex molecular genetics of familial hypercholesterolaemia. Nat. Rev. Cardiol. 2019;16:9–20. doi: 10.1038/s41569-018-0052-6. [DOI] [PubMed] [Google Scholar]
- 4.Beheshti S.O., Madsen C.M., Varbo A., Nordestgaard B.G. Worldwide Prevalence of Familial Hypercholesterolemia: Meta-Analyses of 11 Million Subjects. J. Am. Coll. Cardiol. 2020;75:2553–2566. doi: 10.1016/j.jacc.2020.03.057. [DOI] [PubMed] [Google Scholar]
- 5.Defesche J.C., Gidding S.S., Harada-Shiba M., Hegele R.A., Santos R.D., Wierzbicki A.S. Familial hypercholesterolaemia. Nat. Rev. Dis. Primer. 2017;3:17093. doi: 10.1038/nrdp.2017.93. [DOI] [PubMed] [Google Scholar]
- 6.Rabès J.-P., Béliard S., Carrié A. Familial hypercholesterolemia: Experience from France. Curr. Opin. Lipidol. 2018;29:65–71. doi: 10.1097/MOL.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 7.Talmud P.J., Shah S., Whittall R., Futema M., Howard P., Cooper J.A., Harrison S.C., Li K., Drenos F., Karpe F., et al. Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolaemia: A case-control study. Lancet. 2013;381:1293–1301. doi: 10.1016/S0140-6736(12)62127-8. [DOI] [PubMed] [Google Scholar]
- 8.Varret M., Abifadel M., Rabès J.-P., Boileau C. Genetic heterogeneity of autosomal dominant hypercholesterolemia. Clin. Genet. 2008;73:1–13. doi: 10.1111/j.1399-0004.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- 9.Marduel M., Ouguerram K., Serre V., Bonnefont-Rousselot D., Marques-Pinheiro A., Erik Berge K., Devillers M., Luc G., Lecerf J.-M., Tosolini L., et al. Description of a large family with autosomal dominant hypercholesterolemia associated with the APOE p.Leu167del mutation. Hum. Mutat. 2013;34:83–87. doi: 10.1002/humu.22215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wintjens R., Bozon D., Belabbas K., MBou F., Girardet J.-P., Tounian P., Jolly M., Boccara F., Cohen A., Karsenty A., et al. Global molecular analysis and APOE mutations in a cohort of autosomal dominant hypercholesterolemia patients in France. J. Lipid Res. 2016;57:482–491. doi: 10.1194/jlr.P055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Versmissen J., Oosterveer D.M., Yazdanpanah M., Defesche J.C., Basart D.C.G., Liem A.H., Heeringa J., Witteman J.C., Lansberg P.J., Kastelein J.J.P., et al. Efficacy of statins in familial hypercholesterolaemia: A long term cohort study. BMJ. 2008;337:a2423. doi: 10.1136/bmj.a2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bea A.M., Lamiquiz-Moneo I., Marco-Benedí V., Mateo-Gallego R., Pérez-Calahorra S., Jarauta E., Martín C., Cenarro A., Civeira F. Lipid-lowering response in subjects with the p.(Leu167del) mutation in the APOE gene. Atherosclerosis. 2019;282:143–147. doi: 10.1016/j.atherosclerosis.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 13.Bello-Chavolla O.Y., Kuri-García A., Ríos-Ríos M., Vargas-Vázquez A., Cortés-Arroyo J.E., Tapia-González G., Cruz-Bautista I., Aguilar-Salinas C.A. Familial combined hyperlipidemia: Current knowledge, perspectives, and controversies. Rev. Investig. Clin. Organo Hosp. Enferm. Nutr. 2018;70:224–236. doi: 10.24875/RIC.18002575. [DOI] [PubMed] [Google Scholar]
- 14.Brouwers M.C.G.J., van Greevenbroek M.M.J., Stehouwer C.D.A., de Graaf J., Stalenhoef A.F.H. The genetics of familial combined hyperlipidaemia. Nat. Rev. Endocrinol. 2012;8:352–362. doi: 10.1038/nrendo.2012.15. [DOI] [PubMed] [Google Scholar]
- 15.Civeira F., Jarauta E., Cenarro A., García-Otín A.L., Tejedor D., Zambón D., Mallen M., Ros E., Pocoví M. Frequency of low-density lipoprotein receptor gene mutations in patients with a clinical diagnosis of familial combined hyperlipidemia in a clinical setting. J. Am. Coll. Cardiol. 2008;52:1546–1553. doi: 10.1016/j.jacc.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 16.Solanas-Barca M., de Castro-Orós I., Mateo-Gallego R., Cofán M., Plana N., Puzo J., Burillo E., Martín-Fuentes P., Ros E., Masana L., et al. Apolipoprotein E gene mutations in subjects with mixed hyperlipidemia and a clinical diagnosis of familial combined hyperlipidemia. Atherosclerosis. 2012;222:449–455. doi: 10.1016/j.atherosclerosis.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Marais A.D. Apolipoprotein E in lipoprotein metabolism, health and cardiovascular disease. Pathology. 2019;51:165–176. doi: 10.1016/j.pathol.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y., Mahley R.W. Apolipoprotein E: Structure and function in lipid metabolism, neurobiology, and Alzheimer’s diseases. Neurobiol. Dis. 2014;72:3–12. doi: 10.1016/j.nbd.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breslow J.L., Zannis V.I., SanGiacomo T.R., Third J.L., Tracy T., Glueck C.J. Studies of familial type III hyperlipoproteinemia using as a genetic marker the apoE phenotype E2/2. J. Lipid Res. 1982;23:1224–1235. doi: 10.1016/S0022-2275(20)38060-3. [DOI] [PubMed] [Google Scholar]
- 20.Bennet A.M., Di Angelantonio E., Ye Z., Wensley F., Dahlin A., Ahlbom A., Keavney B., Collins R., Wiman B., de Faire U., et al. Association of apolipoprotein E genotypes with lipid levels and coronary risk. JAMA. 2007;298:1300–1311. doi: 10.1001/jama.298.11.1300. [DOI] [PubMed] [Google Scholar]
- 21.Teslovich T.M., Musunuru K., Smith A.V., Edmondson A.C., Stylianou I.M., Koseki M., Pirruccello J.P., Ripatti S., Chasman D.I., Willer C.J., et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahlab-Aviv S., Zohar K., Cohen Y., Peretz A.R., Eliyahu T., Linial M., Sperling R. Spliceosome-Associated microRNAs Signify Breast Cancer Cells and Portray Potential Novel Nuclear Targets. Int. J. Mol. Sci. 2020;21:8132. doi: 10.3390/ijms21218132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen K.L., Tybjaerg-Hansen A., Nordestgaard B.G., Frikke-Schmidt R. APOE and dementia—Resequencing and genotyping in 105,597 individuals. Alzheimers Dement. J. Alzheimers Assoc. 2020;16:1624–1637. doi: 10.1002/alz.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orth M., Weng W., Funke H., Steinmetz A., Assmann G., Nauck M., Dierkes J., Ambrosch A., Weisgraber K.H., Mahley R.W., et al. Effects of a frequent apolipoprotein E isoform, ApoE4Freiburg (Leu28-- > Pro), on lipoproteins and the prevalence of coronary artery disease in whites. Arterioscler. Thromb. Vasc. Biol. 1999;19:1306–1315. doi: 10.1161/01.ATV.19.5.1306. [DOI] [PubMed] [Google Scholar]
- 25.Biesecker L.G. Correspondence on: Homozygous familial hypercholesterolemia in Italy: Clinical and molecular features. Atherosclerosis. 2021;326:63–64. doi: 10.1016/j.atherosclerosis.2021.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Yue P., Averna M., Lin X., Schonfeld G. The c.43_44insCTG variation in PCSK9 is associated with low plasma LDL-cholesterol in a Caucasian population. Hum. Mutat. 2006;27:460–466. doi: 10.1002/humu.20316. [DOI] [PubMed] [Google Scholar]
- 27.Richard P., Beucler I., Pascual De Zulueta M., Biteau N., De Gennes J.L., Iron A. Compound heterozygote for both rare apolipoprotein E1 (Gly127-- > Asp, Arg158-- > Cys) and E3(Cys112-- > Arg, Arg251-- > Gly) alleles in a multigeneration pedigree with hyperlipoproteinaemia. Clin. Sci. Lond. Engl. 1979. 1997;93:89–95. doi: 10.1042/cs0930089. [DOI] [PubMed] [Google Scholar]
- 28.Cenarro A., Etxebarria A., de Castro-Orós I., Stef M., Bea A.M., Palacios L., Mateo-Gallego R., Benito-Vicente A., Ostolaza H., Tejedor T., et al. The p.Leu167del Mutation in APOE Gene Causes Autosomal Dominant Hypercholesterolemia by Down-regulation of LDL Receptor Expression in Hepatocytes. J. Clin. Endocrinol. Metab. 2016;101:2113–2121. doi: 10.1210/jc.2015-3874. [DOI] [PubMed] [Google Scholar]
- 29.Faivre L., Saugier-Veber P., Pais de Barros J.-P., Verges B., Couret B., Lorcerie B., Thauvin C., Charbonnier F., Huet F., Gambert P., et al. Variable expressivity of the clinical and biochemical phenotype associated with the apolipoprotein E p.Leu149del mutation. Eur. J. Hum. Genet. 2005;13:1186–1191. doi: 10.1038/sj.ejhg.5201480. [DOI] [PubMed] [Google Scholar]
- 30.Awan Z., Choi H.Y., Stitziel N., Ruel I., Bamimore M.A., Husa R., Gagnon M.-H., Wang R.-H.L., Peloso G.M., Hegele R.A., et al. APOE p.Leu167del mutation in familial hypercholesterolemia. Atherosclerosis. 2013;231:218–222. doi: 10.1016/j.atherosclerosis.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Abou Khalil Y., Rabès J.-P., Boileau C., Varret M. APOE gene variants in primary dyslipidemia. Atherosclerosis. 2021;328:11–22. doi: 10.1016/j.atherosclerosis.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Argyri L., Dafnis I., Theodossiou T.A., Gantz D., Stratikos E., Chroni A. Molecular basis for increased risk for late-onset Alzheimer disease due to the naturally occurring L28P mutation in apolipoprotein E4. J. Biol. Chem. 2014;289:12931–12945. doi: 10.1074/jbc.M113.538124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen L.H., Miserez A.R., Ahmad Z., Andersen R.L. Familial defective apolipoprotein B-100: A review. J. Clin. Lipidol. 2016;10:1297–1302. doi: 10.1016/j.jacl.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Haralambos K., Whatley S.D., Edwards R., Gingell R., Townsend D., Ashfield-Watt P., Lansberg P., Datta D.B.N., McDowell I.F.W. Clinical experience of scoring criteria for Familial Hypercholesterolaemia (FH) genetic testing in Wales. Atherosclerosis. 2015;240:190–196. doi: 10.1016/j.atherosclerosis.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Wardell M.R., Rall S.C., Schaefer E.J., Kane J.P., Weisgraber K.H. Two apolipoprotein E5 variants illustrate the importance of the position of additional positive charge on receptor-binding activity. J. Lipid Res. 1991;32:521–528. doi: 10.1016/S0022-2275(20)42076-0. [DOI] [PubMed] [Google Scholar]
- 36.Weisgraber K.H., Rall S.C., Innerarity T.L., Mahley R.W., Kuusi T., Ehnholm C. A novel electrophoretic variant of human apolipoprotein E. Identification and characterization of apolipoprotein E1. J. Clin. Investig. 1984;73:1024–1033. doi: 10.1172/JCI111287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whiffin N., Minikel E., Walsh R., O’Donnell-Luria A.H., Karczewski K., Ing A.Y., Barton P.J.R., Funke B., Cook S.A., MacArthur D., et al. Using high-resolution variant frequencies to empower clinical genome interpretation. Genet. Med. Off. J. Am. Coll. Med. Genet. 2017;19:1151–1158. doi: 10.1038/gim.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chora J.R., Medeiros A.M., Alves A.C., Bourbon M. Analysis of publicly available LDLR, APOB, and PCSK9 variants associated with familial hypercholesterolemia: Application of ACMG guidelines and implications for familial hypercholesterolemia diagnosis. Genet. Med. Off. J. Am. Coll. Med. Genet. 2018;20:591–598. doi: 10.1038/gim.2017.151. [DOI] [PubMed] [Google Scholar]
- 39.Masana L., Ibarretxe D., Rodríguez-Borjabad C., Plana N., Valdivielso P., Pedro-Botet J., Civeira F., López-Miranda J., Guijarro C., Mostaza J., et al. Toward a new clinical classification of patients with familial hypercholesterolemia: One perspective from Spain. Atherosclerosis. 2019;287:89–92. doi: 10.1016/j.atherosclerosis.2019.06.905. [DOI] [PubMed] [Google Scholar]
- 40.Decourt C., Janin A., Moindrot M., Chatron N., Nony S., Muntaner M., Dumont S., Divry E., Dauchet L., Meirhaeghe A., et al. PCSK9 post-transcriptional regulation: Role of a 3’UTR microRNA-binding site variant in linkage disequilibrium with c.1420G. Atherosclerosis. 2020;314:63–70. doi: 10.1016/j.atherosclerosis.2020.10.010. [DOI] [PubMed] [Google Scholar]
- 41.Mellerio H., Alberti C., Druet C., Capelier F., Mercat I., Josserand E., Vol S., Tichet J., Lévy-Marchal C. Novel modeling of reference values of cardiovascular risk factors in children aged 7 to 20 years. Pediatrics. 2012;129:e1020–e1029. doi: 10.1542/peds.2011-0449. [DOI] [PubMed] [Google Scholar]
- 42.Balder J.W., de Vries J.K., Nolte I.M., Lansberg P.J., Kuivenhoven J.A., Kamphuisen P.W. Lipid and lipoprotein reference values from 133,450 Dutch Lifelines participants: Age- and gender-specific baseline lipid values and percentiles. J. Clin. Lipidol. 2017;11:1055–1064. doi: 10.1016/j.jacl.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Marmontel O., Charrière S., Simonet T., Bonnet V., Dumont S., Mahl M., Jacobs C., Nony S., Chabane K., Bozon D., et al. Single, short in-del, and copy number variations detection in monogenic dyslipidemia using a next-generation sequencing strategy. Clin. Genet. 2018;94:132–140. doi: 10.1111/cge.13250. [DOI] [PubMed] [Google Scholar]
- 44.Marmontel O., Rollat-Farnier P.A., Wozny A.-S., Charrière S., Vanhoye X., Simonet T., Chatron N., Collin-Chavagnac D., Nony S., Dumont S., et al. Development of a new expanded next-generation sequencing panel for genetic diseases involved in dyslipidemia. Clin. Genet. 2020;98:589–594. doi: 10.1111/cge.13832. [DOI] [PubMed] [Google Scholar]
- 45.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–423. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrett J.C., Fry B., Maller J., Daly M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinforma. Oxf. Engl. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 47.Purcell S., Neale B., Todd-Brown K., Thomas L., Ferreira M.A.R., Bender D., Maller J., Sklar P., de Bakker P.I.W., Daly M.J., et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request to the corresponding author.